
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CORRECTION article
Front. Pharmacol. , 12 May 2023
Sec. Inflammation Pharmacology
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1199951
This article is a correction to:
Deferoxamine Alleviates Osteoarthritis by Inhibiting Chondrocyte Ferroptosis and Activating the Nrf2 Pathway
 Zhou Guo1†
Zhou Guo1† Jiamin Lin1†
Jiamin Lin1† Kai Sun1
Kai Sun1 Jiayou Guo2
Jiayou Guo2 Xudong Yao3
Xudong Yao3 Genchun Wang1
Genchun Wang1 Liangcai Hou1
Liangcai Hou1 Jingting Xu1
Jingting Xu1 Jiachao Guo4
Jiachao Guo4 Fengjing Guo1*
Fengjing Guo1*A Corrigendum on
Deferoxamine alleviates osteoarthritis by inhibiting chondrocyte ferroptosis and activating the Nrf2 pathway
by Guo Z, Lin J, Sun K, Guo J, Yao X, Wang G, Hou L, Xu J, Guo J and Guo F (2022). Front. Pharmacol. 13:791376. doi: 10.3389/fphar.2022.791376
In the published article, there was an error in (Figure 3H) as published. (Due to the error of data filing, the fluorescence images of the Erastin group in Figure 3H was inserted incorrectly). The corrected (Figure 3H) and its caption (Erastin initiated inflammation responses and ECM degradation in chondrocytes that could be alleviated by Ferrostatin-1) appear below.
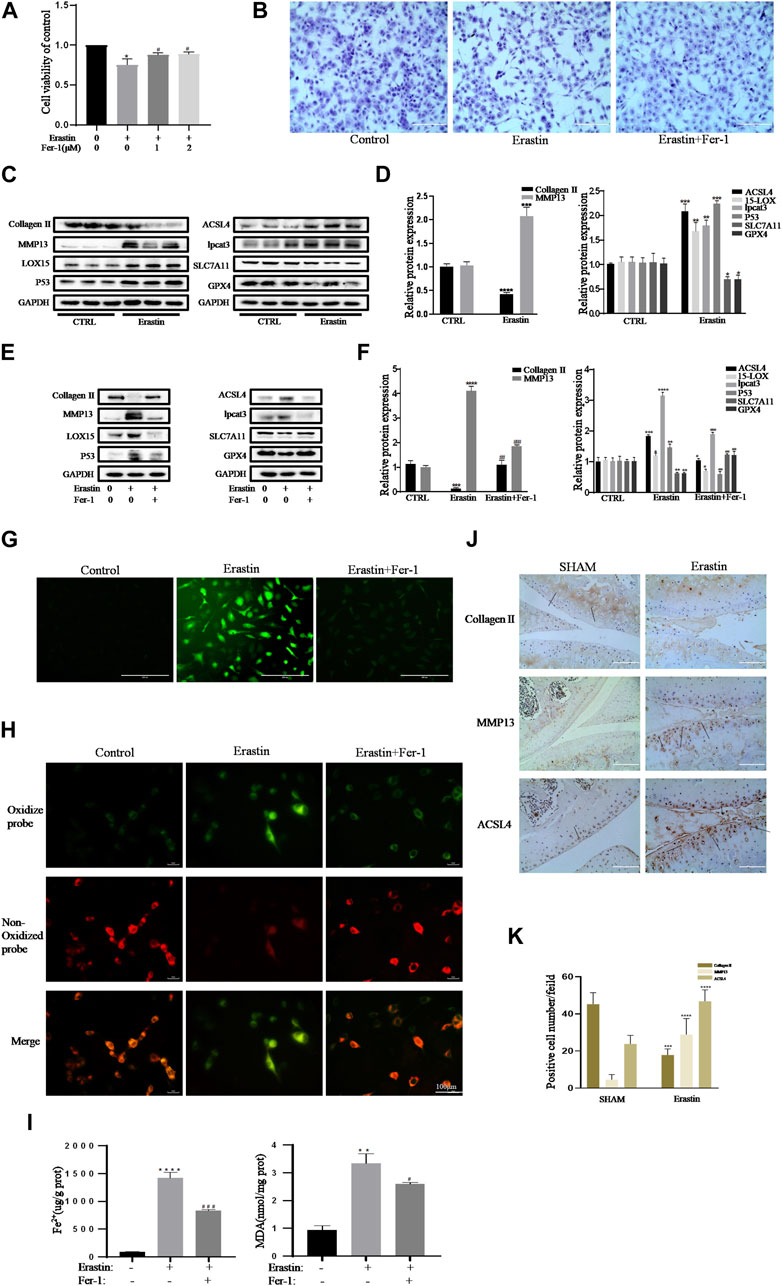
FIGURE 3. Erastin initiated inflammation responses and ECM degradation in chondrocytes that could be alleviated by Ferrostatin-1. (A,B) Cell viability determined by CCK-8 assay and toluidine blue staining. (C,D) The protein expression levels of collagen Ⅱ, MMP13, ACSL4, LOX15, lpcat3, P53, and SLC7A11 GPX4 when treated by erastin (5 μM) were detected by Western blot, and band density ratios of collagen Ⅱ, MMP13, ACSL4, LOX15, lpcat3, P53, and SLC7A11 GPX4 to GAPDH in the Western blots were quantified by densitometry (n = 3). (E,F) The protein expression levels of collagen Ⅱ, MMP13, ACSL4, LOX15, lpcat3, P53, and SLC7A11 GPX4 when treated by Erastin (5 μM) with fer-1 (1 μM) or equal volume of DMSO were detected by Western blot, and band density ratios of collagen Ⅱ, MMP13, ACSL4, LOX15, lpcat3, P53, and SLC7A11 GPX4 to GAPDH in the Western blots were quantified by densitometry (n = 3). (G) Intracellular ROS level detected by DCFH-DA fluorescent probe (scale bar: 200 µm). (H) Intracellular lipid-ROS level detected by C11 BODIPY fluorescent probe (scale bar: 100 µm). Red, reduced form of C11-BODIPY; green, oxidized form of C11-BODIPY. (I) The intracellular level of MDA and Fe2+ was determined using the MDA assay kit and iron assay kit (n = 3). (J) The collagen Ⅱ, MMP13, and ACSL4 expression levels in the cartilage samples were measured using immunohistochemistry staining. Dotted arrows indicate positive cells for MMP13 and ACSL4 and positive staining of collagen Ⅱ (scale bar: 100 µm). (K) Quantification of MMP13- and ACSL4-positive cells and collagen Ⅱ–positive staining in vivo. *p < 0.05 versus control or the sham group, **p < 0.01 versus control or the sham group, ***p < 0.001 versus control or the sham group, ****p < 0.0001 versus control or the sham group, #p < 0.05 versus IL-1β–treated group, ##p < 0.01 versus IL-1β–treated group, and ###p < 0.001 versus IL-1β–treated group. Error bars represent SD.
