- 1Department of Pharmacy, National Cancer Center Hospital East, Kashiwa, Japan
- 2Research Promotion Committee, Japanese Society for Pharmaceutical Palliative Care and Sciences (JSPPCS), Osaka, Japan
- 3Department of Education and Research Center for Pharmacy Practice, Faculty of Pharmaceutical Sciences, Doshisha Women’s College of Liberal Arts, Kyoto, Japan
- 4Department of Pharmacy, Kagoshima University Hospital, Kagoshima, Japan
- 5Department of Clinical Drug Informatics, Faculty of Pharmacy, Institute of Medical, Pharmaceutical and Health Science, Kanazawa University, Kanazawa, Japan
- 6Department of Clinical Pharmacology and Therapeutics, Kyoto University Hospital, Kyoto, Japan
- 7Department of Clinical Pharmacology and Pharmacotherapy, Faculty of Pharmaceutical Sciences, Wakayama Medical University, Wakayama, Japan
- 8Department of Pharmacy, Nippon Medical School Tama-Nagayama Hospital, Tokyo, Japan
Background: In Japan, the involvement of hospital pharmacists in inappropriate medications (IMs) practices has not been sufficiently reported. Therefore, this prospective study described the interventions of hospital pharmacists in discontinuing inappropriate drugs or reducing drug doses.
Methods: We conducted a prospective, multicenter, observational study to investigate the intervention of hospital pharmacists in inappropriate prescriptions for inpatients in September 2018. Fifty pharmacists from 45 hospitals in Japan participated in this study. IMs were defined as medications that pharmacists deemed inappropriate for patient treatment. The subjects of the study were patients who interacted with the participating pharmacists.
Results: During the study period, the median number of beds in hospitals where the 50 participating pharmacists worked was 380, and the average number of beds for which the pharmacists were responsible was 49. The enrolled hospital pharmacists recommended that doctors discontinue or reduce the doses of their regular drugs for 347 out of 1,415 (24.5%) patients. Among the 391 pharmacists’ recommendations to reduce IMs for 347 patients, physicians accepted 368 (94.1%) recommendations, and 523 drugs were discontinued as a result. Pharmacist intervention also led to improvements in hypnotic sedation, delirium, and hypotension. The most common reasons for IMs identified by pharmacists were “long-term administration of irresponsible or aimless medications” (44.5%), “adverse effects caused by medications” (31.5%), and “medications-mediated duplication of the pharmacological effect” (15.3%). Approximately 90% of pharmacists’ suggestions to reduce medications were accepted for each reason. The average number of regular medications used by patients involved in drug reduction was 8.2, and the average number of medications reduced was 1.7. A sub-analysis showed that patients using opioids tended to take more medications, and these patients were able to reduce the amount of medications taken. Interventions by pharmacists certified in palliative pharmacies tended to reduce adverse drug events.
Conclusion: This was the first multicenter prospective observational study conducted in Japan to demonstrate hospital pharmacist intervention’s effectiveness in promoting appropriate prescription and, consequently, a reduction in the number of medications in use and polypharmacy.
1 Introduction
Prescription errors are clinically meaningful errors that occur when prescribing decisions or formulary processes, resulting in an unintentional and significant decrease in the probability of timely and effective treatment or an increased risk of harm compared to generally accepted practice (Abdel-Qader et al., 2010) and can occur in daily clinical practice (Bates et al., 1995; Noguchi et al., 2016). Therefore, preventing prescription errors is essential for hospitals, and hospital pharmacists play a vital role in comprehensively intervening in patient care to prevent adverse drug events (ADEs) (Nester and Hale, 2002; Bond and Raehl, 2007; Abu-Naser, 2021).
The number of medications prescribed to patients increases with age and comorbidities (Park et al., 2016). Polypharmacy, defined as the inappropriate use of multiple medicines in patients in the 1960s (Canadian Medical Association, 1966), is associated with various problems, such as drug interactions, ADEs, increased medical expenses, and decreased medication adherence (Hersh et al., 2017). Studies have found that there is a dose-dependent relationship between polypharmacy and mortality, and excessive polypharmacy, such as regular use of ten or more medications, can lead to death (Leelakanok et al., 2017). However, increasing the number of drugs essential for a patient’s health can result in polypharmacy. Several studies have defined polypharmacy as the use of more drugs than is clinically indicated (Fulton and Allen, 2005). Recent research suggests that deprescribing, a process of identifying and discontinuing inappropriate medications (IMs), can reduce inappropriate polypharmacy in older patients (Reeve et al., 2015). However, it remains unclear whether deprescribing can improve clinical outcomes (Reeve et al., 2014; Scott et al., 2015). The use of multiple drugs in various healthcare settings is associated with potentially inappropriate medication (Nothelle et al., 2017; Nothelle et al., 2019).
According to Japan’s guidelines for the appropriate use of drugs for older patients, polypharmacy is a condition in which various problems occur due to the use of multiple drugs (Ooi, 2019). In Japan, there have been reports on the management of polypharmacy in a specific area of a single hospital (Hashimoto and Tensho, 2016; Horii and Atsuda, 2020). However, it resulted from medical staff with high-level knowledge, and it was difficult to generalize the data. As such, there is insufficient evidence of pharmacists’ contributions to addressing this problem in daily practice. To fill this gap, the Japanese Society for Pharmaceutical Palliative Care and Sciences (JSPPCS) Research Committee previously conducted a questionnaire survey on polypharmacy for members who worked as hospital pharmacists (Uchida et al., 2019) and community pharmacists (Suzuki et al., 2019). These results show the prevalence of polypharmacy and the benefits of pharmacy interventions for drug-related problems (Suzuki et al., 2019; Uchida et al., 2019).
However, since the findings were obtained from a retrospective observational study, the data lacked information on the patient background (age, disease, etc.), the actual number of cases of pharmacist recommendations and acceptance, detailed information such as discontinuation of IMs or reduction of drug doses following pharmacist recommendations, and information linking pharmacist interventions to each patient to improve the symptoms of ADEs. Furthermore, to date, no multicenter prospective studies have been conducted on deprescribing interventions by pharmacists. Therefore, to bridge this gap, the JSPPCS conducted a multicenter, prospective study to clarify the benefits of pharmacy interventions in reducing IMs in Japan. In our previous study, the questionnaire included analyses based on drug use status and professional certification. In this prospective study, we utilized the data obtained to perform a subanalysis, examining the impact of pharmacist intervention based on these factors (Suzuki et al., 2019; Uchida et al., 2019).
2 Materials and methods
2.1 Study design
A multi-center, prospective, observational study of pharmacists’ interventions on inappropriate prescriptions in a hospital was conducted in September 2018.
2.2 Participants
Hospital pharmacists who were members of the JSPPCS were recruited from the JSPPCS homepage to cooperate in this study. The recruitment method involved publicizing the prospective observational study on the JSPPCS website and inviting pharmacists who were interested in participating. A total of 50 pharmacists from 45 hospitals in Japan volunteered to take part in the study. Pharmacists who participated in the study reported information on patients who had pharmacy interventions that resulted in a reduction in the medications the patients were using.
2.3 Variables and data source
We conducted an analysis of various aspects, including the characteristics of the participating pharmacists and pharmacies, the nature of deprescribing through pharmacist interventions, the reasons behind and acceptance rates of pharmacists’ recommendations to reduce medications, the average number of drugs discontinued or reduced in dose, functional classification of discontinued drugs, and their pharmacological categories. The count of drugs discontinued or reduced in dose represents the total “number of cases” in which each drug was either discontinued or reduced, respectively.
At the outset of the survey, the participating pharmacists provided the following personal information: gender, years of experience as a pharmacist, working hours, duration dedicated to patient medication counseling, time spent on clinical pharmacy-related education, and details about their pharmacy, including the daily prescription count, total number of pharmacists employed, number of full-time staff pharmacists, percentage of patients with cancer, and number of patients availing pharmacy services. The pharmacist participants were provided with an electronic survey record form developed and validated by a research committee member of the JSPPCS (Supplementary Tables S1–S3). As part of their regular duties, pharmacists recorded their interventions during each patient visit. In addition, hospital pharmacists were required to record their work status and reduce the number of medicines used or doses administered during the study period.
