
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 13 July 2023
Sec. Drugs Outcomes Research and Policies
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1194537
 Kun-Pin Hsieh1,2
Kun-Pin Hsieh1,2 Ru-Yu Huang3
Ru-Yu Huang3 Yi-Hsin Yang1,3
Yi-Hsin Yang1,3 Pei-Shan Ho4,5,6
Pei-Shan Ho4,5,6 Kuang-Peng Chen7
Kuang-Peng Chen7 Chun-Liong Tung7
Chun-Liong Tung7 Ya-Lan Chu8*
Ya-Lan Chu8* Jui-Hsiu Tsai7,9*
Jui-Hsiu Tsai7,9*Background: Multimorbidity and polypharmacy increase the risk of hospitalization in older adults receiving potentially inappropriate medication (PIM). The current study compared the ability of PIM-Taiwan, PRISCUS, and Beers criteria to predict 90-day rehospitalization in older patients with and without PIM.
Methods: The retrospective cohort study used Taiwan’s Longitudinal Health Insurance Database to retrieve quarterly information about prescribed medication for adults aged ≥65 years hospitalized between 2001 and 2018. We analyzed the association of PIM with 90-day rehospitalization using logistic regression.
Results: The study cohort included 206,058 older adults (mean age: 72.5 years). In the analysis, 133,201 (64.6%), 97,790 (47.5%), and 147,450 (71.6%), were identified as having PIM exposure in PIM-Taiwan, PRICUS, and Beers criteria, respectively. PIM-Taiwan criteria found exposure to PIM affecting the cardiovascular (adjusted OR [aOR] 1.37, 95% confidence interval [CI] = 1.32–1.41), gastrointestinal (aOR 1.26, 95% CI = 1.23–1.30), central nervous (aOR 1.11, 95% CI = 1.08–1.14), and respiratory (aOR 1.16, 95% CI = 1.12–1.20) systems significantly increased the risk of 90-day rehospitalization, after adjustment for covariates. In PRISCUS criteria, exposure to PIM affecting the respiratory (aOR 1.48, 95% CI = 1.41–1.56), central nervous (aOR 1.12, 95% CI = 1.09–1.15), and cardiovascular (aOR 1.20, 95% CI = 1.16–1.24) systems significantly increased the risk. In Beers criteria, exposure to PIM affecting the cardiovascular (aOR 1.37, 95% CI = 1.32–1.41), gastrointestinal (aOR 1.38, 95% CI = 1.35–1.42), central nervous (aOR 1.18, 95% CI = 1.15–1.21), endocrine (aOR 1.10, 95% CI = 1.06–1.15), and respiratory (aOR 1.09, 95% CI = 1.04–1.13) systems significantly increased the risk. Patients with 90-day rehospitalization had higher rates of the potentially harmful drug-drug interaction (DDI) pairs of serotonin syndrome (n = 19; 48.8%), QT prolongation (n = 4; 30.8%), extrapyramidal symptoms (EPS) (n = 102; 24.5%), and hypokalemia (n = 275; 20.1%).
Conclusion: Beers criteria was more efficient in predicting 90-day rehospitalization among older adults experiencing PIM in Taiwan than either PIM-Taiwan or PRISCUS. The risk of 90-day rehospitalization was associated with the potentially harmful DDI classes of serotonin syndrome, QT prolongation, EPS, and hypokalemia.
World population aging is growing rapidly in both developed and developing countries. In Taiwan, the proportion of the older adults increased from 7% in 1993 (an aging country) to 14% in 2018 (an aged country), and is predicted to reach 20% in 2025 (a super-aged country) (National Development Council, 2023). This accelerated aging brings a heavy burden of healthcare for older adults in Taiwan (Chen, 2010). In addition, multimorbidity and polypharmacy among older adults are associated with high rates of unplanned hospitalization, particularly for adverse drug reactions (ADRs) due to age-related alterations in pharmacokinetics, pharmacodynamics, and drug-disease interactions (Leendertse et al., 2008; Olivier et al., 2009; Hamilton et al., 2011; Dalleur et al., 2012). Therefore, adequate drug treatment is paramount to forestall preventable hospitalizations in older adults, particularly the frail and those with multiple morbidities.
Potentially inappropriate medication (PIM) use is not a rare event in older adults, and is associated in this population with ADRs, hospitalization, functional decline, and even death (Lau et al., 2005; Passarelli et al., 2005; Lin et al., 2008; Bradley et al., 2012). For this reason, many countries have built their own criteria systems for PIM, because national pharmacotherapeutic guidelines vary in terms of the specific drugs approved (Chang et al., 2014). The different criteria may lead to wide variations in reported PIM prevalence and their associated health-related outcomes (Chang et al., 2014). Of all PIM criteria the Beers criteria, first established in 1991, is the most widely used to detect PIM in older adults as an indicator of geriatric healthcare quality (Wenger and Shekelle, 2001; Pugh et al., 2006; Chang et al., 2011; Dimitrow et al., 2011; Corsonello et al., 2012). In Taiwan, the PIM-Taiwan, introduced in 2012, outlines the relevant and country-specific PIM criteria (Chang et al., 2012; Chang et al., 2019). The PIM-Taiwan criteria have proven their applicability in several cross-sectional studies among older Taiwanese adults (Chang et al., 2014; Chang et al., 2015; Chang et al., 2018). In comparison with the Beers criteria and PRISCUS criteria, PIM-Taiwan can detect a similar number of PIMs across different populations in Taiwan. PIM users had higher health resource utilization and higher costs of medications than non-PIM users (Chang et al., 2014; Chang et al., 2015). Therefore, PIM-Taiwan criteria can be an important tool to reduce PIM-related adverse events in older Taiwanese adults. In Europe, the PRISCUS criteria, based on expert knowledge developed in Germany, helps physicians make individualized therapeutic decisions for their patients (Holt et al., 2010; Fromm et al., 2013; Schubert et al., 2013). Most studies related to PIM criteria have focused on the prevalence of PIM in limited small-to-medium-sized samples, such as inpatient populations or nursing home residents (Reich et al., 2014; Garcia et al., 2015). However, few studies have assessed the association of PIM with health-related outcomes using nationwide samples (Mekonnen et al., 2021).
Based on National Health Insurance (NHI) claims data, the current study was conducted to estimate the prevalence and changes in PIM among older patients before and after a hospitalization as measured by PIM-Taiwan, PRISCUS, and Beers criteria. We sought to investigate the association of PIM via these criteria systems with the risk of 90-day rehospitalization in this population. Furthermore, we also analyzed the risk of hospitalization associated with different classes of potentially harmful drug-drug interactions (DDIs).
