- 1Chengdu Hi-tech Nanxili Jiuzheng Clinic, Chengdu, Sichuan, China
- 2Department of Respiratory and Critical Care Medicine, Institute of Respiratory Health, Precision Medicine Key Laboratory, West China Hospital, Sichuan University, Chengdu, Sichuan, China
The flavonoids baicalin and baicalein were discovered in the root of Scutellaria baicalensis Georgi and are primarily used in traditional Chinese medicine, herbal supplements and healthcare. Recently, accumulated investigations have demonstrated the therapeutic benefits of baicalin in treating various lung diseases due to its antioxidant, anti-inflammatory, immunomodulatory, antiapoptotic, anticancer, and antiviral effects. In this review, the PubMed database and ClinicalTrials website were searched with the search string “baicalin” and “lung” for articles published between September 1970 and March 2023. We summarized the therapeutic role that baicalin plays in a variety of lung diseases, such as chronic obstructive pulmonary disease, asthma, pulmonary fibrosis, pulmonary hypertension, pulmonary infections, acute lung injury/acute respiratory distress syndrome, and lung cancer. We also discussed the underlying mechanisms of baicalin targeting in these lung diseases.
Introduction
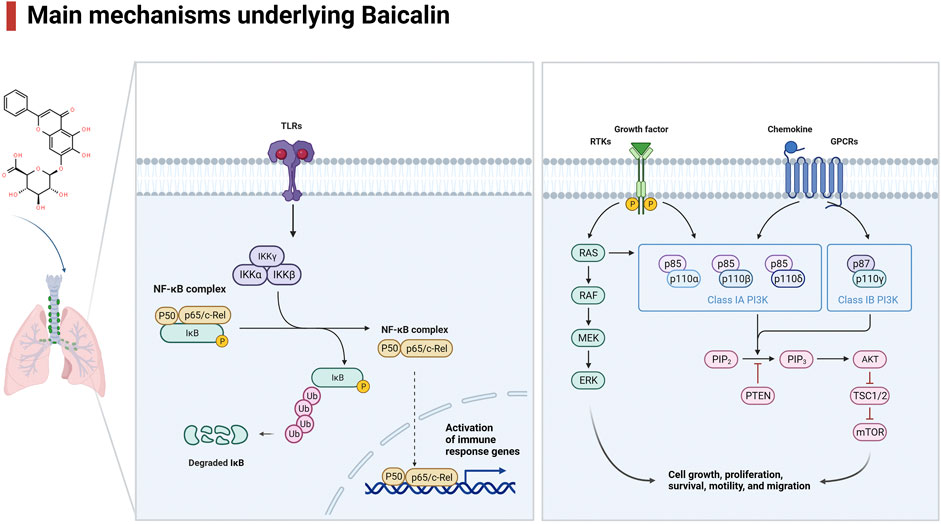
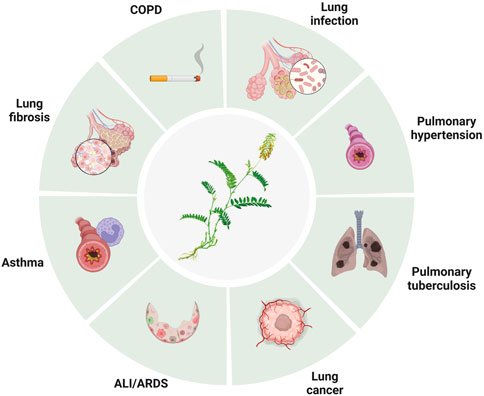
Lung disease is a major global health concern, affecting millions of people worldwide. Baicalin is a flavonoid compound isolated from the root of Scutellaria baicalensis Georgi. Baicalein is a flavone, a type of polyphenolic flavonoid, while baicalin is a flavone glycoside, the glucuronide of baicalein, which is obtained through the binding of glucuronic acid to baicalein. As a natural medicine, baicalin has thus been widely used in the treatment of clinical diseases such as cardiovascular and liver disease, diabetes, and neurodegenerative disorders. Baicalin has been shown to have potent antioxidant, anti-inflammatory, immunomodulatory, and antiapoptotic effects, making it a promising candidate for the treatment of several disease conditions. It thus has wide applications in medicine, healthcare, and food industries and has become a focused issue and trend in research worldwide in recent years. It has also been widely studied for its potential therapeutic benefits in treating various lung diseases, including chronic obstructive pulmonary disease (COPD), asthma and acute lung injury (ALI). The pharmacological contributions of baicalin to multiple lung diseases are being revealed (He et al., 2021) but are not fully understood. Baicalin exhibits anti-inflammatory and immunomodulatory effects by targeting several signaling pathways, including nuclear factor-κB (NF-κB), phosphatidylinositol-3-kinase (PI3K)/AKT), mitogen-activated protein kinases (MAPKs), and Toll-like receptors (TLRs), leading to a reduction in the production of proinflammatory cytokines and chemokines and subsequent development of inflammation in lung diseases (Figure 1, Table 1). In addition, baicalin has been found to have antioxidant and anti-apoptotic properties, which help prevent oxidative damage to cells and tissues. In addition, baicalin has been shown to have anticancer effects through its ability to inhibit the proliferation, migration, and invasion of cancer cells, including lung cancer cells, by inducing apoptosis and cell cycle arrest. Moreover, baicalin also exhibits antiviral effects against respiratory viruses such as influenza and severe acute respiratory syndrome coronavirus (SARS-CoV) by suppressing replication. In conclusion, baicalin shows great potential in the treatment of various lung diseases due to its anti-inflammatory, antioxidant, anticancer and antiviral properties. In this review, we will outline the recent understanding of baicalin treatment in lung diseases and its underlying mechanisms (Figure 2).
Methods
The PubMed database and ClinicalTrials website were searched for articles published between September 1970 and March 2023 using the following search string: (baicalin) and (lung). Studies of all designs that were accessible online were included if they met the following criteria: 1) published in English, 2) randomized control trials, other controlled trials, descriptive and comparative studies, evidence-based practice and 3) full-text available.
COPD
The prevalence of COPD, which is currently the fourth largest cause of morbidity and mortality worldwide, is rising (Barnes et al., 2015; Barnes et al., 2019). Chronic airway inflammation, lung damage, and remodeling are its hallmarks, all of which lead to an irreversible blockage of airflow. Baicalin has been shown in studies to have anti-inflammatory and antioxidant effects that can help lessen the intensity of COPD symptoms. Moreover, baicalin has been shown to enhance lung health and lessen mucus formation in clinical practice, although there have been no reports to date. Additionally, it might protect the lungs from the harm caused by cigarette smoke and other environmental factors.
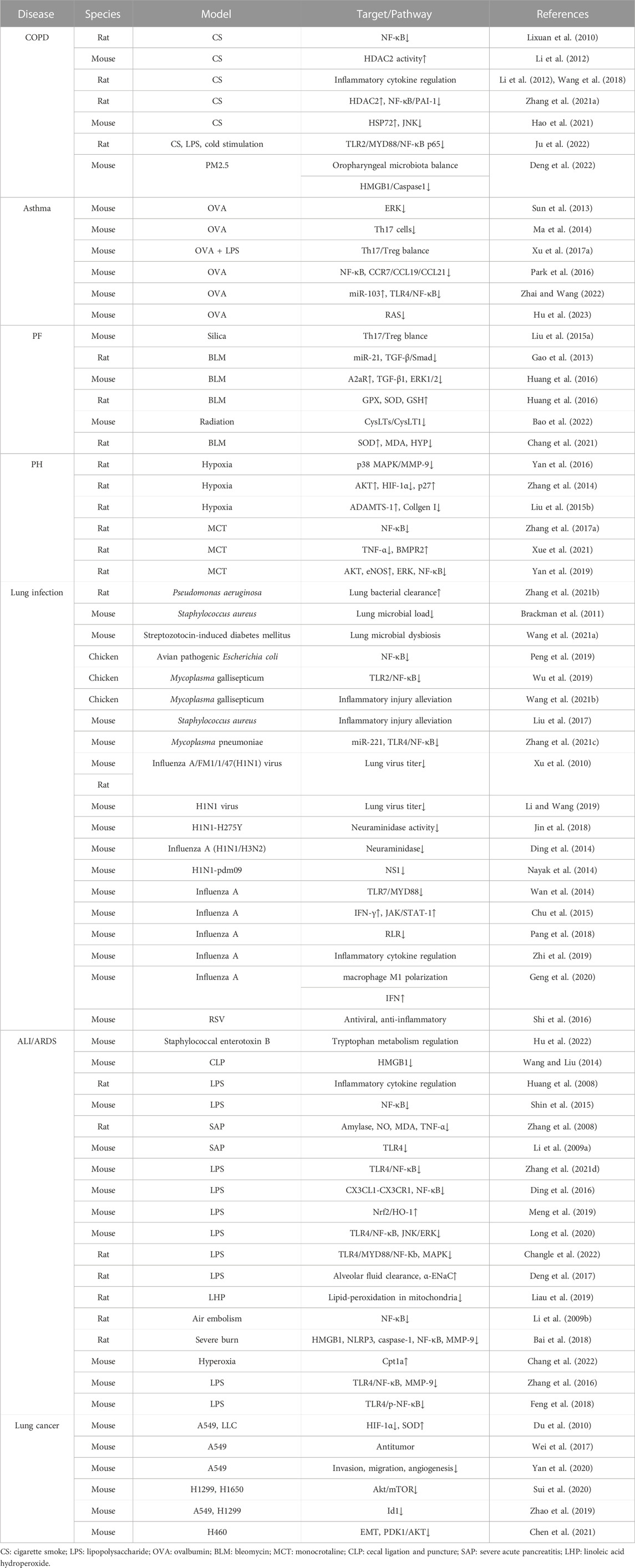
Baicalin has a variety of biological effects, including antioxidant and anti-inflammatory properties (Li et al., 2022). Mouse and cell models were stimulated by cigarette smoke (CS) and CS extract to investigate the effects and underlying mechanisms of baicalin on COPD. According to the findings, baicalin may control the balance between pro- and anti-inflammatory responses and significantly improve lung function in COPD patients (Lixuan et al., 2010; Li et al., 2012; Wang et al., 2018; Zhang et al., 2021a; Hao et al., 2021). The anti-inflammatory effect was probably caused by the inhibition of NF-κB activation (Lixuan et al., 2010), the upregulation of histone deacetylase 2 (HDAC2) activity (Li et al., 2012), and the modulation of the HDAC2/NF-κB/plasminogen activator inhibitor 1 (PAI-1) signaling pathways (Zhang et al., 2021a). Hao et al. (2021) demonstrated that baicalin upregulated the expression of heat shock protein 72 (HSP72), resulting in the inhibition of c-Jun N-terminal kinase (JNK) signaling activation and ultimately relieving COPD. Recently, Ju et al. (2022) proved that baicalin controlled the TLR2/myeloid differentiation primary response gene 88 (MYD88)/NF-κB p65 signaling pathway to reduce oxidative stress and the inflammatory response in COPD rats. Through regulating oropharyngeal microbiota and influencing the expression of the High Mobility Group Protein 1 (HMGB1)/Caspase1 pathway, baicalin may also mitigate mouse lung inflammatory injury caused by exposure to PM2.5 (Deng et al., 2022).
