
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 13 July 2023
Sec. Pharmacology of Anti-Cancer Drugs
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1211506
 Ioana Creanga-Murariu1,2
Ioana Creanga-Murariu1,2 Leontina Elena Filipiuc1
Leontina Elena Filipiuc1 Magda Cuciureanu3
Magda Cuciureanu3 Bogdan-Ionel Tamba1,3*
Bogdan-Ionel Tamba1,3* Teodora Alexa-Stratulat2
Teodora Alexa-Stratulat2Cannabis enjoyed a “golden age” as a medicinal product in the late 19th, early 20th century, but the increased risk of overdose and abuse led to its criminalization. However, the 21st century have witnessed a resurgence of interest and a large body of literature regarding the benefits of cannabinoids have emerged. As legalization and decriminalization have spread around the world, cancer patients are increasingly interested in the potential utility of cannabinoids. Although eager to discuss cannabis use with their oncologist, patients often find them to be reluctant, mainly because clinicians are still not convinced by the existing evidence-based data to guide their treatment plans. Physicians should prescribe cannabis only if a careful explanation can be provided and follow up response evaluation ensured, making it mandatory for them to be up to date with the positive and also negative aspects of the cannabis in the case of cancer patients. Consequently, this article aims to bring some clarifications to clinicians regarding the sometimes-confusing various nomenclature under which this plant is mentioned, current legislation and the existing evidence (both preclinical and clinical) for the utility of cannabinoids in cancer patients, for either palliation of the associated symptoms or even the potential antitumor effects that cannabinoids may have.
A natural remedy known for millennia, the cannabis plant has tranquilizing, hypnotic and aphrodisiac effects, used since ancient times to treat various conditions. The root, was recommended for treating inflammation, gout, arthritis, fever, skin burns, infections, postpartum hemorrhage, gastrointestinal diseases and tumors (Ryz et al., 2017). Cannabis inflorescence and leaves have also been used to treat epilepsy, glaucoma, insomnia, and pain (Brunetti et al., 2020; Jin et al., 2020). In addition to its medicinal role, cannabis has also been used as food, due to its high content of fiber and oils that are very nutritious (Zuardi, 2006). At the beginning of the 20th century, cannabinoids were used in various conditions worldwide. However, their side effects and increased use as recreational drugs led to the criminalization of cannabis in the US in 1937, thus greatly impacting further research that could offer a modern scientific understanding of its medicinal potential (Victor et al., 2022). The first decades of the 21st century have witnessed a resurging interest (Walsh et al., 2017) and more literature regarding the benefits of cannabinoids in various diseases has become available; however, because “cannabis” is in fact an umbrella term for many drugs, the various nomenclature under which this plant is mentioned can sometimes be confusing and lead to hindrances in data reproducibility and direct cross-study comparisons.
The popular term “cannabis” refers in general to all products (herbal or synthetic) derived from the plants belonging to the Cannabis genus, with cannabinoids being several active classes of chemical compounds found in the plant. More than 110 natural compounds, called phytocannabinoids, have been identified and over 100 lipophilic molecules have been isolated within their structure. Δ9-tetrahydrocannabinol (Δ9-THC) and cannabidiol (CBD) are perhaps the best-known representatives. CBD was first extracted in 1940 (Adams et al., 1940) and its full chemical structure was unraveled in 1963 by Mechoulam and others (Mechoulam and ShvoHashish, 1963), and Δ9-THC followed shortly after, being first isolated and structurally elucidated by the same team in 1964 (Gaoni and Mechoulam, 1964).
The term “herbal cannabis” commonly refers to the harvested dried female flowering tops of Cannabis Sativa L, which contain the highest concentrations of natural cannabinoids, mainly Δ9-THC, CBD, cannabigerol, cannabichromene, cannabidivarin and tetrahydrocannabivarin (Lewis et al., 2017; Odieka et al., 2022). Each strain of cannabis plant can have variations in the concentration of the substances within and also contains over 500 other chemical compounds such as cannabinoid phenols, non-cannabinoid phenols, alcohols, aldehydes, n-alkanes, alkaloids, flavonoids, terpenoids, wax esters and steroids, which in turn may modulate the effect of cannabinoids (the “entourage effect”) (Lewis et al., 2017; Odieka et al., 2022). Over time, growers have developed and selected specific cannabis strains due either to their high Δ9-THC content (greater psychoactive effect, source for marijuana and hashish), their high CBD content (most often used for medicinal marijuana) or their high fiber and oil content (hemp) (Hilderbrand, 2018), which lead to the great variability of legal and illegal cannabis-based produce available today.
Different proportions of major active ingredients along with smaller proportions of minor components, that enhance the effects of major phytocannabinoids, of cannabis offer different effects, so they can be used in different conditions. For example, in anorexia, nausea and vomiting it is recommended to use a product with a content of THC higher than CBD (THC > CBD); for insomnia and pain, approximately equal proportions of THC and CBD (THC ≅ CBD); and for anxiety and depression a content of THC lower than CBD (THC < CBD) (Brunetti et al., 2020).
The identification of the first endogenous cannabinoids and cannabinoid ligands (cannabinoid receptor 1 and 2—CB1R and CB2R) (Shahbazi et al., 2020) led to the development of so-called “cannabinoid probes” initially used for better characterizing the endocannabinoid system. Thus, synthetic cannabinoids (SCs) were created. When compared to phytocannabinoids, SCs have significantly more affinity–up to 4 times higher for CB1R and 10 times higher for CB2R and, as a consequence, may have greater psychoactive effects (Bukke et al., 2021). As such, SCs quickly entered the illegal drug market and were sold as recreational drugs. Their popularity has likely been enhanced by the lack of detection in typical urine drug screens for THC (Schneir et al., 2011). Most SC’s are sprayed onto herbal substances so they can be smoked or sold as liquids to be vaporized and inhaled (Tamba et al., 2020). This has led to a significant increase in cannabis-related hospital admissions, especially since acute, severe or unpredictable side effects have a higher incidence in SC users than in natural cannabinoids users (Forrester et al., 2012).
Another important challenge for the progress of cannabis research is the immense variability of both natural and synthetic cannabinoids. Each cannabis plant has a vast assortment of active compounds that vary in composition, concentration and ratio with environmental factors, genetic background and even morpho-spatial position of the plant (Danziger and Bernstein, 2021). Unfortunately, there are only few rigorous studies assessing the effect of plant architecture modulation treatments and other methods for obtaining standardized cannabis cultures (Kocjan Ačko et al., 2019). As such, preclinical studies usually use SCs or purified cannabis oil and clinical studies are usually forced to use the few cannabis-based produce that are already approved and standardized. Most countries have efficient regulatory bodies for cannabis based medicinal products that have undergone a regular marketing authorization process, which ensure the compliance with the Good Manufacturing Practices (GMP), however some products go through more simplified authorization processes, or even exempted from specific regulatory authorizations, thus no guarantee that these aspects are met (Seddon and Floodgate, 2020; de Souza et al., 2022). Illicit SCs have no production standards whatsoever, so two drugs sold under the same street name can in fact have completely different doses, compositions or can contain poisonous or carcinogenic compounds.
Despite having enjoyed a “golden age” as a medicinal product in the late 19th, early 20th century, cannabis began being replaced by other drugs and its use progressively restricted mainly due to the increased risk of overdose and abuse (Crocq, 2020). In 1961, the United Nations Single Convention on Narcotic Drugs classified cannabis as belonging to 2 categories: schedule I, substances that are highly addictive and liable to abuse, and schedule IV, substances that are highly addictive, liable to abuse and lack therapeutic value. This meant that cannabis use for all purposes, including medical use, was to be prohibited (Bayer and Ghodse, 1999). However, in time, a significant body of data that outlined the therapeutic effects of cannabis has been published. In consequence, in December 2020, the United Nations’ Commission for Narcotic Drugs recommendations were revised, concluding that cannabis should be listed only in schedule I of the system, because of its demonstrated therapeutic potential (Mahabir et al., 2020). As a consequence, different countries have adopted more permissive laws regarding cannabis use for either medicinal or recreational purpose. However, the legalization and decriminalization of cannabis are two terms that have different meaning and should not be confused. Legalization allows the production and sale of either recreational or medical marijuana and marijuana-infused products. Decriminalization of cannabis means that while its use is still illegal, it is rather considered a minor infraction with less severe punitive consequences.
The first country to legalize Cannabis use for medical and recreational use was Uruguay in 2013 (Seddon and Floodgate, 2020). Several European states also revised their legislation for medical cannabis use in recent years (Figure 1):
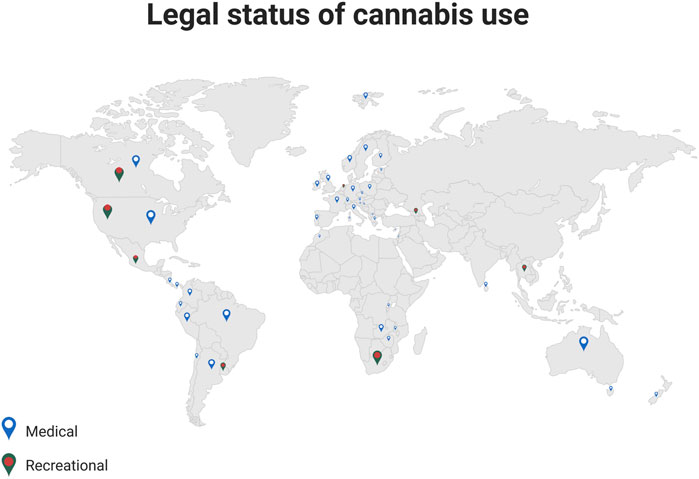
FIGURE 1. Legal status of cannabis use. The illustration provides information on a worldwide scale, regarding the current regulations for prescribing medical cannabis, and for the recreational use (Adapted from Bio Render).
In the United States, 18 states have legalized, so far, the use of Cannabis for medicinal purposes (Mahabir et al., 2020). Despite the fact that the recreational use is still illegal in most of the states, some 48 million people, or about 18% of Americans, have used Cannabis at least once (Compton et al., 2016), with cannabis-related illicit sales reaching $17.5 billion in 2020 (Yakowicz, 2023).
Although recent years have seen a trend towards decriminalizing marijuana use, the Foods and Drugs Administration (FDA) and The European Medicines Agency (EMA) have not yet approved the use of the cannabis plant for any medical purpose. However, both the FDA and EMA have approved the use of drugs that contain individual cannabinoids (Whiting et al., 2015). Some examples include Epidiolex, a purified form of CBD used for the treatment of seizures associated with Lennox-Gastaut syndrome or Dravet syndrome (Sekar and Pack, 2019) (FDA and EMA-approved) or Dronabinol (FDA-approved)—a synthetic THC used as an appetite stimulant in AIDS-related cachexia (Badowski and Yanful, 2018) and for alleviating chemotherapy-induced nausea and vomit in cancer patients (May and Glode, 2016). Another area of interest is the treatment of post-traumatic stress disorder (PTSD) in military personnel with THC or nabilone (Sholler et al., 2020). Nabilone (a synthetic analogue of THC, Cesamet® or Canemes®), ten times more potent than natural THC, is FDA-approved for the treatment of nausea and vomiting for patients receiving chemotherapy who have failed to respond to conventional antiemetics (Vinette et al., 2022). Recently, the effectiveness of nabilone has been assessed in the treatment of neuropathic and chronic pain, and in spasticity related to multiple sclerosis (Manera and Bertini, 2021). Nabiximols (Sativex®), available as an oral spray, that contains both THC and CBD is approved in the United Kingdom, Germany, and Switzerland for multiple sclerosis (MS)-related spasticity and in Canada for pain associated with MS and cancer (Keating, 2017).
Marketing authorization of cannabinoids in Europe can be obtained either with the centralized procedure, via the EMA, or through the non-centralized route where medication may be authorized in individual EU countries through the national competent authorities. Figure 1 shows the countries in which medical cannabis consumption is permitted. Nabiximols is available in most EU states, and even if Dronabinol and Nabilone are less widespread, they are still available in around one third of EU.
Along with the renewed interest in cannabinoids as drugs for chronic neurological disorders, an increased focus on cannabis and its potential applications in oncology has also resurged. Cannabinoids are frequently used both on- and off-label by cancer patients and several multicenter studies have suggested a 18%–40% prevalence of use in this populational group (Abrams, 2022).
However, oncologists seem to be reluctant to follow this trend. A 2018-study assessing 400 oncologists’ perceptions on the issue reported that 70% of them think they have concerning gaps in data regarding cannabinoid use in cancer patients, even though most of them (80%) reported conducting discussions with patients. This reluctance could be because physicians consider that available data about cannabinoids as alternative therapy is contradictory and difficult to evaluate given the sparse randomized controlled trial data (Braun et al., 2018). The same attitude was seen in a recent systematic review, where 21 different studies conducted in United States, Canada, Europe, Australia, and Israel, were included. The lack of knowledge of its clinical effects varied between clinicians, however the physicians experienced in prescribing medical cannabis were less worried about the adverse effects (Rønne et al., 2021). Additionally, the lack of standardization of most cannabinoids used outside standard recommendations are a major drawback for large database analysis, thus hindering data reproducibility and the possibility of identifying clinical benefits.
The motivation of the present article is that a physician should prescribe cannabis only if a careful explanation can be provided, and a follow up response evaluation, ensured. Oncologists should be up to date with the positive and negative aspects of the administration of cannabis in cancer patients as both prevalence and demand has increased in the past years. A better understanding of cannabinoids’ pharmacokinetics, side effects and efficacy profiles as well as their formulations is thus required.
The unique qualities of each cannabis variety or chemovar are the result of distinct concentrations of numerous classes of bioactive molecules, most notably, cannabinoids, terpenoids and flavonoids. As such, cannabinoids have various effects that depend on the dose and the compound used. The plethora of effects can also be explained by the fact that cannabinoids bind to both CB1R and CB2R, and non-cannabinoid receptors, such as the adrenergic receptors, vanilloid receptor 1 (TVRP1), transient receptor ankyrin 1 potential (TRPA1), peroxisome proliferator-activated receptor-gamma or glitazone receptor (PPAR-γ), G55 protein-coupled receptor (GPR55), and nuclear receptor (NRs) (Huang et al., 2020). This broader view of ligands and enzymes involved in the endocannabinoid system led to the concept of the endocannabinoidome, which encompasses hundreds of lipid mediators and tens of enzymes and molecular targets (Huestis et al., 2019; Pryimak et al., 2021; Hinz and Ramer, 2022). As such, the potential effect of cannabinoids on the most prominent cancer-associated symptoms, such as pain, nausea, vomiting, cachexia, anorexia, depression or anxiety has been investigated in several clinical and preclinical studies.
Pain is an unpleasant sensory and emotional experience associated with actual or potential tissue damage, as defined by the International Association for the Study of Pain (IASP) (Raja et al., 2020). In cancer patients, pain is reported as one of the most distressing aspects of their condition and has been linked to numerous physical and mental ailments that contribute to high healthcare costs and loss of productivity (Stoorvogel et al., 2022). Available data indicate that chronic pain generates considerable pressure, being one of the most common reasons adults seek medical care (Rodriguez et al., 2019), with an estimated annual cost of more than $635 billion (Casey and Vaughan, 2018). Of note, current findings indicate that the burden of chronic pain in cancer patients is particularly alarming, with prevalence rates of 39.3% after curative treatment; 55.0% during anticancer treatment; and 66.4% in advanced, metastatic, or terminal disease (van den Beuken-van Everdingen et al., 2016). Moreover, more than one-third of cancer patients do not receive analgesic treatment proportional to or adequate for the intensity of their pain (Gress et al., 2020).
Neuropathic pain accounts for almost 40% of cancer pain cases (Oosterling et al., 2016). It is attributable to the cancer per se and/or because of treatment-related events, including chemotherapy-induced peripheral neuropathy (CIPN), post radiation plexopathies, and surgery for tumor resection (Haroun et al., 2022). Also, current treatment options for neuropathic pain are often ineffective, usually requiring association of several classes of drugs (Kachrani et al., 2020).
Opioids have been the mainstay of severe cancer pain treatment in the past decades. However, it is increasingly clear that these drugs should be carefully prescribed due to side effects and tendency to create dependence (Wang et al., 2021). Furthermore, the number of deaths secondary to opioid overdose is on the rise, with more than 65% of drug-overdose deaths involving at least one opioid, most often morphine or fentanyl (Saloner et al., 2018). Last, but not least, some types of chronic pain are refractory to opioids, requiring invasive techniques that are not always accepted by the patient or anesthetics (Fallon et al., 2018), thus revealing the need for new or improved analgesic drugs.
Recent data suggests that patients are increasingly using cannabis as a substitute for prescription opioids, with various reported outcomes (Casey and Vaughan, 2018). Other studies have focused on the potential synergistic effects of opioids and cannabinoids, the so-called “opioid-sparing effect” (Nielsen et al., 2022), which could address the opioid epidemic by significantly decreasing the analgesic doses required for refractory cancer-related pain.
a) Cannabinoids mechanisms on pain
The effect of exogenous cannabinoids on different types of acute and chronic pain depends on the drug’s interaction with the endocannabinoid system, whose components are expressed almost ubiquitously throughout the nociceptive pathways (Finn et al., 2021). The activation of CB1R from primary afferent neurons, dorsal horn of the spinal cord and brain regions involved in pain processing is associated with a decrease in neuronal excitability and a dampening of neurotransmission (Brown and Farquhar-Smith, 2018). The activation of CB2R, mostly expressed on immune cells, has a plethora of suppressive effects including reduced cytokine release and decreased antigen presentation (Eisenstein and Meissler, 2015), which is extremely relevant for several physiological processes that can modulate pain perception, such as mood, stress response, immune and inflammatory response and neurotransmission (Mangal et al., 2021). Additionally, CB2R have been occasionally identified on neurons, especially after injury, thus making CB2 a potential target in neuropathic pain (Brown and Farquhar-Smith, 2018). Both endogenous and exogenous cannabinoids bind to one or both CB receptors with different affinity, which explains their different effects. For example, both 2-Arachidonoylglycerol (2-AG) and N-arachidonoylethanolamine (AEA), the best-known endogenous cannabinoids, and Δ9-THC are CB1R and CB2R agonists, but with different affinities, whereas CBD is a CB1R and CB2R antagonist. Synthetic cannabinoids are usually more specific, binding to either CB1R or CB2R; examples include AM1710 (CB2 agonist), AM630 (CB2 antagonist) and AM251 (CB1 antagonist) (Rahn et al., 2014; Blake et al., 2017). Additionally, the endocannabinoid network also contains G protein-coupled receptors, members of the transient receptor potential cation channel subfamily, 5-hydroxytryptamine receptor 1A, 2A, and 3A, peroxisome proliferator-activated receptor gamma receptors and others, all of which modulate pain or pain-related processes (Balachandran et al., 2021; Lord et al., 2022). Last, but not least, studies have identified changes in endocannabinoid levels after exposure to neuropathic and nociceptive stimuli, suggesting a dysregulation of the system could be involved in several types of chronic pain (Masocha, 2018). Although the understanding of this system has greatly advanced in recent years, there are still a lot of controversies surrounding the exact mechanism by which cannabinoids modulate different types of pain, which, when coupled with the large variety and heterogenicity of the investigated compounds, leads to mostly contradicting results in observational clinical studies.
b) Cancer pain-preclinical and clinical data
There is a broad spectrum of pain models in which cannabinoids have been tested in the past decades, mostly with good results, especially in chronic neuropathic and inflammatory pain, which have a high prevalence in cancer. Interestingly, a study on a murine model of cancer pain assessed synthetic CB1R and CB2R agonists individually and in combination and concluded that binding of any CB receptor is associated with pain alleviation comparable to that of morphine and the co-administration of the two agonists has synergistical effects (Khasabova et al., 2011), which is why several different natural and synthetic CB1R and CB2R agonists have been investigated in this setting. Intraperitoneal administration of WIN 55212-2, was shown to alleviate pain in both carrageenan-evoked and tumor-evoked hyperalgesia in a time and dose-dependent manner, although the authors noted that the effect on inflammatory pain was more potent than on cancer pain (Kehl et al., 2003). In a model of cancer-induced allodynia, systemic administration of WIN 55212-2, ACEA (CB1R selective) or AM1241 (CB2 selective) significantly decreased pain (Saghafi et al., 2011). More recently, MJN110 (a monoacylglycerol lipase inhibitor) administration alleviated cancer-induced bone pain in an animal model, most likely by increasing the endogenous cannabinoid concentration (Thompson et al., 2020). In the clinical setting, two trials from the 80’s performed by Noyes et al. compared THC to codeine (Noyes et al., 1975a; Noyes et al., 1975b) with good outcomes despite notable side-effects at high doses. Several other subsequent clinical trials have had conflicting results, most likely due to small sample size, varying types of cannabinoids used and different endpoints analyzed. Several systematic reviews and meta-analysis have focused on gathering these data and assessing the effect of medical cannabis and cannabinoids on pain. Of note, Wan et al. included all randomized clinical trials with more than 20 patients that compared medical cannabis or cannabinoids to any non-cannabis control for chronic pain at more than 1 month follow-up. The authors identified 32 studies, of which four specifically focused on cancer-related pain. The analysis of data from over 5,000 patients identified a small improvement of chronic pain as assessed by the visual analog scale compared to placebo (Wang et al., 2021). A recently published clinical study enrolled cancer patients undergoing treatment with medical cannabis and assessed multiple cancer-related symptoms at different timepoints. The study included 324 patients, of which 126 were available for all follow-ups. The authors concluded that most outcome measures improved significantly with medical cannabis treatment, with an 18% decline in symptom burden at the 6-months follow-up. Of note, the average weekly pain intensity was reduced with a median of 20% for approximately 80% of the study participants (Aviram et al., 2022).
