
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 01 June 2023
Sec. Ethnopharmacology
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1185809
This article is part of the Research Topic Real-World Evidence of Natural Products, Herbal Medicines, and Traditional Chinese Medicine Treatments View all 15 articles
Objectives: To investigate the association between traditional Chinese medicine (TCM) therapy and the risk of pneumonia in patients with systemic lupus erythematosus (SLE).
Methods: This population-based control study analyzed the data retrieved from the National Health Insurance Research database in Taiwan. From a cohort of 2 million records of the 2000–2018 period, 9,714 newly diagnosed patients with SLE were initially included. 532 patients with pneumonia and 532 patients without pneumonia were matched 1:1 based on age, sex, and year of SLE diagnosis using propensity score matching. The use of TCM therapy was considered from the SLE diagnosis date to the index date and the cumulative days of TCM therapy were used to calculate the dose effect. Conditional logistic regression was used to investigate the risk of pneumonia infection. Furthermore, to explore the severity of pneumonia in SLE, sensitivity analyses were performed after stratification using the parameters of emergency room visit, admission time, and antibiotic use.
Results: TCM therapy for >60 days could significantly reduce the risk of pneumonia in patients with SLE (95% CI = 0.46–0.91; p = 0.012). Stratified analysis showed that TCM use also reduced the risk of pneumonia in younger and female patients with SLE by 34% and 35%, respectively. TCM for >60 days significantly reduced the risk of pneumonia in the follow-up periods of >2, >3, >7, and >8 years. In addition, the exposure of TCM for >60 days reduced the risk of pneumonia in patients with SLE who were treated with antibiotics for moderate or severe pneumonia. Finally, the study found that using formulae to tonify the kidney for more than 90 days and formulae to activate blood circulation for less than 30 days could significantly reduce the risk of pneumonia infection in patients with SLE.
Conclusion: TCM use is associated with a lower risk of pneumonia among patients with SLE.
Systemic lupus erythematosus (SLE) is an autoimmune disease that presents with complex clinical manifestations. SLE can affect all systems of the body, including the kidneys and nervous system (Kiriakidou and Ching, 2020). The estimated worldwide prevalence of SLE is approximately 30–50/100,000 people, and the prevalence is higher in developing countries (Ingvarsson et al., 2016; Tsioni et al., 2015; Li et al., 2012). The 10-year survival rate is approximately 90%, 15-year survival rate is 85%, and 20-year survival rate is 78% (Durcan et al., 2019), indicating that the disease seriously affects the physical and mental health of the patients as well as their quality of life. The current management of SLE is still dominated by glucocorticoids and immunosuppressive agents. However, these agents often cause several side effects, such as a secondary infection (27%), hypertension (11.3%), and osteoporosis (7.5%) (Tektonidou et al., 2015). Among the side effects, infection and lupus nephritis are the main causes of death in SLE (Sciascia et al., 2017). Clinical studies have found that belimumab, the first biologic approved for the treatment of SLE by the Food and Drug Administration, combined with standard therapy can alleviate the disease activity in some patients with SLE and has been shown to have a safety profile similar to a placebo (Stohl et al., 2017). Although pre-emptive antimicrobial therapy and vaccination has become the consensus for preventing infections, the incidence of certain infections, such as pneumonia, remains high and inevitable (Oku et al., 2021). Therefore, there is an urgent need for the development of clinical treatments or drugs with high safety and good efficacy for alleviating SLE and preventing infections.
Traditional Chinese medicine (TCM) has been used for treating several autoimmune diseases (called Bi syndrome in TCM), including SLE, and has shown significant clinical efficacy for thousands of years (Wang et al., 2019). We previously conducted a meta-analysis of 13 randomized, placebo-controlled trials that included 856 participants and revealed that TCM could control disease activity and reduce glucocorticoid dose used among patients with SLE (Wang et al., 2021). A population-based cohort study based on the National Health Insurance Research Database (NHIRD) in Taiwan provided evidence that regular treatment combined with Chinese herbal medicine improves the survival of patients with SLE. The study included several useful TCM formulae, including Zhi Bo Di Huang Wan, Jia Wei Xiao Yao San, Liu Wei Di Huang Wan, Gan Lu Yin, and Yin Qiao San (Ma et al., 2016). However, few studies have evaluated whether TCM can reduce the risk of infections in patients with SLE.
To the best of our knowledge, no large-scale study has evaluated the association between TCM therapy and the risk of pneumonia in patients with SLE. Thus, the present study aimed to provide some evidence regarding the aforementioned association.
The research data of 2 million people from 1 January 2000, to 31 December 2018, were retrieved from NHIRD, Taiwan, out of the 23 million people included in the database. The random sampling method was employed, with serial numbers assigned to each of the 23 million insurance beneficiaries. NHIRD contains information on patient demographics, age, sex, disease diagnoses, number of clinical visits and hospitalizations, prescribed medications (with dosages), and TCM treatment agents administered by registered TCM physicians. Diseases were defined as per the International Classification of Diseases (ICD) Ninth Revision (ICD-9) and 10th Edition (ICD-10) codes (Goulielmos and Zervou, 2020).