The retrospective cohort study was performed using claim-based data from the 2010 Longitudinal Generation Tracking Database (LGTD 2010), the Catastrophic Illness Registry Dataset (CIRD), the Taiwan Cancer Registry (TCR) database, and Multiple Causes of Death data from 2000 to 2018. Detailed descriptions of the aforementioned 3-dataset sample and study procedures have previously been published and the representativeness of LGTD and TCR has been validated (Chang et al., 2015; Chiang et al., 2015; Hu et al., 2016; Chiang et al., 2017; Center for Biomedical Resources of NHRI, 2023). The LHTG 2010 is a subset of the National Health Insurance Research Database (NHIRD) composed of 2 million randomly-sampled beneficiaries (nearly 10% of the total Taiwanese population) drawn in 2010. The NHIRD was developed and managed by Taiwan’s National Insurance program, which was introduced in 1995 and provides for approximately 99.9% of Taiwanese residents. The LGTD 2010 includes comprehensive claims data for reimbursements for ambulatory, inpatient, emergency, and Chinese medicine visits, and is used to gather information on prescriptions and comorbidities (Center for Biomedical Resources of NHRI; Chiang et al., 2017; Hu et al., 2016). The CIRD contains information about catastrophic illness in patients who suffered from at least one of 30 specific severe medical conditions such as cancer, chronic mental illness (schizophrenia, bipolar I disorder, major depressive disorder), hemodialysis, systemic autoimmune diseases, and stroke (Chang et al., 2015). The TCR provides archives of information on cancer diagnosis and additional Supplementary Material (Chang et al., 2015). The Multiple Causes of Death data provides information on the cause(s) and date of death.
We enrolled patients aged 65 years or older at the first hospitalization from 2001 to 2018 in Taiwan, excluding those with a history of concomitant cancer or catastrophic illness based on the CIRD and TCR records. Patients with at least one hospitalization recorded during the 2001–2018 study period were considered for the first hospitalization. We followed those patients from the first hospitalization until 90 days after discharge or until rehospitalization within this period.
The study outcome of 90-day rehospitalization was defined as one of the following events within 90 days after the first discharge: readmittance to the hospital, emergency department visit, hospitalization visa in the emergency department, or death.
We assessed medication use within 30 days before the first hospitalization (prehospitalization) and within 30 days after the first discharge (post-discharge). Postdischarge was defined as the 30 days following the discharge date, or the date of readmittance within 30 days after discharge, whichever came first. The medications used were classified as PIM or not PIM according to three criteria systems: the updated 2018 PIM-Taiwan criteria (Chang et al., 2018), the updated 2019 Beers criteria (By the American Geriatrics Society Beers Criteria® Update Expert Panel, 2019), and the entire PRISCUS criteria (Holt et al., 2010) (Supplementary Tables S1–S3) (Table 1). Exposure to PIM was considered if the prescribed drug was represented in any of the criteria, and PIM items were also counted. PIM was identified in the database via Anatomical Therapeutic Chemical code and further classified by human body systems, including cardiovascular, endocrine, gastrointestinal, genitourinary, musculoskeletal, central nervous, and respiratory systems; and the additional classifications (not used in all criteria systems) of sex hormones, anti-infective, and blood and blood-forming organs.
The DDI pairs were taken from those listed by McEvoy et al. (2017) and the National Institute of Health and Care Excellence (NICE) clinical guidelines (Chang et al., 2015). The DDI pairs in Micromedex® provided information on the severity of contraindication; UptoDate® provided DDI pairs in categories in X (Shariff et al., 2021). The DDI pairs were classified by human body systems based on the potential DDI-induced ADRs, including the cardiovascular system (bradycardia, QT prolongation, ventricular arrhythmias, and hypotensive), central nervous system (hypotensive, extrapyramidal symptoms [EPS], and central nervous system toxicity), blood (bleeding, hypoglycemia, hyperkalemia, hypokalemia, thrombotic events, and change in drug concentrations), gastrointestinal system (gastrointestinal lesions), and musculoskeletal system (myopathy). The patients’ DDI pairs were determined for the prehospitalization and post-discharge periods. The number of DDI pairs was also identified.
Patients’ demographic (age and gender), socioeconomic (residential areas of NHI division, monthly income of NHI registration), Charlson Comorbidity Index (CCI), and type of comorbidity were retrieved at the first hospitalization. The insurance income ranking was used as a proxy for the monthly income level. A patient had a high or low monthly income if they received New Taiwan dollars (NT$) < 22,000 or NT$ ≥ 22,000 monthly, respectively (in January 2023, US$ 1 = approximately NT$ 30). Patients’ comorbidities were identified within the year preceding the first discharge to calculate the CCI score (Charlson et al., 1987) and further categorized into four groups (i.e., 0, 1, 2, and ≥3 comorbidities).
To determine polypharmacy, we selected only those drugs supplied for more than 28 days and prescribed within 30 days of the first discharge. Polypharmacy was defined as the concomitant use of 5 or more drugs (Lu et al., 2015).
Logistic regression was used to evaluate the odds ratios (ORs) of clinical outcomes in the patients prescribed PIM after discharge, after adjusting for the covariates age at hospitalization, gender, geographic region, income, CCI score, admitted year, DDI (post-discharge), and polypharmacy. The results were reported as adjusted odds ratios (aORs) with 95% confidence intervals (CIs). Data management, computation, and analysis were performed using SAS software, version 9.4 (SAS Institute, Inc., Cary, NC, United States).
As shown in Table 1, a total of 206,058 older patients (mean age: 72.5 years) were enrolled in this study. Of these, 50.2% were female, 33.4% lived in the Taipei district, and 75.3% had high income. In the analysis, PIM-Taiwan, Beers, and PRICUS criteria identified 133,201 (64.6%), 147,450 (71.6%), and 97,790 (47.5%), respectively, as having PIM exposure. The following percentage of patients had a CCI score of 0: 48.6% (in PIM-Taiwan), 47.9% (in Beers), and 46.9% (in PRISCUS). However, those assessed via PRISCUS criteria had a higher proportion of CCI scores of 2 and ≥3 (13.5% and 10.1%, respectively) than those assessed via PIM-Taiwan (12.8% and 9.3%, respectively) or Beers criteria (12.9% and 9.5%, respectively). In all three cohorts, more than 10% had the comorbidities of cerebrovascular disease, chronic pulmonary disease, peptic ulcer disease, and diabetes without chronic complications. In all three cohorts, more than one-fourth of the patients had DDIs. PIM-Taiwan, Beers, and PRICUS criteria identified polypharmacy in 32.1%, 32.5%, and 36.7% of the cohort, respectively.
