
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 14 August 2023
Sec. Pharmacology of Anti-Cancer Drugs
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1224151
 Alireza Mafi1,2†
Alireza Mafi1,2† Hamidreza Rismanchi3†
Hamidreza Rismanchi3† Yasaman Gholinezhad4†
Yasaman Gholinezhad4† Mohaddese Malek Mohammadi3
Mohaddese Malek Mohammadi3 Vahide Mousavi5
Vahide Mousavi5 Seyed Ali Hosseini6
Seyed Ali Hosseini6 Yaser Eshaghi Milasi1
Yaser Eshaghi Milasi1 Russel J. Reiter7
Russel J. Reiter7 Behrooz Ghezelbash8*
Behrooz Ghezelbash8* Malihe Rezaee4,9*
Malihe Rezaee4,9* Amirhossein Sheida10
Amirhossein Sheida10 Fatemeh Zarepour10
Fatemeh Zarepour10 Zatollah Asemi11
Zatollah Asemi11 Mohammad Ali Mansournia12
Mohammad Ali Mansournia12 Hamed Mirzaei11*
Hamed Mirzaei11*Leukaemia is a dangerous malignancy that causes thousands of deaths every year throughout the world. The rate of morbidity and mortality is significant despite many advancements in therapy strategies for affected individuals. Most antitumour medications used now in clinical oncology use apoptotic signalling pathways to induce cancer cell death. Accumulated data have shown a direct correlation between inducing apoptosis in cancer cells with higher tumour regression and survival. Until now, the efficacy of melatonin as a powerful antitumour agent has been firmly established. A change in melatonin concentrations has been reported in multiple tumours such as endometrial, hematopoietic, and breast cancers. Findings show that melatonin’s anticancer properties, such as its prooxidation function and ability to promote apoptosis, indicate the possibility of utilizing this natural substance as a promising agent in innovative cancer therapy approaches. Melatonin stimulates cell apoptosis via the regulation of many apoptosis facilitators, including mitochondria, cytochrome c, Bcl-2, production of reactive oxygen species, and apoptosis receptors. This paper aimed to further assess the anticancer effects of melatonin through the apoptotic pathway, considering the role that cellular apoptosis plays in the pathogenesis of cancer. The effect of melatonin may mean that it is appropriate for use as an adjuvant, along with other therapeutic approaches such as radiotherapy and chemotherapy.
Leukaemia is a type of malignancy that arises from the abnormal differentiation of hematopoietic cells and results in the large-scale proliferation of leukemic cells in the bone marrow, blood circulation, and lymph nodes (Juliusson and Hough, 2016). Despite improvement in the prognosis of patients with leukaemia because of novel progress in treatment strategies, it is still a life-threatening malignancy (Strati et al., 2018; Thol and Ganser, 2020). As reported by GLOBOCAN, leukaemia is the 15th highest new-case-detected cancer and the 11th highest cause of cancer mortality, with an estimated 474,519 cases and 311,594 deaths worldwide in 2020 (Sung et al., 2021). Two types of leukaemia, acute myeloid leukaemia (AML) and acute lymphoblastic leukaemia (ALL) are the most common cancers in children and bring about mortality and morbidity cancer-related causes in patients under 16 years old (Seth and Singh, 2015). Radiation, contact with specific chemicals, genetic mutations, familial positive history, and lifestyle are predisposing factors for leukaemia (Bispo et al., 2020). According to the World Health Organization (fifth edition) categorization of hematolymphoid tumours, the common classification of leukaemia is based on cell lineage, including lymphoid and myeloid, and the first category includes B-cell lymphoma and T/NK-cell lymphoma. Leukaemia can also be classified through the maturity of cells, clinical manifestations or genetic characterizations, and other subtype categories. Acute lymphoblastic leukaemia (ALL), acute myeloid leukaemia (AML), chronic lymphoid leukaemia (CLL), and chronic myeloid leukaemia (CML) are the four main kinds of leukaemia (Li and Li, 2022). Treatment for leukaemia has been improved over the years, and recent research focuses on targeted therapy and individualized treatment strategies (Sharma and Rai, 2019; Fleischmann et al., 2021). In addition to the type of leukaemia, leukocyte count, symptoms, and age as risk factors for treatment, epigenetic characterizations, immunologic profile, and assessment for treatment response are recently changed elements in the planning treatment programs for each patient (Lee and Cho, 2017; Kato and Manabe, 2018). Despite advances, lack of response to treatment, adverse effects of chemotherapy, illness recurrence, and medication resistance are the main concerns that stand in the way of leukaemia (Sharma and Rai, 2019; Zhang et al., 2019; Bárcenas-López et al., 2021).
N-acetyl-5-methoxy tryptamine, or melatonin, is an indoleamine that is secreted via the pineal gland, which was discovered for the first time in 1958 (Lerner et al., 1958; Hardeland et al., 2006). The rhythmic secretion of melatonin is controlled by hypothalamic suprachiasmatic nuclei, resulting in an increase in the level of melatonin in response to darkness (Vasey et al., 2021). Melatonin is distributed to blood and other body fluids and plays a protective role for the body by regulating homeostatic metabolisms with a concentration of 5–200 pg/mL per day (Hickie and Rogers, 2011). Melatonin is synthesized in different organs, including the retina, skin, gastrointestinal tract lymphocytes, and bone marrow (Acuña-Castroviejo et al., 2014). In addition to its circadian rhythm regulatory effect, melatonin has other functions, such as antioxidant, immunomodulatory, and oncostatin activity (Mafi et al., 2023a). The beneficial role of melatonin in the inhibition of the formation of various diseases has been proven for diabetes (Karamitri and Jockers, 2019), heart disease (Sun et al., 2016), neurodegeneration (Prodhan et al., 2021), depression (Tonon et al., 2021), and reproductive pathologies (Carlomagno et al., 2018). Melatonin has anticancer features through the induction of mechanisms including apoptosis, oxidation, and stop cell cycle and also inhibition of processes like angiogenesis, metastasis, and energy production (Reiter et al., 2018). Considering these mechanisms, the influence of melatonin in leukaemia, ovarian cancer, breast cancer, colorectal cancer, prostatic cancer, and others has been shown (Reiter et al., 2017; Kubatka et al., 2018; Mirza-Aghazadeh-Attari et al., 2020; Shafabakhsh et al., 2020; Shen et al., 2021). In addition, melatonin’s adjuvant properties, when used with other chemotherapy drugs, have been widely described (Mafi et al., 2023b). Studies have shown that melatonin has a preserving impact on lymphocytes and other hematopoietic cells, and promising responses in combination with chemotherapy and melatonin have been seen in leukemic patients (Rubio et al., 2007; Casado-Zapico et al., 2011; Lee et al., 2013). Thus, a comprehensive inspection of the antitumour pathways of melatonin is essential to utilize this hormone in leukaemia therapy. This essay aims to analyse and summarise the significance of melatonin in leukaemia’s apoptotic process.
Apoptosis is programmed cell death that differs from other types of cell death like necrosis. In apoptosis, the cell pursues a suicidal process that results in special morphological and biochemical alterations in the cell (Figure 1) (Wong, 2011; Bertheloot et al., 2021). Apoptosis is an essential biological mechanism in the regulation of embryonic development, differentiation and proliferation, homeostasis, and elimination of damaged and unwanted cells (Elmore, 2007; Voss and Strasser, 2020; Yaghoobi et al., 2023). Dysregulation of apoptosis is associated with uncontrolled cell proliferation and accumulation of mutated cells, eventually developing and progressing the cancer and also the resistance to cancer therapy (Fulda, 2009; Garg et al., 2018). Therefore, exploring the molecular components of apoptotic pathways is crucial when developing therapeutic targets and decreasing resistance during cancer treatment (Gerl and Vaux, 2005; Giménez-Bonafé et al., 2009).
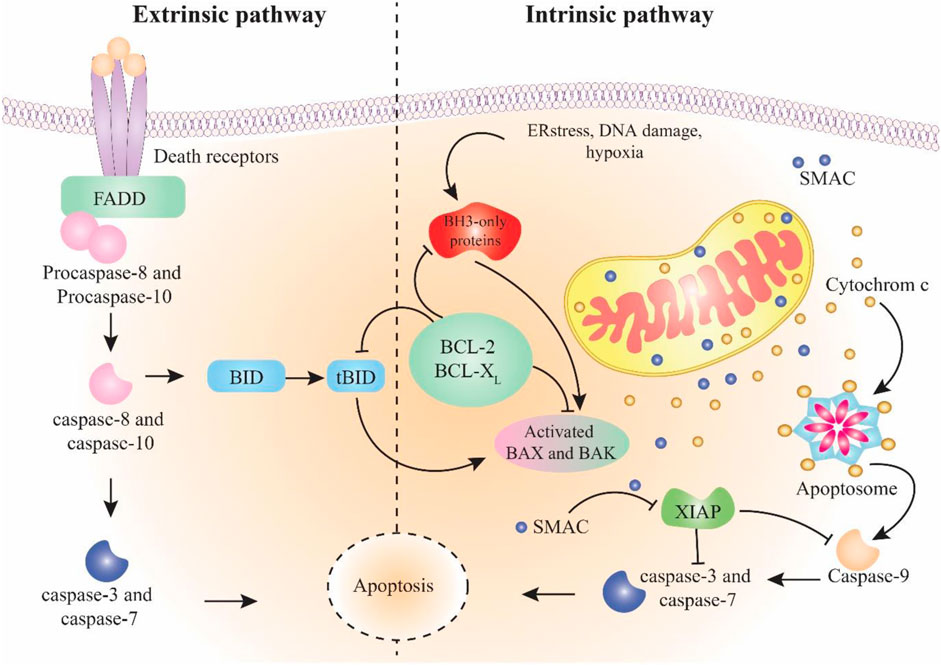
FIGURE 1. Schema of intrinsic and extrinsic pathways of apoptosis. The extrinsic pathway starts with the stimulation of death receptors followed by activation of caspase-8 and -10. The intrinsic pathway initiates in response to cell damage and continues by activation of caspase-9. Both pathways join in caspase-3 activation, which results in apoptosis.