As shown in Figure 1 total of 2 million patients were randomly selected from NHIRD. The included patients were those who were newly diagnosed with SLE (ICD-9 code = 710.0; ICD-10 code = M32) from January 2001 to December 2016 (n = 9,714). A stringent criterion of requiring at least three outpatient visits or one hospital admission was used for recognizing SLE. In addition, pneumonia (ICD-9 code = 480–486; ICD-10 code = J12–J18) was chosen as the representative infection with SLE. After excluding the diagnosis of pneumonia before the first SLE diagnosis date and 1 year after SLE diagnosis, a total of 7,813 patients with SLE were finally selected as the study group. A total of 739 pneumonia patients with SLE were selected from the emergency or admission group. The index date was defined as the pneumonia onset date (Supplementary Figure S1). Moreover, 6,216 patients with SLE were never diagnosed with pneumonia. After 1:1 propensity score matching using the parameters of age, sex, and SLE diagnosis year, 532 pneumonia and 532 non-pneumonia patients with SLE were included in the analysis.
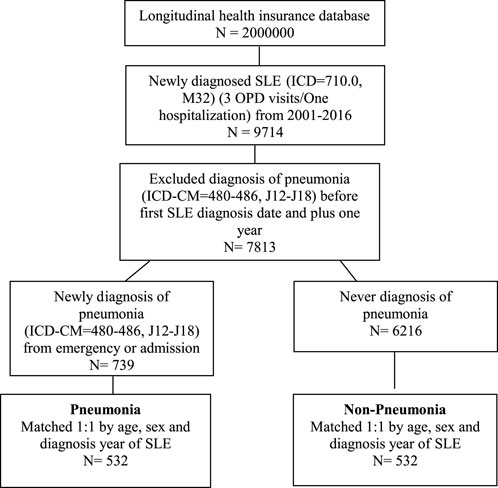
FIGURE 1. Flow chart of the study design. SLE, systemic lupus erythematosus; OPD, Outpatient department.
TCM use was calculated from the SLE diagnosis date to the index date, and the cumulative days of TCM therapy were used to calculate the dose effect. TCM users were defined as patients who used TCM more than three times. In addition, the cumulative days of TCM therapy were classified as 30, 60, and 90 days. TCM considers SLE as a systemic disease associated with the state of the entire body and implicates toxic heat, blood stasis, and kidney yin deficiency in the pathogenesis of SLE (Sun et al., 2018). Therefore, the TCM formulae were initially classified into three types: those tonifying the kidney (KF), those activating blood circulation (BF), and the “other TCM therapy” group. The classification standards were followed as per the classification criteria described in a previous study (Lin et al., 2021). As a result, the top 20 frequently used Chinese medicine formulae in NHIRD were selected and classified into two groups: the KF and BF groups. The KF group included Guilu Erxian Jiao, Jisheng Shenqi Pill, Qiju Dihuang Pill, Zhibo Dihuang Pill, Liuwei Dihuang Wan, and Zuo Guiwan, whereas the BF group comprised Duhuo Jisheng Decoction, Shujing Huoxue Decoction, Danggui Niantong Decoction, Shaoyao Gancao Decoction, Shentong Zhuyu Decoction, Xuefu Zhuyu Decoction, Xiao Huoluo Dan, and Guizhi Shaoyao Zhimu Decoction (Supplementary Table S1). The baseline comorbidities included hypertension (ICD-9 code = 401–405; ICD-10 code = I10–I15), hyperlipidemia (ICD-9 code = 272; ICD-10 code = E78), chronic liver disease (ICD-9 code = 571; ICD-10 code = K70, K73, K74, K75.4, K75.81, K76.0, K76.89, and K76.9), chronic kidney disease (ICD-9 code = 585; ICD-10 code = N184, N185, N186, and N189), diabetes (ICD-9 code = 250; ICD-10 code = E10, E11, E12, E13, and E14), chronic obstructive pulmonary disease (ICD-9 code = 491, 492, and 496; ICD-10 code = J41–J44), rheumatoid arthritis (ICD-9 code = 714.0; ICD-10 code = M05 and M06), ankylosing spondylitis (ICD-9 code = 720.0; ICD-10 code = M45 and M46), hepatitis B (ICD-9 code = 070.2, 070.3, and V02.61; ICD-10 code = B16.0, B16.1, B16.2, B16.9, B18.0, B18.1, B19.10, B19.11, and Z22.51), hepatitis C (ICD-9 code = 070.41, 070.44, 070.51, 070.54, 070.7, and V02.62; ICD-10 code = B17.10, B17.11, B18.2, B19.20, B19.21, and Z22.52), endocarditis (ICD-9 code = 424.91; ICD-10 code = I39), nephritis (ICD-9 code = 583.81; ICD-10 code = E10.21, E11.21, and N16), glomerulonephritis (ICD-9 code = 580.81, 581.81, and 582.81; ICD-10 code = N08). These comorbidities were identified during at least three outpatient visits or one hospital admission between the SLE diagnosis date and index date. The Anatomical Therapeutic Chemical (ATC) Classification System codes were used for defining drugs. The use of corticosteroids, hydroxychloroquine (ATC code = P01BA02), and methotrexate (ATC code = L04AX03) for at least three times prescription in each kind of medication between the SLE diagnosis date and index date was evaluated.
