
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 05 September 2023
Sec. Pharmacology of Anti-Cancer Drugs
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1234342
This article is part of the Research TopicNovel Therapeutic Approaches for Biliary Tract Cancer and Hepatocellular CarcinomaView all 11 articles
 Zhenyun Yang1,2†
Zhenyun Yang1,2† Yizhen Fu1,2†
Yizhen Fu1,2† Weijie Wu1,2†
Weijie Wu1,2† Zili Hu1,2
Zili Hu1,2 Yangxun Pan1,2
Yangxun Pan1,2 Juncheng Wang1,2
Juncheng Wang1,2 Jinbin Chen1,2
Jinbin Chen1,2 Dandan Hu1,2
Dandan Hu1,2 Zhongguo Zhou1,2
Zhongguo Zhou1,2 Minshan Chen1,2*
Minshan Chen1,2* Yaojun Zhang1,2*
Yaojun Zhang1,2*Background: Systemic chemotherapy (SC) remains the only first-line treatment for unresectable intrahepatic cholangiocarcinoma (iCCA). Hepatic arterial infusion chemotherapy (HAIC) has been recently proven to be effective in managing hepatocellular carcinoma (HCC). Hence, our study aims to investigate the safety and efficacy of HAIC in treating unresectable iCCA patients.
Methods: We reviewed 146 patients with unresectable iCCA who had received HAIC or SC between March 2016 and March 2022 in a retrospective manner. Outcomes of patients and safety were compared between the HAIC and SC groups.
Results: There were 75 and 71 patients in the HAIC and SC groups, respectively. The median OS in the HAIC and SC groups was 18.0 and 17.8 months (p = 0.84), respectively. The median PFS in the HAIC and SC groups was 10.8 and 11.4 months (p = 0.59), respectively. However, the HAIC group had significantly longer intrahepatic progression-free survival (IPFS) than the SC group (p = 0.035). The median IPFS in the HAIC and SC groups was 13.7 and 11.4 months, respectively. According to the OS (p = 0.047) and PFS (p = 0.009), single-tumor patients in the HAIC group appeared to benefit more. In addition, the overall incidence of adverse events (AEs) was lower in the HAIC group than that in the SC group.
Conclusion: Our study revealed that HAIC was a safe and effective therapeutic regimen for unresectable iCCA with better intrahepatic tumor control when compared to SC. Meanwhile, patients with single tumor were more likely to benefit from HAIC than SC.
Intrahepatic cholangiocarcinoma (iCCA) is the second most frequent primary liver cancer with a poor prognosis and high level of malignancy (Bridgewater et al., 2014; Sirica et al., 2019; Valle et al., 2021). The incidence of iCCA is higher in Thailand and China (6 per 100,000 people) than that in Western Europe and North America (0·35 to 2 per 100,000 people) (Banales et al., 2016; Oh et al., 2022). Over the next 20–30 years, the incidence of iCCA will increase ten-fold worldwide (Rodriguez and Pennington, 2018; Dong et al., 2022). Surgical resection is currently the first-line and curative therapy for iCCA management. However, most iCCA patients are diagnosed at a late stage as a result of the absence of specific clinical symptoms and limited treatment modalities for iCCA (Rizvi and Gores, 2013; Bupathi et al., 2017; Rizvi et al., 2018).
Currently, the first-line systemic chemotherapy (SC) for biliary tract cancer is gemcitabine plus cisplatin (GEMCIS), with a median overall survival (OS) of 11.7 months (Valle et al., 2010). Oxaliplatin plus gemcitabine (GEMOX) is also a common treatment regimen for biliary tract cancer patients in Asia, with a similar median OS compared to GEMCIS (Fiteni et al., 2014; Kim et al., 2019). The FOLFOX regimen may be an option for the palliative treatment of advanced cholangiocarcinoma (Nehls et al., 2002; Caparica et al., 2019; Lamarca et al., 2021).
Hepatic arterial infusion chemotherapy (HAIC) enables the delivery of chemotherapy drugs directly into the liver. Tumors derive most of their nutrients from the arteries, whereas the liver derives nutrients from the portal vein, which may reduce systemic adverse events (AEs) from systemic chemotherapy (Kemeny et al., 1984; Cercek et al., 2020). Meanwhile, previous studies have clarified that HAIC is useful for advanced iCCA and has shown higher tumor control rates compared to systemic chemotherapy (Kasai et al., 2014; Cercek et al., 2020). However, there was no study comparing HAIC with FOLFOX and first-line systemic chemotherapy in relation to patients’ outcomes and AEs.
Herein, the current study compares the clinical outcomes and tumor response of patients with unresectable iCCA treated with HAIC and SC. In addition, the assessment of safety and AEs were also vital in this retrospective study.