Several preclinical studies have specifically focused on CIPN, a significant long-term issue in cancer survivors. Both AM1710, a selective CB-2 agonist and Δ9-THC were shown to be effective in treating paclitaxel-induced neuropathy, most likely via a CB2-linked mechanism (Deng et al., 2015). Intraperitoneal WIN 55212-2 significantly reduced thermal hyperalgesia and tactile allodynia in both sciatic nerve constriction and paclitaxel-induced neuropathy models with little to no side effects (Pascual et al., 2005). Vaporized cannabis plant (10.3% THC/0.05% CBD) was also effective in a similar setting, decreasing cold allodynia in paclitaxel-treated rats (Alkislar et al., 2021). Targeting the enzymes that degrade endocannabinoids has also been shown to be a valid approach for treating CIPN in several models of vincristine or cisplatin-induced allodynia (Masocha, 2018). Cannabinoids were even shown to have efficacy in preventing CIPN as reported by a 2014-study on paclitaxel-induced neuropathy where both WIN 55212-2 and AM1710 suppressed mechanical allodynia and cold allodynia at varying doses while the animals were receiving chemotherapy (Rahn et al., 2014).
In the clinical setting, there are some data pointing towards the efficacy of cannabinoids in treating CIPN. A small crossover pilot trial published in 2014 assessed nabiximols in cancer patients with CIPN and found that five of the 16 individuals enrolled experienced an average decrease of 2.6 points on a 11-point numeric rating scale for CIPN assessment (Lynch and Campbell, 2011). This was confirmed by a retrospective clinical analysis of cancer patients undergoing oxaliplatin-based chemotherapy. This retrospective study included over 500 patients and divided them based on their exposure to medicinal cannabis (prescribed for other conditions such as nausea or anorexia) in: a. cannabis exposure prior to oxaliplatin chemotherapy, b. cannabis exposure following the initiation of oxaliplatin treatment, and c. no exposure (control). The authors noted a decrease in neuropathy incidence in cannabis users, especially in those that had been exposed to cannabis prior to starting chemotherapy, suggesting a protective effect for cannabis in this setting (Waissengrin et al., 2021).
Topical cannabinoids have also been reported in several papers as being effective in CIPN. When applied to the tail, a solution of DMSO containing WIN 55212-2 was shown to significantly prolong response time in the tail-flick test, most likely through a CB1R-dependent mechanism (Yesilyurt et al., 2003). A case series published in 2021 included all cancer patients that reported the use of topical cannabinoids for alleviating CIPN in a US-based hospital between 2019 and 2020. Of the 26 patients included, 22 reported experiencing a benefit and 4 did not report any improvement in CIPN. Patients used a variety of cannabinoid-containing creams, mostly CBD or a mixture of CBD and THC and overall reported feeling an improvement minutes after topical administration with no side effects (D’Andre et al., 2021).
c) Opioid sparring effect—preclinical and clinical data
There is a plethora of preclinical studies exploring the effect of combining different cannabinoids with well-known analgesics, most often opioids (Schurman et al., 2020). For acute pain, Δ9-THC was shown to have a synergic effect with morphine in a rat model of arthritis (Cox et al., 2007) and in normal rodents undergoing the tail-flick test (Smith et al., 1998). The association between transdermal fentanyl or buprenorphine and Δ9-THC also yielded good results in hairless guinea pigs as assessed by the pin prick test. Intraperitoneal Δ9-THC (50 mg/kg) enhanced the potency of fentanyl 6.7-fold. More interestingly, transdermal Δ9-THC had even better results, with a 5.8-fold increase in potency for fentanyl and 7.2-fold increase for buprenorphine at 4 hours, suggesting significant benefits for a mixt opioid/cannabinoid analgesic patch (Cichewicz et al., 2005).
As such, there is abundant preclinical evidence for the opioid-sparing effect of over 20 natural and synthetic cannabinoids (Nielsen et al., 2022). Of note, this effect was reported irrespective of the type of opioid used. A large meta-analysis of available preclinical studies concluded that cannabinoids, in particular Δ9-THC could potentially have an opioid-sparing effect (Nielsen et al., 2017), thus prompting the design of several clinical trials.
One of the first randomized clinical trials to explore the potential synergic effect of opioids and cannabinoids was published in 2012. Adult cancer patients with moderate or severe cancer pain (an intensity of 4-8 on the numeric rating scale - NRS) despite adequate opioid treatment were randomized to receive an oro-mucosal spray containing either nabiximol (Sativex®) (low-dose, medium-dose or high-dose) or placebo for a period of 5 weeks. Over 250 patients were included in the study and results indicated a statistically significant analgesic effect of cannabinoids which was greatest in the low-dose group that received 1-4 sprays of 100 µL Nabiximols (1.6 points pain reduction on the NRS) (Portenoy et al., 2012). A larger clinical trial (phase three) with a similar design compared a self-titrated regimen of Nabiximols oromucosal spray (Sativex®) to placebo in opioid-treated cancer patients. Although cannabinoids were not found to be more effective than placebo in the statistical analysis of the primary efficacy endpoint, there was a numerical benefit of Nabiximols treatment, with an approximate decrease of 0.8–0.9 points on the NRS in the worst pain score (Lichtman et al., 2018). Two other phase three trials reported similar findings, with no clear-cut benefit for Nabiximols as a co-analgesic, especially in cancer patients requiring large doses of opioids (Fallon et al., 2018). However, a THC:CBD extract was shown to be effective in reducing pain when compared to placebo in cancer patients with intractable pain receiving opioids in a parallel group randomized study. A reduction of over 1.3 on the NRS was noted, with 43% of patients in the cannabis group reporting a reduction of more than 30% of their baseline pain score (Johnson et al., 2010). Despite this, a systematic review published by Hauser in 2019 concluded that when analyzing available data together for cancer patients receiving opioids, the addition of cannabinoids does not improve pain and does not reduce opioid usage (Häuser et al., 2019). More recently, the results of the MedCan1-CBD phase IIb study were published and the authors concluded that cannabidiol oil does not decrease opioid use in cancer patients (Hardy et al., 2023). Due to numerical improvement in NRS and a good safety profile, medical cannabis and cannabinoids have been classified in consensus statements in the “weak recommendation” category for patients with chronic cancer and non-cancer pain (Busse et al., 2021).
d) Approved medication
Although a number of countries authorized the use of Nabiximols (Sativex®) for multiple sclerosis-associated neuropathic pain, to date, there are no cannabinoid-based medications approved by FDA or EMA for the treatment of cancer pain.
As such, American Society of Clinical Oncology (ASCO), National Comprehensive Cancer Network (NCCN) and European Society for Medical Oncology (ESMO) have similar opinions for the clinical use of cannabinoids for this purpose. Clinicians should not offer cannabinoids for cancer pain, because it has no benefits, however also no harm (Loprinzi et al., 2020). Furthermore, there is need for further double-blind, placebo-controlled clinical trials with large sample size in order to establish proper indications (Fallon et al., 2018).
Chemotherapy-induced nausea and vomiting (CINV) is a significant issue for cancer patients, with 45%–65% of patients experiencing nausea and 15%–25% vomiting (Grassi et al., 2015). Although there are several effective antiemetic treatments and also several classes of drugs useful for preventing CINV, it remains an area of unmet need due to high costs, symptom under-reporting and challenges associated with treating the refractory forms of CINV (Gupta et al., 2021). Nausea is amongst the most feared side-effects of chemotherapy and can have a significant impact on patients’ quality of life (Sommariva et al., 2016). Aside from affecting regular activities, nausea and vomiting can also have serious somatic effects, such as dehydration, electrolyte imbalance, anorexia, esophageal tears, or fractures. It can also affect a patient’s willingness to continue a potentially curative anticancer treatment and, in time, it can lead to the deterioration of the patient’s physical and mental status (Lorusso et al., 2017).
The pathophysiology of vomiting involves a multistep process controlled by the cortex, triggered by afferent impulses to the vomiting center. Chemotherapy drugs trigger vomiting through several pathways, most often activating receptors in the trigger zone or stimulating intestinal vagal afferents (Navari and Aapro, 2016). The main neurotransmitters involved in the emetic response bind to serotonin, dopamine, acetylcholine, histamine, opioid and cannabinoid receptors (Gupta et al., 2021). There is a high concentration of endocannabinoids and CB-1R in both the chemoreceptor trigger zone and the dorsal vagal complexes (Behl et al., 2022), which has led researchers to investigate the anti-emetic properties of cannabinoids in the late 1970s, before the discovery of the 5-HT3 antagonists so broadly used nowadays. To date, CINV represents one of the therapeutic areas where the efficacy of cannabinoids has been clearly proven by both preclinical and clinical studies.
a) Preclinical and clinical studies
Reproducing CINV in an animal model is somewhat difficult, since mice and rats do not vomit in response to toxin exposure, thus requiring either the use of other species or evaluating other signs of chemotherapy-induced emesis. McCarthy and Borison used a feline model (cat) to compare the efficacy of cannabinoids (N-metyllevonantradol, nabilone) to prochlorperazine, the standard anti-emetic treatment in 1981. The authors concluded that cannabinoids have a significant dose-dependent effect on Cisplatin-induced vomiting which is superior to the effect of prochlorperazine (Colby et al., 1981). A dose of 0.05–1 mg/kg intraperitoneal THC was shown to inhibit CINV in a dose-dependent manner in a ferret model (Van Sickle et al., 2003), results that were confirmed in a Cisplatin-treated least shrew animal model (Ray et al., 2009). A 2004-study on house musk shrews compared Δ9-THC to ondansetron in CINV and found a comparable anti-emetic effect between the drugs. Interestingly, the authors also assessed the effect of THC/ondansetron combination and concluded that low, ineffective doses of these drugs become highly effective when administered together, thus suggesting synergy (Kwiatkowska et al., 2004). However, few years later, a somewhat similar study compared the anti-emetic efficacy of Δ9-THC, dexamethasone and tropisetron both individually and in combination in a Cisplatin-treated least shrew animal model (Wang et al., 2008). The authors reported that both Δ9-THC (59%–97% attenuation) and tropisetron (79%–100% attenuation) were effective individually, but their combination was not associated with synergic effects, results that do not concur with those of the former study, most likely due to different doses used.
Several synthetic cannabinoids with little or no psychotropic effect have also been assessed in CINV preclinical models with very good results. HU-210 was shown to be effective in alleviating both Cisplatin-induced and Emetine-induced symptoms in a pigeon CINV model (Ferrari et al., 1999). HU-211 was compared to Δ1-THC in a similar animal model and the authors concluded that pretreatment with 2.5 mg/kg of HU-211 decreased CINV by over 90%, whereas Δ1-THC failed to inhibit emesis in 50% of all animals tested and also had psychotropic effects (Feigenbaum et al., 1989). More recently, WIN 55212-2 was shown to prevent opioid-induced vomiting in a ferret model (SimoneauHamza et al., 2001).
One of the first clinical studies that showed the efficacy of cannabinoids dates back to the 90’s, when Gralla et al. (1999) compared THC to the anti-emetic standard of the time, which was high-dose metoclopramide. Results showed that THC was superior for CINV control in patients receiving Cisplatin. At the time, the body of research favoring the use of cannabinoids in CINV was very strong, which led to the approval of Dronabinol (synthetic THC) in this setting. Since its approval, there have been at least ten randomized, placebo-controlled trials assessing Dronabinol in cancer and non-cancer patients, most often with good outcomes and a satisfactory safety profile (Bajtel et al., 2022). Similarly, Nabilone was evaluated in several clinical trials and was found to be effective in the management of CINV, although most studies were somewhat underpowered (Cherkasova et al., 2022).
Several new cannabinoid-derived drugs have been tested in the clinical setting in recent years. Of those, Grimison et al. performed a phase II crossover study (81 participants) comparing THC and CBD mixture (capsules) to placebo in patients experiencing CINV during moderate-to-high emetogenic intravenous chemotherapy despite guideline-consistent antiemetic prophylaxis. The authors concluded that there is an important patient preference (85%) for THC-CBD capsules compared to placebo and a statistically significant improvement in nausea and vomiting for the experimental arm of the study (Grimison et al., 2020).
b) Approved medication
Dronabinol (Marinol® or Syndros®), was approved by the FDA in 1985 for treatment of nausea and vomiting associated with cancer chemotherapy, mostly after the failure of previous therapies (Warr and Hesketh, 2020). The Europeans are more restrictive with the use of Dronabinol, with some countries allowing its use only by special permit, or a temporary use authorization (Abuhasira et al., 2018).
Nabilone (Cesamet® or Canemes®), is approved for nausea associated with cancer chemotherapy. In comparison to Dronabinol, there are more European countries allowing the use of Nabilone in case of cancer patients (Abuhasira et al., 2018).
Oncology practice guidelines for clinicians, NCCN and ASCO include the indication of Nabilone and Dronabinol for nausea and vomiting refractory to conventional therapy. 2.5–5 mg oral solution Dronabinol should be used every 4–6 h 1–2 mg of Nabilone can be used twice daily as rescue therapy (May and Glode, 2016).
Cancer-related cachexia and anorexia syndrome (CACS) is a complex wasting syndrome, commonly diagnosed in patients with advanced cancer, which leads to severe fat and muscle mass losses. The prevalence of cachexia in cancer patients increases from 50% to more than 80% as the disease progresses (Lim et al., 2020). Additionally, CACS is reported to be responsible for more than 20% of all cancer-related deaths (Onesti and Guttridge, 2014), thus representing one of the main challenges in oncology clinical practice.
Cachexia not only involves weight loss, but also anemia, electrolyte and water abnormalities, and is often accompanied by anorexia, defined as the loss of appetite and early satiety. Although anorexia is commonly associated with cachexia, the decrease in food intake is not the main culprit in CACS, as attempts to increase dietary intake through dietary counseling or nutritional supplementation are usually inefficient for stopping the wasting process (van de Worp et al., 2020). Chemotherapy can cause significant nausea and vomiting and subsequently lead to anorexia and weight loss. Additionally, oncology patients often experience psychological distress during their cancer journey, which is a known regulator of food intake.
Thus, CACS remains an extremely complex condition that has both objective and subjective features, encompassing a variety of alterations that range from physiological to behavioral and somatic distress (Arends et al., 2021). However, addressing CACS in all oncology patients is essential due to the important prognostic value and impact on the Quality of Life (QoL) of this condition (Kasvis et al., 2019). CACS management poses a significant challenge, as physicians have to address the low patient adherence and high drop-out rate of exercise and nutritional intervention strategies (Nishikawa et al., 2021), combined with the reduced efficacy and high incidence of side-effects of pharmacological treatments such as corticosteroids, progestins or antipsychotics (Arends et al., 2021). Moreover, because several mechanisms are involved, the likelihood of any one intervention to be successful is low (Argilés et al., 2019), opening the avenue for combined treatment approaches.
Historically, cannabis has been used as food, given it is an excellent source of fibers and oils. The oil from hempseed is very nutritious and contains a high quantity of omega-3-type fatty acids (Klumpers and Thacker, 2019), while hemp sprouts are rich in antioxidants (Cerino et al., 2021). Anecdotal evidence has long suggested that cannabinoids can increase appetite, with recreational cannabis users even reporting an enhancement of the sensory and hedonic properties of food (Kirkham, 2009). When studied in small trials and case series, THC appeared to improve appetite and attenuate weight loss. Studies suggest that cannabis could be used in anorexia and cachexia, mainly by stimulating appetite (due to cannabinoid receptors located in the CNS), but also by inhibiting the nausea mechanisms.
a) Preclinical and clinical data
Several animal models of CACS have been established, even though none can completely replicate all aspects of cancer cachexia that occurs in humans (Ng et al., 2023), most likely due to the syndrome’s complexity and the important emotional and psychological feature of CACS. Additionally, considering the large body of clinical data available, there are only few preclinical studies specifically designed for assessing the effect of cannabinoids on CACS. In an acute model of cachexia (rats that received 6 mg/kg intraperitoneal cisplatin), cannabigerol was shown to both increase food intake and prevent weight loss at 72 h, most likely by protecting against/reversing cisplatin-induced dysregulation (Brierley et al., 2019). A more recent translational study measured the serum levels of six CACS-related cytokines in colorectal cancer patients and in a CACS mouse model and found a negative correlation with body mass index. The authors also found that Δ9-THC treatment attenuated CACS-related muscle atrophy via an anti-inflammatory mechanism, together with decreasing the concentration of the afore-mentioned inflammatory cytokines, thus suggesting a CB2-mediated pathway of CACS (Ng et al., 2023).
Of note, preclinical data vary greatly in terms of anorexic/anti-anorexic effects of cannabinoids, most likely because the effect of cannabinoids on food intake is highly dependent on both dose and type of cannabinoid used. For example, 100 μg/kg of CP 55,940 (a synthetic cannabinoid) had significant anorexic effects and caused a decrease in body weight in rats (McGregor et al., 1996), whereas WIN 55212-2 (2 mg/kg) prevented the acceleration of gastric emptying induced by cachexia in rats (de Sousa Cavalcante et al., 2020). However, some of the candidates with positive results in the preclinical studies have translated towards clinical trials in both cancer and non-cancer patients.
Due to promising results in AIDS-related cachexia, Dronabinol has been assessed in several small clinical trials as an appetite stimulant in patients with CACS. A comprehensive review included all clinical data in the field and concluded that available evidence supports the use of Dronabinol in this setting (Voth and Schwartz, 1997). Subsequently, the North Central Cancer Treatment Group designed a large three-arm study to assess the effect of Dronabinol in cancer patients with CACS (Jatoi et al., 2002). The 469 patients enrolled were randomized to receive oral megestrol acetate, oral dronabinol or both and followed-up. The results, however, showed that megestrol acetate was superior to Dronabinol and that the combination of the two was not better than single drug megestrol therapy. Another phase III clinical study designed by the Cannabis-in-Cachexia-Study-Group compared oral cannabis extract, THC and placebo and found no significant difference between groups in terms of appetite or QoL (Cannabis-In-Cachexia-Study-GroupStrasser et al., 2006).
Despite these disappointing results, several other cannabinoids have been tested in clinical trials, some with positive outcomes. These significant variations most likely derive from differences in the active substance used, the dose and the administration route. There is no comprehensive data on the pharmacokinetic parameters of CBD in humans. Tmax is shorter in smoking/inhalator and sublingual forms than in oral forms (1–6.12 h) (Lord et al., 2022). Cmax is higher in the fed state than in the fasting state or in patients with liver dysfunction. Therefore, caution should be exercised in advanced cancer patients with low dietary intake (Millar et al., 2018). Another potential factor is the severity of cachexia, as more advanced or refractory cases are less likely to have benefits from any treatment. A retrospective analysis of cases from Santé Cannabis, a medical cannabis clinic in Canada, identified 54 individuals (43% of them had cancer) requiring cannabis for appetite improvement. Although there were no significant changes in weight between baseline and the 3-month assessment, patients reported a significant improvement in appetite, especially with nabilone and inhaled cannabis-based produce (Kasvis et al., 2019). In a more specific setting, Nabilone was compared to placebo in advanced lung cancer patients diagnosed with CACS and was shown to significantly increase caloric intake, double the carbohydrates intake, together with a significant increase in QoL (Turcott et al., 2018). A small pilot study enrolled advanced cancer patients with documented CACS and assessed the effect of cannabis capsules (95% THC and 5% CBD) on weight gain and several other patient-reported outcomes. Although the drop-off rate was quite high (11 out of the 24 patients that signed the consent form received the treatment for more than 2 weeks), the authors concluded that almost 20% of patients had a weight increase of more than 10% and almost 40% had stable weight at the time of study completion. Additionally, while receiving the capsules, cancer patients reported improvements in mood, pain and fatigue, as assessed by the EORTC QLQ-C30 questionnaire, albeit with some side effects (Bar-Sela et al., 2020).
There are a lot of uncertainties surrounding the potential effect of cannabinoids in CACS, as emphasized by a recently published systematic review and meta-analysis. After including all clinical trials with cannabinoids as appetite stimulants, the authors concluded that the level of evidence for different cannabinoids is either low or very low, underlining the need for additional studies and the high likelihood of recommendation changes if new data becomes available (Bilbao and Spanagel, 2022).
b) Approved medication
Dronabinol (Marinol® or Syndros®), is approved for anorexia associated with weight loss in patients with AIDS, but not for cancer patients. Current ASCO guidelines state that the administration of cannabinoids for anorexia/cachexia brings no benefits, given the fact that the strength of the evidence from the studies conducted until now is low (Roeland et al., 2020). Similarly, ESMO considers the existing literature data insufficient to justify medical cannabis or its derivatives use for treating anorexia or cachexia in cancer patients (Arends et al., 2021). However, the NCCN guidelines include the possibility of cannabinoid administration in the case of patients with a very low life expectancy (weeks, days until death) to stimulate the appetite.