The Chi-squared test and Student’s t-test were used to compare continuous and dichotomous data, respectively, between the pneumonia and non-pneumonia groups. Multivariate conditional logistic regression analysis was performed to evaluate the risk of pneumonia infection in patients with SLE after prescribing TCM therapy. The risk was estimated using adjusted odds ratios (aORs) and 95% confidence intervals (CIs). Statistical significance was defined as p-value <0.05, and all data analyses were performed using the statistical package SAS for Windows (Version 9.4, SAS Institute Inc., Carey, NC, United States).
Subgroup analyses were performed to determine how the risk of pneumonia infection in patients with SLE patients differed as per sex, age, and days of TCM use. In addition, the association between the use of different TCM formulae and risk of pneumonia infection was evaluated. Antibiotic use and hospitalization period were analyzed to estimate the severity of pneumonia.
The demographic characteristics of included patients with SLE in the pneumonia and non-pneumonia groups are shown in Table 1. After 1:1 propensity score matching using the parameters of age, sex, and SLE diagnosis year, 532 pneumonia and 532 non-pneumonia patients with SLE were finally included in the study. SLE patients with pneumonia had several comorbidities, including hypertension (p = 0.0014), chronic liver disease (p = 0.0312), chronic kidney disease (p < 0.001), diabetes (p = 0.0002), chronic obstructive pulmonary disease (COPD) (p = 0.0102), and rheumatoid arthritis (p = 0.0308), except ankylosing spondylitis. Moreover, corticosteroids, nonsteroidal anti-inflammatory drugs, and hydroxychloroquine were used more frequently by patients with SLE in the pneumonia group. Finally, TCM was prescribed more frequently to SLE patients with pneumonia in the 60 days stratification groups (p = 0.0268).
Conditional logistic regression analysis revealed that TCM use reduced the risk of pneumonia in patients with SLE (aOR (95% CI) = 0.73 (0.55–0.96); p = 0.026). Among the comorbidities, chronic kidney disease (aOR (95% CI) = 2.23 (1.25–3.97); p = 0.007), diabetes (aOR (95% CI) = 1.84 (1.18–2.87); p = 0.007), and COPD (aOR (95% CI) = 1.72 (1.06–2.79); p = 0.028) were associated with a higher risk of pneumonia in patients with SLE. Moreover, the use of corticosteroids (aOR (95% CI) = 2.99 (1.31–6.83); p = 0.009) and hydroxychloroquine (aOR (95% CI) = 2.02 (1.48–2.74); p < 0.001) increased the risk of pneumonia in patients with SLE (Table 2).
The number of days of TCM use was considered as per our previous research (Liang et al., 2021; Lin et al., 2021). Thus, 30 days was selected as an interval, and 30, 60, and 90 days were selected to estimate the risk. When 60 days of TCM use was used for stratification, the statistical difference between the two groups was evident (Table 1). Thus, patients with SLE who cumulatively received TCM for >60 days had a significantly lower risk of pneumonia (aOR (95% CI) = 0.64 (0.45–0.90); p = 0.011) (Table 3).
Conditional logistic regression analysis was performed to evaluate the association between TCM use and the risk of pneumonia in patients with SLE according to age, sex, and follow-up duration, which was defined from the SLE diagnosis date to index date. As shown in Table 4, using TCM for >60 days reduced the risk of pneumonia in younger patients with SLE (age <65 years) (aOR (95% CI) = 0.66 (0.44–0.99); p = 0.042). In addition, female patients with SLE had a lower risk of pneumonia after using TCM for >60 days (aOR (95% CI) = 0.65 (0.45–0.93); p = 0.019). Moreover, patients who used TCM for >60 days and followed-up for >2 (aOR (95% CI) = 0.66 (0.46–0.93); p = 0.034), >3 (aOR (95% CI) = 0.64 (0.44–0.93); p = 0.019), >7 (aOR (95% CI) = 0.57 (0.35–0.94); p = 0.028), and >8 years (aOR (95% CI) = 0.55 (0.32–0.95); p = 0.032) had a lower risk of pneumonia (Figure 2; Supplementary Table S2).
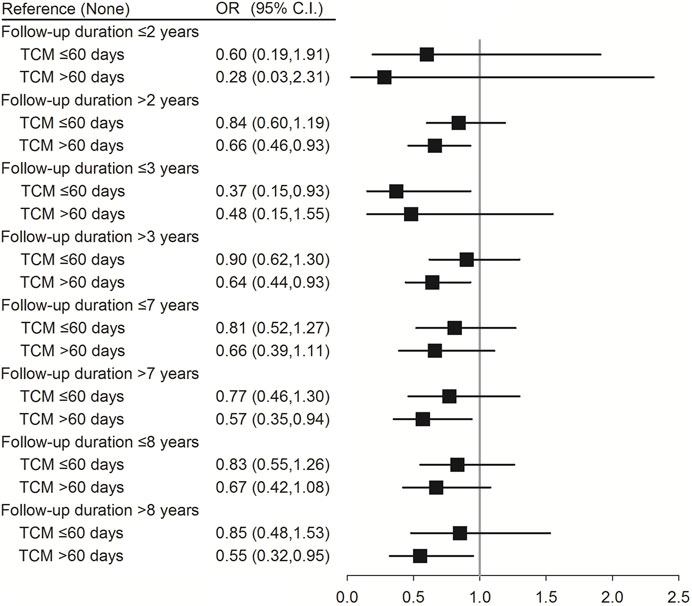
FIGURE 2. Conditional logistic regression of risk of pneumonia by follow-up duration stratification (60 days). †Adjusted for hypertension, hyperlipidemia, chronic liver disease, chronic kidney disease, rheumatoid arthritis, corticosteroids, NSAIDs, and hydroxychloroquine.