As shown in Figure 1, all three PIM criteria showed a lower percentage of patients with PIM exposure at post-discharge than before hospitalization. Compared to before hospitalization, fewer patients post-discharge had PIM exposure in all categories (i.e., ≥10, 6–10, or 1-5 items). As shown in Table 2, PIM-Taiwan criteria found the greatest rates of PIM exposure in all patients after their hospitalization in the central nervous (46.2%), musculoskeletal (45.6%), and gastrointestinal (23.3%) systems. Beers criteria found similar risk for PIM exposure to the musculoskeletal (63.4%), central nervous (33.6%), and gastrointestinal (21.8%) systems. In the PRISCUS criteria, the central nervous (47.0%), cardiovascular (43.2%), and musculoskeletal (27.6%) systems had the highest PIM exposure.
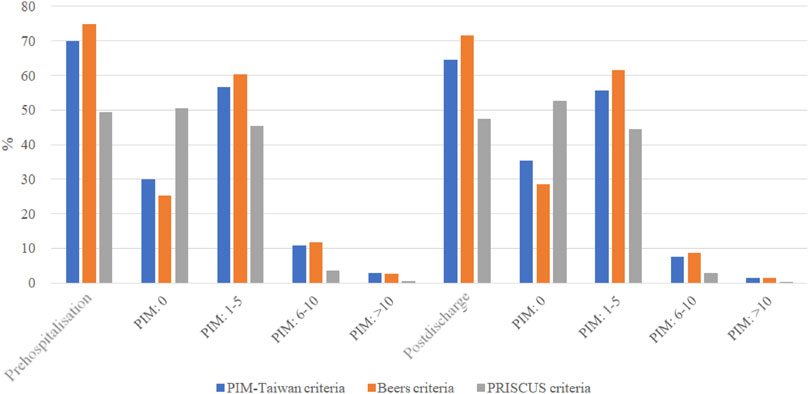
FIGURE 1. The distributions of potentially inappropriate medication at the prehospitalization and those at the postdischarge. PIM, potentially inappropriate medication; Prehospitalization, within 30 days before the first hospitalization; Postdischarge , within 30 days after the first discharge.
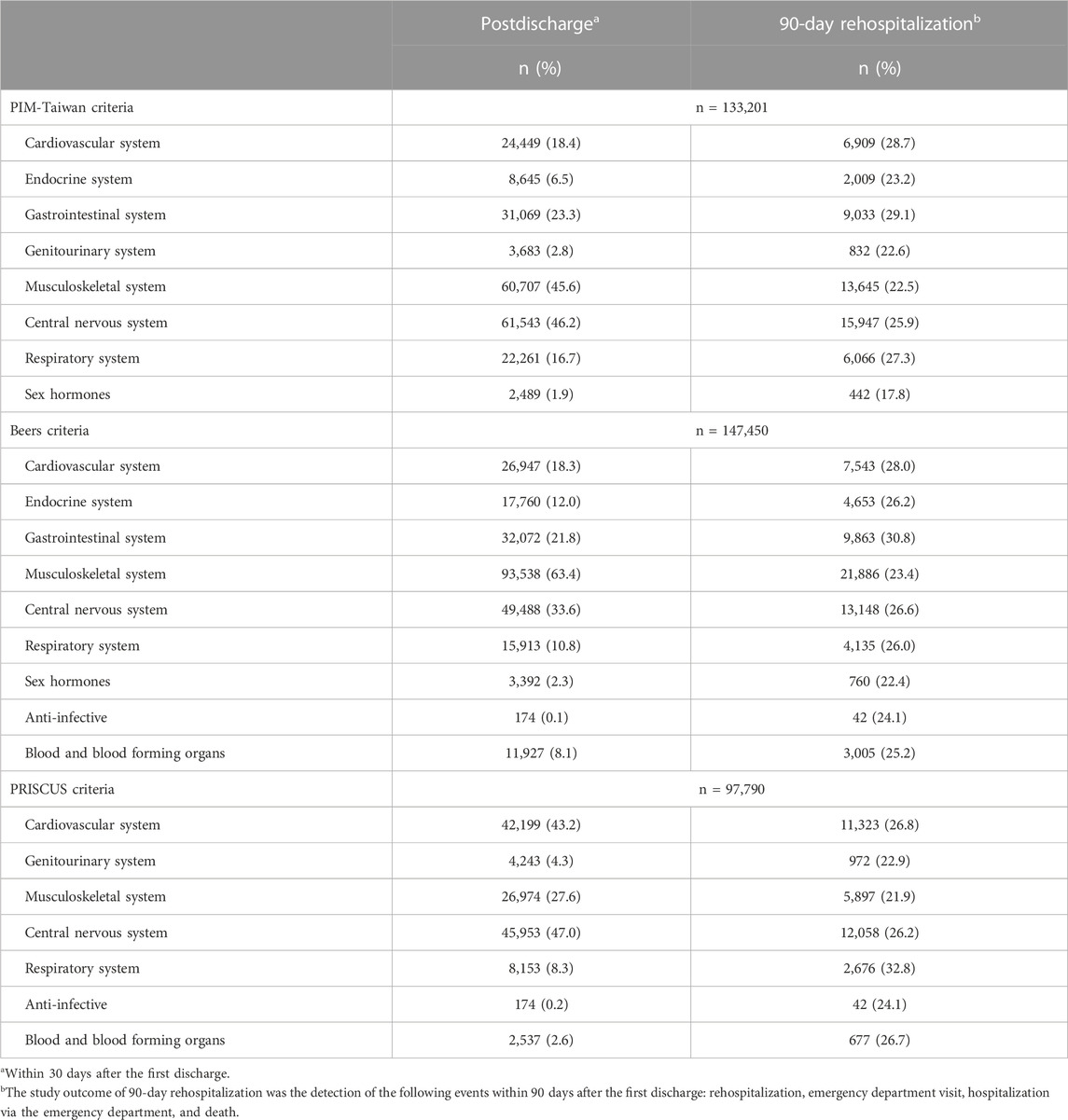
TABLE 2. Potentially inappropriate medication (PIM) exposure between all patients at postdischarge and those with 90-day rehospitalization at postdischarge.
The older patients with the greatest risk of 90-day rehospitalization were those exposed to PIM affecting the gastrointestinal (29.1%), cardiovascular (28.7%), and respiratory (27.3%) systems, according to PIM-Taiwan criteria; for Beers criteria, it was the gastrointestinal (30.8%), cardiovascular (28.0%), and central nervous (26.6%) systems; PRISCUS criteria found the greatest risk of 90-day rehospitalization in those with exposure to PIM affecting the respiratory (32.8%) and cardiovascular (26.8%) system, and the blood and blood-forming organs (26.7%) (Table 2).
