- 1Research Center of National Drug Policy and Ecosystem, School of International Pharmaceutical Business, China Pharmaceutical University, Nanjing, China
- 2West China School of Pharmacy, Sichuan University, Chengdu, China
Introduction: Chinese hospitals still face various barriers to implementing pharmaceutical care. The quantitative instrument for measuring these barriers in China is scarce. This study aims to develop and validate a scale for measuring barriers to providing pharmaceutical care in Chinese hospitals from the perspective of clinical pharmacists.
Methods: The scale was developed based on existing literature and qualitative interviews with 20 experts. The scale was included in a small-range pilot survey and then administered to a validation survey in 31 provinces in China. Exploratory factor analysis was used to identify the structure of the scale. Confirmatory factor analysis was applied to verify the structure of the scale and to validate the scale’s convergent and discriminative validity. Known-group validity was also examined. Cronbach’s alpha examined the internal consistency reliability of the scale.
Results: 292 scales were completed and returned for a response rate of 85.6% in the pilot study. Exploratory factor analysis of the scale suggested a five-factor solution (Cognition and attitude, Knowledge and skills, Objective conditions, External cooperation, and Support from managers) accounting for 66.03% of the total variance. 443 scales were sent out in the validation study, with a response rate of 81.0%. Confirmatory factor analysis demonstrated a good fit of the structural model for pharmaceutical care barriers. It showed the scale’s good convergent and discriminative validity (The average variance extracted >0.5 and composite reliability >0.7). The scale could also identify the differences in total score among the clinical pharmacists from different hospital grades (p < 0.05). Cronbach’s alpha is between 0.658 and 0.896, indicating good internal consistency.
Conclusion: From the perspective of clinical pharmacists, this study has developed a scale to assess obstacles to pharmaceutical care. The scale comprehensively encompasses barriers to clinical pharmacists’ cognitive and ability-related aspects, hindrances encountered in collaborating with other health professionals and patients, and barriers to the working environment. The reliability and validity have been established through verification.
1 Introduction
Since Charles Hepler and Linda Strand systemically introduced the concept of pharmaceutical care in the 1990s (Hepler and Strand, 1990), pharmacists have been gradually integrated into the clinical staff as the professionals of pharmacotherapy, by which the role of clinical pharmacist appeared, becoming an indispensable part of medical teams. Up to today, pharmaceutical care is globally performed, benefiting the patients, the physicians, and the pharmacists simultaneously (da Costa et al., 2019).
Similar to other health services, pharmaceutical care is provided depending on mutual collaboration, adequate supply, and efficient utilization of health resources, and thus, it could be hindered by various kinds of barriers (pharmaceutical care barriers) (Onozato et al., 2020). Clinical pharmacists are recognized as the main providers of pharmaceutical care, making their perception of pharmaceutical care barriers both direct and prominent (Zheng et al., 2021). For policymakers and hospital administrators, obtaining feedback from clinical pharmacists provides valuable insights into the pharmaceutical care deficiencies present within hospitals, which is significant for their decision-making on the improvement and development of the pharmaceutical care system, and its proper functioning.
Currently, studies on the pharmaceutical care barriers of most countries focused on community pharmacies, such as those of Europe (Van Mil et al., 2001), Iran (Mehralian et al., 2014), Australia (Culverhouse and Wohlmuth, 2012), Jordan (Elayeh et al., 2017) and New Zealand (Scahill et al., 2009). However, in China, pharmaceutical care in community pharmacies differs from that in hospitals regarding the receiver, the contents, and the standards (Yao et al., 2017), so the conclusions reached by most current studies may not apply to hospitals in China.
Pharmaceutical care barriers in hospitals were only discussed in a few studies (Ngorsuraches and Li, 2006; Lounsbery et al., 2009; Okonta et al., 2012; Katoue et al., 2014; El Hajj et al., 2016; Elsadig et al., 2017; Omar et al., 2020). Due to the diversity of pharmaceutical care barriers around the globe, the appropriate instrument may tremendously affect the robustness and feasibility of the results and conclusions. Detailed information on the instruments used to measure pharmaceutical care barriers, as proposed in these studies, is presented in Table 1. The studies above provided valuable evidence, but given the significant differences between the health system of China and the countries above (Li and Li, 2018), the applicability of those instruments are doubtable. As for the studies on pharmaceutical care barriers in hospitals in China, three studies discussed the factors that may facilitate or obstacle the development of the pharmaceutical care system based on literature and their practical experiences (Wen et al., 2006; Park, 2007; Dong and Wang, 2013). Though these studies generally found that the imperfect laws and regulations, the conventional “drug-orientation” concept of hospital pharmacists, and the clinical incompetence of pharmacists were the major barriers in China, their conclusions were significantly distinct from and incomparable to each other’s and the primary cause of it might be the absence of a valid and applicable instrument for the evaluation of the pharmaceutical care barriers in China.
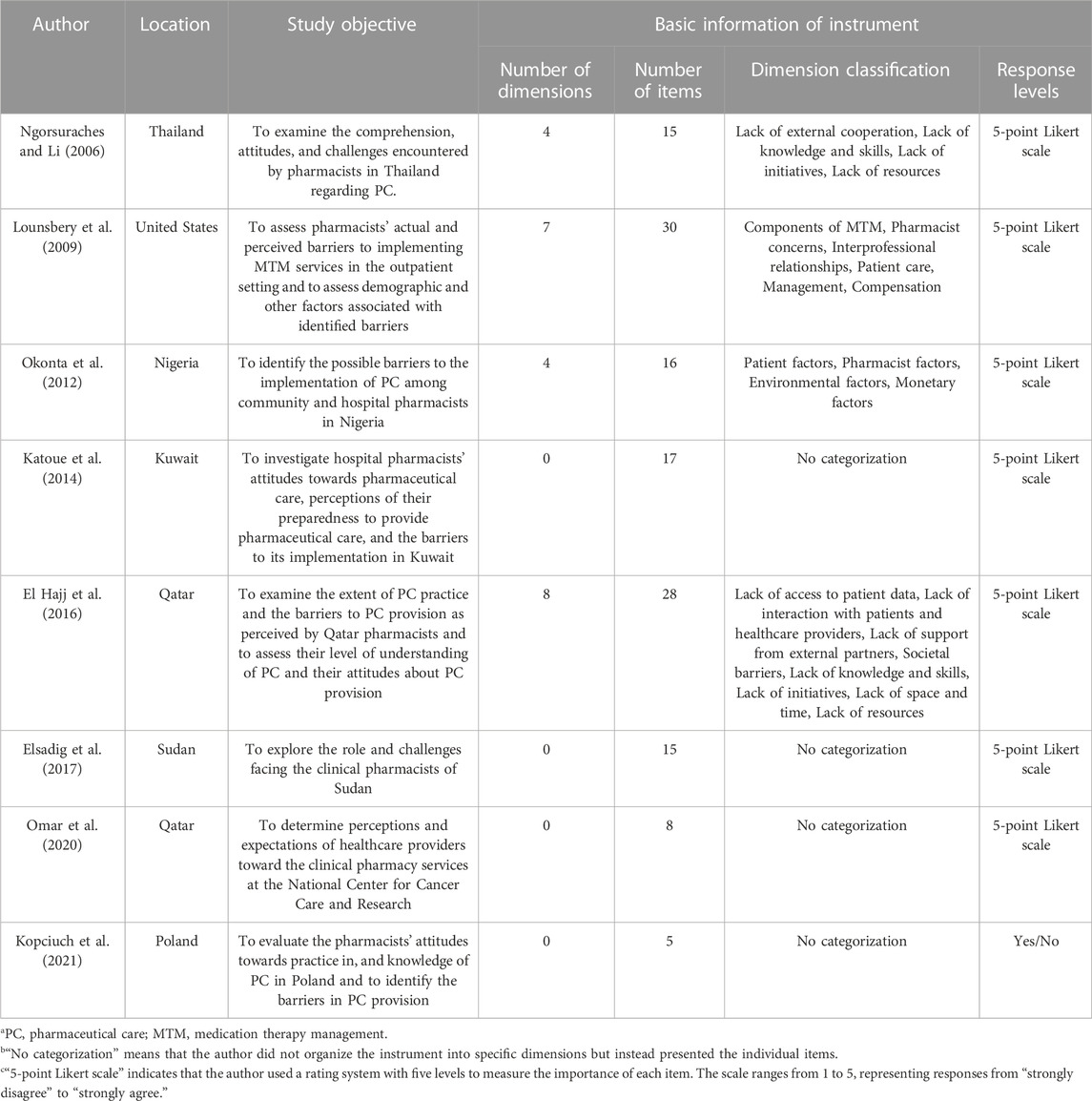
TABLE 1. The basic information of the literature encompassed the instrument for evaluating the pharmaceutical care barriers in hospitals.
