- 1School of Science and the Environment, Memorial University of Newfoundland, Corner Brook, NL, Canada
- 2Biotron Experimental Climate Change Research Centre/Department of Biology, University of Western Ontario, London, ON, Canada
Cannabis sativa, also known as “hemp” or “weed,” is a versatile plant with various uses in medicine, agriculture, food, and cosmetics. This review attempts to evaluate the available literature on the ecology, chemical composition, phytochemistry, pharmacology, traditional uses, industrial uses, and toxicology of Cannabis sativa. So far, 566 chemical compounds have been isolated from Cannabis, including 125 cannabinoids and 198 non-cannabinoids. The psychoactive and physiologically active part of the plant is a cannabinoid, mostly found in the flowers, but also present in smaller amounts in the leaves, stems, and seeds. Of all phytochemicals, terpenes form the largest composition in the plant. Pharmacological evidence reveals that the plants contain cannabinoids which exhibit potential as antioxidants, antibacterial agents, anticancer agents, and anti-inflammatory agents. Furthermore, the compounds in the plants have reported applications in the food and cosmetic industries. Significantly, Cannabis cultivation has a minimal negative impact on the environment in terms of cultivation. Most of the studies focused on the chemical make-up, phytochemistry, and pharmacological effects, but not much is known about the toxic effects. Overall, the Cannabis plant has enormous potential for biological and industrial uses, as well as traditional and other medicinal uses. However, further research is necessary to fully understand and explore the uses and beneficial properties of Cannabis sativa.
1 Introduction
Throughout human civilization, there has been a pursuit of plants for their unique potential, including medicinal use. Evidence of this dates back to 60,000 years, with a recent discovery of a 5,000-year-old Sumerian clay tablet that confirms the use of medicinal plants in drug production (Sumner, 2000). Natural resources like medicinal plants, also known as green medicine, are gaining popularity worldwide due to their safety, effectiveness, cultural acceptance, and lower risk of adverse effects compared to synthetic medications (Mustafa et al., 2017). Today, traditional botanical medicines are widely used to treat human health problems, with over 80% of the global population depending on them (Mander and Liu, 2010).
Cannabis sativa L. (2n ¼ 20) is a well-known plant that has been around since the beginning of time (Small, 2017). This annual plant is a member of the family Cannabaceae and a widespread plant found in varied environments (Andre et al., 2016). It has been used by humans for over 5,000 years and is one of the oldest plant sources of food and fiber (Appendino et al., 2008). The botanical types of Cannabis sativa differ in terms of their chemical content, plant growth habits, agronomic requirements, and processing (Datwyler and Weiblen, 2006). Cannabis flowers and leaves have a distinctive aroma, and the plant’s extracts include a variety of beneficial flavonoids, terpenes, and other compounds that are efficient insecticides, fungicides, and therapeutic agents (Pellati et al., 2018). The flower, leaves, oil, and trichome of the plant have been shown to be cytotoxic, antimicrobial, antioxidant, antihypertensive, antipyretic, and appetite-stimulating (Russo and Marcu, 2017). The flower extracts with antioxidant activity have been shown to have health-promoting and anti-aging properties, and are utilized to treat a variety of metabolic and chronic disorders, including glaucoma, pain, depression, cancer, liver disease, cardiovascular diseases, inflammation, and metabolic syndrome (Nallathambi et al., 2017). As an agricultural crop, industrial Cannabis (hemp), is a plant that may be harvested for its fiber (Johnson, 2014). While in the cosmetic industry, it is used for skincare products such as anti-aging creams and hair food (Schettino et al., 2021). Traditionally, the seeds are used for making oil, while the leaves were the second most consumed part of the plant and were used in various ways, such as seasoning, baking, flour, and added to meals (Iftikhar et al., 2021; Kuppuram, 2022; Xu et al., 2022).
Even though Cannabis is used in many ways, the drug’s unclear legal status worldwide has made it hard to study for the last century (Smith et al., 2014). In addition, there has not been much information about comprehensive analysis of the plant that can show the plant’s usefulness in all aspects. In this review, Cannabis’ potential is discussed in length to provide thorough and up-to-date information on the Cannabis plants.
2 Ethnobotany of Cannabis
2.1 Ecology and distribution
Cannabis sativa’s origin is unknown, but it is believed to have come from temperate regions in Asia, specifically the southern Caspian region, Siberia, China, or the Himalayas (Minelli, 2015). However, due to widespread transportation and modification by humans over the past 6,000 years, it is challenging to determine its original geographic range or whether a plant collected in nature is a primitive wild type or has been influenced by human domestication (Sharma, 1979). “Weed” is the most common informal name for the marijuana form of Cannabis sativa, and it accurately describes the species as a weed that grows primarily in habitats created or modified by humans (Small, 2015). It can be found in various places such as fields, trash heaps, vacant lots, pastures, ditches, creeks, and open woods. However, it is poorly adapted to infiltrating established perennial stands and typically invades only after the soil has been recently disturbed or plowed (Small, 2017).
Except in drainage channels, where it is extremely well suited, weedy Cannabis sativa is a slow colonizer, spreading slowly throughout the landscape. It is possible to judge the ecology of Cannabis sativa prior to human intervention based on the circumstances and adaptations of existing wild-growing populations of this plant species (Small, 2017). By examining the circumstances and adaptations of these populations, researchers can gain insight into the plant’s natural habitat, growth patterns, and environmental interactions. For example, studying the genetic diversity of wild-growing Cannabis sativa populations can provide information on the plant’s evolutionary history and geographic distribution (Small, 2015). Additionally, analyzing the physical characteristics of wild Cannabis sativa plants, such as their size, leaf shape, and stem structure, can provide clues about their adaptation to various environmental conditions (Ren et al., 2021).
2.2 Taxonomic classification and common names
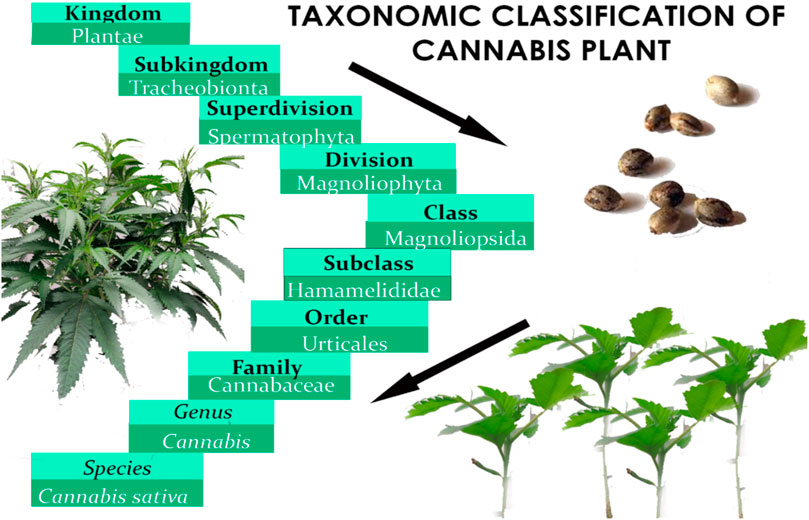
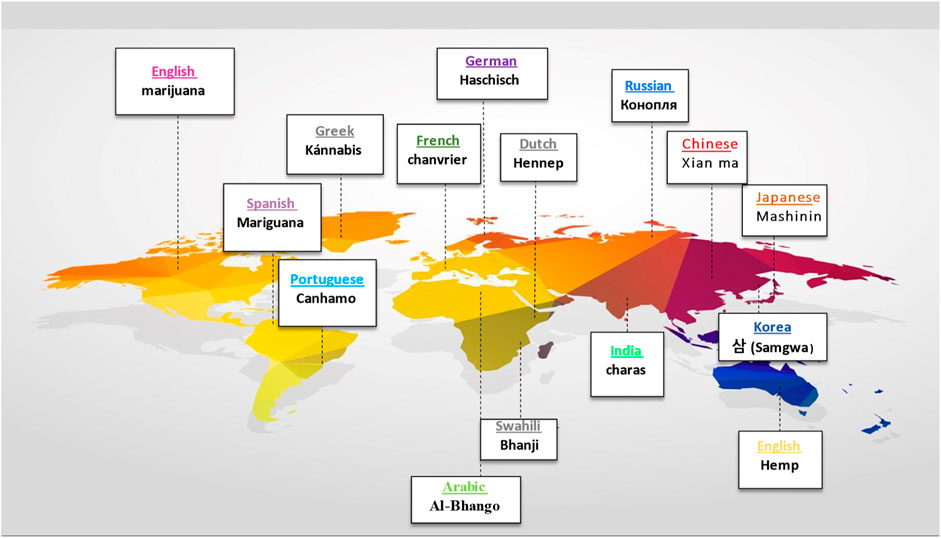
Before Linnaeus published Species Plantarum in the 18th century, domestic hemp was known by various names, including Cannabis angustifolia, Cannabis sativa, and Cannabis indica (Linnaeus, 1753). Later, Jean-Baptiste Lamarck proposed a division between extensively cultivated Cannabis species in western continents and the wild variety found in India (Erkelens and Hazekamp, 2014). After 50 years, Lindley reclassified Cannabis under Linnaeus’ classification system, affirming the plant’s monospecific status for the rest of the century (Lindley, 2011). Below is the botanical classification of Cannabis plant (Figure 1) (Small, 2017). In the early 20th century, a new species called Cannabis ruderalis emerged, but it was not until 1975 that the restoration of the Cannabis indica species to its current name was proposed (Holland, 2010). Figure 2 presents the name of Cannabis in some popular languages.
Cannabis is a polymorphic plant, and chemotaxonomic markers are effective in differentiating between different Cannabis germplasms and screening for hybrids (Piomelli and Russo, 2016). Small and Cronquist (1976) used biphasic techniques (use of distinct approaches) to identify the four subspecies of Cannabis sativa, including sativa var. sativa, sativa var. spontanea, indica var. indica, and indica var. kafiristanica based on morphological and chemical characteristics such as fruit morphology and THC content (Schultes et al., 1975; Pollio, 2016). Both variants of the subspecies sativa are widely cultivated in North America, Europe, and Asia, and have low intoxicating potential when compared to other Cannabis cultivars (Small and Cronquist, 1976). Meanwhile, the subspecies Indica’s variants have a strong intoxicating potential and are primarily found in the Asiatic Continent (Pollio, 2016; Small, 2017).
2.3 Legality-based classification
Despite being an arbitrary term that does not reflect the drug’s properties, Cannabis is classified as a “narcotic” (i.e., illegal drug) in the legal world (Small, 2017). An illegal drug is defined as a chemical or preparation associated with severe punishments due to its actual or suspected detrimental properties (Smith et al., 2014). Cannabis has been criminalized since the Second World War due to its popular use as a recreational substance, leading to limited research and commercial development in the sector. As a result, research and commercial development on the plant was prohibited for most of the 20th century (Appendino et al., 2014). Cannabis sativa became the most commonly cultivated black market crop in the Western world after World War II, leading to the allocation of significant law enforcement resources to remove the plants (Chouvy, 2019). Scientific investigations in Western countries were mostly approved for criminal justice-related forensic studies to assist law enforcement or medical and social-related studies to document and alleviate negative consequences (Chandra et al., 2008).
Criminalizing Cannabis has led to high law enforcement costs and social instability, and many jurisdictions are looking to reduce penalties for its possession and consumption (Small, 2015). The legalization of medical Cannabis is widely accepted, but recreational use is still under debate (Cruz et al., 2016). While punishments for illegal drug use have softened in several countries due to increased public acceptance, although, capital punishment is still a possibility in some Asian countries (Chandra et al., 2008; Small, 2017). The decriminalization of Cannabis use is not unique to the North American continent. More than forty countries have legalized the use of marijuana for medical or recreational purposes. Among these countries are Argentina, Germany, Chile, Colombia, and South Africa (Chouvy, 2019). Additionally, Canada, 18 United States states, and two territories—the District of Columbia and the Australian Capital Territory—have legalized Cannabis. New strains are approved for use in Canada until 2023, and Health Canada has issued regulations amending the Cannabis Act and Cannabis Regulations to ensure proper regulation of Cannabis (Canada, 2018; Caplan, 2018).
2.4 Therapeutic based classification
The cannabinoids in Cannabis are unique terpene phenolic substances. Approximately 100 cannabinoids are produced in epidermal trichomes but in small quantities (Mölleken and Theimer, 1997). As discussed by Small (2017), Cannabis’ psychological effects have been ambiguously called “narcotic” in popular, legal, and scientific contexts. Cannabis and opioids are legally grouped, but they are pharmacologically distinct. “Narcotic” comes from “narcosis,” a substance that induces sleep, but it is used to refer to any medicine that induces sleep, stupor, or insensibility (Macdonald and Rotermann, 2017). In moderate amounts, psychoactive cannabinoids such as THC and CBD in Cannabis can induce sedation (Piomelli and Russo, 2016). CBD has a stimulant effect in low and moderate concentrations, and only in high concentrations has a soothing effect (Piomelli and Russo, 2016). Cannabis sativa’s abundant myrcene is likewise sedative (Russo, 2014). There is still some disagreement on how Cannabis should be pharmacologically classified (Kalant, 2010). In some cases, Cannabis has been classified as a sedative-hypnotic-general anesthetic, a mixed stimulant-depressant, a mild hallucinogen, and a psychedelic (Degenhardt et al., 2015). In surgical and dental procedures, it is referred to as a sedative-hypnotic general anesthetic. Cannabis’s psychedelic, hallucinogenic, psychotomimetic, and psychotic properties are misrepresented by terms like “psychedelic” (Brewster, 2019). While “hallucinogenic” is no longer acceptable, “psychoactive,” “euphoric,” or “intoxicating” are the best pharmacological names for Cannabis (Small, 2017; Brewster, 2019). According to Troutt and DiDonato (2015), medical Cannabis users in the United States are characterized by daily dosing and weekly consumption of 6–9 g (Ko et al., 2016). In Canada, 42% of medical marijuana patients consume 2 to 3 times a day, and 40% consume more than 14 g per week. In Canada and the United States, most patients inhale (Ilgen et al., 2013; Bonn-Miller et al., 2014). Surprisingly, only 53% of adult Cannabis users in the United States use Cannabis purely for recreational purposes, while 47% use it “in part or totally for medicinal purposes,” and 10% use it solely for medicinal purposes (Ko et al., 2016). Research shows that in 2004, about 4% of Canadians over the age of 14 reported using Cannabis in the past year for self-identified medical problems (Schauer et al., 2016). Cannabis remains the most commonly used drug globally, with more than 4% of the global population aged 15–64 (approximately 209 million people) using Cannabis in 2020, a 23% increase from 170 million in 2010 (Richards et al., 2020). Approximately 27% of Israeli adults consumed Cannabis in 2020, making it the country with the highest incidence of Cannabis use as of that year. (Bar-Or et al., 2021). Comparatively, the United States has a lower incidence of Cannabis use, with approximately 17% of the adult population reported to have consumed Cannabis within the same period (Sarvet et al., 2018). In Europe, Czechia has the highest incidence of Cannabis use of 11.1% among their adult population (Arnarsson et al., 2018). Forecasts put the global Cannabis market at $82.3 billion in 2027, a significant projection of 24.3% with $27.7 billion recorded in 2022 (Chen et al., 2021). The United Nations Office on Drugs and Crime (UNODC) identifies Morocco as the largest producer of ‘psychoactive marijuana plants’ worldwide (Kitchen et al., 2022). However, in terms of revenue generation, the United States leads in terms of the sale of medical Cannabis, with an annual total of 10 billion dollars, a significant portion of which comes from therapeutic marijuana (Kilmer and MacCoun, 2017). In Europe, Germany leads in the sale of medical marijuana, with an estimate 87.2 million dollars (Häuser et al., 2018). Between 1995 and 2005, 19 African countries reported the cultivation of Cannabis within their borders. In 2005, worldwide, Cannabis production was estimated at 42,000 metric tons, with Africa alone accounting for 25% of the total (Akyeampong, 2005).
2.5 Morphological characteristics of Cannabis
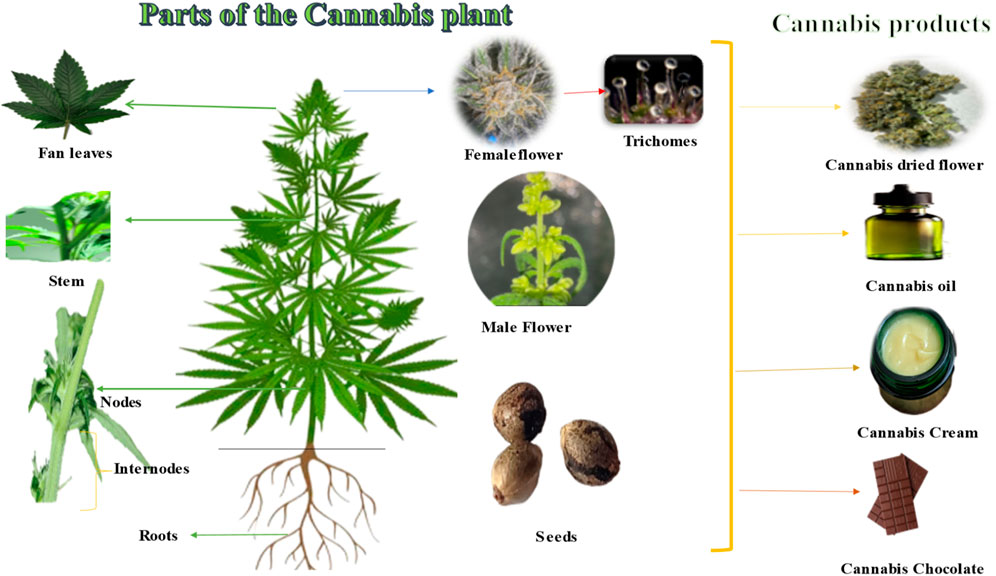
Cannabis sativa L. is an annual plant that can reach up to 5 m in height and has upright stems with palmate leaves consisting of 5–7 linear-lanceolate leaflets (Figure 3). Male flowers lack petals and grow in axillary or terminal panicles, while female flowers have a single ovule and a perianth that is tightly attached (Farag and Kayser, 2017; Bonini et al., 2018). Trichomes, which are glandular protuberances that cover the plant’s leaves, bracts, and stems, are present in high concentrations (Bonini et al., 2018). The fruit of each flower is a single small smooth light brownish-grey fruit that is then passed on to the next-generation. Female flowers grow at the end of the stem and in the axils. They have one ovule and a perianth that is tightly connected. Male flowers, on the other hand, have five yellowish petals and five anthers (Farag and Kayser, 2017).
3 Phytochemistry of Cannabis
The number of natural chemicals isolated from Cannabis sativa L. has not significantly increased in recent years, despite over 500 compounds being discovered so far (Pellati et al., 2018; Al Ubeed et al., 2022). In 1980, 423 compounds were discovered, which grew to 483 by 1995 (Matsuda et al., 1990; Dos Santos and Romão, 2023). Currently, 566 compounds have been identified and isolated which constitutes over 18 classes of different secondary metabolites found in the plant. These substances have been found to be highly abundant in the flowers and leaves of the plant (Kopustinskiene et al., 2022; Odieka et al., 2022). Out of this number, 125 are cannabinoids, 198 are non-cannabinoids and 120 are terpenes, constituting a total of 443. The rest of the substances identified in the plant in 2021 include 2 alkaloids, 34 flavonoids, 42 phenols and 3 sterols (Al Ubeed et al., 2022). The aromatic quality of female Cannabis plants is due to the terpenes they produce, such as pinene, limonene, terpineol, and borneol (McPartland et al., 2001). These terpenes have insect-repellent properties and inhibit the growth of neighboring vegetation. The glandular trichomes on the plant produce a resin that acts as a sophisticated defense mechanism against insects and has the potential to serve as an antibiotic and antifungal agent. These trichomes contain secondary metabolites like phytocannabinoids and terpenoids that are responsible for the plant’s defense and interaction with herbivores and pests, as well as its characteristic scent (Andre et al., 2016). The various phytochemicals are summarized below.