Table 3 provides the risk of 90-day rehospitalization in older patients according to each PIM criteria. According to PIM-Taiwan criteria, exposure to PIM affecting the cardiovascular (aOR 1.37, 95% CI = 1.32–1.41), gastrointestinal (aOR 1.26, 95% CI = 1.23–1.30), central nervous (aOR 1.11, 95% CI = 1.08–1.14), and respiratory (aOR 1.16, 95% CI = 1.12–1.20) systems was significantly associated with increased risk of having 90-day rehospitalization, after adjustment for covariates; conversely, exposure to PIM affecting the sex hormones (aOR 0.74, 95% CI = 0.66–0.82), genitourinary (aOR 0.82, 95% CI = 0.76–0.89), endocrine (aOR 0.91, 95% CI = 0.86–0.96), and musculoskeletal (aOR 0.96, 95% CI = 0.93–0.98) systems was significantly associated with decreased risk. In Beers criteria, exposure to PIM affecting the cardiovascular (aOR 1.37, 95% CI = 1.32–1.41), gastrointestinal (aOR 1.38, 95% CI = 1.35–1.42), central nervous (aOR 1.18, 95% CI = 1.15–1.21), endocrine (aOR 1.10, 95% CI = 1.06–1.15), and respiratory (aOR 1.09, 95% CI = 1.04–1.13) systems was significantly positively associated with the risk of readmission, after adjustment for covariates. In PRISCUS criteria, exposure to PIM affecting the respiratory (aOR 1.48, 95% CI = 1.41–1.56), central nervous (aOR 1.12, 95% CI = 1.09–1.15), and cardiovascular (aOR 1.20, 95% CI = 1.16–1.24) systems was significantly positively associated with 90-day readmission, after adjustment for covariates; conversely, exposure to PIM affecting the genitourinary (aOR 0.82, 95% CI = 0.76–0.88) and musculoskeletal (aOR 0.86, 95% CI = 0.83–0.89) systems was significantly negatively associated with readmission.
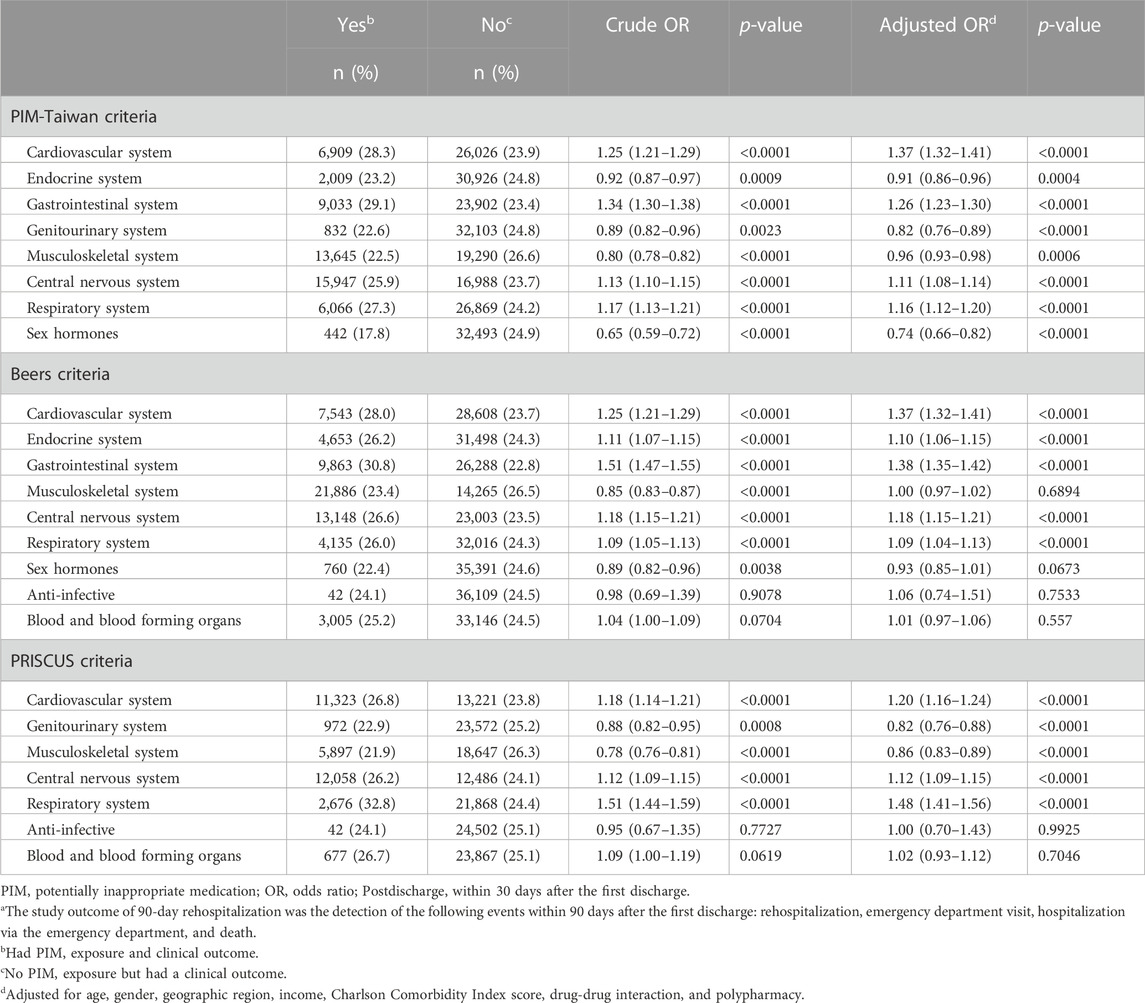
TABLE 3. Risk of 90-day rehospitalizationa in older patients due to the systems of three PIM criteria after the first discharge.
Table 4 shows the patterns of DDIs among older adults at prehospitalization, all patients post-discharge, and at post-discharge in those with 90-day rehospitalization. The prehospitalized older adults had a slightly higher percentage of DDIs than those at post-discharge (21.6% vs. 21.4%). Subsequently, it was found that the rate of 90-day rehospitalization among individuals with DDIs at post-discharge was 14.2%. Notably, patients with three or more DDI pairs at post-discharge had a higher 90-day rehospitalization rate of 18.3% compared to those with one or two DDI pairs. The potentially harmful DDI pairs occurring at more than 1% post-discharge were those affecting bleeding (10.7%), hypotension (8.2%), myopathy (3.7%), bradycardia (1.7%), change in drug concentrations (1.6%), and hyperkalemia (1.5%) (Table 4). The most frequent types of potentially harmful DDI pairs in patients with 90-day rehospitalization were those affecting bleeding (n = 2,959; 13.4%), hypotension (n = 2,645; 15.7%), myopathy (n = 1,031; 13.6%), bradycardia (n = 539; 15.5%), change in drug concentrations (n = 556; 16.4%), and hyperkalemia (n = 546; 18.3%). However, patients with 90-day rehospitalization had higher rates of the potentially harmful DDI pairs of serotonin syndrome (n = 19; 48.8%), QT prolongation (n = 4; 30.8%), EPS (n = 102; 24.5%), and hypokalemia (n = 275; 20.1%) (Table 4).