With early detection and new cancer therapies available, the life expectancy of cancer patients has increased, and many of them will live with cancer as a chronic disease controlled by ongoing therapy. Current estimates indicate that by 2040 approximately 26 million individuals will be living with and beyond cancer in the United States alone (Emery et al., 2022).
The prevalence of common mental disorders among people with cancer varies widely in the published literature. The mean prevalence of depression is believed to be around 13%, with literature data varying from 4% to 49% (Niedzwiedz et al., 2019).
Depression leads to a poorer QoL and compromises patient outcomes, with depression resulting in higher rates of mortality in cancer (Wang et al., 2020). A meta-analysis revealed that minor or major depression increases mortality rates by up to 39%, and that patients displaying even few depressive symptoms may be at a 25% increased risk of mortality (Satin et al., 2009). Additionally, depression triples the risk of nonadherence, further contributing to increased mortality (Fann et al., 2008).
Another important issue to consider is that depression often coexists with symptoms of anxiety in patients with cancer, with fewer available statistical data that examine anxiety alone. In case of cancer survivors, many of them experience cancer-related fear of recurrence, post-traumatic stress symptoms, anxiety, or depression after completing oncological treatment. In the study conducted by Götze et al. (2020) on 1,000 cancer survivors of mixed tumor entities 10 years after the initial diagnosis, results showed a prevalence as high as 17% and 9% for depression and anxiety, respectively. Similar to the other conditions described previously, cannabis has a long history of use as an anxiolytic and antidepressant remedy (Zuardi, 2006), (Russo and Pertwee, 2014).
a) Preclinical and clinical data
Recent experimental data seem to confirm the physiological substrate of these effects. The endocannabinoid system is heavily involved in mood regulation and high levels of CB receptors have been described in the limbic system and prefrontal cortical areas (Maldonado et al., 2020). In a preclinical model for assessing anxiety, low doses of THC (0.3 mg/kg) were associated with anxiolytic-like responses in male CD1 mice (Berrendero and Maldonado, 2002). Similar results were observed after intraperitoneal administration of varying THC doses (0.75 mg/kg being the most effective) in rats, most likely linked to CB1R activation (Rubino et al., 2007).
Additionally, antidepressant effects of cannabinoid administration have been reported in preclinical studies. WIN 55212-2 significantly improved results in the rat forced-swim test (a murine model of depression) when administered intraperitoneally 0.75, 5 and 23 h before the test (Bambico et al., 2007). The preclinical data that supports potential benefits of CBD on both depression and anxiety is perhaps even more convincing, as highlighted in a comprehensive review performed by de Mello Schier et al. (2014).
Of note, the effect of cannabinoids on anxiety and depression seems to be dose-dependent and cannabinoid-dependent, similar to results reviewed for other symptoms. For example, a large dose of the synthetic cannabinoid CP 55940 (75 μg/kg) induced anxiogenic effects in male rats; however, the 10 μg/kg dose did not yield the same effect (Marco et al., 2003) and a dose of 1 μg/kg was associated with behavior suggestive for anxiolysis (Marco et al., 2004).
The dose- and strain-dependent anxiogenic or anxiolytic effect of cannabinoids are similar to data from real-world reports of recreational cannabis use, where some individuals report feeling relaxed, whereas others experience paranoia, panic and anxiety (Freeman et al., 2015), most likely depending on both individual variations and specific CBD/THC content of the drug. Although there are several large on-going randomized clinical trials assessing the effectiveness of different cannabinoids on depression and anxiety, currently available clinical data are sparse and heterogenous. Nonetheless, most authors concur that CBD does indeed have anxiolytic properties and can even reverse some of the negative psychological effects of smoking marijuana. A study on over 100 cannabis users assessed both memory and psychotomimetic symptoms in acutely intoxicated and drug-free states. The authors also analyzed the content of both CBD and THC within the smoked cannabis. Results showed that while there were no significant differences in terms of THC, CBD concentrations varied significantly within different types of cannabis. The low-CBD group had significantly higher ratings of anxiety compared to the high-CBD group and higher levels of CBD seemed to be protective against cannabis-induced memory impairment (Morgan et al., 2010). A more recent study, however, assessed distress-related symptoms in patients using inhaled cannabis and reported that over 95% of the 670 individuals enrolled experienced a statistically significant decrease in symptom intensity, particularly for agitation and anxiety, results that correlated with THC and not CBD concentrations (Stith et al., 2020). A recent comprehensive review identified ten clinical studies that assess the relationship between cannabis and anxiety and concluded that cannabinoid-based treatment, especially that which contains CBD, could represent a therapeutic option for people with pre-existing anxiety (Sharpe et al., 2020).
The effect of cannabinoids on depression is less clear in the clinical setting, as increased cannabis use and depression seem to co-occur quite often (Feingold and Weinstein, 2021). Additionally, there are a lot of hindrances for this area of research, especially due to concurrent anti-depressant medication, patient adherence and various cannabinoids/preparations/plant strains being used, which has led to contradictory results. For example, a small study on 40 multiple sclerosis patients with depression and chronic cannabis use concluded that withdrawal from cannabis improved depression scores at the 4-week landmark assessment (Feinstein et al., 2021). On the other side, a recently published observational trial of cannabis users versus controls concluded that medicinal cannabis users had lower depression scores. Moreover, the individuals in the control group that initiated treatment with medicinal cannabis whilst on the study reported decreased depression and anxiety scores (Martin et al., 2021).
b) Approved medication
Currently, no cannabinoid is approved for managing anxiety or depression outside a clinical trial, with no information regarding the use of cannabinoids for this indication in the previously mentioned guidelines. However, a recently published paper presented the Multinational Association of Supportive Care in Cancer (MASCC) guidelines for cannabis and insomnia, anxiety, or depression. The authors concluded that currently it is impossible to have a guideline about the use of cannabinoids in the treatment of depression and anxiety in patients with cancer due to insufficient data. However, they recommend that patients should not be routinely advised to stop cannabis if already on cannabis and are experiencing benefits with no bothersome adverse effects (De Feo et al., 2023).
Cancer-attributed medical care costs in the United States are substantial and projected to increase dramatically by 2030 to 246$ billion, mainly due to population growth, reflecting the rising burden of cancer care among cancer survivors (Mariotto et al., 2020). As a result, scientists are trying to identify new therapies with maximum antitumor effect and with the least adverse effects, to ensure the QoL of these patients.
Among chat groups, social media or documentaries for cancer patients, a lot of information is shared according to which cannabis can cure cancer. Indeed, the literature provides a consistent trail of in vitro and in vivo studies in which cannabinoids can alter tumor dynamics. Preclinical data shows that through receptors located on the surface of tumor cells, cannabinoids modulate a series of intracellular signaling pathways that trigger a wide range of anti-oncogenic effects. These include inducing programmed cell death by apoptosis, blocking cell proliferation, decreasing tumor angiogenesis and inhibiting cancer cell migration, invasiveness and metastasis. Ultimately, these actions can indeed lead to a reduction of tumor growth, observed in both in vitro and in vivo studies (Velasco et al., 2012).
However, it is known that promising preclinical results do not always readily or easily translate into real world clinical benefit. Rising prevalence and demand for cannabis in recent years has driven some patients to shun conventional cancer treatments in favor of different cannabinoid preparations, in hopes of curing their disease. Given the insufficient scientifically validated clinical data, the empirical administration of cannabinoids, to the detriment of conventional therapy, is truly worrisome. When discussing treatment possibilities with patients, clinicians should have enough information in order to correctly and completely inform the patient about the real benefits and potential risks of using cannabinoids together with or instead of approved anti-cancer treatments.
The relationship between the endocannabinoid system (ECS) and carcinogenesis, tumor growth and metastasis is extremely complex and not fully understood, especially since the definition of the ECS is constantly expanding to include enzymes involved in endocannabinoid synthesis and degradation, endocannabinoid-like lipid mediators and non-CB receptors that bind to cannabinoids, such as the TRPV channel subfamily or the G protein-coupled receptors (GCPR) (Filipiuc et al., 2021).
Both CB receptors and endogenous cannabinoid levels are altered in several cancer cell lines. An increase in CB2R expression in the tumor has been linked to increased aggressiveness and recurrence risk in breast, prostate, pancreas, thyroid and colon cancer, whereas the relationship between CB1 expression and tumor progression is less clear (Pagano et al., 2021).
A large body of available data shows that cannabinoids can reduce cancer cell proliferation through protein kinase B (Akt) inhibition (Hinz and Ramer, 2022). Akt is a serine/threonine kinase that plays a key role in growth factor-induced cell survival. It is a critical mediator of the canonical PI3K signaling pathway, which has long been recognized for its major oncogenic role within the cell (Revathidevi and Munirajan, 2019). Other important mechanisms include retinoblastoma protein hypophosphorylation (Hinz and Ramer, 2022), promotion of reactive oxygen species (Dando et al., 2013), modulation of the mitogen-activated protein kinases (MAPK) pathway and apoptosis signaling. Several preclinical studies report that cannabinoids induce cell cycle arrest by downregulation or inactivation of cyclin-dependent kinases (CDK) and cyclin modulation (Thoma et al., 2021). Depending on the cancer cell line, the type of drug and the dose used, available data indicate that cannabinoids can downregulate CDK2 (Caffarel et al., 2006), which in turn induces cell cycle arrest and affects proliferation. Another relevant mechanism in ECS signaling is ceramide synthesis. Ceramide is a sphingolipid that acts as a second messenger in activating the apoptotic cascade, thus playing a key role in programmed cell death (Mullen and Obeid, 2012). Several cannabinoids and endocannabinoids can elicit ceramide production from membrane phospholipids, which determines cell autophagy (Brown et al., 2013).
Cannabinoids can also modulate cancer progression via the increase of intercellular adhesion molecule-1 (ICAM-1) expression, decreasing metalloproteinase expression and modulating several other extracellular matrix components (Ramer et al., 2012). The ECS system has also been shown to downregulate a number of angiogenesis-inducing factors such as angiopoietin-2, placental growth factor and vascular endothelial growth factor (VEGF) that play a key role in tumor growth and new vessel formation (Hinz and Ramer, 2022).
However, more research is needed to define the ECS’ role in tumor heterogeneity and how its signaling impacts the activation of other signaling pathways involved in cancer dynamics. How cannabinoids are involved in host metabolism via their GPCR receptors, as they present a role in activating numerous receptor tyrosine kinases and Toll-like receptors in the induction of altered epigenetic landscape in cancer cells, represents an exciting area for investigation, as it could modify cancer metabolism and epigenetic reprogramming to a metastatic phenotype. This means that in the future, focus on epigenetics could be an innovative target for preventing and treating cancer.
While most reports indicate an anti-tumor effect, others have also shown that in certain cell lines and in certain concentrations, some cannabinoids can also have the opposite effect, promoting cancer progression via MAPK pathway (Liu et al., 2020). Moreover, over-activation of the ECS has been demonstrated in several tumor types, thus further emphasizing the potential pro-tumorigenic effect of cannabinoids. Last, but not least, the complex relationship between the ECS and the immune system is another potential hindrance for the clinical use of cannabinoids as anti-cancer drugs, since they might induce immunosuppression (Moreno et al., 2019).
However, more specific mechanisms of the endocannabinoid system on host metabolism and energy homeostasis remain to be elucidated. In addition to agonists, antagonists, and inverse agonists as targets for cannabinoids in oncology, allosteric modulators have been identified. They have been shown to bind topographically distinct sites from the classical orthosteric sites, and as in consequence, can contribute to the tempering of the cannabinoid receptor signaling without the desensitization, tolerance and dependence (Shore et al., 2014). Allosteric modulators have numerous advantages, as they act only on tissues where ECs (lipid mediators, e.g., anandamide (AEA) and 2-arachidonoylglycerol (2-AG)) are present. Especially in cancer, where these components of the endocannabinoid system can vary between so many factors and consequently between each patient, the use of allosteric modulators is even more advantageous, as the entity and the duration of their effect will depend on the ECs tone at that time and selective to that area, consequently minimizing the off target side-effects (Burford et al., 2015). Currently some CB1R allosteric modulators have been discovered and studied, while very few compounds have been identified as CB2R allosteric modulators (Gado et al., 2019).
Emerging data regarding the effect of cannabinoids on key cancer signaling pathways has generated significant research interest over the past decades. While a lot of data comes from glioma cell lines, several other tumors have since been assessed via in vitro models. Table 1 summarizes the current state of preclinical research on antitumor effects.
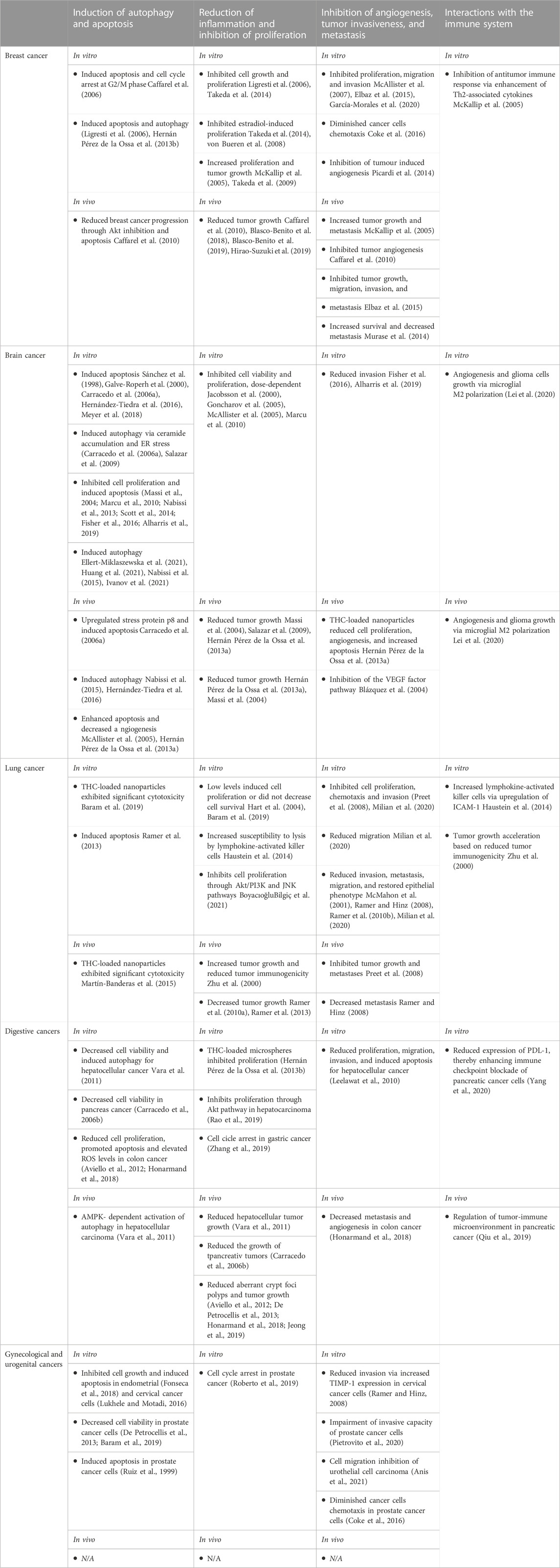
TABLE 1. Current state of the preclinical research about the antitumoral effects of cannabinoids, both in vitro and in vivo.
Munson et al. (1975) were among the first researchers, who in 1975 observed the antitumor effects of cannabinoids. They administered Δ9-THC, Δ8-THC and cannabinol to Lewis lung adenocarcinoma and observed a decrease in cell proliferation.
Since then, more studies documented the antiproliferative effects of cannabinoids. Kolbe et al. (2021) recently presented the effects of Δ9-THC in glioblastoma multiforme (GBM) and the ability to modify the number of Ki67+ cells of human patient-derived GBM cells, particularly through the activation of the orphan receptor GPR55 without affecting CB1R and CB2R as usual targets. Their findings suggest that the sensitivity of cannabinoids and receptor-dependent signaling pathways should be considered to reflect the heterogeneity amongst GBM forms which is critical for when evaluating this translationally to clinic.
Synthetic cannabinoid WIN 55212–2 was also used in case of patient-derived glioblastoma multiforme cells, and was associated with the induction of autophagy and apoptotic cell death, at doses between 0.1 nM and 2 μM (Ellert-Miklaszewska et al., 2021).
Cell death via proapoptotic and anti-proliferative activity of 24 cannabis extracts on head and neck squamous cell carcinoma, had an effect proportional to the CBD content. Extract with the highest CBD content possessed the highest cytotoxic effect, while extracts with minimal CBD had a neglectable cytotoxic effect (Blal et al., 2023).
In case of breast cancer, doses as low as 1–5 µM of CBD induced significant cell death (Kosgodage et al., 2018). CBD’s IC50 values for most cell lines are consistently low, indicating that breast cancer cell lines are generally sensitive to CBD’s anti-proliferative effects (Shrivastava et al., 2011). CBD induced inhibition of cell proliferation and invasion on a human breast adenocarcinoma cell line, with inhibitory concentrations of only 1.5 μM (Nallathambi et al., 2018). In hepatocellular carcinoma cell lines, treatment with Δ9-THC and JWH-015 reduced cell viability through activation of the CB2R (Vara et al., 2011).
In addition to the in vitro testing, researchers explored the possibility that cannabinoids could influence the tumor dynamics, in vivo studies. The ability of THC to treat ErbB2-positive breast cancer, has been evaluated in some recent studies. For example, in a mouse model of ErbB2-driven metastatic breast cancer, THC treatment was able to reduce tumor growth, as well as the amount and severity of lung metastases. THC treatment also induced apoptosis and limited tumor angiogenesis (Caffarel et al., 2010). In vivo mouse models of lung cancer showed that treatment with 10 mg/kg/day of CBD resulted in reduced cell viability, decreased overall tumor growth and decreased metastasis (Ramer et al., 2010a). Similarly, murine models of glioblastoma multiforme revealed that treatment with CBD was able to inhibit tumor growth, enhance apoptosis and significantly prolong mouse survival (Hernán Pérez de la Ossa et al., 2013a; Singer et al., 2015).
Even though recent results in preclinical studies are increasing in number, the existing clinical trials investigating the anti-cancer effects of cannabinoids as single agents are very few and all so far have failed to show any high-quality positive effects (Table 2). However, the study conducted by Guggisberg&collab, includes data from 77 peer-reviewed case reports studies, and concluded that 81% of cases lacked sufficient evidence to support claims that cannabis had an anticancer effect (Guggisberg et al., 2022).
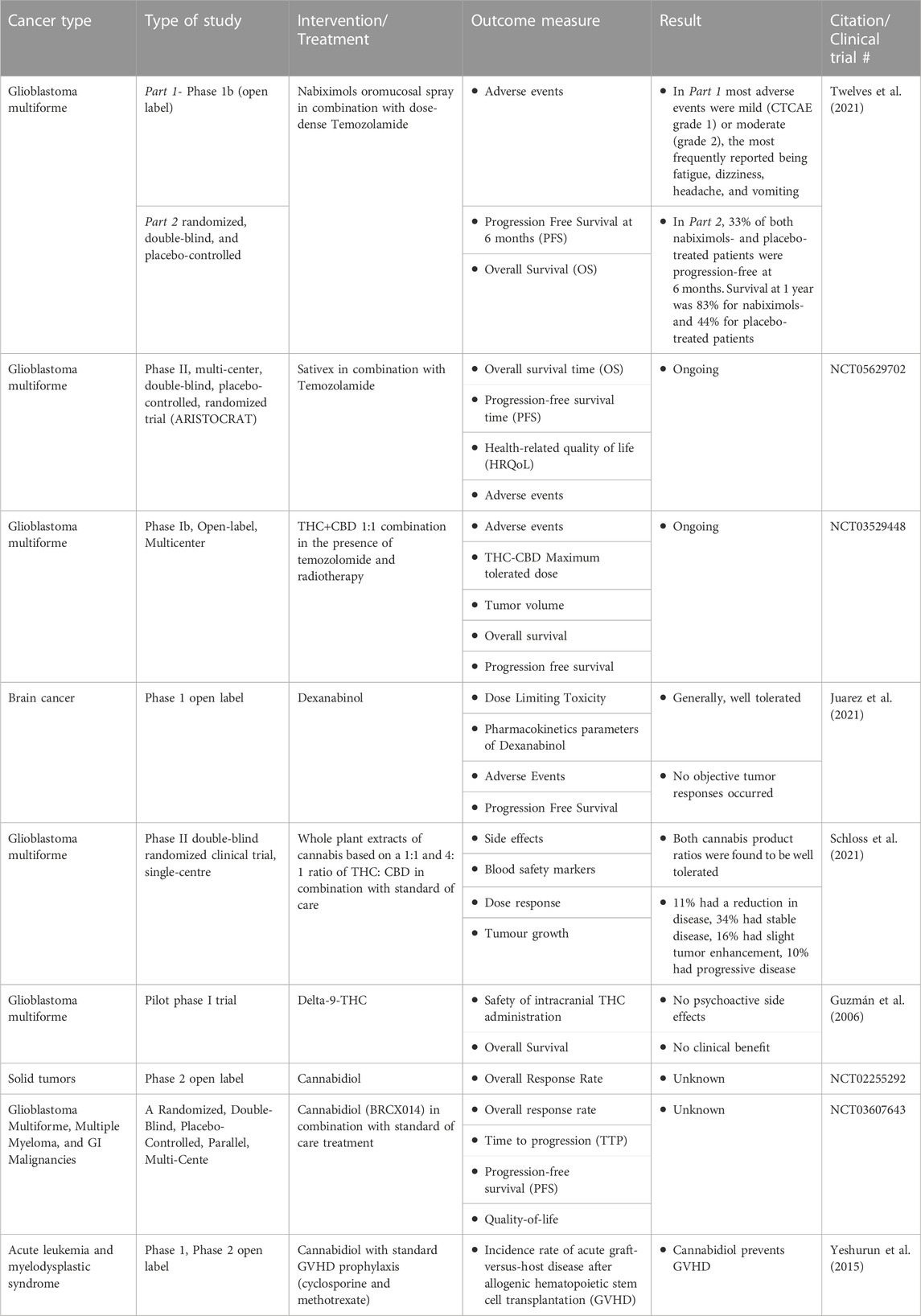
TABLE 2. Clinical trials (finished or ongoing) for potential use of cannabinoids for their antitumor effects.
