- 1International Education College of Zhejiang Chinese Medical University, Hangzhou, China
- 2Department of Cardiology, Hangzhou Hospital of Traditional Chinese Medicine, Hangzhou, China
- 3Hangzhou Clinical Medical College Internal Medicine of Traditional Chinese Medicine of Zhejiang Chinese Medical University, Hangzhou, China
- 4Department of Oncology, The Fourth Affiliated Hospital, International Institutes of Medicine, Zhejiang University School of Medicine, Yiwu, China
- 5Key Laboratory of Combined Multi-Organ Transplantation, Ministry of Public Health, First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China
Background and Objective: Alzheimer’s disease (AD) is a progressive neurodegenerative disorder, that is, characterized by cognitive decline. To date, there are no effective treatments for AD. Therefore, the objective of this study was to map new perspectives on the effects of pharmacological treatment on cognitive function and the overall psychological state in patients with AD.
Methods: Two independent researchers searched for randomized clinical trials (RCTs) exploring new pharmacological approaches related to cognition in Alzheimer’s disease in adults from 2018 to 2023 in PubMed, Web of Science, Scopus, and Cochrane Library databases. A total of 17 RCTs were included in this review.
Results: The results show that in recent years, new drugs have been tested in patients with Alzheimer’s disease, including masitinib, methylphenidate, levetiracetam, Jiannao Yizhi, and Huannao Yicong formulas. Most studies have been conducted in populations with mild to moderate Alzheimer’s disease.
Conclusion: Although some of the drugs found suggested improvement in cognitive function, the scarcity of available studies highlights the need for further research in this area.
Systematic review registration: [www.crd.york.ac.uk/prospero], identifier [CRD42023409986].
1 Introduction
Alzheimer’s disease (AD) is a multifactorial progressive neurodegenerative disorder characterized by memory loss, disorientation, and gradual decline in intellectual ability (Korabecny et al., 2019). It affects approximately 46 million people worldwide and accounts for 60%–80% of all cases of dementia (Ghaffari et al., 2020). The etiology of the disease has not yet been fully elucidated, and only one approved therapeutic approach currently exists for its treatment. The accumulation of beta-amyloid (Aβ) peptides is considered to be one of the fundamental neuropathological pillars of the disease (Dubois et al., 2023), and its dishomeostasis plays a crucial role in its onset (Jeremic et al., 2021; Torres-Mendoza et al., 2022; Tosatti et al., 2022). Researchers are investigating various therapies to combat this disease, including the modulation of targets such as Aβ aggregation, neuroinflammation, and oxidative stress, as well as the use of enhanced multiple biomarkers and risk prediction methods to detect the disease at early stages (Koh et al., 2021; Phadke et al., 2021; Levine et al., 2022). Additionally, research on miRNAs as a possible avenue for AD diagnosis, treatment, and prevention is being conducted (Ghaffari et al., 2020). The efficacy of pharmacological treatment may vary according to the characteristics of different population groups, such as age, disease severity, sex, and presence of other medical conditions (Cardinali et al., 2021).
AD is a progressive neurodegenerative pathology for which there is no definitive cure. However, there are different types of treatments that can help delay its progression and improve the quality of life of affected patients. Treatment options include medication, occupational therapy, cognitive stimulation therapy, physical exercise, massage therapy, music therapy, and nutritional supplements (Pisani et al., 2021). Pharmacological treatments available for AD include donepezil, rivastigmine, galantamine, and memantine (Miculas et al., 2023). These drugs help improve symptoms and delay the progression of the disease in some patients (Rong et al., 2020). Donepezil, rivastigmine, and galantamine are cholinesterase inhibitors used to treat mild to moderate symptoms, whereas memantine is an N-methyl-D-aspartate (NMDA) receptor antagonist used to treat moderate to severe AD symptoms (Birks and Harvey, 2018; Li et al., 2019; Thancharoen et al., 2019). The activity of cholinesterase inhibitors is characterized by the inhibition of the acetylcholinesterase enzyme, responsible mainly for the breakdown of acetylcholine in the nervous system. This allows for the prolonged action of the deficient neurotransmitter in the brain. Rivastigmine has a relatively low protein binding affinity and a more selective action with less possibility of interactions with other drugs. It is important to maintain the balance of different neurotransmitter systems, such as acetylcholine, norepinephrine, dopamine, serotonin, and glutamate, for proper brain function. Although AD is a chronic disease, most research has a limited duration of 6 months, which limits knowledge of the effectiveness of drugs in the long term (Marucci et al., 2021).
Despite advances in available treatments for AD, there is still no definitive cure. Therefore, new treatment approaches are constantly being investigated, such as immunotherapy, gene therapy, light therapy, diet, and physical exercise. Research on new approaches aims to find a more effective and specific therapy than the current treatment options, with the goal of finding a cure for this disease that affects millions of people worldwide (Liang et al., 2018a).
Several psychometric instruments have been used to assess the cognitive and functional performance of patients with AD and other related dementias. The Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-cog) evaluates memory, attention, reasoning, language, orientation, and praxis (Craft et al., 2020). The Clinical Dementia Rating (CDR) scale measures memory, orientation, judgment and problem-solving, community affairs, home and hobbies, and personal care (Gibson et al., 2020). The Neuropsychiatric Inventory (NPI) assesses a wide range of behaviors in patients with dementia (Gibson et al., 2020). The Alzheimer’s Disease Cooperative Study-Activities of Daily Living (ADCS-ADL) scale is used to evaluate the patients’ performance in basic and instrumental activities of daily living (Gibson et al., 2020). In addition, other global assessment scales, such as the Clinical Dementia Rating-Sum of Boxes (CDR-SB), Clinician’s Interview-Based Impression Plus Caregiver Input (CIBI plus), and Clinical Global Impression (CGI), are used in patients with AD. The Mini-Mental State Examination (MMSE), ADAS-cog, and Severe Impairment Battery (SIB) scales are widely used to evaluate cognition in patients with this disease (Levine et al., 2021).
This study aimed to map out new perspectives on the effect of pharmacological treatment on cognition and overall psychological state in patients with AD.
2 Materials and method
2.1 Search strategy and data sources
From February 2023 to March 2023, a search was conducted across four databases, (PubMed, Web of Science, Scopus, and the Cochrane Library) to identify documents published within the past 5 years. To achieve the most comprehensive results possible, the search strategy employed was “Alzheimer Disease/drug therapy” [Mesh].
2.2 Inclusion criteria
The inclusion criteria for this search were as follows: 1) original articles of randomized clinical trials (RCT); 2) studies conducted in living humans with AD; 3) focusing on cognitive state or psychological aspects, such as memory, mood, etc.; 4) AD must be established at the start of the intervention; 5) adult population over 18 years, both men and women; 6) intervention must be a pharmacological treatment; 7) articles published in English; 8) published within the last 5 years, specifically from 2018 to 2023; 9) with full text available; and 10) methodological quality must score greater than three points on the JADAD scale (Jadad Bechara, 1996).
2.3 Exclusion criteria
The exclusion criteria were as follows: 1) studies conducted in animal models, in vitro, in vivo, and/or post-mortem; 2) studies on biochemical composition or biomarkers; 3) studies on nutritional aspects, or other therapeutic alternatives; and 4) studies on the prevention of AD.
Two researchers conducted the search and screening of the documents, and any discrepancies in the selected documents were resolved through consensus between the researchers. This study was registered in the International Prospective Register of Systematic Reviews (PROSPERO) with the code [CRD42023409986].
3 Results
3.1 Study characteristics
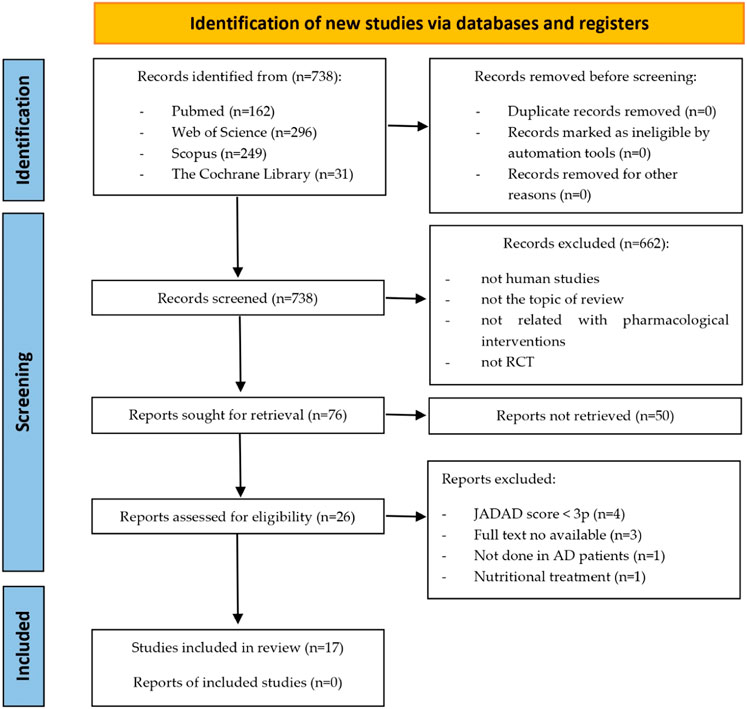
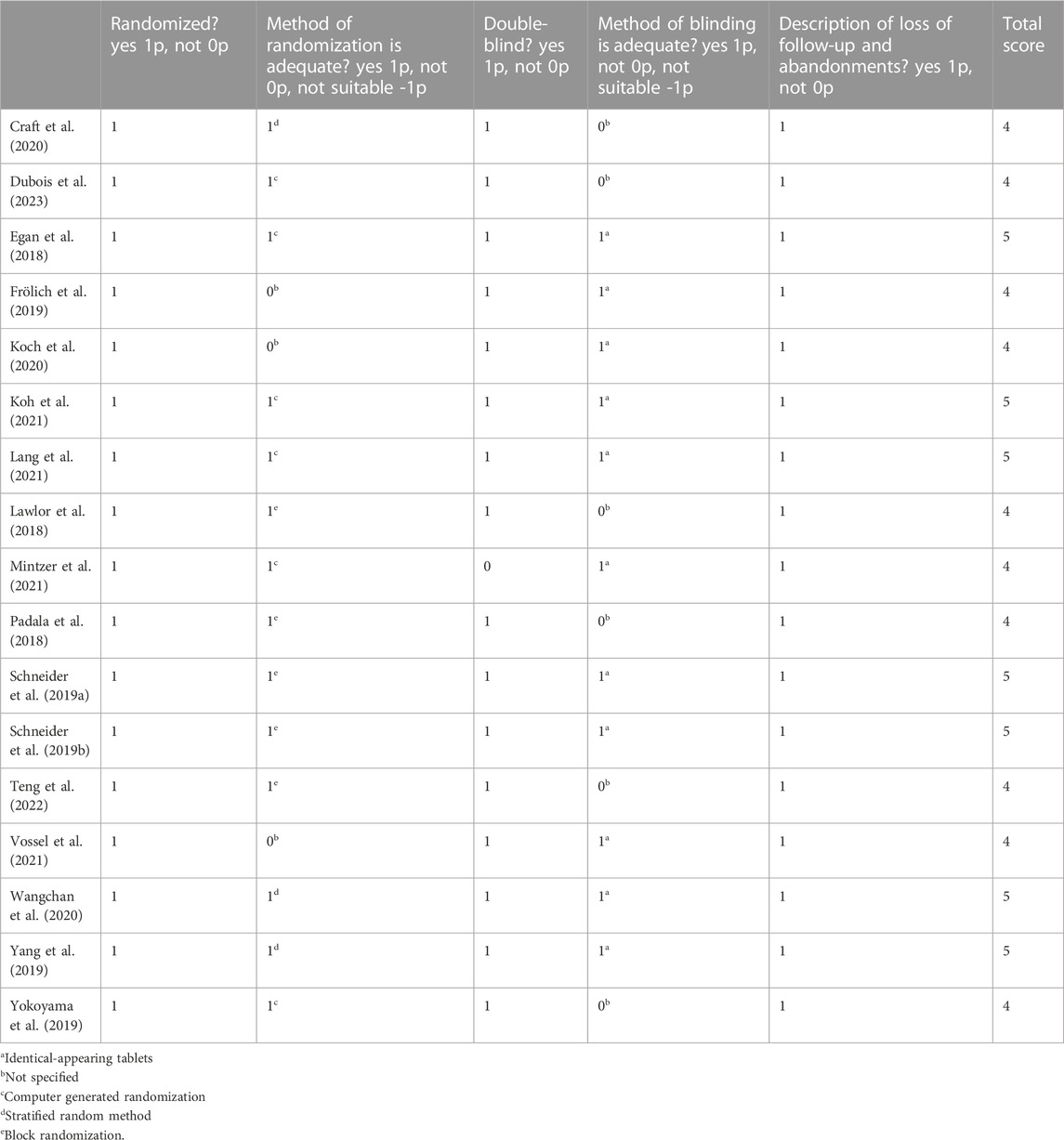
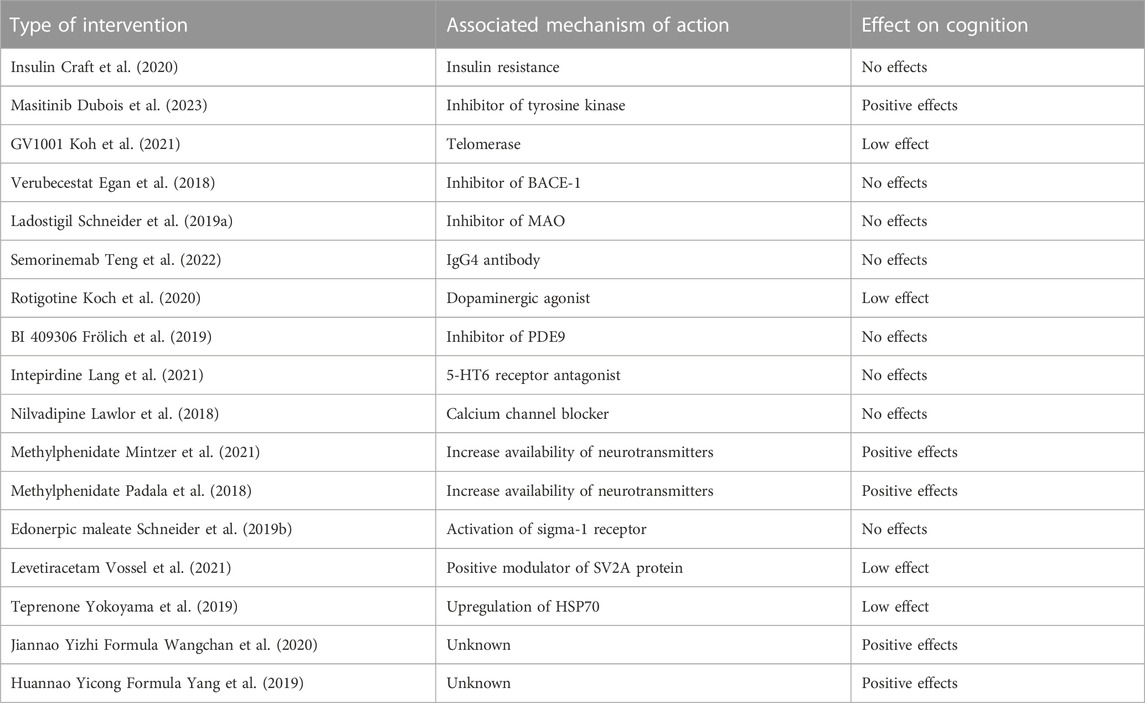
A total of 738 documents were initially identified, 17 of which were included in the study. The selection process of the studies included in this review is summarized in Figure 1. The main characteristics of the studies included in this review are presented in Table 1. Additionally, Table 2 displays the JADAD scores of the included articles. Due to the high variability of interventions in the studies included in our systematic review, we have created Table 3 outlining the type of intervention, associated mechanism of action, and effect on cognition. Since the articles included in this systematic review investigate completely different drugs, they have been classified according to their mechanism of action and therapeutic use.