Asthma
Asthma is a chronic inflammatory respiratory disorder that results in intermittent episodes of wheezing, breathlessness, chest stuffiness, and cough. Chronic bronchial inflammation, bronchial smooth muscle cell hypertrophy and hyperreactivity, as well as increased mucus output, are the disease’s defining characteristics (Papi et al., 2018).
In animal models of asthma, baicalin has been demonstrated to lower airway inflammation and enhance lung function (Sun et al., 2013), in part by modulating the Th17/Treg imbalance (Ma et al., 2014; Xu et al., 2017a). Additionally, baicalin has also been demonstrated to lessen mucus production in the airways and inhibit the contraction of bronchial smooth muscle, which might lessen airway narrowing and improve breathing (Xu et al., 2017a). In a previous study, baicalin pretreatment inhibited the MAPK signaling pathway, significantly reducing the proliferation and migration of airway smooth muscle cells (ASMCs) stimulated by platelet-derived growth factor (PDGF) (Yang et al., 2015). Baicalin administration reduced inflammatory cell infiltration and tumor necrosis factor-α (TNF-α) levels in bronchoalveolar lavage fluids in an animal model of allergic asthma, demonstrating that the anti-inflammatory actions of baicalin in vivo are due to its capacity to inhibit phosphodiesterase 4 (PDE4) (Park et al., 2016). By inhibiting NF-κB and reducing CC-chemokine receptor 7 (CCR7)/C-C motif chemokine ligand 19 (CCL19)/CCL21, Liu et al. showed that oral treatment with baicalin greatly enhanced pulmonary function and reduced inflammatory cell infiltration into the lungs (Liu et al., 2016), and Zhai et al. found that baicalin increased miRNA-103 and mediated the TLR4/NF-κB pathway to successfully reverse ovalbumin (OVA)-induced oxidative stress, inflammation, and changes in the amount of total cells, eosinophils, and neutrophils in bronchoalveolar lavage fluid (BALF) as well as collagen deposition (Zhai and Wang, 2022).
Baicalin significantly decreased the infiltration of inflammatory cells in lung tissue, attenuated airway resistance, and reduced the levels of remodeling-related cytokines such as interleukin (IL-13), vascular endothelial growth factor (VEGF), transforming growth factor-β1 (TGF-β1), matrix metalloproteinase 9 (MMP9), and tissue inhibitor of metallopeptidase 1 (TIMP1) at both the mRNA and protein levels. In an OVA-induced asthmatic mouse model, baicalin administration suppressed the RAS signaling pathway to prevent airway remodeling and ASMC proliferation by regulating the activation of protein kinase C-α (PKC-α), A-rapidly accelerated fibrosarcoma (A-RAF), mitogen activated protein kinase 2 (MEK2), extracellular regulated MAP kinase (ERK), MAPK interacting serine/threonine kinase 1 (MNK1), and ETS transcription factor 1 (ELK1) (Hu et al., 2023). Baicalin has recently been demonstrated to inhibit type 2 immunity by severing the interaction between mast cells and airway epithelial cells, suggesting that it may be a useful alternative therapy for the management of asthma (Yoshida et al., 2021). Baicalin may therefore be an effective medication for treating allergic and asthmatic disorders in humans by regulating NF-κB activity and other signaling pathways.
Pulmonary fibrosis
Pulmonary fibrosis (PF) is a progressive, resistant pulmonary fibrotic condition with no known cause. Patchy but progressive bilateral interstitial fibrosis is the defining feature of PF, and in advanced cases, it can cause severe hypoxia and cyanosis (Lederer et al., 2018).
Treatment with baicalin in a mouse model of silicosis reduced the buildup of inflammatory cells by balancing the Th17 and Treg responses, which also resulted in fewer clinical inflammatory and fibrotic alterations in lung tissues (Liu et al., 2015a). Baicalein oral administration significantly reduced miR-21 levels, increased TGF-β1 and p-smad2/3 expression, and decreased hydroxyproline content and α-smooth muscle actin (α-SMA) levels in lung tissue, which is important for myofibroblast activation and collagen deposition in the extracellular matrix (Gao et al., 2013). In a different study, Huang et al. hypothesized that baicalin exerts its antifibrotic effects by modulating the expression of the adenosine A2a receptor (A2aR) gene, which regulates inflammation by lowering the levels of increased TGF-β1 and p-ERK1/2 (Huang et al., 2016). Subsequent research showed that baicalin considerably boosted serum levels of glutathione peroxidase (GPX), superoxide dismutase (SOD), and glutathione (GSH) while significantly lowering serum levels of malondialdehyde (MDA). Baicalin also controlled cyclin A, D, and E, proliferating cell nuclear antigen, p-AKT, and p-calcium/calmodulin dependent protein kinase type, suppressing the transition of cells from the G0/G1 phase to the G2/M and S phases and lowering the intracellular Ca2+ concentration to suppress bleomycin-induced pulmonary fibrosis and fibroblast proliferation (Zhao et al., 2020). By modulating the TGF-β and ERK/glycogen synthase kinase (GSK3β) signaling pathways (Lu et al., 2017), as well as the cysteinyl leukotriene (CysLTs)/CysLT1 pathway (Bao et al., 2022), baicalin alleviates radiation-induced epithelial-mesenchymal transition of primary type II alveolar epithelial cells. Hong et al. revealed the antifibrotic mechanisms of baicalin, which involve the regulation of four key biomarkers involved in the metabolism of taurine, hypotaurine, glutathione and glycerophospholipids (Chang et al., 2021). Studies have shown that baicalin treatment can improve lung function and reduce symptoms; thus, it may be a useful alternative therapy for the management of PF.
Pulmonary hypertension
Pulmonary hypertension (PH) is usually secondary to a reduction in vessel diameter or an increase in blood flow in the pulmonary vascular bed (Vonk Noordegraaf et al., 2016). Most kinds of PH are thought to have a potential basis in the malfunctioning of pulmonary endothelial cells and/or vascular smooth muscle cells. The entire pulmonary arterial tree thickens the intima and media while narrowing the lumen as a result of endothelial and smooth muscle cell proliferation (Cui et al., 2022). Pretreatment with baicalin in chronic hypoxic rats attenuated PH and right-sided heart dysfunction by reducing p38 MAPK activation, reducing the elevated levels of the proinflammatory cytokines IL-1, IL-6 and TNF-α and downregulating the expression of MMP9 in the pulmonary arteriole walls (Yan et al., 2016).
Baicalin decreased hypoxia inducible factor-1 (HIF-1) production in a model of hypoxia-induced PH by modulating the AKT signaling pathway to stop p27 degradation. Increased p27 levels thereby inhibited pulmonary artery smooth muscle cell (PASMC) proliferation, preventing hypoxia-induced increased pulmonary arterial pressure and pulmonary vascular remodeling (Zhang et al., 2014). Another study found that baicalin suppressed the HIF-1α and aryl hydrocarbon receptor (AhR) pathways, which prevented TGF-β1-induced phenotypic switching and consequently the excessive growth of pulmonary arterial smooth muscle cells (Huang et al., 2014). One study indicated that baicalin provided protection for rats suffering from hypoxic PH. The mechanism may involve an increase in ADAM metallopeptidase with thrombospondin type 1 motif 1 (ADAMTS-1) expression, which inhibits collagen I synthesis and expression (Liu et al., 2015b).
