- 1Chengdu Second People’s Hospital, Chengdu, Sichuan, China
- 2Department of Pharmacy, Personalized Drug Therapy Key Laboratory of Sichuan Province, Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, Sichuan, China
- 3School of Chinese Medicine, The University of Hong Kong, Shatin, Hong Kong SAR, China
Rheumatoid arthritis (RA) is a type of chronic autoimmune and inflammatory disease. In the pathological process of RA, the alteration of fibroblast-like synoviocyte (FLS) and its related factors is the main influence in the clinic and fundamental research. In RA, FLS exhibits a uniquely aggressive phenotype, leading to synovial hyperplasia, destruction of the cartilage and bone, and a pro-inflammatory environment in the synovial tissue for perpetuation and progression. Evidently, it is a highly promising way to target the pathological function of FLS for new anti-RA drugs. Based on this, we summed up the pathological mechanism of RA-FLS and reviewed the recent progress of small molecule drugs, including the synthetic small molecule compounds and natural products targeting RA-FLS. In the end, there were some views for further action. Compared with MAPK and NF-κB signaling pathways, the JAK/STAT signaling pathway has great potential for research as targets. A small number of synthetic small molecule compounds have entered the clinic to treat RA and are often used in combination with other drugs. Meanwhile, most natural products are currently in the experimental stage, not the clinical trial stage, such as triptolide. There is an urgent need to unremittingly develop new agents for RA.
1 Introduction
Rheumatoid arthritis (RA) is a type of autoimmune joint disease. It often occurs in women and the elderly. RA might affect 0.5%–1% of the global population (Zhang et al., 2022). Among the multiple factors, genetic and autoimmune along with environmental factors might be the primary causes. It shows the clinical presentation of joint pain, thickening of the synovial membrane, pannus formation, and infiltration of various inflammatory cells in the joint space, leading to the damage of the cartilage as well as bone tissue, even remarkably joint deformity and dysfunction (Smolen et al., 2018). A lot of attention is paid to the treatment of RA because it has high morbidity, might lead to disability, and has poor prognosis (Davis et al., 2012; Almutairi et al., 2021). Currently, non-steroidal anti-inflammatory drugs (NSAIDs), disease-modifying anti-rheumatic drugs (DMARDs) (synthetic or biologic agents), and glucocorticoids (Lampropoulos et al., 2015; Zhang et al., 2022) are popular in the treatment of RA. With the use of NSAIDs, the risk of cardiovascular disease might occur as well as gastrointestinal side effects, so a comprehensive evaluation is needed (O'Shea et al., 2013). DMARDs such as methotrexate (MTX), while suppressing inflammation and joint destruction, might cause nausea, anorexia, stomatitis, alopecia, myelosuppression, and even liver and pulmonary toxicity in severe cases, which requires careful monitoring. In addition, there are also problems of high expense and gastrointestinal adverse effects for DMARDs (Zhang et al., 2019). Biologic disease-modifying anti-rheumatic drugs (bDMARDs) show therapeutic effects for RA, but there are some individual differences because of different genetic backgrounds and environmental stimuli (Lampropoulos et al., 2015), and they do not cure the disease (Yamada, 2023). There is an urgent need to continuously develop new anti-RA drugs.
The synovium is considered to be a structure of connective soft-tissue membrane located in the joint cavity and the fibrocartilage, around arthrosis to provide nutrition and lubrication (Jay et al., 2000). The fibroblast-like synoviocytes (FLSs) are highly specialized mesenchymal cells found in the synovial membrane. In normal physiological regulation, FLS produces joint lubricants, for example, hyaluronic acid which nourishes the cartilage surface and shapes the synovial extracellular matrix (ECM). However, in RA, FLS exhibits a distinctive aggressive phenotype, with this aggressive behavior toward the ECM further exacerbating joint damage (Nygaard and Firestein, 2020). For this reason, one potential strategy for treating RA is the creation of medicines that target FLS (Bartok and Firestein, 2010). It is important to note that several of their monomers appear to have a positive impact on preventing arthritic synovial hyperplasia. They are mainly related to the induction of apoptosis and the inhibition of FLS proliferation. In this review, taking the state of FLS as a starting point, we summarize and discuss the literature on the small molecule drugs of FLS from PubMed, Embase, and other databases in the recent 3 years until 28 February 2023. Specific keywords used are “RA,” “FLS,” “MAPK,” “NF-κB,” “JAK/STAT,” “Wnt,” and “signaling pathways.” The small molecule drugs contain organic compounds with low molecular weights, typically ≤1000 Da. Also, these include both synthetic compounds and natural products derived mainly from plants and animals. Publications with incomplete data or conclusions and those not directly related to RA and small molecule compounds are excluded. Here, first, there is an introduction of the pathological mechanisms of RA-FLS. Second, according to the signaling pathways controlling the abnormal behavior of FLS, small molecule drugs of related pathways, especially drugs with high anti-RA-FLS potential, are analyzed in depth. Finally, we list our comments, which we hope will provide directions to developing targeted anti-rheumatic drugs for clinics.
2 FLS involved in the pathogenesis of RA
In RA, FLS proliferation releases several anti-inflammatory cytokines and growth factors, among which are tumor necrosis factor (TNF), interleukin (IL) (such as IL-6, IL-1β, and IL-17), chemokines, and inflammatory enzymes [such as nitric oxide synthase (NOS) and cyclooxygenase-2 (COX-2)]. Meanwhile, it provides the inflammatory microenvironment and potentially contributes to the initiation of chronic inflammation in the preliminary stage of RA. In addition, FLS produces large amounts of receptor activator of NF-κB ligand (RANKL), vascular endothelial growth factor (VEGF), matrix metalloproteinases (MMPs), and so on, which causes synovial hyperplasia and arthritic joint destruction (Wang et al., 2012). Worse still, the activated FLS migrates to the cartilage and bone. This migration occurs not only at local sites but also through the bloodstream into distant areas and joints, destroying the cartilage, activating osteoclasts, and enhancing joint destruction in RA (Neumann et al., 2010; Hu et al., 2019). Here, we review the pathological mechanisms of RA from the three perspectives shown in Figure 1: synovial hyperplasia, joint damage, and immune inflammation.
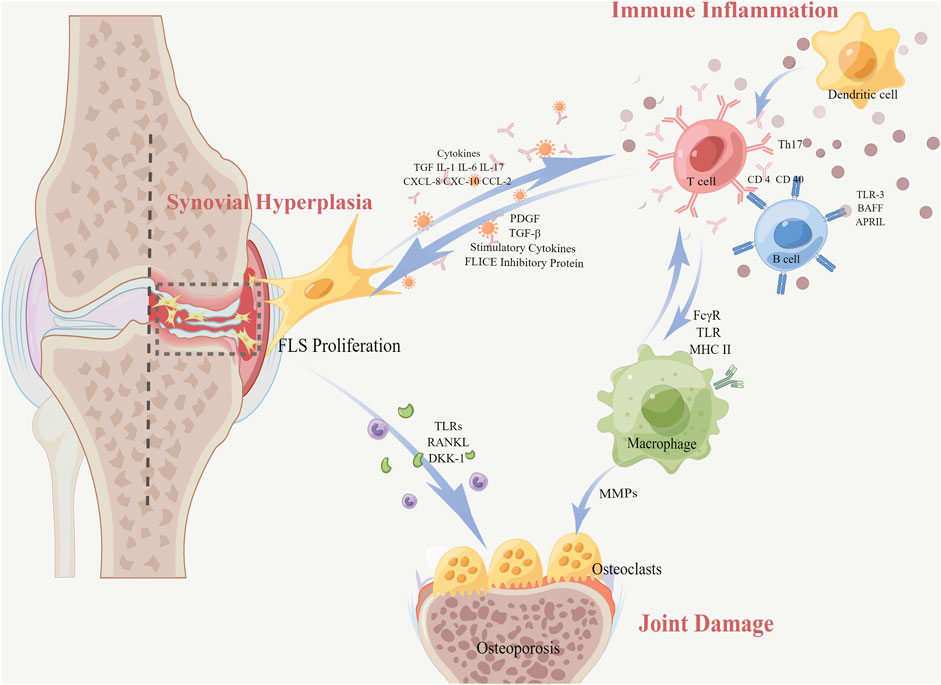
FIGURE 1. Pathological mechanisms of RA with FLS (In RA, the proliferation of FLS resulting from synovial hyperplasia releases various anti-inflammatory cytokines and growth factors. Meanwhile, the interaction between FLS and immune cells causes a transformation of regular FLS into an aggressive phenotype, resulting in an abnormal situation of T-cell and B-cell functions related to immune inflammation. Furthermore, FLS secretes pro-inflammatory cytokines into the joint space and invades the adjacent bone tissue through migration, inducing bone erosion and joint destruction. Macrophages also differentiate directly into mature osteoclasts).
2.1 Synovial hyperplasia
The synovium of RA exhibits endothelial hyperplasia and transformation into pannus tissue that destroys the articular cartilage and bone, with occasional lymphatic-like aggregates. A large number of inflammatory cytokines (IL-1β, TNF-ɑ, etc.) stimulate FLS to proliferate abnormally and exhibit anti-apoptosis. The imbalance between FLS anti-apoptotic and pro-apoptotic factors increases the number of FLS considerably, which directly leads to synovial hyperplasia. The FLS in the synovial lining layer is increased from the normal 1–3 to 10–15 cell layers (Neumann et al., 2010). The proliferated FLS develops into lymphoid-like structures, interacting with immune cells to form lymphoid organs and releasing pro-inflammatory factors and inflammatory mediators. Growth factors, such as platelet-derived growth factor (PDGF), transforming growth factor-β (TGF-β), and stimulatory cytokines in the synovial tissue, induce FLS proliferation through the activation of the signaling pathway. Along with the in situ proliferative capacity of FLS, the expression of anti-apoptotic molecules is also increased. The anti-apoptotic molecule FLICE inhibitory protein (FLIP) suppresses intracellular apoptosis-triggering cystatase-8, decreasing apoptosis and causing synovial proliferation (Bartok and Firestein, 2010).
2.2 Joint damage
Cartilage and bone destruction are hallmarks of RA. MMPs expressed by FLS degrade the chondral matrix, leading to impaired nutrient supply to the articular cartilage and tissue joint destruction.
2.2.1 Chondral matrix degradation
FLS mediates the overproduction of MMPs that interrupts the joint tissue, which contains a structure abundant in collagen and facilitates FLS infestation into the cartilage surface. Mediated by pro-inflammatory cytokines and toll-like receptors (TLRs), FLS upregulates the expression of MMPs, which activate osteoclasts and directly erode the bone, causing cartilage and bone destruction. Activated osteoclasts can reduce bone mass in the periarticular bone early in the lesion, leading to osteoporosis. In addition, the extra expression of MMPs upregulates the levels of inflammatory factors and soluble mediators in the synovial tissue. Also, the factors are bound to receptors of MAPK, JAK/STAT, etc., signaling pathways, promoting and maintaining joint inflammation (Firestein, 2003).
2.2.2 Bone destruction
The migration of FLS is also the process of bone destruction. Due to the cytokines, FLS can migrate into the cartilage and bone, thus exacerbating cartilage destruction (Zeng et al., 2017). FLS produces RANKL in the cartilage or bone. Then, RANKL binds to the receptor activator of NF-κB (RANK) on osteoclast precursors, inducing osteoclast differentiation, activation, and production. A large number of osteoclasts erode the surface of the adjacent articular cartilage membrane and induce bone destruction. Not only that, RA-FLS hinders the recovery process of bone erosion by hindering osteoblast activation through the secretion of dickkopf-1 (DKK-1). DKK-1 is a crucial regulatory molecule within the Wnt pathway, acting as an inhibitor of osteoclast function (Miao et al., 2013). Under specific microenvironmental conditions, macrophages can also differentiate directly into mature osteoclasts. In addition, inflammatory macrophages are a consistent source of matrix metalloproteinases, such as MMP-1, MMP-3, MMP-7, MMP-10, MMP-12, MMP-14, and MMP-25, which participate in connective tissue transformation and joint surface erosion observed in RA.
2.3 Immune inflammation
FLS are known to contribute significantly to RA by secreting inflammatory chemokines that interact with synovial infiltrating cells. The chemokines secreted by FLS, including, CXC motif chemokine 8 (CXCL-8), CXCL-10, and CC motif chemokine ligand 2 (CCL2), can recruit a range of immune cells into the synovial tissue. Then, the inflammatory mediators, for example, IL, TNF-α, and TGF-β1, from these immune cells in turn stimulate FLS activation, resulting in a vicious circle. Macrophages are constantly affected by inflammatory stimuli and participate in the development of chronic synovitis, bone erosion, and cartilage erosion. Macrophages express a lot of molecules on their surface, such as Fc-gamma receptors (FcγRs), TLR, and the major histocompatibility complex class II (MHCII), which in turn, regulate their own activities, activate other cells in the local microenvironment, or attract immune cells outside the joint. TNF-α, IL-6, IL-1β, IL-23, and a wide range of CXCL and CCL chemokines promote and maintain inflammation by recruiting and activating polymorphonuclear leukocytes, T cells, B cells, or monocytes.