Antibiotic use and days of hospitalization may vary as per the severity of pneumonia in patients with SLE. Compared with patients with SLE in the non-pneumonia group, TCM use for >60 days reduced the risk of pneumonia with antibiotic use (aOR (95% CI) = 0.60 (0.43–0.84); p = 0.005) in pneumonia group. Moreover, TCM use for >60 days reduced the risk of pneumonia in patients with SLE having moderate (pneumonia admission <7 days) (aOR (95% CI) = 0.43 (0.25–0.74); p = 0.002) or severe pneumonia [(pneumonia admission ≥7 days) (aOR (95% CI) = 0.42 (0.23–0.74); p = 0.003) and (pneumonia admission ≥8 days) (aOR (95% CI) = 0.46 (0.25–0.83); p = 0.010)]. However, there were no significant differences between pneumonia patients from the emergency room and non-pneumonia patients (p = 0.859 or p = 0.241) (Figure 3; Supplementary Table S3).
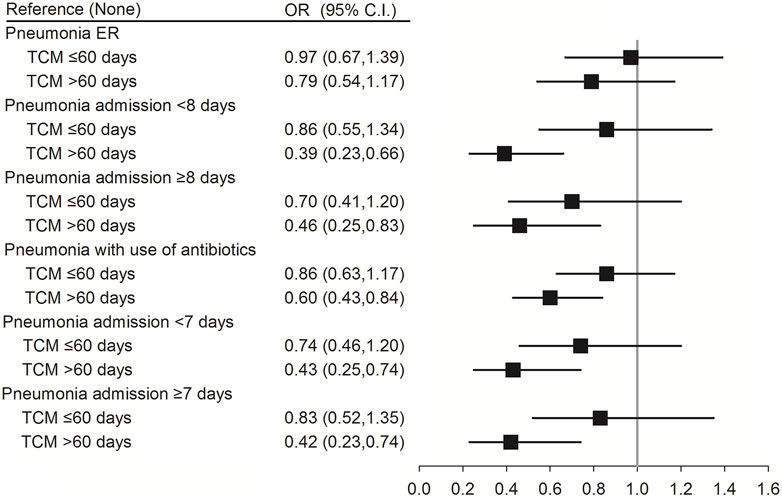
FIGURE 3. Conditional logistic regression of risk of pneumonia by antibiotics use, severity of pneumonia and days of hospitalization. ER, emergency room. †Adjusted for all variables.
Conditional logistic regression analysis was performed to evaluate the association between the use of different TCM formulae and risk of pneumonia infection. The results showed that patients with SLE who used the formulae of the KF group for >30 days (OR (95% CI) = 0.49 (0.29–0.82); p = 0.007), >60 days (OR (95% CI) = 0.45 (0.25–0.82); p = 0.008), and >90 days (OR (95% CI) = 0.50 (0.26–0.96); p = 0.038) had a reduced risk of pneumonia. Moreover, patients with SLE who used the formulae of the BF group for ≤30 days had a reduced risk of pneumonia (OR (95% CI) = 0.48 (0.31–0.74); p < 0.001) (Figure 4; Supplementary Table S4).
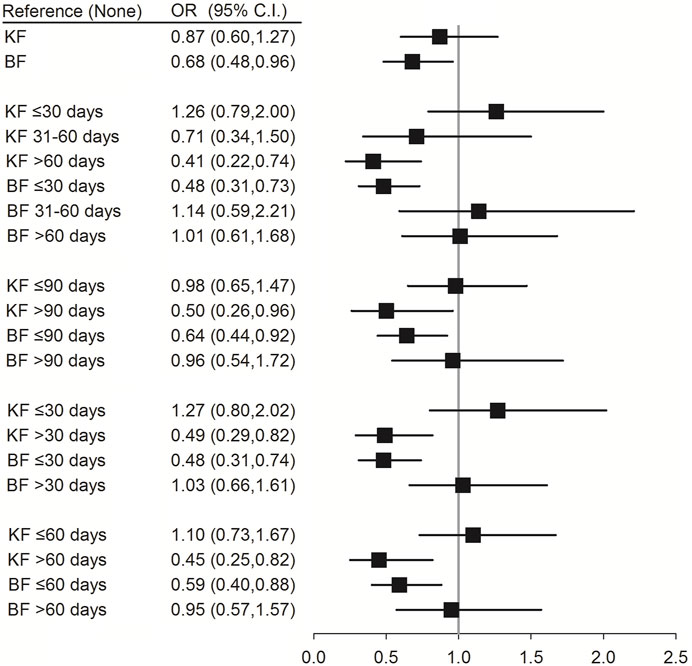
FIGURE 4. Conditional logistic regression of risk of pneumonia by different formulae of TCM. † Adjusted for hypertension, hyperlipidemia, chronic liver disease, chronic kidney disease, diabetes, chronic obstructive pulmonary disease, rheumatoid arthritis, ankylosing spondylitis, corticosteroids, NSAIDs, hydroxychloroquine, and methotrexate. KF: Chinese formulae tonifying the kidney; BF: Chinese formulae activating blood circulation.
