
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol., 22 August 2023
Sec. Experimental Pharmacology and Drug Discovery
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1221719
This article is part of the Research TopicRecent Pharmacological Innovation for the Opioid CrisisView all 9 articles
The United States is entering its fourth decade of the opioid epidemic with no clear end in sight. At the center of the epidemic is an increase in opioid use disorder (OUD), a complex condition encompassing physical addiction, psychological comorbidities, and socioeconomic and legal travails associated with the misuse and abuse of opioids. Existing behavioral and medication-assisted therapies show limited efficacy as they are hampered by lack of access, strict regimens, and failure to fully address the non-pharmacological aspects of the disease. A growing body of research has indicated the potential of hallucinogens to efficaciously and expeditiously treat addictions, including OUD, by a novel combination of pharmacology, neuroplasticity, and psychological mechanisms. Nonetheless, research into these compounds has been hindered due to legal, social, and safety concerns. This review will examine the preclinical and clinical evidence that psychoplastogens, such as ibogaine, ketamine, and classic psychedelics, may offer a unique, holistic alternative for the treatment of OUD while acknowledging that further research is needed to establish long-term efficacy along with proper safety and ethical guidelines.
According to the 2021 National Survey on Drug Use and Health, an estimated 9.8 million individuals misused pain relievers and/or heroin and approximately 5.6 million individuals suffered from opioid use disorder (OUD) in the previous year alone (SAMHSA, 2022). Since the 1990s, opioid use in the United States has skyrocketed from an initial wave caused by an increase in opioid prescriptions to a rapid surge in heroin use a decade later, and a steady influx of fentanyl and other synthetic opioids over the past 10 years. Overdose deaths involving opioids have significantly increased along with their use and abuse, rising from 50,000 to over 80,000 annually from 2019 to 2021, respectively (NIDA, 2023). Along with preventable deaths, the misuse and abuse of opioids is associated with comorbid mental health disorders beyond addiction, legal and financial difficulties, and significant disruptions in familial and social relationships. Indeed, of the 7.7 million Americans over the age of 18 suffering from severe mental illnesses, 6.4 million people had a comorbid substance use disorder (SUD), with 10.3% of those involving the misuse of opioids (SAMHSA, 2022). These numbers make it abundantly clear that any treatment of OUD must also address the possible concomitant mental illness(es) affecting the patient in order to be successful.
Current treatments for OUD mainly focus on alleviating withdrawal and craving symptoms through the use of opioid agonists. Despite these opioid agonist therapies (OATs) being considered the gold standard treatment for OUD, there is an extraordinarily high rate of relapse (Weiss et al., 2011; Amato et al., 2013; Bentzley et al., 2015; Hser et al., 2016). Indeed, a review of buprenorphine maintenance therapy studies found that relapse to illicit opioid use was greater than 50% in every study reviewed, while those with a comparable tapering duration of 4 weeks varied widely, demonstrating the maintenance of abstinence rates from 9.6% to 50% (Bentzley et al., 2015). Similarly, a comparison of buprenorphine/naloxone vs. methadone indicated that 50.9% and 41.1% of patients, respectively, had used heroin or opiates during the 60-month follow-up (Hser et al., 2016). Extending the maintenance and tapering phases of treatment has been shown to increase initial success rates, but the addition of opioid drug counseling failed to significantly improve outcomes either immediately following the treatment (46.7% vs. 51.7%) or after an 8-week follow-up (7.2% vs. 10%) (Weiss et al., 2011). Although there are a number of reasons for the failure rate of these treatments, the most common themes seem to be the required long-term, strict adherence to the regimen and the inability of the pharmacological intervention, even with adjunct counseling, to address other aspects of OUD beyond addiction. Additionally, opioid agonist therapy itself is not without risk, as indicated by the ∼4% of monthly overdose deaths that involve methadone itself (Jones C. M. et al., 2022). A new approach is therefore needed to treat and meet the needs of those suffering from OUD, for whom OATs have either failed or are incompatible.
The following question is then raised: what would an idealized treatment for OUD look like? Beyond the standards of safety and efficacy, an ideal treatment would reduce or eliminate withdrawals and cravings; be more accessible via time, effort, and financial considerations; and provide patients with assistance in the more ineffable and variable aspects of the disease, such as comorbid mental health conditions and addiction-related relationship difficulties. Although there is not likely to be a magic-bullet, one-size-fits-all treatment that meets all of these requirements, there is a class of drugs that, along with psychotherapy, does offer hope to those suffering with OUD.
Drugs that rapidly promote induced neuroplasticity, termed “psychoplastogens,” may hold the key to the future of OUD treatment. Although a number of drugs are capable of inducing neuro-, dendrito-, and spinogenesis, the term “psychoplastogen” was coined by David Olson’s lab in 2018 from the Greek roots for mind (psyche), molding (plast), and producing (gen) to highlight the ability of certain drugs to promote both structural and functional neuroplasticity in a (presumably) therapeutic manner. Compounds in this category include hallucinogens, such as ibogaine, ketamine, and classic psychedelics, along with non-hallucinogenic analogs of these molecules (Ly et al., 2018). Although each of these drugs has its own unique pharmacology, a number of recent preclinical reports highlight their similarity in the ability to increase structural and functional connectivity, specifically within the prefrontal cortex (PFC) (Ly et al., 2018; Olson, 2022; van Elk and Yaden, 2022; Vargas et al., 2023). Atrophy and dysfunction of neurons within the prefrontal cortex are hallmarks of a variety of neuropsychiatric disorders, particularly those affected by stress, such as depression and addiction (Goldstein and Volkow, 2011; Vargas-Perez et al., 2014; Hare and Duman, 2020; Duman et al., 2021). Although a smattering of anecdotal and clinical evidence for the rapid efficacy of these compounds in treating depression, anxiety, and substance use disorders arose as early as the 1950s, their recreational use and the resulting legal crackdown have stifled research in this area until recently. Indeed, it remains to be seen if psychoplastogen-induced neuroplasticity (or part of it) is the driving force behind the apparent clinical efficacy of these compounds.
Within the past 25 years, there has been a resurgence of interest in these compounds for the treatment of a number of neuropsychiatric diseases, with preliminary clinical trials indicating the promise they hold for depression (Ahmed et al., 2023; Goodwin et al., 2023), post-traumatic stress disorder (Mitchell et al., 2021; Abdallah et al., 2022), and addiction (Brown and Alper, 2018; Noller et al., 2018; Garcia-Romeu et al., 2019; O'Shaughnessy et al., 2021; Bogenschutz et al., 2022), among others. The appeal and potential efficacy of psychoplastogens for these disorders is related to their rapid, long-lasting effects on not only the symptoms associated with the primary disorder but also to the alterations in patients’ attitude and approach to daily life and relationships, following as little as a single dosing session. In this review, therefore, we examine the preclinical and clinical evidence of the potential of psychoplastogens, such as ibogaine, ketamine, and classic psychedelics (lysergic acid diethylamide (LSD), psilocybin, and ayahuasca), in addressing not only the primary addiction but also the multi-faceted complexities associated with OUD in order to treat the patient as a whole.
Perhaps the most well-studied psychoplastogen for the treatment of OUD, specifically, ibogaine is an indole alkaloid found in the root bark of the African shrub Tabernanthe iboga. Utilized for centuries by those who follow the Bwiti religion of Central and Western Africa; shavings of the iboga root bark are taken in small amounts to stave off fatigue, thirst, or hunger, while larger amounts are used to induce a trance-like state during religious and initiation ceremonies (Brown, 2013). The iboga root was brought to Europe in the early 20th century, where ibogaine was isolated, purified, and eventually sold in France as the psychostimulant “Lambarene” to treat fatigue and depression.
The potential of ibogaine for combating opioid dependence was discovered (from a non-indigenous perspective) not in the clinic but, like so many hallucinogenic drugs in the 1960s, through recreational experimentation. Hoping to experience a psychoactive trip, Howard Lotsof and 19 other individuals, seven of whom were dependent on heroin, ingested hallucinogenic doses of ibogaine (Alper et al., 2001). Noting that withdrawals and cravings for heroin did not occur during or immediately after the experience, five of the heroin-dependent seven people remained abstinent from opioids for at least 6 months. Nevertheless, despite the advocacy of Lotsof, and scientists and psychiatrists around the country, who were utilizing ibogaine for a variety of psychiatric conditions, the possession of ibogaine became illegal in 1967, followed shortly after by its designation as a Schedule I drug with the passage of the Controlled Substances Act in 1970 (Alper et al., 2001).