2.3.1 FLS and B cells
There is a bidirectional signaling between FLS and B cells. On one hand, FLS affects the maturation and growth of B cells by secreting cytokines. The etiology of autoimmune disorders involves both humoral immunity and B lymphocytes as significant contributors. The preservation of the B-cell pool and humoral immunity depend on the B-cell-activating factor of the TNF family (BAFF, also known as BLYS) and a proliferation-inducing ligand (APRIL). Taking TLR-3 as an example, TLR-3 triggers not only B-cell-activating BAFF but also APRIL. Both of them participate in the stimulation of B cells, thus prolonging B-cell survival (Bombardieri et al., 2011; Leah, 2011). On the other hand, B cells in turn induce the FLS inflammatory phenotype. In the FLS co-culture experiments with age-associated B cells (ABCs), ABCs induce FLS phenotype excitation through TNF-α inducing the activation of ERK1/2 and JAK-STAT1 signaling pathways, consequently promoting the persistence of RA (Qin et al., 2022).
2.3.2 FLS and T cells
T-cell infiltration and excessive proliferation of FLS are significantly upregulated in RA patients. Both interact during RA inflammation to perpetuate inflammation. RA-FLS can present peptides of inflammatory antigens to antigen-specific T cells, contributing to the auto-reactive immune response in RA (Tran et al., 2007). Then, FLS expresses adhesion molecules, transmitting signals to CD4 T cells, such as vascular cell adhesion molecule-1 (VCAM-1) and intercellular cell adhesion molecule-1 (ICAM-1). Finally, these adhesive molecules interact with integrins, for instance, lymphocyte function-associated antigen 1 (LFA-1), resulting in CD4 T-cell proliferation and IL-17 secretion and exacerbation of the inflammatory response (Mori et al., 2017). At the same time, macrophages express MHCII as antigen-presenting cells, thereby participating in the activation and recruitment of pathogenic T cells. So, there is also an interaction between T cells and FLS (Tran et al., 2008; Tu et al., 2022).
To sum up, FLS can secrete pro-inflammatory cytokines such as TNF-α, IL-1β, IL-6, and MMP, in the joint space of RA patients and invade the adjacent bone tissue through migration, inducing bone erosion and joint destruction. The interaction between FLS and immune cells causes a transformation of regular FLS into an aggressive phenotype, resulting in abnormal T- and B-cell functions. Also, our body gradually loses its normal immune regulatory and protective ability (Ding et al., 2023). It is evident that FLS is the central effector cell in the pathogenesis. Given that there is no effective treatment targeted at FLS, the inhibition FLS-mediated pro-inflammatory response and subsequent tissue destruction seems to be a feasible strategy for RA (Nygaard and Firestein, 2020). In the next part, we summarize the results in the recent 3 years of small molecule drugs targeted at FLS.
3 Small molecule drugs regulating FLS
In the previous sections, we have clarified that RA-FLS are activated by multiple cytokines involved in the activation of FLS. Targeted pathways of FLS might simultaneously block multiple signaling of cytokine receptors, inhibiting the activation, proliferation, and invasion of FLS and, thus, significantly controlling RA synovial inflammation and joint damage (Mavers et al., 2009; Wendling et al., 2010; Pan et al., 2016). Despite significant breakthroughs in RA therapy, many people with RA have persistent disease. The current RA therapy plans emphasize reducing T-cell and B-cell activity as well as cytokine signaling (Mahmoud et al., 2022). In RA, targeting signal transduction pathways is an emerging treatment option. According to the signaling pathway interacted with FLS, there are mainly MAPK, NF-κB, JAK/STAT, PI3K/Akt, and Wnt signaling pathways in Figure 2. So, we present the drugs’ research progress which regulates FLS function on the signaling pathways, including the small molecule compounds and natural products. It is aimed to explore promising novel drug development directions and broaden the path of novel targeted FLS.
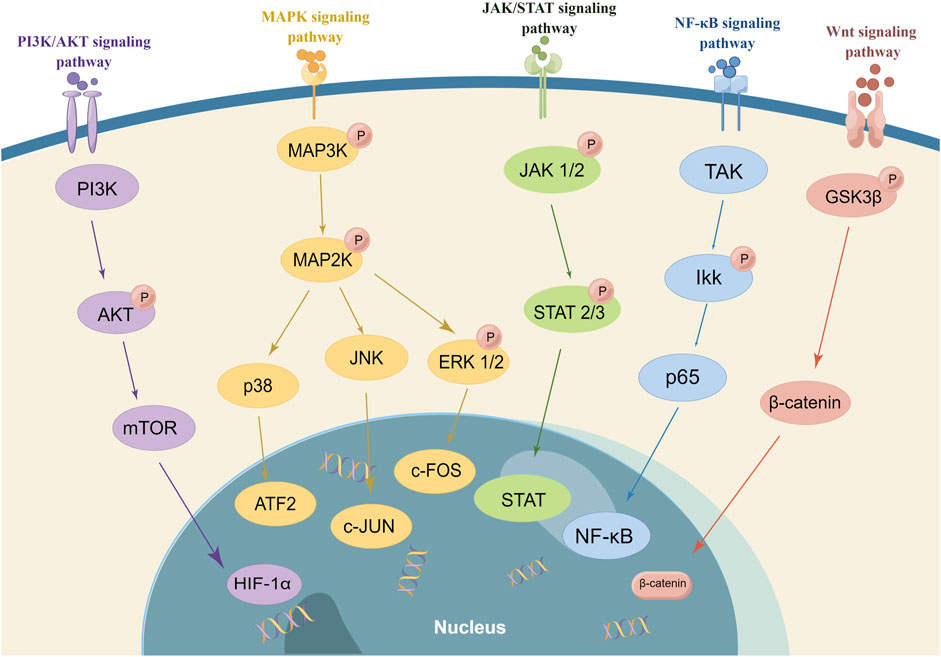
FIGURE 2. Signaling pathway regulating FLS (In RA, targeting signal transduction pathways is an emerging treatment option. The small molecule compounds and natural products interact with FLS in the different signaling pathways. There are mainly MAPK, NF-κB, JAK/STAT, PI3K/Akt, and Wnt signaling pathways. It is important to note that the majority of drugs affected numerous signaling pathways and multiple targets).
3.1 Small molecule drugs targeting MAPK regulating FLS
The MAPK signaling pathway is associated with various kinases, such as P38, c-Jun N-terminal kinase (JNK), and extracellular regulated protein kinases (ERKs), which are involved in the proliferation, apoptosis, and migration of FLS, with the addition of cytokine secretion (Harigai et al., 2004; Tang et al., 2019). ERK is involved in the secretion of certain cytokines and cell proliferation and differentiation through the regulation of B-cell lymphoma 2 (Bcl-2). JNK decreases proteoglycan synthesis and enhances MMP-13 synthesis, which are necessary for bone deterioration and joint inflammation. p38 is associated with the cytokine secretion of MMP. Through inhibiting p38, MMP reduces cartilage degradation and inhibits osteoclast formation. Additionally, the MAPK pathway contributes to the FLS’s increase in TNF-α expression, amplifying inflammatory signals, inducing FLS proliferation, aggravating inflammation, and damaging joints (Zuo et al., 2015; Kadkhoda et al., 2016). An increasing number of studies have shown that the MAPK pathway is activated in immune and autoimmune response conditions, regulating the cell responses of division, differentiation, apoptosis, inflammation, and stress and also participating in the activation of FLS (Müller-Ladner et al., 2007; Bustamante et al., 2017). In addition, MAPK activates downstream transcription factors that promote synovial cell proliferation and chondrocyte apoptosis. It also leads to high expression of multiple MMPs in synovial cells and chondrocytes and overhydrolysis of the extracellular matrix, resulting in joint damage. Therefore, MAPK is one of the most studied targets to inhibit RA-FLS (Wang et al., 2010).
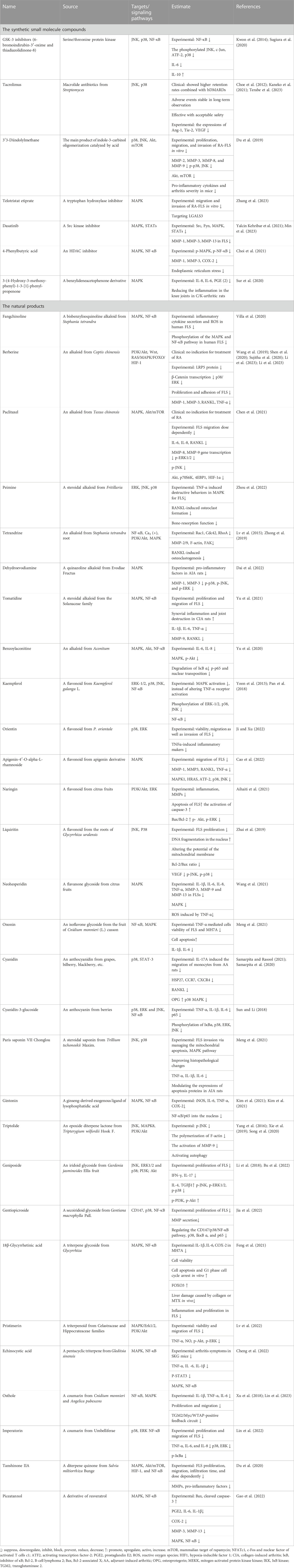
Here, we review the synthetic small molecule compounds and natural products in the recent 3 years targeted to MAPK for FLS in Table 1, and the natural products regulating MAPK are shown in Figure 3. It is important to note that the majority of drugs affected numerous signaling pathways and multiple targets. As an MAPK downstream effector, p38 is considered a possible target for RA, but only few p38 inhibitors have been tested in humans. Tacrolimus as a macrolide calcineurin inhibitor immunosuppressant drug decreased the production of angiopoietin-1 (Ang1), tyrosine-protein kinase receptor (Tie-2), and VEGF in human FLS by preventing the activation of the IL-1β-mediated JNK and p38 MAPK pathways. Sugiura et al.’s (2020) study was very interesting. They found that glycogen synthase kinase 3 (GSK-3) inhibitors significantly reduced synovial fibroblast migration after 72 h and decreased Akt phosphorylation [Ser (473)] after 48 h in vitro, which might have therapeutic efficacy targeting the invasion and migration of synovial fibroblasts. Also, 3′3-diindolylmethane exhibited the possibility of anti-RA-FLS activitiy in vivo and in vitro (Du et al., 2019). The small molecule compounds reported in recent years that could alter FLS in vivo and in vitro were elutriated extirpate, dasatinib, 4-phenylbutyric acid, and 3-(4-hydroxy-3-methoxy-phenyl)-1-3-[1]-phenyl-propenone. Unfortunately, these medications are still in the laboratory stage. Because of their poor performance, p38 inhibitors have limited efficacy in RA treatment. Also, blocking p38’s downstream had a compensatory effect on other kinases, so alternative options for p38 have been progressively explored (Guma et al., 2012). Regulation of MAPK kinases upstream of p38, the human mitogen-activated protein kinase kinase (MKK), such as MKK6 and MKK1, could selectively block the production of MMPs and pro-inflammatory cytokines in FLS (Hammaker et al., 2012). In addition, ubiquitin D might be considered a possible therapeutic target for RA-FLS (Chen et al., 2023).
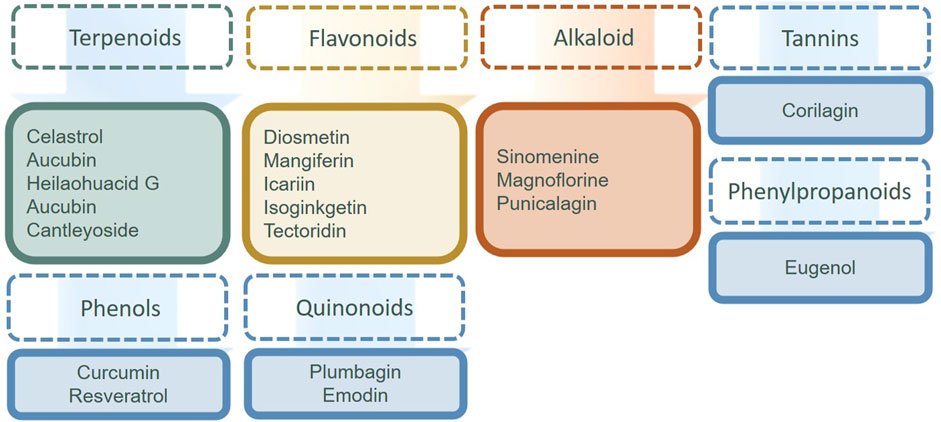
In natural products in Table 1 and Figure 3, alkaloids and flavonoids were more frequently reported and studied for their effects on the MAPK signaling pathway of FLS. Other categories, such as iridoids and saponins, were also found to have an impact. It is well known that flavonoids possess anti-oxidant and anti-inflammatory properties. Flavonoids can inhibit the inflammatory response and reduce the symptoms of inflammation while scavenging free radicals, reducing oxidative stress, and protecting cells from oxidative damage. Flavonoids usually inhibit FLS proliferation, migration, and invasion by inhibiting p38 and JNK. To our surprise, alkaloids also showed up significantly in the treatment of FLS. Preparations of berberine and paclitaxel were available for clinical use, but they have no indication for the treatment of RA.
Triptolide and tetrandrine from Tripterygium wilfordii Hook F. and Stephania tetrandra root, respectively, have anti-rheumatic effects in the classic sense. Tripterygium glycoside preparations have been clinically used for the treatment of RA. As the representative, we concentrate on triptolide, which has been studied more and has been proven to have multiple signaling pathways. The treatment with triptolide decreased the expression of phosphorylated JNK that TNF-α-produced, but it had no effect on the expression of phosphorylated p38 or ERK (Yang et al., 2016) and reduced FLS migration and invasion by targeting the JNK/MAPK signaling pathway (Tang et al., 2020). Triptolide dramatically increased the p-Akt/Akt ratio, and inhibiting the PI3K/Akt signaling pathway in MH7A cells caused autophagy to be triggered, indicating that triptolide repressed autophagy via activating p-Akt/Akt (Xie et al., 2019). Other natural products, such as Paris saponin VII/Chonglou, geniposide, and gentiopicroside, shown in Table 1, also have the potential to regulate FLS against RA. However, it is currently in the experimental stage.