To the best of our knowledge, this is the first large-scale cohort study to demonstrate that TCM decreases the risk of pneumonia in patients with SLE. The study results revealed that patients with SLE who cummulatively used TCM for >60 days had a significantly lower risk of pneumonia. A 34% and 35% reduced risk of pneumonia, respectively, was observed in younger and female patients with SLE. TCM use for >60 days significantly reduced the risk of pneumonia in the follow-up periods of >2, >3, >7, and >8 years. In addition, TCM use for >60 days reduced the risk of pneumonia in patients with SLE who also used antibiotics for moderate or severe pneumonia. Furthermore, the use of formulae of the KF group for >90 days and use of formulae of the BF group for <30 days significantly reduced the risk of pneumonia infection in patients with SLE.
SLE is a chronic autoimmune disease with multiorgan manifestations. On the one hand, infection is a common triggering factor that activates SLE and also a common cause of mortality in SLE. The Epstein–Barr virus (Truszewska et al., 2021)and some bacterial lipopolysaccharides (Jung and Suh, 2017) have been reported as pivotal factors for inducing SLE. Bonometti et al. illustrated the case of a patient in whom SLE was triggered by coronavirus disease 2019 infection (Bonometti et al., 2020). On the other hand, a secondary infection has become a common cause of mortality (25%–50%) in SLE (Wang et al., 2015; Kedves et al., 2020). A previous study revealed that lung, cutaneous, and urinary tract infections account for more than two-thirds of all infections in SLE (Jeong et al., 2009). The risk factors of infection in patients with SLE include the overall disease activity, higher C-reactive protein levels, higher anti-dsDNA levels, low complement levels, nephritis, daily dose of prednisone>10 mg, and others (Duffy et al., 1991; Suh et al., 2001; Bosch et al., 2006; Jeong et al., 2009). In the present study, the comorbidities of chronic kidney disease, diabetes, and COPD increased the risk of pneumonia infection in patients with SLE. Diabetes itself has been proven to be a major risk factor in pnemonia (for example, coronavirus disease) infection (Abdi et al., 2020). In terms of COPD, the incidence of lower respiratory tract infections was higher in patients with exacerbated COPD (Sethi, 2010). The use of corticosteroids and hydroxychloroquine also significantly increased the risk of pnemonia infection in patients with SLE, which correlated with a previous study (Kang and Park, 2003). This is mainly because immunosuppressive drugs strongly suppress immune responses against microorganisms, thereby facilitating the onset of pneumonia.
SLE affects females more frequently than males, with a ratio of nearly 9:1 (Tsang and Bultink, 2021). SLE is most commonly diagnosed during the reproductive age, which may be owing to endogenous estrogen production, failure in X chromosome inactivation, increased expression of Toll-like receptors, and alterations in microRNA function (Nusbaum et al., 2020; Leosuthamas et al., 2023). Men with SLE have a more aggressive clinical course, which include cardiovascular disease and nephritis. However, musculoskeletal involvement appears to be more common in women patients (Andrade et al., 2007; Crosslin and Wiginton, 2011). In the present study, female patients with SLE who used TCM for >60 days had a 35% reduced risk of pneumonia infection. SLE usually occurs in the women of reproductive age (Harden and Hammad, 2020); therefore, TCM use for >60 days could significantly reduce the risk of pneumonia infection in younger patients with SLE (age <65 years).
The formulae of the KF group have been used for treating chronic diseases, COPD, bone marrow suppression, and osteoprosis (Shi et al., 2014; He et al., 2017; You et al., 2019; Gao et al., 2020). In the present study, the use of formulae of the KF group for >60 days significantly reduced the risk of pneumonia infection in patients with SLE. A similar result was observed with the use of the same formulae for >90 days. Lupus nephritis affects nearly half of the patients with SLE and is one of the most commom contributors of patient mortality in SLE. In addition, SLE usually occurs in female patients during adolescence and pregnancy who are predisposed toward kidney yin deficiency (for patients with deficiency syndrome) and stagnation in the blood (for patients with excess syndrome). Furthermore, female patients easily develop kidney and liver yin deficiency during and after menopause. As per TCM, these deficiencies should be treated with a long course of TCM therapy. The formulae of the BF group have been used for treating rheumatoid arthritis, psoriasis, and coronary heart disease (Qiu et al., 2005; Gong et al., 2021; Liu et al., 2021). In the present study, a 46% reduction in the risk of pneumonia infection was observed in patients with SLE who received BF formulae for <30 days (OR = 0.48; p ≤ 0.001). BF formulae are usually prescribed for patients with SLE who also have arthritis, facial erythema, and erythema nodosa. Furthermore, these diseases, which belong to excess syndrome, can be ameliorated in a short period of time. However, using these formulae for a long period would consume healthy Qi. Jieduquyuziyin prescription, which contains both herbs from KF and BF formulae, has been shown to ameliorate SLE in MRL/lpr mice by inhibiting the expression of the IRAK1-NF-κB and PI3K/Akt/PGC-1α signaling pathways (Ji et al., 2020; Ji et al., 2022). Therefore, the underlying mechanisms by which TCM therapies may reduce the risk of pneumonia in patients with SLE need further investigations in the future. Moreover, more randomized clinical trials using TCM and therapies and conventional treatment would be the better way to provide the robust evidence.
