- 1Department of Pathology, Division of Pharmacogenomics and Personalized Medicine, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
- 2Laboratory for Pharmacogenomics, Somdech Phra Debaratana Medical Center (SDMC), Ramathibodi Hospital, Bangkok, Thailand
- 3Department of Pharmacy, Nhan Dan Gia Dinh Hospital, Ho ChiMinh City, Vietnam
- 4Pharmacogenomics and Precision Medicine Clinic, Bumrungrad International Hospital, Bangkok, Thailand
- 5Bumrungrad Genomic Medicine Institute (BGMI), Bumrungrad International Hospital, Bangkok, Thailand
- 6Department of Pharmacy, Nguyen Tat Thanh University, Ho ChiMinh City, Vietnam
Background: The relationship between HLA-B*15:02 and Severe Cutaneous Adverse Reactions was rigorously examined in Japanese, Han Chinese, Thais, and Caucasians. However, the number of studies about this topic in Vietnamese population is still limited and mostly focuses on the North of Vietnam.
Objective: This study aims to clarify the genetic culprit of SCARs in Vietnamese population, particularly in the South of Vietnam, and to validate our result by a meta-analysis about this topic in Vietnamese.
Method: A retrospective case-control study with 37 patients treated with carbamazepine monotherapy. Statistical calculation and meta-analysis were performed by R software.
Result: HLA-B*15:02 increases the risk of SJS 12.5 times higher in CBZ-treated patients (p-value = 0.017). However, this allele has no impact on MCARs (Mild Cutaneous Adverse Reactions) of CBZ. The number needed to test and the number needed to genotype is two and nine patients respectively.
Conclusion: This study recommends more investigations about the cost-effectiveness of this test to accelerate the protection of Southern Vietnamese from SCARs.
1 Introduction
George Snell (Snell, 1981), a Nobel laureate, discovered the Major Histocompatibility Complex (MCH) in 1948. Ten years later, the first Human Leucocyte Antigen (HLA) was detected (Thorsby, 2009). Since then, HLA genes have been gradually unveiled to be one of the most sophisticated genes with over 35,000 alleles confirmed by the time of this study (Barker et al., 2023). Different alleles render different amino acids in MCH molecules, where the antigen presentation happens in a manner of specificity. MCH contains two major classes designated as HLA class I and HLA class II, which are respectively responsible for the endogenous and exogenous pathways. Moreover, the polymorphism of HLA genes plays a crucial role in the development of Steven-Johnson (SJS) and Toxic epidermal necrolysis (TEN).
The diversity of genes encrypted for HLA protein is not only reflected in one single ethnicity, where this diversity allows a wide range of antigens to be recognized and responded to, but also reflected in the differences between ethnicities. Most of the genetic causes of Carbamazepine-induced SCARs were investigated in developed Asian countries, such as Han Chinese (Wang et al., 2011; Zhang et al., 2011), Thais (Tassaneeyakul et al., 2010; Sukasem et al., 2018), and Taiwanese (Chen et al., 2011). However, Southeast Asian populations have received less attention, especially in the South of Vietnam where the flow of immigration created an admixture of crowded population. The South of Vietnam is the home of around 40 million people (2023) with diverse ethnicities such as Kinh Vietnamese, Khmer Krom, Cham Vietnamese, etc. In Caucasians, Japanese, and Koreans, even though a myriad of meticulous research has been conducted about HLA-B*1502, this allele is reported to be rare and not associated with statistically significant risk in these populations. In contrast, the HLA-B*15:02 allele was believed to have the highest allele frequency and fatal risk in the general population of Southeast Asia (Moutaouakkil et al., 2019).
Indeed, the relationship between HLA-*B-15:02 and SCARs was rigorously examined in Japanese, Han Chinese, Thais, and Caucasians. However, the number of studies about this topic in Vietnamese population is still limited and mostly focuses on the North of Vietnam (Van Nguyen et al., 2015; Van Nguyen et al., 2022). Given that Vietnam is a highly populated and racially diverse country, which can create genetic heterogeneity, more research is needed to fully understand the genetic predisposition of Vietnamese, especially those in the south of Vietnam. Unfortunately, as an economically developing area, Vietnam faces tremendous financial barriers in scientific research, which may result in the most vulnerable population possibly receiving the least pre-emptive protection.
HLA-B*15:02 is not only important in the treatment of Carbamazepine, but also in its structural analog, Oxcarbazepine (Phillips et al., 2018). The cross-reactivity of HLA-B*1502 contributes to the improvement in cost-effectiveness, making this allele a worthy investment for personalized therapy.
In this study, we aimed to clarify the genetic culprit of SCARs in the Vietnamese population, particularly in the South of Vietnam. We also compare the associated risk and genetic patterns in the population between the south and the north of Vietnam or other countries.