The apoptotic mechanism occurs in two main pathways: intrinsic and extrinsic (Wong, 2011). Less known pathways include the apoptosis-like activation of the endoplasmic reticulum pathway (Szegezdi et al., 2006) and the perforin/granzyme pathway involved by granzyme A (GzmA) and granzyme B (GzmB) (Osińska et al., 2014). Eventually, these pathways enter into the common execution pathway of apoptosis (Kist and Vucic, 2021). The extrinsic apoptosis pathway begins with the external activation of death ligands binding to death receptors. Death receptors like tumour necrosis factor receptors (TNFR), Fas receptors, and TNF-related apoptosis-inducing ligand receptors (TRAILR) are found on the surface of cells (Contassot et al., 2007). Fas-associated death domain (FADD) and TNF receptor-associated death domain (TRADD) are two examples of intracellular domains used by death receptors (Boatright and Salvesen, 2003). Coherence of death ligands to death receptors (TNFα/TNFR1, (CD95, APO-1)/FasL, and TRAIL/TRAILR (DR5)) leads to revealing the death domain (DD) and binding to cell adaptors, which results in the fabrication of death-inducing signalling complex (DISC) (Seyrek et al., 2020). DISC begins the caspase-8 activation (member of the cysteine-protease family (Fritsch et al., 2019)). Finally, the activated caspase-8 enzyme starts the caspase cleavage and activates caspase-3 and inducts apoptosis through the execution pathway (Stennicke et al., 1998). The intrinsic pathway begins when the cell goes under stimulation, such as hypoxia, oxidative stress, genetic defects, or hyperaccumulation of intracellular Ca2+ (Kroemer et al., 2007). These provocations lead to disrupting the balance among proapoptotic proteins (like Bax, Bcl-Xs, and Bak) as well as antiapoptotic proteins (like Bcl-XL and Bcl-2) of the Bcl-2 family that are present in the mitochondrial membrane (García-Sáez, 2012). The predominance of proapoptotic protein impact alters the mitochondrial membrane outer membrane permeability (MOMP) (Dadsena et al., 2021). These modifications allow the mitochondrial inter-membrane gap to transfer cytochrome C into the cytoplasm and, in combination with Apaf-1 and caspase-9, develop a compound called apoptosome that continues the process through the caspase-3 activation and entrance in the execution pathway (Dorstyn et al., 2018). Additionally, the direct inhibitor of apoptosis proteins binding protein with low pI (DIABLO) and the second mitochondria-derived activator of caspase (Smac) are other components released from the perturbed mitochondrial membrane that link to the inhibitor of apoptosis proteins (IAP) and result in caspase’s separation to IAP. This mechanism causes the caspase-9 and -3 activations (Chai et al., 2000; Derakhshan et al., 2017). The common pathway is the last part of apoptosis that initiates via caspase-3 activation. Caspase-3 is responsible for the cleavage of proteins that are responsible for cytoskeletal construction and DNA repair. As a result, nuclear apoptosis and defects in signalling pathways occur, which eventually result in the cell becoming fragmented (Wong, 2011).
Alterations in every component of the mechanisms discussed previously can be associated with the reduction of apoptosis and resistance in cancer cells and the promotion of carcinogenesis. Pathological alterations defined include the death receptor signalling defect (Müschen et al., 2002), downregulation of caspase (Shen et al., 2010), variation in balance among proapoptotic and antiapoptotic proteins (Krajewska et al., 1996), and the loss of function of p53 because of gene mutations (Shen et al., 2010).
Melatonin plays a crucial role in different biological pathways in mammals. Because of its high lipid and water solubility, it can cross all layers of a cell and the cell’s nucleus and acts as a chemical mediator. Melatonin functions as a hormone, synchronizing the circadian rhythms of many organs and their functions (Alonso-Vale et al., 2008). Melatonin functionality can be classified into two major categories: receptor-mediated and non-receptor-mediated actions (Amaral et al., 2019). The latter can be defined as an interaction between melatonin and intracellular molecules, such as the antioxidative effects and scavenging reactive oxygen species (ROS). The double bonding in the carbon atoms of the melatonin structure gives it a high reduction ability, making it a powerful antioxidative agent. By directly scavenging free radicals or by subtly activating endogenous antioxidants, melatonin may reduce the effects of oxidative stress (Tiwari et al., 2021). Another example is the contact with enzymes like quinone reductase 2 and especially, type II calcium-calmodulin kinase, which melatonin directly inhibits. Through its impact on MT1 and MT2 receptors, melatonin synchronizes circadian rhythms, namely, the sleep–wake cycle and body temperature cycles (Liu et al., 2016). Furthermore, melatonin acts on MT3 receptors with several physiological activities, including the detoxification of free radicals along with antioxidant effects that protect the brain from oxidative stress (Hardeland, 2018). Melatonin exhibits both direct and indirect immunomodulatory effects, making it a potential pro- or anti-inflammatory agent in various conditions. Melatonin could stimulate the production of inflammatory cytokines and other inflammatory factors. Melatonin increases T-helper immune responses. Melatonin may act as an indirect immunoregulatory agent by decreasing nitric oxide formation, which eases the decrease of the inflammatory response. Many studies have shown the oncostatic property of melatonin in cancers such as colon, prostate, ovarian, oral, gastric, and breast cancers (Li et al., 2017a). The anticancer impacts of melatonin were observed to be mediated via various mechanisms, especially via antiapoptotic, antioxidative, and immunomodulatory signalling pathways, which all play an essential part in the growth and evolution of tumours (Reiter et al., 2018; Bhattacharya et al., 2019).
Melatonin’s mechanism of action in inducing apoptosis has been examined in different types of cancer. These studies have consistently demonstrated that melatonin exerts its effects by suppressing cell proliferation and inducing apoptosis through the regulation of crucial signalling pathways involved in the apoptotic cascade (Majidinia et al., 2017).
It has been revealed that melatonin can exert a prooxidant effect on tumour cell lines, as evidenced by its ability to stimulate intracellular ROS production in human promyelocytic leukaemia cells. This prooxidant activity of melatonin has been associated with cytotoxic and proapoptotic outcomes. The significant increase in intracellular ROS production serves as a potential mechanism that could facilitate the apoptotic death of tumour cells (Bejarano et al., 2011). Melatonin’s antioxidant effects are the consequence of several intricate mechanisms, such as the activation of antioxidant enzymes and the prooxidant enzymes’ suppression, the inhibition of mitochondrial radical production, and the scavenging of free radicals (Hardeland et al., 2003).
Most of the positive outcomes associated with the administration of melatonin can be attributed to its impact on mitochondrial physiology (Acuna-Castroviejo et al., 2007; Acuña Castroviejo et al., 2011). Studies have shown that melatonin acts upon mitochondria and regulates the mitochondrial permeability transition pore (mPTP). The fabrication of mPTP results from high concentrations of Ca2+ or due to oxidative stress; it is also regarded as the first apoptosis stage (Halestrap, 2005). Ca2+ causes the pores to open, which depolarizes the inner membrane, interrupts the phosphorylation and respiration procedures, and causes the mitochondria to enlarge. This process also triggers the release of proapoptotic factors (Halestrap, 2009).
Multiple research studies have indicated a correlation between the suppression of p53 and the avoidance of apoptosis by both malignant and pre-malignant cells, resulting in the beginning and the advancement of tumorigenesis (Brown and Wouters, 1999; Yamamoto et al., 1999). The advancement of both the intrinsic and extrinsic routes of apoptosis depends heavily on p53. It is essential for controlling FasL and triggering caspase-8 that then causes Bid activation (Malki et al., 2006). By activating Bax as a result of the translocation of Bid to the mitochondrial membrane, cytochrome c is made more accessible for release (Schuler et al., 2000; Haupt et al., 2003). In contrast, activation of p53 can potentially lead to the inhibition of Bcl-2, an antiapoptotic protein regulated by p53 and located downstream of AKT (Miyashita et al., 1994). p53 promotes the activation of the caspases and the progression of extrinsic apoptosis by suppressing IAPs (Fridman and Lowe, 2003). Alonso-González et al. demonstrated that radiation exposure promotes the activation of p53, and treatment with melatonin enhances its effects (Alonso-González et al., 2016). Both endogenous DNA damage and chemotherapy-induced DNA damage are repaired more quickly when p53 is activated (Santoro et al., 2012; Santoro et al., 2013). Melatonin is a direct phosphorylating agent of p53, according to descriptions of its actions, resulting in the stimulation of DNA repair processes (Santoro et al., 2012). Melatonin action is inhibited by blocking melatonin receptors, and that inhibition can negatively impact the p53-dependent DNA repair reaction (Santoro et al., 2013). Melatonin treatment might increase p53 activity and promote late apoptosis (Alonso-González et al., 2018).
Research has indicated that the activation of MAPKs is highly correlated with the onset and growth of several forms of cancer. JNK, ERK, and p38 are only a few of the subfamilies that control MAPK signalling, which plays a role in regulating vital processes like apoptosis and proliferation via contact with pathways like PI3K and AKT (Burotto et al., 2014; Johnson et al., 2014). Additionally, p53’s proapoptosis activity may be impacted by MAPKs (Brown and Benchimol, 2006). Recently, many studies have focused on melatonin’s dual impact on the MAPK cascades and the PI3K/AKT axis in both cancerous and healthy cells. In normal cells, melatonin activates the AKT pathway, providing neuroprotective properties. However, when melatonin is administered to cancer cells, it inhibits the same pathway (Anhe et al., 2004; Klement et al., 2012; Kim et al., 2014). Several experimental studies have demonstrated that through the suppression of MAPK genes, melatonin can cause apoptosis and lessen the resilience of some cancer cells. However, in some cancers like SGC7901 gastric cancer cells, melatonin might upregulate MAPKs, and it might prevent cell proliferation through additional signalling mechanisms (Li et al., 2017b). Additionally, melatonin might boost redox activity, causing cell death, by inhibiting NFκB and activating MAPKs (Li et al., 2016).
PI3K is essential for many kinds of cancer cells to resist apoptosis. Survivin and other apoptosis inhibitors are activated by PI3K upregulation, making anticancer therapies less effective (Asechi et al., 2010). By focusing on the PI3K pathway, melatonin helps some cancer cells undergo apoptosis and lose viability. Melatonin can decrease the expression of IAP proteins like survivin, cIAP-1, XIAP, and cIAP-2 via decreasing PI3K (Fan et al., 2013). Melatonin was able to inhibit AKT phosphorylation in LoVo and SW480 cells when they were given 5-FU and melatonin, according to the research. Additional analyses showed that PI3K-specific inhibitor LY294002 selectively inhibits PI3K, which increases the suppression of cell viability, demonstrating the critical function of this pathway in limiting the growth of tumour cells. Downregulation of the PI3K/AKT pathway in the cancer cells described is correlated with an increase in PARP and proapoptotic caspases, according to in vivo xerography research (Gao et al., 2016).
