- Department of Pharmacy, West China Hospital, Sichuan University, Chengdu, Sichuan, China
Background: Older patients with dementia always need multiple drugs due to comorbidities and cognitive impairment, further complicating drug treatment and increasing the risk of potentially inappropriate medication. The objective of our study is to estimate the global prevalence of polypharmacy and potentially inappropriate medication (PIM) and explore the factors of PIM for older patients with dementia.
Methods: We searched PubMed, Embase (Ovid), and Web of Science databases to identify eligible studies from inception to 16 June 2023. We conducted a meta-analysis for observational studies reporting the prevalence of potentially inappropriate medication and polypharmacy in older patients with dementia using a random-effect model. The factors associated with PIM were meta-analyzed.
Results: Overall, 62 eligible studies were included, of which 53 studies reported the prevalence of PIM and 28 studies reported the prevalence of polypharmacy. The pooled estimate of PIM and polypharmacy was 43% (95% CI 38–48) and 62% (95% CI 52–71), respectively. Sixteen studies referred to factors associated with PIM use, and 15 factors were further pooled. Polypharmacy (2.83, 95% CI 1.80–4.44), diabetes (1.31, 95% CI 1.04–1.65), heart failure (1.17, 95% CI 1.00–1.37), depression (1.45, 95% CI 1.14–1.88), history of cancer (1.20, 95% CI 1.09–1.32), hypertension (1.46, 95% CI 1.05–2.03), ischemic heart disease (1.55, 95% CI 0.77–3.12), any cardiovascular disease (1.11, 95% CI 1.06–1.17), vascular dementia (1.09, 95% CI 1.03–1.16), chronic obstructive pulmonary disease (1.39, 95% CI 1.13–1.72), and psychosis (1.91, 95% CI 1.04–3.53) are positively associated with PIM use.
Conclusion: PIM and polypharmacy were highly prevalent in older patients with dementia. Among different regions, the pooled estimate of PIM use and polypharmacy varied widely. Increasing PIM in older patients with dementia was closely associated with polypharmacy. For other comorbidities such as heart failure and diabetes, prescribing should be cautioned.
1 Introduction
The statistics of epidemiology revealed that in 2019, there were 703 million individuals aged 65 years or above living in the world, and the number was expected to reach 1.5 billion by 2050 (He and Kinsella, 2020). The global population aging would further accelerate the increase in the geriatric population, which imposed significant demands on the healthcare system. Meanwhile, increased medication use is one of the important challenges (Chiatti et al., 2012; Johnell, 2015).
Polypharmacy is defined as the concurrent use of multiple drugs, generally taking five or more drugs (Masnoon et al., 2017; Rankin et al., 2018). The older population often suffered multiple diseases, and polypharmacy was insufficient for controlling or curing diseases. A cross-sectional study performed by Chandrasekhar reported that the prevalence of polypharmacy in 210 inpatients aged 65 years or above was up to 60% and that of hyperpolypharmacy (ten or more drugs) was 35.7% (Chandrasekhar et al., 2019). Moreover, aging-related alteration in pharmacokinetics and pharmacodynamics might lead to stronger drug effects and prolongation of drug action time (Shi et al., 2008). Thus, the management of adverse effects on multiple drugs and potential drug–drug interaction among the older population was rather complicated and challenging.
Dementia, a degenerative nervous system disorder, features irreversible decline in cognitive function (Prince et al., 2013). Patients diagnosed with dementia were especially sensitive to adverse effects of central nervous system (CNS) drugs (Bell et al., 2012). Communication disorder caused by cognitive impairment and concomitant mental symptoms would lead to more complicated drug use in patients with dementia (Johnell, 2015). Furthermore, compared with non-dementia, dementia was more likely to be accompanied by other chronic diseases, such as hypertension and diabetes, exposing a higher risk of polypharmacy (Clague et al., 2017). Banta et al. indicated older patients with dementia are more likely to have five or more current prescriptions (Banta, 2017). Therefore, we should attach great importance to drug medications for older patients with dementia.
Potentially inappropriate medication (PIM) is an important concept to assess the quality of drug use. The term was first proposed by the American Panel in 1991, defined as those drugs with potential risks outweighing the benefits (Renom-Guiteras et al., 2015). A large number of studies have demonstrated PIM was associated with drug-related problems and adverse outcomes, such as increasing risk of hospitalization and death and incurring extra medical expenditure (Hagstrom et al., 2015; Hyttinen et al., 2017; Murphy et al., 2020). In order to evaluate PIM use and avoid the occurrence of adverse events, several explicit tools based on expert census were developed. A systematic review showed a total of 46 screening lists of PIM in the world that were identified, covering four continents and 13 countries (Kaufmann et al., 2014). The most frequently used criteria were Beers criteria and STOPP/START criteria (American Geriatrics Society Beers Criteria® Update Expert Panel, 2019; O'Mahony et al., 2018), including drugs that should be avoided for treating common systemic diseases in elderly patients, possible adverse reactions, drug–drug interactions, drug–disease interactions, and risky drug use based on the renal function level. PIM use among older people was prevalent, especially in frail patients with dementia who need long-term care (Kristensen et al., 2018). Due to the difference of medical habits in each country and screening tools, the prevalence of PIM in patients with dementia varied widely. In European countries, 60% of older patients with dementia had at least one PIM based on the European Union (7)-PIM list (Renom-Guiteras et al., 2018). In China, the prevalence of PIM was 39.43% evaluated using 2019 Beers criteria (Zhao et al., 2022). Thus, a pooled analysis is necessary to conduct for evaluating the PIM and polypharmacy in patients with dementia, further providing a reference for countries that have not yet carried out a relevant study about drug burden in older patients with dementia.
To date, several reviews about PIM use and polypharmacy in dementia have been published (Johnell, 2015; Disalvo et al., 2016; Redston et al., 2018; Hukins et al., 2019; Delgado et al., 2020), but limited to a specific type of dementia or specific population (such as community or inpatients), or only qualitatively described the prevalence of PIM use or polypharmacy. In the systematic review and meta-analysis, we first summarized the pooled estimate of PIM and polypharmacy in older patients with dementia (not including mild cognitive impairment) across different regions and explored the association between PIM and polypharmacy and reviewed other factors associated with PIM use.
2 Methods
The study protocol of this systematic review and meta-analysis was registered on PROSPERO (CRD42022368310). The study was conducted based on MOOSE (Meta-analysis of Observational Studies in Epidemiology) guidelines (Stroup et al., 2000) and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (Moher et al., 2015).
2.1 Search strategy
A comprehensive search of PubMed, Embase (Ovid), and Web of Science was performed from inception to 16 June 2023. The search strategy was using a combination of Medical Subject Headings (MeSH) and free text words. The specific search details in different databases are listed in Supplementary Table S1. In addition, we performed manual searching of selected published full-text reviews, identifying other potential relevant articles.
2.2 Selection criteria
Studies were included if they recruited older adults with dementia (≥65 years, or mean age ≥70 years), reported the prevalence of polypharmacy (five or more) or PIM in dementia, used explicit criteria to identify PIM, and wrote the manuscript in English. The diagnosis of dementia was based on medical records, DSM criteria, ICD code, or other criteria. In addition, the study design of the included articles was observational studies (cross-sectional study or cohort study).
Studies were excluded if study subjects had mild cognitive impairment, or if those studies were conference abstracts, reviews, and comments.
2.3 Data extraction
Two reviewers (MN Zhao and ZY Chen) independently extracted and verified the data. We extracted information including study characteristics (first author, year of publication, and country), basic information of study subjects (age, sex, and sample size), and study design (setting, prevalence of polypharmacy or PIM, and explicit criteria to evaluate PIM). Any discrepancy between two reviewers was resolved by consultation with a third reviewer (FY, Tian).
2.4 Selection of studies
Two reviewers (MN Zhao and ZY Chen) independently screened the titles and abstracts of initially included literature according to the inclusion and exclusion criteria. The full text was further assessed if the eligibility of the study was not clearly determined from the abstract. Any inconsistency in the process of screening was resolved by consulting a third senior investigator (Ting Xu).
2.5 Quality assessment
The methodological quality of the cross-sectional study was evaluated using the Agency for Healthcare Research and Quality (AHRQ) (Rostom et al., 2004; Hu et al., 2015). A total of 11 items were listed in AHRQ, including 1) the source of data; 2) eligible criteria for study subjects; 3) time period for included population; 4) whether or not subjects were consecutive; 5) whether the outcome indicators are affected by other factors; 6) any assessments for quality assurance; 7) explanation for excluding any patients from the analysis; 8) measurements taken for controlling confounding factors; 9) description for the handing of missing data; 10) summary for patient response rate and completeness of data collection; 11) clarification of follow-up results. The highest score was 11, while the lowest score was 0. If the score was 8 or above, this study was considered high quality. If the score was 3 or below, this study was considered low quality. If the score was between 3 and 8, this study was considered medium quality. The methodological quality of the cohort study was evaluated by the Newcastle–Ottawa scale (Stang, 2010). If the NOS score ≥8, this study was considered high quality. If the NOS score ≤5, this study was considered low quality. If the NOS score was 6 or 7, this study was considered medium quality (Bedaso and Duko, 2022).
2.6 Statistical analysis
We applied STATA, version 16 (Stata Corporation, College Station, Texas, United States) to perform a meta-analysis for polypharmacy and PIM. The pooled prevalence estimate was reported as a proportion with 95% confidence intervals (CI). The I2 statistics was used to assess the magnitude of heterogeneity. When I2 >50%, heterogeneity among studies was considered large and the DerSimonian–Laird random-effect model was applied in analysis. In case of significant heterogeneity, subgroup analysis (e.g., regions, the proportion of females, criteria, and severity of dementia)) was performed to investigate the source of heterogeneity. We also estimated the 95% prediction interval, which further accounts for between-study heterogeneity and evaluates the uncertainty for the effect that would be expected in a new study addressing that same association (Higgins et al., 2009; Migliavaca et al., 2022). Furthermore, a pooled odds ratio was used to analyze the association between PIM and factors when two or more studies reported the same and adjusted odds ratio. Regarding the risk of publication bias, we adopted Egger’s and Begg’s tests for evaluation.