Compared with the health care system in developed countries, those in developing countries such as China are still in an early stage of development. A considerable amount of health resources was grossly underused due to frequent occurrence of irrational drug use (Mao et al., 2015), such as arbitrary changes in drug dosage, abuse of antibiotics and injections (Wang et al., 2014), pointless combined use of drugs (Guan et al., 2019), etc. In China, healthcare institutions, especially hospitals, are the principal setting of drug use or drug information obtainment, making it essential to improve the pharmaceutical care system, aiming at optimal drug use. In the past 2 decades, the Chinese government has issued several policies concerning the construction of the pharmaceutical care system in Chinese public-based hospitals (Guo et al., 2020). Though China has made remarkable achievements, a noticeable gap in the pharmaceutical care quality remains between China and other developed countries, which could be minimized by identifying and eliminating the pharmaceutical care barriers.
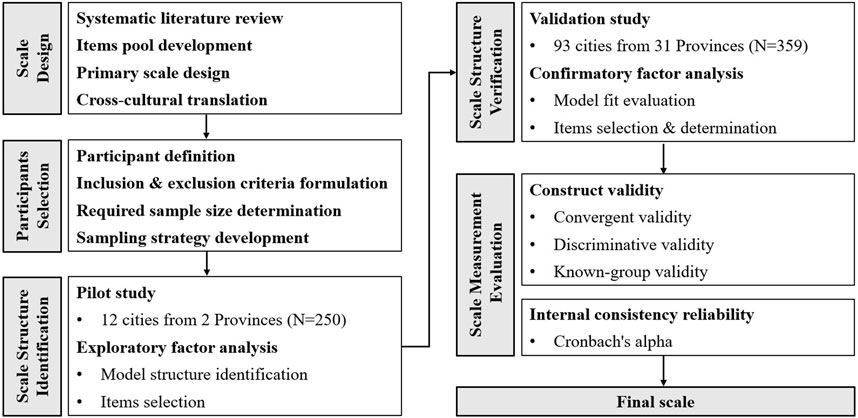
This study aims to develop and validate the Pharmaceutical Care Barriers Scale in Chinese Hospitals (PCBS-CH), an instrument to measure barriers to providing pharmaceutical care in Chinese hospitals from the perspective of clinical pharmacists. The flow of this study is shown in Figure 1.
2 Methods
2.1 Development of the instrument
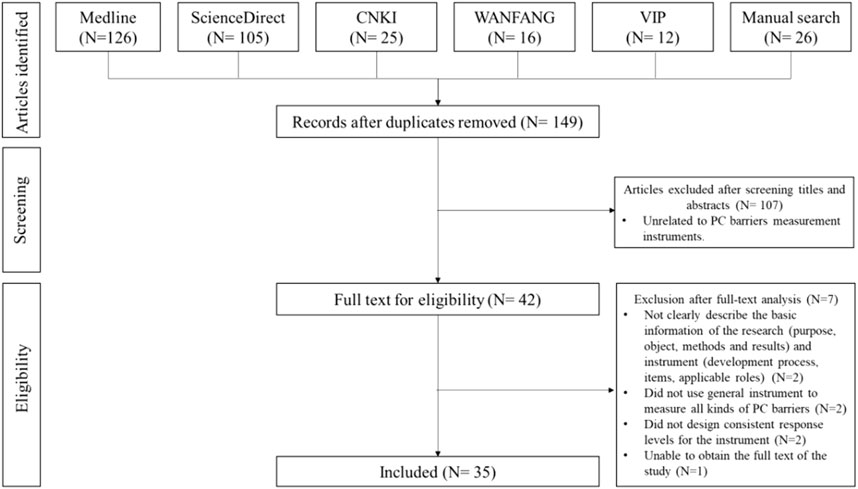
This study first summarized pharmaceutical care barriers based on a systematic literature review, and an initial pool consisting of the pharmaceutical care barriers was then developed. Related literature was retrieved from Medline, ScienceDirect, China National Knowledge Infrastructure (CNKI), Chinese Scientific Journal Database (VIP) and Wanfang database (WANFANG) during database establishment to Jan. 2019, using “Hospital”, “Medical Institution”, “community pharmacy”, “Pharmaceutical Care”, “Pharmacy Service”, “Pharmaceutical Service”, “Barrier”, “Restriction” and “Obstacle” as keywords. Thirty-five pieces of literature were included after a two-step screening process performed by two researchers. The complete search process is available in Figure 2. All the items were extracted by the researchers and included in the initial pool.
