- 1Division of Head and Neck Tumor Multimodality Treatment, Cancer Center, West China Hospital, Sichuan University, Chengdu, China
- 2Department of Medical Administration, West China Hospital, Sichuan University, Chengdu, China
- 3Department of Evidence Based Medicine and Clinical Epidemiology, West China Hospital, Sichuan University, Chengdu, China
Objective: Clinical trials play an important role in the development of healthcare. However, the current status of clinical trials on anti-PD-1/PD-L1 for nasopharyngeal carcinoma remains unclear. Therefore, this study aims to provide a comprehensive analysis of the registered trials related to anti-PD-1/PD-L1 for nasopharyngeal carcinoma on ClinicalTrials.gov.
Methods: A search was conducted on the ClinicalTrials.gov database to identify all registered trials related to anti-PD-1/PD-L1 for nasopharyngeal carcinoma up to 26 February 2023. The characteristics of the trials were examined, and the studied drugs, disease conditions, as well as details of trials with available results were analyzed. Publication status was assessed by a PubMed search using the ClinicalTrials.gov NCT number.
Results: A total of 112 interventional clinical trials registered between 2015 and 2023 were included. Of the trials, 90 were carried out in Asia, 72 were in phase 2, and 31 trials had either companies or universities as sponsors/collaborators. The sample sizes across the trials varied greatly, with a median of 71.5 participants per trial. The majority of trials were recruiting participants, with only 6 had posted results. PD-1 inhibitors were preferred over PD-L1, and Toripalimab emerged as the most extensively studied drug. About one-third (33.9%) of the studies looked into recurrent/metastatic nasopharyngeal cancer.
Conclusion: This study provides an overview of all registered trials of anti-PD-1/PD-L1 for NPC. It is needed to improve the completeness, outcome selection, randomization and masking of trials and to be transparent and timely in reporting of results.
Introduction
Nasopharyngeal carcinoma (NPC) is a type of epithelial carcinoma that arises from the mucosal lining of the nasopharynx (Chin et al., 2016). Compared to other malignancies, it is relatively infrequent. According to the report of the International Agency for Research on Cancer, there were 133,354 new cases of NPC worldwide in 2020, representing about 0.7% of all newly diagnosed cancers (Sung et al., 2021). The incidence of NPC is significantly unbalanced worldwide, with a significantly higher prevalence observed in East and Southeast Asia, accounting for over 70% of new cases (Chen et al., 2019). Therefore, standardized and comprehensive treatment of NPC is particularly important in high-risk areas and populations.
With the implementation of intensity-modulated radiotherapy (IMRT), a precision radiotherapy technique enabling conformation of high doses to concave-shaped tumors while protecting normal tissue, excellent loco-regional control rates have been achieved in the treatment of NPC(Lee et al., 2019; Killock, 2023). However, despite of patients diagnosed at early stages (stages I and II) with a favorable long-term survival, the majority of patients (∼80%) diagnosed at later stages due to the lack of early symptom, only have a 5-year survival rate of 70%–80% after appropriate therapy (Zhang et al., 2022). Most treatment failures, including recurrences and distant metastases, occur within 1–2 years after IMRT, indicating that a proportion of NPC may be radioresistant (Liu et al., 2017), suggesting that additional treatment approaches are needed for NPC.
Triggered by promising advances in immunotherapy, there has been growing interest in the use of immune checkpoint inhibitors (ICI), specifically anti-programmed death-1 or programmed death-1 ligand (PD-1/PD-L1) therapies in the treatment of NPC. Activated T lymphocytes express immune checkpoints such as PD-1 on the surface, which, when bound to ligands, transmit a “stop” signal to T cells and suppress the anti-tumor immune response (Pardoll, 2012). When tumor cells evade T-cell-mediated immune killing by over-expressing programmed cell death protein ligand 1 (PD-L1), they form immune escape by binding to immune checkpoints to disengage the receptor-ligand interaction between tumor cells and T cells (Renkvist et al., 2001). PD-1/PD-L1 therapies allows T cells to be effectively activated, thus restoring the body’s immune function to achieve anti-tumor effects, which is also believed to be a radiation enhancement factor (Wang et al., 2019; Vanneste et al., 2020). Being the commonest histological cell type, non-keratinizing NPC and is almost always associated with Epstein-Barr virus (EBV) infection (Lee et al., 2021). Non-keratinizing EBV+ NPC is characterized by a higher PD-L1 expression level and a pronounced lymphocytic infiltration in the tumor cell culture, rendering it a promising target for immunotherapy (Larbcharoensub et al., 2018; Outh-Gauer et al., 2018; Le et al., 2019). The approval of PD-1 inhibitors toripalimab and camrelizumab has facilitated the establishment of a standard treatment approach for patients with refractory recurrent/metastatic NPC (Xu et al., 2022).
Clinical trials play a vital role in evidence-based medicine and have been instrumental in driving the development of healthcare (Stensland et al., 2022). To ensure transparency in the clinical trial process, the International Committee of Medical Journal Editors (ICMJE) reached a consensus in 2004 that all clinical trials should be registered in a public registry before recruiting patients (De Angelis et al., 2004). This led to the establishment of ClinicalTrials.gov by the U.S. National Library of Medicine and the U.S. Food and Drug Administration in 2005, which currently holds over 446,966 research studies across 221 countries (https://clinicaltrials.gov/ct2/resources/trends). Consequently, it is considered as one of the most reliable sources of information for clinical trials. Accessing the information on ClinicalTrials.gov is expected to provide valuable insights into the current state of research and potential areas for further analysis. Despite previous studies conducted in other fields (Chen et al., 2018; Wang et al., 2020; Riddell et al., 2021), the status of registered trials of anti-PD-1/PD-L1 for NPC remains unknown.
Therefore, to gain a comprehensive overview of current clinical research progression on anti-PD-1/PD-L1 therapies for NPC, the study intends to undertake a thorough analysis of trials registered on ClinicalTrials.gov. The analysis focused on the current landscape of clinical trials on the use of anti-PD-1/PD-L1 therapies in NPC, including characteristics of trials and the study design, the distribution of drugs, as well as the disease conditions being targeted. By synthesizing this information, it is to provide a valuable resource for researchers, clinicians, and patients interested in the latest developments in the field of NPC immunotherapy.
Materials and methods
Data source and search strategy
A cross-sectional study was conducted to analyze clinical trials on anti-PD-1/PD-L1 for nasopharyngeal carcinoma (NPC) registered on ClinicalTrials.gov up to 26 February 2023. The trials were obtained using the advanced search function with the search term nasopharyngeal carcinoma OR NPC for “condition,” and PD-1 OR PD-L1 OR pembrolizumab OR nivolumab OR dostarlimab OR atezolizumab OR avelumab OR durvalumab OR toripalimab OR tislelizumab OR camrelizumab OR cemiplimab OR spartalizumab OR sintilimab OR sugemalimab or penpulimab for “intervention” with results limited to “interventional studies” (Vaddepally et al., 2020). Publication status was then assessed by a PubMed search and oncology conferences search, including ASCO, AACR, ESMO, and SITC. All the ClinicalTrials.gov NCT number were examined, and reports of the trials were analyzed.
Inclusion and exclusion criteria
The inclusion criteria of the registered trials were clinical trials on anti-PD-1/PD-L1 therapies for NPC. To ensure the validity and accuracy of the analysis, trials meeting any of the following criteria were excluded: 1) trials involving patients with various solid tumors without separate data for the NPC patients; 2) trials lacked critical information or had incomplete data; or 3) trials with a non-interventional design that did not involve the administration of anti-PD-1/PD-L1 therapies.
