- 1Department of Medical Oncology, Shaoxing Second Hospital, Shaoxing, Zhejiang, China
- 2The Second Clinical Medical College, Wenzhou Medical University, Wenzhou, Zhejiang, China
- 3Zhejiang Key Laboratory of Diagnosis & Treatment Technology on Thoracic Oncology (Lung and Esophagus), The Cancer Hospital of the University of Chinese Academy of Sciences (Zhejiang Cancer Hospital), Institute of Basic Medicine and Cancer (IBMC), Chinese Academy of Sciences, Hangzhou, Zhejiang, China
- 4Department of Radiation Oncology, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai, China
Acquired anaplastic lymphoma kinase (ALK) mutation is the major resistant mechanism to ALK tyrosine kinase inhibitors (TKIs) in non-small cell lung cancer (NSCLC) patients. At present, treatment options after acquiring secondary ALK mutations are still limited. Here, we report on a patient with metastatic ALK-rearranged NSCLC who was sequentially treated with ALK TKIs, from crizotinib to lorlatinib, and developed rare acquired compound ALK mutations (L1196M and D1203N) that confer resistance to lorlatinib. Moreover, our report describes the clinical response of an NSCLC patient with these compound mutations to multiple anti-tumor therapies. Among them, the patient was treated with SAF-189s 120 mg daily and had a stable disease lasting 3 months. Chemotherapy (pemetrexed-carboplatin) combined with bevacizumab was then administered. She achieved a partial response, which was maintained for 7 months as the best response. Since both SAF-189s and chemotherapy have shown a clear antitumor effect, they may be viable therapeutic options for these patients. Thus, our study can provide some reference in the treatment of NSCLC patients with ALK L1196M/D1203N compound mutations.
Introduction
Lung cancer remains the leading cause of cancer-related mortality worldwide, with a less than 20% 5-year survival rate (Siegel et al., 2020). Non-small cell lung cancer (NSCLC) is the major histological subtype of lung cancer, of which 3%–7% of cases harbor oncogenic anaplastic lymphoma kinase (ALK) gene rearrangements (Tsao et al., 2016). Lorlatinib, a highly potent third-generation ALK tyrosine kinase inhibitor (TKI), can effectively overcome most secondary resistance mutations caused by first/second-generation ALK-TKIs, and possesses high blood-barrier penetration by reducing the P-glycoprotein-dependent efflux (Bauer et al., 2020; Naito et al., 2021). Unfortunately, similar to other ALK-TKIs, acquired resistance to lorlatinib is also inevitable. The major resistance mechanisms are the acquisition of two or more ALK secondary mutations, gene amplification, bypass signaling activation (such as EGFR, Met, KRAS, and c-KIT), and histological and/or phenotypical changes (such as small cell lung cancer transformation and EMT) (Gainor et al., 2016; Yanagitani et al., 2020; Remon et al., 2021).
Treatment-refractory compound ALK mutations are more common in NSCLC patients resistant to lorlatinib, and the most frequent combinations are G1202R/L1196M and D1203N/1171N (Dagogo-Jack et al., 2019). Recent studies have reported that the ALK L1196M/D1203N compound mutations confer high resistance to lorlatinb; however, the mechanism of acquired resistance and the therapeutic strategy for lorlatinib-relapsed patients with these novel compound mutations remain to be elucidated (Recondo et al., 2020; Hua et al., 2022; Katayama et al., 2023). Here, we report rare acquired ALK compound mutations (ALK L1196M and D1203N) conferring resistance to lorlatinib, and we are the first to describe the clinical responses of an NSCLC patient with these compound mutations to multiple anti-tumor therapies. Among them, SAF-189s and chemotherapy showed a clear antitumor effect.