Different combinations between cannabinoids and anti-cancer drugs are being explored based on cannabinoid’s potential for increasing the sensitivity of cancer cells to therapy, thus significantly increasing drug efficacy. There are several small clinical trials/case series available in the literature that suggest a potential role for this combined approach. For example, CBD/gemcitabine combination is more effective than single-drug gemcitabine, most likely because CBD can modulate ERK activation, a common mechanism to acquire chemoresistance both in vitro and in vivo (Adamska et al., 2018; Domenichini et al., 2019).
Co-administration of Doxorubicin and CBD in hepatocarcinoma (known for its chemoresistance) was shown to enable the internalization of Doxorubicin into the tumor cells, thus enhancing its action. This facilitated uptake might allow the use of lower Doxorubicin doses and therefore may improve the therapeutic index, decreasing chemotherapy-associated resistance (Neumann-Raizel et al., 2019). A similar result was reported for glioblastoma cell lines. Nabissi et al. (2013) provided evidence that addition of CBD increased the susceptibility of human glioblastoma cells to chemotherapeutic agents carmustine (BCNU), doxorubicin (DOXO) and temozolomide (TMZ).
In case of myeloma cell line, Bardabo et al. showed that synthetic cannabinoid WIN 55212–2 increased the sensitivity of the cells that were resistant to melphalan or dexamethasone, consequently increasing the anti-cancer activity of the chemotherapy (Barbado et al., 2017).
In a glioma xenograft model, Torres et al. (2011) demonstrated that the combination of THC with temozolamide (TMZ) inhibited tumor growth. Furthermore, combined treatment with these two agents exhibited much higher growth inhibition that each of them alone. In line with this study, Valero et al. showed that oral administration of Sativex, enhanced the effect of TMZ in glioma xenograft models (López-Valero et al., 2018).
Evidence from in vitro and in vivo studies can indicate which cannabinoid combination has the strongest possibility for successful translation to clinic. However, controlled clinical trials are required to test the value of these combinations in the cancer setting. There are several clinical trials which evaluate the antitumor capacity of different cannabinoids, when paired with standard treatment.
Table 2 summarizes some of the completed or ongoing clinical trials regarding the potential use of cannabinoids for their antitumor effects.
Of these, most are centered around glioblastoma patients due to good preclinical and clinical data already available. A recently published randomized, double-blind, and placebo-controlled phase 1b clinical study investigated the safety and preliminary efficacy of nabiximols oromucosal cannabinoid spray and dose intense (DIT) TMZ in patients with first recurrence glioblastoma. Nine of the 12 nabiximols and 6 of the 9 placebo recipients progressed by 6 months. Despite the similar progression rates, 1-year survival was 83% for the nabiximols patients and 44% for the placebo group (p = .042). Moreover, the trend persisted so that at 2 years, 50% of the nabiximols recipients were still alive compared to 22% of the placebo group (p = .134) (Twelves et al., 2021).
An ongoing clinical study, with 21 patients enrolled, investigates the antitumor activity of orally administered Sativex, in addition to dose-intense Temozolamide in recurrent glioblastoma patients. This open-label study also assesses the frequency and severity of adverse events in patients receiving Sativex in combination with standard chemotherapy compared to placebo-chemotherapy (NCT01812616). ARISTOCRAT is a phase II, multi-center, double-blind, placebo-controlled, randomized trial that aims to compare Sativex with placebo in patients with recurrent MGMT methylated glioblastoma treated with temozolomide (TMZ). The trial will randomize a target number of 234 patients on a 2:1 basis to receive either Sativex or Sativex-matched placebo, in combination with standard TMZ (NCT05629702). Another ongoing phase Ib, open-label, multicenter, intrapatient dose-escalation clinical trial investigates the safety profile of the THC+CBD combination at a 1:1 ratio, adding temozolomide and radiotherapy in patients with newly diagnosed glioblastoma (NCT01812603).
As previously mentioned, several key elements of the endocannabinoid system are expressed throughout the immune system and cannabinoids play an important role in immunomodulation. High concentrations of THC suppress the activity of T and B cells, while low concentrations have been associated with immunostimulatory effects (Pryimak et al., 2021). The possibility of using cannabinoids as neoadjuvant therapy together with biologics represents a fruitful avenue for future research (Pryimak et al., 2021). It has been shown that immunotherapy alters serum concentrations of endogenous CB independently of cannabinoid administration (Bar-Sela et al., 2020). However, the scarce clinical data available more likely indicate a deleterious effect of cannabinoids on tumors exposed to immunotherapy. A study by Taha et al. concluded that concomitant administration of cannabinoids and nivolumab immunotherapy reduces response rates (Taha et al., 2019). Other reports underlining the poor clinical outcome and reduced survival in cases where checkpoint inhibitors and cannabinoids were combined have been published (Bar-Sela et al., 2020; Xiong et al., 2022) Some data suggest that THC stimulates breast cancer growth by reducing antineoplastic immune responses (Hinz and Ramer, 2022).
Regarding the potential application of cannabinoids in combination with radiation therapy, even fewer studies have been published. Scott et al. investigated whether cannabinoids could enhance the cytotoxic effects of irradiation. Results were observed in vivo, where the triple combination of CBD, THC and irradiation significantly reduced the tumor size in an orthotopic syngeneic glioma mouse model, with the schedule of administration being of utmost importance (Scott et al., 2014).
Despite an ever-increasing body of evidence that cannabinoids may have anti-tumor activity, it seems that this effect is highly dependent on several factors. One possible explanation for this variation is the high heterogeneity of the ECS system and its change in relationship to cancer progression. Additional factors to consider are the lack of consensus on anti-cancer cannabinoid responses, different drugs and doses used or even the experimental variability among scientists.
Cannabinoids have been shown to produce a biphasic effect depending on the concentration of the compound used. This translates into a delicate balance between the pro- and antitumor effects, with high concentrations (micro-molar) of endogenous cannabinoids displaying an inhibitory effect on tumor growth, whereas low concentrations (nano-molar) induce cell proliferation (Katsidoni et al., 2013).
In case of a triple negative breast cancer cell line, McAllister et al. (2007) demonstrated that higher concentrations of CBD caused a significantly higher decrease in cell proliferation and invasion as opposed to lower concentrations. Another study evaluating the effect of CBD on the tumor microenvironment revealed that lower concentrations of CBD had less of a direct effect on proliferation in comparison to higher concentrations (Elbaz et al., 2015). Further, Hart et al. (2004) showed that treatment of cancer cells with THC, HU-210, WIN 55212-2 and AEA stimulated mitogenic signaling.
On the other hand, the antitumor effects of cannabinoids may vary not only based on the type of cannabinoid, but also depending on certain characteristics of the targeted cells. The study conducted by Baram et al. (2019) assessed the antitumor effects of 12 whole cannabis extracts containing significant amounts of different phytocannabinoids on 12 different cancer lines from various tumor origins. They found a heterogenous antitumoral response between the Cannabis extracts and also between the cancer cell lines derived from the same organ.
There is also data mentioning that smoking cannabis could increase the risk of developing cancer. Two proto-oncogenes are overexpressed in the bronchial epithelium of cannabis-only smokers when compared with tobacco-only smokers (Barsky et al., 1998). The net association between cannabis use and developing cancer is, however, unclear. Cannabis and tobacco smoke share carcinogens, including toxic gases, reactive oxygen species, and polycyclic aromatic hydrocarbons (Hoffmann et al., 1975), believed to be 20 times higher in unfiltered marijuana than in cigarette smoke (Moir et al., 2008). Maertens et al. (2013) found that both cannabis and tobacco smoke influence the same molecular processes, but have differences in terms of activation of pathways, which could explain the contradicting information in the literature.
Studies of large databases have also yielded different results. 64,855 members of the Kaiser Permanente Medical Care Program, former or current cannabis users, were included in a study on the incidence of cancer. After an 8.6 years follow up, it was concluded that there was no increased risk of respiratory cancer (Sidney et al., 1997). By contrast, Zhang et al. (1999) found a correlation between cannabis users and squamous cell carcinoma of the head and neck.
The systematic review and metanalysis developed in 2019 by Ghasemiesfe et al. (2019) tried to find out the association between marijuana use and cancer development in adults with at least 1 year exposure. Low-strength evidence suggests that more than 10 years of cannabis smoking is associated with an increased risk of developing testicular germ cell tumor. However, its association with other cancers, such as lung and head and neck cancers are of poor quality and inconclusive and limited by low exposure and duration of follow-up.
Oral administration of herbal Cannabis has a lower bioavailability (5%–20%) than inhalation, due to the gastric degradation of cannabinoids and first-pass hepatic metabolism (Le Boisselier et al., 2017). The pharmacological effects after oral administration range from 30 min to 3 h and the maximum concentration of cannabinoids in the blood is usually reached within 2 h (Maida and Daeninck, 2016; Brunetti et al., 2020).
Cannabis administration by inhalation seems to be better tolerated and with more predictable effects than orally (Romero-Sandoval et al., 2017). Administration of medicinal cannabis by inhalation can be done in two ways, by vaporization or by smoking, “cannabis vaping” gaining the most popularity in recent years because it eliminates inhalation of smoke by-products such as tar (Ghasemiesfe et al., 2019). Recent studies have shown that vaping products could lead to lung injury, often referred to with the acronym EVALI and is predominately associated with cannabis products (Boyd et al., 2021). Ghasemiesfe & collab showed in a recent meta-analysis that short-term cannabis vaporization has a minimal impact on pulmonary function, however the data regarding long-term exposure is still insufficient and conflicting (Ghasemiesfe et al., 2019).
As for medical cannabis decoctions, and cannabis extract in oil, there are no specific guidelines for a standardized method of preparation provided by any European or international pharmacopoeias. When refrigerated for several days, the cannabinoid concentration decreases, so that extemporaneous preparation is recommended, therefore there are no industrial products available (Hazekamp et al., 2007).
Again, no European or international pharmacopoeia offers a standardized method for preparing cannabis extract in oil. In general, manufacturers extract CBD or THC from the C. Sativa plant, then dilute it with a nontoxic oil. At the moment, there are no FDA or EMA approved cannabinoid oil for treating symptoms associated to cancer, however Epidiolex® it is approved for Lennox-Gastaut and Dravet syndrome (Sekar and Pack, 2019).
Topical administration does not allow the entering of the compounds into the bloodstream, thus considered nonpsychoactive. Their onset of action is 15–30 min after application and effects can last for 3–6 h. Transdermals include patches and gels, have a similar bioavailability as topicals, but can be absorbed into the bloodstream, however, can be time-realeased (Bruni et al., 2018).
Although there is increasing evidence for the therapeutic potential of the cannabinoids in oncology, a clinician should always be aware of the potential side effects associated. Literature available has a significant level of uncertainty regarding the safety of cannabinoids. This is not a result of the lack of research, but rather caused by the extreme variability in study methodology and quality.
Some of the cannabinoids’ adverse effects are summarized in Table 3.
Cannabinoids can have cardiovascular effects in a dose dependent manner, which can be managed through symptomatic treatment, without the need for hospitalization of the young patient (Ravi et al., 2018). However, senior patients with a history of heart diseases can develop life-threatening cardiovascular side effects, such as myocardial infarction (Tait et al., 2016). The main route of administration of illicitly obtained cannabis is through smoking; obviously, it is not without adverse effects on the respiratory tract.
Regarding safety in pregnancy, it is recommended to avoid the administration, given the possibility of increased risk for stillbirth, preterm birth, fetal growth restriction, miscarriage, and adverse neurodevelopmental consequences. However, much of the existing research was performed in the 1980s, when quantities of THC were lower and the frequency of use was less (Thompson et al., 2019).
Another concern regarding cannabis uses it is about the effects on cognition. It seems it can vary depending on the frequency of use and cumulative dose of the compound. The majority are relatively short lived and diminish over time with abstinence. However, the risk of long-term exposure effects of cannabis use appears to increase with earlier age of onset. Bolla et al. (2002) found in their study that dose-dependent neurocognitive impairment persisted after 28 days of abstinence in heavy young users. Furthermore, there is also evidence that adults who initiated regular cannabis use in adolescence may have structural and functional alterations of the CNS (Wieghorst et al., 2022).
Another important aspect of cannabinoid use is the increasing body of evidence aimed at the adverse effects that they may have causing or precipitating existing psychiatric disorders, highly dependent on dosage, time, potency and patient’s age (Johnson et al., 2021). Several studies suggest that acute cannabis exposure may induce temporary psychosis. Regarding chronic consumption, special attention must be paid to patients diagnosed with psychiatric conditions, because cannabis may exacerbate pre-existing symptoms of psychosis and schizophrenia (Ganesh and D’Souza, 2022; Gibbs et al., 2015).
Any health effects of increased potency cannabinoids depend on whether patients are able and willing to titrate their dose of THC. This may also vary with the experience of users. Among naive users, higher THC content may increase the likelihood of adverse psychological effects, while increasing the risk of dependence and psychotic symptoms if regular users do not titrate their dose. However, there is some evidence that cannabis with a high CBD and low THC content may decrease the risk of psychosis (Mechoulam et al., 2007). In the case of the general population, cannabis use may trigger psychosis and schizophrenia in cases of a predisposition or genetic susceptibility to mental illness, again, depending on the factors previously listed (Grotenhermen, 2007). This risk appears especially in the case of young people, also demonstrated by Andréasson et al. (1987) where 50,465 Swedish male conscripts reported that those who had tried cannabis by age 18 years were 2–4 times more likely to be diagnosed with schizophrenia than those who had not.
One of the main concerns that leads to a reserved attitude regarding the medicinal use of cannabis, both on the side of patients and doctors, is represented by the risk of addiction. Approximately 17% of adolescent users develop addiction, however after 25 years old, the addiction pattern follows a downward trajectory, being rarely addictive (Shekhar, 2014). Data between the 70s–90s have shown some correlation between cannabis and other illicit drug use. Adolescents with addictive behavior (as in use of “heavy drugs”, alcohol, tobacco) were more likely to develop addiction following cannabis consumption. The possible explanation for this was believed to be the opportunity to procure cannabis and other illicit drugs from the same black market or the fact that young consumers have a predisposition for combining cannabis with other substances (Mechoulam et al., 2007).
However, it is important for oncologists to control patients by measures of medical history (vulnerability to mental illness and addiction), and periodic clinical exams, with attention to the proportions of active molecules for the desired clinical effects, to prevent undesired psychoactive effects.
Cannabinoids have been used as an almost universal remedy for millennia, but their immense inter-strain variability, combined with a high potential of abuse have hindered research until recently. As more countries legalize or decriminalize cannabis use, patients with chronic conditions, especially cancer patients are more and more interested in using these drugs as palliative or curative treatment. Despite this trend, except for their effect on nausea and vomiting cannabinoids are not currently approved in any cancer-related indication. One of the major issues when interpreting available real-world data and translating them in clinical practice is the lack of good practice standards, which are essential especially for this compound. Still, cannabinoids do appear to have some effect in cancer-related pain, especially as co-analgesics and if started early during treatment, and overall seem to improve patient wellbeing by slightly improving different cancer-related symptoms such as mood, appetite or anxiety. The anti-tumor effect of cannabinoids is also currently a matter of debate, and these drugs seem to act as a double-edged sword in relationship to cancer progression due to the plethora of effects of the endocannabinoid system. However, most current data seem to favor the anti-tumor effect of cannabinoids, suggesting they could be used in the future in conjunction with some anti-cancer systemic therapies with the notable exception of immunotherapy.
However, before prescribing, clinicians should take into consideration the profile of the patient to which prescription is made. Young patients, pregnant, with a history of mental health or substance abuse, or elderly with a history of severe cardiovascular diseases should avoid the use of cannabinoids, until more data regarding the safety are available. Another important aspect is the ability of the clinician to choose the right combination of active molecules for the desired effect, the dosage and form of administration, to prevent undesired effects.
Still, a lot of additional data regarding their mechanism of action, posology, interactions with other drugs, or assessing adverse effects is required to access the full potential of these drugs, along with high quality clinical trials, so that oncologists would be more confident when prescribing them to the patients.
IC-M, TA-S, LF, and MC wrote the original draft of the manuscript; IC-M, TA-S, and B-IT revised and edited the manuscript; IC-M and B-IT—conceptualization, visualization and project administration. All authors contributed to the article and approved the submitted version.
This work was supported by a grant of the Romanian Ministry of Education and Research, CNCS–UEFISCDI, project number PN-III-P4-ID-PCE-2020-1247, within PNCDI III.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abrams, D. I. (2022). Cannabis, cannabinoids and cannabis-based medicines in cancer care. Integr. Cancer Ther. 21, 15347354221081772. doi:10.1177/15347354221081772
Abuhasira, R., Shbiro, L., and Landschaft, Y. (2018). Medical use of cannabis and cannabinoids containing products - regulations in Europe and North America. Eur. J. Intern Med. 49, 2–6. doi:10.1016/j.ejim.2018.01.001
Adams, R., Hunt, M., and Clark, J. H. (1940). Structure of cannabidiol, a product isolated from the marihuana extract of Minnesota wild hemp. I. . J. Am. Chem. Soc. 62 (1), 196–200. doi:10.1021/ja01858a058
Adamska, A., Elaskalani, O., Emmanouilidi, A., Kim, M., Abdol Razak, N. B., Metharom, P., et al. (2018). Molecular and cellular mechanisms of chemoresistance in pancreatic cancer. Adv. Biol. Regul. 68, 77–87. doi:10.1016/j.jbior.2017.11.007
Alharris, E., Singh, N. P., Nagarkatti, P. S., and Nagarkatti, M. (2019). Role of miRNA in the regulation of cannabidiol-mediated apoptosis in neuroblastoma cells. Oncotarget 10 (1), 45–59. doi:10.18632/oncotarget.26534
Alkislar, I., Miller, A. R., Hohmann, A. G., Sadaka, A. H., Cai, X., Kulkarni, P., et al. (2021). Inhaled cannabis suppresses chemotherapy-induced neuropathic nociception by decoupling the raphe nucleus: A functional imaging study in rats. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 6 (4), 479–489. doi:10.1016/j.bpsc.2020.11.015
Andréasson, S., Allebeck, P., Engström, A., and Rydberg, U. (1987). Cannabis and schizophrenia. A longitudinal study of Swedish conscripts. Lancet 2 (8574), 1483–1486. doi:10.1016/s0140-6736(87)92620-1
Anis, O., Vinayaka, A. C., Shalev, N., Namdar, D., Nadarajan, S., Anil, S. M., et al. (2021). Cannabis-derived compounds cannabichromene and d9-tetrahydrocannabinol interact and exhibit cytotoxic activity against urothelial cell carcinoma correlated with inhibition of cell migration and cytoskeleton organization. Molecules 26 (2), 465. doi:10.3390/molecules26020465
Arends, J., Strasser, F., Gonella, S., Solheim, T. S., Madeddu, C., Ravasco, P., et al. (2012) Cancer cachexia in adult patients: ESMO clinical practice guidelines☆. ESMO Open. 6(3):100092. doi:10.1016/j.esmoop.2021.100092
Argilés, J. M., López-Soriano, F. J., and Busquets, S. (2019). Mediators of cachexia in cancer patients. Nutrition 66, 11–15. doi:10.1016/j.nut.2019.03.012
Arseneault, L., Cannon, M., Poulton, R., Murray, R., Caspi, A., and Moffitt, T. E. (2002). Cannabis use in adolescence and risk for adult psychosis: Longitudinal prospective study. BMJ 325 (7374), 1212–1213. doi:10.1136/bmj.325.7374.1212
Aviello, G., Romano, B., Borrelli, F., Capasso, R., Gallo, L., Piscitelli, F., et al. (2012). Chemopreventive effect of the non-psychotropic phytocannabinoid cannabidiol on experimental colon cancer. J. Mol. Med. 90 (8), 925–934. doi:10.1007/s00109-011-0856-x
Aviram, J., Lewitus, G. M., Vysotski, Y., Amna, M. A., Ouryvaev, A., Procaccia, S., et al. (2022). The effectiveness and safety of medical cannabis for treating cancer related symptoms in oncology patients. Front. Pain Res. (Lausanne) 3, 861037. doi:10.3389/fpain.2022.861037
Badowski, M. E., and Yanful, P. K. (2018). Dronabinol oral solution in the management of anorexia and weight loss in AIDS and cancer. Ther. Clin. Risk Manag. 14, 643–651. doi:10.2147/TCRM.S126849
Bajtel, Á., Kiss, T., Tóth, B., Kiss, S., Hegyi, P., Vörhendi, N., et al. (2022). The safety of dronabinol and nabilone: A systematic review and meta-analysis of clinical trials. Pharm. (Basel) 15 (1), 100. doi:10.3390/ph15010100
Balachandran, P., Elsohly, M., and Hill, K. P. (2021). Cannabidiol interactions with medications, illicit substances, and alcohol: A comprehensive review. J. Gen. Intern Med. 36 (7), 2074–2084. doi:10.1007/s11606-020-06504-8
Bambico, F. R., Katz, N., Debonnel, G., and Gobbi, G. (2007). Cannabinoids elicit antidepressant-like behavior and activate serotonergic neurons through the medial prefrontal cortex. J. Neurosci. 27 (43), 11700–11711. doi:10.1523/JNEUROSCI.1636-07.2007
Bar-Sela, G., Cohen, I., Campisi-Pinto, S., Lewitus, G. M., Oz-Ari, L., Jehassi, A., et al. (2020). Cannabis consumption used by cancer patients during immunotherapy correlates with poor clinical outcome. Cancers (Basel) 12 (9), 2447. doi:10.3390/cancers12092447
Baram, L., Peled, E., Berman, P., Yellin, B., Besser, E., Benami, M., et al. (2019). The heterogeneity and complexity of Cannabis extracts as antitumor agents. Oncotarget 10 (41), 4091–4106. doi:10.18632/oncotarget.26983
Barbado, M. V., Medrano, M., Caballero-Velázquez, T., Álvarez-Laderas, I., Sánchez-Abarca, L. I., García-Guerrero, E., et al. (2017). Cannabinoid derivatives exert a potent anti-myeloma activity both in vitro and in vivo. Int. J. Cancer 140 (3), 674–685. doi:10.1002/ijc.30483
Barsky, S. H., Roth, M. D., Kleerup, E. C., Simmons, M., and Tashkin, D. P. (1998). Histopathologic and molecular alterations in bronchial epithelium in habitual smokers of marijuana, cocaine, and/or tobacco. J. Natl. Cancer Inst. 90 (16), 1198–1205. doi:10.1093/jnci/90.16.1198
Bayer, I. S., and Ghodse, H. (1999). Evolution of international drug control, 1945-1995. New York: United Nation.