This is a retrospective study, and the study subjects consisted of 146 patients diagnosed with iCCA who were initially treated with HAIC or first-line SC between March 2016 and March 2022 at Sun Yat-sen University Cancer Center, China. Participants were included if they conformed to the following criteria: (Bridgewater et al., 2014) age 18 years old or elder; (Sirica et al., 2019) histopathological evidence confirmation of iCCA; (Valle et al., 2021) confirmed records of primary HAIC or first-line SC; (Oh et al., 2022) an Eastern Cooperative Oncology Group (ECOG) score of 2 or below; and (Banales et al., 2016) complete medical follow-up data. Patients were excluded based on the following exclusion criteria: (Bridgewater et al., 2014) patients with any other malignant tumor and (Sirica et al., 2019) patients who had contraindications to HAIC and SC.
HAIC was performed according to our previously reported protocol (Li et al., 2022). Femoral artery puncture and catheterization were performed on day 1 of the HAIC cycle, and the patient was transferred to the inpatient ward for drug infusion through the hepatic artery. Oxaliplatin was administered at 130 mg/m2 from 0 to 2 h on day 1; leucovorin was administered at 400 mg/m2 from 2 to 3 h on day 1; fluorouracil was administered at 400 mg/m2 from hour 3 on day 1. Infusional fluorouracil was given at 2400 mg/m2 over 23 h or 46 h. HAIC cycles were performed every 3 weeks. In the GEMCIS group, each cycle comprised cisplatin (25 mg per square meter of body-surface area), followed by gemcitabine (1,000 mg per square meter), which was administered on days 1 and 8 every 3 weeks. In the GEMOX group, each cycle comprised oxaliplatin (85 mg/m2) on day 1 and gemcitabine (1,000 mg per square meter) between days 1 and 8 every 3 weeks. HAIC or SC was suspended at 24 weeks or because of disease progression, unacceptable toxic effects, or patient’s own choice. As a part of treatment, HAIC or SC may be combined with the PD-1 inhibitor or tyrosine kinase inhibitor according to the needs of the condition and patient’s own choice.
All clinical data were obtained from the medical records of the Sun Yat-sen University Cancer Center. Demographic and clinical characteristics included age, sex, hepatitis infection status, ECOG, aspartate aminotransferase (AST), alanine transaminase (ALT), albumin (ALB), total bilirubin (TBIL), carcinoembryonic antigen (CEA), carbohydrate antigen 19–9 (CA19–9), white blood cell count (WBC), platelet count (PLT), creatinine (CRE), largest tumor size, tumor number, macroscopic vascular invasion, lymph node metastasis, extra-hepatic metastasis, and tumor–node–metastasis (TNM) stages. A summary of demographic and clinical characteristics is presented in Table 1. The blood tests and tumor burdens were measured within 5 days before the treatment. After treatment had been initiated, the radiological response was evaluated by magnetic resonance imaging (MRI) or computed tomography (CT) performed at baseline and every 6 weeks. Response Evaluation Criteria in Solid Tumors (RECIST)1.1 and modified RECIST (mRECIST) were used for evaluating the tumor response (Eisenhauer et al., 2009; Llovet and Lencioni, 2020).
Overall survival (OS) was defined as the time interval from first-line treatment to cancer-related death. Progression-free survival (PFS) was defined as the interval from first-line treatment to disease progression, iCCA relapse, or the date of death from iCCA or the date of the last follow-up. Intrahepatic progression-free survival (IPFS) was defined as the interval from the first-line treatment to intrahepatic tumor progression, iCCA relapse, or the date of death from iCCA or the date of the last follow-up, regardless of extrahepatic metastasis.
Non-normally distributed data were expressed as medians and ranges. Continuous parametric variables were analyzed by the unpaired Student’s t-test, and continuous non-parametric variables were analyzed by the Mann–Whitney U test. Categorical data were analyzed by Pearson’s correlation coefficient, chi-squared test with continuity corrections, or Fisher’s exact probability method. Forward LR-based univariate and multivariate Cox regression analyses were conducted to identify independent predictive variables. The OS and PFS were shown by Kaplan–Meier curves, and differences between the groups were compared using the results of the log-rank test. The p-value <0.05 was considered statistically significant. All the analyses were performed using SPSS 25.0 software (SPSS Inc., Chicago, IL) and R version 4.0.1.
Between March 2016 and March 2022, 146 patients diagnosed with iCCA who initially received HAIC or first-line SC were selected at Sun Yat-sen University Cancer Center, China. There were 75 patients in the HAIC group and 71 patients in the SC group (Figure 1). Detailed characteristics of each group are shown in Table 1. No significant baseline differences existed between the HAIC and SC groups.

FIGURE 1. Flowchart for patient inclusion. Abbreviations: iCCA, intrahepatic cholangiocarcinoma; HAIC, hepatic arterial infusion chemotherapy; SC, systemic chemotherapy.
