- 1Department of Pharmacy, Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, Zhejiang, China
- 2Department of Pharmacy, 903 Hospital of the Joint Logistic Support Force of the PLA, Hangzhou, Zhejiang, China
- 3Department of Pharmacy, Sir Run Run Shaw Hospital, College of Medicine, Zhejiang University, Hangzhou, Zhejiang, China
- 4Department of Pediatrics, Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, Zhejiang, China
Perampanel is a promising option for the treatment of pediatric epilepsy, but its plasma concentration varies among patients. This retrospective study aimed to investigate the initial target attainment of perampanel plasma concentration in pediatric patients with epilepsy in China. Inpatients admitted from January 2020 to December 2021 in a tertiary hospital were retrospectively included according to pre-set criteria. Demographic characteristics of patients and dosing strategies and therapeutic drug monitoring results were collected. A total of 137 pediatric patients (84 females and 53 males, aged from 0.6 to 16.4 years) were include for analysis. The perampanel concentrations varied greatly from 60 to 1,560 mg/L among patients, but 89.8% had suitable perampanel concentrations (100–1,000 ng/mL). The concomitant use of enzyme-inductive antiepileptic drugs (AEDs) was the only identified risk factor associated with target nonattainment (OR = 5.92, 95% confidence interval 1.68–20.9). Initial perampanel target attainment in pediatric patients is satisfactory. Routine therapeutic drug monitoring to achieved the suggested concentration range for these patients may be unnecessary, except for those receiving combined enzyme inductive AEDs.
Introduction
Epilepsy is a common condition affecting children with a high prevalence. It has been reported that 1 out of 150 children will have a diagnosis of epilepsy in the first 10 years of life (Aaberg et al., 2017). More seriously, approximately 25% of patients with epilepsy are drug resistant (Sultana et al., 2021). Many different comorbidities may affect these patients. Cognitive and neuropsychiatric disorders such as attention deficit with hyperactivity disorder, autism spectrum disorders, and neurobehavioral problems in children are more common than in the general population (Coppola et al., 2019). The heterogeneity of seizures and epilepsies, the coexistence of comorbidities, and the broad spectrum of efficacy, safety, and tolerability related to the antiepileptic drugs (AEDs), make the management of these patients actually challenging (Fattorusso et al., 2021). Perampanel (PER) is a selective, noncompetitive antagonist of the ionotropic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid glutamate (AMPA) receptor on postsynaptic neurons. It has broad anti-seizure effects and has shown good clinical efficacy in adolescents and children, including those with drug-resistant epilepsy (Chang et al., 2020; Gao et al., 2022). Oral PER is rapidly and almost completely absorbed, with low systemic clearance and high relative bioavailability in humans (Rogawski and Hanada, 2013). PER is approximately 95%–97% bound to plasma proteins in a wide concentration range, and only 5% free PER exerted pharmacologic effect. The distribution volume of PER is large (approximately 1.1 L/kg) and the half-life is also long (105 h). PER is extensively metabolized via primary oxidation, which is mediated by CYP3A4 and/or CYP3A5, and sequential glucuronidation (de Biase et al., 2019). However, potential drug interactions, as well as other individual factors, may contribute to large fluctuations in plasma drug concentrations and, therefore, clinical response. Therapeutic drug monitoring (TDM) is an essential tool to address this complexity, enabling the definition of individual therapeutic concentrations and adaptive control of dosing to minimize drug interactions and prevent loss of efficacy or toxicity (Krasowski, 2010). Some studies have shown that PER plasma levels were affected by otherAEDs in routine TDM practice, including carbamazepine, phenobarbital, valproate and topiramate (Patsalos et al., 2016; Contin et al., 2018; Silva et al., 2023). A recent study indicated that enzyme-inductive AED could increase PER clearance (Fujita et al., 2022). However, it is still unknown whether these drug interactions have impact on PER therapeutic target nonattainment. Thus, we carried out this study to evaluate the initial therapeutic target attainment of PER and identify any independent risk factors associated with target nonattainment in Chinese pediatric patients with epilepsy.