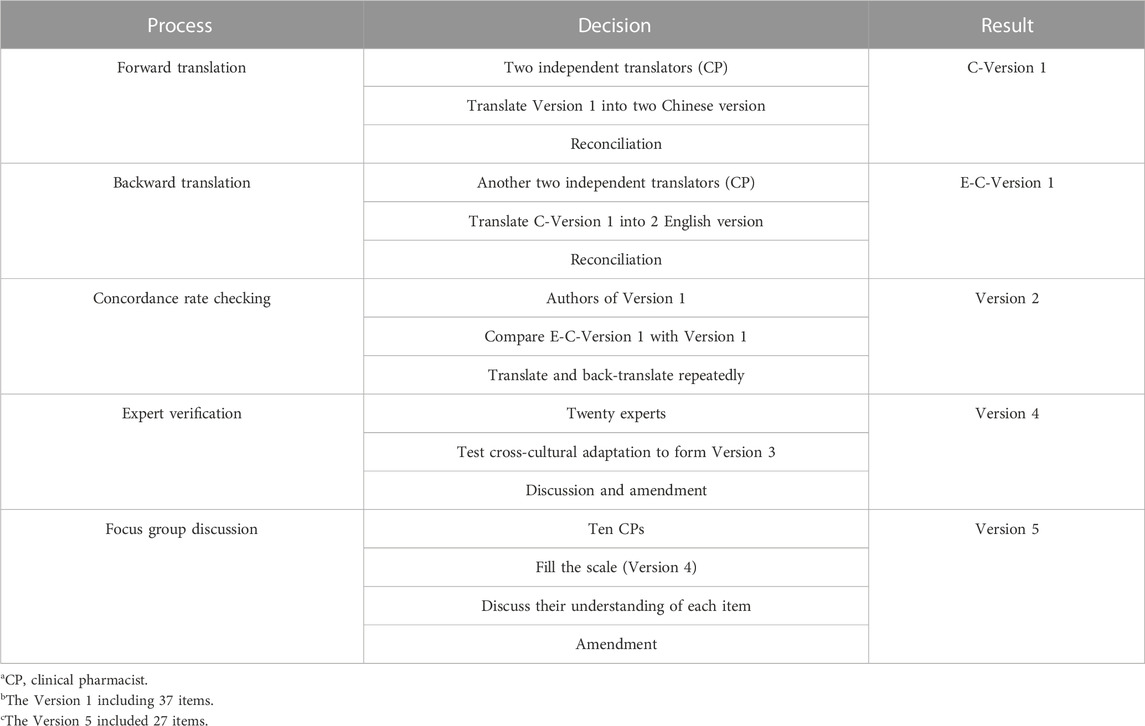
According to the guideline for questionnaire design and scale development (Watson et al., 1988), the primary English scale (Version 1), including 37 items, was developed based on the initial pool. The items comprehensively encompass barriers related to the clinical pharmacists’ cognitive and ability-related aspects, impediments encountered in collaborating with other health professionals and patients, and barriers linked to the working environment. Version 1 was then translated and revised by two translation groups. To ensure the semantical consistency of the original and the translated items (Beaton et al., 2002), a forward translation group was formed by two clinical pharmacists who were fluent in English to translate the Version 1 independently and developed the first Chinese version scale (C-Version 1) while two clinical pharmacists blind to the scales developed the back-translation group to translate C-Version 1 into English (E-C-Version 1). The authors of the primary English scale (Version 1) reviewed and compared E-C-Version 1 and Version 1. To ensure the semantic equivalency, the items of E-C-Version 1 whose concordance rate was less than 70% should be repeatedly translated and back-translated to form the second Chinese version scale (Version 2). Then, experts from a cross-cultural perspective should examine version 2, and the third Chinese version scale was developed (Version 3). Version 3 was sent to an expert panel, including 5 heads of hospital pharmacy departments, 5 front-line clinical pharmacists, 5 staff of pharmaceutical care-related government departments, and 5 university professors engaged in pharmaceutical care-related research from the east, central, and west of China. Experts were required to screen out items confirming China’s national conditions, merge similar items, exclude minor items, and add missing items to synthesize the fourth Chinese version scale (Version 4). Finally, the research team conducted a three-round focus group discussion in February 2019. A total of 10 clinical pharmacists who had participated in the practice of pharmaceutical care were included randomly in each round to revising the difficult-to-understand and ambiguous items of Version 4, and the primary scale for the pilot survey was developed (Version 5). The translation processes can be found in Table 2.
The primary version scale (Version 5) included 27 items, which basically covered all barriers to performing pharmaceutical care in Chinese hospitals. A 5-point Likert scale, where 1 = strongly disagree and 5 = strongly agree, was used to elicit responses to 27 possible barriers to providing pharmaceutical care. The scale is available online as Supplementary Table S1. The sum of each item calculated the total score. Meanwhile, respondents need to fill out a basic information questionnaire to collect data on sex, age, education, and years of practicing as a clinical pharmacist.
2.2 Participants
First-line clinical pharmacists from all parts of China were recruited in this survey. The inclusion criteria for the sample were: 1) Be a full-time clinical pharmacist working in the sampled hospital. 2) Have been working for more than 1 year. 3) Be willing to sign the informed consent. The exclusion criteria were: 1) Clinical pharmacist on vacation or training. 2) Clinical pharmacist who had not provided pharmaceutical care.
Based on the principle that the sample size should be 5 to 10 times of the number of items (27 items) to ensure the results of Exploratory Factor Analysis (EFA) are more precise estimates of factor loadings and are also more stable (Bentler et al., 1987; Gorsuch, 2013), assuming the missing rate of the scale response (20%), a minimum sample size of 169 was estimated to be sufficient for the pilot study, while a sample size of 338 would be even more desirable.
A multi-stage sampling strategy was adopted to ensure sample representativeness of the validation study: 1) The sample covered all 31 provincial administrative regions in mainland China. 2) Cities in each provincial administrative region were divided into three groups according to their 2018 per capita gross domestic product, which is associated with the city’s medical resources possession, such as the quality of healthcare techniques, thereby generating 93 groups. 1 city or district was selected using the random number method in each group, thereby 93 cities or districts were selected. 3) In each selected city or district, 1 secondary hospital and 1 tertiary hospital were surveyed by convenience based on the hospital administrators’ permission to conduct the survey, with the hospital level verified by consulting the hospital information tool by the National Health Commission of China. This ensured that 186 hospitals would be selected. 4) In each surveyed hospital, 1 to 2 clinical pharmacists in secondary hospitals and 2 to 4 clinical pharmacists in tertiary hospitals were selected by convenience, recommended by the hospital administrator(s), or another participant who completed the survey. Overall, at least 279 scales should be distributed during the validation study, and the number of total scales should not exceed 558.
2.3 Pilot study
A three-round pilot study was conducted in 64 secondary hospitals and 71 tertiary hospitals in 6 cities of Jiangsu Province and 6 cities of Anhui Province, from March to April 2019. The investigator explained the intention to the clinical pharmacists to sign an informed consent, and an appropriate time and place was determined for a face-to-face interview. Researchers collated questions from investigators and respondents in the pilot study, such as respondents’ comments, unanswered items, the time spent answering each item, and the total time required to accomplish the scale. EFA was performed on the pilot study data to obtain a general model of dimensions of pharmaceutical care barriers. Researchers revised the scale again according to the feedback from the pilot study. The eventual scale for the validation study was divided into 5 dimensions, including 21 items.
2.4 Validation study
A total of 31 undergraduate students majoring in pharmacy or clinical pharmacy were recruited as data collectors and trained. The validation study was carried out from July to September 2019. After obtaining the hospital administrators’ consent, during the non-working hours of the hospital, the investigators asked the sampled clinical pharmacists for their basic information to determine whether they met the inclusion criteria and then provided the eligible clinical pharmacists with the purposes, the contents and the requirements of our survey and confirmed their willingness to participate in again. Those willing to participate signed the consent form and decided the time and a quiet place for the survey with the investigators. The investigator stated the requirements for each item, the description of the items, and the options of each item and recorded the oral answers of the respondents through an online survey system. After the interview, the data were immediately uploaded to the researcher’s server and converted into a database file that the data analysis software could recognize. A team of 5 postgraduate students was recruited to review the uploaded data and immediately return the documents with errors or damaged data for correction through return visits.