2 Methods
2.1 Study design and participants
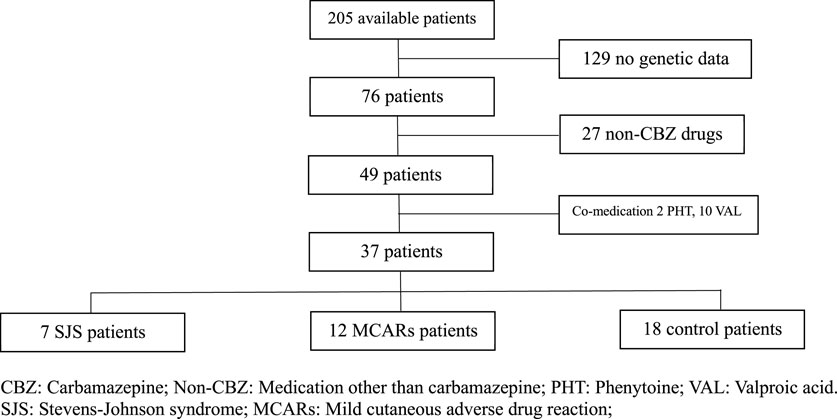
This is a case-control study with the control group defined by patients tolerant with CBZ. The study was conducted in the Department of Neurology at NDGD Hospital from 1 January 2019 to 31 December 2020. The analysis included a total of 7 cases of CBZ-induced SJS, 12 cases of CBZ-induced MCARs, and 18 cases of CBZ-tolerant control as shown in Figure 1.
All patients were recruited retrospectively with the definitions based on the information written in the medical records. The definition of CBZ tolerant is the patients who administered CBZ and the attending physician did not write any diagnosis of SJS or other skin manifestations of allergy. The definition of CBZ-induced SJS is the patients who administered CBZ and the attending physician wrote the diagnosis of SJS. The definition of CBZ-induced MCARs is the patients who administered CBZ and the attending physician wrote the diagnosis of itching skin, hypersensitivity reaction, CBZ allergy, anaphylaxis level 1, skin reaction, or symptomatic allergy. The patients who administered both CBZ and PHT were excluded from this study, only epileptic patients treated with monotherapy CBZ were included. Patients without information of genotype were also excluded from this study. The diagnosis of SJS/TEN was performed by certified dermatologists in accordance with the hospital’s guidelines, which included the following criteria: 1) Onset of symptoms within 2 months of initiating CBZ. 2) Presence of a rash affecting the face, upper body, limbs, or spreading extensively across the entire body. 3) Mucosal damage observed in at least two natural cavities, such as the eyes, nose, mouth, vagina, or anus. 4) Skin detachment (10% for SJS, 30% for TEN) or the presence of Nikolsky’s sign.
2.2 Genotyping methods
Within 76 patients with available DNA, 30 DNA samples were sent to the genotyping service at the laboratory of Pharmacogenomic and Personalize Medicine (PPM) of Ramathibodi Hospital Mahidol University, Bangkok, Thailand, 46 DNA samples were sent to the genotyping service at University Medical Center (UMC), University of Medicine and Pharmacy at Ho Chi Minh city. The genotyping method used at PPM was Luminex™ flow cytometry. In brief, the sample DNA binds complementarily to a panel of probes, which was designed with known nucleic sequences. The fluorescence detection technology was used to identify successful binding complexes, and hence, identify the sequence and genotype of the sample. The genotyping method used at UMC was real-time Polymerase Chain Reaction (real-time PCR), using Tagman™ genotyping assay.
2.3 Statistical analysis
All statistical analyses were done by R software version 4.2.3 (R Foundation for Statistical Computing, Vienna, Austria). Student’s t-test was used to compare the differences between 2 independent groups. Chi-squared tests were used to compare the ratio of males and females between 2 groups; Fisher’s exact test was employed to compare the risk of alleles between the cases and the controls.