By inhibiting the inflammatory response and downregulating the NF-κB signaling pathway, baicalin can considerably lower the expression of TGF-β1 in lung tissues and pulmonary arterial pressure, lessen right ventricular hypertrophy and injury, and attenuate pulmonary vascular remodeling (Luan et al., 2015). Baicalin has been found in numerous studies to significantly reduce P38 MAPK and MMP-9 expression. It effectively improved hypoxia-induced PH in a rat model by blocking the p38 MAPK signaling pathway and MMP-9 in the small pulmonary arteries (Yan et al., 2016).
An earlier study revealed that baicalin had a therapeutic effect on the hypoxia-induced PH rat model, at least in part because it activates peroxisome proliferator-activated receptor γ (PPARγ) and blocks the HMGB1/receptor for advanced glycation end-products (RAGE) inflammatory signaling pathway (Chen and Wang, 2017). In another study, baicalin exhibited increased A2aR activity and decreased stromal cell derived factor-1 (SDF-1)/C-X-C motif chemokine receptor 4 (CXCR4)-induced PI3K/AKT signaling to protect against hypoxia-induced PH (Huang et al., 2017).
Baicalin can inhibit monocrotaline (MCT)-induced PH in rats by upregulating bone morphogenetic protein 4 (BMP4), BMP9, BMP receptor 2 (BMPR2) and p-Smad1/5/8 expression, according to recent research. Baicalin greatly reduces the expression of NF-κB, TNF-α, IL-6 and IL-1. Baicalin, on the other hand, may reduce pulmonary vascular remodeling by preventing ERK and NF-κB phosphorylation and expression, as well as by suppressing endothelial-to-mesenchymal transition (EndMT) via the BMP/Smad axis and NF-κB signaling (Zhang et al., 2017a; Xue et al., 2021). By decreasing p-p65 and p-ERK expression and encouraging p-AKT and p-endothelial nitric oxide synthase (eNOS) expression, baicalin ameliorated pulmonary vascular remodeling and cardiorespiratory injury in the development of pulmonary arterial hypertension through the AKT/eNOS, ERK and NF-κB signaling pathways (Yan et al., 2019; Xue et al., 2021). By boosting the expression of ADAMTS-1, which inhibits the synthesis of type I collagen and its mRNA expression, baicalin administration significantly decreased pulmonary artery pressure and slowed the remodeling of the pulmonary artery under hypoxic conditions, according to Liu et al. (Liu et al., 2015b).
Pulmonary infections
Pneumonia-related deaths from lung infections are common worldwide (McAllister et al., 2019). The lung’s epithelial surfaces are constantly exposed to microbial pollutants in the open air, and other frequent lung conditions and bad lifestyle choices, such smoking and drinking, make the lung parenchyma susceptible to pathogenic organisms.
Treatment with baicalin considerably lessened the severity of lung pathology and sped up the clearance of Pseudomonas aeruginosa from the lungs. After baicalin treatment, the Th1-induced inflammatory response and decreased cell infiltration in the lung around the implants were observed (Zhang et al., 2021b). In a different investigation, tobramycin treatment in combination with baicalin hydrate reduced the microbial load in mouse lungs infected with Burkholderia cenocepacia more than tobramycin treatment alone (Brackman et al., 2011). Via an NF-κB signaling pathway, baicalin might also treat the microbial dysbiosis of the lungs and the subsequent fibrogenesis in streptozotocin-induced diabetic mice (Wang et al., 2021a). Baicalin has also been shown to control the same pathway in avian pathogenic Escherichia coli-induced acute lung injury (Peng et al., 2019). Baicalin alleviates lung inflammatory injury in Mycoplasma gallisepticum infection models by inhibiting the TLR2/NF-κB pathway (Wu et al., 2019) and regulates gut microbiota and phenylalanine metabolism by modulating the gga-miR-190a-3p-Fas-associated death domain (FADD) axis in HD11 macrophages (Wang et al., 2021b). Baicalin also inhibits the development of Staphylococcus aureus pneumonia (Liu et al., 2017).
Among chronic pneumonias, pulmonary tuberculosis is a dangerous infectious illness that poses a substantial threat to human health. According to the World Health Organization, it kills 6% of people worldwide and is now getting worse. Th1 cells are primarily responsible for driving immunity against tuberculosis infection by causing macrophages to kill bacteria (Jasenosky et al., 2015). Re-exposure to Mycobacterium tuberculosis (Mtb) or the reactivation of the infection in a previously sensitized host triggers a rapid defense response, although hypersensitivity also accelerates tissue necrosis and destruction. The findings showed that baicalin did not affect the phosphorylation of p38, JNK, or ERK in either Raw264.7 or primary peritoneal macrophages but did decrease the levels of p-AKT and p-mammalian target of rapamycin (mTOR) at Ser473 and Ser2448, respectively. Moreover, baicalin increased the colocalization of inflammasomes with autophagosomes to exert an autophagic degradative effect on reducing inflammasome activation and exerted an inhibitory effect on NF-κB activity. Via the PI3K/AKT/mTOR pathway, baicalin causes autophagy activation in Mtb-infected macrophages. Moreover, baicalin inhibited the PI3K/AKT/NF-κB signaling pathway, and both autophagy induction and NF-κB inhibition contributed to limiting the activity of the NOD-like receptor thermal protein domain associated protein 3 (NLRP3) inflammasome and the resultant release of the proinflammatory cytokine IL-1β (Zhang et al., 2017b).
Another study showed that baicalin might limit protein kinase R-like endoplasmic reticulum kinase (PERK)/eukaryotic translation initiation factor 2 (eIF2) pathway activation, which would then downregulate thioredoxin interacting protein (TXNIP) expression and reduce the activation of the NLRP3 inflammasome, resulting in reduced pyroptosis in macrophages with Mtb infection (Fu et al., 2021). Baicalin relieves Mycoplasma pneumoniae infection-induced lung injury by blocking miRNA-221 to regulate the TLR4/NF-κB signaling pathway (Zhang et al., 2021c).
Baicalin exhibits inhibitory effects on various strains of influenza virus and SARS-CoV, both in vitro and in vivo (Limanaqi et al., 2020). Oral administration of baicalein to mice infected with the influenza virus increased the average survival time, reduced lung inflammation, and dose-dependently decreased the lung viral titer. These effects are probably caused by baicalin, which has been demonstrated to impede the replication of SARS-CoV and influenza virus in vitro (Chen et al., 2004; Xu et al., 2010). Via the TNF receptor associated factor 6 (TRAF6)-dependent production of Type-I interferons (IFNs), baicalin has been demonstrated to suppress influenza virus replication, which correlates with protection from acute lung injury in infected mice (Li and Wang, 2019). This is noteworthy because, in SARS-CoV, similar to what was observed in the influenza virus, alterations in mitochondrial homeostasis and autophagy were eventually caused by abnormal ubiquitin proteasome system (UPS)-dependent degradation, which was related to the suppression of inhibition of TRAF6-dependent expression of Type-I IFNs (Shi et al., 2014).
Baicalin suppresses influenza virus infection both in vitro and in vivo by directly inhibiting the neuraminidase surface glycoprotein, which is necessary for viral replication and the release of virions from infected cells (Ding et al., 2014; Jin et al., 2018). While baicalein strengthens the antiviral activity of the neuraminidase inhibitor zanamivir, sodium baicalin is also effective against oseltamivir-resistant mutant influenza virus strains (Sithisarn et al., 2013). The anti-neuraminidase activity of baicalein is accompanied by a reduction in TNF-α, IL-6, and IL-8, which is associated with inhibition of the NF-κB and PI3K/AKT pathways and may indicate autophagy is being activated (Sithisarn et al., 2013). This makes sense given that baicalin has been demonstrated to directly target the NS1 protein of the influenza virus, which has been proven to impair autophagy by activating PI3K/AKT (Nayak et al., 2014). Baicalin inhibited virus replication and reduced the activity of major factors of the RIG-I-like receptor (RLR) signaling pathway components, including retinoic-acid-inducible gene I (RIG-I), IFN regulatory factor 3 (IRF3), IRF7, NF-κB, inflammatory responses, and macrophage polarization, in an influenza A virus infection model (Wan et al., 2014; Chu et al., 2015; Zhu et al., 2015; Pang et al., 2018; Zhi et al., 2019; Geng et al., 2020). Treatment with baicalin can also moderately lower respiratory syncytial virus titers recovered from lung tissues with a decrease in T lymphocyte infiltration and proinflammatory factor gene expression (Shi et al., 2016).
The pathologic mechanism of the coronavirus disease (COVID-19) outbreak caused by SARS-CoV-2 is still not completely clear (Huang et al., 2020; Wang et al., 2020). The most common cause of death in severe COVID-19 cases is respiratory failure, and conditions such as acute respiratory distress syndrome (ARDS), septic shock, severe metabolic acidosis, and a hypercoagulable state can be fatal. Baicalin, herbacetin, and pectolinarin have been found to effectively inhibit the proteolytic activity of the main protease, 3-chymotrypsin-like protease (3CLpro), and show effective inhibitory activity against SARS-CoV-2 3CLpro (Jo et al., 2020). It was predicted that baicalin would bind to papain-like protease (PLpro) (Lin et al., 2021) and the S protein (Boozari and Hosseinzadeh, 2021) with considerable affinity. Baicalin is an intriguing prospective therapeutic candidate for future study against SARS-CoV-2 that has been shown to bind the N-terminus and C-terminus of the homology model of the SARS-CoV-2 proteins non-structural protein 14 (Nsp14) and 3CLpro (Su et al., 2020; Liu et al., 2021). Baicalin and ascorbic acid can work together to reduce SARS-CoV-2 entrance by inhibiting the production of angiotensin-converting enzyme II in human small alveolar epithelial cells (Lai et al., 2021). When used to treat COVID-19, certain traditional Chinese medications containing baicalin have been shown to have immunological modulation, anti-infection, anti-inflammation, and multiorgan protection mechanisms (Su et al., 2020; Zhao et al., 2021a; Huang et al., 2022; Wei et al., 2022).