3.2 Small molecule drugs targeting NF-κB regulating FLS
As a major signaling transcription factor, NF-κB contributes to synovial inflammation, proliferation, and decay in bones in RA and regulates inflammatory gene expression and cell proliferation. Both innate and adaptive immune cells include NF-B, which is a key mediator of the stimulation of pro-inflammatory genes (Liu et al., 2017). In a normal situation, NF-κB is bound to its repressor protein IκB and not activated. The nuclear-localization sequence (NLS) that belongs to NF-κB is covered by the IκB unable to undergo nuclear translocation. However, in RA due to the activators (TNF-α, IL-17, etc.), IκB is phosphorylated, ubiquitinated by IκB kinase, and eventually degraded by the enzyme, releasing NF-κB. Following that, NF-κB p65 enters the nucleus and combines with target genes (Aupperle et al., 1999). The production of inflammatory mediators such as TNF-α, COX-2, and IL-1β increases as a result of this nuclear translocation in the synovium. Those activated sustaining states lead to massive abnormal activation of FLS (Saravanan et al., 2014). NF-κB p65 regulates apoptosis and inhibits protein expression, which has an antagonistic effect on apoptosis in FLS (Kadkhoda et al., 2016), leading to synovial hyperplasia and aggravating joint destruction (Yin et al., 2015). In addition, p38 mediates IκB phosphorylation, which is involved in regulating NF-κB activation (Carter et al., 1999; Kaminska, 2005).
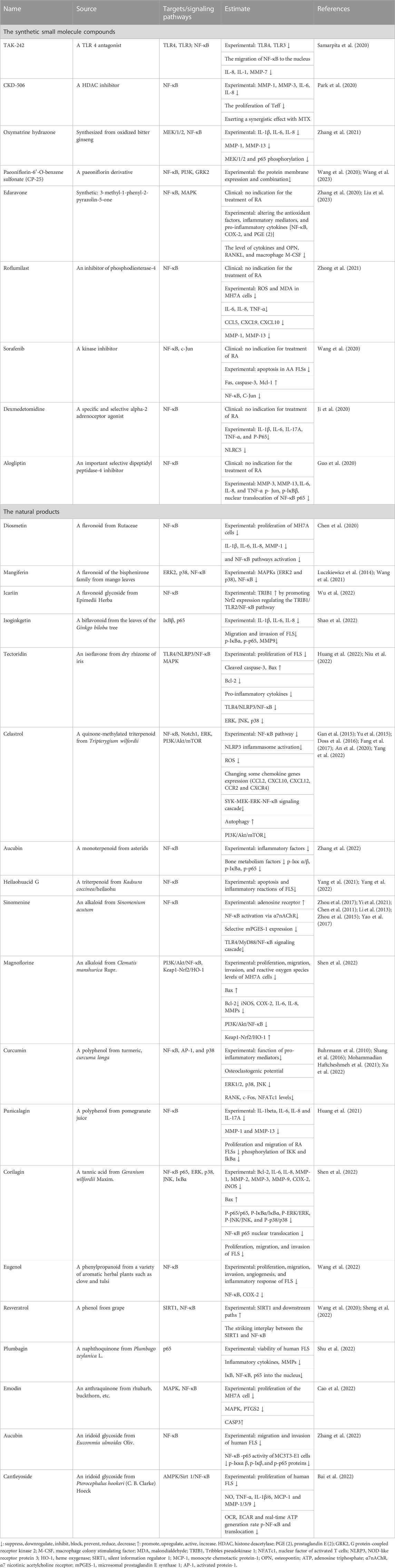
The small molecule drugs and natural products targeted at NF-κB in recent 3 years are summarized in Table 2, and the classification of the natural products is in Figure 3. There have been many studies on small molecule compounds that modulate FLS in the NF-κB signaling pathway, such as TAK-242 (Samarpita et al., 2020), CKD-506 (Park et al., 2020), and synthetic derivatives from natural products that also showed the activity of inhibiting proliferation. For example, oxymatrine hydrazone synthesized from oxidized bitter ginseng induced apoptosis and prevented TNF-α-mediated enhanced viability of RA-FLS (Zhang et al., 2021). Paeoniflorin-6′-O-benzene sulfonate (CP-25), a paeoniflorin derivative, had the ability to decrease membrane expression and the combination of these proteins (Wang et al., 2020; Wang et al., 2023). Edaravone, roflumilast, sorafenib, dexmedetomidine, and alogliptin have been used clinically, without the indication for the treatment of RA. The existing experiments showed that they have the anti-proliferation ability of FLS and were worthy of inclusion in the secondary development of drugs. In the natural products in Figure 4, flavonoids still predominated, such as diosmetin, icariin, isoginkgetin, and tectoridin. In a similar situation with the MAPK inhibitions for RA-FLS, these natural products were in the experimental stage. In addition, some inhibitors modulated both NF-κB and MAPK pathways to regulate FLS activity, such as tectoridin and corilagin.
3.3 Small molecule drugs targeting JAK/STAT regulating FLS
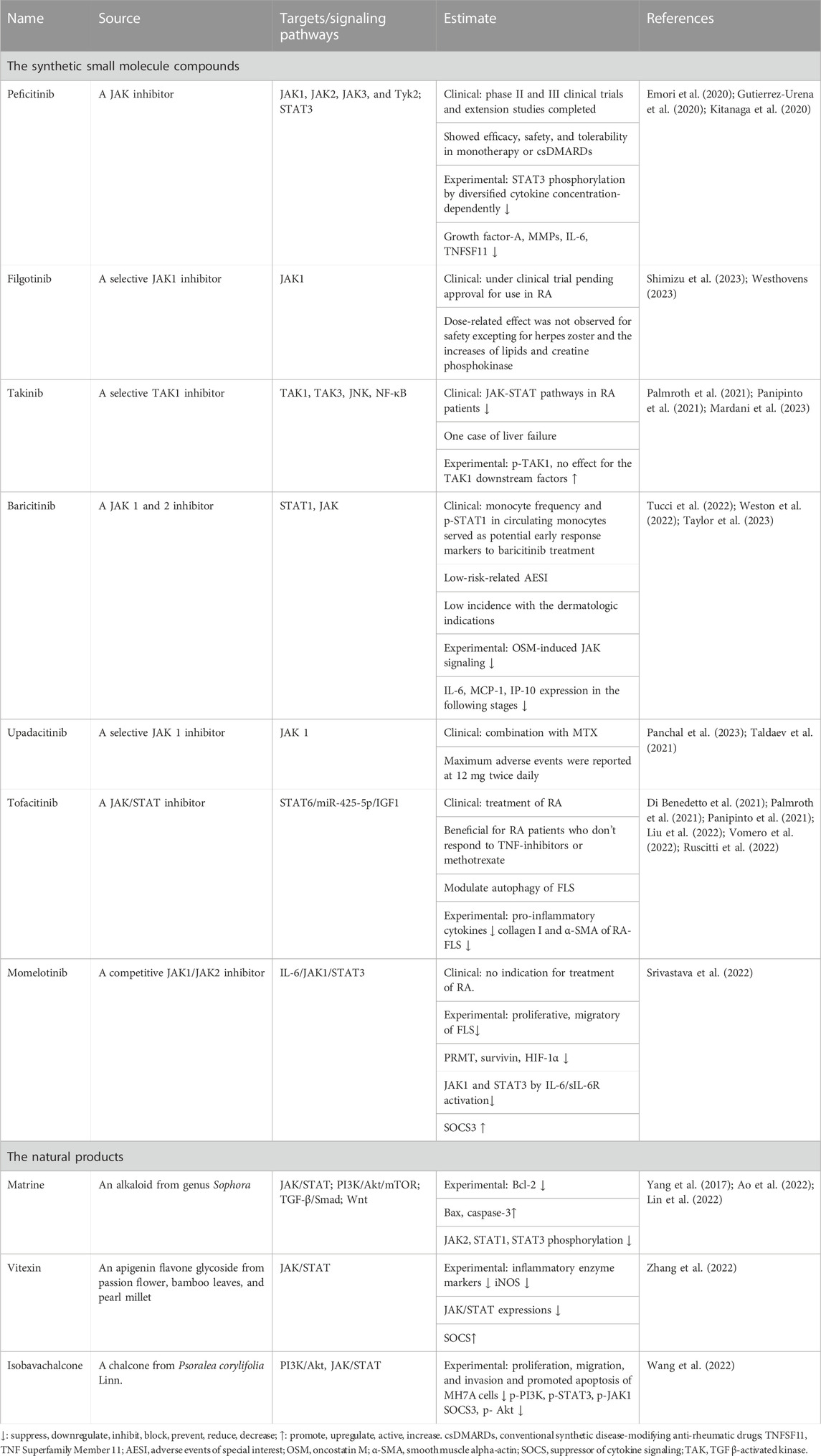
JAK/STAT signaling has been instrumental in regulating immune and inflammatory responses. The JAK/STAT pathway can be segmented into three components: receptor-associated tyrosine kinase, JAK tyrosine kinase, and STAT transcription factor. The JAK kinase activates JAK upon receptor binding, leading to JAK-mediated phosphorylation of STAT. Among the STAT family, STAT1 and STAT3 serve as the primary activators (Kim et al., 2011). The expression and activity of STAT1 are elevated in the initial synovial tissue of RA, while STAT3 facilitates the survival of synovial fibroblasts. Elevated STAT3 expression contributes to the inhibition of programmed cell death-induced anti-apoptotic molecule expression, blocks apoptosis in RA-FLS, and promotes RA synovial thickening (Yang et al., 2017). The JAK/STAT pathway is also involved in regulating the response of RA-FLS to pro-inflammatory cytokines and plays an essential role in the pro-inflammatory response and invasive behavior of FLS (Diller et al., 2019).
Inhibitors of JAKs could block the activation of STATs in RA-LS in the synthesis of various drugs and in the study of natural products. We included the synthetic small molecule compounds and natural products in the last 3 years in Table 3. Tofacitinib is a Food and Drug Administration (FDA)- and European Medicines Agency (EMA)-approved JAK inhibitor that effectively treats RA (Vomero et al., 2022). The synthetic small molecule compounds of peficitinib, fingolitinib, takinib, tolvamycin, baricitinib, and abatinib all demonstrated monotherapy effectiveness in clinical trials in RA. The synthetic JAK inhibitors appeared to be an important treatment choice for difficult-to-treat RA patients and researchers (Kubo et al., 2023). Momelotinib had no indication for the treatment of RA in the clinic, but could inhibit the proliferation and migration of FLS (Srivastava et al., 2022). On the contrary, there are few research reports on the natural products in the JAK/STAT signal pathway.
3.4 Small molecule drugs targeting PI3k/Akt regulating FLS
The PI3K/Akt signaling pathway is involved in regulating cell growth, proliferation, differentiation, and survival and is associated with the production of pro-inflammatory cytokines, degrading enzymes of the extracellular matrix, and other factors in FLS. The activation of PI3K induces the phosphorylation of Akt and p-Akt. As a downstream effector, it can be involved in FLS invasion by regulating the transcriptional levels of MMPs. The Akt phosphorylation also activates downstream mTOR complex 1 (mTORC1). mTORC1 translates mRNA into proteins to regulate the cell activities of metabolism, growth, and differentiation and is involved in RA-FLS proliferation and survival (Wendel et al., 2004; Malemud, 2013).
Table 4 is a summary of the synthetic small molecules and natural drugs that have been developed recently that target PI3k/Akt. Metformin, a drug used to treat type 2 diabetes, has been shown to have a protective effect against the development of RA (Liang et al., 2023), and RA-FLS proliferation is inhibited by metformin in a dose- and time-dependent manner (Chen et al., 2019). The natural products targeted at PI3k/Akt regulating FLS came from a variety of sources. Against the development of inflammatory arthritis, ginger is a preventive substance. There was evidence that ginger helped reduce RA-related joint pain (Al-Nahain et al., 2014). The active ingredients of ginger, 6-shogaol, and 8-shogaol reduced the production of TNF-α, IL-1β, IL-6, etc., prevented migration, invasion, and population growth, and ameliorated joint destruction in mice (N. Li et al., 2023; Jo et al., 2022).
3.5 Wnt signaling pathway and relevant drugs regulating FLS
The Wnt signaling cascade participates in regulating the growth, differentiation, production, and apoptosis of osteoblasts. The conventional Wnt/β-catenin cascade, Wnt/Ca2+ signaling cascade, and Wnt/JNK signaling cascade coordinate with each other to regulate the dynamic balance between osteoclasts and osteoblasts. Once the balance is disturbed, it might lead to bone erosion and bone destruction (Walsh et al., 2009; De, 2011; Deal, 2012). Studies had shown that the growth Wnt3a/5a proteins could activate the Wnt signaling cascade as well as downstream genes, thus increasing fibronectin expression and promoting FLS function. The aforementioned processes also promoted the proliferation of RA synovial tissue without pro-inflammatory factors (Kim et al., 2010; Rabelo Fde et al., 2010; Maeda et al., 2013). Researchers (Cici et al., 2019) suggested that the inflammatory activation of the Wnt pathway might inhibit T-cell function and exacerbate the immune response [181]. In the recent 3 years, we inquired natural products, including paeoniflorin (Yang et al., 2022), 7-hydroxycoumarin (Umbelliferone) (Cai et al., 2022; Cai et al., 2022), and penta-acetyl geniposide (Cai et al., 2021).