3 Results
3.1 Study selection
Overall, 5,642 records were initially obtained through PubMed, Embase, and Web of Science databases. After removing duplication (n = 1,302), 4,337 records were used to screen the title and abstract. Finally, 62 studies (Zuckerman et al., 2005; Raivio et al., 2006; Holmes et al., 2008; Chan et al., 2009; Lau et al., 2010; Somers et al., 2010; Tjia et al., 2010; Andersen et al., 2011; Lau et al., 2011; Bosboom et al., 2012; Colloca et al., 2012; Parsons et al., 2012; Thorpe et al., 2012; Fiss et al., 2013; Koyama et al., 2013; Montastruc et al., 2013; Toscani et al., 2013; Tjia et al., 2014; Hanlon et al., 2015; Skoldunger et al., 2015; Barry et al., 2016; Cross et al., 2016; Walsh et al., 2016; Chuang et al., 2017; Clague et al., 2017; Hyttinen et al., 2017; Kanagaratnam et al., 2017; Oesterhus et al., 2017; Ramsey et al., 2017; Wucherer et al., 2017; Kristensen et al., 2018; Nguyen et al., 2018; Renom-Guiteras et al., 2018; Bala et al., 2019; Brimelow et al., 2019; Denholm et al., 2019; Eshetie et al., 2019a; Kristensen et al., 2019; Soysal et al., 2019; Eshetie et al., 2020; Eshetie et al., 2020; Forgerini et al., 2020; Murphy et al., 2020; Rausch and Hoffmann, 2020; Ruangritchankul et al., 2020; Delgado et al., 2021; Ferreira et al., 2021; Gareri et al., 2021; Growdon et al., 2021; Jaramillo-Hidalgo et al., 2021; Kristensen et al., 2021; Thapaliya et al., 2021; Vickers et al., 2021; Buckley et al., 2022; Chao et al., 2022; Delgado et al., 2022; Rangfast et al., 2022; Riedl et al., 2022; Yoon et al., 2022; Zhao et al., 2022; Bae-Shaaw et al., 2023; Ryskina et al., 2023) were included based on the eligibility criteria after thoroughly reading the full text (n = 122). The flow diagram of literature screening is shown in Figure 1.
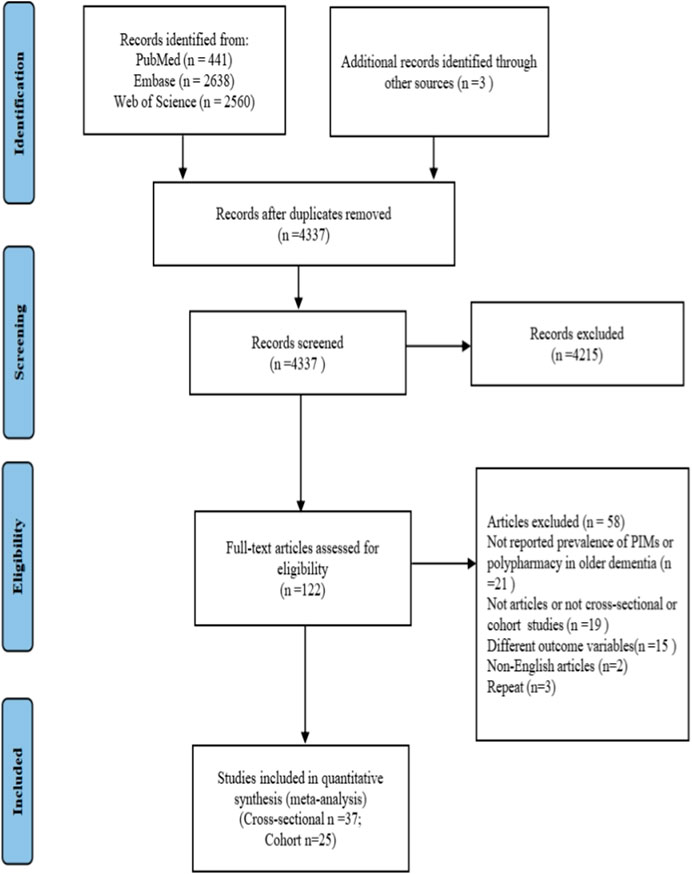
FIGURE 1. PRISMA flow diagram. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; PIM, potentially inappropriate medication.
3.2 Characteristics of included studies
Of all included studies, 53 studies reported the prevalence of PIM (Zuckerman et al., 2005; Raivio et al., 2006; Holmes et al., 2008; Chan et al., 2009; Lau et al., 2010; Somers et al., 2010; Tjia et al., 2010; Andersen et al., 2011; Lau et al., 2011; Bosboom et al., 2012; Colloca et al., 2012; Parsons et al., 2012; Thorpe et al., 2012; Fiss et al., 2013; Koyama et al., 2013; Montastruc et al., 2013; Toscani et al., 2013; Tjia et al., 2014; Hanlon et al., 2015; Skoldunger et al., 2015; Barry et al., 2016; Cross et al., 2016; Chuang et al., 2017; Hyttinen et al., 2017; Oesterhus et al., 2017; Ramsey et al., 2017; Wucherer et al., 2017; Kristensen et al., 2018; Nguyen et al., 2018; Renom-Guiteras et al., 2018; Eshetie et al., 2019a; Bala et al., 2019; Brimelow et al., 2019; Denholm et al., 2019; Eshetie et al., 2020; Eshetie et al., 2020; Forgerini et al., 2020; Murphy et al., 2020; Rausch and Hoffmann, 2020; Ruangritchankul et al., 2020; Delgado et al., 2021; Ferreira et al., 2021; Jaramillo-Hidalgo et al., 2021; Kristensen et al., 2021; Vickers et al., 2021; Buckley et al., 2022; Delgado et al., 2022; Rangfast et al., 2022; Riedl et al., 2022; Yoon et al., 2022; Zhao et al., 2022; Bae-Shaaw et al., 2023; Ryskina et al., 2023), and 28 studies reported the prevalence of polypharmacy (Lau et al., 2010; Somers et al., 2010; Lau et al., 2011; Bosboom et al., 2012; Montastruc et al., 2013; Hanlon et al., 2015; Cross et al., 2016; Walsh et al., 2016; Clague et al., 2017; Kanagaratnam et al., 2017; Oesterhus et al., 2017; Kristensen et al., 2018; Nguyen et al., 2018; Kristensen et al., 2019; Soysal et al., 2019; Forgerini et al., 2020; Rausch and Hoffmann, 2020; Ruangritchankul et al., 2020; Ferreira et al., 2021; Gareri et al., 2021; Growdon et al., 2021; Jaramillo-Hidalgo et al., 2021; Thapaliya et al., 2021; Vickers et al., 2021; Chao et al., 2022; Delgado et al., 2022; Riedl et al., 2022; Zhao et al., 2022). The sample size ranged from 34 to 259,291, comprising a total of 658,431 study subjects. Most studies (n = 30) were conducted in Europe (Raivio et al., 2006; Andersen et al., 2011; Colloca et al., 2012; Parsons et al., 2012; Fiss et al., 2013; Montastruc et al., 2013; Toscani et al., 2013; Skoldunger et al., 2015; Barry et al., 2016; Hyttinen et al., 2016; Walsh et al., 2016; Clague et al., 2017; Kanagaratnam et al., 2017; Oesterhus et al., 2017; Wucherer et al., 2017; Kristensen et al., 2018; Renom-Guiteras et al., 2018; Denholm et al., 2019; Kristensen et al., 2019; Soysal et al., 2019; Murphy et al., 2020; Rausch and Hoffmann, 2020; Delgado et al., 2021; Gareri et al., 2021; Jaramillo-Hidalgo et al., 2021; Kristensen et al., 2021; Buckley et al., 2022; Delgado et al., 2022; Rangfast et al., 2022; Riedl et al., 2022), only two studies were conducted in South America (Forgerini et al., 2020; Ferreira et al., 2021), and the rest of the studies conducted in Oceania (Somers et al., 2010; Bosboom et al., 2012; Cross et al., 2016; Bala et al., 2019; Eshetie et al., 2019b; Brimelow et al., 2019; Eshetie et al., 2020; Eshetie et al., 2020; Ruangritchankul et al., 2020; Thapaliya et al., 2021) (n = 10), North America (Zuckerman et al., 2005; Holmes et al., 2008; Chan et al., 2009; Lau et al., 2010; Tjia et al., 2010; Lau et al., 2011; Thorpe et al., 2012; Koyama et al., 2013; Tjia et al., 2014; Hanlon et al., 2015; Ramsey et al., 2017; Growdon et al., 2021; Vickers et al., 2021; Bae-Shaaw et al., 2023; Ryskina et al., 2023) (n = 15), and Asia (Chuang et al., 2017; Nguyen et al., 2018; Chao et al., 2022; Yoon et al., 2022; Zhao et al., 2022) (n = 5). Table 1 presents the characteristics of the included studies.
3.3 Quality of the included studies
The results of quality assessment are presented in Supplementary Table S2, 3. For the cross-sectional study, we found that the lowest score was 4, and the highest score was 8. Seven research articles were of high methodological quality (AHRQ score ≥8), and 30 articles were of moderate methodological quality (AHRQ score 3–8). For the cohort studies, 20 research articles were of high methodological quality (NOS score ≥8), four articles were of moderate methodological quality (NOS score 6–7), and one article was of low quality (NOS score ≤5).