In the published article, there was an error in (Figure 5F) as published. (Due to the error of data filing, the fluorescence images of the Control group in Figure 5F was inserted incorrectly). The corrected (Figure 5F) and its caption (DFO alleviated chondrocytes ferroptosis and OA progress induced by erastin) appear below.

FIGURE 5. DFO alleviated chondrocytes ferroptosis and OA progress induced by erastin. (A,B) Cell viability determined by CCK-8 assay and toluidine blue staining. (C,D) The protein expression levels of collagen Ⅱ, MMP13, ACSL4, LOX15, lpcat3, and P53 when treated by Erastin (5 μM) with 50 and 100 μM DFO or equa volume of DMSO were detected by Western blot, and band density ratios of collagen Ⅱ, MMP13, ACSL4, LOX15, lpcat3, and P53 to GAPDH in the Western blots were quantified by densitometry (n = 3). (E) Intracellular ROS level detected by DCFH-DA fluorescent probe (scale bar: 200 µm). (F) Intracellular lipid-ROS level detected by C11 BODIPY fluorescent probe (scale bar: 200 µm). Red, reduced form of C11-BODIPY; green, oxidized form of C11-BODIPY. (G) The ultrastucture of mitochondria observed via transmission electron microscopy (scale bar: 5 µm). (H) The intracellular level of MDA and Fe2+ was determined using the MDA assay kit and iron assay kit n = 3). (I) The collagen Ⅱ, MMP13, and ACSL4 expression levels in the cartilage samples were measured using immunohistochemistry staining. Dotted arrows indicate positive cells for MMP13 and ACSL4 and positive staining of collagen Ⅱ (scale bar: 100 µm). (J) Quantification of MMP13- and ACSL4-positive cells and collagen Ⅱ–positive staining in vivo. *p < 0.05 versus control or the sham group, **p < 0.01 versus control or the sham group, ***p < 0.001 versus control or the sham group, ****p < 0.0001 versus control or the sham group, #p < 0.05 versus IL-1β–treated group or the DMM group, ##p < 0.01 versus IL-1β–treated group or the DMM group, ###p < 0.001 versus IL-1β–treated group or the DMM group, and ####p < 0.0001 versus IL-1β–treated group or the DMM group. Error bars represent SD.
In the published article, there was an error. (There is a clerical error in the Materials and Methods section).
A correction has been made to (Materials and Methods), (Animal experiment). This sentence previously stated:
“(the sham group, DMM group, DMM + DFO (10 mg/kg) group, DMM + DFO (100 mg/kg) group, erastin group, erastin (15 mg/kg) + DFO (10 mg/kg) group, and erastin (15 mg/kg) + DFO (100 mg/kg) group.)”
The corrected sentence appears below:
“(the sham group, DMM group, DMM + DFO (10 mg/kg) group, DMM + DFO (100 mg/kg) group, erastin group, erastin (5 mg/kg) + DFO (10 mg/kg) group, and erastin (5 mg/kg) + DFO (100 mg/kg) group.)”
The authors apologize for these errors and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: osteoarthritis, chondrocytes, ferroptosis, deferoxamine, Nrf2
Citation: Guo Z, Lin J, Sun K, Guo J, Yao X, Wang G, Hou L, Xu J, Guo J and Guo F (2023) Corrigendum: Deferoxamine alleviates osteoarthritis by inhibiting chondrocyte ferroptosis and activating the Nrf2 pathway. Front. Pharmacol. 14:1199951. doi: 10.3389/fphar.2023.1199951
Received: 04 April 2023; Accepted: 20 April 2023;
Published: 12 May 2023.
Edited and reviewed by:
Dieter Steinhilber, Goethe University Frankfurt, GermanyCopyright © 2023 Guo, Lin, Sun, Guo, Yao, Wang, Hou, Xu, Guo and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fengjing Guo, Z3VvZmpkb2NAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.