3.2 Synthesis of clinical trials investigating various treatments for Alzheimer’s disease
3.2.1 Therapeutic potential of anticancer agents in Alzheimer’s disease
Anticancer agents have garnered growing interest in the context of repurposed therapies for AD. To date, results have been controversial. For example, some anticancer drugs such as tyrosine kinase inhibitors and retinoid X receptor agonists can modulate cellular signaling pathways and reduce inflammation, which may be beneficial in the context of AD. Additionally, it has been shown that some anticancer drugs, such as histone deacetylase inhibitors, can improve cognitive function and synaptic plasticity in animal models of AD (Ancidoni et al., 2021). Masitinib is an anti-tumor drug that has been investigated for potential use in the treatment of AD. Its mechanism of action is believed to be related to its ability to inhibit certain enzymes that can contribute to neuronal damage and inflammation in the brain, which in turn may reduce AD symptoms. Masitinib is an inhibitor of tyrosine kinase, an enzyme that plays a role in the activation of inflammatory cells and some brain cells involved in AD progression. By inhibiting this enzyme, masitinib may reduce inflammation and activation of these cells, which could help protect brain cells and reduce AD symptoms (Piscopo et al., 2022; Villain, 2022). Masitinib has demonstrated neuroprotective effects in neurodegenerative diseases by inhibiting mast cell and microglia/macrophage activity and its ability to accumulate in the central nervous system at therapeutically relevant concentrations. Administration of Masitinib at doses of 4.5–6.0 mg/kg/day in patients over the age of 50 with mild to moderate AD-associated dementia has been shown to result in significant improvement in ADAS-cog scores (p < 0.001), indicating improved cognition. Masitinib also improved overall function, as assessed by ADCS-ADL (p = 0.038). However, this drug has potential safety concerns, including maculopapular rash, neutropenia, and hypoalbuminemia (Dubois et al., 2023). GV1001 is a peptide composed of 16 amino acids corresponding to a fragment of the catalytic site of human telomerase reverse transcriptase. This peptide possesses neuroprotective properties, as it protects neural cells against neurotoxicity, apoptosis, and reactive oxygen species (ROS) induced by Aβ and oxidative stress. These neuroprotective effects are mediated through a variety of mechanisms, such as anti-apoptotic effects, mitochondrial stabilizers, anti-inflammatory, anti-aging, and antioxidant properties. In a clinical study conducted on patients with moderate to severe AD, a dose of 1.12 mg of GV1001 was administered for 24 weeks, resulting in a significant reduction in changes in SIB scores compared to placebo treatment (p < 0.05). However, changes in the ADCS-ADL and CDR-SB scores were not significant. Furthermore, GV1001 was well-tolerated without safety issues (Koh et al., 2021).
3.2.2 A novel therapeutic agent for Alzheimer’s disease
The Aβ protein is produced through the sequential action of the β-site amyloid precursor protein (APP) cleaving enzyme (BACE-1) and γ-secretase on the amyloid precursor protein (APP). Inhibition of BACE-1 in preclinical models has been shown to reduce Aβ production and amyloid plaque deposition, which may potentially delay the progression of AD. Verubecestat is a selective inhibitor of BACE-1 that reduces Aβ levels in the cerebrospinal fluid of healthy individuals and AD patients by more than 60% (Egan et al., 2018). However, clinical studies have shown that this inhibitor is ineffective and produces numerous negative side effects, such as rash, dermatitis, sleep disorders, weight loss, and coughing (Miranda et al., 2021). In Egan et al. (2018) RCT, it was found that verubecestat administration for 104 months did not improve clinical dementia symptoms in patients between 50 and 85 years of age. In addition, cognitive and daily function scores were worse in the intervention group than in the placebo group, as measured by the CDR-SB. Adverse effects were also more frequent in the intervention group compared to the placebo group (Egan et al., 2018).
Ladostigil is a novel therapeutic agent that acts as an inhibitor of both monoamine oxidase (MAO) and acetylcholinesterase (AChE) in the brain. It has also been found to possess neuroprotective and antiapoptotic properties by preventing oxidative-nitrate stress and gliosis (Uddin et al., 2020). MAO is an enzyme that degrades important neurotransmitters such as dopamine, noradrenaline, and serotonin. Inhibition of MAO by ladostigil increases the amount of these neurotransmitters in the brain, which could improve cognitive function. Ladostigil also prevents the decline of mitochondrial potential caused by oxidative stress and the release of proinflammatory cytokines from activated microglia. In patients with mild cognitive impairment (MCI), treatment with ladostigil may reduce ROS and proinflammatory changes, suggesting a potential slow action in disease progression. However, in the clinical trial by Schneider et al. (2019b), a dose of 10 mg of ladostigil was found to have no significant effects on cognitive function, daily activity, or depressive symptoms in patients with dementia, as evaluated by NTB, DAD, and GDS, respectively. However, total brain volume and hippocampal volume decreased significantly less in the ladostigil-treated group than in the placebo group, suggesting a potential effect on cerebral atrophy (Schneider et al., 2019a).
Semorinemab is a humanized monoclonal IgG4 antibody that targets the N-terminal domain of tau protein. Its mechanism of action is based on its ability to bind and eliminate Aβ protein fragments, which accumulate as plaques in the brains of patients with AD. Semorinemab was selected for development because of its high affinity and specificity for all known isoforms of full-length tau. Neurofibrillary tangles composed of aggregated tau protein are a hallmark neuropathological feature of AD and are correlated with the clinical severity of the disease. Monoclonal antibodies targeting tau protein may have the potential to slow or stop the spread and accumulation of pathological tau, thereby improving the progression of AD. However, a 73-week treatment with semorinemab in patients with prodromal to mild AD did not result in significant improvements in CDR-SB, ADAS-cog13, and ADCS-ADL. The treatment also did not reduce the rate of cerebral tau accumulation or clinical decline in patients with prodromal-to-mild AD. The safety profile of semorinemab was found to be acceptable and well-tolerated (Teng et al., 2022).
3.2.3 Mitochondrial electron transport inhibitors for Alzheimer’s disease
Dopamine is a crucial neuromodulator that influences several distinct synaptic processes and plays an important role in controlling higher cognitive functions such as memory, learning, and decision-making. Dopaminergic dysfunction may contribute to cognitive impairment in patients with AD. Intervention with a transdermal rotigotine patch, a dopaminergic agonist, did not have an effect on global cognitive dysfunction in patients with mild to moderate AD, as evaluated by the ADAS-cog. However, a significant improvement was observed in the deterioration of activities of daily living, as measured by the ADCS-ADL. This effect appears to be related to minor cognitive dysfunction in the frontal lobe (Koch et al., 2020).
3.2.4 The serotonin 5-HT6 receptor as a therapeutic target for Alzheimer’s disease
One of the key features of AD is an abnormality in glutamatergic neurotransmission related to the function of the N-methyl-D-aspartate (NMDA) receptor in the cortex and hippocampus. Activation of the NMDA receptor signaling pathway produces postsynaptic signaling events through the elevation of second messengers such as cyclic guanosine monophosphate (cGMP). In conditions of NMDA receptor hypofunction, such as in AD, it is hypothesized that inhibition of phosphodiesterase type 9 (PDE9), which hydrolyzes cGMP, may increase cGMP levels and improve NMDA receptor signaling. This could lead to increased plasticity and synaptic stabilization through enhanced long-term potentiation (LTP), thus potentially improving cognitive functions. BI 409306 is a selective and potent inhibitor of PDE9 that has been investigated for the symptomatic treatment of AD. However, administration of this drug at various doses for 12 weeks did not improve NTB, CDR-SB, ADAS-cog11, or ADCS-ADL scores in patients with mild AD. It has not been demonstrated to be effective in improving cognition in patients with prodromal or mild AD (Frölich et al., 2019).
The serotonin 5-HT6 receptor, which is found in critical areas of the brain involved in memory, learning, mood, and behavior, has been studied as a potential therapeutic target for AD. Inhibition of this receptor has been shown to improve the release of important neurotransmitters in AD, which could improve cognition in preclinical models. Specifically, the 5-HT6 receptor antagonist, Intepirdine, has been evaluated in phase 2 clinical trials in AD patients and has been suggested as a possible oral treatment to improve cognition. Since the 5-HT6 receptor is primarily located in the central nervous system, antagonism of this receptor may increase the release of important neurotransmitters and minimize peripheral side effects (Lang et al., 2021; Nirogi et al., 2023). However, Lang et al. (2021) RCT, which evaluated intervention with 35 mg/day of Intepirdine for 24 weeks in patients with mild to moderate AD receiving donepezil as the baseline treatment, did not show significant improvements in ADAS-cog scores (p = 0.2249) or ADCS-ADL scores (p = 0.8260). Nevertheless, it was observed that Intepirdine demonstrated a favorable safety profile, similar to placebo (Lang et al., 2021).
3.2.5 The neuroprotective mechanisms of calcium channel blockers in Alzheimer’s disease
Nilvadipine is a dihydropyridine calcium channel blocker drug used to treat hypertension (Lawlor et al., 2018; Ling et al., 2021; Dhapola et al., 2022). In addition to its direct blocking action on calcium channels and maintenance of intracellular calcium homeostasis, nilvadipine has been shown to have a number of neuroprotective mechanisms of action. These include reducing the production of amyloid beta 40 and 42 amino acid peptides (Aβ40 and Aβ42) in vitro and in vivo in transgenic mouse models of AD, and improving Aβ clearance across the blood-brain barrier in vivo mouse models (Lawlor et al., 2018). It is believed that these protective effects could have a dual effect on AD pathogenesis, reducing both mitochondrial dysfunction and beta-amyloid accumulation (Ling et al., 2021; Dhapola et al., 2022). However, the results of a RCT conducted by Lawlor et al. (2018) indicate that nilvadipine did not produce significant changes in cognitive decline in patients with mild to moderate AD after receiving 8 mg of nilvadipine for 18 months (Lawlor et al., 2018).
3.2.6 The role of central nervous system stimulants (methylphenidate) treating apathy in AD patients
There are studies suggesting that methylphenidate, a medication used to treat ADHD, may have beneficial effects on patients with AD, such as improving memory and cognition. However, further research is needed to determine its efficacy and safety in individuals with AD. Additionally, it is important to note that methylphenidate is not suitable for all patients and may have side effects. The exact mechanism of action of methylphenidate is not fully known, but it is believed to act by increasing the availability of neurotransmitters such as dopamine and norepinephrine in the brain. These neurotransmitters are involved in the regulation of attention, mood, and cognition. By increasing their availability, methylphenidate may improve attention and memory in patients with ADHD and possibly in those with AD. Furthermore, it is believed that methylphenidate may have neuroprotective effects, protecting nerve cells from oxidative damage and inflammation (Sassi et al., 2020; van Dyck et al., 2021; Andrade, 2022). Regarding apathy in AD, although no treatment has been shown to be effective, catecholaminergic agents such as methylphenidate are promising. It has been proposed that methylphenidate could act as a cognitive enhancer by increasing dopaminergic and noradrenergic neurotransmission, which are diminished in AD (Padala et al., 2018; Mintzer et al., 2021). Although there is still not enough evidence to confirm its efficacy in this regard, studies have shown that intervention with 10 mg methylphenidate for 6 months significantly improved apathy in AD patients, with no significant differences in safety profiles between treatment groups (Mintzer et al., 2021). Additionally, after 12 weeks of treatment with methylphenidate, apathy also significantly improved in patients with mild AD compared to the placebo group, with improvements in cognition, functional status, caregiver burden, CGI scores, and depression (Padala et al., 2018).
3.2.7 The therapeutic effect of neurotransmitter modulators in AD patients
The edonerpic maleate can exert its effects through different mechanisms, including activation of the sigma-1 receptor, modulation of microglial function, and interaction with the collapsin response mediator protein 2 (CRMP2), which facilitates the administration of the AMPA synaptic receptor. According to Schneider et al. (2019a), edonerpic maleate can protect against Aβ-induced neurotoxicity and memory deficits, promote neurite growth, and preserve hippocampal synapses and spatial memory. However, intervention with edonerpic maleate for 52 weeks in patients with mild to moderate AD had no significant effects on the ADAS-cog or ADCS-CGIC scales (Schneider et al., 2019b).