Acute lung injury/acute respiratory distress syndrome
Damage to the alveolar capillary membrane, which is made up of the microvascular endothelium and the alveolar epithelium, results in pulmonary infiltrates in ALI. In the presence of sepsis, severe trauma, or extensive lung infection, ALI can progress to ARDS, which is more serious diffuse alveolar injury (Matthay et al., 2012).
By regulating the composition of the gut microbiota, increasing the production of short-chain fatty acids, and altering the fecal metabolite profiles via the lung-gut axis, baicalin can ameliorate staphylococcal enterotoxin B-induced ARDS (Hu et al., 2022). Baicalin inhibits the release of HMGB1 and cytokines from macrophages, improves survival and reduces tissue injury in septic mice induced by lipopolysaccharide (LPS) (Wang and Liu, 2014). By inhibiting TLR4, baicalin has a therapeutic impact on LPS-induced ALI (Huang et al., 2008; Shin et al., 2015) as well as acute pancreatitis-associated lung injury (Zhang et al., 2008; Li et al., 2009a; Zhang et al., 2021d). Ding et al. (2016) explained the crosstalk between the C-X3-C motif chemokine ligand 1 (CX3CL1)- C-X3-C motif chemokine receptor 1 (CX3CR1) axis and NF-κB pathway, while Meng et al. revealed that oxidative stress and inflammation were reduced via the activation of the nuclear factor erythroid 2-related factor 2 (NRF2)-mediated heme oxygenase 1 (HO-1) signaling pathway (Meng et al., 2019). Meanwhile, Long and Zhu et al. demonstrated that the mechanism involves the inhibition of the TLR4/NF-κB p65 and ERK/JNK signaling pathways (Long et al., 2020) as well as the TLR4/MyD88/NF-κB/NLRP3 signaling pathway and the MAPK signaling pathway (Changle et al., 2022). Duan et al. (2021) demonstrated that it can attenuate follistatin-like protein 1 (FSTL1) and the ERK/JNK signaling pathway by upregulating miR-200b-3p expression. It may also prevent LPS-induced reduction of alveolar fluid clearance by upregulating epithelial sodium channel α-epithelial sodium channel (α-ENaC) protein through activation of the cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA) signaling pathway to attenuate lung edema (Deng et al., 2017). Another investigation demonstrated that baicalin can reduce lung mitochondrial lipid peroxidation and antioxidant activity induced by linoleic acid hydroperoxide (LHP) in vitro (Liau et al., 2019).
Baicalin attenuated air embolism-induced acute lung injury (Li et al., 2009b) and severe burn-induced remote acute lung injury through the NLRP3 signaling pathway (Bai et al., 2018) and attenuated neonatal hyperoxia-induced endothelial cell dysfunction and alveolar and vascular simplification in adult mice by upregulating carnitine palmitoyltransferase 1a (Cpt1a) (Chang et al., 2022). These results suggest possible treatment approaches for employing baicalin to prevent persistent lung injury in some illnesses and injuries.
Pharmacological studies have shown suppression of TLR4-mediated NF-κB activation (Zhang et al., 2016; Feng et al., 2018), downregulation of MMP9 expression (Zhang et al., 2016), inhibition of p-Src in LPS-activated neutrophils and formation of neutrophil extracellular traps (NETs) in Phorbol 12-myristate 13-acetate (PMA)-induced neutrophils (Xiao et al., 2022) in response to traditional Chinese medications containing baicalin.
Lung cancer
Baicalin has inhibitory effects on the proliferation and migration of various tumor cells. It can promote tumor cell apoptosis through multiple pathways and enhance the effectiveness of chemotherapy and radiotherapy (Zhang et al., 2017c; Singh et al., 2021). Baicalin can prevent lung cancer cells from growing and spreading by inducing apoptosis and cell cycle arrest and inhibiting the production of proinflammatory and protumor cytokines, according to research conducted on animals and in cell culture (Mizushima et al., 1995; Cheng et al., 2003; Himeji et al., 2007; Du et al., 2010; Jangid et al., 2020), and formulations and derivatives are emerging (Zhang et al., 2017c; Li et al., 2017; Wei et al., 2017; Jangid et al., 2020). By activating p38 MAPK and generating intracellular reactive oxygen species, baicalin enhances TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis (Zhang et al., 2017c).
The antitumor effects of baicalin mainly include inhibiting the proliferation of tumor cells by blocking the cell cycle, inducing apoptosis in tumor cells by producing cytotoxicity, and suppressing the erosion and metastasis of tumor cells (Sui et al., 2020; Yan et al., 2020). According to reports, baicalin can block cell cycle progression in the S phase by suppressing cyclin A, but in SKLU1, SKMES1 and DU145 cells, baicalin suppresses the expression of cyclin D1, leading to cell cycle arrest in the G1 phase (Gao et al., 2011). Baicalin stimulates the sirtuin 1 (SIRT1)/AMP-activated protein kinase (AMPK) signaling pathway (You et al., 2018), inhibits p-AKT in tumor cells, and attenuates cisplatin resistance in lung cancer by downregulating MARK2 and p-AKT (Xu et al., 2017b). Yin et al. reported that baicalin attenuates X-ray cross complementing 1 (XRCC1)-mediated DNA repair to enhance the sensitivity of lung cancer cells to cisplatin. Baicalin activated Rap1-GTP binding and dephosphorylated AKT and Src by suppressing a7 nicotinic acetylcholine receptor (a7nAChR), consequently triggering inhibition of inhibitor of differentiation factor 1 (Id1) (Zhao et al., 2019). Diao et al. demonstrated that baicalin inhibits lung cancer growth by targeting PDZ-binding kinase/T-LAK cell-originated protein kinase (PBK/TOPK) (Yan et al., 2020), while Chen et al. revealed that baicalin prevents epithelial-mesenchymal transition (EMT) by blocking the pyruvate dehydrogenase kinase 1 (PDK1)/AKT pathway in human non-small cell lung cancer (NSCLC) (Chen et al., 2021). Baicalin’s antitumor effects were shown by Zhao et al. to be mediated through the miR-340-5p-neuroepithelial cell transforming 1 (NET1) axis (Zhao et al., 2021b). Follow-up research can carry out more effective and precise interventions in the above and other regulatory pathways, inhibiting the growth and metastasis of lung cancer cells and prolonging the survival of patients.
Conclusion and future directions
The evidence thus far suggests baicalin may be a promising option for managing symptoms and preventing disease progression of various lung diseases due to its anti-inflammatory, antioxidant, anticancer and antiviral properties, although research on baicalin as a treatment for lung disease is still ongoing. While research on baicalin for the treatment of lung disease holds potential, there are still limitations to be addressed. First, many of the studies have focused on animal models; thus, more human clinical trials are needed to evaluate the efficacy and safety of baicalin in treating lung diseases. Baicalin has been found to be a safe and well-tolerated treatment for lung disease with a low incidence of adverse effects. Further research is needed to confirm its long-term safety and therapeutic benefits and to optimize its use as a treatment for lung disease. Additionally, the optimal dose and duration of treatment need to be determined, as well as the potential for drug interactions with other medications. With greater research into its mechanisms of action, long-term effects, application domains, and the continual expansion of its application, its value will continue to be unearthed and investigated.
Author contributions
DW collected and analyzed the data and wrote the manuscript. YL collected and analyzed the data and conceived the study. All authors commented on previous versions of the manuscript and read and approved the final manuscript.
