- Department of Rheumatology, The First Affiliated Hospital of Soochow University, Suzhou, Jiangsu, China
Background: Biologics and small-molecule drugs have become increasingly accepted worldwide in the treatment of axial spondyloarthritis (axSpA), including ankylosing spondylitis (AS) and non-radiographic axial spondyloarthritis (nr-axSpA). However, a quantitative multiple comparison of their efficacy and safety is lacking. This study aims to provide an integrated assessment of the relative benefits and safety profiles of these drugs in axSpA treatment.
Methods: We included randomized clinical trials that compared biologics and small-molecule drugs in the treatment of axSpA patients. The primary outcomes assessed were efficacy, including the Assessment of SpondyloArthritis International Society (ASAS) improvement of 20% (ASAS20) and 40% (ASAS40). Safety outcomes included treatment-emergent adverse events (TEAEs) and serious adverse events (SAEs). We used the surface under the cumulative ranking (SUCRA) curve value and ranking plot to evaluate and rank clinical outcomes and safety profiles of different treatments. The two-dimensional graphs were illustrated to visually assess both the efficacy (horizontal axis) and safety (vertical axis) of each intervention.
Results: Our analysis included 57 randomized clinical trials involving a total of 11,787 axSpA patients. We found that seven drugs (TNFRFc, TNFmAb, IL17Ai, IL17A/Fi, IL17RAi, JAK1/3i, and JAK1i) were significantly more effective in achieving ASAS20 response compared to the placebo (PLA). Except for IL17RAi, these drugs were also associated with higher ASAS40 responses. TNFmAb demonstrated the highest clinical response efficacy among all the drugs. Subgroup analyses for AS and nr-axSpA patients yielded similar results. IL17A/Fi emerged as a promising choice, effectively balancing efficacy and safety, as indicated by its position in the upper right corner of the two-dimensional graphs.
Conclusion: Our findings highlight TNFmAb as the most effective biologic across all evaluated efficacy outcomes in this network meta-analysis. Meanwhile, IL17A/Fi stands out for its lower risk and superior performance in achieving a balance between efficacy and safety in the treatment of axSpA patients.
1 Introduction
Axial spondyloarthritis (axSpA), characterized by inflammatory back pain and stiffness, is one of the most prevalent rheumatic conditions (Danve and Deodhar, 2022). AxSpA includes radiographic axSpA, commonly known as ankylosing spondylitis (AS), and non-radiographic axSpA (nr-axSpA) (Sieper and Poddubnyy, 2017). Current guidelines recommend non-pharmacological therapies as the primary approach to managing axSpA, alongside pharmacological treatments such as non-steroidal anti-inflammatory drugs (NSAIDs) or conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs) (Ramiro et al., 2022). Although these interventions may offer palliation of signs and symptoms, they have shown limited efficacy in reducing radiographic damage and modifying disease progression (Danve and Deodhar, 2022).
The development of targeted biologic therapies, including biologics, such as TNF-α inhibitors and IL-17 inhibitors, and small-molecule drugs, primarily JAK inhibitors, has revolutionized the clinical management of axSpA (Sunzini et al., 2022; Webers et al., 2022; Caso et al., 2023). Recent clinical trials and pairwise meta-analyses have demonstrated that these drugs offer significant clinical benefits to patients by promptly suppressing inflammation and targeting molecules that stimulate bone formation (Sieper and Poddubnyy, 2017; Yin et al., 2020; Lawson et al., 2021; Li et al., 2022). However, it is worth noting that, to date, there has been a notable lack of comprehensive head-to-head comparisons between these drugs (Giardina et al., 2010; van der Heijde et al., 2018b). This limitation leaves clinicians with a multitude of options to consider when prescribing pharmacotherapy (Cantini et al., 2017).
To bridge this gap, network meta-analysis is often employed to support evidence-based decision-making (Li et al., 2011). Network meta-analysis extends the principles of pairwise meta-analysis to evaluate multiple treatments by combining both direct and indirect comparisons across trials that share a common comparator, such as placebo (PLA) (Li et al., 2011). Several network meta-analyses have already been conducted to assess the performance of biologics and small-molecule drugs in axSpA (Betts et al., 2016; Deodhar et al., 2020a; Cao et al., 2022; Lee, 2022). However, more recent clinical trials have introduced additional drugs, including brodalumab (an IL-17 receptor A antibody, IL17RAi) (Wei et al., 2021a), upadacitinib (a JAK1-specific inhibitor, JAK1i) (Deodhar et al., 2022), and apremilast (a phosphodiesterase 4 inhibitor, PDE4i) (Taylor et al., 2021). Moreover, there exists a dearth of comparative efficacy studies for these drugs in the management of nr-axSpA.
Our study aimed to comprehensively evaluate the efficacy and safety of biologics and small-molecule drugs in axSpA patients, including both AS and nr-axSpA, by analyzing data from randomized clinical trials with placebo or active controls.
2 Methods
2.1 Registration and ethics
This study was designed and performed based on the methods and recommendations from the Preferred Reporting Items for Systematic Reviews and Meta-analyses for Network Meta-analysis (PRISMA-NMA) reporting guidelines (Hutton et al., 2015). The study protocol has been drafted a priori and registered in PROSPERO (CRD42022378343). We declare that all included data are available within the article and Supplementary Material.
2.2 Search strategy
The eligible studies were identified through systematic searches of MEDLINE via PubMed, Embase, and the Cochrane Central Register of Controlled Trials (CENTRAL). Our search strategy was based on Medical Subject Headings (MeSH) or Emtree terms and followed the PICOS format: Population (P): patients with AxSpA, including nr-axSpA and AS. Intervention (I): biologics, including TNF-α receptor Fc fusion protein (TNFRFc), TNF-α monoclonal antibodies (TNFmAb), IL17A inhibitor (IL17Ai), IL17A/F dual inhibitor (IL17A/Fi), IL17RAi, JAK inhibitors, including JAK1/3i and JAK1i, IL-6 inhibitor (IL6i), IL-12 and/or IL-23 inhibitor (IL12/23i), and PDE4i, across all treatment durations. Comparison (C): the aforementioned biologics, PLA, and/or sulfasalazine (SSZ). Outcomes (O): clinical response rate and safety. Study design (S): randomized placebo- or active-controlled clinical trials.
We conducted searches from the inception of each database until 20 October 2022 and considered studies published in English. The complete search strategy is provided in Supplementary Table S1. Additionally, we scanned the citations in the included articles to identify studies meeting our inclusion criteria.
2.3 Eligibility criteria
We included randomized clinical trials published in peer-reviewed scientific journals. Eligible patients in each study had a documented diagnosis of axial spondyloarthritis (axSpA), which includes two subtypes: AS and nr-axSpA. AS patients met both the Assessment of SpondyloArthritis International Society (ASAS) classification criteria for axSpA (Rudwaleit et al., 2011) and the imaging criterion (sacroiliitis) of the modified New York classification criteria for AS (van der Linden et al., 1984). Nr-axSpA patients met the ASAS classification criteria but did not meet the imaging criterion in the modified New York criteria. Studies recruiting patients with other subforms of axSpA, such as psoriatic arthritis (PsA), reactive arthritis (ReA), and inflammatory bowel disease-associated spondyloarthritis (IBD-SpA), were excluded.
2.4 Study selection and data extraction
The retrieved studies were imported into EndNote software (version 20.0). After duplicates were removed, two investigators (Y Yin and E Zhou) independently screened the titles and abstracts to determine the potential of eligibility for inclusion based on the predefined inclusion and exclusion criteria. The full text of the identified studies will be examined. Areas of disagreement or uncertainty were settled by consensus among the investigators. The detailed variables from the eligible studies were extracted. The efficacy outcome measures were ASAS response criteria, including ASAS20 and ASAS40, the improvement of 50% Bath Ankylosing Spondylitis Disease Activity Index (BASDAI50), and Ankylosing Spondylitis Disease Activity Score Inactive Disease (ASDAS-ID). For safety outcomes, treatment-emergent adverse events (TEAEs) were defined as any unfavorable medical occurrence during treatment, regardless of causality. Serious adverse events (SAEs) were defined as TEAEs that resulted in death, hospital admission or prolongation of existing hospital stay, persistent or significant disability, or life-threatening events.