During their intervention, pharmacists recorded the patient’s age and disease status. In cases in which a patient had more than one disease, the pharmacist recorded the comorbidities. In addition, the pharmacists recorded the following information on the survey record form daily for each case: the number of regularly used drugs in patients treated with inappropriate drugs, the timing when the pharmacist was aware of inappropriate prescriptions, the reasons for pharmacists’ recommendations to reduce medications, and the content of deprescribing, including the number and name of drugs discontinued and dose-reduced drugs by doctors. The study did not provide participating pharmacists with specific criteria for assessing ADEs, but they were instructed to use the CTCAEv4.0 as the criterion for assessing ADEs in their case report forms (CRFs). CTCAE stands for Common Terminology Criteria for Adverse Events; these criteria are also called “common toxicity criteria.” In CTCAE, an adverse event is defined as any abnormal clinical finding temporally associated with the use of a therapy. Specifically, pharmacists assessed whether ADEs were improved after deprescribing.
Subsequently, the JSPPCS office collected the records for analysis. We only collected data from the pharmacists who participated in the study. Even if they worked at the same hospital, those who did not participate in the study were excluded. After the observational period, we collected the data recorded by the enrolled pharmacists. The protocol was approved by the Institutional Review Board of the Ethics Committee of Osaka University of Pharmaceutical Sciences (approval no. 0052), and since the study used only administrative data to improve regular clinical practice, patient consent was waived. Consequently, while the patient data were anonymized for analysis, the pharmacists who conducted the pharmacy interventions were not anonymized.
2.4 Settings
This prospective, observational study focused on the 50 participating and the scope of practice with their respective institutions, where they implemented the pharmacy intervention.
2.5 Definition of inappropriate medications (IMs)
In this study, we categorized IMs as long-term administration of irresponsible or aimless medications, ADEs caused by medications, medication-mediated duplication of the pharmacological effect, medication-induced drug–drug interactions, inappropriate drugs for older patients, and inappropriate drugs or doses considering the patient’s organ function. The study also defined ‘regular drug’ as a prescribed medication to be taken on schedule, excluding drugs taken only when symptoms occurred.
2.6 Data assessment
2.6.1 Data availability and collection
After the survey period, records were collected from the JSPPCS office.
2.6.2 Data analysis
Descriptive analyses were performed to characterize the study population. Bivariate analyses were employed to examine the differences in demographic characteristics using t-tests for continuous variables and chi-square tests or Fisher’s exact probability tests for categorical variables. The study also analyzed the relationship between pharmacist interventions, of pharmacists both with or without Board Certified Pharmacist in Palliative Pharmacy (BCPPP), and the rate of improvement in ADEs in patients who used or did not use opioids. All data were analyzed using SPSS software (version 22.0; SPSS Inc., Chicago, IL, United States). Statistical significance was set at p < 0.05.
3 Results
3.1 Characteristics of 50 hospital pharmacists
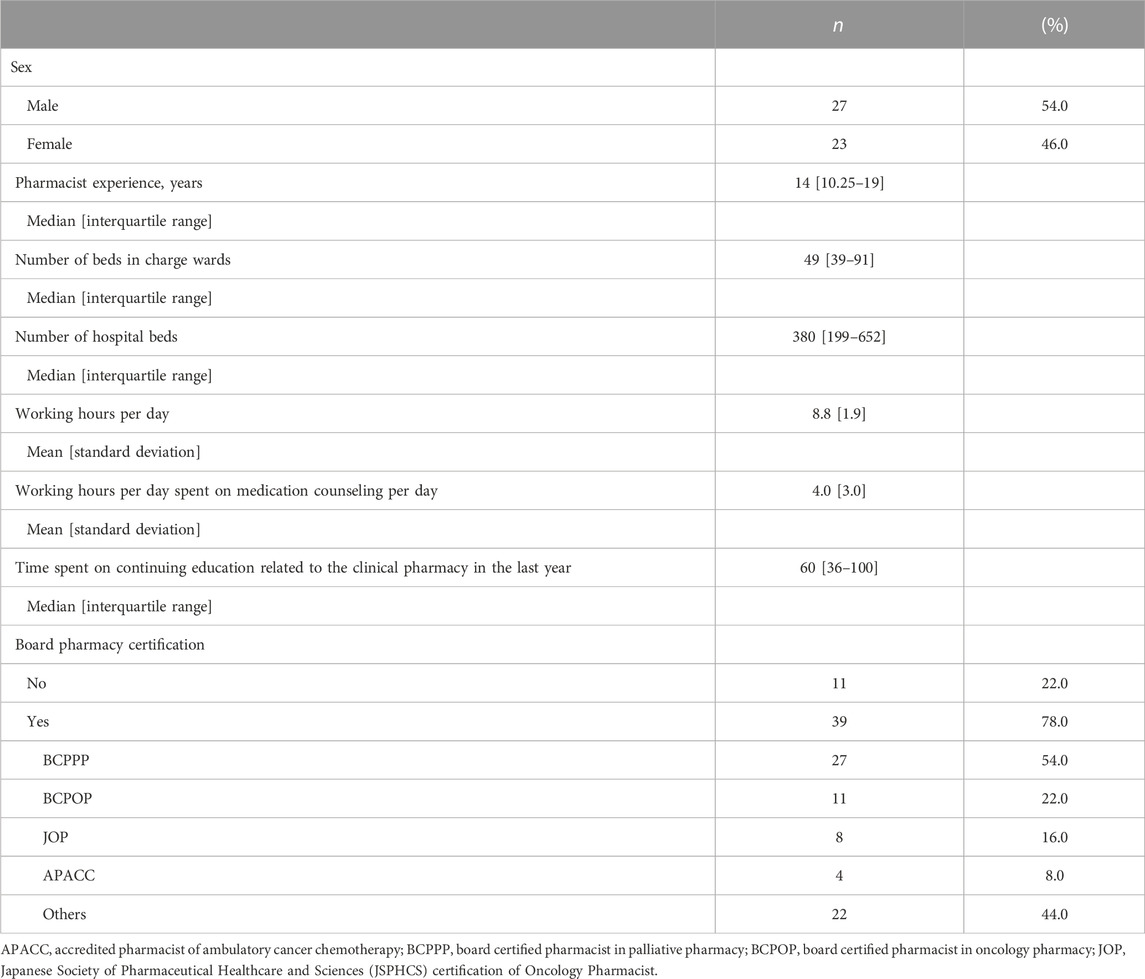
The background information on pharmacists is presented in Table 1. The median pharmacist’s experience was 14 years. The median number of beds in hospitals where the pharmacists worked was 380, and the average number of beds for which the pharmacists were responsible was 49. During the study period, the pharmacists worked an average of 8.8 h per day, with an average of 4.0 h spent on clinical practice for patients. Fifty-four pharmacists (54.0%) had a BCPPP accredited by the JSPPCS.
