
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 31 August 2023
Sec. Ethnopharmacology
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1229963
 Xin Zhu1,2
Xin Zhu1,2 Yun-yun Quan1
Yun-yun Quan1 Zhu-jun Yin1
Zhu-jun Yin1 Min Li2
Min Li2 Ting Wang1
Ting Wang1 Lu-yao Zheng2
Lu-yao Zheng2 Shi-qi Feng1
Shi-qi Feng1 Jun-ning Zhao1,2
Jun-ning Zhao1,2 Li Li1*
Li Li1*Curcumae Longae Rhizoma (turmeric), Curcumae Radix and Curcumae Rhizoma are derived from the Curcuma species, and have gradually become three of the most commonly used medicinal herbs in China due to their different origins, processing methods and medicinal part. These three herbs have certain similarities in morphology, chemical composition, and pharmacological effects. All three of these herbs contain curcuminoids and volatile oil compounds, which exhibit anti-inflammatory, anti-tumor, antioxidant, and neuroprotective properties, although modern clinical applications have their own requirements. At present, there is no systematic guidelines for the clinical application of these three of Curcuma species; consequently, there is a high risk of unwanted phenomena associated with the mixing and indiscriminate use of these herbs. In this review, we focus predominantly on morphology, chemical composition, and the pharmacological activity of these three Curcuma herbs and summarize the current status of research in this field. Our goal is to provide a better understanding of clinical value of these Curcuma species so that we can provide reference guidelines for their further development, utilization and rational clinical application.
The commonly used traditional Chinese medicines Curcumae Longae Rhizoma, Curcumae Radix and Curcumae Rhizoma are derived from medicinal plants of the Curcuma species in the Zingiberaceae family and have been the focus of much research due to their commercial and medicinal values. Curcumae Longae Rhizoma has been used in Asia for thousands of years; indeed, the Curcuma species has been used in traditional Chinese medicine for more than a thousand years. These three forms of medicinal herbs were clearly distinguished during the Ming and Qing dynasties, and continue to be widely applied due to their medicinal properties (Chen, 1981). The 2020 edition of the Chinese Pharmacopoeia reported that Curcumae Longae Rhizoma is the dried rhizomes of Curcuma Longa L. of the Zingiberaceae family, and that Sichuan is the main Taoist producing area for Curcumae Longae Rhizoma in China. Curcumae Radix is the dried tuberous roots of Curcuma wenyujin (Y. H. Chen and C. Ling), Curcuma Longa L., Curcuma kwangsiensis (S. G. Lee and C. F. Liang), and Curcuma phaeocaulis Val. of the ginger plant family; these herbs are habitually known as “Wenyu-jin,” “Huangyujin,” “Guiyujin” and “lvyu-jin,” and are mainly produced in Jiangsu, Zhejiang, Guangxi, Sichuan, Yunnan and other places in China. Curcumae Rhizoma is the dried rhizome of C. phaeocaulis VaL., C. kwangsiensis (S. G. Lee and C. F. Liang), C. wenyujin (Y. H. Chen and C. Ling) of the family of Zingiberaceae (Chinese Pharmacopoeia Commission, 2020), and is mainly produced in Fujian, Guangdong, Zhejiang, Sichuan, and Yunnan in China. Curcuma phaeocaulis, C. kwangsiensis, and C. wenyujin can be used for multiple purposes at the same time; a common phenomenon in traditional Chinese Medicine. The tuberous roots of these four Curcuma species can be used as Curcumae Radix, while the rhizomes of C. phaeocaulis, C. kwangsiensis, and C. wenyujin can be used as Curcumae Rhizoma; the rhizomes of Curcuma Longa L. are used as Curcumae Longae Rhizoma. Consequently, it is highly evident that Curcumae Longae Rhizoma, Curcumae Radix and Curcumae Rhizoma are inextricably linked to each other, and demonstrate both correlations and differences in terms of their properties and abilities; the relationships that exist between these three herbs is shown in Figure 1A.
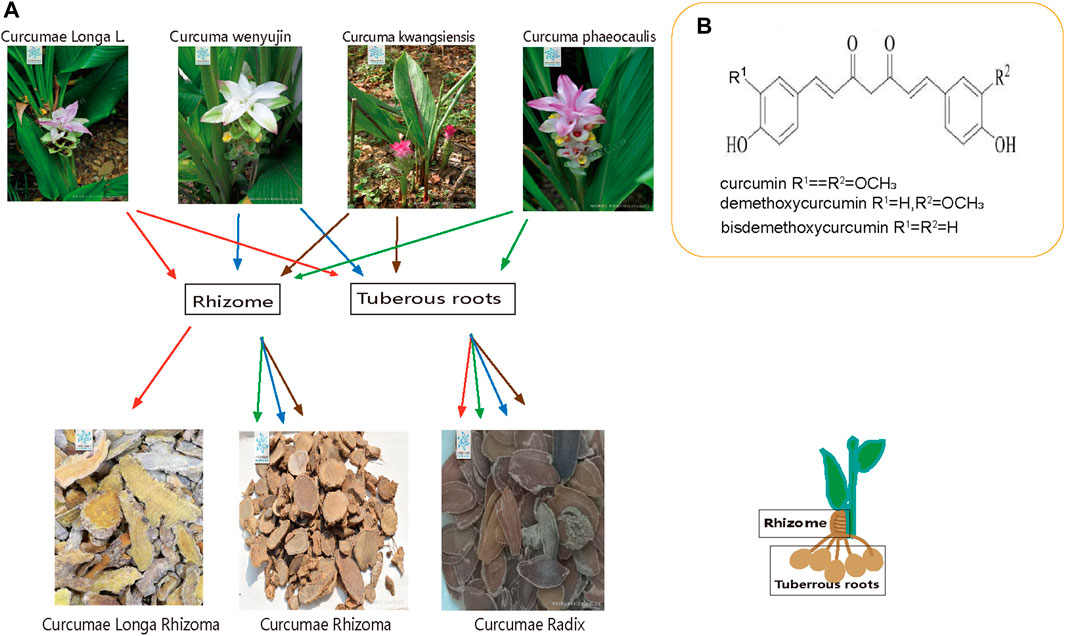
FIGURE 1. Overview of the three plants. (A) Relationship between Curcumae Longae Rhizoma, Curcumae Radix and Curcumae Rhizoma (B) chemical structure formula of Curcumin, demethoxycurcumin and bisdemethoxycurcumin.
The curcuminoids and volatile oil compounds possessed by Curcuma species have been widely studied due to their significant biological activities, their numerous pharmacological effects, including (anti-inflammatory, antitumor, antioxidant, and antimicrobial), their ability to reduce blood glucose levels, and their therapeutic effects on the nervous, digestive and cardiovascular systems (Anand et al., 2008; Kunnumakkara et al., 2008; Rathore et al., 2008; Lekshmi et al., 2012; Gadnayak et al., 2022; Alameri et al., 2023; Balakumar et al., 2023; Erarslan et al., 2023; Jabbar et al., 2023; Louisa et al., 2023; Mohanty et al., 2023; Wei et al., 2023). Differences in the chemical composition and pharmacological effects of Curcuma species are caused by variations in growth environment, medicinal components, cultivation methods and processing, and a range of other factors. These differences allow us to utilize the resources of these three medicinal herbs more effectively and eliminate the phenomena associated with the mixing and indiscriminate use of such herbs. In this review, we summarize the literature relating to plant sources, chemical composition, pharmacological effects and other aspects of Curcumae Longae Rhizoma, Curcumae Radix and Curcumae Rhizoma. In addition, we analyze the similarities and differences between the three herbs and attempt to provide specific guidelines for the future investigation and utilization of Curcuma species and to provide a basis for the rational use of these herbs in clinical scenarios.
Curcumae Longae Rhizoma, Curcumae Radix and Curcumae Rhizoma have their own features in character identification and microscopic identification. The Chinese Pharmacopoeia describes the character identification and microscopic identification features of Curcumae Longae Rhizoma, Curcumae Radix and Curcumae Rhizoma (Chinese Pharmacopoeia Commission, 2020). The differences in the character identification and microscopic identification features between the three of herbs are shown in Table 1.

TABLE 1. The character identification and microscopic identification of Curcumae Longae Rhizoma, Curcumae Rhizoma and Curcumae Radix.
There are several chemical constituents that are common to Curcumae Longae Rhizoma Curcumae Radix and Curcumae Rhizoma; these predominantly include curcuminoids, volatile oils, sugars, and sterols. Curcumae Longae Rhizoma and Curcumae Radix also contain flavonoids, organic acids, alkaloids, polypeptides, and a variety of trace elements, including Ca, K, Na, Zn, Cu, Mn, Pb, and Cd (Zeiner et al., 2022; Yin et al., 2012). Curcuminoids and volatile oils are the major active constituents of Curcuma species and account for the largest proportion of all components. Curcuminoids is a collective term used to describe the diarylheptanoids that feature in the chemical structural formula. This term is also used to refer to the structural similarity of the diarylheptanoids; curcumin, demethoxycurcumin and bisdemethoxycurcumin account for the three largest proportions of active ingredients in this class of compounds. Chemical formulas for the three chemical compounds are shown in Figure 1B. The content of curcuminoids in Curcumae Longae Rhizoma is higher than that in the other two herbs. A previous study found that the content of curcumin was highest in Curcumae Longae Rhizoma; this was followed by Curcumae Rhizoma; the lowest content was in Curcumae Radix (Sun et al., 2017). More than 40 curcuminoids have been isolated and identified (Claeson et al., 1993; Jurgens et al., 1994; Lin and Lin-Shiau, 2001; Zeng et al., 2007; Jiang et al., 2009; Xing et al., 2009); of these, 30 have been detected in Curcumae Longae Rhizoma (Zhou, 2015), 20 in Curcumae Radix (Hu and Shao, 2007), and 36 curcuminoids in Curcumae Rhizoma. More than 39 types of volatile oil compounds have been identified in Curcumae Longae Rhizoma (Ge et al., 2007; Li et al., 2009; Wu et al., 2011; Cui et al., 2016; Lu, 2018; Wei et al., 2020); these are mostly sesquiterpenes or monoterpenes, such as ar-turmerone, α-turmerone, α-curcumene, and β-sesquiphellandrene. The volatile oil compounds of Curcumae Radix contain 69 terpenes, mostly sesquiterpenes, monoterpenes, and diterpenes; of these, sesquiterpenes account for the largest proportion (Yin et al., 2012; Liu et al., 2021), including germacrone, furanodiene, β-pinene, isoborneol, and α-terpineol. Curcumae Rhizoma contains 63 volatile oil compounds, predominantly including curcumol, curcumone, furanodiene, curdione, and β-elemene (Huang et al., 2020; Li et al., 2021). The main chemical constituents of Curcumae Longae Rhizoma, Curcumae Radix and Curcumae Rhizoma are shown in Table 2.
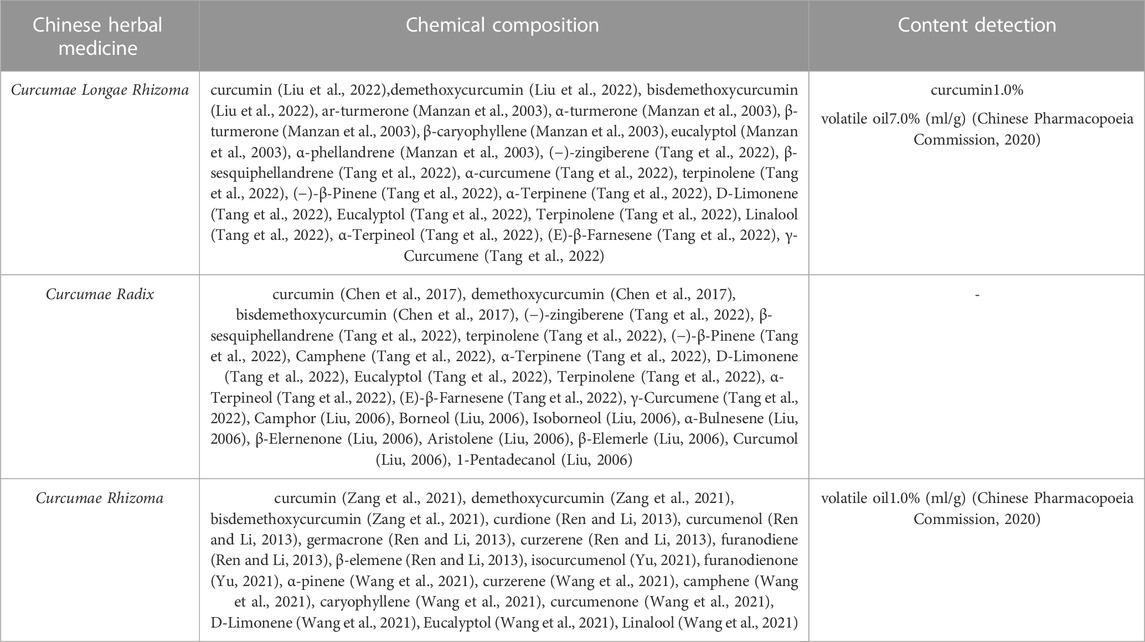
TABLE 2. Study on chemical constituents of Curcumae Longae Rhizoma, Curcumae Radix and Curcumae Rhizoma.
Inflammation is a defense response of the body to stimuli and is manifested by redness, swelling, heat, pain and dysfunction. In the normal human body, the levels of cytokines and chemokines are always low; it is only when the body repairs the physical damage caused by injury or infection that the levels of cytokines in the body, such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and nitric oxide (NO), increase, ultimately leading to the development of inflammation (Dell'Agli et al., 2013). Curcuminoids and volatile oil compounds exert significant anti-inflammatory effects that are comparable to those of steroidal or non-steroidal anti-inflammatory drugs (Guo et al., 2022). In a previous study, Wang et al. (Wang Z. et al., 2022) explored the effect of curcumin on Mycoplasma pneumoniae (MP)-infected pneumonic BALB/c mice based on the NF-κB pathway; analysis showed that the expression of NF-κBp65 protein was reduced in the lung tissues of the mice in the medium-dose and high-dose groups of curcumin, and that the levels of IL-6, IL-8 and TNF-α were significantly reduced in the bronchial lavage fluid (p < 0.01), thus alleviating the symptoms of pneumonic mice. In another study, Funk et al. (Funk et al., 2010) explored the therapeutic effect of turmeric essential oil on streptococcal cell (SCW)-induced arthritis in female Lewis rats, and ultimately found that turmeric volatile oil significantly alleviated SCW-induced arthritis in female Lewis rats; the mechanism of action was considered to involve blockade of the intra-articular inflammatory cells (neutrophil chemokine GRO/KC and monocyte chemokine MCP-1) and the expression levels of the key inflammatory cytokine interleukin-1β (IL-1β) as well as other downstream joint inflammatory mediators (e.g., COX-2). In other research, Li et al. (Liju et al., 2011) investigated the anti-inflammatory and analgesic effects of turmeric volatile oil and used gas chromatography-mass spectrometry (GC-MS) to analyze the material basis of the anti-inflammatory and analgesic effects generated by the volatile oil of turmeric, which showed better anti-inflammatory and analgesic effects when compared with aspirin and diclofenac. The main components of turmeric volatile oil were ar-turmerone, curlone and ar-curcumene. In a previous study, Henrotin et al. (Henrotin et al., 2019) compared the therapeutic effects of different doses of Curcumae Longae Rhizoma extract on knee osteoarthritis (OA) and found that both low and high doses of Curcumae Longae Rhizoma extract led to a significant improvement in the patients’ general assessment of disease activity (PGADA), knee injury and osteoarthritis outcome score (KOOS), and reduced the serum levels of collagen 2-1 (Coll2-1) in patients with OA. Mukophadhyay et al. (Mukhopadhyay et al., 1982) demonstrated the anti-inflammatory activity of curcumin and other semi-synthetic analogs (sodium curcuminate NaC, diacetyl curcumin DAC, triethyl curcumin TEC, and tetrahydro curcumin THC) by investigating carrageenan-induced paw edema and cotton pellet granuloma in experimental rats.