However, research, advocacy, and even unregulated treatments, involving the use of ibogaine to treat addiction, quietly continued in various parts of the world. To date, it is estimated that over 10,000 patients have been treated with ibogaine worldwide (Luz and Mash, 2021). A number of published reports, including some clinical trials, have shown that Lotsof was correct—ibogaine does appear to reduce withdrawal and craving while allowing a significant number of patients to remain abstinent from opioids for an extended period of time after a single treatment (Table 1).
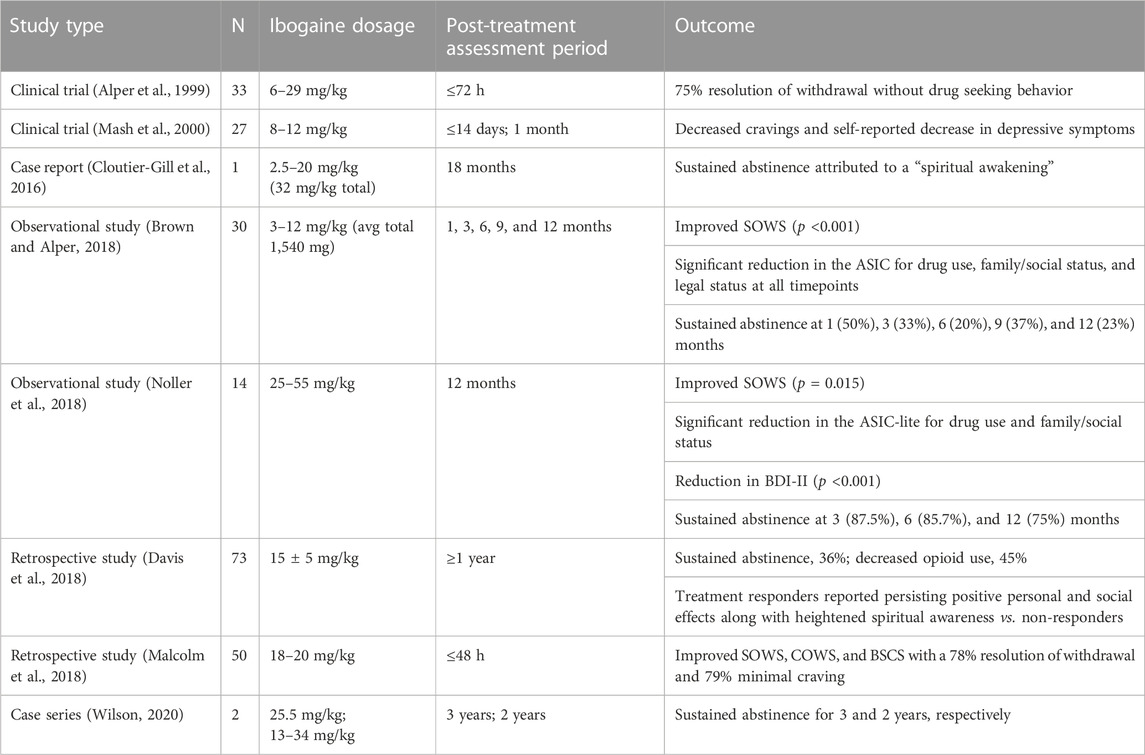
TABLE 1. Selected published reports of ibogaine administration in patients with OUD. SOWS, Subjective Opioid Withdrawal Scale; ASIC, Addiction Severity Index composite; BDI, Beck Depression Inventory; COWS, Clinical Opioid Withdrawal Scale; BSCS, Brief Substance Craving Scale.
So how is ibogaine able to affect such a rapid, drastic change in OUD patients, many of whom had previously tried and failed to remain abstinent using the gold-standard opioid agonist treatment? Researchers are still trying to answer that question, with most data supporting a complex blend of pharmacological action, neuroplasticity, and oneirophrenic or holotropic experiences.
Ibogaine exhibits complex pharmacological action at a wide range of neurotransmitter receptors, with a low binding affinity for the serotonin reuptake transporter; sodium channels; nicotinic acetylcholine; N-methyl-D-aspartate (NMDA) glutamate; and kappa-opioid, mu-opioid, and sigma-2 receptors (Brown, 2013). Although it is an agonist for 5-HT2A, 5-HT3, and muscarinic receptors, ibogaine is also an antagonist for NMDA glutamate and nicotinic acetylcholine receptors. Ibogaine, which is highly lipophilic, therefore, concentrates in the brain and adipose tissue and slowly metabolizes via demethylation to form the active metabolite, noribogaine, which has its own unique pharmacological profile. Noribogaine, with a circulating half-life of 28–49 h, exhibits less affinity for NMDA receptors and a greater affinity for mu-opioid receptors than its parent compound (Mash et al., 2000). This agonism of opioid receptors by noribogaine may contribute to the observed reductions in withdrawals and cravings.
Although noribogaine’s actions at opioid receptors may mediate initial therapeutic effects relating to craving and withdrawal symptoms, they do not fully explain the long-lasting anti-addiction effects often noted after a single session of ibogaine treatment for opioid dependence. It has been postulated that the enduring anti-addictive properties of ibogaine relate instead to a rewiring of neural circuitry associated with addiction. Indeed, both ibogaine and noribogaine increase the glial-derived neurotrophic factor (GDNF) in the ventral tegmental area, which has been shown in preclinical studies to reduce self-administration of alcohol (He et al., 2005). Interestingly, however, 18-methoxycoronardine (18-MC), a structural analog developed to mitigate the negative cardiovascular and hallucinogenic side effects of ibogaine, does not increase the GDNF despite demonstrating efficacy in reducing cocaine and morphine self-administration (Brown, 2013). Noribogaine, but not ibogaine, on the other hand, has been shown to increase dendritic arbor complexity and promote neuritogenesis in cortical neurons in vivo. This rapidly induced neuroplasticity is mediated via the stimulation of TrkB, mTOR, and intracellular 5-HT2A signaling pathways and is thought to be essential to the therapeutic effects of ibogaine and other psychoplastogens on complex mental health conditions, such as depression and SUD (Ly et al., 2018; Vargas et al., 2023).
Similar to classic psychedelics, the activation of the 5-HT2A receptors is also thought to be responsible for the hallucinatory effects of ibogaine. The ibogaine experience has been described both as oneirophrenic, or a waking dream-like state, and holotropic, a healing transformation of the consciousness (Underwood et al., 2021). Although Bwiti practitioners describe the ibogaine visionary experience in four phases, current researchers typically identify three distinct stages. The four phases of the Bwiti ibogaine initiation ritual are broken down by the type of vision and its attached significance: 1) random images lacking meaning; 2) fractal animal or natural images; 3) mythical images promoting peace; and 4) encounters with spiritual entities or ancestors. The three stages identified by researchers in conjunction with the treatment for SUD, in contrast, are characterized by both physical and psychological effects: 1) an acute phase defined by ataxia, possible GI distress, and changes in the heart rate while revisiting memories; 2) a reflective phase focusing inward while examining alternative pasts and futures; and 3) a residual stimulation phase with a decreased intensity of visions and a gradual return to the external environment (Alper et al., 2001; Underwood et al., 2021). It is possible that this altered state of consciousness is a necessary component to ibogaine’s anti-addictive therapeutic effects. Although the vast majority of ibogaine patients claim the experience to be both psychologically difficult and physically draining, objective and subjective reports from these same patients describe how it increased empathy, self-reflection, and appreciation, and decreased their feelings of anxiety and depression, improving familial and social relations (Brown and Alper, 2018; Camlin et al., 2018; Davis et al., 2018; Malcolm et al., 2018; Noller et al., 2018; Brown et al., 2019; Wilson et al., 2020). As one patient reflected, “I was also able to begin to see how, because I felt so unlovable, I paid in one way or another for love. I was able to stop that and open myself up to real love for maybe the first time in my adult life” (Brown et al., 2019). Consideration of these reports is increasingly important as researchers develop new compounds based off of hallucinogens, such as 18-MC and tabernanthalog (TBG). Although these novel molecules are showing some promise in preclinical studies regarding neuroplasticity and reduced addiction-seeking behaviors (Brown, 2013; Cameron et al., 2021), the lack of an altered state of consciousness could render them less effective or even completely ineffective when administered to human patients. This is an active area of research that requires further study.