3.1 Cannabinoids
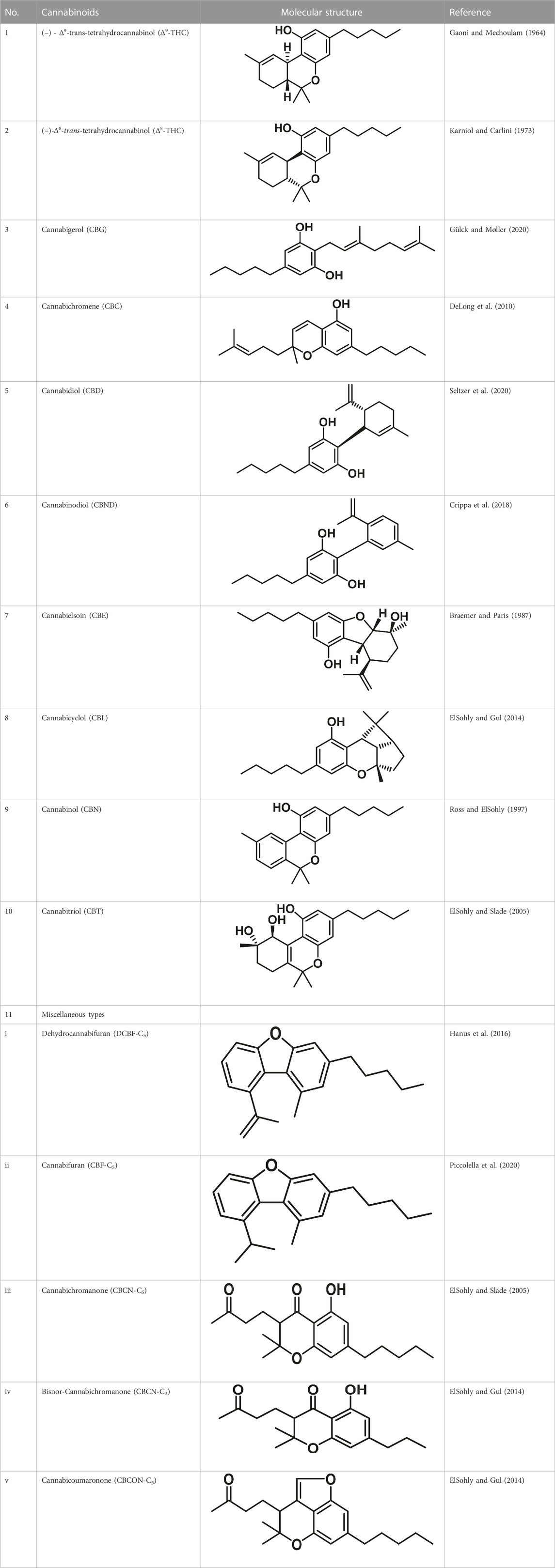
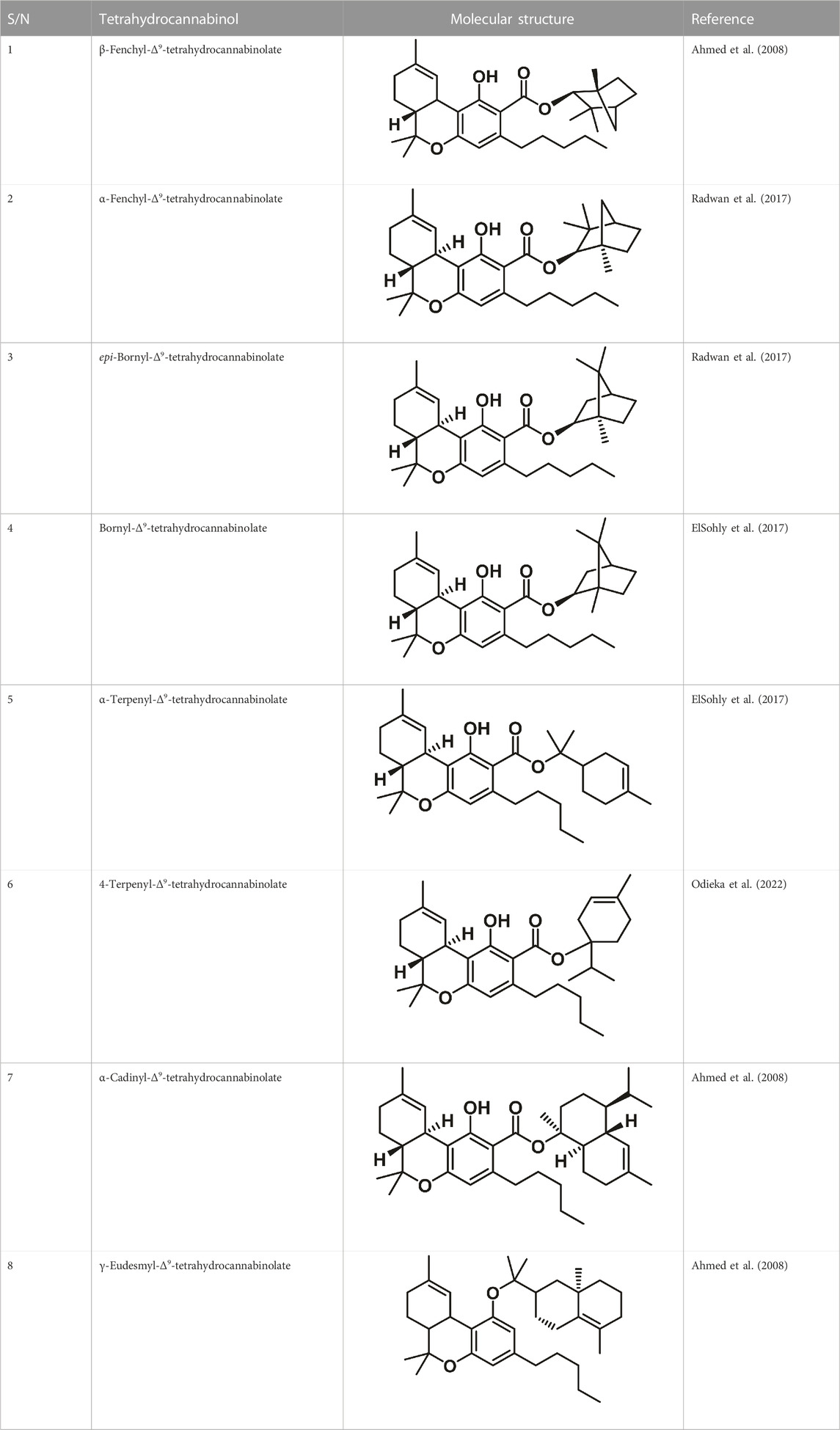
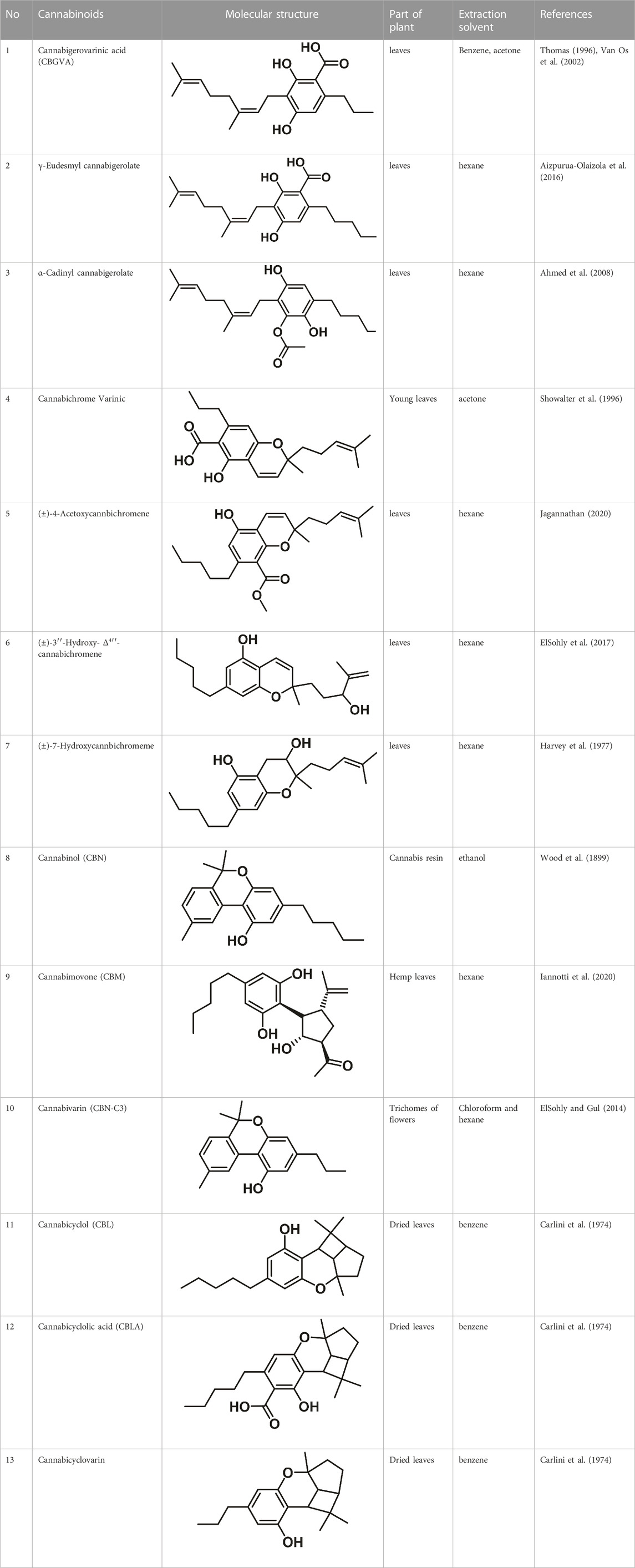
Therapeutic marijuana has a high level of tetrahydrocannabinol (THC), but minimal levels of cannabidiolic acid (CBDA) and cannabidiol (CBD). Cannabinoids undergo decarboxylation during drying, storage, and thermal processing, converting from an acidic to a neutral state. There are now many types of cannabinoids, not just those found in Cannabis, and the term “phytocannabinoids” has been used for those that naturally come from the plant (Radwan et al., 2021). A total of 120 phytocannabinoids have been identified and divided into 11 categories (Berman et al., 2018; Bonn-Miller et al., 2018). Table 1 lists the 11 subclasses of 120 phytocannabinoids.
3.1.1 (−)-Delta-9-trans-tetrahydrocannabinol (Δ9-THC) type
Gaoni and Mechoulam (1971) discovered the structure of Δ9-THC and explained its psychoactive properties. Rhee et al. (1997) used X-ray and proton magnetic resonance (1H NMR) studies to determine the precise conformation of Δ9-THC (Rhee et al., 1997). Dewey (1986) identified Δ9-THCA-A from Cannabis extract, which is photosensitive and cannot form crystals (structure as compound 2 shown in Table 1) (Dewey, 1986). Devane et al. (1988) discovered Δ9-THCA-B (compound 3 in Table 1) from Cannabis. Cannabis sole, a flat form of illicit Cannabis, was eluted from the silicic acid matrix using a 1:1 diethyl ether/petroleum ether solution. Δ9-THCA-B was shown to be more polar than Δ9-THCA-A in thin-layer chromatography (TLC). The determination of the crystalline structure of Δ9-THCA-B was due to the differences in biochemical properties between Δ9-THCA-B and Δ9-THCA-A (Galal et al., 2009).
Romano and Hazekamp (2019) isolated Δ9-tetrahydrocannabivarin (Δ9-THCV) using a mixture of 5 g of Cannabis and 200 mL of petroleum ether and dissolved it in 100 mL of absolute ethyl alcohol (EtOH) (Romano and Hazekamp, 2019). Spectroscopic evidence for Δ9-trans-tetrahydrocannabidiolic acid (Δ9-THCVA) was reported by Matsuda et al. (1990), followed by mass spectrometric evidence data (Pate, 1994). The analysis of 51 samples sourced from various geographic regions led to research on the C3 homologs of Cannabis (Turner et al., 1973). Balcke et al. (2014) discovered a new homologue of Δ9-THC with a methyl side chain, 9-tetrahydrocannabiorcol (Δ9-THC-C1), in an extract of Brazilian Cannabis (Balcke et al., 2014). The concentration of Δ9-THC-C1 was low, so it was not expected to have a significant impact on the drug’s biological action. Dewey (1986) identified Δ9-trans-THCA-C4 and Δ9-trans-THC-C4 using GC-MS, as well as Δ9-trans-tetrahydrocannabiorcolic acid (Δ9-THCA-C1) (Balcke et al., 2014). Several techniques, including NMR spectroscopy and Gas Chromatography-Mass Spectrometry (GC-MS), were used to identify monoterpene or sesquiterpene esters of 9-tetrahydrocannabinolic acid A in Cannabis sativa L. These esters were found to be precursors to Δ9-THC and were broken down into their constituents when subjected to high temperatures during GC-MS analysis (Caspi et al., 2005). Chromatographic methods, such as vacuum liquid chromatography (VLC), High-performance liquid chromatography (HPLC), and Supercritical fluid chromatography (SPC) were used to isolate these cannabinoid esters from high-potency C. sativa varieties. Cannabisol, a dimeric cannabinoid, was also isolated using flash silica gel column chromatography from Cannabis samples that contained a significant amount of CBG (Costa et al., 2007). Eight new substances of the tetrahydrocannabinol family are listed in Table 2.
3.1.2 Cannabigerol (CBG) type
Cannabigerol (CBG) is the first substance purified from Cannabis resin (CBG-C5, compound 5 in Table 1) (Mechoulam and Shvo, 1963). Mechoulam et al. (1995) were the first to describe the condensation of geranyl pyrophosphate in the formation of CBG. Mechoulam et al. (1995) discovered that cannabidiolic acid (CBGA) was the most polar acid component. They also found the methyl ester of CBGA in the acidic part of a single extract of Cannabis (Mechoulam et al., 1995).
Cannabigerovarinic acid (CBGVA–the structure of compound 1 in Table 3) isolated from an extract of the dried leaves of Thai Cannabis was found to be a minor component of the extract (Thomas, 1996; Van Os et al., 2002). After extraction of the acid fraction from the leaves using silica gel column chromatography, the acid fraction was eluted from the dried leaves using a mixture of hexane, ethyl acetate, and a ratio of 5:1 of benzene to acetone. The transparent needle-like CBGVA crystals were obtained following recrystallization in hexane: ethyl acetate solution in a ratio of 3:1.
Cannabinerolic acid (CBRA) and cannabigerolic acid (CBGA) are both acidic cannabinoids that are produced in the Cannabis plant. The primary difference between the two is the location of the double bond in their molecular structures (Taura et al., 1996). CBGA is the precursor to many of the other cannabinoids found in Cannabis, including THC and CBD. It is synthesized by the plant from olivetolic acid and geranyl pyrophosphate. CBGA can be further converted into THCA, CBDA, or CBCA, which are then decarboxylated to produce THC, CBD, or CBC (cannabichromene) (Morimoto et al., 1998). Taura et al. (1995) described a procedure to purify cannabinerolic acid from an air-dried Mexican strain of C. sativa by extracting the leaves with benzene. The extraction was concentrated and loaded onto a silica gel column, then extracted with a 9:1 (v/v) benzene/acetone mixture after dissolving the residue in acetone and removing any insoluble particulates. High-potency cannabigerolic acid esters, i.e., γ-eudesmyl cannabigerolate and α-cadinyl cannabigerolate were also recovered from C. sativa in another study (Kinghorn et al., 2017). The hexane extract of Cannabis was purified by chromatography to obtain the two cannabigerolic acid esters. Both γ-eudesmyl cannabigerolate and α-cadinyl cannabigerolate were shown to be esters of CBGA by the data obtained from their respective spectroscopic analyses (Wallace et al., 2001). van Winkel (2011) identified six substances using flash silica gel analysis of a hexane extract, including 5-acetyl-4-hydroxycannabigerol, 4-acetoxy-2-geranyl-5-hydroxy-3-n-pentylphenol, (±)-6,7-trans-epoxycannabigerolic acid, (±)-6,7-cis-epoxycannabigerolic acid and (±)-6,7-cis-epoxycannabigerol (Van Waes et al., 2012). Appendino et al. (2008) isolated a novel, polar dihydroxy cannabigerol derivative (carmagerol) from the Cannabis leaves. Taylor et al. (2010) identified sesquicannabigerol, a lipophilic analogue of cannabigerol, in the waxy section of the fiber hemp cultivar Carma. Methanolic potassium hydroxide (·KOH) was used to hydrolyze the wax, and it was purified using gravity silica gel column chromatography before being subjected to flash chromatography over neutral alumina (Taylor et al., 2010).
3.1.3 Cannabichromene (CBC) type
Matsuda et al. (1990) reported the independent discovery of cannabichromene (CBC-C5), which is listed as compound 6 in Table 1. Later, CBC-C5 was isolated from dried the leaves at a yield of 1.5% using a method outlined by Mechoulam et al. (1995) and extracted cannabichromenic acid (CBCA) from the benzene percolate. The production of CBCA using a solvent system of 1:1 hexane and ethyl acetate was confirmed using NMR spectroscopy (Borah and Bordoloi, 2020).
The study found that cannabichromenic acid (CBCA) showed similarities to the structure of THCA in its infrared (IR) spectra due to the placement of the carboxyl group and the presence of intermolecular hydrogen bonding. The researchers isolated cannabichromevarin (CBCV), a brownish-red cannabinoid, from neutral cannabinoids obtained from Thai Cannabis leaves through multiple passes through a silica gel column and elution with benzene and 20:10:1 benzene-hexane (Showalter et al., 1996). Cannabichromevarinic acid (CBCVA), was isolated in young leaves of Cannabis, using acetone (Showalter et al., 1996). Synthesis was used to validate the structure of natural CBCVA. Lakhan and Rowland (2009) reported the isolation of three new cannabichromene type cannabinoids from high-potency benzene extract of the flowers (trichomes). These cannabinoids are named (±)-4-acetoxycannabichromene, (±)-3″-hydroxy-Δ4″-cannabichromene and (±)-7-hydroxycannabichromeme (Lakhan and Rowland, 2009).
3.1.4 Cannabidiol (CBD) type
The two main metabolites of non-psychotropic (fiber-type) Cannabis cultivars are cannabidiol (CBD) and cannabidiolic acid (CBDA), their structures were shown in Table 1 as compounds 7 and 8, respectively. CBD was isolated, from the ethanol extract of leaves, and after being left for several weeks, the oily CBD was crystallized (Karniol and Carlini, 1973). Li (1974) reported its synthesis and absolute configuration as (−)-trans-1R, 6R. Cannabidavarin (CBDV) was isolated from an ethanol extract of Cannabis olein (flower), which was chromatographed on silica gel (Iwamura et al., 2001). Howlett et al. (2002) extracted neutral cannabinoids from the ethanol extract of leaves to produce cannabidiol monomethyl ether (CBDM) (M-1). Benzene was used to elute the cannabinoids after they had been chromatographed on Florisil. To produce CBDM, the eluted fraction was rechromatographed on silica gel and eluted with a ratio of 3:1 hexane/benzene.
Cannabis resin and leaves that had been crushed were percolated with ethyl acetate to produce a residue that was filtered and concentrated. This residue was derivatized prior to GC-MS analysis. The mass and methylene unit of cannabidiol-C4 allowed for its identification (Harvey, 1976). Hall and Degenhardt (2007) extracted cannabidiolic acid (CBDA) from the benzene extract of Thailand Cannabis. Cannabimovone (CBM) is a polar cannabinoid that was isolated from an acetone extract of Cannabis sativa L. leaves which is not psychoactive (Hayakawa et al., 2008).
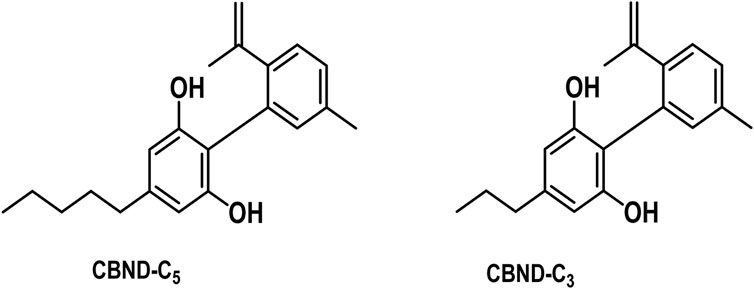
3.1.5 Cannabidiol (CBND) type
The aromatized derivatives of CBD are called CBND-type cannabinoids (Figure 4). The two compounds in this subclass that are characterized are cannabidiol (CBND-C5) and cannabidiol (CBND-C3) (Gaoni and Mechoulam, 1964). By using a hexane-ether extract of Lebanese Cannabis (resinous trichomes), Gorelick and Heishman (2006) were able to successfully isolate cannabidiol. GC-MS analysis revealed the presence of cannabidiol-C3, the propyl homolog of cannabidiol-C5 (Bhattacharyya et al., 2010).
3.1.6 Cannabielsoin (CBE) type
The Cannabielsoin (CBE-C5), Cannabielsoic acid A (CBEA-C5 A), Cannabielsoic acid B (CBEA-C5 B), Cannabielsoin-C3 (CBE-C3), and Cannabielsoic-C3 acid B (CBEA-C3 B) are the five cannabielsoin-type cannabinoids present in Cannabis (Mechoulam et al., 1995). CBE was isolated from an ethanolic extract of hashish (resinous trichomes of flowers) originating in Lebanon (Di Forti et al., 2009). CBEA-C5 A and CBEA-C5 B were extracted from a benzene extract of Cannabis (resinous trichomes) that was grown in Lebanon (Mechoulam and Shvo, 1963).
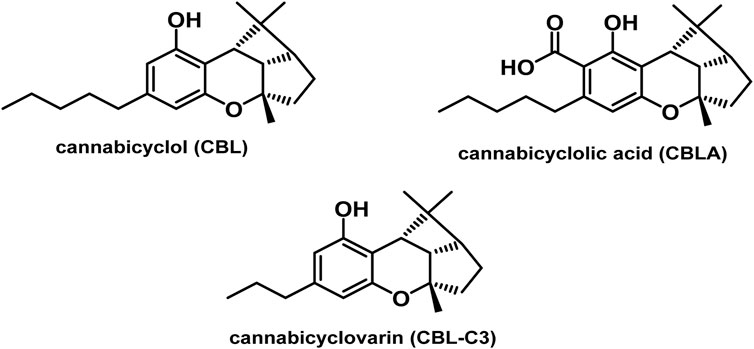
3.1.7 Cannabicyclol (CBL) type
The only compounds that have been identified from this subclass are known as cannabicyclol (CBL), cannabicyclolic acid (CBLA), and cannabicyclovarin (CBL-C3) (Figure 5) (Carlini et al., 1974). Benowitz and Jones (1981) are credited with being the first to identify CBL. They used TLC to isolate CBL from a variety of benzene extract of dried leaves of Cannabis samples.
3.1.8 Cannabinol (CBN) type
Cannabinol (CBN) was given its name for the first time in 1896 (Wood et al., 1899). CBN was made into oil by extracting Cannabis resin using ethanol and heating it. After some time, the oil was acetylated to get pure CBN in the form of its acetate. Bhattacharyya et al. (2009) were able to correctly estimate the structure of CBN. A crude acidic fraction of hashish was used to isolate cannabinolic acid A (CBNA), which was then esterified with diazomethane and purified as its methyl ester on an acid-washed alumina column (Mechoulam and Shvo, 1963). Cannabivarin (CBN-C3) was extracted using a mixture of chloroform and hexane from Nepalese hashish (resin trichomes of flowers), and the structure of the compound was validated by mass spectrum data (ElSohly and Gul, 2014). A summary of some isolated cannabinoids is presented in Table 3.
3.2 Other phytochemicals in Cannabis
3.2.1 Terpenes
Terpenes are aromatic compounds that are found in many plants, and they perform various biological roles, such as attracting pollinators and protecting against predation (Tetali, 2019). In the Cannabis plant, terpenes are stored as essential oils. Currently, over 200 distinct terpenes have been identified in Cannabis, with most of them being discovered through steam distillation (Booth and Bohlmann, 2019).
Terpene concentrations can vary due to various genetic factors. In Cannabis flowers, terpenoid concentrations were found to range from 1% up to 10% within the trichomes as of 2009. However, selective breeding has led to an increase in terpenoid concentrations found in flowers in recent years, with some chemovars exhibiting concentrations of 3.5% or higher (Feder et al., 2021). Currently, over 50 different terpenes have been identified in Cannabis, with a few dominating compounds classified as the “terpene super class,” including linalool, ocimene, limonene, myrcene, α-pinene, humulene, β-caryophyllene, and terpinolene (Liktor-Busa et al., 2021). Similarly, Fischedick and others (2017) analyzed Cannabis samples and classified them into five distinct groups based on the above terpenoid classifications (Fischedick, 2017).