Methods
Ethics and informed consent
This study was approved by the Ethics Committee of the Second Affiliated Hospital, School of Medicine, Zhejiang University (reference number 2021-YAN0406). Informed consent was waived due to the retrospective nature of the study and was in accordance with regional regulation requirements.
Patients and data collection
Inpatients admitted from January 2020 to December 2021 in our hospital were retrospectively included according to the following criteria: (a) patients aged less than 18 years; (b) patients treated with PER as a monotherapy or adjunctive therapy for epilepsy; and (c) patients who underwent PER TDM during the study period. However, patients with poor adherence to PER were excluded. Poor adherence was defined as prescription-based proportion of days covered lower than 0.8 in the first 3 months (Osterberg and Blaschke, 2005; Chen et al., 2023). The patients’ medical records, dosage of PER, age, sex, body weight, and comedications (other AEDs). The dosing strategy was set by the physician according to its label, which was detailly described in our previous publication (Miao et al., 2023). In our hospital, PER TDM was performed 3 weeks after initiation of PER treatment, as PER would achieve steady states after 19 days of dosing. Blood samples were collected in the morning, approximately 12 h after the previous dose. The plasma PER concentration was determined by a validated HPLC method (Franco et al., 2016). Only the first TDM result was included for analysis. A range of 100–1,000 mg/L was considered the target therapeutic range in our center.
Data analysis
The continuous results are presented as the mean and standard deviation, and the categorical results are presented as numbers and percentages. The concentration-to-dose ratio (C/D) of PER was calculated as follows: C/D (kg/L) = [PER concentration (mg/L)/PER dose per weight (mg/kg)]. To evaluate the effect of concomitant AEDs and other factors on the plasma concentration of PER, a simple univariate