Despite the large numbers of patients seeking ibogaine treatment for SUDs like OUD, its status as a Schedule I drug in the United States has hindered the ability of researchers to properly study its efficacy and safety and to create guidelines for its use. Ibogaine, while non-addictive and generally considered safe in the doses utilized for SUD treatment, does have known adverse and potentially dangerous side effects. It is known to inhibit cardiac hERG potassium channels, leading to QT interval prolongation and an increased risk for potentially fatal arrhythmias, including torsades de pointes (TdP) (Koenig and Hilber, 2015; Luz and Mash, 2021). The IC50 concentration for hERG channels is approximately four times greater than the estimated therapeutic levels of ibogaine. However, one must consider that ibogaine is metabolized to noribogaine primarily by cytochrome P450 2D6 (CYP2D6) enzymes, which not only plays an important role in terms of drug–drug interactions but also in terms of genetic variations. For instance, approximately 10% of the Caucasian population lacks CYP2D6, which could significantly put them more at risk for adverse cardiovascular effects due to unexpectedly high plasma levels of ibogaine (Koenig and Hilber, 2015).
Since the beginning of the “vast uncontrolled experiment” of patients flocking to (mostly) unregulated clinics for ibogaine treatment, at least 32 ibogaine-related deaths have been reported (Luz and Mash, 2021). Although most of the reported fatalities were attributed to the aforementioned adverse cardiovascular effects, it is important to note that they also occurred in unsafe and unregulated settings with improper medical screening and dosing along with a lack of suitable medical care on site for monitoring and emergencies. In response to these severe and potentially dangerous side effects, guidelines for the use of ibogaine to treat SUDs have been published (Dickinson et al., 2016). These days, despite the continued lack of federal regulation, most providers understand the importance of careful patient screening for medical (e.g., EKG and liver function) and psychiatric exclusion criteria prior to treatment, as well as the proper monitoring and availability of medical assistance during and after treatment. Utilizing this careful approach, a recent study by Knuijver et al. was able to demonstrate that, while ibogaine treatment for OUD may produce clinically relevant bradycardia, QTc prolongation, and severe ataxia, these side effects were all manageable and reversible (Knuijver et al., 2022).
Further research is necessary before ibogaine treatment for SUDs in general or OUD, specifically, can be approved and made available to the American public, but it is undeniable that this unique, holotropic psychoplastogen holds promise for those suffering from these conditions. As previously mentioned, researchers are attempting to synthesize ibogaine analogs (18-MC and TBG) with improved safety profiles, but, to date, there has only been a single human trial with either of these compounds (NIH, 2023). There are currently two clinical trials in the recruiting stages for ibogaine for the treatment of opioid withdrawal and detoxification (Table 2). It will be extremely interesting to see if the non-hallucinogenic analogs produce the same efficacy as their hallucinogenic counterpart and if a regulated study design can mitigate any adverse effects of ibogaine itself.
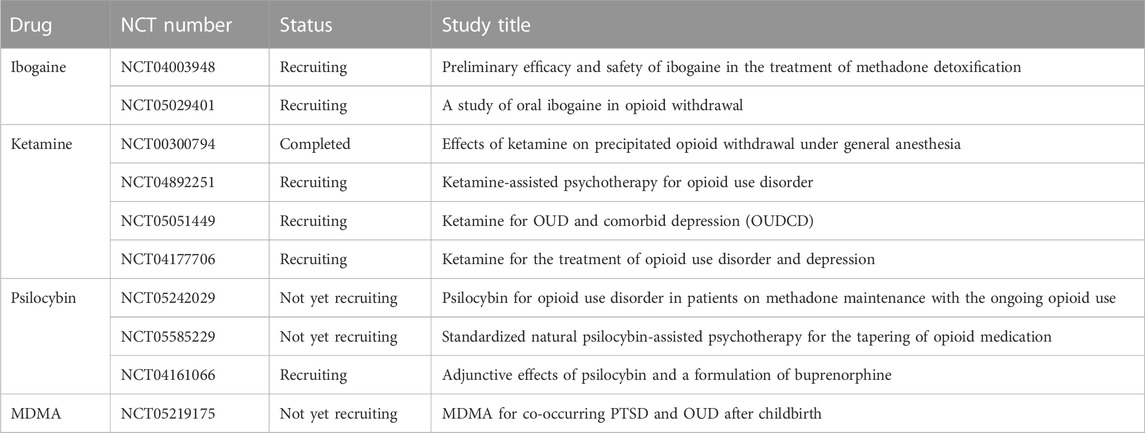
TABLE 2. Current clinical trials of psychoplastogens for the treatment of OUD (NIH, 2023).
During the 1950s, phencyclidine (PCP) was widely synthesized and used as a dissociative anesthetic (Naughton et al., 2014). The drug was removed from the market in 1978 because of its high abuse potential along with psychodysleptic side effects (delusions, hallucinations, and out-of-body experiences). Ketamine, a derivative of PCP, gradually replaced PCP as a clinical anesthetic during the 1970s (Dundee et al., 1970). As with PCP, ketamine does induce psychodysleptic effects, but to a lesser extent, while maintaining hemodynamic stability (Zanos et al., 2018). Because of these unique side effects, ketamine is primarily used as an anesthetic in veterinary medicine, although it is also utilized as one of the preferred anesthetic agents in humans for certain specific applications, such as pediatrics, burn victims, and hemodynamically compromised patients (Morgan et al., 2012; Nowacka and Borczyk, 2019). Beyond its anesthetic uses, ketamine is also commonly used as an analgesic. Adding ketamine to opioids reduces respiratory depression and prevents hyperalgesia during acute pain management (Chawarski et al., 2020). In this way, ketamine is already being utilized to lower the dosage of prescription opioids needed in treatment, which may assist in preventing more patients from becoming dependent on opioids in the first place.
The most notable and recent application of ketamine as a therapeutic agent, however, is its utilization as an antidepressant in conjunction with psychotherapy. Ketamine has been shown to be highly effective in treating refractory major depressive disorder along with depression-resistant suicidal thoughts, with a rapid and long-lasting remission or reduction in the symptoms (Serafini et al., 2014; Reinstatler and Youssef, 2015; Loo et al., 2016). Additionally, it has become evident that ketamine shows potential to treat opioid, alcohol, and cocaine addiction (Krupitsky and Grinenko, 1997; Krupitsky et al., 2007; Dakwar et al., 2014). In an early study by Kruptisky and Grinenko, 70 detoxified heroin-addicted patients were randomized to receive high-dose ketamine (2 mg/kg intramuscularly) or a sub-psychedelic low dose of ketamine (0.2 mg/kg intramuscularly) alongside psychotherapy. The purpose of these psychotherapy sessions was to connect the ketamine experience with the patients’ interpersonal problems and carrying out a life without heroin usage. High-dose ketamine showed a significantly higher reduction in abstinence at 24 months vs. the low-dose group in addition to a significant reduction in cravings immediately after ketamine infusion (Krupitsky and Grinenko, 1997). A follow-up study compared the efficacy of a single dose vs. multiple sessions of ketamine-assisted psychotherapy for heroin addiction (Krupitsky et al., 2007). After one year, 50% percent of patients remained abstinent in the multiple-dose group vs. 22% in the single-dose group (p <0.05). These initial studies serve to indicate that ketamine shows potential to treat OUD, although higher (psychedelic) doses and multiple follow-up sessions may be necessary to maintain abstinence.