Data extraction and statistical analysis
A data extraction form was built to keep a record of the main characteristics of included trials. The following information was extracted: NCT number, title, status, study results, conditions, interventions, primary outcome measures, gender, age, phases, enrollment, funders, study type, allocation, intervention model, masking, start date, primary completion date, completion date, locations, etc. Descriptive statistics were utilized to characterize each trial, and categorical data were presented as frequencies and percentages.
Between-group comparisons for study design elements were performed using the Pearson χ2 test or the Fisher exact test if the number of studies in any single category was less than 10. Statistical analyses were completed on 20 June 2023, using SPSS Statistics Subscription Build, version 1.0.0.1461 (IBM Corp). Statistical significance was set at 2-sided p < .05. Since this study was based on publicly available data, ethical approval was not required.
Results
The characteristics of included trials
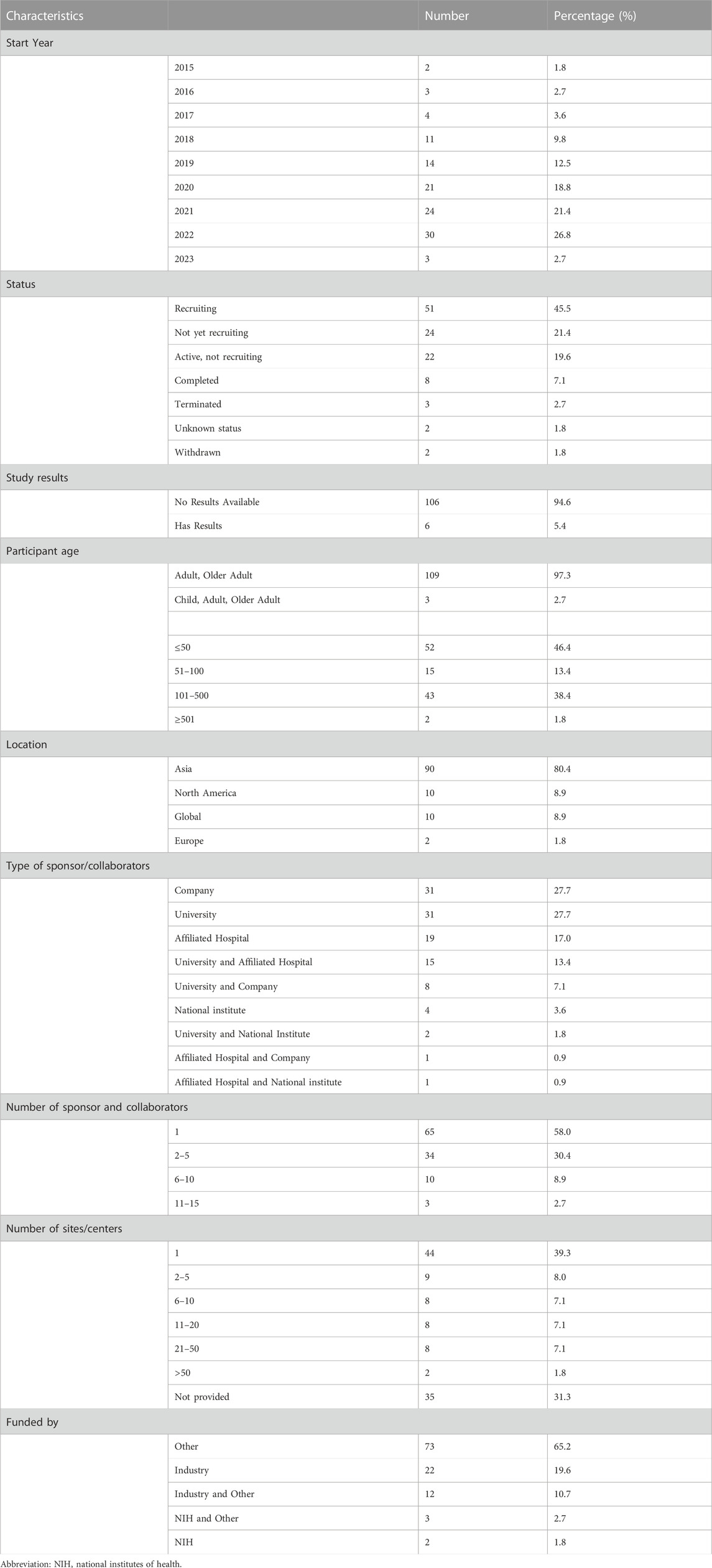
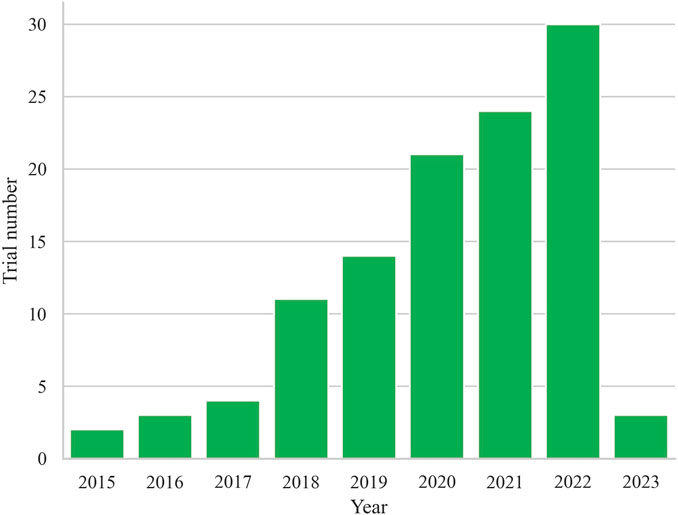
Finally, a total of 112 registered trials were included. Table 1 shows the basic characteristics of the included 112 clinical trials. The annual trends of the number of registered trials, as depicted in Figure 1, reveal a steady increase since 2015, reaching their peak with the highest number of trials registered in 2022 (n = 30, 26.8%). Of the included trials, 51 (45.5%) were currently recruiting participants, while 24 (21.4%) had not yet started recruitment, and 22 (19.6%) were active but not recruiting. A small number of trials had completed (n = 8, 7.1%), terminated (n = 3, 2.7%), withdrawn (n = 2, 1.8%), or had an unknown status (n = 2, 1.8%). The majority of trials did not have available results (n = 106, 94.6%), while only 6 trials (5.4%) had available results.
In terms of participant demographics, most trials included only adult and older adult participants (n = 109, 97.3%), with only a few trials including children participants (n = 3, 2.7%). Most trials would enroll 101–500 participants (n = 43, 38.4%), while 52 trials (46.4%) would enroll ≤ 50 participants, and 15 trials (13.4%) would enroll 51–100 participants. Only 2 trials would enroll ≥ 501 participants. Geographically, most trials would conduct in Asian (n = 90, 80.4%), followed by North America (n = 10, 8.9%), Global (n = 10, 8.9%), Europe (n = 2, 1.8%).
It appears that the most common sponsors/collaborators were companies and universities alone, each comprising 31 trials (27.7%). Hospitals were the third most common sponsor/collaborator, with 19 trials (17% of the trials). Interestingly, a significant portion of the trials (n = 15, 13.4%) had both a university and hospital as sponsors/collaborators. Regarding the number of sponsors and collaborators, the majority of trials (n = 65, 58%) had only one sponsor or collaborator, while 34 trials (30.4%) had between 2–5 sponsors or collaborators. A smaller proportion of trials had between 6–10 (n = 10, 8.9%) or 11–15 (n = 3, 2.7%) sponsors/collaborators.