2.5 Quality evaluation
We assessed the risk of bias for each included study using the revised Cochrane Risk-of-Bias 2 (Rob2.0) tool (Sterne et al., 2019). The evaluation covered several aspects, including the randomization process, deviations from the intended intervention, missing outcome data, measurement of the outcome, and selection of the reported result. The certainty of the evidence was categorized into three levels: low risk of bias, some concerns, and high risk of bias. Two reviewers independently conducted the bias assessment, and any disagreements were resolved through consensus.
2.6 Statistical analysis
We conducted a network meta-analysis using Stata/SE (version 17.0) and R (version 4.2.2), employing a random-effects model. The analysis was based on frequency theory and a multivariate framework. To visualize the comparisons between different interventions, we created evidence network diagrams for various outcome indicators. Consistency testing was performed using both global (Wald test) and local (node-splitting method) approaches within the network (Hoaglin et al., 2011; van Valkenhoef et al., 2016). The global test assessed inconsistency between comparisons, while the local test assessed inconsistency between direct and indirect evidence within each comparison. We calculated summary odds ratios (ORs) with corresponding 95% confidence intervals (95% CIs) for all outcome indicators and presented these estimates in league charts. To assess the potential effectiveness of future trials, we calculated 95% predictive intervals (95% PrIs) of ORs and displayed them on forest plots alongside meta-analysis estimates. To identify interventions with the highest probability of effectiveness, we used the surface under cumulative ranking (SUCRA) curve. SUCRA values, expressed as percentages ranging from 0% to 100%, indicate the probability of achieving the endpoint. We also used a two-dimensional graph to visually assess both efficacy and safety for each intervention. Finally, we employed funnel plots to detect the presence of a small sample effect and assess publication bias in the analysis. Statistical significance was set at p < 0.05.
3 Results
3.1 Search strategy and quality assessment
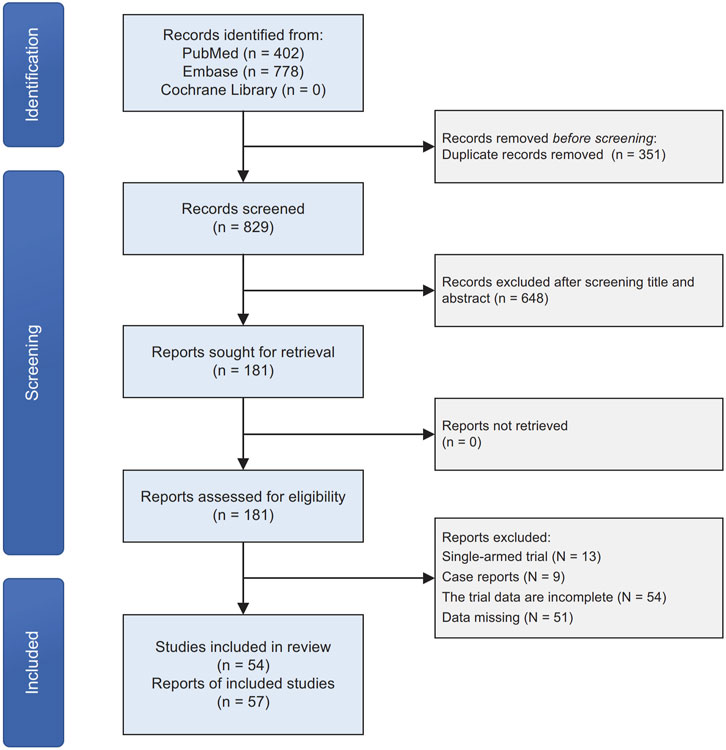
We initially identified 1,180 original records through our search strategies in electronic databases. After removing 351 duplicates and screening titles and abstracts, 448 articles were excluded. Following a detailed examination of the full text of the remaining 181 publications, 127 studies were excluded. These exclusions were primarily due to the study type being single-armed trials, case reports, or incomplete data. Ultimately, we included 54 articles, encompassing 57 clinical trials, in our quantitative network meta-analysis (Figure 1). The majority of the included studies exhibited a low-to-moderate risk of bias (Supplementary Table S2).
3.2 Basic characteristics
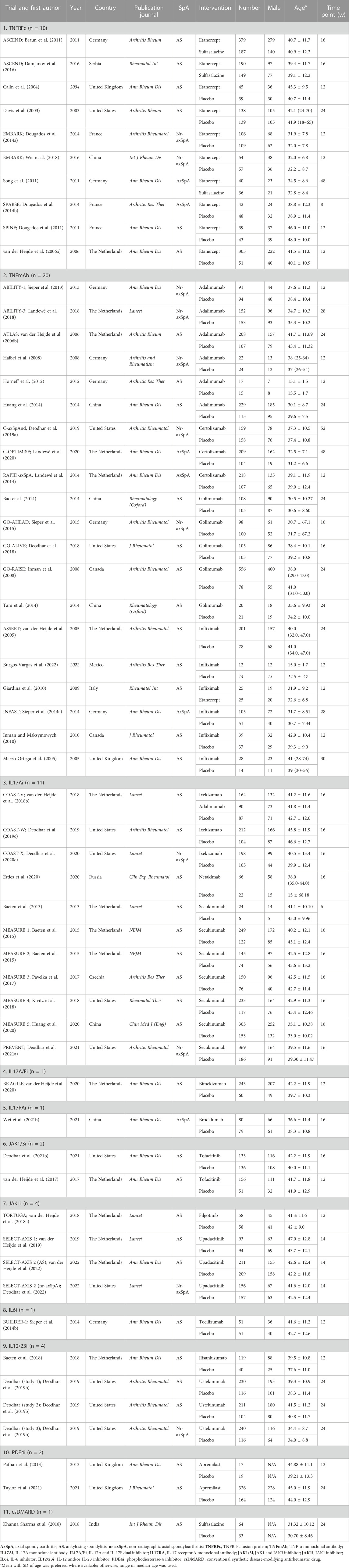
The basic characteristics of the included studies are summarized in Table 1. The data represent 57 clinical trials published between 2013 and 2022. A total of 11,787 patients ( 9,057 with AS and 2,730 with nr-axSpA) were recruited and followed for 6–52 weeks. Similar large variations were observed among intervention and control groups for male individuals (ranging from 18.3% to 94.9%) and age (ranging from 31.2 ± 6.6 years to 48.0 ± 10.0 years).
All articles involved biologics, including TNFRFc [10 studies involving etanercept (Davis et al., 2003; Calin et al., 2004; van der Heijde et al., 2006a; Braun et al., 2011; Dougados et al., 2011; Song et al., 2011; Dougados et al., 2014a; Dougados et al., 2014b; Damjanov et al., 2016; Wei et al., 2018)], TNFmAb [six studies involving adalimumab (van der Heijde et al., 2006b; Haibel et al., 2008; Horneff et al., 2012; Sieper et al., 2013; Huang et al., 2014; Landewé et al., 2018), three studies involving certolizumab (Landewé et al., 2014; Deodhar et al., 2019a; Landewé et al., 2020), five studies involving golimumab (Inman et al., 2008; Bao et al., 2014; Tam et al., 2014; Sieper et al., 2015; Deodhar et al., 2018), and six studies involving infliximab (Marzo-Ortega et al., 2005; van der Heijde et al., 2005; Giardina et al., 2010; Inman and Maksymowych, 2010; Sieper et al., 2014a; Burgos-Vargas et al., 2022)], IL17Ai [three studies involving ixekizumab (van der Heijde et al., 2018b; Deodhar et al., 2019c; Deodhar et al., 2020c), one study involving netakimab (Erdes et al., 2020), and seven studies involving secukinumab (Baeten et al., 2013; Baeten et al., 2015; Pavelka et al., 2017; Kivitz et al., 2018; Huang et al., 2020; Deodhar et al., 2021a)], IL17A/Fi [one study involving bimekizumab (van der Heijde et al., 2020)], IL17RAi [one study involving brodalumab (Wei et al., 2021b)], IL6i [one study involving tocilizumab (Sieper et al., 2014b)], IL12/23i [one study involving risankizumab (Baeten et al., 2018) and three studies involving ustekinumab (Deodhar et al., 2019b)], and PDE4i [two studies involving apremilast (Pathan et al., 2013; Taylor et al., 2021)], small-molecule drugs, including JAK1/3i [two studies involving tofacitinib (van der Heijde et al., 2017; Deodhar et al., 2021b)] and JAK1i [one study involving filgotinib (van der Heijde et al., 2018a) and three studies involving upadacitinib (van der Heijde et al., 2019; Deodhar et al., 2022; van der Heijde et al., 2022)], and csDMARD [one study involving SSZ (Khanna Sharma et al., 2018)]. All studies included at least one outcome measure for comparison. The network plots of outcomes to exhibit all the available evidence of each treatment are displayed in Figure 2.