2.4 Meta-analysis
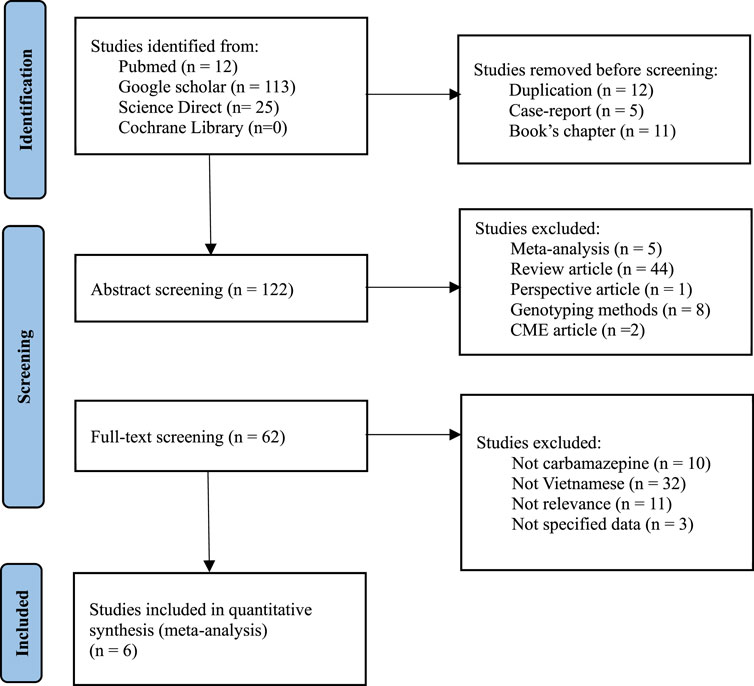
This analysis aimed to compare our conclusion with the Vietnamese population in the both the North and the South by evaluating the impact of HLA-B*15:02 in Vietnamese epileptic patients treated with CBZ, utilizing various databases such as Pubmed, Google Scholar, Cochrane Library, and ScienceDirect (Figure 2). The keywords used were “HLA-B*15:02 Vietnamese”, “HLA-B*15:02 Vietnam’’, “SJS”, “Steven-Johnson Syndrome”. To select the suitable studies, the selection criteria were: 1) Containing the case-control analysis of HLA-B*15:02 and SJS, 2) Targeting Vietnamese epileptic patients, 3) Including the clear definitions of case and control with corresponding numbers, and the exclusion criteria were: 1) Duplicating studies, 2) Researching about the development of genotyping methods in Vietnam, 3) Being a case report or cross-sectional study. Data analysis and visualization were performed on Rstudio, using random effect with a p-value lower than 0.05 is considered statistically significant, and with I2 higher than 50% was defined as heterogeneous. The estimation method used for the random effect is restricted maximum likelihood.
2.5 Ethics approval
This study was approved by the Ethics Committee of NDGD Hospital, Ho Chi Minh City, Vietnam, under approval number 23-2015/CN-HĐĐĐ, on 13 October 2015. As we used only retrospective data from health records while also maintaining patient confidentiality, no informed consent was required for this study.
3 Result
3.1 Genetic and demographic information of patients
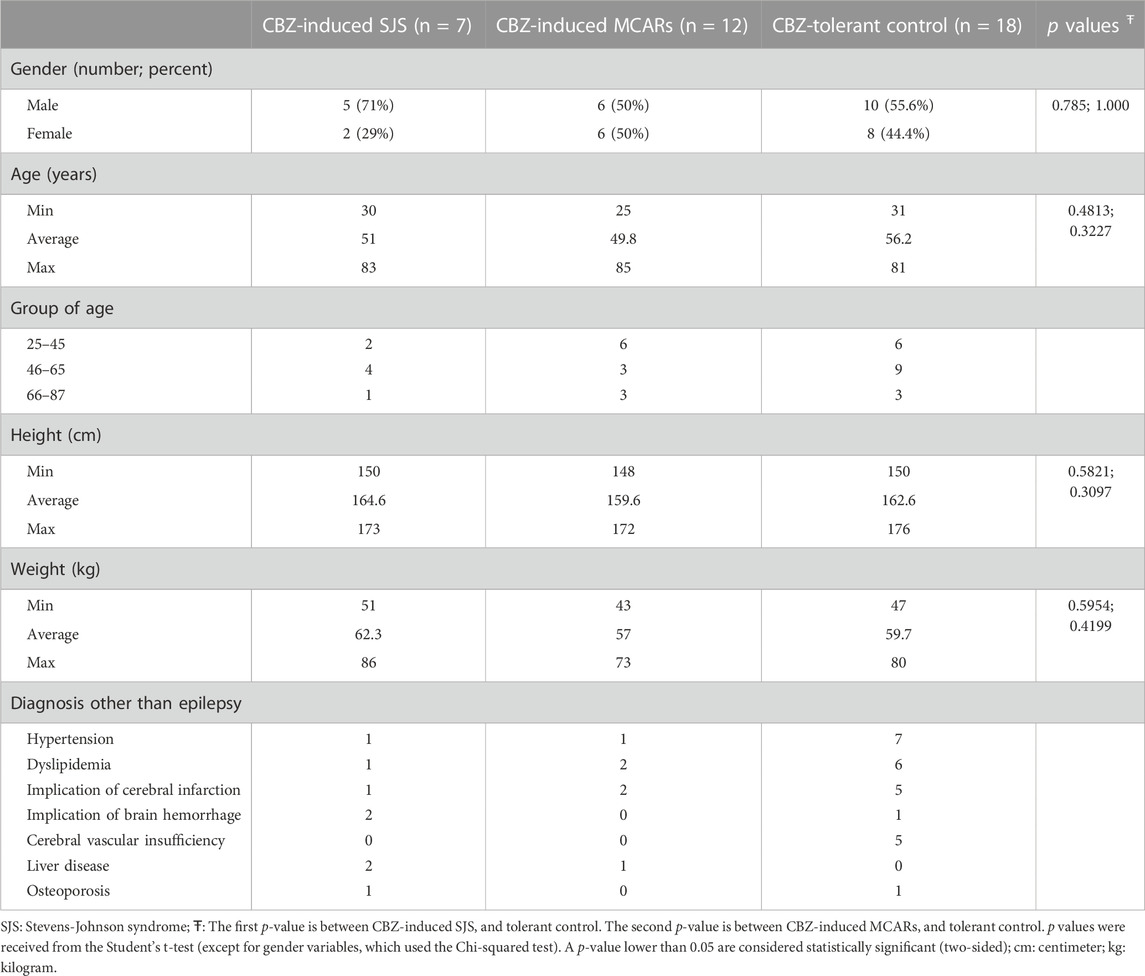
Table 1 describes the characteristic of the participants. The age of patients was widely distributed from 25 to 85 years old. The separation of gender was fairly equal between males (21 patients) and females (16 patients) in total participants. However, there was a higher proportion of males in the SJS group. The percentage of males respectively was 50% and 55.6% in the group of CBZ-induced MCARs and CBZ-tolerant control. All three groups contained patients with chronic diseases, such as hypertension or dyslipidemia. Nearly 30% of epileptic patients had a medical history of cerebral infarction or brain hemorrhage.