Among the trials included in the analysis, 44 trials (39.3% of the total) were conducted at a single site/center. 9 trials (8.0%) were conducted at 2 to 5 sites/centers, 8 trials (7.1%) were conducted at 6 to 10 sites/centers, 8 trials (7.1%) were conducted at 11 to 20 sites/centers, and 8 trials (7.1%) were conducted at 21 to 50 sites/centers. There were 2 trials (1.8%) that involved more than 50 sites/centers. The information regarding the sites/centers was not provided for 35 trials (31.3%).
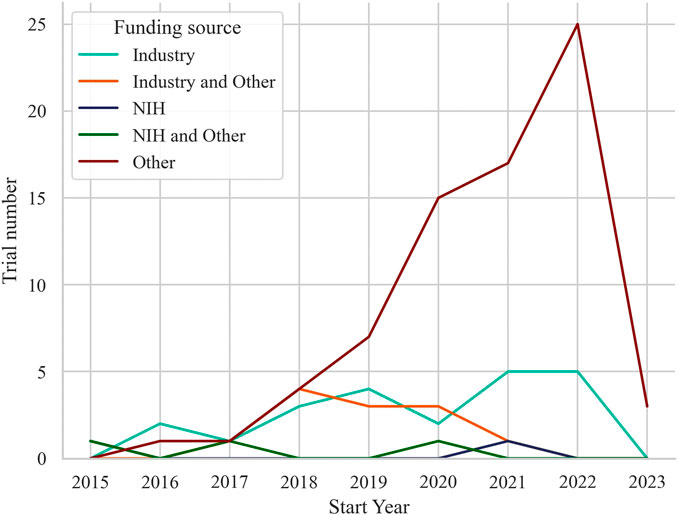
Funding for the trials was primarily from other sources (n = 73, 65.2%), with 22 trials (19.6%) funded by industry, 12 trials (10.7%) funded by industry and other, and 3 trials (2.7%) funded by National Institutes of Health (NIH) and other. Only 2 trials (1.8%) were funded by NIH alone. Annually, the distribution of trials also varied across different funding sources as displayed in Figure 2. Other-funded trials raised along with the trend of all trials, from 1 trial in 2016 to 25 trials in 2022. Industry-funded trials showed fluctuating numbers over the years, ranging from 2 trials in 2016 to 5 trials in both 2021 and 2022. Trials funded by industry and other sources had 1 trial in 2017, increasing to 4 trials in 2018, and then ranging from 1 to 3 trials in subsequent years. The 3 trials received funding from both the NIH and other sources distributed across 2015, 2017, and 2020, and the 2 trials solely funded by the NIH distributed with one in 2015 and the other in 2021.
Characteristics of study design
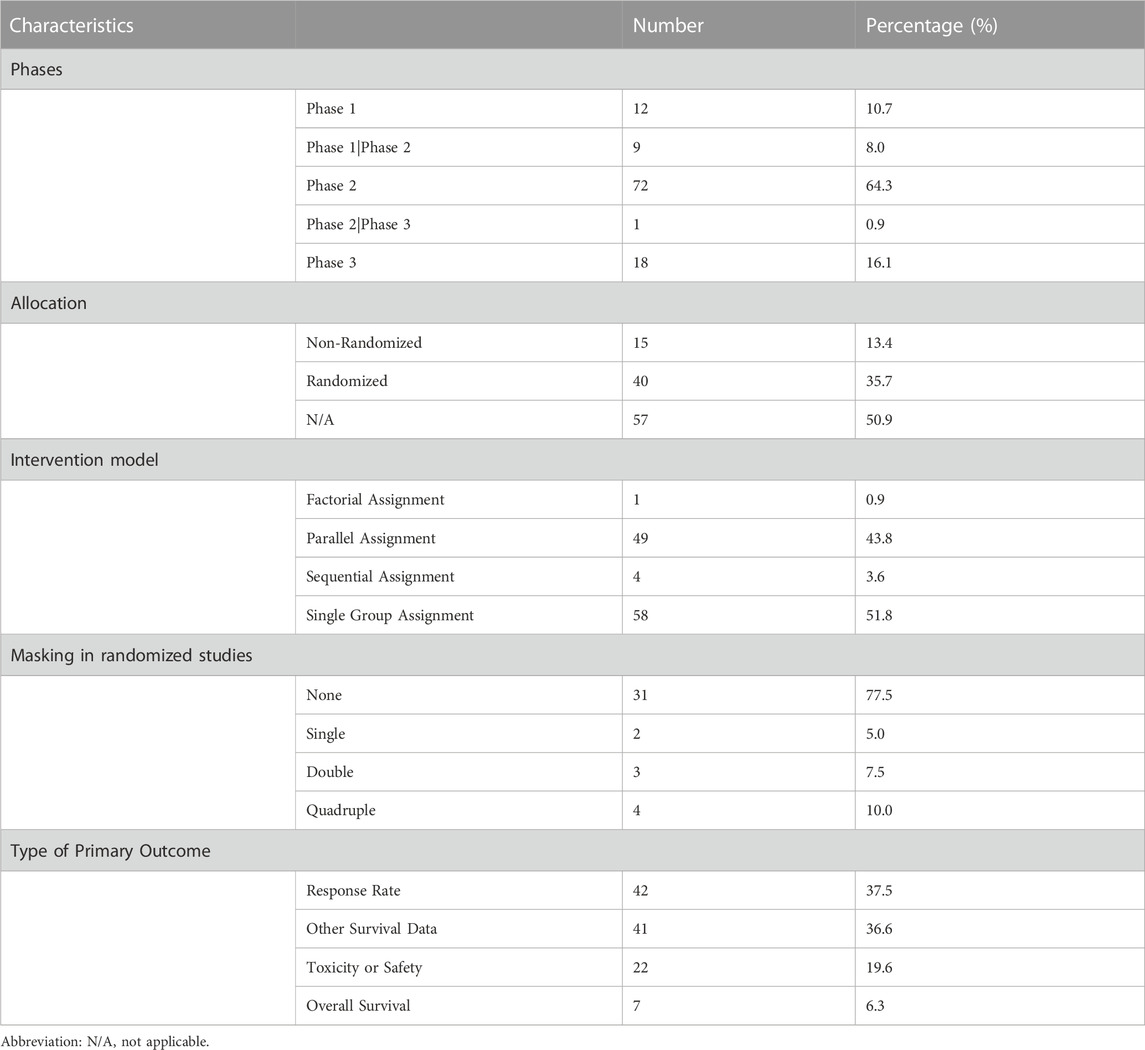
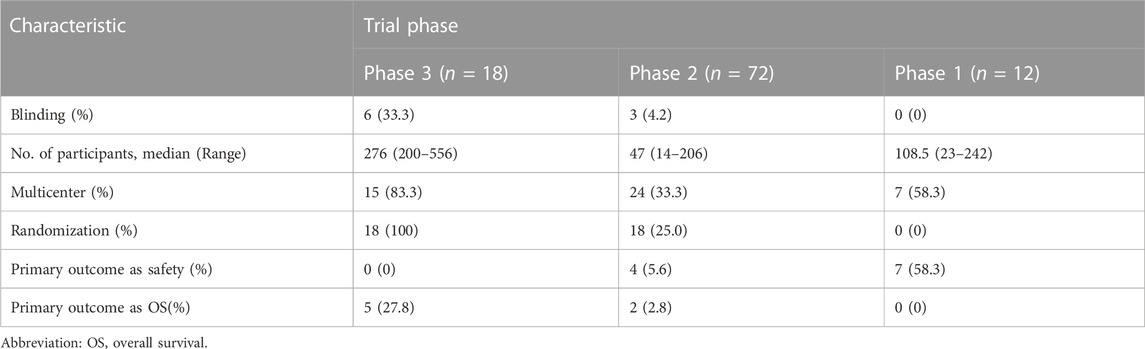
Study design characteristics of included trials are displayed in Table 2. Among the 112 trials, phase 2 trials were the most common (n = 72, 64.3%), followed by phase 3 trials (n = 18, 16.1%), and phase 1 trials (n = 12, 10.7%). In terms of allocation, 40 trials (35.7%) were randomized, 15 (13.4%) were non-randomized, and 57 (50.9%) did not report allocation information. Single-group assignment was the most common intervention model (n = 58, 51.8%), followed by parallel assignment (n = 49, 43.8%), and sequential assignment (n = 4, 3.6%). The majority of trials (n = 103, 92%) had no masking, while a small number of trials had single (n = 2, 1.8%), double (n = 3, 2.7%), or quadruple (n = 4, 3.6%) masking. Among the 112 trials, phase 2 trials were the most common (n = 72, 64.3%), followed by phase 3 trials (n = 18, 16.1%), and phase 1 trials (n = 12, 10.7%). In terms of allocation, 40 trials (35.7%) were randomized, 15 (13.4%) were non-randomized, and 57 (50.9%) did not report allocation information. Single-group assignment was the most common intervention model (n = 58, 51.8%), followed by parallel assignment (n = 49, 43.8%), and sequential assignment (n = 4, 3.6%). The type of primary outcomes varied, with response rate (Objective response rate (ORR), best overall response rate (BORR), and complete response (CR)) and survival data other than overall survival (progress-free survival (PFS), prolong one-year disease free survival (DFS-1y), failure-free survival (FFS), disease-free survival, etc.) being the most common outcomes (37.5% and 36.6%, respectively). Toxicity or safety/tolerability were also primary outcomes for a considerable proportion of trials (n = 22, 19.6%), and only 7 trials (6.3%) set OS as the primary outcome.