Secondly, several terpenes found in Cannabis exist as hydrocarbons which are direct products of terpene synthase enzymes as compared to complex terpenes that require adjustments by other enzymes such as cytochrome P450 (ElSohly, 2002). It can be concluded that the chemical diversity of terpenes in Cannabis is a direct reflection of the encoding enzymes in Cannabis. Other common terpenes in Cannabis are bisabolol, sesquiterpenes, and β-farnesene, (Booth, 2020). Monoterpenes have a ten-carbon isoprenoid precursor known as the geranyl diphosphate (GPP), while sesquiterpenes have a fifteen-carbon isoprenoid farenesyl diphosphate (FPP) (Stasiłowicz et al., 2021). Therefore, in the synthesis of sesquiterpenes and monoterpenes, GPP and FPP act as substrates in producing different structures of terpenes.
3.2.2 Flavonoids
At least 20 flavonoids have been found in Cannabis, most of which are flavanols and flavones (Li et al., 2022). In 2011, three geranylated flavones known as cannflavin A, B, and C were found in the plant (Bautista et al., 2021). Currently, the leaves, flowers, seedlings, and fruits of C. sativa have been found to contain flavonoids that remain undetected in roots and seeds (Eggers et al., 2019). Apart from finding this compound in specific regions of the plant, flavonoids have been identified to vary in bracts during plant development (Ross et al., 2005).
Since several flavonoids have protective functions, their production is dependent on environmental factors that have been found in several plants as well as Cannabis. For instance, the accumulation of cannflavin A is predisposed to genetic variations, as well as environmental factors such as temperature, rainfall, and humidity in the environment (Kumar and Pandey, 2013). Besides, the contents of cannflavin A, B, and C in cloned species of C. sativa vary at different altitudes (Wiles et al., 2022). With these findings, it can be postulated that identifying unknown flavonoids in the plant, is reliant on certain environmental conditions or stresses. Another study by Pavlovic et al. (2019) confirms that certain flavonoids are produced in significant quantities in hexane extracts of flowers of C. sativa chemovars like cannflavcin C. Thus, identifying more flavonoids in C. sativa will provide a comprehensive understanding of its biosynthesis and functions in the plant.
Cannabis has 26 distinct flavonoids (ElSohly, 2007). There are various flavonoids in Cannabis, but the most important ones are orientin, vitexin, luteolin-7-O-glucoside, and apigenin-7-O-glucoside (ElSohly, 2007). Moreover, it contains the potent antioxidant, quercetin (Mnekin and Ripoll, 2021). Cannabinoids are a new type of flavonoid. They are made up of three chemicals found only in Cannabis, i.e., Cannflavin A, B, and C. Cannflavins were discovered in hemp’s leaves and blooms (Werz et al., 2014). Cannflavin A is 30 times more anti-inflammatory than aspirin (Barrett et al., 1985). This anti-inflammatory activity can be explained by the reduction of mPGES-1 and 5-LO (Erridge et al., 2020).
3.2.3 Steroids
Presently, steroid compounds such as campesterol, sitosterol, and stigmasterol have been identified in Cannabis roots (Ryz et al., 2017). Moreover, eleven phytosterols have been found in the plant which belongs to the groups stated above (Farinon et al., 2020). A trifecta of sterols (campesterol, stigmasterol, and sitosterol) was extracted using hexane from the seed oil of the Indian Cannabis strain (Jurgoński et al., 2020). These three phytosterols were also found in Cannabis smoke, according to research by Kumar et al. (2021). Furthermore, β-sitosterol-3-O—Dglucopyranosyl-60-acetate, a known sterol, was first isolated from roots, stem bark, and leaves of Cannabis by using a mixture of methanol and chloroform solvent in the ratio 9:1 (Jin et al., 2020). Sitosterol and sitosterol-D-glucoside were extracted from the plant’s roots in the same study using a mixture of methanol and chloroform solvent in a ratio of 9:1 (Jin et al., 2020). Recent research by Ferrini et al. (2021), found that higher concentrations of campesterol, stigmasterol, and sitosterol were associated with higher total sterol levels in flowers, leaves, roots, and stems (Ferrini et al., 2021).
3.2.4 Alkaloids
Alkaloids are part of the chemical defense mechanism used by plants to ward off herbivores (Walters, 2011). It has been shown that Cannabis contains endogenous indole alkaloids (Fasakin et al., 2022). For example, alkaloids may be used as analgesics, antibiotics, anticancer drugs, antiarrhythmics, asthma medications, antimalarials, anticholinergics, bronchodilators, laxatives, miotics, oxytocics, vasodilators, psychotropics, and stimulants (Manske and Holmes, 2014). Included in this group of chemicals are morphine, cocaine, nicotine, caffeine, quinine, ephedrine, and many more (Yan et al., 2016).
A group of researchers led by Klein in 1971 researched Cannabis alkaloid combinations and reported the isolation of four different alkaloids, which they called cannabimines A-D (Garcia-Romeu et al., 2016). In the study of cannabinoids, Cannabisativine was the pioneering alkaloid. In 1975, it was extracted from the roots of a Mexican variety of Cannabis sativa that was growing in Mississippi, United States. The compound was extracted using methanol as a solvent. (Chandra et al., 2017). These alkaloids were shown to have diuretic, analgesic, anticancer, antipyretic, and antiemetic effects (Lata et al., 2016).
In 1881, Siebold and Bradbury presented their findings on the separation of the alkaloid cannabinine at the British Pharmaceutical Conference (Warden, 1885). The next year, in 1883, Hay discovered tetanocannabin, another physiologically active alkaloid. Due to its ability to induce convulsions in amphibians similar to those caused by strychnine, the compound earned its name, a “cannabine alkaloid” product, that was marketed by Merck (of Darmstadt) as early as 1986 (Lowe et al., 2021).
3.2.5 Fatty acids
Fatty acids carry out their physiological activities due to the involvement of their functional groups in various chemical processes. Some of the fatty acids produced by Cannabis sativa L. can be identified based on their chemical structure (Mölleken and Theimer, 1997). The fatty acids produced by Cannabis sativa L. have a specific chemical structure that can be distinguished from other fatty acids based on their unique features (Babiker et al., 2021). In 1996, Ross and others investigated the fatty acid profile of lipid matter in commercialized Cannabis seeds from several geographical locations. Omega-3 fatty acids such as linolenic acid, isolinolenic acid, and eicosapentaenoic acid; omega-6 fatty acids such as linoleic acid and others such as Caproic acid, caprylic acid, myristic acid, palmitoleic acid, palmitic acid, margaric acid, oleic acid, stearic acid, arachidic acid, isoarachidic acid, and behenic acid are just some of the fatty acids found in commercial Cannabis sativa (Kriese et al., 2004). The oil content of the Cannabis plant varies depending on various factors such as the cultivar, growing conditions, and the part of the plant that is being analyzed. In general, the oil content of the seeds of the Cannabis sativa plant is typically around 30%–35% on a dry weight basis (Mihoc et al., 2012). However, the oil content of other parts of the Cannabis plant, such as the flowers or leaves, is generally much lower (8%–16%) than that of the seeds (Novak et al., 2001). Therefore, the seeds are the primary source of oil extracted from the Cannabis plant for both industrial and nutritional purposes (Potter, 2014). Cannabis is not typically considered a significant dietary source of fatty acids. While Cannabis does contain various fatty acids, the concentrations are relatively low compared to other food sources (vegetable oils) that have high concentrations of fatty acids (Callaway, 2004). Almost half of hulled Cannabis seeds are made up of fat (triglycerides), and the oil that is extracted from them is unique among cooking oils because its triglycerides include very low levels of saturated fatty acids (0.9%) and extremely high levels of polyunsaturated fatty acids (80%) (Rasool, 2018; Krist, 2020). Linoleic acid (18:2ω6, 54%–60%), α-linolenic acid (18:3ω3, 18%–23%), and oleic acid (18:1ω9, 7%–12%) are the three primary fatty acids found in Cannabis seed oil.”
Additionally, C. sativa L. seeds, offer nutritional value, since they are composed of around 25% highly nutritious protein and 35% fat (Farinon et al., 2020). Hemp oil, pressed from the seeds of the Cannabis plant, is rich in a wide variety of nutrients, including essential fatty acids (EFAs) such as omega-3 and omega-6 fatty acids, vitamins (C, E, B1, B2, B6, B12, and folate), minerals (including calcium, magnesium, potassium, phosphorus, iron, zinc, sodium, and copper), and macronutrients (fat, carbohydrates, fiber, and protein) (Silver et al., 2021).
3.2.6 Waxes
Plants create waxes, a class of non-volatile, larger molecular weight, hydrophobic chemicals, to shield their leaves and stem from dehydration and disease (Ribeiro et al., 2021). They may also serve to stabilize defense chemicals, like the phytocannabinoids and terpenes found in Cannabis, which are produced on plant inflorescence (flower heads) (Romero et al., 2020). If compared to Cannabis leaves, the wax content in Cannabis inflorescence is three times higher (Tipple et al., 2016). The initial stage in the creation of therapeutic Cannabis products is commonly the extraction of Cannabis inflorescence using organic solvents or supercritical Carbon dioxide (CO2), often resulting in a ‘resin’ with a high wax concentration (Ramirez et al., 2019; Sawicka et al., 2021). As a result, waxes are a crucial category of phytochemicals to consider during such production. N-pentacosane (C25H52), n-heptacosane (C27H56), n-nonacosane (C29H60), and n-hentriacontane (C31H64) are the most prevalent straight-chain hydrocarbons in Cannabis waxes (Adams and Jones, 1973). A summary of the structures of other important phytochemicals are provided in (Table 4).
4 Traditional and psychedelic properties of Cannabis
The Cannabis plant has been used for centuries and is one of the most beneficial plant genera. Historically, the seeds were used for making oil and pickles, while the leaves were the second most consumed part of the plant and were used in various ways, such as seasoning, flour, and added to meals (Balant et al., 2021). Historically, the psychedelic and recreational use of Cannabis dates to the early 1800 s in tropical parts of the world such as South America and Africa. However, the psychedelic use of the plant did not make it to Europe and America until after the 1800 s (Balant et al., 2021). In countries outside the tropics, the psychoactive components of Cannabis are not present in the variants grown. Cannabis has a long history of medicinal and psychoactive use in India, and it became known in America and Europe in the 19th century for its narcotic and stimulant properties (Tipparat et al., 2012).
Cannabis cultivation, commercialization, and use as a recreational drug has a significant incidence on a global basis, and often fall within the realm of illicit activities (Carnevale et al., 2017). However, the comparatively low proportion of psychotropic use does not match the significance of these activities (Carnevale et al., 2017). The CANNUSE database documents various methods of using Cannabis for psychoactive purposes, including smoking leaves or inflorescences, ingesting preparations made from leaves, inflorescences, and shoots, and consuming preparations of varying intensity such as charas, attar, hashish, ganja, and plant powder (Boniotti and Griffith, 2002; Balant et al., 2021).
The most common ways of administering Cannabis for psychoactive purposes are smoking (56.6%), drinking (37.74%), and eating (5.66%) (Balant et al., 2021). The leaf is the most commonly used part of the plant, accounting for 46.44% of use, even though inflorescences have the highest concentration of THC and other cannabinoids (Hasan, 1975).
The Cannabis-based food industry mostly uses seeds and derivatives, but other plant parts like sprouts, leaves, and flowers are consumed raw in dishes and drinks. These plant parts contain higher levels of bioactive phytochemicals like polyphenols and cannabinoids than seeds. Ingesting Cannabis makes up 7.29% of all uses, with 58.72% corresponding to traditional meals and 41.28% to traditional beverages. Seeds are the most common plant component used for food, and this association is statistically significant. Seeds are popular among Asian senior citizens due to their high protein content and low glycemic index (Haldar et al., 2019). Cannabis seeds can be found in different forms such as energy bars, chocolates, flour, baked products, milk, and flavoring sauce. The plant’s sprouts, leaves, and flowers are also consumed raw in dishes and drinks (Iftikhar et al., 2021; Kuppuram, 2022; Xu et al., 2022).
5 Pharmacological potential of Cannabis
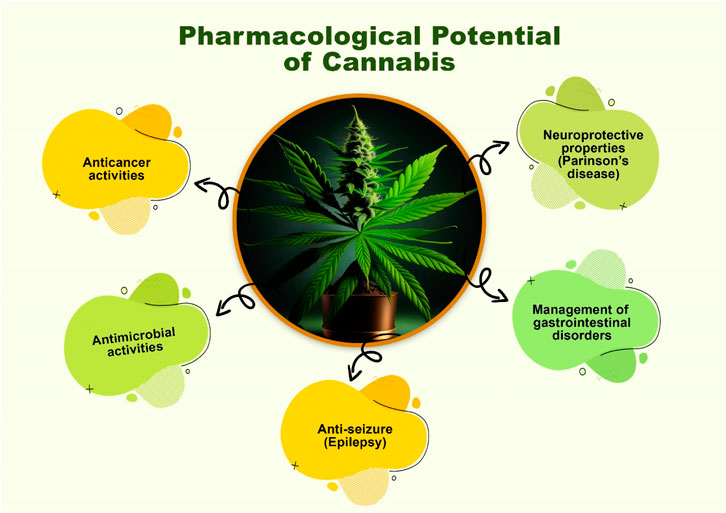
The prospect of using Cannabis for the treatment of a wide variety of diseases is promising now that the Δ9-THC and endocannabinoid systems, receptors, enzymatic systems, and physiological effects have been identified (Maroon and Bost, 2018). It has been effective in the treatment of gastrointestinal disorders, infections, psychosis, anxiety, depression, anorexia, and cachexia, as well as in the treatment of asthma (bronchiectasis), pain, musculoskeletal disorders, tumor, and arthritis (Śmiarowska et al., 2022). It also has antiglaucoma, antimicrobial, and antiemetic properties. Additionally, it has anti-obesity and anti-cancer properties (De Meijer et al., 2003). Clinical studies have investigated the effects of Δ9-tetrahydrocannabinol (THC) on a variety of diseases, such as AIDS, advanced cancer, glaucoma, nausea, chemotherapy-induced vomiting, itching, allergies, psychiatric symptoms, and movement abnormalities (Barrales-Cureño et al., 2020). Some of these pharmacological potentials are summarized below in Figure 6.
5.1 Antimicrobial activities of Cannabis sativa extracts
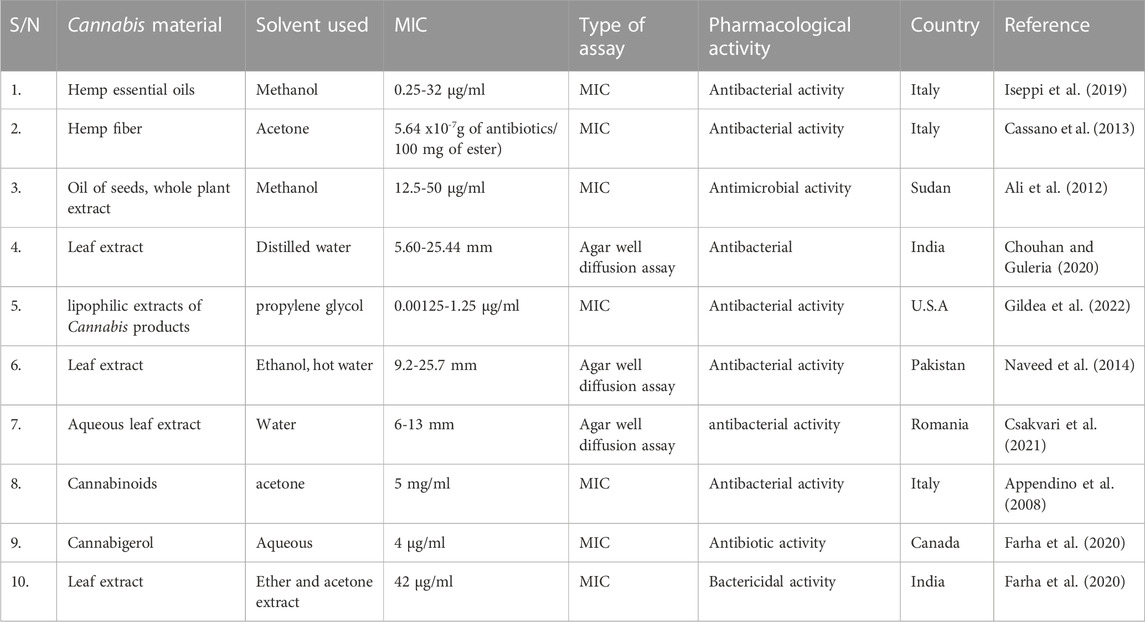
Cannabinoids’ antimicrobial property has been known since the 1950s when the first reports appeared (Baswan et al., 2020). The bactericidal activity of C. sativa could not be linked to a single compound since these trials were performed before the phytochemistry of Cannabis was extensively known (Zheljazkov et al., 2020). It was accomplished in 1976 when it was discovered that both Δ9-THC and CBD are bacteriostatic and bactericidal against a panel of Gram-positive bacteria (De Vita et al., 2022). Antibacterial activities of C. sativa extracts, including essential oils and those obtained from petroleum ether, methanol, and hot water, have also garnered significant attention (Taghinasab and Jabaji, 2020). The oil from the seeds of the plant was extracted using petroleum ether and methanol and was found to exhibit antibacterial activity against Gram-positive bacteria. Interestingly, while the petroleum ether extract was found to be ineffective against Pseudomonas aeruginosa, it was observed to have some mild activity against Gram-negative bacteria (Ali et al., 2012). The principal components of C. sativa ethanol extracts showed moderate effectiveness exclusively against both clinical samples and non-clinical methicillin-resistant staphylococcus aureus (MRSA) infection isolates, as was earlier shown by Laidlaw (2016).
Figuerêdo et al. (2022) found that ethanol Cannabis. sativa seed extracts inhibited S. aureus biofilm development, suggesting that these extracts may have significant use as food and cosmetic preservatives. Also, Karas et al. (2020) discovered that cannabinoids are more successful than commercial toothpaste like Oral B and Colgate in reducing the bacterial colony count in dental plaque, suggesting that C. sativa-derived chemicals might be employed for oral care applications. Medical, aesthetic, veterinary, agricultural, and culinary uses of a Δ9-THC-free essential oil of C. sativa are all possible and are now being researched. Naringenin, a flavanone, was shown to contribute to the oil’s mild antibacterial efficacy and antibiofilm activity when tested against many strains of S. aureus (Zengin et al., 2018). Helicobacter pylori, a Gram-negative bacteria, was likewise shown to be susceptible to the antimicrobial effect, although no antifungal activity was detected (Fufa, 2019).
Several S. aureus and Streptococcus isolates were shown to be susceptible to CBD and Δ9-THC, with MIC in the range of 1–5 g/mL (Lohar and Rathore, 2013). This bactericidal action, however, was diminished in the presence of horse serum, most likely because of the cannabinoids binding to plasma proteins (Lohar and Rathore, 2013). Blaskovich et al. (2021) examined a variety of cannabichromene analogues for their antimicrobial and antifungal effects. Activity against B. subtilis and S. aureus seems to depend on the n-pentyl chain meta to the alcohol group (Blaskovich et al., 2021).
In addition to reducing biofilm formation, the use of certain Cannabis-infused medicines was found to modify other biofilm-associated virulence factors such as cell aggregation, hydrophobicity, membrane potential, and spreading ability (Feldman et al., 2018). These medicines can be used in conjunction with standard antibiotics like ampicillin and gentamicin to treat MRSA biofilm infections that have shown resistance to other treatments. CBD has also been shown to enhance the antibacterial action of the peptide medication bacitracin against Staphylococcus species, L. monocytogenes, and E. faecalis (Wanmakok et al., 2018). Several Cannabis analogues were tested by Wassmann and Klitgaard (2021) and proved to be effective against MRSA USA300 and E. coli. Several commonly used cannabinoids showed moderate to excellent activity; these findings were generally consistent with those of prior research (vide supra). The MIC values increased by up to a factor of four (Ranieri et al., 2015).
CBD was also found by Sionov and Steinberg (2022) to be a synergistic agent when combined with different antibiotics. CBD was found to significantly inhibit the release of membrane vesicles by Gram-negative pathogens, which are involved in bacterial communication. When used in conjunction with erythromycin, vancomycin, rifampicin, kanamycin, or colistin, CBD’s antibacterial action was amplified against E. coli VCS257. These findings suggest that these cannabinoids may be used to increase efficiency and broaden the action of currently available antibiotics, which is an important development in the field of antibiotic resistance.
The Cannabis plant and its secondary metabolites have also been studied for their antifungal capabilities. Some articles claim that Cannabis extracts may be effectively utilized in the control of pathogenic fungi, albeit this impact has not been as widely explored as its antibacterial properties (Głodowska and Łyszcz, 2017). Popescu-Spineni et al. (2021) showed that ethanol and petroleum extract from Cannabis leaves effectively inhibited the growth and development of Candida albicans, Candida krusei, and Aspergillus niger. In both instances, the concentration of the leaf extract was 10 times greater compared to the antifungal antibiotic (Nystatin), although the zone of inhibition was substantially larger with the antifungal antibiotic. The antifungal properties of Cannabis sativa L. seed oil and whole-plant extracts of petroleum ether and methanol were investigated by Głodowska and Łyszcz (2017). However, while the whole-plant petroleum ether extract exhibited some action against C. albicans, the seed extract and whole-plant methanol extract were ineffective against the two fungi tested. For their ability to prevent the spread of the seed-borne phytopathogenic fungus Alternaria spp.; Akhtar et al. (2016) examined the antifungal properties of extracts from 11 weed varieties. Although all plants showed some antifungal activity, some were far more effective than others. The percentage of mycelial development that Cannabis sativa L. was able to halt was not the highest among the plants analyzed, but it was still rather high. The acetone-based extract proved to be the most effective antifungal agent among 5 distinct extract types. Some properties are summarized in Table 5 below.