linear regression was performed using C/D as the dependent variable. Then, factors with p < 0.2 were put into a multivariate linear regression model to seek influencing factors of C/D. The included patients were subsequently divided into groups by factors. Differences in the C/D between groups were tested using Student’s t-test. To analyze independent variables associated with PER target nonattainment, a univariate logistic analysis was performed, and any factors with p < 0.2 were put into a backward multivariate logistic analysis. All statistical analyses were run in SPSS. Statistical significance was set at p < 0.05.
Results
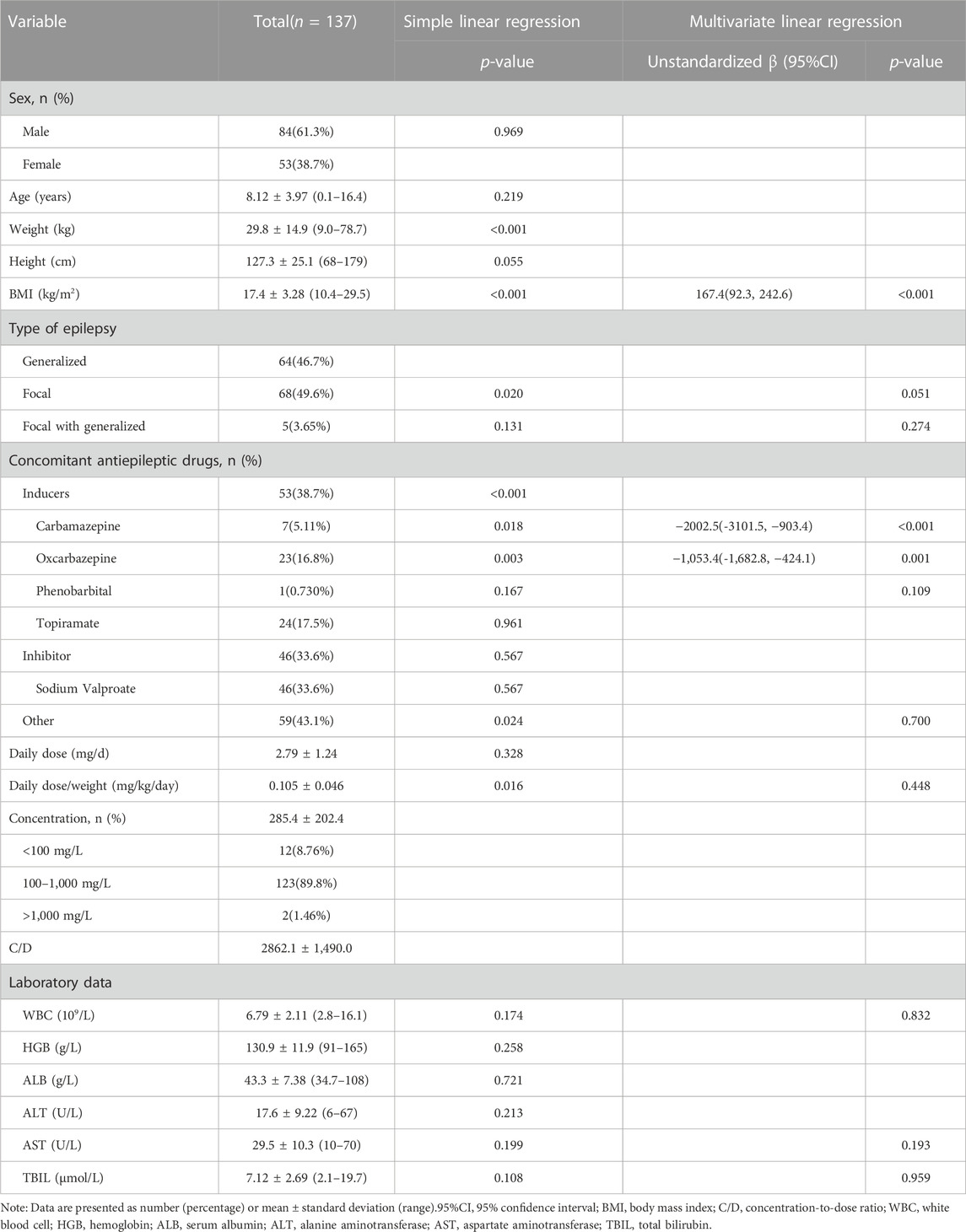
As a result, 137 pediatric patients with epilepsy were included in this study. The aged of patients ranged from 0.6 to 16.4 years and the detailed demographics are shown in Table 1. Although the concentration of PER varied greatly from 60 to 1,560 mg/L among patients, the overall therapeutic target attainment was as high as 89.8%. As shown in Table 1, the combined use of enzyme-inductive AEDs (carbamazepine and oxcarbazepine) and BMI were factors that were included in the multivariate linear regression model, thus indicating that these factors have a significant influence on the C/D of PER. When we divided the patients by concomitant AEDs, it can be seen that patients receiving carbamazepine or oxcarbazepine had significantly lower levels than other patients (Figure 1). However, from the perspective of target nonattainment, the combined use of enzyme-inductive AEDs was the only independent risk factor associated with target nonattainment (Table 2).
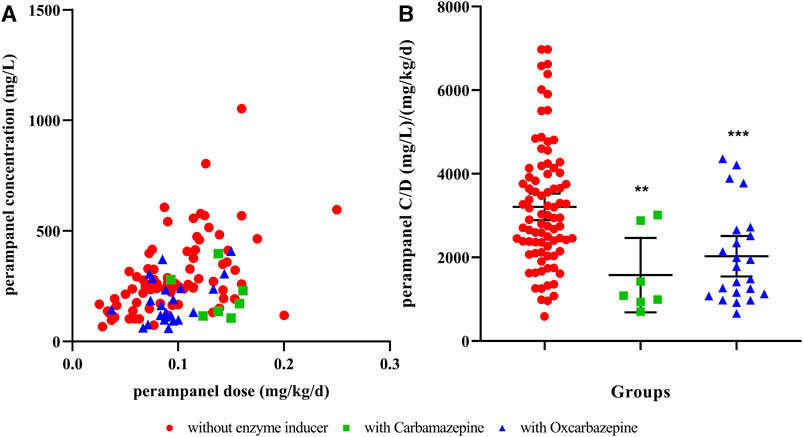
FIGURE 1. Influence of concomitant enzyme inducers on the perampanel concentration-to-dose ratio (A) relationship to perampanel concentration and dose; (B) perampanel concentration distribution in different groups. ** indicates p <0.01, *** indicates p <0.001, compared to those without enzyme inducers. C/D, concentration-to-dose ratio.