2.5 Ethical approval
The ethical approval to conduct the pilot survey and validation study was granted by the Ethics Committee of China Pharmaceutical University (Project Number: CPU2019015). Written consent to participate was obtained from each participant before data collection. No sensitive and personal data were recorded, while confidentiality of data was assured during data analysis and reporting.
2.6 Statistical analysis
EFA with principal components analysis and varimax rotation was carried out to obtain a general model of the dimension of the pharmaceutical care barriers and determine the formal scale. Confirmatory factor analysis (CFA) was applied to verify the structural validity of the scale and goodness of fit for the model. One-way ANOVA was used to determine the structural validity in a known group. Shapiro-Wilk test and Levene’s test were applied to test the normality and homogeneous variance of the sample before the one-way ANOVA. Internal consistency reliability was examined using Cronbach’s alpha.
For EFA, the KMO value (Kaiser–Meyer–Olkin) is used to determine whether factor analysis is possible. The closer to 1 the KMO is, the more suitable the variable for factor analysis is (Batistelli Vignola and Tucci, 2014). The following criteria are mainly used when selecting the items: 1) the load value of a variable on a factor is greater than 0.5; 2) the cross load value between variables is low; 3) the characteristic value of the variable is greater than 1; 4) items with the same factor have the same meaning (Fabrigar et al., 1999). Questionnaires that are more reliable possess a higher value of Cronbach’s alpha. A value of 0.7 is regarded as an acceptable value (Peill et al., 2022). For CFA, the absolute fitting index, relative fitting index, and goodness-of-fit index were used to check the model’s fit (Alamer and Marsh, 2022). The average variance extracted (AVE) > 0.5, Composite Reliability (CR) > 0.7 (Bacon et al., 1995) implies good aggregation validity; The AVE root value of each factor is greater than the maximum value of the correlation coefficient, indicating good discriminative validity (Dash and Paul, 2021). For one-way ANOVA, p < 0.05 represents a significant difference (Lix et al., 1996).
The CFA used IBM SPSS AMOS version 24.0 (IBM SPSS Statistics, Armonk, NY). All other statistical analyses were performed using IBM SPSS statistics version 26.0 (SPSS Inc., Chicago, IL).
3 Results
3.1 Participant characteristics
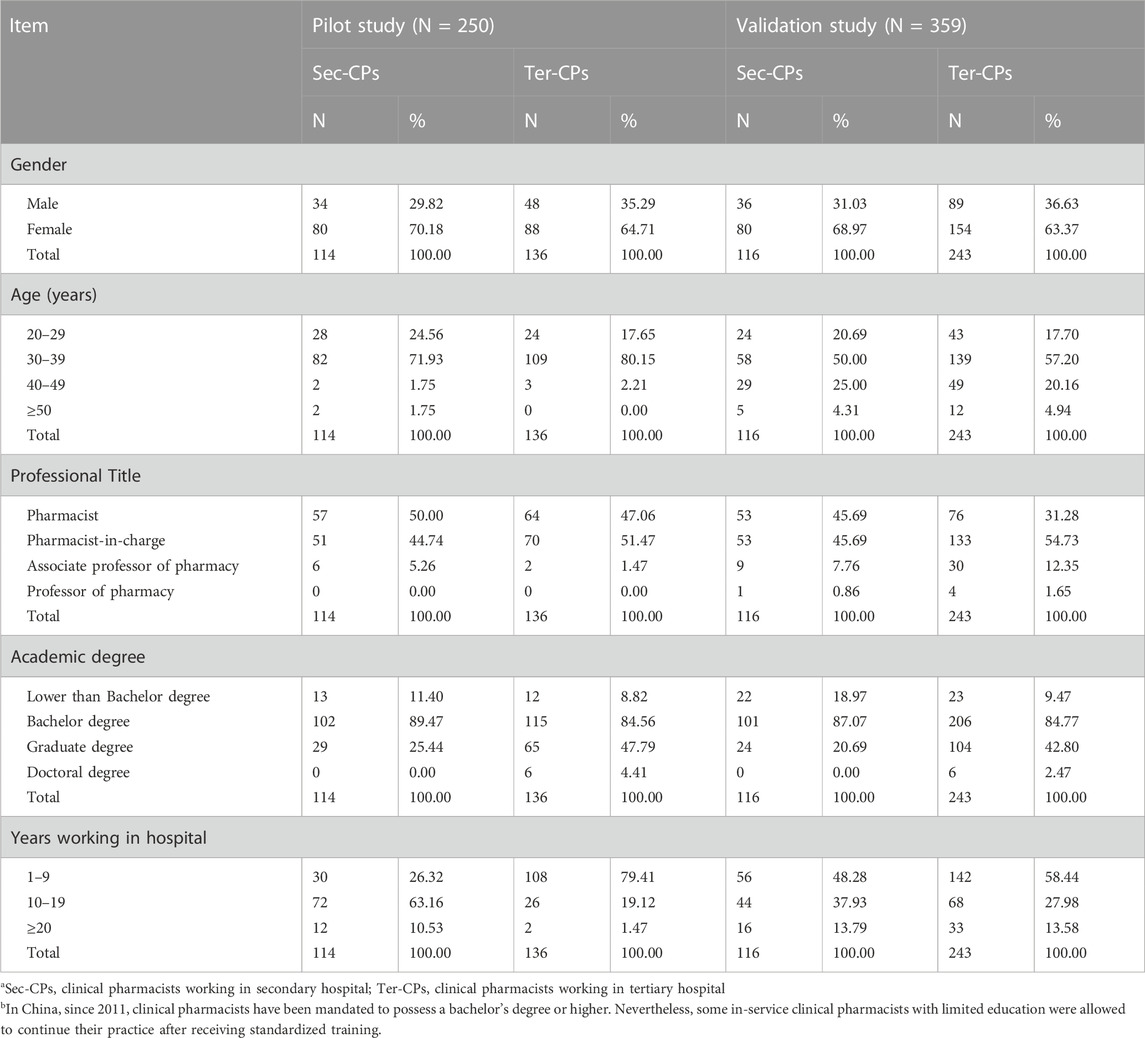
The pilot study involved the participation of 292 clinical pharmacists, with 250 valid scales recovered, resulting in a recovery rate of 85.6%. Out of the 443 scales distributed in the validation study, we received a response rate of 81.0%, yielding 359 effective scales. Females accounted for more than half of the study population. The respondents’ age, professional title, and education were concentrated in the bracket of 30–39, intermediate titles, and undergraduates. The demographic information is consistent with the previous survey of pharmaceutical care in China (Xi et al., 2017), except for the distribution of academic qualifications, which may be attributed to a convenient sampling method to select hospitals and clinical pharmacists (Table 3).