Among twelve carbamazepine-induced MCARs patients, five patients were diagnosed with itching skin and hypersensitivity reactions, six patients were diagnosed with symptomatic allergy and skin reactions of allergy, and one patient was diagnosed with anaphylaxis level 1 due to CBZ allergy. It is important to note that none of the twelve patients exhibited symptoms specific enough to be classified as SJS/TEN.
3.2 The frequencies of HLA-B alleles detected in Southern Vietnamese
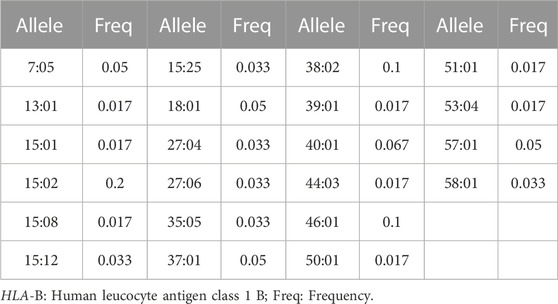
Table 2 shows the HLA allele polymorphism in the epileptic southern population of Vietnam, where a total of 22 alleles were observed. The most popular HLA-B alleles are 15:02 (20%), 38:02 (10%), and 46:01 (10%). There is a 5% of frequency in each of the following alleles: 7:05; 18:01, 37:01, 57:01. The rest 15 alleles account for around 1.7%–6.7% of the whole population.
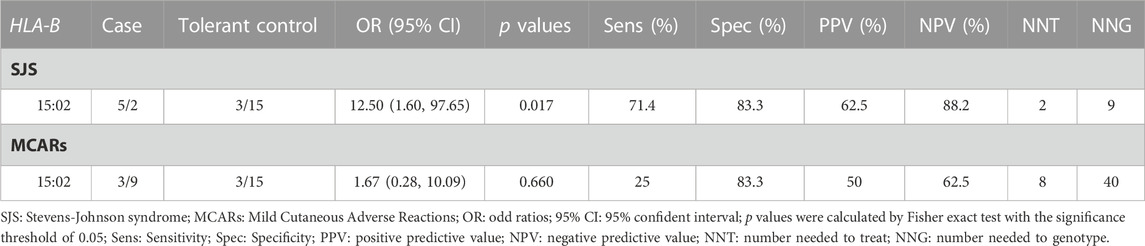
3.3 The increased risk patients carrying HLA-B*15:02 in Southern Vietnamese
Table 3 illustrates that in the Southern Vietnamese population, the HLA-B*15:02 allele is a statistically significant risk factor for SJS (p = 0.017), but not for MCARs (p = 0.660). The odd ratio of the HLA-B*15:02 allele is 12.5. In other words, the carriers of this allele have 12.5 times higher risk than non-carriers, in terms of SJS. The sensitivity and specificity are 71.4% and 83.3% respectively, meaning that the hospital’s protocol can correctly identify 71.4% of SJS patients, and correctly identify 83.3% of non-SJS patients. Besides, the PPV and NPV are 62.5% and 88.2% respectively. These two parameters are interpreted in the context that the results of HLA-B*15:02 are available. In these cases, if the result of a patient is positive, the possibility for this patient to have SJS is 62.5%, and if the result of a patient is negative, the possibility for this patient to not have SJS is 88.3%. Moreover, the NNT and NNG are respectively 2 and 9, which confirms that to protect 1 patient from SJS, the intervention of replacing CBZ with an alternative drug has to be done in 2 patients, or the genetic test has to be done in 9 patients.