4 Conclusion
In this review, we summarized as much as possible the involvement of FLS, covering the RA-FLS pathogenesis, synthetic small molecular compounds, and natural products targeting primary signaling pathways in the last 3 years. Natural products comprise a range of substances derived from diverse natural sources, such as plants, animals, and microorganism. These sources provided valuable resources for the design and development of drugs. From the results, the content of this paper could be continuously extended in the following aspects. 1) For the synthetic small molecule compounds, the popular targeting signaling pathways are still MAPK and NF-κB in the current research stage. We cannot ignore that JAK/STAT has great potential for research studies, due to the fact that several drugs have appeared in the clinic. Moreover, modulation of Wnt signaling might not only repair articular bone damage but also inhibit the production of pro-inflammatory cytokines, showing a new strategy for RA treatment (Miao et al., 2013; Liu et al., 2019). Typically, these signaling pathways interacted with each other. A small molecule could act through multiple pathways. 2) For the natural products, there was great potential. Researchers have tried to explore drugs targeted to activate FLS to treat RA using traditional human experience and herbs. For example, triptolide has been a hot area of research for several years. Most of the results are currently in the experimental stage, not the clinical trial stage. Fortunately, the source plants of these natural products have been used for RA in clinical studies. 3) The natural products derived from herbal medicine that can regulate RA-FLS abnormalities are mainly alkaloids, flavonoids, saponins, phenols, and quinones (Smolen et al., 2018). 4) In addition, we have found many reports on the mechanisms of herbal extract, Chinese herbal compound prescription, and traditional Chinese patent medicines in RA that were worthy of further research.
Author contributions
YT was responsible for writing and drawing by Figdraw. XL drafted the original framework and figures. QD collected and sorted materials. JS and YF provided guidance. LB reviewed writing and drawing. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the National Natural Science Foundation of China (82073311), Sichuan Science and Technology Program (2022JDTD0025 and 2023NSFSC0665), and Scientific Research Project of Sichuan Medical Association (S22084). This work was supported by the Personalized Drug Therapy Key Laboratory of Sichuan Province, and part of the figures were drawn by Figdraw.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aihaiti, Y., Song Cai, Y., Tuerhong, X., Ni Yang, Y., Ma, Y., Shi Zheng, H., et al. (2021). Therapeutic effects of naringin in rheumatoid arthritis: Network pharmacology and experimental validation. Front. Pharmacol. 12, 672054. doi:10.3389/fphar.2021.672054
Al-Nahain, A., Jahan, R., and Rahmatullah, M. (2014). Zingiber officinale: A potential plant against rheumatoid arthritis. Arthritis 2014, 159089–159098. doi:10.1155/2014/159089
Almutairi, K., Nossent, J., Preen, D., Keen, H., and Inderjeeth, C. (2021). The global prevalence of rheumatoid arthritis: A meta-analysis based on a systematic review. Rheumatol. Int. 41 (5), 863–877. doi:10.1007/s00296-020-04731-0
An, L., Li, Z., Shi, L., Wang, L., Wang, Y., Jin, L., et al. (2020). Inflammation-targeted celastrol nanodrug attenuates collagen-induced arthritis through NF-κB and Notch1 pathways. Nano Lett. 20 (10), 7728–7736. doi:10.1021/acs.nanolett.0c03279
Ao, L., Gao, H., Jia, L., Liu, S., Guo, J., Liu, B., et al. (2022). Matrine inhibits synovial angiogenesis in collagen-induced arthritis rats by regulating HIF-VEGF-Ang and inhibiting the PI3K/Akt signaling pathway. Mol. Immunol. 141, 13–20. doi:10.1016/j.molimm.2021.11.002
Aupperle, K. R., Bennett, B. L., Boyle, D. L., Tak, P. P., Manning, A. M., and Firestein, G. S. (1999). NF-kappa B regulation by I kappa B kinase in primary fibroblast-like synoviocytes. J. Immunol. 163 (1), 427–433. doi:10.4049/jimmunol.163.1.427
Bai, J., Xie, N., Hou, Y., Chen, X., Hu, Y., Zhang, Y., et al. (2022). The enhanced mitochondrial dysfunction by cantleyoside confines inflammatory response and promotes apoptosis of human HFLS-RA cell line via AMPK/Sirt 1/NF-κB pathway activation. Biomed. Pharmacother. 149, 112847. doi:10.1016/j.biopha.2022.112847
Bartok, B., and Firestein, G. S. (2010). Fibroblast-like synoviocytes: Key effector cells in rheumatoid arthritis. Immunol. Rev. 233 (1), 233–255. doi:10.1111/j.0105-2896.2009.00859.x
Bombardieri, M., Kam, N. W., Brentano, F., Choi, K., Filer, A., Kyburz, D., et al. (2011). A BAFF/APRIL-dependent TLR3-stimulated pathway enhances the capacity of rheumatoid synovial fibroblasts to induce AID expression and Ig class-switching in B cells. Ann. Rheum. Dis. 70 (10), 1857–1865. doi:10.1136/ard.2011.150219
Bu, Y., Wu, H., Deng, R., and Wang, Y. (2022). The anti-angiogenesis mechanism of Geniposide on rheumatoid arthritis is related to the regulation of PTEN. Inflammopharmacology 30 (3), 1047–1062. doi:10.1007/s10787-022-00975-3
Buhrmann, C., Mobasheri, A., Matis, U., and Shakibaei, M. (2010). Curcumin mediated suppression of nuclear factor-κB promotes chondrogenic differentiation of mesenchymal stem cells in a high-density co-culture microenvironment. Arthritis Res. Ther. 12 (4), R127. doi:10.1186/ar3065
Bustamante, M. F., Garcia-Carbonell, R., Whisenant, K. D., and Guma, M. (2017). Fibroblast-like synoviocyte metabolism in the pathogenesis of rheumatoid arthritis. Arthritis Res. Ther. 19 (1), 110. doi:10.1186/s13075-017-1303-3
Cai, L., Mu, Y. R., Liu, M. M., Zhou, M. Y., Meng, B., Liu, F. Y., et al. (2021). Penta-acetyl Geniposide suppresses migration, invasion, and inflammation of TNF-α-stimulated rheumatoid arthritis fibroblast-like synoviocytes involving wnt/β-catenin signaling pathway. Inflammation 44 (6), 2232–2245. doi:10.1007/s10753-021-01495-y
Cai, L., Zhou, M. Y., Hu, S., Liu, F. Y., Wang, M. Q., Wang, X. H., et al. (2022). Umbelliferone inhibits migration, invasion and inflammation of rheumatoid arthritis fibroblast-like synoviocytes and relieves adjuvant-induced arthritis in rats by blockade of wnt/β-catenin signaling pathway. Am. J. Chin. Med. 50 (7), 1945–1962. doi:10.1142/S0192415X22500835
Cai, L., Zong, P., Zhou, M. Y., Liu, F. Y., Meng, B., Liu, M. M., et al. (2022). 7-Hydroxycoumarin mitigates the severity of collagen-induced arthritis in rats by inhibiting proliferation and inducing apoptosis of fibroblast-like synoviocytes via suppression of Wnt/β-catenin signaling pathway. Phytomedicine 94, 153841. doi:10.1016/j.phymed.2021.153841
Cao, C., Zeng, L., and Rong, X. (2022). Therapeutic mechanism of emodin for treatment of rheumatoid arthritis: A network pharmacology-based analysis. Nan Fang. Yi Ke Da Xue Xue Bao 42 (6), 913–921. doi:10.12122/j.issn.1673-4254.2022.06.16
Cao, D., Fan, Q., Li, Z., Chen, M., Jiang, Y., Lin, R., et al. (2022). Transcriptomic profiling revealed the role of apigenin-4'-O-α-L-rhamnoside in inhibiting the activation of rheumatoid arthritis fibroblast-like synoviocytes via MAPK signaling pathway. Phytomedicine 102, 154201. doi:10.1016/j.phymed.2022.154201
Carter, A. B., Knudtson, K. L., Monick, M. M., and Hunninghake, G. W. (1999). The p38 mitogen-activated protein kinase is required for NF-kappaB-dependent gene expression. The role of TATA-binding protein (TBP). J. Biol. Chem. 274 (43), 30858–30863. doi:10.1074/jbc.274.43.30858
Chen, D. P., Wong, C. K., Leung, P. C., Fung, K. P., Lau, C. B., Lau, C. P., et al. (2011). Anti-inflammatory activities of Chinese herbal medicine sinomenine and Liang Miao San on tumor necrosis factor-α-activated human fibroblast-like synoviocytes in rheumatoid arthritis. J. Ethnopharmacol. 137 (1), 457–468. doi:10.1016/j.jep.2011.05.048
Chen, H., Tao, L., Liang, J., Pan, C., and Wei, H. (2023). Ubiquitin D promotes the progression of rheumatoid arthritis via activation of the p38 MAPK pathway. Mol. Med. Rep. 27 (2), 53. doi:10.3892/mmr.2023.12940
Chen, J., Lin, X., He, J., Liu, D., He, L., Zhang, M., et al. (2022). Artemisitene suppresses rheumatoid arthritis progression via modulating METTL3-mediated N6-methyladenosine modification of ICAM2 mRNA in fibroblast-like synoviocytes. Clin. Transl. Med. 12 (12), e1148. doi:10.1002/ctm2.1148
Chen, K., Lin, Z. W., He, S. M., Wang, C. Q., Yang, J. C., Lu, Y., et al. (2019). Metformin inhibits the proliferation of rheumatoid arthritis fibroblast-like synoviocytes through IGF-IR/PI3K/AKT/m-TOR pathway. Biomed. Pharmacother. 115, 108875. doi:10.1016/j.biopha.2019.108875
Chen, X., Lin, H., Chen, J., Wu, L., Zhu, J., Ye, Y., et al. (2021). Paclitaxel inhibits synoviocyte migration and inflammatory mediator production in rheumatoid arthritis. Front. Pharmacol. 12, 714566. doi:10.3389/fphar.2021.714566
Chen, Y., Wang, Y., Liu, M., Zhou, B., and Yang, G. (2020). Diosmetin exhibits anti-proliferative and anti-inflammatory effects on TNF-α-stimulated human rheumatoid arthritis fibroblast-like synoviocytes through regulating the Akt and NF-κB signaling pathways. Phytother. Res. 34 (6), 1310–1319. doi:10.1002/ptr.6596
Cheng, Y. C., Zhang, X., Lin, S. C., Li, S., Chang, Y. K., Chen, H. H., et al. (2022). Echinocystic acid ameliorates arthritis in SKG mice by suppressing Th17 cell differentiation and human rheumatoid arthritis fibroblast-like synoviocytes inflammation. J. Agric. Food Chem. 70 (51), 16176–16187. doi:10.1021/acs.jafc.2c05802
Choe, J. Y., Lee, S. J., Park, S. H., and Kim, S. K. (2012). Tacrolimus (FK506) inhibits interleukin-1β-induced angiopoietin-1, Tie-2 receptor, and vascular endothelial growth factor through down-regulation of JNK and p38 pathway in human rheumatoid fibroblast-like synoviocytes. Jt. Bone Spine 79 (2), 137–143. doi:10.1016/j.jbspin.2011.03.018
Choi, Y., Lee, E. G., Jeong, J. H., and Yoo, W. H. (2021). 4-Phenylbutyric acid, a potent endoplasmic reticulum stress inhibitor, attenuates the severity of collagen-induced arthritis in mice via inhibition of proliferation and inflammatory responses of synovial fibroblasts. Kaohsiung J. Med. Sci. 37 (7), 604–615. doi:10.1002/kjm2.12376
Cici, D., Corrado, A., Rotondo, C., and Cantatore, F. P. (2019). Wnt signaling and biological therapy in rheumatoid arthritis and spondyloarthritis. Int. J. Mol. Sci. 20 (22), 5552. doi:10.3390/ijms20225552
Dai, Y., Sheng, J., He, S., Wu, Q., Wang, Y., and Su, L. (2022). Dehydroevodiamine suppresses inflammatory responses in adjuvant-induced arthritis rats and human fibroblast-like synoviocytes. Bioengineered 13 (1), 268–279. doi:10.1080/21655979.2021.1999554