5.2 Anticancer activities
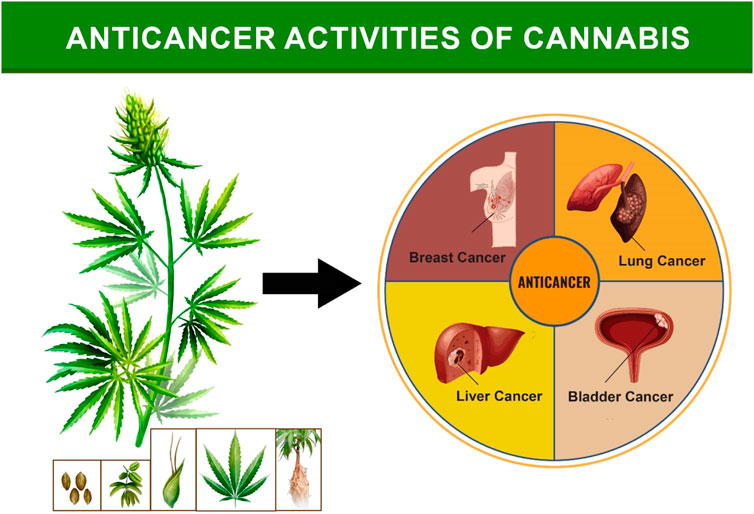
Cannabinoids (CBs) are active metabolites in Cannabis sativa, and they are responsible for the plant’s medical effectiveness (Kumar et al., 2021). CB derivatives have been shown to suppress the growth and survival of multiple forms of cancer cells. The underlying processes of the effects may be unique to each type of cell, and CBs can target tumors specifically to disrupt signaling and biological processes, leading to growth pause, cell death, and migratory blockage (Alexander et al., 2009). CBs may also have indirect effects on the tumor microenvironment, immune response, and vascularization suppression. Both direct and indirect anticancer effects of CBs have been studied (Hellmich and Szabo, 2015). In recent decades, there have been significant studies on the purity, efficacy, and therapeutic utility of Cannabis and cannabinoids (CBs) in preclinical and clinical cancer models. CBs have shown promise in treating and diagnosing cancer-related symptoms. THC, a type of CB, has been observed to accelerate the death of tumor cells compared to healthy cells. Long-term rat models have shown that Δ9-THC exhibits little toxicity and has no discernible impact on hematological parameters, general health, or mortality (Russo, 2016; Hartsel et al., 2019). Non-psychoactive CBD has been studied as a potential anticancer drug due to its action in vitro and in vivo against tumor cells produced from CBs found in C. sativa. Meanwhile, THC was administered to terminal cancer patients. However, the precise chemical pathways by which CBs exert their anticancer effects are not completely understood suggesting that more research involving the antitumor effects of CBs should be done. (Chung et al., 2021; Kumar et al., 2021). Secondly, regulation of the proinflammatory nuclear factor kappa B pathway has been linked to a tumor’s prosurvival impact, as well as chemoresistance in cancer cells, although the route is independent of Akt. Epidermal growth factor (EGF) signaling activation is essential for tumor cell growth, survival, and progression (Lau et al., 2018). In addition to its ability to destroy cancer cells, Cannabis sativa extract has anti-nausea and anti-vomiting properties that are beneficial for cancer patients. CBD has been found to reduce proinflammatory pathways by decreasing EGF signaling pathway activity (Pellati et al., 2018). By activating the TRPV channel and increasing endoplasmic reticulum stress, CBD can induce cancer cells to self-destruct. CBD also binds to and activates GPR55, which suppresses ERK pathway activation and halts cancer cell growth. As a result, multinational corporations are now offering medications containing cannabinoids in the form of plant extracts or volatile oils. Sativex, a standardized extract of Cannabis sativa L., has been licensed in Canada for the treatment of pain (Farag and Kayser, 2015). Various anticancer activities of cannabis found in liver, breast, bladder and lung are summarized below (Figure 7).
5.2.1 Liver cancer
Liver cancer is a leading cause of mortality and suffering worldwide (Torre et al., 2015). CBs have been found to have anticancer effects by triggering apoptosis and suppressing telomerase activity. Low molecular weight hemp peptides have been shown to induce apoptosis, decrease cell viability, and reduce cell motility in Hep3B human liver cancer cells without modifying the baseline overexpression of cleaved caspase 3 and Bad, or downregulation of antiapoptotic Bcl-2 (Salamat et al., 2022). This strategy induced Akt and GSK-3 phosphorylation, followed by downregulation of β-catenin, demonstrating that β-catenin’s signaling modulation is the mechanism governing the anticancer activity. While further research is needed, these results suggest that hemp peptides may serve as a promising therapy for liver cancer (Kocatürk et al., 2021).
5.2.2 Breast cancer
Breast cancer is the deadliest disease affecting females worldwide (Akram et al., 2017; Alsaraireh and Darawad, 2019). CBs have been found to significantly inhibit the proliferation of breast cancer cell lines, including human MDAMB231-luc-D3H2LN cells, which are sensitive to both 9-THC and CBD. CBs inhibited tumor invasion and metastasis in animal models and reduced EGF-induced proliferation and chemotaxis in triple-negative breast cancer cells (Arkell et al., 2019). CBD inhibited Id-1 receptor expression, cell proliferation, and invasion in breast cancer cells. In an athymic nude mouse model of breast cancer cells, CBD and CBG decreased tumor volume and promoted apoptosis. Δ9-THC was also found to reduce the growth of breast cancer cell lines (Kumar et al., 2021). At a concentration of 5 mM, CBD destroyed breast cancer cells in cell culture by cell-autonomous apoptosis and autophagy without harming normal cells, with relatively insignificant effects on TRPV1, CB1, and CB2 receptors (Perucca, 2017; Gaston and Szaflarski, 2018).
CBD’s in vivo antimetastatic activity was evaluated in syngeneic BALB/c mice by injecting 4 T1 breast cancer cells into the tail vein. CBD at 1 and 5 mg/kg suppressed both primary tumor growth and the number of metastatic foci by regulating cell migration through the activation of the ERK enzyme (Zhelyazkova et al., 2020). CBD at 5 mg/kg body weight also suppressed tumor development and lowered tumor volume in athymic nude mice with breast tumor xenografts, increasing the animals’ median survival time (Sakarin et al., 2022).
5.2.3 Bladder cancer
Smoking cigarettes is a major contributor to the development of bladder cancer (Brennan et al., 2001). Numerous polls have found that a large percentage of cigarette smokers also regularly consume Cannabis (Hindocha et al., 2021). To determine the link between Cannabis and cigarette use and the development of bladder cancer in men in California, Thomas et al. (2015) conducted epidemiological research. They interviewed 84,170 males to find out about their habits like smoking and using Cannabis. The study indicated that while smoking alone was linked to a 15% increased risk of getting bladder cancer, Cannabis use alone was linked to a 45% lower risk. However, more rigorous studies are needed to thoroughly assess the plant’s medical potential in the treatment of bladder cancer.
5.2.4 Lung cancer
Small-cell and non-small-cell lung cancers are the most common forms of lung cancer, with tobacco use, family history, and exposure to radon gas increasing the likelihood of developing the disease (Zou et al., 2021). CBs have been studied in vivo for their efficacy against lung cancer, and CBD therapy has been found to inhibit tumor growth, invasion, and metastasis in mice bearing xenografts of A549 cells (Solinas et al., 2015; Laezza et al., 2020). In vitro, investigations using lung cancer cell lines A549, H358, and H460 showed that CBD upregulated the antimetastatic protein ICAM-1, which is hypothesized to reduce tumor development through an immunosurveillance mechanism (Benedicto et al., 2017).
CBD was found to increase TIMP-1 and ICAM-1 expression in a dose-dependent manner in lung cancer cell lines and inhibited the spread and invasion of human lung cancer xenografts in mice, partially due to the increase in ICAM-1 and TIMP-1. It triggered apoptosis through PPAR-g and COX-2 in human metastatic lung cancer cells and caused tumor regression in A549 xenografted mice (Mrowka and Glodkowska-Mrowka, 2020). In human metastatic lung cancer cells and cancer cell lines A549 and H460, CBD and THC boosted ICAM-mediated lymphokine-activated killer cell adhesion and cancer cell lysis, increasing the lung cancer cells’ susceptibility to being lysed by LAK cells. Further research is needed to confirm Cannabis’ protective effect against lung cancer (Seltzer et al., 2020).
5.3 Epilepsy
As a neurological condition, epilepsy is characterized by aberrant brain activity and frequent seizures (Rana and Musto, 2018). During the first decade of life, this affects about 1 in 150 children (Ramantani et al., 2013). Epileptic encephalopathies are characterized by refractory seizures, severe electroencephalographic abnormalities, and developmental impairment (Khan and Al Baradie, 2012). Clinical evidence for the use of CBs in the management of epilepsy has been backed up by preliminary studies (Antonarakis et al., 2020; Oberbarnscheidt and Miller, 2020). Ellen et al. (2018) reported that using CBs in a mouse model of Dravet syndrome led to a decrease in autistic-like social deficiencies, suggesting that these drugs’ benefits extend beyond seizure control.
Clinical investigations have shown that CBD and cannabidivarin (CBD’s propyl version) have anticonvulsant qualities, although the particular processes by which they do so remain unknown (Devinsky et al., 2014). A large, prospective, single-center, open-label study of CBD for the treatment of medication-resistant epilepsy in children and adults showed striking improvements in disease phenotype in response to CBD therapy for 72 children and 60 adults (Sher and Maldonado, 2015). In addition, EPIDIOLEX®, a CBD medicine derived from Cannabis, was recently licensed by the FDA for the first time to treat Lennox-Gastaut syndrome and Dravet syndrome, two extremely uncommon but extremely serious forms of epilepsy (Lattanzi et al., 2021). These results suggest that CBs including CBD may be useful for the treatment of epilepsy and other neurological disorders (Kaplan et al., 2017). Furthermore, CBD proved beneficial in reducing seizure frequency in a comprehensive trial and meta-analysis of its efficacy for treatment-resistant epilepsy (Pamplona et al., 2018).
5.4 Parkinson’s disease
There are motor and non-motor symptoms associated with this kind of neurodegenerative disease. Non-motor symptoms of Parkinson’s disease, such as constipation, sleep issues, anxiety, and sleep instability, were studied by Sauerbier et al. (2016), who reviewed the data of many trials to determine whether CBD may be helpful. Dopamine-containing neurons in the basal ganglia were shown to deteriorate in Parkinson’s disease, which may be linked to mitochondrial malfunction, oxidative stress, and impaired protein breakdown in the affected cells (Poewe et al., 2017). Several studies have found a correlation between the endocannabinoid system (ECS) and Parkinson’s disease (Han et al., 2020). The ECS consists of cannabinoids (CB) receptors, i.e., CB1 and CB2, their ligands, and the enzymes responsible for their production and metabolism (Iannotti et al., 2016). The basal ganglia of the brain are where endocannabinoids are most concentrated. By activating or inhibiting CB1 or CB2, CBD contributes to the lowering of dopamine levels. CBD’s sedative action has also been studied, with mixed results (depending on dosage and mode of administration) including enhanced sleep delay and alertness (Sarris et al., 2020). Rats given either high or moderate dosages of CBD in an experiment by Silvestro et al. (2019) slept longer and for longer periods thereafter.
One study found the effectiveness of Cannabis-based medicinal extracts v/s placebo for the treatment of individuals with spinal cord injury (SCI) (Grotenhermen, 2004). Peppermint oil, 0.05% (v/v), ethanol, and propylene glycol (50:50) were the excipients in a THC (27 mg/mL): CBD (25 mg/mL) extract of Cannabis sativa L. After receiving treatment, patients reported significantly higher ratings of central neuropathic pain on the 11-point numerical rating scale, with a negative number indicating an increase in pain from pre-treatment levels. Thomas et al. (2021) also did a scoping assessment of the literature on Cannabis’s effect on SCI pain severity. Variations in methodology, such as the lack of standardized dosage regimens, modes of use, and trial length, led to contradictory findings across the study’s articles reporting on five treatment studies. Consistent and sufficient data is, therefore, lacking to form accurate conclusions on the efficacy of Cannabis in lowering the pain intensity associated with SCI, indicating that more study is needed in this area.
5.4 Gastrointestinal disorders
Cannabis is used by inflammatory Bowel Disease (IBD) patients to alleviate symptoms and improve their quality of life. Endocannabinoids (ECS) help in maintaining intestinal homeostasis, which requires a combination of centrally and peripherally mediated actions (Russia et al., 2015). The ECS consists of endocannabinoids, enzymes that make and break down endocannabinoids, and CB receptors that mediate endocannabinoid effects (Kilaru and Chapman, 2020). The enzymes responsible for the breakdown of endocannabinoids are fat acid amide hydrolase (FAAH) and monoacylglycerol lipase (MGL). However, more research is needed to understand the role of the ECS in IBD and the effects of Cannabis on these conditions (Gil-Ordóñez et al., 2018).
The endocannabinoid system (ECS) is present throughout the gut and controls several digestive processes such as GI motility, inflammation, and immune response. Cannabis can potentially impact these processes by activating the receptors in the ECS, leading to an increase in food intake and metabolic processes like lipolysis and glucose metabolism (Sergi et al., 2021).
Cannabis is used for various gastrointestinal (GI) ailments, including enteric infections, inflammation, motility difficulties, emesis, and stomach discomfort. Endocannabinoids can inhibit proinflammatory mediators such as IL-1β, TNF-α, and nitric oxide, reducing the cellular pathways leading to the coordinated inflammatory reactions in IBD (Saqib et al., 2017; Bennett et al., 2018). Cannabis formulations have been shown to significantly reduce the severity of colitis in experimental animal models of IBD. CBs can regulate GI motility, which has paved the way for the development of a new class of antibiotics that can treat a wide range of GI conditions, including colitis, Crohn’s disease, gastric ulcers, paralytic ileus, IBS, colon cancer, and others (Tyakht et al., 2018).
The ECS can be useful in managing irritable bowel syndrome-diarrhea (IBS-D) and irritable bowel syndrome-constipation (IBS-C) by affecting motility and secretion through CB1 agonists. CB2 receptors can be used to treat IBS-D because they are overexpressed during stomach inflammation (Uranga et al., 2018). The ECS plays an inhibitory role in the GI tract by suppressing motility and secretion and controlling pain perception. CB receptor activation can protect against colitis, and inhibiting breakdown enzymes (FAAH or MAGL) can reduce inflammation. Inhibiting the enzyme that produces 2-AG (DAGL) can regularize feces in constipation-prone animals and reduce 2-AG levels (Karwad et al., 2017).
Studies have shown that patients with Crohn’s disease have increased CB receptors and/or endocannabinoids in their intestines. CB1 and/or CB2 agonist treatment has been found to reduce colitis in animal models of inflammatory bowel disease (Hryhorowicz et al., 2021). Clinical research on the use of Δ9-THC to treat Crohn’s disease has shown promising results, but further studies are needed to determine appropriate doses, modalities of usage, patient populations that would benefit, and long-term exposure risks through randomized, controlled, and prospective clinical studies (Ziemssen and Thomas, 2017).
6 Cannabis’ potential in the food industry
According to Kanabus et al. (2021), Cannabis cultivars can be used in food production in Europe if the amount of ∆9-THC and ∆9-THCA in unrecognized blooming or fruiting plant tips is less than 0.2% dry matter. This requirement is in place to prevent the production of edible Cannabis products, but the consumption of hemp seeds is still allowed. In November 2015, the European Union passed Regulation 2015/2283, which designates certain hemp extracts and parts as “novel foods" (Kanabus et al., 2021).
There’s a wide variety of baked goods, pizza, oil, beer, milk, chocolate, ice cream, and snacks made with Cannabis seeds (Kanabus et al., 2021; Sorrentino, 2021). Cannabis-related food exports from Italy are worth 50 billion euros and make a big difference in the country’s economy (Sorrentino, 2021). The hemp-based agri-food chain may be the biggest step forward for this industry. Demand for hemp-based foods has increased fivefold from 2017 levels (Moliterni et al., 2022). Hemp seed flour is a healthy alternative to wheat flour. Although it tastes rough and rustic, hemp flour has 21% fewer calories than regular oat flour (Albala, 2003). Celiacs can eat it without worry because it does not contain gluten (Sorrentino, 2021). According to Sorrentino (2021), hemp has only 25% protein and 65% edestin. Recent studies by Bao et al. (2014) have shown that, like animal proteins, edestin provides all eight essential amino acids. These findings reveal that Cannabis has the potential to make the world’s food supply much safer by lowering the need for animal protein.
7 Cannabis’ potential in the Cosmetics industry
Cannabis seeds are frequently used in traditional cosmetic treatments, especially for hair care, due to their high oil content. Michailidis et al. (2021) studied the effect of Cannabis seed oil on the strength of hair and nails. Cannabis stems are also highly valued, particularly in Pakistan (Michailidis et al., 2021).
Moreover, Cannabis seed oil is often used as a hair food due to its rich nutritional profile. The oil is particularly high in polyunsaturated fatty acids, such as omega-3 and omega-6 fatty acids, which are essential for maintaining healthy hair (Crini et al., 2020). These fatty acids help to nourish and moisturize the hair, making it softer, smoother, and more manageable. They also help to prevent hair breakage, split ends, and dryness, which can lead to hair loss over time (Callaway, 2004).
In addition to its fatty acid content, Cannabis seed oil is also high in carotenoids, which have been shown to promote hair growth and improve hair health (Baral et al., 2020). Cannabis seed oil can be used in a variety of ways as a hair treatment. It can be applied directly to the scalp and hair as a hair mask, left on for several minutes or overnight, and then rinsed off with shampoo and conditioner. The oil can also be added to shampoos and conditioners to enhance their moisturizing and nourishing properties (Baral et al., 2020). Carotenoids, such as β-carotene, can help to strengthen the hair shaft and protect it from damage caused by UV radiation and other environmental stressors. They can also help to improve the elasticity and overall appearance of the hair (Nahhas et al., 2019).
The endocannabinoid system (ECS) in the skin plays an important role in regulating cell differentiation, development, survival, inflammation and immune responses, pain perception, and hair growth (Mnekin and Ripoll, 2021). Disruption of the ECS can lead to various dermatological problems (Del Río et al., 2018). The ECS is controlled by CB1 and CB2 receptors, which are found in different cells in the skin. CB1 is expressed in hair follicle cells, immune cells, and keratinocytes and regulates pain, neuronal activity, and inflammation. CB2 is found in sensory neurons, immune cells, sebaceous glands, and keratinocytes and also regulates inflammation (Baswan et al., 2020). Activating CB1 has been shown to prevent keratinocytes from producing pro-inflammatory cytokines and maintaining the integrity of the epidermal barrier (Gaffal et al., 2013).
Cannabis seed oil is a rich source of carotenoids, such as β-carotene, lutein, and zeaxanthin, which are easily absorbed by the skin (Irakli et al., 2019). These carotenoids have antioxidant properties that can combat free radicals and protect against UV light (Wisniewska et al., 2006). β-carotene can also prevent the activation of pro-inflammatory cytokines by UV-B radiation, thus exhibiting anti-inflammatory effects. Carotenoids can also improve skin hydration, promote wound healing, and stimulate the production of collagen and elastin by activating fibroblasts (Baswan et al., 2021). The Cannabis seed oil has a high concentration of chlorophyll, which can range from 100 μg/g to 230 μg/g, depending on the extraction process (Stephens et al., 2015). Chlorophyll has been shown to promote tissue growth and have antibacterial properties, making it potentially useful in wound healing and treating skin problems like acne, eczema, and ulcers. The green pigment in hemp seed oil comes from chlorophyll (ElSohly, 2007).
The Cannabis seed oil contains flavonoids, terpenes, carotenoids, chlorophylls, and phytosterols that contribute to its anti-inflammatory and anti-aging properties. The oil is quickly absorbed and does not clog pores, making it useful in formulations designed to soothe the skin, such as sunscreen creams and lotions. The natural presence of chlorophyll makes it potentially effective for wound healing and treating skin problems. Topical creams and ointments containing Cannabis seed oil have potential applications in anti-aging skincare (Baral et al., 2020). The various uses are summarized below in Figure 8 below.
8 Significance of Cannabis cultivation to agriculture and environment
Cannabis sativa L. was first cultivated for textile fiber in Western Asia and Egypt. It later spread to Europe and was eventually brought to North America in 1,606, beginning with Port Royal in Canada (Small and Marcus, 2002). Cannabis farming has a low negative environmental impact because it can proliferate, kill weeds, and does not require pesticides. It does not have parasites that are only beneficial for one plant, which helps with pollination and improves soil fertility (Zheng et al., 2021). Although Cannabis sativa was traditionally used as a source of stem fiber and was rarely considered a narcotic, it has been one of the world’s oldest sources of textile fibers for more than 6,000 years. Its use as an oil crop was limited for most of its existence (Small, 2015).
Hemp was introduced for fiber production in Western Asia and Egypt between 1,000 and 2000 BC and later spread to Europe. After 500 AD, the cultivation of hemp became widespread throughout Europe (Clarke and Watson, 2002). Clarke and Merlin (2013) also provide an excellent overview of the historical and cultural use of Cannabis. Cannabis is considered an environmentally friendly crop in recent times, with added interest in its cultivation due to its potential to help combat climate change and desertification. As a result, the EU has proposed Cannabis cultivation as a potential new star in European agriculture, aligning with EU 2030 goals of reducing greenhouse gas emissions by 40% from 1990 levels. (Sorrentino, 2021; Zheng et al., 2021).
Cannabis can reduce the amount of Carbon dioxide (CO2) in the atmosphere, and it is particularly effective due to its high rate of growth. This makes it a valuable agricultural species for reducing greenhouse gases. However, the current atmospheric CO2 level is still much higher than pre-industrial levels, so further research is needed to find more effective ways to reduce carbon emissions (Showalter et al., 1996; Thomas and Elsohly, 2015). The use of slow-release nitrogen fertilizers in which urea is combined with an aldehyde like nitroform, methylene urea, or urea formaldehyde is recommended for Cannabis farming due to their positive impact on plant growth and seed quality (Butsic et al., 2018). In contrast, the use of synthetic fertilizers like ammonium nitrate can increase greenhouse gas emissions like nitrous oxide (N2O), which contributes to global warming and is a significant source of emissions in some countries (Sorrentino, 2021). Cannabis has a different eco-physiological trait than cotton and kenaf, where it is not as efficient in using nitric nitrogen. However, it excels at photosynthetic metabolism at low nitrogen levels (Dilley and Morrison, 2014). Slow-release nitrogen fertilizers, such as urea-formaldehyde, can reduce the amount of N2O released during the growth cycle, which is a significant contributor to greenhouse gases. Additionally, Cannabis stores CO2 in its biomass, making it a potentially climate-friendly crop that can help prevent climate change (Stone, 2011).
Growing Cannabis has the potential to set up new supply chains due to the versatility of the different plant parts. This could be beneficial for farmers, the environment, and human health, making it an important plant for the new green economy (Sorrentino, 2021). Various industries can use different parts of the plant: seeds for the agri-food industry, canapulo for the green building sector, fiber for the textile industry, and inflorescences and roots for the pharmaceutical and para-pharmaceutical industry through the extraction of bioactive molecules (Akhtar et al., 2016).