3.2 Hospital pharmacist interventions
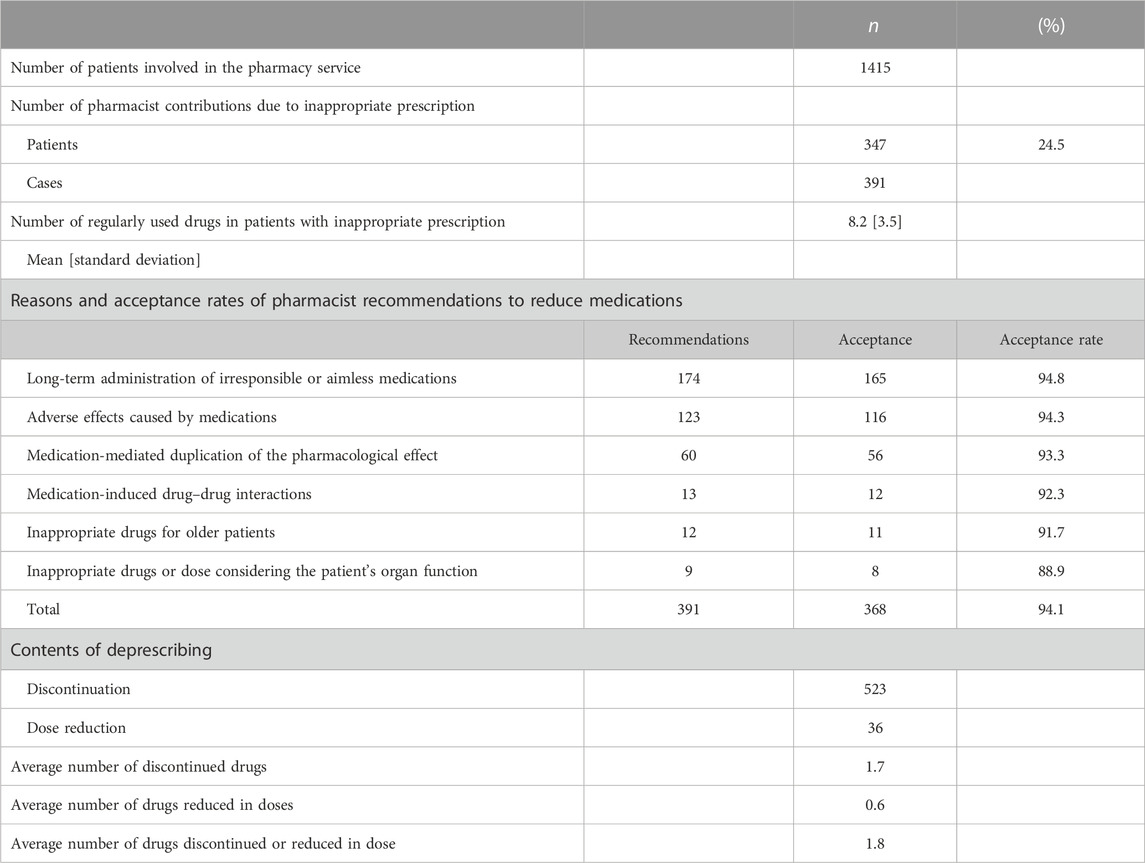
Hospital pharmacists’ interventions are shown in Table 2. A total of 1,415 patients underwent interventions by 50 pharmacists. More than 80% of the patients had cancer. Out of the total 1,415 patients, 347 individuals (24.5%) received pharmacist interventions specifically aimed at reducing medication usage or decreasing the dosage of their current medications. Of the 347 patients, pharmacists provided 391 deprescribing suggestions. The most common reasons for IMs identified by pharmacists were “long-term administration of irresponsible or aimless medications” (44.5%), “adverse effects caused by medications” (31.5%), and “medications-mediated duplication of the pharmacological effect” (15.3%). Approximately 90% of all pharmacists’ recommendations were accepted for each reason to reduce medications, and consequently, physicians accepted 368 recommendations (94.1%). The average number of regular medications used by patients involved in drug reduction was 8.2 [standard deviation (S.D.): 3.5], and the average number of medications reduced was 1.7 (S.D. 1.4).
3.3 Characteristics of 347 patients with pharmacy interventions
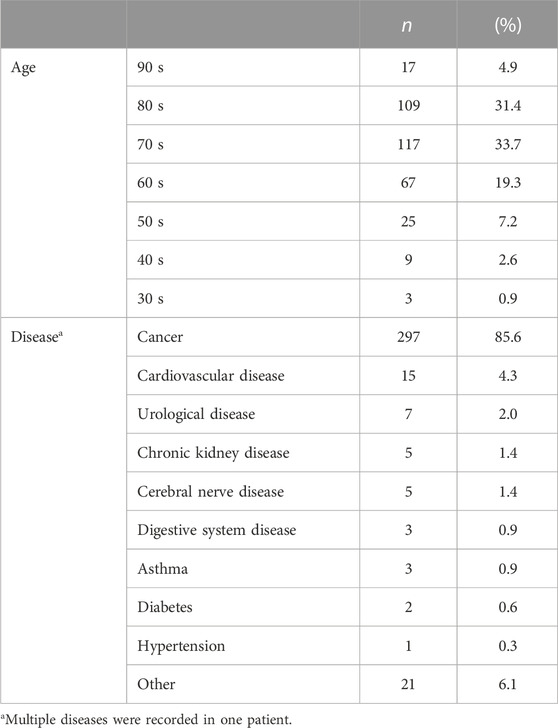
The backgrounds of the patients who underwent the intervention are presented in Table 3. The most common age group encompassed 310 patients (89.3%) in their 60 s or older. Cancer was the most common disease (297 patients, 85.6%), followed by cardiovascular (15 patients, 4.3%) and urological (7 patients, 2.0%) diseases. Twelve patients (42.7%) received at least one treatment during their current hospitalization.
3.4 Drugs discontinued following pharmacist recommendations
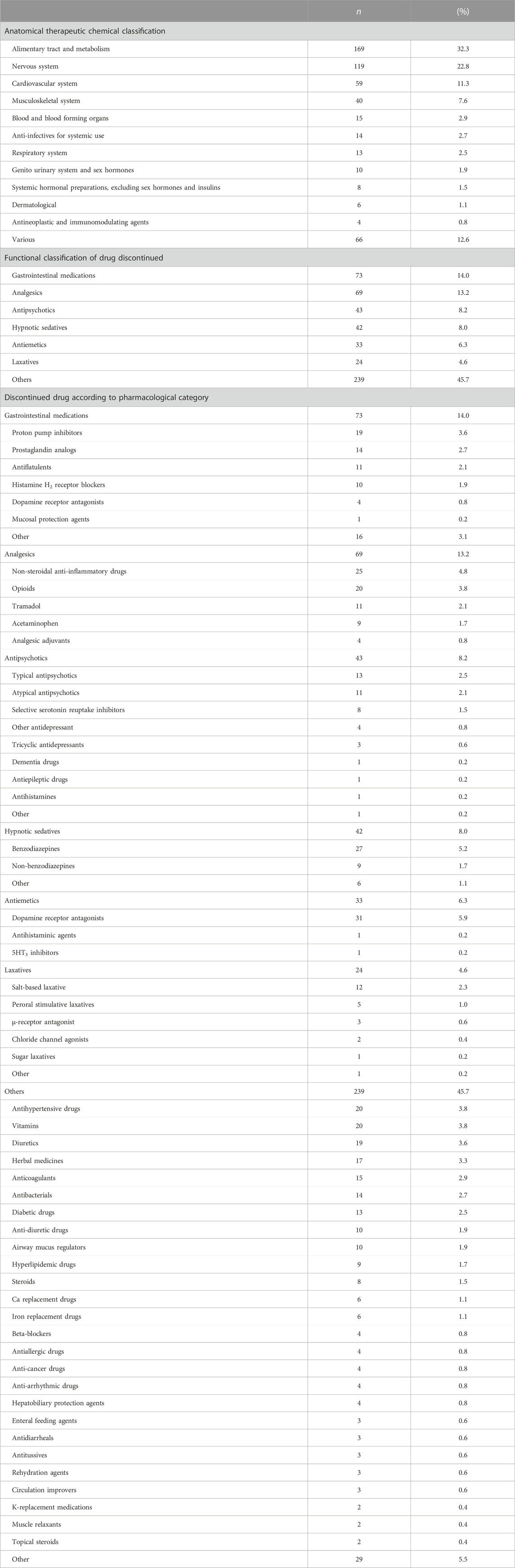
A total of 523 drugs were discontinued following the recommendations of 50 pharmacists during the study period, as shown in Table 4. The most common drug categories, according to the Anatomical Therapeutic Chemical Classification, were the alimentary tract and metabolism (32.3%), nervous system (22.8%), and cardiovascular system (11.3%). The most common drug categories according to functional classification were gastrointestinal medications (14.0%), analgesics (13.2%), and antipsychotics (8.2%). Table 4 also shows the pharmacological categories of drugs that were discontinued following pharmacists’ recommendations.