Levetiracetam is an antiepileptic drug that acts as a positive modulator of the SV2A protein, which is a member of the SV2 protein family involved in neurotransmission and is found in most nerve terminals. Levetiracetam binds to the SV2A protein and modulates its function to inhibit neurotransmitter release and reduce neuronal activity (Stout et al., 2019). Decreased levels of SV2A in the brains of patients with AD have been demonstrated, which may contribute to synaptic dysfunction and neuronal loss in AD. SV2 is an important target for new PET tracers that have been developed to visualize synaptic density in the brain (Stout et al., 2019; Carson et al., 2022; Samudra et al., 2023). Although the precise function of SV2 is not fully understood, some possible functions include vesicular transport, stabilization of vesicular neurotransmitter load, anchoring of vesicular proteins, regulation of calcium sensitivity, and interaction with the extracellular matrix. The hypothesis is discussed that SV2 does not directly transport calcium but makes prepared vesicles more sensitive to calcium (Stout et al., 2019). The mechanism of action of Levetiracetam is not fully understood, but it is believed to act by inhibiting the release of glutamate, a neurotransmitter that has been linked to neuronal death in AD. In addition, Levetiracetam has been shown to have neuroprotective effects, helping to prevent brain cell death and neuroinflammation (Musaeus et al., 2021; Vossel et al., 2021). Although Levetiracetam did not improve cognitive function in AD patients in a 12-week intervention study, it improved performance in spatial memory tasks and executive function. It has been studied as a possible treatment to improve cognition in AD patients, but more studies are needed to confirm its effectiveness in this field. Levetiracetam is considered safe and well-tolerated at low doses (Vossel et al., 2021).
3.2.8 Antidiabetics in AD
Currently, research is being conducted to determine the effectiveness of antidiabetic treatments in improving AD due to the possible relationship between type 2 diabetes and AD. Type 2 diabetes is a metabolic disease that affects insulin activity in regulating blood glucose levels. Insulin resistance in the brain, which is an important factor in the development of type 2 diabetes, has also been shown to be related to a higher risk of developing AD (Chen et al., 2022; Malin et al., 2022). Several epidemiological and clinical studies have suggested a possible shared pathophysiology between diabetes and AD, and the administration of certain antidiabetic medications, such as intranasal insulin, metformin, incretins, and thiazolidinediones, has been shown to improve cognition and memory in patients with mild cognitive impairment and AD. As a result, the term “type 3 diabetes” has been proposed for AD, considering it a metabolic disease caused by insulin resistance and insulin-like growth factor in the brain (Čater and Hölter, 2022). Additionally, antidiabetic drugs such as metformin have been observed to improve insulin sensitivity and reduce inflammation in the brain, suggesting that they could slow or reverse the process of cognitive decline in patients with AD. It has been shown that insulin signaling is reduced in the brains of patients with AD, known as cerebral insulin resistance, and is related to the accumulation of beta-amyloid plaques and the formation of neurofibrillary tangles (Michailidis et al., 2022). Although brain glucose metabolism does not depend on insulin, it can alter its use through interactions with the neuronal glucose transporter type 4 (GLUT4) in key cognitive circuits and by promoting glycogen uptake in astrocytes, processes that are considered important during times of high energy demand. Additionally, insulin improves synaptic viability and dendritic spine formation, and modulates levels of key neurotransmitters such as dopamine. Although previous studies have demonstrated that intranasal insulin administration improves performance in the ADAS-cog test and brain glucose metabolism in patients with AD, a randomized clinical trial by Craft et al. (2020) did not find significant effects of intranasal insulin administration on ADAS-cog-12 scores in AD patients, possibly due to the inadequate use of some devices in the study (Craft et al., 2020).
3.2.9 Gastric protectors HSP70 overexpression and teprenone administration as a neuroprotective strategies for AD
It has been reported that elevating HSP70 levels in the brain via genetic modification or teprenone administration in mouse models of AD inhibits the accumulation of Aβ, senile plaque formation, neuronal death, and neurodegeneration, while significantly enhancing memory capacity. Its mechanism of action is believed to involve multiple protective effects, such as reducing Aβ protein production and decreasing cerebral inflammation. HSP70 overexpression leads to positive regulation of Aβ degrading enzyme and TGF-β1 expression, both in vitro and in vivo. Additionally, teprenone is an antiulcer agent that can inhibit Aβ increase, senile plaque formation, neuronal degeneration, and improve memory. However, a 12-month intervention study in patients with mild to moderate AD who received a combination of donepezil and teprenone did not significantly affect the ADAS-Jcog score (p = 0.861), but did impact the MMSE score (p = 0.044) (Yokoyama et al., 2019).
3.2.10 Traditional Chinese medicine: a natural alternative for treating Alzheimer’s disease
Traditional Chinese Medicine (TCM) employs a variety of herbal medicines to treat various diseases, including AD, and is considered a natural alternative to synthetic drugs. The mechanisms of action of these herbal medicines have been investigated, and it is believed that they may have beneficial effects in the prevention and treatment of AD. Some herbal medicines may reduce inflammation in the brain, protect nerve cells from damage, and improve cognitive function by modulating different signaling pathways such as NF-κB, Nrf2, JAK/STAT, ubiquitin-proteasome pathway, AMPK/mTOR related to the autophagy-lysosome pathway, GSK-3/mTOR, and PI3K/Akt/mTOR, as well as the SIRT1 and PPARα pathways (Soheili et al., 2021; Ding et al., 2022; Tan et al., 2022). Herbal medicines can also modulate multiple signaling pathways associated with Aβ deposition, protein tau phosphorylation, and chronic inflammation. Some herbal medicines may prevent excessive apoptosis and reduce AChE activity (Fang et al., 2020; Pei et al., 2020; Li et al., 2021). A 6-month study with the Jiannao Yizhi formula increased MoCA and MMSE scores and decreased ADAS-cog and CM-SS scores (p < 0.05) in patients with AD. There were no significant differences in the group receiving donepezil, suggesting that the effect of the Jiannao Yizhi formula is not inferior to that of donepezil. The Jiannao Yizhi formula had a favorable safety profile, and no serious adverse effects were found (Wangchan et al., 2020). Similarly, a 6-month study with the Huannao Yicong formula increased MoCA and MMSE scores and decreased ADAS-cog and CM-SS scores (p < 0.01) in patients with mild to moderate AD. No serious adverse effects were observed during the study. The Huannao Yicong formula may prevent neuronal apoptosis in the CA1 area of the hippocampus, inhibit secretase activity, and reduce neurotoxicity caused by Aβ peptide in rat models of AD (Yang et al., 2019).
4 Discussion
Our systematic review was limited by the scarcity of studies available for discussion of the findings. However, we presented some of the discoveries obtained during the literature search, which could not be included in the systematic review because their failure to meet the established inclusion criteria.
Lithium is considered to be a potential treatment for improving neurotrophic responses and protecting the brain. In patients with amnestic cognitive impairment, treatment with lithium carbonate showed cognitive and functional stability for 2 years, with better performance in memory and attention tests compared to the placebo group (Forlenza et al., 2019). Unlike the expensive aducanumab approved by the FDA in 2021 for patients with mild dementia caused by AD, lithium is more cost-effective and has been shown to be effective for both mild cognitive impairment and AD. In addition, a recent meta-analysis found that lithium is more effective than aducanumab in reducing cognitive decline, as measured by MMSE (Terao et al., 2022). However, some studies indicate that lithium has no significant effect on cognitive performance, and it is important to carefully monitor its administration and follow-up due to its toxicity (Restrepo-Martínez et al., 2022).
Insulin not only regulates glucose homeostasis, but it also has functions in the brain. It has been shown to improve synaptic viability, modulate neurotransmitter levels, such as dopamine, and protect against the toxic effects of Aβ peptide (Craft et al., 2020; Kellar et al., 2022). Unlike the results reported by Craft et al. (2020) (Craft et al., 2020), low insulin levels could be related to AD, and intranasal insulin administration has been shown to improve in cognition in patients with this disease. Intranasal insulin can also reduce the progression of white matter hyperintensity and improve verbal memory (Avgerinos et al., 2018; Zhang et al., 2018; Kellar et al., 2021). Antidiabetic drugs such as intranasal insulin, pioglitazone, rosiglitazone, metformin, sitagliptin, and liraglutide can significantly improve the cognition of patients with AD and mild cognitive impairment; However, metformin does not seem to reduce the risk of AD, and its consumption in the Asian population is associated with a higher risk of this disease, although causality is unknown (Cao et al., 2018; Munõz-Jiménez et al., 2021; Luo et al., 2022).
In the last decade, research has being conducted to evaluate the effect of vaccination and immunotherapy in the treatment of AD (Foroutan et al., 2019; Vaz and Silvestre, 2020). Most of these investigations are in the safety and tolerability phase or in phase I. However, the efficacy of this type of treatment for managing neurodegenerative disease is still unknown (Lacosta et al., 2018). On the other hand, intravenous administration of immunoglobulins has been shown to be ineffective in the treatment of AD, according to reports from previous studies (Okuya et al., 2018; Manolopoulos et al., 2019). Currently, one of the most investigated goals is the treatment of AD with monoclonal antibodies. Despite promising results from clinical trials, the risk-benefit profile of these drugs remains uncertain (Lacorte et al., 2022). Monoclonal antibodies aducanumab and solanezumab have been investigated for their effect on Aβ accumulation and cognitive function. It has been shown that these antibodies can improve cognitive outcomes, evaluated by the ADAS-cog scale (Avgerinos et al., 2021; Budd Haeberlein et al., 2022). Lecanemab (BAN2401) is a humanized monoclonal IgG1 antibody that selectively targets soluble aggregated Aβ species, including oligomers, protofibrils, and insoluble fibrils. Phase II clinical trials have demonstrated that this antibody can reduce brain amyloid burden and clinical decline. However, given that the available evidence is still limited, further studies are needed to fully evaluate its efficacy and safety (Swanson et al., 2021; Dhadda et al., 2022).
Given that pharmacology and nutrition have some points in common, we will also discuss some data we have found regarding the relationship between nutrition and AD. Various nutrients and nutraceuticals have been linked to improvements in cognition and other psychological aspects related to AD (Guzman-Martinez et al., 2021; Abduljawad et al., 2022; Mahnashi et al., 2022; Xu Lou et al., 2023) Some examples include Gingko Biloba (Liao et al., 2020), Melissa Officinalis (Noguchi-Shinohara et al., 2020; Noguchi-Shinohara et al., 2022), Ginseng (Ahmad et al., 2023), anti-inflammatory fatty acids (Albrahim, 2020), medium-chain fatty acids (Juby et al., 2022), ketone bodies (Avgerinos et al., 2020a), saffron (Avgerinos et al., 2020b; Talebi et al., 2021), fenugreek seed (Foroumandi et al., 2023), genistein (Viña et al., 2022), sodium oligomannate (Xiao et al., 2021), anthocyanin (Suresh et al., 2022), microbiota and probiotics (Den et al., 2020; Maitre et al., 2021; Liu et al., 2022; Naomi et al., 2022), benfotiamine (Gibson et al., 2020), omega-3 fatty acids (Canhada et al., 2018; Jernerén et al., 2019), resveratrol (Gu et al., 2021; Buglio et al., 2022; Fang et al., 2022; Tosatti et al., 2022), melatonin (Tseng et al., 2022), citicoline (Bonvicini et al., 2023), folic acid, vitamin B12 (Chen et al., 2021), vitamins and minerals (Mccleery et al., 2018; Karthika et al., 2022), selenium (Pereira et al., 2022), vitamin D (Jia et al., 2019), and mangosteen (Muangpaisan et al., 2022). However, some studies do not support the efficacy of certain nutrients (Zhu et al., 2018; Thancharoen et al., 2019; Araya-Quintanilla et al., 2020; Burckhardt et al., 2020; Du et al., 2020; Prabhakar et al., 2020; Shim et al., 2021; Tofiq et al., 2021; Takada et al., 2022). The effects of these nutrients and micronutrients appear to be related to their anti-apoptotic, antioxidant, and anti-inflammatory properties (de Andrade Teles et al., 2018; Summers et al., 2018; Tamtaji et al., 2019; Zamanian et al., 2021; Rasi Marzabadi et al., 2022). The response to nutritional interventions is greater in the early stages of AD (Moreira et al., 2020), and this response is linked to the APOE genotype (Xu et al., 2020). The maximum benefit of probiotics has been observed in individuals with early cognitive dysfunction and no effect has been found in those with advanced disease or no apparent disease (Akhgarjand et al., 2022; Sánchez-De-Lara-Sánchez and Sánchez-Pérez, 2022). Caprylic acid is a ketone that, when metabolized, produces beta-hydroxybutyrate and acetoacetate ketones, that can cross the blood-brain barrier. Caprylic acid improves cognition in patients with mild-to-moderate AD. This is associated with an increased blood flow in specific brain regions. However, only patients who lack the APOE ε4 allele benefit from this effect (Torosyan et al., 2018).
AD is commonly treated with drugs such as donepezil, rivastigmine, galantamine, and memantine (Li et al., 2019). Although these drugs do not generally cause serious adverse events (Hong et al., 2019), common side effects include headache, diarrhea, nausea, and vomiting (Tricco et al., 2018). Although donepezil is the first-line drug for AD treatment (Birks and Harvey, 2018; Thancharoen et al., 2019), high doses should be administered with caution due to an increased risk of gastrointestinal and cardiac problems (Espiritu and Cenina, 2020; Wang et al., 2022). Both donepezil and memantine are widely used for the treatment of moderate AD (Matsunaga et al., 2018; Rozankovic et al., 2021; Youn et al., 2021). It has been observed that memantine may have a more significant effects on cognition than other commonly used AD medications (Liang et al., 2018b). However, a meta-analysis suggested that the efficacy of memantine is limited in some cases and does not differ significantly compared to placebo (Blanco-Silvente et al., 2018). Although memantine is associated with a lower incidence of AD progression, it also increases the incidence of somnolence (Kishi et al., 2018). Additionally, it is important to note that most available studies have a duration of less than 6 months, and participants usually have mild AD. Optimal pharmacological treatmenst often includes multiple drugs (McShane et al., 2019).