Funding
The research leading to these results has received funding from Sichuan University Full-time Postdoctoral Research and Development Fund, Grant/Award Number: 2020SCU12023; PostDoctor Research Project, West China Hospital, Sichuan University, Grant/Award Number: 2018HXBH041. Financial support was received by YL.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bai, C., Li, T., Sun, Q., Xin, Q., Xu, T., Yu, J., et al. (2018). Protective effect of baicalin against severe burn-induced remote acute lung injury in rats. Mol. Med. Rep. 17 (2), 2689–2694. doi:10.3892/mmr.2017.8120
Bao, W. A., Wang, Y. Z., Zhu, X., Lin, J., Fan, J. F., Yang, Y., et al. (2022). Baicalin ameliorates radiation-induced lung injury by inhibiting the CysLTs/CysLT1 signaling pathway. Evid. Based Complement. Altern. Med. 2022, 2765354. doi:10.1155/2022/2765354
Barnes, P. J., Baker, J., and Donnelly, L. E. (2019). Cellular senescence as a mechanism and target in chronic lung diseases. Am. J. Respir. Crit. Care Med. 200 (5), 556–564. doi:10.1164/rccm.201810-1975TR
Barnes, P. J., Burney, P. G. J., Silverman, E. K., Celli, B. R., Vestbo, J., Wedzicha, J. A., et al. (2015). Chronic obstructive pulmonary disease. Nat. Rev. Dis. Prim. 1 (1), 15076. doi:10.1038/nrdp.2015.76
Boozari, M., and Hosseinzadeh, H. (2021). Natural products for COVID-19 prevention and treatment regarding to previous coronavirus infections and novel studies. Phytother. Res. 35 (2), 864–876. doi:10.1002/ptr.6873
Brackman, G., Cos, P., Maes, L., Nelis, H. J., and Coenye, T. (2011). Quorum sensing inhibitors increase the susceptibility of bacterial biofilms to antibiotics in vitro and in vivo. Antimicrob. Agents Chemother. 55 (6), 2655–2661. doi:10.1128/AAC.00045-11
Chang, H., Meng, H. Y., Bai, W. F., and Meng, Q. G. (2021). A metabolomic approach to elucidate the inhibitory effects of baicalin in pulmonary fibrosis. Pharm. Biol. 59 (1), 1016–1025. doi:10.1080/13880209.2021.1950192
Chang, J. L., Gong, J., Rizal, S., Peterson, A. L., Yao, C., Dennery, P. A., et al. (2022). Upregulating carnitine palmitoyltransferase 1 attenuates hyperoxia-induced endothelial cell dysfunction and persistent lung injury. Respir. Res. 23 (1), 205. doi:10.1186/s12931-022-02135-1
Changle, Z., Cuiling, F., Feng, F., Xiaoqin, Y., Guishu, W., Liangtian, S., et al. (2022). Baicalin inhibits inflammation of lipopolysaccharide-induced acute lung injury toll like receptor-4/myeloid differentiation primary response 88/nuclear factor-kappa B signaling pathway. J. Tradit. Chin. Med. 42 (2), 200–212. doi:10.19852/j.cnki.jtcm.20211214.004
Chen, F., Chan, K. H., Jiang, Y., Kao, R. Y. T., Lu, H. T., Fan, K. W., et al. (2004). In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J. Clin. Virol. 31 (1), 69–75. doi:10.1016/j.jcv.2004.03.003
Chen, J., Yuan, C. B., Yang, B., and Zhou, X. (2021). Baicalin inhibits EMT through PDK1/AKT signaling in human nonsmall cell lung cancer. J. Oncol. 2021, 4391581. doi:10.1155/2021/4391581
Chen, Z., and Wang, Q. (2017). Activation of PPARγ by baicalin attenuates pulmonary hypertension in an infant rat model by suppressing HMGB1/RAGE signaling. FEBS Open Bio 7 (4), 477–484. doi:10.1002/2211-5463.12180
Cheng, K. T., Hou, W. C., Huang, Y. C., and Wang, L. F. (2003). Baicalin induces differential expression of cytochrome C oxidase in human lung H441 cell. J. Agric. Food Chem. 51 (25), 7276–7279. doi:10.1021/jf0301549
Chu, M., Xu, L., Zhang, M. B., Chu, Z. Y., and Wang, Y. D. (2015). Role of baicalin in anti-influenza virus A as a potent inducer of IFN-gamma. Biomed. Res. Int. 2015, 263630. doi:10.1155/2015/263630
Cui, L., Yuan, T., Zeng, Z., Liu, D., Liu, C., Guo, J., et al. (2022). Mechanistic and therapeutic perspectives of baicalin and baicalein on pulmonary hypertension: A comprehensive review. Biomed. Pharmacother. 151, 113191. doi:10.1016/j.biopha.2022.113191
Deng, J., Wang, D. X., Liang, A. L., Tang, J., and Xiang, D. K. (2017). Effects of baicalin on alveolar fluid clearance and α-ENaC expression in rats with LPS-induced acute lung injury. Can. J. Physiol. Pharmacol. 95 (2), 122–128. doi:10.1139/cjpp-2016-0212
Deng, L., Ma, M., Li, S., Zhou, L., Ye, S., Wang, J., et al. (2022). Protective effect and mechanism of baicalin on lung inflammatory injury in BALB/cJ mice induced by PM2.5. Ecotoxicol. Environ. Saf. 248, 114329. doi:10.1016/j.ecoenv.2022.114329
Ding, X. M., Pan, L., Wang, Y., and Xu, Q. Z. (2016). Baicalin exerts protective effects against lipopolysaccharide-induced acute lung injury by regulating the crosstalk between the CX3CL1-CX3CR1 axis and NF-κB pathway in CX3CL1-knockout mice. Int. J. Mol. Med. 37 (3), 703–715. doi:10.3892/ijmm.2016.2456
Ding, Y., Dou, J., Teng, Z., Yu, J., Wang, T., Lu, N., et al. (2014). Antiviral activity of baicalin against influenza A (H1N1/H3N2) virus in cell culture and in mice and its inhibition of neuraminidase. Arch. Virol. 159 (12), 3269–3278. doi:10.1007/s00705-014-2192-2
Du, G., Han, G., Zhang, S., Lin, H., Wu, X., Wang, M., et al. (2010). Baicalin suppresses lung carcinoma and lung metastasis by SOD mimic and HIF-1alpha inhibition. Eur. J. Pharmacol. 630 (1-3), 121–130. doi:10.1016/j.ejphar.2009.12.014
Duan, X. Y., Sun, Y., Zhao, Z. F., Shi, Y. Q., Ma, X. Y., Tao, L., et al. (2021). Baicalin attenuates LPS-induced alveolar type II epithelial cell A549 injury by attenuation of the FSTL1 signaling pathway via increasing miR-200b-3p expression. Innate Immun. 27 (4), 294–312. doi:10.1177/17534259211013887
Feng, L., Yang, N., Li, C., Tian, G., Wang, J., Dong, Z. B., et al. (2018). Pudilan xiaoyan oral liquid alleviates LPS-induced respiratory injury through decreasing nitroxidative stress and blocking TLR4 activation along with NF-ΚB phosphorylation in mice. J. Ethnopharmacol. 214, 292–300. doi:10.1016/j.jep.2017.07.009
Fu, Y., Shen, J., Li, Y., Liu, F., Ning, B., Zheng, Y., et al. (2021). Inhibition of the PERK/TXNIP/NLRP3 Axis by baicalin reduces NLRP3 inflammasome-mediated pyroptosis in macrophages infected with Mycobacterium tuberculosis. Mediat. Inflamm. 2021, 1805147. doi:10.1155/2021/1805147
Gao, J., Morgan, W. A., Sanchez-Medina, A., and Corcoran, O. (2011). The ethanol extract of Scutellaria baicalensis and the active compounds induce cell cycle arrest and apoptosis including upregulation of p53 and Bax in human lung cancer cells. Toxicol. Appl. Pharmacol. 254 (3), 221–228. doi:10.1016/j.taap.2011.03.016
Gao, Y., Lu, J., Zhang, Y., Chen, Y., Gu, Z., and Jiang, X. (2013). Baicalein attenuates bleomycin-induced pulmonary fibrosis in rats through inhibition of miR-21. Pulm. Pharmacol. Ther. 26 (6), 649–654. doi:10.1016/j.pupt.2013.03.006
Geng, P., Zhu, H., Zhou, W., Su, C., Chen, M., Huang, C., et al. (2020). Baicalin inhibits influenza A virus infection via promotion of M1 macrophage polarization. Front. Pharmacol. 11, 01298. doi:10.3389/fphar.2020.01298
Hao, D., Li, Y., Shi, J., and Jiang, J. (2021). Baicalin alleviates chronic obstructive pulmonary disease through regulation of HSP72-mediated JNK pathway. Mol. Med. 27 (1), 53. doi:10.1186/s10020-021-00309-z
He, Y. Q., Zhou, C. C., Yu, L. Y., Wang, L., Deng, J. L., Tao, Y. L., et al. (2021). Natural product derived phytochemicals in managing acute lung injury by multiple mechanisms. Pharmacol. Res. 163, 105224. doi:10.1016/j.phrs.2020.105224
Himeji, M., Ohtsuki, T., Fukazawa, H., Tanaka, M., Yazaki, S. I., Ui, S., et al. (2007). Difference of growth-inhibitory effect of Scutellaria baicalensis-producing flavonoid wogonin among human cancer cells and normal diploid cell. Cancer Lett. 245 (1-2), 269–274. doi:10.1016/j.canlet.2006.01.011
Hu, L., Li, L., Yan, C., Cao, Y., Duan, X., and Sun, J. (2023). Baicalin inhibits airway smooth muscle cells proliferation through the RAS signaling pathway in murine asthmatic airway remodeling model. Oxid. Med. Cell Longev. 2023, 4144138. doi:10.1155/2023/4144138
Hu, T., Zhu, Y., Zhu, J., Yang, M., Wang, Y., and Zheng, Q. (2022). Wine-processed radix scutellariae alleviates ARDS by regulating tryptophan metabolism through gut microbiota. Front. Pharmacol. 13, 1104280. doi:10.3389/fphar.2022.1104280
Huang, C., Wang, Y., Li, X., Ren, L., Zhao, J., Hu, Y., et al. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395 (10223), 497–506. doi:10.1016/S0140-6736(20)30183-5