Case presentation
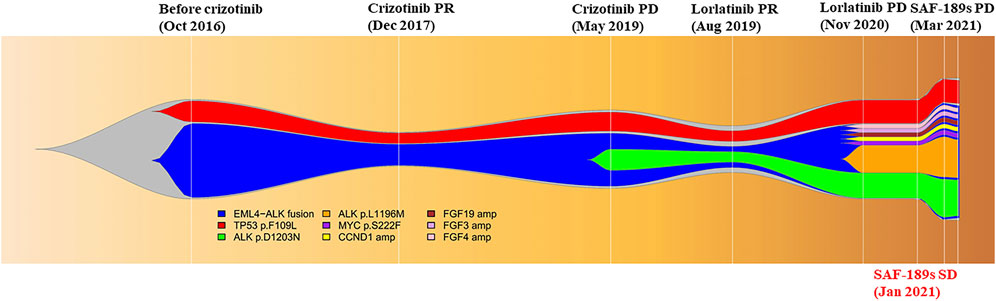
A 29-year-old young woman was diagnosed with left lung adenocarcinoma and bone metastases (cT1N3M1, IV). She had received two cycles of chemotherapy consisting of pemetrexed and carboplatin from August 2016 to October 2016 at another hospital. Her Eastern Cooperative Oncology Group performance score (ECOG-PS) was 1. She came to our clinic for the following therapy. Before treatment, a biopsy of the enlarged cervical lymph nodes was performed, and next-generation sequencing (NGS) identified the rearrangement of ALK gene and the mutation of TP53 gene in the biopsy specimen. Thus, she started crizotinib 250 mg twice daily and had confirmed partial response (PR) as the best response. After 30 months, the patient presented with progression of metastases in the sternum, vertebrae, ribs, and ilium. Plasma NGS revealed the emergence of ALK D1203N. Then, the third-generation ALK inhibitor lorlatinib was administered at a dose of 100 mg daily from May 2019 and she also achieved PR. However, a new liver metastasis and enlarged abdominal and inguinal lymph nodes were detected at a regular CT scan in November 2020, while pulmonary lesions were well controlled. Ultrasound-guided biopsy of the left inguinal lymph node confirmed that the nature of the lesion was lung adenocarcinoma metastasis, and NGS of the biopsy specimen revealed two acquired mutations, ALK L1196M (mutation allelic frequency (MAF): 14.62%) and ALK D1203N (MAF: 13.22%). Thus, progressive disease (PD) was confirmed and the patient reached a PFS of 19 months to lorlatinib treatment.
The patient was then enrolled into a phase I/II trial of SAF-189s (ClinicalTrials.gov identifier: NCT04237805), and was treated with SAF-189s 120 mg daily in December 2020. After 6 weeks of treatment, the first restaging CT scan showed a stable disease (SD) based on RECIST 1.1. NGS of a liver biopsy specimen was also performed and revealed the persistence of the same acquired ALK mutations. However, in March 2021, a CT scan showed worsening liver metastases and new supraclavicular lymphadenopathy, suggesting PD. The response was maintained for 3 months. The toxicities of SAF-189s for the patient were tolerable, with only grade 3 hypercholesterolemia. Her ECOG-PS was 2 throughout the treatment period. After progression with SAF-189s therapy, NGS of a plasma and supraclavicular lymph node biopsy specimen revealed the continual persistence of ALK L1196M and ALK D1203N.
The therapy was then switched to ensartinib 225 mg daily. A brain MRI in April 2021 revealed metastases in the right frontal and parietal lobes, which indicated that the disease had progressed again, with a PFS of only 1 month. After whole-brain radiotherapy (30 Gy/10 F), chemotherapy (pemetrexed-carboplatin) combined with bevacizumab was administered, she achieved PR, and lasted for 7 months. After the cancer relapsed, she received another two therapies, ceritinib and anlotinib, but responded to neither. Later on, the patient become increasingly frail and died on 20 January 2022, with an overall survival of 66 months (Figures 1, 2).

FIGURE 1. Illustrated summary of the treatment received by the patient, including best response and progression-free survival. Mutations and their corresponding allelic fractions detected by NGS are also presented at the bottom of the figure.
Discussion
Sequential treatment of ALK-TKIs could lead to the stepwise accumulation of mutations mediating the high resistance to ALK inhibitors (Yoda et al., 2018). Our patient was sequentially treated with first-generation (crizotinib) and third-generation (lorlatinib) ALK inhibitors, and acquired ALK compound mutations (L1196M and D1203N) conferring high-level resistance to lorlatinib. In post-lorlatinib progressed patients, ≥2 ALK mutations can be detected in 48% of plasma specimens, the most frequent combinations being ALK G1202R/L1196M and ALK D1203N/1171N (Dagogo-Jack et al., 2019). Some compound mutations, such as ALK C1156Y/L1198F and ALK I1171N/L1256F, are resistant to lorlatinib but re-sensitize to crizotinib and alectinib, suggesting that patients with these mutations can be re-treated with first- or second-generation ALK-TKIs. However, compound mutations such as ALK G1202R/L1196M confer high resistance to all ALK-TKIs (Mizuta et al., 2021). Thus, the identification of compound ALK mutations is important for developing therapeutic strategies and predicting prognosis. At present, only a few studies have reported ALK L1196M/D1203N compound mutations in lorlatinib-resistant patients, while there is no relevant report on the subsequent therapeutic strategy.