Autophagy is an intracellular process in which damaged organelles or proteins are hydrolyzed by lysosomal enzymes. This process could both have prosurvival and promortality effects on cancer cells. On one hand, it can maintain cancer cell survival by hindering the caspase-dependent apoptosis, and it can lead to programmed cell death with over-activating of autophagy (Mizushima and Komatsu, 2011; Mariño et al., 2014; Chen et al., 2016). Several studies examined the potential role of melatonin on apoptosis through modulating autophagy in tumoral cells (Mehrzadi et al., 2021). For instance, in cervical cancer, adding melatonin to HeLa cells under cisplatin treatment enhances the function of the damaged mitochondrial membrane and hinders mitophagy through blockade of the JNK/Parkin pathway. Mitophagy is a process that preserves mitochondrial quality and homeostasis by selectively removing damaged mitochondria (Tan and Wong, 2017). The effect of melatonin on mitophagy leads to increased cytochrome-c releases from the mitochondrial membrane and activation of the caspase-9-dependent apoptosis pathway (Chen et al., 2018). The role of melatonin in inducing apoptosis through modulating autophagy in leukaemia has been examined. In one potential pathway, Lomovsky et al. showed that the combination therapy of melatonin and navitoclax in acute promyelocytic leukaemia increases the endoplasmic reticulum stress and release of autophagy-related proteins like protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK). The increase in PERK levels increases the expression of C/EBP homologous protein (CHOP). The expression of CHOP is associated with activation of the apoptosis cascade by controlling antiapoptotic and proapoptotic proteins (Fernández et al., 2015). Moreover, CHOP promotes the release of Ca2+ from the endoplasmic reticulum. Changes in the Ca2+ capacities of cells affect the mitochondrial membrane permeability, which leads to ROS formation and initiation of apoptosis (Lomovsky et al., 2020a).
Acute promyelocytic leukaemia (APL) is a subtype of acute myeloid leukaemia (AML) that is different in the morphologic, clinical, and genetic aspects (Ghiaur et al., 2022). Wei et al. have illustrated a substantially lower APL cell viability when using arsenic trioxide (ATO), an approved drug for APL, together with melatonin, compared with either melatonin or ATO used separately (Wei et al., 2019).
ATO-induced apoptosis can occur through caspase signalling. Many proteins, including Bcl-2, an antiapoptotic, and Bax, a proapoptotic protein, are crucial in controlling caspase-dependent apoptosis (Wei et al., 2019). In the combination of ATO + melatonin, the caspase-9, caspase-3, and Bax expressions have been substantially increased. However, the level of prosurvival Bcl-2 proteins was lower in the ATO + melatonin group than in the ATO group. In addition, the ATO + melatonin group showed increased caspase-3 cleavage by regulating ATG-7-related autophagy. As a result, the apoptosis rate was higher while using melatonin as an adjuvant therapy combination with ATO than while using ATO alone. These findings all show that melatonin modifies autophagy to increase the cytotoxicity brought on by ATO in APL cells (Wei et al., 2019).
Mixed lineage leukaemia-rearranged (MLL-r) leukaemia occurs in 5%–10% of pediatric acute leukaemia cases with poor prognosis, relapsing, and therapeutic failure (Feng et al., 2016; Khaw et al., 2016). Human telomerase reverse transcriptase (hTERT) and COX-2 have been expressed in MLL-r leukaemia, and their expression levels have roles in tumorigenicity and poor prognosis (Calvello et al., 2018). The COX-2 signalling pathway might enhance the growth, metastasis, and invasion of cancer cells (Woo et al., 2015).
Melatonin has the potential to regulate the COX-2 and hTERT signalling pathways. RBFOX3 (RNA-binding protein fox-1 homolog 3) is one of the transcription factors located on the hTERT promoter region that regulates hTERT expression. It has been seen that melatonin significantly reduced RBFOX3’s ability to bind to the hTERT promoter and repressed the production of the hTERT protein. In addition, melatonin hindered the RBFOX3 expression in MLL-r cell lines, and, in lower RBFOX3 expression, a significant reduction in the hTERT expression was seen (Tang et al., 2019).
In many malignancies, the binding of numerous transcription factors, including NF-κB p65, to the COX-2 promoter region controls COX-2 production (Lu et al., 2016; Shrestha et al., 2017). It was observed that melatonin reduced the NF-κB p65 expression in the nucleus of the MLL-r cell lines. Moreover, melatonin prevented NF-κB p65 from attaching to the COX-2 promoter. As a result, melatonin could inhibit the NF-κB p65/COX-2 signalling pathway in the MLL-r cell lines. Furthermore, melatonin has increased caspase-9 and caspase-3 activities in the MLL-r cell lines. Therefore, melatonin could induce leukemic cell apoptosis by enhancing the caspase-dependent apoptotic pathway. Tang et al. compared the melatonin-treated mice group with the control group and stated that melatonin reduces the expression of the proteins such as RBFOX3, COX-2, and hTERT and lowers the growth of cancer cells in vivo (Tang et al., 2019).
Acute myeloid leukaemia (AML) is characterized by the most prevalent genetic alteration, the type III receptor Fms-like tyrosine kinase 3 (FLT3) mutation, which affects around one-third of AML patients (Kennedy and Smith, 2020). Despite it being a renowned antioxidant, some cancer cells are also made to produce ROS by melatonin (Florido et al., 2022). Overproduction of ROS causes mitochondrial malfunction, which eventually results in the death of cancer cells (Chen et al., 2020). It has been discovered that melatonin caused FLT3/ITD human leukaemia cells to undergo apoptosis via transferring cytochrome c from the mitochondria into the cytosol. As was mentioned in earlier studies, mitochondrial cytochrome c is a key molecule that causes apoptosis in cells (Tian et al., 2019; Xiang et al., 2019).
Sorafenib is a treatment strategy that has the potential to kill leukaemia blasts with FLT3/ITD (Bae et al., 2022). Sorafenib exerts its effect by blocking tyrosine receptor signalling and inducing ROS in tumour cells (Wan et al., 2013). When melatonin and sorafenib are used together, more cytochrome c is transferred from mitochondria into the cytosol and more intracellular ROS are accumulated than when either drug is used alone. Melatonin thus increases sorafenib’s anticancer effects in FLT3/ITD cells. According to Tian et al., melatonin and sorafenib together showed much stronger proapoptosis and antiproliferation actions than either drug alone, in vitro and in vivo. Melatonin and sorafenib together led to greater leukaemia cell depletion in peripheral blood smears and greater reductions in spleen and liver weight in mice as compared to mice treated with sorafenib alone (Tian et al., 2019).
5′-Adenosine monophosphate-activated kinase (AMPK) is a cellular enzyme that regulates energy homeostasis (Dengler, 2020). It was stated in numerous studies that activation of this enzyme could regulate apoptosis. Thereby, the therapeutic target AMPK for cancer may be worth considering (Jiang et al., 2020; Dehnavi et al., 2021). The combination of melatonin with puromycin, an unselective anticancer drug, increased AMPK’s phosphorylation in HL-60 human APL cells. As a result, melatonin enhanced puromycin-induced apoptosis by raising the AMPK activation in HL-60 cells. In addition, this resulted in an increase in caspase-3 activation and the cleavage of poly ADP-ribose polymerase (PARP), both of which are crucial for HL-60 cells to undergo apoptosis (Koh et al., 2011). DNA strand nicks and breaks stimulate PARP activation. In minimal DNA damage, PARP is a survival factor that causes DNA repair. In a high-DNA damage condition, PARP induces apoptosis (Cherian et al., 2008). In addition to the caspase-3 activation, melatonin synergistically hindered the expression of the antiapoptotic proteins Bcl-xL and Bcl-2 (Koh et al., 2011).
Melatonin was observed to cause apoptosis in HL-60 cells by depolarizing the mitochondrial membrane, inducing permeability transition pores, and activating caspase-9 and -3. Cytochrome c was transferred into the cytosol as a result of permeability transition pore and mitochondrial membrane depolarization stimulation. Additionally, melatonin may greatly increase the expression of proapoptotic proteins like Bax and Bid in the leukaemia HL-60 cell lines (Bejarano et al., 2009).
Cytarabine is an antitumor drug that is utilized in APL therapy, non-Hodgkin lymphoma (NHL), and chronic myelocytic lymphoma (CML) (Liao et al., 2021). Lomovsky et al. demonstrated that melatonin substantially increased the effects of cytarabine when they were used together. Using cytarabine and melatonin together could reduce the mitotic index (MI) and the level of Bcl-2 more than using cytarabine alone (Lomovsky et al., 2020b).
An important factor for the beginning of apoptosis is the formation of the mitochondrial permeability transition pore (mPTP) in the inner mitochondrial membrane. After the mPTP is formed, the outer mitochondrial membrane becomes more permeable, causing the mitochondria to enlarge and release cytochrome c into the cytosol from the inter-membrane gap (Lee and Lee, 2018; Aslam et al., 2021). The voltage-dependent anion channel (VDAC) and the translocator protein (TSPO) are factors that play a role in mPTP regulation (Bayrhuber et al., 2008; Lee et al., 2020). According to reports, the quantity of VDAC is said to increase in certain tumours, making it a potential target for anticancer treatments (Mathupala and Pedersen, 2010). In addition, it has been seen that the level of TSPO increased in many tumours and played a substantial role in the development of cancer cells. Therefore, TSPO expression could be considered a tumorigenicity agent (Veenman et al., 2004; Wu and Gallo, 2013; Shehadeh et al., 2019).
It is reported that low (10–6–10–8 M) concentrations of melatonin increased intracellular glutathione, which is an antioxidant agent in the cells, and ultimately enhanced cell viability. In contrast, in high (10–3–10–4 M) concentrations, melatonin would induce the production of intracellular ROS and the reduction of glutathione, which contribute to apoptosis induction in the leukaemia CMK, HL-60, Jurkat, and MOLT-4 cell lines (Büyükavcı et al., 2006; Büyükavci et al., 2011; Estaras et al., 2021).
Radiotherapy irradiations influence both tumour cells and normal cells via ROS production induction and DNA damage that leads to apoptosis (Qin et al., 2021). When exposed to radiation, the tumour suppressor gene p53 is activated, which causes apoptosis (Sisakht et al., 2020). Activated p53 induces cell cycle arrest to give damaged DNA a repair opportunity or promotes apoptosis to prevent the propagation of cells with damaged DNA (Feroz and Sheikh, 2020). Jang et al. compared the radiation-induced apoptosis in mouse normal and leukaemia cells treated with melatonin with control groups. They observed that in the irradiated normal cells that were incubated with melatonin, the expression of p53 was reduced; in contrast, Bcl-2 expression increased compared with control groups. As a result, melatonin had the potential ability to reduce radiation-induced apoptosis in normal cells. In contrast, they found different results in the leukaemia cells. Melatonin enhanced the expression of the p53 protein in the melatonin-treated, irradiated-leukaemia cells compared to controls. Therefore, melatonin increased radiation-induced apoptosis in leukaemia cells, unlike normal cells (Jang et al., 2009).
