- 1Department of Pharmaceutics, Shanghai Eighth People’s Hospital, Shanghai, China
- 2Department of Pharmacy, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Cyclin-dependent kinase 4/6 inhibitors (CDK4/6i) play a crucial role in cancer treatment, particularly in breast cancer, and their mechanism of drug resistance is a topic of global interest in research. Hence, it is vital to comprehend the distinctions between various CDK4/6i, including their mechanisms of action and resistance mechanisms. This article aims to summarize the metabolic and transport variations as well as the differences in resistance among the three FDA-approved CDK4/6 inhibitors: Abemaciclib, Palbociclib, and Ribociclib. It also aims to discuss how these differences impact the effectiveness and safety of anticancer drugs. It was conducted in March 2023 to search PubMed, Embase, and Web of Science for literature related to this topic. Despite all being CDK4/6i, differences in their metabolism and transport were found, which are related to their chemical structure. Moreover, there are variations in preclinical pharmacology, pharmacokinetics, and clinical safety and efficacy of the different inhibitors. Genetic mutations, drug tolerance, and other factors may influence CDK4/6 resistance mechanisms. Currently, the resistance mechanisms differences of the three drugs remain largely unknown, and there are differences in the resistance mechanisms among them, necessitating further exploration and research.
1 Introduction
Dysregulation of the cell cycle and persistent cell proliferation due to cyclin-dependent kinase (CDK) activation are important markers of tumorigenesis. Currently marketed CDK4/6 inhibitors (CDK4/6i) belong to the third generation of CDK inhibitors. In compared to the non-selective CDK inhibitors such as Flavopiridol, Roscovitine and Olomucine, as well as broad-spectrum CDK inhibitors including Dinaciclib and AG-024322, CDK4/6i inhibitors demonstrate the ability to impede the cell cycle from G1 to S phase and, while also maintaining a superior equilibrium between antitumor efficacy and safety (Chen et al., 2016). Until now, a total of 5 CDK4/6i have been approved for marketing worldwide, and multiple CDK4/6i are still in the clinical research. Abemaciclib, Ribociclib, Palbociclib, and Trilaciclib have been approved by the Food and Drug Administration (FDA) for marketing, and all except Trilaciclib, which is used for chemotherapy-induced bone marrow suppression, are used for cancer treatment. Dalpiciclib (SHR6390) was approved by the National Medical Products Administration (NMPA) on 31 December 2021, in China.
CDK4/6 inhibitors play a crucial role in cancer treatment, particularly in breast cancer, and their mechanism of drug resistance is a topic of global interest in research (Pang et al., 2022). The latest domestic and foreign guidelines recommend the use of CDK4/6i for adjuvant and advanced treatment of hormone receptor-positive (HR+) breast cancer patients (Gradishar et al., 2022; Li and Jiang, 2022). Even though CDK4/6i have shown significant clinical benefits in HR + breast cancer (Scheidemann and Shajahan-Haq, 2021; Xu et al., 2021). Studies indicate that approximately 20% of HR + breast cancer treated with CDK4/6i experience primary resistance (Kong et al., 2019), while over 30% of patients develop secondary resistance (O'Leary et al., 2018). The development of resistance in almost all patients during treatment poses new challenges for managing this disease (Scheidemann and Shajahan-Haq, 2021). Multi-omics analysis has revealed that distinct CDK4/6i exhibit pharmacological and clinical activity differences. Moreover, these CDK4/6i drugs induce significant variations in transcriptional, proteomic, and phenotypic changes (Hafner et al., 2019). The toxicity and adverse effects of CDK4/6i differ significantly. For instance, Abemaciclib causes gastrointestinal toxicity, Palbociclib is mainly hemotoxic, and Ribociclib increases the risk of cardiotoxicity (Gao et al., 2020). A study has demonstrated that patients who exhibit resistance to Palbociclib with a high expression of cyclin E1 (CCNE1) may derive greater benefits from treatment with Abemaciclib as opposed to Ribociclib (Turner et al., 2019). It is vital to comprehend the distinctions between various CDK4/6i, including their mechanisms of action and resistance mechanisms. This article aims to provide a summary of the metabolic and transport variations and resistance differences of the three CDK4/6i (Abemaciclib, Palbociclib, and Ribociclib) that have been approved by the FDA for treating tumors. It also aims to discuss how these differences impact the effectiveness and safety of anticancer drugs.
2 Metabolic and transport differences
CDK4/6i are oral medications, which makes them a convenient treatment option for patients. Metabolic and transport difference have been identified for Abemaciclib, Palbociclib, and Ribociclib in Table 1, which may influence the efficacy and safety. By understanding these genetic differences, healthcare professionals can tailor the treatment to the individual patient, potentially improving outcomes and reducing adverse effects. The three CDK4/6i are primarily metabolized in the liver via the cytochrome P4503A4 enzyme (CYP3A4) (Yu et al., 2017; Sorf et al., 2018; Yu et al., 2019). Abemaciclib is a sensitive substrate for CYP3A4 (Yu et al., 2019), which means that the activity of this enzyme can significantly impact the drug’s metabolism and elimination from the body. On the other hand, Ribociclib is a strong inhibitor of CYP3A (Sorf et al., 2018; Yu et al., 2019), which can potentially increase the concentration of other drugs that are metabolized through this pathway. It is important to note that Palbociclib is also affected by CYP3A4, but it does not appear to be a sensitive substrate or a strong inhibitor. The CYP3A4 mediated interaction can increase the risk of adverse effects, such as toxicity or drug interactions, and may require dose adjustments or avoidance of co-administration with other CYP3A substrates or inhibitors for Palbociclib (Martínez-Chávez et al., 2019; Molenaar-Kuijsten et al., 2022; Patil et al., 2022) and Ribociclib (Patil et al., 2022). A Study has shown that transgenic CYP3A4 drastically reduces the plasma concentration of Abemaciclib (Martínez-Chávez et al., 2022). However, there is also another study suggested that Abemaciclib does not influence CYP3A4 substrates pharmacokinetics which has used mild inhibitors (Turner et al., 2020). In short, the three CDK4/6i are primarily metabolized by CYP3A4 in vivo, but the extent of this influence may differ between the drugs. Therefore, it is important to consider potential drug interactions and the patient’s individual characteristics when selecting and dosing CDK4/6i to optimize treatment outcomes and minimize adverse effects.