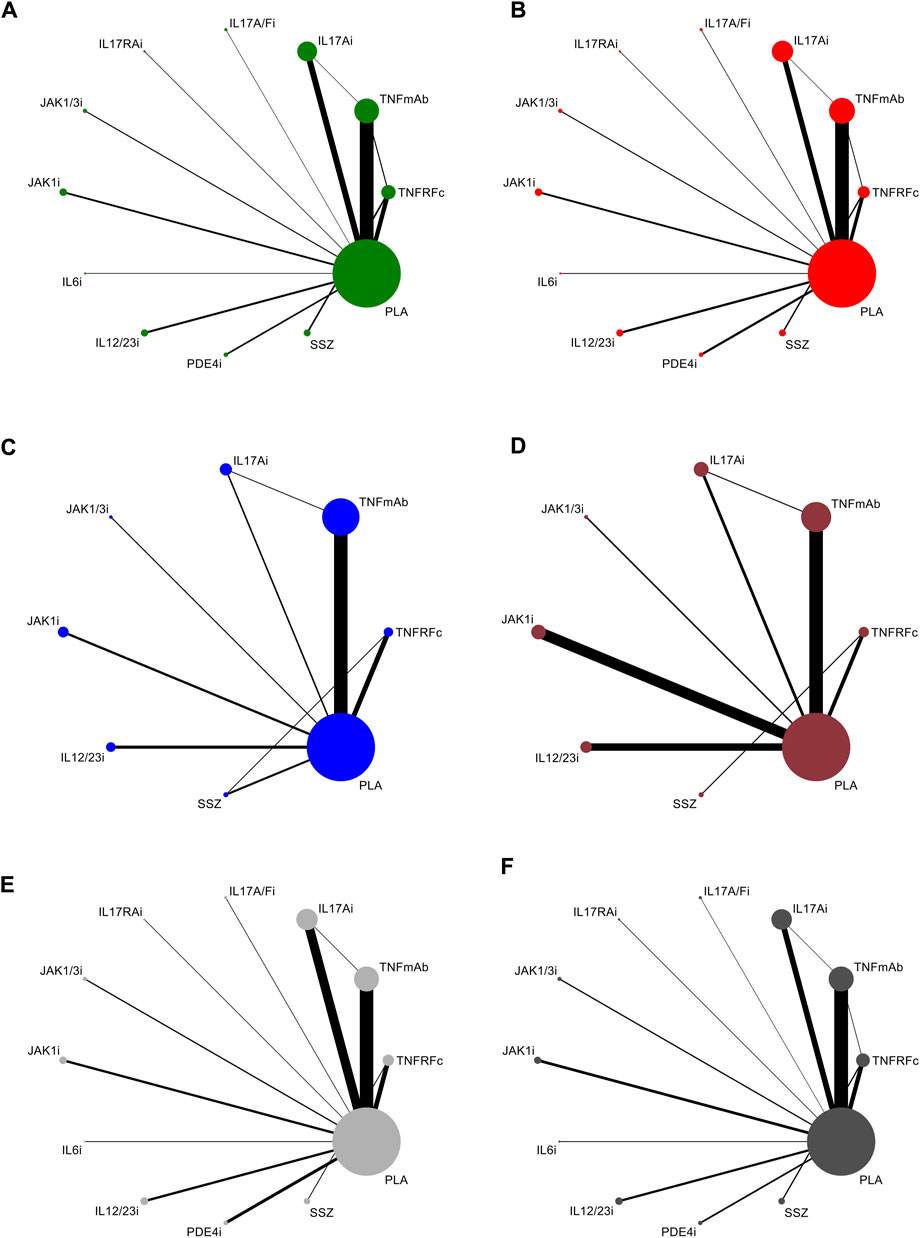
FIGURE 2. Evidence network plots for the analysis of (A) ASAS20, (B) ASAS40, (C) BASDAI50, (D) ASDAS-ID, (E) TEAEs, and (F) SAEs. Line thickness corresponds to the number of trials comparing each pair of treatments. Node size is proportional to the number of randomized participants receiving the treatment. TNFRFc, TNFR-Fc fusion protein; TNFmAb, TNF-α monoclonal antibody; IL17Ai, IL-17A monoclonal antibody; IL17A/Fi, IL-17A and IL-17F dual inhibitor; IL17RA, IL-17 receptor A monoclonal antibody; JAK1/3i, JAK1 and JAK3 inhibitor; JAK1i, JAK1 inhibitor; IL6i, IL-6 inhibitor; IL12/23i, IL-12 and/or IL-23 inhibitor; PDE4i, phosphodiesterase-4 inhibitor; PLA: placebo.
3.3 Efficacy analysis
The league plot in Figure 3 illustrates the relative efficacy of different treatments. When compared to PLA, seven treatments showed significantly greater efficacy in achieving an ASAS20 response: TNFRFc (OR, 3.00; 95% CI, 2.10–4.29), TNFmAb (OR, 3.93; 95% CI, 3.16–4.90), IL17Ai (OR, 2.65; 95% CI, 2.01–3.48), IL17A/Fi (OR, 3.56; 95% CI, 1.45–8.74), IL17RAi (OR, 2.90; 95% CI, 1.15–7.27), JAK1/3i (OR, 2.84; 95% CI, 1.54–5.26), and JAK1i (OR, 3.04; 95% CI, 1.98–4.65). Regarding head-to-head comparisons, statistically significant improvements in achieving ASAS20 response were observed in comparisons such as TNFRFc or TNFmAb vs. IL12/23i, PDE4i, or SSZ; IL17Ai or JAK1i vs. IL12/23i or SSZ; and IL17A/Fi or JAK1/3i vs. SSZ (Figure 3).
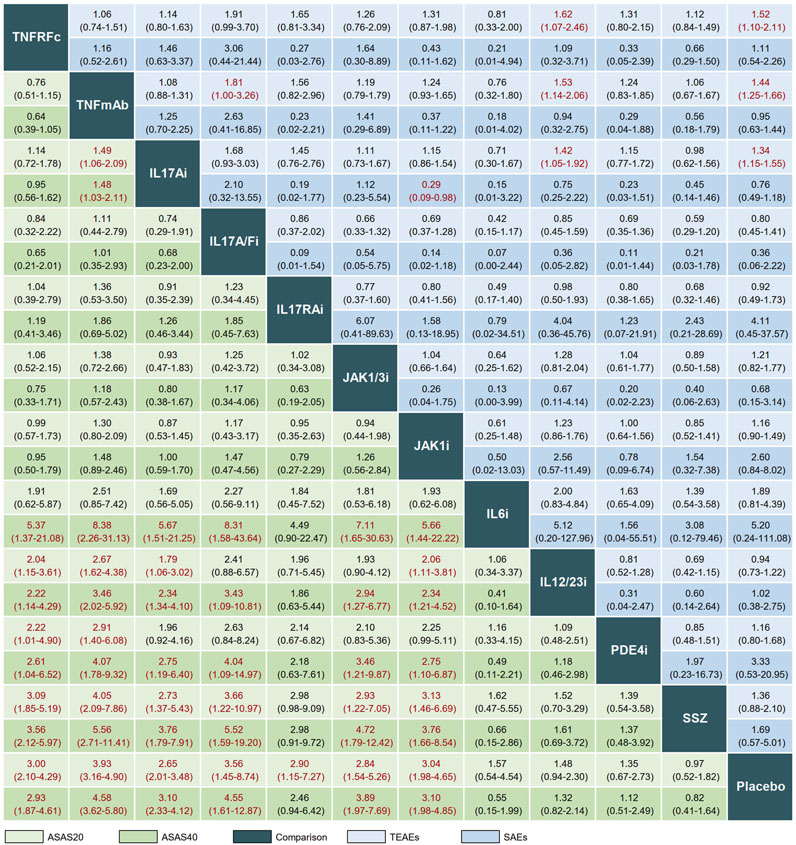
FIGURE 3. League plot comparing efficacy and safety across interventions. Treatment comparisons should be read from left to right. Efficacy data are presented as odds ratios with 95% confidence intervals. Values above 1 favor the column-defining treatment. TNFRFc, TNFR-Fc fusion protein; TNFmAb, TNF-α monoclonal antibody; IL17Ai, IL-17A monoclonal antibody; IL17A/Fi, IL-17A and IL-17F dual inhibitor; IL17RA, IL-17 receptor A monoclonal antibody; JAK1/3i, JAK1 and JAK3 inhibitor; JAK1i, JAK1 inhibitor; IL6i, IL-6 inhibitor; IL12/23i, IL-12 and/or IL-23 inhibitor; PDE4i, phosphodiesterase-4 inhibitor; PLA, placebo.