Behl, T., Makkar, R., Sehgal, A., Singh, S., Makeen, H. A., Albratty, M., et al. (2022). Exploration of multiverse activities of endocannabinoids in biological systems. Int. J. Mol. Sci. 23 (10), 5734. doi:10.3390/ijms23105734
Berrendero, F., and Maldonado, R. (2002). Involvement of the opioid system in the anxiolytic-like effects induced by Delta(9)-tetrahydrocannabinol. Psychopharmacol. Berl. 163 (1), 111–117. doi:10.1007/s00213-002-1144-9
Bilbao, A., and Spanagel, R. (2022). Medical cannabinoids: A pharmacology-based systematic review and meta-analysis for all relevant medical indications. BMC Med. 20 (1), 259. doi:10.1186/s12916-022-02459-1
Blake, A., Wan, B. A., Malek, L., DeAngelis, C., Diaz, P., Lao, N., et al. (2017). A selective review of medical cannabis in cancer pain management. Ann. Palliat. Med. 6 (2), S215–S222. doi:10.21037/apm.2017.08.05
Blal, K., Besser, E., Procaccia, S., Schwob, O., Lerenthal, Y., Abu Tair, J., et al. (2023). The effect of cannabis plant extracts on head and neck squamous cell carcinoma and the quest for cannabis-based personalized therapy. Cancers (Basel) 15 (2), 497. doi:10.3390/cancers15020497
Blasco-Benito, S., Moreno, E., Seijo-Vila, M., Tundidor, I., Andradas, C., Caffarel, M. M., et al. (2019). Therapeutic targeting of HER2-CB2R heteromers in HER2-positive breast cancer. Proc. Natl. Acad. Sci. U. S. A. 116 (9), 3863–3872. doi:10.1073/pnas.1815034116
Blasco-Benito, S., Seijo-Vila, M., Caro-Villalobos, M., Tundidor, I., Andradas, C., García-Taboada, E., et al. (2018). Appraising the “entourage effect”: Antitumor action of a pure cannabinoid versus a botanical drug preparation in preclinical models of breast cancer. Biochem. Pharmacol. 157, 285–293. doi:10.1016/j.bcp.2018.06.025
Blázquez, C., González-Feria, L., Alvarez, L., Haro, A., Casanova, M. L., and Guzmán, M. (2004). Cannabinoids inhibit the vascular endothelial growth factor pathway in gliomas. Cancer Res. 64 (16), 5617–5623. doi:10.1158/0008-5472.CAN-03-3927
Bolla, K. I., Brown, K., Eldreth, D., Tate, K., and Cadet, J. L. (2002). Dose-related neurocognitive effects of marijuana use. Neurology 59 (9), 1337–1343. doi:10.1212/01.wnl.0000031422.66442.49
Borgelt, L. M., Franson, K. L., Nussbaum, A. M., and Wang, G. S. (2013). The pharmacologic and clinical effects of medical cannabis. Pharmacotherapy 33 (2), 195–209. doi:10.1002/phar.1187
Boyacıoğlu, Ö., Bilgiç, E., Varan, C., Bilensoy, E., Nemutlu, E., Sevim, D., et al. (2021). ACPA decreases non-small cell lung cancer line growth through Akt/PI3K and JNK pathways in vitro. Cell Death Dis. 12 (1), 56. doi:10.1038/s41419-020-03274-3
Boyd, C. J., McCabe, S. E., Evans-Polce, R. J., and Veliz, P. T. (2021). Cannabis, vaping, and respiratory symptoms in a probability sample of U. S. youth. J. Adolesc. Health 69 (1), 149–152. doi:10.1016/j.jadohealth.2021.01.019
Braun, I. M., Wright, A., Peteet, J., Meyer, F. L., Yuppa, D. P., Bolcic-Jankovic, D., et al. (2018). Medical oncologists’ beliefs, practices, and knowledge regarding marijuana used therapeutically: A nationally representative survey study. J. Clin. Oncol. 36 (19), 1957–1962. doi:10.1200/JCO.2017.76.1221
Brierley, D. I., Harman, J. R., Giallourou, N., Leishman, E., Roashan, A. E., Mellows, B. A. D., et al. (2019). Chemotherapy-induced cachexia dysregulates hypothalamic and systemic lipoamines and is attenuated by cannabigerol. J. Cachexia Sarcopenia Muscle 10 (4), 844–859. doi:10.1002/jcsm.12426
Brown, I., Cascio, M. G., Rotondo, D., Pertwee, R. G., Heys, S. D., and Wahle, K. W. J. (2013). Cannabinoids and omega-3/6 endocannabinoids as cell death and anticancer modulators. Prog. Lipid Res. 52 (1), 80–109. doi:10.1016/j.plipres.2012.10.001
Brown, M. R. D., and Farquhar-Smith, W. P. (2018). Cannabinoids and cancer pain: A new hope or a false dawn? Eur. J. Intern Med. 49, 30–36. doi:10.1016/j.ejim.2018.01.020
Broyd, S. J., van Hell, H. H., Beale, C., Yücel, M., and Solowij, N. (2016). Acute and chronic effects of cannabinoids on human cognition-A systematic review. Biol. Psychiatry 79 (7), 557–567. doi:10.1016/j.biopsych.2015.12.002
Brunetti, P., Pichini, S., Pacifici, R., Busardò, F. P., and Del Rio, A. (2020). Herbal preparations of medical cannabis: A vademecum for prescribing doctors. Med. Kaunas. 56 (5), 237. doi:10.3390/medicina56050237
Bruni, N., Della Pepa, C., Oliaro-Bosso, S., Pessione, E., Gastaldi, D., and Dosio, F. (2018). Cannabinoid delivery systems for pain and inflammation treatment. Molecules 23 (10), 2478. doi:10.3390/molecules23102478
Bukke, V. N., Archana, M., Villani, R., Serviddio, G., and Cassano, T. (2021). Pharmacological and toxicological effects of phytocannabinoids and recreational synthetic cannabinoids: Increasing risk of public health. Pharm. (Basel) 14 (10), 965. doi:10.3390/ph14100965
Burford, N. T., Traynor, J. R., and Alt, A. (2015). Positive allosteric modulators of the μ-opioid receptor: A novel approach for future pain medications. Br. J. Pharmacol. 172 (2), 277–286. doi:10.1111/bph.12599
Busse, J. W., Vankrunkelsven, P., Zeng, L., Heen, A. F., Merglen, A., Campbell, F., et al. (2021). Medical cannabis or cannabinoids for chronic pain: A clinical practice guideline. BMJ 374, n2040. doi:10.1136/bmj.n2040
Caffarel, M. M., Andradas, C., Mira, E., Pérez-Gómez, E., Cerutti, C., Moreno-Bueno, G., et al. (2010). Cannabinoids reduce ErbB2-driven breast cancer progression through Akt inhibition. Mol. Cancer 9, 196. doi:10.1186/1476-4598-9-196
Caffarel, M. M., Sarrió, D., Palacios, J., Guzmán, M., and Sánchez, C. (2006). Delta9-tetrahydrocannabinol inhibits cell cycle progression in human breast cancer cells through Cdc2 regulation. Cancer Res. 66 (13), 6615–6621. doi:10.1158/0008-5472.CAN-05-4566
Cannabis-In-Cachexia-Study-Group, , , Strasser, F., Luftner, D., Possinger, K., Ernst, G., Ruhstaller, T., et al. (2006). Comparison of orally administered cannabis extract and delta-9-tetrahydrocannabinol in treating patients with cancer-related anorexia-cachexia syndrome: A multicenter, phase III, randomized, double-blind, placebo-controlled clinical trial from the cannabis-in-cachexia-study-group. J. Clin. Oncol. 24 (21), 3394–3400. doi:10.1200/JCO.2005.05.1847
Carracedo, A., Gironella, M., Lorente, M., Garcia, S., Guzmán, M., Velasco, G., et al. (2006b). Cannabinoids induce apoptosis of pancreatic tumor cells via endoplasmic reticulum stress-related genes. Cancer Res. 66 (13), 6748–6755. doi:10.1158/0008-5472.CAN-06-0169
Carracedo, A., Lorente, M., Egia, A., Blázquez, C., García, S., Giroux, V., et al. (2006a). The stress-regulated protein p8 mediates cannabinoid-induced apoptosis of tumor cells. Cancer Cell 9 (4), 301–312. doi:10.1016/j.ccr.2006.03.005
Casey, S. L., and Vaughan, C. W. (2018). Plant-based cannabinoids for the treatment of chronic neuropathic pain. Med. (Basel) 5 (3), 67. doi:10.3390/medicines5030067
Cerino, P., Buonerba, C., Cannazza, G., D’Auria, J., Ottoni, E., Fulgione, A., et al. (2021). A review of hemp as food and nutritional supplement. Cannabis Cannabinoid Res. 6 (1), 19–27. doi:10.1089/can.2020.0001
Cherkasova, V., Wang, B., Gerasymchuk, M., Fiselier, A., Kovalchuk, O., and Kovalchuk, I. (2022). Use of cannabis and cannabinoids for treatment of cancer. Cancers (Basel) 14 (20), 5142. doi:10.3390/cancers14205142
Cichewicz, D. L., Welch, S. P., and Smith, F. L. (2005). Enhancement of transdermal fentanyl and buprenorphine antinociception by transdermal delta9-tetrahydrocannabinol. Eur. J. Pharmacol. 525 (1–3), 74–82. doi:10.1016/j.ejphar.2005.09.039
Coke, C. J., Scarlett, K. A., Chetram, M. A., Jones, K. J., Sandifer, B. J., Davis, A. S., et al. (2016). Simultaneous activation of induced heterodimerization between CXCR4 chemokine receptor and cannabinoid receptor 2 (CB2) reveals a mechanism for regulation of tumor progression. J. Biol. Chem. 291 (19), 9991–10005. doi:10.1074/jbc.M115.712661
Colby, E. D., McCarthy, L. E., and Borison, H. L. (1981). Emetic action of xylazine on the chemoreceptor trigger zone for vomiting in cats. J. Vet. Pharmacol. Ther. 4 (2), 93–96. doi:10.1111/j.1365-2885.1981.tb00716.x
Compton, W. M., Han, B., Jones, C. M., Blanco, C., and Hughes, A. (2016). Marijuana use and use disorders in adults in the USA, 2002-14: Analysis of annual cross-sectional surveys. Lancet Psychiatry 3 (10), 954–964. doi:10.1016/S2215-0366(16)30208-5
Connor, J. P., Stjepanović, D., Le Foll, B., Hoch, E., Budney, A. J., and Hall, W. D. (2021). Cannabis use and cannabis use disorder. Nat. Rev. Dis. Prim. 7 (1), 16–17. doi:10.1038/s41572-021-00247-4
Cousijn, J., Wiers, R. W., Ridderinkhof, K. R., van den Brink, W., Veltman, D. J., and Goudriaan, A. E. (2012). Grey matter alterations associated with cannabis use: Results of a VBM study in heavy cannabis users and healthy controls. Neuroimage 59 (4), 3845–3851. doi:10.1016/j.neuroimage.2011.09.046
Cox, M. L., Haller, V. L., and Welch, S. P. (2007). Synergy between delta9-tetrahydrocannabinol and morphine in the arthritic rat. Eur. J. Pharmacol. 567 (1–2), 125–130. doi:10.1016/j.ejphar.2007.04.010
Crocq, M-A. (2020). History of cannabis and the endocannabinoid system. Dialogues Clin. Neurosci. 22 (3), 223–228. doi:10.31887/DCNS.2020.22.3/mcrocq
Dando, I., Donadelli, M., Costanzo, C., Dalla Pozza, E., D’Alessandro, A., Zolla, L., et al. (2013). Cannabinoids inhibit energetic metabolism and induce AMPK-dependent autophagy in pancreatic cancer cells. Cell Death Dis. 4 (6), e664. doi:10.1038/cddis.2013.151
D’Andre, S., McAllister, S., Nagi, J., Giridhar, K. V., Ruiz-Macias, E., and Loprinzi, C. (2021). Topical cannabinoids for treating chemotherapy-induced neuropathy: A case series. Integr. Cancer Ther. 20, 15347354211061739. doi:10.1177/15347354211061739
Danziger, N., and Bernstein, N. (2021). Shape matters: Plant architecture affects chemical uniformity in large-size medical cannabis plants. Plants 10 (9), 1834. doi:10.3390/plants10091834
De Feo, G., Case, A. A., Crawford, G. B., Hui, D., To, J., Sbrana, A., et al. (2023). Multinational association of supportive care in cancer (MASCC) guidelines: Cannabis for psychological symptoms including insomnia, anxiety, and depression. Support Care Cancer 31 (3), 176. doi:10.1007/s00520-023-07628-3
de Mello Schier, A. R., de Oliveira Ribeiro, N. P., Coutinho, D. S., Machado, S., Arias-Carrión, O., Crippa, J. A., et al. (2014). Antidepressant-like and anxiolytic-like effects of cannabidiol: A chemical compound of cannabis sativa. CNS Neurol. Disord. Drug Targets 13 (6), 953–960. doi:10.2174/1871527313666140612114838
De Petrocellis, L., Ligresti, A., Schiano Moriello, A., Iappelli, M., Verde, R., Stott, C. G., et al. (2013). Non-THC cannabinoids inhibit prostate carcinoma growth in vitro and in vivo: Pro-apoptotic effects and underlying mechanisms. Br. J. Pharmacol. 168 (1), 79–102. doi:10.1111/j.1476-5381.2012.02027.x
de Sousa Cavalcante, M. L., Silva, M. S., Cavalcante, A. K. M., de Oliveira Santos, R., Nunes, D. D. T., Busquets, S., et al. (2020). Win 55,212-2, atenolol and subdiaphragmatic vagotomy prevent acceleration of gastric emptying induced by cachexia via Yoshida-AH-130 cells in rats. Eur. J. Pharmacol. 877, 173087. doi:10.1016/j.ejphar.2020.173087
de Souza, M. R., Henriques, A. T., and Limberger, R. P. (2022). Medical cannabis regulation: An overview of models around the world with emphasis on the Brazilian scenario. J. Cannabis Res. 4 (1), 33. doi:10.1186/s42238-022-00142-z
Deng, L., Guindon, J., Cornett, B. L., Makriyannis, A., Mackie, K., and Hohmann, A. G. (2015). Chronic cannabinoid receptor 2 activation reverses paclitaxel neuropathy without tolerance or cannabinoid receptor 1-dependent withdrawal. Biol. Psychiatry 77 (5), 475–487. doi:10.1016/j.biopsych.2014.04.009
Di Forti, M., Marconi, A., Carra, E., Fraietta, S., Trotta, A., Bonomo, M., et al. (2015). Proportion of patients in south london with first-episode psychosis attributable to use of high potency cannabis: A case-control study. Lancet Psychiatry 2 (3), 233–238. doi:10.1016/S2215-0366(14)00117-5
Domenichini, A., Edmands, J. S., Adamska, A., Begicevic, R-R., Paternoster, S., and Falasca, M. (2019). Pancreatic cancer tumorspheres are cancer stem-like cells with increased chemoresistance and reduced metabolic potential. Adv. Biol. Regul. 72, 63–77. doi:10.1016/j.jbior.2019.02.001
Eisenstein, T. K., and Meissler, J. J. (2015). Effects of cannabinoids on T-cell function and resistance to infection. J. Neuroimmune Pharmacol. 10 (2), 204–216. doi:10.1007/s11481-015-9603-3
Elbaz, M., Nasser, M. W., Ravi, J., Wani, N. A., Ahirwar, D. K., Zhao, H., et al. (2015). Modulation of the tumor microenvironment and inhibition of EGF/EGFR pathway: Novel anti-tumor mechanisms of cannabidiol in breast cancer. Mol. Oncol. 9 (4), 906–919. doi:10.1016/j.molonc.2014.12.010
Ellert-Miklaszewska, A., Ciechomska, I. A., and Kaminska, B. (2021). Synthetic cannabinoids induce autophagy and mitochondrial apoptotic pathways in human glioblastoma cells independently of deficiency in TP53 or PTEN tumor suppressors. Cancers (Basel) 13 (3), 419. doi:10.3390/cancers13030419
Emery, J., Butow, P., Lai-Kwon, J., Nekhlyudov, L., Rynderman, M., and Jefford, M. (2022). Management of common clinical problems experienced by survivors of cancer. Lancet 399 (10334), 1537–1550. doi:10.1016/S0140-6736(22)00242-2
Fallon, M., Giusti, R., Aielli, F., Hoskin, P., Rolke, R., Sharma, M., et al. (2018). Management of cancer pain in adult patients: ESMO clinical practice guidelines. Ann. Oncol. 29 (4), iv166–91. doi:10.1093/annonc/mdy152
Fann, J. R., Thomas-Rich, A. M., Katon, W. J., Cowley, D., Pepping, M., McGregor, B. A., et al. (2008). Major depression after breast cancer: A review of epidemiology and treatment. Gen. Hosp. Psychiatry 30 (2), 112–126. doi:10.1016/j.genhosppsych.2007.10.008
Feigenbaum, J. J., Richmond, S. A., Weissman, Y., and Mechoulam, R. (1989). Inhibition of cisplatin-induced emesis in the pigeon by a non-psychotropic synthetic cannabinoid. Eur. J. Pharmacol. 169 (1), 159–165. doi:10.1016/0014-2999(89)90828-5
Feingold, D., and Weinstein, A. (2021). Cannabis and depression. Adv. Exp. Med. Biol. 1264, 67–80. doi:10.1007/978-3-030-57369-0_5
Feinstein, A., Meza, C., Stefan, C., and Staines, W. R. (2021). Discontinuing cannabis improves depression in people with multiple sclerosis: A short report. Mult. Scler. 27 (4), 636–639. doi:10.1177/1352458520934070
Ferrari, F., Ottani, A., and Giuliani, D. (1999). Cannabimimetic activity in rats and pigeons of HU 210, a potent antiemetic drug. Pharmacol. Biochem. Behav. 62 (1), 75–80. doi:10.1016/s0091-3057(98)00114-2
Filipiuc, L. E., Ababei, D. C., Alexa-Stratulat, T., Pricope, C. V., Bild, V., Stefanescu, R., et al. (2021). Major phytocannabinoids and their related compounds: Should we only search for drugs that act on cannabinoid receptors? Pharmaceutics 13 (11), 1823. doi:10.3390/pharmaceutics13111823
Finn, D. P., Haroutounian, S., Hohmann, A. G., Krane, E., Soliman, N., and Rice, A. S. C. (2021). Cannabinoids, the endocannabinoid system, and pain: A review of preclinical studies. Pain 162 (1), S5–S25. doi:10.1097/j.pain.0000000000002268
Fisher, T., Golan, H., Schiby, G., PriChen, S., Smoum, R., Moshe, I., et al. (2016). In vitro and in vivo efficacy of non-psychoactive cannabidiol in neuroblastoma. Curr. Oncol. 23 (2), S15–S22. doi:10.3747/co.23.2893
Fonseca, B. M., Correia-da-Silva, G., and Teixeira, N. A. (2018). Cannabinoid-induced cell death in endometrial cancer cells: Involvement of TRPV1 receptors in apoptosis. J. Physiol. Biochem. 74 (2), 261–272. doi:10.1007/s13105-018-0611-7
Forrester, M. B., Kleinschmidt, K., Schwarz, E., and Young, A. (2012). Synthetic cannabinoid and marijuana exposures reported to poison centers. Hum. Exp. Toxicol. 31 (10), 1006–1011. doi:10.1177/0960327111421945
Freeman, D., Dunn, G., Murray, R. M., Evans, N., Lister, R., Antley, A., et al. (2015). How cannabis causes paranoia: Using the intravenous administration of ∆9-tetrahydrocannabinol (THC) to identify key cognitive mechanisms leading to paranoia. Schizophr. Bull. 41 (2), 391–399. doi:10.1093/schbul/sbu098
Frost, L., Mostofsky, E., Rosenbloom, J. I., Mukamal, K. J., and Mittleman, M. A. (2013). Marijuana use and long-term mortality among survivors of acute myocardial infarction. Am. Heart J. 165 (2), 170–175. doi:10.1016/j.ahj.2012.11.007
Gado, F., Meini, S., Bertini, S., Digiacomo, M., Macchia, M., and Manera, C. (2019). Allosteric modulators targeting cannabinoid cb1 and cb2 receptors: Implications for drug discovery. Future Med. Chem. 11 (15), 2019–2037. doi:10.4155/fmc-2019-0005
Galve-Roperh, I., Sánchez, C., Cortés, M. L., Gómez del Pulgar, T., Izquierdo, M., and Guzmán, M. (2000). Anti-tumoral action of cannabinoids: Involvement of sustained ceramide accumulation and extracellular signal-regulated kinase activation. Nat. Med. 6 (3), 313–319. doi:10.1038/73171
Ganesh, S., and D’Souza, D. C. (2022). Cannabis and psychosis: Recent epidemiological findings continuing the “causality debate”. Am. J. Psychiatry 179 (1), 8–10. doi:10.1176/appi.ajp.2021.21111126
Gaoni, Y., and Mechoulam, R. (1964). Isolation, structure, and partial synthesis of an active constituent of hashish. J. Am. Chem. Soc. 86 (8), 1646–1647. doi:10.1021/ja01062a046
García-Morales, L., Castillo, A. M., Tapia Ramírez, J., Zamudio-Meza, H., Domínguez-Robles, M. D. C., and Meza, I. (2020). CBD reverts the mesenchymal invasive phenotype of breast cancer cells induced by the inflammatory cytokine IL-1β. Int. J. Mol. Sci. 21 (7), 2429. doi:10.3390/ijms21072429
Ghasemiesfe, M., Barrow, B., Leonard, S., Keyhani, S., and Korenstein, D. (2019). Association between marijuana use and risk of cancer: A systematic review and meta-analysis. JAMA Netw. Open 2 (11), e1916318. doi:10.1001/jamanetworkopen.2019.16318
Gibbs, M., Winsper, C., Marwaha, S., Gilbert, E., Broome, M., and Singh, S. P. (2015). Cannabis use and mania symptoms: A systematic review and meta-analysis. J. Affect Disord. 171, 39–47. doi:10.1016/j.jad.2014.09.016
Goncharov, I., Weiner, L., and Vogel, Z. (2005). Delta9-tetrahydrocannabinol increases C6 glioma cell death produced by oxidative stress. Neuroscience 134 (2), 567–574. doi:10.1016/j.neuroscience.2005.04.042
Götze, H., Friedrich, M., Taubenheim, S., Dietz, A., Lordick, F., and Mehnert, A. (2020). Depression and anxiety in long-term survivors 5 and 10 years after cancer diagnosis. Support Care Cancer 28 (1), 211–220. doi:10.1007/s00520-019-04805-1
Gralla, R. J. (1999). “Cannabinoids and the control of chemotherapy-induced nausea and vomiting,” in Marihuana and medicine. Editors G. G. Nahas, K. M. Sutin, D. Harvey, S. Agurell, N. Pace, and R. Cancro (Totowa, NJ: Humana Press), 599–610.