Cannabis requires nutrients and water to grow, with varying daily water use depending on location, soil, weather, and growing methods (Carah et al., 2015; Zheng et al., 2021). Outdoor Cannabis cultivation in California uses an average of 5.5 gallons of water per day per plant, according to a survey (Wilson et al., 2019). Agricultural usage, population growth, and climate change are expected to worsen water shortages, which will affect the Cannabis industry and harm the environment (Schlenker et al., 2007; Zheng et al., 2021). The amount of water needed for Cannabis plants to survive and thrive is a concern for the industry (Moyle, 2002). Cannabis cultivation, particularly illegal cultivation, can lead to water contamination. The plant requires increased levels of nitrogen, phosphorus, and potassium for optimal growth, but little research has been done on how this affects water quality globally (Saloner et al., 2019). The use of pesticides, such as herbicides, insecticides, fungicides, nematocides, and rodenticides, can also contribute to water contamination when not properly checked, posing a threat to the environment (Gabriel et al., 2013). Cannabis cultivation can lead to the contamination of soil, surface water, and groundwater due to the leakage of nitrogen and pesticides from runoff or rain (Thompson et al., 2014). This can harm both humans and crops that consume these chemicals. The contamination of water caused by Cannabis cultivation can also impact the environment where other important irrigated crops are grown (Thompson et al., 2014). However, it is difficult to link Cannabis farming practices to water pollution without proper measurement of water quality and chemical levels. Thus, legislation is needed to protect the environment from pollutions arising from commercial Cannabis cultivation. In this regard, Canada has some of the strictest environmental regulations for growing Cannabis indoors to mitigate the impact on the environment.
Zheng et al. (2021), report that Cannabis production is directly linked to soil erosion, especially on steep slopes that are more prone to erosion. The cutting down of trees and clearing of forests for Cannabis cultivation exacerbates soil erosion. However, durable greenhouses can help prevent soil erosion by avoiding the need for massive clearings that expose soil to erosion (Bauer et al., 2015).
Cannabis can help address biodiversity loss by attracting pollinating insects due to its terpenoid essence, and its pollen can be blown up to 3 km, increasing the diversity of agroecosystems (Sorrentino, 2021). The plant blooms at different times and provides ample pollen, making it an important source of food for bees (Balcke et al., 2014). By creating a microclimate that is beneficial for pollinators, Cannabis contributes to the conservation of biodiversity, which is essential to the health of the planet (Flicker et al., 2020). Another potential application of Cannabis is a bioremediation crop that can absorb and store heavy metals from the soil, making it effective in cleaning contaminated soil (McPartland and McKernan, 2017). Tainted soil fertilizer is a common source of arsenic, cadmium, lead, and mercury. Singani and Ahmadi (2012) found that Cannabis sativa may absorb lead and cadmium from manure-contaminated soils.
9 Risk and safety of Cannabis
The widespread acceptance of medical and recreational Cannabis usage in recent years has contributed to a surge in the drug’s popularity in several countries (Hajizadeh, 2016). There is an ongoing debate among experts regarding the safety of the plant. While in some parts of the world, it is viewed as a helpful medicine, in other parts it is seen as a dangerous substance if taken in large amounts, particularly when it comes to the consumption of Cannabis-containing foods or beverages (Lindblom, 2019). Research suggests that certain genetic variants may increase the risk of mental health problems in individuals who use Cannabis due to its impact on brain development, including neuroanatomical alterations, respiratory problems, metabolic and neurotransmitter functioning, and neuronal activation (Gage et al., 2016; Connor et al., 2021).
Second, Cannabis has been linked with negative effects on conditions like cardiopulmonary arrest, coronary artery disease, transient ischemic attack, and cannabis arteritis. Additionally, exposure to high amounts of THC for recreational use has been found to negatively affect various physiological systems, including ophthalmological, gastrointestinal, respiratory, immunological, and hormonal systems (Le Boisselier et al., 2017). However, serious poisoning is rare among adults and negative side effects are reported by only a small percentage of users. A study found that only 3.24% of participants reported negative side effects from using Cannabis. The flower was used in 42.86% of cases, and the leaf was used in 40.8% of cases. The study identified 45 different side effects, but only one mention of death (Boakye et al., 2021).
Furthermore, ancient medicine recognizes both the benefits of Cannabis and the risks of overuse (Iber et al., 2022). Research has found that Cannabis has both stimulating and sedative effects, including increased appetite and aphrodisiac effects as well as the ability to cool the body (Seltenrich, 2019). However, according to Volkow et al. (2014), long-term use can have unintended side effects such as stomach pain, thinning skin, depression, inability to work, and dropsy (a buildup of water in the body) (Volkow et al., 2014). Using the right amount of Cannabis is important for both medical and recreational purposes. In 2021, the United Nations Office on Drugs and Crime (UNDOC) removed Cannabis from Schedule IV, but it remains on the Schedule I list due to insufficient data on its effects (Riboulet-Zemouli and Krawitz, 2022). For instance, reports have linked Cannabis use to the growth of tumors, including in children whose mothers’ used marijuana during pregnancy. Depending on the dose and length of use, Cannabis can also cause cancer, birth defects, and genetic changes. In addition to harming mental health, Cannabis use can negatively impact respiratory, cardiovascular, and bone functions (Sharma et al., 2012).
One of the main risks associated with Cannabis use is the potential for overconsumption, particularly when consuming edibles or other foods infused with Cannabis (Lin et al., 2022). This is because the effects of ingested Cannabis can take longer to manifest and last longer as compared to when Cannabis is smoked or vaporized, leading users to inadvertently consume more than intended (Grant and Bélanger, 2017). Overconsumption of Cannabis can cause severe side effects, including vomiting, nausea, anxiety, paranoia, and, in the extreme cases can lead to hospitalization (Ford et al., 2017). A study found that the rate of emergency department visits related to Cannabis-containing edibles increased significantly after legalization in Colorado (Heard et al., 2017).
Moreover, one of the major safety concerns associated with Cannabis use is the potential for contamination with biological, physical, or chemical contaminants (Rather et al., 2017). Microbial contamination of Cannabis-containing foods for instance can lead to foodborne illness, especially in persons with weak immune systems or other underlying health conditions. In one study, researchers analyzed a variety of Cannabis-infused food products and found that many were contaminated with high levels of bacteria including E. coli and Salmonella. In other studies, a significant percentage of Cannabis products tested were found to be contaminated with pesticides, mycotoxins, and heavy metals above the legal limit (Seltenrich, 2019; Peng and Shahidi, 2021; López-Ruiz et al., 2022). In addition to these risks, there is also the potential for adverse drug interactions between Cannabis and other medications, particularly those that are metabolized by the liver (Brown and Winterstein, 2019). Cannabis can interact with certain medications, such as blood thinners and antidepressants, leading to unintended side effects or reduced effectiveness of these drugs (Polson et al., 2021). Furthermore, there are concerns about the potential for Cannabis to interact with other medications or supplements (Wheeler et al., 2020). For example, a study found that consuming grapefruit juice with Cannabis-containing products can increase the levels of THC in the blood, potentially leading to an increased risk of adverse effects (Tireki, 2021).
Another issue is the lack of standardized dosing guidelines for Cannabis-containing foods (Nyland and Moyer, 2022). Because the potency of these products can vary widely, it can be difficult for consumers to know how much of a particular product they should consume to achieve the desired effects without risking overconsumption (Potter, 2014). This has led to instances of accidental overconsumption and adverse effects, particularly in the case of edibles, which can be deceivingly potent (Larkin Jr and Madras, 2019). Another concern is the potential for Cannabis-containing foods to be appealing to children and young people. As these products become more widely available, there is a risk that they could be mistaken for regular food items and ingested by children, potentially leading to serious adverse effects (Karbakhsh et al., 2018).
To address this issue, some jurisdictions have implemented packaging and labeling regulations for Cannabis-containing products to make them less appealing to children. In the United States, for example, the FDA requires that all Cannabis-containing food products be labeled with the statement “Keep out of reach of children” and include a warning that the product contains Cannabis (Nyland and Moyer, 2022). Some states such as Colorado, California, and Washington have gone further, requiring that products be packaged in child-resistant containers or that the packaging be opaque or non-descript to reduce their appeal to children. Similarly, in Canada, the Cannabis Act requires that all Cannabis-containing products be packaged in child-resistant containers and display a standardized warning label that includes the THC content and other relevant information (Leos-Toro, 2019). In addition, the act prohibits the use of branding and labeling that may appeal to children, such as cartoon characters or bright colors (Leos-Toro, 2019). In Australia, Cannabis-containing products must be packaged in opaque, child-resistant packaging and display warnings about the potential health risks associated with consumption (Rasera et al., 2021). In the Netherlands, all Cannabis-containing products must be labeled with a warning that they are not intended for consumption by children or minors (Rasera et al., 2021).
10 Conclusion and future perspectives
Cannabis is a versatile plant with many therapeutic uses. The current review has shown that it contains compounds with numerous therapeutic benefits, such as antioxidants, cytotoxic agents, and antibacterial, antifungal, anticancer, antidiarrheal, neuroprotective, and hepatoprotective properties. Cannabis sativa can be used in a variety of industries including biomedicine, agriculture, food, and cosmetics.
These bioactivities are attributed to its phytoconstituents, including cannabinoids, terpenes, flavonoids, alkaloids, and steroids, which highlight its potential as a source of medicinal agents. In addition to its medicinal uses, Cannabis has diverse applications in agriculture as fibre, food (as a source of protein, fiber, and functional foods), and cosmetics (as an active ingredient or oil). Although the available literature demonstrates the endless potential of Cannabis in these areas, more extensive research is needed to uncover the many unknown chemical substances with medical value. While empirical investigations of the plant have already been established, further studies should aim to identify and test these substances for their therapeutic benefits in treating various ailments.
In addition, Cannabis sativa L. is often overexploited as a recreational drug despite its potential uses in various fields due to its easy accessibility. Studies have confirmed its toxicity to brain development and the nervous system, but its extensive traditional use presents challenges in controlling its impact. Engaging users to understand the plant’s potential and training growers in cultivation, extraction, and production can help reduce overexploitation. Policies are also needed to protect and utilize the benefits of Cannabis plants. Community engagement, planning, monitoring, evaluation, and implementation can all help in this effort.
Author contributions
Conceptualization, EF; methodology, EF; resources, EF; writing original draft preparation, EF; writing—review and editing, EF, MC, AS, RJ, TP, CM, and RT; supervision, CM and RT; funding acquisition, CM and RT. All authors contributed to the article and approved the submitted version.
Funding
This research received external funding from Beehigh Vital Elements Inc., NL, Canada.
Conflict of interest
The authors declare that this study received funding from Beehigh Vital Elements Inc. Corner Brook, NL, Canada The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adams, J. R. T. C., and Jones, L. A. (1973). Long-chain hydrocarbons of cannabis and its smoke. J. Agric. Food Chem. 21, 1129–1131. doi:10.1021/jf60190a005
Ahmed, S. A., Ross, S. A., Slade, D., Radwan, M. M., Zulfiqar, F., Elsohly, M. A., et al. (2008). Cannabinoid ester constituents from high-potency Cannabis sativa. J. Nat. Prod. 71, 536–542. doi:10.1021/np070454a
Aizpurua-Olaizola, O., Soydaner, U., ÖZtüRk, E., Schibano, D., Simsir, Y., Navarro, P., et al. (2016). Evolution of the cannabinoid and terpene content during the growth of Cannabis sativa plants from different chemotypes. J. Nat. Prod. 79, 324–331. doi:10.1021/acs.jnatprod.5b00949
Akhtar, N., Ali, A., Bashir, U., and Haider, M. S. (2016). Bactericidal action of crude leaf extracts of common weeds. Pak. J. Weed Sci. Res. 22, 157–167.
Akram, M., Iqbal, M., Daniyal, M., and Khan, A. U. (2017). Awareness and current knowledge of breast cancer. J. Biol. Res. 50, 33–23. doi:10.1186/s40659-017-0140-9
Akyeampong, E. (2005). Diaspora and drug trafficking in west Africa: A case study of Ghana. Afr. Aff. 104, 429–447. doi:10.1093/afraf/adi015
Al Ubeed, H. M. S., Bhuyan, D. J., Alsherbiny, M. A., Basu, A., and Vuong, Q. V. (2022). A comprehensive review on the techniques for extraction of bioactive compounds from medicinal cannabis. Molecules 27, 604. doi:10.3390/molecules27030604
Albala, K. (2003). Food in early modern Europe. Westport, Connecticut, USA: Greenwood Publishing Group.
Alexander, A., Smith, P. F., and Rosengren, R. J. (2009). Cannabinoids in the treatment of cancer. Cancer Lett. 285, 6–12. doi:10.1016/j.canlet.2009.04.005
Ali, E. M., Almagboul, A. Z., Khogali, S. M., and Gergeir, U. M. (2012). Antimicrobial activity of Cannabis sativa L. Sci. Res. J. 3, 61–64. doi:10.4236/cm.2012.31010
Alsaraireh, A., and Darawad, M. W. (2019). Impact of a breast cancer educational program on female University students’ knowledge, attitudes, and practices. J. Cancer Educ. 34, 315–322. doi:10.1007/s13187-017-1304-6
Andre, C. M., Hausman, J.-F., and Guerriero, G. (2016). Cannabis sativa: The plant of the thousand and one molecules. Front. plant Sci. 7, 19. doi:10.3389/fpls.2016.00019
Antonarakis, S. E., Skotko, B. G., Rafii, M. S., Strydom, A., Pape, S. E., Bianchi, D. W., et al. (2020). Down syndrome. J. Nat. Rev. Dis. Prim. 6, 9–20. doi:10.1038/s41572-019-0143-7
Appendino, G., Gibbons, S., Giana, A., Pagani, A., Grassi, G., Stavri, M., et al. (2008). Antibacterial cannabinoids from cannabis sativa: A structure− activity study. J. Nat. Prod. 71, 1427–1430. doi:10.1021/np8002673
Appendino, G., Minassi, A., and Taglialatela-Scafati, O. (2014). Recreational drug discovery: Natural products as lead structures for the synthesis of smart drugs. Nat. Product. Rep. 31, 880–904. doi:10.1039/c4np00010b
Arkell, T. R., Kevin, R. C., Stuart, J., Lintzeris, N., Haber, P. S., Ramaekers, J. G., et al. (2019). Detection of Δ9 THC in oral fluid following vaporized cannabis with varied cannabidiol (CBD) content: An evaluation of two point-of-collection testing devices. Drug Test. analysis J. 11, 1486–1497. doi:10.1002/dta.2687
Arnarsson, A., Kristofersson, G. K., and Bjarnason, T. (2018). Adolescent alcohol and cannabis use in Iceland 1995–2015. Drug Alcohol Rev. 37, S49–S57. doi:10.1111/dar.12587
Babiker, E. E., Uslu, N., Al Juhaimi, F., Ahmed, I. A. M., Ghafoor, K., Özcan, M. M., et al. (2021). Effect of roasting on antioxidative properties, polyphenol profile and fatty acids composition of hemp (Cannabis sativa L) seeds. LWT 139, 110537. doi:10.1016/j.lwt.2020.110537
Balant, M., Gras, A., Ruz, M., Valles, J., Vitales, D., and Garnatje, T. (2021). Traditional uses of Cannabis: An analysis of the CANNUSE database. J. Ethnopharmacol. 279, 114362. doi:10.1016/j.jep.2021.114362
Balcke, G. U., Bennewitz, S., Zabel, S., and Tissier, A. (2014). Isoprenoid and metabolite profiling of plant trichomes. Methods Mol. Biol. 1153, 189–202. Plant Isoprenoids. Springer. doi:10.1007/978-1-4939-0606-2_13
Bao, Q., Liu, H., Fu, K., Zhang, C., Wang, C., and Feng, Y. (2014). Hemp bast fiber extract with antibacterial activity, preparation method and application of hemp bast fiber extract. USA patent application.
Bar-Or, R. L., Kor, A., Jaljuli, I., and Lev-Ran, S. (2021). The epidemiology of substance use disorders among the adult Jewish population in Israel. Eur. Addict. Res. 27, 362–370. doi:10.1159/000513776
Baral, P., Bagul, V., and Gajbhiye, S. (2020). Hemp seed oil for skin care (non-drug cannabis sativa L): A review. World J. Pharm. Res. 9, 2534–2556.
Barrales-Cureño, H. J., López-Valdez, L. G., Reyes, C., Cetina-Alcalá, V. M., Vasquez-García, I., Diaz-Lira, O. F., et al. (2020). Chemical characteristics, therapeutic uses, and legal aspects of the cannabinoids of cannabis sativa: A review. Braz. Archives Biol. Technol. 63, 1–14. doi:10.1590/1678-4324-2020190222
Barrett, M., Gordon, D., and Evans, F. (1985). Isolation from cannabis sativa L. Of cannflavin—a novel inhibitor of prostaglandin production. Biochem. Pharmacol. 34, 2019–2024. doi:10.1016/0006-2952(85)90325-9
Baswan, S. M., Klosner, A. E., Glynn, K., Rajgopal, A., Malik, K., Yim, S., et al. (2020). Therapeutic potential of cannabidiol (CBD) for skin health and disorders. Clin. Cosmet. investigational dermatology 13, 927–942. doi:10.2147/CCID.S286411
Baswan, S. M., Klosner, A. E., Weir, C., Salter-Venzon, D., Gellenbeck, K. W., Leverett, J., et al. (2021). Role of ingestible carotenoids in skin protection: A review of clinical evidence. Photodermatol. Photoimmunol. Photomed. 37, 490–504. doi:10.1111/phpp.12690
Bauer, S., Olson, J., Cockrill, A., Van Hattem, M., Miller, L., Tauzer, M., et al. (2015). Impacts of surface water diversions for marijuana cultivation on aquatic habitat in four northwestern California watersheds. PloS one 10, e0120016. doi:10.1371/journal.pone.0120016
Bautista, J. L., Yu, S., and Tian, L. (2021). Flavonoids in Cannabis sativa: Biosynthesis, bioactivities, and biotechnology. ACS Omega J. 6, 5119–5123. doi:10.1021/acsomega.1c00318
Benedicto, A., Romayor, I., and Arteta, B. (2017). Role of liver ICAM-1 in metastasis. J. Oncol. Lett. 14, 3883–3892. doi:10.3892/ol.2017.6700
Bennett, J. M., Reeves, G., Billman, G. E., and Sturmberg, J. P. (2018). Inflammation–nature's way to efficiently respond to all types of challenges: Implications for understanding and managing “the epidemic” of chronic diseases. J Front. Med. 316, 316. doi:10.3389/fmed.2018.00316
Benowitz, N. L., and Jones, R. T. (1981). Cardiovascular and metabolic considerations in prolonged cannabinoid administration in man. J. Clin. Pharmacol. 21, 214S–223S. doi:10.1002/j.1552-4604.1981.tb02598.x
Berman, P., Futoran, K., Lewitus, G. M., Mukha, D., Benami, M., Shlomi, T., et al. (2018). A new ESI-LC/MS approach for comprehensive metabolic profiling of phytocannabinoids in Cannabis. Sci. Rep. 8, 14280. doi:10.1038/s41598-018-32651-4
Bhattacharyya, S., Fusar-Poli, P., Borgwardt, S., Martin-Santos, R., Nosarti, C., O’Carroll, C., et al. (2009). Modulation of mediotemporal and ventrostriatal function in humans by delta9-tetrahydrocannabinol: A neural basis for the effects of cannabis sativa on learning and psychosis. Archives general psychiatry 66, 442–451. doi:10.1001/archgenpsychiatry.2009.17
Bhattacharyya, S., Morrison, P. D., Fusar-Poli, P., Martin-Santos, R., Borgwardt, S., Winton-Brown, T., et al. (2010). Opposite effects of Δ-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology 35, 764–774. doi:10.1038/npp.2009.184
Blaskovich, M. A., Kavanagh, A. M., Elliott, A. G., Zhang, B., Ramu, S., Amado, M., et al. (2021). The antimicrobial potential of cannabidiol. J. Commun. Biol. 4, 7–18. doi:10.1038/s42003-020-01530-y
Boakye, E., Obisesan, O. H., Uddin, S. I., El-Shahawy, O., Dzaye, O., Osei, A. D., et al. (2021). Cannabis vaping among adults in the United States: Prevalence, trends, and association with high-risk behaviors and adverse respiratory conditions. Prev. Med. 153, 106800. doi:10.1016/j.ypmed.2021.106800
Bonini, S. A., Premoli, M., Tambaro, S., Kumar, A., Maccarinelli, G., Memo, M., et al. (2018). Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharmacol. 227, 300–315. doi:10.1016/j.jep.2018.09.004
Boniotti, M. B., and Griffith, M. E. (2002). "Cross-talk" between cell division cycle and development in plants. Plant Cell 14, 11–16. doi:10.1105/tpc.140121
Bonn-Miller, M. O., Boden, M. T., Bucossi, M. M., and Babson, K. A. (2014). Self-reported cannabis use characteristics, patterns and helpfulness among medical cannabis users. Am. J. drug alcohol abuse 40, 23–30. doi:10.3109/00952990.2013.821477
Bonn-Miller, M. O., Elsohly, M. A., Loflin, M. J., Chandra, S., and Vandrey, R. (2018). Cannabis and cannabinoid drug development: Evaluating botanical versus single molecule approaches. Int. Rev. Psychiatry 30, 277–284. doi:10.1080/09540261.2018.1474730
Booth, J. K., and Bohlmann, J. (2019). Terpenes in Cannabis sativa–From plant genome to humans. J. Plant Sci. 284, 67–72. doi:10.1016/j.plantsci.2019.03.022
Booth, J. (2020). Terpene and isoprenoid biosynthesis in Cannabis sativa. Kelowna: University of British Columbia.
Borah, H. J., and Bordoloi, N. (2020). Chemical constituents of cannabis sativa L(marijuana). Cannabis 18, 1.
Braemer, R., and Paris, M. (1987). Biotransformation of cannabinoids by a cell suspension culture of Cannabis sativa L. Plant Cell Rep. 6, 150–152. doi:10.1007/BF00276675
Brennan, P., Bogillot, O., Greiser, E., Chang-Claude, J., Wahrendorf, J., Cordier, S., et al. (2001). The contribution of cigarette smoking to bladder cancer in women (pooled European data). Cancer Causes Control 12, 411–417. doi:10.1023/a:1011214222810
Brewster, J. M. (2019). A century of cannabis research in Canada. Can. J. Addict. 10, 6–9. doi:10.1097/cxa.0000000000000066
Brown, J. D., and Winterstein, A. G. (2019). Potential adverse drug events and drug–drug interactions with medical and consumer cannabidiol (CBD) use. J. Clin. Med. 8, 989. doi:10.3390/jcm8070989
Butsic, V., Carah, J. K., Baumann, M., Stephens, C., and Brenner, J. C. (2018). The emergence of cannabis agriculture frontiers as environmental threats. Environ. Res. Lett. 13, 124017. doi:10.1088/1748-9326/aaeade
Callaway, J. C. (2004). Hempseed as a nutritional resource: An overview. Euphytica 140, 65–72. doi:10.1007/s10681-004-4811-6
Canada, G. O. (2018). Regulations amending the cannabis regulations (new classes of cannabis): SOR/2019-206. Can. Gaz. 153, 3558–3728.