In terms of ASAS40, significant differences in clinical response were observed after treatment with six drugs (TNFRFc, TNFmAb, IL17Ai, IL17A/Fi, JAK1/3i, and JAK1i) in comparison with PLA. The better clinical efficacy in achieving ASAS40 response were achieved by TNFRFc, TNFmAb, IL17Ai, IL17A/Fi, JAK1/3i, and JAK1i compared to IL6i, IL12/23i, PDE4i, SSZ, or PLA (Figure 3).
As for BASDAI50, there are four treatments (TNFRFc, TNFmAb, IL17Ai, and JAK1i) that showed better response rates compared to PLA, and head-to-head comparison indicates that three (TNFRFc, TNFmAb, and IL17Ai) of these four treatments are effective compared to IL12/23i; similar results are obtained in the evaluation of ASDAS-ID response (Supplementary Figure S1). The forest plots of the relative mean effects of treatments, along with 95% CIs and 95% PrIs, are shown in Supplementary Figure S2.
According to the SUCRA-based relative ranking of treatments, TNFmAb (SUCRA, 89.3%) had the highest probability to achieve ASAS20 response, and the efficacy of the remaining treatments were ranked from high to low in the following order: IL17A/Fi (SUCRA, 76.8%) > JAK1i (SUCRA, 70.5%) > TNFRFc (SUCRA, 68.7%) > JAK1/3i (SUCRA, 66.0%) > IL17RAi (SUCRA, 64.3%) > IL17Ai (SUCRA, 59.5%) > IL6i (SUCRA, 33.3%) > IL12/23i (SUCRA, 28.1%) > PDE4i (SUCRA, 24.3%) > SSZ (SUCRA, 10.2%) > PLA (SUCRA, 9.1%) (Figure 4). In the following analysis, TNFmAb still ranked the highest probability for achieving efficacy in ASAS40, BASDAI50, and ASDAS-ID (Figure 4). The detailed ranking plots for a single outcome using probabilities are shown in Supplementary Figure S3.

FIGURE 4. SUCRA ranking plots for (A) ASAS20, (B) ASAS40, (C) BASDAI50, (D) ASDAS-ID, (E) TEAEs, and (F) SAEs. Treatments located toward the upper right corner exhibit the most favorable ranking for that outcome compared to other options. TNFRFc, TNFR-Fc fusion protein; TNFmAb, TNF-α monoclonal antibody; IL17Ai, IL-17A monoclonal antibody; IL17A/Fi, IL-17A and IL-17F dual inhibitor; IL17RA, IL-17 receptor A monoclonal antibody; JAK1/3i, JAK1 and JAK3 inhibitor; JAK1i, JAK1 inhibitor; IL6i, IL-6 inhibitor; IL12/23i, IL-12 and/or IL-23 inhibitor; PDE4i, phosphodiesterase-4 inhibitor; PLA, placebo.
3.4 Subgroup analysis
Because two categories of patients were included, we evaluated whether the efficacy outcomes of drugs varied in different patient populations (AS and nr-axSpA). Considering efficacy of both ASAS20 and ASAS40 responses, six treatments (TNFRFc, TNFmAb, IL17Ai, IL17A/Fi, JAK1/3i, and JAK1i) and four treatments (TNFRFc, TNFmAb, IL17Ai, and JAK1i) were more effective than PLA in patients with AS and nr-axSpA, respectively; other treatments (IL6i, IL12/23i, PDE4i, and SSZ) had no effect in these patients, being similar to the results in axSpA patients (Supplementary Figures S5, S6). TNFmAb was ranked the most effective treatment for patients with AS; this result was also found in patients with nr-axSpA (Supplementary Figure S7). Note that IL12/23i (OR, 1.54; 95% CI, 1.03–2.29) had a higher ASAS20 response than PLA in patients with AS. In the original article, three studies recruiting patients with nr-axSpA were prematurely discontinued due to failure in receiving endpoints in a concurrent study (Deodhar et al., 2019b). Therefore, these data should be interpreted with caution.
3.5 Safety analysis
A total of 49 and 55 articles reported the occurrence of TEAEs and SAEs, respectively. Our results showed that TNFRFc (OR, 1.52; 95% CI, 1.10–2.11), TNFmAb (OR, 1.44; 95% CI, 1.25–1.66), and IL17Ai (OR, 1.34; 95% CI, 1.15–1.55) had a higher incidence of increasing risk of TEAEs compared with PLA. Additionally, TNFmAb had a higher risk of TEAEs compared to IL17A/Fi (OR, 1.81; 95% CI, 1.00–3.26). For the analysis of SAEs, the overwhelming majority of treatments showed no significant advantage or disadvantage compared to PLA or among each other, and only IL17Ai treatment had a lower risk of SAEs compared with JAK1i (OR, 0.29; 95% CI, 0.09–0.98) (Figure 3). The forest plots of the relative mean effects of treatments are shown in Supplementary Figure S2. A lower incidence of TEAEs and SAEs was observed in patients treated with IL17A/Fi (SUCRA, 10.6) and IL17RAi (SUCRA, 10.7), respectively, compared to those undergoing other treatments (Figure 4).
Two-dimensional graphs were illustrated to evaluate the overall performance (Figure 5). For the comprehensive assessment using ASAS20 and TEAEs, IL17A/Fi might be the best choice in balancing efficacy and safety. Similar results were also observed in the comprehensive assessment using ASAS40 and SAEs (Figure 5).
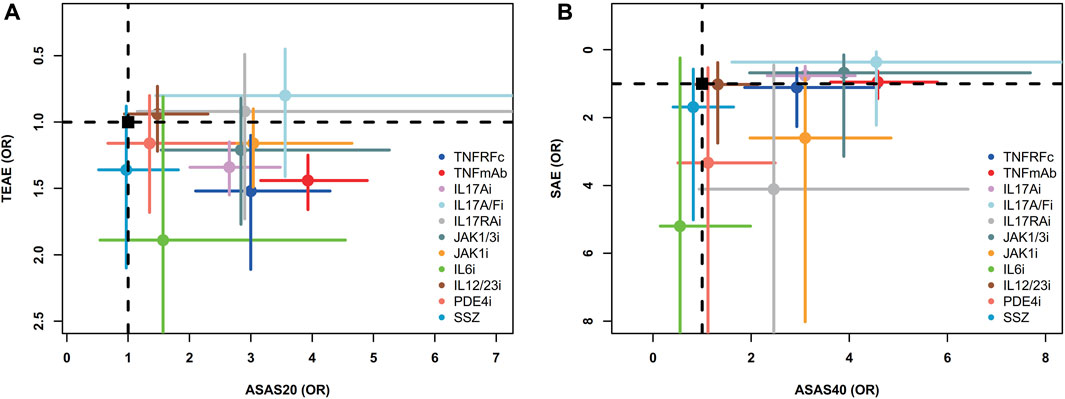
FIGURE 5. Two-dimensional graphs for (A) TEAEs versus ASAS20 and (B) SAEs versus ASAS40. Individual treatments are nodes, with placebo as a black square. Data are mean odds ratios with error bars representing 95% confidence intervals. Nodes in the upper right corner indicate treatments with high efficacy and low adverse events. TNFRFc, TNFR-Fc fusion protein; TNFmAb, TNF-α monoclonal antibody; IL17Ai, IL-17A monoclonal antibody; IL17A/Fi, IL-17A and IL-17F dual inhibitor; IL17RA, IL-17 receptor A monoclonal antibody; JAK1/3i, JAK1 and JAK3 inhibitor; JAK1i, JAK1 inhibitor; IL6i, IL-6 inhibitor; IL12/23i, IL-12 and/or IL-23 inhibitor; PDE4i, phosphodiesterase-4 inhibitor; PLA, placebo.