In the HAIC group, the median age was 54 years old, 52 patients were male subjects, the largest tumor size of 25 (33.3%) patients was longer than 10 cm, a majority of patients had multiple tumors (66.7%), a total of 23 (30.7%) patients had macrovascular invasion, 51 (68%) patients had lymph node metastasis, and 17 (22.7%) patients had extra-hepatic metastasis. In the SC group, the median age was 57 years old, and 40 patients were male subjects, the largest tumor size of 14 (19.7%) patients was longer than 10 cm, a majority of patients had multiple tumors (69%), a total of 18 (25.4%) patients had macrovascular invasion, 47 (66.2%) patients had lymph node metastasis, and 24 (33.8%) patients had extra-hepatic metastasis. According to characteristics of a tumor, most patients in this study had large tumor burden and advanced iCCA.
Prognostic factors of all clinical variables were analyzed in univariate analysis. Univariate analyses showed that ECOG, tumor number, extra-hepatic metastasis, and TNM stages were significant risk factors for patients’ OS. Univariate analysis for PFS showed that ECOG, CA19–9, and extra-hepatic metastasis were significant risk factors. More details are described in Table 2. The multivariate Cox proportional analysis revealed that ECOG (p < 0.001) and extra-hepatic metastasis (p = 0.026) were significant and independent prognostic factors of OS (Table 2). The multivariate Cox proportional analysis revealed that ECOG (p < 0.001), CA19–9 (p = 0.02), macrovascular invasion (p = 0.02), and extra-hepatic metastasis (p = 0.001) were significant and independent prognostic factors of PFS (Table 2).

TABLE 2. Univariate and multivariate Cox regression analyses of risk factors for overall survival and progression-free survival.
The median OS in the HAIC and SC groups was 18.0 and 17.8 months, respectively. Meanwhile, the median PFS times in the HAIC and SC groups were 10.8 and 11.4 months, respectively. There was no significant difference between the two groups in OS (p = 0.84; Figure 2A) and PFS (p = 0.59; Figure 2B). However, patients in the HAIC group had significantly longer IPFS than patients in the SC group (p = 0.035; Figure 2C). The median IPFS in the HAIC and SC groups was 13.7 and 11.4 months, respectively. The median follow-up in the HAIC and SC group was 16.8 and 17.7 months, respectively (Supplementary Figure S1). Patients in the SC group were divided into two subgroups (GEMCIS and GEMOX). GEMCIS and GEMOX were compared with HAIC in OS and PFS (Supplementary Figure S2).
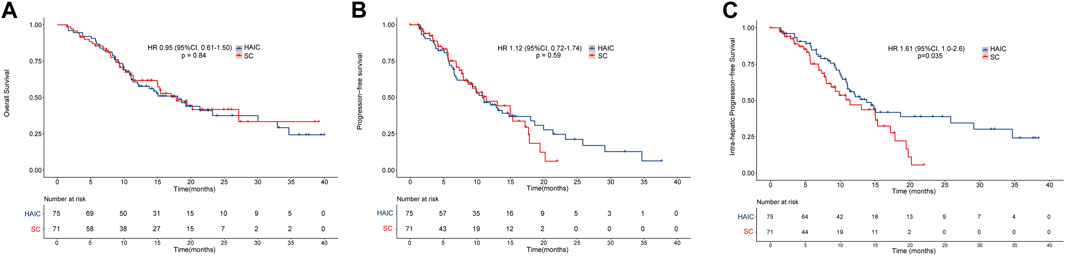
FIGURE 2. Overall survival and progression-free survival of the two groups of patients. Kaplan–Meier curves of (A) overall survival, (B) progression-free survival, and (C) intrahepatic progression-free survival for patients in the HAIC and SC groups.
The subgroup analyses of OS and PFS are shown in Figure 3. HAIC provided a clinical benefit for OS and PFS in tumor number subgroups. Single-tumor patients appeared to benefit more from it in terms of OS (p = 0.047; Supplementary Figure S3A) and PFS (p = 0.009; Supplementary Figure S3B). The intrahepatic tumor responses of the patients are shown in Table 3. On the basis of RECIST1.1 and mRECIST criteria, HAIC showed an ORR two times higher than SC (40% vs. 16.9%, p = 0.002, RECIST1.1; 45.3% vs. 21.2%, p = 0.002, mRECIST). The optimal response for intrahepatic target lesions by patients according to RECIST1.1 criteria is shown in the waterfall plot in Figure 4.