3.5 Improved ADEs due to pharmacy interventions
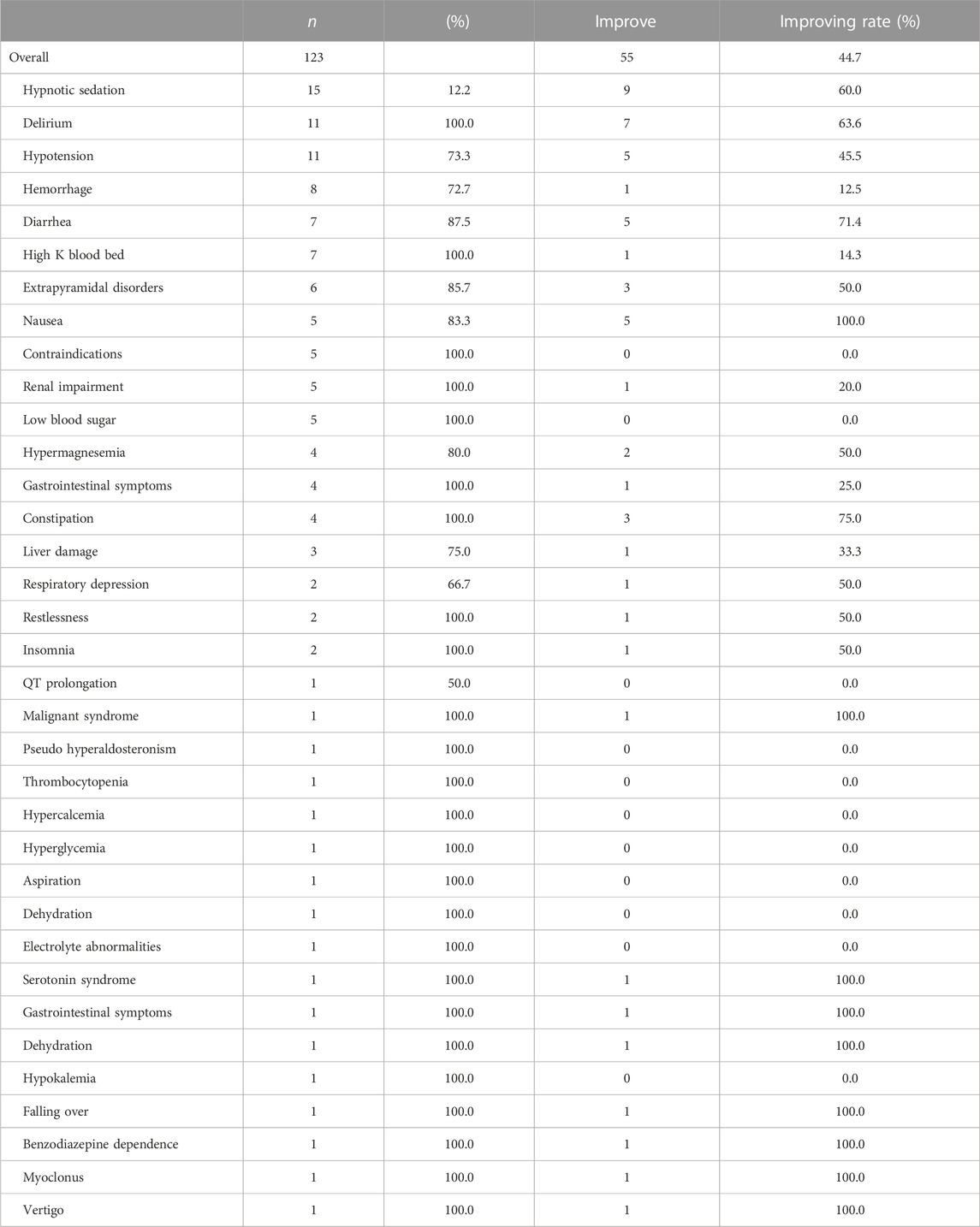
Table 5 shows the ADEs potentially avoided according to pharmacists’ recommendations. Out of the 123 pharmacy interventions for IMs that considered potential ADEs, physicians accepted 116 of these interventions (94.3%). The three most common ADEs were “hypnotic sedation” (12.2%), “delirium” (8.9%), and “hypotension” (8.9%). Of the 123 symptoms, 55 (44.7%) improved after deprescription. Improve rates of the top three symptoms of ADEs reduced due to pharmacist interventions were “hypnotic sedation” (60.0%), “delirium” (63.6%), and “hypotension” (45.5%).
3.6 Differences between cancer patients using and not using opioids
Table 6 presents a comparison between the 391 interventions performed by pharmacists on patients who used opioids with those who did not. All patients receiving opioid analgesics suffered from cancer. The average number of regularly used medications was significantly higher among opioid-using patients than among non-opioid-using patients (8.9 vs. 7.3, p < 0.001). The average number of deprescribed medications was significantly higher among opioid-using patients than among opioid-non-using patients (1.9 vs. 1.5, p < 0.01). There were 210 pharmacy interventions in 180 opioid-using cases and 181 pharmacy interventions in 167 non-opioid-using cases. The top three reasons for IMs were “long-term administration of irresponsible or aimless medications” (n = 99), “adverse effects caused by medications” (n = 65), and “medication-mediated duplication of the pharmacological effect” (n = 33) in opioid-using patients. In patients who did not use opioids, the top three reasons for IMs were similar to those for pharmacy interventions in opioid-using patients. The acceptance rate of opioid users was significantly higher than that of non-opioid users (96.7% vs. 91.2%, p = 0.02). The rate of symptom improvement after deprescription was also significantly higher in opioid-using patients than in non-using patients (62.5% vs. 25.0%, p < 0.001).
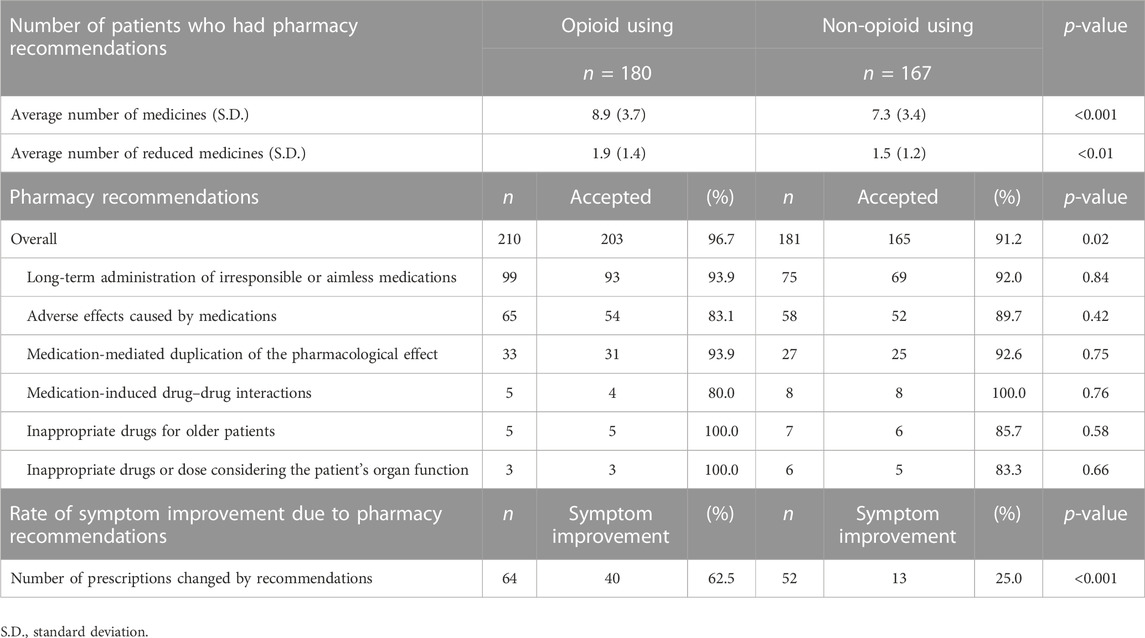
TABLE 6. Comparison of interventions implemented by pharmacists in patients using opioids and those not using opioids.