Perdomo et al. evaluated the probable potential of melatonin in apoptosis of Molt-3 leukaemia cells, and interestingly, they found that melatonin significantly enhances the activation of caspase-3, -6, -7, and -9 but only found slight enhancement in caspase-8. Therefore, they suggested that melatonin induced apoptosis by the intrinsic pathway in Molt-3 cells. As the Bcl-2 protein family is an important agent to control the intrinsic pathway via mitochondria outer membrane permeability control, they evaluated this family and realized that melatonin induced the upregulation of proapoptotic factor Bax while it reduced Bcl-2 and Bcl-XL, two prosurvival proteins that are expressed contrarily. As a result, melatonin increased the Bax/Bcl-2 ratio that was contributing to apoptosis induction (Perdomo et al., 2013). Collectively, the summarized information shows that melatonin alone and/or together with chemotherapeutic agents can induce apoptosis in leukaemia through different signalling pathways (Figure 2).
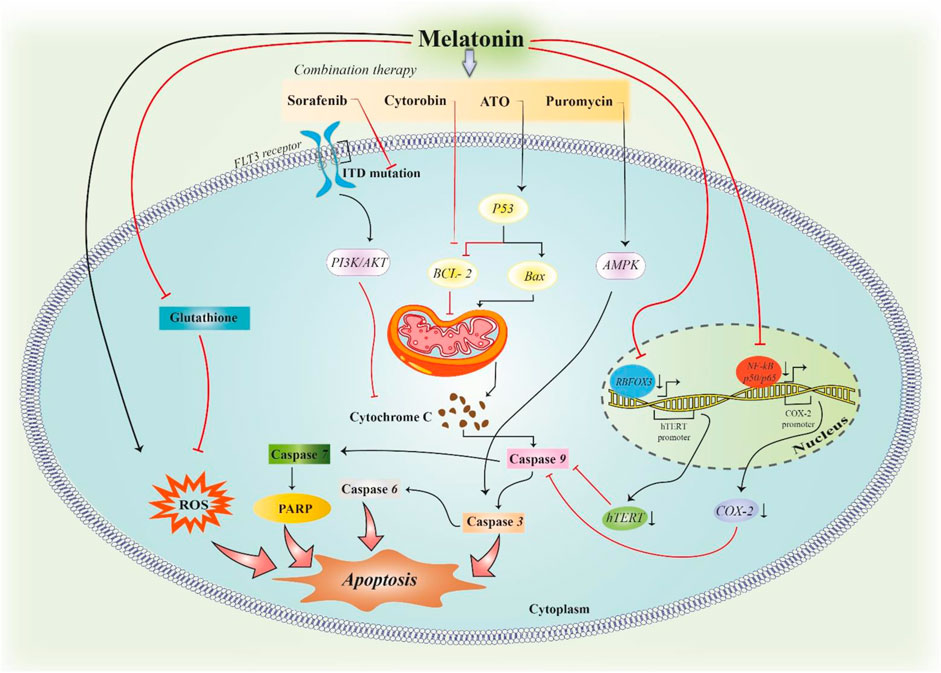
FIGURE 2. Induction of apoptosis in leukaemia by melatonin. Melatonin can induce apoptosis in leukaemic cells by inducing ROS formation. In addition, melatonin can downregulate the expression of Cox-2 and hTERT genes, which leads to hindering the inhibitory effect of these two proteins on the caspase-dependent pathway of apoptosis. The combination therapy of melatonin with antitumour drugs in leukaemia can synergize the induction of apoptosis in tumoral cells. Each pathway modulates the function of proteins in the waterfall of apoptosis.
Studies demonstrated that melatonin has a dose-dependent cytotoxicity potential. Zhelev et al. evaluated the leukaemia cytotoxic feature of several anticancer drugs in solo applications compared to melatonin additive usage. It has been found that melatonin has a greater cytotoxic capacity to kill leukaemia cells when combined with anticancer medications, including everolimus, lonafarnib, palbociclib, MG-132, bortezomib, and barasertib, than when these medications were used separately (Lee et al., 2013).
APL is principally defined by the chromosomal translocation of the gene of the retinoic acid receptor-alpha (RARA) (Mannan et al., 2020). Retinoic acid is mainly used in the treatment of APL, but it has adverse effects on the human body (Afacan Ozturk et al., 2021; Zhang et al., 2022). The simultaneous use of melatonin with retinoic acid enhances its effects on HL-60. Therefore, a lower dose of retinoic acid is needed in the combination therapy than the current common therapeutic dose; subsequently, its side effects are reduced (Krestinina et al., 2018). According to a study conducted by Krestinina et al., the combined impact of melatonin and retinoic acid with a much lower content than that used for retinoic acid alone previously has similar effects on the reduction of the antiapoptotic Bcl-2 protein and VDAC expressions. It also caused a further decline in TSPO expression and, thus, tumorigenicity suppression (Krestinina et al., 2018).
Everolimus is an immunosuppressant drug that also possesses anticancer activity through inhibition of the mammalian target of the rapamycin (mTOR) pathway that is important for controlling cell viability, translation start, and cell cycle progression (Ramírez-Valle et al., 2010; Zhu et al., 2020). Everolimus had a slight cytotoxic effect on leukaemia lymphocytes through the induction of apoptosis. It has been observed that melatonin enhanced the cytotoxic effect of everolimus by exhibiting a strong induction of apoptosis, although not on normal lymphocytes. This apoptosis was not dependent on ROS production because the level of ROS was reduced when melatonin was added to everolimus compared to the use of everolimus alone (Zhelev et al., 2017). It might occur because of the downregulation of mTOR expression and other oncogenes due to melatonin (Lee et al., 2013).
Based on the Buyukavci et al. study, melatonin did not enhance the cytotoxic effects of antileukaemia medications like cytarabine, etoposide, and daunorubicin in leukaemia cells in vitro. However, melatonin has protective potential on normal cells against chemotherapeutic drugs in vitro (Büyükavci et al.). For instance, melatonin could reduce the cardiac cytotoxicity side effect of daunorubicin by its indirect antioxidant activity (Zare et al., 2021).
When combined with chemotherapy and radiotherapy, hyperthermia is a powerful approach to treating cancer. Hyperthermia has the potential to decrease cell growth and cause alterations in the nuclei of the cells that are exposed to it, which leads to apoptosis. (Kang et al., 2020; Zastko et al., 2021). Melatonin could enhance the number of apoptotic cells when this hormone is added to hyperthermia treatment, which is used on both U937 and HL-60 AML cell lines. Interestingly, the combination of melatonin with hyperthermia increased the caspase-9, -8, -3, and caspase-2 activation in the caspase-dependent pathway apoptosis and release of cytochrome c to the cytosol more than the use of hyperthermia alone (Quintana et al., 2016).
Various studies have proven that melatonin has a wide range of positive effects in the treatment of leukaemia by influencing several cellular and molecular pathways in leukaemia cells. Melatonin also improves the effects of other chemotherapy medications in combination. Melatonin’s proapoptotic and prooxidant characteristics, together with its powerful influence on DNA damage, are its key therapeutic benefits in the treatment of leukaemia. Melatonin can decrease DNA damage, improve DNA repair, and promote antioxidant enzymes in normal cells and tissues, particularly chemo/radiosensitive cells like bone marrow and lymphocytes. This may lessen apoptosis and improve tissue tolerance. Antiapoptotic genes like NF-B and Bcl-2 are often highly expressed in cancer cells, and, in contrast, p53 function may be reduced. Through the upregulation and activation of proapoptotic molecules, including p53 and Bax, melatonin plays a part in the control of cancer cell death. Melatonin plays a critical part in boosting ROS formation through mitochondria in different cancer cells. When used with different chemotherapeutic drugs, melatonin was indicated to increase superoxide and depolarize the mitochondrial membrane. An increase in the rate of mitophagy after melatonin administration has been proposed as an indicator for mitochondrial impairment and cell death. Melatonin has been found to enhance apoptosis via suppressing mitophagy in some cancerous cells. Overall, one of the most powerful properties of melatonin that can increase the sensitivity of tumour cells to treatment methods like chemotherapy and radiotherapy appears to be the regulation of apoptotic signalling pathways.
HM involved in conception, design, statistical analysis and drafting of the manuscript. AM, HRR, YGH, MMM, VM, SAH, YEM, RJR, BGH, MR, AS, FZ, ZA, MAM contributed in involved in the conception, interpretation of data, drafting and critically revised manuscript. This author approved the final version of manuscript. Also, they agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acuña Castroviejo, D., López, L. C., Escames, G., López, A., García, J. A., and Reiter, R. J. (2011). Melatonin-mitochondria interplay in health and disease. Curr. Top. Med. Chem. 11 (2), 221–240. doi:10.2174/156802611794863517
Acuna-Castroviejo, D., Escames, G., Rodriguez, M. I., and Lopez, L. C. (2007). Melatonin role in the mitochondrial function. Front. Biosci. 12, 947–963. doi:10.2741/2116
Acuña-Castroviejo, D., Escames, G., Venegas, C., Díaz-Casado, M. E., Lima-Cabello, E., López, L. C., et al. (2014). Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol. Life Sci. 71 (16), 2997–3025. doi:10.1007/s00018-014-1579-2
Afacan Ozturk, H. B., Albayrak, M., Maral, S., Reis Aras, M., Yilmaz, F., Akyol, P., et al. (2021). Hypercalcemia associated with the interaction between all trans retinoic acid and posaconazole in an acute promyelocytic leukemia case. J. Oncol. Pharm. Pract. 27 (8), 2027–2029. doi:10.1177/10781552211007889
Alonso-González, C., González, A., Martínez-Campa, C., Menéndez-Menéndez, J., Gómez-Arozamena, J., García-Vidal, A., et al. (2016). Melatonin enhancement of the radiosensitivity of human breast cancer cells is associated with the modulation of proteins involved in estrogen biosynthesis. Cancer Lett. 370 (1), 145–152. doi:10.1016/j.canlet.2015.10.015
Alonso-González, C., Menéndez-Menéndez, J., González-González, A., González, A., Cos, S., and Martínez-Campa, C. (2018). Melatonin enhances the apoptotic effects and modulates the changes in gene expression induced by docetaxel in MCF-7 human breast cancer cells. Int. J. Oncol. 52 (2), 560–570. doi:10.3892/ijo.2017.4213
Alonso-Vale, M. I. C., Andreotti, S., Mukai, P. Y., Borges-Silva, C. N., Peres, S. B., Cipolla-Neto, J., et al. (2008). Melatonin and the circadian entrainment of metabolic and hormonal activities in primary isolated adipocytes. J. pineal Res. 45 (4), 422–429. doi:10.1111/j.1600-079X.2008.00610.x
Amaral, F. G. D., Andrade-Silva, J., Kuwabara, W. M. T., and Cipolla-Neto, J. (2019). New insights into the function of melatonin and its role in metabolic disturbances. Expert Rev. Endocrinol. Metabolism 14 (4), 293–300. doi:10.1080/17446651.2019.1631158
Anhe, G., Caperuto, L., Pereira-Da-Silva, M., Souza, L., Hirata, A., Velloso, L., et al. (2004). In vivo activation of insulin receptor tyrosine kinase by melatonin in the rat hypothalamus. J. Neurochem. 90, 559–566. doi:10.1111/j.1471-4159.2004.02514.x
Asechi, H., Hatano, E., Nitta, T., Tada, M., Iwaisako, K., Tamaki, N., et al. (2010). Resistance to cisplatin-induced apoptosis via PI3K-dependent survivin expression in a rat hepatoma cell line. Int. J. Oncol. 37, 89–96.