Chen et al. (Chen et al., 2019) used GC-MS to analyze the chemical composition of the active components of Curcumae Radix extracts that relate to blood activation and analgesic efficacy; ultimately, curcumin and its derivatives were found to be the main bioactive components responsible for these effects. Qin et al. (Qin et al., 2022a) revealed that Curcumae Radix extract could treat dysmenorrhea by regulating the inflammatory reaction, relaxing smooth muscle and endocrine by curcumenone, 13-hydroxygermacrone (+)-cuparene, caryophyllene oxide, zederone, and isocurcumenol. In another study, Qiu (Qiu et al., 2014) found that the ethyl acetate extract of Wenyu-jin reduced the levels of TNF-α, IL-6, and IL-1β in ear tissues, and inhibited acute inflammation and the pain triggered by physical and chemical factors, inflammation-causing agent-induced ear swelling, and changes in capillary permeability; the inhibitory effect of this extract was stronger when the dosages was increased. Dong et al. (Dong et al., 2015) isolated a sesquiterpenoid compound (curcumolide) from Wenyu-jin with a unique structure that exhibited significant anti-inflammatory effects by inhibiting lipopolysaccharide (LPS)-induced NF-κB activation in RAW264.7 macrophages, and by reducing the production of TNF-α, IL-6, IL-1β, NO, and reactive oxygen species (ROS). In addition, Huang et al. (Huang et al., 2013) found that diterpenoid compound C from Wenyu-jin inhibited the secretion of pro-inflammatory factor IL-8, the expression of Ik Kα and Ik Kβ, and promoted the secretion of anti-inflammatory factor IL-4 in GES-1 gastric cells; these effects were enhanced when drug dosage was increased. Furthermore, this compound exerted an inhibitory effect on the inflammation induced by Helicobacter pylori (Hp) in human gastric GES-1 epithelial cells. Zheng et al. (Zhen et al., 2022) used Western blotting, immunofluorescence, enzyme linked immunosorbent assay (ELISA) and reverse transcription-PCR (RT-PCR) to investigate the effect of curcumol on macrophage M1/M2 phenotypic differentiation and its mechanism of action; analysis showed that curcumol could inhibit the inflammatory factors IL-1β and TNF-α, the M1 macrophage markers iNOS, NF-κB, and NLRP3 in activated RAW264.7 macrophages, and increased the expression of the M2 type macrophage marker CD206; these events, inhibited the inflammatory response by regulating macrophage phenotypic shift. Another investigation showed studied that a high dose extract group of Curcumae Rhizoma, and the high and low dose groups of Curcumae Rhizoma vinegar, significantly increased the levels of PGE2, 6-keto-PGF-1α, NO, and β-EP in uterine tissues, and reduced the levels of TXB2, PGF2α, IL-6, TNF-α, and Ca2+; these events exerted analgesic effects by regulating the levels of these pain factors (Tong, 2021). Information relating to the parameters described in pharmacological studies on the anti-inflammatory and analgesic effects of Curcumae Longae Rhizoma, Curcumae Radix and Curcumae Rhizoma is shown in Table 3.
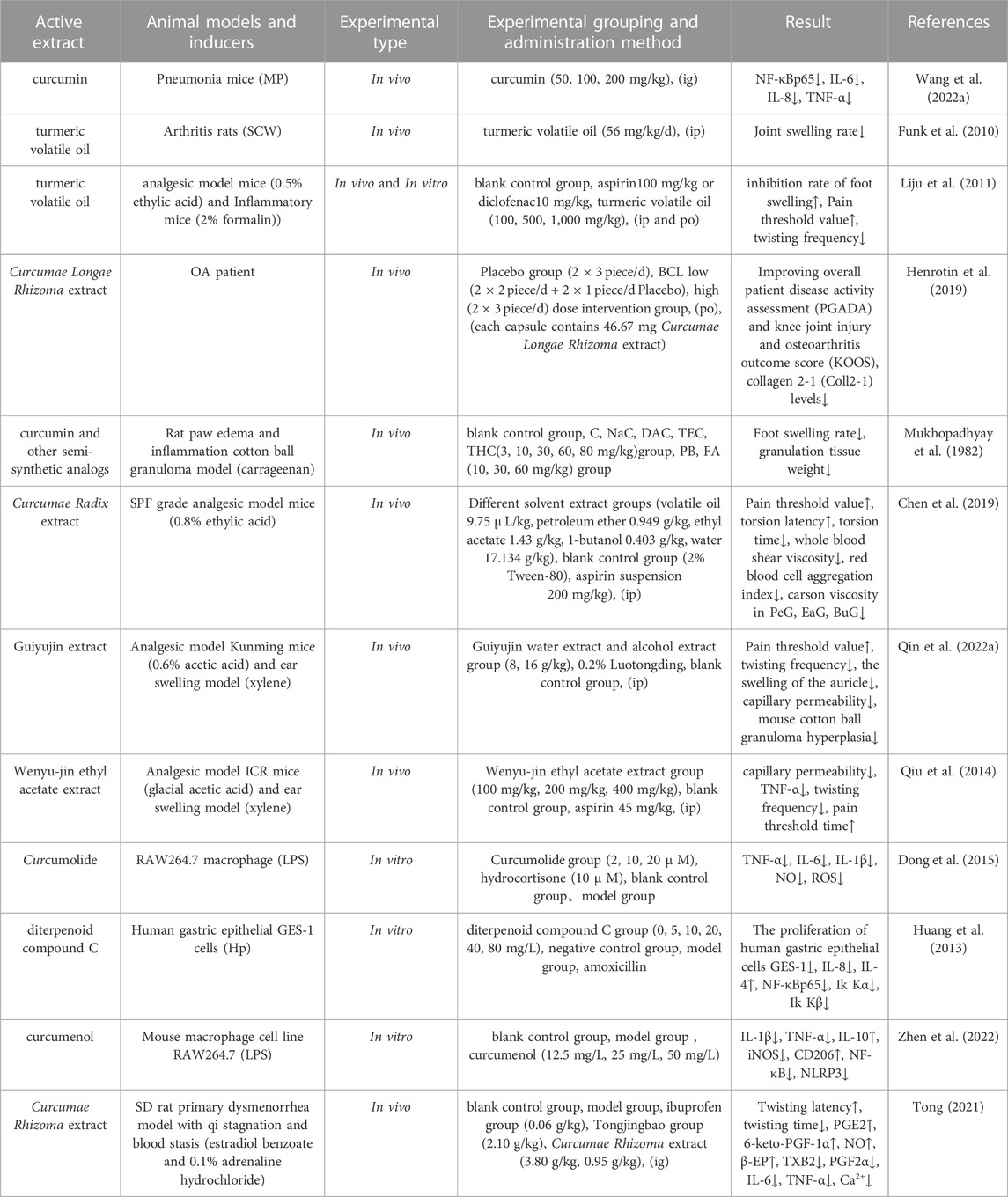
TABLE 3. Pharmacological parameters of anti-inflammatory and analgesic effects of Curcumae Longae Rhizoma, Curcumae Radix and Curcumae Rhizoma.
Oxidative stress refers to an imbalance between the production of oxidants and antioxidants; when ROS is produced by the body, it can directly oxidize a range of macromolecules, including membrane lipids, structural proteins, enzymes and nucleic acids, thus leading to impaired cell function and cell death (Zhang W. et al., 2023). Kim et al. (Kim et al., 2021) found that water extracts of turmeric helped to inhibit the reduction of grip strength and muscle mass caused by muscle atrophy while reducing the activity of antioxidant enzymes as well as the protein levels of the muscle atrophy-related genes MuRF-1 and Atrogin1 in mice. In addition, this water extract reduced the levels of malondialdehyde (MDA) in muscle tissues, effectively alleviating the symptoms of Dexamethasone-induced muscular atrophy. In another study, Li et al. (Liju et al., 2011) investigated the antioxidant activities of the components of volatile oil from turmeric and discovered that the volatile oil of turmeric could significantly increase the levels of antioxidant enzymes, superoxide dismutase (SOD), glutathione (GSH), and GSH-Px in the blood, as well as the levels of glutathione sulfotransferase and SOD in the liver of experiment mice (p < 0.01); in addition, these components exerted antioxidant effects. Oxidative stress is a key pathogenic factor in osteoporosis; Zhao et al. (Zhao et al., 2022) cultured osteoblasts in vitro to create a model of oxidative stress for osteoblasts and explored the effect of curcumin on osteoblasts under oxidative stress. Analysis showed that the curcumin-treated groups showed enhanced proliferation of osteoblasts under oxidative stress, and also showed an inhibition of oxidative stress, as manifested by an increase in T-AOC, SOD and CAT levels; in addition, MDA and p-p38 MAPK levels were significantly reduced (p < 0.01) while the levels of Wnt5a were upregulated. The mechanism of action of curcumin may be to inhibit the activation of p38MAPK and promote the expression of the Wnt signaling pathway; these events may play a protective role in osteoblasts. In addition, other studies have shown that curcumin is mainly involved in skeletal muscle reconstruction and actus through the oxidative pathways by inhibiting fibrosis and the apoptosis of skeletal muscle cells (Deng, 2022; Hu, 2022).
Li et al. (Li Y. et al., 2022) evaluated the antioxidant activity of eight neo-sesquiterpenoids isolated from Wenyu-jin with regards to activation of the Nrf2-ARE pathway in human embryonic kidney (HEK293) cells. Analysis showed that procurcumenol and 9-oxo-neoprocurcumenol possessed certain antioxidant activities which were mainly exerted through the activation of the Nrf2-ARE pathway in a dose-dependent manner. In addition, both Wenyu-jin extract and vitamin E have been shown to completely inhibit radiation-induced lipid peroxidation, and when compared with the traditional antioxidant vitamin E, Wenyu-jin extract had a stronger antioxidant effect (Wang et al., 1996). He et al. (He et al., 2013) established a model of H2O2-induced oxidative stress injury in human umbilical vein endothelial cell (HUVEC) cells and demonstrated the antioxidant effect of Curcumae Radix extract; analysis showed that Curcumae Radix extract exerted antioxidative stress activity and protective effected against endothelial injury. Gou et al. (Gou et al., 2015) investigated the scavenging ability and reducing ability of C. phaeocaulis polysaccharides by performing in vitro antioxidant experiments. Analysis showed that C. phaeocaulis polysaccharides had a significant scavenging effect and strong reducing ability on DPPH (1,1-Diphenyl-2-picrylhydrazyl radical) radicals, hydroxyl radicals and superoxide anions. Zhang et al. (Zhang et al., 2015) compared the antioxidant activities of Curcumae Longae Rhizoma and Curcumae Rhizoma by Folin-Ciocaheu and Aluminum Salt Chromatography and found that both herbs exhibited strong antioxidant capacity although the antioxidant capacity of Curcumae Rhizoma was slightly stronger than Curcumae Longae Rhizoma. Information on the parameters of pharmacological studies relating to the antioxidant effects of Curcumae Longae Rhizoma, Curcumae Radix and Curcumae Rhizoma are shown in Table 4.
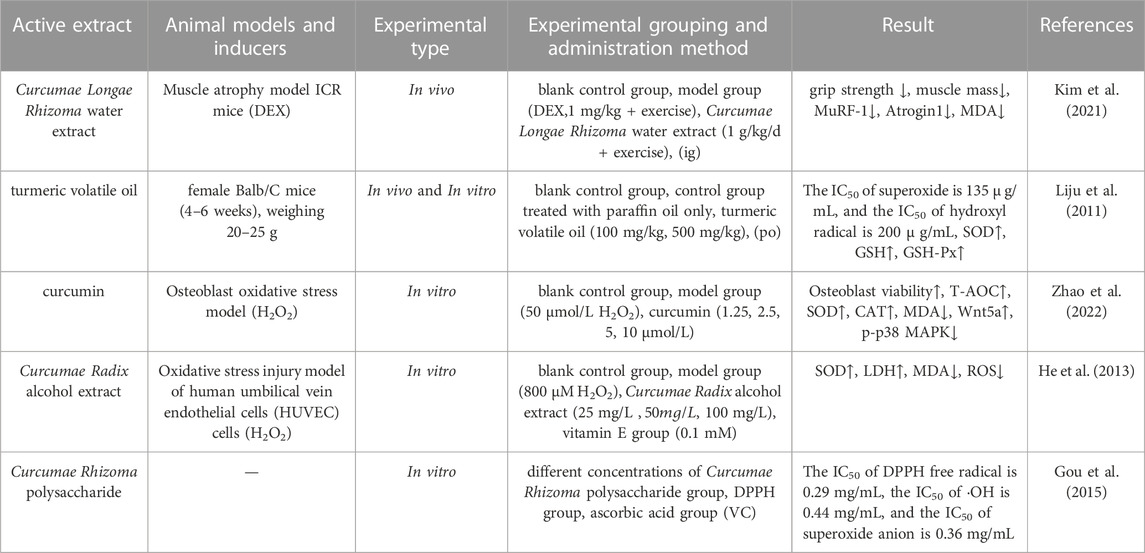
TABLE 4. Pharmacological parameters of antioxidant effects of Curcumae Longae Rhizoma, Curcumae Radix and Curcumae Rhizoma.
Cancer is one of the leading causes of death globally, and lung, liver, stomach, breast, and colon cancers are the top five leading causes of cancer-related deaths (Zhang Y. et al., 2023). Liver cancer rose had the third-highest cancer mortality rate in 2018; this rose to the second-highest in 2020. It was estimated that China will account for 24% of the world’s newly diagnosed cases and 30% of cancer-related deaths in 2020 (Cao et al., 2021). Many studies have now shown that curcumin can be used as a safe and effective anticancer drug that exhibits inhibitory effects in a variety of cancers such as gastric (Zhang X. et al., 2020), breast (Ma et al., 2022), liver (Zuo et al., 2022), and prostate cancer (Cheng and Gao, 2022), and that its anti-tumor mechanism of action is through the effective induction of apoptosis, the prevention of metastasis and invasion, and by exerting influence on a variety of growth factor receptors and cell adhesion molecules (Wilken et al., 2011; Yallapu et al., 2014; Qiao et al., 2020). Lei (Lei, 2013) reported that turmeric volatile oil inhibited the proliferation of liver cancer cells (Bel-7402, HepG2, and SMMC-7721) mainly by activating the mitochondrial apoptotic pathway, down-regulating the expression of Bcl-2 in the Bel-7402 liver cancer cells, reducing the levels of cytochrome C in the mitochondria, and by up-regulating Bax and activating the expression of caspase-9- and caspase-3-induced apoptosis in liver cancer cells. Lee et al. (Lee et al., 2018) revealed that curcumin can effectively reduce the protein levels of SIRT1. With regards to mechanisms, the electrophilic α- and β-unsaturated carbonyl portion of curcumin has been shown to exert certain antioxidant activities. When combined with the SIRT1 protein, curcumin can promote the effects of SIRT1 on the proteasomal degradation of colorectal cancer and exert anti-tumor effects. In another study, Jiang et al. (Jiang et al., 2012) measured the anti-tumor inhibition activity of curcuminoids extracts on Hela cells of cervical cancer and identified the main active components that could effectively inhibit the tumor cells from 26 species of curcuminoids extracts. Analysis showed that 13 types of curcuminoids (including cyclo-curcumin, cyclo-demethoxycurcumin and cyclo-bisdemethoxycurcumin) could effectively inhibit the proliferation and metastasis of HeLa cells and concluded that the antitumor effect was more significant when the structural formula featured a conjugated system, carbonyl or hydroxyl group. Liu et al. (Liu et al., 2019) investigated the effects of Wenyu-jin extracts on A498 and Sw-156 cells (renal cell carcinoma) and analyzed its mechanism of action; analysis showed that Wenyu-jin extracts caused mitochondrial dysfunction in A498 renal cell carcinoma cells, thus promoting the release of cytochrome C enzymes and inducing apoptosis in A498 cells. β-Elemene is a natural compound; Deng et al. (Deng et al., 2019) investigated the inhibitory effects of β-elemene on tumor cells and found that β-elemene effectively inhibited the peritoneal spreading and metastatic ability of gastric carcinoma. It was considered that the mechanism of action of β-elemene involves the downregulation of FAK phosphorylation and by influencing the expression of Claudin-1.