Huang, K. L., Chen, C. S., Hsu, C. W., Li, M. H., Chang, H., Tsai, S. H., et al. (2008). Therapeutic effects of baicalin on lipopolysaccharide-induced acute lung injury in rats. Am. J. Chin. Med. 36 (2), 301–311. doi:10.1142/S0192415X08005783
Huang, S., Chen, P., Shui, X., He, Y., Wang, H., Zheng, J., et al. (2014). Baicalin attenuates transforming growth factor-β1-induced human pulmonary artery smooth muscle cell proliferation and phenotypic switch by inhibiting hypoxia inducible factor-1α and aryl hydrocarbon receptor expression. J. Pharm. Pharmacol. 66 (10), 1469–1477. doi:10.1111/jphp.12273
Huang, X., He, Y., Chen, Y., Wu, P., Gui, D., Cai, H., et al. (2016). Baicalin attenuates bleomycin-induced pulmonary fibrosis via adenosine A2a receptor related TGF-β1-induced ERK1/2 signaling pathway. BMC Pulm. Med. 16 (1), 132. doi:10.1186/s12890-016-0294-1
Huang, X., Wu, P., Huang, F., Xu, M., Chen, M., Huang, K., et al. (2017). Baicalin attenuates chronic hypoxia-induced pulmonary hypertension via adenosine A(2A) receptor-induced SDF-1/CXCR4/PI3K/AKT signaling. J. Biomed. Sci. 24 (1), 52. doi:10.1186/s12929-017-0359-3
Huang, Y. X., Li, N. F., Li, C. Y., Zheng, F. P., Yao, X. Y., Lin, B. H., et al. (2022). Clinical features and effectiveness of Chinese medicine in patients with COVID-19 from overseas: A retrospective study in xiamen, China. Front. Public Health 10, 1038017. doi:10.3389/fpubh.2022.1038017
Jangid, A. K., Agraval, H., Rai, D. B., Jain, P., Yadav, U. C., Pooja, D., et al. (2020). Baicalin encapsulating lipid-surfactant conjugate based nanomicelles: Preparation, characterization and anticancer activity. Chem. Phys. Lipids 233, 104978. doi:10.1016/j.chemphyslip.2020.104978
Jasenosky, L. D., Scriba, T. J., Hanekom, W. A., and Goldfeld, A. E. (2015). T cells and adaptive immunity to Mycobacterium tuberculosis in humans. Immunol. Rev. 264 (1), 74–87. doi:10.1111/imr.12274
Jin, J., Chen, Y., Wang, D., Ma, L., Guo, M., Zhou, C., et al. (2018). The inhibitory effect of sodium baicalin on oseltamivir-resistant influenza A virus via reduction of neuraminidase activity. Arch. Pharm. Res. 41 (6), 664–676. doi:10.1007/s12272-018-1022-6
Jo, S., Kim, S., Kim, D. Y., Kim, M. S., and Shin, D. H. (2020). Flavonoids with inhibitory activity against SARS-CoV-2 3CLpro. J. Enzyme Inhib. Med. Chem. 35 (1), 1539–1544. doi:10.1080/14756366.2020.1801672
Ju, J., Li, Z., and Shi, Q. (2022). Baicalin inhibits inflammation in rats with chronic obstructive pulmonary disease by the TLR2/MYD88/NF-κBp65 signaling pathway. Evid. Based Complement. Altern. Med. 2022, 7273387. doi:10.1155/2022/7273387
Lai, Y. J., Chang, H. S., Yang, Y. P., Lin, T. W., Lai, W. Y., Lin, Y. Y., et al. (2021). The role of micronutrient and immunomodulation effect in the vaccine era of COVID-19. J. Chin. Med. Assoc. 84 (9), 821–826. doi:10.1097/JCMA.0000000000000587
Lederer, D. J., Longo, D. L., and Martinez, F. J. (2018). Idiopathic pulmonary fibrosis. N. Engl. J. Med. 378 (19), 1811–1823. doi:10.1056/NEJMra1705751
Li, L., Bao, H., Wu, J., Duan, X., Liu, B., Sun, J., et al. (2012). Baicalin is anti-inflammatory in cigarette smoke-induced inflammatory models in vivo and in vitro: A possible role for HDAC2 activity. Int. Immunopharmacol. 13 (1), 15–22. doi:10.1016/j.intimp.2012.03.001
Li, L. Y., Zhang, C. T., Zhu, F. Y., Zheng, G., Liu, Y. F., Liu, K., et al. (2022). Potential natural small molecular compounds for the treatment of chronic obstructive pulmonary disease: An overview. Front. Pharmacol. 13, 821941. doi:10.3389/fphar.2022.821941
Li, M. H., Huang, K. L., Wu, S. Y., Chen, C. W., Yan, H. C., Hsu, K., et al. (2009). Baicalin attenuates air embolism-induced acute lung injury in rat isolated lungs. Br. J. Pharmacol. 157 (2), 244–251. doi:10.1111/j.1476-5381.2009.00139.x
Li, R., and Wang, L. (2019). Baicalin inhibits influenza virus A replication via activation of type I IFN signaling by reducing miR-146a. Mol. Med. Rep. 20 (6), 5041–5049. doi:10.3892/mmr.2019.10743
Li, S., Wang, L., Liu, Y., and Su, H. (2017). Combination lung cancer chemotherapy: Design of a pH-sensitive transferrin-PEG-Hz-lipid conjugate for the co-delivery of docetaxel and baicalin. Biomed. Pharmacother. 95, 548–555. doi:10.1016/j.biopha.2017.08.090
Li, Z., Xia, X., Zhang, S., Zhang, A., Bo, W., and Zhou, R. (2009). Up-regulation of Toll-like receptor 4 was suppressed by emodin and baicalin in the setting of acute pancreatitis. Biomed. Pharmacother. 63 (2), 120–128. doi:10.1016/j.biopha.2008.01.003
Liau, P. R., Wu, M. S., and Lee, C. K. (2019). Inhibitory effects of Scutellaria baicalensis root extract on linoleic acid hydroperoxide-induced lung mitochondrial lipid peroxidation and antioxidant activities. Molecules 24 (11), 2143. doi:10.3390/molecules24112143
Limanaqi, F., Busceti, C. L., Biagioni, F., Lazzeri, G., Forte, M., Schiavon, S., et al. (2020). Cell clearing systems as targets of polyphenols in viral infections: Potential implications for COVID-19 pathogenesis. Antioxidants (Basel) 9 (11), 1105. doi:10.3390/antiox9111105
Lin, C., Tsai, F. J., Hsu, Y. M., Ho, T. J., Wang, G. K., Chiu, Y. J., et al. (2021). Study of baicalin toward COVID-19 treatment: In silico target analysis and in vitro inhibitory effects on SARS-CoV-2 proteases. Biomed. Hub. 6 (3), 122–137. doi:10.1159/000519564
Liu, C., Zhu, X., Lu, Y., Zhang, X., Jia, X., and Yang, T. (2021). Potential treatment with Chinese and Western medicine targeting NSP14 of SARS-CoV-2. J. Pharm. Anal. 11 (3), 272–277. doi:10.1016/j.jpha.2020.08.002
Liu, J., Wei, Y., Luo, Q., Xu, F., Zhao, Z., Zhang, H., et al. (2016). Baicalin attenuates inflammation in mice with OVA-induced asthma by inhibiting NF-κB and suppressing CCR7/CCL19/CCL21. Int. J. Mol. Med. 38 (5), 1541–1548. doi:10.3892/ijmm.2016.2743
Liu, P., Yan, S., Chen, M., Chen, A., Yao, D., Xu, X., et al. (2015). Effects of baicalin on collagen Ι and collagen ΙΙΙ expression in pulmonary arteries of rats with hypoxic pulmonary hypertension. Int. J. Mol. Med. 35 (4), 901–908. doi:10.3892/ijmm.2015.2110
Liu, S., Liu, B., Luo, Z. Q., Qiu, J., Zhou, X., Li, G., et al. (2017). The combination of osthole with baicalin protects mice from Staphylococcus aureus pneumonia. World J. Microbiol. Biotechnol. 33 (1), 11. doi:10.1007/s11274-016-2162-9
Liu, T., Dai, W., Liu, F., Chen, Y., Weng, D., et al. (2015). Baicalin alleviates silica-induced lung inflammation and fibrosis by inhibiting the Th17 response in C57bl/6 mice. J. Nat. Prod. 78 (12), 3049–3057. doi:10.1021/acs.jnatprod.5b00868
Lixuan, Z., Jingcheng, D., Wenqin, Y., Jianhua, H., Baojun, L., and Xiaotao, F. (2010). Baicalin attenuates inflammation by inhibiting NF-kappaB activation in cigarette smoke induced inflammatory models. Pulm. Pharmacol. Ther. 23 (5), 411–419. doi:10.1016/j.pupt.2010.05.004
Long, Y., Xiang, Y., Liu, S., Zhang, Y., Wan, J., Yang, Q., et al. (2020). Baicalin liposome alleviates lipopolysaccharide-induced acute lung injury in mice via inhibiting TLR4/JNK/ERK/NF-κB pathway. Mediat. Inflamm. 2020, 8414062. doi:10.1155/2020/8414062
Lu, J., Zhong, Y., Lin, Z., Lin, X., Chen, Z., Wu, X., et al. (2017). Baicalin alleviates radiation-induced epithelial-mesenchymal transition of primary type II alveolar epithelial cells via TGF-β and ERK/GSK3β signaling pathways. Biomed. Pharmacother. 95, 1219–1224. doi:10.1016/j.biopha.2017.09.037
Luan, Y., Chao, S., Ju, Z. Y., Wang, J., Xue, X., Qi, T. G., et al. (2015). Therapeutic effects of baicalin on monocrotaline-induced pulmonary arterial hypertension by inhibiting inflammatory response. Int. Immunopharmacol. 26 (1), 188–193. doi:10.1016/j.intimp.2015.01.009
Ma, C., Ma, Z., and Fu, Q. (2014). Anti-asthmatic effects of baicalin in a mouse model of allergic asthma. Phytother. Res. 28 (2), 231–237. doi:10.1002/ptr.4983
Matthay, M. A., Ware, L. B., and Zimmerman, G. A. (2012). The acute respiratory distress syndrome. J. Clin. investigation 122 (8), 2731–2740. doi:10.1172/JCI60331
McAllister, D. A., Liu, L., Shi, T., Chu, Y., Reed, C., Burrows, J., et al. (2019). Global, regional, and national estimates of pneumonia morbidity and mortality in children younger than 5 years between 2000 and 2015: A systematic analysis. Lancet Glob. Health 7 (1), e47–e57. doi:10.1016/S2214-109X(18)30408-X