Aslam, M., Kanthlal, S., and Panonummal, R. (2021). Peptides: a supercilious candidate for activating intrinsic apoptosis by targeting mitochondrial membrane permeability for cancer therapy. Int. J. Peptide Res. Ther. 27, 2883–2893. doi:10.1007/s10989-021-10297-7
Bae, K. H., Lai, F., Mong, J., Niibori-Nambu, A., Chan, K. H., Her, Z., et al. (2022). Bone marrow-targetable green tea catechin-based micellar nanocomplex for synergistic therapy of acute myeloid leukemia. J. Nanobiotechnology 20 (1), 481–518. doi:10.1186/s12951-022-01683-4
Bárcenas-López, D. A., Mendiola-Soto, D. K., Núñez-Enríquez, J. C., Mejía-Aranguré, J. M., Hidalgo-Miranda, A., and Jiménez-Morales, S. (2021). Promising genes and variants to reduce chemotherapy adverse effects in acute lymphoblastic leukemia. Transl. Oncol. 14 (1), 100978. doi:10.1016/j.tranon.2020.100978
Bayrhuber, M., Meins, T., Habeck, M., Becker, S., Giller, K., Villinger, S., et al. (2008). Structure of the human voltage-dependent anion channel. Proc. Natl. Acad. Sci. 105 (40), 15370–15375. doi:10.1073/pnas.0808115105
Bejarano, I., Espino, J., Barriga, C., Reiter, R. J., Pariente, J. A., and Rodríguez, A. B. (2011). Pro-oxidant effect of melatonin in tumour leucocytes: relation with its cytotoxic and pro-apoptotic effects. Basic Clin. Pharmacol. Toxicol. 108 (1), 14–20. doi:10.1111/j.1742-7843.2010.00619.x
Bejarano, I., Redondo, P. C., Espino, J., Rosado, J. A., Paredes, S. D., Barriga, C., et al. (2009). Melatonin induces mitochondrial-mediated apoptosis in human myeloid HL-60 cells. J. pineal Res. 46 (4), 392–400. doi:10.1111/j.1600-079X.2009.00675.x
Bertheloot, D., Latz, E., and Franklin, B. S. (2021). Necroptosis, pyroptosis and apoptosis: an intricate game of cell death. Cell Mol. Immunol. 18 (5), 1106–1121. doi:10.1038/s41423-020-00630-3
Bhattacharya, S., Patel, K. K., Dehari, D., Agrawal, A. K., and Singh, S. (2019). Melatonin and its ubiquitous anticancer effects. Mol. Cell. Biochem. 462, 133–155. doi:10.1007/s11010-019-03617-5
Bispo, J. A. B., Pinheiro, P. S., and Kobetz, E. K. (2020). Epidemiology and etiology of leukemia and lymphoma. Cold Spring Harb. Perspect. Med. 10 (6), a034819. doi:10.1101/cshperspect.a034819
Boatright, K. M., and Salvesen, G. S. (2003). Mechanisms of caspase activation. Curr. Opin. Cell Biol. 15 (6), 725–731. doi:10.1016/j.ceb.2003.10.009
Brown, J. M., and Wouters, B. G. (1999). Apoptosis, p53, and tumor cell sensitivity to anticancer agents. Cancer Res. 59 (7), 1391–1399.
Brown, L., and Benchimol, S. (2006). The involvement of MAPK signaling pathways in determining the cellular response to p53 activation: cell cycle arrest or apoptosis. J. Biol. Chem. 281 (7), 3832–3840. doi:10.1074/jbc.M507951200
Burotto, M., Chiou, V. L., Lee, J. M., and Kohn, E. C. (2014). The MAPK pathway across different malignancies: a new perspective. Cancer 120 (22), 3446–3456. doi:10.1002/cncr.28864
Büyükavci, M., Özdemir, Ö., Buck, S., Ravindranath, Y., and Savaşan, S. (2011). Effect of melatonin on the cytotoxicity of chemotherapeutic drugs in human leukemia cells. vivo 25 (3), 405–409.
Büyükavcı, M., Özdemir, Ö., Buck, S., Stout, M., Ravindranath, Y., and Savaşan, S. (2006). Melatonin cytotoxicity in human leukemia cells: relation with its pro-oxidant effect. Fundam. Clin. Pharmacol. 20 (1), 73–79. doi:10.1111/j.1472-8206.2005.00389.x
Calvello, C., Rocca, B., Klersy, C., Zappatore, R., Giardini, I., Dambruoso, I., et al. (2018). Alternative splicing of hTERT: a further mechanism for the control of active hTERT in acute myeloid leukemia. Leukemia lymphoma 59 (3), 702–709. doi:10.1080/10428194.2017.1346252
Carlomagno, G., Minini, M., Tilotta, M., and Unfer, V. (2018). From implantation to birth: insight into molecular melatonin functions. Int. J. Mol. Sci. 19 (9), 2802. doi:10.3390/ijms19092802
Casado-Zapico, S., Martín, V., García-Santos, G., Rodríguez-Blanco, J., Sánchez-Sánchez, A. M., Luño, E., et al. (2011). Regulation of the expression of death receptors and their ligands by melatonin in haematological cancer cell lines and in leukaemia cells from patients. J. Pineal Res. 50 (3), 345–355. doi:10.1111/j.1600-079X.2010.00850.x
Chai, J., Du, C., Wu, J. W., Kyin, S., Wang, X., and Shi, Y. (2000). Structural and biochemical basis of apoptotic activation by Smac/DIABLO. Nature 406 (6798), 855–862. doi:10.1038/35022514
Chen, L., Liu, L., Li, Y., and Gao, J. (2018). Melatonin increases human cervical cancer HeLa cells apoptosis induced by cisplatin via inhibition of JNK/Parkin/mitophagy axis. Vitro Cell Dev. Biol. Anim. 54 (1), 1–10. doi:10.1007/s11626-017-0200-z
Chen, Y., Henson, E. S., Xiao, W., Huang, D., McMillan-Ward, E. M., Israels, S. J., et al. (2016). Tyrosine kinase receptor EGFR regulates the switch in cancer cells between cell survival and cell death induced by autophagy in hypoxia. Autophagy 12 (6), 1029–1046. doi:10.1080/15548627.2016.1164357
Chen, Y-C., Wang, P-Y., Huang, B-M., Chen, Y-J., Lee, W-C., and Chen, Y-C. (2020). 16-Hydroxycleroda-3, 13-dien-15, 16-olide induces apoptosis in human bladder cancer cells through cell cycle arrest, mitochondria ROS overproduction, and inactivation of EGFR-related signalling pathways. Molecules 25 (17), 3958. doi:10.3390/molecules25173958
Cherian, P. P., Schenker, S., and Henderson, G. I. (2008). Ethanol-mediated DNA damage and PARP-1 apoptotic responses in cultured fetal cortical neurons. Alcohol. Clin. Exp. Res. 32 (11), 1884–1892. doi:10.1111/j.1530-0277.2008.00769.x
Contassot, E., Gaide, O., and French, L. E. (2007). Death receptors and apoptosis. Dermatol Clin. 25 (4), 487–501. doi:10.1016/j.det.2007.06.010
Dadsena, S., King, L. E., and García-Sáez, A. J. (2021). Apoptosis regulation at the mitochondria membrane level. Biochim. Biophys. Acta Biomembr. 1863 (12), 183716. doi:10.1016/j.bbamem.2021.183716
Dehnavi, S., Kiani, A., Sadeghi, M., Biregani, A. F., Banach, M., Atkin, S. L., et al. (2021). Targeting AMPK by statins: A potential therapeutic approach. Drugs 81 (8), 923–933. doi:10.1007/s40265-021-01510-4
Dengler, F. (2020). Activation of AMPK under hypoxia: many roads leading to rome. Int. J. Mol. Sci. 21 (7), 2428. doi:10.3390/ijms21072428
Derakhshan, A., Chen, Z., and Van Waes, C. (2017). Therapeutic small molecules target inhibitor of apoptosis proteins in cancers with deregulation of extrinsic and intrinsic cell death pathways. Clin. Cancer Res. 23 (6), 1379–1387. doi:10.1158/1078-0432.CCR-16-2172
Dorstyn, L., Akey, C. W., and Kumar, S. (2018). New insights into apoptosome structure and function. Cell Death Differ. 25 (7), 1194–1208. doi:10.1038/s41418-017-0025-z
Elmore, S. (2007). Apoptosis: A review of programmed cell death. Toxicol. Pathol. 35 (4), 495–516. doi:10.1080/01926230701320337
Estaras, M., Gonzalez-Portillo, M. R., Martinez, R., Garcia, A., Estevez, M., Fernandez-Bermejo, M., et al. (2021). Melatonin modulates the antioxidant defenses and the expression of proinflammatory mediators in pancreatic stellate cells subjected to hypoxia. Antioxidants 10 (4), 577. doi:10.3390/antiox10040577
Fan, L., Sun, G., Ma, T., Zhong, F., and Wei, W. (2013). Melatonin overcomes apoptosis resistance in human hepatocellular carcinoma by targeting Survivin and XIAP. J. pineal Res. 55, 174–183. doi:10.1111/jpi.12060
Feng, Z., Yao, Y., Zhou, C., Chen, F., Wu, F., Wei, L., et al. (2016). Pharmacological inhibition of LSD1 for the treatment of MLL-rearranged leukemia. J. Hematol. Oncol. 9 (1), 24–13. doi:10.1186/s13045-016-0252-7
Fernández, A., Ordóñez, R., Reiter, R. J., González-Gallego, J., and Mauriz, J. L. (2015). Melatonin and endoplasmic reticulum stress: relation to autophagy and apoptosis. J. Pineal Res. 59 (3), 292–307. doi:10.1111/jpi.12264
Feroz, W., and Sheikh, A. M. A. (2020). Exploring the multiple roles of guardian of the genome: P53. Egypt. J. Med. Hum. Genet. 21 (1), 49. doi:10.1186/s43042-020-00089-x
Florido, J., Rodriguez-Santana, C., Martinez-Ruiz, L., López-Rodríguez, A., Acuña-Castroviejo, D., Rusanova, I., et al. (2022). Understanding the mechanism of action of melatonin, which induces ROS production in cancer cells. Antioxidants 11 (8), 1621. doi:10.3390/antiox11081621
Fleischmann, M., Schnetzke, U., Hochhaus, A., and Scholl, S. (2021). Management of acute myeloid leukemia: current treatment options and future perspectives. Cancers (Basel) 13 (22), 5722. doi:10.3390/cancers13225722
Fridman, J. S., and Lowe, S. W. (2003). Control of apoptosis by p53. Oncogene 22 (56), 9030–9040. doi:10.1038/sj.onc.1207116
Fritsch, M., Günther, S. D., Schwarzer, R., Albert, M. C., Schorn, F., Werthenbach, J. P., et al. (2019). Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature 575 (7784), 683–687. doi:10.1038/s41586-019-1770-6
Fulda, S. (2009). Tumor resistance to apoptosis. Int. J. Cancer 124 (3), 511–515. doi:10.1002/ijc.24064
Gao, Y., Xiao, X., Zhang, C., Yu, W., Guo, W., Zhang, Z., et al. (2016). Melatonin synergizes the chemotherapeutic effect of 5-fluorouracil in colon cancer by suppressing PI3K/AKT and NF-κB/iNOS signaling pathways. J. pineal Res. 62, e12380. doi:10.1111/jpi.12380
García-Sáez, A. J. (2012). The secrets of the Bcl-2 family. Cell Death Differ. 19 (11), 1733–1740. doi:10.1038/cdd.2012.105
Garg, V. K., Kashyap, D. B., and Tuli, H. S. (2018). Targeting telomerase and topoisomerase-II by natural moieties: an anti-cancer approach. Semin. Cancer Biol. 21 (6), 349–353.