Although CYP3A4 is the primary enzyme responsible for the metabolism of CDK4/6i, other liver enzyme metabolism genes may also play a role in drug efficacy and safety. CYP3A5 is a closely related enzyme to CYP3A4 (Hlavica, 2017). One study found that the CYP3A5 *1/*3 genotype may associated with lower plasma concentrations of Palbociclib, suggesting that individuals with the CYP3A5 1/*3 genotype may exhibit a more effective metabolism of Palbociclib (Roncato et al., 2022). Nevertheless, there is insufficient data regarding the potential influence of CYP3A5 genetic variations on the metabolism and pharmacokinetics of Abemaciclib and Ribociclib. Some studies have explored the effects of other metabolism genes on the pharmacokinetics and pharmacodynamics of CDK4/6i. One study suggested that Ribociclib may have the potential for drug-drug interactions by inhibiting CYP1A2 and CYP2C9 (Sorf et al., 2018), which are important enzymes in drug metabolism. On the other hand, another study found that Abemaciclib did not influence the pharmacokinetics of CYP1A2, CYP2C9, and CYP2D6 substrates (Turner et al., 2020). However, there is limited data available on the potential impact of metabolism genes on the pharmacokinetics and pharmacodynamics of Palbociclib. It has been reported that approximately 26% of Palbociclib is metabolized by sulfotransferase family 2A member 1 (SULT2A1) (Yu et al., 2017). However, there is currently no information available regarding the potential role of SULT2A1 in the metabolism and pharmacokinetics of Abemaciclib and Ribociclib. These findings suggest that genetic variations may influence the metabolism and response to CDK4/6i, and personalized dosing strategies may be necessary to optimize treatment outcomes. A deeper understanding of the clinical implications of genetic variability in CDK4/6i metabolism depends on further studies to confirm these findings.
There are still some differences in the metabolism and transport that have not been fully elucidated. Studies have shown that Palbociclib is a substrate for ATP Binding Cassette Subfamily B Member 1 (ABCB1, also known as P-glycoprotein) and ATP binding cassette subfamily G member 2 (ABCG2, protein BCRP) (de Gooijer et al., 2015; Martínez-Chávez et al., 2019; Fu et al., 2022). Ribociclib is a substrate of ABCB1 and a potent inhibitor of ABCB1 and ABCG2 (Sorf et al., 2018), while Abemaciclib acts as both substrate (Martínez-Chávez et al., 2022) and inhibitor (Wu et al., 2017). Therefore, Abemaciclib has been shown to be less efflux efficient than Palbociclib and penetrates the central nervous system better (Raub et al., 2015). Studies have investigated the relationship between CDK4/6i and efflux transporters. Abemaciclib concentrations are higher when ABCB1 2677G>T/A is homozygous (Maeda et al., 2022; Maeda et al., 2023). Palbociclib oral absorption and plasma levels may be affected by ABCB1-rs1128503, rs1045642, and rs2032582 (Roncato et al., 2022). ABCB1_rs1128503 is potential risk factor for Palbociclib grade 3/4 neutropenia in non-Asian patients (Iwata et al., 2021). Fatal Statin-induced rhabdomyolysis may be a consequence of the interaction with Palbociclib (Nelson et al., 2017) and Ribociclib (Streicher et al., 2021) by organic anion transporting polypeptides OATP1B1 and OATP1B3. Some studies have suggested that the differences in efflux transport of the three CDK4/6i may be related to their chemical structures (Abdelmalak et al., 2022), while others have suggested that there may be other factors involved that are not yet fully understood. Additionally, there is some controversy in the literature regarding the role of efflux transporters in the pharmacokinetics and pharmacodynamics of CDK4/6i. It is still necessary to conduct further research to fully understand the mechanisms of CDK4/6i metabolism, transport and to identify potential strategies to optimize their efficacy and safety.
3 Chemical structure, preclinical pharmacology, pharmacokinetic differences
The CDK4/6i have different chemical structures, preclinical pharmacology, and pharmacokinetic differences which may affect their efficacy and safety profiles (George et al., 2021). Abemaciclib, Palbociclib, and Ribociclib were derivatives of pyrimidine, pyridopyrimidine, triazolopyridine, respectively. Abemaciclib is unique among the three drugs in that it contains two fluorine atoms (Chen et al., 2016). Since Ribociclib and Palbociclib have larger substituents, they are more lipophilic and have larger binding sites than Abemaciclib (Marra and Curigliano, 2019). This can affect the drugs’ pharmacokinetics and pharmacodynamics, including their absorption, distribution, metabolism, and elimination in the body, as well as their specific interactions with target proteins. Palbociclib, Ribociclib, and Abemaciclib have slightly different selectivity for different cyclin-dependent kinases (Braal et al., 2021). A study has shown that Abemaciclib inhibits CDK4 more selectively than CDK6, with a selectivity ratio of about 5:1, which may result in lower hematological toxicity than Palbociclib and Ribociclib. However, Abemaciclib also has inhibitory effects on other kinases (Kim et al., 2018), including CDK2/Cyclin A/E, CDK4/6, and CDK1/Cyclin B which is associated with CDK resistance (Hafner et al., 2019), resulting in specific gastrointestinal toxicity of Abemaciclib. In contrast, Ribociclib has a higher selectivity for inhibiting CDK4 with weaker inhibition of other kinases, However, there are some adverse effects in the electrocardiogram. The transcriptional, proteome, and phenotypic changes induced simultaneously by Palbociclib, Ribociclib, and Abemaciclib are significantly different (Hafner et al., 2019). Palbociclib, Ribociclib, and Abemaciclib have different pharmacokinetic profiles, including their half-life. According to the drug labels and clinical studies, the different pharmacokinetic profiles of these drugs may affect their dosing schedules, frequency of administration, and potential drug interactions.
Abemaciclib is typically administered twice daily at a dosage of 150 mg, whereas Palbociclib is given once daily at a 125 mg dosage for 21 consecutive days, followed by a 7-day interval. Ribociclib, on the other hand, is prescribed at a daily dosage of 600 mg for a 3-weeks period, followed by a 1-week break, with the option of adjusting the dosage based on individual patient considerations. The varying dosing schedules may impact treatment adherence and patient quality of life (Higano and Hafron, 2023; Koni et al., 2023), as some patients may prefer less frequent dosing while others may tolerate daily dosing better. Furthermore, the expenses and resource allocation linked to the administration of these drugs may be influenced by the dosing regimens (Srivastava et al., 2013; Patel et al., 2023), particularly in outpatient environment where frequent monitoring and interventions may be required. It is important to note that while the endorsed doses and schedules for these agents founded on clinical trials and practical experience, the heterogeneity in drug exposure due to variations in metabolism and clearance among patients may lead to inter-patient differences in effectiveness and adverse effects. Consequently, the utilization of pharmacodynamic biomarkers or serum drug levels for therapeutic drug monitoring may facilitate the optimization of dosing and reduction of toxicity in specific patient cohorts. Additionally, clinical investigations have demonstrated that these medications may exhibit distinct profiles of adverse effect, necessitating the implementation of dose reductions or interruptions to mitigate toxicities. As a result, drug recommendations may differ across populations (El Rassy et al., 2018), underscoring the importance of meticulously evaluating each drug’s dosing regimen, potential side effects, and patient-specific factors to optimize the efficacy and safety of CDK4/6 inhibitor therapy. In addition, ongoing research is required to examine the most effective administration schedules, sequencing, and amalgamations of these agents, along with their enduring toxicity and efficacy profiles.