Meng, X., Hu, L., and Li, W. (2019). Baicalin ameliorates lipopolysaccharide-induced acute lung injury in mice by suppressing oxidative stress and inflammation via the activation of the Nrf2-mediated HO-1 signaling pathway. Naunyn Schmiedeb. Arch. Pharmacol. 392 (11), 1421–1433. doi:10.1007/s00210-019-01680-9
Mizushima, Y., Kashii, T., Tokimitsu, Y., and Kobayashi, M. (1995). Cytotoxic effect of herbal medicine sho-saiko-to on human lung-cancer cell-lines in-vitro. Oncol. Rep. 2 (1), 91–94. doi:10.3892/or.2.1.91
Nayak, M. K., Agrawal, A. S., Bose, S., Naskar, S., Bhowmick, R., Chakrabarti, S., et al. (2014). Antiviral activity of baicalin against influenza virus H1N1-pdm09 is due to modulation of NS1-mediated cellular innate immune responses. J. Antimicrob. Chemother. 69 (5), 1298–1310. doi:10.1093/jac/dkt534
Pang, P., Zheng, K., Wu, S., Xu, H., Deng, L., Shi, Y., et al. (2018). Baicalin downregulates RLRs signaling pathway to control influenza A virus infection and improve the prognosis. Evid. Based Complement. Altern. Med. 2018, 4923062. doi:10.1155/2018/4923062
Papi, A., Brightling, C., Pedersen, S. E., and Reddel, H. K. (2018). Asthma. Lancet 391 (10122), 783–800. doi:10.1016/S0140-6736(17)33311-1
Park, K., Lee, J. S., Choi, J. S., Nam, Y. J., Han, J. H., Byun, H. D., et al. (2016). Identification and characterization of baicalin as a phosphodiesterase 4 inhibitor. Phytother. Res. 30 (1), 144–151. doi:10.1002/ptr.5515
Peng, L. Y., Yuan, M., Song, K., Yu, J. L., Li, J. H., Huang, J. N., et al. (2019). Baicalin alleviated APEC-induced acute lung injury in chicken by inhibiting NF-κB pathway activation. Int. Immunopharmacol. 72, 467–472. doi:10.1016/j.intimp.2019.04.046
Shi, C. S., Qi, H. Y., Boularan, C., Huang, N. N., Abu-Asab, M., Shelhamer, J. H., et al. (2014). SARS-coronavirus open reading frame-9b suppresses innate immunity by targeting mitochondria and the MAVS/TRAF3/TRAF6 signalosome. J. Immunol. 193 (6), 3080–3089. doi:10.4049/jimmunol.1303196
Shi, H., Ren, K., Lv, B., Zhang, W., Zhao, Y., Tan, R. X., et al. (2016). Baicalin from Scutellaria baicalensis blocks respiratory syncytial virus (RSV) infection and reduces inflammatory cell infiltration and lung injury in mice. Sci. Rep. 6, 35851. doi:10.1038/srep35851
Shin, Y. O., Park, C. H., Lee, G. H., Yokozawa, T., Roh, S. S., and Rhee, M. H. (2015). Heat-Processed scutellariae radix enhances anti-inflammatory effect against lipopolysaccharide-induced acute lung injury in mice via NF- κ B signaling. Evid. Based Complement. Altern. Med. 2015, 456846. doi:10.1155/2015/456846
Singh, S., Meena, A., and Luqman, S. (2021). Baicalin mediated regulation of key signaling pathways in cancer. Pharmacol. Res. 164, 105387. doi:10.1016/j.phrs.2020.105387
Sithisarn, P., Michaelis, M., Schubert-Zsilavecz, M., and Cinatl, J. (2013). Differential antiviral and anti-inflammatory mechanisms of the flavonoids biochanin A and baicalein in H5N1 influenza A virus-infected cells. Antivir. Res. 97 (1), 41–48. doi:10.1016/j.antiviral.2012.10.004
Su, H. X., Yao, S., Zhao, W. F., Li, M. J., Liu, J., Shang, W. J., et al. (2020). Anti-SARS-CoV-2 activities in vitro of Shuanghuanglian preparations and bioactive ingredients. Acta Pharmacol. Sin. 41 (9), 1167–1177. doi:10.1038/s41401-020-0483-6
Sui, X., Han, X., Chen, P., Wu, Q., Feng, J., Duan, T., et al. (2020). Baicalin induces apoptosis and suppresses the cell cycle progression of lung cancer cells through downregulating akt/mTOR signaling pathway. Front. Mol. Biosci. 7, 602282. doi:10.3389/fmolb.2020.602282
Sun, J., Li, L., Wu, J., Liu, B., Gong, W., Lv, Y., et al. (2013). Effects of baicalin on airway remodeling in asthmatic mice. Planta Med. 79 (3-4), 199–206. doi:10.1055/s-0032-1328197
Vonk Noordegraaf, A., Groeneveldt, J. A., and Bogaard, H. J. (2016). Pulmonary hypertension. Eur. Respir. Rev. 25 (139), 4–11. doi:10.1183/16000617.0096-2015
Wan, Q., Wang, H., Han, X., Lin, Y., Yang, Y., Gu, L., et al. (2014). Baicalin inhibits TLR7/MYD88 signaling pathway activation to suppress lung inflammation in mice infected with influenza A virus. Biomed. Rep. 2 (3), 437–441. doi:10.3892/br.2014.253
Wang, C., Horby, P. W., Hayden, F. G., and Gao, G. F. (2020). A novel coronavirus outbreak of global health concern. Lancet 395 (10223), 470–473. doi:10.1016/S0140-6736(20)30185-9
Wang, G., Hu, Y. X., He, M. Y., Xie, Y. H., Su, W., Long, D., et al. (2021). Gut-lung dysbiosis accompanied by diabetes mellitus leads to pulmonary fibrotic change through the NF-κB signaling pathway. Am. J. Pathol. 191 (5), 838–856. doi:10.1016/j.ajpath.2021.02.019
Wang, G., Mohammadtursun, N., Lv, Y., Zhang, H., Sun, J., and Dong, J. (2018). Baicalin exerts anti-airway inflammation and anti-remodelling effects in severe stage rat model of chronic obstructive pulmonary disease. Evid. Based Complement. Altern. Med. 2018, 7591348. doi:10.1155/2018/7591348
Wang, H., and Liu, D. (2014). Baicalin inhibits high-mobility group box 1 release and improves survival in experimental sepsis. Shock 41 (4), 324–330. doi:10.1097/SHK.0000000000000122
Wang, J., Ishfaq, M., and Li, J. (2021). Baicalin ameliorates Mycoplasma gallisepticum-induced inflammatory injury in the chicken lung through regulating the intestinal microbiota and phenylalanine metabolism. Food Funct. 12 (9), 4092–4104. doi:10.1039/d1fo00055a
Wei, W. C., Liaw, C. C., Tsai, K. C., Chiou, C. T., Tseng, Y. H., Chiou, W. F., et al. (2022). Targeting spike protein-induced TLR/NET axis by COVID-19 therapeutic NRICM102 ameliorates pulmonary embolism and fibrosis. Pharmacol. Res. 184, 106424. doi:10.1016/j.phrs.2022.106424
Wei, Y., Liang, J., Zheng, X., Pi, C., Liu, H., Yang, H., et al. (2017). Lung-targeting drug delivery system of baicalin-loaded nanoliposomes: Development, biodistribution in rabbits, and pharmacodynamics in nude mice bearing orthotopic human lung cancer. Int. J. Nanomedicine 12, 251–261. doi:10.2147/IJN.S119895
Wu, Z., Chen, C., Miao, Y., Liu, Y., Zhang, Q., Li, R., et al. (2019). Baicalin attenuates Mycoplasma gallisepticum-induced inflammation via inhibition of the TLR2-NF-κb pathway in chicken and DF-1 cells. Infect. Drug Resist 12, 3911–3923. doi:10.2147/IDR.S231908
Xiao, S., Liu, L., Sun, Z., Liu, X., Xu, J., Guo, Z., et al. (2022). Network Pharmacology and experimental validation to explore the mechanism of qing-jin-hua-tan-decoction against acute lung injury. Front. Pharmacol. 13, 891889. doi:10.3389/fphar.2022.891889
Xu, G., Dou, J., Zhang, L., Guo, Q., and Zhou, C. (2010). Inhibitory effects of baicalein on the influenza virus in vivo is determined by baicalin in the serum. Biol. Pharm. Bull. 33 (2), 238–243. doi:10.1248/bpb.33.238
Xu, L., Li, J., Zhang, Y., Zhao, P., and Zhang, X. (2017). Regulatory effect of baicalin on the imbalance of Th17/Treg responses in mice with allergic asthma. J. Ethnopharmacol. 208, 199–206. doi:10.1016/j.jep.2017.07.013
Xu, Z., Mei, J., and Tan, Y. (2017). Baicalin attenuates DDP (cisplatin) resistance in lung cancer by downregulating MARK2 and p-Akt. Int. J. Oncol. 50 (1), 93–100. doi:10.3892/ijo.2016.3768
Xue, X., Zhang, S., Jiang, W., Wang, J., Xin, Q., Sun, C., et al. (2021). Protective effect of baicalin against pulmonary arterial hypertension vascular remodeling through regulation of TNF-α signaling pathway. Pharmacol. Res. Perspect. 9 (1), e00703. doi:10.1002/prp2.703
Yan, G., Wang, J., Yi, T., Cheng, J., Guo, H., He, Y., et al. (2019). Baicalin prevents pulmonary arterial remodeling in vivo via the AKT/ERK/NF-κB signaling pathways. Pulm. Circ. 9 (4), 2045894019878599. doi:10.1177/2045894019878599
Yan, S., Wang, Y., Liu, P., Chen, A., Chen, M., Yao, D., et al. (2016). Baicalin attenuates hypoxia-induced pulmonary arterial hypertension to improve hypoxic cor pulmonale by reducing the activity of the p38 MAPK signaling pathway and MMP-9. Evid. Based Complement. Altern. Med. 2016, 2546402. doi:10.1155/2016/2546402
Yan, Y., Yao, L., Sun, H., Pang, S., Kong, X., Zhao, S., et al. (2020). Effects of wogonoside on invasion and migration of lung cancer A549 cells and angiogenesis in xenograft tumors of nude mice. J. Thorac. Dis. 12 (4), 1552–1560. doi:10.21037/jtd-20-1555
Yang, G., Li, J., Bo, J. P., Wang, B., Tian, X. R., Liu, T. Z., et al. (2015). Baicalin inhibits PDGF-induced proliferation and migration of airway smooth muscle cells. Int. J. Clin. Exp. Med. 8 (11), 20532–20539.