As a dissociative anesthetic and analgesic, ketamine works by inhibiting open NMDA receptors in a non-competitive manner, which is considered to play a role in its utility in both depression and SUDs (Franks and Lieb, 1994; Naughton et al., 2014; Strasburger et al., 2017). NMDA normally serves as a binding site for glutamate. Glutamate dysfunction is thought to be one of the pathways involved in SUDs and other neuropsychiatric disorders. Addiction research has shown that metabotropic glutamate receptor 2 (mGlu2), which is abundant in the mesocorticolimbic system, medial PFC, and the nucleus accumbens (nAC), has a reduced function in response to prolonged exposure to drugs, such as alcohol, resulting in altered reward pathways and increased drug-seeking behaviors (Domanegg et al., 2023). Utilizing compounds with NMDA receptor antagonist activity, such as ketamine or ibogaine, works to restore the potential glutamate imbalance and produce synaptic improvements, ultimately allowing one to learn new behaviors (Browne and Lucki, 2013; Strasburger et al., 2017). The rapidity and relatively long-term efficacy of ketamine for depression (and potentially SUDs) have been linked, like ibogaine/noribogaine and classic psychedelics, to a rapid synthesis of brain- and/or glial-derived growth factors along with the activation of the mTOR signaling pathway, leading to synaptogenesis (Li et al., 2010; Autry et al., 2011; Ly et al., 2018; Aleksandrova and Phillips, 2021). In addition to ketamine’s activity at NMDA receptors, recent studies have shown that its activation of µ-opioid receptors, in particular, may be essential to its initial antidepressant effects (Heifets et al., 2021). Although a recent clinical trial investigating the effects of ketamine on precipitated opioid withdrawal (NCT00300794, Table 2) may help answer whether this opioid activity assists with craving and withdrawal symptoms similar to ibogaine, this is an active area of research. Interestingly, other NMDA antagonists that have been developed in order to treat depression, similar to ketamine, without either the opioid or hallucinogenic activity, have not shown as much efficacy in either neurogenesis or long-lasting symptom reduction (Autry et al., 2011; Browne and Lucki, 2013). As with both ibogaine and classic psychedelics, ketamine pharmacology is complex, interacting with multiple neurotransmitters and receptors (Kohrs and Durieux, 1998; Udesky et al., 2005; Mion and Villevieille, 2013; Pacheco Dda et al., 2014). Additionally, similar to the other psychoplastogens discussed here, researchers are still unable to determine how much of ketamine’s efficacy in depression (and possibly SUDs) is reliant on the hallucinogenic and psychotherapeutic components of ketamine-assisted psychotherapy. In Krupitsky’s two-year follow-up study, it was noted that high-dose ketamine, while significantly improving heroin abstinence rates and decreasing cravings, also increased non-verbal emotional attitudes, as compared with a low dose (Krupitsky et al., 2002). Interestingly, both low and high doses improved patients’ symptoms of anxiety, depression, and anhedonia, while increasing their spirituality. Additionally, a study of the effects of ketamine on motivation to quit and cue-induced cravings in cocaine-dependent individuals found that ketamine significantly enhanced motivation for changing problematic behaviors (Dakwar et al., 2014). This is particularly notable as these patients were only subject to a short mindfulness exercise prior to treatment, as opposed to a full psychotherapy session. Moving forward, it is imperative that researchers seeking to develop new compounds, whether to improve safety or to eliminate the altered states of consciousness from the equation, thoroughly investigate the activity of these older medicines. If researchers can identify the pharmacological action and chemical moiety responsible for the enhanced motivation seen in ketamine, for example, it will ensure that this aspect of the potential therapeutic effect can be incorporated into the new compound or can at least be methodically studied by alternate incorporation and removal.
Despite the lower potency and shorter half-life when compared to its parent drug PCP, ketamine is still used recreationally as well to produce feelings of weightlessness, out-of-body experiences, and visions (Curran and Morgan, 2000). Interestingly, regardless of this fact, unlike ibogaine and classic psychedelics, ketamine is a Schedule III drug in the United States. Therefore, it has some advantages, in terms of our knowledge, of the safety profile and clinical guidelines as compared with other psychoplastogens (Sanacora et al., 2017; Cohen et al., 2018; Schwenk et al., 2018). Although it remains to be determined what the ideal dose of ketamine for OUD would be, the known side effects of doses currently used in treating depression include transient cardiovascular alterations, such as an increased heart rate and blood pressure, mild respiratory depression, and dissociative or hallucinogenic effects (Mion and Villevieille, 2013; Sanacora et al., 2017). Several clinical trials of ketamine for OUD alone and with comorbid depression are already underway (Table 2). The results of these trials will help further establish the safety and efficacy of this potential treatment, and hopefully pave the way for future research and trials with other psychoplastogens.
5-HT2A agonists, otherwise known as “classic psychedelics,” including lysergic acid diethylamide, psilocybin, and ayahuasca, have been known for nearly three-quarters of a century to provide benefit to those suffering from mental health disorders, including addiction (Abuzzahab et al., 1971; Savage and McCabe, 1973; Krebs and Johansen, 2012; Dos Santos et al., 2016). Unfortunately, a rise in the recreational use of these compounds and the resulting social and legal fallout led to the inability of researchers to study, and subsequently patients to benefit from, these drugs for 30+ years. In the past 25 years, however, there has been a resurgence of interest in psychedelics and the potential they hold for the rising incidence of mental health conditions in the United States and throughout the world. This renewed interest is not only capturing the attention of researchers and medical or mental health providers, it is also gripping the public, with 7.4 million people nationwide aged ≥18 years using hallucinogens in 2021 (SAMHSA, 2022).
The recent public interest, contrary to that of the 1960s, is being harnessed by researchers interested in furthering the therapeutic potential of psychedelics. Utilizing data from the National Survey of Drug Use and Health (NSDUH) from 2008–2013, Pisano et al. found that psychedelic use decreased the odds of past year opioid dependence by 27% and opioid abuse by 40% (Pisano et al., 2017). This was surpassed only by cannabis use, which reduced the risk of past year opioid abuse by 55%. In a follow-up study, using NSDUH data from 2015–2019, another group found that, of classic psychedelics, only psilocybin reduced the risk of OUD by 30%, while both cannabis and MDMA increased the risk (Jones G. et al., 2022). In Canada, psychedelic use among marginalized women who used opioids was found to significantly decrease the hazards of suicidal ideation or attempt (Argento et al., 2018), while recent psychedelic use was associated with 55% reduced odds of daily opioid use among a population of people who use illicit drugs (Argento et al., 2022). These studies, combined with early reports and recent clinical trials of classic psychedelics for the treatment of depression (Becker et al., 2022; Goodwin et al., 2023), anxiety (Uthaug et al., 2021; Holze et al., 2023), PTSD (Mitchell et al., 2021), and addiction (Abuzzahab et al., 1971; Dos Santos et al., 2016; Johnson et al., 2017; O'Shaughnessy et al., 2021; Sessa et al., 2021; Bogenschutz et al., 2022; Nicholas et al., 2022), all indicate that this class of drugs shows potential to be a novel alternative therapy for OUD.
As with ibogaine, LSD, psilocybin, and ayahuasca each exhibit their own unique pharmacological profile, interacting with a wide variety of neurotransmitters and receptors within the brain. They are typically classed together, however, as they are all 5-HT2A receptor agonists, and as such, they produce an altered state of consciousness together with rapid neuroplasticity. As potent psychoplastogens, LSD, psilocin (the active metabolite of psilocybin), and N-N-dimethyltryptamine (DMT; the main psychoactive component of ayahuasca) have all been shown to increase structural and functional neuritogenesis, spinogenesis, and synaptogenesis within cortical neurons (Ly et al., 2018; Shao et al., 2021; Olson, 2022). This effect, purportedly driven by intracellular 5-HT2A receptors and mTOR and TrkB signaling pathways, appears to be both rapid and persisting (Shao et al., 2021; Vargas et al., 2023). This psychoplastogen effect could account for the reported long-lasting efficacy of these compounds, as noted after only one to three doses. Additionally, as with ketamine, psychedelics may restore glutamate transmission in the PFC. In the case of 5-HT2A agonists, it has been found that 5-HT2A and mGlu2 are capable of regulating each other’s downstream signaling pathways via monovalent crosstalk or heteromer formation (Duman et al., 2021). One theory for neuroplastic-induced efficacy with regards to SUDs is a top–down approach, wherein changes to the PFC via neurogenesis, increasing mGlu2 and decreasing 5-HT2A, leads to increased inhibitory control and decreased stress reactivity within dorsal raphe nuclei and the extended amygdala with a subsequent decreased stress response and stress-induced neurodegeneration at the hypothalamus–pituitary–adrenal axis (Urban et al., 2023). The neuroplastic changes in the PFC may additionally signal to the ventral tegmental area and the nAC to decrease corticoaccumbal transmission, thereby reducing impulsivity and drug-seeking behaviors.