3.7 Differences between BCPPP and non-BCPPP
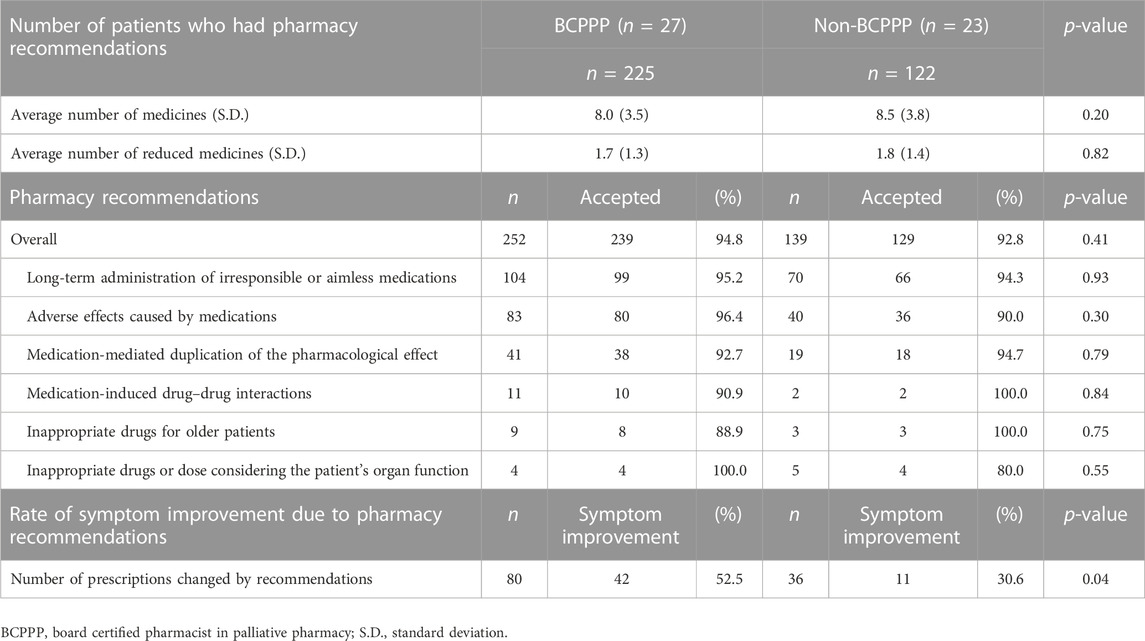
Table 7 presents a comparison of 391 interventions conducted by pharmacists with and without the BCPPP. The average number of regularly used and reduced medicines due to pharmacy interventions was not significantly different between patients who received pharmacy interventions by the BCPPP and those who received pharmacy interventions by the non-BCPPP. The top three reasons for the IMs detected by BCPPP were “long-term administration of irresponsible or aimless medications” (n = 104), “adverse effects caused by medications” (n = 83), and “medication-mediated duplication of the pharmacological effect” (n = 41). In the non-BCPPP pharmacy interventions, the top three reasons for IMs were similar to those of the BCPPP pharmacy interventions. In addition, the acceptance rate by doctors was not significantly different between pharmacy interventions using the BCPPP and those using the pharmacy interventions by non-BCPPP (94.8% vs. 92.8%, p = 0.41). The rate of improvement in symptoms after deprescribing was also significantly higher in pharmacy interventions by the BCPPP than in pharmacy interventions by the non-BCPPP (52.5% vs. 30.6%, p = 0.04).
4 Discussion
This was the first multicenter prospective observational study conducted in Japan to demonstrate how hospital pharmacists contribute to eliminating polypharmacy among inpatients. Of the 1,415 patients involved in the study, the pharmacists recommended discontinuing or reducing the dosage for 347 patients (24.5%). A total of 391 patients received recommendations from pharmacists’ to reduce IMs use, of which 368 (94.1%) were accepted. A total of 523 drugs were discontinued following the pharmacist’s recommendations. Pharmacist intervention improved hypnotic sedation, delirium, and hypotension. Although the trends in IMs were similar to those of a previous questionnaire survey (Uchida et al., 2019), we were able to reveal the results of medication reduction that the questionnaire survey could not clarify. The average number of 8.0 regular medications may reflect the actual situation of patients with cancer in clinical practice. This also reveals polypharmacy and the fact that pharmacists can reduce the number of medications. The pattern of deprescribing due to pharmacy intervention in the questionnaire survey (Uchida et al., 2019) differed from that in the current prospective observational study, indicating a difference between responses based on general knowledge and cases encountered in actual clinical practice. The main reasons for drug reduction were “less meaningful drug use” and “ADEs,” which were apparently associated with patient-related disadvantages. This result also differs from that of a questionnaire survey (Uchida et al., 2019). However, our prospective study of community pharmacists confirmed a similar trend (Uchida et al., 2022). Compared with prospective studies of interventions by community pharmacists, the rate of prescribing interventions by hospital pharmacists was clearly higher and more diverse. This observation could be attributed to the closer working relationships between hospital pharmacists and prescribing doctors. The proximity and collaboration between hospital pharmacists and physicians may have facilitated prescribing interventions, making it easier for hospital pharmacists to intervene compared to pharmacists working in community pharmacies.
This study provided a case report form through which hospital pharmacists could document whether the symptoms of ADEs improved after the intervention. Although the symptoms of ADEs varied widely, approximately 40% of the patients’ adverse symptoms improved after the intervention. Interventions aimed at reducing ADEs are the mainstay of IMs use. A multicenter study in Japan reported that 29 of 100 hospitalized patients had ADEs, of which 4.9% were severe and 1.6% were life-threatening (Morimoto et al., 2011). Patients who experience drug reactions have higher mortality rates and longer hospital stays than those who do not (Bond and Raehl, 2006). Half of the adverse drug reactions are preventable (Chan et al., 2001; Leendertse et al., 2008; Zed et al., 2008), and this intervention can improve patient quality of life, healthcare costs, and treatment. Especially in older individuals, renal dysfunction is a cause of adverse drug reactions due to unintentional overdose caused by delayed elimination of many drugs, such as water-soluble antibacterials, diuretics, and non-steroidal anti-inflammatory drugs (Mangoni and Jackson, 2004). A prospective observational study reported that the most common types of drugs causing adverse drug reactions were anti-infectives, steroids, anticoagulants, non-steroidal anti-inflammatory drugs, and diuretics (Geer et al., 2016). In this study, the reasons for drug reduction varied, leading to a corresponding reduction in the number of medications prescribed to patients. The diversity of reasons contributed to the decision to decrease the usage or dosage of specific drugs in order to address various concerns or optimize the patients’ medication regimens. ADEs were also diverse; however, because many of the patients were in the palliative medicine field, such as oncology, symptom improvement in hypnotic sedation, hypotension, and delirium were achieved in many cases.
Although the number of drug-drug interactions in the breakdown of IMs was small, the acceptance rate was high. Drug interactions are a potential concern for most medications, but it is important to note that the majority of interactions are not absolute contraindications. Instead, they often serve as guidelines for cautious administration. The use of medications with cautious administration can indeed present challenges, and even with knowledge and expertise, it can be difficult for pharmacists to determine in actual practice whether a patient’s condition is adversely affected by a drug interaction or whether to continue a medication that has been identified as causing a potential drug interaction. Although the number of interactions identified by pharmacists in this study was low, the acceptance rate by doctors was high because the interactions identified by pharmacists were clearly unfavorable to the patient. A Swedish drug registry study reported a strong relationship between the number of drugs and drug-drug interactions (Hajjar et al., 2007; Johnell and Klarin, 2007). Since the average number of regularly used drugs in the study was 8.2, it is expected that the number of drug-sensory interactions was high and consequently the associated potential for patient disadvantage was also high. In polypharmacy, drug interactions are more potentially concerning, and education or training on how to deal with them is necessary. Therefore, in Japan, continuing education to detect drug-drug interactions in routine clinical practice is necessary. As suggested by Scott et al., incorporating education on polypharmacy and deprescribing into pharmacists’ training would be advantageous for the success of future pharmacists (Scott et al., 2023).