3.6 Inconsistency and publication bias
There was no global inconsistency for most outcomes except for BASDAI50 (χ2, 11.78; p = 0.0082) in our results (Supplementary Table S3). The local inconsistency test implied that there was no difference between most of the direct comparison and indirect comparison, except for ASAS40 (TNFmAb vs. IL17Ai and IL17Ai vs. PLA) and BASDAI50 (TNFRFc vs. SSZ, TNFRFc vs. PLA, and SSZ vs. PLA), which suggests low overall inconsistency (Supplementary Table S4). Comparison-adjusted funnel plots were used to examine publication bias. No significant visual asymmetry was found in the plots of the efficacy and safety outcomes, showing no obvious publication bias among the aforementioned analyses (Supplementary Figure S8).
4 Discussion
The primary objective in treating axSpA is to enhance long-term health-related quality of life (Ramiro et al., 2022). The introduction of biologics, followed by the release of small-molecule drugs, has played a crucial role in achieving this objective (Ramiro et al., 2022). While various types of these drugs have been approved and have shown clear efficacy in these patients, their differing performance in clinical response rates and potential adverse events have garnered significant attention. Therefore, a comprehensive assessment of various treatment regimens may be beneficial for clinicians when selecting the most appropriate treatment for these patients.
Our network meta-analysis provides the most comprehensive summary to date by comparing the efficacy and safety of 11 classes of biologics and small-molecule drugs in patients with axSpA. Furthermore, this study offers the first insights into the relative efficacy of these drugs in nr-axSpA patients. The results indicate that seven treatments (TNFmAb, IL17A/Fi, JAK1i, TNFRFc, JAK1/3i, IL17RAi, and IL17Ai) were associated with superior clinical response compared to PLA. Among them, TNFmAb demonstrated the best response across all efficacy outcomes included in this study. Safety analyses suggested that IL17A/Fi might carry the lowest risk of TEAEs and SAEs. TNFmAb had the third highest SUCRA value for TEAEs, suggesting that its remarkable efficacy might be accompanied by a slightly higher rate of adverse events. Finally, most treatments showed no significant advantage or disadvantage regarding SAEs.
Several scholars have attempted comparative comparisons of treatment efficacy in ankylosing spondylitis (Deodhar et al., 2020a; Cao et al., 2022). Deodhar et al. (2020a) evaluated the relative efficacy of four types of biologics (IL17Ai, JAK inhibitors, TNF inhibitors, and PDE4i) across 28 interventions in 30 included studies. Their study identified tofacitinib (JAK1/3i) as the top-ranked treatment for ASAS20 response, followed by golimumab (TNFmAb) and filgotinib (JAK1i). However, safety outcomes were not evaluated in this study. Results from the study by Cao et al. (2022) showed the highest ASAS20 and ASAS40 response rates in patients treated with IL17A/Fi. In our study, IL17A/Fi was ranked the second highest for these clinical response rates among active treatments, which differs slightly from this finding. These discrepancies may be attributed to the broader scope of our study, which included both AS and nr-axSpA patients, incorporated more recently published trials (e.g., PDE4i and JAK1i), and evaluated more promisingly effective drugs (e.g., IL17RAi) for treating axSpA, compared to previous analyses. Regarding safety, no significant increase in the risk of SAEs was observed for any of the drugs compared to PLA, consistent with previous studies (Betts et al., 2016; Deodhar et al., 2020a; Cao et al., 2022; Lee, 2022).
Nr-axSpA is considered to represent an early stage of AS or just an abortive form of axSpA (Baraliakos and Braun, 2015). Correspondingly, patients with nr-axSpA are less likely to be treated with biologics (Hunter et al., 2021). Registry and clinical trial data suggest that patients with AS and nr-axSpA exhibit similar clinical manifestations, disease activity, disease burden, and treatment needs, regardless of the presence of radiographic damage (Rudwaleit et al., 2009; López-Medina et al., 2019). Currently, few biologics have been approved for managing nr-axSpA (Deodhar et al., 2020b; Ramiro et al., 2022). Several other drugs are used for these patients, but off-label. Another novel finding of this study is that TNFmAb also ranked the highest for efficacy outcomes in patients with nr-axSpA. These findings could serve as a reference for the development of further management recommendations and the approval of additional drugs in this field.
5 Limitations
This study has several limitations. First, drugs with the same mechanism of action were grouped together for analysis regardless of molecular structure differences, which may not fully reflect the heterogeneity in efficacy. Second, concomitant medications like NSAIDs and csDMARDs were allowed in some included trials, which could influence results. However, baseline medication use was balanced between arms within each trial. Together with the consistent results from inconsistency and publication bias assessments, the relative treatment effects observed in this analysis are considered reliable. Third, patients across a wide range of blinded periods from 6 to 52 weeks were analyzed together, precluding conclusions about specific time points. However, these findings still provide meaningful evidence regarding axSpA treatment, especially in the short-to-medium term. Longer follow-up is necessary to fully evaluate rare adverse events like malignancy. Therefore, while informative for clinical decision-making, the results should be interpreted judiciously considering the study limitations.
6 Conclusion
This network meta-analysis evaluated the efficacy and safety of various biologics and small-molecule drugs in patients with axSpA. Our findings suggest that TNFmAb may provide the greatest efficacy based on the outcomes assessed, while IL17A/Fi was associated with the relatively lowest risk and had the best performance in balancing efficacy and safety. Clinicians should discuss the balance between benefit and harm with individual patients when considering treatment options.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Author contributions
YY conceived the project and designed the study. EZ, JW, and MW contributed to data extraction. EZ and YY conducted the statistical analysis and wrote the manuscript. EZ, KZ, and YY reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1226528/full#supplementary-material
References
Baeten, D., Baraliakos, X., Braun, J., Sieper, J., Emery, P., Van Der Heijde, D., et al. (2013). Anti-interleukin-17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: a randomised, double-blind, placebo-controlled trial. Lancet 382, 1705–1713. doi:10.1016/S0140-6736(13)61134-4
Baeten, D., Østergaard, M., Wei, J. C., Sieper, J., Järvinen, P., Tam, L. S., et al. (2018). Risankizumab, an IL-23 inhibitor, for ankylosing spondylitis: results of a randomised, double-blind, placebo-controlled, proof-of-concept, dose-finding phase 2 study. Ann. Rheum. Dis. 77, 1295–1302. doi:10.1136/annrheumdis-2018-213328
Baeten, D., Sieper, J., Braun, J., Baraliakos, X., Dougados, M., Emery, P., et al. (2015). Secukinumab, an interleukin-17a inhibitor, in ankylosing spondylitis. N. Engl. J. Med. 373, 2534–2548. doi:10.1056/NEJMoa1505066