Davis, J. M., and Matteson, E. L.American College of RheumatologyEuropean League Against Rheumatism (2012). My treatment approach to rheumatoid arthritis. Mayo Clin. Proc. 87 (7), 659–673. doi:10.1016/j.mayocp.2012.03.011
De, A. (2011). Wnt/Ca2+ signaling pathway: A brief overview. Acta Biochim. Biophys. Sin. (Shanghai) 43 (10), 745–756. doi:10.1093/abbs/gmr079
Deal, C. (2012). Bone loss in rheumatoid arthritis: Systemic, periarticular, and focal. Curr. Rheumatol. Rep. 14 (3), 231–237. doi:10.1007/s11926-012-0253-7
Deng, H., Zheng, M., Hu, Z., Zeng, X., Kuang, N., and Fu, Y. (2020). Effects of daphnetin on the autophagy signaling pathway of fibroblast-like synoviocytes in rats with collagen-induced arthritis (CIA) induced by TNF-α. Cytokine 127, 154952. doi:10.1016/j.cyto.2019.154952
Di Benedetto, P., Ruscitti, P., Berardicurti, O., Panzera, N., Grazia, N., Di Vito Nolfi, M., et al. (2021). Blocking Jak/STAT signalling using tofacitinib inhibits angiogenesis in experimental arthritis. Arthritis Res. Ther. 23 (1), 213. doi:10.1186/s13075-021-02587-8
Diller, M., Hasseli, R., Hülser, M. L., Aykara, I., Frommer, K., Rehart, S., et al. (2019). Targeting activated synovial fibroblasts in rheumatoid arthritis by peficitinib. Front. Immunol. 10, 541. doi:10.3389/fimmu.2019.00541
Ding, Q., Hu, W., Wang, R., Yang, Q., Zhu, M., Li, M., et al. (2023). Signaling pathways in rheumatoid arthritis: Implications for targeted therapy. Signal Transduct. Target Ther. 8 (1), 68. doi:10.1038/s41392-023-01331-9
Doss, H. M., Ganesan, R., and Rasool, M. (2016). Trikatu, an herbal compound ameliorates rheumatoid arthritis by the suppression of inflammatory immune responses in rats with adjuvant-induced arthritis and on cultured fibroblast like synoviocytes via the inhibition of the NFκB signaling pathway. Chem. Biol. Interact. 258, 175–186. doi:10.1016/j.cbi.2016.09.003
Du, H., Wang, Y., Zeng, Y., Huang, X., Liu, D., Ye, L., et al. (2020). Tanshinone IIA suppresses proliferation and inflammatory cytokine production of synovial fibroblasts from rheumatoid arthritis patients induced by TNF-α and attenuates the inflammatory response in AIA mice. Front. Pharmacol. 11, 568. doi:10.3389/fphar.2020.00568
Du, H., Zhang, X., Zeng, Y., Huang, X., Chen, H., Wang, S., et al. (2019). A novel phytochemical, DIM, inhibits proliferation, migration, invasion and TNF-α induced inflammatory cytokine production of synovial fibroblasts from rheumatoid arthritis patients by targeting MAPK and AKT/mTOR signal pathway. Front. Immunol. 10, 1620. doi:10.3389/fimmu.2019.01620
Emori, T., Kasahara, M., Sugahara, S., Hashimoto, M., Ito, H., Narumiya, S., et al. (2020). Role of JAK-STAT signaling in the pathogenic behavior of fibroblast-like synoviocytes in rheumatoid arthritis: Effect of the novel JAK inhibitor peficitinib. Eur. J. Pharmacol. 882, 173238. doi:10.1016/j.ejphar.2020.173238
Fang, Z., He, D., Yu, B., Liu, F., Zuo, J., Li, Y., et al. (2017). High-throughput study of the effects of celastrol on activated fibroblast-like synoviocytes from patients with rheumatoid arthritis. Genes (Basel) 8 (9), 221. doi:10.3390/genes8090221
Feng, Y., Mei, L., Wang, M., Huang, Q., and Huang, R. (2021). Anti-inflammatory and pro-apoptotic effects of 18beta-glycyrrhetinic acid in vitro and in vivo models of rheumatoid arthritis. Front. Pharmacol. 12, 681525. doi:10.3389/fphar.2021.681525
Firestein, G. S. (2003). Evolving concepts of rheumatoid arthritis. Nature 423 (6937), 356–361. doi:10.1038/nature01661
Gan, K., Xu, L., Feng, X., Zhang, Q., Wang, F., Zhang, M., et al. (2015). Celastrol attenuates bone erosion in collagen-Induced arthritis mice and inhibits osteoclast differentiation and function in RANKL-induced RAW264.7. Int. Immunopharmacol. 24 (2), 239–246. doi:10.1016/j.intimp.2014.12.012
Gao, X., Kang, X., Lu, H., Xue, E., Chen, R., Pan, J., et al. (2022). Piceatannol suppresses inflammation and promotes apoptosis in rheumatoid arthritis-fibroblast-like synoviocytes by inhibiting the NF-κB and MAPK signaling pathways. Mol. Med. Rep. 25 (5), 180. doi:10.3892/mmr.2022.12696
Gharib, M., Elbaz, W., Darweesh, E., Sabri, N. A., and Shawki, M. A. (2021). Efficacy and safety of Metformin use in rheumatoid arthritis: A randomized controlled study. Front. Pharmacol. 12, 726490. doi:10.3389/fphar.2021.726490
Guma, M., Hammaker, D., Topolewski, K., Corr, M., Boyle, D. L., Karin, M., et al. (2012). Antiinflammatory functions of p38 in mouse models of rheumatoid arthritis: Advantages of targeting upstream kinases MKK-3 or MKK-6. Arthritis Rheum. 64 (9), 2887–2895. doi:10.1002/art.34489
Guo, Q., Zhang, S., Huang, J., and Liu, K. (2020). Alogliptin inhibits IL-1β-induced inflammatory response in fibroblast-like synoviocytes. Int. Immunopharmacol. 83, 106372. doi:10.1016/j.intimp.2020.106372
Gutierrez-Urena, S. R., Amaya-Cabrera, E. L., Uribe-Martinez, J. F., Ventura-Valenzuela, M. E., Rosal-Arteaga, C., Martinez-Bonilla, G. E., et al. (2020). Peficitinib hydrobromide to treat rheumatoid arthritis. Drugs Today (Barc) 56 (8), 505–514. doi:10.1358/dot.2020.56.8.3123469
Hammaker, D., Topolewski, K., Edgar, M., Yoshizawa, T., Fukushima, A., Boyle, D. L., et al. (2012). Decreased collagen-induced arthritis severity and adaptive immunity in MKK-6-deficient mice. Arthritis Rheum. 64 (3), 678–687. doi:10.1002/art.33359
Harigai, M., Hara, M., Kawamoto, M., Kawaguchi, Y., Sugiura, T., Tanaka, M., et al. (2004). Amplification of the synovial inflammatory response through activation of mitogen-activated protein kinases and nuclear factor kappaB using ligation of CD40 on CD14+ synovial cells from patients with rheumatoid arthritis. Arthritis Rheum. 50 (7), 2167–2177. doi:10.1002/art.20340
Hu, X., Tang, J., Zeng, G., Hu, X., Bao, P., Wu, J., et al. (2019). RGS1 silencing inhibits the inflammatory response and angiogenesis in rheumatoid arthritis rats through the inactivation of Toll-like receptor signaling pathway. J. Cell Physiol. 234 (11), 20432–20442. doi:10.1002/jcp.28645
Huang, M., Wu, K., Zeng, S., Liu, W., Cui, T., Chen, Z., et al. (2021). Punicalagin inhibited inflammation and migration of fibroblast-like synoviocytes through NF-κB pathway in the experimental study of rheumatoid arthritis. J. Inflamm. Res. 14, 1901–1913. doi:10.2147/JIR.S302929
Huang, Q., Xiao, X., Yu, J., Yang, Y., Yu, J., Liu, Y., et al. (2022). Tectoridin exhibits anti-rheumatoid arthritis activity through the inhibition of the inflammatory response and the MAPK pathway in vivo and in vitro. Arch. Biochem. Biophys. 727, 109328. doi:10.1016/j.abb.2022.109328
Jay, G. D., Britt, D. E., and Cha, C. J. (2000). Lubricin is a product of megakaryocyte stimulating factor gene expression by human synovial fibroblasts. J. Rheumatol. 27 (3), 594–600.
Ji, W., and Xu, W. (2022). Orientin inhibits the progression of fibroblast-like synovial cells in rheumatoid arthritis by regulating MAPK-signaling pathway. Allergol. Immunopathol. Madr. 50 (6), 154–162. doi:10.15586/aei.v50i6.742
Ji, Y. R., Chen, Y., Chen, Y. N., Qiu, G. L., Wen, J. G., Zheng, Y., et al. (2020). Dexmedetomidine inhibits the invasion, migration, and inflammation of rheumatoid arthritis fibroblast-like synoviocytes by reducing the expression of NLRC5. Int. Immunopharmacol. 82, 106374. doi:10.1016/j.intimp.2020.106374
Jia, N., Ma, H., Zhang, T., Wang, L., Cui, J., Zha, Y., et al. (2022). Gentiopicroside attenuates collagen-induced arthritis in mice via modulating the CD147/p38/NF-κB pathway. Int. Immunopharmacol. 108, 108854. doi:10.1016/j.intimp.2022.108854
Jo, S., Samarpita, S., Lee, J. S., Lee, Y. J., Son, J. E., Jeong, M., et al. (2022). 8-Shogaol inhibits rheumatoid arthritis through targeting TAK1. Pharmacol. Res. 178, 106176. doi:10.1016/j.phrs.2022.106176
Kadkhoda, Z., Amirzargar, A., Esmaili, Z., Vojdanian, M., and Akbari, S. (2016). Effect of TNF-α blockade in gingival crevicular fluid on periodontal condition of patients with rheumatoid arthritis. Iran. J. Immunol. 13 (3), 197–203.
Kaminska, B. (2005). MAPK signalling pathways as molecular targets for anti-inflammatory therapy--from molecular mechanisms to therapeutic benefits. Biochim. Biophys. Acta 1754 (1-2), 253–262. doi:10.1016/j.bbapap.2005.08.017
Kaneko, Y., Kawahito, Y., Kojima, M., Nakayama, T., Hirata, S., Kishimoto, M., et al. (2021). Efficacy and safety of tacrolimus in patients with rheumatoid arthritis - a systematic review and meta-analysis. Mod. Rheumatol. 31 (1), 61–69. doi:10.1080/14397595.2020.1719607
Kim, J., Kim, J., Kim, D. W., Ha, Y., Ihm, M. H., Kim, H., et al. (2010). Wnt5a induces endothelial inflammation via beta-catenin-independent signaling. J. Immunol. 185 (2), 1274–1282. doi:10.4049/jimmunol.1000181
Kim, M., Sur, B., Villa, T., Nah, S. Y., and Oh, S. (2021). Inhibitory activity of gintonin on inflammation in human IL-1β-stimulated fibroblast-like synoviocytes and collagen-induced arthritis in mice. J. Ginseng Res. 45 (4), 510–518. doi:10.1016/j.jgr.2020.12.001
Kim, M., Sur, B., Villa, T., Yun, J., Nah, S. Y., and Oh, S. (2021). Gintonin regulates inflammation in human IL-1β-stimulated fibroblast-like synoviocytes and carrageenan/kaolin-induced arthritis in rats through LPAR2. J. Ginseng Res. 45 (5), 575–582. doi:10.1016/j.jgr.2021.02.001
Kim, S. K., Park, K. Y., Yoon, W. C., Park, S. H., Park, K. K., Yoo, D. H., et al. (2011). Melittin enhances apoptosis through suppression of IL-6/sIL-6R complex-induced NF-κB and STAT3 activation and Bcl-2 expression for human fibroblast-like synoviocytes in rheumatoid arthritis. Jt. Bone Spine 78 (5), 471–477. doi:10.1016/j.jbspin.2011.01.004
Kitanaga, Y., Imamura, E., Nakahara, Y., Fukahori, H., Fujii, Y., Kubo, S., et al. (2020). In vitro pharmacological effects of peficitinib on lymphocyte activation: A potential treatment for systemic sclerosis with JAK inhibitors. Rheumatol. Oxf. 59 (8), 1957–1968. doi:10.1093/rheumatology/kez526
Kubo, S., Nakayamada, S., and Tanaka, Y. (2023). JAK inhibitors for rheumatoid arthritis. Expert Opin. Investig. Drugs 32 (4), 333–344. doi:10.1080/13543784.2023.2199919
Kwon, Y. J., Yoon, C. H., Lee, S. W., Park, Y. B., Lee, S. K., and Park, M. C. (2014). Inhibition of glycogen synthase kinase-3β suppresses inflammatory responses in rheumatoid arthritis fibroblast-like synoviocytes and collagen-induced arthritis. Jt. Bone Spine 81 (3), 240–246. doi:10.1016/j.jbspin.2013.09.006
Lampropoulos, C. E., Orfanos, P., Bournia, V. K., Karatsourakis, T., Mavragani, C., Pikazis, D., et al. (2015). Adverse events and infections in patients with rheumatoid arthritis treated with conventional drugs or biologic agents: A real world study. Clin. Exp. Rheumatol. 33 (2), 216–224.