4 The resistance mechanisms to different CDK4/6i
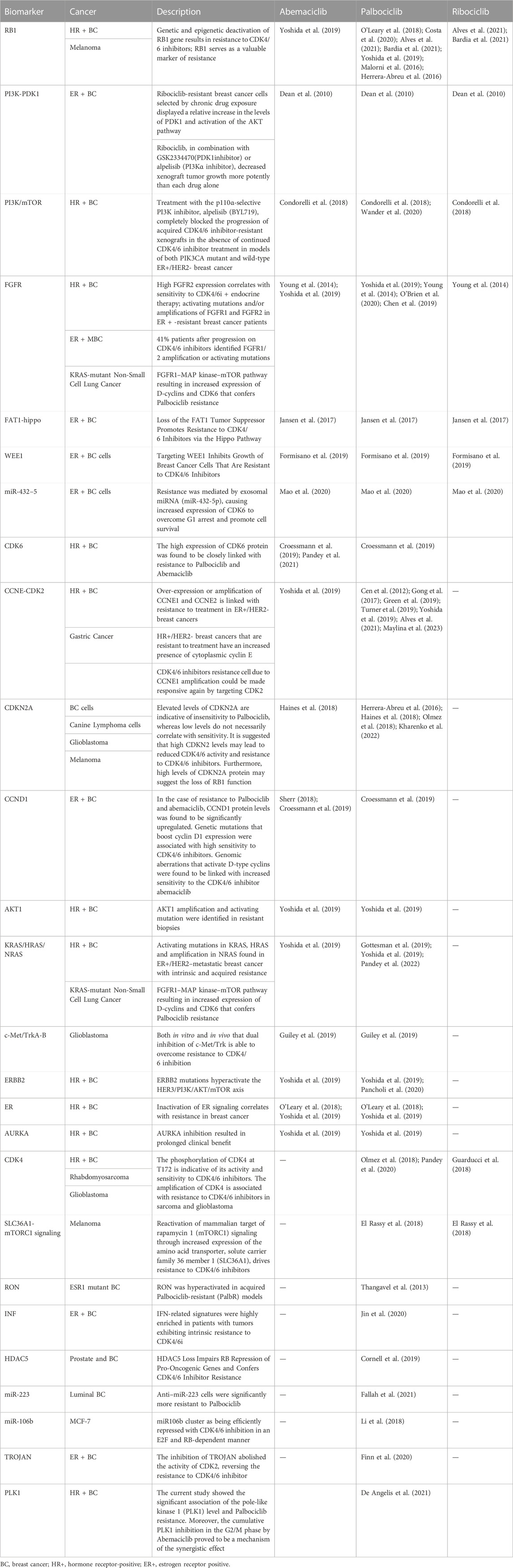
The mechanism of resistance to CDK4/6i can be influenced by various factors, such as genetic mutations, drug tolerance, cellular environment, drug resistance genes, and drug metabolism. Including abnormal CDK activity, loss of PTEN function (Costa et al., 2020), AKT1 (Alves et al., 2021), PI3K mutation (Bardia et al., 2021), mTOR (Yoshida et al., 2019), CDK2 activation, etc. According to studies, Abemaciclib cross-resistance is incomplete with Palbociclib or Ribociclib. In spite of the fact that they are all CDK4/6i, their chemical structures and inhibitory profiles differ slightly from one another, which may lead to different activities and resistance mechanisms in different cells and tumor types. In this article we subdivided the different resistance mechanisms (Table 2).
4.1 Resistance mechanisms that are shared in Palbociclib, Ribociclib, and Abemaciclib
There are several pathways and mechanisms that have been confirmed to mediate the resistance of Palbociclib, Ribociclib, and Abemaciclib. Loss of RB1 function is a common mechanism of CDK4/6i resistance (O'Leary et al., 2018; Malorni et al., 2016; Herrera-Abreu et al., 2016; Condorelli et al., 2018; Wander et al., 2020; Dean et al., 2010; Young et al., 2014). PI3K inhibitor alpelisib completely blocked the CDK4/6i resistant xenografts progression in both PIK3CA mutant and ER+/HER2-breast cancer (O'Brien et al., 2020; Chen et al., 2019). Kinome-wide siRNA screen identified 3-Phosphoinositide-dependent protein kinase 1 (PDK1) as a key modulator of Ribociclib sensitivity in MCF-7 and exhibited cross-resistance to Palbociclib and Abemaciclib (Jansen et al., 2017). ER + breast cancer cells transduced with Fibroblast growth factor receptor 1 (FGFR1) was resistant to CDK4/6i. Breast cancer cells expressing FGFR1, transduced with FGFR1 or amplified with FGFR1 were resistant to CDK4/6i (O'Leary et al., 2018; Wander et al., 2020; Formisano et al., 2019; Mao et al., 2020; Finn et al., 2020). A loss of FAT1 promotes CDK4/6i resistance via the Hippo Pathway (Li et al., 2018). When WEE1 is targeted, it inhibits the growth of CDK4/6i-resistant breast cancer cells (Fallah et al., 2021). CDK6 expression was increased in response to exosomal miRNAs (miR-432-5p), allowing cells to survive and overcome G1 arrest (Cornell et al., 2019). These pathways and mechanisms are complex and interconnected, and their involvement in mediating resistance to CDK4/6i may vary depending on the specific cancer type and individual patient characteristics. The shared presence of these mechanisms across the three CDK4/6i implies their role as common determinants of resistance to CDK4/6 Inhibition.
4.2 Resistance mechanisms may not be shared
Palbociclib, the first CDK4/6i to be approved, has been more thoroughly investigated for its resistance mechanism compared to other CDK4/6i. The following mechanisms were only studied in Palbociclib and not in Abemaciclib and Ribociclib. LncRNA TROJAN bind to NKRF and inhibits the interaction with RELA, restoring CDK2 expression and reversing CDK4/6 resistance (Jin et al., 2020). The miR106b (Thangavel et al., 2013) and miR-223 (Citron et al., 2020) were efficiently repressed with Palbociclib in an E2F and RB-dependent manner. Lack of HDAC5 impairs RB repression of pro-oncogenic genes and confers resistance to Palbociclib (Zhou et al., 2021). Palbociclib-resistant tumors exhibited high levels of IFN-related signatures (De Angelis et al., 2021) and RON hyperactivated (Dustin et al., 2021). The increase of TK1 and CDK9 are associated with clinical resistance to Palbociclib (Del Re et al., 2019). The development of resistance to Palbociclib caused elevated expression of genes including CDK7, the master regulator of the cell cycle. Additionally, loss of ER and RB1 has been shown to increase sensitivity to CDK7 inhibition (Pancholi et al., 2020). Inhibition of PLK1 cumulatively by eribulin or abemaciclib in the G2/M phase proved to be one mechanism for synergistic effects in Palbociclib resistance (Pandey et al., 2022). It is observed that high levels of CDKN1B may predict resistance to Palbociclib (Gottesman et al., 2019; Guiley et al., 2019). Indeed, the resistance mechanisms mentioned above have only been extensively studied in Palbociclib, and their relevance to resistance to Abemaciclib and Ribociclib is still not clear. However, it is worth noting that Palbociclib was the first CDK4/6i to receive FDA approval, and its specific resistance mechanisms may differ from those of Abemaciclib and Ribociclib. Further research is needed to elucidate the resistance mechanisms of all CDK4/6i.