3.4 Prevalence of polypharmacy
Out of 62 included studies, 28 studies, comprising 4,813,226 older patients with dementia, reported the prevalence of polypharmacy, ranging from 14.10% to 92%. The pooled estimate of polypharmacy was 62% (95% CI 52–71). After weighing the population size by region, a significant difference among different regions was observed (df = 4, p < 0.0001). The pooled prevalence in Oceania was highest (79%, 95% CI 69–90) and lowest in Asia (36%, 95% CI 8–64). The detailed data about regions are shown in Figure 2.
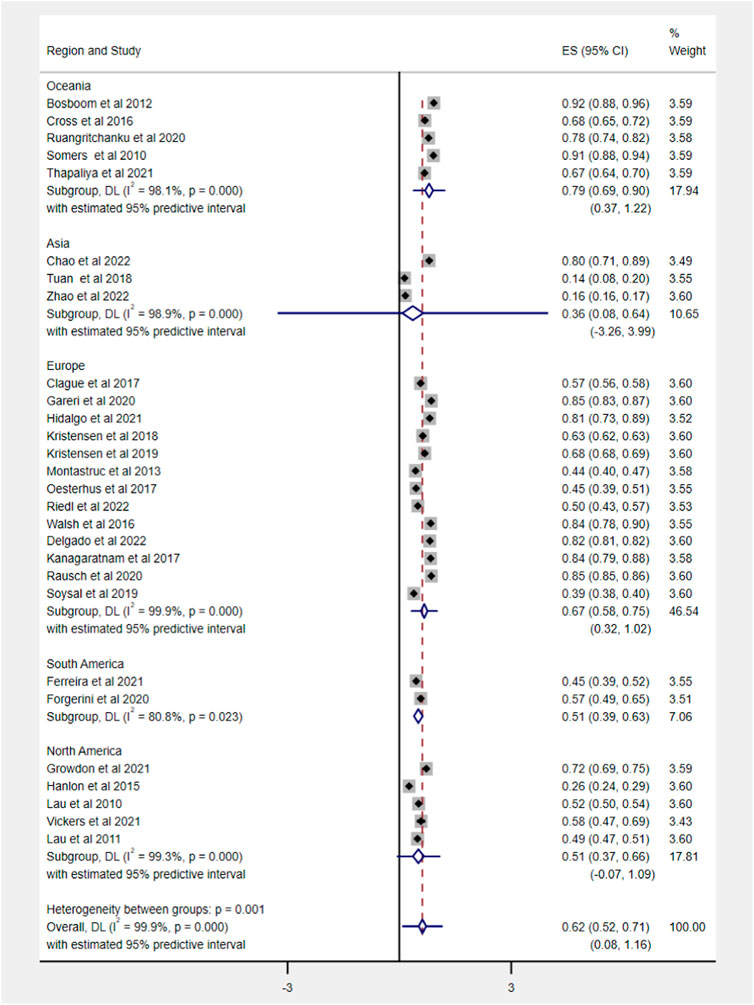
FIGURE 2. Prevalence of polypharmacy in older people with dementia across various geographic regions. Note that with <3 studies, the distribution is inestimable and hence not displayed.
3.5 Prevalence of potentially inappropriate medication
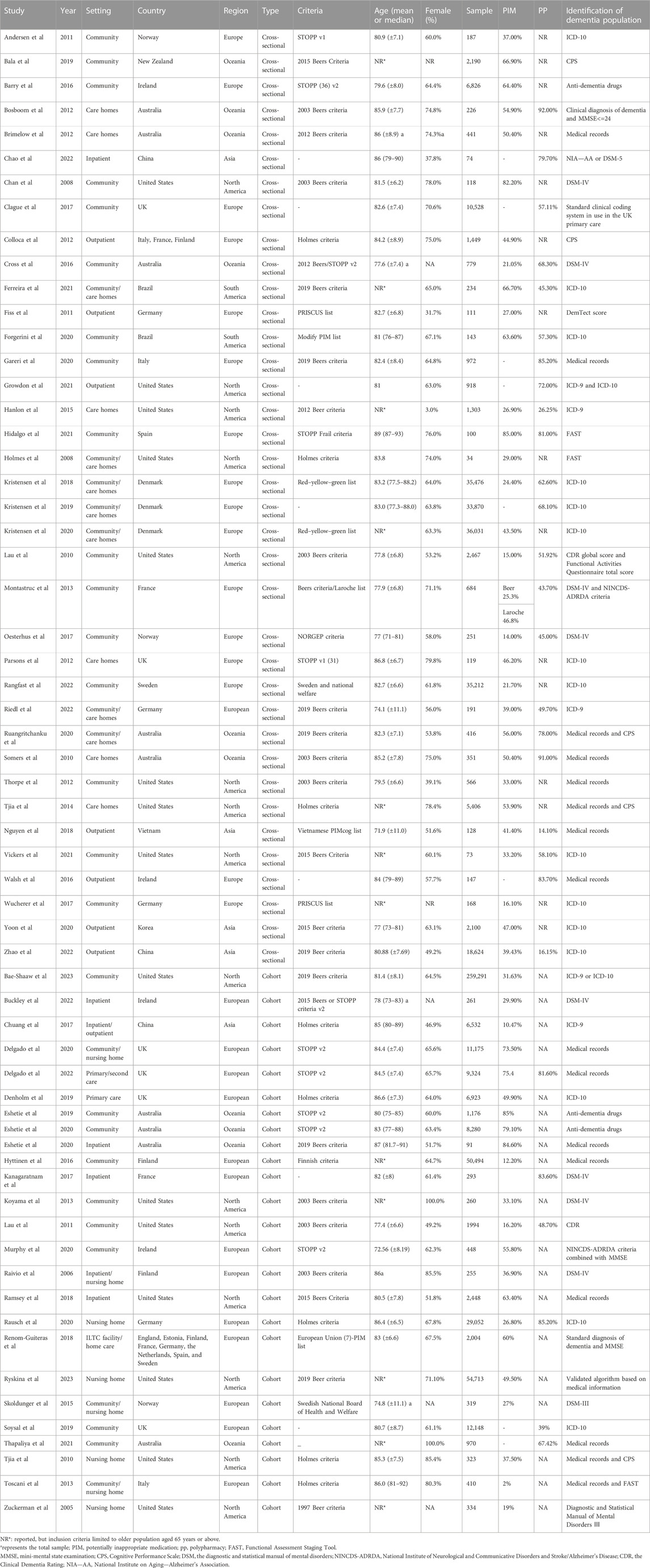
Fifty-three studies evaluated the prevalence of PIM based on different criteria, ranging from 2% to 85.1%. The pooled prevalence estimate of PIM was 43% (95% CI 38–48), as shown in Figure 3. Among regions, the prevalence of PIM showed a significant difference in statistics (Q = 59.5, df = 4, p < 0.0001), ranging from 34% in Asia (95% CI 15–54) to 66% in South America (95% CI 61–70).
3.6 Stratified analysis
We applied the stratified analysis for estimating the substantial heterogeneity in pooled prevalence of polypharmacy and PIM. Based on various basic characteristics, such as the proportion of females, year of publication, study design, and severity of dementia, we stratified the studies.
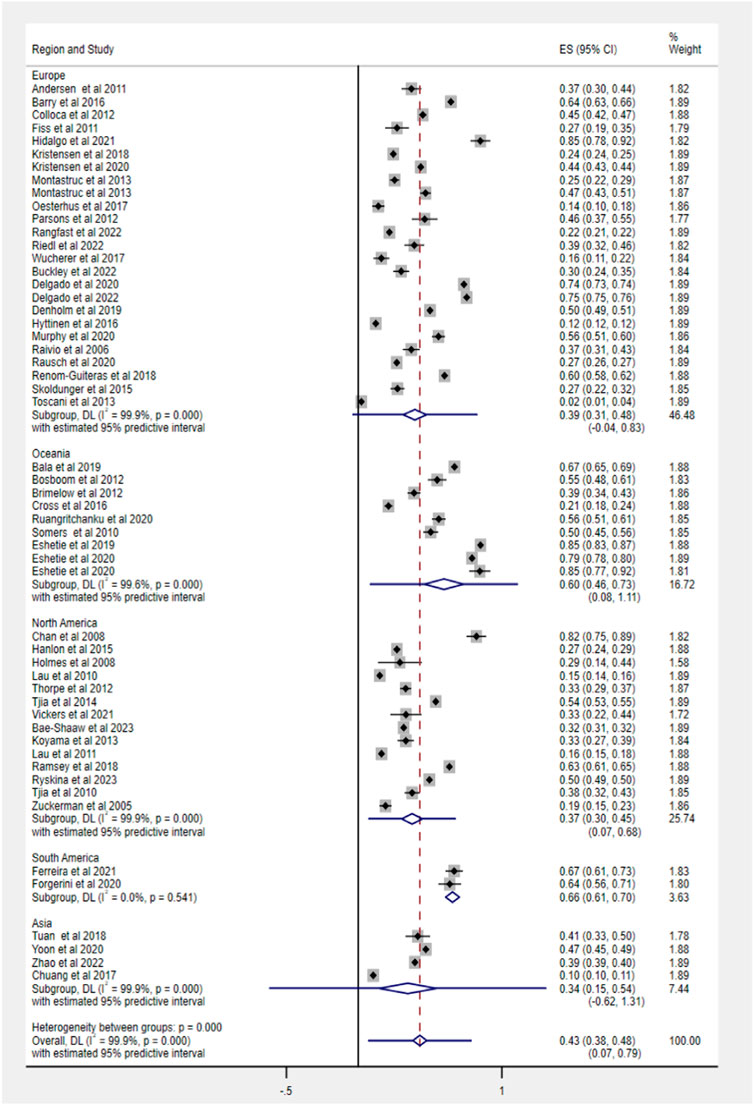
The subgroup analysis based on study design found no significant heterogeneity between groups in PIM use (cross-sectional: 42%, 95% CI 37–48; cohort: 44%, 95% CI 35–52). According to PIM criteria, we estimated that the pooled prevalence of PIM using the STOPP tool was highest (68%, 95% CI 63–73), following Beers criteria (44%, 95% CI 39–49) and other screening tools (32%, 95% CI 26–38). The specific data information in each subgroup for PIM use and polypharmacy is summarized in Table 2.