Table 3 presents several key trial characteristics observed across phases of clinical research. In terms of blinding, Phase 3 trials exhibited blinding in 33.3% of cases, contrasting with Phase 2 trials where blinding was employed in only 4.2% of studies; conspicuously, blinding was entirely absent in Phase 1 trials. The median number of participants in these trials displayed notable variations: Phase 3 trials featured a median of 276 participants, encompassing a range of 200–556, while Phase 2 trials had a median of 47 participants, spanning a range of 14–206. Phase 1 trials, on the other hand, had a median of 108.5 participants. The participant numbers in the Phase 1 trials included in the study exhibited significant variation, ranging from 23 to 242, owing to the inclusion of various solid tumors. As for multicenter involvement, Phase 3 trials were notably prevalent in this regard, with 83.3% of trials engaging multiple centers, in contrast to Phase 2 where only 33.3% of trials embraced multicenter collaboration; Phase 1 trials exhibited intermediate multicenter participation, accounting for 58.3% of the trials. In terms of the utilization of randomization, Phase 3 trials consistently employed randomization in all cases, totaling 100% implementation, reflecting a rigorous allocation approach. Phase 2 trials showed a more modest utilization of randomization at 25.0%, while none of the Phase 1 trials employed randomization. For outcome measures, overwhelmingly more Phase 1 trials focused on safety than other phases, while more Phase 3 trials set OS as their primary outcome.
Overview of drugs
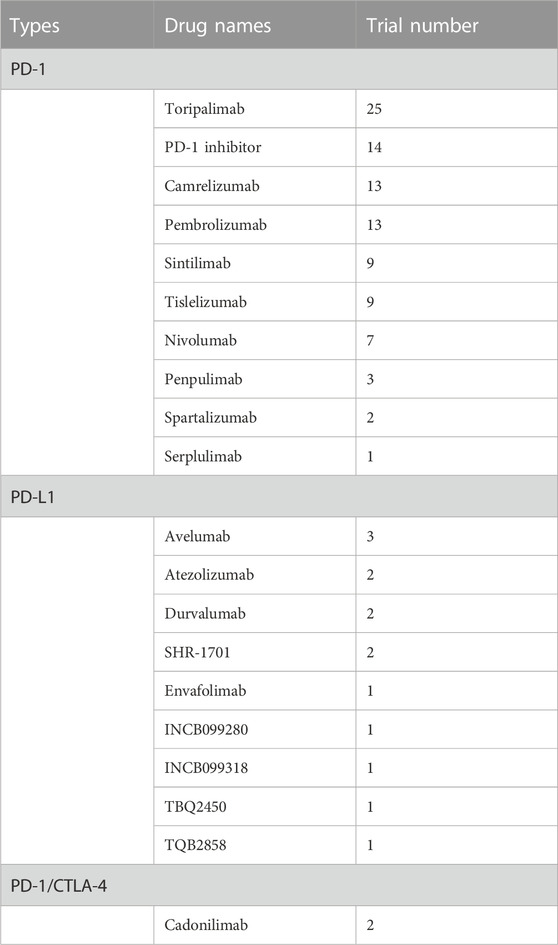
Table 4 shows the types and number of drugs used in clinical trials targeting the PD-1 and PD-L1 pathways. PD-1 inhibitors were the most studied drugs, with Toripalimab being used in the highest number of trials (n = 25), followed by Camrelizumab (n = 13), Pembrolizumab (n = 13), Sintilimab (n = 9), Tislelizumab (n = 9), and Nivolumab (n = 7), while Penpulimab, Spartalizumab, and Serplulimab were used in a small number of trials. Among PD-L1 inhibitors, Avelumab was used in the highest number of trials (3), followed by Atezolizumab, Durvalumab, SHR-1701, Envafolimab, INCB099280, INCB099318, TBQ2450, and TQB2858, each used in 1–2 trials. It is worthy to know that the Cadonilimab, a PD-1/CTLA-4 bi-specific antibody, has been analyzed in 2 clinical trials for the treatment of NPC.
In the top five most studied PD-1 inhibitors, four were developed by Chinese pharmaceutical companies, with Toripalimab by Junshi Biosciences being used in the highest number of trials, followed by Camrelizumab by Jiangsu Hengrui Medicine, Sintilimab by Innovent Biologics, and Tislelizumab by BeiGene. The only non-Chinese PD-1 inhibitor in the top five was Pembrolizumab, developed by Merck & Co., Inc.