Inactivation of the ER signaling pathway (O'Leary et al., 2018; Wander et al., 2020) and ERBB2 mutations (Croessmann et al., 2019; Wander et al., 2020) is closely associated with Abemaciclib and Palbociclib resistance. The presence of activating mutations in KRAS and HRAS, as well as amplification in NRAS, have been identified in ER+/HER2-metastatic breast cancer cases exhibiting both intrinsic and acquired resistance for Abemaciclib (Wander et al., 2020) and Palbociclib (Haines et al., 2018; Sherr, 2018; Wander et al., 2020). AKT1 amplification and activating mutation were identified in Abemaciclib and Palbociclib resistant biopsies (Wander et al., 2020). Experiments confirmed resistant cells were sensitive to Aurora Kinase (AURKA) inhibition LY3295668 (Wander et al., 2020) and inhibition of c-Met/Trk (Olmez et al., 2018). In the case of resistance to abemaciclib (Gong et al., 2017; Kharenko et al., 2022) and Palbociclib (Kharenko et al., 2022), CCND1 protein levels were found to be significantly upregulated. Genetic mutations that boost cyclin D1 expression were associated with high sensitivity to CDK4/6i. Elevated levels of CDKN2A are indicative of insensitivity whereas low levels do not necessarily correlate with sensitivity. Furthermore, high levels of CDKN2A protein may suggest the loss of RB1 function. It is suggested that high CDKN2 levels may lead to reduced activity and resistance to Abemaciclib (Maylina et al., 2023) and Palbociclib (Cen et al., 2012; Young et al., 2014; Green et al., 2019; Maylina et al., 2023). CDK2 Targeting can be used to reactivate resistance cells to Abemaciclib (Wander et al., 2020) and Palbociclib (Herrera-Abreu et al., 2016; Guarducci et al., 2018; Min et al., 2018; Turner et al., 2019; Pandey et al., 2020; Wander et al., 2020; Pandey et al., 2021) due to CCNE1 amplification. The high expression of CDK6 protein was found to be closely linked with resistance to Palbociclib (Kharenko et al., 2022) and Abemaciclib (Yang et al., 2017; Kharenko et al., 2022), which is not seen in Ribociclib resistant cell lines.
While Abemaciclib and Ribociclib were both approved by the FDA around the same time, the specific mechanisms of Ribociclib resistance may be less well-studied. The phosphorylation of CDK4 at T172 is indicative of its activity and sensitivity to Palbociclib in breast cancer, sarcoma and glioblastoma (Cen et al., 2012; Raspé et al., 2017). CDK4 Amplification Reduces Sensitivity to Ribociclib in fusion-positive rhabdomyosarcoma (Olanich et al., 2015). A reactivation of mammalian target of rapamycin 1 (mTORC1) signaling by increasing expression of solute carrier family 36 member 1 (SLC36A1), contributes Palbociclib and Ribociclib resistance in Melanoma (Yoshida et al., 2019). There is currently no evidence of CDK4 or mTORC1 mediated abemaciclib resistance.
All the mechanisms involved in resistance are mainly studied in breast cancer, but there are also Melanoma, Non-Small Cell Lung Cancer, Rhabdomyosarcoma, Glioblastoma, Gastric Cancer, Canine Lymphoma cells, Prostate, Glioblastoma, Which Remind us that CDK4/6i are being widely studied and applied in various fields.
5 Conclusion and perspectives
CDK4/6i will still be primarily used against breast cancer in the next 5 years, and its clinical applications will expand to include a range of tumor types. It is important to note, however, that as use of the drug increases, the incidence of drug resistance increases, and new combination regimens and other targeted drugs may become available as a result. Currently, the resistance mechanisms of the three drugs remain largely unknown, and there are differences in the resistance mechanisms among them, necessitating further exploration and research.
Author contributions
QZ was responsible for the conceptualization, methodology and writing-original draft preparation and funding acquisition. ZZ was responsible for supervision, writing reviewing and editing. All authors contributed to the article and approved the submitted version.
Funding
This work was Sponsored by Shanghai Sailing Program (20YF1427000); Ruijin Youth NSCF Cultivation Fund (KY20194238).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdelmalak, M., Singh, R., Anwer, M., Ivanchenko, P., Randhawa, A., Ahmed, M., et al. (2022). The renaissance of CDK inhibitors in breast cancer therapy: An update on clinical trials and therapy resistance. Cancers 14, 5388. doi:10.3390/cancers14215388
Alves, C. L., Ehmsen, S., Terp, M. G., Portman, N., Tuttolomondo, M., Gammelgaard, O. L., et al. (2021). Co-targeting CDK4/6 and AKT with endocrine therapy prevents progression in CDK4/6 inhibitor and endocrine therapy-resistant breast cancer. Nat. Commun. 12, 5112. doi:10.1038/s41467-021-25422-9
Bardia, A., Hurvitz, S. A., DeMichele, A., Clark, A. S., Zelnak, A., Yardley, D. A., et al. (2021). Phase I/II trial of exemestane, ribociclib, and everolimus in women with HR(+)/HER2(-) advanced breast cancer after progression on CDK4/6 inhibitors (TRINITI-1). Clin. cancer Res. official J. Am. Assoc. Cancer Res. 27, 4177–4185. doi:10.1158/1078-0432.CCR-20-2114
Braal, C. L., Jongbloed, E. M., Wilting, S. M., Mathijssen, R. H. J., Koolen, S. L. W., and Jager, A. (2021). Inhibiting CDK4/6 in breast cancer with palbociclib, ribociclib, and abemaciclib: Similarities and differences. Drugs 81, 317–331. doi:10.1007/s40265-020-01461-2
Cen, L., Carlson, B. L., Schroeder, M. A., Ostrem, J. L., Kitange, G. J., Mladek, A. C., et al. (2012). p16-Cdk4-Rb axis controls sensitivity to a cyclin-dependent kinase inhibitor PD0332991 in glioblastoma xenograft cells. Neuro-oncology 14, 870–881. doi:10.1093/neuonc/nos114
Chen, L., Yang, G., Dong, H., Luo, Y., Xu, Q., Han, C., et al. (2019). Competitive interaction with keystone taxa induced negative priming under biochar amendments. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 25, 77–86. doi:10.1186/s40168-019-0693-7
Chen, P., Lee, N. V., Hu, W., Xu, M., Ferre, R. A., Lam, H., et al. (2016). Spectrum and degree of CDK drug interactions predicts clinical performance. Mol. cancer Ther. 15, 2273–2281. doi:10.1158/1535-7163.MCT-16-0300