Zolpidem and Zopiclone have been studied for their use in AD patients with insomnia, as insomnia is a frequent problem in these patients (Louzada et al., 2022). Depression is a condition associated with dementia and AD, and sertraline and mirtazapine are antidepressant drugs that have been evaluated in RCTs in patients with AD who also suffer from depression. However, they have not been shown to be effective in these cases (Zuidersma et al., 2019). In contrast, vortioxetine could have beneficial effects on the cognition and mood in elderly patients with AD (Cumbo et al., 2019). While in animal models antidepressants have been shown to delay cognitive decline in animal models, there is still insufficient evidence to support these results in humans (Qin et al., 2022). Agitation and aggression are common symptoms in patients with dementia (Ruthirakuhan et al., 2019; Supasitthumrong et al., 2019), but there are no effective drugs for their treatment. Typical and atypical antipsychotics are commonly used to treat agitation and psychosis in dementia, although their effect on psychosis is insignificant (Mühlbauer et al., 2021). Nabilone may improve agitation; however, more studies are needed to confirm these results (Herrmann et al., 2019). On the other hand, pimavanserin may improve both agitation and aggression in patients with AD (Ballard et al., 2020).
Compared to donepezil, TCM shows no significant difference in effectiveness for treating AD (Hang-kun et al., 2018), although some of its formulas may help improve disease progression (May et al., 2018). For example, Danggui-Shaoyao-San has been found to significantly reduce symptoms in patients with vascular dementia (Kim and Cho, 2020). Furthermore, some studies suggest that a combination of TCM and Western medicine may offer greater benefits than using only one of them (Huang et al., 2021). Traditional Chinese and Japanese medicines have become important sources for drug discovery, and their efficacy in modern drug discovery needs to be investigated (Paudel et al., 2020).
AD is a common type of dementia that has caused a significant global economic and health burden, and there has been a wide debate on the use of statins as a treatment for this disease. Although a systematic review by Mejias-Trueba et al. (2018) did not find statins to improve cognition in AD patients (Mejias-Trueba et al., 2018), a more recent meta-analysis by Xuan et al. (2020) found that statins used in AD patients had beneficial short-term effects on MMSE scores, delaying the deterioration of neuropsychiatric status and significantly improving activities of daily living. However, no benefits were found in the ADAS-cog scores (Xuan et al., 2020).
Animal studies have suggested that TNF-α inhibitors may improve cognition and behavior. However, human studies have been limited (Ekert et al., 2018). Sodium benzoate has been shown to be a cognitive enhancer in patients with AD, schizophrenia, or late-life depression (Lane et al., 2022). On the other hand, estrogen has been shown to delay disease progression and minimize cognitive decline in AD patients, especially in women. However, hormone replacement therapy should be carefully considered due to its potential side effects (Zhou et al., 2020). The new selective glycine transporter-1 inhibitor, BI 425809, has not shown significant clinical improvement in patients with probable AD dementia (Wunderlich et al., 2023). Anti-inflammatory drugs may be beneficial in preventing dementia, although there is no evidence to support the use of aspirin or other NSAIDs (Jordan et al., 2020; Davis et al., 2021). According to epidemiological and laboratory studies, anti-inflammatory drugs may delay or prevent the onset of AD. In observational studies, the use of NSAIDs is significantly associated with a lower risk of AD, especially in long-term users. However, there is no support from RCTs. Neuroinflammation participates in the pathogenic cascades of AD. One possible mode of action for the effectiveness of NSAIDs is through the blocking of COX-2 in the brain. In addition, NSAIDs can also function by activating peroxisome proliferator-activated nuclear receptors, a group of nuclear hormone receptors that act to negatively inhibit the transcription of proinflammatory genes such as IL-6, TNF-α, COX-2, NOS, and cytokines (Wang et al., 2015).
Benzodiazepines and related drugs have been associated with an increased risk of AD in old age and adverse events in patients with mild to moderate AD (Dyer et al., 2020). Eszopiclone may improve sleep quality and cognitive function in elderly patients with AD and sleep disorders (Huo et al., 2022). The combination of various treatments has a better effect than the use of a single treatment or monotherapy in patients with AD, both in moderate and severe stages (Glinz et al., 2019; Guo et al., 2020; Knorz and Quante, 2022). Although our results suggest that methylphenidate has positive effects on apathy associated with AD (Padala et al., 2018; Mintzer et al., 2021), longer follow-up studies are needed to evaluate its efficacy (Lee et al., 2022). Idalopirdine, a selective 5-hydroxytryptamine6 receptor antagonist, has not demonstrated significant efficacy in patients with AD and has been associated with a higher incidence of adverse events (Matsunaga et al., 2019). Current evidence suggests that anti-tau drugs have little impact on slowing cognitive decline (Zheng et al., 2022). The presence of the APOE ε4 genotype, the main genetic risk factor for AD, does not influence the therapeutic effect of acetylcholinesterase inhibitors, but its relationship with other types of drugs is unknown (Cheng et al., 2018). Preclinical studies in transgenic models have suggested that DHP1401 has neuroprotective effects and improves memory. Although studies in humans with this drug are limited (Shim et al., 2022). According to a systematic review by Fink et al. (2018), the use of pharmacological treatments for cognitive protection in individuals with normal cognition or mild cognitive impairment has not been supported. Instead, most studies have been conducted in mild to moderate AD populations, and very few studies have been conducted in more advanced stages (Fink et al., 2018). Verubecestat and lanabecestat have been shown to worsen the cognitive status of patients with AD, although they may improve verbal fluency tasks (Wessels et al., 2020). A relationship has been found between hypertension and an increased risk of AD. Antihypertensive drugs can improve cognition and behavioral symptoms in patients with AD and reduce the incidence of cognitive disorders. Angiotensin receptor blockers are associated with a lower risk of AD, although their potential mechanisms remains unknown (Oscanoa et al., 2021; Rahimi et al., 2021). Epichaperomes play an important role in neuronal pathology, and their inhibition is a promising therapeutic approach for treating neurodegenerative diseases, including AD. However, drugs of this type are still in phase I, and their efficacy is unknown (Silverman et al., 2022). The effectiveness of thiazolidinediones in treating AD is influenced by APOE gene polymorphisms (Iketani et al., 2018). Finally, cannabis-based drugs may inhibit the progression of AD by modulating Aβ modifications. However, more research is needed to determine their efficacy in treating psychiatric manifestations of AD (Farkhondeh et al., 2020; Paunescu et al., 2020).
5 Limitations of the systematic review
Although RCTs are methodologically sound according to the JADAD scale, there is still significant variability in the types of interventions used. Only two studies (Padala et al., 2018; Mintzer et al., 2021) have investigated the same active ingredient, highlighting the need for further exploration in this area of research. Additionally, the majority of studies on drugs for AD are conducted in Caucasian populations, despite ethnicity being a factor that can affect treatment efficacy. Ethnic diversity in AD clinical trials remains inadequate (Franzen et al., 2022). Presently, there are no pharmacological therapies that modify the natural progression of AD. Clinical trials in this field typically involve only patients in the early stages of the disease, with those in the advanced stages underrepresented (Ruiz et al., 2022). Symptomatic anti-Alzheimer’s drugs are commonly employed in the treatment of the disease (Watanabe et al., 2019). Overall, our findings suggest that Alzheimer’s pharmacology is a constantly evolving field with significant implications for the development of new pharmacological therapies. We emphasize the critical need for further investigation in this area to advance our understanding of this disease. The findings of our systematic review on the latest advances in Alzheimer’s research indicate that there is still no definitive cure for this disease, at best, its progression can be slowed down. We believe that research into biogenetics and bioengineering could possibly pave the way for new lines of research for the treatment and cure of currently incurable diseases. Many of the drugs included in this systematic review are still in phase III, and therefore, little is currently known about them. Our review opens up new avenues for research into the treatment of AD. While we acknowledge that some of the studies included in our analysis have shown no significant difference between pharmacological intervention and placebo and, in some cases, even worsened the situation, we believe that reporting both positive and negative outcomes is crucial in scientific research to provide a reliable representation of reality.
6 Conclusion
In conclusion, although a potential improvement in cognitive function has been observed with some of the evaluated drugs, the limited number of available studies necessitates further research to determine their effectiveness and safety in treating cognitive impairments in Alzheimer’s disease.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
All the authors listed have made substantial, direct, and intellectual contributions to the work and approved it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abduljawad, A. A., Elawad, M. A., Elkhalifa, M. E. M., Ahmed, A., Hamdoon, A. A. E., Salim, L. H. M., et al. (2022). Alzheimer’s disease as a major public health concern: Role of dietary saponins in mitigating neurodegenerative disorders and their underlying mechanisms. Molecules 27 (20), 6804–6823. doi:10.3390/molecules27206804
Ahmad, S., Ahmed, S. B., Khan, A., Wasim, M., Tabassum, S., Haider, S., et al. (2023). Natural remedies for Alzheimer’s disease: A systematic review of randomized controlled trials. Metab. Brain Dis. 38 (1), 17–44. doi:10.1007/s11011-022-01063-9
Akhgarjand, C., Vahabi, Z., Shab-Bidar, S., Etesam, F., and Djafarian, K. (2022). Effects of probiotic supplements on cognition, anxiety, and physical activity in subjects with mild and moderate Alzheimer’s disease: A randomized, double-blind, and placebo-controlled study. Front. Aging Neurosci. 14, 1032494. doi:10.3389/fnagi.2022.1032494
Albrahim, T. (2020). The potential role of nutritional components in improving brain function among patients with alzheimer’s disease: A meta-analysis of RCT studies. Neurosciences 25 (1), 4–17. doi:10.17712/nsj.2020.1.20190037
Ancidoni, A., Bacigalupo, I., Remoli, G., Lacorte, E., Piscopo, P., Sarti, G., et al. (2021). Anticancer drugs repurposed for Alzheimer’s disease: A systematic review. Alzheimer’s Res. Ther. 13 (1), 96. doi:10.1186/s13195-021-00831-6
Andrade, C. (2022). Methylphenidate and other pharmacologic treatments for apathy in alzheimer’s disease. J. Clin. Psychiatry 83 (1), 14398. doi:10.4088/jcp.22f14398
Araya-Quintanilla, F., Gutiérrez-Espinoza, H., Sánchez-Montoya, U., Muñoz-Yañez, M. J., Baeza-Vergara, A., Petersen-Yanjarí, M., et al. (2020). Effectiveness of omega-3 fatty acid supplementation in patients with alzheimer disease: A systematic review and meta-analysis. Neurologia 35 (2), 105–114. doi:10.1016/j.nrl.2017.07.009
Avgerinos, K. I., Egan, J. M., Mattson, M. P., and Kapogiannis, D. (2020a). Medium Chain Triglycerides induce mild ketosis and may improve cognition in Alzheimer’s disease. A systematic review and meta-analysis of human studies. Ageing Res. Rev. 58, 101001. doi:10.1016/j.arr.2019.101001
Avgerinos, K. I., Ferrucci, L., Kapogiannis, D., Avgerinos, I. K., Ferrucci, L., and Kapogiannis, D. (2021). Effects of monoclonal antibodies against amyloid-beta on clinical and biomarker outcomes and adverse event risks: A systematic review and meta-analysis of phase III RCTs in alzheimer’s disease. AGEING Res. Rev. 68, 101339. doi:10.1016/j.arr.2021.101339
Avgerinos, K. I., Kalaitzidis, G., Malli, A., Kalaitzoglou, D., Myserlis, P. G., and Lioutas, V.-A. (2018). Intranasal insulin in alzheimer’s dementia or mild cognitive impairment: A systematic review. J. Neurol. 265 (7), 1497–1510. doi:10.1007/s00415-018-8768-0
Avgerinos, K. I., Vrysis, C., Chaitidis, N., Kolotsiou, K., Myserlis, P. G., and Kapogiannis, D. (2020b). Effects of saffron (crocus sativus L) on cognitive function: A systematic review of RCTs. Neurol. Sci. 41 (10), 2747–2754. doi:10.1007/s10072-020-04427-0
Ballard, C. G., Coate, B., Abler, V., Stankovic, S., and Foff, E. (2020). Evaluation of the efficacy of pimavanserin in the treatment of agitation and aggression in patients with alzheimer’s disease psychosis: A post hoc analysis. Int. J. Geriatr. Psychiatry 35 (11), 1402–1408. doi:10.1002/gps.5381