Caplan, D. M. (2018). Propagation and root zone management for controlled environment Cannabis production. Guelph: University of Guelph.
Carah, J. K., Howard, J. K., Thompson, S. E., Short Gianotti, A. G., Bauer, S. D., Carlson, S. M., et al. (2015). High time for conservation: Adding the environment to the debate on marijuana liberalization. BioScience 65, 822–829. doi:10.1093/biosci/biv083
Carlini, E. A., Karniol, I. G., Renault, P., and Schuster, C. (1974). Effects of marihuana in laboratory animals and in man. Br. J. Pharmacol. 50, 299–309. doi:10.1111/j.1476-5381.1974.tb08576.x
Carnevale, J. T., Kagan, R., Murphy, P. J., and Esrick, J. (2017). A practical framework for regulating for-profit recreational marijuana in US states: Lessons from Colorado and Washington. Int. J. Drug Policy 42, 71–85. doi:10.1016/j.drugpo.2017.03.001
Caspi, A., Moffitt, T. E., Cannon, M., Mcclay, J., Murray, R., Harrington, H., et al. (2005). Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: Longitudinal evidence of a gene X environment interaction. Biol. psychiatry 57, 1117–1127. doi:10.1016/j.biopsych.2005.01.026
Cassano, R., Trombino, S., Ferrarelli, T., Nicoletta, F. P., Mauro, M. V., Giraldi, C., et al. (2013). Hemp fiber (Cannabis sativa L) derivatives with antibacterial and chelating properties. Cellulose 20, 547–557. doi:10.1007/s10570-012-9804-3
Chandra, S., Lata, H., Khan, I. A., and Elsohly, M. A. (2017). Cannabis sativa L.: Botany and horticulture. Cannabis sativa l.-botany and biotechnology. Cham, Switzerland: Springer.
Chandra, S., Lata, H., Khan, I. A., and Elsohly, M. A. (2008). Photosynthetic response of Cannabis sativa L. to variations in photosynthetic photon flux densities, temperature and CO 2 conditions. Physiology Mol. Biol. Plants 14, 299–306. doi:10.1007/s12298-008-0027-x
Chen, F., Choi, S., Fu, C., and Nycholat, J. (2021). Too high to get it right: The effect of cannabis legalization on the performance of cannabis-related stocks. Econ. Analysis Policy 72, 715–734. doi:10.1016/j.eap.2021.10.001
Chouhan, S., and Guleria, S. (2020). Green synthesis of AgNPs using Cannabis sativa leaf extract: Characterization, antibacterial, anti-yeast and α-amylase inhibitory activity. Mater. Sci. Energy Technol. 3, 536–544. doi:10.1016/j.mset.2020.05.004
Chouvy, P.-A. (2019). Cannabis cultivation in the world: Heritages, trends and challenges. Morocco: EchoGéo, 48, 3–10.
Chung, M., Benkli, B., Hirani, S., and Le-Short, C. (2021). Cannabinoids and cancer pain. Cannabinoids and pain. Cham, Switzerland: Springer.
Clark, M. N., and Bohm, B. A. (1979). Flavonoid variation in cannabis L. Botanical J. Linn. Soc. 79, 249–257. doi:10.1111/j.1095-8339.1979.tb01517.x
Clarke, R. C., and Watson, D. P. (2002). Botany of natural cannabis medicines. Cannabis and cannabinoids: Pharmacology, toxicology and therapeutic potential, 3–13.
Clarke, R., and Merlin, M. (2013). Cannabis. Evolution and ethnobotany. LA, USA: University of California Press.
Connor, J. P., Stjepanović, D., Le Foll, B., Hoch, E., Budney, A. J., and Hall, W. D. (2021). Cannabis use and cannabis use disorder. Nat. Rev. Dis. Prim. 7, 16–24. doi:10.1038/s41572-021-00247-4
Costa, B., Trovato, A. E., Comelli, F., Giagnoni, G., and Colleoni, M. (2007). The non-psychoactive cannabis constituent cannabidiol is an orally effective therapeutic agent in rat chronic inflammatory and neuropathic pain. Eur. J. Pharmacol. 556, 75–83. doi:10.1016/j.ejphar.2006.11.006
Crini, G., Lichtfouse, E., Chanet, G., and Morin-Crini, N. (2020). Applications of hemp in textiles, paper industry, insulation and building materials, horticulture, animal nutrition, food and beverages, nutraceuticals, cosmetics and hygiene, medicine, agrochemistry, energy production and environment: A review. Environ. Chem. Lett. 18, 1451–1476. doi:10.1007/s10311-020-01029-2
Crippa, J. A., Guimarães, F. S., Campos, A. C., and Zuardi, A. W. (2018). Translational investigation of the therapeutic potential of cannabidiol (CBD): Toward a new age. Front. Immunol. 9, 2009. doi:10.3389/fimmu.2018.02009
Cruz, J. M., Queirolo, R., and Boidi, M. F. (2016). Determinants of public support for marijuana legalization in Uruguay, the United States, and El Salvador. J. Drug Issues 46, 308–325. doi:10.1177/0022042616649005
Csakvari, A. C., Moisa, C., Radu, D. G., Olariu, L. M., Lupitu, A. I., Panda, A. O., et al. (2021). Green synthesis, characterization, and antibacterial properties of silver nanoparticles obtained by using diverse varieties of Cannabis sativa leaf extracts. Molecules 26, 4041. doi:10.3390/molecules26134041
Datwyler, S. L., and Weiblen, G. D. (2006). Genetic variation in hemp and marijuana (Cannabis sativa L) according to amplified fragment length polymorphisms. J. Forensic Sci. 51, 371–375. doi:10.1111/j.1556-4029.2006.00061.x
Delong, G. T., Wolf, C. E., Poklis, A., and Lichtman, A. H. (2010). Pharmacological evaluation of the natural constituent of Cannabis sativa cannabichromene and its modulation by Δ9-tetrahydrocannabinol. Drug alcohol dependence 112, 126–133. doi:10.1016/j.drugalcdep.2010.05.019
De Meijer, E. P., Bagatta, M., Carboni, A., Crucitti, P., Moliterni, V. C., Ranalli, P., et al. (2003). The inheritance of chemical phenotype in Cannabis sativa L. Genetics 163, 335–346. doi:10.1093/genetics/163.1.335
De Vita, S., Finamore, C., Chini, M. G., Saviano, G., De Felice, V., De Marino, S., et al. (2022). Phytochemical analysis of the methanolic extract and essential oil from leaves of industrial hemp futura 75 cultivar: Isolation of a new cannabinoid derivative and biological profile using computational approaches. Plants 11, 1671. doi:10.3390/plants11131671
Degenhardt, L., Bruno, R., Lintzeris, N., Hall, W., Nielsen, S., Larance, B., et al. (2015). Agreement between definitions of pharmaceutical opioid use disorders and dependence in people taking opioids for chronic non-cancer pain (POINT): A cohort study. Lancet Psychiatry 2, 314–322. doi:10.1016/S2215-0366(15)00005-X
Del Río, C., Millán, E., García, V., Appendino, G., Demesa, J., and Muñoz, E. (2018). The endocannabinoid system of the skin. A potential approach for the treatment of skin disorders. Biochem. Pharmacol. 157, 122–133. doi:10.1016/j.bcp.2018.08.022
Devane, W. A., Dysarz, F. R., Johnson, M. R., Melvin, L. S., and Howlett, A. C. (1988). Determination and characterization of a cannabinoid receptor in rat brain. Mol. Pharmacol. 34, 605–613.
Devinsky, O., Cilio, M. R., Cross, H., Fernandez-Ruiz, J., French, J., Hill, C., et al. (2014). Cannabidiol: Pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia 55, 791–802. doi:10.1111/epi.12631
Di Forti, M., Morgan, C., Dazzan, P., Pariante, C., Mondelli, V., Marques, T. R., et al. (2009). High-potency cannabis and the risk of psychosis. Br. J. Psychiatry 195, 488–491. doi:10.1192/bjp.bp.109.064220
Dilley, R. J., and Morrison, W. A. (2014). Vascularisation to improve translational potential of tissue engineering systems for cardiac repair. Int. J. Biochem. Cell Biol. 56, 38–46. doi:10.1016/j.biocel.2014.10.020
Dos Santos, N. A., and Romão, W. (2023). Cannabis-a state of the art about the millenary plant: Part I. Forensic Chem. 32, 100470. doi:10.1016/j.forc.2023.100470
Eggers, C., Fujitani, M., Kato, R., and Smid, S. (2019). Novel cannabis flavonoid, cannflavin A displays both a hormetic and neuroprotective profile against amyloid β-mediated neurotoxicity in PC12 cells: Comparison with geranylated flavonoids, mimulone and diplacone. Biochem. Pharmacol. 169, 113609. doi:10.1016/j.bcp.2019.08.011
Ellen, E., Van Der Sluis, M., De, H., and Rodenburg, T. (2018). “Using sensor technologies in animal breeding: Reducing damaging behaviour of animals kept in groups,” in 11th International Conference on Methods and Techniques in Behavioral Research- Measuring behavior 2018, Manchester, UK, 5 June 2018-8 June 2018, 181–182.
Elsohly, M. A., and Slade, D. (2005). Chemical constituents of marijuana: The complex mixture of natural cannabinoids. Life Sci. 78, 539–548. doi:10.1016/j.lfs.2005.09.011
Elsohly, M. A. (2007). Marijuana and the cannabinoids. Totowa, NJ, USA: Springer Science and Business Media.
Elsohly, M. A., Radwan, M. M., Gul, W., Chandra, S., and Galal, A. (2017). “Phytochemistry of cannabis sativa L,” in Phytocannabinoids: unraveling the complex chemistry and pharmacology of cannabis sativa (New York, USA: Springer). 1-36.
Erkelens, J. L., and Hazekamp, A. (2014). That which we call Indica, by any other name would smell as sweet. Cannabinoids 9, 9–15.
Erridge, S., Mangal, N., Salazar, O., Pacchetti, B., and Sodergren, M. H. (2020). Cannflavins–from plant to patient: A scoping review. Fitoterapia 146, 104712. doi:10.1016/j.fitote.2020.104712
Farag, S., and Kayser, O. (2015). Cultivation and breeding of Cannabis sativa L. for preparation of standardized extracts for medicinal purposes. J. Med. aromatic plants world, 165–186.
Farag, S., and Kayser, O. (2017). “The cannabis plant: Botanical aspects,” in Handbook of cannabis and related pathologies (Dortmund, Germany: Elsevier). Technical University Dortmund.
Farha, M. A., El-Halfawy, O. M., Gale, R. T., Macnair, C. R., Carfrae, L. A., Zhang, X., et al. (2020). Uncovering the hidden antibiotic potential of cannabis. ACS Infect. Dis. 6, 338–346. doi:10.1021/acsinfecdis.9b00419
Farinon, B., Molinari, R., Costantini, L., and Merendino, N. (2020). The seed of industrial hemp (Cannabis sativa L): Nutritional quality and potential functionality for human health and nutrition. J. Nutrients 12, 1935. doi:10.3390/nu12071935
Fasakin, O. W., Oboh, G., Ademosun, A. O., and Lawal, A. O. (2022). The modulatory effects of alkaloid extracts of Cannabis sativa, Datura stramonium, Nicotiana tabacum and male Carica papaya on neurotransmitter, neurotrophic and neuroinflammatory systems linked to anxiety and depression. Inflammopharmacology 30, 2447–2476. doi:10.1007/s10787-022-01006-x
Feder, C. L., Cohen, O., Shapira, A., Katzir, I., Peer, R., Guberman, O., et al. (2021). Fertilization following pollination predominantly decreases phytocannabinoids accumulation and alters the accumulation of terpenoids in cannabis inflorescences. Front. Plant Sci. J. 12, 753847. doi:10.3389/fpls.2021.753847
Feldman, M., Smoum, R., Mechoulam, R., and Steinberg, D. (2018). Antimicrobial potential of endocannabinoid and endocannabinoid-like compounds against methicillin-resistant Staphylococcus aureus. Sci. Rep. 8, 17696. doi:10.1038/s41598-018-35793-7
Ferrini, F., Fraternale, D., Donati Zeppa, S., Verardo, G., Gorassini, A., Carrabs, V., et al. (2021). Yield, characterization, and possible exploitation of Cannabis sativa L. roots grown under aeroponics cultivation. Molecules 26, 4889. doi:10.3390/molecules26164889
Figuerêdo, J. S. D., Santos, F. P., Furtado, P. V., Andrade, T. J., Júnior, J. S., Lima, N. M., et al. (2022). “Natural products from plants with antimicrobial action,” in Promising antimicrobials from natural products (Cham, Switzerland: Springer).
Fischedick, J. T. (2017). Identification of terpenoid chemotypes among high (−)-trans-Δ9-tetrahydrocannabinol-producing Cannabis sativa L. cultivars. Cannabis cannabinoid Res. 2, 34–47. doi:10.1089/can.2016.0040
Flicker, N. R., Poveda, K., and Grab, H. (2020). The bee community of cannabis sativa and corresponding effects of landscape composition. Environ. Entomol. 49, 197–202. doi:10.1093/ee/nvz141
Ford, T. C., Hayley, A. C., Downey, L. A., and Parrott, A. C. (2017). Cannabis: An overview of its adverse acute and chronic effects and its implications. Curr. drug abuse Rev. 10, 6–18. doi:10.2174/1874473710666170712113042
Fufa, B. K. (2019). Anti-bacterial and anti-fungal properties of garlic extract (allium sativum): A review. Int. J. Microbiol. 28, 1–5. doi:10.9734/mrji/2019/v28i330133
Gabriel, M. W., Wengert, G. M., Higley, J. M., Krogan, S., Sargent, W., and Clifford, D. L. (2013). Silent forests. Rodenticides on illegal marijuana crops harm wildlife. [Online], 7. Available at: https://www.iercecology.org/wp-content/uploads/2013/03/Silent_Forests_by_Mourad_W._Gabriel_et_al.TWP_Spring_2013.pdf.
Gaffal, E., Cron, M., Glodde, N., and Tüting, T. (2013). Anti-inflammatory activity of topical THC in DNFB-mediated mouse allergic contact dermatitis independent of CB 1 and CB 2 receptors. Allergy 68, 994–1000. doi:10.1111/all.12183
Gage, S. H., Hickman, M., and Zammit, S. (2016). Association between cannabis and psychosis: Epidemiologic evidence. J. Biol. psychiatry 79, 549–556. doi:10.1016/j.biopsych.2015.08.001
Galal, A. M., Slade, D., Gul, W., El-Alfy, A. T., Ferreira, D., and Elsohly, M. A. (2009). Naturally occurring and related synthetic cannabinoids and their potential therapeutic applications. Recent Pat. CNS Drug Discov. Discontin. 4, 112–136. doi:10.2174/157488909788453031
Gaoni, Y., and Mechoulam, R. (1964). Isolation, structure, and partial synthesis of an active constituent of hashish. J. Am. Chem. Soc. 86, 1646–1647. doi:10.1021/ja01062a046
Gaoni, Y., and Mechoulam, R. (1971). The isolation and structure of delta-1-tetrahydrocannabinol and other neutral cannabinoids from hashish. J. Am. Chem. Soc. 93, 217–224. doi:10.1021/ja00730a036
Garcia-Romeu, A., Kersgaard, B., and Addy, P. H. (2016). Clinical applications of hallucinogens: A review. J. Exp. Clin. Psychopharmacol. 24, 229–268. doi:10.1037/pha0000084
Gaston, T. E., and Szaflarski, J. P. (2018). Cannabis for the treatment of epilepsy: An update. J. Curr. Neurol. Neurosci. Rep. 18, 73–79. doi:10.1007/s11910-018-0882-y
Gildea, L., Ayariga, J. A., Ajayi, O. S., Xu, J., Villafane, R., and Samuel-Foo, M. (2022). Cannabis sativa CBD extract shows promising antibacterial activity against Salmonella typhimurium and S. Newington. Molecules 27, 2669. doi:10.3390/molecules27092669
Gil-Ordóñez, A., Martín-Fontecha, M., Ortega-Gutiérrez, S., and López-Rodríguez, M. L. (2018). Monoacylglycerol lipase (MAGL) as a promising therapeutic target. J. Biochem. Pharmacol. 157, 18–32. doi:10.1016/j.bcp.2018.07.036
Głodowska, M., and Łyszcz, M. (2017). Cannabis sativa L. and its antimicrobial properties–A review. Agron. Sukcesku 11, 77–82.
Gorelick, D. A., and Heishman, S. J. (2006). Methods for clinical research involving cannabis administration. Methods Mol. Med. 123, 235–253. doi:10.1385/1-59259-999-0:235
Grant, C. N., and Bélanger, R. E. (2017). Cannabis and Canada’s children and youth. Paediatr. child health 22, 98–102. doi:10.1093/pch/pxx017
Grotenhermen, F. (2004). Clinical pharmacodynamics of cannabinoids. J. Cannabis Ther. 4, 29–78. doi:10.1300/j175v04n01_03
Gülck, T., and Møller, B. L. (2020). Phytocannabinoids: Origins and biosynthesis. Trends plant Sci. 25, 985–1004. doi:10.1016/j.tplants.2020.05.005
Hajizadeh, M. (2016). Legalizing and regulating marijuana in Canada: Review of potential economic, social, and health impacts. Int. J. health policy Manag. 5, 453–456. doi:10.15171/ijhpm.2016.63
Haldar, S., Gan, L., Tay, S. L., Ponnalagu, S., and Henry, C. J. (2019). Postprandial glycemic and insulinemic effects of the addition of aqueous extracts of dried corn silk, cumin seed powder or tamarind pulp, in two forms, consumed with high glycemic index rice. Foods J. 8, 437. doi:10.3390/foods8100437
Hall, W., and Degenhardt, L. (2007). Prevalence and correlates of cannabis use in developed and developing countries. Curr. Opin. Psychiatry 20, 393–397. doi:10.1097/YCO.0b013e32812144cc
Han, Q.-W., Yuan, Y.-H., and Chen, N.-H. (2020). The therapeutic role of cannabinoid receptors and its agonists or antagonists in Parkinson's disease. Prog. Neuro-Psychopharmacology Biol. Psychiatry 96, 109745. doi:10.1016/j.pnpbp.2019.109745
Hanus, L. O., Meyer, S. M., Munoz, E., Taglialatela-Scafati, O., and Appendino, G. (2016). Phytocannabinoids: A unified critical inventory. Nat. Prod. Rep. 33, 1357–1392. doi:10.1039/c6np00074f
Hartsel, J. A., Boyar, K., Pham, A., Silver, R. J., and Makriyannis, A. (2019). “Cannabis in veterinary medicine: Cannabinoid therapies for animals,” in Nutraceuticals in veterinary medicine (Cham, Switzerland: Springer).
Harvey, D. (1976). Characterization of the butyl homologues of delta1-tetrahydrocannabinol, cannabinol and cannabidiol in samples of cannabis by combined gas chromatography and mass spectrometry. J. Pharm. Pharmacol. 28, 280–285. doi:10.1111/j.2042-7158.1976.tb04153.x
Harvey, D. J., Martin, B., and Paton, W. (1977). Identification of glucuronides as in vivo liver conjugates of seven cannabinoids and some of their hydroxy and acid metabolites. Res. Commun. Chem. Pathology Pharmacol. 16, 265–279.
Hasan, K. A. (1975). “Social aspects of the use of cannabis in India,” in Cannabis and culture (Hague, Paris: Mouton Publishers), 235–246.
Häuser, W., Finn, D. P., Kalso, E., Krcevski-Skvarc, N., Kress, H. G., Morlion, B., et al. (2018). European Pain Federation (EFIC) position paper on appropriate use of cannabis-based medicines and medical cannabis for chronic pain management. Eur. J. Pain 22, 1547–1564. doi:10.1002/ejp.1297
Hayakawa, K., Mishima, K., Hazekawa, M., Sano, K., Irie, K., Orito, K., et al. (2008). Cannabidiol potentiates pharmacological effects of Delta(9)-tetrahydrocannabinol via CB(1) receptor-dependent mechanism. Brain Res. 1188, 157–164. doi:10.1016/j.brainres.2007.09.090
Heard, K., Marlin, M. B., Nappe, T., and Hoyte, C. O. (2017). Common marijuana-related cases encountered in the emergency department. Am. J. Health-System Pharm. 74, 1904–1908. doi:10.2146/ajhp160715
Hellmich, M. R., and Szabo, C. (2015). Hydrogen sulfide and cancer. Chem. Biochem. Pharmacol. Hydrogen Sulfide 230, 233–241. doi:10.1007/978-3-319-18144-8_12
Hindocha, C., Brose, L. S., Walsh, H., and Cheeseman, H. (2021). Cannabis use and co-use in tobacco smokers and non-smokers: Prevalence and associations with mental health in a cross-sectional, nationally representative sample of adults in great britain, 2020. J. Addict. 116, 2209–2219. doi:10.1111/add.15381
Holland, J. (2010). The pot book: A complete guide to cannabis. Toronto, Canada: Simon and Schuster.
Howlett, A., Barth, F., Bonner, T., Cabral, G., Casellas, P., Devane, W., et al. (2002). International union of pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 54, 161–202. doi:10.1124/pr.54.2.161
Hryhorowicz, S., Kaczmarek-Ryś, M., Zielińska, A., Scott, R. J., Słomski, R., and Pławski, A. (2021). Endocannabinoid system as a promising therapeutic target in inflammatory bowel disease–A systematic review. J Front. Immunol. 12, 790803. doi:10.3389/fimmu.2021.790803
Iannotti, F. A., Di Marzo, V., and Petrosino, S. (2016). Endocannabinoids and endocannabinoid-related mediators: Targets, metabolism and role in neurological disorders. J Prog. lipid Res. 62, 107–128. doi:10.1016/j.plipres.2016.02.002
Iannotti, F. A., De Maio, F., Panza, E., Appendino, G., Taglialatela-Scafati, O., De Petrocellis, L., et al. (2020). Identification and characterization of cannabimovone, a cannabinoid from Cannabis sativa, as a novel PPARγ agonist via a combined computational and functional study. Molecules 25, 1119. doi:10.3390/molecules25051119
Iber, B. T., Kasan, N. A., Torsabo, D., and Omuwa, J. W. (2022). A review of various sources of chitin and chitosan in nature. J. Renew. Mater. 10, 1097–1123. doi:10.32604/jrm.2022.018142
Iftikhar, A., Zafar, U., Ahmed, W., Shabbir, M. A., Sameen, A., Sahar, A., et al. (2021). Applications of cannabis sativa L. In food and its therapeutic potential: From a prohibited drug to a nutritional supplement. Molecules 26, 7699. doi:10.3390/molecules26247699
Ilgen, M. A., Bohnert, K., Kleinberg, F., Jannausch, M., Bohnert, A. S., Walton, M., et al. (2013). Characteristics of adults seeking medical marijuana certification. Drug alcohol dependence 132, 654–659. doi:10.1016/j.drugalcdep.2013.04.019
Irakli, M., Tsaliki, E., Kalivas, A., Kleisiaris, F., Sarrou, E., and Cook, C. M. (2019). Effect οf genotype and growing year on the nutritional, phytochemical, and antioxidant properties of industrial hemp (Cannabis sativa L) seeds. Antioxidants 8, 491. doi:10.3390/antiox8100491
Iseppi, R., Brighenti, V., Licata, M., Lambertini, A., Sabia, C., Messi, P., et al. (2019). Chemical characterization and evaluation of the antibacterial activity of essential oils from fibre-type Cannabis sativa L(Hemp). Molecules 24, 2302. doi:10.3390/molecules24122302
Iwamura, H., Suzuki, H., Ueda, Y., Kaya, T., and Inaba, T. (2001). In vitro and in vivo pharmacological characterization of JTE-907, a novel selective ligand for cannabinoid CB2 receptor. J. Pharmacol. Exp. Ther. 296, 420–425.
