
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CORRECTION article
Front. Pharmacol. , 03 May 2023
Sec. Respiratory Pharmacology
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1207356
This article is a correction to:
Elexacaftor-Tezacaftor-Ivacaftor Treatment Reduces Abdominal Symptoms in Cystic Fibrosis-Early results Obtained With the CF-Specific CFAbd-Score
 Jochen G. Mainz1,2*†
Jochen G. Mainz1,2*† Carlos Zagoya1†
Carlos Zagoya1† Louise Polte1
Louise Polte1 Lutz Naehrlich3,4
Lutz Naehrlich3,4 Lenny Sasse3
Lenny Sasse3 Olaf Eickmeier5
Olaf Eickmeier5 Christina Smaczny5
Christina Smaczny5 Anton Barucha1
Anton Barucha1 Lilith Bechinger1
Lilith Bechinger1 Franziska Duckstein1
Franziska Duckstein1 Ludwik Kurzidim1,6
Ludwik Kurzidim1,6 Patience Eschenhagen6
Patience Eschenhagen6 Laura Caley7
Laura Caley7 Daniel Peckham7,8‡
Daniel Peckham7,8‡ Carsten Schwarz6‡
Carsten Schwarz6‡A Corrigendum on
Elexacaftor-Tezacaftor-Ivacaftor treatment reduces abdominal symptoms in cystic fibrosis-early results obtained with the CF-specific CFAbd-Score
by Mainz JG, Zagoya C, Polte L, Naehrlich L, Sasse L, Eickmeier O, Smaczny C, Barucha A, Bechinger L, Duckstein F, Kurzidim L, Eschenhagen P, Caley L, Peckham D and Schwarz C (2022). Front. Pharmacol. 13:877118. doi: 10.3389/fphar.2022.877118
In the published article, there was an error in Figure 1 as published. The figure itself including the proportion of changes is correct. However, the numbers representing percent changes in reduction of symptoms do not correspond to those mentioned in the text but rather to a preliminary calculation from a smaller cohort of patients. Noteworthy, the improvement in abdominal symptoms assessed with the CFAbd-Score during ETI is in fact markedly higher in the now corrected version. The corrected Figure 1 and its caption appear below.
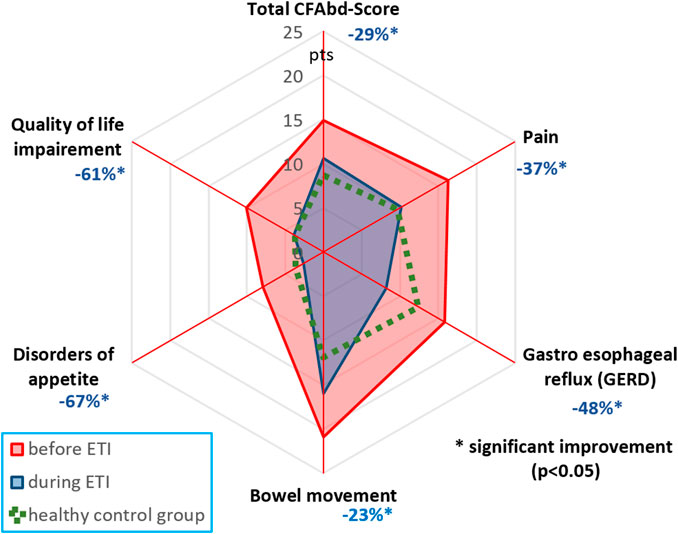
FIGURE 1. CFAbd-Score changes for the whole cohort and its 5 domains after therapy initiation (Table 1). Percent changes are calculated from estimated marginal means (EMMs) at week 24 of ETI therapy.
The authors apologize for this error and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: gastrointestinal, patient reported outcome measure, CFTR modulators, elexacaftor, symptom score
Citation: Mainz JG, Zagoya C, Polte L, Naehrlich L, Sasse L, Eickmeier O, Smaczny C, Barucha A, Bechinger L, Duckstein F, Kurzidim L, Eschenhagen P, Caley L, Peckham D and Schwarz C (2023) Corrigendum: Elexacaftor-Tezacaftor-Ivacaftor treatment reduces abdominal symptoms in cystic fibrosis-early results obtained with the CF-specific CFAbd-Score. Front. Pharmacol. 14:1207356. doi: 10.3389/fphar.2023.1207356
Received: 17 April 2023; Accepted: 21 April 2023;
Published: 03 May 2023.
Edited and reviewed by:
Frederic Becq, University of Poitiers, FranceCopyright © 2023 Mainz, Zagoya, Polte, Naehrlich, Sasse, Eickmeier, Smaczny, Barucha, Bechinger, Duckstein, Kurzidim, Eschenhagen, Caley, Peckham and Schwarz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jochen G. Mainz, ai5tYWluekBrbGluaWt1bS1icmFuZGVuYnVyZy5kZQ==
†These authors have contributed equally to this work
‡These authors share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.