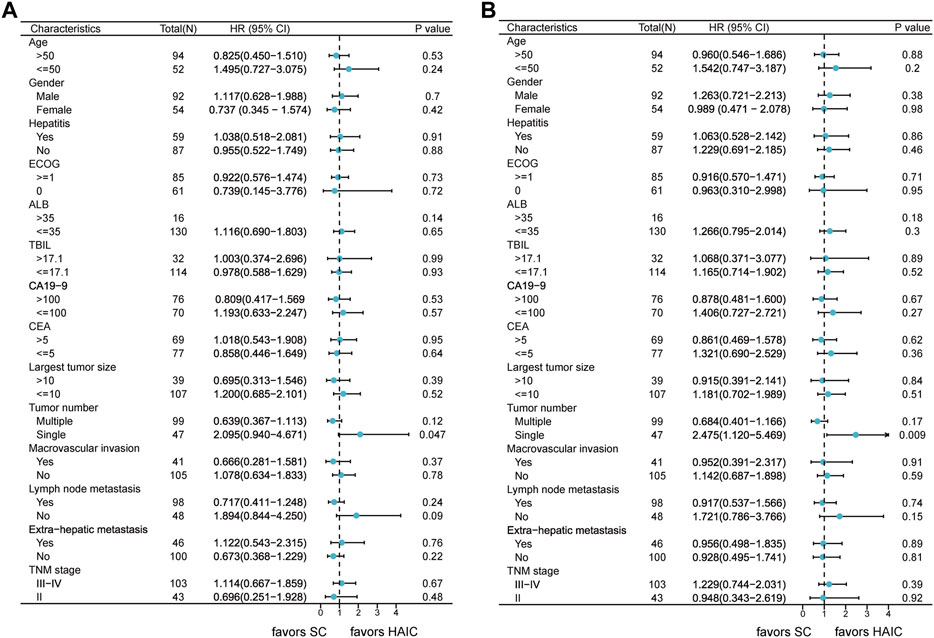
FIGURE 3. Forest plots of (A) overall survival and (B) progression-free survival in different patient subgroups. Abbreviations: HR, hazard ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; ALB, albumin; TBIL, total bilirubin; CA19–9, carbohydrate antigen 19–9; CEA, carcinoembryonic antigen; TNM, tumor–node–metastasis.
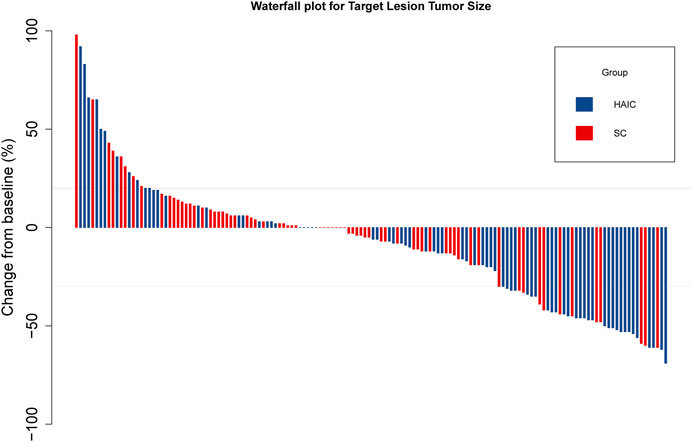
FIGURE 4. Waterfall plot for tumor size changes in intrahepatic target lesions. Abbreviations: PD, progressive disease; PR, partial response.
In general, the SC resulted in more AEs than those in HAIC (Table 4). The frequencies of rash (3 [4%] vs. 20 [28.2%]; p < 0.001), vomiting (27 [36%] vs. 51 [71.8%]; p < 0.001), fatigue (19 [25.3%] vs. 35 [49.3%]; p < 0.001), leukopenia (9 [12%] vs. 20 [28.2%]; p = 0.014), anemia (13 [17.3%] vs. 33 [46.5%]; p < 0.001), and sensory neuropathy (9 [12%] vs. 18 [25.4%]; p = 0.038) were lower in the HAIC group. Meanwhile, the overall incidence of serious AEs was higher in the SC group than that in the HAIC group. The frequencies of grades 3–4 vomiting (1 [1.3%] vs. 8 [11.2%]; p = 0.032), leukopenia (0 [0] vs. 5 [7%]; p = 0.025), and anemia (0 [0] vs. 6 [8.5%]; p = 0.012) were significantly higher in the SC group than those in the HAIC group. There were no significant differences in the frequencies of fever (15 [20%] vs. 10 [14.1%]; p = 0.343), abdominal pain (19 [25.3%] vs. 13 [18.3%]; p = 0.305), diarrhea (2 [2.7%] vs. 2 [2.8%]; p = 1.000), neutropenia (6 [8%] vs. 9 [12.7%]; p = 0.352), thrombocytopenia (8 [10.7%] vs. 16 [22.5%]; p = 0.053), elevated ALT (20 [26.7%] vs. 16 [22.5%]; p = 0.563), elevated AST (30 [40%] vs. 24 [33.8%]; p = 0.438), hyperbilirubinemia (12 [16%] vs. 10 [14.1%]; p = 0.746), hypoalbuminemia (37 [49.3%] vs. 34 [47.9%]; p = 0.861), and elevated creatinine (8 [10.7%] vs. 6 [8.5%]; p = 0.649). In the HAIC group, three (4%) patients delayed and discontinued treatment because of AEs. In the SC group, seven (9.86%) patients delayed and discontinued the treatment because of AEs.