Xu et al. (Xu et al., 2018) investigated the effects of Curcumae Rhizoma oil on the protein expression of death factor receptor (Fas), secreted immune factors (Toll-like receptor 2 (TLR2), Toll-like receptor 4 (TLR4), interleukin-10 (IL-10)), and the oncogene C-Raf transforming growth factor-beta 1 (TGF-β1) in SW1463 rectal cancer cells and found that the protein expression of IL-10, TLR2, TLR4, and C-Raf was significantly reduced in cells from the Curcumae Rhizoma oil concentration group (p < 0.01). In addition, the protein expression of TGF-β1 was significantly reduced (p < 0.05); an increasing dose of Curcumae Rhizoma oil exerted a stronger inhibitory effect on the proliferation of rectal cancer cells. It is possible that Curcumae Rhizoma oil may inhibit the proliferation of SW1463 rectal cancer cells by down-regulating the Fas/FasL pathway. The tumor suppressor microRNA-30a-5p is known to inhibit the proliferation of colon cancer cells; however, whether curcumol can inhibit colon cancer, and whether microRNA-30a-5p is involved, remains unknown. Yu et al. (Yu et al., 2021) investigated the effect of curcumol on colon cancer by MTT, Western blotting and PCR. With regards to the mechanism of action of curcumol on colon cancer, research showed that a reduction in the expression of the tumor suppressor microRNA-30a-5p, accompanied by inactivation of the Hippo signaling pathway, led to an improvement in the viability and migration rate of colon cancer cells. Furthermore, curcumol increased the expression of miR-30a-5p in cells and activated the Hippo signaling pathway, thus inhibiting the expression of YAP1, β-catenin and MMP2 in HCT116 cells. Furthermore, curcumol increased the expression of E-cadherin, MST-1, LATS1 and p-YAP1, and inhibited the proliferation and migration of colon cancer cells. Pharmacological parameters of the antitumor effects of Curcumae Longae Rhizoma, Curcumae Radix and Curcumae Rhizoma are shown in Table 5. In summary, it is highly evident that Curcumae Longae Rhizoma, Curcumae Radix and Curcumae Rhizoma can exert anticancer effects via complex signaling pathways, including the Hippo and Fas/FasL pathways, by the upregulation of LATS1, p-YAP1, Bid, and Bax levels, and by the downregulation of IL-10, TLR2, and TLR4 levels. These herbs can exhibit antitumor effects on several cancers, but particularly cervical, colon, renal and liver cancer; the anticancer mechanisms of these herbs are shown in Figure 2.
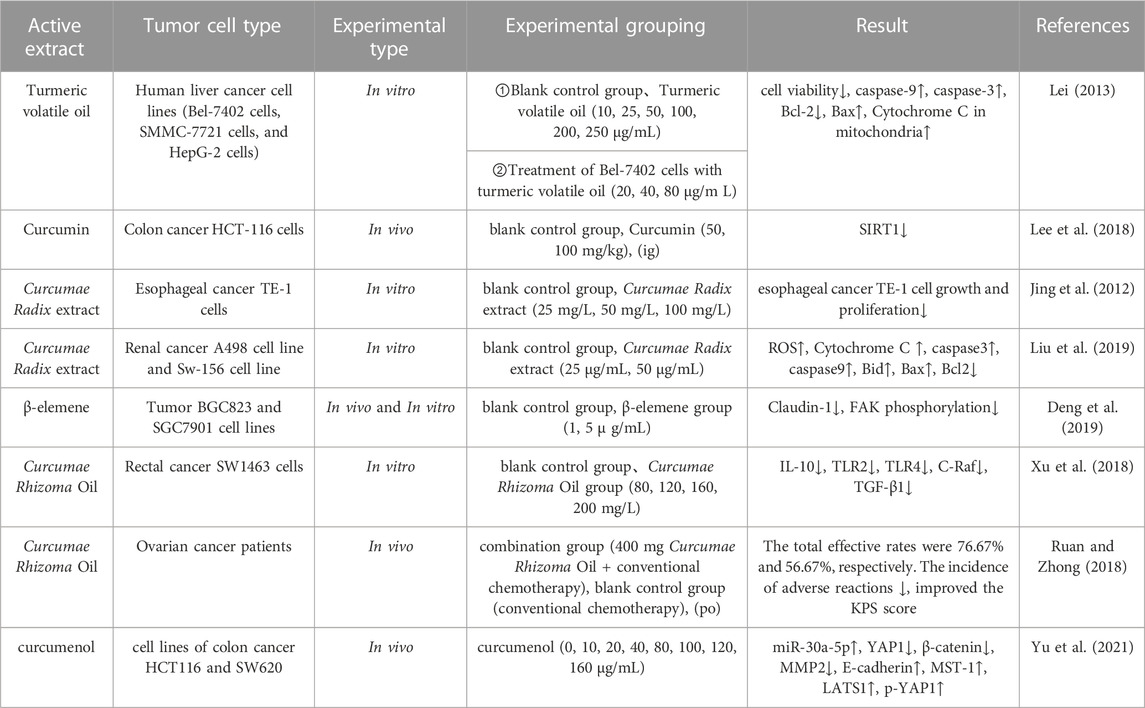
TABLE 5. Pharmacological parameters of antitumor effects of Curcumae Longae Rhizoma, Curcumae Radix and Curcumae Rhizoma.
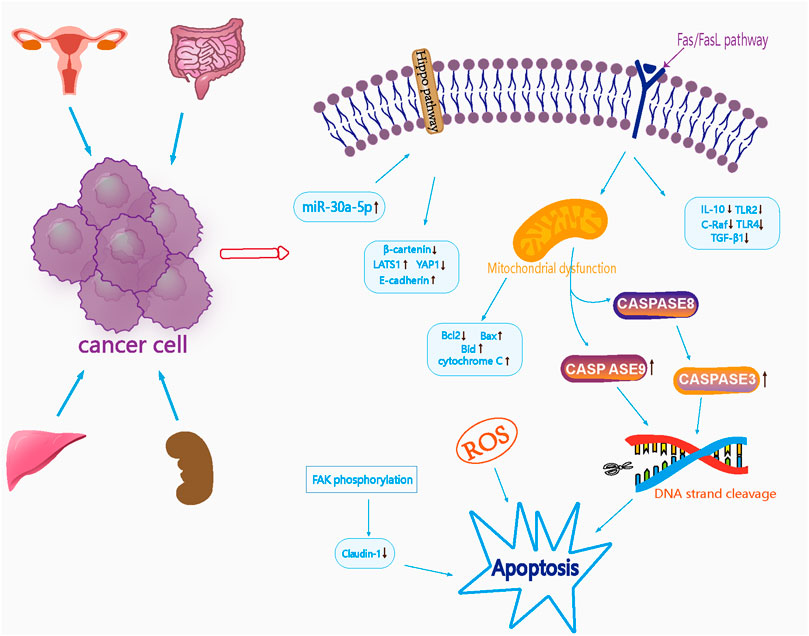
FIGURE 2. Mechanism diagram of antitumor effects of Curcumae Longae Rhizoma, Curcumae Radix and Curcumae Rhizoma.
Microorganisms affect the human defense system in many different ways, with their impact ranging from mild to life-threatening conditions. These infections occur when harmful bacteria invade the body, leading to a host immune response that can result in localized or systemic symptoms (de Souza et al., 2023). Figueira et al. (W Figueira et al., 2020) investigated the effect of turmeric volatile oil on Gram-positive bacteria, Gram-negative bacteria and fungi. Analysis showed that the minimum inhibitory concentration (MIC) of Staphyloccocus aureus Rosenbach and Pseudomonas aeruginosa was 25 mg/mL while the MIC of Candida albicans was 12.5 mg/mL; thus, turmeric volatile oil was demonstrated to possess significant antimicrobial activity. In another study, Hu et al. (Hu et al., 2017) explored the antimicrobial effect and mechanisms of turmeric volatile oil and found that turmeric volatile oil had significant inhibitory effects on the growth of Aspergillus flavus with a certain dose-dependence. Paw et al. (Paw et al., 2020) concluded that the antimicrobial activity of turmeric volatile oil was superior to that of antimicrobial drugs such as fluconazole and ciprofloxacin, and had strong inhibitory effects on Bacillus subtilis (MIC: 7.5
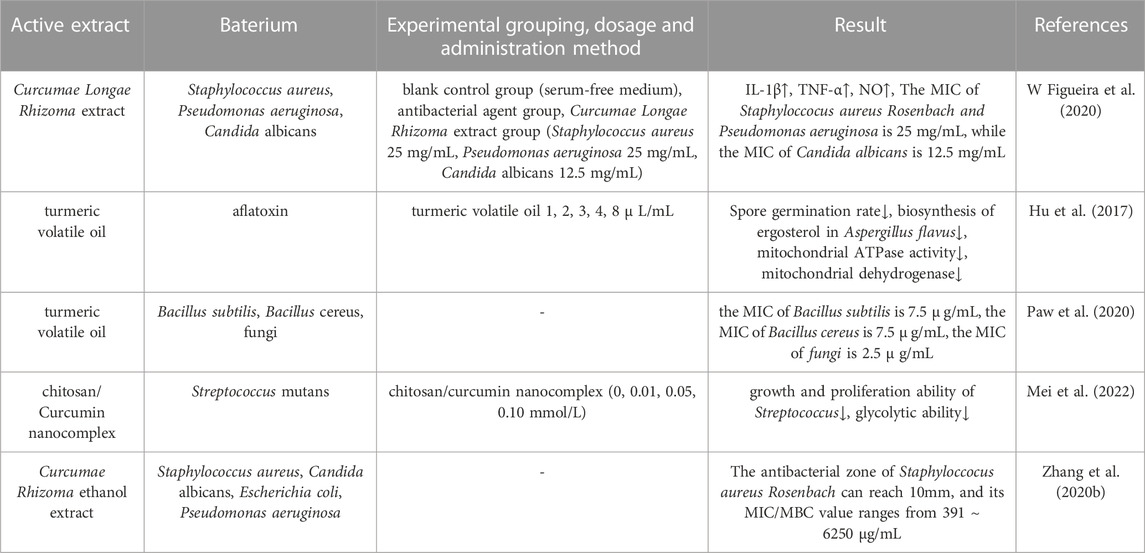
TABLE 6. Pharmacological parameters of antibacterial effects of Curcumae Longae Rhizoma, Curcumae Radix and Curcumae Rhizoma.
Viral infections are prevalent in humans and can cause permanent injury to various degrees and by different mechanisms (Hammarström and Nyström, 2023). Liu et al. (Liu X. et al., 2023) investigated the effect of curcumin on porcine epidemic diarrhea virus (PEDV) infection by using VERO cells as experimental objects. Analysis showed that curcumin enhanced the antiviral effects of antiviral cytokines in VERO cells and inhibited the proliferation of PEDV in VERO cells by increasing the levels of IFN-β, MX1, ISG15, and ZAP. In addition, curcumin also inhibited a variety of intracellular signaling pathways, including mitogen-activated protein kinases (MAPKs), casein kinase II (CKII), and COP9 signalosome (CSN). Coxsackievirus (CVB3) infection has been associated with a variety of diseases, including myocarditis and dilated cardiomyopathy. The search for new antiviral drugs against CVB3 found that curcumin could significantly reduce viral RNA expression, protein synthesis, and viral titer, and to protect against virus-induced cytopathic effects and apoptosis. The mechanism by which curcumin acts might involve regulation of the dysregulated ubiquitin-proteasome system (UPS) to effectively inhibit the replication of CVB3 (Si et al., 2007). In another study, Dong et al. (Dong et al., 2013) conducted in vitro antiviral assays with sesquiterpenoids isolated from Wenyu-jin and found that Wenyu-jin extracts exerted significant antiviral activity against the influenza A virus and HIVI with IC50 values ranging from 6.80 to 39.97
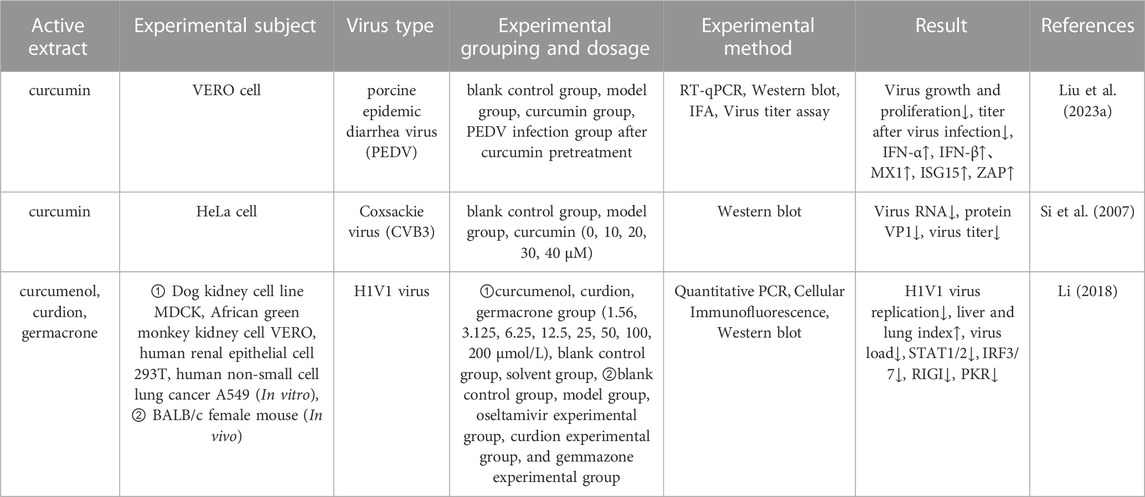
TABLE 7. Pharmacological parameters of antiviral effects of Curcumae Longae Rhizoma, Curcumae Radix and Curcumae Rhizoma.
According to the China Cardiovascular Health and Disease Report 2021, the number of individuals suffering from cardiovascular disease (CVD) in China is currently around 330 million. In addition, the prevalence of CVD is still increasing year-by-year, with CVD responsible for 46.74% and 44.26% of deaths in rural and urban areas in 2019 alone. This has led to new requirements for CVD prevention and treatment strategies and healthcare resource allocation in China (China Cardiovascular Health and Disease, 2022; Fu et al., 2023). Qiao et al. (Lei, 2013) explored the effects of turmeric extract on the cardiovascular system in New Zealand rabbits and diastolic isolated rat thoracic aorta, and concluded that turmeric extract could significantly inhibit platelet aggregation in rabbits (p < 0.05) and had a certain inhibitory effect on vascular ring contraction of the thoracic aorta in isolated rats caused by potassium chloride (KCl). Other research (Kim et al., 2012) demonstrated that curcumin and bisdemethoxycurcumin significantly prolonged the activated partialthromboplastin time (APTT) and prothrombin time (PT), and inhibited the activity of thrombin and activated factor X (FXa). The effect of curcumin was stronger than that of bisdemethoxycurcumin. Furthermore, it was deduced that methoxylation of the chemical structural formula enhanced its anticoagulant effect. Shah et al. (Shah et al., 1999) found that curcumin had a significant anticoagulant effect on blood vessels in the isolated rats, and inhibited the platelet aggregation induced by platelet-activating factor (PAF) and arachidonic acid (AA) in human blood, mainly by inhibiting the synthesis of thromboxane A2 (TXA2) and the Ca2+ signaling pathway. However, there was no significant inhibitory effect on the platelet aggregation induced by protein kinase C (PKC).