3.2 Exploratory factor analysis
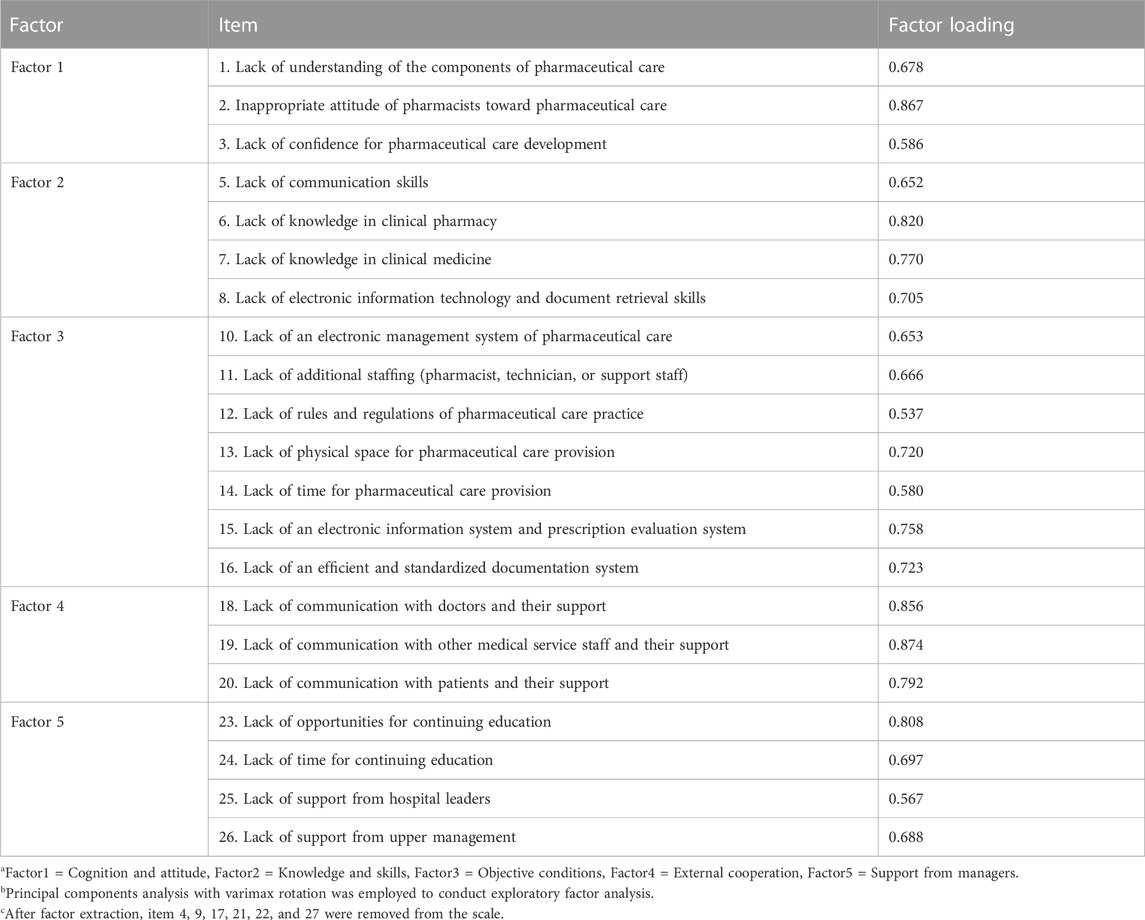
EFA was adopted to identify the structure of the PCBS-CH. For EFA, The KMO value was 0.89, and Bartlett’s test of sphericity reached statistical significance (chi-square = 2,615.41, p < 0.05), supporting the factorability of the correlation matrix. According to the above criteria (see statistical analysis), 6 items were eliminated after factor extraction, and the factor loading of each item reached the standard. Table 4 presents the items with their loadings in each factor. The eigenvalue of each factor was greater than 1, and the cumulative interpretation rate was 66.026%. The factors were labeled after closely examining the content of the items constituting each factor. Factor 1, termed “cognition and attitude,” comprised 3 items and explained 5.091% of the variance. Factor 2, labeled “knowledge and skills,” consisted of 4 items, accounting for 7.978%. Factor 3, denoted as “objective conditions,” encompassed 7 items and explained a substantial 37.249%. Factor 4, designated as “external cooperation,’” comprised 3 items, accounting for 7.093%. Lastly, Factor 5, labeled “support from managers,” comprised 4 items and explained 8.615% of the variance.
3.3 Confirmatory factor analysis
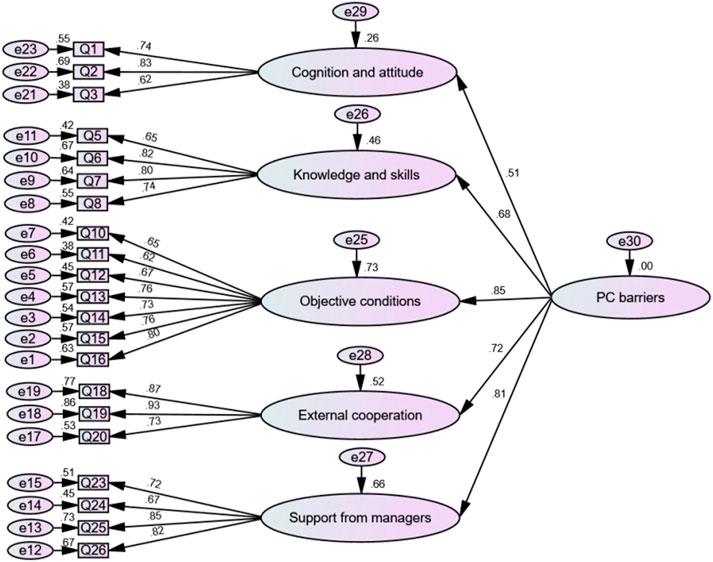
CFA with the method of maximum likelihood was applied to the sample of the validation study (n = 359) and resulted in the same five-factor structure as above. Figure 3 shows the structural model with the individual items of the PCBS-CH and standardized coefficients of each path; the highest and the lowest coefficients were related to “Objective conditions” and “Cognition and attitude”. The path diagram provides a clear representation of the factor structure of the theoretical concept, with each variable loading exclusively on one factor. In short, Figure 3 indicates that the structural model, consisting of 21 items and organized into five factors, serves as an appropriate measure of pharmaceutical care barriers in the present sample.