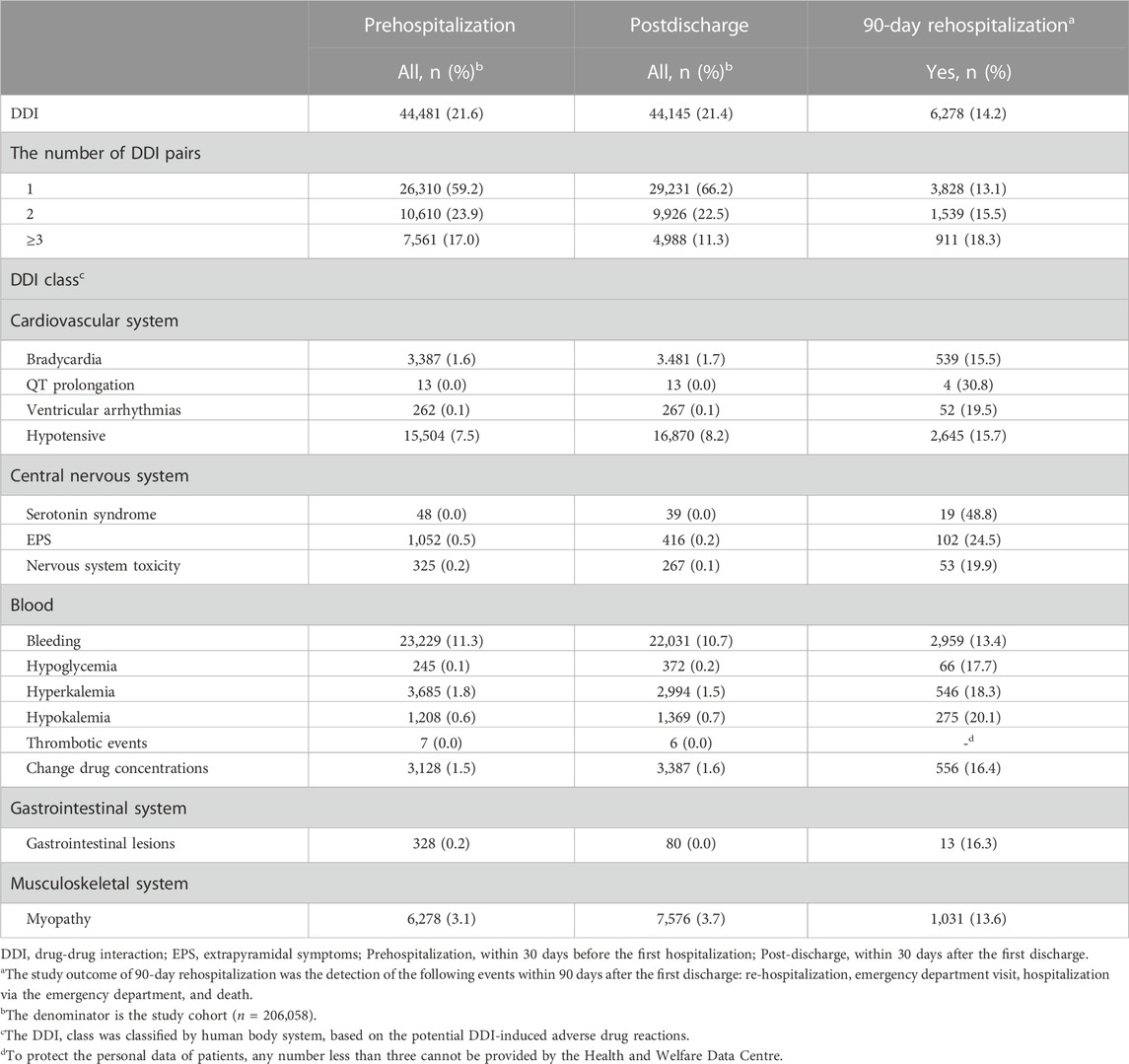
TABLE 4. Drug-drug interaction patterns among older adults at prehospitalization, postdischarge, and those with 90-day rehospitalizationa after discharge.
To our knowledge, this is the first nationally representative evaluation of the association between exposure to PIM and health-related outcomes among older adults in Taiwan. We compared exposure to PIM and the risk of 90-day rehospitalization in this population using the PIM-Taiwan, PRISCUS, and Beers criteria systems. The PIM criteria identified a high percentage of older adults who had been prescribed at least one PIM at the first hospitalization; meanwhile, the greatest incidence of PIM was found by Beers, then PIM-Taiwan, and then PRISCUS criteria. The findings proved that Beers criteria are more efficient in predicting 90-day rehospitalization among older adults experiencing PIM in Taiwan than either PIM-Taiwan or PRISCUS criteria.
This retrospective cohort study, examining 206,058 older adults at the first hospitalization, found varying PIM rates from 48% to 72% using the PRICUS, PIM-Taiwan, and Beers criteria systems. Our findings are similar to those of previous studies (Chang et al., 2014; Storms et al., 2017). Approximately one-half of the older patients in our study had a CCI score of 0 at the first hospitalization; however, those assessed via PRISCUS criteria had a higher proportion of CCI scores of 2 and ≥3 than those evaluated using the other two criteria. The data showed that about one-half of our older participants felt well in physical condition; the results implied that the PRICUS criteria had greater sensitivity than the other two criteria in identifying participants with poor physical condition (Fromm et al., 2013; Chang et al., 2019). In addition, over one-third of our patients had polypharmacy (taking >5 medications) at the first hospitalization. Our finding was similar to that of the other study in Australia (36%) (Lu et al., 2015).
As was shown in Figure 1, older adults in Taiwan had fewer PIM prescribed after their first hospital discharge. According to Table 2, older adults in Taiwan after their first discharge had PIM exposure most commonly in the musculoskeletal, central nervous, gastrointestinal, and cardiovascular systems. The result of high PIM exposure in the musculoskeletal and gastrointestinal systems is not consistent with the results of other studies (Ye et al., 2016; Saboor et al., 2019). This discrepancy may be related to differences in medication prescribing habits in different regions. Furthermore, we initially assessed the association of PIM with 90-day rehospitalization among older adults in Taiwan. Table 2 shows that the older patients with 90-day rehospitalization still had PIM exposure in the musculoskeletal, central nervous, gastrointestinal, and cardiovascular systems after their hospitalization. We further evaluated the association of exposure to PIM with the risk of 90-day rehospitalization. According to the Beers criteria, exposure to PIM affecting the cardiovascular, gastrointestinal, central nervous, endocrine, and respiratory systems was positively associated with an increased risk of 90-day rehospitalization among older adults in Taiwan; hence, lack of PIM exposure was negatively associated with the risk. In PIM-Taiwan, PIM exposure affecting the cardiovascular, gastrointestinal, central nervous, and respiratory systems was positively associated with the risk; conversely, exposure to PIM affecting the sex hormones, genitourinary, endocrine, and musculoskeletal systems was negatively associated with the risk. In PRISCUS criteria, exposure to PIM affecting the respiratory, central nervous, and cardiovascular systems was positively associated with the risk of rehospitalization; conversely, exposed to PIM affecting the genitourinary and musculoskeletal systems had a negative association. Our findings tended to corroborate those of most studies. In a systematic review and meta-analysis including 63 studies, the pooled estimates for PIM and all-cause hospitalization were not statistically significant (aOR 1.11, 95% CI 0.76–1.63; adjusted Hazard Ratio 1.02, 95% CI 0.89–1.18) (Garcia et al., 2015). In brief, Beers criteria was proven more efficient in predicting 90-day rehospitalization among older adults experiencing PIM in Taiwan, compared to PIM-Taiwan and PRISCUS criteria.