It is widely acknowledged that iCCA is a gastrointestinal adenocarcinoma with a high level of malignancy and poor prognosis. In addition, most of the patients with iCCA cannot receive surgery because of advanced disease in iCCA, and these patients with unresectable iCCA undergo chemotherapy to control tumor development. Over the past years, GEMCIS and GEMOX have become the standard first-line chemotherapy regimen (Okusaka et al., 2010; Valle et al., 2010; Fiteni et al., 2014; Grenader et al., 2015). However, the occurrence of AEs is an urgent problem to be solved for SC. There is also an urgent need to find a regimen to reduce the occurrence of AEs while achieving similar survival benefits. Localized arterial treatment such as HAIC, TACE, and transarterial radioembolization (TARE) might be important treatment options for advanced cholangiocarcinoma (Mosconi et al., 2021; Ishii et al., 2022; Schaarschmidt et al., 2023). A previous study clarified that patients receiving TARE as first-line therapy had a 68.6% disease control rate and a median OS of 12 months (Schaarschmidt et al., 2023). In addition, a systemic review and meta-analysis demonstrated that the median OS after TACE was 14.2 months, while after TARE, it was 13.5 months for advanced iCCA (Mosconi et al., 2021). Meanwhile, few previous studies indicated that HAIC combined with systemic gemcitabine (GEM) and oxaliplatin may be an effective therapy for patients with advanced iCCA (Marumoto et al., 2014; Cercek et al., 2020). A retrospective study indicated the mFOLFOX regimen used in HAIC could be a new option for patients with iCCA (Cai et al., 2021). Some prospective studies demonstrated that HAIC with mFOLFOX had relatively low toxicity for hepatocellular carcinoma (HCC) (He et al., 2019; Li et al., 2022; Lyu et al., 2022; Li et al., 2023). Although these studies focused on HCC patients, the safety of HAIC with mFOLFOX was still of clinical significance for patients with iCCA, and HAIC with FOLFOX might be a feasible and promising regimen for treating iCCA patients.
In the current study of 146 patients, we compared HAIC with the first-line SC (GEMCIS and GEMOX) and found that patients in the HAIC group had significantly longer IPFS than patients in the SC group and that HAIC showed an ORR higher than SC. In subgroup analyses, single-tumor patients appeared to benefit from considering HAIC in terms of OS and PFS, indicating that HAIC might have a better efficacy than SC in relatively early-stage unresectable iCCA patients and that HAIC could control liver lesions better than SC. One potential explanation for this is that HAIC can provide higher concentrations of the chemotherapeutic agents in the liver than SC, therefore contributing to control tumor in the liver. As is known to all, the liver possesses a dual blood supply. In detail, the hepatic artery provides nearly all of the tumor’s blood flow, and the portal vein supplies blood to the non-neoplastic liver parenchyma. HAIC could preferentially deliver more chemotherapeutic agents to the hepatic artery, which contributes to controlling tumors in the liver.
We also found that patients with unresectable iCCA had similar OS and PFS after HAIC or SC treatment, suggesting that HAIC had a similar clinical efficiency to SC in the outcomes of patients. Although HAIC could better control intrahepatic tumors compared to SC, there were no significant differences in the outcome of patients. It could be explained by the fact that in this study, most patients were at the advanced stage and had extrahepatic metastases. The progression of extrahepatic lesions resulted in the death of patients, and HAIC had a poor control effect on extrahepatic lesions. Therefore, it would be an excellent clinical treatment strategy to add immune therapy and targeted therapy or SC on the basis of HAIC for those patients with extrahepatic metastasis.