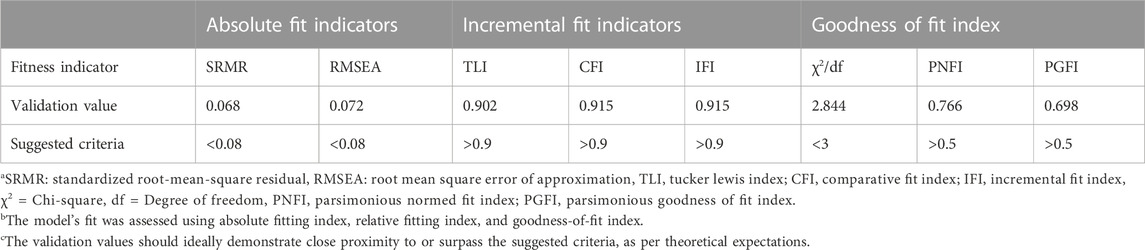
The structural model showed an overall satisfactory fit in the CFA, as indicated by the following values for the goodness of fit indicators: SRMR and RMSEA were 0.068 and 0.072, respectively, below the threshold of 0.08. TLI, CFI, and IFI values were 0.902, 0.915, and 0.915, respectively, approaching the desired level of 0.9. PNFI and PGFI values were 0.766 and 0.698, respectively, exceeding the 0.50 threshold. In addition, the χ2/df value was 2.844, indicating a good fit (less than 3). Overall, all indicators met the requirements for the goodness of fit, which implied that the developed five-factor PCBS-CH had enough validity to show a relationship between latent and visible variables amongst the Chinese clinical pharmacist population (Table 5).
3.4 Convergent and discriminative validity
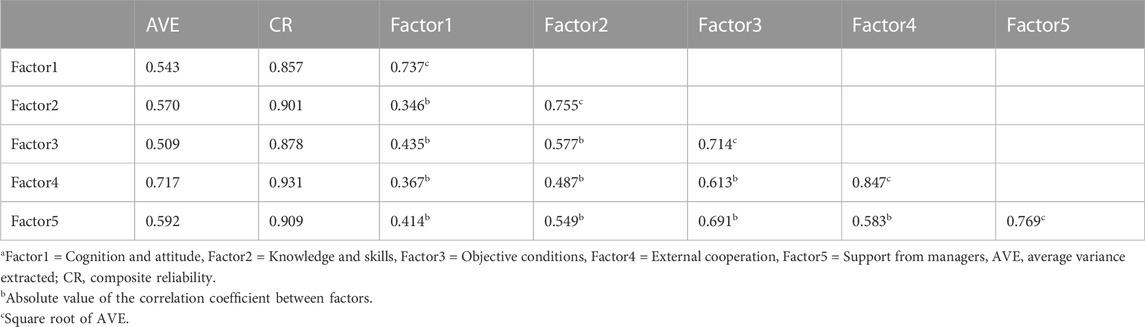
For CFA, AVE >0.5 and CR > 0.7 for all factors indicated good convergent validity. The square root of AVE of each factor was all larger than the absolute value of the correlation coefficient between factors, indicating that the internal correlation was greater than the external correlation, reflecting good discriminative validity (Table 6).
3.5 Known-group validity
Known-group validity was also examined regarding the difference between respondents’ total score from different hospital grades. The total score of each subgroup was expressed in the form of mean ± standard deviation: secondary hospital (55.02 ± 12.43), tertiary hospital (49.98 ± 12.77). The difference between various categories of ‘hospital grade’ was statistically significant (F (1,357) = 12.431, p < 0.05).
3.6 Internal consistency reliability
Cronbach’s alpha tests yielded favorable results for the PCBS-CH. The overall alpha coefficient was 0.865, while the individual dimensions demonstrated internal consistency reliability with Cronbach’s alpha values of 0.658, 0.830, 0.862, 0.896, and 0.788, respectively. These results indicate acceptable levels of internal consistency reliability for both the instrument and its sub-dimensions.
4 Discussion
This study developed an instrument for measuring the pharmaceutical care barriers in hospitals in China. Under this topic, PCBS-CH is the first instrument developed and validated using psychometric methods. The results proved the good reliability and construct validity of the 21-item scale. PCBS-CH is an applicable instrument that allows policymakers and researchers to measure the pharmaceutical care barriers in hospitals in China on a unified scale. This evidence can be helpful for them to fully understand all the types of existing barriers, accurately assess the importance of different barriers, and formulate strategies to eliminate barriers in the pharmaceutical care practice.
A systemic approach identified five dimensions of pharmaceutical care barriers in hospitals in China and then validated them. Among them, the “Cognition and attitude” combined the dimension “Components of MTM’ and “Attitude and vision”, which were mentioned in the instrument from US and Europe (Van Mil et al., 2001; Lounsbery et al., 2009). “Knowledge and skills” combined the dimensions “Lack of knowledge and skills”, “Skills” and ‘Interprofessional relationships” which was mentioned in the instrument from Thailand, Europe and US (Van Mil et al., 2001; Ngorsuraches and Li, 2006; Lounsbery et al., 2009). “Objective conditions” combined the “Lack of resources” and “Resources” dimensions in the Thai and European instrument (Van Mil et al., 2001; Ngorsuraches and Li, 2006). “External cooperation” and “Support from managers” were respectively similar to “Lack of external cooperation” and “management” dimensions referred to in the Thai and American instrument (Ngorsuraches and Li, 2006; Lounsbery et al., 2009). However, the items in dimension “Regulatory and environment” in Iranian scale was excluded into the initial pool (Mehralian et al., 2014), because the Chinese government has gradually attached importance to the development of pharmaceutical care, and there is a good regulatory and legal environment in China (Sun et al., 2008).
EFA indicated that most items were validated to be loaded on the dimension as they were in the primary version scale (Version 5), suggesting that most items were strongly related to the corresponding factor as expected. One exception is the item “Lack of sufficient compensation for pharmaceutical care provision”, of which the factor loading on the factor “objective conditions” was smaller than the acceptable threshold (0.5), indicating that this item had no practical significance, so this item was removed. However, there is a growing call from Chinese clinical pharmacists to introduce ‘pharmaceutical care fees’ to recognize the value of their work (Zhang et al., 2020; Zhang et al., 2021). Although pharmaceutical care does not depend on these fees because there is no specific provision in Chinese law requiring patients to pay for pharmaceutical care, their implementation could increase clinical pharmacists’ motivation to provide higher-quality services. Therefore, in this study, the absence of financial compensation was not considered a barrier but rather an appropriate facilitator and was therefore excluded from the scope of this scale. Furthermore, with the enactment of the Pharmacists Act, the issue of ‘pharmaceutical care fees’ is expected to be adequately addressed.
The item “lack of opportunities for continuing education” and item “lack of time for continuing education” were validated to be loaded on the factor of “Support from managers” rather than “Knowledge and skills” as initially hypothesized. This may be because administrators of hospitals or specific clinical departments in China have great decision-making power on the staffing and in-service education of their subordinates for the convenience and feasibility of personnel management (Penm et al., 2014). Thus, pharmacists tend to believe that the lack of opportunities for continuing education is in the scope of the administrators’ responsibility.
The items 21 and 22 are also noteworthy. Both of them were removed according to EFA because they had no acceptable factor loading on any factor extracted. Besides reflecting the pharmacist’s power to review and modify the prescriptions, these two items seem to represent the degree of the pharmacist’s participation in the clinical decision-making regarding a patient’s medication. Clinical pharmacists in China are required to implement effective interventions in prescriptions audit and drug dispensing according to ‘Management standard of hospital prescription comment’. Therefore, relevant barriers cannot be perceived easily by clinical pharmacists.