In other research, Jiang (Jiang et al., 2015) found that Curcumae Radix extract significantly inhibited platelet aggregation in rabbits and reduced the levels of TBIL, DBIL and AST in the serum of an experimental model of jaundice induced by α-naphthyl isothiocyanate (ANIT). Research has shown that the improvement of blood stagnation by Wenyu-jin extract is mainly related to lipid metabolism (linoleic acid metabolism, ether lipid metabolism, sphingolipid metabolism, glycerophospholipid metabolism, AA metabolism, and amino acid metabolism (including tryptophan metabolism and lysine degradation) (Hao et al., 2018). Su et al. (Su et al., 2019) found that the aqueous extract of Guiyujin (WECK) could maintain equilibrium of the ratio of TXA2 and PGI2 levels in the plasma, reduce the synthesis of TXA2 to inhibit platelet hyperfunction, promote the secretion of PGI2 by endothelial cells, prolong the activity of PT and APTT, induce vascular dilatation, and inhibit platelet aggregation (p < 0.01). Furthermore, the levels of TXB2 in the low-dose and medium-dose WECK groups were significantly elevated (p < 0.01); this was accompanied by a significant reduction in the levels of 6-K-PGF1α (p < 0.01). Furthermore, Guiyujin exerted antithrombotic effect by inhibiting the rate of thrombus formation by 38.23% and platelet aggregation by up to 72.10%. Chen et al. (Chen et al., 2018a; Chen et al., 2018b) demonstrated that the extract of Curcumae Rhizoma had a significant protective effect on death and hemiparesis caused by collagen-adrenaline-induced thrombosis in mice, significantly inhibited the formation of thrombi in the tail of mice caused by carrageenan, and reduced the number of animals that developed thrombosed black tails and the length of the black tails (p < 0.05, p < 0.01). These authors also demonstrated significant antithrombotic effects in venous blood vessels in vivo; the higher the concentration of Curcumae Rhizoma extract administered, the stronger the inhibitory effect. The antithrombotic effect of Curcumae Rhizoma extract may be related to increased levels of NO and 6-keto-PGF1α in the blood, reduced levels of ET-1 and TXB2, and reduced whole blood viscosity and plasma viscosity. Information on the parameters reported by pharmacological studies on the effect of Curcumae Longae Rhizoma, Curcumae Radix and Curcumae Rhizoma on blood rheology is shown in Table 8.
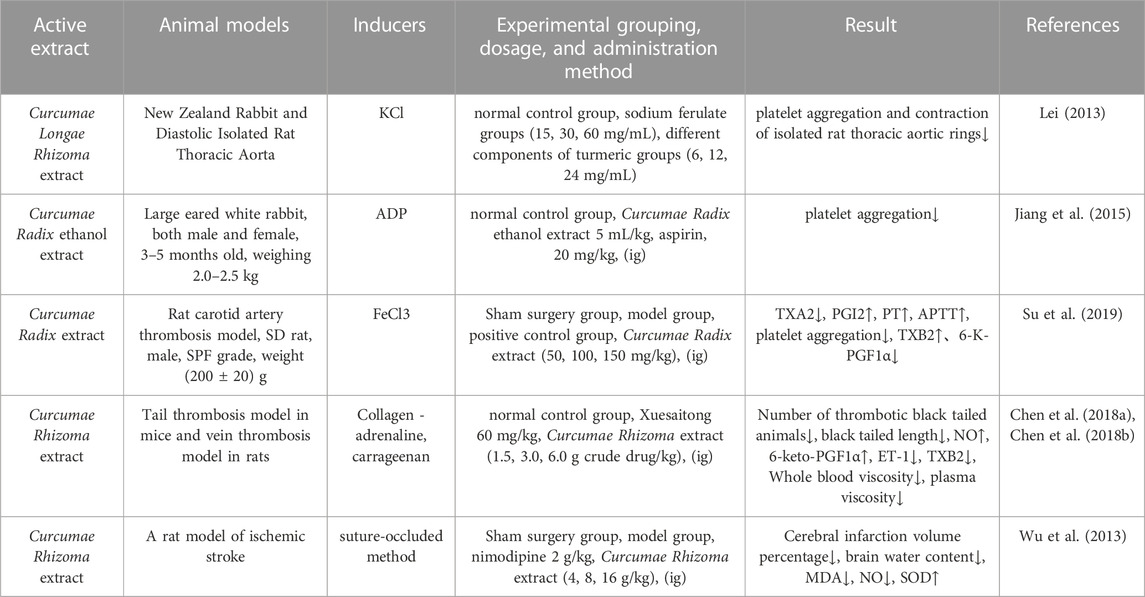
TABLE 8. Pharmacological parameters of effects of Curcumae Longae Rhizoma, Curcumae Radix and Curcumae Rhizoma on hemology.
It is estimated that 20%–25% of the global population have been diagnosed with metabolic syndrome (MetS); this is a chronic disease characterized by abnormalities in lipid metabolism, hypertension, hyperglycemia, and obesity. Furthermore, MetS can lead to abnormalities in the metabolic system, which can in turn induce the development of other diseases (Alberti et al., 2005). Lekshmi et al. (Lekshmi et al., 2012) evaluated the antidiabetic capacity of turmeric volatile oil by the α-glucosidase inhibition test and amylase inhibition test. Analysis showed that turmeric volatile oil increased the inhibitory ability of α-glucosidase (IC50 = 0.28
Cai et al. (Cai et al., 2017) investigated the effects and mechanism of action of the sesquiterpenoid compound curcumolide from Wenyu-jin on a streptomycin-induced mouse model of diabetes. Diabetes was induced by an intraperitoneal (ip) injection of freshly prepared streptomycin (55 mg/kg). Analysis showed that curcumolide activated the NF-κB pathway and attenuated diabetic retinal vascular permeability and vascular white matter sludge, and reduced the overexpression of TNF-α and ICAM-1 in the diabetic retina. In another study, Xu et al. (Xu et al., 2009) investigated the antioxidant capacity of Curcumae Rhizoma polysaccharide and lovastatin. Experiments were divided into a blank control group, a lovastatin group (3.0 mg/kg), and Curcumae Rhizoma polysaccharide Ⅰ (300 mg/kg), II (600 mg/kg), Ⅲ (900 mg/kg) dose groups for 3 weeks. The blank control group was injected with the same volume of saline. Analysis showed that compared with the model control group, the levels of TC, TG, and LDL-c in the rats in the Curcumae Rhizoma polysaccharide-treated groups were reduced to close to normal level while the levels of HDL-c in the serum, and the activity of antioxidant enzymes, were significantly elevated. These results indicated that the protective effect of Curcumae Rhizoma polysaccharide on oxidative damage was comparable to that of lovastatin, and that this herb might play a protective role by regulating the degree of lipid peroxidation and by enhancing the antioxidant defense system. The pharmacological parameters describing the effects of Curcumae Longae Rhizoma, Curcumae Radix and Curcumae Rhizoma on blood glucose and blood lipids are shown in Table 9. In summary, it is highly evident that Curcumae Longae Rhizoma, Curcumae Radix and Curcumae Rhizoma can induce hypoglycemic and hypolipidemic effects by reducing the absorption of carbohydrates in the small intestine, vascular permeability, cholesterol synthesis, and apoptosis of β-cells; the associated mechanisms are shown in Figure 3.
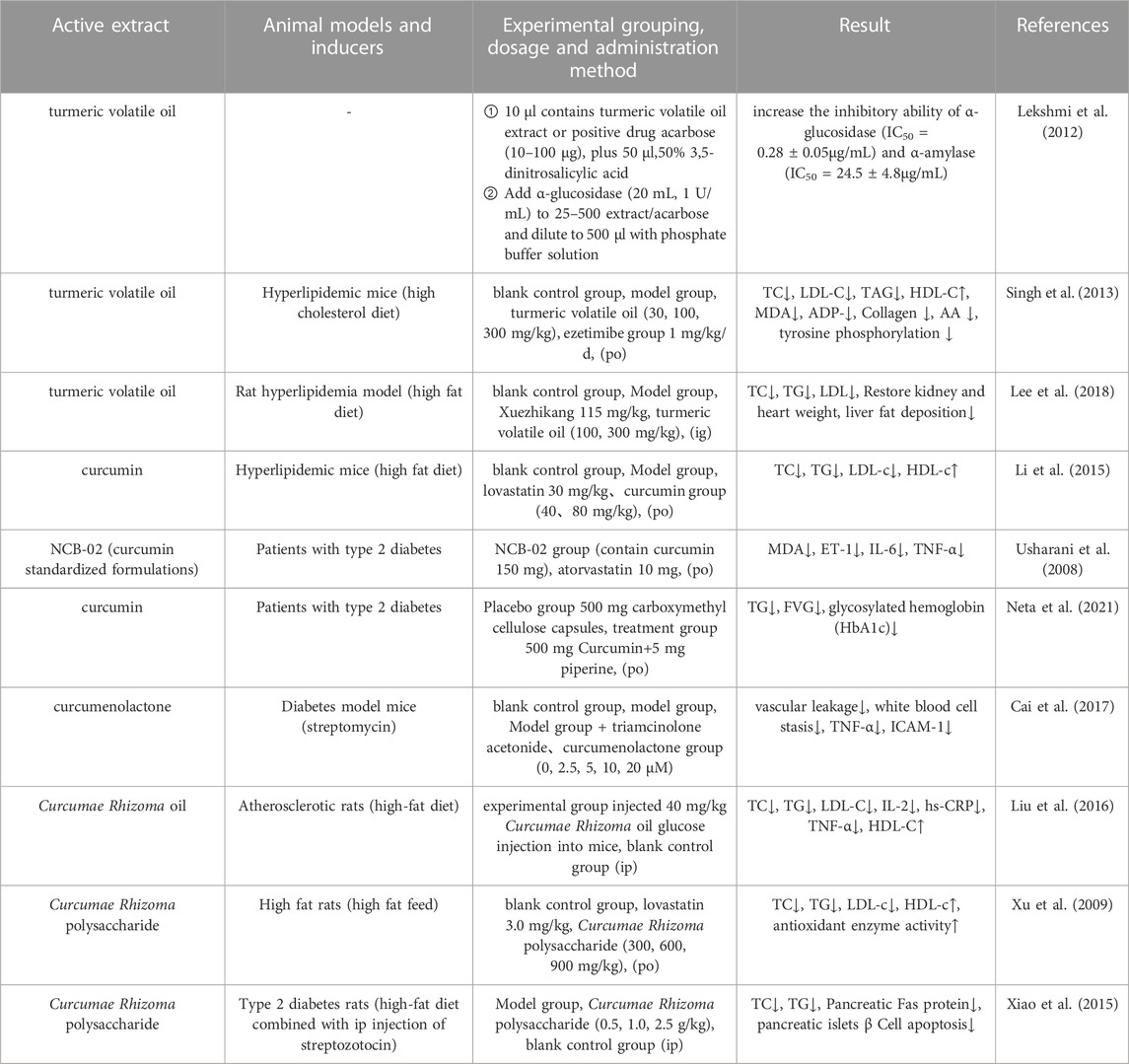
TABLE 9. Pharmacological parameters of effects of Curcumae Longae Rhizoma, Curcumae Radix and Curcumae Rhizoma on blood glucose and blood lipid.
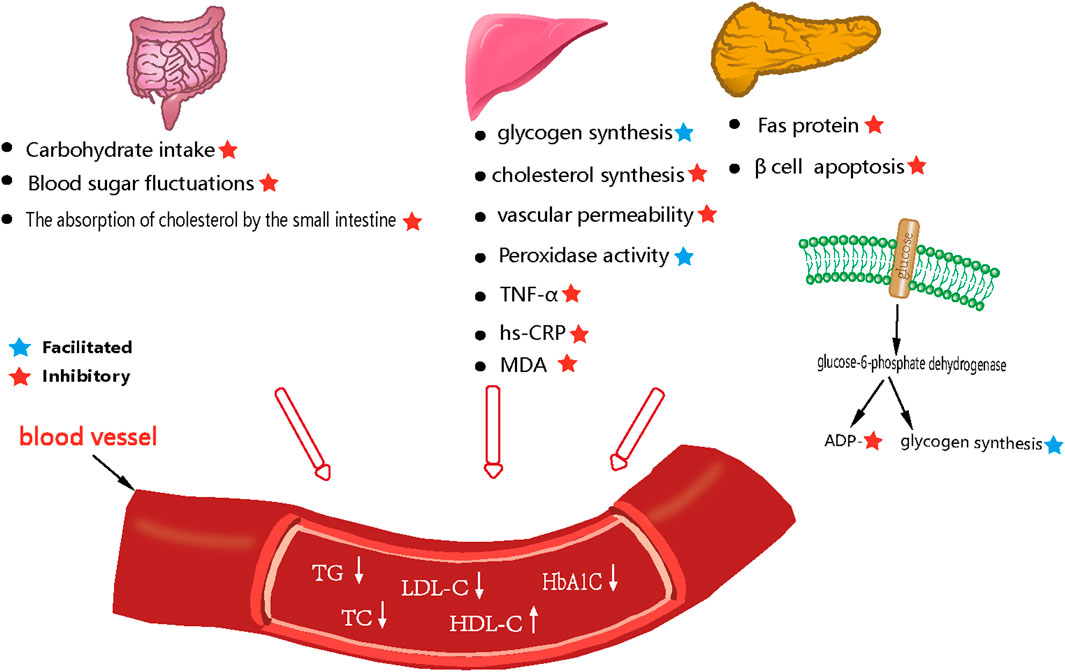
FIGURE 3. Mechanism diagram of reducing blood glucose and blood lipid levels in Curcumae Longae Rhizoma, Curcumae Radix and Curcumae Rhizoma.
Neurodegenerative diseases, such as epilepsy, Alzheimer’s disease (AD), depression, Parkinson’s disease (PD) and others, are characterized by a complex pathogenesis and rapid onset; these diseases represent a significant challenge for both the patient and clinician. Eun et al. (Eun et al., 2017) investigated the effects of turmeric extract on memory dysfunction in two brain cell lines (C6 rat glioma cells and BV2 mouse microglial cells) and a scopolamine-induced mouse model of memory dysfunction. Turmeric extract inhibited the time taken for t-BHP and H2O2-induced cellular damage to occur while also reducing the production of pro-inflammatory mediators, including NO, TNF-α, PGE2, iNOS, and COX-2. Furthermore, turmeric extract attenuated scopolamine-induced memory impairment, inhibited the activity of AChE, and promoted the activation of CREB and the expression of brain-derived neurotrophic factor in experimental mice. Li et al. (Li H. et al., 2022) found that curcumin improved sevoflurane-induced neuronal cell injury mainly by inhibiting the inflammation, oxidative stress and neuronal apoptosis of the hippocampus. Yu et al. (Su et al., 2022) used the Morris water maze test and Western blotting to investigate the effects of curcumin on memory function and GSK3β protein expression in the hippocampus of AD rats. Analysis showed that the evasion latency time in the curcumin group was significantly increased, the number of crossing platforms was reduced (p < 0.05), and the expression of GSK3β protein in the hippocampus was reduced, thus significantly ameliorating neuronal cell damage in the rat model of AD. In another study, Fikry et al. (Fikry et al., 2022) investigated the potential protective influence of curcumin on the cerebellum of rats with rotenone-induced PD. Analysis showed that curcumin attenuated neurotoxic effects and degenerative histological changes while alleviating induced oxidative stress in the cerebellar cortex of the rat model of PD. Therefore, curcumin may exhibit neuroprotective effects against the development of cerebellum-related PD symptoms.
Wang et al. (Wang et al., 2016) used β-amyloid (Aβ25-35) to establish an Aβ25-35 SPF-grade mouse model of dementia and investigated the effects of Curcumae Radix extracts with different solvents. Analysis showed that aqueous, volatile oil and ethanol extracts of Curcumae Radix significantly improved the symptoms of AD mice. Qi et al. (Qi et al., 2017) investigated the effect of Wenyu-jin volatile oil on tau protein phosphorylation in a SPF mouse model of AD and also studied the mechanisms involved. Analysis showed that the phosphorylation level of tau protein (Thr231 and Ser404) in the hippocampus of the rats in the high-dose group of Wenyu-jin volatile oil was significantly reduced (p < 0.05), and that the levels of phosphorylation of PI3K and Akt were significantly increased (p < 0.05), thus demonstrating that the protective effects of the volatile oil of Wenyu-jin on AD mice might be related to the PI3K/Akt signaling pathway. Li et al. (Li et al., 2010) investigated the effect of an aqueous extract of Curcumae Rhizoma on monoamine neurotransmitters in different parts of the male rat brain. Analysis concluded that the aqueous extract of Curcumae Rhizoma promoted the secretion of catecholamine (CA), norepinephrine (NE), and 5-hydroxytryptophan (5-HT) in different parts of the brain tissue. Yu et al. (Yu et al., 2016) investigated the effect of an aqueous extract of Curcumae Rhizoma on the expression of neural cell adhesion molecule (NCAM), sialyltransferase ST8SiaII, ST8SiaIV, Fyn, and focal adhesion kinase (FAK) mRNA in the brains of offspring derived from rats with blood stasis or normal pregnancy when compared with a normal control group. The expression levels of ST8SiaII, p-FAK, NCAM, and p-Fyn protein in the hippocampus were significantly downregulated (all p < 0.05); this may represent one of the mechanisms underlying neurodevelopmental toxicity. Information on the parameters reported by pharmacological studies on the effects of Curcumae Longae Rhizoma, Curcumae Radix and Curcumae Rhizoma on the nervous system is shown in Table 10.