Bao, C., Huang, F., Khan, M. A., Fei, K., Wu, Z., Han, C., et al. (2014). Safety and efficacy of golimumab in Chinese patients with active ankylosing spondylitis: 1-year results of a multicentre, randomized, double-blind, placebo-controlled phase III trial. Rheumatol. Oxf. 53, 1654–1663. doi:10.1093/rheumatology/keu132
Baraliakos, X., and Braun, J. (2015). Non-radiographic axial spondyloarthritis and ankylosing spondylitis: what are the similarities and differences? RMD Open 1, e000053. doi:10.1136/rmdopen-2015-000053
Betts, K. A., Griffith, J., Song, Y., Mittal, M., Joshi, A., Wu, E. Q., et al. (2016). Network meta-analysis and cost per responder of tumor necrosis factor-α and interleukin inhibitors in the treatment of active ankylosing spondylitis. Rheumatol. Ther. 3, 323–336. doi:10.1007/s40744-016-0038-y
Braun, J., Van Der Horst-Bruinsma, I. E., Huang, F., Burgos-Vargas, R., Vlahos, B., Koenig, A. S., et al. (2011). Clinical efficacy and safety of etanercept versus sulfasalazine in patients with ankylosing spondylitis: a randomized, double-blind trial. Arthritis Rheum. 63, 1543–1551. doi:10.1002/art.30223
Burgos-Vargas, R., Loyola-Sanchez, A., Ramiro, S., Reding-Bernal, A., Alvarez-Hernandez, E., Van Der Heijde, D., et al. (2022). A randomized, double-blind, placebo-controlled 12-week trial of infliximab in patients with juvenile-onset spondyloarthritis. Arthritis Res. Ther. 24, 187. doi:10.1186/s13075-022-02877-9
Calin, A., Dijkmans, B. A., Emery, P., Hakala, M., Kalden, J., Leirisalo-Repo, M., et al. (2004). Outcomes of a multicentre randomised clinical trial of etanercept to treat ankylosing spondylitis. Ann. Rheum. Dis. 63, 1594–1600. doi:10.1136/ard.2004.020875
Cantini, F., Niccoli, L., Nannini, C., Cassarà, E., Kaloudi, O., Giulio Favalli, E., et al. (2017). Second-line biologic therapy optimization in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. Semin. Arthritis Rheum. 47, 183–192. doi:10.1016/j.semarthrit.2017.03.008
Cao, Z., Guo, J., Li, Q., Li, Y., and Wu, J. (2022). Optimal biologic drugs for the treatment of ankylosing spondylitis: results from a network meta-analysis and network metaregression. Biomed. Res. Int. 2022, 8316106. doi:10.1155/2022/8316106
Caso, F., Costa, L., Triggianese, P., Maione, F., Bertolini, N., Vastarella, M., et al. (2023). Recent developments for new investigational JAK inhibitors in psoriatic arthritis. Expert Opin. Investig. Drugs 32, 361–371. doi:10.1080/13543784.2023.2207737
Damjanov, N., Shehhi, W. A., Huang, F., Kotak, S., Burgos-Vargas, R., Shirazy, K., et al. (2016). Assessment of clinical efficacy and safety in a randomized double-blind study of etanercept and sulfasalazine in patients with ankylosing spondylitis from Eastern/Central Europe, Latin America, and Asia. Rheumatol. Int. 36, 643–651. doi:10.1007/s00296-016-3452-0
Danve, A., and Deodhar, A. (2022). Treatment of axial spondyloarthritis: an update. Nat. Rev. Rheumatol. 18, 205–216. doi:10.1038/s41584-022-00761-z
Davis, J. C., Van Der Heijde, D., Braun, J., Dougados, M., Cush, J., Clegg, D. O., et al. (2003). Recombinant human tumor necrosis factor receptor (etanercept) for treating ankylosing spondylitis: a randomized, controlled trial. Arthritis Rheum. 48, 3230–3236. doi:10.1002/art.11325
Deodhar, A., Blanco, R., Dokoupilová, E., Hall, S., Kameda, H., Kivitz, A. J., et al. (2021a). Improvement of signs and symptoms of nonradiographic axial spondyloarthritis in patients treated with secukinumab: primary results of a randomized, placebo-controlled phase III study. Arthritis Rheumatol. 73, 110–120. doi:10.1002/art.41477
Deodhar, A., Chakravarty, S. D., Cameron, C., Peterson, S., Hensman, R., Fogarty, S., et al. (2020a). A systematic review and network meta-analysis of current and investigational treatments for active ankylosing spondylitis. Clin. Rheumatol. 39, 2307–2315. doi:10.1007/s10067-020-04970-3
Deodhar, A., Gensler, L. S., Kay, J., Maksymowych, W. P., Haroon, N., Landewé, R., et al. (2019a). A fifty-two-week, randomized, placebo-controlled trial of certolizumab pegol in nonradiographic axial spondyloarthritis. Arthritis Rheumatol. 71, 1101–1111. doi:10.1002/art.40866
Deodhar, A., Gensler, L. S., Sieper, J., Clark, M., Calderon, C., Wang, Y., et al. (2019b). Three multicenter, randomized, double-blind, placebo-controlled studies evaluating the efficacy and safety of ustekinumab in axial spondyloarthritis. Arthritis Rheumatol. 71, 258–270. doi:10.1002/art.40728
Deodhar, A., Poddubnyy, D., Pacheco-Tena, C., Salvarani, C., Lespessailles, E., Rahman, P., et al. (2019c). Efficacy and safety of ixekizumab in the treatment of radiographic axial spondyloarthritis: sixteen-week results from a phase III randomized, double-blind, placebo-controlled trial in patients with prior inadequate response to or intolerance of tumor necrosis factor inhibitors. Arthritis Rheumatol. 71, 599–611. doi:10.1002/art.40753
Deodhar, A., Reveille, J. D., Harrison, D. D., Kim, L., Lo, K. H., Leu, J. H., et al. (2018). Safety and efficacy of golimumab administered intravenously in adults with ankylosing spondylitis: results through week 28 of the GO-ALIVE study. J. Rheumatol. 45, 341–348. doi:10.3899/jrheum.170487
Deodhar, A., Sandoval, D., Holdsworth, E., Booth, N., and Hunter, T. (2020b). Use and switching of biologic therapy in patients with non-radiographic axial spondyloarthritis: a patient and provider survey in the United States. Rheumatol. Ther. 7, 415–423. doi:10.1007/s40744-020-00208-5
Deodhar, A., Sliwinska-Stanczyk, P., Xu, H., Baraliakos, X., Gensler, L. S., Fleishaker, D., et al. (2021b). Tofacitinib for the treatment of ankylosing spondylitis: a phase III, randomised, double-blind, placebo-controlled study. Ann. Rheum. Dis. 80, 1004–1013. doi:10.1136/annrheumdis-2020-219601
Deodhar, A., Van Den Bosch, F., Poddubnyy, D., Maksymowych, W. P., Van Der Heijde, D., Kim, T. H., et al. (2022). Upadacitinib for the treatment of active non-radiographic axial spondyloarthritis (SELECT-AXIS 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 400, 369–379. doi:10.1016/S0140-6736(22)01212-0
Deodhar, A., Van Der Heijde, D., Gensler, L. S., Kim, T. H., Maksymowych, W. P., Østergaard, M., et al. (2020c). Ixekizumab for patients with non-radiographic axial spondyloarthritis (COAST-X): a randomised, placebo-controlled trial. Lancet 395, 53–64. doi:10.1016/S0140-6736(19)32971-X
Dougados, M., Braun, J., Szanto, S., Combe, B., Elbaz, M., Geher, P., et al. (2011). Efficacy of etanercept on rheumatic signs and pulmonary function tests in advanced ankylosing spondylitis: results of a randomised double-blind placebo-controlled study (SPINE). Ann. Rheum. Dis. 70, 799–804. doi:10.1136/ard.2010.139261
Dougados, M., Van Der Heijde, D., Sieper, J., Braun, J., Maksymowych, W. P., Citera, G., et al. (2014a). Symptomatic efficacy of etanercept and its effects on objective signs of inflammation in early nonradiographic axial spondyloarthritis: a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol. 66, 2091–2102. doi:10.1002/art.38721
Dougados, M., Wood, E., Combe, B., Schaeverbeke, T., Miceli-Richard, C., Berenbaum, F., et al. (2014b). Evaluation of the nonsteroidal anti-inflammatory drug-sparing effect of etanercept in axial spondyloarthritis: results of the multicenter, randomized, double-blind, placebo-controlled SPARSE study. Arthritis Res. Ther. 16, 481. doi:10.1186/s13075-014-0481-5
Erdes, S., Nasonov, E., Kunder, E., Pristrom, A., Soroka, N., Shesternya, P., et al. (2020). Primary efficacy of netakimab, a novel interleukin-17 inhibitor, in the treatment of active ankylosing spondylitis in adults. Clin. Exp. Rheumatol. 38, 27–34.