Out of the twelve cases of allopurinol-induced MCARs, three patients tested positive for HLA-B*15:02. While the strength of evidence is not large enough to be statistically significant, it is worth noting that the rate of HLA-B*15:02 in the MCARs group is higher compared to the group of tolerant controls.
3.4 Meta-analysis
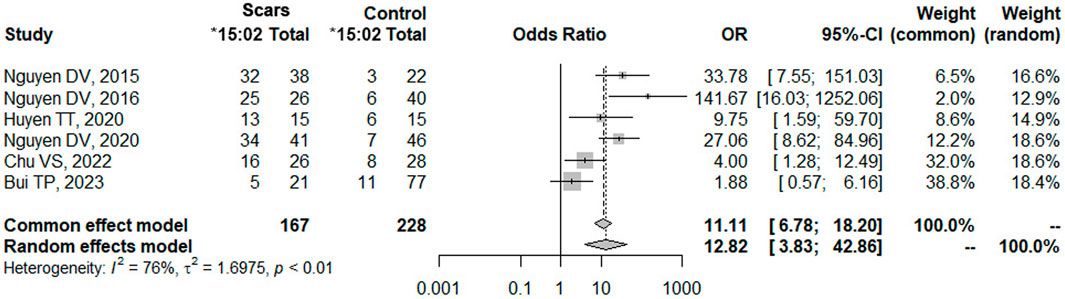
A meta-analysis was performed about the risk of HLA-B*15:02 and SCARs in Vietnamese with a total of 6 studies (Van Nguyen et al., 2015; Van Nguyen et al., 2017; Huyen et al., 2020; van Nguyen et al., 2021; Chu, 2022; Bui et al., 2023). All these 6 studies were conducted and recruited patients exclusively in the northern region of Vietnam, specifically at Bach Mai Hospital, Tam Anh Hospital (Ha Noi branch), and the National Hospital of Dermatology and Venerology. Surprisingly, no eligible studies conducted in the southern region of Vietnam were identified or included in this meta-analysis (Supplementary Table S1). Interestingly, a recent study by Bui TP et al. (Bui et al., 2023) reported a negative association between HLA-*B15:02 and SCARs in Vietnamese in January 2023. However, our analysis using pooled data from all six studies showed a strong positive association between HLA-B*15:02 and SCARs, as determined by both common effect and random effects models (Figure 3). Carriers of HLA-B*15:02 had 12.82 times higher odds of developing SCARs compared to non-carriers (p-value <0.01). The odd ratio calculated from previous studies in the meta-analysis was consistent with the odd ratio in the current study.
4 Discussion
This is the first case-control study to investigate the pharmacogenomics of CBZ-induced SJS in Southern Vietnam. Based on the evidence that the pharmacogenomic test can protect one case of SJS with every two interventions or every nine genetic tests, we recommend implementing this test in the population of Southern Vietnamese.
In the population of Southern Vietnamese, our study reported a higher frequency of the HLA-B*15:02 allele, 20% compared to 11.88% in a study of the general population of Southern Vietnamese by Do MD et al. (Do et al., 2020). In the population of Northern Vietnamese, the frequency of HLA-B*15:02 in epileptic patients varies between studies 13.5% (Van Nguyen et al., 2015), 15.2% (van Nguyen et al., 2021), 17.7% (Bui et al., 2023), and 41.7% (Huyen et al., 2020). The possible explanation may be due to the different regions or the small sample size, which can only reflect a part of the whole population. Interestingly enough, Que TN et al. (Que et al., 2022) reported a frequency of 15.11% of HLA-B*15:02 with a sample size of 3750 participants, who were recruited in Northern and North-central Vietnam (Que et al., 2022). Besides, we found the frequencies of HLA-B*38:02 and *46:01 to be equally 10%. Comparatively, Do MD et al. (Do et al., 2020) reported these two alleles to be 7.92% and 9.41% respectively. Que TN et al. (Que et al., 2022) also reported these two alleles to be 7.29% and 10.7% respectively.
Regarding global populations, Southeast Asians are regarded as having the highest frequency of HLA-B*15:02 in the world (Moutaouakkil et al., 2019; Van Nguyen et al., 2019). Indeed, numerous studies published the frequency of HLA-B*15:02 in their own country, such as 15.11% in Thailand (Zhang et al., 2011), 32.8% in Indonesia (Khosama, 2017), 8.3% in Malaysia (Chang et al., 2011), and 5.7% in Singapore (Middleton et al., 2004). Moving toward northeast Asia, this frequency gradually decreases, such as 7.3% in Southern Chinese (Trachtenberg et al., 2007), 1.9% in Northern Chinese (Hong et al., 2005), 7.7% in Taiwanese (Chen et al., 2011), 10.2% in Hongkong (Middleton et al., 2004), 1.7% in Japanese (Ikeda et al., 2010) and 0.4% in South Koreans (Kim et al., 2011). In contrast, HLA-B*15:02 is seemingly absent in Caucasians, such as French (Gourraud et al., 2015), and the United States (Maiers et al., 2007).
