
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 14 August 2023
Sec. Pharmacology of Anti-Cancer Drugs
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1230395
This article is part of the Research TopicThe Biomarkers, Mechanism, and Therapeutic Strategies of Cancer Immunotherapy ResistanceView all 14 articles
Objective: Our study aims to assess the effectiveness and safety profile of Disitamab Vedotin (DV, RC48-ADC), an innovative humanized anti-HER2 antibody conjugated with tubulin-disrupting antimitotic drug monomethyl auristatin E (MMAE) via a cleavable peptide linker. This treatment combined immune checkpoint inhibitors as part of the bladder sparing approach for selected patients suffering from locally and locally advanced bladder urothelial carcinoma.
Patients and methods: We conducted a two-center, real-world study involving locally advanced urothelial carcinoma (UC) patients. Patients were classified based on HER2 expression (IHC 3+/2+/1+) or lack of HER2 expression (IHC 0). The primary endpoint was the objective response rate (ORR), assessed by the investigator following the criteria of RECIST V1.1. Secondary endpoints encompassed the pathological complete response rate (pCR), pathological partial response rate (pPR), and pathological stable disease (pSD), along with recurrence-free survival (RFS), the pathological downstaging rate, and the safety profile of the treatment.
Results: In this study, nine patients were enrolled, with a median follow-up duration of 12.0 months. The overall confirmed ORR was 88.9%, Five patients achieved a complete response (CR), and three patients achieved a partial response (PR). The radiological complete response (rCR) aligned perfectly with pCR. The median radiological progression-free survival (rPFS) spanned 12.0 months (range from 8.0 to 17.0 months). One patient diagnosed with disease progression (PD) underwent a radical cystectomy. The pathological stage evolved from T2N0M0 to T3aN2M0, followed by adjuvant chemotherapy with a gemcitabine-cisplatin (GC) combination radiotherapy. At the 9-month follow-up, neither recurrence nor metastasis was observed. The rate and intensity of complications were manageable among these patients, with no evidence of grade 4 and 5 adverse events.
Conclusion: The combination of DV and PD-1 demonstrated considerable activity in the objective response rate (ORR) in patients with HER2 IHC 0/1+/2+/3+ muscle-invasive bladder cancer (MIBC), along with the longest reported median radiological progression-free survival (rPFS) to date. With an extended duration of treatment, the safety profile of DV plus PD-1 was also confirmed to be manageable.
Urothelial bladder cancer (UC) ranks the ninth most common cancer worldwide, with an annual incidence of over 500,000 new cases and 200,000 deaths attributable to the disease (Bray et al., 2018). Only 30% of these newly diagnosed cases involve muscle-invasive bladder cancer (MIBC), where the tumor invades the detrusor muscle (Charlton et al., 2014). A combination of Neoadjuvant chemotherapy plus Radical cystectomy (RC) has traditionally been the standard of care for MIBC. Current RC methodologies have achieved 5-year overall survival (OS) rates ranging from 56% to 66% (Grossman et al., 2003; Sherif et al., 2004; Stein et al., 2001; Zehnder et al., 2011).
Over recent decades, there has been a growing trend toward organ-preserving therapies in treating numerous cancers. Within the sphere of bladder cancer, introducing a disciplinary approach has led to the development of bladder-sparing approaches. These approaches combine maximal transurethral resection (TURBT) with radiotherapy and concurrent radio-sensitizing chemotherapy to treat MIBC. While no definitive randomized studies have compared RC and this bladder-sparing trimodal therapy (TMT), multiple series suggest that TMT may produce favorable outcomes for carefully chosen patients (Hoskin et al., 2010; Efstathiou et al., 2012; James et al., 2012a; Mak et al., 2014).