3.7 Factors associated with potentially inappropriate medications
A total of sixteen studies (Lau et al., 2010; Colloca et al., 2012; Fiss et al., 2013; Montastruc et al., 2013; Barry et al., 2016; Chuang et al., 2017; Hyttinen et al., 2017; Oesterhus et al., 2017; Renom-Guiteras et al., 2018; Murphy et al., 2020; Delgado et al., 2021; Ferreira et al., 2021; Rangfast et al., 2022; Tuan et al., 2018; Yoon et al., 2022; Zhao et al., 2022) referred to potential confounding factors with PIM in older patients with dementia. The specific details of each study reporting factors are shown in Supplementary Table S4. In the study, 15 factors, namely, age, female, polypharmacy, type of dementia, diabetes, heart failure, depression, psychosis, epilepsy, hypertension, stoke, ischemic heart disease, history of cancer, and chronic obstructive pulmonary disease or asthma were further analyzed (Figure 4).
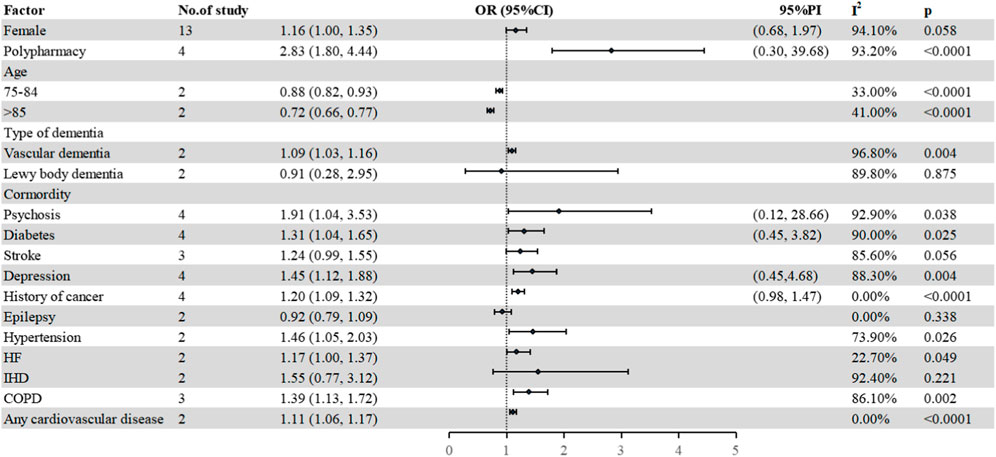
FIGURE 4. Factors associated with PIM use. HF, heart failure; IHD, ischemic heart disease; COPD, chronic obstructive pulmonary disease; PI, prediction interval.
Four studies (Montastruc et al., 2013; Ferreira et al., 2021; Yoon et al., 2022; Zhao et al., 2022) investigated the relationship between polypharmacy (five or more) and the risk of PIMs in older patients with dementia. The pooled estimate was 2.83 (95% CI 1.80–4.44), which indicated that increasing PIM risk was related with polypharmacy.
Regarding gender, a total of thirteen studies (Colloca et al., 2012; Fiss et al., 2013; Montastruc et al., 2013; Barry et al., 2016; Chuang et al., 2017; Hyttinen et al., 2017; Oesterhus et al., 2017; Nguyen et al., 2018; Murphy et al., 2020; Ferreira et al., 2021; Rangfast et al., 2022; Yoon et al., 2022; Zhao et al., 2022) were pooled to explore the association between females and PIM in older patients with dementia, in which six studies (Fiss et al., 2013; Montastruc et al., 2013; Barry et al., 2016; Oesterhus et al., 2017; Yoon et al., 2022; Zhao et al., 2022) showed that women were positively associated with PIM use, six studies (Colloca et al., 2012; Chuang et al., 2017; Murphy et al., 2020; Ferreira et al., 2021; Tuan et al., 2018; Rangfast et al., 2022) showed no correlation in statistics, and one study showed a negative association with PIM use (Hyttinen et al., 2017). The pooled estimate was 1.16 (95% CI 1.00–1.35). Three studies (Lau et al., 2010; Rangfast et al., 2022; Tuan et al., 2018) mentioned the impact of the type of dementia on PIM use. Two studies (Rangfast et al., 2022; Tuan et al., 2018) were further pooled to explore the relationship between the type of dementia and PIM use. Compared with Alzheimer’s disease (AD), patients with vascular dementia were more likely to suffer PIM, while in case of Lewy body dementia, there was no difference in statistics (vascular dementia: 1.09, 95% CI 1.03–1.16; Lewy body dementia: 0.91, 95% CI 0.28–2.95). For other potential confounding factors (age, diabetes, hypertension, psychosis, and heart failure), the details are shown in Figure 4.
3.8 Publication bias assessment
Egger’s and Begg’s tests were used to assess the publication bias in the pooled estimate of polypharmacy and PIM. The results for the pooled estimate of polypharmacy were 0.855 (Egger’s test) and 0.767 (Begg’s test), indicating no publication bias. Regarding PIM, no statistically significant publication bias was observed (Egger test: p = 0.058; Begg’s test: p = 0.765).
4 Discussion
To our knowledge, the current study first comprehensively summarized the pooled prevalence of PIM and polypharmacy in older patients with dementia across different regions and analyzed potential confounding factors associated with PIM use. This review may provide evidence for healthcare decision-makers in avoiding adverse drug use events in elderly with dementia.
For the included studies, the prevalence of PIM varied widely, ranging from 2% to 85.1%. Several reasons could explain the phenomenon well. First, the difference in marketing drugs and medical habits and the gap in healthcare systems in different regions might significantly affect the prevalence of PIM. Based on the pooled results by region, we clearly found a vast difference in PIM prevalence. Renom-Guiteras et al. evaluated the PIM prevalence of eight European countries using the European Union (7)-PIM list and found the PIM prevalence ranging 47%–67.5% (Renom-Guiteras et al., 2018). Zhao et al. also reported differences in PIM prevalence across different cities in China, ranging from 28.48% to 44.79% (Zhao et al., 2022). Second, the screening tools were considered a factor resulting in differences in PIM. To date, many screening tools have been applied to evaluate PIM, such as Beers criteria, STOPP/START criteria, and Holmes criteria (American Geriatrics Society Beers Criteria® Update Expert Panel, 2019; O'Mahony et al., 2018; Holmes et al., 2008). In our study, a total of 13 criteria were used, of which the most frequently used was Beers criteria, accounting for nearly 50%, followed by STOPP/START criteria. Published PIM lists have important differences in terms of contents and number (e.g., 81 items in STOPP criteria, while 91 in Beers criteria), which might lead to different prevalence of PIM. Target population and clinical practice among various PIM lists might also affect PIM prevalence, such as Holmes criteria mainly focusing on advanced dementia and NORGEP criteria for ambulatory patients (Kaufmann et al., 2014). The stratified analysis based on different criteria in our study also found differences in PIM prevalence. Although the same tool was used, the proportion of patients receiving PIM still varied, which were mainly attributed to how the tools were applied and the edition of the criteria. For instance, several studies just used part of the items of Beers criteria due to the absence of diagnostic information or other laboratory indicators, underestimating PIM to some extent (Chan et al., 2009; Lau et al., 2010; Thorpe et al., 2012; Montastruc et al., 2013). Therefore, before conducting research, researchers must consider how to select appropriate tools and how to apply them based on the collected information and diagnoses. In addition to the impact of region and screening tools on PIM, severity of dementia should be considered. In the analysis, we found that advanced dementia has a lower pooled estimate of PIM than mild–moderate dementia. Despite that patients living with advanced dementia depended completely on others, suffering from a series of distressing symptoms, such as neuropsychiatric symptoms and pain (Moens et al., 2014; Hendriks et al., 2015; Sampson et al., 2018), the emphasis of therapy for those was on ensuring patient comfort and symptom management and reducing polypharmacy (Disalvo et al., 2016). A review by Parsons summarized a viewpoint of physicians about drug use for advanced dementia, recommending discontinuation of anticholinesterase inhibitors, memantine, quetiapine, and simvastatin (Parsons et al., 2010). This may lead to a lower prevalence of PIM in advanced dementia. Despite the vast difference in PIM prevalence among included studies, the pooled estimate of PIM use in our analysis was up to 43%, which was higher than the PIM estimate for older patients in worldwide as given in Tian et al. (2023). Thus, we should pay great attention on the PIM use of older patients with dementia.