Grassi, L., Berardi, M. A., Ruffilli, F., Meggiolaro, E., Andritsch, E., Sirgo, A., et al. (2015). Role of psychosocial variables on chemotherapy-induced nausea and vomiting and health-related quality of life among cancer patients: A European study. Psychother. Psychosom. 84 (6), 339–347. doi:10.1159/000431256
Gress, K. L., Charipova, K., Kaye, A. D., Viswanath, O., and Urits, I. (2020). An overview of current recommendations and options for the management of cancer pain: A comprehensive review. Oncol. Ther. 8 (2), 251–259. doi:10.1007/s40487-020-00128-y
Grimison, P., Mersiades, A., Kirby, A., Lintzeris, N., Morton, R., Haber, P., et al. (2020). Oral THC:CBD cannabis extract for refractory chemotherapy-induced nausea and vomiting: A randomised, placebo-controlled, phase II crossover trial. Ann. Oncol. 31 (11), 1553–1560. doi:10.1016/j.annonc.2020.07.020
Grotenhermen, F., and Müller-Vahl, K. (2012). The therapeutic potential of cannabis and cannabinoids. Dtsch. Arztebl Int. 109 (29–30), 495–501. doi:10.3238/arztebl.2012.0495
Grotenhermen, F. (2007). The toxicology of cannabis and cannabis prohibition. Chem. Biodivers. 4 (8), 1744–1769. doi:10.1002/cbdv.200790151
Guggisberg, J., Schumacher, M., Gilmore, G., and Zylla, D. M. (2022). Cannabis as an anticancer agent: A review of clinical data and assessment of case reports. Cannabis Cannabinoid Res. 7 (1), 24–33. doi:10.1089/can.2021.0045
Gupta, K., Walton, R., and Kataria, S. P. (2021). Chemotherapy-induced nausea and vomiting: Pathogenesis, recommendations, and new trends. Cancer Treat. Res. Commun. 26, 100278. doi:10.1016/j.ctarc.2020.100278
Guzmán, M., Duarte, M. J., Blázquez, C., Ravina, J., Rosa, M. C., Galve-Roperh, I., et al. (2006). A pilot clinical study of Delta9-tetrahydrocannabinol in patients with recurrent glioblastoma multiforme. Br. J. Cancer 95 (2), 197–203. doi:10.1038/sj.bjc.6603236
Hancox, R. J., Poulton, R., Ely, M., Welch, D., Taylor, D. R., McLachlan, C. R., et al. (2010). Effects of cannabis on lung function: A population-based cohort study. Eur. Respir. J. 35 (1), 42–47. doi:10.1183/09031936.00065009
Hardy, J., Greer, R., Huggett, G., Kearney, A., Gurgenci, T., and Good, P. (2023). Phase IIb randomized, placebo-controlled, dose-escalating, double-blind study of cannabidiol oil for the relief of symptoms in advanced cancer (MedCan1-CBD). J. Clin. Oncol. 41 (7), 1444–1452. doi:10.1200/JCO.22.01632
Haroun, R., Wood, J. N., and Sikandar, S. (2022). Mechanisms of cancer pain. Front. Pain Res. Lausanne 3, 1030899. doi:10.3389/fpain.2022.1030899
Hart, S., Fischer, O. M., and Ullrich, A. (2004). Cannabinoids induce cancer cell proliferation via tumor necrosis factor alpha-converting enzyme (TACE/ADAM17)-mediated transactivation of the epidermal growth factor receptor. Cancer Res. 64 (6), 1943–1950. doi:10.1158/0008-5472.can-03-3720
Häuser, W., Welsch, P., Klose, P., Radbruch, L., and Fitzcharles, M-A. (2019). Efficacy, tolerability and safety of cannabis-based medicines for cancer pain: A systematic review with meta-analysis of randomised controlled trials. Schmerz 33 (5), 424–436. doi:10.1007/s00482-019-0373-3
Haustein, M., Ramer, R., Linnebacher, M., Manda, K., and Hinz, B. (2014). Cannabinoids increase lung cancer cell lysis by lymphokine-activated killer cells via upregulation of ICAM-1. Biochem. Pharmacol. 92 (2), 312–325. doi:10.1016/j.bcp.2014.07.014
Hazekamp, A., Bastola, K., Rashidi, H., Bender, J., and Verpoorte, R. (2007). Cannabis tea revisited: A systematic evaluation of the cannabinoid composition of cannabis tea. J. Ethnopharmacol. 113 (1), 85–90. doi:10.1016/j.jep.2007.05.019
Hernán Pérez de la Ossa, D., Gil-Alegre, M. E., Ligresti, A., Aberturas, M. D. R., Molpeceres, J., Torres, A. I., et al. (2013b). Preparation and characterization of Δ(9)-tetrahydrocannabinol-loaded biodegradable polymeric microparticles and their antitumoral efficacy on cancer cell lines. J. Drug Target 21 (8), 710–718. doi:10.3109/1061186X.2013.809089
Hernán Pérez de la Ossa, D., Lorente, M., Gil-Alegre, M. E., Torres, S., García-Taboada, E., Aberturas, M. D. R., et al. (2013a). Local delivery of cannabinoid-loaded microparticles inhibits tumor growth in a murine xenograft model of glioblastoma multiforme. PLoS ONE 8 (1), e54795. doi:10.1371/journal.pone.0054795
Hernández-Tiedra, S., Fabriàs, G., Dávila, D., Salanueva, Í. J., Casas, J., Montes, L. R., et al. (2016). Dihydroceramide accumulation mediates cytotoxic autophagy of cancer cells via autolysosome destabilization. Autophagy 12 (11), 2213–2229. doi:10.1080/15548627.2016.1213927
Hinz, B., and Ramer, R. (2022). Cannabinoids as anticancer drugs: Current status of preclinical research. Br. J. Cancer 127 (1), 1–13. doi:10.1038/s41416-022-01727-4
Hirao-Suzuki, M., Takeda, S., Watanabe, K., Takiguchi, M., and Aramaki, H. (2019). Δ9-Tetrahydrocannabinol upregulates fatty acid 2-hydroxylase (FA2H) via PPARα induction: A possible evidence for the cancellation of pparβ/δ-mediated inhibition of PPARα in MDA-MB-231 cells. Arch. Biochem. Biophys. 662, 219–225. doi:10.1016/j.abb.2018.12.011
Hoffmann, D., Brunnemann, K. D., Gori, G. B., and Wynder, E. L. (1975). “On the carcinogenicity of marijuana smoke,” in Recent advances in phytochemistry. Editor V. C. Runeckles (Boston, MA: Springer US), 63–81.
Honarmand, M., Namazi, F., Mohammadi, A., and Nazifi, S. (2018). Can cannabidiol inhibit angiogenesis in colon cancer? Comp. Clin. Path 28 (1), 165–172. doi:10.1007/s00580-018-2810-6
Huang, S., Xiao, P., and Sun, J. (2020). Structural basis of signaling of cannabinoids receptors: Paving a way for rational drug design in controling mutiple neurological and immune diseases. Signal Transduct. Target Ther. 5 (1), 127. doi:10.1038/s41392-020-00240-5
Huang, T., Xu, T., Wang, Y., Zhou, Y., Yu, D., Wang, Z., et al. (2021). Cannabidiol inhibits human glioma by induction of lethal mitophagy through activating TRPV4. Autophagy 17 (11), 3592–3606. doi:10.1080/15548627.2021.1885203
Huestis, M. A., Solimini, R., Pichini, S., Pacifici, R., Carlier, J., and Busardò, F. P. (2019). Cannabidiol adverse effects and toxicity. Curr. Neuropharmacol. 17 (10), 974–989. doi:10.2174/1570159X17666190603171901
Ivanov, V. N., Grabham, P. W., Wu, C-C., and Hei, T. K. (2021). Author Correction: Inhibition of autophagic flux differently modulates cannabidiol-induced death in 2D and 3D glioblastoma cell cultures. Sci. Rep. 11 (1), 18616. doi:10.1038/s41598-021-98292-2
Jacobsson, S. O., Rongård, E., Stridh, M., Tiger, G., and Fowler, C. J. (2000). Serum-dependent effects of tamoxifen and cannabinoids upon C6 glioma cell viability. Biochem. Pharmacol. 60 (12), 1807–1813. doi:10.1016/s0006-2952(00)00492-5
Jatoi, A., Windschitl, H. E., Loprinzi, C. L., Sloan, J. A., Dakhil, S. R., Mailliard, J. A., et al. (2002). Dronabinol versus megestrol acetate versus combination therapy for cancer-associated anorexia: A North Central cancer treatment group study. J. Clin. Oncol. 20 (2), 567–573. doi:10.1200/JCO.2002.20.2.567
Jeong, S., Yun, H. K., Jeong, Y. A., Jo, M. J., Kang, S. H., Kim, J. L., et al. (2019). Cannabidiol-induced apoptosis is mediated by activation of Noxa in human colorectal cancer cells. Cancer Lett. 447, 12–23. doi:10.1016/j.canlet.2019.01.011
Jin, D., Dai, K., Xie, Z., and Chen, J. (2020). Secondary metabolites profiled in cannabis inflorescences, leaves, stem barks, and roots for medicinal purposes. Sci. Rep. 10 (1), 3309. doi:10.1038/s41598-020-60172-6
Johnson, E. C., Hatoum, A. S., Deak, J. D., Polimanti, R., Murray, R. M., Edenberg, H. J., et al. (2021). The relationship between cannabis and schizophrenia: A genetically informed perspective. Addiction 116 (11), 3227–3234. doi:10.1111/add.15534
Johnson, J. R., Burnell-Nugent, M., Lossignol, D., Ganae-Motan, E. D., Potts, R., and Fallon, M. T. (2010). Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. J. Pain Symptom Manage 39 (2), 167–179. doi:10.1016/j.jpainsymman.2009.06.008
Joshi, M., Joshi, A., and Bartter, T. (2022). Marijuana and the lung: Evolving understandings. Med. Clin. North Am. 106 (6), 1093–1107. doi:10.1016/j.mcna.2022.07.010
Jouanjus, E., Leymarie, F., Tubery, M., and Lapeyre-Mestre, M. (2011). Cannabis-related hospitalizations: Unexpected serious events identified through hospital databases. Br. J. Clin. Pharmacol. 71 (5), 758–765. doi:10.1111/j.1365-2125.2010.03897.x
Juarez, T. M., Piccioni, D., Rose, L., Nguyen, A., Brown, B., and Kesari, S. (2021). Phase I dose-escalation, safety, and CNS pharmacokinetic study of dexanabinol in patients with brain cancer. Neurooncol Adv. 3 (1), vdab006. doi:10.1093/noajnl/vdab006
Kachrani, R., Santana, A., Rogala, B., and Pawasauskas, J. (2020). Chemotherapy-induced peripheral neuropathy: Causative agents, preventative strategies, and treatment approaches. J. Pain Palliat. Care Pharmacother. 34 (3), 141–152. doi:10.1080/15360288.2020.1734144
Kasvis, P., Vigano, M., and Vigano, A. (2019). Health-related quality of life across cancer cachexia stages. Ann. Palliat. Med. 8 (1), 33–42. doi:10.21037/apm.2018.08.04
Katsidoni, V., Kastellakis, A., and Panagis, G. (2013). Biphasic effects of Δ9-tetrahydrocannabinol on brain stimulation reward and motor activity. Int. J. Neuropsychopharmacol. 16 (10), 2273–2284. doi:10.1017/S1461145713000709
Keating, G. M. (2017). Delta-9-Tetrahydrocannabinol/Cannabidiol oromucosal spray (Sativex®): A review in multiple sclerosis-related spasticity. Drugs 77 (5), 563–574. doi:10.1007/s40265-017-0720-6
Kehl, L. J., Hamamoto, D. T., Wacnik, P. W., Croft, D. L., Norsted, B. D., Wilcox, G. L., et al. (2003). A cannabinoid agonist differentially attenuates deep tissue hyperalgesia in animal models of cancer and inflammatory muscle pain. Pain 103 (1–2), 175–186. doi:10.1016/s0304-3959(02)00450-5
Khasabova, I. A., Gielissen, J., Chandiramani, A., Harding-Rose, C., Odeh, D. A., Simone, D. A., et al. (2011). CB1 and CB2 receptor agonists promote analgesia through synergy in a murine model of tumor pain. Behav. Pharmacol. 22 (5–6), 607–616. doi:10.1097/FBP.0b013e3283474a6d
Kirkham, T. C. (2009). Cannabinoids and appetite: Food craving and food pleasure. Int. Rev. Psychiatry 21 (2), 163–171. doi:10.1080/09540260902782810
Klumpers, L. E., and Thacker, D. L. (2019). A brief background on cannabis: From plant to medical indications. J. AOAC Int. 102 (2), 412–420. doi:10.5740/jaoacint.18-0208
Kocjan Ačko, D., Flajšman, M., and Trdan, S. (2019). Apical bud removal increased seed yield in hemp (Cannabis sativa L). Sect. B — Soil and Plant Sci. 69 (4), 317–323. doi:10.1080/09064710.2019.1568540
Kolbe, M. R., Hohmann, T., Hohmann, U., Ghadban, C., Mackie, K., Zöller, C., et al. (2021). THC reduces ki67-immunoreactive cells derived from human primary glioblastoma in a GPR55-dependent manner. Cancers (Basel) 13 (5), 1064. doi:10.3390/cancers13051064
Kosgodage, U. S., Mould, R., Henley, A. B., Nunn, A. V., Guy, G. W., Thomas, E. L., et al. (2018). Cannabidiol (CBD) is a novel inhibitor for exosome and microvesicle (EMV) release in cancer. Front. Pharmacol. 9, 889. doi:10.3389/fphar.2018.00889
Kuhathasan, N., Dufort, A., MacKillop, J., Gottschalk, R., Minuzzi, L., and Frey, B. N. (2019). The use of cannabinoids for sleep: A critical review on clinical trials. Exp. Clin. Psychopharmacol. 27 (4), 383–401. doi:10.1037/pha0000285
Kwiatkowska, M., Parker, L. A., Burton, P., and Mechoulam, R. (2004). A comparative analysis of the potential of cannabinoids and ondansetron to suppress cisplatin-induced emesis in the Suncus murinus (house musk shrew). Psychopharmacol. Berl. 174 (2), 254–259. doi:10.1007/s00213-003-1739-9
Le Boisselier, R., Alexandre, J., Lelong-Boulouard, V., and Debruyne, D. (2017). Focus on cannabinoids and synthetic cannabinoids. Clin. Pharmacol. Ther. 101 (2), 220–229. doi:10.1002/cpt.563
Leelawat, S., Leelawat, K., Narong, S., and Matangkasombut, O. (2010). The dual effects of delta(9)-tetrahydrocannabinol on cholangiocarcinoma cells: Anti-invasion activity at low concentration and apoptosis induction at high concentration. Cancer Invest. 28 (4), 357–363. doi:10.3109/07357900903405934
Lei, X., Chen, X., Quan, Y., Tao, Y., and Li, J. (2020). Targeting CYP2J2 to enhance the anti-glioma efficacy of cannabinoid receptor 2 stimulation by inhibiting the pro-angiogenesis function of M2 microglia. Front. Oncol. 10, 574277. doi:10.3389/fonc.2020.574277
Lewis, M. M., Yang, Y., Wasilewski, E., Clarke, H. A., and Kotra, L. P. (2017). Chemical profiling of medical cannabis extracts. ACS Omega 2 (9), 6091–6103. doi:10.1021/acsomega.7b00996
Lichtman, A. H., Lux, E. A., McQuade, R., Rossetti, S., Sanchez, R., Sun, W., et al. (2018). Results of a double-blind, randomized, placebo-controlled study of nabiximols oromucosal spray as an adjunctive therapy in advanced cancer patients with chronic uncontrolled pain. J. Pain Symptom Manage 55 (2), 179–188.e1. doi:10.1016/j.jpainsymman.2017.09.001
Ligresti, A., Moriello, A. S., Starowicz, K., Matias, I., Pisanti, S., De Petrocellis, L., et al. (2006). Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. J. Pharmacol. Exp. Ther. 318 (3), 1375–1387. doi:10.1124/jpet.106.105247
Lim, S., Brown, J. L., Washington, T. A., and Greene, N. P. (2020). Development and progression of cancer cachexia: Perspectives from bench to bedside. Sports Med. Health Sci. 2 (4), 177–185. doi:10.1016/j.smhs.2020.10.003
Liu, Y., Mikrani, R., He, Y., Faran Ashraf Baig, M. M., Abbas, M., Naveed, M., et al. (2020). TRPM8 channels: A review of distribution and clinical role. Eur. J. Pharmacol. 882, 173312, 173312. doi:10.1016/j.ejphar.2020.173312
López-Valero, I., Saiz-Ladera, C., Torres, S., Hernández-Tiedra, S., García-Taboada, E., Rodríguez-Fornés, F., et al. (2018). Targeting Glioma Initiating Cells with A combined therapy of cannabinoids and temozolomide. Biochem. Pharmacol. 157, 266–274. doi:10.1016/j.bcp.2018.09.007
Loprinzi, C. L., Lacchetti, C., Bleeker, J., Cavaletti, G., Chauhan, C., Hertz, D. L., et al. (2020). Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: ASCO guideline update. J. Clin. Oncol. 38 (28), 3325–3348. doi:10.1200/JCO.20.01399
Lord, S., Hardy, J., and Good, P. (2022). Does cannabidiol have a benefit as a supportive care drug in cancer? Curr. Treat. Options Oncol. 23 (4), 514–525. doi:10.1007/s11864-021-00934-0
Lorenzetti, V., Solowij, N., and Yücel, M. (2016). The role of cannabinoids in neuroanatomic alterations in cannabis users. Biol. Psychiatry 79 (7), e17–e31. doi:10.1016/j.biopsych.2015.11.013
Lorusso, D., Bria, E., Costantini, A., Di Maio, M., Rosti, G., and Mancuso, A. (2017). Patients’ perception of chemotherapy side effects: Expectations, doctor-patient communication and impact on quality of life - an Italian survey. Eur. J. Cancer Care (Engl). 26 (2), e12618. doi:10.1111/ecc.12618
Lukhele, S. T., and Motadi, L. R. (2016). Cannabidiol rather than Cannabis sativa extracts inhibit cell growth and induce apoptosis in cervical cancer cells. BMC Complement. Altern. Med. 16 (1), 335. doi:10.1186/s12906-016-1280-0
Lynch, M. E., and Campbell, F. (2011). Cannabinoids for treatment of chronic non-cancer pain; a systematic review of randomized trials. Br. J. Clin. Pharmacol. 72 (5), 735–744. doi:10.1111/j.1365-2125.2011.03970.x
Maertens, R. M., White, P. A., Williams, A., and Yauk, C. L. (2013). A global toxicogenomic analysis investigating the mechanistic differences between tobacco and marijuana smoke condensates in vitro. Toxicology 308, 60–73. doi:10.1016/j.tox.2013.03.008
Mahabir, V. K., Merchant, J. J., Smith, C., and Garibaldi, A. (2020). Medical cannabis use in the United States: A retrospective database study. J. Cannabis Res. 2 (1), 32. doi:10.1186/s42238-020-00038-w
Maida, V., and Daeninck, P. J. (2016). A user’s guide to cannabinoid therapies in oncology. Curr. Oncol. 23 (6), 398–406. doi:10.3747/co.23.3487
Maldonado, R., Cabañero, D., and Martín-García, E. (2020). The endocannabinoid system in modulating fear, anxiety, and stress. Dialogues Clin. Neurosci. 22 (3), 229–239. doi:10.31887/DCNS.2020.22.3/rmaldonado
Manera, C., and Bertini, S. (2021). Cannabinoid-based medicines and multiple sclerosis. Adv. Exp. Med. Biol. 1264, 111–129. doi:10.1007/978-3-030-57369-0_8
Mangal, N., Erridge, S., Habib, N., Sadanandam, A., Reebye, V., and Sodergren, M. H. (2021). Cannabinoids in the landscape of cancer. J. Cancer Res. Clin. Oncol. 147 (9), 2507–2534. doi:10.1007/s00432-021-03710-7