Giardina, A. R., Ferrante, A., Ciccia, F., Impastato, R., Miceli, M. C., Principato, A., et al. (2010). A 2-year comparative open label randomized study of efficacy and safety of etanercept and infliximab in patients with ankylosing spondylitis. Rheumatol. Int. 30, 1437–1440. doi:10.1007/s00296-009-1157-3
Haibel, H., Rudwaleit, M., Listing, J., Heldmann, F., Wong, R. L., Kupper, H., et al. (2008). Efficacy of adalimumab in the treatment of axial spondylarthritis without radiographically defined sacroiliitis: results of a twelve-week randomized, double-blind, placebo-controlled trial followed by an open-label extension up to week fifty-two. Arthritis Rheum. 58, 1981–1991. doi:10.1002/art.23606
Hoaglin, D. C., Hawkins, N., Jansen, J. P., Scott, D. A., Itzler, R., Cappelleri, J. C., et al. (2011). Conducting indirect-treatment-comparison and network-meta-analysis studies: report of the ISPOR task force on indirect treatment comparisons good research practices: part 2. Value Health 14, 429–437. doi:10.1016/j.jval.2011.01.011
Horneff, G., Fitter, S., Foeldvari, I., Minden, K., Kuemmerle-Deschner, J., Tzaribacev, N., et al. (2012). Double-blind, placebo-controlled randomized trial with adalimumab for treatment of juvenile onset ankylosing spondylitis (JoAS): significant short term improvement. Arthritis Res. Ther. 14, R230. doi:10.1186/ar4072
Huang, F., Gu, J., Zhu, P., Bao, C., Xu, J., Xu, H., et al. (2014). Efficacy and safety of adalimumab in Chinese adults with active ankylosing spondylitis: results of a randomised, controlled trial. Ann. Rheum. Dis. 73, 587–594. doi:10.1136/annrheumdis-2012-202533
Huang, F., Sun, F., Wan, W. G., Wu, L. J., Dong, L. L., Zhang, X., et al. (2020). Secukinumab provided significant and sustained improvement in the signs and symptoms of ankylosing spondylitis: results from the 52-week, Phase III China-centric study, MEASURE 5. Chin. Med. J. Engl. 133, 2521–2531. doi:10.1097/CM9.0000000000001099
Hunter, T., Sandoval, D., Booth, N., Holdsworth, E., and Deodhar, A. (2021). Comparing symptoms, treatment patterns, and quality of life of ankylosing spondylitis and non-radiographic axial spondyloarthritis patients in the USA: findings from a patient and rheumatologist Survey. Clin. Rheumatol. 40, 3161–3167. doi:10.1007/s10067-021-05642-6
Hutton, B., Salanti, G., Caldwell, D. M., Chaimani, A., Schmid, C. H., Cameron, C., et al. (2015). The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann. Intern Med. 162, 777–784. doi:10.7326/M14-2385
Inman, R. D., Davis, J. C., Heijde, D., Diekman, L., Sieper, J., Kim, S. I., et al. (2008). Efficacy and safety of golimumab in patients with ankylosing spondylitis: results of a randomized, double-blind, placebo-controlled, phase III trial. Arthritis Rheum. 58, 3402–3412. doi:10.1002/art.23969
Inman, R. D., and Maksymowych, W. P.CANDLE Study Group (2010). A double-blind, placebo-controlled trial of low dose infliximab in ankylosing spondylitis. J. Rheumatol. 37, 1203–1210. doi:10.3899/jrheum.091042
Khanna Sharma, S., Kadiyala, V., Naidu, G., and Dhir, V. (2018). A randomized controlled trial to study the efficacy of sulfasalazine for axial disease in ankylosing spondylitis. Int. J. Rheum. Dis. 21, 308–314. doi:10.1111/1756-185X.13124
Kivitz, A. J., Wagner, U., Dokoupilova, E., Supronik, J., Martin, R., Talloczy, Z., et al. (2018). Efficacy and safety of secukinumab 150 mg with and without loading regimen in ankylosing spondylitis: 104-week results from MEASURE 4 study. Rheumatol. Ther. 5, 447–462. doi:10.1007/s40744-018-0123-5
Landewé, R., Braun, J., Deodhar, A., Dougados, M., Maksymowych, W. P., Mease, P. J., et al. (2014). Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled Phase 3 study. Ann. Rheum. Dis. 73, 39–47. doi:10.1136/annrheumdis-2013-204231
Landewé, R. B., Van Der Heijde, D., Dougados, M., Baraliakos, X., Van Den Bosch, F. E., Gaffney, K., et al. (2020). Maintenance of clinical remission in early axial spondyloarthritis following certolizumab pegol dose reduction. Ann. Rheum. Dis. 79, 920–928. doi:10.1136/annrheumdis-2019-216839
Landewé, R., Sieper, J., Mease, P., Inman, R. D., Lambert, R. G., Deodhar, A., et al. (2018). Efficacy and safety of continuing versus withdrawing adalimumab therapy in maintaining remission in patients with non-radiographic axial spondyloarthritis (ABILITY-3): a multicentre, randomised, double-blind study. Lancet 392, 134–144. doi:10.1016/S0140-6736(18)31362-X
Lawson, D. O., Eraso, M., Mbuagbaw, L., Joanes, M., Aves, T., Leenus, A., et al. (2021). Tumor necrosis factor inhibitor dose reduction for axial spondyloarthritis: a systematic review and meta-analysis of randomized controlled trials. Arthritis Care Res. Hob. 73, 861–872. doi:10.1002/acr.24184
Lee, Y. H. (2022). Comparative efficacy and safety of janus kinase inhibitors and secukinumab in patients with active ankylosing spondylitis: a systematic review and meta-analysis. Pharmacology 107, 537–544. doi:10.1159/000525627
Li, S., Li, F., Mao, N., Wang, J., and Xie, X. (2022). Efficacy and safety of Janus kinase inhibitors in patients with ankylosing spondylitis: a systematic review and meta-analysis. Eur. J. Intern Med. 102, 47–53. doi:10.1016/j.ejim.2022.04.007
Li, T., Puhan, M. A., Vedula, S. S., Singh, S., and Dickersin, K.Ad Hoc Network Meta-analysis Methods Meeting Working Group (2011). Network meta-analysis-highly attractive but more methodological research is needed. BMC Med. 9, 79. doi:10.1186/1741-7015-9-79
López-Medina, C., Ramiro, S., Van Der Heijde, D., Sieper, J., Dougados, M., and Molto, A. (2019). Characteristics and burden of disease in patients with radiographic and non-radiographic axial Spondyloarthritis: a comparison by systematic literature review and meta-analysis. RMD Open 5, e001108. doi:10.1136/rmdopen-2019-001108
Marzo-Ortega, H., Mcgonagle, D., Jarrett, S., Haugeberg, G., Hensor, E., O'connor, P., et al. (2005). Infliximab in combination with methotrexate in active ankylosing spondylitis: a clinical and imaging study. Ann. Rheum. Dis. 64, 1568–1575. doi:10.1136/ard.2004.022582
Pathan, E., Abraham, S., Van Rossen, E., Withrington, R., Keat, A., Charles, P. J., et al. (2013). Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in ankylosing spondylitis. Ann. Rheum. Dis. 72, 1475–1480. doi:10.1136/annrheumdis-2012-201915
Pavelka, K., Kivitz, A., Dokoupilova, E., Blanco, R., Maradiaga, M., Tahir, H., et al. (2017). Efficacy, safety, and tolerability of secukinumab in patients with active ankylosing spondylitis: a randomized, double-blind phase 3 study, MEASURE 3. Arthritis Res. Ther. 19, 285. doi:10.1186/s13075-017-1490-y
Ramiro, S., Nikiphorou, E., Sepriano, A., Ortolan, A., Webers, C., Baraliakos, X., et al. (2022). Response to: correspondence on "ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update" by Braun et al. Ann. Rheum. Dis. 82, e206. doi:10.1136/ard-2023-223937
Rudwaleit, M., Haibel, H., Baraliakos, X., Listing, J., Märker-Hermann, E., Zeidler, H., et al. (2009). The early disease stage in axial spondylarthritis: results from the German Spondyloarthritis Inception Cohort. Arthritis Rheum. 60, 717–727. doi:10.1002/art.24483
Rudwaleit, M., Van Der Heijde, D., Landewé, R., Akkoc, N., Brandt, J., Chou, C. T., et al. (2011). The Assessment of SpondyloArthritis international Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann. Rheumatic Dis. 70, 25–31. doi:10.1136/ard.2010.133645
Sieper, J., Lenaerts, J., Wollenhaupt, J., Rudwaleit, M., Mazurov, V. I., Myasoutova, L., et al. (2014a). Efficacy and safety of infliximab plus naproxen versus naproxen alone in patients with early, active axial spondyloarthritis: results from the double-blind, placebo-controlled INFAST study, Part 1. Ann. Rheum. Dis. 73, 101–107. doi:10.1136/annrheumdis-2012-203201