Leah, E. (2011). Crosstalk in RA synovia-TLR3-BAFF axis sustains B-cell activation. Nat. Rev. Rheumatol. 7 (10), 559. doi:10.1038/nrrheum.2011.122
Li, F., Dai, M., Wu, H., Deng, R., Fu, J., Zhang, Z., et al. (2018). Immunosuppressive effect of Geniposide on mitogen-activated protein kinase signalling pathway and their cross-talk in fibroblast-like synoviocytes of adjuvant arthritis rats. Molecules 23 (1), 91. doi:10.3390/molecules23010091
Li, J., Pang, J., Liu, Z., Ge, X., Zhen, Y., Jiang, C. C., et al. (2021). Shikonin induces programmed death of fibroblast synovial cells in rheumatoid arthritis by inhibiting energy pathways. Sci. Rep. 11 (1), 18263. doi:10.1038/s41598-021-97713-6
Li, N., Li, X., Deng, L., Yang, H., Gong, Z., Wang, Q., et al. (2023). 6-Shogaol inhibits the proliferation, apoptosis, and migration of rheumatoid arthritis fibroblast-like synoviocytes via the PI3K/AKT/NF-κB pathway. Phytomedicine 109, 154562. doi:10.1016/j.phymed.2022.154562
Li, X., He, L., Hu, Y., Duan, H., Li, X., Tan, S., et al. (2013). Sinomenine suppresses osteoclast formation and Mycobacterium tuberculosis H37Ra-induced bone loss by modulating RANKL signaling pathways. PLoS One 8 (9), e74274. doi:10.1371/journal.pone.0074274
Li, X., and Wang, Y. (2020). Cinnamaldehyde attenuates the progression of rheumatoid arthritis through down-regulation of PI3K/AKT signaling pathway. Inflammation 43 (5), 1729–1741. doi:10.1007/s10753-020-01246-5
Li, Z., Chen, M., Wang, Z., Fan, Q., Lin, Z., Tao, X., et al. (2023). Berberine inhibits RA-FLS cell proliferation and adhesion by regulating RAS/MAPK/FOXO/HIF-1 signal pathway in the treatment of rheumatoid arthritis. Bone Jt. Res. 12 (2), 91–102. doi:10.1302/2046-3758.122.BJR-2022-0269.R1
Lian-Hua, H. E., Qian-Qian, W., Cong-Cong, S., Na, L., and Chun-Fang, L. (2020). Effect of shikonin on function of rheumatoid arthritis fibroblast like synoviocytes. Zhongguo Zhong Yao Za Zhi 45 (19), 4712–4718. doi:10.19540/j.cnki.cjcmm.20200506.401
Liang, J., Cai, Y., Zhang, J., Jing, Z., Lv, L., Zhang, G., et al. (2023). Metformin treatment reduces the incidence of rheumatoid arthritis: A two-sample mendelian randomized study. J. Clin. Med. 12 (7), 2461. doi:10.3390/jcm12072461
Lin, W., Chen, G., Mao, Y., Ma, X., Zhou, J., Yu, X., et al. (2022). Imperatorin inhibits proliferation, migration, and inflammation via blocking the NF-κB and MAPK pathways in rheumatoid fibroblast-like synoviocytes. ACS Omega 7 (34), 29868–29876. doi:10.1021/acsomega.2c02766
Lin, X., Chen, J., Tao, C., Luo, L., He, J., and Wang, Q. (2023). Osthole regulates N6-methyladenosine-modified TGM2 to inhibit the progression of rheumatoid arthritis and associated interstitial lung disease. MedComm 4(2), e219. doi:10.1002/mco2.219
Lin, Y., He, F., Wu, L., Xu, Y., and Du, Q. (2022). Matrine exerts pharmacological effects through multiple signaling pathways: A comprehensive review. Drug Des. Devel Ther. 16, 533–569. doi:10.2147/DDDT.S349678
Liu, J., Zhao, N., Su, S. H., Gao, Y., and Qi, B. (2023). Anti-arthritic effect of edaravone against complete freund adjuvant induced arthritis via osteoclast differentiation and HIF-1α-VEGF-ANG-1 Axis. Drug Des. Devel Ther. 17, 519–534. doi:10.2147/DDDT.S391606
Liu, R., Song, Y., Li, C., Zhang, Z., Xue, Z., Huang, Q., et al. (2022). The naturally occurring flavonoid nobiletin reverses methotrexate resistance via inhibition of P-glycoprotein synthesis. J. Biol. Chem. 298 (4), 101756. doi:10.1016/j.jbc.2022.101756
Liu, T., Zhang, L., Joo, D., and Sun, S. C. (2017). NF-κB signaling in inflammation. Signal Transduct. Target Ther. 2, 17023. doi:10.1038/sigtrans.2017.23
Liu, X. G., Zhang, Y., Ju, W. F., Li, C. Y., and Mu, Y. C. (2019). MiR-21 relieves rheumatoid arthritis in rats via targeting Wnt signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 23 (3), 96–103. doi:10.26355/eurrev_201908_18635
Liu, Y., Peng, J., Xiong, X., Cheng, L., and Cheng, X. (2022). Tofacitinib enhances IGF1 via inhibiting STAT6 transcriptionally activated-miR-425-5p to ameliorate inflammation in RA-FLS. Mol. Cell Biochem. 477 (10), 2335–2344. doi:10.1007/s11010-022-04444-x
Luczkiewicz, P., Kokotkiewicz, A., Dampc, A., and Luczkiewicz, M. (2014). Mangiferin: A promising therapeutic agent for rheumatoid arthritis treatment. Med. Hypotheses 83 (5), 570–574. doi:10.1016/j.mehy.2014.08.021
Lv, M., Liang, Q., Luo, Z., Han, B., Ni, T., Wang, Y., et al. (2022). UPLC-LTQ-Orbitrap-Based cell metabolomics and network pharmacology analysis to reveal the potential antiarthritic effects of pristimerin: In vitro, in silico and in vivo study. Metabolites 12 (9), 839. doi:10.3390/metabo12090839
Lv, Q., Zhu, X. Y., Xia, Y. F., Dai, Y., and Wei, Z. F. (2015). Tetrandrine inhibits migration and invasion of rheumatoid arthritis fibroblast-like synoviocytes through down-regulating the expressions of Rac1, Cdc42, and RhoA GTPases and activation of the PI3K/Akt and JNK signaling pathways. Chin. J. Nat. Med. 13 (11), 831–841. doi:10.1016/S1875-5364(15)30087-X
Maeda, K., Takahashi, N., and Kobayashi, Y. (2013). Roles of Wnt signals in bone resorption during physiological and pathological states. J. Mol. Med. Berl. 91 (1), 15–23. doi:10.1007/s00109-012-0974-0
Mahmoud, D. E., Kaabachi, W., Sassi, N., Tarhouni, L., Rekik, S., Jemmali, S., et al. (2022). The synovial fluid fibroblast-like synoviocyte: A long-neglected piece in the puzzle of rheumatoid arthritis pathogenesis. Front. Immunol. 13, 942417. doi:10.3389/fimmu.2022.942417
Malemud, C. J. (2013). Intracellular signaling pathways in rheumatoid arthritis. J. Clin. Cell Immunol. 4, 160. doi:10.4172/2155-9899.1000160
Mardani, M., Mohammadshahi, J., Abolghasemi, S., and Teimourpour, R. (2023). Drug-induced liver injury due to tofacitinib: A case report. J. Med. Case Rep. 17 (1), 97. doi:10.1186/s13256-023-03821-4
Mavers, M., Ruderman, E. M., and Perlman, H. (2009). Intracellular signal pathways: Potential for therapies. Curr. Rheumatol. Rep. 11 (5), 378–385. doi:10.1007/s11926-009-0054-9
Meng, M., Yue, Z., Chang, L., Liu, Y., Hu, J., Song, Z., et al. (2021). Anti-rheumatoid arthritic effects of Paris saponin VII in human rheumatoid arthritis fibroblast-like synoviocytes and adjuvant-induced arthritis in rats. Front. Pharmacol. 12, 683698. doi:10.3389/fphar.2021.683698
Meng, Y., Ji, J., Xiao, X., Li, M., Niu, S., He, Y., et al. (2021). Ononin induces cell apoptosis and reduces inflammation in rheumatoid arthritis fibroblast-like synoviocytes by alleviating MAPK and NF-κB signaling pathways. Acta Biochim. Pol. 68 (2), 239–245. doi:10.18388/abp.2020_5528
Miao, C. G., Yang, Y. Y., He, X., Li, X. F., Huang, C., Huang, Y., et al. (2013). Wnt signaling pathway in rheumatoid arthritis, with special emphasis on the different roles in synovial inflammation and bone remodeling. Cell Signal 25 (10), 2069–2078. doi:10.1016/j.cellsig.2013.04.002
Min, H. K., Kim, S. H., Won, J. Y., Kim, K. W., Lee, J. Y., Lee, S. H., et al. (2023). Dasatinib, a selective tyrosine kinase inhibitor, prevents joint destruction in rheumatoid arthritis animal model. Int. J. Rheum. Dis. 26 (4), 718–726. doi:10.1111/1756-185X.14627
Mohammadian Haftcheshmeh, S., Khosrojerdi, A., Aliabadi, A., Lotfi, S., Mohammadi, A., and Momtazi-Borojeni, A. A. (2021). Immunomodulatory effects of curcumin in rheumatoid arthritis: Evidence from molecular mechanisms to clinical outcomes. Rev. Physiol. Biochem. Pharmacol. 179, 1–29. doi:10.1007/112_2020_54
Mori, M., Hashimoto, M., Matsuo, T., Fujii, T., Furu, M., Ito, H., et al. (2017). Cell-contact-dependent activation of CD4(+) T cells by adhesion molecules on synovial fibroblasts. Mod. Rheumatol. 27 (3), 448–456. doi:10.1080/14397595.2016.1220353
Müller-Ladner, U., Ospelt, C., Gay, S., Distler, O., and Pap, T. (2007). Cells of the synovium in rheumatoid arthritis. Synovial fibroblasts. Arthritis Res. Ther. 9 (6), 223. doi:10.1186/ar2337
Neumann, E., Lefèvre, S., Zimmermann, B., Gay, S., and Müller-Ladner, U. (2010). Rheumatoid arthritis progression mediated by activated synovial fibroblasts. Trends Mol. Med. 16 (10), 458–468. doi:10.1016/j.molmed.2010.07.004
Niu, X., Song, H., Xiao, X., Yang, Y., Huang, Q., Yu, J., et al. (2022). Tectoridin ameliorates proliferation and inflammation in TNF-α-induced HFLS-RA cells via suppressing the TLR4/NLRP3/NF-κB signaling pathway. Tissue Cell 77, 101826. doi:10.1016/j.tice.2022.101826
Nygaard, G., and Firestein, G. S. (2020). Restoring synovial homeostasis in rheumatoid arthritis by targeting fibroblast-like synoviocytes. Nat. Rev. Rheumatol. 16 (6), 316–333. doi:10.1038/s41584-020-0413-5
O'Shea, J. J., Laurence, A., and McInnes, I. B. (2013). Back to the future: Oral targeted therapy for RA and other autoimmune diseases. Nat. Rev. Rheumatol. 9 (3), 173–182. doi:10.1038/nrrheum.2013.7
Palmroth, M., Kuuliala, K., Peltomaa, R., Virtanen, A., Kuuliala, A., Kurttila, A., et al. (2021). Tofacitinib suppresses several JAK-STAT pathways in rheumatoid arthritis in vivo and baseline signaling profile associates with treatment response. Front. Immunol. 12, 738481. doi:10.3389/fimmu.2021.738481
Pan, D., Li, N., Liu, Y., Xu, Q., Liu, Q., You, Y., et al. (2018). Kaempferol inhibits the migration and invasion of rheumatoid arthritis fibroblast-like synoviocytes by blocking activation of the MAPK pathway. Int. Immunopharmacol. 55, 174–182. doi:10.1016/j.intimp.2017.12.011
Pan, F., Zhu, L., Lv, H., and Pei, C. (2016). Quercetin promotes the apoptosis of fibroblast-like synoviocytes in rheumatoid arthritis by upregulating lncRNA MALAT1. Int. J. Mol. Med. 38 (5), 1507–1514. doi:10.3892/ijmm.2016.2755
Panchal, V., Vyas, B. H., Sivasubramanian, B. P., Panchal, K., and Patel, H. (2023). A meta-analysis evaluating the effectiveness and safety of upadacitinib in treating rheumatoid arthritis in patients with inadequate response to disease-modifying anti-rheumatic drugs. Cureus 15 (1), e34384. doi:10.7759/cureus.34384
Panipinto, P. M., Singh, A. K., Shaikh, F. S., Siegel, R. J., Chourasia, M., and Ahmed, S. (2021). Takinib inhibits inflammation in human rheumatoid arthritis synovial fibroblasts by targeting the janus kinase-signal transducer and activator of transcription 3 (JAK/STAT3) pathway. Int. J. Mol. Sci. 22 (22), 12580. doi:10.3390/ijms222212580
Park, J. K., Jang, Y. J., Oh, B. R., Shin, J., Bae, D., Ha, N., et al. (2020). Therapeutic potential of CKD-506, a novel selective histone deacetylase 6 inhibitor, in a murine model of rheumatoid arthritis. Arthritis Res. Ther. 22 (1), 176. doi:10.1186/s13075-020-02258-0
Qin, Y., Cai, M. L., Jin, H. Z., Huang, W., Zhu, C., Bozec, A., et al. (2022). Age-associated B cells contribute to the pathogenesis of rheumatoid arthritis by inducing activation of fibroblast-like synoviocytes via TNF-α-mediated ERK1/2 and JAK-STAT1 pathways. Ann. Rheum. Dis. 81 (11), 1504–1514. doi:10.1136/ard-2022-222605
Rabelo Fde, S., da Mota, L. M., Lima, R. A., Lima, F. A., Barra, G. B., de Carvalho, J. F., et al. (2010). The Wnt signaling pathway and rheumatoid arthritis. Autoimmun. Rev. 9 (4), 207–210. doi:10.1016/j.autrev.2009.08.003
Ruscitti, P., Liakouli, V., Panzera, N., Angelucci, A., Berardicurti, O., Di Nino, E., et al. (2022). Tofacitinib may inhibit myofibroblast differentiation from rheumatoid-fibroblast-like synoviocytes induced by TGF-beta and IL-6. Pharm. (Basel) 15 (5), 622. doi:10.3390/ph15050622
Samarpita, S., Ganesan, R., and Rasool, M. (2020). Cyanidin prevents the hyperproliferative potential of fibroblast-like synoviocytes and disease progression via targeting IL-17A cytokine signalling in rheumatoid arthritis. Toxicol. Appl. Pharmacol. 391, 114917. doi:10.1016/j.taap.2020.114917
Samarpita, S., Kim, J. Y., Rasool, M. K., and Kim, K. S. (2020). Investigation of toll-like receptor (TLR) 4 inhibitor TAK-242 as a new potential anti-rheumatoid arthritis drug. Arthritis Res. Ther. 22 (1), 16. doi:10.1186/s13075-020-2097-2
Samarpita, S., and Rasool, M. (2021). Cyanidin attenuates IL-17A cytokine signaling mediated monocyte migration and differentiation into mature osteoclasts in rheumatoid arthritis. Cytokine 142, 155502. doi:10.1016/j.cyto.2021.155502
Saravanan, S., Islam, V. I., Babu, N. P., Pandikumar, P., Thirugnanasambantham, K., Chellappandian, M., et al. (2014). Swertiamarin attenuates inflammation mediators via modulating NF-κB/I κB and JAK2/STAT3 transcription factors in adjuvant induced arthritis. Eur. J. Pharm. Sci. 56, 70–86. doi:10.1016/j.ejps.2014.02.005