After lorlatinib resistance, our patient was first enrolled into a phase I/II trial of SAF-189s and treated with SAF-189s 120 mg daily. The best response was SD and the PFS was 3 months. The adverse event of SAF-189s was hypercholesterolemia, indicating that the lipid profile should be tested periodically during the treatment. Moreover, she also had a clinical response to the chemotherapy treatment (pemetrexed-carboplatin) and bevacizumab, achieving a PFS of 7 months with a best response of PR. SAF-189s is a new-generation ALK inhibitor that can overcome various drug-resistant mutations. In the multicenter phase I/II trial of SAF-189s, 47.6% of crizotinib-/ceritinib-resistant patients achieved PR, showing a significant antitumor effect (Xia et al., 2021; Ma et al., 2022). In this case, the patient also exhibited a clinical response to SAF-189s. However, due to the large tumor burden at the late stage of the disease, she could not achieve a satisfactory PFS. Given the effectiveness of both SAF-189s and chemotherapy, we hypothesize that combining these therapies might yield greater survival benefits, which could be a viable therapeutic option for patients with ALK L1196M and D1203N compound mutations. Overall, our report can provide guidance and reference in the treatment of patient with this acquired compound mutations in the future.
The acquisition of intra-tyrosine kinase secondary mutations is the main mechanism of resistance to ALK TKIs (Remon et al., 2021). Therefore, it is critical to dynamically monitor tumor genomic evolution during the targeted therapy. NGS is a feasible way to detect molecular profiling, and was recommended by the European Society for Medical Oncology (ESMO) in routine clinical practice for advanced NSCLC patients in 2020 (Mosele et al., 2020). With the routine use of NGS analysis, we can better understand the molecular mechanisms of resistance and formulate more effective and reasonable therapeutic strategies with ALK TKIs, particularly when administered sequentially.
Conclusion
In conclusion, we report a metastatic ALK-rearranged NSCLC patient who was treated with sequential ALK TKIs and developed rare acquired ALK compound mutations (L1196M and D1203N) resulting in resistance to lorlatinib. Moreover, we are the first to describe the clinical responses of a patient with these ALK compound mutations to multiple therapies. Among them, SAF-189s and chemotherapy showed a clear antitumor effect, which may be viable therapeutic options. Thus, our study can provide some reference in the treatment of patients with ALK L1196M/D1203N compound mutations in the future.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the ethical standards of the Ethics Committee of Zhejiang Cancer Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
NL wrote the first draft of the manuscript; HL, DW, and XX contributed to the conception and design of the study; NL, HL, and DW collected data and wrote sections of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bauer, T. M., Shaw, A. T., Johnson, M. L., Navarro, A., Gainor, J. F., Thurm, H., et al. (2020). Brain penetration of lorlatinib: cumulative incidences of CNS and non-CNS progression with lorlatinib in patients with previously treated ALK-positive non-small-cell lung cancer. Target Oncol. 15 (1), 55–65. doi:10.1007/s11523-020-00702-4
Dagogo-Jack, I., Rooney, M., Lin, J. J., Nagy, R. J., Yeap, B. Y., Hubbeling, H., et al. (2019). Treatment with next-generation ALK inhibitors fuels plasma ALK mutation diversity. Clin. Cancer Res. 25 (22), 6662–6670. doi:10.1158/1078-0432.CCR-19-1436
Gainor, J. F., Dardaei, L., Yoda, S., Friboulet, L., Leshchiner, I., Katayama, R., et al. (2016). Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov. 6 (10), 1118–1133. doi:10.1158/2159-8290.CD-16-0596