However, the study had several limitations, which are inherent to observational studies and registries. For instance, detailed information regarding the SLE disease activity and the laboratory results of parameters such as anti-dsDNA, C3, and C4 were lacking. Moreover, the medication doses for each traditional Chinese herbal formula could not be fully accessed in the database. This study used a set of ICD codes to capture all types of pneumonia that occurred 1 year after the diagnosis of SLE, which may have inevitably included pneumonia caused by opportunistic pathogens as well as common pathogens, thereby confounding the results. In addition, the included study population was Taiwanese, and there may be regional differences in other regions of China and other eastern countries, including Korea and Japan, where TCM is popular for treating certain chronic diseases. Finally, the lack of some individual factors, such as the smoking status, blood pressure, body mass index, patient lifestyle, and environmental factors, may have potentially led to unmeasured confounding.
The present population-based cohort study revealed that the use of TCM could reduce the risk of pneumonia in patients with SLE. In particular, the use of KF formulae for >90 days and BF formulae for <30 days are recommended in the TCM treatment of SLE. Further randomized clinical trials are needed to confirm these results.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Institutional Review Board of the Chung Shan Medical University Hospital, No. CS1-20201. The patients/participants provided their written informed consent to participate in this study.
Study conception: WW and JC-CW. Statistical expertise: Y-HW. Data interpretation: WW and JC-CW. Drafting of article: WW. Collecting references: KY and XY. Critical revision of article: XW and JC-CW. All authors contributed to the article and approved the submitted version.
This research was supported by National Natural Science Foundation of China (grant number: 82004238), Natural Science Foundation of Zhejiang Province (grant number: LBY21H270001), China Postdoctoral Fund (grant number: 2019M660952), Zhejiang Medicine and Health Science and Technology Project (grant number: 2021KY843) and Young Elite Scientists Sponsorship Program by CACM (grant number: CACM2021-QNRC2-B01).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1185809/full#supplementary-material
Abdi, A., Jalilian, M., Sarbarzeh, P. A., and Vlaisavljevic, Z. (2020). Diabetes and COVID-19: A systematic review on the current evidences. Diabetes Res. Clin. Pract. 166, 108347. doi:10.1016/j.diabres.2020.108347
Andrade, R. M., Alarcón, G. S., Fernández, M., Apte, M., Vilá, L. M., and Reveille, J. D. (2007). Accelerated damage accrual among men with systemic lupus erythematosus: XLIV. Results from a multiethnic US cohort. Arthritis Rheum. 56, 622–630. doi:10.1002/art.22375
Bonometti, R., Sacchi, M. C., Stobbione, P., Lauritano, E. C., Tamiazzo, S., Marchegiani, A., et al. (2020). The first case of systemic lupus erythematosus (SLE) triggered by COVID-19 infection. Eur. Rev. Med. Pharmacol. Sci. 24, 9695–9697. doi:10.26355/eurrev_202009_23060
Bosch, X., Guilabert, A., Pallarés, L., Cerveral, R., Ramos-Casals, M., Bové, A., et al. (2006). Infections in systemic lupus erythematosus: A prospective and controlled study of 110 patients. Lupus 15, 584–589. doi:10.1177/0961203306071919
Crosslin, K. L., and Wiginton, K. L. (2011). Sex differences in disease severity among patients with systemic lupus erythematosus. Gend. Med. 8, 365–371. doi:10.1016/j.genm.2011.10.003
Duffy, K. N., Duffy, C. M., and Gladman, D. D. (1991). Infection and disease activity in systemic lupus erythematosus: A review of hospitalized patients. J. Rheumatol. 18, 1180–1184.
Durcan, L., O'Dwyer, T., and Petri, M. (2019). Management strategies and future directions for systemic lupus erythematosus in adults. Lancet 393, 2332–2343. doi:10.1016/s0140-6736(19)30237-5
Gao, Z., Liu, Y., Xu, Y., and Dong, J. (2020). Tonifying kidney therapy for stable chronic obstructive pulmonary disease: A systematic review. J. Tradit. Chin. Med. 40, 188–196.