The recent application of immune checkpoint inhibitors (ICIs) has yielded significant clinical outcomes, thereby establishing a role in treating metastatic UC (mUC) and MIBC. This includes inhibitors such as pembrolizumab, nivolumab, avelumab, tislelizumab, and toripalimab. Using neoadjuvant cisplatin-based chemotherapy (NAC) and PD-1/PD-L1-based immunotherapy has demonstrated pathologic complete response rates of approximately 30%–40% and 30%–50%, respectively, (APOLO et al., 2017; SHARMA et al., 2017; Powles et al., 2019; Guptal et al., 2020; Sheng X. et al., 2021; Necchi et al., 2019; Bellmunt et al., 2021; Ye et al., 2021; Christopher et al., 2022). Furthermore, immunotherapy has been linked to a notably high complete response rate (up to 80%) in conjunction with bladder-sparing treatment, an improvement compared to traditional TMT, which achieves 60%–70% (COPPIN et al., 1996; SAUER et al., 1998; JAMES et al., 2012b; Balar et al., 2021; Garcia delMuro et al., 2021). The observed outcomes underscore the need for additional research into the application of ICIs in combination therapies for the curative treatment of MIBC patients.
In bladder cancer, human epidermal growth factor receptor 2 (HER2) overexpression is strongly linked with tumor progression and poor prognosis, although HER2 genomic amplification is not a typical mechanism (Chow et al., 2001; Jimenez et al., 2001; Kruger et al., 2002; Fleischmann et al., 2011). Disitamab vedotin (DV, RC48-ADC) is an innovative humanized anti-HER2 antibody, conjugated with MMAE via a cleavable linker. DV has demonstrated promising antitumor activity and a tolerable safety profile toward mUC, exhibiting an objective response rate (ORR) of 51.2% and median progression-free survival (PFS) of 6.9 months (Sheng Xinan et al., 2021). In the RC48-C014 trial, 32 patients with metastatic UC were treated with DV and toripalimab, resulting in an ORR of 71.8% in the overall population and 73.9% in first-line previously untreated patients (Sheng et al., 2022). Additionally, DV combined with PD-1 inhibitors has yielded satisfactory efficacy in patients with MIBC and non-muscle Invasive Bladder Cancer (NMIBC) (Wen, 2022; Hu et al., 2023; Huang et al., 2023). Nevertheless, real-world studies, which provide crucial information on a drug’s efficacy and safety within the actual patient population, are lacking for the role of DV in MIBC. Therefore, this study aims to explore the use of DV for the treatment of locally advanced bladder urothelial carcinoma, utilizing real-world data.
This real-world study retrospectively analyzed the clinicopathological and follow-up results of patients with locally or locally advanced primary urothelial carcinoma of the bladder, treated with DV and immunotherapy at Fujian Provincial Hospital and the Union Hospital Affiliated with Fujian Medical University. The inclusion criteria should meet the following requirements, patients without DV or immunotherapy contraindications; ECOG score were 0 or 1; aged between 18 and 85 years; patients without underlying severe medical conditions. In addition to the following requirements, they should also meet the criteria as follows:
1. Diagnosis and treatment were managed comprehensively within the two centers.
2. Detailed clinicopathological data were available.
3. Primary urothelial cell carcinoma of the bladder was confirmed pathologically, excluding specific differentiation, such as sarcoma or clear cell.
4. The study only included locally and locally advanced UC, with the staging at cT2-T4aN0-2M0.
5. At least two or more courses of this combined neoadjuvant therapy were included.
6. The therapeutic response could be evaluated.
DV was administered according to the RC48-C014 study protocol, i.e., DV 2 mg/kg (Equivalent to dose of 1.5 mg/kg using DV-based extinction coefficient outside of China) every 2 weeks (Q2W), and the immunotherapy consisted of either tislelizumab 200 mg every 3 weeks (Q3W) or toripalimab 3 mg/kg Q2W. The study was approved by the ethics committees of both centers and received written informed consent from the patients’ families.
Patients enrolled in this study started drug combination therapy in December 2021, with follow-ups extending until July 2023. The collected data encompassed demographic information, bladder cancer history, pathological data, details of neoadjuvant drugs and course of treatment, surgical interventions, and primary and secondary study endpoints. The pathological grade was classified into Grade 1, grade 2, and Grade 3 according to WHO 1973 criteria. HER2 expression was evaluated by immunohistochemistry (IHC), with categories being IHC 0; 1+; 2+; 3+, or positive or negative as determined by the FISH gene testing. The expression of programmed cell death protein 1 (PDL-1) was evaluated and was classified as ≥1% and <1%. All patients had a pelvic Magnetic Resonance Imaging (MRI) for bladder measurable lesions in baseline before combination therapy.