Citron, F., Segatto, I., Vinciguerra, G. L. R., Musco, L., Russo, F., Mungo, G., et al. (2020). Downregulation of miR-223 expression is an early event during mammary transformation and confers resistance to CDK4/6 inhibitors in luminal breast cancer. Cancer Res. 80, 1064–1077. doi:10.1158/0008-5472.CAN-19-1793
Condorelli, R., Spring, L., O'Shaughnessy, J., Lacroix, L., Bailleux, C., Scott, V., et al. (2018). Polyclonal RB1 mutations and acquired resistance to CDK 4/6 inhibitors in patients with metastatic breast cancer. Ann. Oncol. official J. Eur. Soc. Med. Oncol. 29, 640–645. doi:10.1093/annonc/mdx784
Cornell, L., Wander, S. A., Visal, T., Wagle, N., and Shapiro, G. I. (2019). MicroRNA-mediated suppression of the TGF-β pathway confers transmissible and reversible CDK4/6 inhibitor resistance. Cell. Rep. 26, 2667–2680.e7. doi:10.1016/j.celrep.2019.02.023
Costa, C., Wang, Y., Ly, A., Hosono, Y., Murchie, E., Walmsley, C. S., et al. (2020). PTEN loss mediates clinical cross-resistance to CDK4/6 and PI3Kα inhibitors in breast cancer. Cancer Discov. 10, 72–85. doi:10.1158/2159-8290.CD-18-0830
Croessmann, S., Formisano, L., Kinch, L. N., Gonzalez-Ericsson, P. I., Sudhan, D. R., Nagy, R. J., et al. (2019). Combined blockade of activating ERBB2 mutations and ER results in synthetic lethality of ER+/HER2 mutant breast cancer. Clin. cancer Res. official J. Am. Assoc. Cancer Res. 25, 277–289. doi:10.1158/1078-0432.CCR-18-1544
De Angelis, C., Fu, X., Cataldo, M. L., Nardone, A., Pereira, R., Veeraraghavan, J., et al. (2021). Activation of the IFN signaling pathway is associated with resistance to CDK4/6 inhibitors and immune checkpoint activation in ER-positive breast cancer. Clin. cancer Res. official J. Am. Assoc. Cancer Res. 27, 4870–4882. doi:10.1158/1078-0432.CCR-19-4191
de Gooijer, M. C., Zhang, P., Thota, N., Mayayo-Peralta, I., Buil, L. C., Beijnen, J. H., et al. (2015). P-glycoprotein and breast cancer resistance protein restrict the brain penetration of the CDK4/6 inhibitor palbociclib. Investig. new drugs 33, 1012–1019. doi:10.1007/s10637-015-0266-y
Dean, J. L., Thangavel, C., McClendon, A. K., Reed, C. A., and Knudsen, E. S. (2010). Therapeutic CDK4/6 inhibition in breast cancer: Key mechanisms of response and failure. Oncogene 29, 4018–4032. doi:10.1038/onc.2010.154
Del Re, M., Bertolini, I., Crucitta, S., Fontanelli, L., Rofi, E., De Angelis, C., et al. (2019). Overexpression of TK1 and CDK9 in plasma-derived exosomes is associated with clinical resistance to CDK4/6 inhibitors in metastatic breast cancer patients. Breast cancer Res. Treat. 178, 57–62. doi:10.1007/s10549-019-05365-y
Dustin, D., Gu, G., Beyer, A. R., Herzog, S. K., Edwards, D. G., Lin, H., et al. (2021). RON signalling promotes therapeutic resistance in ESR1 mutant breast cancer. Br. J. cancer 124, 191–206. doi:10.1038/s41416-020-01174-z
El Rassy, E., Bakouny, Z., Assi, T., and Kattan, J. (2018). Different inhibitors for the same target in metastatic luminal breast cancer: Is there any difference? Future Oncol. Lond. Engl. 14, 891–895. doi:10.2217/fon-2017-0532
Fallah, Y., Demas, D. M., Jin, L., He, W., and Shajahan-Haq, A. N. (2021). Targeting WEE1 inhibits growth of breast cancer cells that are resistant to endocrine therapy and CDK4/6 inhibitors. Front. Oncol. 11, 681530. doi:10.3389/fonc.2021.681530
Finn, R. S., Liu, Y., Zhu, Z., Martin, M., Rugo, H. S., Diéras, V., et al. (2020). Biomarker analyses of response to cyclin-dependent kinase 4/6 inhibition and endocrine therapy in women with treatment-naïve metastatic breast cancer. Clin. cancer Res. official J. Am. Assoc. Cancer Res. 26, 110–121. doi:10.1158/1078-0432.CCR-19-0751
Formisano, L., Lu, Y., Servetto, A., Hanker, A. B., Jansen, V. M., Bauer, J. A., et al. (2019). Aberrant FGFR signaling mediates resistance to CDK4/6 inhibitors in ER+ breast cancer. Nat. Commun. 10, 1373. doi:10.1038/s41467-019-09068-2
Fu, H., Wu, Z. X., Lei, Z. N., Teng, Q. X., Yang, Y., Ashby, C. R., et al. (2022). The resistance of cancer cells to palbociclib, a cyclin-dependent kinase 4/6 inhibitor, is mediated by the ABCB1 transporter. Front. Pharmacol. 13, 861642. doi:10.3389/fphar.2022.861642
Gao, J. J., Cheng, J., Bloomquist, E., Sanchez, J., Wedam, S. B., Singh, H., et al. (2020). CDK4/6 inhibitor treatment for patients with hormone receptor-positive, HER2-negative, advanced or metastatic breast cancer: A US Food and drug administration pooled analysis. Lancet Oncol. 21, 250–260. doi:10.1016/S1470-2045(19)30804-6
George, M. A., Qureshi, S., Omene, C., Toppmeyer, D. L., and Ganesan, S. (2021). Clinical and pharmacologic differences of CDK4/6 inhibitors in breast cancer. Front. Oncol. 11, 693104. doi:10.3389/fonc.2021.693104
Gong, X., Litchfield, L. M., Webster, Y., Chio, L. C., Wong, S. S., Stewart, T. R., et al. (2017). Genomic aberrations that activate D-type cyclins are associated with enhanced sensitivity to the CDK4 and CDK6 inhibitor abemaciclib. Cancer Cell. 32, 761–776.e6. doi:10.1016/j.ccell.2017.11.006
Gottesman, S. R. S., Somma, J., Tsiperson, V., Dresner, L., Govindarajulu, U., Patel, P., et al. (2019). Tyrosine phosphorylation of p27Kip1 correlates with palbociclib responsiveness in breast cancer tumor cells grown in explant culture. Mol. cancer Res. MCR 17, 669–675. doi:10.1158/1541-7786.MCR-18-0188
Gradishar, W. J., Moran, M. S., Abraham, J., Aft, R., Agnese, D., Allison, K. H., et al. (2022). Breast cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. JNCCN. 20, 691–722. doi:10.6004/jnccn.2022.0030
Green, J. L., Okerberg, E. S., Sejd, J., Palafox, M., Monserrat, L., Alemayehu, S., et al. (2019). Direct CDKN2 modulation of CDK4 alters target engagement of CDK4 inhibitor drugs. Mol. cancer Ther. 18, 771–779. doi:10.1158/1535-7163.MCT-18-0755
Guarducci, C., Bonechi, M., Benelli, M., Biagioni, C., Boccalini, G., Romagnoli, D., et al. (2018). Cyclin E1 and Rb modulation as common events at time of resistance to palbociclib in hormone receptor-positive breast cancer. NPJ breast cancer 4, 38. doi:10.1038/s41523-018-0092-4
Guiley, K. Z., Stevenson, J. W., Lou, K., Barkovich, K. J., Kumarasamy, V., Wijeratne, T. U., et al. (2019). p27 allosterically activates cyclin-dependent kinase 4 and antagonizes palbociclib inhibition. New York, NY: Science, 366.