Gong, X., Liu, W. X., Tang, X. P., Wang, J., Liu, J., Huang, Q. C., et al. (2021). Traditional Chinese medicine qingre huoxue treatment vs. the combination of methotrexate and hydroxychloroquine for active rheumatoid arthritis: A multicenter, double-blind, randomized controlled trial. Front. Pharmacol. 12, 679588. doi:10.3389/fphar.2021.679588
Goulielmos, G. N., and Zervou, M. I. (2020). Risk of systemic lupus erythematosus in patients with idiopathic thrombocytopenic purpura: Population-based cohort study. Ann. Rheum. Dis. doi:10.1136/annrheumdis-2020-218128
Harden, O. C., and Hammad, S. M. (2020). Sphingolipids and diagnosis, prognosis, and organ damage in systemic lupus erythematosus. Front. Immunol. 11, 586737. doi:10.3389/fimmu.2020.586737
He, J. B., Chen, M. H., and Lin, D. K. (2017). New insights into the tonifying kidney-yin herbs and formulas for the treatment of osteoporosis. Arch. Osteoporos. 12, 14. doi:10.1007/s11657-016-0301-4
Ingvarsson, R. F., Bengtsson, A. A., and Jönsen, A. (2016). Variations in the epidemiology of systemic lupus erythematosus in southern Sweden. Lupus 25, 772–780. doi:10.1177/0961203316635288
Jeong, S. J., Choi, H., Lee, H. S., Han, S. H., Chin, B. S., Baek, J. H., et al. (2009). Incidence and risk factors of infection in a single cohort of 110 adults with systemic lupus erythematosus. Scand. J. Infect. Dis. 41, 268–274. doi:10.1080/00365540902744741
Ji, L., Fan, X., Hou, X., Fu, D., Bao, J., Zhuang, A., et al. (2020). Jieduquyuziyin prescription suppresses inflammatory activity of MRL/lpr mice and their bone marrow-derived macrophages via inhibiting expression of IRAK1-NF-κb signaling pathway. Front. Pharmacol. 11, 1049. doi:10.3389/fphar.2020.01049
Ji, L. N., Wu, S., Fu, D. Q., Fang, S. J., Xie, G. Q., Fan, Y. S., et al. (2022). Jieduquyuziyin Prescription alleviates hepatic gluconeogenesis via PI3K/Akt/PGC-1α pathway in glucocorticoid-induced MRL/lpr mice. J. Ethnopharmacol. 284, 114815. doi:10.1016/j.jep.2021.114815
Jung, J. Y., and Suh, C. H. (2017). Infection in systemic lupus erythematosus, similarities, and differences with lupus flare. Korean J. Intern Med. 32, 429–438. doi:10.3904/kjim.2016.234
Kang, I., and Park, S. H. (2003). Infectious complications in SLE after immunosuppressive therapies. Curr. Opin. Rheumatol. 15, 528–534. doi:10.1097/00002281-200309000-00002
Kedves, M., Kósa, F., Kunovszki, P., Takács, P., Szabó, M. Z., Karyekar, C., et al. (2020). Large-scale mortality gap between SLE and control population is associated with increased infection-related mortality in lupus. Rheumatol. Oxf. 59, 3443–3451. doi:10.1093/rheumatology/keaa188
Kiriakidou, M., and Ching, C. L. (2020). Systemic lupus erythematosus. Ann. Intern Med. 172, Itc81–itc96. doi:10.7326/aitc202006020
Leosuthamas, P., Narongroeknawin, P., Chaiamnuay, S., Asavatanabodee, P., and Pakchotanon, R. (2023). Performance of systemic lupus erythematosus responder index for detecting clinician-rated responders in patients with active systemic lupus erythematosus. Int. J. Rheum. Dis. 26, 667–672. doi:10.1111/1756-185x.14606
Li, R., Sun, J., Ren, L. M., Wang, H. Y., Liu, W. H., Zhang, X. W., et al. (2012). Epidemiology of eight common rheumatic diseases in China: A large-scale cross-sectional survey in beijing. Rheumatol. Oxf. 51, 721–729. doi:10.1093/rheumatology/ker370
Liang, Y. T., Lin, C. Y., Wang, Y. H., Chou, H. H., and Wei, J. C. (2021). Associations of Chinese herbal medicine usage with risk of dementia in patients with Parkinson's disease: A population-based, nested case-control study. J. Altern. Complement. Med. 27, 606–612. doi:10.1089/acm.2020.0422
Lin, S. K., Wang, P. H., Huang, C. H., Kuo, Y. H., Lai, J. N., and Cheng-Chung Wei, J. (2021). Association between traditional Chinese medicine and a lower risk of dementia in patients with major depression: A case-control study. J. Ethnopharmacol. 278, 114291. doi:10.1016/j.jep.2021.114291
Liu, L. C., Mao, Q. Y., Liu, C., Hu, J., Duan, L., and Wang, J. (2021). The effectiveness and safety of bushen huoxue decoction on treating coronary heart disease: A meta-analysis. Evid. Based Complement. Altern. Med., 2021, 5541228. doi:10.1155/2021/5541228
Ma, Y. C., Lin, C. C., Li, C. I., Chiang, J. H., Li, T. C., and Lin, J. G. (2016). Traditional Chinese medicine therapy improves the survival of systemic lupus erythematosus patients. Semin. Arthritis Rheum. 45, 596–603. doi:10.1016/j.semarthrit.2015.09.006
Nusbaum, J. S., Mirza, I., Shum, J., Freilich, R. W., Cohen, R. E., Pillinger, M. H., et al. (2020). Sex differences in systemic lupus erythematosus: Epidemiology, clinical considerations, and disease pathogenesis. Mayo Clin. Proc. 95, 384–394. doi:10.1016/j.mayocp.2019.09.012
Oku, K., Hamijoyo, L., Kasitanon, N., Li, M. T., Navarra, S., Morand, E., et al. (2021). Prevention of infective complications in systemic lupus erythematosus: A systematic literature review for the aplar consensus statements. Int. J. Rheum. Dis. 24, 880–895. doi:10.1111/1756-185x.14125
Qiu, S., Tan, S., Zhang, J., Liu, P., Ran, L., and Lei, X. (2005). Effect of liangxue huoxue xiaoyin tang on serum levels of TNF-alpha, IFN-gamma and IL-6 in psoriasis of blood-heat type. J. Tradit. Chin. Med. 25, 292–295.