Neoadjuvant immunotherapy drugs included tirelizumab, administered at 200 mg every 3 weeks (Q3W), or toripalimab, dosed as per literature at 3 mg/kg every 2 weeks (Q2W) for 12 weeks. The RECIST 1.1 standard evaluated the efficacy of neoadjuvant therapy. We assessed the imaging efficacy (categorized as rCR, rPR, rSD, and rPD) of bladder lesions in patients by MRI, and assessed pathological efficacy (categorized as pCR, pPR, and pSD) by MRI combined with bladder biopsy or TURBT, and evaluated the lungs and abdomen by Computed Tomography (CT) to assess the presence of distant metastases at a frequency of every 3–6 months.
After neoadjuvant therapy, patients underwent a second surgical treatment, including diagnostic radical transurethral resection of bladder tumor (TURBT) and/or bladder biopsy, partial cystectomy, or radical cystectomy. Secondary study endpoints such as pCR, pPR, and pSD were determined based on pathological findings. Secondary study endpoints included the rPFS in months following neoadjuvant therapy and pathological degradation. Concurrently, side effects and their severity were evaluated according to the adverse reaction classification.
We screened 53 patients, and nine patients with primary bladder urothelial carcinoma from two institutions were finally included in this study, among which the ratio of males to females was 8:1 (Table 1). All of these patients were platinum-tolerable. Still, after detailed explanation and communication, these patients accepted DV combined immunotherapy as a new treatment. These patients had a median age of 72 and a median BMI of 22.3 kg/m2. Eight patients had an Eastern Cooperative Oncology Group (ECOG) performance status score of 0, while one scored 1. Among the participants, six patients were newly diagnosed, and three experienced recurrences after bladder sparing treatment (two for the third time and one for the second time).
All patients have pathologically diagnosed with bladder urothelial carcinoma: eight were diagnosed with diagnostic TURBT, and one was diagnosed through laparoscopic radical ureteral carcinoma resection (during which ureteral carcinoma was found to involve the bladder wall during the operation with positive incisal margin, postoperative MRI review showed bladder wall thickening and enhancement, which was considered as residual bladder cancer). Staging for all cases was T2-T4aN0-3M0, with four cases each at stages T2 and T3. All pathological grades were Grade 3. Four patients accepted some cycles of gemcitabine-cisplatin (GC) treatment; these patients had a recurrence of bladder lesions or lesions remaining after GC treatment and then received DV combined immunization as neoadjuvant or bladder-sparing therapy.
Immunohistochemistry demonstrated that three cases were HER2 (2+), four cases were (3+), and the remaining two cases were (1+) and (0+), respectively. Roche VENTANA PD-L1 (SP263) tests all resulted in a combined positive score of <1%. Of these, three cases presented with underlying comorbidities, including three with hypertension, one with diabetes, and one with a lower risk of prostate cancer.
The median follow-up duration for these patients was 12.0 months (range from 8.0 to 17.0 months), calculated from the initiation of combination therapy to the end of follow-up.
All patients underwent treatment with DV combined with PD-1 inhibitors. This included six patients who received three courses of combined therapy, two patients who underwent four or more courses of treatment, and one patient who underwent two courses of treatment. Regarding the choice of PD-1 inhibitors, five patients were treated with tislelizumab 200 mg every 3 weeks (Q3W), and four patients were treated with toripalimab 3 mg/kg every 2 weeks (Q2W) (Table 1).
The treatment efficacy was finally evaluated by comparing imaging changes, urine cytological alterations, and results from transurethral resection. An ORR was achieved in eight cases, which included CR in five cases (Figure 1) and PR in three cases. rCR was entirely consistent with pCR.