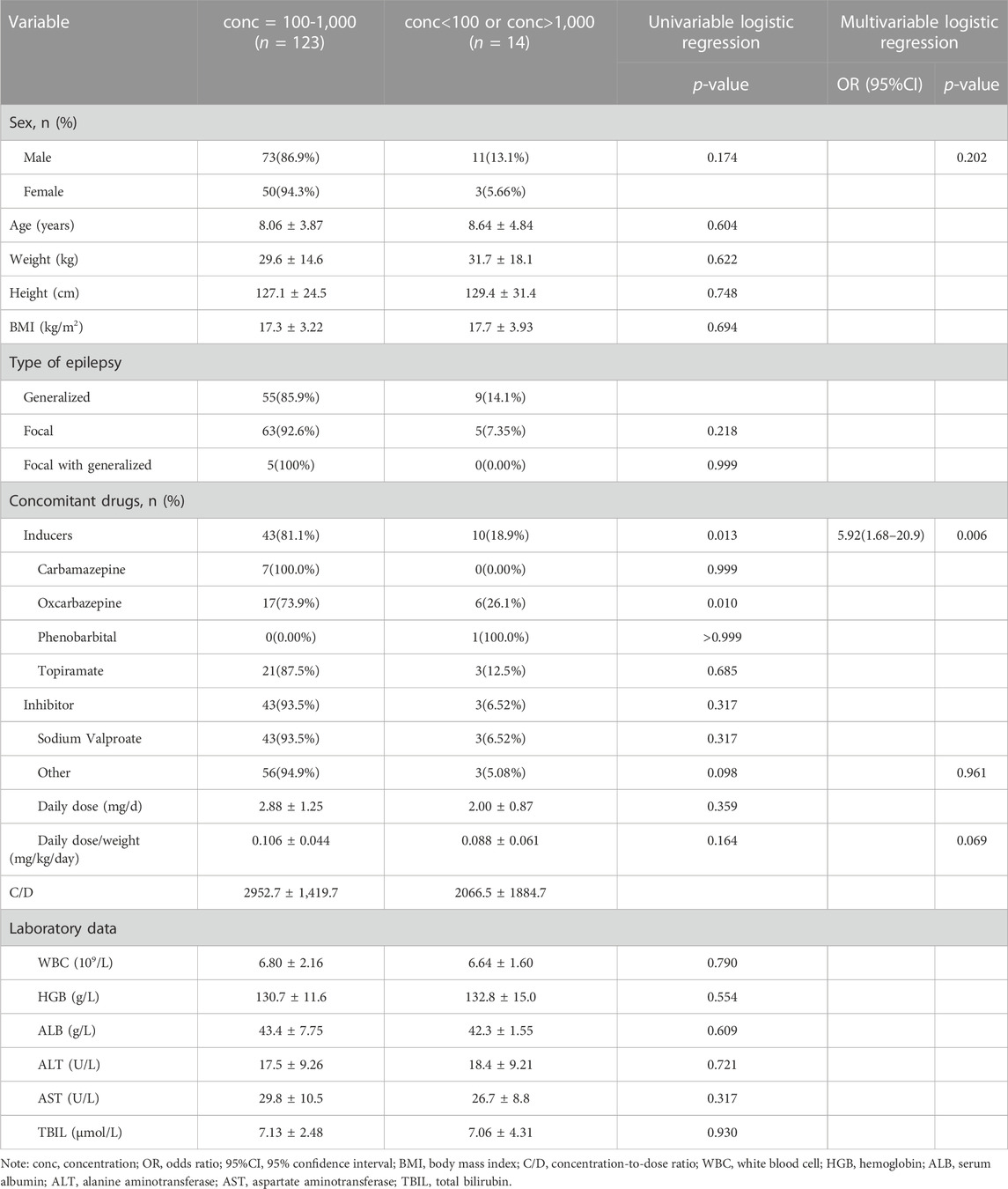
TABLE 2. Patient demographic characteristics of patients with or without concentrations of 100–1,000 mg/L.