Jagannathan, R. (2020). Identification of psychoactive metabolites from Cannabis sativa, its smoke, and other phytocannabinoids using machine learning and multivariate methods. ACS omega 5, 281–295. doi:10.1021/acsomega.9b02663
Jin, D., Dai, K., Xie, Z., and Chen, J. (2020). Secondary metabolites profiled in cannabis inflorescences, leaves, stem barks, and roots for medicinal purposes. Sci. Rep. 10, 3309–3314. doi:10.1038/s41598-020-60172-6
Johnson, R. (2014). Hemp as an agricultural commodity. Washington: Library of Congress Washington DC Congressional Research Service, 34.
Jurgoński, A., Opyd, P. M., and Fotschki, B. (2020). Effects of native or partially defatted hemp seeds on hindgut function, antioxidant status and lipid metabolism in diet-induced obese rats. J. Funct. Foods 72, 104071. doi:10.1016/j.jff.2020.104071
Kalant, H. (2010). Drug classification: Science, politics, both or neither? Addiction 105, 1146–1149. doi:10.1111/j.1360-0443.2009.02830.x
Kanabus, J., Bryła, M., Roszko, M., Modrzewska, M., and Pierzgalski, A. (2021). Cannabinoids—characteristics and potential for use in food production. Molecules 26, 6723. doi:10.3390/molecules26216723
Kaplan, J. S., Stella, N., Catterall, W. A., and Westenbroek, R. E. (2017). Cannabidiol attenuates seizures and social deficits in a mouse model of Dravet syndrome. J Proc. Natl. Acad. Sci. 114, 11229–11234. doi:10.1073/pnas.1711351114
Karas, J. A., Wong, L. J., Paulin, O. K., Mazeh, A. C., Hussein, M. H., Li, J., et al. (2020). The antimicrobial activity of cannabinoids. J. Antibiotics 9, 406. doi:10.3390/antibiotics9070406
Karbakhsh, M., Smith, J., and Pike, I. (2018). Where does the high road lead? Potential implications of cannabis legalization for pediatric injuries in Canada. Can. J. public health 109, 752–755. doi:10.17269/s41997-018-0137-3
Karniol, I., and Carlini, E. (1973). Comparative studies in man and in laboratory animals on▵ 8-and▵ 9-trans-Tetrahydrocannabinol. Pharmacology 9, 115–126. doi:10.1159/000136375
Karwad, M. A., Couch, D. G., Theophilidou, E., Sarmad, S., Barrett, D. A., Larvin, M., et al. (2017). The role of CB1 in intestinal permeability and inflammation. J FASEB J. 31, 3267–3277. doi:10.1096/fj.201601346R
Khan, S., and Al Baradie, R. (2012). Epileptic encephalopathies: An overview. Epilepsy Res. Treat. 2012, 403592. doi:10.1155/2012/403592
Kilaru, A., and Chapman, K. D. (2020). The endocannabinoid system. J Essays Biochem. 64, 485–499. doi:10.1042/EBC20190086
Kilmer, B., and Maccoun, R. J. (2017). How medical marijuana smoothed the transition to marijuana legalization in the United States. Annu. Rev. Law Soc. Sci. 13, 181–202. doi:10.1146/annurev-lawsocsci-110615-084851
Kinghorn, A. D., Falk, H., Gibbons, S., and Kobayashi, J. I. (2017). Phytocannabinoids. Cham, Switzerland: Springer.
Kitchen, C., Kabba, J. A., and Fang, Y. (2022). Status and impacts of recreational and medicinal cannabis policies in Africa: A systematic review and thematic analysis of published and “gray” literature. Cannabis cannabinoid Res. 7, 239–261. doi:10.1089/can.2021.0110
Ko, G. D., Bober, S. L., Mindra, S., and Moreau, J. M. (2016). Medical cannabis–the Canadian perspective. J. pain Res. 9, 735–744. doi:10.2147/JPR.S98182
Kocatürk, R. R., Zelka, F. Z., Özcan, Ö. Ö., and Canbolat, F. (2021). “Antioxidant effects of peptides,” in Synthetic peptide vaccine models (Boca Raton, FL, USA: CRC Press).
Kopustinskiene, D. M., Masteikova, R., Lazauskas, R., and Bernatoniene, J. (2022). Cannabis sativa L. Bioactive compounds and their protective role in oxidative stress and inflammation. Antioxidants 11, 660. doi:10.3390/antiox11040660
Kriese, U., Schumann, E., Weber, W., Beyer, M., Brühl, L., and Matthäus, B. (2004). Oil content, tocopherol composition and fatty acid patterns of the seeds of 51 Cannabis sativa L. genotypes. Euphytica 137, 339–351. doi:10.1023/b:euph.0000040473.23941.76
Kumar, P., Mahato, D. K., Kamle, M., Borah, R., Sharma, B., Pandhi, S., et al. (2021). Pharmacological properties, therapeutic potential, and legal status of Cannabis sativa L.: An overview. Phytotherapy Res. 35, 6010–6029. doi:10.1002/ptr.7213
Kumar, S., and Pandey, A. K. (2013). Chemistry and biological activities of flavonoids: An overview. Sci. world J. 2013, 162750. doi:10.1155/2013/162750
Kuppuram, G. (2022). The nutritional and therapeutical value of Cannabis Sattiva. J. Assoc. Res. 27, 18–23.
Laezza, C., Pagano, C., Navarra, G., Pastorino, O., Proto, M. C., Fiore, D., et al. (2020). The endocannabinoid system: A target for cancer treatment. Int. J. Mol. Sci. 21, 747. doi:10.3390/ijms21030747
Laidlaw, A. M. (2016). Control and detection of enterohaemorrhagic Escherichia coli. Alberta: University of Alberta.
Lakhan, S. E., and Rowland, M. (2009). Whole plant cannabis extracts in the treatment of spasticity in multiple sclerosis: A systematic review. BMC Neurol. 9, 1–6. doi:10.1186/1471-2377-9-59
Larkin, P. J., and Madras, B. K. (2019). Opioids, overdoses, and cannabis: Is marijuana an effective therapeutic response to the opioid abuse epidemic. Geo. JL Pub. Pol'y 17, 555.
Lata, H., Chandra, S., Techen, N., Khan, I. A., and Elsohly, M. A. (2016). In vitro mass propagation of cannabis sativa L.: A protocol refinement using novel aromatic cytokinin meta-topolin and the assessment of eco-physiological, biochemical and genetic fidelity of micropropagated plants. J. Appl. Res. Med. Aromatic Plants 3, 18–26. doi:10.1016/j.jarmap.2015.12.001
Lattanzi, S., Zaccara, G., Russo, E., La Neve, A., Lodi, M. A. M., and Striano, P. (2021). Practical use of pharmaceutically purified oral cannabidiol in Dravet syndrome and Lennox-Gastaut syndrome. Expert Rev. Neurother. 21, 99–110. doi:10.1080/14737175.2021.1834383
Lau, W. M., Teng, E., Huang, K. K., Tan, J. W., Das, K., Zang, Z., et al. (2018). Acquired resistance to FGFR inhibitor in diffuse-type gastric cancer through an AKT-independent PKC-mediated phosphorylation of GSK3β. J. Mol. Cancer Ther. 17, 232–242. doi:10.1158/1535-7163.MCT-17-0367
Le Boisselier, R., Alexandre, J., Lelong-Boulouard, V., and Debruyne, D. (2017). Focus on cannabinoids and synthetic cannabinoids. Clin. Pharmacol. Ther. 101, 220–229. doi:10.1002/cpt.563
Leos-Toro, C. (2019). Health warnings, cannabis marketing and perceptions among youth and young adults in Canada.
Li, C.-R., Yang, L.-X., Guo, Z.-F., Yang, H., Zhang, Y., Wang, Y.-M., et al. (2022). LC-MS-based untargeted metabolomics reveals chemical differences of Cannabis leaves from different regions of China. Industrial Crops Prod. 176, 114411. doi:10.1016/j.indcrop.2021.114411
Li, H.-L. (1974). An archaeological and historical account of cannabis in China. Econ. Bot. 28, 437–448. doi:10.1007/bf02862859
Liktor-Busa, E., Keresztes, A., Lavigne, J., Streicher, J. M., and Largent-Milnes, T. M. (2021). Analgesic potential of terpenes derived from cannabis sativa. J. Pharmacol. Rev. 73, 98–126. doi:10.1124/pharmrev.120.000046
Lin, A., O’Connor, M., Behnam, R., Hatef, C., and Milanaik, R. (2022). Edible marijuana products and potential risks for pediatric populations. Curr. Opin. Pediatr. 34, 279–287. doi:10.1097/MOP.0000000000001132
Lindblom, E. N. (2019). How FDA could use its existing authorities to make state legalization of cannabis more safe and effective. Food and Drug LJ 74, 191.
Lindley, J. (2011). “Flora medica: A botanical account of all the more important plants used in medicine,” in Different parts of the world (Cambridge, UK: Cambridge University Press).
Lohar, V., and Rathore, A. S. (2013). Cannabinoids: Pharmacological profile of promising molecules. Phytopharm. J. 4, 41–52.
López-Ruiz, R., Marín-Sáez, J., Garrido Frenich, A., and Romero-González, R. (2022). Recent applications of chromatography for analysis of contaminants in cannabis products: A review. Pest Manag. Sci. 78, 19–29. doi:10.1002/ps.6599
Lowe, H., Steele, B., Bryant, J., Toyang, N., and Ngwa, W. (2021). Non-cannabinoid metabolites of Cannabis sativa L. with therapeutic potential. Plants J. 10, 400. doi:10.3390/plants10020400
Macdonald, R., and Rotermann, M. (2017). Experimental estimates of cannabis consumption in Canada, 1960 to 2015 [Online]. Statistics Canada= Statistique Canada. Available at: https://www150.statcan.gc.ca/n1/pub/11-626-x/11-626-x2017077-eng.htm (Accessed December 20, 2022).
Mander, L., and Liu, H.-W. (2010). Comprehensive natural products II: Chemistry and biology. Oxford, U.K: Elsevier.
Manske, R. H. F., and Holmes, H. L. (2014). The alkaloids: Chemistry and physiology. Guelph, Ontario: Elsevier.
Maroon, J., and Bost, J. (2018). Review of the neurological benefits of phytocannabinoids. Surg. Neurol. Int. 9, 91. doi:10.4103/sni.sni_45_18
Matsuda, L. A., Lolait, S. J., Brownstein, M. J., Young, A. C., and Bonner, T. I. (1990). Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346, 561–564. doi:10.1038/346561a0
Mcpartland, J., Di Marzo, V., De Petrocellis, L., Mercer, A., and Glass, M. (2001). Cannabinoid receptors are absent in insects. J. Comp. Neurology 436, 423–429. doi:10.1002/cne.1078
Mcpartland, J. M., and Mckernan, K. J. (2017). “Contaminants of concern in cannabis: Microbes, heavy metals and pesticides,” in Cannabis sativa L.-Botany and biotechnology (Cham, Switzerland: Springer).
Mechoulam, R., Ben-Shabat, S., Hanus, L., Ligumsky, M., Kaminski, N. E., Schatz, A. R., et al. (1995). Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 50, 83–90. doi:10.1016/0006-2952(95)00109-d
Mechoulam, R., and Shvo, Y. (1963). Hashish—I: The structure of cannabidiol. Tetrahedron 19, 2073–2078. doi:10.1016/0040-4020(63)85022-x
Mechoulam, R., and Hanuš, L. R. (2000). A historical overview of chemical research on cannabinoids. Chem. Phys. Lipids 108, 1–13. doi:10.1016/s0009-3084(00)00184-5
Michailidis, D., Angelis, A., Nikolaou, P. E., Mitakou, S., and Skaltsounis, A. L. (2021). Exploitation of vitis vinifera, foeniculum vulgare, cannabis sativa and punica granatum by-product seeds as dermo-cosmetic agents. Molecules 26, 731. doi:10.3390/molecules26030731
Mihoc, M., Pop, G., Alexa, E., and Radulov, I. (2012). Nutritive quality of Romanian hemp varieties (Cannabis sativa L) with special focus on oil and metal contents of seeds. Chem. Central J. 6, 1–12.
Minelli, A. (2015). “Morphological misfits and the architecture of development,” in Macroevolution (Cham, Switzerland: Springer), 329–343.
Mnekin, L., and Ripoll, L. (2021). Topical use of cannabis sativa L. Biochemicals. Cosmetics 8, 85. doi:10.3390/cosmetics8030085
Moliterni, V. M. C., Pojić, M., and Tiwari, B. (2022). “Industrial hemp by-product valorization,” in Industrial hemp (Massachusetts, USA: Elsevier).
Mölleken, H., and Theimer, R. R. (1997). Survey of minor fatty acids in Cannabis sativa L. fruits of various origins. J. Int. Hemp Assoc. 4, 13–17.
Morimoto, S., Komatsu, K., Taura, F., and Shoyama, Y. (1998). Purification and characterization of cannabichromenic acid synthase from Cannabis sativa. Phytochemistry 49, 1525–1529. doi:10.1016/s0031-9422(98)00278-7
Moyle, P. B. (2002). Inland fishes of California: Revised and expanded. LA, USA: Univ of California Press.
Mrowka, P., and Glodkowska-Mrowka, E. (2020). PPARγ agonists in combination cancer therapies. J. Curr. Cancer Drug Targets 20, 197–215. doi:10.2174/1568009619666191209102015
Mustafa, G., Arif, R., Atta, A., Sharif, S., and Jamil, A. (2017). Bioactive compounds from medicinal plants and their importance in drug discovery in Pakistan. Matrix Sci. Pharma 1, 17–26. doi:10.26480/msp.01.2017.17.26
Nahhas, A. F., Abdel-Malek, Z. A., Kohli, I., Braunberger, T. L., Lim, H. W., and Hamzavi, I. H. (2019). The potential role of antioxidants in mitigating skin hyperpigmentation resulting from ultraviolet and visible light-induced oxidative stress. Photodermatol. Photoimmunol. Photomed. 35, 420–428. doi:10.1111/phpp.12423
Nallathambi, R., Mazuz, M., Ion, A., Selvaraj, G., Weininger, S., Fridlender, M., et al. (2017). Anti-inflammatory activity in colon models is derived from δ9-tetrahydrocannabinolic acid that interacts with additional compounds in cannabis extracts. Cannabis cannabinoid Res. 2, 167–182. doi:10.1089/can.2017.0027
Naveed, M., Khan, T. A., Ali, I., Hassan, A., Ali, H., Ud, Z., et al. (2014). In vitro antibacterial activity of Cannabis sativa leaf extracts to some selective pathogenicbacterial strains. Int. J. Biosci. 4, 65–70.
Novak, J., Zitterl-Eglseer, K., Deans, S. G., and Franz, C. M. (2001). Essential oils of different cultivars of Cannabis sativa L. and their antimicrobial activity. Flavour Fragr. J. 16, 259–262. doi:10.1002/ffj.993
Nyland, C. R., and Moyer, D. C. (2022). Regulating for safety: Cannabidiol dose in food: A review. J. food Prot. 85, 1355–1369. doi:10.4315/JFP-21-374
Oberbarnscheidt, T., and Miller, N. S. (2020). The impact of cannabidiol on psychiatric and medical conditions. J. Clin. Med. Res. 12, 393–403. doi:10.14740/jocmr4159
Odieka, A. E., Obuzor, G. U., Oyedeji, O. O., Gondwe, M., Hosu, Y. S., and Oyedeji, A. O. (2022). The medicinal natural products of cannabis sativa Linn.: A review. Molecules 27, 1689. doi:10.3390/molecules27051689
Pamplona, F. A., Da Silva, L. R., and Coan, A. C. (2018). Potential clinical benefits of CBD-rich cannabis extracts over purified CBD in treatment-resistant epilepsy: Observational data meta-analysis. Front. neurology 9, 759. doi:10.3389/fneur.2018.00759
Pavlovic, R., Panseri, S., Giupponi, L., Leoni, V., Citti, C., Cattaneo, C., et al. (2019). Phytochemical and ecological analysis of two varieties of hemp (Cannabis sativa L) grown in a mountain environment of Italian Alps. Front. Plant Sci. 10, 1265. doi:10.3389/fpls.2019.01265
Pellati, F., Borgonetti, V., Brighenti, V., Biagi, M., Benvenuti, S., and Corsi, L. (2018). Cannabis sativa L. And nonpsychoactive cannabinoids: Their chemistry and role against oxidative stress, inflammation, and cancer. J. BioMed Res. Int. 2018, 1691428. doi:10.1155/2018/1691428
Peng, H., and Shahidi, F. (2021). Cannabis and cannabis edibles: A review. J. Agric. Food Chem. 69, 1751–1774. doi:10.1021/acs.jafc.0c07472
Perucca, E. (2017). Cannabinoids in the treatment of epilepsy: Hard evidence at last? J. epilepsy Res. 7, 61–76. doi:10.14581/jer.17012
Piccolella, S., Crescente, G., Formato, M., and Pacifico, S. (2020). A cup of hemp coffee by moka pot from Southern Italy: An UHPLC-HRMS investigation. Foods 9, 1123. doi:10.3390/foods9081123
Piomelli, D., and Russo, E. B. (2016). The cannabis sativa versus cannabis indica debate: An interview with ethan Russo, MD. Cannabis cannabinoid Res. 1, 44–46. doi:10.1089/can.2015.29003.ebr
Pollastro, F., Minassi, A., and Fresu, L. G. (2018). Cannabis phenolics and their bioactivities. Curr. Med. Chem. 25, 1160–1185. doi:10.2174/0929867324666170810164636
Poewe, W., Seppi, K., Tanner, C. M., Halliday, G. M., Brundin, P., Volkmann, J., et al. (2017). Parkinson disease. Nat. Rev. Dis. Prim. 3, 17013–17021. doi:10.1038/nrdp.2017.13
Pollio, A. (2016). The name of cannabis: A short guide for nonbotanists. Cannabis cannabinoid Res. 1, 234–238. doi:10.1089/can.2016.0027
Polson, G., Chung, M., Hirani, S., and Le-Short, C. (2021). “Cannabis drug interactions,” in Cannabinoids and pain (Cham, Switzerland: Springer).
Popescu-Spineni, D. M., Moldoveneau, A. C., Armean, P., Ionescu-Tirgoviste, C., Militaru, C., and Munteanu, A. M. (2021). Antimicobial effect of cannabis sativa L. Rev. Roum. Chim J. 66, 417–422.
Potter, D. J. (2014). A review of the cultivation and processing of cannabis (Cannabis sativa L) for production of prescription medicines in the UK. Drug Test. analysis 6, 31–38. doi:10.1002/dta.1531
Radwan, M. M., Wanas, A. S., Chandra, S., and Elsohly, M. A. (2017). Natural cannabinoids of cannabis and methods of analysis. Cannabis sativa l.-botany and biotechnology. New York, USA: Springer.
Radwan, M. M., Chandra, S., Gul, S., and Elsohly, M. A. (2021). Cannabinoids, phenolics, terpenes and alkaloids of cannabis. Molecules 26, 2774. doi:10.3390/molecules26092774
Ramantani, G., Kadish, N. E., Strobl, K., Brandt, A., Stathi, A., Mayer, H., et al. (2013). Seizure and cognitive outcomes of epilepsy surgery in infancy and early childhood. Eur. J. Paediatr. neurology 17, 498–506. doi:10.1016/j.ejpn.2013.03.009
Ramirez, C. L., Fanovich, M. A., and Churio, M. S. (2019). Cannabinoids: Extraction methods, analysis, and physicochemical characterization. J. Stud. Nat. Prod. Chem. 61, 143–173. doi:10.1016/B978-0-444-64183-0.00004-X
Rana, A., and Musto, A. E. (2018). The role of inflammation in the development of epilepsy. J. Neuroinflammation 15, 1–12. doi:10.1186/s12974-018-1192-7
Ranieri, R., Marasco, D., Bifulco, M., and M Malfitano, A. (2015). Phytocannabinoids and cannabimimetic drugs: Recent patents in central nervous system disorders. J. Recent Pat. CNS Drug Discov. 10, 157–177. doi:10.2174/1574889810666160517123938
Rasera, G. B., Ohara, A., and De Castro, R. J. S. (2021). Innovative and emerging applications of cannabis in food and beverage products: From an illicit drug to a potential ingredient for health promotion. Trends Food Sci. Technol. 115, 31–41. doi:10.1016/j.tifs.2021.06.035
Rasool, U. (2018). Safety and efficacy of hemp products in broiler production. Winnipeg: University of Manitoba.