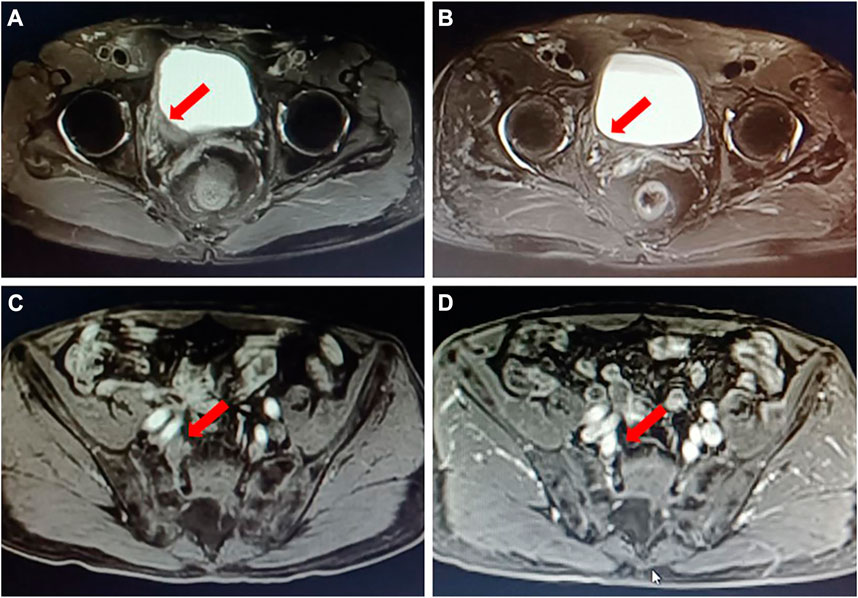
FIGURE 1. A typical case of neoadjuvant therapy of DV in combination with an immune checkpoint inhibitor. A 72 years old male patient with “gross hematuria” was admitted; MRI showed that a bladder tumor in the right wall of the bladder with the inner segment of the right ureteral bladder wall involved (A); multiple mildly enlarged lymph nodes paravascular were found on the right iliac with a maximum of 1.1 cm, and lymph nodes metastasis were considered (C). Pathology of biopsy confirmed bladder high-grade urothelial carcinoma, with PD-L1 low expression (IHC) and HER2 (2+) (IHC). DV 2 mg/kg plus toripalimab 3 mg/kg, Q3w was given as neoadjuvant therapy for three circles. Then MRI was performed to evaluate the outcomes of the neoadjuvant treatment; it showed a radiological complete response (B, D). After laparoscopic right pelvic lymph node dissection with partial bladder incision and right ureteral bladder replantation, the postoperative pathology confirmed no evidence of cancer, suggesting a pathological complete response.
By the end of the follow-up period, the median rPFS of these patients was 12.0 months (range: 8.0–17.0 months) (Table 2). One patient with HER2 (0+) was diagnosed with PD and underwent radical cystectomy; the pathological stage progressed from ypT2N0M0 to ypT3aN2M0. The treatment was then switched to GC combined with radiotherapy as adjuvant chemotherapy and primary radiotherapy. No tumor recurrence or metastasis was observed at the 9-month follow-up.
In general, the incidence and severity of complications were manageable in these patients, and no grade 4 or 5 adverse events were observed. Treatment-related adverse reactions were reported in eight patients. The most observed symptoms included loss of appetite, rash, and fatigue, each occurring in three cases, all classified as grade I or II.
Additional adverse reactions such as hypothyroidism, fatigue, abnormal liver function, and peripheral sensory neuropathy were reported in two cases, grade I or II. A single instance of each of the following was reported: immune pneumonia (grade I), abdominal pain (grade I), nausea (grade II), joint congestion (grade II), gastrointestinal bleeding (grade III), and intestinal obstruction (grade III) (Table 3).
This real-world study explores the application of DV combined with PD-1 inhibitors in locally advanced bladder urothelial carcinoma. In this study, DV combined with either tislelizumab or toripalimab demonstrated promising responses in patients with locally advanced urothelial carcinoma. The study enrolled nine patients with locally advanced urothelial carcinoma, of which five achieved confirmed CR and three achieved PR, resulting in an ORR of 88.9%. rCR was entirely consistent with pCR. The median rPFS was 12.0 months (range: 8.0–17.0 months).