Discussion
This was the first study to evaluate the initial therapeutic target attainment of PER in pediatric patients and risk factors associated with target nonattainment. In this study, we found that the initial target attainment of PER in pediatric patients was high (89.8%), and the couse of enzyme-inductive AEDs was an independent risk factor for PER target nonattainment. These findings would benefit the clinical application of PER in pediatric patients.
The variance in plasma PER concentrations was large in our study, which was also elucidated by other studies. Steinhoff et al. found a large concentration range of 19–2436 ng/mL in adults (Steinhoff et al., 2019). However, the target attainment of PER in our study was as high as 89.8%. Li et al. also found a high therapeutic target attainment in pediatric patients (75%) using a narrower reference range (180–610 mg/L) (Li et al., 2022). Several reference ranges for PER have been reported. A previous pharmacokinetic-pharmacodynamic study suggested a PER concentration of 70 ng/mL or greater for efficacy in adults and adolescents, which was lower than our lower boundary (Gidal et al., 2013). However, PER concentrations have ranged from 180 to 980 μg/L in the patients who responded to PER in these trials, and this range was used as a putative reference range (Patsalos et al., 2018). Ranges of 50–400 and 200-600 were also suggested, but without sufficient support (Johannessen Landmark et al., 2020; Yamamoto et al., 2020). The Norwegian Association of Clinical Pharmacology established national guidelines about AED TDM for Norway and suggested a reference range of 100–1,000 ng/mL for PER based on current available evidence (Reimers et al., 2018). Previous study also confirmed good tolerability in pediatric patients in middle and long-term therapy of PER (Operto et al., 2020). Thus, the reference range used in the current study was reasonable. From the results of the study, routine TDM in pediatric patients to ensure that the PER concentration is in the reference range may be unnecessary. However, using a TDM method to identify individual optimized PER levels or investigate patient adherence to PER is still meaningful.
PER is extensively metabolized by CYP3A, and naturally, its pharmacokinetics are affected by enzyme inducers or inhibitors (Rogawski and Hanada, 2013). Concomitant use of carbamazepine and oxcarbazepine was negatively correlated to PER C/D in our study. This is in accordance with previous reports that the concomitant use of enzyme-inducing AEDs could result in lower PER concentrations or C/D, both in children and adults (Patsalos et al., 2016; Gaudio et al., 2019; Ikemoto et al., 2019; Ishikawa et al., 2019). Carbamazepine could increase the clearance of PER in a population pharmacokinetic analysis (Fujita et al., 2022). However, the influence of the enzyme inhibitor AED, valproic acid, was not identified in this study. Valproic acid was reported to affect PER C/D in adults and adolescents. However, a previous study also showed that the effect of coadministration of the CYP3A inhibitor ketoconazole on the pharmacokinetics of PER could be neglected (Gidal et al., 2017). Our study found that only one personnel characteristic, BMI, was related to C/D. While age was also reported to be a factor of C/D (younger and older than 12 years) (Ikemoto et al., 2019), Patsalos et al. also found that age was independent of PER plasma concentration in adults (Patsalos et al., 2016). Although numerous factors can affect PER C/D, it is uncertain whether this influence had clinical significance until this report.
For now, there is no ethnic-related differences founded in the pharmacokinetics of PER (de Biase et al., 2019). A population pharmacokinetic analysis including phase Ⅱ/Ⅲ trails data found that the difference of clearance between Asian race and other the was small and clinical irrelevant (Takenaka et al., 2018). Another pharmacokinetic study also concluded that there were no clinically relevant ethnic differences in PK following multiple doses of perampanel between Korean, white, or Japanese subjects (Tabuchi et al., 2018).
The advantage of our study is that concomitant use of AEDs was found to be the only risk factor associated with PER therapeutic target nonattainment in pediatric patients. This would be helpful in clinical practice. Physicians need to be aware that when prescribing PER, the dose should be elevated or adjusted according to TDM results if concomitant enzyme-inductive AEDs are present. It should be noted that in the multivariate logistic regression, concomitant use of inducers and use of oxcarbazepine were related, and we selected the former to perform the final analysis. It is reasonable that carbamazepine was reported to have a larger inductive effect on PER metabolism than oxcarbazepine (Patsalos, 2015).