Rather, I. A., Koh, W. Y., Paek, W. K., and Lim, J. (2017). The sources of chemical contaminants in food and their health implications. Front. Pharmacol. 8, 830. doi:10.3389/fphar.2017.00830
Ren, G., Zhang, X., Li, Y., Ridout, K., Serrano-Serrano, M. L., Yang, Y., et al. (2021). Large-scale whole-genome resequencing unravels the domestication history of Cannabis sativa. Sci. Adv. 7, eabg2286. doi:10.1126/sciadv.abg2286
Rhee, M.-H., Vogel, Z., Barg, J., Bayewitch, M., Levy, R., Hanuš, L., et al. (1997). Cannabinol derivatives: Binding to cannabinoid receptors and inhibition of adenylylcyclase. J. Med. Chem. 40, 3228–3233. doi:10.1021/jm970126f
Ribeiro, A. M., Estevinho, B. N., Rocha, F. J. F., and Technology, B. (2021). Preparation and incorporation of functional ingredients in edible films and coatings. Food bioproc. Tech. 14, 209–231. doi:10.1007/s11947-020-02528-4
Riboulet-Zemouli, K., and Krawitz, M. A. (2022). WHO’s first scientific review of medicinal cannabis: From global struggle to patient implications. J. Drugs, Habits Soc. Policy 23 (1), 5–21. doi:10.1108/dhs-11-2021-0060
Richards, J. R., Blohm, E., Toles, K. A., Jarman, A. F., Ely, D. F., and Elder, J. W. (2020). The association of cannabis use and cardiac dysrhythmias: A systematic review. Clin. Toxicol. 58, 861–869. doi:10.1080/15563650.2020.1743847
Romano, L., and Hazekamp, A. (2019). An overview of galenic preparation methods for medicinal cannabis. Curr. Bioact. Compd. 15, 174–195. doi:10.2174/1573407214666180612080412
Romero, P., Peris, A., Vergara, K., and Matus, J. T. (2020). Comprehending and improving cannabis specialized metabolism in the systems biology era. Plant Sci. 298, 110571. doi:10.1016/j.plantsci.2020.110571
Ross, S. A., Elsohly, H. N., Elkashoury, E. A., and Elsohly, M. A. (1996). Fatty acids of cannabis seeds. Phytochem. Anal. 7, 279–283. doi:10.1002/(sici)1099-1565(199611)7:6<279::aid-pca322>3.0.co;2-p
Ross, S., and Elsohly, M. (1997). CBN and∆ 9-THC concentration ratio as an indicator of the age of stored marijuana samples. Bull. Narcotics 49, 139.
Ross, S. A., Elsohly, M. A., Sultana, G. N., Mehmedic, Z., Hossain, C. F., and Chandra, S. (2005). Flavonoid glycosides and cannabinoids from the pollen of Cannabis sativa L. Int. J. Plant Chem. Biochem. Tech. 16, 45–48. doi:10.1002/pca.809
Russia, I. K., Sweden, G. L., Pakistan, Z. A., Argentina, L. B. F., India, S. J. B., Mexico, M. S., et al. (2015). Irritable bowel syndrome: A global perspective. J. Update.
Russo, E. B., and Marcu, J. (2017). Cannabis pharmacology: The usual suspects and a few promising leads. Adv. Pharmacol. 80, 67–134. doi:10.1016/bs.apha.2017.03.004
Russo, E. B. (2014). The pharmacological history of Cannabis. Handb. cannabis, 23–43. doi:10.1093/acprof:oso/9780199662685.003.0002
Russo, E. B. (2016). “The solution to the medicinal cannabis problem,” in Ethical issues in chronic pain management (Boca Raton: CRC Press).
Ryz, N. R., Remillard, D. J., and Russo, E. B. (2017). Cannabis roots: A traditional therapy with future potential for treating inflammation and pain. Cannabis cannabinoid Res. 2, 210–216. doi:10.1089/can.2017.0028
Sakarin, S., Meesiripan, N., Sangrajrang, S., Suwanpidokkul, N., Prayakprom, P., Bodhibukkana, C., et al. (2022). Antitumor effects of cannabinoids in human pancreatic ductal adenocarcinoma cell line (Capan-2)-Derived xenograft mouse model. J Front. Veterinary Sci. 9, 867575. doi:10.3389/fvets.2022.867575
Salamat, J. M., Ledbetter, E. L., Abbott, K. L., Thungrat, K., Flannery, P. C., Huang, C.-C. J., et al. (2022). “Cannabinoids in cancer: Cross-talk between cannabinoids and miRNAs,” in Cannabis/marijuana for healthcare (Cham, Switzerland: Springer).
Saloner, A., Sacks, M. M., and Bernstein, N. (2019). Response of medical cannabis (Cannabis sativa L) genotypes to K supply under long photoperiod. Front. Plant Sci. 10, 1369. doi:10.3389/fpls.2019.01369
Saqib, U., Sarkar, S., and Baig, M. (2017). Inflammation and its disease consequences. J. Immun. Res. 4, 1027.
Sarris, J., Sinclair, J., Karamacoska, D., Davidson, M., and Firth, J. (2020). Medicinal cannabis for psychiatric disorders: A clinically-focused systematic review. J. BMC psychiatry 20, 24–14. doi:10.1186/s12888-019-2409-8
Sarvet, A. L., Wall, M. M., Fink, D. S., Greene, E., Le, A., Boustead, A. E., et al. (2018). Medical marijuana laws and adolescent marijuana use in the United States: A systematic review and meta-analysis. Addiction 113, 1003–1016. doi:10.1111/add.14136
Sauerbier, A., Jenner, P., Todorova, A., and Chaudhuri, K. R. (2016). Non motor subtypes and Parkinson's disease. J. Park. Relat. Disord. 22, S41–S46. doi:10.1016/j.parkreldis.2015.09.027
Sawicka, B., Skiba, D., Umachandran, K., and Dickson, A. (2021). “Alternative and new plants,” in Preparation of phytopharmaceuticals for the management of disorders (London, UK: Elsevier).
Schauer, G. L., King, B. A., Bunnell, R. E., Promoff, G., and Mcafee, T. A. (2016). Toking, vaping, and eating for health or fun: Marijuana use patterns in adults, US, 2014. Am. J. Prev. Med. 50, 1–8. doi:10.1016/j.amepre.2015.05.027
Schettino, L., Prieto, M., Benedé, J. L., Chisvert, A., and Salvador, A. (2021). A rapid and sensitive method for the determination of cannabidiol in cosmetic products by liquid chromatography–tandem mass spectrometry. Cosmetics 8, 30. doi:10.3390/cosmetics8020030
Schlenker, W., Hanemann, W. M., and Fisher, A. C. (2007). Water availability, degree days, and the potential impact of climate change on irrigated agriculture in California. Clim. Change 81, 19–38. doi:10.1007/s10584-005-9008-z
Schultes, R. E., Klein, W. M., Plowman, T., and Lockwood, T. E. (1975). Cannabis: An example of taxonomic neglect. Cannabis Cult., 21–38. doi:10.1515/9783110812060.21
Seltenrich, N. (2019). Cannabis contaminants: Regulating solvents, microbes, and metals in legal weed. Environ. Health Perspect. 127 (8), 82001. doi:10.1289/EHP5785
Seltzer, E. S., Watters, A. K., Mackenzie, D., Granat, L. M., and Zhang, D. (2020). Cannabidiol (CBD) as a promising anti-cancer drug. J. Cancers 12, 3203. doi:10.3390/cancers12113203
Sergi, D., Boulestin, H., Campbell, F. M., and Williams, L. M. (2021). The role of dietary advanced glycation end products in metabolic dysfunction. J Mol. Nutr. Food Res. 65, 1900934. doi:10.1002/mnfr.201900934
Sharma, G. (1979). Significance of eco-chemical studies in cannabis [hemp (drug plant), USA]. Sci. Cult. 45 (8), 303–307.
Sharma, P., Murthy, P., and Bharath, M. S. (2012). Chemistry, metabolism, and toxicology of cannabis: Clinical implications. Iran. J. psychiatry 7, 149–156.
Sher, Y., and Maldonado, J. R. (2015). “Major neurocognitive disorders (dementias),” in Handbook of consultation-liaison psychiatry (Cham, Switzerland: Springer).
Showalter, V. M., Compton, D. R., Martin, B. R., and Abood, M. E. (1996). Evaluation of binding in a transfected cell line expressing a peripheral cannabinoid receptor (CB2): Identification of cannabinoid receptor subtype selective ligands. J. Pharmacol. Exp. Ther. 278, 989–999.
Silver, R., Wakshalg, J., Wynn, S., and Kramer, K. (2021). “Nutritional analysis of cannabis,” in Cannabis therapy in veterinary medicine: A complete guide (Berlin: Springer Nature), 271–293.
Silvestro, S., Mammana, S., Cavalli, E., Bramanti, P., and Mazzon, E. (2019). Use of cannabidiol in the treatment of epilepsy: Efficacy and security in clinical trials. J. Mol. 24, 1459. doi:10.3390/molecules24081459
Singani, A. A. S., and Ahmadi, P. (2012). Manure application and cannabis cultivation influence on speciation of lead and cadmium by selective sequential extraction. Soil Sediment Contam. Int. J. 21, 305–321. doi:10.1080/15320383.2012.664186
Sionov, R. V., and Steinberg, D. (2022). Anti-microbial activity of phytocannabinoids and endocannabinoids in the light of their physiological and pathophysiological roles. Biomedicines 10, 631. doi:10.3390/biomedicines10030631
Small, E. (2017). “Classification of cannabis sativa L,” in Relation to agricultural, biotechnological, medical and recreational utilization. Cannabis sativa L.-Botany and biotechnology (Ottawa, Canada: Springer).
Small, E., and Cronquist, A. (1976). A practical and natural taxonomy for Cannabis. Taxon 25, 405–435. doi:10.2307/1220524
Small, E. (2015). Evolution and classification of Cannabis sativa (marijuana, hemp) in relation to human utilization. botanical Rev. 81, 189–294. doi:10.1007/s12229-015-9157-3
Small, E., and Marcus, D. (2002). Hemp: A new crop with new uses for North America. Trends new crops new uses 24, 284–326.
Śmiarowska, M., Białecka, M., and Machoy-Mokrzyńska, A. (2022). Cannabis and cannabinoids: Pharmacology and therapeutic potential. Neurol. i Neurochir. Pol. 56, 4–13. doi:10.5603/PJNNS.a2022.0015
Smith, J. L., Mattick, R. P., Jamadar, S. D., and Iredale, J. M. (2014). Deficits in behavioural inhibition in substance abuse and addiction: A meta-analysis. Drug alcohol dependence 145, 1–33. doi:10.1016/j.drugalcdep.2014.08.009
Solinas, M., Cinquina, V., and Parolaro, D. (2015). “Cannabidiol and cancer—An overview of the preclinical data,” in Molecular considerations and evolving surgical management issues in the treatment of patients with a brain tumor (Norderstedt: Books on Demand), 434.
Sommano, S. R., Chittasupho, C., Ruksiriwanich, W., and Jantrawut, P. (2020). The cannabis terpenes. Molecules 25, 5792. doi:10.3390/molecules25245792
Sorrentino, G. (2021). Introduction to emerging industrial applications of cannabis (Cannabis sativa L). Rendiconti Lincei. Sci. Fis. Nat. 32, 233–243. doi:10.1007/s12210-021-00979-1
Stasiłowicz, A., Tomala, A., Podolak, I., and Cielecka-Piontek, J. (2021). Cannabis sativa L. as a natural drug meeting the criteria of a multitarget approach to treatment. Int. J. Mol. Sci. 22, 778. doi:10.3390/ijms22020778
Stephens, T. J., Mccook, J. P., and Herndon, J. R. J. H. (2015). Pilot study of topical copper chlorophyllin complex in subjects with facial acne and large pores. J. Drugs Dermatology 14, 589–592.
Stone, J. M. (2011). Glutamatergic antipsychotic drugs: A new dawn in the treatment of schizophrenia? Ther. Adv. Psychopharmacol. 1, 5–18. doi:10.1177/2045125311400779
Taghinasab, M., and Jabaji, S. (2020). Cannabis microbiome and the role of endophytes in modulating the production of secondary metabolites: An overview. Microorganisms 8, 355. doi:10.3390/microorganisms8030355
Taura, F., Morimoto, S., and Shoyama, Y. (1995). Cannabinerolic acid, a cannabinoid from Cannabis sativa. Phytochemistry 39, 457–458. doi:10.1016/0031-9422(94)00887-y
Taura, F., Morimoto, S., and Shoyama, Y. (1996). Purification and characterization of cannabidiolic-acid synthase from Cannabis sativa L.: Biochemical analysis of a novel enzyme that catalyzes the oxidocyclization of cannabigerolic acid to cannabidiolic acid. J. Biol. Chem. 271, 17411–17416. doi:10.1074/jbc.271.29.17411
Taylor, A. H., Amoako, A. A., Bambang, K., Karasu, T., Gebeh, A., Lam, P. M., et al. (2010). Endocannabinoids and pregnancy. Clin. Chim. acta 411, 921–930. doi:10.1016/j.cca.2010.03.012
Tetali, S. D. (2019). Terpenes and isoprenoids: A wealth of compounds for global use. J. Plants 249, 1–8. doi:10.1007/s00425-018-3056-x
Thomas, A. A., Wallner, L. P., Quinn, V. P., Slezak, J., Van Den Eeden, S. K., Chien, G. W., et al. (2015). Association between cannabis use and the risk of bladder cancer: Results from the California men's health study. J. Urol. 85, 388–392. doi:10.1016/j.urology.2014.08.060
Thomas, B. F., and Elsohly, M. (2015). The analytical chemistry of cannabis: Quality assessment, assurance, and regulation of medicinal marijuana and cannabinoid preparations. Massachesetts, USA: Elsevier.
Thomas, H. (1996). A community survey of adverse effects of cannabis use. Drug alcohol dependence 42, 201–207. doi:10.1016/s0376-8716(96)01277-x
Thomas, P. A., Carter, G. T., and Bombardier, C. H. (2021). A scoping review on the effect of cannabis on pain intensity in people with spinal cord injury. J. spinal cord Med. 45, 656–667. doi:10.1080/10790268.2020.1865709
Thompson, C., Sweitzer, R., Gabriel, M., Purcell, K., Barrett, R., and Poppenga, R. (2014). Impacts of rodenticide and insecticide toxicants from marijuana cultivation sites on Fisher survival rates in the Sierra National Forest, California. Conserv. Lett. 7, 91–102. doi:10.1111/conl.12038
Tipparat, P., Natakankitkul, S., Chamnivikaipong, P., and Chutiwat, S. (2012). Characteristics of cannabinoids composition of Cannabis plants grown in Northern Thailand and its forensic application. Forensic Sci. Int. 215, 164–170. doi:10.1016/j.forsciint.2011.05.006
Tipple, B. J., Hambach, B., Barnette, J. E., Chesson, L. A., and Ehleringer, J. R. (2016). The influences of cultivation setting on inflorescence lipid distributions, concentrations, and carbon isotope ratios of Cannabis sp. Forensic Sci. Int. 262, 233–241. doi:10.1016/j.forsciint.2016.03.029
Tireki, S. (2021). A review on packed non-alcoholic beverages: Ingredients, production, trends and future opportunities for functional product development. Trends Food Sci. Technol. 112, 442–454. doi:10.1016/j.tifs.2021.03.058
Tomko, A. M., Whynot, E. G., Ellis, L. D., and Dupre, D. J. (2020). Anti-Cancer potential of cannabinoids, terpenes, and flavonoids present in cannabis. Cancers (Basel) 12, 1985. doi:10.3390/cancers12071985
Torre, L. A., Bray, F., Siegel, R. L., Ferlay, J., Lortet-Tieulent, J., and Jemal, A. (2015). Global cancer statistics, 2012. CA Cancer J. Clin. 65, 87–108. doi:10.3322/caac.21262
Troutt, W. D., and Didonato, M. D. (2015). Medical cannabis in Arizona: Patient characteristics, perceptions, and impressions of medical cannabis legalization. J. Psychoact. drugs 47, 259–266. doi:10.1080/02791072.2015.1074766
Turner, C. E., Hadley, K., and Fetterman, P. S. (1973). Constituents of cannabis sativa L. VI: Propyl homologs in samples of known geographical origin. J. Pharm. Sci. 62, 1739–1741. doi:10.1002/jps.2600621045
Tyakht, A. V., Manolov, A. I., Kanygina, A. V., Ischenko, D. S., Kovarsky, B. A., Popenko, A. S., et al. (2018). Genetic diversity of Escherichia coli in gut microbiota of patients with Crohn’s disease discovered using metagenomic and genomic analyses. J. BMC genomics 19, 968. doi:10.1186/s12864-018-5306-5
Uranga, J., Vera, G., and Abalo, R. (2018). Cannabinoid pharmacology and therapy in gut disorders. Biochem. Pharmacol. 157, 134–147. doi:10.1016/j.bcp.2018.07.048
Van Os, J., Bak, M., Hanssen, M., Bijl, R., De Graaf, R., and Verdoux, H. (2002). Cannabis use and psychosis: A longitudinal population-based study. Am. J. Epidemiol. 156, 319–327. doi:10.1093/aje/kwf043
Van Waes, V., Beverley, J. A., Siman, H., Tseng, K. Y., and Steiner, H. (2012). CB1 cannabinoid receptor expression in the striatum: Association with corticostriatal circuits and developmental regulation. Front. Pharmacol. 3, 21. doi:10.3389/fphar.2012.00021
Van Winkel, R.Genetic Risk and Outcome of Psychosis GROUP Investigators (2011). Family-based analysis of genetic variation underlying psychosis-inducing effects of cannabis: Sibling analysis and proband follow-up. Archives general psychiatry 68, 148–157. doi:10.1001/archgenpsychiatry.2010.152
Volkow, N. D., Baler, R. D., Compton, W. M., and Weiss, S. R. (2014). Adverse health effects of marijuana use. N. Engl. J. Med. 370, 879–2227. doi:10.1056/NEJMc1407928
Wallace, M. J., Wiley, J. L., Martin, B. R., and Delorenzo, R. J. (2001). Assessment of the role of CB1 receptors in cannabinoid anticonvulsant effects. Eur. J. Pharmacol. 428, 51–57. doi:10.1016/s0014-2999(01)01243-2
Walters, D. (2011). Plant defense: Warding off attack by pathogens, herbivores and parasitic plants. Edinburgh, UK: John Wiley and Sons.
Wanmakok, M., Orrapin, S., Intorasoot, A., and Intorasoot, S. (2018). Expression in Escherichia coli of novel recombinant hybrid antimicrobial peptide AL32-P113 with enhanced antimicrobial activity in vitro. Gene 671, 1–9. doi:10.1016/j.gene.2018.05.106
Wassmann, C. S., and Klitgaard, J. K. (2021). Combination of cannabidiol and bacitracin against bacterial infections. Odense: University of Southern Denmark.
Werz, O., Seegers, J., Schaible, A. M., Weinigel, C., Barz, D., Koeberle, A., et al. (2014). Cannflavins from hemp sprouts, a novel cannabinoid-free hemp food product, target microsomal prostaglandin E2 synthase-1 and 5-lipoxygenase. PharmaNutrition 2, 53–60. doi:10.1016/j.phanu.2014.05.001
Wheeler, M., Merten, J. W., Gordon, B. T., and Hamadi, H. (2020). CBD (cannabidiol) product attitudes, knowledge, and use among young adults. Subst. use misuse 55, 1138–1145. doi:10.1080/10826084.2020.1729201
Wiles, D., Shanbhag, B. K., O'Brien, M., Doblin, M. S., Bacic, A., and Beddoe, T. (2022). Heterologous production of Cannabis sativa-derived specialised metabolites of medicinal significance–Insights into engineering strategies. J. Phytochemistry 203, 113380. doi:10.1016/j.phytochem.2022.113380
Wilson, S., Bodwitch, H., Carah, J., Daane, K., Getz, C., Grantham, T., et al. (2019). First known survey of cannabis production practices in California. Calif. Agric. 73, 119–127. doi:10.3733/ca.2019a0015
Wisniewska, A., Widomska, J., and Subczynski, W. K. (2006). Carotenoid-membrane interactions in liposomes: Effect of dipolar, monopolar, and nonpolar carotenoids. Acta Biochim. Pol. 53, 475–484. doi:10.18388/abp.2006_3318
Wood, T. B., Spivey, W. N., and Easterfield, T. H. (1899). III.—cannabinol. Part I. J. Chem. Soc. Trans. 75, 20–36. doi:10.1039/ct8997500020
Xu, J., Bai, M., Song, H., Yang, L., Zhu, D., and Liu, H. (2022). Hemp (cannabis sativa subsp. sativa) chemical composition and the application of hempseeds in food formulations. J. Plant Foods Hum. Nutr. 77, 504–513. doi:10.1007/s11130-022-01013-x
Yan, X., Zhou, Y., Tang, J., Ji, M., Lou, H., and Fan, P. (2016). Diketopiperazine indole alkaloids from hemp seed. Phytochem. Lett. 18, 77–82. doi:10.1016/j.phytol.2016.09.001
Zengin, G., Menghini, L., Di Sotto, A., Mancinelli, R., Sisto, F., Carradori, S., et al. (2018). Chromatographic analyses, in vitro biological activities, and cytotoxicity of cannabis sativa L. essential oil: A multidisciplinary study. Molecules 23, 3266. doi:10.3390/molecules23123266
Zheljazkov, V. D., Sikora, V., Dincheva, I., Kačániová, M., Astatkie, T., Semerdjieva, I. B., et al. (2020). Industrial, CBD, and wild hemp: How different are their essential oil profile and antimicrobial activity? Molecules 25, 4631. doi:10.3390/molecules25204631
Zhelyazkova, M., Kirilov, B., and Momekov, G. (2020). The pharmacological basis for application of cannabidiol in cancer chemotherapy. J. Pharm. 67, 239–252. doi:10.3897/pharmacia.67.e51304
Zheng, Z., Fiddes, K., and Yang, L. (2021). A narrative review on environmental impacts of cannabis cultivation. J. Cannabis Res. 3, 35–10. doi:10.1186/s42238-021-00090-0
Ziemssen, T., and Thomas, K. (2017). Alemtuzumab in the long-term treatment of relapsing-remitting multiple sclerosis: An update on the clinical trial evidence and data from the real world. J Ther. Adv. neurological Disord. 10, 343–359. doi:10.1177/1756285617722706
Keywords: marijuana, cannabiniods, pharmacology, ethnobotany, phytochemistry
Citation: Fordjour E, Manful CF, Sey AA, Javed R, Pham TH, Thomas R and Cheema M (2023) Cannabis: a multifaceted plant with endless potentials. Front. Pharmacol. 14:1200269. doi: 10.3389/fphar.2023.1200269
Received: 05 April 2023; Accepted: 30 May 2023;
Published: 15 June 2023.
Edited by:
Agnieszka Szopa, Jagiellonian University Medical College, PolandReviewed by:
Jahan Marcu, University of the Sciences, United StatesPiotr Szymczyk, Medical University of Lodz, Poland
Suman Chandra, University of Mississippi, United States
Copyright © 2023 Fordjour, Manful, Sey, Javed, Pham, Thomas and Cheema. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eric Fordjour, efordjour@grenfell.mun.ca; Mumtaz Cheema, mcheema@grenfell.mun.ca
 Eric Fordjour
Eric Fordjour