The clinical implication of HLA-B-*15:02 is specifically related to SJS and not MCARs, even though both are the cutaneous manifestations of allergy. This observation can be explained by recent immunologic findings that the CBZ hyperactivity reaction is not solely dependent on the specificity of HLA, but also the specificity of the T-cells and their receptors (Ko et al., 2011; Ko and Chen, 2012). This evidence also explains why three patients in our study tested positive for HLA-B*15:02 but did not develop SJS.
Pharmacogenomics and personalized medicine are well-known to be ethnicity-specific. Even though in the same country, the genetic diversity could be significant and should not be ignored. This is especially true in the case of Vietnam, with a population of 100 million or 54 ethnicities, concentrated mainly in two metropolitan and healthcare centers of the north and the south. The absence of medical evidence in an ethnic group can impede the clinical implementation of a beneficial intervention. This is not only a medical issue, but also an ethical problem to have an equitable healthcare system (Patrinos et al., 2023). Besides the genetic differences, the socioeconomic and academic differences between regions also play an important role in pharmacogenomic research and implementation to ensure cost-effectiveness in resource-limited hospitals (Nagar et al., 2019).
Several limitations need to be rectified by future research. Firstly, the patients were genotyped by two genotyping methods, which may have increased the potential for bias. The decision to employ these methods was necessitated by the challenging circumstances presented by the COVID-19 pandemic. As a result, the HLA-B*15:02 genotyping had to be conducted at a different hospital. Therefore, the better choice would be to use only one genotyping method. Secondly, the sample size is relatively small, larger studies should be conducted to clarify the finding in this study more thoroughly, especially for the outcome of MCARs in Southern Vietnamese. Thirdly, the exclusion of patients without genotyping results is another limitation, and future endeavors with more financial support should aim to include more patients to obtain a more comprehensive understanding of the real-world population.
In conclusion, this study confirms the association between CBZ-induced SJS and HLA-B*15:02 in Vietnamese, particularly in the Southern population. Therefore, it is recommended to perform further studies about the cost-effectiveness of this test to accelerate the protection of Southern Vietnamese from SCARs.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by The Ethics Committee of NDGD Hospital, Ho Chi Minh City, Vietnam, under approval number 23-2015/CN-HĐĐĐ, on 13 October, 2015. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
Conceived and designed the experiments: CS, HP; Collect demographic data, performed the experiment, and analyzed the data: QN, A-HN; Interpretated the finding: CS, HP, A-HN; Drafted the manuscript: A-HN; Adjusted the manuscript: HP, CS. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by Nguyen Tat Thanh University, Ho Chi Minh City, Vietnam.
Acknowledgments
The author would like to show gratitude to the clinical pharmacists and administrative staff of Nhan dan Gia Dinh Hospital for their support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1217516/full#supplementary-material
References
Barker, D. J., Maccari, G., Georgiou, X., Cooper, M. A., Flicek, P., Robinson, J., et al. (2023). The IPD-IMGT/HLA database. Nucleic Acids Res. 51 (D1), D1053–D1060. doi:10.1093/nar/gkac1011
Bui, T. P., Nguyen, L. T. T., Le, P. L., Le, N. T. T., Nguyen, T. D., Van Nguyen, L., et al. (2023). Next-generation sequencing-based HLA typing reveals the association of HLA-B* 46: 01: 01 and HLA-DRB1* 09: 01: 02 alleles with carbamazepine-induced hypersensitivity reactions in Vietnamese patients with epilepsy. Hum. Immunol. 84 (3), 186–195. doi:10.1016/j.humimm.2023.01.005
Chang, C. C., Too, C. L., Murad, S., and Hussein, S. H. (2011). Association of HLA-B* 1502 allele with carbamazepine-induced toxic epidermal necrolysis and Stevens–Johnson syndrome in the multi-ethnic Malaysian population. Int. J. dermatology 50 (2), 221–224. doi:10.1111/j.1365-4632.2010.04745.x