This study also has some limitations. The international consensus about the therapeutic range of PER has not yet been reached; thus, the target attainment of PER would change when the reference range changes. PER is highly bounded to plasma proteins but free PER concentrations were not determined in this study. Clinical outcome, as well as the relationship between PER concentration and outcome, were not evaluated. Patients receiving some enzyme-inductive AEDs, especially weak enzyme-inductive AEDs such as topiramate, are few, and the influence of these drugs on PER target attainment should be evaluated in future studies.
Conclusion
In pediatric patients with epilepsy, PER concentrations varied to a great extent, but the target attainment was high if using the reference range of 100–1,000 ng/mL. BMI and the couse of enzyme-inductive AEDs significantly affected the C/D of PER. However, only the concomitant use of enzyme-inductive AEDs was associated with PER therapeutic target nonattainment. Routine TDM for these patients to ensure PER concentration may be unnecessary, except for those receiving combined enzyme inductive AEDs.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Second Affiliated Hospital, School of Medicine, Zhejiang University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’; legal guardians/next of kin because Informed consent was waived due to the retrospective nature of the study and was in accordance with regional regulation requirements.
Author contributions
Conceptualization, JF and HD; Data curation, LY and MC; Formal analysis, LY, MC, and JL; Funding acquisition, LY; Investigation, LY; Methodology, LY and ZY; Project administration, JF and HD; Resources, LY; Software, LY and JL; Supervision, JF and HD; Validation, ZY; Visualization, LY and JL; Roles/Writing–original draft, LY, ZY; Writing–review and editing, JF and HD. All authors contributed to the article and approved the submitted version.
Funding
The work was supported by Zhejiang Medical Association (2021ZYC-A03) and Zhejiang Medical Doctors Association (YS2022-1-001).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aaberg, K. M., Gunnes, N., Bakken, I. J., Lund Søraas, C., Berntsen, A., Magnus, P., et al. (2017). Incidence and prevalence of childhood epilepsy: a nationwide cohort study. Pediatrics 139, e20163908. doi:10.1542/peds.2016-3908
Chang, F. M., Fan, P. C., Weng, W. C., Chang, C. H., and Lee, W. T. (2020). The efficacy of perampanel in young children with drug-resistant epilepsy. Seizure 75, 82–86. doi:10.1016/j.seizure.2019.12.024
Chen, S., Fukasawa, T., Ikeda, A., Takeuchi, M., Shimotake, A., Yoshida, S., et al. (2023). Adherence to and persistence with lacosamide, perampanel, lamotrigine, and levetiracetam in adult patients with focal epilepsy in Japan: a descriptive cohort study using a claims database. Heliyon 9 (4), e15017. doi:10.1016/j.heliyon.2023.e15017
Contin, M., Bisulli, F., Santucci, M., Riva, R., Tonon, F., Mohamed, S., et al. (2018). Effect of valproic acid on perampanel pharmacokinetics in patients with epilepsy. Epilepsia 59, e103–e108. doi:10.1111/epi.14446
Coppola, G., Operto, F. F., Matricardi, S., and Verrotti, A. (2019). Monitoring and managing depression in adolescents with epilepsy: current perspectives. Neuropsychiatr. Dis. Treat. 15, 2773–2780. doi:10.2147/NDT.S192714
de Biase, S., Gigli, G. L., Nilo, A., Romano, G., and Valente, M. (2019). Pharmacokinetic and pharmacodynamic considerations for the clinical efficacy of perampanel in focal onset seizures. Expert Opin. Drug Metab. Toxicol. 15 (2), 93–102. doi:10.1080/17425255.2019.1560420