An open-label, single-arm, multicenter phase Ib/II clinical trial demonstrated that four patients with locally advanced urothelial carcinoma and HER2 IHC 1+/2+/3+ achieved a cCR rate of 100% after receiving DV plus tislelizumab as neoadjuvant therapy (Wen, 2022). However, our study involved a more diverse patient population: nine patients, including three recurrent cases and one with HER2 (0). In another retrospective study, seven patients with HER2 overexpressing (IHC 2+ or 3+) NMIBC, who could neither have their bladder tumor completely resected nor tolerate surgery, were treated with either DV or a combination of DV and ICIs, showing promising efficacy and an ORR of 85.7% for all patients (Hu et al., 2023). The patient populations in these studies were distinct; the previous study enrolled all NMIBC patients, while our study focused on MIBC, which is associated with poorer survival outcomes for patients with urothelial carcinoma. Overall, this retrospective study represents a successful exploration of neoadjuvant or bladder-preserving therapy in the real-world setting using DV combined with immunotherapy.
Besides short-term efficacy, the safety of DV combined with PD-1 inhibitors during therapy in patients with MIBC is of utmost importance. In this study, no adverse events (AEs) of grades 4 or 5 were observed, with grade 1 or 2 AEs accounting for 88.9% of the total. Anorexia, rash, and fatigue were the most frequently reported AEs. Importantly, these events were of grade 1 or 2 severity, transient, and could be effectively managed in the outpatient setting without necessitating any dose modification or interruption. No new AEs were reported, and all observed AEs were manageable. Furthermore, there were no recorded fatalities during the therapy period.
An essential finding of this study was the anti-tumor activity of DV combined with PD-1 inhibitors in patients with HER2 IHC1+, IHC 0, and PD-L1< 1%. This effect is not solely due to the nonspecific bystander effects of the cytotoxic drugs released by DV. Our data strongly support a T cell-dependent mechanism and the efficacy of the combination therapy. This effect is achieved through non-redundant yet complementary mechanisms. Specifically, DV enhances T cell infiltration into the tumor by inducing tumor-specific, adaptive anti-tumor immunity, while PD-1 blockade rejuvenates exhausted T cells (Huang et al., 2022). Other chemotherapy regimens have had similar therapeutic benefits (Haratani et al., 2020; D’Amico et al., 2019; Wang et al., 2018).
Some limitations to our study should be acknowledged. The most significant was the lack of a mature follow-up period to provide survival outcomes. Extended follow-up was required, particularly focusing on long-term bladder preservation and survival. Another limitation was the small sample size of patients with cT2-T3bN0-2M0 tumors included in our study; we will embark on a prospective, case-control multicenter study, including evaluating the efficacy of the combination therapy in specific subgroups based on HER2 expression levels, to further validate our findings and provide new approaches to guide neoadjuvant therapy or bladder-sparing treatment for bladder cancer. Furthermore, we could not obtain data on survival rates with our limited observation period and sample size; we will include this subset of patients to observe survival data in future studies.
The promising safety profile and anti-tumor activity demonstrated by the combination of DV and PD-1 inhibitors in this real-world study lay a solid foundation for a phase 2 clinical trial exploring DV in combination with PD-1 inhibitors in patients with MIBC. Our study data were the most extended median follow-up time for DV plus immunotherapy to date, and our findings provided a new possibility for DV plus immunotherapy as neoadjuvant therapy or treatment of sparing bladder.
The combination of DV and PD-1 inhibitors demonstrated significant improvements in ORR in patients with MIBC across all HER2 IHC levels (0/1+/2+/3+). This regimen also reported the longest median rPFS to date, solidifying the combination of DV and PD-1 inhibitors as a compelling treatment choice for patients with T2-T4aN0-3M0 staging. Furthermore, this study confirms that the combination treatment maintains a manageable safety profile even with extended treatment duration.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the individuals for the publication of any potentially identifiable images or data included in this article.
Conceptualization: YW and JC. Formal analysis and investigation: RZ, CY, ZH, LL, and TL. Writing—original draft preparation: YW. Writing—review and editing: YW and JC. All authors contributed to the article and approved the submitted version.
This study was supported by the Natural Science Foundation of Fujian Province (2021J01359, YW; 2022J05211, RZ; and 2022J01408, TL), China Urological Oncology Research Fund (#027) (YW) and and Training Program for Young and Middle-aged elite Talents Sponsored by Fujian provincial health technology project (2021GGA014, JC).