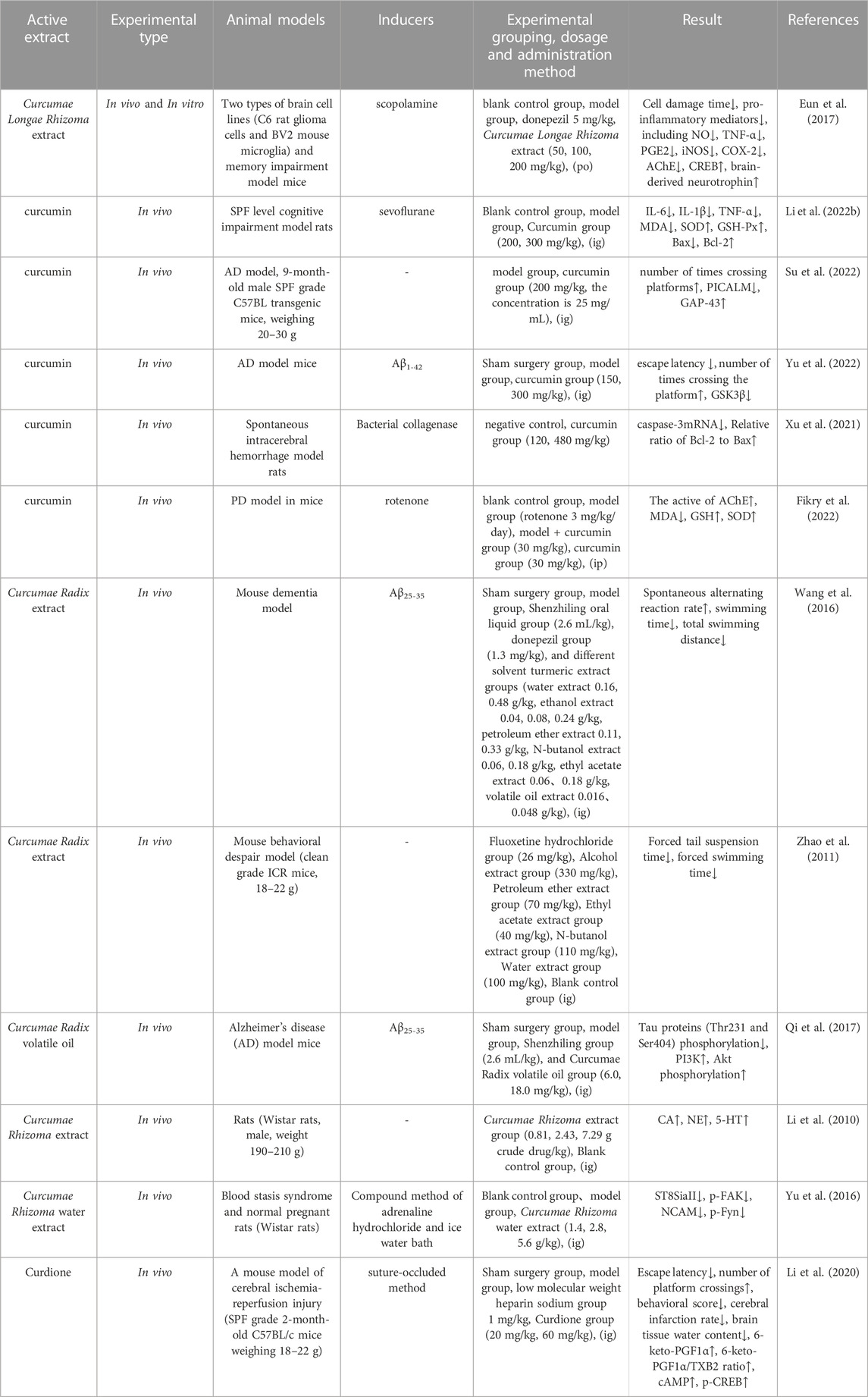
TABLE 10. Pharmacological parameters of effects of Curcumae Longae Rhizoma, Curcumae Radix and Curcumae Rhizoma on nervous system.
Respiratory diseases mainly include asthma, chronic obstructive pulmonary disease, and pneumonia. Most of the current treatments involve leukotriene inhibitors, glucocorticoids and bronchodilators, although these methods do not solve the main causative problem. Thus, the discovery of safe and effective therapeutic drugs has become a major challenge (Glass and Rosenthal, 2018; Liu Y. et al., 2023). Manarin et al. (Manarin et al., 2019) investigated the effects of turmeric extract on asthma; patients received either turmeric extract or placebo (approximately 30 mg/kg/d). After 6 months of treatment, analysis showed that the treatment group taking turmeric extract had a lower frequency of nocturnal awakenings and a lower frequency of short-acting β-adrenergic agonists when compared to the placebo group of asthma patients; thus, turmeric extract was effective in controlling asthma exacerbations. In addition, Houssen et al. (Houssen et al., 2010) evaluated the efficacy of Boswellia serrata, Glycyrrhiza glabra, and Curcumae Longae Rhizoma as a combination of natural leukotriene inhibitors, anti-inflammatory agents, and antioxidants, respectively, for the control of bronchial asthma by randomizing patients with bronchial asthma into a treatment group (150 mg boswellic acid, 50 mg licorice extract, and 15 mg curcumin) and placebo groups. After 4 weeks, 5 mL of blood were collected from each patient, and lung function was assessed by measuring the levels of leukotrienes, NO, and MDA in the plasma of all the patients participating in the stud. Compared to the placebo group, patients of the treatment group showed a significant reduction in the levels of leukotrienes, NO, and MDA in plasma; thus, Boswellia serrata, glycyrrza glabra, and Curcumae Longae Rhizoma extracts had significant therapeutic effects on bronchial asthma. In another study, Sun et al. (Sun, 2014) investigated the effects and mechanisms of curcumin on lung injury induced by one-lung ventilation in rabbits. Twenty-four New Zealand white rabbits were randomly divided into a two-lung ventilation group (TLV group), a one-lung ventilation group (OLV group), and a curcumin group (40 mg/kg). The TLV group and the OLV group were given 2 mL of 1% carboxymethylcellulosesodium and the administration was begun with the corresponding dose 7 days prior to the experiment, and was administered twice every day. Analysis showed that the oxygenation index at T3 was elevated in the curcumin group when compared with the OLV group (p < 0.05), the wet/dry weight ratio of the right and left lungs was reduced in the curcumin group (p < 0.05), SOD activity was elevated in the lung tissues of the curcumin intervention group (p < 0.05), and the levels of MDA were reduced (p < 0.05). Moreover, the levels of Nrf2 protein in the lung tissue of the curcumin group were elevated (p < 0.05); thus, curcumin exerted a protective effect on single-lung ventilation-induced lung injury in rabbits by regulating the Nrf2/ARE signaling pathway. Information on the parameters reported by pharmacological studies on the effects of Curcumae Longae Rhizoma, Curcumae Radix and Curcumae Rhizoma on the respiratory system is shown in Table 11.
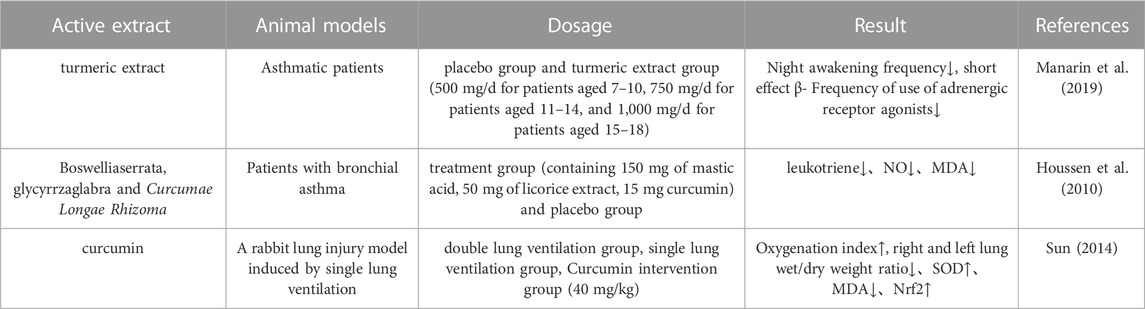
TABLE 11. Pharmacological parameters of effects of Curcumae Longae Rhizoma, Curcumae Radix and Curcumae Rhizoma on respiratory system.
The digestive system, as one of the eight major systems in the human body, consists of two parts: the digestive tract and the digestive glands. The basic physiological functions of this system are the ingestion, transit and digestion of food, the absorption of nutrients, and the excretion of wastes. Digestive diseases include gastrointestinal disorders and disorders of the liver, gallbladder, spleen, and pancreas (Li et al., 2023). Li (Li, 2020) used a high-fat diet to establish an animal model of gallbladder cholesterol stones in C57BL/6 mice and then investigated the role and mechanism of curcumin in the prevention of cholecystolithiasis. Analysis showed that curcumin acted by inhibiting the hydrolysis of SREBP-2 precursor protein to modulate the expression of NPC1L1 in cells of the small intestine. Hanai et al. (Hanai et al., 2006) evaluated the therapeutic effect of curcumin on ulcerative colitis (UC) in clinical trials and found that curcumin combined with mesalamine had significantly better clinical efficacy than placebo + mesalamine in preventing recurrence, significantly relieving the clinical symptoms of UC and reducing the recurrence rate. Sugimoto et al. (Sugimoto et al., 2002) investigated the effect and mechanism of curcumin on a trinitrobenzene sulfonic acid-induced mouse model of colitis; 0.5%, 2.0%, and 5.0% curcumin was added to the feed of mice in the curcumin intervention group. Then, the levels of NF-κB in the colonic mucosa were detected by immunohistochemistry while RT-PCR was used to detect the expression of cytokine mRNA in colon tissues. When compared to the model group, curcumin reduced mortality in mice with colitis, inhibited IκB, induced the nuclear translocation of NF-κB into the epithelial nucleus, and reduced the infiltration of CD4+ T-cells. Thus, the authors concluded that curcumin could exert significant clinical therapeutic effects on mice with colitis by inhibiting the expression of pro-inflammatory cytokine mRNA and NF-κB activation in the colonic mucosa. Information on the parameters reported by pharmacological studies on the effects of Curcumae Longae Rhizoma, Curcumae Radix and Curcumae Rhizoma on the digestive system is shown in Table 12.
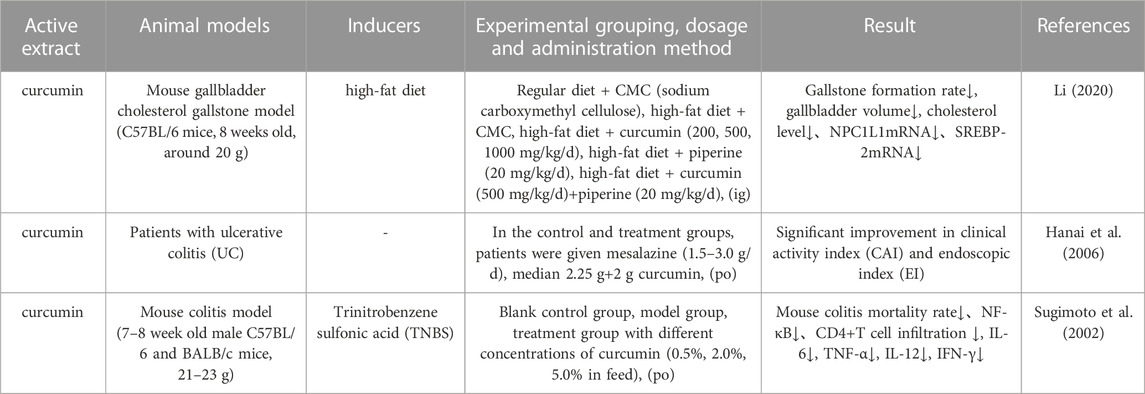
TABLE 12. Pharmacological parameters of effects of Curcumae Longae Rhizoma, Curcumae Radix and Curcumae Rhizoma on digestive system.
Curcumin has been shown to have a therapeutic effect on damage incurred by several organs, including the liver, kidney, skin, hands, feet, and mouth (Hu et al., 2018), and has also been shown to have antiprotozoal, antispasmodic, and antidepressant effects (Araújo and Leon, 2001; Wang Y. et al., 2022). Gong et al. (Gong et al., 2022) found that curcumin protected retinal Müller cells from damage caused by ultraviolet light, mainly through the activation of the Nrf2 signaling pathway in Müller cells; this attenuated damage in a mouse model. Some researchers have proposed that curcumin can significantly elevate weight, reduce viral load and plasma D-dimer (D-D), the levels of neuron-specific enolase (NSE) and neuropeptide Y (NPY) in rats with severe hand-foot-and-mouth disease (Ming et al., 2022). Li et al. (Li et al., 2018) investigated the effects and mechanisms of ar-turmerone in a mouse model of imiquimod-induced psoriasis and concluded that ar-turmerone inhibited CD8+ T cell metastasis in the epidermis, decreased the expression of NF-κB, COX-2 and IL-6 levels, reduced the phosphorylation of p38MAPK, and downregulated the expression of IL-17, IL-22, and IL-23 mRNA.
The extracts of Wenyu-jin were shown to have a terminating effect on all periods of mouse pregnancy, although oral administration was ineffective. In addition, Wenyu-jin extract had a significant excitatory effect on the isolated uterus of non-pregnant or mice in the early stages of pregnancy; as the dose of Wenyu-jin extract increased, the stronger the excitatory effect on the uterus became (Zhang et al., 1983). In another study, Xie et al. (Xie et al., 2020) used biochemical and histopathological methods to evaluate the anti-liver fibrosis effect of Wenyu-jin extract and showed that Wenyu-jin extract inhibited the activation and proliferation of hepatic stellate cells and induced apoptosis by blocking the TGF-β/Smad signaling pathway and significantly up-regulating the level of MMP-2/TIMP-1. These data indicated that Wenyu-jin extract was involved in the degradation of the extracellular matrix, and also maintained the formation and production of the extracellular matrix. Liu et al. (Liu and Cheng, 2012) confirmed that the traditional Chinese medicine Curcumae Rhizoma can delay renal interstitial fibrosis in rats with unilateral ureteral obstruction, reduce the content of N-acetyl-β-aminoglucosidase and urinary protein in urine; its effect on the kidneys were comparable to that of Losartan. Liu et al. (Liu et al., 2018) investigated the therapeutic effect and mechanism of C. kwangsiensis extracts on psoriasis and showed that C. kwangsiensis extract reduced the dendritic cell expression of lymphatic homing chemokine receptor CCR7 and its ligand CCL21 while also significantly reducing the activity of pro-inflammatory cytokines (IL-12, IL-6 and IL-1β), as well as the proliferation of T-cells and the differentiation of Th1 and Th17 cells. Collectively, this data suggested that C. kwangsiensis extract could represent a potential drug for psoriasis.
Curcumae Longae Rhizoma, Curcumae Radix and Curcumae Rhizoma all belong to the Curcuma species. Their chemical compositions are similar, although each has its own characteristics due to differences in medicinal components, geographic factors, and other factors. For example, Curcumae Longae Rhizoma is native to subtropical regions, and its curcuminoid content is much higher than that of Curcumae Radix and Curcumae Rhizoma; C. kwangsiensis contains the least amount of curcumin. Research has shown that the curcumin content of Curcumae Longae Rhizoma is higher than that of Curcumae Radix (Wang, 2014). Generally, rhizomes of the same species contain more curcuminoids and volatile oil compounds than their tubers.