Gerl, R., and Vaux, D. L. (2005). Apoptosis in the development and treatment of cancer. Carcinogenesis 26 (2), 263–270. doi:10.1093/carcin/bgh283
Ghiaur, A., Ionescu, B., Constantin, C., Vasilache, D., Dragomir, M., and Coriu, D. (2022). AML-372 acute promyelocytic leukemia: a case of pseudotumor cerebri syndrome during treatment consolidation. Clin. Lymphoma Myeloma Leukemia 22, S241–S242. doi:10.1016/s2152-2650(22)01278-2
Giménez-Bonafé, P., Tortosa, A., and Pérez-Tomás, R. (2009). Overcoming drug resistance by enhancing apoptosis of tumor cells. Curr. Cancer Drug Targets 9 (3), 320–340. doi:10.2174/156800909788166600
Halestrap, A. (2005). Biochemistry: a pore way to die. Nature 434 (7033), 578–579. doi:10.1038/434578a
Halestrap, A. P. (2009). What is the mitochondrial permeability transition pore? J. Mol. Cell Cardiol. 46 (6), 821–831. doi:10.1016/j.yjmcc.2009.02.021
Hardeland, R., Coto-Montes, A., and Poeggeler, B. (2003). Circadian rhythms, oxidative stress, and antioxidative defense mechanisms. Chronobiol Int. 20 (6), 921–962. doi:10.1081/cbi-120025245
Hardeland, R., Pandi-Perumal, S. R., and Cardinali, D. P. (2006). Melat. Int. J. Biochem. Cell Biol. 38 (3), 313–316. doi:10.1016/j.biocel.2005.08.020
Hardeland, R. (2018). Melatonin and inflammation—story of a double-edged blade. J. Pineal Res. 65 (4), e12525. doi:10.1111/jpi.12525
Haupt, S., Berger, M., Goldberg, Z., and Haupt, Y. (2003). Apoptosis - the p53 network. J. Cell Sci. 116 (20), 4077–4085. doi:10.1242/jcs.00739
Hickie, I. B., and Rogers, N. L. (2011). Novel melatonin-based therapies: potential advances in the treatment of major depression. Lancet 378 (9791), 621–631. doi:10.1016/S0140-6736(11)60095-0
Jang, S. S., Kim, W. D., and Park, W. Y. (2009). Melatonin exerts differential actions on X-ray radiation-induced apoptosis in normal mice splenocytes and Jurkat leukemia cells. J. pineal Res. 47 (2), 147–155. doi:10.1111/j.1600-079X.2009.00694.x
Jiang, S., Shi, F., Lin, H., Ying, Y., Luo, L., Huang, D., et al. (2020). Inonotus obliquus polysaccharides induces apoptosis of lung cancer cells and alters energy metabolism via the LKB1/AMPK axis. Int. J. Biol. Macromol. 151, 1277–1286. doi:10.1016/j.ijbiomac.2019.10.174
Johnson, G., Stuhlmiller, T., Angus, S., Zawistowski, J., and Graves, L. (2014). Molecular pathways: adaptive kinome reprogramming in response to targeted inhibition of the BRAF-MEK-ERK pathway in cancer. Clin. cancer Res. 20, 2516–2522. doi:10.1158/1078-0432.CCR-13-1081
Kang, J. K., Kim, J. C., Shin, Y., Han, S. M., Won, W. R., Her, J., et al. (2020). Principles and applications of nanomaterial-based hyperthermia in cancer therapy. Archives pharmacal Res. 43 (1), 46–57. doi:10.1007/s12272-020-01206-5
Karamitri, A., and Jockers, R. (2019). Melatonin in type 2 diabetes mellitus and obesity. Nat. Rev. Endocrinol. 15 (2), 105–125. doi:10.1038/s41574-018-0130-1
Kato, M., and Manabe, A. (2018). Treatment and biology of pediatric acute lymphoblastic leukemia. Pediatr. Int. 60 (1), 4–12. doi:10.1111/ped.13457
Kennedy, V. E., and Smith, C. C. (2020). FLT3 mutations in acute myeloid leukemia: key concepts and emerging controversies. Front. Oncol. 10, 612880. doi:10.3389/fonc.2020.612880
Khaw, S. L., Suryani, S., Evans, K., Richmond, J., Robbins, A., Kurmasheva, R. T., et al. (2016). Venetoclax responses of pediatric ALL xenografts reveal sensitivity of MLL-rearranged leukemia. Blood, J. Am. Soc. Hematol. 128 (10), 1382–1395. doi:10.1182/blood-2016-03-707414
Kim, H., Kim, T-J., and Yoo, Y-M. (2014). Melatonin combined with endoplasmic reticulum stress induces cell death via the PI3K/Akt/mTOR pathway in B16F10 melanoma cells. PloS one 9, e92627. doi:10.1371/journal.pone.0092627
Kist, M., and Vucic, D. (2021). Cell death pathways: intricate connections and disease implications. Embo J. 40 (5), e106700. doi:10.15252/embj.2020106700
Klement, G. L., Goukassian, D., Hlatky, L., Carrozza, J., Morgan, J. P., and Yan, X. (2012). Cancer therapy targeting the HER2-PI3K pathway: potential impact on the heart. Front. Pharmacol. 3, 113. doi:10.3389/fphar.2012.00113
Koh, W., Jeong, S. J., Lee, H. J., Ryu, H. G., Lee, E. O., Ahn, K. S., et al. (2011). Melatonin promotes puromycin-induced apoptosis with activation of caspase-3 and 5′-adenosine monophosphate-activated kinase-alpha in human leukemia HL-60 cells. J. pineal Res. 50 (4), 367–373. doi:10.1111/j.1600-079X.2010.00852.x
Krajewska, M., Moss, S. F., Krajewski, S., Song, K., Holt, P. R., and Reed, J. C. (1996). Elevated expression of Bcl-X and reduced Bak in primary colorectal adenocarcinomas. Cancer Res. 56 (10), 2422–2427.
Krestinina, O., Fadeev, R., Lomovsky, A., Baburina, Y., Kobyakova, M., and Akatov, V. (2018). Melatonin can strengthen the effect of retinoic acid in HL-60 cells. Int. J. Mol. Sci. 19 (10), 2873. doi:10.3390/ijms19102873
Kroemer, G., Galluzzi, L., and Brenner, C. (2007). Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 87 (1), 99–163. doi:10.1152/physrev.00013.2006
Kubatka, P., Zubor, P., Busselberg, D., Kwon, T. K., Adamek, M., Petrovic, D., et al. (2018). Melatonin and breast cancer: evidences from preclinical and human studies. Crit. Rev. Oncol. Hematol. 122, 133–143. doi:10.1016/j.critrevonc.2017.12.018
Lee, J. W., and Cho, B. (2017). Prognostic factors and treatment of pediatric acute lymphoblastic leukemia. Korean J. Pediatr. 60 (5), 129–137. doi:10.3345/kjp.2017.60.5.129
Lee, S. E., Kim, S. J., Yoon, H. J., Yu, S. Y., Yang, H., Jeong, S. I., et al. (2013). Genome-wide profiling in melatonin-exposed human breast cancer cell lines identifies differentially methylated genes involved in the anticancer effect of melatonin. J. pineal Res. 54 (1), 80–88. doi:10.1111/j.1600-079X.2012.01027.x
Lee, Y., Park, Y., Nam, H., Lee, J-W., and Yu, S-W. (2020). Translocator protein (TSPO): the new story of the old protein in neuroinflammation. BMB Rep. 53 (1), 20–27. doi:10.5483/BMBRep.2020.53.1.273
Lee, Y. J., and Lee, C. (2018). Porcine deltacoronavirus induces caspase-dependent apoptosis through activation of the cytochrome c-mediated intrinsic mitochondrial pathway. Virus Res. 253, 112–123. doi:10.1016/j.virusres.2018.06.008
Lerner, A. B., Case, J. D., Takahashi, Y., Lee, T. H., and Mori, W. (1958). Isolation of melatonin, the pineal gland factor that lightens melanocyteS1. J. Am. Chem. Soc. 80 (10), 2587. doi:10.1021/ja01543a060
Li, W., Wang, Z., Chen, Y., Wang, K., Lu, T., Ying, F., et al. (2017b). Melatonin treatment induces apoptosis through regulating the nuclear factor-κB and mitogen-activated protein kinase signaling pathways in human gastric cancer SGC7901 cells. Oncol. Lett. 13 (4), 2737–2744. doi:10.3892/ol.2017.5785
Li, W., Wu, J., Li, Z., Zhou, Z., Zheng, C., Lin, L., et al. (2016). Melatonin induces cell apoptosis in mia PaCa-2 cells via the suppression of nuclear factor-κB and activation of ERK and JNK: a novel therapeutic implication for pancreatic cancer. Oncol. Rep. 36, 2861–2867. doi:10.3892/or.2016.5100
Li, W. (2022). “The 5(th) edition of the World health organization classification of hematolymphoid tumors,” in Leukemia. Editor W. Li (Brisbane: Exon Publications).