Birks, J. S., and Harvey, R. J. (2018). Donepezil for dementia due to Alzheimer’s disease. Cochrane Database Syst. Rev. 6 (6), CD001190. doi:10.1002/14651858.CD001190.pub3
Blanco-Silvente, L. L., Capellà, D., Garre-Olmo, J., Vilalta-Franch, J., Castells, X., Capella, D., et al. (2018). Predictors of discontinuation, efficacy, and safety of memantine treatment for alzheimer’s disease: meta-analysis and meta-regression of 18 randomized clinical trials involving 5004 patients. BMC Geriatr. 18 (1), 168. doi:10.1186/s12877-018-0857-5
Bonvicini, M., Travaglini, S., Lelli, D., Antonelli Incalzi, R., and Pedone, C. (2023). Is citicoline effective in preventing and slowing down dementia?—a systematic review and a meta-analysis. Nutrients 15 (2), 386. doi:10.3390/nu15020386
Budd Haeberlein, S., Aisen, P. S., Barkhof, F., Chalkias, S., Chen, T., Cohen, S., et al. (2022). Two randomized phase 3 studies of aducanumab in early alzheimer’s disease. J. Prev. Alzheimer’s Dis. 9 (2), 197–210. doi:10.14283/jpad.2022.30
Buglio, D. S., Marton, L. T., Laurindo, L. F., Guiguer, E. L., Araújo, A. C., Buchaim, R. L., et al. (2022). The role of resveratrol in mild cognitive impairment and alzheimer’s disease: A systematic review. J. Med. Food 25 (8), 797–806. doi:10.1089/jmf.2021.0084
Burckhardt, M., Watzke, S., Wienke, A., Langer, G., and Fink, A. (2020). Souvenaid for Alzheimer’s disease. Cochrane Database Syst. Rev. 2020 (12), 2020. doi:10.1002/14651858.cd011679.pub2
Canhada, S., Castro, K., Perry, I. S., and Luft, V. C. (2018). Omega-3 fatty acids’ supplementation in alzheimer’s disease: A systematic review. Nutr. Neurosci. 21 (8), 529–538. doi:10.1080/1028415X.2017.1321813
Cao, B., Rosenblat, J. D., Brietzke, E., Park, C., Lee, Y., Musial, N., et al. (2018). Comparative efficacy and acceptability of antidiabetic agents for alzheimer’s disease and mild cognitive impairment: A systematic review and network meta-analysis. Diabetes, Obes. Metab. 20 (10), 2467–2471. doi:10.1111/dom.13373
Cardinali, C. A. E. F., Martins, Y. A., and Torrão, A. S. (2021). Use of hormone therapy in postmenopausal women with alzheimer’s disease: A systematic review. Drugs Aging 38 (9), 769–791. doi:10.1007/s40266-021-00878-y
Carson, R. E., Naganawa, M., Toyonaga, T., Koohsari, S., Yang, Y., Chen, M. K., et al. (2022). Imaging of synaptic density in neurodegenerative disorders. J. Nucl. Med. 63 (1), 60S–67S. doi:10.2967/jnumed.121.263201
Čater, M., and Hölter, S. M. (2022). A pathophysiological intersection of diabetes and alzheimer’s disease. Int. J. Mol. Sci. 23 (19), 11562. doi:10.3390/ijms231911562
Chen, H., Liu, S., Ge, B., Zhou, D., Li, M., Li, W., et al. (2021). Effects of folic acid and vitamin B12 supplementation on cognitive impairment and inflammation in patients with alzheimer’s disease: A randomized, single-blinded, placebo-controlled trial. J. Prev. Alzheimer’s Dis. 8 (3), 249–256. doi:10.14283/jpad.2021.22
Chen, W., Cai, W., Hoover, B., and Kahn, C. R. (2022). Insulin action in the brain: Cell types, circuits, and diseases. Trends Neurosci. 45 (5), 384–400. doi:10.1016/j.tins.2022.03.001
Cheng, Y.-C., Huang, Y.-C., and Liu, H.-C. (2018). Effect of apolipoprotein E epsilon 4 carrier status on cognitive response to acetylcholinesterase inhibitors in patients with alzheimer’s disease: A systematic review and meta-analysis. Dement. Geriatr. Cogn. Disord. 45 (5–6), 335–352. doi:10.1159/000490175
Craft, S., Raman, R., Chow, T. W., Rafii, M. S., Sun, C.-K. K., Rissman, R. A., et al. (2020). Safety, efficacy, and feasibility of intranasal insulin for the treatment of mild cognitive impairment and alzheimer disease dementia A randomized clinical trial. JAMA Neurol. 77 (9), 1099–1109. doi:10.1001/jamaneurol.2020.1840
Cumbo, E., Cumbo, S., Torregrossa, S., and Migliore, D. (2019). Treatment effects of vortioxetine on cognitive functions in mild alzheimer’s disease patients with depressive symptoms: A 12 Month, open-label, observational study. J. Prev. Alzheimer’s Dis. 6 (3), 192–197. doi:10.14283/jpad.2019.24
Davis, K. A. S., Bishara, D., Molokhia, M., Mueller, C., Perera, G., and Stewart, R. J. (2021). Aspirin in people with dementia, long-term benefits, and harms: A systematic review. Eur. J. Clin. Pharmacol. 77 (7), 943–954. doi:10.1007/s00228-021-03089-x
de Andrade Teles, R. B., Diniz, T. C. T. C., Costa Pinto, T. C., De Oliveira, R. G., e Silva, M. G., De Lavor, É. M., et al. (2018). Flavonoids as therapeutic agents in alzheimer’s and Parkinson’s diseases: A systematic review of preclinical evidences. Oxid. Med. Cell Longev. 2018, 7043213. doi:10.1155/2018/7043213
Den, H., Dong, X., Chen, M., Zou, Z., Deng, H., Dong, X., et al. (2020). Efficacy of probiotics on cognition, and biomarkers of inflammation and oxidative stress in adults with Alzheimer’s disease or mild cognitive impairment - a meta-analysis of randomized controlled trials. Aging (Albany NY) 12 (4), 4010–4039. doi:10.18632/aging.102810
Dhadda, S., Kanekiyo, M., Li, D., Swanson, C. J., Irizarry, M., Berry, S., et al. (2022). Consistency of efficacy results across various clinical measures and statistical methods in the lecanemab phase 2 trial of early Alzheimer’s disease. Alzheimers Res. Ther. 14 (1), 182. doi:10.1186/s13195-022-01129-x
Dhapola, R., Sarma, P., Medhi, B., Prakash, A., and Reddy, D. H. K. (2022). Recent advances in molecular pathways and therapeutic implications targeting mitochondrial dysfunction for alzheimer’s disease. Mol. Neurobiol. 59 (1), 535–555. doi:10.1007/s12035-021-02612-6
Ding, M. R., Qu, Y. J., Hu, B., and An, H. M. (2022). Signal pathways in the treatment of Alzheimer’s disease with traditional Chinese medicine. Biomed. Pharmacother. 152, 113208. doi:10.1016/j.biopha.2022.113208
Du, Y., Liang, F., Zhang, L., Liu, J., and Dou, H. (2020). Vitamin D supplement for prevention of alzheimer’s disease: A systematic review and meta-analysis. Am. J. Ther. 28 (6), e638–e648. doi:10.1097/MJT.0000000000001302
Dubois, B., Lopez-Arrieta, J., Lipschitz, S., Triantafyllos, D., Spiru, L., Moroz, S., et al. (2023). Masitinib for mild-to-moderate alzheimer’s disease: Results from a randomized, placebo-controlled, phase 3, clinical trial. Alzheimers Res. Ther. 15 (1), 39. doi:10.1186/s13195-023-01169-x
Dyer, A. H., Murphy, C., Lawlor, B., Kennelly, S., Segurado, R., Olde Rikkert, M. G. M., et al. (2020). Cognitive outcomes of long-term benzodiazepine and related drug (BDZR) use in people living with mild to moderate alzheimer’s disease: Results from NILVAD. J. Am. Med. Dir. Assoc. 21 (2), 194–200. doi:10.1016/j.jamda.2019.08.006
Egan, M. F., Kost, J., Tariot, P. N., Aisen, P. S., Cummings, J. L., Vellas, B., et al. (2018). Randomized trial of verubecestat for mild-to-moderate alzheimer’s disease. N. Engl. J. Med. 378 (18), 1691–1703. doi:10.1056/NEJMoa1706441
Ekert, J. O., Gould, R. L., Reynolds, G., and Howard, R. J. (2018). TNF alpha inhibitors in alzheimer’s disease: A systematic review. Int. J. Geriatr. Psychiatry 33 (5), 688–694. doi:10.1002/gps.4871
Espiritu, A. I., and Cenina, A. R. F. (2020). The effectiveness and tolerability of the high dose donepezil at 23 mg tablet per day for alzheimer’s disease: A meta-analysis of randomized controlled trials. Acta Med. Philipp. 54 (3), 296–304. doi:10.47895/amp.v54i3.1669
Fang, X., Zhang, J., Zhao, J., and Wang, L. (2022). Effect of resveratrol combined with donepezil hydrochloride on inflammatory factor level and cognitive function level of patients with alzheimer’s disease. J. Healthc. Eng. 2022, 1–7. doi:10.1155/2022/9148650
Fang, Z., Tang, Y., Ying, J., Tang, C., and Wang, Q. (2020). Traditional Chinese medicine for anti-alzheimer’s disease: Berberine and evodiamine from evodia rutaecarpa. Chin. Med. 15 (1), 82. doi:10.1186/s13020-020-00359-1
Farkhondeh, T., Khan, H., Aschner, M., Samini, F., Pourbagher-Shahri, A. M., Aramjoo, H., et al. (2020). Impact of cannabis-based medicine on alzheimer’s disease by focusing on the amyloid β-modifications: A systematic study. CNS Neurol. Disord. - Drug Targets. 19 (5), 334–343. doi:10.2174/1871527319666200708130745
Fink, H. A., Jutkowitz, E., McCarten, J. R., Hemmy, L. S., Butler, M., Davila, H., et al. (2018). Pharmacologic interventions to prevent cognitive decline, mild cognitive impairment, and clinical alzheimer-type dementia A systematic review. Ann. Intern Med. 168 (1), 39–51. doi:10.7326/M17-1529
Forlenza, O. V., Radanovic, M., Talib, L. L., and Gattaz, W. F. (2019). Clinical and biological effects of long-term lithium treatment in older adults with amnestic mild cognitive impairment: Randomised clinical trial. Br. J. Psychiatry 215 (5), 668–674. doi:10.1192/bjp.2019.76
Foroumandi, E., Javan, R., Moayed, L., Fahimi, H., Kheirabadi, F., Neamatshahi, M., et al. (2023). The effects of fenugreek seed extract supplementation in patients with alzheimer’s disease: A randomized, double-blind, placebo-controlled trial. Phyther Res. 37 (1), 285–294. doi:10.1002/ptr.7612
Foroutan, N., Hopkins, R. B., Tarride, J.-E. E., Florez, I. D., and Levine, M. (2019). Safety and efficacy of active and passive immunotherapy in mild-to-moderate alzheimer’s disease: A systematic review and network meta-analysis. Clin. Investig. Med. 42 (1), E53–E65. doi:10.25011/cim.v42i1.32393
Franzen, S., Smith, J. E., van den Berg, E., Rivera Mindt, M., van Bruchem-Visser, R. L., Abner, E. L., et al. (2022). Diversity in Alzheimer’s disease drug trials: The importance of eligibility criteria. Alzheimer’s Dement. 18 (4), 810–823. doi:10.1002/alz.12433
Frölich, L., Wunderlich, G., Thamer, C., Roehrle, M., Garcia, M., Dubois, B., et al. (2019). Evaluation of the efficacy, safety and tolerability of orally administered BI 409306, a novel phosphodiesterase type 9 inhibitor, in two randomised controlled phase II studies in patients with prodromal and mild Alzheimer’s disease. ALZHEIMERS Res. Ther. 11 (1), 18. doi:10.1186/s13195-019-0467-2
Ghaffari, M., Sanadgol, N., and Abdollahi, M. (2020). A systematic review of current progresses in the nucleic acid-based therapies for neurodegeneration with implications for alzheimer’s disease. Mini-Reviews Med. Chem. 20 (15), 1499–1517. doi:10.2174/1389557520666200513122357
Gibson, G. E., Luchsinger, J. A., Cirio, R., Chen, H., Franchino-Elder, J., Hirsch, J. A., et al. (2020). Benfotiamine and cognitive decline in alzheimer’s disease: Results of a randomized placebo-controlled phase IIa clinical trial. J. Alzheimer’s Dis. 78 (3), 989–1010. doi:10.3233/JAD-200896
Glinz, D., Gloy, V. L., Monsch, A. U., Kressig, R. W., Patel, C., McCord, K. A., et al. (2019). Acetylcholinesterase inhibitors combined with memantine for moderate to severe alzheimer’s disease: A meta-analysis. Swiss Med. Wkly. 149 (25–26), w20093. doi:10.4414/smw.2019.20093
Gu, J., Li, Z., Chen, H., Xu, X., Li, Y., and Gui, Y. (2021). Neuroprotective effect of trans-resveratrol in mild to moderate alzheimer disease: A randomized, double-blind trial. Neurol. Ther. 10 (2), 905–917. doi:10.1007/s40120-021-00271-2
Guo, J., Wang, Z., Liu, R., Huang, Y., Zhang, N., and Zhang, R. (2020). Memantine, donepezil, or combination therapy—what is the best therapy for alzheimer’s disease? A network meta-analysis. Brain Behav. 10 (11), e01831. doi:10.1002/brb3.1831
Guzman-Martinez, L., Farías, G. A., Tapia, J. P., Sánchez, M. P., Fuentes, P., Gloger, S., et al. (2021). Interventional study to evaluate the clinical effects and safety of the nutraceutical compound BrainUp-10® in a cohort of patients with alzheimer’s disease: A multicenter, randomized, double-blind, and placebo-controlled trial. J. Alzheimer’s Dis. 81 (3), 1231–1241. doi:10.3233/JAD-201501
Hang-kun, M., Yue, L., Bo, L., Ying, Z., Lin-juan, S., and Feng-qin, X. (2018). Chinese medicine for alzheimer’s disease: A meta-analysis of randomized controlled trials. Chin. J. Integr. Med. 24 (12), 938–943. doi:10.1007/s11655-018-2567-4
Herrmann, N., Ruthirakuhan, M., Gallagher, D., Verhoeff, N. P. L. G. G., Kiss, A., Black, S. E., et al. (2019). Randomized placebo-controlled trial of nabilone for agitation in alzheimer’s disease. Am. J. Geriatr. PSYCHIATRY 27 (11), 1161–1173. doi:10.1016/j.jagp.2019.05.002
Hong, Y. J., Han, H. J., Youn, Y. C., Park, K. W., Yang, D. W., Kim, S., et al. (2019). Safety and tolerability of donepezil 23mg with or without intermediate dose titration in patients with alzheimer’s disease taking donepezil 10mg: A multicenter, randomized, open-label, parallel-design, three-arm, prospective trial. ALZHEIMERS Res. Ther. 11 (1), 37. doi:10.1186/s13195-019-0492-1
Huang, P., He, X.-Y. Y., and Xu, M. (2021). Dengzhan shengmai capsule combined with donepezil hydrochloride in the treatment of alzheimer’s disease: Preliminary findings, randomized and controlled clinical trial. Rev. Assoc. Med. Bras. 67 (2), 190–194. doi:10.1590/1806-9282.67.02.20200378
Huo, S., Cheng, L., Li, S., and Xu, F. (2022). Effects of eszopiclone on sleep quality and cognitive function in elderly patients with alzheimer’s disease and sleep disorder: A randomized controlled trial. Brain Behav. 12 (2), e2488. doi:10.1002/brb3.2488
Iketani, R., Ohno, K., Kawasaki, Y., Matsumoto, K., Yamada, H., and Kishino, S. (2018). Apolipoprotein E gene polymorphisms affect the efficacy of thiazolidinediones for alzheimer’s disease: A systematic review and meta-analysis. Biol. Pharm. Bull. 41 (7), 1017–1023. doi:10.1248/bpb.b17-00929
Jadad Bechara, A. (1996). Jadad scale, journal of controlled clinical trials. Available at: https://es.scribd.com/doc/191898470/Escala-de-Jadad.