Safety and the incidence of AEs are also important indicators for evaluating the chemotherapy regimen apart from the therapeutic effect. The common objective treatment-related AEs observed in this study were rash, fever, abdominal pain, vomiting, fatigue, diarrhea, leukopenia, neutropenia, anemia, thrombocytopenia, elevated ALT, elevated AST, hyperbilirubinemia, hypoalbuminemia, elevated creatinine, and sensory neuropathy. In general, the ratio of AEs in the HAIC group was lower than that in the SC group. The frequencies of rash, vomiting, fatigue, leukopenia, anemia, and sensory neuropathy were also lower in the HAIC group. Hematologic toxicity and liver function damage were the main grade 3-4 AEs in this study. In addition, the frequencies of grade 3–4 AEs were lower in the HAIC group. One possible reason for this is that HAIC enables the delivery of chemotherapy drugs directly into the liver, causing a relatively low systemic blood concentration of drugs. However, SC is the intravenous administration of chemotherapy drugs. In order to achieve the effect of killing liver tumors, the systemic blood concentration of the drug must be at a high level to cause damage to various systems in the body. It is also possible that the liver could clear the drugs via first-pass metabolism to approach diminish systemic toxic effects (Ensminger and Gyves, 1983; Cohen and Kemeny, 2003; Cercek et al., 2020). Meanwhile, most of these AEs were controlled after symptomatic treatment for the HAIC group and would not affect the next session. Therefore, HAIC may be a safe and effective therapeutic regimen for treating patients with unresectable iCCA.
This study also had few limitations. First, it was a retrospective study, and all of the patients came from a single center; thus, further prospective, large-sample, and randomized studies are needed to confirm our findings. Second, the relatively small sample size was limited by the generalizability of our results, and there was a risk of type II error. Finally, more bench-scale research studies are needed to determine the intrinsic mechanism guiding HAIC for patients with iCCA.
In conclusion, this study demonstrated that HAIC was a safe and effective therapeutic regimen in the cohort of 146 patients with unresectable iCCA. Meanwhile, our study indicated that patients with single tumor are most likely to benefit from HAIC than SC.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Institutional Review Board of the Sun Yat-sen University Cancer Center. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
ZY: conceptualization, methodology, software, formal analysis, and writing—original draft; YF: methodology, software, and formal analysis; WW: conceptualization, software, resources, and data curation; ZH: resources and investigation; YP: resources and investigation; DH: resources and investigation; ZZ: supervision and data curation; MC: conceptualization, funding acquisition, project administration, and supervision; YZ: conceptualization, methodology, project administration, supervision, and writing—review and editing. All authors contributed to the article and approved the submitted version.
This work is funded by the National Natural Science Foundation of China (No: 82103566).
The authors would like to thank Bullet Edits Limited for the linguistic editing and proofreading of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1234342/full#supplementary-material
SUPPLEMENTARY FIGURE S1 | Median follow-up times of the (A) HAIC and (B) SC groups calculated by the reversed Kaplan–Meier method.
SUPPLEMENTARY FIGURE S2 | Overall survival and progression-free survival of the two groups of patients. Kaplan–Meier curves of (A) overall survival and (B) progression-free survival for patients in the HAIC, GEMCIS, and GEMOX groups.
SUPPLEMENTARY FIGURE S3 | Subgroup analysis for the overall survival and progression-free survival of the two groups of patients. Kaplan–Meier curves of (A) overall survival and (B) progression-free survival for patients with single tumor.
Banales, J. M., Cardinale, V., Carpino, G., Marzioni, M., Andersen, J. B., Invernizzi, P., et al. (2016). Expert consensus document: cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European network for the study of cholangiocarcinoma (ENS-CCA). Nat. Rev. Gastroenterol. Hepatol. 13 (5), 261–280. doi:10.1038/nrgastro.2016.51
Bridgewater, J., Galle, P. R., Khan, S. A., Llovet, J. M., Park, J. W., Patel, T., et al. (2014). Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J. Hepatol. 60 (6), 1268–1289. doi:10.1016/j.jhep.2014.01.021
Bupathi, M., Ahn, D. H., and Bekaii-Saab, T. (2017). Therapeutic options for intrahepatic cholangiocarcinoma. Hepatobiliary Surg. Nutr. 6 (2), 91–100. doi:10.21037/hbsn.2016.12.12
Cai, Z., He, C., Zhao, C., and Lin, X. (2021). Survival comparisons of hepatic arterial infusion chemotherapy with mFOLFOX and transarterial chemoembolization in patients with unresectable intrahepatic cholangiocarcinoma. Front. Oncol. 11, 611118. doi:10.3389/fonc.2021.611118
Caparica, R., Lengelé, A., Bekolo, W., and Hendlisz, A. (2019). FOLFIRI as second-line treatment of metastatic biliary tract cancer patients. Autops. Case Rep. 9 (2), e2019087. doi:10.4322/acr.2019.087
Cercek, A., Boerner, T., Tan, B. R., Chou, J. F., Gönen, M., Boucher, T. M., et al. (2020). Assessment of hepatic arterial infusion of floxuridine in combination with systemic gemcitabine and oxaliplatin in patients with unresectable intrahepatic cholangiocarcinoma: a phase 2 clinical trial. JAMA Oncol. 6 (1), 60–67. doi:10.1001/jamaoncol.2019.3718
Cohen, A. D., and Kemeny, N. E. (2003). An update on hepatic arterial infusion chemotherapy for colorectal cancer. Oncologist 8 (6), 553–566. doi:10.1634/theoncologist.8-6-553
Dong, L., Lu, D., Chen, R., Lin, Y., Zhu, H., Zhang, Z., et al. (2022). Proteogenomic characterization identifies clinically relevant subgroups of intrahepatic cholangiocarcinoma. Cancer Cell 40 (1), 70–87.e15. doi:10.1016/j.ccell.2021.12.006
Eisenhauer, E. A., Therasse, P., Bogaerts, J., Schwartz, L. H., Sargent, D., Ford, R., et al. (2009). New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45 (2), 228–247. doi:10.1016/j.ejca.2008.10.026
Ensminger, W. D., and Gyves, J. W. (1983). Clinical pharmacology of hepatic arterial chemotherapy. Semin. Oncol. 10 (2), 176–182.