Hua, G., Zhang, X., Zhang, M., Wang, Q., Chen, X., Yu, R., et al. (2022). Real-world circulating tumor DNA analysis depicts resistance mechanism and clonal evolution in ALK inhibitor-treated lung adenocarcinoma patients. ESMO Open 7 (1), 100337. doi:10.1016/j.esmoop.2021.100337
Katayama, Y., Yamada, T., Tanimura, K., Tokuda, S., Morimoto, K., Hirai, S., et al. (2023). Adaptive resistance to lorlatinib via EGFR signaling in ALK-rearranged lung cancer. NPJ Precis. Oncol. 7 (1), 12. doi:10.1038/s41698-023-00350-7
Ma, L., Xiao, J., Guan, Y., Wu, D., Gu, T., and Wang, J. (2022). SDK1-ALK fusion in a lung adenocarcinoma patient with excellent response to ALK inhibitor treatment: a case report. Front. Oncol. 12, 860060. doi:10.3389/fonc.2022.860060
Mizuta, H., Okada, K., Araki, M., Adachi, J., Takemoto, A., Kutkowska, J., et al. (2021). Gilteritinib overcomes lorlatinib resistance in ALK-rearranged cancer. Nat. Commun. 12 (1), 1261. doi:10.1038/s41467-021-21396-w
Mosele, F., Remon, J., Mateo, J., Westphalen, C. B., Barlesi, F., Lolkema, M. P., et al. (2020). Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: a report from the ESMO Precision Medicine Working Group. Ann. Oncol. 31 (11), 1491–1505. doi:10.1016/j.annonc.2020.07.014
Naito, T., Shiraishi, H., and Fujiwara, Y. (2021). Brigatinib and lorlatinib: their effect on ALK inhibitors in NSCLC focusing on resistant mutations and central nervous system metastases. Jpn. J. Clin. Oncol. 51 (1), 37–44. doi:10.1093/jjco/hyaa192
Recondo, G., Mezquita, L., Facchinetti, F., Planchard, D., Gazzah, A., Bigot, L., et al. (2020). Diverse resistance mechanisms to the third-generation ALK inhibitor lorlatinib in ALK-rearranged lung cancer. Clin. Cancer Res. 26 (1), 242–255. doi:10.1158/1078-0432.CCR-19-1104
Remon, J., Pignataro, D., Novello, S., and Passiglia, F. (2021). Current treatment and future challenges in ROS1- and ALK-rearranged advanced non-small cell lung cancer. Cancer Treat. Rev. 95, 102178. doi:10.1016/j.ctrv.2021.102178
Siegel, R. L., Miller, K. D., and Jemal, A. (2020). Cancer statistics, 2020. CA Cancer J. Clin. 70 (1), 7–30. doi:10.3322/caac.21590
Tsao, A. S., Scagliotti, G. V., Bunn, P. A., Carbone, D. P., Warren, G. W., Bai, C., et al. (2016). Scientific advances in lung cancer 2015. J. Thorac. Oncol. 11 (5), 613–638. doi:10.1016/j.jtho.2016.03.012
Xia, Z. J., Ji, Y. C., Sun, D. Q., Peng, X., Gao, Y. L., Fang, Y. F., et al. (2021). SAF-189s, a potent new-generation ROS1 inhibitor, is active against crizotinib-resistant ROS1 mutant-driven tumors. Acta Pharmacol. Sin. 42 (6), 998–1004. doi:10.1038/s41401-020-00513-3
Yanagitani, N., Uchibori, K., Koike, S., Tsukahara, M., Kitazono, S., Yoshizawa, T., et al. (2020). Drug resistance mechanisms in Japanese anaplastic lymphoma kinase-positive non-small cell lung cancer and the clinical responses based on the resistant mechanisms. Cancer Sci. 111 (3), 932–939. doi:10.1111/cas.14314
Keywords: SAF-189s, ALK L1196M, ALK D1203N, lorlatinib resistance, treatment
Citation: Li N, Li H, Wang D and Xu X (2023) Case report: SAF-189s is a potent inhibitor in a lorlatinib-resistant NSCLC patient with acquired compound mutations ALK L1196M and D1203N. Front. Pharmacol. 14:1197163. doi: 10.3389/fphar.2023.1197163
Received: 30 March 2023; Accepted: 27 November 2023;
Published: 11 December 2023.
Edited by:
Wei Li, University of Tennessee Health Science Center (UTHSC), United StatesReviewed by:
Magesh Muthu, Wayne State University, United StatesShaodong Hong, Sun Yat-sen University Cancer Center (SYSUCC), China
Copyright © 2023 Li, Li, Wang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoling Xu, eHV4bEB6amNjLm9yZy5jbg==
†These authors have contributed equally to this work and share first authorship
 Na Li
Na Li Huihui Li
Huihui Li Ding Wang
Ding Wang Xiaoling Xu
Xiaoling Xu