Approximately 10%–30% of all hospitalizations in older adults are due to ADRs, of which almost 50% are potentially preventable (Leendertse et al., 2008; Salvi et al., 2012; Parameswaran Nair et al., 2016; Oscanoa et al., 2017). PIM criteria can help physicians and pharmacologists detect DDIs early and reduce DDI-induced ADRs, even though PIM does not seem to be an important cause of ADRs in older adults (Laroche et al., 2007). The DDI pairs have been classified according to major human body systems based on the potential for DDI-induced ADRs. Although the entire cohort of patients had a higher rate of DDIs than those with 90-day rehospitalization after the first discharge, the rate of ≥3 DDI pairs occurring in those with 90-day rehospitalization obviously increased from 11.3% in the whole cohort to 18.3% in those rehospitalized. This result showed that the risk of 90-day rehospitalization in older adults may be associated with the number of DDI pairs occurring, but not the number of those who experienced a DDI. We further analyzed the DDI classes in our study; the entire cohort of patients after discharge and those who experienced 90-day rehospitalization had the same 5 most common classes of DDI: bleeding, hypotension, myopathy, bradycardia, and change in drug concentrations. A greater rate of 90-day rehospitalization was associated with the potentially harmful DDI classes of serotonin syndrome, QT prolongation, EPS, and hypokalemia, as shown in Table 4. Notably, physicians and pharmacists should be well aware of the association of the above drug-related adverse effects with rehospitalization in older adults, despite their low incidence.
The primary strength of the current study was its use of a large population-based cohort that enabled analysis of the association between three PIM criteria and rehospitalization among older adults experiencing PIM. The findings may help prevent medication-related hospitalization in older adults, particularly in ethnic Chinese older adults. Furthermore, this study captured almost all prescribed medications reimbursed by the NHI, meaning our database contains detailed nationwide information on medications, including the use of drug combinations.
Of course, we acknowledge several possible limitations in this study. First, it is impossible to clearly demonstrate causality between a high incidence of PIMs and the outcome measure of rehospitalization based on this retrospective data. Second, patients’ adherence to medications could not be evaluated, due to the limitations of the prescription claims databases. However, medication nonadherence would most likely have resulted in a nondifferentiated exposure misclassification, leading to possible underestimation of PIM prevalence and rehospitalization. Third, the use of some drugs, such as over-the-counter medications, alternative remedies, and herbal supplements, could not be included, because these drugs are not covered by the NHI. Fourth, three different lists were used in three different cohort of older adults. This method may have some disadvantages such as case-to-case variance bias. Although those cohorts had minor variations, we considered these factors as variables to be adjusted in the regression analysis. Finally, we did not have access to other potentially confounding factors for hospitalization among older patients such as disease severity, biochemistry data, and patient habits, such as alcohol use and tobacco consumption.
This study made several important findings. First, all three instruments found high PIM rates in older adults in Taiwan, but the greatest incidence of PIM was found by Beers, then PIM-Taiwan, and then PRISCUS criteria. Second, the 5 common potential DDI-induced adverse effects in older adults in Taiwan were bleeding, hypotension, myopathy, bradycardia, and change in drug concentrations. Despite their low incidence, the potential DDI-induced adverse effects of serotonin syndrome, QT prolongation, EPS, and hypokalemia were associated with high rates of rehospitalization among older adults. Finally, Beers criteria proved most efficient in predicting 90-day rehospitalization in older adults with PIM in Taiwan, compared to PIM-Taiwan and PRISCUS criteria. The current study findings permit several recommendations to be made. Physicians and pharmacists should seek to prevent and carefully manage ADR in older adults via the PIM criteria. In particular, they should look for PIM particularly in the DDI classes of serotonin syndrome, QT prolongation, EPS, and hypokalemia, which have the greatest potential for serious adverse events, despite their low incidence.
The original contributions presented in the study are included in the article/Supplementary Material, data are available from the National Health Insurance Research Database (NHIRD) published by Taiwan National Health Insurance (NHI) Bureau. Due to legal restrictions imposed by the government of Taiwan in relation to the “Personal Information Protection Act,” data cannot be made publicly available. Requests for data may be sent as a formal proposal to the NHIRD (http://nhird.nhri.org.tw).
The study was performed in accordance with the Declaration of Helsinki. All methods were performed in accordance with the relevant guidelines and regulations. Ethical approval was obtained from the Ethics Committee of the Dalin Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Chia-Yi, Taiwan (IRB No. B10901009) and Kaohsiung Medical University, Kaohsiung, Taiwan (IRB No. KMUHIRB-E(I)-20190112). The need for informed consent was waived because we used de-identified medical information from the NHIRD.
K-PH, Y-LC, and J-HT conceptualized and designed the study, conducted the statistical analyses, drafted the initial article, and reviewed and revised the article. K-PH and R-YH supervised the data collection. R-YH, Y-HY, and P-SH assisted with the statistical analyses and interpretation of data. All authors contributed to the article and approved the submitted version.
This study was supported by the Dalin Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation Chia-Yi, Taiwan (Grants No. DTCRD109-I-19) and Kaohsiung Medical University, Kaohsiung, Taiwan (Grants No. KMU-M109026).
We would like to thank Miss Mei-Hsin Ho for her assistance. The authors thank the Center for Medical Informatics and Statistics of Kaohsiung Medical University for providing administrative support. All authors acknowledge the Taiwan NHRI and BNHI for the use of data. The interpretation and conclusions described in this paper do not represent those of the NHRI and BNHI.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1194537/full#supplementary-material
Bradley, M. C., Fahey, T., Cahir, C., Bennett, K., O’Reilly, D., Parsons, C., et al. (2012). Potentially inappropriate prescribing and cost outcomes for older people: Across-sectional study using the Northern Ireland Enhanced Prescribing Database. Eur. J. Clin. Pharmacol. 68, 1425–1433. doi:10.1007/s00228-012-1249-y
By the American Geriatrics Society Beers Criteria® Update Expert Panel (2019). American geriatrics society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J. Am. Geriatr. Soc. 67, 674–694. doi:10.1111/jgs.15767
Center for Biomedical Resources of NHRI (2023). National health insurance research database, taiwan. Available at: https://nhird.nhri.org.tw/en/Data_Subsets.html (Accessed January 20, 2023).