Overview of NPC conditions
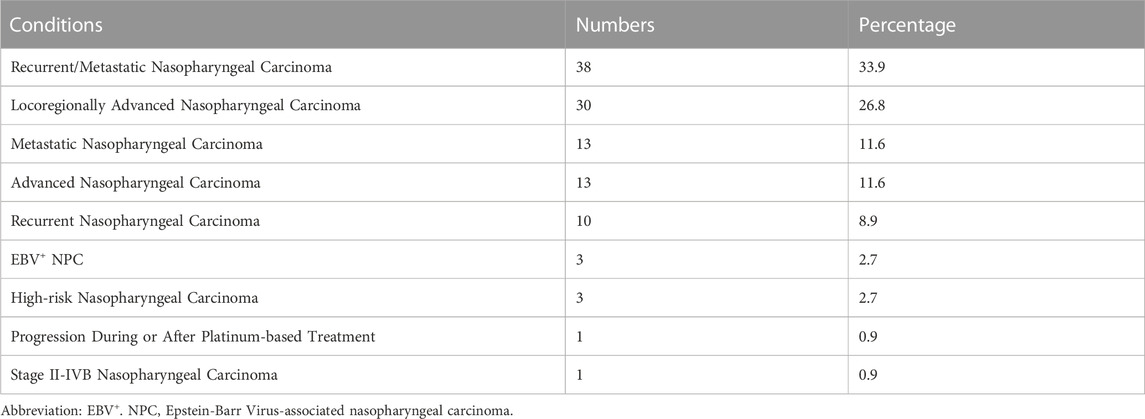
Table 5 displays the distribution of different conditions in the included interventional trials. The most common condition studied was recurrent/metastatic nasopharyngeal cancer, with 38 trials (33.9%) of this condition. This was followed by locoregionally advanced nasopharyngeal carcinoma (26.8%). Other conditions included metastatic nasopharyngeal carcinoma (11.6%), advanced nasopharyngeal carcinoma (11.6%), recurrent nasopharyngeal carcinoma (8.9%). Lastly, 3 (2.7%) trials were conducted on EBV+ NPC, and 1 (0.9%) each on stage II-IVB nasopharyngeal carcinoma and progression during or after platinum-based treatment.
Characteristics of trials with results available on ClinicalTrials.gov
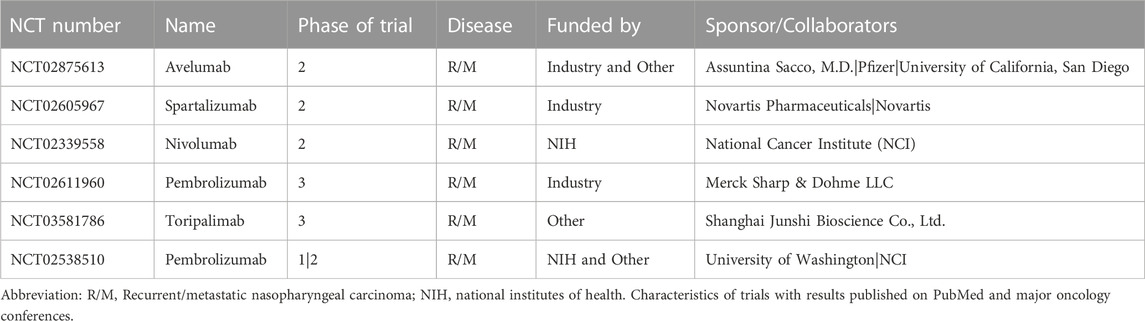
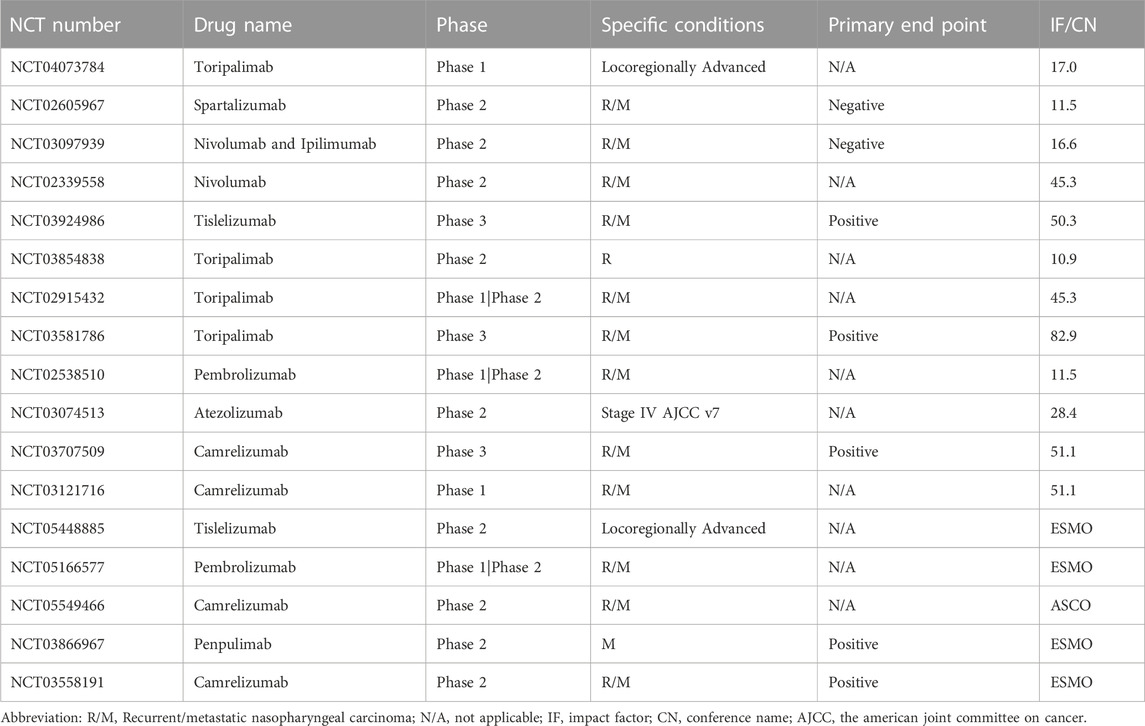
Table 6 provides the information on 6 clinical trials with available results, including the drug name, phase of the trial, disease condition studied, funding source, and sponsor/collaborators. Notably, 5 out of the 6 trials analyzed PD-1 inhibitors (Avelumab, Nivolumab, Pembrolizumab, and Toripalimab), while the remaining one analyzed a PD-L1 inhibitor (Atezolizumab). Of the 5 PD-1 inhibitor trials, 3 were phase 2 trials (Avelumab, Nivolumab, and Spartalizumab), while the remaining 2 were phase 3 trials (Pembrolizumab and Toripalimab). Among these trials, only the Nivolumab phase 2 trial was funded by the NIH, while the other 4 trials were funded by industry and other sources. It is also worth noting that all 5 trials targeted recurrent/metastatic NPC. For Pembrolizumab, both phase 1/2 and phase 3 trials were registered, with the phase 1/2 trial being funded by the NIH and other sources and the phase 3 trial being funded by Merck Sharp & Dohme LLC.
Table 7 provides the information on 12 clinical trials with available results on PubMed and 5 trials with results presented on major oncology conferences, including the drug name, phase of the trial, disease condition studied, whether the primary end point was positive or not, and the impact factor (IF) of the journal according to Journal Citation Reports Social Sciences Edition (Clarivate Analytics, 2023) in which the studies were published, or the name of the posted conference. It can be noticed that all trials focused on late-stage, especially R/M NPC. Two phase 3 trials, NCT03581786 and NCT03707509 both met the primary endpoint, and were published on journals with highest IF, reaching 82.9 and 51.1 respectively. In contrast, trials that did not met positive findings on the primary end point (NCT02605967 and NCT03097939) were published on journals with relatively lower IF of 11.5 and 16.6.