Observed differences in total PCBS-CH score between sub-groups of pharacists were consistent with the known differences in the development of pharmaceutical care systems of those sub-groups (Xi et al., 2017), and it validated the construct validity of the PCBS-CH as external evidence. Consistent with the status quo of China’s pharmaceutical care system development, clinical pharmacists in tertiary hospitals have significantly lower degrees of perceived barriers than that in secondary hospitals. The average total score was 5 points higher in secondary hospitals, indicating more potential barriers in certain domains. In the clinical application of the scale, PCBS-CH demonstrates enough sensitivity in capturing variations in pharmaceutical care barriers across hospitals, enabling policymakersto identify the source and severity of these barriers accurately. They can then formulate targeted strategies accordingly.
Though the PCBS-CH was designed to measure the pharmaceutical care barriers of hospitals in China, it may have the potential to be applied in community pharmacies or primary healthcare institutions after necessary validations or adaptions. From a global perspective, the development of pharmaceutical care systems in all types of healthcare institutions is universally in the early stage and may be facing common barriers, such as lack of objective conditions and external cooperation.
Several limitations to the study should be noted: firstly, owing to limited resources, this study resorted to a convenient sampling approach for selecting hospitals and clinical pharmacists, potentially impacting the representativeness of the samples. Although the study results suggest relative balance across dimensions, it is worth noting that clinical pharmacists with Doctoral degrees were underrepresented in the sample. Therefore, future research should consider quota sampling based on the demographic characteristics of clinical pharmacists to enhance sample representativeness and ensure a more comprehensive evaluation of the population. Secondly, due to time constraints, it was a cross-sectional survey, so the retest reliability cannot be checked. Thus, further longitudinal studies are warranted to test their measurement properties.
5 Conclusion
This study developed and validated an instrument (PCBS-CH) for measuring the pharmaceutical care barriers in hospitals in China. This instrument was shown to have good reliability and construct validity. It provided the policymakers and hospital administrators with an applicable and reliable tool for finding the direction for improving the current pharmaceutical care system. This instrument may be suitable for other settings of pharmaceutical care in China, such as community pharmacies or primary healthcare institutions, for which validations and adaptions are needed.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
LC: Conception or design of the work, Data analysis and interpretation, Drafting the article, Critical revision of the article. NY: Data collection, Data analysis and interpretation, Critical revision of the article. YH: Data collection. XX: Conception or design of the work. All authors contributed to the article and approved the submitted version.
Funding
The study was supported by a grant from the National Natural Science Foundation of China (Grant No. 72004230).
Acknowledgments
The authors thank the expert panel for valuable advice related to study design, as well as the pharmacists and students who participated in the survey. This paper would not be accomplished without their efforts.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1194901/full#supplementary-material
References
Alamer, A., and Marsh, H. (2022). Exploratory structural equation modeling in second language research an applied example using the dualistic model of passion. Stud. Second Lang. Acquis. 44 (5), 1477–1500. doi:10.1017/s0272263121000863
Bacon, D. R., Sauer, P. L., Young, M. J. E., and Measurement, P. (1995). Composite reliability in structural equations modeling. Educ. Psychol. Meas. 55 (3), 394–406. doi:10.1177/0013164495055003003
Batistelli Vignola, R. C., and Tucci, A. M. (2014). Adaptation and validation of the depression, anxiety and stress scale (DASS) to Brazilian Portuguese. J. Affect. Disord. 155, 104–109. doi:10.1016/j.jad.2013.10.031
Beaton, D., Bombardier, C., Guillemin, F., and Ferraz, M. (2002). Recommendations for the cross-cultural adaptation of health status measures. Keele, England: Keele University.
Bentler, P. M., and Chou, C.-P. J. S. m. (1987). Practical issues in structural modeling. Sociol. Methods Res. 16 (1), 78–117. doi:10.1177/0049124187016001004
Culverhouse, S. E., and Wohlmuth, H. (2012). Factors affecting pharmacists' recommendation of complementary medicines - a qualitative pilot study of Australian pharmacists. BMC Complement. Altern. Med. 12, 183. doi:10.1186/1472-6882-12-183
da Costa, F. A., Van Mil, J. F., and Alvarez-Risco, A. (2019). The pharmacist guide to implementing pharmaceutical care. Berlin, Germany: Springer.
Dash, G., and Paul, J. (2021). CB-SEM vs PLS-SEM methods for research in social sciences and technology forecasting. Technol. Forecast. Soc. Change 173, 121092. doi:10.1016/j.techfore.2021.121092
Dong, H., and Wang, J. (2013). Investigation the status of the pharmacy technicians’ cognition on pharmaceutical care in county hospitals in Xinjiang. Mod. Med. Health 29 (17), 2606–2607. doi:10.3969/j.issn.1009-5519.2013.17.023
El Hajj, M. S., Al-Saeed, H. S., and Khaja, M. (2016). Qatar pharmacists' understanding, attitudes, practice and perceived barriers related to providing pharmaceutical care. Int. J. Clin. Pharm. 38 (2), 330–343. doi:10.1007/s11096-016-0246-0
Elayeh, E., Akour, A., Almadaeen, S., AlQhewii, T., and Basheti, I. A. (2017). Practice of pharmaceutical care in community pharmacies in Jordan. Trop. J. Pharm. Res. 16 (2), 463–470. doi:10.4314/tjpr.v16i2.27
Elsadig, H., Weiss, M., Scott, J., and Laaksonen, R. (2017). Exploring the challenges for clinical pharmacists in Sudan. Int. J. Clin. Pharm. 39 (5), 1047–1054. doi:10.1007/s11096-017-0521-8
Fabrigar, L. R., Wegener, D. T., MacCallum, R. C., and Strahan, E. J. (1999). Evaluating the use of exploratory factor analysis in psychological research. Psychol. Methods 4 (3), 272–299. doi:10.1037/1082-989x.4.3.272
Guan, X., Tian, Y., Song, J., Zhu, D., and Shi, L. (2019). Effect of physicians' knowledge on antibiotics rational use in China's county hospitals. Soc. Sci. Med. 224, 149–155. doi:10.1016/j.socscimed.2019.01.049
Guo, X., Yao, D., Liu, J., Huang, Y., Wang, Y., and Yao, W. (2020). The current status of pharmaceutical care provision in tertiary hospitals: Results of a cross-sectional survey in China. BMC Health Serv. Res. 20 (1), 518. doi:10.1186/s12913-020-05371-7