Nevertheless, as it is with ibogaine, it is currently not possible to separate the known pharmacological effects from the psychological effects caused by psychedelics, producing altered states of consciousness. Although each of the psychedelics produce their own unique “trips” in terms of duration, physical side effects, and hallucinatory manifestations, the impact of the experience on the user tends to be profound and, for the most part, highly beneficial. Higher psychedelic doses, mystical-type experiences, and greater personal insights are all correlated with a more favorable outcome (Argento et al., 2019; Garcia-Romeu et al., 2019; Hamill et al., 2019). Indeed, even when controlling the intensity of the drug’s effects, mystical-type subjective effects have been found in some studies to be “necessary” for the enduring beneficial efficacy of the drug (Garcia-Romeu et al., 2014; Yaden and Griffiths, 2021). In the psilocybin tobacco cessation study, the authors likened the effect of the mystical experience to an “inverse PTSD-like experience,” in which a singular event was capable of causing lasting behavioral and likely biological benefits to the patients, with one patient stating that it left them “feeling complete as a person and physically part of all things” (Garcia-Romeu et al., 2014). It should be noted that the studies investigating these drugs for changes in mental health or addiction nearly always involve a strong psychotherapeutic component, whether in the form of a religious ceremony or Western psychotherapy. Indeed, ritual users, such as those who partake of ayahuasca as a sacrament in the Uniao do Vegetal (UDV) church, acknowledge that, for them, the two cannot be separated (Grob et al., 1996). Ayahuasca served as a catalyst for change in their lives, but the ritual and community helped solidify their resolutions and outlook on life. Similarly, post-treatment psychotherapy employed by modern researchers serves to help patients integrate the visionary experience into their lives moving forward.
Despite multiple recent clinical trials demonstrating the efficacy of classic psychedelics for a number of neuropsychiatric conditions, these compounds remain as Schedule I drugs. Therefore, there are no set clinical guidelines with regards to patient exclusion criteria, dosing, administration, or medical monitoring, although individual states are beginning to establish these details (OHA, 2023). Likewise, the FDA recently released a draft of the guidelines for the industry to address the necessary and complicated components of conducting clinical investigations with psychedelic drugs (FDA, 2023). Nevertheless, 5-HT2A agonists are generally considered safe and non-addictive. DMT has been shown to increase the heart rate, blood pressure, and anxiety, but these adverse events are mild and self-limited (D'Souza et al., 2022). Ayahuasca, which contains monoamine oxidase inhibitors, harmine and harmaline, in addition to DMT, causes similar short-term cardiovascular responses as DMT alone, along with typically inducing nausea and vomiting shortly after ingestion (Hamill et al., 2019). The emesis portion of the ayahuasca experience is often portrayed as a “cleansing” to prepare the participant for the visions to come. Adverse events reported with psilocybin include headache, nausea, dizziness, and a transient increase in BP and HR, but, as with ayahuasca and DMT, these are self-limited (Goodwin et al., 2022). A recent clinical trial of LSD for anxiety reported mild and transient adverse events during treatment, including anxiety, nausea, and headache (Holze et al., 2023). Overall, the adverse effects of psychedelics tend to be mild and transitory, while the positive benefits to neuropsychiatric disorders and a general outlook on life are rapid and long-lasting. To date, despite the potential, there have been no published results of clinical trials on classic psychedelics for the treatment of OUD, although three trials involving psilocybin and one of MDMA are in the initial and recruiting phases (Table 2). The results of these trials will hopefully add to the growing evidence of the safety and efficacy of classic psychedelics for the treatment of not only neuropsychiatric disorders and substance abuse but also of the person as a whole. Indeed, a couple of these trials (NCT05242029 and NCT05585229) are notable in the sense that they are investigating the potential of psilocybin in patients who are currently using OATs for OUD. Although many patients who seek out treatments like ibogaine, ketamine, or psychedelics for OUD do so because they have found OATs to either be ineffectual or too difficult to adhere to, it is possible that the combination of therapy may be synergistic pharmacologically or that psychoplastogens may enhance the motivation of patients to adhere to the regimen of OATs. On the other hand, the use of these compounds may assist patients in transitioning off of OATs. This is an interesting concept requiring further study.
The opioid epidemic is a crisis at the national level that the government and public health authorities are attempting to combat by increasing funding and access to existing evidence-based prevention and treatment programs while alongside addressing socioeconomic and mental health factors. For patients with OUD, it is a personal battle—one that encompasses their physical and mental health, their finances, their relationships, and their whole lives. New treatment options are desperately needed that can address not only the physical addiction but also patients’ mental health and overall outlook on life. Psychoplastogens, like ibogaine, ketamine, and classic psychedelics, present a novel approach with the potential to treat the patient as a whole with rapid, long-lasting efficacy. As we continue to reevaluate these compounds as medicines rather than drugs of abuse themselves, future clinical trials are needed to establish best-practice guidelines along with their long-term efficacy and safety. Nevertheless, for those suffering with OUD, as well as their friends and family, the potential of these therapies provides hope for a better future.
MH conceived the manuscript. MH and AS wrote sections of the manuscript, contributed to manuscript revision, and read and approved the submitted version. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdallah, C. G., Roache, J. D., Gueorguieva, R., Averill, L. A., Young-McCaughan, S., Shiroma, P. R., et al. (2022). Dose-related effects of ketamine for antidepressant-resistant symptoms of posttraumatic stress disorder in veterans and active duty military: a double-blind, randomized, placebo-controlled multi-center clinical trial. Neuropsychopharmacology 47, 1574–1581. doi:10.1038/s41386-022-01266-9
Abuzzahab, F. S., Sr, , and Anderson, B. J. (1971). A review of LSD treatment in alcoholism. Int. Pharmacopsychiatry 6, 223–235. doi:10.1159/000468273
Ahmed, G. K., Elserogy, Y. M., Elfadl, G. M. A., Ghada Abdelsalam, K., and Ali, M. A. (2023). Antidepressant and anti-suicidal effects of ketamine in treatment-resistant depression associated with psychiatric and personality comorbidities: a double-blind randomized trial. J. Affect Disord. 325, 127–134. doi:10.1016/j.jad.2023.01.005