Chen, P., Lin, J. J., Lu, C. S., Ong, C. T., Hsieh, P. F., Yang, C. C., et al. (2011). Carbamazepine-induced toxic effects and HLA-B* 1502 screening in Taiwan. N. Engl. J. Med. 364 (12), 1126–1133. doi:10.1056/NEJMoa1009717
Chu, V. S. (2022). Carbamazepine allergy and its association with the presence of HLA-B*15:02 and HLA-A*31:01 alleles in Tam Anh hospital. Tạp chí Y học Việt Nam 518 (2). doi:10.51298/vmj.v518i2.3458
Do, M. D., Le, L. G. H., Nguyen, V. T., Dang, T. N., Nguyen, N. H., Vu, H. A., et al. (2020). High-Resolution HLA typing of HLA-A, -B, -C, -DRB1, and -DQB1 in Kinh Vietnamese by using Next-generation sequencing. Front. Genet. 11, 11. doi:10.3389/fgene.2020.00383
Gourraud, P.-A., Pappas, D. J., Baouz, A., Balère, M. L., Garnier, F., and Marry, E. (2015). High-resolution HLA-A, HLA-B, and HLA-DRB1 haplotype frequencies from the French bone marrow donor registry. Hum. Immunol. 76 (5), 381–384. doi:10.1016/j.humimm.2015.01.028
Hong, W., Fu, Y., Chen, S., Wang, F., Ren, X., and Xu, A. (2005). Distributions of HLA class I alleles and haplotypes in northern han Chinese. Tissue antigens 66 (4), 297–304. doi:10.1111/j.1399-0039.2005.00474.x
Huyen, T. T., Hoa, P. D., Trang, T. M., Khanh, N. B., Que, T. N., Phuong, N. H., et al. (2020). The link between HLA-B alleles and causative drugs in Vietnamese patients with stevens-johnson syndrome/toxic epidermal necrolysis. Open Access Macedonian J. Med. Sci. 8 (B), 395–400. doi:10.3889/oamjms.2020.4906
Ikeda, H., Takahashi, Y., Yamazaki, E., Fujiwara, T., Kaniwa, N., Saito, Y., et al. (2010). HLA class I markers in Japanese patients with carbamazepine-induced cutaneous adverse reactions. Epilepsia 51 (2), 297–300. doi:10.1111/j.1528-1167.2009.02269.x
Khosama, H. (2017). HLA-B* 1502 and carbamazepine induced Stevens-Johnson syndrome/toxic epidermal necrolysis in Indonesia. Neurol. Asia 22 (2).
Kim, S. H., Lee, K. W., Song, W. J., Kim, S. H., Jee, Y. K., Lee, S. M., et al. (2011). Carbamazepine-induced severe cutaneous adverse reactions and HLA genotypes in Koreans. Epilepsy Res. 97 (1-2), 190–197. doi:10.1016/j.eplepsyres.2011.08.010
Ko, T.-M., and Chen, Y.-T. (2012). T-Cell receptor and carbamazepine-induced stevens–johnson syndrome and toxic epidermal necrolysis: Understanding a hypersensitivity reaction. Expert Rev. Clin. Immunol. 8 (5), 467–477. doi:10.1586/eci.12.31
Ko, T.-M., Chung, W. H., Wei, C. Y., Shih, H. Y., Chen, J. K., Lin, C. H., et al. (2011). Shared and restricted T-cell receptor use is crucial for carbamazepine-induced Stevens-Johnson syndrome. J. Allergy Clin. Immunol. 128 (6), 1266–1276. e11. doi:10.1016/j.jaci.2011.08.013
Maiers, M., Gragert, L., and Klitz, W. (2007). High-resolution HLA alleles and haplotypes in the United States population. Hum. Immunol. 68 (9), 779–788. doi:10.1016/j.humimm.2007.04.005
Middleton, D., Hawkins, B. R., Williams, F., Meenagh, A., Moscoso, J., Zamora, J., et al. (2004). HLA class I allele distribution of a Hong Kong Chinese population based on high-resolution PCR-SSOP typing. Tissue antigens 63 (6), 555–561. doi:10.1111/j.0001-2815.2004.00234.x
Moutaouakkil, Y., Adouani, B., Cherrah, Y., Lamsaouri, J., and Bousliman, Y. (2019). Diagnostic utility of human leukocyte antigen B* 15: 02 screening in severe carbamazepine hypersensitivity syndrome. Ann. Indian Acad. Neurology 22 (4), 377–383. doi:10.4103/aian.AIAN_492_18
Nagar, S. D., Moreno, A. M., Norris, E. T., Rishishwar, L., Conley, A. B., O'Neal, K. L., et al. (2019). Population pharmacogenomics for precision public health in Colombia. Front. Genet. 10, 241. doi:10.3389/fgene.2019.00241
Patrinos, G. P., Quinones, L. A., and Sukasem, C. (2023). Editorial: Pharmacogenomics and ethnicity: Prevalence and clinical significance of pharmacogenomic biomarkers in indigenous and other populations. Front. Pharmacol. 14, 1180487. doi:10.3389/fphar.2023.1180487
Phillips, E. J., Sukasem, C., Whirl-Carrillo, M., Müller, D. J., Dunnenberger, H. M., Chantratita, W., et al. (2018). Clinical pharmacogenetics implementation consortium guideline for HLA genotype and use of carbamazepine and oxcarbazepine: 2017 update. Clin. Pharmacol. Ther. 103 (4), 574–581. doi:10.1002/cpt.1004