Hafner, M., Mills, C. E., Subramanian, K., Chen, C., Chung, M., Boswell, S. A., et al. (2019). Multiomics profiling establishes the polypharmacology of FDA-approved CDK4/6 inhibitors and the potential for differential clinical activity. Cell. Chem. Biol. 26, 1067–1080.e8. doi:10.1016/j.chembiol.2019.05.005
Haines, E., Chen, T., Kommajosyula, N., Chen, Z., Herter-Sprie, G. S., Cornell, L., et al. (2018). Palbociclib resistance confers dependence on an FGFR-MAP kinase-mTOR-driven pathway in KRAS-mutant non-small cell lung cancer. Oncotarget 9, 31572–31589. doi:10.18632/oncotarget.25803
Herrera-Abreu, M. T., Palafox, M., Asghar, U., Rivas, M. A., Cutts, R. J., Garcia-Murillas, I., et al. (2016). Early adaptation and acquired resistance to CDK4/6 inhibition in estrogen receptor-positive breast cancer. Cancer Res. 76, 2301–2313. doi:10.1158/0008-5472.CAN-15-0728
Higano, C. S., and Hafron, J. (2023). Adherence with oral anticancer therapies: Clinical trial vs real-world experiences with a focus on prostate cancer. J. Urol. 209, 485–493. doi:10.1097/JU.0000000000003081
Hlavica, P. (2017). Challenges in assignment of allosteric effects in cytochrome P450-catalyzed substrate oxidations to structural dynamics in the hemoprotein architecture. J. Inorg. Biochem. 167, 100–115. doi:10.1016/j.jinorgbio.2016.11.025
Iwata, H., Umeyama, Y., Liu, Y., Zhang, Z., Schnell, P., Mori, Y., et al. (2021). Evaluation of the association of polymorphisms with palbociclib-induced neutropenia: Pharmacogenetic analysis of PALOMA-2/-3. Oncol. 26, e1143–e1155. doi:10.1002/onco.13811
Jansen, V. M., Bhola, N. E., Bauer, J. A., Formisano, L., Lee, K. M., Hutchinson, K. E., et al. (2017). Kinome-wide RNA interference screen reveals a role for PDK1 in acquired resistance to CDK4/6 inhibition in ER-positive breast cancer. Cancer Res. 77, 2488–2499. doi:10.1158/0008-5472.CAN-16-2653
Jin, X., Ge, L. P., Li, D. Q., Shao, Z. M., Di, G. H., Xu, X. E., et al. (2020). LncRNA TROJAN promotes proliferation and resistance to CDK4/6 inhibitor via CDK2 transcriptional activation in ER+ breast cancer. Mol. cancer 19, 87. doi:10.1186/s12943-020-01210-9
Kharenko, O. A., Patel, R. G., Calosing, C., and van der Horst, E. H. (2022). Combination of ZEN-3694 with CDK4/6 inhibitors reverses acquired resistance to CDK4/6 inhibitors in ER-positive breast cancer. Cancer gene Ther. 29, 859–869. doi:10.1038/s41417-021-00375-9
Kim, S., Tiedt, R., Loo, A., Horn, T., Delach, S., Kovats, S., et al. (2018). The potent and selective cyclin-dependent kinases 4 and 6 inhibitor ribociclib (LEE011) is a versatile combination partner in preclinical cancer models. Oncotarget 9, 35226–35240. doi:10.18632/oncotarget.26215
Kong, T., Xue, Y., Cencic, R., Zhu, X., Monast, A., Fu, Z., et al. (2019). eIF4A inhibitors suppress cell-cycle feedback response and acquired resistance to CDK4/6 inhibition in cancer. Mol. cancer Ther. 18, 2158–2170. doi:10.1158/1535-7163.MCT-19-0162
Koni, A. A., Suwan, B. A., Nazzal, M. A., Sleem, A., Daifallah, A., Allah, M. H., et al. (2023). Adherence to oral anticancer hormonal therapy in breast cancer patients and its relationship with treatment satisfaction: An important insight from a developing country. BMC Womens Health 23, 114. doi:10.1186/s12905-023-02276-5
Li, J., and Jiang, Z. (2022). Chinese society of clinical oncology breast cancer (CSCO BC) guidelines in 2022: Stratification and classification. Cancer Biol. Med. 19, 769–773. doi:10.20892/j.issn.2095-3941.2022.0277
Li, Z., Razavi, P., Li, Q., Toy, W., Liu, B., Ping, C., et al. (2018). Loss of the FAT1 tumor suppressor promotes resistance to CDK4/6 inhibitors via the Hippo pathway. Cancer Cell. 34, 893–905.e8. doi:10.1016/j.ccell.2018.11.006
Maeda, A., Ando, H., Irie, K., Hashimoto, N., Morishige, J. I., Fukushima, S., et al. (2023). Effects of ABCB1 and ABCG2 polymorphisms on the pharmacokinetics of abemaciclib metabolites (M2, M20, M18). Anticancer Res. 43, 1283–1289. doi:10.21873/anticanres.16275
Maeda, A., Ando, H., Irie, K., Hashimoto, N., Morishige, J. I., Fukushima, S., et al. (2022). Effects of ABCB1 and ABCG2 polymorphisms on the pharmacokinetics of abemaciclib. Eur. J. Clin. Pharmacol. 78, 1239–1247. doi:10.1007/s00228-022-03331-0
Malorni, L., Piazza, S., Ciani, Y., Guarducci, C., Bonechi, M., Biagioni, C., et al. (2016). A gene expression signature of retinoblastoma loss-of-function is a predictive biomarker of resistance to palbociclib in breast cancer cell lines and is prognostic in patients with ER positive early breast cancer. Oncotarget 7, 68012–68022. doi:10.18632/oncotarget.12010
Mao, P., Cohen, O., Kowalski, K. J., Kusiel, J. G., Buendia-Buendia, J. E., Cuoco, M. S., et al. (2020). Acquired FGFR and FGF alterations confer resistance to estrogen receptor (ER) targeted therapy in ER(+) metastatic breast cancer. Clin. cancer Res. official J. Am. Assoc. Cancer Res. 26, 5974–5989. doi:10.1158/1078-0432.CCR-19-3958
Marra, A., and Curigliano, G. (2019). Are all cyclin-dependent kinases 4/6 inhibitors created equal? NPJ breast cancer 5, 27. doi:10.1038/s41523-019-0121-y