Marco, E. M., Pérez-Alvarez, L., Borcel, E., Rubio, M., Guaza, C., Ambrosio, E., et al. (2004). Involvement of 5-HT1A receptors in behavioural effects of the cannabinoid receptor agonist CP 55,940 in male rats. Behav. Pharmacol. 15 (1), 21–27. doi:10.1097/00008877-200402000-00003
Marcu, J. P., Christian, R. T., Lau, D., Zielinski, A. J., Horowitz, M. P., Lee, J., et al. (2010). Cannabidiol enhances the inhibitory effects of delta9-tetrahydrocannabinol on human glioblastoma cell proliferation and survival. Mol. Cancer Ther. 9 (1), 180–189. doi:10.1158/1535-7163.MCT-09-0407
Marin, S., Marco, E., Biscaia, M., Fernández, B., Rubio, M., Guaza, C., et al. (2003). Involvement of the κ-opioid receptor in the anxiogenic-like effect of CP 55,940 in male rats. Pharmacol. Biochem. Behav. 74 (3), 649–656. doi:10.1016/s0091-3057(02)01041-9
Mariotto, A. B., Enewold, L., Zhao, J., Zeruto, C. A., and Yabroff, K. R. (2020). Medical care costs associated with cancer survivorship in the United States. Cancer Epidemiol. Biomarkers Prev. 29 (7), 1304–1312. doi:10.1158/1055-9965.EPI-19-1534
Martin, E. L., Strickland, J. C., Schlienz, N. J., Munson, J., Jackson, H., Bonn-Miller, M. O., et al. (2021). Antidepressant and anxiolytic effects of medicinal cannabis use in an observational trial. Front. Psychiatry 12, 729800. doi:10.3389/fpsyt.2021.729800
Martín-Banderas, L., Muñoz-Rubio, I., Prados, J., Álvarez-Fuentes, J., Calderón-Montaño, J. M., López-Lázaro, M., et al. (2015). In vitro and in vivo evaluation of Δ⁹-tetrahidrocannabinol/PLGA nanoparticles for cancer chemotherapy. Int. J. Pharm. 487 (1–2), 205–212. doi:10.1016/j.ijpharm.2015.04.054
Masocha, W. (2018). Targeting the endocannabinoid system for prevention or treatment of chemotherapy-induced neuropathic pain: Studies in animal models. Pain Res. Manag. 2018, 1–9. doi:10.1155/2018/5234943
Massi, P., Vaccani, A., Ceruti, S., Colombo, A., Abbracchio, M. P., and Parolaro, D. (2004). Antitumor effects of cannabidiol, a nonpsychoactive cannabinoid, on human glioma cell lines. J. Pharmacol. Exp. Ther. 308 (3), 838–845. doi:10.1124/jpet.103.061002
May, M. B., and Glode, A. E. (2016). Dronabinol for chemotherapy-induced nausea and vomiting unresponsive to antiemetics. Cancer Manag. Res. 8, 49–55. doi:10.2147/CMAR.S81425
McAllister, S. D., Chan, C., Taft, R. J., Luu, T., Abood, M. E., Moore, D. H., et al. (2005). Cannabinoids selectively inhibit proliferation and induce death of cultured human glioblastoma multiforme cells. J. Neurooncol 74 (1), 31–40. doi:10.1007/s11060-004-5950-2
McAllister, S. D., Christian, R. T., Horowitz, M. P., Garcia, A., and Desprez, P-Y. (2007). Cannabidiol as a novel inhibitor of Id-1 gene expression in aggressive breast cancer cells. Mol. Cancer Ther. 6 (11), 2921–2927. doi:10.1158/1535-7163.MCT-07-0371
McGregor, I. S., Issakidis, C. N., and Prior, G. (1996). Aversive effects of the synthetic cannabinoid CP 55,940 in rats. Pharmacol. Biochem. Behav. 53 (3), 657–664. doi:10.1016/0091-3057(95)02066-7
McKallip, R. J., Nagarkatti, M., and Nagarkatti, P. S. (2005). Delta-9-tetrahydrocannabinol enhances breast cancer growth and metastasis by suppression of the antitumor immune response. J. Immunol. 174 (6), 3281–3289. doi:10.4049/jimmunol.174.6.3281
McMahon, G. A., Petitclerc, E., Stefansson, S., Smith, E., Wong, M. K., Westrick, R. J., et al. (2001). Plasminogen activator inhibitor-1 regulates tumor growth and angiogenesis. J. Biol. Chem. 276 (36), 33964–33968. doi:10.1074/jbc.M105980200
Mechoulam, R., Peters, M., Murillo-Rodriguez, E., and Hanus, L. O. (2007). Cannabidiol--recent advances. Chem. Biodivers. 4 (8), 1678–1692. doi:10.1002/cbdv.200790147
Mechoulam, R., and ShvoHashish, Y. I. (1963). Hashish. I. The structure of cannabidiol. Tetrahedron 19 (12), 2073–2078. doi:10.1016/0040-4020(63)85022-x
Meier, M. H., Caspi, A., R Knodt, A., Hall, W., Ambler, A., Harrington, H., et al. (2022). Long-term cannabis use and cognitive reserves and hippocampal volume in midlife. Am. J. Psychiatry 179 (5), 362–374. doi:10.1176/appi.ajp.2021.21060664
Meyer, N., Zielke, S., Michaelis, J. B., Linder, B., Warnsmann, V., Rakel, S., et al. (2018). AT 101 induces early mitochondrial dysfunction and HMOX1 (heme oxygenase 1) to trigger mitophagic cell death in glioma cells. Autophagy 14 (10), 1693–1709. doi:10.1080/15548627.2018.1476812
Milian, L., Mata, M., Alcacer, J., Oliver, M., Sancho-Tello, M., Martín de Llano, J. J., et al. (2020). Cannabinoid receptor expression in non-small cell lung cancer. Effectiveness of tetrahydrocannabinol and cannabidiol inhibiting cell proliferation and epithelial-mesenchymal transition in vitro. PLoS ONE 15 (2), e0228909. doi:10.1371/journal.pone.0228909
Millar, S. A., Stone, N. L., Yates, A. S., and O’Sullivan, S. E. (2018). A systematic review on the pharmacokinetics of cannabidiol in humans. Front. Pharmacol. 9, 1365. doi:10.3389/fphar.2018.01365
Moir, D., Rickert, W. S., Levasseur, G., Larose, Y., Maertens, R., White, P., et al. (2008). A comparison of mainstream and sidestream marijuana and tobacco cigarette smoke produced under two machine smoking conditions. Chem. Res. Toxicol. 21 (2), 494–502. doi:10.1021/tx700275p
Moreno, E., Cavic, M., Krivokuca, A., Casadó, V., and Canela, E. (2019). The endocannabinoid system as a target in cancer diseases: Are we there yet? Front. Pharmacol. 10, 339. doi:10.3389/fphar.2019.00339
Morgan, C. J. A., Schafer, G., Freeman, T. P., and Curran, H. V. (2010). Impact of cannabidiol on the acute memory and psychotomimetic effects of smoked cannabis: Naturalistic study: Naturalistic study [corrected]. Br. J. Psychiatry 197 (4), 285–290. doi:10.1192/bjp.bp.110.077503
Mullen, T. D., and Obeid, L. M. (2012). Ceramide and apoptosis: Exploring the enigmatic connections between sphingolipid metabolism and programmed cell death. Anticancer Agents Med. Chem. 12 (4), 340–363. doi:10.2174/187152012800228661
Munson, A. E., Harris, L. S., Friedman, M. A., Dewey, W. L., and Carchman, R. A. (1975). Antineoplastic activity of cannabinoids. JNCI J. Natl. Cancer Inst. 55 (3), 597–602. doi:10.1093/jnci/55.3.597
Murase, R., Kawamura, R., Singer, E., Pakdel, A., Sarma, P., Judkins, J., et al. (2014). Targeting multiple cannabinoid anti-tumour pathways with a resorcinol derivative leads to inhibition of advanced stages of breast cancer. Br. J. Pharmacol. 171 (19), 4464–4477. doi:10.1111/bph.12803
Nabissi, M., Morelli, M. B., Amantini, C., Liberati, S., Santoni, M., Ricci-Vitiani, L., et al. (2015). Cannabidiol stimulates Aml-1a-dependent glial differentiation and inhibits glioma stem-like cells proliferation by inducing autophagy in a TRPV2-dependent manner. Int. J. Cancer 137 (8), 1855–1869. doi:10.1002/ijc.29573
Nabissi, M., Morelli, M. B., Santoni, M., and Santoni, G. (2013). Triggering of the TRPV2 channel by cannabidiol sensitizes glioblastoma cells to cytotoxic chemotherapeutic agents. Carcinogenesis 34 (1), 48–57. doi:10.1093/carcin/bgs328
Nallathambi, R., Mazuz, M., Namdar, D., Shik, M., Namintzer, D., Vinayaka, A. C., et al. (2018). Identification of synergistic interaction between cannabis-derived compounds for cytotoxic activity in colorectal cancer cell lines and colon polyps that induces apoptosis-related cell death and distinct gene expression. Cannabis Cannabinoid Res. 3 (1), 120–135. doi:10.1089/can.2018.0010
Navari, R. M., and Aapro, M. (2016). Antiemetic prophylaxis for chemotherapy-induced nausea and vomiting. N. Engl. J. Med. 374 (14), 1356–1367. doi:10.1056/nejmra1515442
Neumann-Raizel, H., Shilo, A., Lev, S., Mogilevsky, M., Katz, B., Shneor, D., et al. (2019). 2-APB and CBD-mediated targeting of charged cytotoxic compounds into tumor cells suggests the involvement of TRPV2 channels. Front. Pharmacol. 10, 1198. doi:10.3389/fphar.2019.01198
Ng, S-K., Chung, D-J., Chang, L-C., Luo, C-K., Jwo, S-H., Lee, Y-H., et al. (2023). The protective effect of cannabinoids against colorectal cancer cachexia through modulation of inflammation and immune responses. Biomed. Pharmacother. 161, 114467. doi:10.1016/j.biopha.2023.114467
Niedzwiedz, C. L., Knifton, L., Robb, K. A., Katikireddi, S. V., and Smith, D. J. (2019). Depression and anxiety among people living with and beyond cancer: A growing clinical and research priority. BMC Cancer 19 (1), 943. doi:10.1186/s12885-019-6181-4
Nielsen, S., Picco, L., Murnion, B., Winters, B., Matheson, J., Graham, M., et al. (2022). Opioid-sparing effect of cannabinoids for analgesia: An updated systematic review and meta-analysis of preclinical and clinical studies. Neuropsychopharmacology 47 (7), 1315–1330. doi:10.1038/s41386-022-01322-4
Nielsen, S., Sabioni, P., Trigo, J. M., Ware, M. A., Betz-Stablein, B. D., Murnion, B., et al. (2017). Opioid-sparing effect of cannabinoids: A systematic review and meta-analysis. Neuropsychopharmacology 42 (9), 1752–1765. doi:10.1038/npp.2017.51
Nishikawa, H., Goto, M., Fukunishi, S., Asai, A., Nishiguchi, S., and Higuchi, K. (2021). Cancer cachexia: Its mechanism and clinical significance. Int. J. Mol. Sci. 22 (16), 8491. doi:10.3390/ijms22168491
Noyes, R., Brunk, S. F., Avery, D. A., and Canter, A. C. (1975a). The analgesic properties of delta-9-tetrahydrocannabinol and codeine. Clin. Pharmacol. Ther. 18 (1), 84–89. doi:10.1002/cpt197518184
Noyes, R., Brunk, S. F., Baram, D. A., and Canter, A. (1975b). Analgesic effect of delta-9-tetrahydrocannabinol. J. Clin. Pharmacol. 15 (2–3), 139–143. doi:10.1002/j.1552-4604.1975.tb02348.x
Odieka, A. E., Obuzor, G. U., Oyedeji, O. O., Gondwe, M., Hosu, Y. S., and Oyedeji, A. O. (2022). The medicinal natural products of Cannabis sativa linn.: A review. A Rev. Mol. 27 (5), 1689. doi:10.3390/molecules27051689
Onesti, J. K., and Guttridge, D. C. (2014). Inflammation based regulation of cancer cachexia. Biomed. Res. Int. 2014, 1–7. doi:10.1155/2014/168407
Oosterling, A., te Boveldt, N., Verhagen, C., van der Graaf, W. T., Van Ham, M., Van der Drift, M., et al. (2016). Neuropathic pain components in patients with cancer: Prevalence, treatment, and interference with daily activities. Pain Pract. 16 (4), 413–421. doi:10.1111/papr.12291
Pagano, C., Navarra, G., Coppola, L., Bifulco, M., and Laezza, C. (2021). Molecular mechanism of cannabinoids in cancer progression. Int. J. Mol. Sci. 22 (7), 3680. doi:10.3390/ijms22073680
Pascual, D., Goicoechea, C., Suardíaz, M., and Martín, M. I. (2005). A cannabinoid agonist, WIN 55,212-2, reduces neuropathic nociception induced by paclitaxel in rats. Pain 118 (1–2), 23–34. doi:10.1016/j.pain.2005.07.008
Picardi, P., Ciaglia, E., Proto, M., and Pisanti, S. (2014). Anandamide inhibits breast tumor-induced angiogenesis. Transl. Med. UniSa 10, 8–12. doi:10.14273/UNISA-438
Pietrovito, L., Iozzo, M., Bacci, M., Giannoni, E., and Chiarugi, P. (2020). Treatment with cannabinoids as a promising approach for impairing fibroblast activation and prostate cancer progression. Int. J. Mol. Sci. 21 (3), 787. doi:10.3390/ijms21030787
Portenoy, R. K., Ganae-Motan, E. D., Allende, S., Yanagihara, R., Shaiova, L., Weinstein, S., et al. (2012). Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: A randomized, placebo-controlled, graded-dose trial. J. Pain 13 (5), 438–449. doi:10.1016/j.jpain.2012.01.003
Preet, A., Ganju, R. K., and Groopman, J. E. (2008). Delta9-Tetrahydrocannabinol inhibits epithelial growth factor-induced lung cancer cell migration in vitro as well as its growth and metastasis in vivo. Oncogene 27 (3), 339–346. doi:10.1038/sj.onc.1210641
Pryimak, N., Zaiachuk, M., Kovalchuk, O., and Kovalchuk, I. (2021). The potential use of cannabis in tissue fibrosis. Front. Cell Dev. Biol. 9, 715380. doi:10.3389/fcell.2021.715380
Qiu, C., Yang, L., Wang, B., Cui, L., Li, C., Zhuo, Y., et al. (2019). The role of 2-arachidonoylglycerol in the regulation of the tumor-immune microenvironment in murine models of pancreatic cancer. Biomed. Pharmacother. 115, 108952. doi:10.1016/j.biopha.2019.108952
Rahn, E. J., Deng, L., Thakur, G. A., Vemuri, K., Zvonok, A. M., Lai, Y. Y., et al. (2014). Prophylactic cannabinoid administration blocks the development of paclitaxel-induced neuropathic nociception during analgesic treatment and following cessation of drug delivery. Mol. Pain 10, 27. doi:10.1186/1744-8069-10-27
Raja, S. N., Carr, D. B., Cohen, M., Finnerup, N. B., Flor, H., Gibson, S., et al. (2020). The revised international association for the study of pain definition of pain: Concepts, challenges, and compromises. Pain 161 (9), 1976–1982. doi:10.1097/j.pain.0000000000001939
Ramer, R., Bublitz, K., Freimuth, N., Merkord, J., Rohde, H., Haustein, M., et al. (2012). Cannabidiol inhibits lung cancer cell invasion and metastasis via intercellular adhesion molecule-1. FASEB J. 26 (4), 1535–1548. doi:10.1096/fj.11-198184
Ramer, R., Heinemann, K., Merkord, J., Rohde, H., Salamon, A., Linnebacher, M., et al. (2013). COX-2 and PPAR-γ confer cannabidiol-induced apoptosis of human lung cancer cells. Mol. Cancer Ther. 12 (1), 69–82. doi:10.1158/1535-7163.MCT-12-0335
Ramer, R., and Hinz, B. (2008). Inhibition of cancer cell invasion by cannabinoids via increased expression of tissue inhibitor of matrix metalloproteinases-1. J. Natl. Cancer Inst. 100 (1), 59–69. doi:10.1093/jnci/djm268
Ramer, R., Merkord, J., Rohde, H., and Hinz, B. (2010a). Cannabidiol inhibits cancer cell invasion via upregulation of tissue inhibitor of matrix metalloproteinases-1. Biochem. Pharmacol. 79 (7), 955–966. doi:10.1016/j.bcp.2009.11.007
Ramer, R., Rohde, A., Merkord, J., Rohde, H., and Hinz, B. (2010b). Decrease of plasminogen activator inhibitor-1 may contribute to the anti-invasive action of cannabidiol on human lung cancer cells. Pharm. Res. 27 (10), 2162–2174. doi:10.1007/s11095-010-0219-2
Rao, M., Chen, D., Zhan, P., and Jiang, J. (2019). MDA19, a novel CB2 agonist, inhibits hepatocellular carcinoma partly through inactivation of AKT signaling pathway. Biol. Direct 14 (1), 9. doi:10.1186/s13062-019-0241-1
Ravi, D., Ghasemiesfe, M., Korenstein, D., Cascino, T., and Keyhani, S. (2018). Associations between marijuana use and cardiovascular risk factors and outcomes: A systematic review. Ann. Intern Med. 168 (3), 187–194. doi:10.7326/M17-1548
Ray, A. P., Griggs, L., and Darmani, N. A. (2009). Delta 9-tetrahydrocannabinol suppresses vomiting behavior and Fos expression in both acute and delayed phases of cisplatin-induced emesis in the least shrew. Behav. Brain Res. 196 (1), 30–36. doi:10.1016/j.bbr.2008.07.028
Revathidevi, S., and Munirajan, A. K. (2019). Akt in cancer: Mediator and more. Semin. Cancer Biol. 59, 80–91. doi:10.1016/j.semcancer.2019.06.002
Roberto, D., Klotz, L. H., and Venkateswaran, V. (2019). Cannabinoid WIN 55,212-2 induces cell cycle arrest and apoptosis, and inhibits proliferation, migration, invasion, and tumor growth in prostate cancer in a cannabinoid-receptor 2 dependent manner. Prostate 79 (2), 151–159. doi:10.1002/pros.23720
Rodriguez, C., Ji, M., Wang, H-L., Padhya, T., and McMillan, S. C. (2019). Cancer pain and quality of life. J. Hosp. Palliat. Nurs. 21 (2), 116–123. doi:10.1097/NJH.0000000000000507
Roeland, E. J., Bohlke, K., Baracos, V. E., Bruera, E., Del Fabbro, E., Dixon, S., et al. (2020). Management of cancer cachexia: ASCO guideline. J. Clin. Oncol. 38 (21), 2438–2453. doi:10.1200/JCO.20.00611
Romero-Sandoval, E. A., Kolano, A. L., and Alvarado-Vázquez, P. A. (2017). Cannabis and cannabinoids for chronic pain. Curr. Rheumatol. Rep. 19 (11), 67. doi:10.1007/s11926-017-0693-1
Rønne, S. T., Rosenbæk, F., Pedersen, L. B., Waldorff, F. B., Nielsen, J. B., Riisgaard, H., et al. (2021). Physicians’ experiences, attitudes, and beliefs towards medical cannabis: A systematic literature review. BMC Fam. Pract. 22 (1), 212. doi:10.1186/s12875-021-01559-w
Rubino, T., Sala, M., Viganò, D., Braida, D., Castiglioni, C., Limonta, V., et al. (2007). Cellular mechanisms underlying the anxiolytic effect of low doses of peripheral Delta9-tetrahydrocannabinol in rats. Neuropsychopharmacology 32 (9), 2036–2045. doi:10.1038/sj.npp.1301330
Ruiz, L., Miguel, A., and Diaz-Laviada, I. (1999). Δ9 -Tetrahydrocannabinol induces apoptosis in human prostate PC-3 cells via a receptor-independent mechanism. FEBS Lett. 458 (3), 400–404. doi:10.1016/s0014-5793(99)01073-x
Russo, E. B. (2014). “The pharmacological history of cannabis,” in Handbook of cannabis. Editor R. Pertwee (Oxford University Press), 23–43.