Jeremic, D., Jiménez-Díaz, L., and Navarro-López, J. D. (2021). Past, present and future of therapeutic strategies against amyloid-β peptides in alzheimer’s disease: A systematic review. Ageing Res. Rev. 72, 101496. doi:10.1016/j.arr.2021.101496
Jernerén, F., Cederholm, T., Refsum, H., Smith, A. D., Turner, C., Palmblad, J., et al. (2019). Homocysteine status modifies the treatment effect of omega-3 fatty acids on cognition in a randomized clinical trial in mild to moderate alzheimer’s disease: The OmegAD study. J. ALZHEIMERS Dis. 69 (1), 189–197. doi:10.3233/JAD-181148
Jia, J., Hu, J., Huo, X., Miao, R., Zhang, Y., and Ma, F. (2019). Effects of vitamin D supplementation on cognitive function and blood aβ-related biomarkers in older adults with alzheimer’s disease: A randomised, double-blind, placebo-controlled trial. J. Neurol. Neurosurg. Psychiatry 90 (12), 1347–1352. doi:10.1136/jnnp-2018-320199
Jordan, F., Quinn, T. J., McGuinness, B., Passmore, P., Kelly, J. P., Tudur Smith, C., et al. (2020). Aspirin and other non-steroidal anti-inflammatory drugs for the prevention of dementia. Cochrane Database Syst. Rev. 4 (4), CD011459. doi:10.1002/14651858.CD011459.pub2
Juby, A. G., Blackburn, T. E., and Mager, D. R. (2022). Use of medium chain triglyceride (mct) oil in subjects with alzheimer’s disease: A randomized, double-blind, placebo-controlled, crossover study, with an open-label extension. Alzheimer’s Dement. Transl. Res. Clin. Interv. 8 (1), e12259. doi:10.1002/trc2.12259
Karthika, C., Appu, A. P., Akter, R., Rahman, M. H., Tagde, P., Ashraf, G. M., et al. (2022). Potential innovation against alzheimer’s disorder: A tricomponent combination of natural antioxidants (vitamin E, quercetin, and basil oil) and the development of its intranasal delivery. Environ. Sci. Pollut. Res. 29 (8), 10950–10965. doi:10.1007/s11356-021-17830-7
Kellar, D., Lockhart, S. N., Aisen, P., Raman, R., Rissman, R. A., Brewer, J., et al. (2021). Intranasal insulin reduces white matter hyperintensity progression in association with improvements in cognition and CSF biomarker profiles in mild cognitive impairment and alzheimer’s disease. J. Prev. Alzheimer’s Dis. 8 (3), 1–9. doi:10.14283/jpad.2021.14
Kellar, D., Register, T., Lockhart, S. N., Aisen, P., Raman, R., Rissman, R. A., et al. (2022). Intranasal insulin modulates cerebrospinal fluid markers of neuroinflammation in mild cognitive impairment and alzheimer’s disease: A randomized trial. Sci. Rep. 12 (1), 1346. doi:10.1038/s41598-022-05165-3
Kim, Y., and Cho, S. H. (2020). Danggui-shaoyao-san for dementia: A PRISMA-compliant systematic review and meta-analysis. Med. (United States) 99 (4), e18507. doi:10.1097/MD.0000000000018507
Kishi, T., Matsunaga, S., and Iwata, N. (2018). Memantine treatment for Japanese patients with moderate to severe alzheimer’s disease: A meta-analysis of double-blind, randomized, placebo-controlled trials. Neuropsychiatr. Dis. Treat. 14, 2915–2922. doi:10.2147/NDT.S187320
Knorz, A. L., and Quante, A. (2022). Alzheimer’s disease: Efficacy of mono- and combination therapy. A systematic review. J. Geriatr. Psychiatry Neurol. 35 (4), 475–486. doi:10.1177/08919887211044746
Koch, G., Motta, C., Bonnì, S., Pellicciari, M. C., Picazio, S., Casula, E. P., et al. (2020). Effect of rotigotine vs placebo on cognitive functions among patients with mild to moderate alzheimer disease A randomized clinical trial. JAMA Netw. OPEN 3 (7), e2010372. doi:10.1001/jamanetworkopen.2020.10372
Koh, S.-H. H., Kwon, H. S., Choi, S. H., Jeong, J. H., Na, H. R., Lee, C. N., et al. (2021). Efficacy and safety of GV1001 in patients with moderate-to-severe alzheimer’s disease already receiving donepezil: A phase 2 randomized, double-blind, placebo-controlled, multicenter clinical trial. ALZHEIMERS Res. Ther. 13 (1), 66. doi:10.1186/s13195-021-00803-w
Korabecny, J., Spilovska, K., Mezeiova, E., Benek, O., Juza, R., Kaping, D., et al. (2019). A systematic review on donepezil-based derivatives as potential cholinesterase inhibitors for alzheimer’s disease. Curr. Med. Chem. 26 (30), 5625–5648. doi:10.2174/0929867325666180517094023
Lacorte, E., Ancidoni, A., Zaccaria, V., Remoli, G., Tariciotti, L., Bellomo, G., et al. (2022). Safety and efficacy of monoclonal antibodies for alzheimer’s disease: A systematic review and meta-analysis of published and unpublished clinical trials. J. ALZHEIMERS Dis. 87 (1), 101–129. doi:10.3233/JAD-220046
Lacosta, A.-M. M., Pascual-Lucas, M. M., Pesini, P., Casabona, D., Pérez-Grijalba, V., Marcos-Campos, I., et al. (2018). Safety, tolerability and immunogenicity of an active anti-aβ40 vaccine (ABvac40) in patients with Alzheimer's disease: A randomised, double-blind, placebo-controlled, phase I trial. ALZHEIMERS Res. Ther. 10 (1), 12. doi:10.1186/s13195-018-0340-8
Lane, H. Y., Wang, S. H., and Lin, C. H. (2022). Endogenous antioxidants predicted outcome and increased after treatment: A benzoate dose-finding, randomized, double-blind, placebo-controlled trial for alzheimer’s disease. Psychiatry Clin. Neurosci. 77, 102–109. doi:10.1111/pcn.13504
Lang, F. M., Mo, Y., Sabbagh, M., Solomon, P., Boada, M., Jones, R. W., et al. (2021). Intepirdine as adjunctive therapy to donepezil for mild-to-moderate alzheimer’s disease: A randomized, placebo-controlled, phase 3 clinical trial (mindset). Alzheimer’s Dement. Transl. Res. Clin. Interv. 7 (1), e12136. doi:10.1002/trc2.12136
Lawlor, B., Segurado, R., Kennelly, S., Olde Rikkert, M. G. M., Howard, R., Pasquier, F., et al. (2018). Nilvadipine in mild to moderate alzheimer disease: A randomised controlled trial. PLOS Med. 15 (9), e1002660. doi:10.1371/journal.pmed.1002660
Lee, C. W., Chen, J. Y., Ko, C. C., Chuang, M. H., Tsai, W. W., Sun, C. K., et al. (2022). Efficacy of methylphenidate for the treatment of apathy in patients with alzheimer’s disease: A systematic review and meta-analysis of randomized controlled studies. Psychopharmacol. Berl. 239 (12), 3743–3753. doi:10.1007/s00213-022-06261-y
Levine, S. Z., Goldberg, Y., Yoshida, K., Samara, M., Cipriani, A., Iwatsubo, T., et al. (2022). Early- and subsequent-response of cognitive functioning in Alzheimer’s disease: Individual-participant data from five pivotal randomized clinical trials of donepezil. J. Psychiatr. Res. 148, 159–164. doi:10.1016/j.jpsychires.2022.01.055
Levine, S. Z., Yoshida, K., Goldberg, Y., Samara, M., Cipriani, A., Efthimiou, O., et al. (2021). Linking the mini-mental state examination, the alzheimer’s disease assessment scale-cognitive Subscale and the severe impairment Battery: Evidence from individual participant data from five randomised clinical trials of donepezil. Evid. Based Ment. Health 24 (2), 56–61. doi:10.1136/ebmental-2020-300184
Li, D. D., Zhang, Y. H., Zhang, W., and Zhao, P. (2019). Meta-analysis of randomized controlled trials on the efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of Alzheimer’s disease. Front. Neurosci. 13, 472. doi:10.3389/fnins.2019.00472
Li, J., Du, Q., Li, N., Du, S., and Sun, Z. (2021). Alpiniae oxyphyllae Fructus and Alzheimer’s disease: An update and current perspective on this traditional Chinese medicine. Biomed. Pharmacother. 135, 111167. doi:10.1016/j.biopha.2020.111167
Liang, J., Li, J., Jia, R., Wang, Y., Wu, R., Zhang, H., et al. (2018b). Identification of the optimal cognitive drugs among alzheimer’s disease: A bayesian meta-analytic review. Clin. Interv. Aging 13, 2061–2073. doi:10.2147/CIA.S184968
Liang, J., Xu, Y., Lin, L., Jia, R., Zhang, H., and Hang, L. (2018a). Comparison of multiple interventions for older adults with alzheimer disease or mild cognitive impairment: A PRISMA-compliant network meta-analysis. Med. Baltim. 97 (20), e10744. doi:10.1097/MD.0000000000010744
Liao, Z., Cheng, L., Li, X., Zhang, M., Wang, S., and Huo, R. (2020). Meta-analysis of ginkgo biloba preparation for the treatment of alzheimer’s disease. Clin. Neuropharmacol. 43 (4), 93–99. doi:10.1097/WNF.0000000000000394
Ling, Y., Hao, Z. Y., Liang, D., Zhang, C. L., Liu, Y. F., and Wang, Y. (2021). The expanding role of pyridine and dihydropyridine scaffolds in drug design. Drug Des. Devel Ther. 15, 4289–4338. doi:10.2147/DDDT.S329547
Liu, C., Guo, X., and Chang, X. (2022). Intestinal flora balance therapy based on probiotic support improves cognitive function and symptoms in patients with alzheimer’s disease: A systematic review and meta-analysis. Biomed. Res. Int. 2022, 4806163. doi:10.1155/2022/4806163
Louzada, L. L., Machado, F. V., Quintas, J. L., Ribeiro, G. A., Silva, M. V., Mendonça-Silva, D. L., et al. (2022). The efficacy and safety of zolpidem and zopiclone to treat insomnia in alzheimer’s disease: A randomized, triple-blind, placebo-controlled trial. Neuropsychopharmacology 47 (2), 570–579. doi:10.1038/s41386-021-01191-3
Luo, A., Ning, P., Lu, H., Huang, H., Shen, Q., Zhang, D., et al. (2022). Association between metformin and alzheimer’s disease: A systematic review and meta-analysis of clinical observational studies. J. Alzheimer’s Dis. 88 (4), 1311–1323. doi:10.3233/JAD-220180
Mahnashi, M. H., Alshahrani, M. A., Nahari, M. H., Hassan, S. S., Jan, M. S., Ayaz, M., et al. (2022). In-vitro, in-vivo, molecular docking and ADMET studies of 2-substituted 3,7-dihydroxy-4H-chromen-4-one for oxidative stress, inflammation and alzheimer’s disease. Metabolites 12 (11), 1055. doi:10.3390/metabo12111055
Maitre, Y., Mahalli, R., Micheneau, P., Delpierre, A., Amador, G., and Denis, F. (2021). Evidence and therapeutic perspectives in the relationship between the oral microbiome and alzheimer’s disease: A systematic review. Int. J. Environ. Res. Public Health 18 (21), 11157. doi:10.3390/ijerph182111157
Malin, S. K., Stewart, N. R., Ude, A. A., and Alderman, B. L. (2022). Brain insulin resistance and cognitive function: Influence of exercise. J. Appl. Physiol. 133 (6), 1368–1380. doi:10.1152/japplphysiol.00375.2022
Manolopoulos, A., Andreadis, P., Malandris, K., Avgerinos, I., Karagiannis, T., Kapogiannis, D., et al. (2019). Intravenous immunoglobulin for patients with alzheimer’s disease: A systematic review and meta-analysis. Am. J. Alzheimers Dis. Other Demen 34 (5), 281–289. doi:10.1177/1533317519843720
Marucci, G., Buccioni, M., Ben, D. D., Lambertucci, C., Volpini, R., and Amenta, F. (2021). Efficacy of acetylcholinesterase inhibitors in Alzheimer’s disease. Neuropharmacology 190, 108352. doi:10.1016/j.neuropharm.2020.108352
Matsunaga, S., Fujishiro, H., and Takechi, H. (2019). Efficacy and safety of idalopirdine for alzheimer’s disease: A systematic review and meta-analysis. Int. Psychogeriatrics 31 (11), 1627–1633. doi:10.1017/S1041610218002156
Matsunaga, S., Kishi, T., Nomura, I., Sakuma, K., Okuya, M., Ikuta, T., et al. (2018). The efficacy and safety of memantine for the treatment of Alzheimer’s disease. Expert Opin. Drug Saf. 17 (10), 1053–1061. doi:10.1080/14740338.2018.1524870
May, B. H., Feng, M., Hyde, A. J., Hugel, H., Chang, S., Dong, L., et al. (2018). Comparisons between traditional medicines and pharmacotherapies for alzheimer disease: A systematic review and meta-analysis of cognitive outcomes. Int. J. Geriatr. Psychiatry 33 (3), 449–458. doi:10.1002/gps.4830
Mccleery, J., Abraham, R. P., Denton, D. A., Rutjes, A. W. S., Chong, L. Y., Al-Assaf, A. S., et al. (2018). Vitamin and mineral supplementation for preventing dementia or delaying cognitive decline in people with mild cognitive impairment. Cochrane Database Syst. Rev. 11 (11), CD011905. doi:10.1002/14651858.CD011905.pub2
McShane, R., Westby, M. J., Roberts, E., Minakaran, N., Schneider, L., Farrimond, L. E., et al. (2019). Memantine for dementia. Cochrane Database Syst. Rev. 2019 (3), CD003154–446. doi:10.1002/14651858.CD003154.pub6
Mejias-Trueba, M., Antonia Perez-Moreno, M., and Angeles Fernandez-Arche, M. (2018). Systematic review of the efficacy of statins for the treatment of Alzheimer’s disease. Clin. Med. N. Il. 18 (1), 54–61. doi:10.7861/clinmedicine.18-1-54
Michailidis, M., Tata, D. A., Moraitou, D., Kavvadas, D., Karachrysafi, S., Papamitsou, T., et al. (2022). Antidiabetic drugs in the treatment of alzheimer’s disease. Int. J. Mol. Sci. 23 (9), 4641. doi:10.3390/ijms23094641
Miculas, D. C., Negru, P. A., Bungau, S. G., Behl, T., Hassan, S. S., and Tit, D. M. (2023). Pharmacotherapy evolution in alzheimer’s disease: Current framework and relevant directions. Cells 12 (1), 131–226. doi:10.3390/cells12010131