We are grateful to all the patients who contributed to this research. In addition, we would like to express our special thanks to Song Zheng, Jun Lin, Junjie Bai and Hanying Jin for their efforts in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Apolo, A. B., Infante, J. R., Balmanoukian, A., Patel, M. R., Wang, D., Kelly, K., et al. (2017). Avelumab, an anti-programmed death-ligand 1 antibody,in patients with refractory metastatic urothelial carcinoma: results from a multicenter,phase ib study. J. Clin. Oncol. 35 (19), 2117–2124. doi:10.1200/JCO.2016.71.6795
Balar, A. V., Milowsky, M. I., and PeterO'Donnell, H. (2021). Pembrolizumab in combination with gemcitabine andconcurrent hypofractionated radiation therapy asbladder sparing treatment for muscle-invasiveurothelial cancer of the bladder: A multicenter phase 2 trial. Virginia, United States: ASCO. abstract No. 4504.
Bellmunt, J., Necchi, A., Wit, R. D., Lee, J. L., Fong, L., Vogelzang, N. J., et al. (2021). Pembrolizumab (pembro) versus investigator’s choice of paclitaxel, docetaxel, or vinflunine in recurrent, advanced urothelial cancer (UC): 5-year follow-up from the phase 3 KEYNOTE-045 trial. J. Clin. Oncol. 39, 4532. 15_suppl. doi:10.1200/jco.2021.39.15_suppl.4532
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., and Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424. doi:10.3322/caac.21492
Charlton, M. E., Adamo, M. P., Sun, L., and Deorah, S. (2014). Bladder cancer collaborative stage variables and their data quality, usage, and clinical implications: A review of SEER data. Cancer 120, 3815–3825. 2004–2010. doi:10.1002/cncr.29047
Chow, N. H., Chan, S. H., Tzai, T. S., Ho, C. L., and Liu, H. S. (2001). Expression profiles of ErbB family receptors and prognosis in primary transitional cell carcinoma of the urinary bladder. Clin. Cancer Res. 7, 1957–1962.
Christopher, J. H., Adra, N., and Fleming, M. T. (2022). GU14-188: Phase 1b/2 neoadjuvant pembrolizumab and chemotherapy for locally advanced urothelial cancer: Final results of the cisplatin-eligible cohort (NCT02365766). Virginia, United States: ASCO. Abstract No. 5047.
Coppin, C. M., Gospodarowicz, M. K., James, K., Tannock, I. F., Zee, B., Carson, J., et al. (1996). Improved local control of invasive bladder cancer by concurrent cisplatin and preoperative or definitive radiation.The National Cancer Institute of Canada Clinical Trials Group. J. Clin. Oncol. 14 (11), 2901–2907. doi:10.1200/JCO.1996.14.11.2901
D’Amico, L., Menzel, U., Prummer, M., Müller, P., Buchi, M., Kashyap, A., et al. (2019). A novel anti-HER2 anthracycline-based antibody-drug conjugate induces adaptive anti-tumor immunity and potentiates PD-1 blockade in breast cancer. J. Immunother. Cancer 7, 16. doi:10.1186/s40425-018-0464-1
Efstathiou, J. A., Spiegel, D. Y., Wu, S., Heney, N. M., Kaufman, D. S., Niemierko, A., et al. (2012). Long-term outcomes of selective bladder preservation by combined-modality therapy for invasive bladder cancer: the MGH experience. Eur. Urol. 61, 705–711. doi:10.1016/j.eururo.2011.11.010
Fleischmann, A., Rotzer, D., Seiler, R., Studer, U. E., and Thalmann, G. N. (2011). Her2 amplification is significantly more frequent in lymph node metastases from urothelial bladder cancer than in the primary tumours. Eur. Urol. 60, 350–357. doi:10.1016/j.eururo.2011.05.035
Garcia del Muro, X., Valderrama, B. P., and Medina, A. (2021). Phase ll trial of durvalumab plus tremelimumab with concurrent radiotherapy in patients with localized muscle invasive bladdercancer treated with a selective bladder preservation approach:IMMUNOPRESERVE-SOGUC trial. Virginia, United States: ASCO. Abstract No.4505.