Martínez-Chávez, A., Loos, N. H. C., Lebre, M. C., Tibben, M. M., Rosing, H., Beijnen, J. H., et al. (2022). ABCB1 and ABCG2 limit brain penetration and, together with CYP3A4, total plasma exposure of abemaciclib and its active metabolites. Pharmacol. Res. 178, 105954. doi:10.1016/j.phrs.2021.105954
Martínez-Chávez, A., van Hoppe, S., Rosing, H., Lebre, M. C., Tibben, M., Beijnen, J. H., et al. (2019). P-Glycoprotein limits ribociclib brain exposure and CYP3A4 restricts its oral bioavailability. Mol. Pharm. 16, 3842–3852. doi:10.1021/acs.molpharmaceut.9b00475
Maylina, L., Kambayashi, S., Baba, K., Igase, M., Mizuno, T., and Okuda, M. (2023). Decreased sensitivity of cyclin-dependent kinase 4/6 inhibitors, palbociclib and abemaciclib to canine lymphoma cells with high p16 protein expression and low retinoblastoma protein phosphorylation. J. veterinary Med. Sci. 85, 99–104. doi:10.1292/jvms.22-0498
Min, A., Kim, J. E., Kim, Y. J., Lim, J. M., Kim, S., Kim, J. W., et al. (2018). Cyclin E overexpression confers resistance to the CDK4/6 specific inhibitor palbociclib in gastric cancer cells. Cancer Lett. 430, 123–132. doi:10.1016/j.canlet.2018.04.037
Molenaar-Kuijsten, L., Braal, C. L., Groenland, S. L., de Vries, N., Rosing, H., Beijnen, J. H., et al. (2022). Effects of the moderate CYP3A4 inhibitor erythromycin on the pharmacokinetics of palbociclib: A randomized crossover trial in patients with breast cancer. Clin. Pharmacol. Ther. 111, 477–484. doi:10.1002/cpt.2455
Nelson, K. L., Stenehjem, D., Driscoll, M., and Gilcrease, G. W. (2017). Fatal statin-induced rhabdomyolysis by possible interaction with palbociclib. Front. Oncol. 7, 150. doi:10.3389/fonc.2017.00150
O'Brien, N. A., McDermott, M. S. J., Conklin, D., Luo, T., Ayala, R., Salgar, S., et al. (2020). Targeting activated PI3K/mTOR signaling overcomes acquired resistance to CDK4/6-based therapies in preclinical models of hormone receptor-positive breast cancer. Breast cancer Res. BCR 22, 89. doi:10.1186/s13058-020-01320-8
O'Leary, B., Cutts, R. J., Liu, Y., Hrebien, S., Huang, X., Fenwick, K., et al. (2018). The genetic landscape and clonal evolution of breast cancer resistance to palbociclib plus fulvestrant in the PALOMA-3 trial. Cancer Discov. 8, 1390–1403. doi:10.1158/2159-8290.CD-18-0264
Olanich, M. E., Sun, W., Hewitt, S. M., Abdullaev, Z., Pack, S. D., and Barr, F. G. (2015). CDK4 amplification reduces sensitivity to CDK4/6 inhibition in fusion-positive rhabdomyosarcoma. Clin. cancer Res. official J. Am. Assoc. Cancer Res. 21, 4947–4959. doi:10.1158/1078-0432.CCR-14-2955
Olmez, I., Zhang, Y., Manigat, L., Benamar, M., Brenneman, B., Nakano, I., et al. (2018). Combined c-met/trk inhibition overcomes resistance to CDK4/6 inhibitors in glioblastoma. Cancer Res. 78, 4360–4369. doi:10.1158/0008-5472.CAN-17-3124
Pancholi, S., Ribas, R., Simigdala, N., Schuster, E., Nikitorowicz-Buniak, J., Ressa, A., et al. (2020). Tumour kinome re-wiring governs resistance to palbociclib in oestrogen receptor positive breast cancers, highlighting new therapeutic modalities. Oncogene 39, 4781–4797. doi:10.1038/s41388-020-1284-6
Pandey, K., Katuwal, N. B., Park, N., Hur, J., Cho, Y. B., Kim, S. K., et al. (2022)., 14. Cancers. doi:10.3390/cancers14010210Combination of abemaciclib following eribulin overcomes palbociclib-resistant breast cancer by inhibiting the G2/M cell cycle phaseCancers (Basel).210
Pandey, K., Lee, E., Park, N., Hur, J., Cho, Y. B., Katuwal, N. B., et al. (2021). Deregulated immune pathway associated with palbociclib resistance in preclinical breast cancer models: Integrative genomics and transcriptomics. Genes. 12, 159. doi:10.3390/genes12020159
Pandey, K., Park, N., Park, K. S., Hur, J., Cho, Y. B., Kang, M., et al. (2020). Combined CDK2 and CDK4/6 inhibition overcomes palbociclib resistance in breast cancer by enhancing senescence. Cancers 12, 3566. doi:10.3390/cancers12123566
Pang, J., Li, H., and Sheng, Y. (2022). CDK4/6 inhibitor resistance: A bibliometric analysis. Front. Oncol. 12, 917707. doi:10.3389/fonc.2022.917707
Patel, J. V., Hughes, D. M., and Ko, N. Y. (2023). OPTIMAL breast cancer care: Effect of an outpatient pharmacy team to improve management and adherence to oral cancer treatment. JCO Oncol. Pract. 19, e306–e314. doi:10.1200/OP.22.00135
Patil, P. H., Birangal, S., Shenoy, G. G., Rao, M., Kadari, S., Wankhede, A., et al. (2022). Molecular dynamics simulation and in vitro evaluation of herb-drug interactions involving dietary polyphenols and CDK inhibitors in breast cancer chemotherapy. Phytotherapy Res. PTR 36, 3988–4001. doi:10.1002/ptr.7547
Raspé, E., Coulonval, K., Pita, J. M., Paternot, S., Rothé, F., Twyffels, L., et al. (2017). CDK4 phosphorylation status and a linked gene expression profile predict sensitivity to palbociclib. EMBO Mol. Med. 9, 1052–1066. doi:10.15252/emmm.201607084
Raub, T. J., Wishart, G. N., Kulanthaivel, P., Staton, B. A., Ajamie, R. T., Sawada, G. A., et al. (2015). Brain exposure of two selective dual CDK4 and CDK6 inhibitors and the antitumor activity of CDK4 and CDK6 inhibition in combination with temozolomide in an intracranial glioblastoma xenograft. Drug metabolism Dispos. Biol. fate Chem. 43, 1360–1371. doi:10.1124/dmd.114.062745