The drug management of older patients living with dementia often takes place in the context of additional comorbidities, which result in a large number of prescriptions for patients with dementia (Blass et al., 2008; Callahan and Schubert, 2014; Amy et al., 2018). In the analysis, polypharmacy was found to be prevalent with an estimated overall prevalence of 62%, slightly higher than that found in the study by Janice et al., with an estimate of 59% (Toh et al., 2023). Overall, significant heterogeneity was observed in the prevalence of polypharmacy. The difference may be attributed to several factors, for example, study subjects from different settings, geographical regions, study design, and year of publication. Although heterogeneity did not decrease by subgroups, significant differences were observed between some groups. In our review, we clearly found that the prevalence of polypharmacy in Asia was lowest compared with other regions. This may be due to socioeconomic-related healthcare inequalities between developing and developed countries in the access to healthcare. A report from the WHO declared that developed regions account for 11.6% of the worldwide burden, but account for 90.2% of health expenditure worldwide (Murray and Lopez, 1997). Different settings might affect the prevalence of polypharmacy. In this study, two out of three studies in the Asian region were outpatient studies, with a proportion of only over 10% for polypharmacy. Of note, the same definition of polypharmacy (five or more) shows a difference in measurement, which also might affect the outcome. Lau et al. declared that medications for topical applications, vitamins, and herbal medications were excluded in polypharmacy, with the prevalence of 51.92%, while Bosboom et al. considered all different medications in prescription were being counted, with the prevalence of 92% (Lau et al., 2011; Bosboom et al., 2012). In addition, the time of exposure to the medications has an impact on polypharmacy estimates. Kristensen et al. and Rausch et al. reported the prevalence of polypharmacy at 3 and 6 months as 62.6% and 85.2%, respectively (Kristensen et al., 2018; Rausch and Hoffmann, 2020). Although differences in distribution of regions, measurement, and exposure of time affect the estimate of polypharmacy, polypharmacy still cannot be ignored.
Several studies have been reported regarding potential confounding factors associated with PIM use. The association between PIM and polypharmacy for older patients with dementia was always discussed by researchers. Due to different definitions of polypharmacy and other factors, different studies concluded different results. Ferreira et al. reported polypharmacy was not related to PIM use (Ferreira et al., 2021), while Yoon et al. declared that a strong association between polypharmacy and PIM use was observed (Yoon et al., 2022). In our study, patients with polypharmacy (five or more) were exposed to a higher risk of PIM use, which was consistent with the observation of Tian et al. (2021) (reported older patients). A growing body of studies reported the impact of gender on PIM use, but a consensus has not yet been reached. According to our meta-analysis, no statistically significant difference was observed in women. Of note, we found vascular dementia was more susceptible to PIM use than Alzheimer’s disease. This may be related to cardiovascular events being the main cause of vascular dementia (Leys et al., 2005). Cardiovascular diseases and well-recognized high-risk factors of cardiovascular diseases, diabetes and hypertension, were associated with PIM use. This may be due to the use of non-steroidal drugs, regular insulin, and sulfonylureas of the PIM list. Other factors, such as comorbidity, psychosis, depression, and history of cancer, were considered to increase the risk of PIM in the current study. The long-term use of antidepressants, antipsychotics, and opioids in order to control symptoms may explain the phenomenon. Although several factors associated with PIM use were identified in our study, more relevant research was still needed for further validation.
PIM and polypharmacy in older patients with dementia are common. A large number of studies have shown PIM use and polypharmacy were related to hospitalization and death (Gnjidic et al., 2012; Maher et al., 2014). Therefore, it is necessary to optimize drug management for older patients with dementia. Deprescribing is an approach to reduce PIM and polypharmacy (Wu et al., 2021). Due to the use of multiple drugs and memory loss in older patients, drug compliance was relatively poor (Dunbar-Jacob and Mortimer-Stephens, 2001; Smith et al., 2017). For older patients with dementia featuring cognitive impairment and communication disorder, the adherence of drug medication was poorer. Deprescribing could not only reduce the number of medications taken but also increase the drug compliance of elderly patients (Basheti et al., 2016; Jäge et al., 2017). Research has confirmed the advantages of deprescribing in reducing PIM and polypharmacy (Ibrahim et al., 2021). Thus, deprescribing can be applied in the clinics to solve polypharmacy and PIM.
Although the analysis quantitatively summarized the prevalence of PIM and polypharmacy and further explored potential factors with PIM use, providing a reference for the prescription of old patients with dementia, we must acknowledge some limitations of the study. First, those studies included were from all over the world, and other factors, such as culture, education level, geographical location, and social status, would affect the results of polypharmacy and PIM. Second, the included studies show substantial heterogeneity, which may be related to the study subject, sample size, and screening tools used. Third, some studies did not specify the living conditions of the study population and type of dementia, so we cannot conduct a subgroup analysis based on living conditions and type of dementia. Fourth, included studies were limited to English articles, leading to results that could either underestimate or overestimate the prevalence. In addition, only factors using multivariate regression were extracted and several factors in our study were only examined in two studies, which gave biased results. Thus, a further study regarding factors affecting PIM use will be explored.
5 Conclusion
The analysis revealed that PIM use and polypharmacy were highly prevalent in older patients with dementia. Among different regions, the pooled estimate of PIM use and polypharmacy varied widely. Increasing PIM was related with polypharmacy, women, and vascular dementia. These findings highlight the necessity of some measures taken to improve the prescription quality of older patients with dementia, and they also imply that more caution should be taken when prescribing for women, polypharmacy, and vascular dementia.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Author contributions
MZ: developing design, literature search and screening, data extraction, and manuscript writing. ZC: literature screening and analysis of results. TX: management and manuscript editing. FT: data extraction and manuscript editing. PF: management and manuscript editing. All authors contributed to the article and approved the submitted version.
Funding
The study was supported by the 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (Project number: ZYJC18028) and the Sichuan Geriatrics Healthcare Program (Project number: 2023-124). This research was supported by the National Key Clinical Specialties Construction Program.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1221069/full#supplementary-material
References
American Geriatrics Society Beers Criteria® Update Expert Panel (2019). American geriatrics society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J. Am. Geriatr. Soc. 67 (4), 674–694. doi:10.1111/jgs.15767
Amy, P., Christopher, E. B., Seubert, L. J., Clark, V., Hill, X., King, S., et al. (2018). Medication use to manage comorbidities for people with dementia: A systematic review. J. Pharm. Pract. Res. 48 (4), 356–367. doi:10.1002/jppr.1403
Andersen, F., Viitanen, M., Halvorsen, D. S., Straume, B., and Engstad, T. A. (2011). Co-morbidity and drug treatment in Alzheimer's disease. A cross sectional study of participants in the dementia study in northern Norway. BMC Geriatr. 11, 58.doi:10.1186/1471-2318-11-58
Bae-Shaaw, Y. H., Shier, V., Sood, N., Seabury, S. A., and Joyce, G. (2023). Potentially inappropriate medication use in community-dwelling older adults living with dementia. J. Alzheimers Dis. 93 (2), 471–481. doi:10.3233/JAD-221168
Bala, S. S., Jamieson, H. A., and Nishtala, P. S. (2019). Determinants of prescribing potentially inappropriate medications in a nationwide cohort of community dwellers with dementia receiving a comprehensive geriatric assessment. Int. J. Geriatr. Psychiatry 34 (1), 153–161. doi:10.1002/gps.5004