Shang, W., Zhao, L. J., Dong, X. L., Zhao, Z. M., Li, J., Zhang, B. B., et al. (2016). Curcumin inhibits osteoclastogenic potential in PBMCs from rheumatoid arthritis patients via the suppression of MAPK/RANK/c-Fos/NFATc1 signaling pathways. Mol. Med. Rep. 14 (4), 3620–3626. doi:10.3892/mmr.2016.5674
Shao, N., Feng, Z., and Li, N. (2022). Isoginkgetin inhibits inflammatory response in the fibroblast-like synoviocytes of rheumatoid arthritis by suppressing matrix metallopeptidase 9 expression. Chem. Biol. Drug Des. 99 (6), 923–929. doi:10.1111/cbdd.14049
Shen, P., Jiao, Y., Miao, L., Chen, J. H., and Momtazi-Borojeni, A. A. (2020). Immunomodulatory effects of berberine on the inflamed joint reveal new therapeutic targets for rheumatoid arthritis management. J. Cell Mol. Med. 24 (21), 12234–12245. doi:10.1111/jcmm.15803
Shen, Y., Fan, X., Qu, Y., Tang, M., Huang, Y., Peng, Y., et al. (2022). Magnoflorine attenuates inflammatory responses in RA by regulating the PI3K/Akt/NF-κB and Keap1-Nrf2/HO-1 signalling pathways in vivo and in vitro. Phytomedicine 104, 154339. doi:10.1016/j.phymed.2022.154339
Shen, Y., Teng, L., Qu, Y., Liu, J., Zhu, X., Chen, S., et al. (2022). Anti-proliferation and anti-inflammation effects of corilagin in rheumatoid arthritis by downregulating NF-κB and MAPK signaling pathways. J. Ethnopharmacol. 284, 114791. doi:10.1016/j.jep.2021.114791
Sheng, S., Wang, X., Liu, X., Hu, X., Shao, Y., Wang, G., et al. (2022). The role of resveratrol on rheumatoid arthritis: From bench to bedside. Front. Pharmacol. 13, 829677. doi:10.3389/fphar.2022.829677
Shimizu, T., Kawashiri, S. Y., Morimoto, S., Kawazoe, Y., Kuroda, S., Kawasaki, R., et al. (2023). Efficacy and safety of selective JAK 1 inhibitor filgotinib in active rheumatoid arthritis patients with inadequate response to methotrexate: Comparative study with filgotinib and tocilizumab examined by clinical index as well as musculoskeletal ultrasound assessment (TRANSFORM study): Study protocol for a randomized, open-label, parallel-group, multicenter, and non-inferiority clinical trial. Trials 24 (1), 161. doi:10.1186/s13063-023-07176-5
Shu, C., Chen, J., Lv, M., Xi, Y., Zheng, J., and Xu, X. (2022). Plumbagin relieves rheumatoid arthritis through nuclear factor kappa-B (NF-κB) pathway. Bioengineered 13 (5), 13632–13642. doi:10.1080/21655979.2022.2081756
Smolen, J. S., Aletaha, D., Barton, A., Burmester, G. R., Emery, P., Firestein, G. S., et al. (2018). Rheumatoid arthritis. Nat. Rev. Dis. Prim. 4, 18001. doi:10.1038/nrdp.2018.1
Song, X., Zhang, Y., Dai, E., Wang, L., and Du, H. (2020). Prediction of triptolide targets in rheumatoid arthritis using network pharmacology and molecular docking. Int. Immunopharmacol. 80, 106179. doi:10.1016/j.intimp.2019.106179
Srivastava, S., Samarpita, S., Ganesan, R., and Rasool, M. (2022). CYT387 inhibits the hyperproliferative potential of fibroblast-like synoviocytes via modulation of IL-6/JAK1/STAT3 signaling in rheumatoid arthritis. Immunol. Invest. 51 (6), 1582–1597. doi:10.1080/08820139.2021.1994589
Sugiura, T., Kamino, H., Nariai, Y., Murakawa, Y., Kondo, M., Kawakami, M., et al. (2020). Screening of a panel of low molecular weight compounds that inhibit synovial fibroblast invasion in rheumatoid arthritis. J. Immunol. 205 (12), 3277–3290. doi:10.4049/jimmunol.1901429
Sujitha, S., Dinesh, P., and Rasool, M. (2020). Berberine encapsulated PEG-coated liposomes attenuate Wnt1/β-catenin signaling in rheumatoid arthritis via miR-23a activation. Eur. J. Pharm. Biopharm. 149, 170–191. doi:10.1016/j.ejpb.2020.02.007
Sun, Y., and Li, L. (2018). Cyanidin-3-glucoside inhibits inflammatory activities in human fibroblast-like synoviocytes and in mice with collagen-induced arthritis. Clin. Exp. Pharmacol. Physiol. 45 (10), 1038–1045. doi:10.1111/1440-1681.12970
Sur, B., Kim, M., Villa, T., and Oh, S. (2020). Benzylideneacetophenone derivative alleviates arthritic symptoms via modulation of the MAPK signaling pathway. Molecules 25 (15), 3319. doi:10.3390/molecules25153319
Taldaev, A., Rudnev, V. R., Nikolsky, K. S., Kulikova, L. I., and Kaysheva, A. L. (2021). Molecular modeling insights into upadacitinib selectivity upon binding to JAK protein family. Pharm. (Basel) 15 (1), 30. doi:10.3390/ph15010030
Tang, M., Zhu, W. J., Yang, Z. C., and He, C. S. (2019). Brucine inhibits TNF-α-induced HFLS-RA cell proliferation by activating the JNK signaling pathway. Exp. Ther. Med. 18 (1), 735–740. doi:10.3892/etm.2019.7582
Tang, Y., Liu, Q., Feng, Y., Zhang, Y., Xu, Z., Wen, C., et al. (2020). Tripterygium ingredients for pathogenicity cells in rheumatoid arthritis. Front. Pharmacol. 11, 583171. doi:10.3389/fphar.2020.583171
Taylor, P. C., Bieber, T., Alten, R., Witte, T., Galloway, J., Deberdt, W., et al. (2023). Baricitinib safety for events of special interest in populations at risk: Analysis from randomised trial data across rheumatologic and dermatologic indications. Adv. Ther. 40 (4), 1867–1883. doi:10.1007/s12325-023-02445-w
Terabe, K., Takahashi, N., Asai, S., Hirano, Y., Kanayama, Y., Yabe, Y., et al. (2023). Effectiveness of tacrolimus concomitant with biological disease-modifying antirheumatic drugs in patients with rheumatoid arthritis. Mod. Rheumatol. 33 (2), 292–301. doi:10.1093/mr/roac025
Tran, C. N., Davis, M. J., Tesmer, L. A., Endres, J. L., Motyl, C. D., Smuda, C., et al. (2007). Presentation of arthritogenic peptide to antigen-specific T cells by fibroblast-like synoviocytes. Arthritis Rheum. 56 (5), 1497–1506. doi:10.1002/art.22573
Tran, C. N., Thacker, S. G., Louie, D. M., Oliver, J., White, P. T., Endres, J. L., et al. (2008). Interactions of T cells with fibroblast-like synoviocytes: Role of the B7 family costimulatory ligand B7-H3. J. Immunol. 180 (5), 2989–2998. doi:10.4049/jimmunol.180.5.2989
Tu, J., Huang, W., Zhang, W., Mei, J., and Zhu, C. (2022). Two main cellular components in rheumatoid arthritis: Communication between T cells and fibroblast-like synoviocytes in the joint synovium. Front. Immunol. 13, 922111. doi:10.3389/fimmu.2022.922111
Tucci, G., Garufi, C., Pacella, I., Zagaglioni, M., Pinzon Grimaldos, A., Ceccarelli, F., et al. (2022). Baricitinib therapy response in rheumatoid arthritis patients associates to STAT1 phosphorylation in monocytes. Front. Immunol. 13, 932240. doi:10.3389/fimmu.2022.932240
Villa, T., Kim, M., and Oh, S. (2020). Fangchinoline has an anti-arthritic effect in two animal models and in IL-1β-stimulated human FLS cells. Biomol. Ther. Seoul. 28 (5), 414–422. doi:10.4062/biomolther.2020.113
Vomero, M., Caliste, M., Barbati, C., Speziali, M., Celia, A. I., Ucci, F., et al. (2022). Tofacitinib decreases autophagy of fibroblast-like synoviocytes from rheumatoid arthritis patients. Front. Pharmacol. 13, 852802. doi:10.3389/fphar.2022.852802
Walsh, N. C., Reinwald, S., Manning, C. A., Condon, K. W., Iwata, K., Burr, D. B., et al. (2009). Osteoblast function is compromised at sites of focal bone erosion in inflammatory arthritis. J. Bone Min. Res. 24 (9), 1572–1585. doi:10.1359/jbmr.090320
Wang, C. H., Yao, H., Chen, L. N., Jia, J. F., Wang, L., Dai, J. Y., et al. (2012). CD147 induces angiogenesis through a vascular endothelial growth factor and hypoxia-inducible transcription factor 1α-mediated pathway in rheumatoid arthritis. Arthritis Rheum. 64 (6), 1818–1827. doi:10.1002/art.34341
Wang, D. D., Jiang, M. Y., Wang, W., Zhou, W. J., Zhang, Y. W., Yang, M., et al. (2020). Paeoniflorin-6'-O-benzene sulfonate down-regulates CXCR4-Gβγ-PI3K/AKT mediated migration in fibroblast-like synoviocytes of rheumatoid arthritis by inhibiting GRK2 translocation. Biochem. Biophys. Res. Commun. 526 (3), 805–812. doi:10.1016/j.bbrc.2020.03.164
Wang, G., Xie, X., Yuan, L., Qiu, J., Duan, W., Xu, B., et al. (2020). Resveratrol ameliorates rheumatoid arthritis via activation of SIRT1-Nrf2 signaling pathway. Biofactors 46 (3), 441–453. doi:10.1002/biof.1599
Wang, H., Mei, D., Liang, F. Q., Xue, Z. Y., Wang, P., Liu, R. J., et al. (2023). BAFF promotes FLS activation through BAFFR-mediated non-canonical NF-κB pathway and the effects of CP-25. Inflammation 46, 861–875. doi:10.1007/s10753-022-01774-2
Wang, H., Tu, S., Yang, S., Shen, P., Huang, Y., Ba, X., et al. (2019). Berberine modulates LPA function to inhibit the proliferation and inflammation of FLS-RA via p38/ERK MAPK pathway mediated by LPA(1). Evid. Based Complement. Altern. Med. 2019, 2580207. doi:10.1155/2019/2580207
Wang, J., Lian, J., Kong, X., and Lin, N. (2010). Effects of triptolide on cell proliferation and regulation of Ras-MAPKs pathway in synoviocytes induced by tumor necrosis factor. Zhongguo Zhong Yao Za Zhi 35 (7), 888–891. doi:10.4268/cjcmm20100719
Wang, M., Dai, T., Li, S., and Wang, W. (2022). Eugenol suppresses the proliferation and invasion of TNF-α-induced fibroblast-like synoviocytes via regulating NF-κB and COX-2. Biochem. Biophys. Res. Commun. 612, 63–69. doi:10.1016/j.bbrc.2022.04.074
Wang, R., Liu, J., Wang, Z., Wu, X., Guo, H., Jiao, X., et al. (2021). Mangiferin exert protective effects on joints of adjuvant-induced arthritis rats by regulating the MAPKs/NF-κB pathway of fibroblast-like synoviocytes. Int. Immunopharmacol. 101, 108352. doi:10.1016/j.intimp.2021.108352
Wang, S., Du, Q., Sun, J., Geng, S., and Zhang, Y. (2022). Investigation of the mechanism of Isobavachalcone in treating rheumatoid arthritis through a combination strategy of network pharmacology and experimental verification. J. Ethnopharmacol. 294, 115342. doi:10.1016/j.jep.2022.115342
Wang, X. H., Dai, C., Wang, J., Liu, R., Li, L., and Yin, Z. S. (2021). Therapeutic effect of neohesperidin on TNF-α-stimulated human rheumatoid arthritis fibroblast-like synoviocytes. Chin. J. Nat. Med. 19 (10), 741–749. doi:10.1016/S1875-5364(21)60107-3
Wang, Z. Z., Huang, T. Y., Gong, Y. F., Zhang, X. M., Feng, W., and Huang, X. Y. (2020). Effects of sorafenib on fibroblast-like synoviocyte apoptosis in rats with adjuvant arthritis. Int. Immunopharmacol. 83, 106418. doi:10.1016/j.intimp.2020.106418
Wendel, H. G., De Stanchina, E., Fridman, J. S., Malina, A., Ray, S., Kogan, S., et al. (2004). Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature 428 (6980), 332–337. doi:10.1038/nature02369
Wendling, D., Prati, C., Toussirot, E., and Herbein, G. (2010). Targeting intracellular signaling pathways to treat rheumatoid arthritis: Pandora's box? Jt. Bone Spine 77 (2), 96–98. doi:10.1016/j.jbspin.2010.01.004
Westhovens, R. (2023). Filgotinib in rheumatoid arthritis. Expert Rev. Clin. Immunol. 19 (2), 135–144. doi:10.1080/1744666X.2023.2149495
Weston, S., Macdonald, J. L., Williams, L. M., Roussou, E., Kang, N. V., Kiriakidis, S., et al. (2022). The JAK inhibitor baricitinib inhibits oncostatin M induction of proinflammatory mediators in ex-vivo synovial derived cells. Clin. Exp. Rheumatol. 40 (9), 1620–1628. doi:10.55563/clinexprheumatol/cfsajk
Wu, Z. M., Xiang, Y. R., Zhu, X. B., Shi, X. D., Chen, S., Wan, X., et al. (2022). Icariin represses the inflammatory responses and survival of rheumatoid arthritis fibroblast-like synoviocytes by regulating the TRIB1/TLR2/NF-kB pathway. Int. Immunopharmacol. 110, 108991. doi:10.1016/j.intimp.2022.108991
Xie, C., Jiang, J., Liu, J., Yuan, G., and Zhao, Z. (2019). Triptolide suppresses human synoviocyte MH7A cells mobility and maintains redox balance by inhibiting autophagy. Biomed. Pharmacother. 115, 108911. doi:10.1016/j.biopha.2019.108911
Xu, R., Liu, Z., Hou, J., Huang, T., and Yang, M. (2018). Osthole improves collagen-induced arthritis in a rat model through inhibiting inflammation and cellular stress. Cell Mol. Biol. Lett. 23, 19. doi:10.1186/s11658-018-0086-0
Xu, Z., Shang, W., Zhao, Z., Zhang, B., Liu, C., and Cai, H. (2022). Curcumin alleviates rheumatoid arthritis progression through the phosphatidylinositol 3-kinase/protein kinase B pathway: An in vitro and in vivo study. Bioengineered 13 (5), 12899–12911. doi:10.1080/21655979.2022.2078942
Yalcin Kehribar, D., Ozgen, M., Yolbas, S., Yildirim, A., Onalan Etem, E., Ciftci, O., et al. (2021). The inhibition of Src kinase suppresses the production of matrix metalloproteinases in from synovial fibroblasts and inhibits MAPK and STATs pathways. Turk J. Med. Sci. 51 (4), 2142–2149. doi:10.3906/sag-2008-274
Yamada, H. (2023). The search for the pathogenic T cells in the joint of rheumatoid arthritis: Which T-cell subset drives autoimmune inflammation? Int. J. Mol. Sci. 24 (8), 6930. doi:10.3390/ijms24086930
Yang, F., Shen, J., and Cai, H. (2022). Paeoniflorin inhibits Wnt1/beta-catenin pathway and promotes apoptosis of fibroblast-like synoviocytes in patients with rheumatoid arthritis by upregulating lncRNA MALAT1. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 38 (8), 692–698.