Roncato, R., Gerratana, L., Palmero, L., Gagno, S., Poetto, A. S., Peruzzi, E., et al. (2022). An integrated pharmacological counselling approach to guide decision-making in the treatment with CDK4/6 inhibitors for metastatic breast cancer. Front. Pharmacol. 13, 897951. doi:10.3389/fphar.2022.897951
Scheidemann, E. R., and Shajahan-Haq, A. N. (2021). Resistance to CDK4/6 inhibitors in estrogen receptor-positive breast cancer. Int. J. Mol. Sci. 22, 12292. doi:10.3390/ijms222212292
Sherr, C. J. (2018). Acquired palbociclib resistance in KRAS-mutant lung cancer. Oncotarget 9, 32734–32735. doi:10.18632/oncotarget.26027
Sorf, A., Hofman, J., Kučera, R., Staud, F., and Ceckova, M. (2018). Ribociclib shows potential for pharmacokinetic drug-drug interactions being a substrate of ABCB1 and potent inhibitor of ABCB1, ABCG2 and CYP450 isoforms in vitro. Biochem. Pharmacol. 154, 10–17. doi:10.1016/j.bcp.2018.04.013
Srivastava, K., Arora, A., Kataria, A., Cappelleri, J. C., Sadosky, A., and Peterson, A. M. (2013). Impact of reducing dosing frequency on adherence to oral therapies: A literature review and meta-analysis. Patient Prefer Adherence 7, 419–434. doi:10.2147/PPA.S44646
Streicher, C., Daulange, A., Madranges, N., and Vayre, L. (2021).Severe rhabdomyolysis induced by possible drug-drug interaction between Ribociclib and Simvastatin. J. Oncol. Pharm. Pract., 27, 722–726. doi:10.1177/1078155220945365
Thangavel, C., Boopathi, E., Ertel, A., Lim, M., Addya, S., Fortina, P., et al. (2013). Regulation of miR106b cluster through the RB pathway: Mechanism and functional targets. Cell. cycleGeorget. Tex) 12, 98–111. doi:10.4161/cc.23029
Turner, N. C., Liu, Y., Zhu, Z., Loi, S., Colleoni, M., Loibl, S., et al. (2019). Cyclin E1 expression and palbociclib efficacy in previously treated hormone receptor-positive metastatic breast cancer. J. Clin. Oncol. official J. Am. Soc. Clin. Oncol. 37, 1169–1178. doi:10.1200/JCO.18.00925
Turner, P. K., Hall, S. D., Chapman, S. C., Rehmel, J. L., Royalty, J. E., Guo, Y., et al. (2020). Abemaciclib does not have a clinically meaningful effect on pharmacokinetics of CYP1A2, CYP2C9, CYP2D6, and CYP3A4 substrates in patients with cancer. Drug metabolism Dispos. Biol. fate Chem. 48, 796–803. doi:10.1124/dmd.119.090092
Wander, S. A., Cohen, O., Gong, X., Johnson, G. N., Buendia-Buendia, J. E., Lloyd, M. R., et al. (2020). The genomic landscape of intrinsic and acquired resistance to cyclin-dependent kinase 4/6 inhibitors in patients with hormone receptor-positive metastatic breast cancer. Cancer Discov. 10, 1174–1193. doi:10.1158/2159-8290.CD-19-1390
Wu, T., Chen, Z., To, K. K. W., Fang, X., Wang, F., Cheng, B., et al. (2017). Effect of abemaciclib (LY2835219) on enhancement of chemotherapeutic agents in ABCB1 and ABCG2 overexpressing cells in vitro and in vivo. Biochem. Pharmacol. 124, 29–42. doi:10.1016/j.bcp.2016.10.015
Xu, X. Q., Pan, X. H., Wang, T. T., Wang, J., Yang, B., He, Q. J., et al. (2021). Intrinsic and acquired resistance to CDK4/6 inhibitors and potential overcoming strategies. Acta Pharmacol. Sin. 42, 171–178. doi:10.1038/s41401-020-0416-4
Yang, C., Li, Z., Bhatt, T., Dickler, M., Giri, D., Scaltriti, M., et al. (2017). Acquired CDK6 amplification promotes breast cancer resistance to CDK4/6 inhibitors and loss of ER signaling and dependence. Oncogene 36, 2255–2264. doi:10.1038/onc.2016.379
Yoshida, A., Bu, Y., Qie, S., Wrangle, J., Camp, E. R., Hazard, E. S., et al. (2019). SLC36A1-mTORC1 signaling drives acquired resistance to CDK4/6 inhibitors. Sci. Adv. 5, eaax6352. doi:10.1126/sciadv.aax6352
Young, R. J., Waldeck, K., Martin, C., Foo, J. H., Cameron, D. P., Kirby, L., et al. (2014). Loss of CDKN2A expression is a frequent event in primary invasive melanoma and correlates with sensitivity to the CDK4/6 inhibitor PD0332991 in melanoma cell lines. Pigment Cell. & melanoma Res. 27, 590–600. doi:10.1111/pcmr.12228
Yu, J., Petrie, I. D., Levy, R. H., and Ragueneau-Majlessi, I. (2019). Mechanisms and clinical significance of pharmacokinetic-based drug-drug interactions with drugs approved by the U.S. Food and drug administration in 2017. Drug metabolism Dispos. Biol. fate Chem. 47, 135–144. doi:10.1124/dmd.118.084905
Yu, Y., Loi, C. M., Hoffman, J., and Wang, D. (2017). Physiologically based pharmacokinetic modeling of palbociclib. J. Clin. Pharmacol. 57, 173–184. doi:10.1002/jcph.792
Keywords: targeted therapy, CDK4/6 inhibitors, metabolizer transport, drug resistance, Abemaciclib, Palbociclib, Ribociclib
Citation: Zhu Z and Zhu Q (2023) Differences in metabolic transport and resistance mechanisms of Abemaciclib, Palbociclib, and Ribociclib. Front. Pharmacol. 14:1212986. doi: 10.3389/fphar.2023.1212986
Received: 27 April 2023; Accepted: 27 June 2023;
Published: 05 July 2023.
Edited by:
Amit Kumar Pandey, National Institute of Pharmaceutical Education and Research, Ahmedabad, IndiaReviewed by:
James M. Rae, University of Michigan, United StatesCopyright © 2023 Zhu and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiongni Zhu, bmh1eGlhb25pQDEyNi5jb20=
 Zhimin Zhu1
Zhimin Zhu1 Qiongni Zhu
Qiongni Zhu