Li, W., Li, S., Zhou, Y., Meng, X., Zhang, J-J., Xu, D-P., et al. (2017a). Melatonin for the prevention and treatment of cancer. Oncotarget 8 (24), 39896–39921. doi:10.18632/oncotarget.16379
Liao, A-M., Zhang, Y., Hou, Y., Huang, J-H., Hui, M., Lee, K-K., et al. (2021). Preparation, characterization, and cytotoxicity evaluation of self-assembled nanoparticles of diosgenin-cytarabine conjugate. Food Chem. Toxicol. 151, 112101. doi:10.1016/j.fct.2021.112101
Liu, J., Clough, S. J., Hutchinson, A. J., Adamah-Biassi, E. B., Popovska-Gorevski, M., and Dubocovich, M. L. (2016). MT1 and MT2 melatonin receptors: a therapeutic perspective. Annu. Rev. Pharmacol. Toxicol. 56, 361–383. doi:10.1146/annurev-pharmtox-010814-124742
Lomovsky, A., Baburina, Y. L., Kobyakova, M., Fadeev, R., Akatov, V., and Krestinina, O. (2020b). Melatonin enhances the chemotherapeutic effect of cytarabin in HL-60 cells. Biochem. Mosc. Suppl. Ser. A Membr. Cell Biol. 14 (2), 140–145. doi:10.1134/s1990747819060072
Lomovsky, A., Baburina, Y., Odinokova, I., Kobyakova, M., Evstratova, Y., Sotnikova, L., et al. (2020a). Melatonin can modulate the effect of navitoclax (ABT-737) in HL-60 cells. Antioxidants (Basel) 9 (11), 1143. doi:10.3390/antiox9111143
Lu, J-J., Fu, L., Tang, Z., Zhang, C., Qin, L., Wang, J., et al. (2016). Melatonin inhibits AP-2β/hTERT, NF-κB/COX-2 and Akt/ERK and activates caspase/Cyto C signaling to enhance the antitumor activity of berberine in lung cancer cells. Oncotarget 7 (3), 2985–3001. doi:10.18632/oncotarget.6407
Mafi, A., Keshavarzmotamed, A., Hedayati, N., Boroujeni, Z. Y., Reiter, R. J., Dehmordi, R. M., et al. (2023a). Melatonin targeting non-coding RNAs in cancer: focus on mechanisms and potential therapeutic targets. Eur. J. Pharmacol. 950, 175755. doi:10.1016/j.ejphar.2023.175755
Mafi, A., Rezaee, M., Hedayati, N., Hogan, S. D., Reiter, R. J., Aarabi, M. H., et al. (2023b). Melatonin and 5-fluorouracil combination chemotherapy: opportunities and efficacy in cancer therapy. Cell Commun. Signal 21 (1), 33. doi:10.1186/s12964-023-01047-x
Majidinia, M., Sadeghpour, A., Mehrzadi, S., Reiter, R. J., Khatami, N., and Yousefi, B. (2017). Melatonin: a pleiotropic molecule that modulates DNA damage response and repair pathways. J. Pineal Res. 63 (1), e12416. doi:10.1111/jpi.12416
Malki, A. M., Gentry, J., and Evans, S. C. (2006). Differential effect of selected methylxanthine derivatives on radiosensitization of lung carcinoma cells. Exp. Oncol. 28 (1), 16–24.
Mannan, A., Muhsen, I. N., Barragán, E., Sanz, M. A., Mohty, M., Hashmi, S. K., et al. (2020). Genotypic and phenotypic characteristics of acute promyelocytic leukemia translocation variants. Hematology/Oncology Stem Cell Ther. 13 (4), 189–201. doi:10.1016/j.hemonc.2020.05.007
Mariño, G., Niso-Santano, M., Baehrecke, E. H., and Kroemer, G. (2014). Self-consumption: the interplay of autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 15 (2), 81–94. doi:10.1038/nrm3735
Mathupala, S. P., and Pedersen, P. L. (2010). Voltage dependent anion channel-1 (VDAC-1) as an anti-cancer target. Cancer Biol. Ther. 9 (12), 1053–1056. doi:10.4161/cbt.9.12.12451
Mehrzadi, S., Pourhanifeh, M. H., Mirzaei, A., Moradian, F., and Hosseinzadeh, A. (2021). An updated review of mechanistic potentials of melatonin against cancer: pivotal roles in angiogenesis, apoptosis, autophagy, endoplasmic reticulum stress and oxidative stress. Cancer Cell Int. 21 (1), 188. doi:10.1186/s12935-021-01892-1
Mirza-Aghazadeh-Attari, M., Mohammadzadeh, A., Mostavafi, S., Mihanfar, A., Ghazizadeh, S., Sadighparvar, S., et al. (2020). Melatonin: an important anticancer agent in colorectal cancer. J. Cell Physiol. 235 (2), 804–817. doi:10.1002/jcp.29049
Miyashita, T., Krajewski, S., Krajewska, M., Wang, H. G., Lin, H. K., Liebermann, D. A., et al. (1994). Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene 9 (6), 1799–1805.
Mizushima, N., and Komatsu, M. (2011). Autophagy: renovation of cells and tissues. Cell 147 (4), 728–741. doi:10.1016/j.cell.2011.10.026
Müschen, M., Rajewsky, K., Krönke, M., and Küppers, R. (2002). The origin of CD95-gene mutations in B-cell lymphoma. Trends Immunol. 23 (2), 75–80. doi:10.1016/s1471-4906(01)02115-9
Osińska, I., Popko, K., and Demkow, U. (2014). Perforin: an important player in immune response. Cent. Eur. J. Immunol. 39 (1), 109–115. doi:10.5114/ceji.2014.42135
Perdomo, J., Cabrera, J., Estévez, F., Loro, J., Reiter, R. J., and Quintana, J. (2013). Melatonin induces apoptosis through a caspase-dependent but reactive oxygen species-independent mechanism in human leukemia Molt-3 cells. J. pineal Res. 55 (2), 195–206. doi:10.1111/jpi.12062
Prodhan, A., Cavestro, C., Kamal, M. A., and Islam, M. A. (2021). Melatonin and sleep disturbances in alzheimer's disease. CNS Neurol. Disord. Drug Targets 20 (8), 736–754. doi:10.2174/1871527320666210804155617
P. Strati, N. Jain, and S. O'Brien (Editors) (2018). Chronic lymphocytic leukemia: Diagnosis and treatment, mayo clinic proceedings (Netherlands: Elsevier).
Qin, X., Yang, C., Xu, H., Zhang, R., Zhang, D., Tu, J., et al. (2021). Cell-Derived biogenetic gold nanoparticles for sensitizing radiotherapy and boosting immune response against cancer. Small 17 (50), 2103984. doi:10.1002/smll.202103984
Quintana, C., Cabrera, J., Perdomo, J., Estévez, F., Loro, J. F., Reiter, R. J., et al. (2016). Melatonin enhances hyperthermia-induced apoptotic cell death in human leukemia cells. J. pineal Res. 61 (3), 381–395. doi:10.1111/jpi.12356
Ramírez-Valle, F., Badura, M. L., Braunstein, S., Narasimhan, M., and Schneider, R. J. (2010). Mitotic raptor promotes mTORC1 activity, G2/M cell cycle progression, and internal ribosome entry site-mediated mRNA translation. Mol. Cell. Biol. 30 (13), 3151–3164. doi:10.1128/MCB.00322-09
Reiter, R. J., Rosales-Corral, S. A., Tan, D. X., Acuna-Castroviejo, D., Qin, L., Yang, S. F., et al. (2017). Melatonin, a full service anti-cancer agent: inhibition of initiation, progression and metastasis. Int. J. Mol. Sci. 18 (4), 843. doi:10.3390/ijms18040843
Reiter, R. J., Tan, D. X., Rosales-Corral, S., Galano, A., Zhou, X. J., and Xu, B. (2018). Mitochondria: central organelles for melatonin′ s antioxidant and anti-aging actions. Molecules 23 (2), 509. doi:10.3390/molecules23020509
Rubio, S., Estévez, F., Cabrera, J., Reiter, R. J., Loro, J., and Quintana, J. (2007). Inhibition of proliferation and induction of apoptosis by melatonin in human myeloid HL-60 cells. J. Pineal Res. 42 (2), 131–138. doi:10.1111/j.1600-079X.2006.00392.x
Santoro, R., Marani, M., Blandino, G., Muti, P., and Strano, S. (2012). Melatonin triggers p53Ser phosphorylation and prevents DNA damage accumulation. Oncogene 31 (24), 2931–2942. doi:10.1038/onc.2011.469
Santoro, R., Mori, F., Marani, M., Grasso, G., Cambria, M. A., Blandino, G., et al. (2013). Blockage of melatonin receptors impairs p53-mediated prevention of DNA damage accumulation. Carcinogenesis 34 (5), 1051–1061. doi:10.1093/carcin/bgt025
Schuler, M., Bossy-Wetzel, E., Goldstein, J. C., Fitzgerald, P., and Green, D. R. (2000). p53 induces apoptosis by caspase activation through mitochondrial cytochrome c release. J. Biol. Chem. 275 (10), 7337–7342. doi:10.1074/jbc.275.10.7337
Seth, R., and Singh, A. (2015). Leukemias in children. Indian J. Pediatr. 82 (9), 817–824. doi:10.1007/s12098-015-1695-5
Seyrek, K., Ivanisenko, N. V., Richter, M., Hillert, L. K., König, C., and Lavrik, I. N. (2020). Controlling cell death through post-translational modifications of DED proteins. Trends Cell Biol. 30 (5), 354–369. doi:10.1016/j.tcb.2020.02.006
Shafabakhsh, R., Mirzaei, H., and Asemi, Z. (2020). Melatonin: A promising agent targeting leukemia. J. Cell Biochem. 121 (4), 2730–2738. doi:10.1002/jcb.29495
Sharma, S., and Rai, K. R. (2019). Chronic lymphocytic leukemia (CLL) treatment: so many choices, such great options. Cancer 125 (9), 1432–1440. doi:10.1002/cncr.31931
Shehadeh, M., Palzur, E., Apel, L., and Soustiel, J. F. (2019). Reduction of traumatic brain damage by tspo ligand etifoxine. Int. J. Mol. Sci. 20 (11), 2639. doi:10.3390/ijms20112639