Que, T. N., Khanh, N. B., Khanh, B. Q., Van Son, C., Van Anh, N. T., Anh, T. T. T., et al. (2022). Allele and haplotype frequencies of HLA-A, -B, -C, and -DRB1 genes in 3,750 cord blood units from a Kinh Vietnamese population. Front. Immunol. 13, 875283. doi:10.3389/fimmu.2022.875283
Snell, G. D. (1981). Studies in histocompatibility. Science 213 (4504), 172–178. doi:10.1126/science.7017931
Sukasem, C., Chaichan, C., Nakkrut, T., Satapornpong, P., Jaruthamsophon, K., Jantararoungtong, T., et al. (2018). Association between HLA-B alleles and carbamazepine-induced maculopapular exanthema and severe cutaneous reactions in Thai patients. J. Immunol. Res. 2018, 2780272. doi:10.1155/2018/2780272
Tassaneeyakul, W., Tiamkao, S., Jantararoungtong, T., Chen, P., Lin, S. Y., Chen, W. H., et al. (2010). Association between HLA-B* 1502 and carbamazepine-induced severe cutaneous adverse drug reactions in a Thai population. Epilepsia 51 (5), 926–930. doi:10.1111/j.1528-1167.2010.02533.x
Thorsby, E. (2009). A short history of HLA. Tissue antigens 74 (2), 101–116. doi:10.1111/j.1399-0039.2009.01291.x
Trachtenberg, E., Vinson, M., Hayes, E., Hsu, Y. M., Houtchens, K., Erlich, H., et al. (2007). HLA class I (A, B, C) and class II (DRB1, DQA1, DQB1, DPB1) alleles and haplotypes in the Han from southern China. Tissue antigens 70 (6), 455–463. doi:10.1111/j.1399-0039.2007.00932.x
Van Nguyen, D., Anderson, J., Vidal, C., Fulton, R., Li, J., and Fernando, S. L. (2022). The utility of surrogate markers in predicting HLA alleles associated with adverse drug reactions in Vietnamese. Asian Pac. J. Allergy Immunol. 40 (2), 134–140. doi:10.12932/AP-170219-0493
Van Nguyen, D., Chu, H. C., Nguyen, D. V., Phan, M. H., Craig, T., Baumgart, K., et al. (2015). HLA-B* 1502 and carbamazepine-induced severe cutaneous adverse drug reactions in Vietnamese. Asia Pac. Allergy 5 (2), 68–77. doi:10.5415/apallergy.2015.5.2.68
van Nguyen, D., Chu, H. C., Vidal, C., Fulton, R. B., Nguyen, N. N., Quynh Do, N. T., et al. (2021). Genetic susceptibilities and prediction modeling of carbamazepine and allopurinol-induced severe cutaneous adverse reactions in Vietnamese. Pharmacogenomics 22 (1), 1–12. doi:10.2217/pgs-2019-0146
Van Nguyen, D., Chu, H. C., Vidal, C., Nguyen, N. N., Quynh Do, N. T., Linh Tran, T. T., et al. (2017). Genetic susceptibility to carbamazepine and allopurinol–induced severe cutaneous adverse reactions in Vietnamese. J. Allergy Clin. Immunol. 139 (2), AB118. doi:10.1016/j.jaci.2016.12.378
Van Nguyen, D., Vidal, C., Chu, H. C., and van Nunen, S. (2019). Developing pharmacogenetic screening methods for an emergent country: Vietnam. World Allergy Organ. J. 12 (5), 100037. doi:10.1016/j.waojou.2019.100037
Wang, Q., Zhou, J. q., Zhou, L. m., Chen, Z. y., Fang, Z. y., Chen, S. d., et al. (2011). Association between HLA-B* 1502 allele and carbamazepine-induced severe cutaneous adverse reactions in Han people of southern China mainland. Seizure 20 (6), 446–448. doi:10.1016/j.seizure.2011.02.003
Zhang, Y., Wang, J., Zhao, L. M., Peng, W., Shen, G. Q., Xue, L., et al. (2011). Strong association between HLA-B* 1502 and carbamazepine-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in mainland Han Chinese patients. Eur. J. Clin. Pharmacol. 67, 885–887. doi:10.1007/s00228-011-1009-4
Keywords: pharmacogenomics, Vietnam, HLA-B*15:02, SJS, MCARs, SCARs, epileptic, carbamazepine
Citation: Nguyen A-H, Sukasem C, Nguyen QN and Pham HT (2023) The pharmacogenomics of carbamazepine-induced cutaneous adverse drug reaction in the South of Vietnam. Front. Pharmacol. 14:1217516. doi: 10.3389/fphar.2023.1217516
Received: 05 May 2023; Accepted: 03 July 2023;
Published: 13 July 2023.
Edited by:
Miriam Saiz-Rodríguez, Hospital Universitario de Burgos, SpainReviewed by:
Abdelbaset A. Elzagallaai, Western University, CanadaZhibin Chen, Monash University, Australia
Copyright © 2023 Nguyen, Sukasem, Nguyen and Pham. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Tham Pham, phtham@ntt.edu.vn
 Ai-Hoc Nguyen
Ai-Hoc Nguyen