Sciascia, S., Mompean, E., Radin, M., Roccatello, D., and Cuadrado, M. J. (2017). Rate of adverse effects of medium-to high-dose glucocorticoid therapy in systemic lupus erythematosus: A systematic review of randomized control trials. Clin. Drug Investig. 37, 519–524. doi:10.1007/s40261-017-0518-z
Sethi, S. (2010). Infection as a comorbidity of COPD. Eur. Respir. J. 35, 1209–1215. doi:10.1183/09031936.00081409
Shi, S. H., Cai, Y. P., Cai, X. J., Zheng, X. Y., Cao, D. S., Ye, F. Q., et al. (2014). A network pharmacology approach to understanding the mechanisms of action of traditional medicine: Bushenhuoxue formula for treatment of chronic kidney disease. PLoS One 9, e89123. doi:10.1371/journal.pone.0089123
Stohl, W., Schwarting, A., Okada, M., Scheinberg, M., Doria, A., Hammer, A. E., et al. (2017). Efficacy and safety of subcutaneous belimumab in systemic lupus erythematosus: A fifty-two-week randomized, double-blind, placebo-controlled study. Arthritis Rheumatol. 69, 1016–1027. doi:10.1002/art.40049
Suh, C. H., Jeong, Y. S., Park, H. C., Lee, C. H., Lee, J., Song, C. H., et al. (2001). Risk factors for infection and role of C-reactive protein in Korean patients with systemic lupus erythematosus. Clin. Exp. Rheumatol. 19, 191–194.
Sun, J., Shao, T. J., Zhang, D. Y., Huang, X. Q., Xie, Z. J., and Wen, C. P. (2018). Effect of lang-chuang-ding decoction () on DNA methylation of CD70 gene promoter in peripheral blood mononuclear cells of female patients with systemic lupus erythematosus. Chin. J. Integr. Med. 24, 348–352. doi:10.1007/s11655-017-2804-2
Tektonidou, M. G., Wang, Z., Dasgupta, A., and Ward, M. M. (2015). Burden of serious infections in adults with systemic lupus erythematosus: A national population-based study, 1996-2011. Arthritis Care Res. Hob. 67, 1078–1085. doi:10.1002/acr.22575
Truszewska, A., Wirkowska, A., Gala, K., Truszewski, P., Krzemień-Ojak, Ł., Mucha, K., et al. (2021). EBV load is associated with cfDNA fragmentation and renal damage in SLE patients. Lupus 30, 1214–1225. doi:10.1177/09612033211010339
Tsang, A. S. M. W. P., and Bultink, I. E. M. (2021). New developments in systemic lupus erythematosus. Rheumatol. Oxf. 60, vi21–vi28. doi:10.1093/rheumatology/keab498
Tsioni, V., Andreoli, L., Meini, A., Frassi, M., Raffetti, E., Airò, P., et al. (2015). The prevalence and incidence of systemic lupus erythematosus in children and adults: A population-based study in a mountain community in northern Italy. Clin. Exp. Rheumatol. 33, 681–687.
Wang, W., Wang, X., Tang, X., Jiang, Q., and Fan, Y. (2019). Classifying rheumatoid arthritis by traditional Chinese medicine zheng: A multi-center cross-sectional study. J. Tradit. Chin. Med. 39, 425–432.
Wang, Y., Han, M., Pedigo, C. E., Xie, Z. M., Wang, W. J., and Liu, J. P. (2021). Chinese herbal medicine for systemic lupus erythematosus: A systematic review and meta-analysis of randomized, placebo-controlled trials. Chin. J. Integr. Med. 27, 778–787. doi:10.1007/s11655-021-3497-0
Wang, Z., Wang, Y., Zhu, R., Tian, X., Xu, D., Wang, Q., et al. (2015). Long-term survival and death causes of systemic lupus erythematosus in China: A systemic review of observational studies. Med. Baltim. 94, e794. doi:10.1097/md.0000000000000794
Keywords: systemic lupus erythematosus (SLE), traditional Chinese medicine (TCM), pneumonia, cohort, infection
Citation: Wang W, Wang Y-H, Yang K, Ye X, Wang X and Wei JC-C (2023) Traditional Chinese medicine use is associated with lower risk of pneumonia in patients with systemic lupus erythematosus: a population-based retrospective cohort study. Front. Pharmacol. 14:1185809. doi: 10.3389/fphar.2023.1185809
Received: 14 March 2023; Accepted: 17 May 2023;
Published: 01 June 2023.
Edited by:
Yiming Li, SWISS TCM University, SwitzerlandReviewed by:
Wared Nour-Eldine, Hamad Bin Khalifa University, QatarCopyright © 2023 Wang, Wang, Yang, Ye, Wang and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinchang Wang, b3NzYW5pQDEyNi5jb20=; James Cheng-Chung Wei, amNjd2VpQGdtYWlsLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.