Shen, D., Ju, L., Zhou, F., Yu, M., Ma, H., Zhang, Y., et al. (2021). The inhibitory effect of melatonin on human prostate cancer. Cell Commun. Signal 19 (1), 34. doi:10.1186/s12964-021-00723-0
Shen, X. G., Wang, C., Li, Y., Wang, L., Zhou, B., Xu, B., et al. (2010). Downregulation of caspase-9 is a frequent event in patients with stage II colorectal cancer and correlates with poor clinical outcome. Colorectal Dis. 12 (12), 1213–1218. doi:10.1111/j.1463-1318.2009.02009.x
Shrestha, S., Zhu, J., Wang, Q., Du, X., Liu, F., Jiang, J., et al. (2017). Melatonin potentiates the antitumor effect of curcumin by inhibiting IKKβ/NF-κB/COX-2 signaling pathway. Int. J. Oncol. 51 (4), 1249–1260. doi:10.3892/ijo.2017.4097
Sisakht, M., Darabian, M., Mahmoodzadeh, A., Bazi, A., Shafiee, S. M., Mokarram, P., et al. (2020). The role of radiation induced oxidative stress as a regulator of radio-adaptive responses. Int. J. Radiat. Biol. 96 (5), 561–576. doi:10.1080/09553002.2020.1721597
Stennicke, H. R., Jürgensmeier, J. M., Shin, H., Deveraux, Q., Wolf, B. B., Yang, X., et al. (1998). Pro-caspase-3 is a major physiologic target of caspase-8. J. Biol. Chem. 273 (42), 27084–27090. doi:10.1074/jbc.273.42.27084
Sun, H., Gusdon, A. M., and Qu, S. (2016). Effects of melatonin on cardiovascular diseases: progress in the past year. Curr. Opin. Lipidol. 27 (4), 408–413. doi:10.1097/MOL.0000000000000314
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71 (3), 209–249. doi:10.3322/caac.21660
Szegezdi, E., Logue, S. E., Gorman, A. M., and Samali, A. (2006). Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 7 (9), 880–885. doi:10.1038/sj.embor.7400779
Tan, S., and Wong, E. (2017). Mitophagy transcriptome: mechanistic insights into polyphenol-mediated mitophagy. Oxid. Med. Cell Longev. 2017, 9028435. doi:10.1155/2017/9028435
Tang, Y-L., Sun, X., Huang, L-B., Liu, X-J., Qin, G., Wang, L-N., et al. (2019). Melatonin inhibits MLL-rearranged leukemia via RBFOX3/hTERT and NF-κB/COX-2 signaling pathways. Cancer Lett. 443, 167–178. doi:10.1016/j.canlet.2018.11.037
Thol, F., and Ganser, A. (2020). Treatment of relapsed acute myeloid leukemia. Curr. Treat. Options Oncol. 21 (8), 66. doi:10.1007/s11864-020-00765-5
Tian, T., Li, J., Li, Y., Lu, Y-X., Tang, Y-L., Wang, H., et al. (2019). Melatonin enhances sorafenib-induced cytotoxicity in FLT3-ITD acute myeloid leukemia cells by redox modification. Theranostics 9 (13), 3768–3779. doi:10.7150/thno.34327
Tiwari, R. K., Lal, M. K., Kumar, R., Mangal, V., Altaf, M. A., Sharma, S., et al. (2021). Insight into melatonin-mediated response and signaling in the regulation of plant defense under biotic stress. Plant Mol. Biol. 109, 385–399. doi:10.1007/s11103-021-01202-3
Tonon, A. C., Pilz, L. K., Markus, R. P., Hidalgo, M. P., and Elisabetsky, E. (2021). Melatonin and depression: a translational perspective from animal models to clinical studies. Front. Psychiatry 12, 638981. doi:10.3389/fpsyt.2021.638981
Vasey, C., McBride, J., and Penta, K. (2021). Circadian rhythm dysregulation and restoration: the role of melatonin. Nutrients 13 (10), 3480. doi:10.3390/nu13103480
Veenman, L., Levin, E., Weisinger, G., Leschiner, S., Spanier, I., Snyder, S. H., et al. (2004). Peripheral-type benzodiazepine receptor density and in vitro tumorigenicity of glioma cell lines. Biochem. Pharmacol. 68 (4), 689–698. doi:10.1016/j.bcp.2004.05.011
Voss, A. K., and Strasser, A. (2020). The essentials of developmental apoptosis. F1000Res 9, 148. doi:10.12688/f1000research.21571.1
Wan, J., Liu, T., Mei, L., Li, J., Gong, K., Yu, C., et al. (2013). Synergistic antitumour activity of sorafenib in combination with tetrandrine is mediated by reactive oxygen species (ROS)/Akt signaling. Br. J. cancer 109 (2), 342–350. doi:10.1038/bjc.2013.334
Wei, X., Pu, X., Yang, S., Meng, X., Chen, X., Zhang, Z., et al. (2019). Melatonin enhances arsenic trioxide-induced cytotoxicity by modulating autophagy in an acute promyelocytic leukemia cell line. Transl. Cancer Res. 8 (5), 2079–2088. doi:10.21037/tcr.2019.09.26
Wong, R. S. (2011). Apoptosis in cancer: from pathogenesis to treatment. J. Exp. Clin. Cancer Res. 30 (1), 87. doi:10.1186/1756-9966-30-87
Woo, S. M., Min, Kj, and Kwon, T. K. (2015). Melatonin-mediated Bim up-regulation and cyclooxygenase-2 (COX-2) down-regulation enhances tunicamycin-induced apoptosis in MDA-MB-231 cells. J. Pineal Res. 58 (3), 310–320. doi:10.1111/jpi.12217
Wu, X., and Gallo, K. A. (2013). The 18-kDa translocator protein (TSPO) disrupts mammary epithelial morphogenesis and promotes breast cancer cell migration. PLoS One 8 (8), e71258. doi:10.1371/journal.pone.0071258
Xiang, F., Ma, S., Lv, Y., Zhang, D., Song, H., and Huang, Y. (2019). Tumor necrosis factor receptor-associated protein 1 regulates hypoxia-induced apoptosis through a mitochondria-dependent pathway mediated by cytochrome c oxidase subunit II. Burns trauma 7, 16. doi:10.1186/s41038-019-0154-3
Yaghoobi, A., Nazerian, Y., Meymand, A. Z., Ansari, A., Nazerian, A., and Niknejad, H. (2023). Hypoxia-sensitive miRNA regulation via CRISPR/dCas9 loaded in hybrid exosomes: a novel strategy to improve embryo implantation and prevent placental insufficiency during pregnancy. Front. Cell Dev. Biol. 10, 1082657. doi:10.3389/fcell.2022.1082657
Yamamoto, M., Maehara, Y., Oda, S., Ichiyoshi, Y., Kusumoto, T., and Sugimachi, K. (1999). The p53 tumor suppressor gene in anticancer agent-induced apoptosis and chemosensitivity of human gastrointestinal cancer cell lines. Cancer Chemother. Pharmacol. 43 (1), 43–49. doi:10.1007/s002800050861
Zare, S., Heydari, F., Hayes, A., Reiter, R., Zirak, M., and Karimi, G. (2021). Melatonin attenuates chemical-induced cardiotoxicity. Hum. Exp. Toxicol. 40 (3), 383–394. doi:10.1177/0960327120959417
Zastko, L., Petrovičová, P., Račková, A., Jakl, L., Jakušová, V., Marková, E., et al. (2021). DNA damage response and apoptosis induced by hyperthermia in human umbilical cord blood lymphocytes. Toxicol. Vitro 73, 105127. doi:10.1016/j.tiv.2021.105127
Zhang, J., Gu, Y., and Chen, B. (2019). Mechanisms of drug resistance in acute myeloid leukemia. Onco Targets Ther. 12, 1937–1945. doi:10.2147/OTT.S191621
Zhang, X., Wu, S., Yang, J., Zhang, G., Su, Y., Zhang, M., et al. (2022). Long-term retrospective study of retinoic acid combined with arsenic and chemotherapy for acute promyelocytic leukemia. Int. J. Hematol. 117, 530–537. doi:10.1007/s12185-022-03507-5
Zhelev, Z., Ivanova, D., Bakalova, R., Aoki, I., and Higashi, T. (2017). Synergistic cytotoxicity of melatonin and new-generation anticancer drugs against leukemia lymphocytes but not normal lymphocytes. Anticancer Res. 37 (1), 149–159. doi:10.21873/anticanres.11300
Zhu, M., Molina, J. R., Dy, G. K., Croghan, G. A., Qi, Y., Glockner, J., et al. (2020). A phase I study of the VEGFR kinase inhibitor vatalanib in combination with the mTOR inhibitor, everolimus, in patients with advanced solid tumors. Investig. New Drugs 38 (6), 1755–1762. doi:10.1007/s10637-020-00936-z
Keywords: melatonin, apoptosis, leukaemia, signalling pathway, therapy
Citation: Mafi A, Rismanchi H, Gholinezhad Y, Mohammadi MM, Mousavi V, Hosseini SA, Milasi YE, Reiter RJ, Ghezelbash B, Rezaee M, Sheida A, Zarepour F, Asemi Z, Mansournia MA and Mirzaei H (2023) Melatonin as a regulator of apoptosis in leukaemia: molecular mechanism and therapeutic perspectives. Front. Pharmacol. 14:1224151. doi: 10.3389/fphar.2023.1224151
Received: 17 May 2023; Accepted: 19 July 2023;
Published: 14 August 2023.
Edited by:
Zhiyu Zhang, Fourth Affiliated Hospital of China Medical University, ChinaReviewed by:
Yee Lian Tiong, International Medical University, MalaysiaCopyright © 2023 Mafi, Rismanchi, Gholinezhad, Mohammadi, Mousavi, Hosseini, Milasi, Reiter, Ghezelbash, Rezaee, Sheida, Zarepour, Asemi, Mansournia and Mirzaei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hamed Mirzaei, aC5taXJ6YWVpMjAwMkBnbWFpbC5jb20=; Behrooz Ghezelbash, YmVocnV6Z2hlemVsYmFzaEB5YWhvby5jb20=; Malihe Rezaee, bWFsaWhlcmV6YWVlODU1QGdtYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.