Characteristics of early discontinued trials
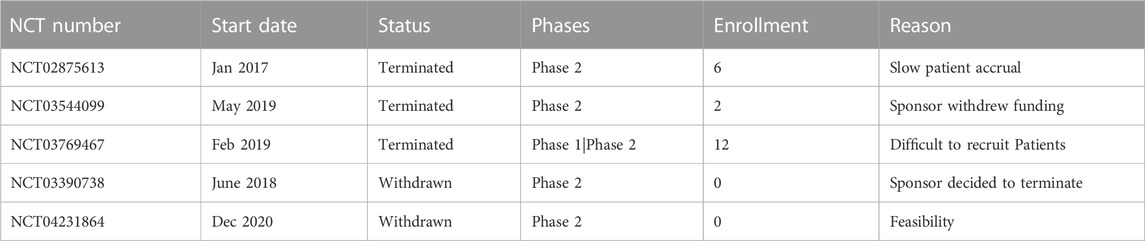
Table 8 provides an overview of the trials included in the analysis that experienced early discontinuation. The phase 2 trial registered under NCT02875613, which began in January 2017, was terminated due to slow patient accrual. It had an enrollment of 6 participants. Similarly, the phase 1/phase 2 trial registered under NCT03769467, which started in February 2019, was terminated with an enrollment of 12 participants for the difficulty in recruiting patients. The phase 2 trial registered under NCT03544099, initiated in May 2019, was terminated because the sponsor withdrew funding. Only 2 participants were enrolled in this trial. On the other hand, two phase 2 trials, NCT03390738 and NCT04231864, were withdrawn without enrolling any participants. The reasons stated for withdrawal were sponsor termination and feasibility concerns respectively.
Diverse treatment approaches across different lines of therapy
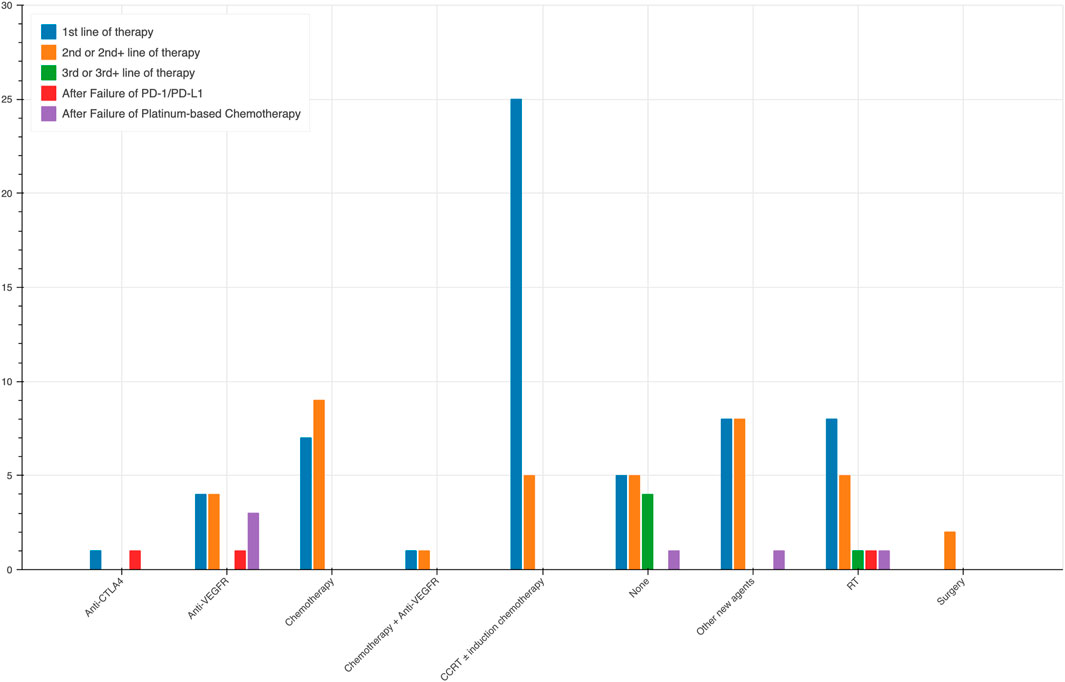
During our analysis, we observed notable trends in the selection of treatment modalities based on the lines of therapy. Apart from 15 trials using PD-1/PD-L1 monotherapy, the rest 97 trials combined it with other agents. The different combination across lines of therapy are shown in Figure 3. In trials that adopted anti-PD-1/PD-L1 as the first line of therapy, induction chemotherapy combined with radiotherapy was the predominant choice, employed in 25 trials, highlighting its significance as an initial strategy in NPC treatment. Furthermore, chemotherapy alone was utilized in 7 trials, while a combination of chemotherapy and anti-VEGFR therapy was employed in 1 trial. Radiation therapy (RT) was chosen in eight trials, and surgery in eight trials. Additionally, one trial each utilized anti-CTLA4 therapy, anti-VEGFR therapy, and other new agents along with anti-PD-1/PD-L1 as the first line of treatment. In the second or second + lines of therapy, CCRT without induction chemotherapy was still a commonly utilized combination, albeit less frequently, being employed in 5 trials. Chemotherapy alone was used in 9 trials, anti-VEGFR therapy alone was opted for 4 trials, while a combination of chemotherapy and anti-VEGFR therapy was employed in one trial. In this context, RT was chosen in five trials, surgery in two trials, and five trials did not involve any other specific treatment.
For the third or third + lines of therapy, the data is limited, with only one trial incorporating RT along with PD-1/PD-L1 therapy. In the post anti-PD-1/PD-L1 and post Platinum-based Chemotherapy settings, the use of anti-VEGFR therapy was more common, being employed in three trials and one trial, respectively. Additionally, single trials in each of these settings utilized chemotherapy and anti-CTLA4 therapy.
Discussion
This study provides an overview of the registered trials on ClinicalTrials.gov regarding anti-PD-1/PD-L1 therapy for NPC. The registered trials increased steadily from 2015 to 2022, with most being conducted in Asia. Only a small number of trials were funded by the NIH, while most of the trials received funding from sources other than the NIH.
Many trials included in this study were either recruiting or not yet recruiting and enrolled between 101 and 500 participants. Phase 2 trials were the most used but also the most withdrawn or terminated study design, and single-group assignment was the most frequently employed intervention. It is demonstrated that current trials on anti-PD-1/PD-L1 therapy for NPC were predominantly incomplete, early-phase studies with a generally high proportion of single-group assignment studies. These findings indicated that the current data of anti-PD-1/PD-L1 immunotherapy for NPC was largely in early-stage discovery. It is worth noting that all 18 phase 3 trials included in the analysis adopted randomization and parallel assignment, with an average enrollment of 325 participants. However, only 2 trials used quadruple masking (participant, care provider, investigator, and outcomes assessor), 1 used double masking (participant and investigator), and 2 used single masking (outcomes assessor), while the remaining 13 trials were open-labelled. Randomization is a crucial aspect of high-quality clinical trials as it ensures that the treatment is received by a certain proportion of patients, and that the treatment groups being compared are comparable in both measured and unmeasured patient characteristics (Bespalov et al., 2020). By minimizing bias and increasing the reliability of evidence, randomization has become a hallmark of high-quality clinical trials (Kunz et al., 2007). Therefore, it is important for researchers to consider adopting randomization and blinding whenever feasible (Broglio, 2018).
Most trials used response rate (mainly ORR) and survival data other than OS (mainly PFS) the primary outcomes. ORR is a widely accepted endpoint in single-arm trials as it allows for measurable tumor response without requiring a control group for direct comparison. PFS, on the other hand, is defined as the time from the start of therapy to the first documented tumor progression or death due to any cause (Kemp and Prasad, 2017). The measurement of PFS is complex, and bias can be introduced in PFS assessment depending on the adequacy of the comparator used. In comparison, OS, defined as the time from treatment initiation to death, remains the gold-standard clinical endpoint for oncology cytotoxic clinical trials (Anagnostou et al., 2017). However, OS measurement can be resource-intensive and time-consuming. More recently, the majority of accelerated approvals have been based on ORR (Beaver et al., 2018; Batta et al., 2020); these approvals are conditional and require subsequent confirmation of benefit, such as PFS or OS, in larger and/or randomized studies.