Aleksandrova, L. R., and Phillips, A. G. (2021). Neuroplasticity as a convergent mechanism of ketamine and classical psychedelics. Trends Pharmacol. Sci. 42, 929–942. doi:10.1016/j.tips.2021.08.003
Alper, K. R., Beal, D., and Kaplan, C. D. (2001). A contemporary history of ibogaine in the United States and Europe. Alkaloids Chem. Biol. 56, 249–281. doi:10.1016/s0099-9598(01)56018-6
Alper, K. R., Lotsof, H. S., Frenken, G. M., Luciano, D. J., and Bastiaans, J. (1999). Treatment of acute opioid withdrawal with ibogaine. Am. J. Addict. 8, 234–242. doi:10.1080/105504999305848
Amato, L., Davoli, M., Minozzi, S., Ferroni, E., Ali, R., and Ferri, M. (2013). Methadone at tapered doses for the management of opioid withdrawal. Cochrane Database Syst. Rev. 2013, Cd003409. doi:10.1002/14651858.CD003409.pub4
Argento, E., Braschel, M., Walsh, Z., Socias, M. E., and Shannon, K. (2018). The moderating effect of psychedelics on the prospective relationship between prescription opioid use and suicide risk among marginalized women. J. Psychopharmacol. 32, 1385–1391. doi:10.1177/0269881118798610
Argento, E., Socias, M. E., Hayashi, K., Choi, J., Mackay, L., Christie, D., et al. (2022). Psychedelic use is associated with reduced daily opioid use among people who use illicit drugs in a Canadian setting. Int. J. Drug Policy 100, 103518. doi:10.1016/j.drugpo.2021.103518
Argento, E., Tupper, K. W., and Socias, M. E. (2019). The tripping point: the potential role of psychedelic-assisted therapy in the response to the opioid crisis. Int. J. Drug Policy 66, 80–81. doi:10.1016/j.drugpo.2018.11.006
Autry, A. E., Adachi, M., Nosyreva, E., Na, E. S., Los, M. F., Cheng, P. F., et al. (2011). NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475, 91–95. doi:10.1038/nature10130
Becker, A. M., Holze, F., Grandinetti, T., Klaiber, A., Toedtli, V. E., Kolaczynska, K. E., et al. (2022). Acute effects of psilocybin after escitalopram or placebo pretreatment in a randomized, double-blind, placebo-controlled, crossover study in healthy subjects. Clin. Pharmacol. Ther. 111, 886–895. doi:10.1002/cpt.2487
Bentzley, B. S., Barth, K. S., Back, S. E., and Book, S. W. (2015). Discontinuation of buprenorphine maintenance therapy: perspectives and outcomes. J. Subst. Abuse Treat. 52, 48–57. doi:10.1016/j.jsat.2014.12.011
Bogenschutz, M. P., Ross, S., Bhatt, S., Baron, T., Forcehimes, A. A., Laska, E., et al. (2022). Percentage of heavy drinking days following psilocybin-assisted psychotherapy vs placebo in the treatment of adult patients with alcohol use disorder: a randomized clinical trial. JAMA Psychiatry 79, 953–962. doi:10.1001/jamapsychiatry.2022.2096
Brown, T. K., and Alper, K. (2018). Treatment of opioid use disorder with ibogaine: detoxification and drug use outcomes. Am. J. Drug Alcohol Abuse 44, 24–36. doi:10.1080/00952990.2017.1320802
Brown, T. K. (2013). Ibogaine in the treatment of substance dependence. Curr. Drug Abuse Rev. 6, 3–16. doi:10.2174/15672050113109990001
Brown, T. K., Noller, G. E., and Denenberg, J. O. (2019). Ibogaine and subjective experience: transformative states and psychopharmacotherapy in the treatment of opioid use disorder. J. Psychoact. Drugs 51, 155–165. doi:10.1080/02791072.2019.1598603
Browne, C. A., and Lucki, I. (2013). Antidepressant effects of ketamine: mechanisms underlying fast-acting novel antidepressants. Front. Pharmacol. 4, 161. doi:10.3389/fphar.2013.00161
Cameron, L. P., Tombari, R. J., Lu, J., Pell, A. J., Hurley, Z. Q., Ehinger, Y., et al. (2021). A non-hallucinogenic psychedelic analogue with therapeutic potential. Nature 589, 474–479. doi:10.1038/s41586-020-3008-z
Camlin, T., Eulert, D., Horvath, A. T., Bucky, S. F., Barsulglia, J. P., and Polanco, M. (2018). A phenomenological investigation into the lived experience of ibogaine and its potential to treat opioid use disorders. J. Psychedelic Stud. 2, 24–35. doi:10.1556/2054.2018.004
Chawarski, M. C., Hawk, K., Edelman, E. J., O'Connor, P., Owens, P., Martel, S., et al. (2020). Use of amphetamine-type stimulants among emergency department patients with untreated opioid use disorder. Ann. Emerg. Med. 76, 782–787. doi:10.1016/j.annemergmed.2020.06.046
Cloutier-Gill, L., Wood, E., Millar, T., Ferris, C., and Eugenia Socias, M. (2016). Remission of severe opioid use disorder with ibogaine: a case report. J. Psychoact. Drugs 48, 214–217. doi:10.1080/02791072.2016.1180467
Cohen, S. P., Bhatia, A., Buvanendran, A., Schwenk, E. S., Wasan, A. D., Hurley, R. W., et al. (2018). Consensus guidelines on the use of intravenous ketamine infusions for chronic pain from the American society of regional anesthesia and pain medicine, the American academy of pain medicine, and the American society of anesthesiologists. Reg. Anesth. Pain Med. 43, 521–546. doi:10.1097/AAP.0000000000000808
Curran, H. V., and Morgan, C. (2000). Cognitive, dissociative and psychotogenic effects of ketamine in recreational users on the night of drug use and 3 days later. Addiction 95, 575–590. doi:10.1046/j.1360-0443.2000.9545759.x
D'Souza, D. C., Syed, S. A., Flynn, L. T., Safi-Aghdam, H., Cozzi, N. V., and Ranganathan, M. (2022). Exploratory study of the dose-related safety, tolerability, and efficacy of dimethyltryptamine (DMT) in healthy volunteers and major depressive disorder. Neuropsychopharmacology 47, 1854–1862. doi:10.1038/s41386-022-01344-y
Dakwar, E., Levin, F., Foltin, R. W., Nunes, E. V., and Hart, C. L. (2014). The effects of subanesthetic ketamine infusions on motivation to quit and cue-induced craving in cocaine-dependent research volunteers. Biol. Psychiatry 76, 40–46. doi:10.1016/j.biopsych.2013.08.009
Davis, A. K., Renn, E., Windham-Herman, A. M., Polanco, M., and Barsuglia, J. P. (2018). A mixed-method analysis of persisting effects associated with positive outcomes following ibogaine detoxification. J. Psychoact. Drugs 50, 287–297. doi:10.1080/02791072.2018.1487607
Dickinson, J., McAlpin, J., Wilkins, C., Fitzsimmons, C., Guion, P., Paterson, T., et al. (2016). Clinical guidelines for ibogaine-assisted detoxification. Montreal, Quebec: Global Ibogaine Therapy Alliance.
Domanegg, K., Sommer, W. H., and Meinhardt, M. W. (2023). Psychedelic targeting of metabotropic glutamate receptor 2 and its implications for the treatment of alcoholism. Alcohol. Cells 12, 963. doi:10.3390/cells12060963
Dos Santos, R. G., Osorio, F. L., Crippa, J. A., Riba, J., Zuardi, A. W., and Hallak, J. E. (2016). Antidepressive, anxiolytic, and antiaddictive effects of ayahuasca, psilocybin and lysergic acid diethylamide (LSD): a systematic review of clinical trials published in the last 25 years. Ther. Adv. Psychopharmacol. 6, 193–213. doi:10.1177/2045125316638008
Duman, R. S., Deyama, S., and Fogaca, M. V. (2021). Role of BDNF in the pathophysiology and treatment of depression: activity-dependent effects distinguish rapid-acting antidepressants. Eur. J. Neurosci. 53, 126–139. doi:10.1111/ejn.14630
Dundee, J. W., Knox, J. W., Black, G. W., Moore, J., Pandit, S. K., Bovill, J., et al. (1970). Ketamine as an induction agent in anaesthetics. Lancet 1, 1370–1371. doi:10.1016/s0140-6736(70)91273-0
FDA (2023). Psychedelic drugs: Considerations for clinical investigations guidance for industry (draft guidance). U. S. D. o. H. a. H. Services.