Banta, J. E. (2017). Dementia: morbidity and medications. Age Ageing 46 (1), 4–5. doi:10.1093/ageing/afw182
Barry, H. E., Cooper, J. A., Ryan, C., Passmore, A. P., Robinson, A. L., Molloy, G. J., et al. (2016). Potentially inappropriate prescribing among people with dementia in primary care: A retrospective cross-sectional study using the enhanced prescribing database. J. Alzheimers Dis. 52 (4), 1503–1513. doi:10.3233/JAD-151177
Basheti, I. A., Al-Qudah, R. A., Obeidat, N. M., and Bulatova, N. R. (2016). Home medication management review in outpatients with chronic diseases in Jordan: A randomized control trial. Int. J. Clin. Pharm. 38 (2), 404–413. doi:10.1007/s11096-016-0266-9
Bedaso, A., and Duko, B. (2022). Epidemiology of depression among displaced people: A systematic review and meta-analysis. Psychiatry Res. 311, 114493. doi:10.1016/j.psychres.2022.114493
Bell, J. S., Mezrani, C., Blacker, N., LeBlanc, T., Frank, O., Alderman, C. P., et al. (2012). Anticholinergic and sedative medicines - prescribing considerations for people with dementia. Aust. Fam. Physician 41 (1-2), 45–49. doi:10.1016/j.aprim.2011.09.009
Blass, D. M., Black, B. S., Phillips, H., Finucane, T., Baker, A., Loreck, D., et al. (2008). Medication use in nursing home residents with advanced dementia. Int. J. Geriatr. Psychiatry 23 (5), 490–496. doi:10.1002/gps.1921
Bosboom, P. R., Alfonso, H., Almeida, O. P., and Beer, C. (2012). Use of potentially harmful medications and health-related quality of life among people with dementia living in residential aged care facilities. Dement. Geriatr. Cogn. Dis. Extra 2 (1), 361–371. doi:10.1159/000342172
Brimelow, R. E., Wollin, J. A., Byrne, G. J., and Dissanayaka, N. N. (2019). Prescribing of psychotropic drugs and indicators for use in residential aged care and residents with dementia. Int. Psychogeriatr. 31 (6), 837–847. doi:10.1017/S1041610218001229
Buckley, E., Jonsson, A., Flood, Z., Lavelle, M., O'Sullivan, N., Nurdin, N., et al. (2022). Potentially inappropriate medication use and mortality in patients with cognitive impairment. Eur. J. Clin. Pharmacol. 78 (12), 2013–2020. doi:10.1007/s00228-022-03410-2
Callahan, C. M., and Schubert, C. C. (2014). Dementia: the complexities of comorbidity in dementia. Nat. Rev. Neurol. 10 (4), 184–186. doi:10.1038/nrneurol.2014.46
Chan, V. T., Woo, B. K., Sewell, D. D., Allen, E. C., Golshan, S., Rice, V., et al. (2009). Reduction of suboptimal prescribing and clinical outcome for dementia patients in a senior behavioral health inpatient unit. Int. Psychogeriatr. 21 (1), 195–199. doi:10.1017/S104161020800803X
Chandrasekhar, D., Samjas, M., and pattani, D. (2019). Evaluation of potentially inappropriate medications among hospitalized geriatric patients in tertiary care referral hospital using STOPP/START criteria. Clin. Epidemiol. Glob. Health 7 (3), 268–273. doi:10.1016/j.cegh.2018.10.008
Chao, Y. T., Kuo, F. H., Lee, Y. S., Huang, Y. H., Weng, S. C., Chou, Y. Y., et al. (2022). Characteristics and outcome determinants of hospitalized older patients with cognitive dysfunction. Int. J. Environ. Res. Public Health 19 (1), 584. Published 2022 Jan 5. doi:10.3390/ijerph19010584
Chiatti, C., Bustacchini, S., Furneri, G., Mantovani, L., Cristiani, M., Misuraca, C., et al. (2012). The economic burden of inappropriate drug prescribing, lack of adherence and compliance, adverse drug events in older people: A systematic review. Drug Saf. 35 (Suppl. 1), 73–87. doi:10.1007/BF03319105
Chuang, H. Y., Wen, Y. W., Chen, L. K., and Hsiao, F. Y. (2017). Medication appropriateness for patients with dementia approaching the end of their life. Geriatr. Gerontol. Int. 17 (Suppl. 1), 65–74. doi:10.1111/ggi.13038
Clague, F., Mercer, S. W., McLean, G., Reynish, E., and Guthrie, B. (2017). Comorbidity and polypharmacy in people with dementia: insights from a large, population-based cross-sectional analysis of primary care data. Age Ageing 46 (1), 33–39. doi:10.1093/ageing/afw176
Colloca, G., Tosato, M., Vetrano, D. L., Topinkova, E., Fialova, D., Gindin, J., et al. (2012). Inappropriate drugs in elderly patients with severe cognitive impairment: results from the shelter study. PLoS One 7 (10), e46669. doi:10.1371/journal.pone.0046669
Cross, A. J., George, J., Woodward, M. C., Ames, D., Brodaty, H., Ilomäki, J., et al. (2016). Potentially inappropriate medications and anticholinergic burden in older people attending memory clinics in Australia. Drugs Aging 33 (1), 37–44. doi:10.1007/s40266-015-0332-3
Delgado, J., Bowman, K., and Clare, L. (2020). Potentially inappropriate prescribing in dementia: A state-of-the-art review since 2007. BMJ Open 10 (1), e029172. Published 2020 Jan 2. doi:10.1136/bmjopen-2019-029172
Delgado, J., Evans, P. H., Gray, D. P., Sidaway-Lee, K., Allan, L., Clare, L., et al. (2022). Continuity of GP care for patients with dementia: impact on prescribing and the health of patients. Br. J. Gen. Pract. 72 (715), e91–e98. Published 2022 Jan 27. doi:10.3399/BJGP.2021.0413
Delgado, J., Jones, L., Bradley, M. C., Allan, L. M., Ballard, C., Clare, L., et al. (2021). Potentially inappropriate prescribing in dementia, multi-morbidity and incidence of adverse health outcomes. Age Ageing 50 (2), 457–464. doi:10.1093/ageing/afaa147
Denholm, R., Morris, R., and Payne, R. (2019). Polypharmacy patterns in the last year of life in patients with dementia. Eur. J. Clin. Pharmacol. 75 (11), 1583–1591. doi:10.1007/s00228-019-02721-1
Disalvo, D., Luckett, T., Agar, M., Bennett, A., and Davidson, P. M. (2016). Systems to identify potentially inappropriate prescribing in people with advanced dementia: A systematic review. BMC Geriatr. 16, 114. doi:10.1186/s12877-016-0289-z
Dunbar-Jacob, J., and Mortimer-Stephens, M. K. (2001). Treatment adherence in chronic disease. J. Clin. Epidemiol. 54 (Suppl. 1), S57–S60. doi:10.1016/s0895-4356(01)00457-7
Eshetie, T. C., Nguyen, T. A., Gillam, M. H., and Kalisch Ellett, L. M. (2019a). Potentially inappropriate prescribing before and after initiation of medicines for dementia: an Australian population-based study. Geriatr. Gerontol. Int. 19 (7), 654–659. doi:10.1111/ggi.13686
Eshetie, T. C., Nguyen, T. A., Gillam, M. H., and Kalisch Ellett, L. M. (2019b). Potentially inappropriate prescribing in people with dementia: an Australian population-based study. Int. J. Geriatr. Psychiatry 34 (10), 1498–1505. doi:10.1002/gps.5160
Eshetie, T. C., Roberts, G., Nguyen, T. A., Gillam, M. H., Maher, D., and Kalisch Ellett, L. M. (2020). Potentially inappropriate medication use and related hospital admissions in aged care residents: the impact of dementia. Br. J. Clin. Pharmacol. 86 (12), 2414–2423. doi:10.1111/bcp.14345
Ferreira, T. R., Lopes, L. C., Motter, F. R., and de Cássia Bergamaschi, C. (2021). Potentially inappropriate prescriptions to Brazilian older people with alzheimer disease: A cross-sectional study. Med. Baltim. 100 (12), e25015. doi:10.1097/MD.0000000000025015
Fiss, T., Thyrian, J. R., Fendrich, K., van den Berg, N., and Hoffmann, W. (2013). Cognitive impairment in primary ambulatory health care: pharmacotherapy and the use of potentially inappropriate medicine. Int. J. Geriatr. Psychiatry 28 (2), 173–181. doi:10.1002/gps.3806
Forgerini, M., Herdeiro, M. T., Galduróz, J. C. F., and Mastroianni, P. C. (2020). Risk factors associated with drug therapy among elderly people with alzheimer's disease: A cross-sectional study. Sao Paulo Med. J. 138 (3), 216–218. doi:10.1590/1516-3180.2019.0461.R2.19022020
Gareri, P., Cotroneo, A. M., Pontieri, M. T., Palleria, C., and De Sarro, G. (2021). The risk of polypharmacy and potentially inappropriate drugs in residential care dementia patients: tips from the PharE study. Aging Clin. Exp. Res. 33 (7), 1909–1917. doi:10.1007/s40520-020-01719-5
Gnjidic, D., Hilmer, S. N., Blyth, F. M., Naganathan, V., Waite, L., Seibel, M. J., et al. (2012). Polypharmacy cutoff and outcomes: five or more medicines were used to identify community-dwelling older men at risk of different adverse outcomes. J. Clin. Epidemiol. 65 (9), 989–995. doi:10.1016/j.jclinepi.2012.02.018
Growdon, M. E., Gan, S., Yaffe, K., and Steinman, M. A. (2021). Polypharmacy among older adults with dementia compared with those without dementia in the United States. J. Am. Geriatr. Soc. 69 (9), 2464–2475. doi:10.1111/jgs.17291
Hagstrom, K., Nailor, M., Lindberg, M., Hobbs, L., and Sobieraj, D. M. (2015). Association between potentially inappropriate medication use in elderly adults and hospital-related outcomes. J. Am. Geriatr. Soc. 63 (1), 185–186. doi:10.1111/jgs.13229
Hanlon, J. T., Aspinall, S. L., Handler, S. M., Gellad, W. F., Stone, R. A., Semla, T. P., et al. (2015). Potentially suboptimal prescribing for older veteran nursing home patients with dementia. Ann. Pharmacother. 49 (1), 20–28. doi:10.1177/1060028014558484
He, W., and Kinsella, K. (2020). “Global aging in the new millennium,” in Cult.Context aging worldwide perspect (Westport: praeger/abc-clio), 27.