Mintzer, J., Lanctot, K. L., Scherer, R. W., Rosenberg, P. B., Herrmann, N., van Dyck, C. H., et al. (2021). Effect of methylphenidate on apathy in patients with alzheimer disease the ADMET 2 randomized clinical trial. JAMA Neurol. 78 (11), 1324–1332. doi:10.1001/jamaneurol.2021.3356
Miranda, A., Montiel, E., Ulrich, H., Paz, C., and Ferreira, S. (2021). Selective secretase targeting for alzheimer’s disease therapy. J. Alzheimers Dis. 81 (1), 1–17. doi:10.3233/JAD-201027
Moreira, S. C., Jansen, A. K., and Silva, F. M. (2020). Dietary interventions and cognition of alzheimer’s disease patients a systematic review of randomized controlled trial. Dement Neuropsychol 14 (3), 258–282. doi:10.1590/1980-57642020dn14-030008
Muangpaisan, W., Wiputhanuphongs, P., Jaisupa, N., Junnu, S., Samer, J., Moongkarndi, P., et al. (2022). Effects of water-soluble mangosteen extract on cognitive function and neuropsychiatric symptoms in patients with mild to moderate alzheimer’s disease (WECAN-AD): A randomized controlled trial. Alzheimer’s Dement. Transl. Res. Clin. Interv. 8 (1), e12292. doi:10.1002/trc2.12292
Mühlbauer, V., Möhler, R., Dichter, M. N., Zuidema, S. U., Köpke, S., and Luijendijk, H. J. (2021). Antipsychotics for agitation and psychosis in people with Alzheimer’s disease and vascular dementia. Cochrane Database Syst. Rev. 12 (12), CD013304. doi:10.1002/14651858.CD013304.pub2
Munõz-Jiménez, M., Zaarkti, A., Garciá-Arnés, J. A., and Garciá-Casares, N. (2021). Antidiabetic drugs in alzheimer’s disease and mild cognitive impairment: A systematic review. Dement. Geriatr. Cogn. Disord. 49 (5), 423–434. doi:10.1159/000510677
Musaeus, C. S., Nilsson, C., Cooper, C., Kramberger, M. G., Verdelho, A., Stefanova, E., et al. (2021). Pharmacological medical treatment of epilepsy in patients with dementia: A systematic review. Curr. Alzheimer Res. 18 (9), 689–694. doi:10.2174/1567205018666211126121529
Naomi, R., Embong, H., Othman, F., Ghazi, H. F., Maruthey, N., and Bahari, H. (2022). Probiotics for alzheimer’s disease: A systematic review. Nutrients 14 (1), 20. doi:10.3390/nu14010020
Nirogi, R., Jayarajan, P., Shinde, A., Mohammed, A. R., Grandhi, V. R., Benade, V., et al. (2023). Progress in investigational agents targeting serotonin-6 receptors for the treatment of brain disorders. Biomolecules 13 (2), 309. doi:10.3390/biom13020309
Noguchi-Shinohara, M., Hamaguchi, T., Sakai, K., Komatsu, J., Iwasa, K., Horimoto, M., et al. (2022). Effects of Melissa officinalis extract containing rosmarinic acid on cognition in older adults without dementia: A randomized controlled trial. J. Alzheimer’s Dis. 91 (2), 805–814. doi:10.3233/JAD-220953
Noguchi-Shinohara, M., Ono, K., Hamaguchi, T., Nagai, T., Kobayashi, S., Komatsu, J., et al. (2020). Safety and efficacy of Melissa officinalis extract containing rosmarinic acid in the prevention of Alzheimer’s disease progression. Sci. Rep. 10 (1), 18627. doi:10.1038/s41598-020-73729-2
Okuya, M., Matsunaga, S., Ikuta, T., Kishi, T., and Iwata, N. (2018). Efficacy, acceptability, and safety of intravenous immunoglobulin administration for mild-to-moderate alzheimer’s disease: A systematic review and meta-analysis. J. Alzheimer’s Dis. 66 (4), 1379–1387. doi:10.3233/JAD-180888
Oscanoa, T. J., Amado, J., Vidal, X., and Romero-Ortuno, R. (2021). Angiotensin-receptor blockers and the risk of alzheimer´s disease: A meta-analysis. Curr. Rev. Clin. Exp. Pharmacol. 16 (1), 73–78. doi:10.2174/1574884715666200131120224
Padala, P. R., Padala, K. P., Lensing, S. Y., Ramirez, D., Monga, V., Bopp, M. M., et al. (2018). Methylphenidate for apathy in community-dwelling older veterans with mild alzheimer’s disease: A double-blind, randomized, placebo-controlled trial. Am. J. Psychiatry 175 (2), 159–168. doi:10.1176/appi.ajp.2017.17030316
Paudel, P., Park, C. H., Jung, H. A., Yokozawa, T., and Choi, J. S. (2020). A systematic review on anti-Alzheimer’s disease activity of prescription Kangen-karyu. DRUG Discov. Ther. 14 (2), 61–66. doi:10.5582/ddt.2020.03013
Paunescu, H., Dima, L., Ghita, I., Coman, L., Ifteni, P. I., Fulga, I., et al. (2020). A systematic review of clinical studies on the effect of psychoactive cannabinoids in psychiatric conditions in alzheimer dementia. Am. J. Ther. 27 (3), e249–e269. doi:10.1097/MJT.0000000000001120
Pei, H., Ma, L., Cao, Y., Wang, F., Li, Z., Liu, N., et al. (2020). Traditional Chinese medicine for alzheimer’s disease and other cognitive impairment: A review. Am. J. Chin. Med. 48 (3), 487–511. doi:10.1142/S0192415X20500251
Pereira, M. E., Souza, J. V. J. V., Galiciolli, M. E. A., Sare, F., Vieira, G. S., Kruk, I. L., et al. (2022). Effects of selenium supplementation in patients with mild cognitive impairment or alzheimer’s disease: A systematic review and meta-analysis. Nutrients 14 (15), 3205. doi:10.3390/nu14153205
Phadke, A. V., Tayade, A. A., and Khambete, M. P. (2021). Therapeutic potential of ferulic acid and its derivatives in alzheimer’s disease—a systematic review. Chem. Biol. Drug Des. 98 (5), 713–721. doi:10.1111/cbdd.13922
Pisani, S., Mueller, C., Huntley, J., Aarsland, D., and Kempton, M. J. (2021). A meta-analysis of randomised controlled trials of physical activity in people with Alzheimer’s disease and mild cognitive impairment with a comparison to donepezil. Int. J. Geriatr. Psychiatry 36 (10), 1471–1487. doi:10.1002/gps.5581
Piscopo, P., Crestini, A., Carbone, E., Rivabene, R., Ancidoni, A., Lo Giudice, M., et al. (2022). A systematic review on drugs for synaptic plasticity in the treatment of dementia. Ageing Res. Rev. 81, 101726. doi:10.1016/j.arr.2022.101726
Prabhakar, S., Vishnu, V. Y., Modi, M., Mohanty, M., Sharma, A., Medhi, B., et al. (2020). Efficacy of bacopa monnieri (brahmi) and donepezil in alzheimer’s disease and mild cognitive impairment: A randomized double-blind parallel phase 2b study. Ann. Indian Acad. Neurol. 23 (6), 767–773. doi:10.4103/aian.AIAN_610_19
Qin, M., Wu, J., Zhou, Q., Liang, Z., and Su, Y. (2022). Global cognitive effects of second-generation antidepressants in patients with alzheimer’s disease: A systematic review and meta-analysis of randomized controlled trials. J. Psychiatr. Res. 155, 371–379. doi:10.1016/j.jpsychires.2022.09.039
Rahimi, R., Nikfar, S., Sadeghi, M., Abdollahi, M., Moghaddam, R. H., and Farzaei, M. H. (2021). Effect of antihypertensive drugs on cognition and behavioral symptoms of patients with alzheimer’s disease: A meta-analysis. Curr. Pharm. Biotechnol. 22 (11), 1511–1519. doi:10.2174/1386207323666201211101720
Rasi Marzabadi, L., Fazljou, S. M. B., Araj-Khodaei, M., Sadigh-Eteghad, S., Naseri, A., and Talebi, M. (2022). Saffron reduces some inflammation and oxidative stress markers in donepezil-treated mild-to-moderate alzheimer’s disease patients: A randomized double-blind placebo-control trial. J. Herb. Med. 34, 100574. doi:10.1016/j.hermed.2022.100574
Restrepo-Martínez, M., Restrepo Bernal, D., and Molina Castaño, C. F. (2022). Can lithium treatment be recommended for mild cognitive impairment and dementia due to alzheimer’s disease? Evidence on its efficacy and safety is lacking: Systematic review and meta-analysis. Rev. Colomb. Psiquiatr. 2022, 1–10.
Rong, X., Jiang, L., Qu, M., Hassan, S. S., and Liu, Z. (2020). Enhancing therapeutic efficacy of donepezil by combined therapy: A comprehensive review. Curr. Pharm. Des. 27 (3), 332–344. doi:10.2174/1381612826666201023144836
Rozankovic, P. B., Rozankovic, M., Badzak, J., Stojic, M., and Sporis, I. S. (2021). Impact of donepezil and memantine on behavioral and psychological symptoms of alzheimer disease: Six-month open-label study. Cogn. Behav. Neurol. 34 (4), 288–294. doi:10.1097/WNN.0000000000000285
Ruiz, A., Sánchez, D., Lafuente, A., Ortega, G., Buendía, M., Papasey, J., et al. (2022). Evaluation of the feasibility, safety and efficacy of the use of intravenous infusions of adenosine triphosphate (atp) in people affected by moderate to severe alzheimer’s disease: A double-blind masked clinical trial for dose finding. J. Prev. Alzheimer’s Dis. 9 (3), 425–434. doi:10.14283/jpad.2022.38
Ruthirakuhan, M., Lanctôt, K. L., Vieira, D., and Herrmann, N. (2019). Natural and synthetic cannabinoids for agitation and aggression in alzheimer’s disease: A meta-analysis. J. Clin. Psychiatry 80 (2), 18r12617. doi:10.4088/JCP.18r12617
Samudra, N., Ranasinghe, K., Kirsch, H., Rankin, K., and Miller, B. (2023). Etiology and clinical significance of network hyperexcitability in alzheimer’s disease: Unanswered questions and next steps. J. Alzheimers Dis. 92 (1), 13–27. doi:10.3233/JAD-220983
Sánchez-De-Lara-Sánchez, S., and Sánchez-Pérez, A. M. (2022). Probiotics treatment can improve cognition in patients with mild cognitive impairment: A systematic review. J. Alzheimer’s Dis. 89 (4), 1173–1191. doi:10.3233/JAD-220615
Sassi, K. L. M., Rocha, N. P., Colpo, G. D., John, V., and Teixeira, A. L. (2020). Amphetamine use in the elderly: A systematic review of the literature. Curr. Neuropharmacol. 18 (2), 126–135. doi:10.2174/1570159X17666191010093021
Schneider, L. S., Geffen, Y., Rabinowitz, J., Thomas, R. G., Schmidt, R., Ropele, S., et al. (2019). Low-dose ladostigil for mild cognitive impairment: A phase 2 placebo-controlled clinical trial. Neurology 93 (15), e1474–e1484. doi:10.1212/WNL.0000000000008239
Schneider, L. S., Thomas, R. G., Hendrix, S., Rissman, R. A., Brewer, J. B., Salmon, D. P., et al. (2019). Safety and efficacy of edonerpic maleate for patients with mild to moderate alzheimer disease: A phase 2 randomized clinical trial. JAMA Neurol. 76 (11), 1330–1339. doi:10.1001/jamaneurol.2019.1868
Shim, Y., Han, H. J., Park, K. W., Kim, B. C., Park, K. H., Park, M. Y., et al. (2022). A multicenter, randomized, double-blind, placebo-controlled, phase IIb clinical study to evaluate the safety and efficacy of DHP1401 in patients with mild to moderate Alzheimer's disease treated with donepezil: DHP1401 randomized trial in mild to moderate Alzheimer's disease (DRAMA). J. Alzheimer’s Dis. 87 (1), 391–403. doi:10.3233/JAD-215277
Shim, Y. S., Yoon, B., Na, S., Lim, E. Y., Hong, Y. J., and Yang, D. W. (2021). A systematic review and meta-analysis of the clinical effects of Souvenaid in patients with Alzheimer’s disease. Asia Pac J. Clin. Nutr. 30 (1), 30–41.
Silverman, M. H., Duggan, S., Bardelli, G., Sadler, B., Key, C., Medlock, M., et al. (2022). Safety, tolerability and pharmacokinetics of icapamespib, a selective epichaperome inhibitor, in healthy adults. J. Prev. Alzheimer’s Dis. 9 (4), 635–645. doi:10.14283/jpad.2022.71
Soheili, M., Karimian, M., Hamidi, G., and Salami, M. (2021). Alzheimer’s disease treatment: The share of herbal medicines. Iran. J. Basic Med. Sci. 24 (2), 123–135. doi:10.22038/IJBMS.2020.50536.11512
Stout, K. A., Dunn, A. R., Hoffman, C., and Miller, G. W. (2019). The synaptic vesicle glycoprotein 2: Structure, function, and disease relevance. ACS Chem. Neurosci. 10 (9), 3927–3938. doi:10.1021/acschemneuro.9b00351
Summers, W. K., Martin, R. L., Liu, Y., Peña, B., and Marsh, G. M. (2018). Complex antioxidants in a randomized single-blinded study of memory in seniors. Aging Clin. Exp. Res. 30 (4), 395–405. doi:10.1007/s40520-017-0788-6
Supasitthumrong, T., Bolea-Alamanac, B. M., Asmer, S., Woo, V. L., Abdool, P. S., and Davies, S. J. C. (2019). Gabapentin and pregabalin to treat aggressivity in dementia: A systematic review and illustrative case report. Br. J. Clin. Pharmacol. 85 (4), 690–703. doi:10.1111/bcp.13844
Suresh, S., Begum, R. F., Singh, A. S., and Chitra, V. (2022). Anthocyanin as a therapeutic in alzheimer’s disease: A systematic review of preclinical evidences. AGEING Res. Rev. 76, 101595. doi:10.1016/j.arr.2022.101595
Swanson, C. J., Zhang, Y., Dhadda, S., Wang, J., Kaplow, J., Lai, R. Y. K., et al. (2021). A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer’s disease with lecanemab, an anti-Aβ protofibril antibody. Alzheimer’s Res. Ther. 13 (1), 80. doi:10.1186/s13195-021-00813-8
Takada, M., Tanaka, S., Tanaka, K., Tsukie, T., Tsukamoto-Yasui, M., Suzuki, K., et al. (2022). Effects of an essential amino acid mixture on behavioral and psychological symptoms of dementia and executive function in patients with alzheimer’s disease: A double-blind, randomized, placebo-controlled exploratory clinical trial. Int. J. Geriatr. Psychiatry 37 (9). doi:10.1002/gps.5782
Talebi, M., Talebi, M., and Samarghandian, S. (2021). Association of crocus sativus with cognitive dysfunctions and alzheimer’s disease: A systematic review. Biointerface Res. Appl. Chem. 11 (1), 7468–7492.