In terms of clinical application, Curcumae Longa Rhizoma and Curcumae Radix are mainly used in raw products, the vinegar products of Curcumae Rhizoma can be used to enhance the role in removing blood stssis and eliminating mass. In terms of efficacy, Curcumae Longae Rhizoma is efficacious in promoting menstruation to relieve pain and is mostly used in the treatment of rheumatic diseases. Curcumae Rhizoma is efficacious at removing accumulation and alleviating pain, activating qi and blood circulation, and treating dyspepsia distending pain. Curcumae Radix is efficacious at activating qi and relieving depression, and activating blood circulation and alleviating pain (Qin et al., 2022b). In terms of medicinal properties, all three herbs can activating blood circulation and removing blood stasis, activating qi and alleviating pain, and can be used to treat blood stagnation, amenorrhea with abdominal mass. However, Curcumae Longae Rhizoma and Curcumae Rhizoma are both efficacious for the cold accumulation causing qi stagnation and blood stasis while Curcumae Radix is suitable for the treatment of depression stagnation due to blood heat. Modern pharmacological studies have shown that Curcumae Longae Rhizoma exerts mainly anti-inflammatory, analgesic, antioxidant, hypoglycemic, hypolipidemic, and antitumor effects while also improving blood rheology, antibacterial properties, protecting the nervous system, and other pharmacological effects (Wang and Xu, 2018). These properties can treat rheumatoid arthritis, cardiovascular and cerebrovascular diseases, neurodegenerative diseases, diabetes mellitus and other diseases with significant therapeutic effects. Curcumae Radix can regulate immune function, exert anti-inflammatory effects, protect the nervous system, protect the liver and promote gallbladder function, thus improving blood rheology and exerting anti-tumor effects. This herb is mainly used in the treatment of hepatobiliary system diseases, chronic gastritis, hepatitis, psoriasis, cardiovascular and cerebrovascular diseases, tissue contusion, lithiasis disease, and cyclomastopathy. Curcumae Rhizoma is mainly used for the clinical treatment of various malignant tumors and has significant therapeutic effects on the uterus. When combined with Carthami flos, Persicae semen and other herbs, it also has significant curative effects on endometriosis, dysmenorrhea, chronic pelvic inflammatory disease and other gynecological diseases. In contrast, Curcumae Rhizoma is also efficacious in removing stagnation; clinical preparations containing Curcumae Rhizoma and “xiao’er Huashi Koufuye” are mainly used for the treatment of nervosa, dyspepsia, and constipation in children (Tong et al., 2020).
Curcuma species exert significant anti-inflammatory and antioxidant effects mainly by modulating oxidative stress as well as NF-κB and other pathways, thus affecting the levels of factors such as TNF-α, IL-6, IL-1β, IL-8, NO, and COX-2 in vivo to exert anti-inflammatory effects. These herbs exert antioxidant effects by regulating the levels of SOD, GSH, MDA, and ROS (Ak and Gülçin, 2008). Curcumae Longae Rhizoma, Curcumae Radix and Curcumae Rhizoma all exhibit some effects on the blood system; however, Curcumae Rhizoma has a stronger blood-activating effect than turmeric. Research has been shown that there was no significant difference between the curcuminoid compounds in turmeric and Curcumae Rhizoma in terms of antiplatelet aggregation and vascular dilatation (Lei, 2013). However, the differences produced by their volatile oil components is very obvious; the inhibitory effects of turmeric volatile oil components on platelet aggregation is weaker, thus showing that the volatile components are the potential material basis for the efficacy of blood-activation. Curcumae Radix mainly exerts its efficacy of purging heart and cooling blood by regulating lipid metabolism, plasma lipoprotein levels, platelet activation, oxidative stress reactions, apoptosis and other processes. Curcuminoids and volatile oil compounds (e.g., curcumin, curcumol, curdione, β-elemene, curzerenone, and germacrone) in Curcuma species have certain anti-tumor effects, of these, β-elemene is considered to be a broad-spectrum antitumor agent and is known to play a therapeutic role in the treatment of a variety of malignant tumors and has few side effects in humans (Chen et al., 2021). Overall, Curcumae Longae Rhizoma is mostly known for its anti-inflammatory and antioxidant effects while Curcumae Radix is mainly known for its effects on the cardiovascular system; Curcumae Rhizoma is mainly known for its anti-tumor effects.
There is no systematic exposition of the herbs of the Curcuma species. Most of the existing research focuses on investigating single pharmacological effects of traditional Chinese medicine or the pharmacological effect of an active ingredient within such medicine (Yuan et al., 2015). Future research should focus on other active ingredients to comprehensively evaluate the pharmacological effects of medicinal materials. In terms of the quality control of medicinal materials, the Chinese Pharmacopoeia only stipulates the content of volatile oil and curcumin of Curcumae Longae Rhizoma, and the content of the volatile oil of Curcumae Rhizoma, but does not stipulate the content of Curcumae Radix. In order to more comprehensively evaluate the quality standards of medicinal materials, it is important to include a wider range of scientific and technical methods.
XZ and Y-yQ contributed the conception of the review. XZ, Y-yQ, Z-jY, TW, L-yZ, S-qF, ML and J-nZ summarized and analyzed the literatures. XZ and Y-yQ drafted the manuscript, prepared tables, and drew the figures. LL critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Science and Technology Department of Sichuan Province, China (A-2021N-Z-3). Sichuan Provincial Administration of Traditional Chinese Medicine, China (2022C009), and Open Project of Translational Chinese Medicine Key Laboratory of Sichuan Province (2022-KFKT-1).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ak, T., and Gülçin, I. (2008). Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interact. 174 (1), 27–37. doi:10.1016/j.cbi.2008.05.003
Alameri, A. A., Ghanni, M. U., Ali, A., Singh, M., Al-Gazally, M. E., Almulla, A. F., et al. (2023). The effects of curcumin on astrocytes in common neurodegenerative conditions. Mini Rev. Med. Chem. 23. doi:10.2174/1389557523666230502143131
Alberti, K. G., Zimmet, P., and Shaw, J.IDF Epidemiology Task Force Consensus Group (2005). The metabolic syndrome-a new worldwide definition. Lancet 366 (9491), 1059–1062. doi:10.1016/S0140-6736(05)67402-8
Anand, P., Thomas, S. G., Kunnumakkara, A. B., Sundaram, C., Harikumar, K. B., Sung, B., et al. (2008). Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem. Pharmacol. 76 (11), 1590–1611. doi:10.1016/j.bcp.2008.08.008
Araújo, C. C., and Leon, L. L. (2001). Biological activities of Curcuma longa L. Mem. Inst. Oswaldo Cruz 96 (5), 723–728. doi:10.1590/s0074-02762001000500026
Balakumar, P., Venkatesan, K., and Abdulla, K. N. (2023). Mechanistic insights into the beneficial effects of curcumin on insulin resistance: opportunities and challenges. Drug Discov. Today 28 (7), 103627. doi:10.1016/j.drudis.2023.103627
Cai, Y., Li, W., and Tu, H. (2017). Curcumolide reduces diabetic retinal vascular leukostasis and leakage partly via inhibition of the p38MAPK/NF-κB signaling. Bioorg Med. Chem. Lett. 27 (8), 1835–1839. doi:10.1016/j.bmcl.2017.02.045
Cao, W., Chen, H. D., and Yu, Y. W. (2021). Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin. Med. J. Engl. 134 (7), 783–791. doi:10.1097/CM9.0000000000001474
Chen, X., Wei, J., and Jian, Z. (2018b). Antithrombotic effects of ethyl acetate fraction of Curcuma kwangsiensis. Chin. Tradit. Pat. Med. 40 (06), 1238–1242. doi:10.3969/j.issn.1001-1528.2018.06.002
Chen, X., Wei, J., and Nong, Y. (2018a). Experimental study on anti-thrombotic effect of aqueous extract of ezhu (curcuma kwangsiensis). Chin. J. Tradit. Med. Sci. Technol. 25 (04), 495–497.
Chen, Y. (1981). Preliminary study on curcuma plants in China - I. Plant identification. Acta Pharm. Sin. 16 (05), 385–389.
Chen, Y., Zhu, Z., Chen, J., Zheng, Y., Limsila, B., Lu, M., et al. (2021). Terpenoids from Curcumae Rhizoma: their anticancer effects and clinical uses on combination and versus drug therapies. Biomed. Pharmacother. 138, 111350. doi:10.1016/j.biopha.2021.111350
Chen, Z., Quan, L., Zhou, H., Zhao, Y., Chen, P., Hu, L., et al. (2019). Screening of active fractions from Curcuma Longa Radix isolated by HPLC and GC-MS for promotion of blood circulation and relief of pain. J. Ethnopharmacol. 24 (234), 68–75. doi:10.1016/j.jep.2018.09.035
Chen, Z., Zhao, Y., Quan, L., Zhou, H., Cao, D., Hu, C., et al. (2017). Study on quality standard of processed curcuma longa radix. Evid. Based Complement. Altern. Med. 2017, 2830754. doi:10.1155/2017/2830754
Cheng, J., and Gao, D. (2022). Effect of curcumin on PC-3 cell growth in vitro. Med. J. W. 34 (06), 808–812. doi:10.3969/j.issn.1672-3511.2022.06.006
China Cardiovascular Health and Disease (2022). China cardiovascular Health and disease Report 2021 summary. Chin. Circ. J. 37 (6), 553–578. doi:10.3969/j.issn.1000-3614.2022.06.001
Chinese Pharmacopoeia Commission (2020). Pharmacopoeia of the people’s Republic of China. Vol. I. Beijing, China: China Med. Sci. Press, 276.
Claeson, P., Panthong, A., Tuchinda, P., Reutrakul, V., Kanjanapothi, D., Taylor, W. C., et al. (1993). Three non-phenolic diarylheptanoids with anti-inflammatory activity from Curcuma xanthorrhiza. Planta Med. 59 (5), 451–454. doi:10.1055/s-2006-959730
Cui, Y., An, X., and Wang, H. (2016). Chemical constituents from rhizomes of Curcuma longa. Chin. Tradit. Herb. Drugs. 47 (7), 1074–1078.doi:10.7501/j.issn.0253-2670.2016.07.002
de Souza, A. B., Pinheirom, J. C. A., and Soares, J. B. (2023). Antibacterial activity and anxiolytic-like effect of Ziziphus joazeiro Mart. leaves in adult zebrafish (Danio rerio). Fish. Shellfish Immunol. Rep. 5, 100108. doi:10.1016/j.fsirep.2023.100108
Dell'Agli, M., Di Lorenzo, C., Badea, M., Sangiovanni, E., Dima, L., Bosisio, E., et al. (2013). Plant food supplements with anti-inflammatory properties: a systematic review (I). Crit. Rev. Food Sci. Nutr. 53 (4), 403–413. doi:10.1080/10408398.2012.682123
Deng, M., Zhang, Y., Liu, B., Chen, Y., Song, H., Yu, R., et al. (2019). β-Elemene inhibits peritoneal metastasis of gastric cancer cells by modulating FAK/Claudin-1 signaling. Phytother. Res. 33 (9), 2448–2456. doi:10.1002/ptr.6436
Deng, W. (2022). Effects of curcumin combined with resistance exercise on skeletal muscle reconstruction. Mol. Plant Breed. 20 (14), 4820–4826. doi:10.13271/j.mpb.020.004820
Dong, J., Shao, W., Yan, P., Cai, X., Fang, L., Zhao, X., et al. (2015). Curcumolide, a unique sesquiterpenoid with anti-inflammatory properties from Curcuma wenyujin. Bioorg Med. Chem. Lett. 25 (2), 198–202. doi:10.1016/j.bmcl.2014.11.075
Dong, J. Y., Ma, X. Y., Cai, X. Q., Yan, P. C., Yue, L., Lin, C., et al. (2013). Sesquiterpenoids from Curcuma wenyujin with anti-influenza viral activities. Phytochemistry 85, 122–128. doi:10.1016/j.phytochem.2012.09.008
Erarslan, A. S., Ozmerdivenli, R., and Sirinyıldız, F. (2023). Therapeutic and prophylactic role of vitamin D and curcumin in acetic acid-induced acute ulcerative colitis model. Toxicol. Mech. Methods 33 (6), 480–489. doi:10.1080/15376516.2023.2187729
Eun, C. S., Lim, J. S., and Lee, J. (2017). The protective effect of fermented Curcuma longa L. on memory dysfunction in oxidative stress-induced C6 gliomal cells, proinflammatory-activated BV2 microglial cells, and scopolamine-induced amnesia model in mice. BMC Complement. Altern. Med. 17 (1), 367. doi:10.1186/s12906-017-1880-3
Fikry, H., Saleh, L. A., and Abdel Gawad, S. (2022). Neuroprotective effects of curcumin on the cerebellum in a rotenone-induced Parkinson's Disease Model. CNS Neurosci. Ther. 28 (5), 732–748. doi:10.1111/cns.13805
Fu, T., Geng, H., and Zhang, D. (2023). Physical activity status of Chinese residents and its relationship with cardiovascular diseases. Chin. Prev. Med. 24 (01), 62–66. doi:10.16506/j.1009-6639.2023.01.012
Funk, J. L., Frye, J. B., Oyarzo, J. N., Zhang, H., and Timmermann, B. N. (2010). Anti-arthritic effects and toxicity of the essential oils of turmeric (Curcuma longa L). J. Agric. Food Chem. 58 (2), 842–849. doi:10.1021/jf9027206
Gadnayak, A., Dehury, B., Nayak, A., and Jena, S. (2022). Mechanistic insights into 5-lipoxygenase inhibition by active principles derived from essential oils of Curcuma species: molecular docking, ADMET analysis and molecular dynamic simulation study. PLoS One 17 (7), e0271956. doi:10.1371/journal.pone.0271956
Ge, Y., Gao, H., and Wang, Z. (2007). Advances in study of genus Curcuma. J. Chin. Mater. Med. 32 (23), 2461–2467.