Hendriks, S. A., Smalbrugge, M., Galindo-Garre, F., Hertogh, C. M., and van der Steen, J. T. (2015). From admission to death: prevalence and course of pain, agitation, and shortness of breath, and treatment of these symptoms in nursing home residents with dementia. J. Am. Med. Dir. Assoc. 16 (6), 475–481. doi:10.1016/j.jamda.2014.12.016
Higgins, J. P., Thompson, S. G., and Spiegelhalter, D. J. (2009). A re-evaluation of random-effects meta-analysis. J. R. Stat. Soc. Ser. A Stat. Soc. 172 (1), 137–159. doi:10.1111/j.1467-985X.2008.00552.x
Holmes, H. M., Sachs, G. A., Shega, J. W., Hougham, G. W., Cox Hayley, D., and Dale, W. (2008). Integrating palliative medicine into the care of persons with advanced dementia: identifying appropriate medication use. J. Am. Geriatr. Soc. 56 (7), 1306–1311. doi:10.1111/j.1532-5415.2008.01741.x
Hu, J., Dong, Y., Chen, X., Liu, Y., Ma, D., Liu, X., et al. (2015). Prevalence of suicide attempts among Chinese adolescents: A meta-analysis of cross-sectional studies. Compr. Psychiatry 61, 78–89. doi:10.1016/j.comppsych.2015.05.001
Hukins, D., Macleod, U., and Boland, J. W. (2019). Identifying potentially inappropriate prescribing in older people with dementia: A systematic review. Eur. J. Clin. Pharmacol. 75 (4), 467–481. doi:10.1007/s00228-018-02612-x
Hyttinen, V., Jyrkkä, J., and Valtonen, H. (2016). A systematic review of the impact of potentially inappropriate medication on health care utilization and costs among older adults. Med. Care 54 (10), 950–964. doi:10.1097/MLR.0000000000000587
Hyttinen, V., Taipale, H., Tanskanen, A., Tiihonen, J., Tolppanen, A. M., Hartikainen, S., et al. (2017). Risk factors for initiation of potentially inappropriate medications in community-dwelling older adults with and without alzheimer's disease. Drugs Aging 34 (1), 67–77. doi:10.1007/s40266-016-0415-9
Ibrahim, K., Cox, N. J., Stevenson, J. M., Lim, S., Fraser, S. D. S., and Roberts, H. C. (2021). A systematic review of the evidence for deprescribing interventions among older people living with frailty. BMC Geriatr. 21 (1), 258. doi:10.1186/s12877-021-02208-8
Jäger, C., Freund, T., Steinhäuser, J., Stock, C., Krisam, J., Kaufmann-Kolle, P., et al. (2017). Impact of a tailored program on the implementation of evidence-based recommendations for multimorbid patients with polypharmacy in primary care practices-results of a cluster-randomized controlled trial. Implement Sci. 12 (1), 8. doi:10.1186/s13012-016-0535-y
Jaramillo-Hidalgo, J., Lozano-Montoya, I., Tornero-Torres, O., Tejada-González, P., Fuentes-Irigoyen, R., and Gómez-Pavón, F. J. (2021). Prevalence of potentially inappropriate prescription in community-dwelling patients with advanced dementia and palliative care needs. Rev. Esp. Geriatr. Gerontol. 56 (4), 203–207. doi:10.1016/j.regg.2021.03.001
Johnell, K. (2015). Inappropriate drug use in people with cognitive impairment and dementia: A systematic review. Curr. Clin. Pharmacol. 10 (3), 178–184. doi:10.2174/1574884710666150609154741
Kanagaratnam, L., Dramé, M., Novella, J. L., Trenque, T., Joachim, C., Nazeyrollas, P., et al. (2017). Risk factors for adverse drug reactions in older subjects hospitalized in a dedicated dementia unit. Am. J. Geriatr. Psychiatry 25 (3), 290–296. doi:10.1016/j.jagp.2016.07.002
Kaufmann, C. P., Tremp, R., Hersberger, K. E., and Lampert, M. L. (2014). Inappropriate prescribing: A systematic overview of published assessment tools. Eur. J. Clin. Pharmacol. 70 (1), 1–11. doi:10.1007/s00228-013-1575-8
Koyama, A., Steinman, M., Ensrud, K., Hillier, T. A., and Yaffe, K. (2013). Ten-year trajectory of potentially inappropriate medications in very old women: importance of cognitive status. J. Am. Geriatr. Soc. 61 (2), 258–263. doi:10.1111/jgs.12093
Kristensen, R. U., Jensen-Dahm, C., Gasse, C., and Waldemar, G. (2021). Declining use of potentially inappropriate medication in people with dementia from 2000 to 2015: A repeated cross-sectional nationwide register-based study. J. Alzheimers Dis. 79 (4), 1459–1470. doi:10.3233/JAD-200627
Kristensen, R. U., Nørgaard, A., Jensen-Dahm, C., Gasse, C., Wimberley, T., and Waldemar, G. (2019). Changes in the prevalence of polypharmacy in people with and without dementia from 2000 to 2014: A nationwide study. J. Alzheimers Dis. 67 (3), 949–960. doi:10.3233/JAD-180427
Kristensen, R. U., Nørgaard, A., Jensen-Dahm, C., Gasse, C., Wimberley, T., and Waldemar, G. (2018). Polypharmacy and potentially inappropriate medication in people with dementia: A nationwide study. J. Alzheimers Dis. 63 (1), 383–394. doi:10.3233/JAD-170905
Lau, D. T., Mercaldo, N. D., Harris, A. T., Trittschuh, E., Shega, J., and Weintraub, S. (2010). Polypharmacy and potentially inappropriate medication use among community-dwelling elders with dementia. Alzheimer Dis. Assoc. Disord. 24 (1), 56–63. doi:10.1097/WAD.0b013e31819d6ec9
Lau, D. T., Mercaldo, N. D., Shega, J. W., Rademaker, A., and Weintraub, S. (2011). Functional decline associated with polypharmacy and potentially inappropriate medications in community-dwelling older adults with dementia. Am. J. Alzheimers Dis. Other Demen 26 (8), 606–615. doi:10.1177/1533317511432734
Leys, D., Hénon, H., Mackowiak-Cordoliani, M. A., and Pasquier, F. (2005). Poststroke dementia. Lancet Neurol. 4 (11), 752–759. doi:10.1016/S1474-4422(05)70221-0
Maher, R. L., Hanlon, J., and Hajjar, E. R. (2014). Clinical consequences of polypharmacy in elderly. Expert Opin. Drug Saf. 13 (1), 57–65. doi:10.1517/14740338.2013.827660
Masnoon, N., Shakib, S., Kalisch-Ellett, L., and Caughey, G. E. (2017). What is polypharmacy? A systematic review of definitions. BMC Geriatr. 17 (1), 230. doi:10.1186/s12877-017-0621-2
Migliavaca, C. B., Stein, C., Colpani, V., Barker, T. H., Ziegelmann, P. K., Munn, Z., et al. (2022). Meta-analysis of prevalence: I2 statistic and how to deal with heterogeneity. Res. Synth. Methods 13 (3), 363–367. doi:10.1002/jrsm.1547
Moens, K., Higginson, I. J., Harding, R., and Euro, I. M. P. A. C. T. (2014). Are there differences in the prevalence of palliative care-related problems in people living with advanced cancer and eight non-cancer conditions? A systematic review. J. Pain Symptom Manage 48 (4), 660–677. doi:10.1016/j.jpainsymman.2013.11.009
Moher, D., Shamseer, L., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., et al. (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 4 (1), 1. doi:10.1186/2046-4053-4-1
Montastruc, F., Gardette, V., Cantet, C., Piau, A., Lapeyre-Mestre, M., Vellas, B., et al. (2013). Potentially inappropriate medication use among patients with alzheimer disease in the REAL.FR cohort: be aware of atropinic and benzodiazepine drugs. Eur. J. Clin. Pharmacol. 69 (8), 1589–1597. doi:10.1007/s00228-013-1506-8
Murphy, C., Dyer, A. H., Lawlor, B., and Kennelly, S. P.NILVAD Study Group (2020). Potentially inappropriate medication use in older adults with mild-moderate alzheimer's disease: prevalence and associations with adverse events. Age Ageing 49 (4), 580–587. doi:10.1093/ageing/afaa067
Murray, C. J., and Lopez, A. D. (1997). Global mortality, disability, and the contribution of risk factors: global Burden of Disease Study. Lancet 349 (9063), 1436–1442. doi:10.1016/S0140-6736(96)07495-8
Nguyen, T. A., Pham, T., Vu, H. T. T., Nguyen, T. X., Vu, T. T., Nguyen, B. T. T., et al. (2018). Use of potentially inappropriate medications in people with dementia in Vietnam and its associated factors. Am. J. Alzheimers Dis. Other Demen 33 (7), 423–432. doi:10.1177/1533317518768999
O'Mahony, D., O'Sullivan, D., Byrne, S., O'Connor, M. N., Ryan, C., and Gallagher, P. (2015). STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing 44 (2), 213–218. doi:10.1093/ageing/afu145
Oesterhus, R., Aarsland, D., Soennesyn, H., Rongve, A., Selbaek, G., and Kjosavik, S. R. (2017). Potentially inappropriate medications and drug-drug interactions in home-dwelling people with mild dementia. Int. J. Geriatr. Psychiatry 32 (2), 183–192. doi:10.1002/gps.4456
Parsons, C., Hughes, C. M., Passmore, A. P., and Lapane, K. L. (2010). Withholding, discontinuing and withdrawing medications in dementia patients at the end of life: A neglected problem in the disadvantaged dying? Drugs Aging 27 (6), 435–449. doi:10.2165/11536760-000000000-00000
Parsons, C., Johnston, S., Mathie, E., Baron, N., Machen, I., Amador, S., et al. (2012). Potentially inappropriate prescribing in older people with dementia in care homes: A retrospective analysis. Drugs Aging 29 (2), 143–155. doi:10.2165/11598560-000000000-00000
Prince, M., Bryce, R., Albanese, E., Wimo, A., Ribeiro, W., and Ferri, C. P. (2013). The global prevalence of dementia: A systematic review and metaanalysis. Alzheimers Dement. 9 (1), 63–75. doi:10.1016/j.jalz.2012.11.007
Raivio, M. M., Laurila, J. V., Strandberg, T. E., Tilvis, R. S., and Pitkälä, K. H. (2006). Use of inappropriate medications and their prognostic significance among in-hospital and nursing home patients with and without dementia in Finland. Drugs Aging 23 (4), 333–343. doi:10.2165/00002512-200623040-00006
Ramsey, C. M., Gnjidic, D., Agogo, G. O., Allore, H., and Moga, D. (2017). Longitudinal patterns of potentially inappropriate medication use following incident dementia diagnosis. Alzheimers Dement. (N Y) 4, 1–10. Published 2017 Nov 26. doi:10.1016/j.trci.2017.10.008
Rangfast, I., Sönnerstam, E., and Gustafsson, M. (2022). Prevalence of potentially inappropriate medications among old people with major neurocognitive disorder in 2012 and 2017. BMC Geriatr. 22 (1), 544. doi:10.1186/s12877-022-03240-y
Rankin, A., Cadogan, C. A., Patterson, S. M., Kerse, N., Cardwell, C. R., Bradley, M. C., et al. (2018). Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst. Rev. 9 (9), CD008165. doi:10.1002/14651858.CD008165.pub4
Rausch, C., and Hoffmann, F. (2020). Prescribing medications of questionable benefit prior to death: A retrospective study on older nursing home residents with and without dementia in Germany. Eur. J. Clin. Pharmacol. 76 (6), 877–885. doi:10.1007/s00228-020-02859-3
Redston, M. R., Hilmer, S. N., McLachlan, A. J., Clough, A. J., and Gnjidic, D. (2018). Prevalence of potentially inappropriate medication use in older inpatients with and without cognitive impairment: A systematic review. J. Alzheimers Dis. 61 (4), 1639–1652. doi:10.3233/JAD-170842
Renom-Guiteras, A., Meyer, G., and Thürmann, P. A. (2015). The EU(7)-PIM list: A list of potentially inappropriate medications for older people consented by experts from seven European countries. Eur. J. Clin. Pharmacol. 71 (7), 861–875. doi:10.1007/s00228-015-1860-9
Renom-Guiteras, A., Thürmann, P. A., Miralles, R., Klaaßen-Mielke, R., Thiem, U., Stephan, A., et al. (2018). Potentially inappropriate medication among people with dementia in eight European countries. Age Ageing 47 (1), 68–74. doi:10.1093/ageing/afx147
Riedl, L., Kiesel, E., Hartmann, J., Fischer, J., Roßmeier, C., Haller, B., et al. (2022). A bitter pill to swallow - polypharmacy and psychotropic treatment in people with advanced dementia. BMC Geriatr. 22 (1), 214. doi:10.1186/s12877-022-02914-x
Rostom, A., Dube, C., Cranney, A., Saloojee, N., Rsy, C., Garritty, C., et al. (2004). Celiac disease. Rockville (MD). United States: Agency for Healthcare Research and Quality. (Evidence Reports/Technology Assessments, No. 104.) Appendix D. Quality Assessment Forms http://www.ncbi.nlm.nih.gov/books/NBK35156.