Pharmacists reduced the number of drugs used in various pharmacological categories. The most common drugs discontinued following pharmacists’ recommendations were gastrointestinal medications (14.0%), followed by analgesics (13.2%), antipsychotics (8.2%), and hypnotic sedatives (8.0%). This was due to long-term administration of irresponsible or aimless medications, adverse drug reactions, duplication of the same type of drug, and indiscriminate prescription. Sleepiness or sedation, cognitive function, and other symptoms improved after discontinuation of the medications. These interventions resulted in the resolution of the ADEs. Our data suggest that the clinical services provided by pharmacists can contribute to reducing the disadvantages faced by outpatients.
Although most patients targeted for pharmacist intervention in this study were cancer patients, it was clear that those using opioids had a higher number of medications and were more likely to be able to reduce their medications. Since the JSPPCS conducted the study, half of the pharmacists who participated in the study had BCPPP, and most of the interventions were for patients with cancer. Comparing patients with and without opioid use showed that patients prescribed opioids use more medications, contributed more to medication reduction, and had a greater rate of improvement in adverse event symptoms. Although pharmacists must consider the use of opioids, the results clearly demonstrate their significance. When comparing pharmacists’ interventions with and without the BCPPP, there was no statistical difference in the number of drugs used between the two groups and no difference in the mean value of medication reduction. There was no statistical difference in the acceptance rate of interventions for IM, but there was a twofold difference in the number of suggestions made by the 27 pharmacists with BCPPP and the 23 without, 252 and 139, respectively. Furthermore, the rate of ADEs improvement was considerably higher in the BCPPP group than in the non-BCPPP group. Hence, the training and certification in BCPPP provided by the JSPPCS holds remarkable value. However, since these results were based on a univariate sub-analysis, a carefully designed prospective intervention study with or without BCPPP is needed to confirm this important clinical question.
Several definitions of inappropriate prescribing exist (O’Connor et al., 2012; O’Mahony et al., 2015; Panel et al., 2015; Levy, 2017; Chun et al., 2018), including the American Geriatrics Society Beers Criteria (Panel et al., 2015) and the Screening Tool of Older People’s Prescriptions (STOPP) (O’Mahony et al., 2015) which are well-known criteria that address multiple elements to reduce polypharmacy. However, to alleviate the burden on hospital pharmacists in their daily practice, we did not use international criteria to detect IMs in this prospective study. Hamilton et al. reported that potential inappropriate medications, as defined by the STOPP criteria, were extensively associated with avoidable drug reactions in older patients (Hamilton et al., 2011). Although the Beers criteria (Panel et al., 2015), the STOPP/START criteria (O’Mahony et al., 2015), and the Medication Appropriateness Index (Hanlon and Schmader, 2013) have clear evidence and should be used for evaluation, only a few facilities in Japan currently implement these criteria in their daily clinical practice. In addition, the use of these criteria requires diagnostic studies and imaging evaluations that pharmacists cannot perform regularly. Therefore, we did not use these criteria in this study. This is further because a Dutch study on outpatients reported that most drug-related problems were not associated with the STOPP/START criteria (Verdoorn et al., 2015); those criteria are not absolute. These criteria should be carefully selected according to the purpose of the study. As the purpose of our study was to clarify pharmacists’ drug reduction activities in actual clinical practice, the study was successful without evaluation using international standard criteria. Polypharmacy is often defined in numbers (Levy, 2017; Masnoon et al., 2017; Wastesson et al., 2018), but there is no unanimous definition of what constitutes polypharmacy (Masnoon et al., 2017). In our study, we define polypharmacy as the use of more drugs than clinically indicated rather than by a specific number (Fulton and Allen, 2005). ADEs cause some symptoms related to the central nervous system and the gastrointestinal tract, often resulting in the prescription of additional drugs to control these symptoms (Budnitz et al., 2011; Carroll and Hassanin, 2017; Wastesson et al., 2018), which is a necessary treatment for patients. Therefore, pharmacists must propose the use of necessary drugs based on the successive assessment of the patient’s treatment and not just the number of drugs. Prescribing multiple medications can negatively impact patient adherence and health-related quality of life (Schenker et al., 2019); it has been reported that medication adherence decreases with the number of medications prescribed (Pasina et al., 2014), making the involvement of pharmacists’ rather crucial.
This prospective study proved that pharmacists could contribute to improving the use of IMs in clinical practice in Japan. This study, however, has few limitations, including the possibility of bias in that most registered pharmacists were JSPPCS members, the small number of participating pharmacists, and the level of interest in polypharmacy among the participating pharmacists. Nonetheless, the results obtained in this study were not influenced by these limitations as they provide a background similar to the current situation in Japan, where there are many older patients suffering from cancer. While it is important to strive for minimizing bias in study design, it is worth noting that complete elimination of bias may not be feasible in certain prospective studies that involve real-world clinical data. We designed this study to minimize the burden of clinical trials by considering the work of pharmacists. In the data analysis, since the pharmacist intervention in actual clinical practice was summarized, its detailed analysis was not designed before the study, and the data obtained could not be analyzed in detail due to the study design, which is similar to a fact-finding survey. Therefore, subsequent studies are required to collect more detailed data. Alternatively, it is desirable to use a study design that clearly demonstrates the effectiveness of pharmacist intervention, such as a two-arm comparison with and without pharmacist intervention, or a pre- and post-pharmacist intervention comparison, rather than a single-arm observational study design. However, pharmacist intervention is a standard practice within Japanese medical care, and it is considered unethical for medical institutions to intentionally create an intervention group without including pharmacist intervention. Unfortunately, the more we aim to collect detailed data, the greater becomes the burden, drifting us away from actual clinical results. In addition, as there are reports of improvement in IMs by multidisciplinary teams led by nurses overseas (Garland et al., 2021), such clinical work should be conducted together with physicians, nurses, and other multidisciplinary teams (Liu, 2014); in terms of collaborative work, the role of clinical pharmacists in Japan is still in the process of development and may not be considered mature. In addition, because of the importance of working with patients to resolve polypharmacy and IMs, Woodward suggested deprescribing principles by reviewing all current medications, identifying medications for discontinuation, planning a deprescribing regimen, and working with patients and caregivers (Woodward, 2003). Therefore, a comprehensive approach is required.
5 Conclusion
This multicenter prospective observational study conducted in Japan is the first to demonstrate the effectiveness of hospital pharmacist interventions in promoting appropriate prescriptions. As a result, there was a significant reduction in the number of medications used and a decrease in polypharmacy. The study also highlighted the active role of hospital pharmacists in addressing polypharmacy by discontinuing inappropriate drugs or reducing the dosage of regularly prescribed medications, which contributed to the mitigation of ADEs.
Data availability statement
The datasets presented in this article are not readily available because this dataset only to raw, anonymized data. Never share participants identifiable data. Requests to access the datasets should be directed to ssuzuki@east.ncc.go.jp.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Review Board of the Ethics Committee of Osaka University of Pharmaceutical Sciences (approval no. 0052). The patients/participants provided their written informed consent to participate in this study.