Based on the analysis of multiple drugs, PD-1 inhibitors were found to have undergone the most extensive research, with Toripalimab being the most commonly utilized agent. Conversely, Avelumab was identified as the most frequently studied PD-L1 inhibitor. Whether anti-PD-1 and anti-PD-L1 deliver different clinical outcomes remains a topic of controversy, and systematic reviews and meta-analyses conducted yielded varying outcomes regarding the clinical performance of different immune check-point inhibitors through indirect comparison (Wu et al., 2018; You et al., 2018). The current study indicated that anti-PD-1 was more favorable by researchers and might demonstrate superior clinical outcomes in NPC patients compared to anti-PD-L1. Nonetheless, head-to-head studies were necessary for a direct comparison between alternative interventions.”
It is notable that among the top studied PD-1 inhibitors for NPC, the majority were developed by Chinese pharmaceutical companies. However, the trials initiated by the United States industry and NIH demonstrated a higher rate of result postings. This suggested a potential need for improvement in the conduct of clinical trials by Chinese universities and companies, particularly in terms of timely reporting of trials’ results. Future efforts should be aimed at ensuring that all trials, regardless of funding source or sponsor, were conducted in accordance with best practices for clinical research and that their results should be reported in a transparent and timely manner. Recurrent/metastatic nasopharyngeal cancer and locoregionally advanced nasopharyngeal carcinoma were the most studied conditions in the study. The finding is noteworthy as it aligns with the unmet clinical needs in the management of nasopharyngeal carcinoma (Le et al., 2019; Wong et al., 2021).
The choice of publication journal can significantly impact the visibility and influence of clinical trial findings. The findings from trials published in high-IF journals have a greater likelihood of influencing clinical practice and shaping future research directions (Evangelou et al., 2012). Our analysis suggests a potential correlation between the positive trial outcomes and the selection of high-IF journals for publication. On the other hand, trials with negative or inconclusive outcomes may face challenges in publication acceptance, particularly in high-IF journals. This phenomenon could stem from a publication bias favoring positive results or a higher threshold for acceptance in prestigious journals. It is important to acknowledge that the IF is just one factor in evaluating the quality and significance of research publications. Other considerations, such as study design, methodology, and scientific rigor, also contribute to the overall credibility and influence of a study.
The choice of treatment modalities in NPC management is influenced by the line of therapy, with induction chemotherapy combined with radiotherapy being notable options in the early lines of treatment, while anti-VEGFR therapy and other therapies become more relevant in later lines and post-treatment scenarios.
The study had several limitations that need to be acknowledged. Firstly, the scope of the analysis was restricted to clinical trials registered in ClinicalTrials.gov, which may not include all clinical trials, as some investigators or sponsors may register their studies in other databases (Yang et al., 2021). Secondly, the study only provided an overview of the registered trials’ characteristics and did not assess the actual strengths and weaknesses of the clinical studies. Thirdly, the search strategy might have missed trials that studied solid tumors if NPC was not mentioned in the inclusion criteria, such as the well-known KEYNOTE-028 and NCI-9742 studies (Ma et al., 2018; Ott et al., 2019). However, given the rarity of these types of large-scale trials, it is unlikely that the absence of these studies would significantly impact the conclusions drawn from the research. Additionally, the validity of the data in ClinicalTrials.gov was contingent on the quality of information provided by the sponsors, and missing data in certain fields might introduce bias into the results. These limitations should be taken into consideration when interpreting the findings.
In conclusion, the current study provides an overview of clinical trials investigating anti-PD-1/PD-L1 therapies for NPC. The analysis highlights the need for improvement in completeness, outcome selection, randomization and masking of trials.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
LL and YZ supervised the study. ZD, NL, and CT contributed to searches, manuscript writing and preparing the manuscript draft. JW, LL, and YZ revised and approved the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Anagnostou, V., Yarchoan, M., Hansen, A. R., Wang, H., Verde, F., Sharon, E., et al. (2017). Immuno-oncology trial endpoints: capturing clinically meaningful activity. Clin. Cancer Res. 23 (17), 4959–4969. doi:10.1158/1078-0432.CCR-16-3065
Batta, A., Kalra, B. S., and Khirasaria, R. (2020). Trends in FDA drug approvals over last 2 decades: an observational study. J. Fam. Med. Prim. Care 9 (1), 105–114. doi:10.4103/jfmpc.jfmpc_578_19
Beaver, J. A., Howie, L. J., Pelosof, L., Kim, T., Liu, J., Goldberg, K. B., et al. (2018). A 25-year experience of US food and drug administration accelerated approval of malignant hematology and oncology drugs and Biologics: a review. JAMA Oncol. 4 (6), 849–856. doi:10.1001/jamaoncol.2017.5618
Bespalov, A., Wicke, K., and Castagné, V. (2020). Blinding and randomization. Handb. Exp. Pharmacol. 257, 81–100. doi:10.1007/164_2019_279
Broglio, K. (2018). Randomization in clinical trials: permuted blocks and stratification. Jama 319 (21), 2223–2224. doi:10.1001/jama.2018.6360
Chen, L., Su, Y., Quan, L., Zhang, Y., and Du, L. (2018). Clinical trials focusing on drug control and prevention of ventilator-associated pneumonia: a comprehensive analysis of trials registered on ClinicalTrials.gov. Front. Pharmacol. 9, 1574. doi:10.3389/fphar.2018.01574
Chen, Y. P., Chan, A. T. C., Le, Q. T., Blanchard, P., Sun, Y., and Ma, J. (2019). Nasopharyngeal carcinoma. Lancet 394 (10192), 64–80. doi:10.1016/S0140-6736(19)30956-0
Chin, Y. M., Tan, L. P., Abdul Aziz, N., Mushiroda, T., Kubo, M., Mohd Kornain, N. K., et al. (2016). Integrated pathway analysis of nasopharyngeal carcinoma implicates the axonemal dynein complex in the Malaysian cohort. Int. J. Cancer 139 (8), 1731–1739. doi:10.1002/ijc.30207
De Angelis, C., Drazen, J. M., Frizelle, F. A., Haug, C., Hoey, J., Horton, R., et al. (2004). Clinical trial registration: a statement from the international committee of medical journal editors. Lancet 364 (9438), 911–912. doi:10.1016/S0140-6736(04)17034-7