Glass, R. I., and Rosenthal, J. P. (2018). International approach to environmental and lung Health. A perspective from the fogarty international center. Ann. Am. Thorac. Soc. 15 (2), S109–S113. doi:10.1513/AnnalsATS.201708-685MG
Gong, Y., Meng, N., and Xi, H. (2022). Protection effect of curcumin on light-induced retinal Müller cell damage and its mechanism. J. Qiqihar Univ. Med. 43 (07), 606–609. doi:10.3969/j.issn.1002-1256.2022.07.002
Gou, X., Wang, Q., and Gao, G. (2015). Antioxidant activities of polysaccharides from rhizomes of the herb Curcuma phaeocaulis in vitro. Sci. Technol. Food Ind. 36 (06), 122–125+130. doi:10.13386/j.issn1002-0306.2015.06.019
Guo, F., Gu, Z., and Jia, X. (2022). Research progress of the medicinal plant turmeric. J. Anhui Agric. Sci. 50 (16), 14–15. doi:10.4244/AIJ-E-22-00001
Hammarström, P., and Nyström, S. (2023). Viruses and amyloids - a vicious liaison. Prion 17 (1), 82–104. doi:10.1080/19336896.2023.2194212
Hanai, H., Iida, T., and Takeuchi, K. (2006). Curcumin maintenance therapy for ulcerative colitis: randomized, multicenter, double-blind, placebo-controlled trial. Clin. Gastroenterol. Hepatol. 4 (12), 1502–1506. doi:10.1016/j.cgh.2006.08.008
Hao, M., Ji, D., Li, L., Su, L., Gu, W., Gu, L., et al. (2018). Mechanism of curcuma wenyujin rhizoma on acute blood stasis in rats based on a UPLC-Q/TOF-MS metabolomics and network approach. Molecules 24 (1), 82. doi:10.3390/molecules24010082
He, J., Wang, R., and He, J. (2013). Protection of ethanol extract from curcumae radix on hydrogen peroxide-induced oxidative stress in human umbilical vein endothelial cell. Chin. J. Exp. Tradit. Med. Formulae. 19 (03), 223–225. doi:10.13422/j.cnki.syfjx.2013.03.073
Henrotin, Y., Malaise, M., and Wittoek, R. (2019). Bio-optimized curcuma longa extract is efficient on knee osteoarthritis pain: a double-blind multicenter randomized placebo controlled three-arm study. Arthritis Res. Ther. 27 (1), 179. doi:10.1186/s13075-019-1960-5
Houssen, M. E., Ragab, A., and Mesbah, A. (2010). Natural anti-inflammatory products and leukotriene inhibitors as complementary therapy for bronchial asthma. Clin. Biochem. 43 (10-11), 887–890. doi:10.1016/j.clinbiochem.2010.04.061
Hu, J. (2022). Study on biomedical function of curcumin extract from Turmeric. Mol. Plant Breed. 20 (02), 683–688. doi:10.13271/j.mpb.020.000683
Hu, L., Wang, H., and Zhang, J. (2018). Research progress in the protective effect of curcumin on injury of multiple organs. Med. Recapitulate. 24 (20), 4097–4102. doi:10.3969/j.issn.1006-2084.2018.20.026
Hu, R., and Shao, Q. (2007). Research advances on chemical constituents of curcuma wenyujin. Lishizhen Med. Mater. Med. Res. 07, 1773–1775. doi:10.3969/j.issn.1008-0805.2007.07.143
Hu, Y., Zhang, J., Kong, W., Zhao, G., and Yang, M. (2017). Mechanisms of antifungal and anti-aflatoxigenic properties of essential oil derived from turmeric (Curcuma longa L) on Aspergillus flavus. Food Chem. 220, 1–8. doi:10.1016/j.foodchem.2016.09.179
Huang, P., Wang, Q., and Zhou, G. (2020). GC-MS differences of volatile components in rhizome, roots and fibrous root of curcuma kwangsiensis. Lishizhen Med. Mater. Med. Res. 31 (10), 2366–2368. doi:10.3969/j.issn.1008-0805.2020.10.018
Huang, X., Lv, B., and Zhao, M. (2013). Effect of diterpenoid C extracted from radix curcumae on inflammation and NF-κB signaling pathway in helicobacter pylori-infected gastric epithelial cells. Chin. Pharm. Bull. 29 (04), 562–567. doi:10.3969/j.issn.1001-1978.2013.04.026
Jabbar, A., Rehman, K., Jabri, T., Kanwal, T., and Perveen, S. (2023). Improving curcumin bactericidal potential against multi-drug resistant bacteria via its loading in polydopamine coated zinc-based metal-organic frameworks. Drug Deliv. 30 (1), 2159587. doi:10.1080/10717544.2022.2159587
Jiang, H., Song, J., and Yan, L. (2015). Comparative study on pharmacological of different varieties of Curcumae Radix. N.a. J. Tradit. Chin. Med. Pharm. 30 (12), 4491–4494.
Jiang, J. L., Jin, X. L., Zhang, H., Su, X., Qiao, B., and Yuan, Y. J. (2012). Identification of antitumor constituents in curcuminoids from Curcuma longa L. based on the composition-activity relationship. J. Pharm. Biomed. Anal. 70, 664–670. doi:10.1016/j.jpba.2012.05.011
Jiang, Y., Yuan, J., and Shen, Z. (2009). Research progress on chemical constituents of Turmeric. Asia-Pac. Tradit. Med. 5 (2), 124∼125.
Jing, Z., Zou, H., and Xu, F. (2012). The molecular mechanisms of curcuma wenyujin extract-mediated inhibitory effects on human esophageal carcinoma cells in vitro. Chin. J. Integr. Tradit. West. Med. 32 (09), 1219–1222.
Jurgens, T. M., Frazier, E. G., Schaeffer, J. M., Jones, T. E., Zink, D. L., Borris, R. P., et al. (1994). Novel nematocidal agents from Curcuma comosa. J. Nat. Prod. 57 (2), 230–235. doi:10.1021/np50104a006
Kim, D. C., Ku, S. K., and Bae, J. S. (2012). Anticoagulant activities of curcumin and its derivative. BMB Rep. 45 (4), 221–226. doi:10.5483/bmbrep.2012.45.4.221
Kim, S., Kim, K., and Park, J. (2021). Curcuma longa L. Water extract improves Dexamethasone- induced sarcopenia by modulating the muscle-related gene and oxidative stress in mice. Antioxidants (Basel) 10 (7), 1000. doi:10.3390/antiox10071000
Kunnumakkara, A. B., Anand, P., and Aggarwal, B. B. (2008). Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 269 (2), 199–225. doi:10.1016/j.canlet.2008.03.009
Lee, Y. H., Song, N. Y., Suh, J., Kim, D. H., Kim, W., Ann, J., et al. (2018). Curcumin suppresses oncogenicity of human colon cancer cells by covalently modifying the cysteine 67 residue of SIRT1. Cancer Lett. 431, 219–229. doi:10.1016/j.canlet.2018.05.036
Lei, Hui. (2013). Studies on anti-hyperliperlipemia and anti-tumor activitives of turmeric oil. Nanjing: Nanjing University Traditional Chin. Med.
Lekshmi, P. C., Arimboor, R., Indulekha, P. S., and Menon, A. N. (2012). Turmeric (Curcuma longa L) volatile oil inhibits key enzymes linked to type 2 diabetes. Int. J. Food Sci. Nutr. 63 (7), 832–834. doi:10.3109/09637486.2011.607156
Li, H., Qi, X., and Mao, S. (2022b). Effects of curcumin on sevoflurane induced cognitive dysfunction in rats. Shenzhen J. Integr. Tradit. Chin. West. Med. 32 (03), 1–5+137. doi:10.16458/j.cnki.1007-0893.2022.03.001
Li, J., Guo, S., and Xiao, S. (2020). Protective effects of curdione on cognitive and neurological function in mice with cerebral ischemia-reperfusion injury. Chin. J. Comp. Med. 30 (02), 84–89. doi:10.3969/j.issn.1671-7856.2020.02.013
Li, J., Zhang, D., and Zhang, X. (2010). Research the traditional Chinese medicine properties by detecting the rats' monoamine neurotransmitter after given two different traditional Chinese medicine. Liaoning J. Tradit. Chin. Med. 37 (11), 2256–2258. doi:10.13192/j.ljtcm.2010.11.181.lijq.069
Li, L. (2018). Study on antiviral activity and mechanism of three main active ingredients from Zedoary Tumeric oil against H1N1. Suzhou: Suzhou University.
Li, W., Feng, J. T., Xiao, Y. S., Wang, Y. Q., Xue, X. Y., and Liang, X. M. (2009). Three novel terpenoids from the rhizomes of Curcuma longa. J. Asian Nat. Prod. Res. 11 (6), 569–575. doi:10.1080/10286020902939182
Li, Y., Chen, L., Hu, X., Liu, G., Hu, H., Ouyang, F., et al. (2023). Hydrogen sulfide ameliorates abdominal aorta coarctation-induced myocardial fibrosis by inhibiting pyroptosis through regulating eukaryotic translation initiation factor 2α phosphorylation and activating PI3K/AKT1 pathway. Shaanxi J. Tradit. Chin. Med. 44 (03), 345–356. doi:10.4196/kjpp.2023.27.4.345
Li, Y. L., Du, Z. Y., and Li, P. H. (2018). Aromatic-turmerone ameliorates imiquimod-induced psoriasis-like inflammation of BALB/c mice. Int. Immunopharmacol. 64, 319–325. doi:10.1016/j.intimp.2018.09.015
Li, Y., Liu, J., Wu, Y., and Guo, F. (2022a). Guaiane-type sesquiterpenes from Curcuma wenyujin. Phytochemistry 198, 113164. doi:10.1016/j.phytochem.2022.113164
Li, Y. N. (2020). Study on the machanism of curcumin in preventing cholesterol stone formation in gallbladder. Med. University.
Li, Z., Cao, R., and Hao, E. (2021). Research progress on chemical constituents and pharmacological effects of Curcuma kwangsiensis and prediction of its quality marker (Q-Marker). Chin. Tradit. Herb. Drugs. 52 (15), 4687–4699. doi:10.7501/j.issn.0253-2670.2021.15.028
Li, Z. Y., Ding, L. L., and Li, J. M. (2015). ¹H-NMR and MS based metabolomics study of the intervention effect of curcumin on hyperlipidemia mice induced by high-fat diet. PLoS One 10 (3), e0120950. doi:10.1371/journal.pone.0120950
Liju, V. B., Jeena, K., and Kuttan, R. (2011). An evaluation of antioxidant, anti-inflammatory, and antinociceptive activities of essential oil from Curcuma longa L. Indian J. Pharmacol. 43 (5), 526–531. doi:10.4103/0253-7613.84961
Lin, J. K., and Lin-Shiau, S. Y. (2001). Mechanisms of cancer chemoprevention by curcumin. Proc. Natl. Sci. Counc. Repub. China B 25 (2), 59–66.
Liu, F., Hua, H., and Liu, L. (2022). Study on HPLC fingerprint of curcumin from Sichuan Province and determination of curcumin components. West n.a. J. Pharm. Sci. 37 (05), 541–544. doi:10.13375/j.cnki.wcjps.2022.05.015
Liu, H. (2006). Ultrasonic assisted extraction of volatile oil from Curcumae Radix and analysis of its chemical components. Lishizhen Med. Mater. Med. Res. 10, 1876–1877. doi:10.3969/j.issn.1008-0805.2006.10.006
Liu, M., Guo, X., and Sun, Q. (2021). Research progress on chemical constituents and pharmacological activity of Curcuma wenyujin. Drugs & Clin. 36 (01), 204–208. doi:10.7501/j.issn.1674-5515.2021.01.041
Liu, Q., Yin, W., and Han, L. (2018). Diarylheptanoid from rhizomes of Curcuma kwangsiensis (DCK) inhibited imiquimod-induced dendritic cells activation and Th1/Th17 differentiation. Int. Immunopharmacol. 56, 339–348. doi:10.1016/j.intimp.2018.01.044
Liu, R., Pei, Q., Shou, T., Zhang, W., Hu, J., and Li, W. (2019). Apoptotic effect of green synthesized gold nanoparticles from Curcuma wenyujin extract against human renal cell carcinoma A498 cells. Int. J. Nanomedicine 14, 4091–4103. doi:10.2147/IJN.S203222
Liu, X., Niu, H., and Gao, J. (2016). Effect of Zedoray Turmeric Oil on serum lipid and inflammatory factors in rats with atherosclerosis. Mod. J. Integr. Tradit. Chin. West. Med. 25 (20), 2183–2185. doi:10.3969/j.issn.1008-8849.2016.20.006
Liu, X., Zhou, Y., and Zhang, S. (2023a). Inhibitory effect of curcumin on PEDV infected VERO cells. Chin. Veterinary Sci. 1-9. doi:10.16656/j.issn.1673-4696.2023.0091
Liu, Y., Chen, X., Liang, F., Li, Y., Xiong, C., Qin, E. Q., et al. (2023b). Progress of researches on anti-inflammatory mechanism of acupuncture underlying amelioration of chronic respiratory diseases. Acupunct. Res. 48 (02), 147–152. doi:10.13702/j.1000-0607.20220760
Liu, Z., and Cheng, J. (2012). Protective effect of Curcuma zedoary on kidney in rats with unilateral ureteral obstruction. Zhejiang J. Tradit. Chin. Med. 47 (02), 138–139. doi:10.3969/j.issn.0411-8421.2012.02.044
Louisa, M., Wanafri, E., Arozal, W., Sandhiutami, N. M. D., and Basalamah, A. M. (2023). Nanocurcumin preserves kidney function and haematology parameters in DMBA-induced ovarian cancer treated with cisplatin via its antioxidative and anti-inflammatory effect in rats. Pharm. Biol. 61 (1), 298–305. doi:10.1080/13880209.2023.2166965
Lu, C. (2018). Composition Analysis and properties of turmeric volatile oil. Shijiazhuang: Hebei University Sci. Technol.
Ma, R., Wang, F., and Li, J. (2022). Mechanism of curcumin improving cisplatin resistance of human ovarian cancer cells based on the targeted regulation of flap-like endonuclidene 1 by microRNA-135b-5p. N.a. Med. 17 (12), 1827–1832. doi:10.3760/j.issn.1673-4777.2022.12.015
Manarin, G., Anderson, D., and Silva, J. M. E. (2019). Curcuma longa L. ameliorates asthma control in children and adolescents: a randomized, double-blind, controlled trial. J. Ethnopharmacol. 238, 111882. doi:10.1016/j.jep.2019.111882
Manzan, A. C., Toniolo, F. S., Bredow, E., and Povh, N. P. (2003). Extraction of essential oil and pigments from Curcuma longa [L] by steam distillation and extraction with volatile solvents. J. Agric. Food Chem. 51 (23), 6802–6807. doi:10.1021/jf030161x
Mei, J., Shi, W., and Guo, T. (2022). Effect of chitosan/curcumin nanocomposite on cariogenic ability of Streptococcus mutans. Stomatology 38 (11), 1058–1063. doi:10.13701/j.cnki.kqyxyj.2022.11.012
Ming, S., Kang, B., and Cheng, J. (2022). Effect of curcumin on rat model with severe HFMD. N.a. J. Chin. Med. 37 (09), 1928–1933. doi:10.16368/j.issn.1674-8999.2022.09.346
Mohanty, S., Ray, A., Naik, P. K., and Sahoo, A. (2023). Variation in yield, chemical composition and biological activities of essential oil of three curcuma species: a comparative evaluation of hydrodistillation and solvent-free microwave extraction methods. Molecules 28 (11), 4434. doi:10.3390/molecules28114434
Mukhopadhyay, A., Basu, N., Ghatak, N., and Gujral, P. K. (1982). Anti-inflammatory and irritant activities of curcumin analogues in rats. Agents Actions 12 (4), 508–515. doi:10.1007/BF01965935
Neta, J. F. F., Veras, V. S., Sousa, D. F., Cunha, M. d. C. D. S. O., Queiroz, M. V. O., Neto, J. C. G. L., et al. (2021). Effectiveness of the piperine-supplemented curcuma longa L. In metabolic control of patients with type 2 diabetes: a randomised double-blind placebo-controlled clinical trial. Int. J. Food Sci. Nutr. 72 (7), 968–977. doi:10.1080/09637486.2021.1885015
Paw, M., Gogoi, R., Sarma, N., Pandey, S. K., Borah, A., Begum, T., et al. (2020). Study of anti-oxidant, anti-inflammatory, genotoxicity, and antimicrobial activities and analysis of different constituents found in rhizome essential oil of curcuma caesia roxb., collected from north east India. Curr. Pharm. Biotechnol. 21 (5), 403–413. doi:10.2174/1389201020666191118121609
Qi, Y., Qin, W., and Kang, K. (2017). Effects of wenyujin essential oil on tau protein phosphorylation in mice with aβ-induced alzheimer disease through PI3k/akt pathway. Chin. J. Inf. Tradit. Chin. Med. 24 (01), 45–48. doi:10.3969/j.issn.1005-5304.2017.01.012
Qiao, M., Xiong, L., and Liu, Y. (2020). Study of material basis on the difference of blood circulation activating efficacy between curcuma longa and curcuma phaecocaulis. World Sci. technol.-mod. Tradit. Chin. Med. 22 (07), 2531–2539. doi:10.11842/wst.20190525003
Qin, Y., Fei, C., Zhang, W., Li, Y., Xu, Z., Su, L. L., et al. (2022b). Research progress on related substances of Curcuma herbs for promoting blood circulation and removing blood stasis. N.a. J. Chin. Mater. Med. 47 (01), 24–35. doi:10.19540/j.cnki.cjcmm.20210817.603
Qin, Y., Fei, C., Zhang, W., and Su, L. (2022a). Based on UPLC/MS/MS and bioinformatics analysis to explore the difference substances and mechanism of curcumae radix (curcuma wenyujin) in dysmenorrhea. Chem. Biodivers. 19 (10), e202200361. doi:10.1002/cbdv.202200361
Qiu, G., Cai, Y., and Fang, L. (2014). Anti-lfammatory and analgesic effects of ethyl acetate extract from Curcuma wenyujin. J. Wenzhou Med. Univ. 44 (09), 660–663. doi:10.13771/j.cnki.33-1386/r.2014.09.010
Rathore, P., Dohare, P., Varma, S., Ray, A., Sharma, U., Jagannathan, N. R., et al. (2008). Curcuma oil: reduces early accumulation of oxidative product and is anti-apoptogenic in transient focal ischemia in rat brain. Neurochem. Res. 33 (9), 1672–1682. doi:10.1007/s11064-007-9515-6
Ren, M., and Li, J. (2013). The contents of curcumenedione, curcumenol, Germarone, curcumenene, furadiene and β-elemene in Curcuma were determined by RP-HPLC. Chin. J. Exp. Tradit. Med. Formulae 19 (21), 155–158. doi:10.11653/syfj2013210155
Ruan, Q., and Zhong, G. (2018). Effect analysis of curcuma zedoary oil combined with conventional chemotherapy on ovarian cancer. J. Med. Theory Pract. 31 (04), 554–555. doi:10.19381/j.issn.1001-7585.2018.04.045
Shah, B. H., Nawaz, Z., and Pertani, S. A. (1999). Inhibitory effect of curcumin, a food spice from turmeric, on platelet-activating factor- and arachidonic acid-mediated platelet aggregation through inhibition of thromboxane formation and Ca2+ signaling. Biochem. Pharmacol. 58 (7), 1167–1172. doi:10.1016/s0006-2952(99)00206-3
Si, X., Wang, Y., Wong, J., Zhang, J., McManus, B. M., and Luo, H. (2007). Dysregulation of the ubiquitin-proteasome system by curcumin suppresses coxsackievirus B3 replication. J. Virol. 81 (7), 3142–3150. doi:10.1128/JVI.02028-06
Singh, V., Jain, M., and Misra, A. (2013). Curcuma oil ameliorates hyperlipidaemia and associated deleterious effects in golden Syrian hamsters. Br. J. Nutr. 110 (3), 437–446. doi:10.1017/S0007114512005363
Su, R., Liu, M., and Wang, Y. (2022). Effects of cureumin on phosphatidylinositol-binding clathrin assembly mediated neuronal endocytosis in Tg2576 transgenic mice. N.a. J. Tradit. Chin. Med. Pharm. 37 (03), 1719–1722.