Yang, J., Liu, J., Li, J., Jing, M., Zhang, L., Sun, M., et al. (2022). Celastrol inhibits rheumatoid arthritis by inducing autophagy via inhibition of the PI3K/AKT/mTOR signaling pathway. Int. Immunopharmacol. 112, 109241. doi:10.1016/j.intimp.2022.109241
Yang, Y., Dong, Q., and Li, R. (2017). Matrine induces the apoptosis of fibroblast-like synoviocytes derived from rats with collagen-induced arthritis by suppressing the activation of the JAK/STAT signaling pathway. Int. J. Mol. Med. 39 (2), 307–316. doi:10.3892/ijmm.2016.2843
Yang, Y. P., Jian, Y. Q., Liu, Y. B., Ismail, M., Xie, Q. L., Yu, H. H., et al. (2021). Triterpenoids from kadsura coccinea with their anti-inflammatory and inhibited proliferation of rheumatoid arthritis-fibroblastoid synovial cells activities. Front. Chem. 9, 808870. doi:10.3389/fchem.2021.808870
Yang, Y. P., Jian, Y. Q., Liu, Y. B., Xie, Q. L., Yu, H. H., Wang, B., et al. (2022). Heilaohuacid G, a new triterpenoid from Kadsura coccinea inhibits proliferation, induces apoptosis, and ameliorates inflammation in RA-FLS and RAW 264.7 cells via suppressing NF-?B pathway. Phytother. Res. 36 (10), 3900–3910. doi:10.1002/ptr.7527
Yang, Y., Ye, Y., Qiu, Q., Xiao, Y., Huang, M., Shi, M., et al. (2016). Triptolide inhibits the migration and invasion of rheumatoid fibroblast-like synoviocytes by blocking the activation of the JNK MAPK pathway. Int. Immunopharmacol. 41, 8–16. doi:10.1016/j.intimp.2016.10.005
Yao, R. B., Zhao, Z. M., Zhao, L. J., and Cai, H. (2017). Sinomenine inhibits the inflammatory responses of human fibroblast-like synoviocytes via the TLR4/MyD88/NF-κB signaling pathway in rheumatoid arthritis. Pharmazie 72 (6), 355–360. doi:10.1691/ph.2017.6946
Yi, L., Ke, J., Liu, J., Lai, H., Lv, Y., Peng, C., et al. (2021). Sinomenine increases adenosine A2A receptor and inhibits NF-κB to inhibit arthritis in adjuvant-induced-arthritis rats and fibroblast-like synoviocytes through α7nAChR. J. Leukoc. Biol. 110 (6), 1113–1120. doi:10.1002/JLB.3MA0121-024RRRR
Yin, G., Wang, Y., Cen, X. M., Yang, M., Liang, Y., and Xie, Q. B. (2015). Lipid peroxidation-mediated inflammation promotes cell apoptosis through activation of NF-κB pathway in rheumatoid arthritis synovial cells. Mediat. Inflamm. 2015, 460310. doi:10.1155/2015/460310
Yoon, H. Y., Lee, E. G., Lee, H., Cho, I. J., Choi, Y. J., Sung, M. S., et al. (2013). Kaempferol inhibits IL-1β-induced proliferation of rheumatoid arthritis synovial fibroblasts and the production of COX-2, PGE2 and MMPs. Int. J. Mol. Med. 32 (4), 971–977. doi:10.3892/ijmm.2013.1468
Yu, H. H., Li, M., Li, Y. B., Lei, B. B., Yuan, X., Xing, X. K., et al. (2020). Benzoylaconitine inhibits production of IL-6 and IL-8 via MAPK, Akt, NF-κB signaling in IL-1β-induced human synovial cells. Biol. Pharm. Bull. 43 (2), 334–339. doi:10.1248/bpb.b19-00719
Yu, X., Zhou, J., Zhao, F., Liu, X., Mao, Y., Diao, L., et al. (2021). Tomatidine suppresses the destructive behaviors of fibroblast-like synoviocytes and ameliorates type II collagen-induced arthritis in rats. Front. Pharmacol. 12, 670707. doi:10.3389/fphar.2021.670707
Yu, Y., Koehn, C. D., Yue, Y., Li, S., Thiele, G. M., Hearth-Holmes, M. P., et al. (2015). Celastrol inhibits inflammatory stimuli-induced neutrophil extracellular trap formation. Curr. Mol. Med. 15 (4), 401–410. doi:10.2174/1566524015666150505160743
Zeng, S., Wang, K., Huang, M., Qiu, Q., Xiao, Y., Shi, M., et al. (2017). Halofuginone inhibits TNF-α-induced the migration and proliferation of fibroblast-like synoviocytes from rheumatoid arthritis patients. Int. Immunopharmacol. 43, 187–194. doi:10.1016/j.intimp.2016.12.016
Zhai, K. F., Duan, H., Cui, C. Y., Cao, Y. Y., Si, J. L., Yang, H. J., et al. (2019). Liquiritin from Glycyrrhiza uralensis attenuating rheumatoid arthritis via reducing inflammation, suppressing angiogenesis, and inhibiting MAPK signaling pathway. J. Agric. Food Chem. 67 (10), 2856–2864. doi:10.1021/acs.jafc.9b00185
Zhang, D., Ning, T., and Wang, H. (2022). Vitexin alleviates inflammation and enhances apoptosis through the regulation of the JAK/STAT/SOCS signaling pathway in the arthritis rat model. J. Biochem. Mol. Toxicol. 36 (12), e23201. doi:10.1002/jbt.23201
Zhang, G., Liu, B., Zeng, Z., Chen, Q., Feng, Y., and Ning, X. (2021). Oxymatrine hydrazone (OMTH) synthesis and its protective effect for rheumatoid arthritis through downregulation of MEK/NF-κB pathway. Environ. Toxicol. 36 (12), 2448–2453. doi:10.1002/tox.23357
Zhang, L., Lin, Y., Xu, X., Liu, H., Wang, X., and Pan, J. (2023). Telotristat Etiprate alleviates rheumatoid arthritis by targeting LGALS3 and affecting MAPK signaling. Intractable Rare Dis. Res. 12 (1), 45–57. doi:10.5582/irdr.2022.01121
Zhang, Q., Liu, J., Zhang, M., Wei, S., Li, R., Gao, Y., et al. (2019). Apoptosis induction of fibroblast-like synoviocytes is an important molecular-mechanism for herbal medicine along with its active components in treating rheumatoid arthritis. Biomolecules 9 (12), 795. doi:10.3390/biom9120795
Zhang, X., Guan, X., Piao, Y., Che, X., Si, M., and Jin, J. (2022). Baicalein induces apoptosis of rheumatoid arthritis synovial fibroblasts through inactivation of the PI3K/Akt/mTOR pathway. Evid. Based Complement. Altern. Med. 2022, 3643265. doi:10.1155/2022/3643265
Zhang, X., Ye, G., Wu, Z., Zou, K., He, X., Xu, X., et al. (2020). The therapeutic effects of edaravone on collagen-induced arthritis in rats. J. Cell Biochem. 121 (2), 1463–1474. doi:10.1002/jcb.29382
Zhang, Y., Tang, L. D., Wang, J. Y., Wang, H., Chen, X. Y., Zhang, L., et al. (2022). Anti-inflammatory effects of aucubin in cellular and animal models of rheumatoid arthritis. Chin. J. Nat. Med. 20 (6), 458–472. doi:10.1016/S1875-5364(22)60182-1
Zhong, B., Guo, S., Yang, Z., Han, L., Du, J., Chen, J., et al. (2021). Roflumilast reduced the IL-18-induced inflammatory response in fibroblast-like synoviocytes (FLS). ACS Omega 6 (3), 2149–2155. doi:10.1021/acsomega.0c05281
Zhong, Z., Qian, Z., Zhang, X., Chen, F., Ni, S., Kang, Z., et al. (2019). Tetrandrine prevents bone loss in ovariectomized mice by inhibiting RANKL-induced osteoclastogenesis. Front. Pharmacol. 10, 1530. doi:10.3389/fphar.2019.01530
Zhou, H., Liu, J. X., Luo, J. F., Cheng, C. S., Leung, E. L., Li, Y., et al. (2017). Suppressing mPGES-1 expression by sinomenine ameliorates inflammation and arthritis. Biochem. Pharmacol. 142, 133–144. doi:10.1016/j.bcp.2017.07.010
Zhou, J., Mao, Y., Shi, X., Zhang, Y., Yu, X., Liu, X., et al. (2022). Peimine suppresses collagen-induced arthritis, activated fibroblast-like synoviocytes and TNFα-induced MAPK pathways. Int. Immunopharmacol. 111, 109181. doi:10.1016/j.intimp.2022.109181
Zhou, Y. R., Zhao, Y., Bao, B. H., and Li, J. X. (2015). SND-117, a sinomenine bivalent alleviates type II collagen-induced arthritis in mice. Int. Immunopharmacol. 26 (2), 423–431. doi:10.1016/j.intimp.2015.04.006
Zuo, J., Xia, Y., Li, X., Ou-Yang, Z., and Chen, J. W. (2015). Selective modulation of MAPKs contribute to the anti-proliferative and anti-inflammatory activities of 1,7-dihydroxy-3,4-dimethoxyxanthone in rheumatoid arthritis-derived fibroblast-like synoviocyte MH7A cells. J. Ethnopharmacol. 168, 248–254. doi:10.1016/j.jep.2015.03.069
Glossary
Keywords: rheumatoid arthritis, fibroblast-like synoviocytes, signaling pathways, small molecule drugs, natural products
Citation: Tong Y, Li X, Deng Q, Shi J, Feng Y and Bai L (2023) Advances of the small molecule drugs regulating fibroblast-like synovial proliferation for rheumatoid arthritis. Front. Pharmacol. 14:1230293. doi: 10.3389/fphar.2023.1230293
Received: 28 May 2023; Accepted: 10 July 2023;
Published: 21 July 2023.
Edited by:
Yan Huang, Anhui Medical University, ChinaReviewed by:
Anil Kumar Singh, Washington State University, United StatesShaohua Qi, Houston Methodist Research Institute, United States
Ke Wang, Xi’an Jiaotong University, China
Copyright © 2023 Tong, Li, Deng, Shi, Feng and Bai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lan Bai, YmxjaUAxNjMuY29t; Yibin Feng, eWZlbmdAaGt1Lmhr; Jianyou Shi, c2hpamlhbnlvdWRlQDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
 Yitong Tong1†
Yitong Tong1† Qichuan Deng
Qichuan Deng Yibin Feng
Yibin Feng Lan Bai
Lan Bai