Sieper, J., and Poddubnyy, D. (2017). Axial spondyloarthritis. Lancet 390, 73–84. doi:10.1016/S0140-6736(16)31591-4
Sieper, J., Porter-Brown, B., Thompson, L., Harari, O., and Dougados, M. (2014b). Assessment of short-term symptomatic efficacy of tocilizumab in ankylosing spondylitis: results of randomised, placebo-controlled trials. Ann. Rheum. Dis. 73, 95–100. doi:10.1136/annrheumdis-2013-203559
Sieper, J., Van Der Heijde, D., Dougados, M., Maksymowych, W. P., Scott, B. B., Boice, J. A., et al. (2015). A randomized, double-blind, placebo-controlled, sixteen-week study of subcutaneous golimumab in patients with active nonradiographic axial spondyloarthritis. Arthritis Rheumatol. 67, 2702–2712. doi:10.1002/art.39257
Sieper, J., Van Der Heijde, D., Dougados, M., Mease, P. J., Maksymowych, W. P., Brown, M. A., et al. (2013). Efficacy and safety of adalimumab in patients with non-radiographic axial spondyloarthritis: results of a randomised placebo-controlled trial (ABILITY-1). Ann. Rheum. Dis. 72, 815–822. doi:10.1136/annrheumdis-2012-201766
Song, I. H., Hermann, K., Haibel, H., Althoff, C. E., Listing, J., Burmester, G., et al. (2011). Effects of etanercept versus sulfasalazine in early axial spondyloarthritis on active inflammatory lesions as detected by whole-body MRI (ESTHER): a 48-week randomised controlled trial. Ann. Rheum. Dis. 70, 590–596. doi:10.1136/ard.2010.139667
Sterne, J. a.C., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: a revised tool for assessing risk of bias in randomised trials. Bmj 366, l4898. doi:10.1136/bmj.l4898
Sunzini, F., D'antonio, A., Fatica, M., Triggianese, P., Conigliaro, P., Greco, E., et al. (2022). What's new and what's next for biological and targeted synthetic treatments in psoriatic arthritis? Expert Opin. Biol. Ther. 22, 1545–1559. doi:10.1080/14712598.2022.2152321
Tam, L. S., Shang, Q., Kun, E. W., Lee, K. L., Yip, M. L., Li, M., et al. (2014). The effects of golimumab on subclinical atherosclerosis and arterial stiffness in ankylosing spondylitis—a randomized, placebo-controlled pilot trial. Rheumatol. Oxf. 53, 1065–1074. doi:10.1093/rheumatology/ket469
Taylor, P. C., Van Der Heijde, D., Landewé, R., Mccue, S., Cheng, S., and Boonen, A. (2021). A phase III randomized study of apremilast, an oral phosphodiesterase 4 inhibitor, for active ankylosing spondylitis. J. Rheumatol. 48, 1259–1267. doi:10.3899/jrheum.201088
Van Der Heijde, D., Baraliakos, X., Gensler, L. S., Maksymowych, W. P., Tseluyko, V., Nadashkevich, O., et al. (2018a). Efficacy and safety of filgotinib, a selective Janus kinase 1 inhibitor, in patients with active ankylosing spondylitis (TORTUGA): results from a randomised, placebo-controlled, phase 2 trial. Lancet 392, 2378–2387. doi:10.1016/S0140-6736(18)32463-2
Van Der Heijde, D., Baraliakos, X., Sieper, J., Deodhar, A., Inman, R. D., Kameda, H., et al. (2022). Efficacy and safety of upadacitinib for active ankylosing spondylitis refractory to biological therapy: a double-blind, randomised, placebo-controlled phase 3 trial. Ann. Rheum. Dis. 81, 1515–1523. doi:10.1136/ard-2022-222608
Van Der Heijde, D., Cheng-Chung Wei, J., Dougados, M., Mease, P., Deodhar, A., Maksymowych, W. P., et al. (2018b). Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (COAST-V): 16 week results of a phase 3 randomised, double-blind, active-controlled and placebo-controlled trial. Lancet 392, 2441–2451. doi:10.1016/S0140-6736(18)31946-9
Van Der Heijde, D., Da Silva, J. C., Dougados, M., Geher, P., Van Der Horst-Bruinsma, I., Juanola, X., et al. (2006a). Etanercept 50 mg once weekly is as effective as 25 mg twice weekly in patients with ankylosing spondylitis. Ann. Rheum. Dis. 65, 1572–1577. doi:10.1136/ard.2006.056747
Van Der Heijde, D., Deodhar, A., Wei, J. C., Drescher, E., Fleishaker, D., Hendrikx, T., et al. (2017). Tofacitinib in patients with ankylosing spondylitis: a phase II, 16-week, randomised, placebo-controlled, dose-ranging study. Ann. Rheum. Dis. 76, 1340–1347. doi:10.1136/annrheumdis-2016-210322
Van Der Heijde, D., Dijkmans, B., Geusens, P., Sieper, J., Dewoody, K., Williamson, P., et al. (2005). Efficacy and safety of infliximab in patients with ankylosing spondylitis: results of a randomized, placebo-controlled trial (ASSERT). Arthritis Rheum. 52, 582–591. doi:10.1002/art.20852
Van Der Heijde, D., Gensler, L. S., Deodhar, A., Baraliakos, X., Poddubnyy, D., Kivitz, A., et al. (2020). Dual neutralisation of interleukin-17A and interleukin-17F with bimekizumab in patients with active ankylosing spondylitis: results from a 48-week phase IIb, randomised, double-blind, placebo-controlled, dose-ranging study. Ann. Rheum. Dis. 79, 595–604. doi:10.1136/annrheumdis-2020-216980
Van Der Heijde, D., Kivitz, A., Schiff, M. H., Sieper, J., Dijkmans, B. A., Braun, J., et al. (2006b). Efficacy and safety of adalimumab in patients with ankylosing spondylitis: results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 54, 2136–2146. doi:10.1002/art.21913
Van Der Heijde, D., Song, I. H., Pangan, A. L., Deodhar, A., Van Den Bosch, F., Maksymowych, W. P., et al. (2019). Efficacy and safety of upadacitinib in patients with active ankylosing spondylitis (SELECT-AXIS 1): a multicentre, randomised, double-blind, placebo-controlled, phase 2/3 trial. Lancet 394, 2108–2117. doi:10.1016/S0140-6736(19)32534-6
Van Der Linden, S., Valkenburg, H. A., and Cats, A. (1984). Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 27, 361–368. doi:10.1002/art.1780270401
Van Valkenhoef, G., Dias, S., Ades, A. E., and Welton, N. J. (2016). Automated generation of node-splitting models for assessment of inconsistency in network meta-analysis. Res. Synth. Methods 7, 80–93. doi:10.1002/jrsm.1167
Webers, C., Ortolan, A., Sepriano, A., Falzon, L., Baraliakos, X., Landewé, R. B. M., et al. (2022). Efficacy and safety of biological DMARDs: a systematic literature review informing the 2022 update of the ASAS-EULAR recommendations for the management of axial spondyloarthritis. Ann. Rheum. Dis. 82, 130–141. doi:10.1136/ard-2022-223298
Wei, J.C.-C., Kim, T.-H., Kishimoto, M., Ogusu, N., Jeong, H., Kobayashi, S., et al. (2021a). Efficacy and safety of brodalumab, an anti-IL17RA monoclonal antibody, in patients with axial spondyloarthritis: 16-week results from a randomised, placebo-controlled, phase 3 trial. Ann. Rheumatic Dis. 80, 1014–1021. doi:10.1136/annrheumdis-2020-219406
Wei, J. C., Kim, T. H., Kishimoto, M., Ogusu, N., Jeong, H., Kobayashi, S., et al. (2021b). Efficacy and safety of brodalumab, an anti-IL17RA monoclonal antibody, in patients with axial spondyloarthritis: 16-week results from a randomised, placebo-controlled, phase 3 trial. Ann. Rheum. Dis. 80, 1014–1021. doi:10.1136/annrheumdis-2020-219406
Wei, J. C., Tsai, W. C., Citera, G., Kotak, S., and Llamado, L. (2018). Efficacy and safety of etanercept in patients from Latin America, Central Europe and Asia with early non-radiographic axial spondyloarthritis. Int. J. Rheum. Dis. 21, 1443–1451. doi:10.1111/1756-185X.12973
Keywords: biologics, small-molecule drugs, axial spondyloarthritis, systematic review, network meta-analysis
Citation: Zhou E, Wu J, Zeng K, Wang M and Yin Y (2023) Comparison of biologics and small-molecule drugs in axial spondyloarthritis: a systematic review and network meta-analysis. Front. Pharmacol. 14:1226528. doi: 10.3389/fphar.2023.1226528
Received: 21 May 2023; Accepted: 06 October 2023;
Published: 24 October 2023.
Edited by:
Lazaros Ignatios Sakkas, University of Thessaly, GreeceReviewed by:
Maria Sole Chimenti, University of Rome Tor Vergata, ItalyEleftherios Pelechas, University Hospital of Ioannina, Greece
Copyright © 2023 Zhou, Wu, Zeng, Wang and Yin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yufeng Yin, eWlueXVmZW5nQDEyNi5jb20=
†These authors have contributed equally to this work
 Erye Zhou†
Erye Zhou† Yufeng Yin
Yufeng Yin