Fattorusso, A., Matricardi, S., Mencaroni, E., Dell'Isola, G. B., Di Cara, G., Striano, P., et al. (2021). The pharmaco-resistant epilepsy: an overview on existant and new emerging therapies. Front. Neurol. 12, 674483. doi:10.3389/fneur.2021.674483
Franco, V., Marchiselli, R., Fattore, C., Tartara, E., De Sarro, G., Russo, E., et al. (2016). Development and validation of an HPLC-UV assay for the therapeutic monitoring of the new antiepileptic drug perampanel in human plasma. Ther. drug Monit. 38 (6), 744–750. doi:10.1097/FTD.0000000000000350
Fujita, Y., Murai, M., Muraki, S., Suetsugu, K., Tsuchiya, Y., Hirota, T., et al. (2022). Population pharmacokinetic analysis of drug–drug interactions between perampanel and carbamazepine using enzyme induction model in epileptic patients. Ther. Drug Monit. 45, 653–659. doi:10.1097/ftd.0000000000001055
Gao, L., Shi, L., and Liu, Q. (2022). Effectiveness and tolerability of adjunctive perampanel in the treatment of pediatric patients with uncontrolled epilepsy: a retrospective, single-center, real-world study. Epilepsy Behav. 137, 108961. doi:10.1016/j.yebeh.2022.108961
Gaudio, E., Gienapp, A. J., and Wheless, J. (2019). Perampanel pharmacokinetics in children: correlation of dose with serum concentrations. J. Child. Neurol. 34, 427–431. doi:10.1177/0883073819837465
Gidal, B. E., Ferry, J., Majid, O., and Hussein, Z. (2013). Concentration-effect relationships with perampanel in patients with pharmacoresistant partial-onset seizures. Epilepsia 54, 1490–1497. doi:10.1111/epi.12240
Gidal, B. E., Maganti, R., Laurenza, A., Yang, H., Verbel, D. A., Schuck, E., et al. (2017). Effect of enzyme inhibition on perampanel pharmacokinetics: why study design matters. Epilepsy Res. 134, 41–48. doi:10.1016/j.eplepsyres.2017.04.018
Ikemoto, S., Hamano, S. I., Hirata, Y., Matsuura, R., and Koichihara, R. (2019). Efficacy and serum concentrations of perampanel for treatment of drug-resistant epilepsy in children, adolescents, and young adults: comparison of patients younger and older than 12 years. Seizure 73, 75–78. doi:10.1016/j.seizure.2019.10.023
Ishikawa, N., Tateishi, Y., Tani, H., Kobayashi, Y., and Kobayashi, M. (2019). Clinical profiles associated with serum perampanel concentrations in children with refractory epilepsy. Epilepsy Behav. 94, 82–86. doi:10.1016/j.yebeh.2019.02.004
Johannessen Landmark, C., Johannessen, S. I., and Patsalos, P. N. (2020). Therapeutic drug monitoring of antiepileptic drugs: current status and future prospects. Expert Opin. Drug Metab. Toxicol. 16, 227–238. doi:10.1080/17425255.2020.1724956
Krasowski, M. D. (2010). Therapeutic drug monitoring of the newer anti-epilepsy medications. Pharmaceuticals 3, 1909–1935. doi:10.3390/ph3061909
Li, Y., Dong, N., Qin, Y. X., Dai, H. R., Hu, Y. H., Zhao, Y. T., et al. (2022). Therapeutic drug monitoring of perampanel in children diagnosed with epilepsy: focus on influencing factors on the plasma concentration-to-dose ratio. Epilepsia Open 7, 737–746. doi:10.1002/epi4.12653
Miao, P., Zhu, X., Jin, W., Yu, L., Li, Y., Wang, Y., et al. (2023). Efficacy of perampanel in pediatric epilepsy with known and presumed genetic etiology. Ann. Clin. Transl. Neurol. 10, 1374–1382. doi:10.1002/acn3.51828