Chang, C. B., Chen, J. H., Wen, C. J., Kuo, H. K., Lu, I. S., Chiu, L. S., et al. (2011). Potentially inappropriate medications in geriatric outpatients with polypharmacy: Application of six sets of published explicit criteria. Br. J. Clin. Pharmacol. 72, 482–489. doi:10.1111/j.1365-2125.2011.04010.x
Chang, C. B., Lai, H. Y., Hwang, S. J., Yang, S. Y., Wu, R. S., Chang, L. Y., et al. (2019). The updated PIM-taiwan criteria: A list of potentially inappropriate medications in older people. Ther. Adv. Chronic Dis. 10, 2040622319879602–2040622319879621. doi:10.1177/2040622319879602
Chang, C. B., Lai, H. Y., Hwang, S. J., Yang, S. Y., Wu, R. S., Liu, H. C., et al. (2018). The application of updating PIM-Taiwan criteria in clinic-visiting older patients with polypharmacy. Ther. Adv. Drug. Saf. 9, 699–709. doi:10.1177/2042098618804493
Chang, C. B., Lai, H. Y., Yang, S. Y., Wu, R. S., Liu, H. C., Hsu, H. Y., et al. (2014). Patient- and clinic visit-related factors associated with potentially inappropriate medication use among older home healthcare service recipients. PLoS One 9, e94350. doi:10.1371/journal.pone.0094350
Chang, C. B., Yang, S. Y., Lai, H. Y., Wu, R. S., Liu, H. C., Hsu, H. Y., et al. (2015). Application of three different sets of explicit criteria for assessing inappropriate prescribing in older patients: A nationwide prevalence study of ambulatory care visits in taiwan. BMJ open 5 (11), e008214. doi:10.1136/bmjopen-2015-008214
Chang, C. B., Yang, S. Y., Lai, H. Y., Wu, R. S., Liu, H. C., Hsu, H. Y., et al. (2012). Using published criteria to develop a list of potentially inappropriate medications for elderly patients in Taiwan. Pharmacoepidemiol. Drug Saf. 21, 1269–1279. doi:10.1002/pds.3274
Charlson, M. E., Pompei, P., Ales, K. L., and MacKenzie, C. R. (1987). A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 40, 373–383. doi:10.1016/0021-9681(87)90171-8
Chen, L. K. (2010). A new era of research on clinical gerontology and geriatrics in Asia. J. Clin. Gerontol. Geriatr. 1, 1. doi:10.1016/j.jcgg.2010.10.001
Cheng, C. L., Chien, H. C., Lee, C. H., Lin, S. J., and Yang, Y. H. (2015). Validity of in-hospital mortality data among patients with acute myocardial infarction or stroke in National Health Insurance Research Database in Taiwan. Int. J. Cardiol. 201, 96–101. doi:10.1016/j.ijcard.2015.07.075
Chiang, C. J., You, S. L., Chen, C. J., Yang, Y. W., Lo, W. C., and Lai, M. S. (2015). Quality assessment and improvement of nationwide cancer registration system in taiwan: A review. Jpn. J. Clin. Oncol. 45, 291–296. doi:10.1093/jjco/hyu211
Chiang, J. K., Lin, C. W., Wang, C. L., Koo, M., and Kao, Y. H. (2017). Cancer studies based on secondary data analysis of the taiwan's national health insurance research database: A computational text analysis and visualization study. Med. Baltim. 96, e6704. doi:10.1097/MD.0000000000006704
Corsonello, A., Onder, G., Abbatecola, A. M., Guffanti, E. E., Gareri, P., and Lattanzio, F. (2012). Explicit criteria for potentially inappropriate medications to reduce the risk of adverse drug reactions in elderly people: From Beers to STOPP/START criteria. Drug Saf. 35 (Suppl. 1), 21–28. doi:10.1007/BF03319100
Dalleur, O., Spinewine, A., Henrard, S., Losseau, C., Speybroeck, N., and Boland, B. (2012). Inappropriate prescribing and related hospital admissions in frail older persons according to the STOPP and START criteria. Drugs Aging 29, 829–837. doi:10.1007/s40266-012-0016-1
Dimitrow, M. S., Airaksinen, M. S., Kivela, S. L., Lyles, A., and Leikola, S. N. (2011). Comparison of prescribing criteria to evaluate the appropriateness of drug treatment in individuals aged 65 and older: A systematic review. J. Am. Geriatr. Soc. 59, 1521–1530. doi:10.1111/j.1532-5415.2011.03497.x
Dzubur, A., Ejubovic, M., Fajkic, A., Dervisevic, A., Durak Nalbantic, A., Avdic Agic, A., et al. (2019). The serum triglyceride to high-density lipoprotein (HDL) ratio in patients with acute coronary syndrome with and without renal dysfunction. Med. Glas. (Zenica) 16, 28–34. doi:10.17392/990-19
Fromm, M. F., Maas, R., Tumena, T., and Gassmann, K. G. (2013). Potentially inappropriate medications in a large cohort of patients in geriatric units: Association with clinical and functional characteristics. Eur. J. Clin. Pharmacol. 69, 975–984. doi:10.1007/s00228-012-1425-0
Garcia, J., Vaz, M., and Poggi, M. (2015). Estimated prevalence of contraindicated, severe and moderate interactions in ambulatory patients with polypharmacy in a healthcare provider in Uruguay. Clin. Ther. 37, e145. doi:10.1016/j.clinthera.2015.05.416
Hamilton, H., Gallagher, P., Ryan, C., Byrne, S., and O'Mahony, D. (2011). Potentially inappropriate medications defined by STOPP criteria and the risk of adverse drug events in older hospitalized patients. Arch. Intern. Med. 171, 1013–1019. doi:10.1001/archinternmed.2011.215
Holt, S., Schmiedl, S., and Thurmann, P. A. (2010). Potentially inappropriate medications in the elderly: the PRISCUS list. Dtsch. Arztebl. Int. 107, 543–551. doi:10.3238/arztebl.2010.0543
Hu, K. F., Chou, Y. H., Wen, Y. H., Hsieh, K. P., Tsai, J. H., Yang, P., et al. (2016). Antipsychotic medications and dental caries in newly diagnosed schizophrenia: A nationwide cohort study. Psychiatry Res. 245, 45–50. doi:10.1016/j.psychres.2016.07.047
Laroche, M. L., Charmes, J. P., Nouaille, Y., Picard, N., and Merle, L. (2007). Is inappropriate medication use a major cause of adverse drug reactions in the elderly? Br. J. Clin. Pharmacol. 63, 177–186. doi:10.1111/j.1365-2125.2006.02831.x