Hepler, C. D., and Strand, L. M. (1990). Opportunities and responsibilities in pharmaceutical care. Am. J. Hosp. Pharm. 47 (3), 533–543. doi:10.1093/ajhp/47.3.533
Katoue, M. G., Awad, A. I., Schwinghammer, T. L., and Kombian, S. B. (2014). Pharmaceutical care in Kuwait: Hospital pharmacists' perspectives. Int. J. Clin. Pharm. 36 (6), 1170–1178. doi:10.1007/s11096-014-0013-z
Kopciuch, D., Paczkowska, A., Zaprutko, T., Ratajczak, P., Nowakowska, E., and Kus, K. (2021). A survey of pharmacists' knowledge, attitudes and barriers in pharmaceutical care concept in Poland. BMC Med. Educ. 21 (1), 458. doi:10.1186/s12909-021-02891-6
Li, J., and Li, Z. (2018). Differences and similarities in clinical pharmacy practice in China and the United States: A narrative review. Eur. J. Hosp. Pharm. 25 (1), 2–5. doi:10.1136/ejhpharm-2016-001195
Lix, L. M., Keselman, J. C., and Keselman, H. J. (1996). Consequences of assumption violations revisited: A quantitative review of alternatives to the one-way analysis of variance F test. Rev. Educ. Res. 66 (4), 579–619. doi:10.2307/1170654
Lounsbery, J. L., Green, C. G., Bennett, M. S., and Pedersen, C. A. (2009). Evaluation of pharmacists' barriers to the implementation of medication therapy management services. J. Am. Pharm. Assoc. 49 (1), 51–58. doi:10.1331/JAPhA.2009.017158
Mao, W., Vu, H., Xie, Z., Chen, W., and Tang, S. J. P. O. (2015). Systematic review on irrational use of medicines in China and Vietnam. PLoS One 10 (3), e0117710. doi:10.1371/journal.pone.0117710
Mehralian, G., Rangchian, M., Javadi, A., and Peiravian, F. (2014). Investigation on barriers to pharmaceutical care in community pharmacies: A structural equation model. Int. J. Clin. Pharm. 36 (5), 1087–1094. doi:10.1007/s11096-014-9998-6
Ngorsuraches, S., and Li, S. C. (2006). Thai pharmacists' understanding, attitudes, and perceived barriers related to providing pharmaceutical care. Am. J. Health-System Pharm. 63 (21), 2144–2150. doi:10.2146/ajhp060054
Okonta, J. M., Okonta, E. O., and Ofoegbu, T. C. (2012). Barriers to implementation of pharmaceutical care by pharmacists in nsukka and enugu metropolis of enugu state. J. basic Clin. Pharm. 3 (2), 295–298. doi:10.4103/0976-0105.103823
Omar, N. E., Elazzazy, S., Abdallah, O., Nashwan, A. J., Eltorki, Y., Afifi, H. M., et al. (2020). Perceptions and expectations of health care providers towards clinical pharmacy services at a tertiary cancer centre in Qatar. J. Oncol. Pharm. Pract. 26 (5), 1086–1096. doi:10.1177/1078155219882076
Onozato, T., Francisca dos Santos Cruz, C., Milhome da Costa Farre, A. G., Silvestre, C. C., de Oliveira Santos Silva, R., Araujo dos Santos Júnior, G., et al. (2020). Factors influencing the implementation of clinical pharmacy services for hospitalized patients: A mixed-methods systematic review. Res. Soc. Adm. Pharm. 16 (4), 437–449. doi:10.1016/j.sapharm.2019.06.018
Park, R. (2007). Obstacles to the transformation of the current hospital clinical pharmaceutical care. Mod. Med. Health 14, 2200–2201. doi:10.3969/j.issn.1009-5519.2007.14.141
Peill, J. M., Trinci, K. E., Kettner, H., Mertens, L. J., Roseman, L., Timmermann, C., et al. (2022). Validation of the psychological insight scale: A new scale to assess psychological insight following a psychedelic experience. J. Psychopharmacol. 36 (1), 31–45. doi:10.1177/02698811211066709
Penm, J., Moles, R., Wang, H., Li, Y., and Chaar, B. (2014). Factors affecting the implementation of clinical pharmacy services in China. Qual. Health Res. 24 (3), 345–356. doi:10.1177/1049732314523680
Scahill, S., Harrison, J., and Sheridan, J. (2009). The ABC of New Zealand's ten year vision for pharmacists: Awareness, barriers and consultation. Int. J. Pharm. Pract. 17 (3), 135–142. doi:10.1211/ijpp.17.03.0003
Sun, Q., Santoro, M. A., Meng, Q., Liu, C., and Eggleston, K. J. H. A. (2008). Pharmaceutical policy in China. Health Aff. (Millwood) 27 (4), 1042–1050. doi:10.1377/hlthaff.27.4.1042
Van Mil, J., De Boer, W., and Tromp, T. (2001). European barriers to the implementation of pharmaceutical care. Int. J. Pharm. Pract. 9 (3), 163–168. doi:10.1111/j.2042-7174.2001.tb01044.x
Wang, J., Wang, P., Wang, X., Zheng, Y., and Xiao, Y. (2014). Use and prescription of antibiotics in primary health care settings in China. JAMA Intern Med. 174 (12), 1914–1920. doi:10.1001/jamainternmed.2014.5214
Watson, D., Clark, L. A., and Tellegen, A. (1988). Development and validation of brief measures of positive and negative affect - the panas scales. J. Personality Soc. Psychol. 54 (6), 1063–1070. doi:10.1037/0022-3514.54.6.1063
Wen, X., Cui, Y., and Han, L. (2006). Thinking about the factors restricting the development of clinical pharmaceutical care in primary hospitals. Chin. Community Physicians Compr. Ed. 23, 152.
Xi, X., Yao, D., Huang, Y., Wang, X., Wang, Y., and Yao, W. (2017). Research on the current status and issues of clinical pharmaceutical services in tertiary hospitals in China (Part 1): Introduction and analysis of basic conditions for clinical pharmaceutical services. Chin. Pharm. J. 52 (19), 1746–1752. doi:10.11669/cpj.2017.19.019
Yao, D., Xi, X., Huang, Y., Hu, H., Hu, Y., Wang, Y., et al. (2017). A national survey of clinical pharmacy services in county hospitals in China. PLoS One 12 (11), e0188354. doi:10.1371/journal.pone.0188354
Zheng, Sq., Yang, L., Zhou, Px., Li, Hb., Liu, F., and Zhao, Rs. (2021). Recommendations and guidance for providing pharmaceutical care services during COVID-19 pandemic: A China perspective. Res. Soc. Adm. Pharm. 17 (1), 1819–1824. doi:10.1016/j.sapharm.2020.03.012
Zhang, C., Xiao, J., Yu, Z., Sun, S., and Liu, D. (2021). Cancer pain management and the roles of pharmacists in China. Int. J. Clin. Pharm. 43 (2), 383–385. doi:10.1007/s11096-021-01230-5
Keywords: barrier, Chinese hospital, clinical pharmacist, pharmaceutical care, scale development
Citation: Chen L, Yang N, Huang Y and Xi X (2023) Development and validation of pharmaceutical care barriers scale in Chinese hospitals: a cross-sectional survey. Front. Pharmacol. 14:1194901. doi: 10.3389/fphar.2023.1194901
Received: 27 March 2023; Accepted: 23 June 2023;
Published: 13 July 2023.
Edited by:
Hao Li, Shanghai Jiao Tong University, ChinaReviewed by:
Yumao Zhang, Sun Yat-sen University, ChinaZaiwei Song, Peking University Third Hospital, China
Copyright © 2023 Chen, Yang, Huang and Xi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoyu Xi, eGl4eUBjcHUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Liangjiang Chen
Liangjiang Chen Nan Yang
Nan Yang Yuankai Huang1
Yuankai Huang1 Xiaoyu Xi
Xiaoyu Xi