Ruangritchankul, S., Peel, N. M., Hanjani, L. S., and Gray, L. C. (2020). Drug related problems in older adults living with dementia. PLoS One 15 (7), e0236830. doi:10.1371/journal.pone.0236830
Ryskina, K., Lo, D., Zhang, T., Gerlach, L., Bynum, J., and Shireman, T. I. (2023). Potentially harmful medication prescribing by the degree of physician specialization in nursing home practice: an observational study. J. Am. Med. Dir. Assoc. S1525-8610 (23), 1240–1246.e2. doi:10.1016/j.jamda.2023.03.017
Sampson, E. L., Candy, B., Davis, S., Gola, A. B., Harrington, J., King, M., et al. (2018). Living and dying with advanced dementia: A prospective cohort study of symptoms, service use and care at the end of life. Palliat. Med. 32 (3), 668–681. doi:10.1177/0269216317726443
Shi, S., Mörike, K., and Klotz, U. (2008). The clinical implications of ageing for rational drug therapy. Eur. J. Clin. Pharmacol. 64 (2), 183–199. doi:10.1007/s00228-007-0422-1
Sköldunger, A., Fastbom, J., Wimo, A., Fratiglioni, L., and Johnell, K. (2015). Impact of inappropriate drug use on hospitalizations, mortality, and costs in older persons and persons with dementia: findings from the SNAC study. Drugs Aging 32 (8), 671–678. doi:10.1007/s40266-015-0287-4
Smith, D., Lovell, J., Weller, C., Kennedy, B., Winbolt, M., Young, C., et al. (2017). A systematic review of medication non-adherence in persons with dementia or cognitive impairment. PLoS One 12 (2), e0170651. doi:10.1371/journal.pone.0170651
Somers, M., Rose, E., Simmonds, D., Whitelaw, C., Calver, J., and Beer, C. (2010). Quality use of medicines in residential aged care. Aust. Fam. Physician 39 (6), 413–416. https://search.informit.org/doi/10.3316/informit.145841247651952
Soysal, P., Perera, G., Isik, A. T., Onder, G., Petrovic, M., Cherubini, A., et al. (2019). The relationship between polypharmacy and trajectories of cognitive decline in people with dementia: A large representative cohort study. Exp. Gerontol. 120, 62–67. doi:10.1016/j.exger.2019.02.019
Stang, A. (2010). Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25 (9), 603–605. doi:10.1007/s10654-010-9491-z
Stroup, D. F., Berlin, J. A., Morton, S. C., Olkin, I., Williamson, G. D., Rennie, D., et al. (2000). Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-Analysis of observational studies in epidemiology (MOOSE) group. JAMA 283 (15), 2008–2012. doi:10.1001/jama.283.15.2008
Thapaliya, K., Harris, M. L., and Byles, J. E. (2021). Polypharmacy trajectories among older women with and without dementia: A longitudinal cohort study. Explor Res. Clin. Soc. Pharm. 3, 100053. doi:10.1016/j.rcsop.2021.100053
ThorpeThorpeKennelty, J. M. C. T. K. A., Gellad, W. F., and Schulz, R. (2012). The impact of family caregivers on potentially inappropriate medication use in noninstitutionalized older adults with dementia. Am. J. geriatric Pharmacother. 10 (4), 230–241. doi:10.1016/j.amjopharm.2012.05.001
Tian, F., Chen, Z., Zeng, Y., Feng, Q., and Chen, X. (2023). Prevalence of use of potentially in appropriate medications among older adults worldwide: A systematic review and meta-analysis. JAMA Netw. Open 6 (8), e2326910. doi:10.1001/jamanetworkopen.2023.26910
Tian, F., Li, H., Chen, Z., and Xu, T. (2021). Potentially inappropriate medications in Chinese older outpatients in tertiary hospitals according to Beers criteria: A cross-sectional study. Int. J. Clin. Pract. 75 (8), e14348. doi:10.1111/ijcp.14348
Tjia, J., Briesacher, B. A., Peterson, D., Liu, Q., Andrade, S. E., and Mitchell, S. L. (2014). Use of medications of questionable benefit in advanced dementia. JAMA Intern Med. 174 (11), 1763–1771. doi:10.1001/jamainternmed.2014.4103
Tjia, J., Rothman, M. R., Kiely, D. K., Shaffer, M. L., Holmes, H. M., Sachs, G. A., et al. (2010). Daily medication use in nursing home residents with advanced dementia. J. Am. Geriatr. Soc. 58 (5), 880–888. doi:10.1111/j.1532-5415.2010.02819.x
Toh, J. J. Y., Zhang, H., Soh, Y. Y., Zhang, Z., and Wu, X. V. (2023). Prevalence and health outcomes of polypharmacy and hyperpolypharmacy in older adults with frailty: A systematic review and meta-analysis. Ageing Res. Rev. 83, 101811. doi:10.1016/j.arr.2022.101811
Toscani, F., Di Giulio, P., Villani, D., Giunco, F., Brunelli, C., Gentile, S., et al. (2013). Treatments and prescriptions in advanced dementia patients residing in long-term care institutions and at home. J. Palliat. Med. 16 (1), 31–37. doi:10.1089/jpm.2012.0165
Vickers, L. E., Martinez, A. I., Wallem, A. M., Johnson, C., and Moga, D. C. (2021). Potentially inappropriate medication use in older adults with alzheimer's disease and related dementias living in the community: A cross-sectional analysis. Drugs Real World Outcomes 8 (4), 519–526. doi:10.1007/s40801-021-00265-4
Walsh, K. A., O'Regan, N. A., Byrne, S., Browne, J., Meagher, D. J., and Timmons, S. (2016). Patterns of psychotropic prescribing and polypharmacy in older hospitalized patients in Ireland: the influence of dementia on prescribing. Int. Psychogeriatr. 28 (11), 1807–1820. doi:10.1017/S1041610216001307
Wu, H., Kouladjian O'Donnell, L., Fujita, K., Masnoon, N., and Hilmer, S. N. (2021). Deprescribing in the older patient: A narrative review of challenges and solutions. Int. J. Gen. Med. 14, 3793–3807. Published 2021 Jul 24. doi:10.2147/IJGM.S253177
Wucherer, D., Eichler, T., Hertel, J., Kilimann, I., Richter, S., Michalowsky, B., et al. (2017). Potentially inappropriate medication in community-dwelling primary care patients who were screened positive for dementia. J. Alzheimers Dis. 55 (2), 691–701. doi:10.3233/JAD-160581
Yoon, K., Kim, J. T., Kwack, W. G., Kim, D., Lee, K. T., Yang, S., et al. (2022). Potentially inappropriate medication use in patients with dementia. Int. J. Environ. Res. Public Health 19 (18), 11426. doi:10.3390/ijerph191811426
Zhao, M., Chen, Z., Tian, F., and Xu, T. (2022). Potentially inappropriate medication among people with dementia in China: A nationwide cross-sectional study. Front. Pharmacol. 13, 929584. doi:10.3389/fphar.2022.929584
Zuckerman, I. H., Hernandez, J. J., Gruber-Baldini, A. L., Hebel, J. R., Stuart, B., Zimmerman, S., et al. (2005). Potentially inappropriate prescribing before and after nursing home admission among patients with and without dementia. Am. J. Geriatr. Pharmacother. 3 (4), 246–254. doi:10.1016/j.amjopharm.2005.12.007
Keywords: polypharmacy, potentially inappropriate medication, older, dementia, meta-analysis, factors
Citation: Zhao M, Chen Z, Xu T, Fan P and Tian F (2023) Global prevalence of polypharmacy and potentially inappropriate medication in older patients with dementia: a systematic review and meta-analysis. Front. Pharmacol. 14:1221069. doi: 10.3389/fphar.2023.1221069
Received: 11 May 2023; Accepted: 03 August 2023;
Published: 24 August 2023.
Edited by:
Tanveer Ahmed Khan, National Institute of Health, PakistanReviewed by:
Daniela Oliveira de Melo, Federal University of São Paulo, BrazilTatiane Da Silva Dal Pizzol, Federal University of Rio Grande do Sul, Brazil
Copyright © 2023 Zhao, Chen, Xu, Fan and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ping Fan, ODI1MzcwMzIwQHFxLmNvbQ==; Fangyuan Tian, dGlhbmZhbmd5dWFuMDYwOEAxNjMuY29t
 Mengnan Zhao
Mengnan Zhao Zhaoyan Chen
Zhaoyan Chen Ting Xu
Ting Xu Ping Fan
Ping Fan Fangyuan Tian
Fangyuan Tian