Fiteni, F., Nguyen, T., Vernerey, D., Paillard, M. J., Kim, S., Demarchi, M., et al. (2014). Cisplatin/gemcitabine or oxaliplatin/gemcitabine in the treatment of advanced biliary tract cancer: a systematic review. Cancer Med. 3 (6), 1502–1511. doi:10.1002/cam4.299
Grenader, T., Nash, S., Plotkin, Y., Furuse, J., Mizuno, N., Okusaka, T., et al. (2015). Derived neutrophil lymphocyte ratio may predict benefit from cisplatin in the advanced biliary cancer: the ABC-02 and BT-22 studies. Ann. Oncol. 26 (9), 1910–1916. doi:10.1093/annonc/mdv253
He, M., Li, Q., Zou, R., Shen, J., Fang, W., Tan, G., et al. (2019). Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion: A randomized clinical trial. JAMA Oncol. 5 (7), 953–960. doi:10.1001/jamaoncol.2019.0250
Ishii, M., Itano, O., Morinaga, J., Shirakawa, H., and Itano, S. (2022). Potential efficacy of hepatic arterial infusion chemotherapy using gemcitabine, cisplatin, and 5-fluorouracil for intrahepatic cholangiocarcinoma. PLoS One 17 (4), e0266707. doi:10.1371/journal.pone.0266707
Kasai, K., Kooka, Y., Suzuki, Y., Suzuki, A., Oikawa, T., Ushio, A., et al. (2014). Efficacy of hepatic arterial infusion chemotherapy using 5-fluorouracil and systemic pegylated interferon α-2b for advanced intrahepatic cholangiocarcinoma. Ann. Surg. Oncol. 21 (11), 3638–3645. doi:10.1245/s10434-014-3766-7
Kemeny, N., Daly, J., Oderman, P., Shike, M., Chun, H., Petroni, G., et al. (1984). Hepatic artery pump infusion: toxicity and results in patients with metastatic colorectal carcinoma. J. Clin. Oncol. 2 (6), 595–600. doi:10.1200/JCO.1984.2.6.595
Kim, S. T., Kang, J. H., Lee, J., Lee, H. W., Oh, S. Y., Jang, J. S., et al. (2019). Capecitabine plus oxaliplatin versus gemcitabine plus oxaliplatin as first-line therapy for advanced biliary tract cancers: a multicenter, open-label, randomized, phase III, noninferiority trial. Ann. Oncol. 30 (5), 788–795. doi:10.1093/annonc/mdz058
Lamarca, A., Palmer, D. H., Wasan, H. S., Ross, P. J., Ma, Y. T., Arora, A., et al. (2021). Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol. 22 (5), 690–701. doi:10.1016/S1470-2045(21)00027-9
Li, Q. J., He, M. K., Chen, H. W., Fang, W. Q., Zhou, Y. M., Xu, L., et al. (2022). Hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin versus transarterial chemoembolization for large hepatocellular carcinoma: a randomized phase III trial. J. Clin. Oncol. 40 (2), 150–160. doi:10.1200/JCO.21.00608
Li, S. H., Mei, J., Cheng, Y., Li, Q., Wang, Q. X., Fang, C. K., et al. (2023). Postoperative adjuvant hepatic arterial infusion chemotherapy with FOLFOX in hepatocellular carcinoma with microvascular invasion: a multicenter, phase III, randomized study. J. Clin. Oncol. 41 (10), 1898–1908. doi:10.1200/JCO.22.01142
Llovet, J. M., and Lencioni, R. (2020). mRECIST for HCC: performance and novel refinements. J. Hepatol. 72 (2), 288–306. doi:10.1016/j.jhep.2019.09.026
Lyu, N., Wang, X., Li, J. B., Lai, J. F., Chen, Q. F., Li, S. L., et al. (2022). Arterial chemotherapy of oxaliplatin plus fluorouracil versus sorafenib in advanced hepatocellular carcinoma: a biomolecular exploratory, randomized, phase III trial (FOHAIC-1). J. Clin. Oncol. 40 (5), 468–480. doi:10.1200/JCO.21.01963
Marumoto, M., Yamasaki, T., Marumoto, Y., Saeki, I., Harima, Y., Urata, Y., et al. (2014). Systemic gemcitabine combined with hepatic arterial infusion chemotherapy with cisplatin, 5-fluorouracil, and isovorin for the treatment of advanced intrahepatic cholangiocarcinoma: a pilot study. Hepatogastroenterology 61 (129), 162–167.