Grossman, H. B., Natale, R. B., Tangen, C. M., Speights, V. O., Vogelzang, N. J., Trump, D. L., et al. (2003). Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N. Engl. J. Med. 349, 859–866. doi:10.1056/NEJMoa022148
Guptal, S., Guru, S., and Christopher, J. W. “Results from BLASST-1(Bladder Cancer Signal Seeking Trial) ofnivolumab, gemcitabine, and cisplatin in muscle invasive bladdercancer patients undergoing cystectomy,” in Genitourinary Cancers Symposium, #GU20, San Francisco, California, February 2020.
Haratani, K., Yonesaka, K., Takamura, S., Maenishi, O., Kato, R., Takegawa, N., et al. (2020). U3–1402 sensitizes HER3-expressing tumors to PD-1 blockade by immune activation. J. Clin. Investig. 130, 374–388. 44. doi:10.1172/JCI126598
Hoskin, P. J., Rojas, A. M., Bentzen, S. M., and Saunders, M. I. (2010). Radiotherapy with concurrent carbogen and nicotinamide in bladder carcinoma. J. Clin. Oncol. 28, 4912–4918. doi:10.1200/JCO.2010.28.4950
Hu, H. L., Guo, S. Z., and Shen, C. (2023). The efficacy and safety of RC48 in patients with non-muscle invasive bladder cancer. SIU Around world Montr. Abstract MP-04.11. doi:10.1200/JCO.2023.41.16_suppl.e16616
Huang, H., Zhang, Y., Chen, Z., Zeng, X., Hu, Z., and Yang, C. (2023). Neoadjuvant therapy with Disitamab vedotin in treating muscle-invasive bladder cancer: A case report. HELIYON 9, e15157. doi:10.1016/j.heliyon.2023.e15157
Huang, L., Wang, R., Xie, K., Zhang, J., Tao, F., Pi, C., et al. (2022). A HER2 target antibody drug conjugate combined with anti-PD-(L)1 treatment eliminates hHER2+tumors in hPD-1 transgenic mouse model and contributes immune memory formation. Breast Cancer Res. Treat. 191, 51–61. doi:10.1007/s10549-021-06384-4
James, N. D., Hussain, S. A., Hall, E., Jenkins, P., Tremlett, J., Rawlings, C., et al. (2012a). Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N. Engl. J. Med. 366, 1477–1488. doi:10.1056/NEJMoa1106106
James, N. D., Hussain, S. A., Hall, E., Jenkins, P., Tremlett, J., Rawlings, C., et al. (2012b). Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N. Engl. J. Med. 366 (16), 1477–1488. doi:10.1056/NEJMoa1106106
Jimenez, R. E., Hussain, M., Bianco, F. J., Vaishampayan, U., Tabazcka, P., Sakr, W. A., et al. (2001). Her-2/neu overexpression in muscle-invasive urothelial carcinoma of the bladder: prognostic significance and comparative analysis in primary and metastatic tumors. Clin. Cancer Res. 7, 2440–2447.
Kruger, S., Weitsch, G., Buttner, H., Matthiensen, A., Böhmer, T., Marquardt, T., et al. (2002). Overexpression of c-erbB-2 oncoprotein in muscle-invasive bladder carcinoma: relationship with gene amplification, clinicopathological parameters and prognostic outcome. Int. J. Oncol. 21, 981–987. doi:10.3892/ijo.21.5.981
Mak, R. H., Hunt, D., Wu, S., Efstathiou, J. A., Tester, W. J., Hagan, M. P., et al. (2014). Long-term outcomes in patients with muscle-invasive bladder cancer after selective bladderpreserving combined-modality therapy: A pooled analysis of radiation therapy Oncology Group protocols 8802, 8903, 9506, 9706, 9906, and 0233. J. Clin. Oncol. 32, 3801–3809. doi:10.1200/JCO.2014.57.5548
Necchi, A., Anichini, A., Raggi, D., Briganti, A., Massa, S., Lucianò, R., et al. (2019). Pembrolizumab as neoadjuvant therapy before radical cystectomy in patients with muscle-invasive urothelial bladder carcinoma (PURE-01): an open-label, single-Arm,Phase II study. J. Clin. Oncol. 36, 3353–3360. doi:10.1200/JCO.18.01148