Evangelou, E., Siontis, K. C., Pfeiffer, T., and Ioannidis, J. P. (2012). Perceived information gain from randomized trials correlates with publication in high-impact factor journals. J. Clin. Epidemiol. 65 (12), 1274–1281. doi:10.1016/j.jclinepi.2012.06.009
Kemp, R., and Prasad, V. (2017). Surrogate endpoints in oncology: when are they acceptable for regulatory and clinical decisions, and are they currently overused? BMC Med. 15 (1), 134. doi:10.1186/s12916-017-0902-9
Killock, D. (2023). Recurrent nasopharyngeal carcinoma: hyperfractionation of IMRT improves outcomes. Nat. Rev. Clin. Oncol. 20, 283. doi:10.1038/s41571-023-00753-2
Kunz, R., Vist, G., and Oxman, A. D. (2007). Randomisation to protect against selection bias in healthcare trials. Cochrane Database Syst. Rev. 2, Mr000012. doi:10.1002/14651858.MR000012.pub2
Larbcharoensub, N., Mahaprom, K., Jiarpinitnun, C., Trachu, N., Tubthong, N., Pattaranutaporn, P., et al. (2018). Characterization of PD-L1 and PD-1 expression and CD8+ tumor-infiltrating lymphocyte in epstein-barr virus-associated nasopharyngeal carcinoma. Am. J. Clin. Oncol. 41 (12), 1204–1210. doi:10.1097/COC.0000000000000449
Le, Q. T., Colevas, A. D., O'Sullivan, B., Lee, A. W. M., Lee, N., Ma, B., et al. (2019). Current treatment landscape of nasopharyngeal carcinoma and potential trials evaluating the value of immunotherapy. J. Natl. Cancer Inst. 111 (7), 655–663. doi:10.1093/jnci/djz044
Lee, A. W. M., Lee, V. H. F., Ng, W. T., Strojan, P., Saba, N. F., Rinaldo, A., et al. (2021). A systematic review and recommendations on the use of plasma EBV DNA for nasopharyngeal carcinoma. Eur. J. Cancer 153, 109–122. doi:10.1016/j.ejca.2021.05.022
Lee, A. W. M., Ng, W. T., Chan, J. Y. W., Corry, J., Mäkitie, A., Mendenhall, W. M., et al. (2019). Management of locally recurrent nasopharyngeal carcinoma. Cancer Treat. Rev. 79, 101890. doi:10.1016/j.ctrv.2019.101890
Liu, X., Tang, L. L., Du, X. J., Li, W. F., Chen, L., Zhou, G. Q., et al. (2017). Changes in disease failure risk of nasopharyngeal carcinoma over time: analysis of 749 patients with long-term follow-up. J. Cancer 8 (3), 455–459. doi:10.7150/jca.17104
Ma, B. B. Y., Lim, W. T., Goh, B. C., Hui, E. P., Lo, K. W., Pettinger, A., et al. (2018). Antitumor activity of nivolumab in recurrent and metastatic nasopharyngeal carcinoma: an international, multicenter study of the mayo clinic phase 2 consortium (NCI-9742). J. Clin. Oncol. 36 (14), 1412–1418. doi:10.1200/JCO.2017.77.0388
Ott, P. A., Bang, Y. J., Piha-Paul, S. A., Razak, A. R. A., Bennouna, J., Soria, J. C., et al. (2019). T-Cell-Inflamed gene-expression profile, programmed death ligand 1 expression, and tumor mutational burden predict efficacy in patients treated with pembrolizumab across 20 cancers: KEYNOTE-028. J. Clin. Oncol. 37 (4), 318–327. doi:10.1200/JCO.2018.78.2276
Outh-Gauer, S., Alt, M., Le Tourneau, C., Augustin, J., Broudin, C., Gasne, C., et al. (2018). Immunotherapy in head and neck cancers: a new challenge for immunologists, pathologists and clinicians. Cancer Treat. Rev. 65, 54–64. doi:10.1016/j.ctrv.2018.02.008
Pardoll, D. M. (2012). The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12 (4), 252–264. doi:10.1038/nrc3239
Renkvist, N., Castelli, C., Robbins, P. F., and Parmiani, G. (2001). A listing of human tumor antigens recognized by T cells. Cancer Immunol. Immunother. 50 (1), 3–15. doi:10.1007/s002620000169
Riddell, M., Lam, K., Funk, A., Lodha, N., Lorenzetti, D. L., and Freedman, S. B. (2021). Comparison of publication of pediatric probiotic vs antibiotic trials registered on ClinicalTrials.gov. JAMA Netw. Open 4 (10), e2125236. doi:10.1001/jamanetworkopen.2021.25236
Stensland, K. D., Damschroder, L. J., Sales, A. E., Schott, A. F., and Skolarus, T. A. (2022). Envisioning clinical trials as complex interventions. Cancer 128 (17), 3145–3151. doi:10.1002/cncr.34357
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71 (3), 209–249. doi:10.3322/caac.21660
Vaddepally, R. K., Kharel, P., Pandey, R., Garje, R., and Chandra, A. B. (2020). Review of indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers (Basel) 12 (3), 738. doi:10.3390/cancers12030738
Vanneste, B. G. L., Van Limbergen, E. J., Dubois, L., Samarska, I. V., Wieten, L., Aarts, M. J. B., et al. (2020). Immunotherapy as sensitizer for local radiotherapy. Oncoimmunology 9 (1), 1832760. doi:10.1080/2162402X.2020.1832760
Wang, Y., Liu, Z. G., Yuan, H., Deng, W., Li, J., Huang, Y., et al. (2019). The reciprocity between radiotherapy and cancer immunotherapy. Clin. Cancer Res. 25 (6), 1709–1717. doi:10.1158/1078-0432.CCR-18-2581
Wang, Y., Zhou, Q., Xu, M., Kang, J., and Chen, Y. (2020). Characteristics of clinical trials relating to COVID-19 registered at ClinicalTrials.gov. J. Clin. Pharm. Ther. 45 (6), 1357–1362. doi:10.1111/jcpt.13222
Wong, K. C. W., Hui, E. P., Lo, K. W., Lam, W. K. J., Johnson, D., Li, L., et al. (2021). Nasopharyngeal carcinoma: an evolving paradigm. Nat. Rev. Clin. Oncol. 18 (11), 679–695. doi:10.1038/s41571-021-00524-x
Wu, Y., Lin, L., Shen, Y., and Wu, H. (2018). Comparison between PD-1/PD-L1 inhibitors (nivolumab, pembrolizumab, and atezolizumab) in pretreated NSCLC patients: evidence from a Bayesian network model. Int. J. Cancer 143 (11), 3038–3040. doi:10.1002/ijc.31733
Xu, J. Y., Wei, X. L., Wang, Y. Q., and Wang, F. H. (2022). Current status and advances of immunotherapy in nasopharyngeal carcinoma. Ther. Adv. Med. Oncol. 14, 17588359221096214. doi:10.1177/17588359221096214
Yang, R., Zhang, Y., Liao, X., Guo, R., Yao, Y., Huang, C., et al. (2021). Cross-sectional survey of clinical trials of stem cell therapy for heart disease registered at ClinicalTrials.gov. Front. Cardiovasc Med. 8, 630231. doi:10.3389/fcvm.2021.630231
You, W., Liu, M., Miao, J. D., Liao, Y. Q., Song, Y. B., Cai, D. K., et al. (2018). A network meta-analysis comparing the efficacy and safety of anti-PD-1 with anti-PD-L1 in non-small cell lung cancer. J. Cancer 9 (7), 1200–1206. doi:10.7150/jca.22361
Keywords: nasopharyngeal carcinoma, anti-PD-1/PD-L1 antibodies, clinical trial, treatment, comprehensive analysis
Citation: Dai Z, Li N, Wang J, Tan C, Zhang Y and Liu L (2023) Anti-PD-1/PD-L1 for nasopharyngeal carcinoma: a comprehensive analysis of registered trials on ClinicalTrials.gov. Front. Pharmacol. 14:1212813. doi: 10.3389/fphar.2023.1212813
Received: 27 April 2023; Accepted: 18 October 2023;
Published: 13 November 2023.
Edited by:
Ye Shen, University of Georgia, United StatesReviewed by:
Guang Chen, The University of Hong Kong, Hong Kong SAR, ChinaJong Chul Park, Massachusetts General Hospital Cancer Center, United States
Copyright © 2023 Dai, Li, Wang, Tan, Zhang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Liu, bGl1bGVpaHhAZ21haWwuY29t
†These authors have contributed equally to this work and share first authorship
 Zelei Dai
Zelei Dai Nian Li
Nian Li Jun Wang1†
Jun Wang1† Yonggang Zhang
Yonggang Zhang Lei Liu
Lei Liu