Author contributions
SS, MU, and TN designed the concept, performed statistical analyses, and wrote the manuscript. SS performed the statistical analyses. HS, YS, TN, and HT interpreted and discussed the data. All authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to thank the 48 hospital pharmacists who volunteered to participate in this study. We express our sincere gratitude to the following participants who agree to consent names and affiliations: Ayako Yamaguchi (Saiseikai Yokohamashi Nanbu Hospital), Ayuko Kano (Kamitsuga General Hospital), Ayumi Kato (Nippon Medical School Hospital), Emi Ryu (Nagasaki University Hospital), Fusako Iharada (Nishi Nara Central Hospital), Hideyuki Katsura (Komatsu Municipal Hospital), Hideyuki Kuroda (Mitaki General Hospital), Hiroki Mochihara (Nozominohana Clinic), Hiroki Sugihara (Onomichi Municipal Hospital), Hiroko Fujii (Murakami Karindoh Hospital), Hironori Kitade (Kaga Medical Center), Hiroyuki Ooya (Saitama Medical Center), Junya Hashizume (Nagasaki University Hospital), Kazuyo Nakamura (Shizuoka General Hospital), Keigo Nagatani (Yao Municipal Hospital), Keiji Seo (Hirosima City Hiroshima Citizens Hospital), Keisuke Inada (Asoka Vihara Hospital), Keisuke Kongo (Kobe Minimally Invasive Cancer Center), Kozue Hata (Kagoshima University Hospital), Makio Imamura (Kurashiki Medical Center), Makoto Akao (Yonezawa City Hospital), Makoto Terao (Kyoto University Hospital), Mamiko Mantani (Aiwa Hospital), Mariko Kawana (Kameda Medical Hospital), Masanori Fujitani (Fuchu Hospital), Masayuki Miyazawa (Shonan Central Hospital), Megumi Kabeya (Nagoya Memorial Hospital), Michiko Nagai (Shonan Central Hospital), Michiko Yamamoto (Isehara Kyodo Hospital), Mizuhiko Yamaguchi (Omihachiman Community Medical Center), Moemi Murano (Nippon Medical School Tama-Nagayama Hospital), Rintaro Ohno (Saiseikai Utsunomiya Hospital), Sadaharu Oike (Shimada Hospital), Satoko Kushibiki (Shonan Central Hospital), Satomi Chihara (Itami City Hospital), Seiko Tonogaki (Kyowakai Medical Corporation Senrichuo Hospital), Shinsuke Tajima (Kyorin University Hospital), Shoichi Miyata (Tokyo Women’s Medical University Hospital), Shota Fujimura (Saitama Medical Center, Saitama Medical University), Taiju Nozoe (Southern Region Hospital), Takashi Nakashima (Tarumizu Municipal Hospital), Takeshi Daizou (Ishikawa Prefectural Central Hospital), Toshiyasu Tsujii (Asago Medical Center), Yasunari Okuda (Jichi Medical University Hospital), Yoshihiro Yamamoto (Komaki City Hospital), Yukiko Kawano (Japanese Red Cross Ogawa Hospital), Yukio Sakata (Hakodate Municipal Hospital), and Yuko Takasu (Takarazuka City Hospital).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1195732/full#supplementary-material
References
Abdel-Qader, D. H., Harper, L., Cantrill, J. A., and Tully, M. P. (2010). Pharmacists' interventions in prescribing errors at hospital discharge: An observational study in the context of an electronic prescribing system in a UK teaching hospital. Drug Saf. 33 (11), 1027–1044. doi:10.2165/11538310-000000000-00000
Abu-Naser, D. (2021). Impact of clinical pharmacist interventions in prescribing errors in hospitalized diabetic patients with major polypharmacy. Hosp. Pharm. 56 (4), 392–399. doi:10.1177/0018578720985428
Bates, D. W., Cullen, D. J., Laird, N., Petersen, L. A., Small, S. D., Servi, D., et al. (1995). Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. JAMA 274 (1), 29–34. doi:10.1001/jama.274.1.29
Bond, C. A., and Raehl, C. L. (2006). Adverse drug reactions in United States hospitals. Pharmacotherapy 26 (5), 601–608. doi:10.1592/phco.26.5.601
Bond, C. A., and Raehl, C. L. (2007). Clinical pharmacy services, pharmacy staffing, and hospital mortality rates. Pharmacotherapy 27 (4), 481–493. doi:10.1592/phco.27.4.481
Budnitz, D. S., Lovegrove, M. C., Shehab, N., and Richards, C. L. (2011). Emergency hospitalizations for adverse drug events in older Americans. N. Engl. J. Med. 365 (21), 2002–2012. doi:10.1056/NEJMsa1103053
Carroll, C., and Hassanin, A. (2017). Polypharmacy in the elderly-when good drugs lead to bad outcomes: A teachable moment. JAMA Intern Med. 177 (6), 871. doi:10.1001/jamainternmed.2017.0911
Chan, M., Nicklason, F., and Vial, J. H. (2001). Adverse drug events as a cause of hospital admission in the elderly. Intern Med. J. 31 (4), 199–205. doi:10.1046/j.1445-5994.2001.00044.x
Chun, J. C., Appel, S. J., and Simmons, S. (2018). 2015 Beers criteria medication review in assisted living facilities. J. Am. Assoc. Nurse Pract. 30 (11), 648–654. doi:10.1097/JXX.0000000000000082
Fulton, M. M., and Allen, E. R. (2005). Polypharmacy in the elderly: A literature review. J. Am. Acad. Nurse Pract. 17 (4), 123–132. doi:10.1111/j.1041-2972.2005.0020.x
Garland, C. T., Guenette, L., Kroger, E., Carmichael, P. H., Rouleau, R., and Sirois, C. (2021). A new care model reduces polypharmacy and potentially inappropriate medications in long-term care. J. Am. Med. Dir. Assoc. 22 (1), 141–147. doi:10.1016/j.jamda.2020.09.039
Geer, M. I., Koul, P. A., Tanki, S. A., and Shah, M. Y. (2016). Frequency, types, severity, preventability and costs of Adverse Drug Reactions at a tertiary care hospital. J. Pharmacol. Toxicol. Methods 81, 323–334. doi:10.1016/j.vascn.2016.04.011
Hajjar, E. R., Cafiero, A. C., and Hanlon, J. T. (2007). Polypharmacy in elderly patients. Am. J. Geriatr. Pharmacother. 5 (4), 345–351. doi:10.1016/j.amjopharm.2007.12.002
Hamilton, H., Gallagher, P., Ryan, C., Byrne, S., and O'Mahony, D. (2011). Potentially inappropriate medications defined by STOPP criteria and the risk of adverse drug events in older hospitalized patients. Arch. Intern Med. 171 (11), 1013–1019. doi:10.1001/archinternmed.2011.215
Hanlon, J. T., and Schmader, K. E. (2013). The medication appropriateness index at 20: Where it started, where it has been, and where it may be going. Drugs Aging 30 (11), 893–900. doi:10.1007/s40266-013-0118-4
Hashimoto, Y., and Tensho, M. (2016). Effect of pharmacist intervention on physician prescribing in patients with chronic schizophrenia: A descriptive pre/post study. BMC Health Serv. Res. 16, 150. doi:10.1186/s12913-016-1408-4
Hersh, L. R., Beldowski, K., and Hajjar, E. R. (2017). Polypharmacy in the geriatric oncology population. Curr. Oncol. Rep. 19 (11), 73. doi:10.1007/s11912-017-0632-3
Horii, T., and Atsuda, K. (2020). Effects of pharmacist intervention on polypharmacy in patients with type 2 diabetes in Japan. BMC Res. Notes 13 (1), 183. doi:10.1186/s13104-020-05032-2
Johnell, K., and Klarin, I. (2007). The relationship between number of drugs and potential drug-drug interactions in the elderly: A study of over 600,000 elderly patients from the Swedish prescribed drug register. Drug Saf. 30 (10), 911–918. doi:10.2165/00002018-200730100-00009
Leelakanok, N., Holcombe, A. L., Lund, B. C., Gu, X., and Schweizer, M. L. (2017). Association between polypharmacy and death: A systematic review and meta-analysis. J. Am. Pharm. Assoc. JAPhA. 57 (6), 729–738. doi:10.1016/j.japh.2017.06.002
Leendertse, A. J., Egberts, A. C., Stoker, L. J., van den Bemt, P. M., and Group, H. S. (2008). Frequency of and risk factors for preventable medication-related hospital admissions in The Netherlands. Arch. Intern Med. 168 (17), 1890–1896. doi:10.1001/archinternmed.2008.3