Mosconi, C., Solaini, L., Vara, G., Brandi, N., Cappelli, A., Modestino, F., et al. (2021). Transarterial chemoembolization and radioembolization for unresectable intrahepatic cholangiocarcinoma-a systemic review and meta-analysis. Cardiovasc Interv. Radiol. 44 (5), 728–738. doi:10.1007/s00270-021-02800-w
Nehls, O., Klump, B., Arkenau, H. T., Hass, H. G., Greschniok, A., Gregor, M., et al. (2002). Oxaliplatin, fluorouracil and leucovorin for advanced biliary system adenocarcinomas: a prospective phase II trial. Br. J. Cancer 87 (7), 702–704. doi:10.1038/sj.bjc.6600543
Oh, D. Y., Lee, K. H., Lee, D. W., Yoon, J., Kim, T. Y., Bang, J. H., et al. (2022). Gemcitabine and cisplatin plus durvalumab with or without tremelimumab in chemotherapy-naive patients with advanced biliary tract cancer: an open-label, single-centre, phase 2 study. Lancet Gastroenterol. Hepatol. 7 (6), 522–532. doi:10.1016/S2468-1253(22)00043-7
Okusaka, T., Nakachi, K., Fukutomi, A., Mizuno, N., Ohkawa, S., Funakoshi, A., et al. (2010). Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br. J. Cancer 103 (4), 469–474. doi:10.1038/sj.bjc.6605779
Rizvi, S., and Gores, G. J. (2013). Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology 145 (6), 1215–1229. doi:10.1053/j.gastro.2013.10.013
Rizvi, S., Khan, S. A., Hallemeier, C. L., Kelley, R. K., and Gores, G. J. (2018). Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat. Rev. Clin. Oncol. 15 (2), 95–111. doi:10.1038/nrclinonc.2017.157
Rodriguez, H., and Pennington, S. R. (2018). Revolutionizing precision oncology through collaborative proteogenomics and data sharing. Cell 173 (3), 535–539. doi:10.1016/j.cell.2018.04.008
Schaarschmidt, B. M., Kloeckner, R., Dertnig, T., Demircioglu, A., Müller, L., Auer, T. A., et al. (2023). Real-life experience in the treatment of intrahepatic cholangiocarcinoma by (90)Y radioembolization: a multicenter retrospective study. J. Nucl. Med. 64 (4), 529–535. doi:10.2967/jnumed.122.264598
Sirica, A. E., Gores, G. J., Groopman, J. D., Selaru, F. M., Strazzabosco, M., Wei Wang, X., et al. (2019). Intrahepatic cholangiocarcinoma: continuing challenges and translational advances. Hepatology 69 (4), 1803–1815. doi:10.1002/hep.30289
Valle, J., Wasan, H., Palmer, D. H., Cunningham, D., Anthoney, A., Maraveyas, A., et al. (2010). Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 362 (14), 1273–1281. doi:10.1056/NEJMoa0908721
Keywords: intrahepatic cholangiocarcinoma, hepatic arterial infusion chemotherapy, systemic chemotherapy, overall survival, progression-free survival, adverse events
Citation: Yang Z, Fu Y, Wu W, Hu Z, Pan Y, Wang J, Chen J, Hu D, Zhou Z, Chen M and Zhang Y (2023) Comparison of hepatic arterial infusion chemotherapy with mFOLFOX vs. first-line systemic chemotherapy in patients with unresectable intrahepatic cholangiocarcinoma. Front. Pharmacol. 14:1234342. doi: 10.3389/fphar.2023.1234342
Received: 04 June 2023; Accepted: 21 August 2023;
Published: 05 September 2023.
Edited by:
Maria Lina Tornesello, G. Pascale National Cancer Institute Foundation (IRCCS), ItalyReviewed by:
Rui Dai, Massachusetts General Hospital and Harvard Medical School, United StatesCopyright © 2023 Yang, Fu, Wu, Hu, Pan, Wang, Chen, Hu, Zhou, Chen and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Minshan Chen, Y2hlbm1zaEBzeXN1Y2Mub3JnLmNu; Yaojun Zhang, emhhbmd5dWpAc3lzdWNjLm9yZy5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.