Franks, N. P., and Lieb, W. R. (1994). Molecular and cellular mechanisms of general anaesthesia. Nature 367, 607–614. doi:10.1038/367607a0
Garcia-Romeu, A., Davis, A. K., Erowid, E., Erowid, F., Griffiths, R. R., and Johnson, M. W. (2019). Persisting reductions in cannabis, opioid, and stimulant misuse after naturalistic psychedelic use: an online Survey. Front. Psychiatry 10, 955. doi:10.3389/fpsyt.2019.00955
Garcia-Romeu, A., Griffiths, R. R., and Johnson, M. W. (2014). Psilocybin-occasioned mystical experiences in the treatment of tobacco addiction. Curr. Drug Abuse Rev. 7, 157–164. doi:10.2174/1874473708666150107121331
Goldstein, R. Z., and Volkow, N. D. (2011). Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat. Rev. Neurosci. 12, 652–669. doi:10.1038/nrn3119
Goodwin, G. M., Aaronson, S. T., Alvarez, O., Arden, P. C., Baker, A., Bennett, J. C., et al. (2022). Single-dose psilocybin for a treatment-resistant episode of major depression. N. Engl. J. Med. 387, 1637–1648. doi:10.1056/NEJMoa2206443
Goodwin, G. M., Aaronson, S. T., Alvarez, O., Atli, M., Bennett, J. C., Croal, M., et al. (2023). Single-dose psilocybin for a treatment-resistant episode of major depression: impact on patient-reported depression severity, anxiety, function, and quality of life. J. Affect Disord. 327, 120–127. doi:10.1016/j.jad.2023.01.108
Grob, C. S., McKenna, D. J., Callaway, J. C., Brito, G. S., Neves, E. S., Oberlaender, G., et al. (1996). Human psychopharmacology of hoasca, a plant hallucinogen used in ritual context in Brazil. J. Nerv. Ment. Dis. 184, 86–94. doi:10.1097/00005053-199602000-00004
Hamill, J., Hallak, J., Dursun, S. M., and Baker, G. (2019). Ayahuasca: psychological and physiologic effects, pharmacology and potential uses in addiction and mental illness. Curr. Neuropharmacol. 17, 108–128. doi:10.2174/1570159X16666180125095902
Hare, B. D., and Duman, R. S. (2020). Prefrontal cortex circuits in depression and anxiety: contribution of discrete neuronal populations and target regions. Mol. Psychiatry 25, 2742–2758. doi:10.1038/s41380-020-0685-9
He, D. Y., McGough, N. N., Ravindranathan, A., Jeanblanc, J., Logrip, M. L., Phamluong, K., et al. (2005). Glial cell line-derived neurotrophic factor mediates the desirable actions of the anti-addiction drug ibogaine against alcohol consumption. J. Neurosci. 25, 619–628. doi:10.1523/JNEUROSCI.3959-04.2005
Heifets, B. D., Bentzley, B. S., Williams, N., and Schatzberg, A. F. (2021). Unraveling the opioid actions of S-ketamine and R-ketamine: comment on bonaventura et al. Mol. Psychiatry 26, 6104–6106. doi:10.1038/s41380-021-01167-1
Holze, F., Gasser, P., Muller, F., Dolder, P. C., and Liechti, M. E. (2023). Lysergic acid diethylamide-assisted therapy in patients with anxiety with and without a life-threatening illness: a randomized, double-blind, placebo-controlled phase II study. Biol. Psychiatry 93, 215–223. doi:10.1016/j.biopsych.2022.08.025
Hser, Y. I., Evans, E., Huang, D., Weiss, R., Saxon, A., Carroll, K. M., et al. (2016). Long-term outcomes after randomization to buprenorphine/naloxone versus methadone in a multi-site trial. Addiction 111, 695–705. doi:10.1111/add.13238
Johnson, M. W., Garcia-Romeu, A., and Griffiths, R. R. (2017). Long-term follow-up of psilocybin-facilitated smoking cessation. Am. J. Drug Alcohol Abuse 43, 55–60. doi:10.3109/00952990.2016.1170135
Jones, C. M., Compton, W. M., Han, B., Baldwin, G., and Volkow, N. D. (2022a). Methadone-involved overdose deaths in the US before and after federal policy changes expanding take-home methadone doses from opioid treatment programs. JAMA Psychiatry 79, 932–934. doi:10.1001/jamapsychiatry.2022.1776
Jones, G., Ricard, J. A., Lipson, J., and Nock, M. K. (2022b). Associations between classic psychedelics and opioid use disorder in a nationally-representative U.S. adult sample. Sci. Rep. 12, 4099. doi:10.1038/s41598-022-08085-4
Knuijver, T., Schellekens, A., Belgers, M., Donders, R., van Oosteren, T., Kramers, K., et al. (2022). Safety of ibogaine administration in detoxification of opioid-dependent individuals: a descriptive open-label observational study. Addiction 117, 118–128. doi:10.1111/add.15448
Koenig, X., and Hilber, K. (2015). The anti-addiction drug ibogaine and the heart: a delicate relation. Molecules 20, 2208–2228. doi:10.3390/molecules20022208
Kohrs, R., and Durieux, M. E. (1998). Ketamine: teaching an old drug new tricks. Anesth. Analg. 87, 1186–1193. doi:10.1097/00000539-199811000-00039
Krebs, T. S., and Johansen, P. O. (2012). Lysergic acid diethylamide (LSD) for alcoholism: meta-analysis of randomized controlled trials. J. Psychopharmacol. 26, 994–1002. doi:10.1177/0269881112439253
Krupitsky, E., Burakov, A., Romanova, T., Dunaevsky, I., Strassman, R., and Grinenko, A. (2002). Ketamine psychotherapy for heroin addiction: immediate effects and two-year follow-up. J. Subst. Abuse Treat. 23, 273–283. doi:10.1016/s0740-5472(02)00275-1
Krupitsky, E. M., Burakov, A. M., Dunaevsky, I. V., Romanova, T. N., Slavina, T. Y., and Grinenko, A. Y. (2007). Single versus repeated sessions of ketamine-assisted psychotherapy for people with heroin dependence. J. Psychoact. Drugs 39, 13–19. doi:10.1080/02791072.2007.10399860
Krupitsky, E. M., and Grinenko, A. Y. (1997). Ketamine psychedelic therapy (KPT): a review of the results of ten years of research. J. Psychoact. Drugs 29, 165–183. doi:10.1080/02791072.1997.10400185
Li, N., Lee, B., Liu, R. J., Banasr, M., Dwyer, J. M., Iwata, M., et al. (2010). mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329, 959–964. doi:10.1126/science.1190287
Loo, C. K., Galvez, V., O'Keefe, E., Mitchell, P. B., Hadzi-Pavlovic, D., Leyden, J., et al. (2016). Placebo-controlled pilot trial testing dose titration and intravenous, intramuscular and subcutaneous routes for ketamine in depression. Acta Psychiatr. Scand. 134, 48–56. doi:10.1111/acps.12572
Luz, M., and Mash, D. C. (2021). Evaluating the toxicity and therapeutic potential of ibogaine in the treatment of chronic opioid abuse. Expert Opin. Drug Metab. Toxicol. 17, 1019–1022. doi:10.1080/17425255.2021.1944099
Ly, C., Greb, A. C., Cameron, L. P., Wong, J. M., Barragan, E. V., Wilson, P. C., et al. (2018). Psychedelics promote structural and functional neural plasticity. Cell Rep. 23, 3170–3182. doi:10.1016/j.celrep.2018.05.022
Malcolm, B. J., Polanco, M., and Barsuglia, J. P. (2018). Changes in withdrawal and craving scores in participants undergoing opioid detoxification utilizing ibogaine. J. Psychoact. Drugs 50, 256–265. doi:10.1080/02791072.2018.1447175
Mash, D. C., Kovera, C. A., Pablo, J., Tyndale, R. F., Ervin, F. D., Williams, I. C., et al. (2000). Ibogaine: complex pharmacokinetics, concerns for safety, and preliminary efficacy measures. Ann. N. Y. Acad. Sci. 914, 394–401. doi:10.1111/j.1749-6632.2000.tb05213.x
Mion, G., and Villevieille, T. (2013). Ketamine pharmacology: an update (pharmacodynamics and molecular aspects, recent findings). CNS Neurosci. Ther. 19, 370–380. doi:10.1111/cns.12099
Mitchell, J. M., Bogenschutz, M., Lilienstein, A., Harrison, C., Kleiman, S., Parker-Guilbert, K., et al. (2021). MDMA-Assisted therapy for severe PTSD: a randomized, double-blind, placebo-controlled phase 3 study. Nat. Med. 27, 1025–1033. doi:10.1038/s41591-021-01336-3
Morgan, C. J., and Curran, H. V.Independent Scientific Committee on Drugs (2012). Ketamine use: a review. Addiction 107, 27–38. doi:10.1111/j.1360-0443.2011.03576.x
Naughton, M., Clarke, G., O'Leary, O. F., Cryan, J. F., and Dinan, T. G. (2014). A review of ketamine in affective disorders: current evidence of clinical efficacy, limitations of use and pre-clinical evidence on proposed mechanisms of action. J. Affect Disord. 156, 24–35. doi:10.1016/j.jad.2013.11.014
Nicholas, C. R., Wang, J. B., Coker, A., Mitchell, J. M., Klaire, S. S., Yazar-Klosinski, B., et al. (2022). The effects of MDMA-assisted therapy on alcohol and substance use in a phase 3 trial for treatment of severe PTSD. Drug Alcohol Depend. 233, 109356. doi:10.1016/j.drugalcdep.2022.109356
NIDA. (2023). "Trends and statistics: drug overdose death rates." Retrieved April 19, 2023, Available at: https://nida.nih.gov/research-topics/trends-statistics/overdose-death-rates.