Lau, D. T., Kasper, J. D., Potter, D. E., Lyles, A., and Bennett, R. G. (2005). Hospitalization and death associated with potentially inappropriate medication prescriptions among elderly nursing home residents. Arch. Intern. Med. 165, 68–74. doi:10.1001/archinte.165.1.68
Leendertse, A. J., Egberts, A. C., Stoker, L. J., and van den Bemt, P. M.HARM Study Group. (2008). Frequency of and risk factors for preventable medication-related hospital admissions in The Netherlands. Arch. Intern. Med. 168, 1890–1896. doi:10.1001/archinternmed.2008.3
Lin, H. Y., Liao, C. C., Cheng, S. H., Wang, P. C., and Hsueh, Y. S. (2008). Association of potentially inappropriate medication use with adverse outcomes in ambulatory elderly patients with chronic diseases: Experience in a Taiwanese medical setting. Drugs Aging 25, 49–59. doi:10.2165/00002512-200825010-00006
Lu, W. H., Wen, Y. W., Chen, L. K., and Hsiao, F. Y. (2015). Effect of polypharmacy, potentially inappropriate medications and anticholinergic burden on clinical outcomes: A retrospective cohort study. CMAJ 187, E130–E137. doi:10.1503/cmaj.141219
McEvoy, D. S., Sittig, D. F., Hickman, T. T., Aaron, S., Ai, A., Amato, M., et al. (2017). Variation in high-priority drug-drug interaction alerts across institutions and electronic health records. J. Am. Med. Inf. Assoc. 24, 331–338. doi:10.1093/jamia/ocw114
Mekonnen, A. B., Redley, B., de Courten, B., and Manias, E. (2021). Potentially inappropriate prescribing and its associations with health-related and system-related outcomes in hospitalised older adults: A systematic review and meta-analysis. Br. J. Clin. Pharmacol. 87, 4150–4172. doi:10.1111/bcp.14870
National Development Council (2023). Population projections for the R.O.C. (Taiwan). Available at: http://pop-proj.ndc.gov.tw/chart.aspx?c=10&uid=66&pid=60 (Accessed January 20, 2023).
Olivier, P., Bertrand, L., Tubery, M., Lauque, D., Montastruc, J. L., and Lapeyre-Mestre, M. (2009). Hospitalizations because of adverse drug reactions in elderly patients admitted through the emergency department: A prospective survey. Drugs Aging 26, 475–482. doi:10.2165/00002512-200926060-00004
Oscanoa, T. J., Lizaraso, F., and Carvajal, A. (2017). Hospital admissions due to adverse drug reactions in the elderly. A meta-analysis. Eur. J. Clin. Pharmacol. 73, 759–770. doi:10.1007/s00228-017-2225-3
Parameswaran Nair, N., Chalmers, L., Peterson, G. M., Bereznicki, B. J., Castelino, R. L., and Bereznicki, L. R. (2016). Hospitalization in older patients due to adverse drug reactions–the need for a prediction tool. Clin. Interv. Aging. 11, 497–505. doi:10.2147/CIA.S99097
Passarelli, M. C., Jacob-Filho, W., and Figueras, A. (2005). Adverse drug reactions in an elderly hospitalised population: Inappropriate prescription is a leading cause. Drugs Aging 22, 767–777. doi:10.2165/00002512-200522090-00005
Pugh, M. J., Hanlon, J. T., Zeber, J. E., Bierman, A., Cornell, J., and Berlowitz, D. R. (2006). Assessing potentially inappropriate prescribing in the elderly Veterans Affairs population using the HEDIS 2006 quality measure. J. Manag. Care Pharm. 12, 537–545. doi:10.18553/jmcp.2006.12.7.537
Reich, O., Rosemann, T., Rapold, R., Blozik, E., and Senn, O. (2014). Potentially inappropriate medication use in older patients in Swiss managed care plans: Prevalence, determinants and association with hospitalization. PLoS One 9, e105425. doi:10.1371/journal.pone.0105425
Saboor, M., Kamrani, A. A., Momtaz, Y. A., and Sahaf, R. (2019). Prevalence and associated factors of potentially inappropriate medications among Iranian older adults. Med. Glas. (Zenica) 16, 121–127. doi:10.17392/989-19
Salvi, F., Marchetti, A., D’Angelo, F., Boemi, M., Lattanzio, F., and Cherubini, A. (2012). Adverse drug events as a cause of hospitalization in older adults. Drug Saf. 35, 29–45. doi:10.1007/BF03319101
Schubert, I., Küpper-Nybelen, J., Ihle, P., and Thurmann, P. (2013). Prescribing potentially inappropriate medication (PIM) in Germany's elderly as indicated by the PRISCUS list. An analysis based on regional claims data. Pharmacoepidemiol. Drug Saf. 22, 719–727. doi:10.1002/pds.3429
Shariff, A., Belagodu Sridhar, S., Abdullah Basha, N. F., Bin Taleth Alshemeil, S. S. H., Ahmed Aljallaf Alzaabi, N. A., and 4th, (2021). Assessing consistency of drug-drug interaction-related information across various drug information resources. Cureus 13 (3), e13766. doi:10.7759/cureus.13766
Storms, H., Marquet, K., Aertgeerts, B., and Claes, N. (2017). Prevalence of inappropriate medication use in residential long-term care facilities for the elderly: A systematic review. Eur. J. Gen. Pract. 23, 69–77. doi:10.1080/13814788.2017.1288211
Wenger, N. S., and Shekelle, P. G. (2001). Assessing care of vulnerable elders: ACOVE project overview. Ann. Intern. Med. 135, 642–646. doi:10.7326/0003-4819-135-8_part_2-200110161-00002
Keywords: potentially inappropriate medication, 90-day rehospitalization, PIM-Taiwan, PRISCUS, Beers criteria, older adults, Taiwan
Citation: Hsieh K-P, Huang R-Y, Yang Y-H, Ho P-S, Chen K-P, Tung C-L, Chu Y-L and Tsai J-H (2023) Using PIM-Taiwan, PRISCUS, and Beers criteria to assess potentially inappropriate medication use among older adults with 90-day rehospitalization: a population-based study in Taiwan. Front. Pharmacol. 14:1194537. doi: 10.3389/fphar.2023.1194537
Received: 27 March 2023; Accepted: 04 July 2023;
Published: 13 July 2023.
Edited by:
Elham Rahme, McGill University, CanadaReviewed by:
Maja Ortner Hadžiabdić, University of Zagreb, CroatiaCopyright © 2023 Hsieh, Huang, Yang, Ho, Chen, Tung, Chu and Tsai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jui-Hsiu Tsai, ZmFhbnZhbmdvZ2hAZ21haWwuY29t; Ya-Lan Chu, Y2h1X3lhbGFuQGhvdG1haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.