Tamtaji, O. R., Heidari-soureshjani, R., Mirhosseini, N., Kouchaki, E., Bahmani, F., Aghadavod, E., et al. (2019). Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in alzheimer’s disease: A randomized, double-blind, controlled trial. Clin. Nutr. 38 (6), 2569–2575. doi:10.1016/j.clnu.2018.11.034
Tan, W., Qi, L., Hu, X., and Tan, Z. (2022). Research progress in traditional Chinese medicine in the treatment of Alzheimer’s disease and related dementias. Front. Pharmacol. 13, 921794. doi:10.3389/fphar.2022.921794
Teng, E., Manser, P. T., Pickthorn, K., Brunstein, F., Blendstrup, M., Sanabria Bohorquez, S., et al. (2022). Safety and efficacy of semorinemab in individuals with prodromal to mild alzheimer disease A randomized clinical trial. JAMA Neurol. 79 (8), 758–767. doi:10.1001/jamaneurol.2022.1375
Terao, I., Honyashiki, M., and Inoue, T. (2022). Comparative efficacy of lithium and aducanumab for cognitive decline in patients with mild cognitive impairment or alzheimer’s disease: A systematic review and network meta-analysis. Ageing Res. Rev. 81, 101709. doi:10.1016/j.arr.2022.101709
Thancharoen, O., Limwattananon, C., Waleekhachonloet, O., Rattanachotphanit, T., Limwattananon, P., and Limpawattana, P. (2019). Ginkgo biloba extract (EGb761), cholinesterase inhibitors, and memantine for the treatment of mild-to-moderate alzheimer’s disease: A network meta-analysis. Drugs Aging 36, 435–452. doi:10.1007/s40266-019-00648-x
Tofiq, A., Zetterberg, H., Blennow, K., Basun, H., Cederholm, T., Eriksdotter, M., et al. (2021). Effects of peroral omega-3 fatty acid supplementation on cerebrospinal fluid biomarkers in patients with alzheimer’s disease: A randomized controlled trial - the OmegAD study. J. Alzheimer’s Dis. 83 (3), 1291–1301. doi:10.3233/JAD-210007
Torosyan, N., Sethanandha, C., Grill, J. D., Dilley, M. L., Lee, J., Cummings, J. L., et al. (2018). Changes in regional cerebral blood flow associated with a 45 day course of the ketogenic agent, caprylidene, in patients with mild to moderate Alzheimer’s disease: Results of a randomized, double-blinded, pilot study. Exp. Gerontol. 111, 118–121. doi:10.1016/j.exger.2018.07.009
Torres-Mendoza, B. M. G., Ortiz, G. G., Sánchez-Romero, L., Delgado-Lara, D. L. C., García Martínez, M. T., Mireles-Ramírez, M., et al. (2022). Dietary fish oil increases catalase activity in patients with probable Alzheimer’s disease. Nutr. Hosp. 39 (6), 1364–1368.
Tosatti, J. A. G., Fontes, A. F. S., Caramelli, P., Gomes, K. B., Goncalves Tosatti, J. A., da Silva Fontes, A. F., et al. (2022). Effects of resveratrol supplementation on the cognitive function of patients with alzheimer’s disease: A systematic review of randomized controlled trials. Drugs Aging 39 (4), 285–295. doi:10.1007/s40266-022-00923-4
Tricco, A. C., Ashoor, H. M., Soobiah, C., Rios, P., Veroniki, A. A., Hamid, J. S., et al. (2018). Comparative effectiveness and safety of cognitive enhancers for treating alzheimer’s disease: Systematic review and network metaanalysis. J. Am. Geriatr. Soc. 66 (1), 170–178. doi:10.1111/jgs.15069
Tseng, P.-T., Zeng, B.-Y., Chen, Y.-W., Yang, C.-P., Su, K.-P., Chen, T.-Y., et al. (2022). The dose and duration-dependent association between melatonin treatment and overall cognition in alzheimer’s dementia: A network meta- analysis of randomized placebo-controlled trials. Curr. Neuropharmacol. 20 (10), 1816–1833. doi:10.2174/1570159X20666220420122322
Uddin, M. S., Kabir, M. T., Rahman, M. M., Mathew, B., Shah, M. A., and Ashraf, G. M. (2020). TV 3326 for Alzheimer's dementia: A novel multimodal ChE and MAO inhibitors to mitigate alzheimer's-like neuropathology. J. Pharm. Pharmacol. 72 (8), 1001–1012. doi:10.1111/jphp.13244
van Dyck, C. H., Arnsten, A. F. T., Padala, P. R., Brawman-Mintzer, O., Lerner, A. J., Porsteinsson, A. P., et al. (2021). Neurobiologic rationale for treatment of apathy in alzheimer’s disease with methylphenidate. Am. J. Geriatr. Psychiatry 29 (1), 51–62. doi:10.1016/j.jagp.2020.04.026
Vaz, M., and Silvestre, S. (2020). Alzheimer’s disease: Recent treatment strategies. Eur. J. Pharmacol. 887, 173554. doi:10.1016/j.ejphar.2020.173554
Villain, N. (2022). Therapeutic news in Alzheimer’s disease: Soon a disease-modifying therapy? Rev. Neurol. Paris. 178 (5), 437–440. doi:10.1016/j.neurol.2022.02.456
Viña, J., Escudero, J., Baquero, M., Cebrián, M., Carbonell-Asíns, J. A., Muñoz, J. E., et al. (2022). Genistein effect on cognition in prodromal Alzheimer’s disease patients. The GENIAL clinical trial. Alzheimer’s Res. Ther. 14 (1), 164. doi:10.1186/s13195-022-01097-2
Vossel, K., Ranasinghe, K. G., Beagle, A. J., La, A., Ah Pook, K., Castro, M., et al. (2021). Effect of levetiracetam on cognition in patients with alzheimer disease with and without epileptiform activity A randomized clinical trial. JAMA Neurol. 78 (11), 1345–1354. doi:10.1001/jamaneurol.2021.3310
Wang, H., Zong, Y., Han, Y., Zhao, J., Liu, H., and Liu, Y. (2022). Compared of efficacy and safety of high-dose donepezil vs standard-dose donepezil among elderly patients with alzheimer’s disease: A systematic review and meta-analysis. Expert Opin. Drug Saf. 21 (3), 407–415. doi:10.1080/14740338.2022.2027905
Wang, J., Tan, L., Wang, H. F., Tan, C. C., Meng, X. F., Wang, C., et al. (2015). Anti-inflammatory drugs and risk of alzheimer’s disease: An updated systematic review and meta-analysis. J. Alzheimers Dis. 44 (2), 385–396. doi:10.3233/JAD-141506
Wangchan, H., yang, L. N., Zhang, S., Yang, Y., Wang, Z. Y., Wei, Y., et al. (2020). Clinical experience in treatment of alzheimer’s disease with Jiannao Yizhi formula (健脑益智方) and routine western medicine. Chin. J. Integr. Med. 26 (3), 212–218. doi:10.1007/s11655-019-2718-2
Watanabe, M., Nakamura, Y., Yoshiyama, Y., Kagimura, T., Kawaguchi, H., Matsuzawa, H., et al. (2019). Analyses of natural courses of Japanese patients with alzheimer’s disease using placebo data from placebo-controlled, randomized clinical trials: Japanese study on the estimation of clinical course of alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 5, 398–408. doi:10.1016/j.trci.2019.07.004
Wessels, A. M., Lines, C., Stern, R. A., Kost, J., Voss, T., Mozley, L. H., et al. (2020). Cognitive outcomes in trials of two BACE inhibitors in Alzheimer’s disease. Alzheimer’s Dement. 16 (11), 1483–1492. doi:10.1002/alz.12164
Wunderlich, G., Blahova, Z., Garcia, M., and Jessen, F. (2023). Efficacy and safety of the novel GlyT1 inhibitor BI 425809 in alzheimer’s dementia: A randomized controlled trial. Alzheimers Res. Ther. 15 (1), 24. doi:10.1186/s13195-023-01163-3
Xiao, S., Chan, P., Wang, T., Hong, Z., Wang, S., Kuang, W., et al. (2021). A 36-week multicenter, randomized, double-blind, placebo-controlled, parallel-group, phase 3 clinical trial of sodium oligomannate for mild-to-moderate Alzheimer’s dementia. ALZHEIMERS Res. Ther. 13 (1), 62. doi:10.1186/s13195-021-00795-7
Xu Lou, I., Ali, K., and Chen, Q. (2023). Effect of nutrition in alzheimer’s disease: A systematic review. Front. Neurosci. 17, 17. doi:10.3389/fnins.2023.1147177
Xu, Q., Zhang, Y., Zhang, X., Liu, L., Zhou, B., Mo, R., et al. (2020). Medium-chain triglycerides improved cognition and lipid metabolomics in mild to moderate alzheimer’s disease patients with APOE4−/−: A double-blind, randomized, placebo-controlled crossover trial. Clin. Nutr. 39 (7), 2092–2105. doi:10.1016/j.clnu.2019.10.017
Xuan, K., Zhao, T., Qu, G., Liu, H., Chen, X., and Sun, Y. (2020). The efficacy of statins in the treatment of alzheimer’s disease: A meta-analysis of randomized controlled trial. Neurol. Sci. 41 (6), 1391–1404. doi:10.1007/s10072-020-04243-6
Yang, Y., Liu, J., yan, F. J., Wangchan, H., Wei, Y., Cao, Y., et al. (2019). Effect and safety of Huannao Yicong formula (还脑益聪方) in patients with mild-to-moderate alzheimer’s disease: A randomized, double-blinded, donepezil-controlled trial. Chin. J. Integr. Med. 25 (8), 574–581. doi:10.1007/s11655-018-3054-7
Yokoyama, S., Yoshinaga, T., Matsuzaki, J., and Suzuki, H. (2019). A randomized, double-blind, placebo-controlled study to evaluate the efficacy of teprenone in patients with alzheimer’s disease. J. Alzheimer’s Dis. 71 (4), 1187–1199. doi:10.3233/JAD-190305
Youn, H., Lee, K. J., Kim, S. G., Cho, S. J., Kim, W. J., Lee, W. J., et al. (2021). The behavioral effects of combination therapy of memantine and acetylcholinesterase inhibitors compared with acetylcholinesterase inhibitors alone in patients with moderate alzheimer’s dementia: A double-blind randomized placebo-controlled trial. Psychiatry Investig. 18 (3), 233–240. doi:10.30773/pi.2020.0329
Zamanian, M. Y., Kujawska, M., Nikbakhtzadeh, M., Hassanshahi, A., Ramezanpour, S., Kamiab, Z., et al. (2021). Carvacrol as a potential neuroprotective agent for neurological diseases: A systematic review article. CNS Neurol. Disord. - Drug Targets. 20 (10), 942–953. doi:10.2174/1871527320666210506185042
Zhang, H., Liu, D., Huang, H., Zhao, Y., and Zhou, H. (2018). Characteristics of insulin-degrading enzyme in alzheimer’s disease: A meta-analysis. Curr. Alzheimer Res. 15 (7), 610–617. doi:10.2174/1567205015666180119105446
Zheng, X., Tang, Y., Yang, Q., Wang, S., Chen, R., Tao, C., et al. (2022). Effectiveness and safety of anti-tau drugs for Alzheimer’s disease: Systematic review and meta-analysis. J. Am. Geriatr. Soc. 70 (11), 3281–3292. doi:10.1111/jgs.18025
Zhou, C. C., Wu, Q., Wang, Z., Wang, Q., Liang, Y., and Liu, S. (2020). The effect of hormone replacement therapy on cognitive function in female patients with alzheimer’s disease: A meta-analysis. Am. J. ALZHEIMERS Dis. OTHER DEMENTIAS 35, 1533317520938585. doi:10.1177/1533317520938585
Zhu, C. W., Grossman, H., Neugroschl, J., Parker, S., Burden, A., Luo, X., et al. (2018). A randomized, double-blind, placebo-controlled trial of resveratrol with glucose and malate (rgm) to slow the progression of alzheimer’s disease: A pilot study. Alzheimer’s Dement. Transl. Res. Clin. Interv. 4, 609–616. doi:10.1016/j.trci.2018.09.009
Keywords: Alzheimer disease, cognition, pharmacology, treatment, latest research, beta-amyloid, immunotherapy
Citation: Xu Lou I, Chen J, Ali K, Shaikh AL and Chen Q (2023) Mapping new pharmacological interventions for cognitive function in Alzheimer’s disease: a systematic review of randomized clinical trials. Front. Pharmacol. 14:1190604. doi: 10.3389/fphar.2023.1190604
Received: 21 March 2023; Accepted: 12 May 2023;
Published: 01 June 2023.
Edited by:
Syed Shams Ul Hassan, Shanghai Jiao Tong University, ChinaReviewed by:
Suet Cheung, Tsinghua University, ChinaSyed Qamar Abbas, Sarhad University of Science & Information Technology (SUIT), Pakistan
Copyright © 2023 Xu Lou, Chen, Ali, Shaikh and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qilan Chen, Y3FsMTM1ODg3NTA5NDFAcXEuY29t
†These authors have contributed equally to this work and share first authorship
 Inmaculada Xu Lou
Inmaculada Xu Lou Jiayue Chen2,3†
Jiayue Chen2,3† Kamran Ali
Kamran Ali