Powles, T., Kockx, M., Rodriguez-Vida, A., Duran, I., Crabb, S. J., Van Der Heijden, M. S., et al. (2019). Clinical efficacy and biomarker analysis of neoadjuvant atezolizumab in operable urothelial carcinoma in the ABACUS trial. Nat. Med. 25, 1706–1714. doi:10.1038/s41591-019-0628-7
Sauer, R., Birkenhake, S., Kuhn, R., Wittekind, C., Schrott, K. M., and Martus, P. (1998). Efficacy of radiochemotherapy with platin derivatives compared to radiotherapy alone in organ-sparing treatment of bladder cancer. Int. J. Radiat. Oncol. Biol. Phys. 40 (1), 121–127. doi:10.1016/s0360-3016(97)00579-8
Sharma, P., Retz, M., Siefker-Radtke, A., Baron, A., Necchi, A., Bedke, J., et al. (2017). Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 18 (3), 312–322. doi:10.1016/S1470-2045(17)30065-7
Sheng, X., Chen, H., Hu, B., Yao, X., Liu, Z., Yao, X., et al. (2021a). Safety, efficacy and biomarker analysis of toripalimab in patients with previously treated advanced urothelial carcinoma: results from a multicenter phase II trial POLARIS-03. Clin. Cancer Res. 28, 489–497. doi:10.1158/1078-0432.CCR-21-2210
Sheng, X., Yan, X., Wang, L., Shi, Y., Yao, X., Luo, H., et al. (2021b). Open-label, multicenter, phase II study of RC48-ADC, a HER2-targeting antibody–drug conjugate, in patients with locally advanced or metastatic urothelial carcinoma. Clin. Cancer Res. 27, 43–51. doi:10.1158/1078-0432.CCR-20-2488
Sheng, X., Zhou, L., and He, Z. (2022). Preliminary results of a phase Ib/ll combination study of RC48-ADC, a novel humanized anti-HER2 antibody-drug conjugate (ADC) with toripalimab, a humanized IgG4 mAb against programmed death-1 (PD-1) in patients with locally advanced or metastatic urothelial carcinoma(La/mUC). Virginia, United States: ASCOASCO. abstract No. 4518.
Sherif, A., Holmberg, L., Rintala, E., Mestad, O., Nilsson, J., Nilsson, S., et al. (2004). Neoadjuvant cisplatinum based combination chemotherapy in patients with invasive bladder cancer: A combined analysis of two nordic studies. Eur. Urol. 45, 297–303. doi:10.1016/j.eururo.2003.09.019
Stein, J. P., Lieskovsky, G., Cote, R., Groshen, S., Feng, A. C., Boyd, S., et al. (2001). Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1054 patients. J. Clin. Oncol. 19, 666–675. doi:10.1200/JCO.2001.19.3.666
Wang, M., Yao, L. C., Cheng, M., Cai, D., Martinek, J., Pan, C. X., et al. (2018). Humanized mice in studying efficacy and mechanisms of PD-1-targeted cancer immunotherapy. FASEB J. 32, 1537–1549. doi:10.1096/fj.201700740R
Wen, F. (2022). A multi-center phase Ib/II study of RC48-ADC combined with tislelizumab as neoadjuvant treatment in patients with HER2 positive locally advanced MIBC. Lugano, Switzerland: ESMO asia. Abstract 1350.
Ye, D., Liu, J., Zhou, A., Zou, Q., and Fu, C. (2021). Tislelizumab in Asian patients with previously treated locally advanced or metastatic urothelial carcinoma. Cancer Sci. 112, 305–313. doi:10.1111/cas.14681
Keywords: bladder cancer, bladder-sparing protocol, RC48, PD-1, HER2, antibody-drug conjugate, immunotherapy
Citation: Wei Y, Zhang R, Yu C, Hong Z, Lin L, Li T and Chen J (2023) Disitamab vedotin in combination with immune checkpoint inhibitors for locally and locally advanced bladder urothelial carcinoma: a two-center’s real-world study. Front. Pharmacol. 14:1230395. doi: 10.3389/fphar.2023.1230395
Received: 28 May 2023; Accepted: 01 August 2023;
Published: 14 August 2023.
Edited by:
Mou Peng, Central South University, ChinaReviewed by:
Zijun Wang, The Rockefeller University, United StatesCopyright © 2023 Wei, Zhang, Yu, Hong, Lin, Li and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianhui Chen, Y2hlbmppYW5odWkxOTgzQHFxLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.