Ryz, N. R., Remillard, D. J., and Russo, E. B. (2017). Cannabis roots: A traditional therapy with future potential for treating inflammation and pain. Cannabis Cannabinoid Res. 2 (1), 210–216. doi:10.1089/can.2017.0028
Sachs, J., McGlade, E., and Yurgelun-Todd, D. (2015). Safety and toxicology of cannabinoids. Neurotherapeutics 12 (4), 735–746. doi:10.1007/s13311-015-0380-8
Saghafi, N., Lam, D. K., and Schmidt, B. L. (2011). Cannabinoids attenuate cancer pain and proliferation in a mouse model. Neurosci. Lett. 488 (3), 247–251. doi:10.1016/j.neulet.2010.11.039
Salazar, M., Carracedo, A., Salanueva, I. J., Hernández-Tiedra, S., Egia, A., Lorente, M., et al. (2009). TRB3 links ER stress to autophagy in cannabinoid anti-tumoral action. Autophagy 5 (7), 1048–1049. doi:10.4161/auto.5.7.9508
Saloner, B., McGinty, E. E., Beletsky, L., Bluthenthal, R., Beyrer, C., Botticelli, M., et al. (2018). A public health strategy for the opioid crisis. Public Health Rep. 133 (1), 24S–34S. doi:10.1177/0033354918793627
Sánchez, C., Galve-Roperh, I., Canova, C., Brachet, P., and Guzmán, M. (1998). Delta9-tetrahydrocannabinol induces apoptosis in C6 glioma cells. FEBS Lett. 436, 6–10. doi:10.1016/s0014-5793(98)01085-0
Satin, J. R., Linden, W., and Phillips, M. J. (2009). Depression as a predictor of disease progression and mortality in cancer patients: A meta-analysis. Cancer 115 (22), 5349–5361. doi:10.1002/cncr.24561
Schloss, J., Lacey, J., Sinclair, J., Steel, A., Sughrue, M., Sibbritt, D., et al. (2021). A phase 2 randomised clinical trial assessing the tolerability of two different ratios of medicinal cannabis in patients with high grade gliomas. Front. Oncol. 11, 649555. doi:10.3389/fonc.2021.649555
Schneir, A. B., Cullen, J., and Ly, B. T. (2011). “Spice” girls: Synthetic cannabinoid intoxication. J. Emerg. Med. 40 (3), 296–299. doi:10.1016/j.jemermed.2010.10.014
Schurman, L. D., Lu, D., Kendall, D. A., Howlett, A. C., and Lichtman, A. H. (2020). Molecular mechanism and cannabinoid pharmacology. Handb. Exp. Pharmacol. 258, 323–353. doi:10.1007/164_2019_298
Scott, K. A., Dalgleish, A. G., and Liu, W. M. (2014). The combination of cannabidiol and Δ9-tetrahydrocannabinol enhances the anticancer effects of radiation in an orthotopic murine glioma model. Mol. Cancer Ther. 13 (12), 2955–2967. doi:10.1158/1535-7163.MCT-14-0402
Seddon, T., and Floodgate, W. (2020). Regulating cannabis: A global review and future directions. Cham: Springer International Publishing.
Sekar, K., and Pack, A. (2019). Epidiolex as adjunct therapy for treatment of refractory epilepsy: A comprehensive review with a focus on adverse effects. F1000Res. 8, F1000 Faculty Rev-234. doi:10.12688/f1000research.16515.1
Shahbazi, F., Grandi, V., Banerjee, A., and Trant, J. F. (2020). Cannabinoids and cannabinoid receptors: The story so far. iScience 23 (7), 101301. doi:10.1016/j.isci.2020.101301
Sharpe, L., Sinclair, J., Kramer, A., de Manincor, M., and Sarris, J. (2020). Cannabis, a cause for anxiety? A critical appraisal of the anxiogenic and anxiolytic properties. J. Transl. Med. 18 (1), 374. doi:10.1186/s12967-020-02518-2
Shekhar, A. (2014). Review of marijuana use in the adolescent population and implications of its legalization in the United States. J. Drug Metab. Toxicol. 05 (01). doi:10.4172/2157-7609.1000165
Sholler, D. J., Huestis, M. A., Amendolara, B., Vandrey, R., and Cooper, Z. D. (2020). Therapeutic potential and safety considerations for the clinical use of synthetic cannabinoids. Pharmacol. Biochem. Behav. 199, 173059. doi:10.1016/j.pbb.2020.173059
Shore, D. M., Baillie, G. L., Hurst, D. H., Navas, F., Seltzman, H. H., Marcu, J. P., et al. (2014). Allosteric modulation of a cannabinoid G protein-coupled receptor: Binding site elucidation and relationship to G protein signaling. J. Biol. Chem. 289 (9), 5828–5845. doi:10.1074/jbc.M113.478495
Shrivastava, A., Kuzontkoski, P. M., Groopman, J. E., and Prasad, A. (2011). Cannabidiol induces programmed cell death in breast cancer cells by coordinating the cross-talk between apoptosis and autophagy. Mol. Cancer Ther. 10 (7), 1161–1172. doi:10.1158/1535-7163.MCT-10-1100
Sidney, S., Quesenberry, C. P., Friedman, G. D., and Tekawa, I. S. (1997). Marijuana use and cancer incidence (California, United States). Cancer Causes Control 8 (5), 722–728. doi:10.1023/a:1018427320658
Simoneau, , , Hamza, M. S., Mata, H. P., Siegel, E. M., Vanderah, T. W., Porreca, F., et al. (2001). The cannabinoid agonist WIN55,212-2 suppresses opioid-induced emesis in ferrets. Anesthesiology 94 (5), 882–887. doi:10.1097/00000542-200105000-00029
Singer, E., Judkins, J., Salomonis, N., Matlaf, L., Soteropoulos, P., McAllister, S., et al. (2015). Reactive oxygen species-mediated therapeutic response and resistance in glioblastoma. Cell Death Dis. 6 (1), e1601. doi:10.1038/cddis.2014.566
Smith, F. L., Cichewicz, D., Martin, Z. L., and Welch, S. P. (1998). The enhancement of morphine antinociception in mice by delta9-tetrahydrocannabinol. Pharmacol. Biochem. Behav. 60 (2), 559–566. doi:10.1016/s0091-3057(98)00012-4
Sommariva, S., Pongiglione, B., and Tarricone, R. (2016). Impact of chemotherapy-induced nausea and vomiting on health-related quality of life and resource utilization: A systematic review. Crit. Rev. Oncol. Hematol. 99, 13–36. doi:10.1016/j.critrevonc.2015.12.001
Stith, S. S., Li, X., Diviant, J. P., Brockelman, F. C., Keeling, K. S., Hall, B., et al. (2020). The effectiveness of inhaled Cannabis flower for the treatment of agitation/irritability, anxiety, and common stress. J. Cannabis Res. 2 (1), 47. doi:10.1186/s42238-020-00051-z
Stoorvogel, H., van Haastregt, J., Theunissen, M., Schoenmaekers, J., Hoeben, A., and van den Beuken-van Everdingen, M. (2022). Unacceptable pain in oncology: The patients’ perspective on reasons for absence of pain interventions. Eur. J. Cancer Care (Engl). 31 (5), e13628. doi:10.1111/ecc.13628
Taha, T., Meiri, D., Talhamy, S., Wollner, M., Peer, A., and Bar-Sela, G. (2019). Cannabis impacts tumor response rate to nivolumab in patients with advanced malignancies. Oncologist 24 (4), 549–554. doi:10.1634/theoncologist.2018-0383
Tait, R. J., Caldicott, D., Mountain, D., Hill, S. L., and Lenton, S. (2016). A systematic review of adverse events arising from the use of synthetic cannabinoids and their associated treatment. Clin. Toxicol. (Phila). 54 (1), 1–13. doi:10.3109/15563650.2015.1110590
Takeda, S., Okazaki, H., Ikeda, E., Abe, S., Yoshioka, Y., Watanabe, K., et al. (2014). Down-regulation of cyclooxygenase-2 (COX-2) by cannabidiolic acid in human breast cancer cells. J. Toxicol. Sci. 39 (5), 711–716. doi:10.2131/jts.39.711
Takeda, S., Yamamoto, I., and Watanabe, K. (2009). Modulation of Delta9-tetrahydrocannabinol-induced MCF-7 breast cancer cell growth by cyclooxygenase and aromatase. Toxicology 259 (1–2), 25–32. doi:10.1016/j.tox.2009.01.024
Tamba, B. I., Stanciu, G. D., Urîtu, C. M., Rezus, E., Stefanescu, R., Mihai, C. T., et al. (2020). Challenges and opportunities in preclinical research of synthetic cannabinoids for pain therapy. Med. Kaunas. 56 (1), 24. doi:10.3390/medicina56010024
Tashkin, D. P., and Tan, W-C. (2022). Inhaled marijuana and the lung. J. Allergy Clin. Immunol. Pract. 10 (11), 2822–2829. doi:10.1016/j.jaip.2022.05.009
Thoma, O-M., Neurath, M. F., and Waldner, M. J. (2021). Cyclin-dependent kinase inhibitors and their therapeutic potential in colorectal cancer treatment. Front. Pharmacol. 12, 757120. doi:10.3389/fphar.2021.757120
Thomas, G., Kloner, R. A., and Rezkalla, S. (2014). Adverse cardiovascular, cerebrovascular, and peripheral vascular effects of marijuana inhalation: What cardiologists need to know. Am. J. Cardiol. 113 (1), 187–190. doi:10.1016/j.amjcard.2013.09.042
Thompson, A. L., Grenald, S. A., Ciccone, H. A., BassiriRad, N., Niphakis, M. J., Cravatt, B. F., et al. (2020). The endocannabinoid system alleviates pain in a murine model of cancer-induced bone pain. J. Pharmacol. Exp. Ther. 373 (2), 230–238. doi:10.1124/jpet.119.262337
Thompson, R., DeJong, K., and Lo, J. (2019). Marijuana use in pregnancy: A review. Obstet. Gynecol. Surv. 74 (7), 415–428. doi:10.1097/OGX.0000000000000685
Torres, S., Lorente, M., Rodríguez-Fornés, F., Hernández-Tiedra, S., Salazar, M., García-Taboada, E., et al. (2011). A combined preclinical therapy of cannabinoids and temozolomide against glioma. Mol. Cancer Ther. 10 (1), 90–103. doi:10.1158/1535-7163.MCT-10-0688
Turcott, J. G., Del Rocío Guillen Núñez, M., Flores-Estrada, D., Oñate-Ocaña, L. F., Zatarain-Barrón, Z. L., Barrón, F., et al. (2018). The effect of nabilone on appetite, nutritional status, and quality of life in lung cancer patients: A randomized, double-blind clinical trial. Support Care Cancer 26 (9), 3029–3038. doi:10.1007/s00520-018-4154-9
Twelves, C., Sabel, M., Checketts, D., Miller, S., Tayo, B., Jove, M., et al. (2021). A phase 1b randomised, placebo-controlled trial of nabiximols cannabinoid oromucosal spray with temozolomide in patients with recurrent glioblastoma. Br. J. Cancer 124 (8), 1379–1387. doi:10.1038/s41416-021-01259-3
van de Worp, W. R. P. H., Schols, A. M. W. J., Theys, J., van Helvoort, A., and Langen, R. C. J. (2020). Nutritional interventions in cancer cachexia: Evidence and perspectives from experimental models. Front. Nutr. 7, 601329. doi:10.3389/fnut.2020.601329
van den Beuken-van Everdingen, M. H. J., Hochstenbach, L. M. J., Joosten, E. A. J., Tjan-Heijnen, V. C. G., and Janssen, D. J. A. (2016). Update on prevalence of pain in patients with cancer: Systematic review and meta-analysis. J. Pain Symptom Manage 51 (6), 1070–1090.e9. doi:10.1016/j.jpainsymman.2015.12.340
Van Sickle, M. D., Oland, L. D., Mackie, K., Davison, J. S., and Sharkey, K. A. (2003). Delta9-tetrahydrocannabinol selectively acts on CB1 receptors in specific regions of dorsal vagal complex to inhibit emesis in ferrets. Am. J. Physiol. Gastrointest. Liver Physiol. 285 (3), G566–G576. doi:10.1152/ajpgi.00113.2003
Vara, D., Salazar, M., Olea-Herrero, N., Guzmán, M., Velasco, G., and Díaz-Laviada, I. (2011). Anti-tumoral action of cannabinoids on hepatocellular carcinoma: Role of AMPK-dependent activation of autophagy. Cell Death Differ. 18 (7), 1099–1111. doi:10.1038/cdd.2011.32
Velasco, G., Sánchez, C., and Guzmán, M. (2012). Towards the use of cannabinoids as antitumour agents. Nat. Rev. Cancer 12 (6), 436–444. doi:10.1038/nrc3247
Victor, B., Hager, K., and Stacy, S. (2022). Re-legalizing cannabis for medical use in the USA. J. Public Health (Oxf). 44 (3), 679–684. doi:10.1093/pubmed/fdab066
Vinette, B., Côté, J., El-Akhras, A., Mrad, H., Chicoine, G., and Bilodeau, K. (2022). Routes of administration, reasons for use, and approved indications of medical cannabis in oncology: A scoping review. BMC Cancer 22 (1), 319. doi:10.1186/s12885-022-09378-7
von Bueren, A. O., Schlumpf, M., and Lichtensteiger, W. (2008). Delta(9)-tetrahydrocannabinol inhibits 17beta-estradiol-induced proliferation and fails to activate androgen and estrogen receptors in MCF7 human breast cancer cells. Anticancer Res. 28 (1A), 85–89.
Voth, E. A., and Schwartz, R. H. (1997). Medicinal applications of delta-9-tetrahydrocannabinol and marijuana. Ann. Intern Med. 126 (10), 791–798. doi:10.7326/0003-4819-126-10-199705150-00008
Waissengrin, B., Mirelman, D., Pelles, S., Bukstein, F., Blumenthal, D. T., Wolf, I., et al. (2021). Effect of cannabis on oxaliplatin-induced peripheral neuropathy among oncology patients: A retrospective analysis. Ther. Adv. Med. Oncol. 13, 1758835921990203. doi:10.1177/1758835921990203
Walsh, Z., Gonzalez, R., Crosby, K., Thiessen, S. M., Carroll, C., and Bonn-Miller, M. O. (2017). Medical cannabis and mental health: A guided systematic review. Clin. Psychol. Rev. 51, 15–29. doi:10.1016/j.cpr.2016.10.002
Wang, D., Wang, H., Ning, W., Backlund, M. G., Dey, S. K., and DuBois, R. N. (2008). Loss of cannabinoid receptor 1 accelerates intestinal tumor growth. Cancer Res. 68 (15), 6468–6476. doi:10.1158/0008-5472.CAN-08-0896
Wang, L., Hong, P. J., May, C., Rehman, Y., Oparin, Y., Hong, C. J., et al. (2021). Medical cannabis or cannabinoids for chronic non-cancer and cancer related pain: A systematic review and meta-analysis of randomised clinical trials. BMJ 374, n1034. doi:10.1136/bmj.n1034
Wang, Y-H., Li, J-Q., Shi, J-F., Que, J-Y., Liu, J-J., Lappin, J. M., et al. (2020). Depression and anxiety in relation to cancer incidence and mortality: A systematic review and meta-analysis of cohort studies. Mol. Psychiatry 25 (7), 1487–1499. doi:10.1038/s41380-019-0595-x
Warr, D., and Hesketh, P. (2020). Cannabinoids as antiemetics: Everything that’s old is new again. Ann. Oncol. 31 (11), 1425–1426. doi:10.1016/j.annonc.2020.08.2104
Whiting, P. F., Wolff, R. F., Deshpande, S., Di Nisio, M., Duffy, S., Hernandez, A. V., et al. (2015). Cannabinoids for medical use: A systematic review and meta-analysis. JAMA 313 (24), 2456–2473. doi:10.1001/jama.2015.6358
Wieghorst, A., Roessler, K. K., Hendricks, O., and Andersen, T. E. (2022). The effect of medical cannabis on cognitive functions: A systematic review. Syst. Rev. 11 (1), 210. doi:10.1186/s13643-022-02073-5
Xiong, X., Chen, S., Shen, J., You, H., Yang, H., Yan, C., et al. (2022). Cannabis suppresses antitumor immunity by inhibiting JAK/STAT signaling in T cells through CNR2. Signal Transduct. Target Ther. 7 (1), 99. doi:10.1038/s41392-022-00918-y
Yakowicz, W. (2023)U.S. Cannabis sales hit record $17.5 billion as Americans consume more marijuana than ever before.
Yang, Y., Huynh, N., Dumesny, C., Wang, K., He, H., and Nikfarjam, M. (2020). Cannabinoids inhibited pancreatic cancer via P-21 activated kinase 1 mediated pathway. Int. J. Mol. Sci. 21 (21), 8035. doi:10.3390/ijms21218035
Yeshurun, M., Shpilberg, O., Herscovici, C., Shargian, L., Dreyer, J., Peck, A., et al. (2015). Cannabidiol for the prevention of graft-versus-host-disease after allogeneic hematopoietic cell transplantation: Results of a phase II study. Biol. Blood Marrow Transpl. 21 (10), 1770–1775. doi:10.1016/j.bbmt.2015.05.018
Yesilyurt, O., Dogrul, A., Gul, H., Seyrek, M., Kusmez, O., Ozkan, Y., et al. (2003). Topical cannabinoid enhances topical morphine antinociception. Pain 105 (1–2), 303–308. doi:10.1016/s0304-3959(03)00245-8
Zhang, X., Qin, Y., Pan, Z., Li, M., Liu, X., Chen, X., et al. (2019). Cannabidiol induces cell cycle arrest and cell apoptosis in human gastric cancer SGC-7901 cells. Biomolecules 9 (8), 302. doi:10.3390/biom9080302
Zhang, Z. F., Morgenstern, H., Spitz, M. R., Tashkin, D. P., Yu, G. P., Marshall, J. R., et al. (1999). Marijuana use and increased risk of squamous cell carcinoma of the head and neck. Cancer Epidemiol. Biomarkers Prev. 8 (12), 1071–1078.
Zhu, L. X., Sharma, S., Stolina, M., Gardner, B., Roth, M. D., Tashkin, D. P., et al. (2000). Delta-9-tetrahydrocannabinol inhibits antitumor immunity by a CB2 receptor-mediated, cytokine-dependent pathway. J. Immunol. 165 (1), 373–380. doi:10.4049/jimmunol.165.1.373
Keywords: cannabis, cannabinoids, cancer, oncology, pain, nausea and vomiting, anorexia and cachexia, anxiety and depression
Citation: Creanga-Murariu I, Filipiuc LE, Cuciureanu M, Tamba B-I and Alexa-Stratulat T (2023) Should oncologists trust cannabinoids?. Front. Pharmacol. 14:1211506. doi: 10.3389/fphar.2023.1211506
Received: 24 April 2023; Accepted: 03 July 2023;
Published: 13 July 2023.
Edited by:
David A. Gewirtz, Virginia Commonwealth University, United StatesReviewed by:
Myron R. Szewczuk, Queen’s University, CanadaCopyright © 2023 Creanga-Murariu, Filipiuc, Cuciureanu, Tamba and Alexa-Stratulat. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bogdan-Ionel Tamba, Ym9nZGFuLnRhbWJhQHVtZmlhc2kucm8=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.