Levy, H. B. (2017). Polypharmacy reduction strategies: Tips on incorporating American Geriatrics society beers and screening Tool of older people's prescriptions criteria. Clin. Geriatr. Med. 33 (2), 177–187. doi:10.1016/j.cger.2017.01.007
Liu, L. M. (2014). Deprescribing: An approach to reducing polypharmacy in nursing home residents. J. Nurse Pract. 10 (2), 136–139. doi:10.1016/j.nurpra.2013.09.010
Mangoni, A. A., and Jackson, S. H. (2004). Age-related changes in pharmacokinetics and pharmacodynamics: Basic principles and practical applications. Br. J. Clin. Pharmacol. 57 (1), 6–14. doi:10.1046/j.1365-2125.2003.02007.x
Masnoon, N., Shakib, S., Kalisch-Ellett, L., and Caughey, G. E. (2017). What is polypharmacy? A systematic review of definitions. BMC Geriatr. 17 (1), 230. doi:10.1186/s12877-017-0621-2
Morimoto, T., Sakuma, M., Matsui, K., Kuramoto, N., Toshiro, J., Murakami, J., et al. (2011). Incidence of adverse drug events and medication errors in Japan: The JADE study. J. Gen. Intern Med. 26 (2), 148–153. doi:10.1007/s11606-010-1518-3
Nester, T. M., and Hale, L. S. (2002). Effectiveness of a pharmacist-acquired medication history in promoting patient safety. Am. J. Health Syst. Pharm. 59 (22), 2221–2225. doi:10.1093/ajhp/59.22.2221
Noguchi, C., Sakuma, M., Ohta, Y., Bates, D. W., and Morimoto, T. (2016). Prevention of medication errors in hospitalized patients: The Japan adverse drug events study. Drug Saf. 39 (11), 1129–1137. doi:10.1007/s40264-016-0458-1
Nothelle, S. K., Sharma, R., Oakes, A., Jackson, M., and Segal, J. B. (2019). Factors associated with potentially inappropriate medication use in community-dwelling older adults in the United States: A systematic review. Int. J. Pharm. Pract. 27 (5), 408–423. doi:10.1111/ijpp.12541
Nothelle, S. K., Sharma, R., Oakes, A. H., Jackson, M., and Segal, J. B. (2017). Determinants of potentially inappropriate medication use in long-term and acute care settings: A systematic review. J. Am. Med. Dir. Assoc. 18 (9), 806 e1–e806. doi:10.1016/j.jamda.2017.06.005
O'Connor, M. N., Gallagher, P., and O'Mahony, D. (2012). Inappropriate prescribing: Criteria, detection and prevention. Drugs Aging 29 (6), 437–452. doi:10.2165/11632610-000000000-00000
O'Mahony, D., O'Sullivan, D., Byrne, S., O'Connor, M. N., Ryan, C., and Gallagher, P. (2015). STOPP/START criteria for potentially inappropriate prescribing in older people: Version 2. Age Ageing 44 (2), 213–218. doi:10.1093/ageing/afu145
Ooi, K. (2019). Appropriate use of medicine and polypharmacy in older patients. Yakugaku Zasshi 139 (4), 571–574. doi:10.1248/yakushi.18-00181-5
Panel, A. G. S. B. C. U. E., Fick, D. M., Semla, T. P., Beizer, J., Brandt, N., Dombrowski, R., et al. (2015). American Geriatrics Society 2015 updated beers criteria for potentially inappropriate medication use in older adults. J. Am. Geriatr. Soc. 63 (11), 2227–2246. doi:10.1111/jgs.13702
Park, H. Y., Ryu, H. N., Shim, M. K., Sohn, H. S., and Kwon, J. W. (2016). Prescribed drugs and polypharmacy in healthcare service users in South Korea: An analysis based on national health insurance claims data. Int. J. Clin. Pharmacol. Ther. 54 (5), 369–377. doi:10.5414/CP202484
Pasina, L., Brucato, A. L., Falcone, C., Cucchi, E., Bresciani, A., Sottocorno, M., et al. (2014). Medication non-adherence among elderly patients newly discharged and receiving polypharmacy. Drugs Aging 31 (4), 283–289. doi:10.1007/s40266-014-0163-7
Reeve, E., Gnjidic, D., Long, J., and Hilmer, S. (2015). A systematic review of the emerging defiition of ‘deprescribing’ with network analysis: Implications for future research and clinical practice. Br. J. Clin. Pharmacol. 80 (6), 1254–1268. doi:10.1111/bcp.12732
Reeve, E., Shakib, S., Hendrix, I., Roberts, M. S., and Wiese, M. D. (2014). Review of deprescribing processes and development of an evidence-based, patient-centred deprescribing process. Br. J. Clin. Pharmacol. 78 (4), 738–747. doi:10.1111/bcp.12386
Schenker, Y., Park, S. Y., Jeong, K., Pruskowski, J., Kavalieratos, D., Resick, J., et al. (2019). Associations between polypharmacy, symptom burden, and quality of life in patients with advanced, life-limiting illness. J. Gen. Intern Med. 34 (4), 559–566. doi:10.1007/s11606-019-04837-7
Scott, D., Cernasev, A., Barenie, R. E., Springer, S. P., and Axon, D. R. (2023). Teaching deprescribing and combating polypharmacy in the pharmacy curriculum: Educational recommendations from thematic analysis of focus groups. Clin. Pract. 13 (2), 442–453. doi:10.3390/clinpract13020040
Scott, I. A., Hilmer, S. N., Reeve, E., Potter, K., Le Couteur, D., Rigby, D., et al. (2015). Reducing inappropriate polypharmacy: The process of deprescribing. JAMA Intern Med. 175 (5), 827–834. doi:10.1001/jamainternmed.2015.0324
Suzuki, S., Uchida, M., Suga, Y., Sugawara, H., Kokubun, H., Uesawa, Y., et al. (2019). A nationwide survey of community pharmacist contributions to polypharmacy in opioid-using and non-using cancer patients in Japan. Biol. Pharm. Bull. 42 (7), 1164–1171. doi:10.1248/bpb.b19-00043
Uchida, M., Suzuki, S., Sugawara, H., Suga, Y., Kokubun, H., Uesawa, Y., et al. (2019). A nationwide survey of hospital pharmacist interventions to improve polypharmacy for patients with cancer in palliative care in Japan. J. Pharm. Health Care Sci. 5, 14. doi:10.1186/s40780-019-0143-5
Uchida, M., Suzuki, S., Sugawara, H., Suga, Y., Nakagawa, T., and Takase, H. (2022). Multicentre prospective observational study on community pharmacist interventions to reduce inappropriate medications. Int. J. Pharm. Pract. 30 (5), 427–433. doi:10.1093/ijpp/riac032
Verdoorn, S., Kwint, H. F., Faber, A., Gussekloo, J., and Bouvy, M. L. (2015). Majority of drug-related problems identified during medication review are not associated with STOPP/START criteria. Eur. J. Clin. Pharmacol. 71 (10), 1255–1262. doi:10.1007/s00228-015-1908-x
Wastesson, J. W., Morin, L., Tan, E. C. K., and Johnell, K. (2018). An update on the clinical consequences of polypharmacy in older adults: A narrative review. Expert Opin. Drug Saf. 17 (12), 1185–1196. doi:10.1080/14740338.2018.1546841
Woodward, M. C. (2003). Deprescribing: Achieving better health outcomes for older people through reducing medications. J. Pharm. Pract. Res. 33 (4), 323–328. doi:10.1002/jppr2003334323
Keywords: hospital pharmacists, intervention, inappropriate medications, polypharmacy, multicenter prospective observational study
Citation: Suzuki S, Uchida M, Sugawara H, Suga Y, Nakagawa T and Takase H (2023) Multicenter prospective observational study on hospital pharmacist interventions to reduce inappropriate medications. Front. Pharmacol. 14:1195732. doi: 10.3389/fphar.2023.1195732
Received: 28 March 2023; Accepted: 12 June 2023;
Published: 29 June 2023.
Edited by:
Hao Li, Shanghai Jiao Tong University, ChinaReviewed by:
Ela Hoti, University of Medicine, Tirana, AlbaniaYumao Zhang, Sun Yat-sen University, China
Marcela Forgerini, São Paulo State University, Brazil
Copyright © 2023 Suzuki, Uchida, Sugawara, Suga, Nakagawa and Takase. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shinya Suzuki, c3N1enVraUBlYXN0Lm5jYy5nby5qcA==
†These authors have contributed equally to this work and share first authorship
 Shinya Suzuki
Shinya Suzuki Mayako Uchida
Mayako Uchida Hideki Sugawara
Hideki Sugawara Yukio Suga2,5
Yukio Suga2,5 Takayuki Nakagawa
Takayuki Nakagawa