Su, Y., Cheng, J., and Liu, Z. (2019). Study on antithrombotic effect and mechanism of water extract of Guiyujin. J. Shaanxi Coll. Tradit. Chin. Med. 42 (06), 80–84. doi:10.13424/j.cnki.jsctcm.2019.06.022
Sugimoto, K., Hanai, H., and Tozawa, K. (2002). Curcumin prevents and ameliorates trinitrobenzene sulfonic acid-induced colitis in mice. Gastroenterology 123 (6), 1912–1922. doi:10.1053/gast.2002.37050
Sun, W., Wang, S., Zhao, W., Wu, C., Guo, S., and Gao, H. (2017). Chemical constituents and biological research on plants in the genus Curcuma. Crit. Rev. Food Sci. Nutr. 57 (7), 1451–1523. doi:10.1080/10408398.2016.1176554
Sun, Z. (2014). Expression of lung tissue nuclear factor NF-E2 related factor 2 and the intervention effect of curcumin under one-lung ventilation inducing lung injury in rabbits. Shijiazhuang: hebei med. university.
Tang, G. M., Shi, Y. T., Gao, W., Li, M. N., Li, P., and Yang, H. (2022). Comparative analysis of volatile constituents in root tuber and rhizome of curcuma longa L. Using fingerprints and chemometrics approaches on gas chromatography-mass spectrometry. Molecules 27 (10), 3196. doi:10.3390/molecules27103196
Tong, H. (2021). Study on the material basis and mechanism of promoting circulation,removing stasis and analgesic effects by different decoction pieces of Curcuma Wenyujin Y.H.Chen et C.Ling. Nanjing: Nanjing University Traditional Chin. Med.
Tong, H., Yu, M., and Lu, T. (2020). Classification and clinical application analysis of formulas containing curcumae rhizoma. Chin. Tradit. Pat. Med. 42 (03), 731–735. doi:10.27253/d.cnki.gnjzu.2021.000068
Usharani, P., Mateen, A. A., and Naidu, M. U. (2008). Effect of NCB-02, atorvastatin and placebo on endothelial function, oxidative stress and inflammatory markers in patients with type 2 diabetes mellitus: a randomized, parallel-group, placebo-controlled, 8-week study. Drugs R. D. 9 (4), 243–250. doi:10.2165/00126839-200809040-00004
W Figueira, L., de Oliveira, J. R., Netto, A. A., S Zamarioli, L. D., Marcucci, M. C., Camargo, S. E., et al. (2020). Curcuma longa L. helps macrophages to control opportunistic micro-organisms during host-microbe interactions. Future Microbiol. 15, 1237–1248. doi:10.2217/fmb-2019-0297
Wang, B., Cao, J., and Li, B. (1996). Effect of Curcuma wenyujin extract on lipid peroxidation induced by radiation. J. Harbin Med. Univ. 02, 128.
Wang, C., and Xu, Z. (2018). Analysis of curcumae Longae rhizoma, curcumae radix and curcumae rhizoma. Nei Mong. J. Tradit. Chin. 37 (09), 95–96. doi:10.16040/j.cnki.cn15-1101.2018.09.063
Wang, Y. (2014). Comparative study on the chemical constituents of traditional Chinese medicine Curcumae Longae rhizoma, Curcumae rhizoma, and Curcumae Radix. Chongqing: chongqing university.
Wang, Y., He, T., and Wang, J. (2021). High performance liquid chromatography fingerprint and headspace gas chromatography-mass spectrometry combined with chemometrics for the species authentication of Curcumae Rhizoma. J. Pharm. Biomed. Anal. 202, 114144. doi:10.1016/j.jpba.2021.114144
Wang, Y., Kang, K., and Qi, Y. (2016). Radix curcumea extract on Alzheimer's disease mice induced by β-amyloid(Aβ25-35). Chin. Arch. Tradit. Chin. Med. 34 (12), 2905–2909. doi:10.13193/j.issn.1673-7717.2016.12.024
Wang, Y., Wang, J., Ma, H., Zhao, L., and He, H. (2022b). Clinical and genetic characteristics for 4 patients with Type Ib pseudohypoparathyroidism. Chin. J. Clin. Pharm. 38 (13), 1461–1466. doi:10.11817/j.issn.1672-7347.2022.220029
Wang, Z., Liu, X., and Li, J. (2022a). A preliminary study on anti-inflammatory effect and mechanism of curcumin on young mice infected with Mycoplasma pneumoniae pneumonia. J. Clin. Pulm. Med. 27 (01), 12–16. doi:10.3969/j.issn.1009-6663.2022.01.003
Wei, Y., Hao, E., and Du, Z. (2020). Analysis of Curcuma longa Q-markers based on traditional efficacy and modern research. Chin. Tradit. Herb. Drugs. 51 (14), 3830–3839. doi:10.7501/j.issn.0253-2670.2020.14.030
Wei, Z., Pinfang, K., Jing, Z., Zhuoya, Y., Shaohuan, Q., and Chao, S. (2023). Curcumin improves diabetic cardiomyopathy by inhibiting pyroptosis through AKT/Nrf2/ARE pathway. Mediat. Inflamm. 2023, 3906043. doi:10.1155/2023/3906043
Wilken, R., Veena, M. S., Wang, M. B., and Srivatsan, E. S. (2011). Curcumin: a review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol. Cancer 10, 12. doi:10.1186/1476-4598-10-12
Wu, G., Wang, L., and Lin, L. (2013). The therapeutic effect and mechanisms of Ezhu on ischemic stroke in rats. Pharm. Clin. Chin. Mater. Med. 4 (06), 34–36.
Wu, H., Li, H., and Tang, S. (2011). Advances in basic research of pharmacodynamic substances of turmeric. Chin. J. Inf. Tradit. Chin. Med. 18 (02), 104–106. doi:10.3969/j.issn.1005-5304.2011.02.054
Xiao, W., Zeng, J., and Chen, X. (2015). Hypoglycemic effect of curcuma kwangsiensis polysaccharides on type 2 diabetes mellitus rats. Chin. J. Exp. Tradit. Med. Formulae. 21 (21), 144–147. doi:10.7666/d.D01051729
Xie, H., Su, D., and Zhang, J. (2020). Raw and vinegar processed Curcuma wenyujin regulates hepatic fibrosis via bloking TGF-β/Smad signaling pathways and up-regulation of MMP-2/TIMP-1 ratio. J. Ethnopharmacol. 246, 111768. doi:10.1016/j.jep.2019.01.045
Xing, B., Shao, Q., and Hu, R. (2009). Progress in curcumin derivatives form curcuma wenyujin. J. Anhui Agric. Sci. 37 (16), 7516∼ 7518. doi:10.1016/j.ijbiomac.2008.11.005
Xu, C., Haiyan, Z., and Hua, Z. (2009). Effect of Curcuma kwangsiensis polysaccharides on blood lipid profiles and oxidative stress in high-fat rats. Int. J. Biol. Macromol. 44 (2), 138–142. doi:10.1016/j.ijbiomac.2008.11.005
Xu, P., Ye, Y., and Qiao, P. (2021). Effects of curcumin on cognitive function and hippocampal cell apoptosis in rats with cerebral hemorrhage. J. Apoplexy Nerv. Dis. 38 (12), 1076–1080. doi:10.19845/j.cnki.zfysjjbzz.2021.0287
Xu, Z., Zhu, S., and Pan, N. (2018). Effect of guizhou zedoary oil on Toll like receptor and related immune factors in SW1463 cell line. Chin. J. Exp. Tradit. Med. Formulae. 24 (05), 137–141. doi:10.13422/j.cnki.syfjx.2018050137
Yallapu, M. M., Khan, S., Maher, D. M., Ebeling, M. C., Sundram, V., Chauhan, N., et al. (2014). Anti-cancer activity of curcumin loaded nanoparticles in prostate cancer. Biomaterials 35 (30), 8635–8648. doi:10.1016/j.biomaterials.2014.06.040
Yin, G., Zhang, Q., and An, Y. (2012). Advance in chemical constituents and pharmacological activity of Curcuma wenyujin. N.a. J. Chin. Mater. Med. 37 (22), 3354–3360.
Yu, B., Ye, M., and Pan, F. (2022). Effect of curcumin on GSK3β protein expression in Hippocampus of Alzheimer's disease model rats. J. Med. Res. 51 (02), 42–46. doi:10.13192/j.issn.1000-1719.2016.07.055
Yu, C., Tang, S., and Li, X. (2016). Effects of Zedoary Turmeric on expression of nerve adhesion molecules and sialic acid transferase in normal and blood-stasis syndrome rat offspring brain tissue. Liaoning J.Traditional Chin. Med. 43 (07), 1510–1512. doi:10.13192/j.issn.1000-1719.2016.07.055
Yu, D., Liu, H., and Qin, J. (2021). Curcumol inhibits the viability and invasion of colorectal cancer cells via miR-30a-5p and Hippo signaling pathway. Oncol. Lett. 21 (4), 299. doi:10.3892/ol.2021.12560
Yu, M. (2021). Study on the quality and analysis techniques of different Curcumae rhizoma pieces. Jiangxi, China: Jiangxi University Traditional Chin. Med.
Yuan, A., Zhao, M., and Li, Y. (2015). The review of pharmacological effects of Lianqiao. Pharm. Clin. Chin. Mater. Med. 6 (05), 56–59.
Zang, Y., Xu, L., and Liu, H. (2021). Determination of 8 main active components in Zedoary by HPLC wavelength switching method. Chin. J. Mod. Appl. Pharm. 38 (18), 2227–2233. doi:10.13748/j.cnki.issn1007-7693.2021.18.006
Zeiner, M., Šoltić, M., Nemet, I., and Juranović Cindrić, I. (2022). Multielement determinationin turmeric (curcuma longa L) using different digestion methods. Molecules 27 (23), 8392. doi:10.3390/molecules27238392
Zeng, Y. C., Qiu, F., Takahashi, K., Liang, J., Qu, G., and Yao, X. (2007). New Sesquiterpenes and calebin derivatives from Curcuma longa. Chem. Pham Bull. 55 (6), 940–943. doi:10.1248/cpb.55.940
Zhang, T., Wu, Y., and Dian, Z. (2020b). Screening of antimicrobial activity of 19 Chinese herbal medicines ethanol extracts in vitro. Guihaia 40 (12), 1712–1720. doi:10.11931/guihaia.gxzw201903004
Zhang, W., Zha, K., and Xiong, Y. (2023a). Glucose-responsive, antioxidative HA-PBA-FA/EN106 hydrogel enhanced diabetic wound healing through modulation of FEM1b-FNIP1 axis and promoting angiogenesis. Bioact. Mater 30, 29–45. doi:10.1016/j.bioactmat.2023.07.006
Zhang, X., Liu, Y., and Cao, X. (2015). Antioxidant activity of 22 Chinese herbal medicines for anti-cancer. Acta Chin. Med. Pharm. 43 (05), 31–35. doi:10.19664/j.cnki.1002-2392.2015.05.009
Zhang, X., Zhang, C., Ren, Z., Zhang, F., and Xu, J. (2020a). Curcumin affects gastric cancer cell migration, invasion and cytoskeletal remodeling through gli1-β-catenin. Cancer Manag. Res. 12, 3795–3806. doi:10.2147/CMAR.S244384
Zhang, Y., Liu, C., Wu, C., and Song, L. (2023b). Natural peptides for immunological regulation in cancer therapy: mechanism, facts and perspectives. Biomed. Pharmacother. 159, 114257. doi:10.1016/j.biopha.2023.114257
Zhang, Y., Cai, N., and Shen, K. (1983). Termination of pregnancy of animals with Curcuma wenyujin. Chin. Tradit. Pat. Med. 06, 29–30.
Zhao, R., Liang, W., and Deng, W. (2022). Study on the mechanism of curcumin in protecting osteoblast function through anti-oxidative stress. Nat. Prod. Res. Dev. 34 (08), 1311–1318. doi:10.16333/j.1001-6880.2022.8.005
Zhao, Z., Zhang, P., and Wu, Y. (2011). Screening of antidepressant fractions of Wenyujin. N.a. J. Tradit. Chin. Med. Pharm. 26 (08), 868–869.
Zhen, Y., Wang, J., and Zhao, T. (2022). Effects of curcumenol on M1/M2 phenotype differentiation and secretion of inflammatory cytokines in RAW264.7 macrophages. Chin. Arch. Tradit. Chin. Med. 40 (11), 77–80+278. doi:10.13193/j.issn.1673-7717.2022.11.019
Zhou, P. (2015). Extraction and separation of curcuminoids from Curcuma longa L. Tianjing: Tianjing University.
Zhu, J., Lower-Nedza, A. D., Hong, M., Jie, S., Wang, Z., Yingmao, D., et al. (2013). Chemical composition and antimicrobial activity of three essential oils from Curcuma wenyujin. Nat. Prod. Commun. 8 (4), 1934578X1300800–6. doi:10.1177/1934578x1300800430
Keywords: Curcumae Longae Rhizoma, Curcumae Radix, Curcumae Rhizoma, pharmacology, curcumin, targets
Citation: Zhu X, Quan Y-y, Yin Z-j, Li M, Wang T, Zheng L-y, Feng S-q, Zhao J-n and Li L (2023) Sources, morphology, phytochemistry, pharmacology of Curcumae Longae Rhizoma, Curcumae Radix, and Curcumae Rhizoma: a review of the literature. Front. Pharmacol. 14:1229963. doi: 10.3389/fphar.2023.1229963
Received: 27 May 2023; Accepted: 15 August 2023;
Published: 31 August 2023.
Edited by:
Matthias F. Melzig, Freie Universität Berlin, GermanyReviewed by:
Serhat Sezai Cicek, University of Kiel, GermanyCopyright © 2023 Zhu, Quan, Yin, Li, Wang, Zheng, Feng, Zhao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Li, Njk1ODU1NTY0QHFxLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.