Operto, F. F., Pastorino, G. M. G., Mazza, R., Di Bonaventura, C., Matricardi, S., Verrotti, A., et al. (2020). Perampanel tolerability in children and adolescents with focal epilepsy: effects on behavior and executive functions. Epilepsy Behav. 103, 106879. doi:10.1016/j.yebeh.2019.106879
Osterberg, L., and Blaschke, T. (2005). Adherence to medication. N. Engl. J. Med. 353 (5), 487–497. doi:10.1056/NEJMra050100
Patsalos, P. N. (2015). The clinical pharmacology profile of the new antiepileptic drug perampanel: a novel noncompetitive AMPA receptor antagonist. Epilepsia 56, 12–27. doi:10.1111/epi.12865
Patsalos, P. N., Gougoulaki, M., and Sander, J. W. (2016). Perampanel serum concentrations in adults with epilepsy: effect of dose, age, sex, and concomitant anti-epileptic drugs. Ther. Drug Monit. 38, 358–364. doi:10.1097/FTD.0000000000000274
Patsalos, P. N., Spencer, E. P., and Berry, D. J. (2018). Therapeutic drug monitoring of antiepileptic drugs in epilepsy: a 2018 update. Ther. Drug Monit. 40, 526–548. doi:10.1097/FTD.0000000000000546
Reimers, A., Berg, J. A., Burns, M. L., Brodtkorb, E., Johannessen, S. I., and Landmark, C. J. (2018). Reference ranges for antiepileptic drugs revisited: a practical approach to establish national guidelines. Drug Des. devel. Ther. 12, 271–280. doi:10.2147/DDDT.S154388
Rogawski, M. A., and Hanada, T. (2013). Preclinical pharmacology of perampanel, a selective non-competitive AMPA receptor antagonist. Acta Neurol. Scand. 127, 19–24. doi:10.1111/ane.12100
Silva, R., Colom, H., Bicker, J., Almeida, A., Silva, A., Sales, F., et al. (2023). Population pharmacokinetic analysis of perampanel in Portuguese patients diagnosed with refractory epilepsy. Pharmaceutics 15 (6), 1704. doi:10.3390/pharmaceutics15061704
Steinhoff, B. J., Hübers, E., Kurth, C., and Jürges (Kehl-Kork), U. (2019). Plasma concentration and clinical effects of perampanel—the Kork experience. Seizure 67, 18–22. doi:10.1016/j.seizure.2019.02.022
Sultana, B., Panzini, M. A., Veilleux Carpentier, A., Comtois, J., Rioux, B., Gore, G., et al. (2021). Incidence and prevalence of drug-resistant epilepsy: a systematic review and meta-analysis. Neurology 96 (17), 805–817. doi:10.1212/WNL.0000000000011839
Tabuchi, H., Shiba, S., Yasuda, S., Ohnishi, A., and Shin, J. G. (2018). Pharmacokinetics of perampanel in healthy Korean, white, and Japanese adult subjects. Clin. Pharmacol. Drug Dev. 7 (6), 613–620. doi:10.1002/cpdd.581
Takenaka, O., Ferry, J., Saeki, K., and Laurenza, A. (2018). Pharmacokinetic/pharmacodynamic analysis of adjunctive perampanel in subjects with partial-onset seizures. Acta neurol. Scand. 137 (4), 400–408. doi:10.1111/ane.12874
Keywords: epilepsy, seizure, pediatric, perampanel, therapeutic drug monitoring
Citation: Yu L, Chen M, Liu J, Yu Z, Feng J and Dai H (2023) Initial therapeutic target attainment of perampanel in pediatric patients with epilepsy. Front. Pharmacol. 14:1209815. doi: 10.3389/fphar.2023.1209815
Received: 21 April 2023; Accepted: 31 October 2023;
Published: 15 November 2023.
Edited by:
Pasquale Parisi, Sapienza University of Rome, ItalyReviewed by:
Yang Xu, First Affiliated Hospital of Wannan Medical College, ChinaAna Fortuna, University of Coimbra, Portugal
Alberto Verrotti, University of L’Aquila, Italy
Copyright © 2023 Yu, Chen, Liu, Yu, Feng and Dai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianhua Feng, aHpoejg3MDgzODg2QHpqdS5lZHUuY24=; Haibin Dai, aGFpYmluZGFpQHpqdS5lZHUuY24=
†These authors have contributed equally to this work
 Lingyan Yu1†
Lingyan Yu1† Meng Chen
Meng Chen Zhenwei Yu
Zhenwei Yu Jianhua Feng
Jianhua Feng Haibin Dai
Haibin Dai