NIH (2023). Retrieved April 24, 2023, Available at: https://clinicaltrials.gov/.
Noller, G. E., Frampton, C. M., and Yazar-Klosinski, B. (2018). Ibogaine treatment outcomes for opioid dependence from a twelve-month follow-up observational study. Am. J. Drug Alcohol Abuse 44, 37–46. doi:10.1080/00952990.2017.1310218
Nowacka, A., and Borczyk, M. (2019). Ketamine applications beyond anesthesia - a literature review. Eur. J. Pharmacol. 860, 172547. doi:10.1016/j.ejphar.2019.172547
O'Shaughnessy, D. M., Berlowitz, I., Rodd, R., Sarnyai, Z., and Quirk, F. (2021). Within-treatment changes in a novel addiction treatment program using traditional Amazonian medicine. Ther. Adv. Psychopharmacol. 11, 2045125320986634. doi:10.1177/2045125320986634
OHA. (2023). "Oregon psilocybin services." Retrieved April 28, 2023, Available at: https://www.oregon.gov/oha/ph/preventionwellness/pages/oregon-psilocybin-services.aspx.
Olson, D. E. (2022). Biochemical mechanisms underlying psychedelic-induced neuroplasticity. Biochemistry 61, 127–136. doi:10.1021/acs.biochem.1c00812
Pacheco Dda, F., Romero, T. R., and Duarte, I. D. (2014). Central antinociception induced by ketamine is mediated by endogenous opioids and mu- and delta-opioid receptors. Brain Res. 1562, 69–75. doi:10.1016/j.brainres.2014.03.026
Pisano, V. D., Putnam, N. P., Kramer, H. M., Franciotti, K. J., Halpern, J. H., and Holden, S. C. (2017). The association of psychedelic use and opioid use disorders among illicit users in the United States. J. Psychopharmacol. 31, 606–613. doi:10.1177/0269881117691453
Reinstatler, L., and Youssef, N. A. (2015). Ketamine as a potential treatment for suicidal ideation: a systematic review of the literature. Drugs R. D. 15, 37–43. doi:10.1007/s40268-015-0081-0
SAMHSA (2022). Key substance use and mental health indicators in the United States: results from the 2021 national Survey on drug use and health. Rockville, MD: M. H. S. Administration.
Sanacora, G., Frye, M. A., McDonald, W., Mathew, S. J., Turner, M. S., Schatzberg, A. F., et al. (2017). A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry 74, 399–405. doi:10.1001/jamapsychiatry.2017.0080
Savage, C., and McCabe, O. L. (1973). Residential psychedelic (LSD) therapy for the narcotic addict. A controlled study. Arch. Gen. Psychiatry 28, 808–814. doi:10.1001/archpsyc.1973.01750360040005
Schwenk, E. S., Viscusi, E. R., Buvanendran, A., Hurley, R. W., Wasan, A. D., Narouze, S., et al. (2018). Consensus guidelines on the use of intravenous ketamine infusions for acute pain management from the American society of regional anesthesia and pain medicine, the American academy of pain medicine, and the American society of anesthesiologists. Reg. Anesth. Pain Med. 43, 456–466. doi:10.1097/AAP.0000000000000806
Serafini, G., Howland, R. H., Rovedi, F., Girardi, P., and Amore, M. (2014). The role of ketamine in treatment-resistant depression: a systematic review. Curr. Neuropharmacol. 12, 444–461. doi:10.2174/1570159X12666140619204251
Sessa, B., Higbed, L., O'Brien, S., Durant, C., Sakal, C., Titheradge, D., et al. (2021). First study of safety and tolerability of 3,4-methylenedioxymethamphetamine-assisted psychotherapy in patients with alcohol use disorder. J. Psychopharmacol. 35, 375–383. doi:10.1177/0269881121991792
Shao, L. X., Liao, C., Gregg, I., Davoudian, P. A., Savalia, N. K., Delagarza, K., et al. (2021). Psilocybin induces rapid and persistent growth of dendritic spines in frontal cortex in vivo. Neuron 109, 2535–2544.e4. doi:10.1016/j.neuron.2021.06.008
Strasburger, S. E., Bhimani, P. M., Kaabe, J. H., Krysiak, J. T., Nanchanatt, D. L., Nguyen, T. N., et al. (2017). What is the mechanism of ketamine's rapid-onset antidepressant effect? A concise overview of the surprisingly large number of possibilities. J. Clin. Pharm. Ther. 42, 147–154. doi:10.1111/jcpt.12497
Udesky, J. O., Spence, N. Z., Achiel, R., Lee, C., and Flood, P. (2005). The role of nicotinic inhibition in ketamine-induced behavior. Anesth. Analg. 101, 407–411. doi:10.1213/01.ANE.0000155291.81338.90
Underwood, M. S., Bright, S. J., and Lancaster, B. L. (2021). A narrative review of the pharmacological, cultural and psychological literature on ibogaine. J. Psychedelic Stud. 5, 44–54. doi:10.1556/2054.2021.00152
Urban, M. M., Stingl, M. R., and Meinhardt, M. W. (2023). Mini-review: the neurobiology of treating substance use disorders with classical psychedelics. Front. Neurosci. 17, 1156319. doi:10.3389/fnins.2023.1156319
Uthaug, M. V., Mason, N. L., Toennes, S. W., Reckweg, J. T., de Sousa Fernandes Perna, E. B., Kuypers, K. P. C., et al. (2021). A placebo-controlled study of the effects of ayahuasca, set and setting on mental health of participants in ayahuasca group retreats. Psychopharmacol. Berl. 238, 1899–1910. doi:10.1007/s00213-021-05817-8
van Elk, M., and Yaden, D. B. (2022). Pharmacological, neural, and psychological mechanisms underlying psychedelics: a critical review. Neurosci. Biobehav Rev. 140, 104793. doi:10.1016/j.neubiorev.2022.104793
Vargas, M. V., Dunlap, L. E., Dong, C., Carter, S. J., Tombari, R. J., Jami, S. A., et al. (2023). Psychedelics promote neuroplasticity through the activation of intracellular 5-HT2A receptors. Science 379, 700–706. doi:10.1126/science.adf0435
Vargas-Perez, H., Bahi, A., Bufalino, M. R., Ting, A. K. R., Maal-Bared, G., Lam, J., et al. (2014). BDNF signaling in the VTA links the drug-dependent state to drug withdrawal aversions. J. Neurosci. 34, 7899–7909. doi:10.1523/JNEUROSCI.3776-13.2014
Weiss, R. D., Potter, J. S., Fiellin, D. A., Byrne, M., Connery, H. S., Dickinson, W., et al. (2011). Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: a 2-phase randomized controlled trial. Arch. Gen. Psychiatry 68, 1238–1246. doi:10.1001/archgenpsychiatry.2011.121
Wilson, C., Millar, T., and Matieschyn, Z. (2020). Novel treatment of opioid use disorder using ibogaine and iboga in two adults. J. Psychedelic Stud. 4, 149–155. doi:10.1556/2054.2020.00133
Yaden, D. B., and Griffiths, R. R. (2021). The subjective effects of psychedelics are necessary for their enduring therapeutic effects. ACS Pharmacol. Transl. Sci. 4, 568–572. doi:10.1021/acsptsci.0c00194
Keywords: opioid use disorder, psychoplastogens, ibogaine, ketamine, psychedelic agents
Citation: Hornick MG and Stefanski A (2023) Hallucinogenic potential: a review of psychoplastogens for the treatment of opioid use disorder. Front. Pharmacol. 14:1221719. doi: 10.3389/fphar.2023.1221719
Received: 12 May 2023; Accepted: 09 August 2023;
Published: 22 August 2023.
Edited by:
Cody Wenthur, University of Wisconsin-Madison, United StatesReviewed by:
Javier Gonzalez-Maeso, Virginia Commonwealth University, United StatesCopyright © 2023 Hornick and Stefanski. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mary G. Hornick, bWhvcm5pY2swMkByb29zZXZlbHQuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.