Yin, Z., Chen, E., Cai, X., Gong, E., Li, Y., Xu, C., et al. (2022). Baicalin attenuates XRCC1-mediated DNA repair to enhance the sensitivity of lung cancer cells to cisplatin. J. Recept Signal Transduct. Res. 42 (3), 215–224. doi:10.1080/10799893.2021.1892132
Yoshida, K., Takabayashi, T., Kaneko, A., Takiyama, M., Sakashita, M., Imoto, Y., et al. (2021). Baicalin suppresses type 2 immunity through breaking off the interplay between mast cell and airway epithelial cell. J. Ethnopharmacol. 267, 113492. doi:10.1016/j.jep.2020.113492
You, J., Cheng, J., Yu, B., Duan, C., and Peng, J. (2018). Baicalin, a Chinese herbal medicine, inhibits the proliferation and migration of human non-small cell lung carcinoma (NSCLC) cells, A549 and H1299, by activating the SIRT1/AMPK signaling pathway. Med. Sci. Monit. 24, 2126–2133. doi:10.12659/msm.909627
Zhai, C., and Wang, D. (2022). Baicalin regulates the development of pediatric asthma via upregulating microRNA-103 and mediating the TLR4/NF-κB pathway. J. Recept Signal Transduct. Res. 42 (3), 230–240. doi:10.1080/10799893.2021.1900865
Zhang, H., Li, X., Wang, J., Cheng, Q., Shang, Y., and Wang, G. (2021). Baicalin relieves Mycoplasma pneumoniae infection-induced lung injury through regulating microRNA-221 to inhibit the TLR4/NF-κB signaling pathway. Mol. Med. Rep. 24 (2), 571. doi:10.3892/mmr.2021.12210
Zhang, H., Liu, B., Jiang, S., Wu, J. F., Qi, C. H., Mohammadtursun, N., et al. (2021). Baicalin ameliorates cigarette smoke-induced airway inflammation in rats by modulating HDAC2/NF-κB/PAI-1 signalling. Pulm. Pharmacol. Ther. 70, 102061. doi:10.1016/j.pupt.2021.102061
Zhang, L., Pu, Z., Wang, J., Zhang, Z., Hu, D., and Wang, J. (2014). Baicalin inhibits hypoxia-induced pulmonary artery smooth muscle cell proliferation via the AKT/HIF-1α/p27-associated pathway. Int. J. Mol. Sci. 15 (5), 8153–8168. doi:10.3390/ijms15058153
Zhang, L., Wang, X., Wang, R., Zheng, X., Li, H., et al. (2017). Baicalin potentiates TRAIL-induced apoptosis through p38 MAPK activation and intracellular reactive oxygen species production. Mol. Med. Rep. 16 (6), 8549–8555. doi:10.3892/mmr.2017.7633
Zhang, L., Yang, L., Xie, X., Zheng, H., Zheng, H., et al. (2021). Baicalin magnesium salt attenuates lipopolysaccharide-induced acute lung injury via inhibiting of TLR4/NF-κB signaling pathway. J. Immunol. Res. 2021, 6629531. doi:10.1155/2021/6629531
Zhang, P., Guo, Q., Wei, Z., Yang, Q., Guo, Z., Shen, L., et al. (2021). Baicalin represses type three secretion system of Pseudomonas aeruginosa through PQS system. Molecules 26 (6), 1497. doi:10.3390/molecules26061497
Zhang, Q., Sun, J., Wang, Y., He, W., Wang, L., Zheng, Y., et al. (2017). Antimycobacterial and anti-inflammatory mechanisms of baicalin via induced autophagy in macrophages infected with Mycobacterium tuberculosis. Front. Microbiol. 8, 2142. doi:10.3389/fmicb.2017.02142
Zhang, X. P., Zhang, L., Yang, P., Zhang, R. P., and Cheng, Q. H. (2008). Protective effects of baicalin and octreotide on multiple organ injury in severe acute pancreatitis. Dig. Dis. Sci. 53 (2), 581–591. doi:10.1007/s10620-007-9868-3
Zhang, X., Sun, C. Y., Zhang, Y. B., Guo, H. Z., Feng, X. X., Peng, S. Z., et al. (2016). Kegan Liyan oral liquid ameliorates lipopolysaccharide-induced acute lung injury through inhibition of TLR4-mediated NF-κB signaling pathway and MMP-9 expression. J. Ethnopharmacol. 186, 91–102. doi:10.1016/j.jep.2016.03.057
Zhang, Z., Zhang, L., Sun, C., Kong, F., Wang, J., Xin, Q., et al. (2017). Baicalin attenuates monocrotaline-induced pulmonary hypertension through bone morphogenetic protein signaling pathway. Oncotarget 8 (38), 63430–63441. doi:10.18632/oncotarget.18825
Zhao, F., Zhao, Z., Han, Y., Li, S., Liu, C., and Jia, K. (2021). Baicalin suppresses lung cancer growth phenotypes via miR-340-5p/NET1 axis. Bioengineered 12 (1), 1699–1707. doi:10.1080/21655979.2021.1922052
Zhao, H., Li, C., Li, L., Liu, J., Gao, Y., Mu, K., et al. (2020). Baicalin alleviates bleomycin-induced pulmonary fibrosis and fibroblast proliferation in rats via the PI3K/AKT signaling pathway. Mol. Med. Rep. 21 (6), 2321–2334. doi:10.3892/mmr.2020.11046
Zhao, J., Tian, S., Lu, D., Yang, J., Zeng, H., Zhang, F., et al. (2021). Systems pharmacological study illustrates the immune regulation, anti-infection, anti-inflammation, and multi-organ protection mechanism of Qing-Fei-Pai-Du decoction in the treatment of COVID-19. Phytomedicine 85, 153315. doi:10.1016/j.phymed.2020.153315
Zhao, Z., Liu, B., Sun, J., Liu, L., Liu, L., Qiu, J., et al. (2019). Scutellaria flavonoids effectively inhibit the malignant phenotypes of non-small cell lung cancer in an id1-dependent manner. Int. J. Biol. Sci. 15 (7), 1500–1513. doi:10.7150/ijbs.33146
Zhi, H. J., Zhu, H. Y., Zhang, Y. Y., Lu, Y., Li, H., and Chen, D. F. (2019). In vivo effect of quantified flavonoids-enriched extract of Scutellaria baicalensis root on acute lung injury induced by influenza A virus. Phytomedicine 57, 105–116. doi:10.1016/j.phymed.2018.12.009
Keywords: baicalin, lung disease, lung infection, lung injury, lung cancer
Citation: Wang D and Li Y (2023) Pharmacological effects of baicalin in lung diseases. Front. Pharmacol. 14:1188202. doi: 10.3389/fphar.2023.1188202
Received: 17 March 2023; Accepted: 10 April 2023;
Published: 24 April 2023.
Edited by:
Ochuko Lucky Erukainure, University of the Free State, South AfricaReviewed by:
Okukwe Obode, Federal Institute of Industrial Research Oshodi, NigeriaMarvellous Acho, Landmark University, Nigeria
Copyright © 2023 Wang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Li, cGVhY2hfYWRvcmVAaG90bWFpbC5jb20vbGl5aUB3Y2hzY3UuY24=
†ORCID: Yi Li, orcid.org/0000-0001-8661-4617
 Duoning Wang1,2
Duoning Wang1,2 Yi Li
Yi Li