
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 28 August 2023
Sec. Gastrointestinal and Hepatic Pharmacology
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1184754
 Qing-Zhou Kong1†
Qing-Zhou Kong1† Cheng Peng1†
Cheng Peng1† Zhen Li1
Zhen Li1 Bao-Ling Tian1
Bao-Ling Tian1 Yue-Yue Li1
Yue-Yue Li1 Fei-Xue Chen1,2,3*
Fei-Xue Chen1,2,3* Xiu-Li Zuo1,2,3
Xiu-Li Zuo1,2,3 Yan-Qing Li1,2,3
Yan-Qing Li1,2,3Goals: To explore factors associated with inadequate gastric preparation for MCE.
Background: Factors associated with inadequate gastric preparation for magnetically controlled capsule endoscopy (MCE) remains unclear.
Study: Data of patients who underwent MCE from June 2021 to July 2022 were prospectively collected. The gastric cleanliness score (GCS) of the six stomach regions (gastric cardia, fundus, body, angulus, antrum, and pylorus) was recorded. Patients with GCS score ≥18 were defined as the adequate preparation. Factors related to inadequate gastric preparation were analyzed using a logistic regression model with estimated odds ratios (OR).
Results: The mean GCS score of 211 patients was 17.01 ± 2.82. In the multivariable analysis, proton pump inhibitor (PPI) use (OR 3.57; 95% CI 1.69–7.95; p < 0.01) and premedication time after administering simethicone <30 min (OR 2.86; 95% CI 1.10–7.39; p = 0.03) were independent risk factors for inadequate gastric preparation. Comparing the gastric cleanliness of different locations, the median GCS of the lower stomach [10.00, IQR (9.50, 11.00)] was significantly higher than that of the upper stomach [7.00, IQR (6.00, 8.00)] (p <0.001).
Conclusion: PPI use and inadequate premedication time (<30 min) may reduce the quality of gastric preparation for MCE. The type, dose, duration of medication, and discontinuation time of PPIs was well worth further exploration. Appropriate control of the type and time of premedication may be the key to improving overall gastric cleanliness.
Magnetically controlled capsule endoscopy (MCE) offers a safe means to screen the gastric mucosa, detect lesions and identify gastric cancer, using a remote magnetic control system to adjust the angle of capsule observation without the need for intubation or sedation (Keller et al., 2011; Liao et al., 2012; Van Cutsem et al., 2016; Kim et al., 2018; Zhang et al., 2018). Multiple large, prospective clinical studies have shown that MCE has considerable diagnostic efficacy in examining gastric lesions compared to gastroscopy (Rey et al., 2012; Zou et al., 2015; Liao et al., 2016; Geropoulos et al., 2021; Li et al., 2021). Due to its minimal invasiveness and lack of sedation requirements, MCE has gained widespread acceptance as a diagnostic modality (Eliakim, 2017; Jiang et al., 2022).
The diagnostic performance of MCE relies on clear visualization of the entire gastric mucosa. However, in clinical practice, the presence of chyme, air bubbles, mucus, and bile in the gastric lumen may obscure microscopic lesions, reduce the cleanliness of the gastric mucosa, compromise the integrity of the visual field, and potentially lead to misdiagnosis or omission (Zhu et al., 2018). Unlike traditional gastroscopy, MCE does not provide the ability to clean the gastric mucosa by spraying water. Therefore, the quality of gastric preparation in MCE greatly affects the diagnostic accuracy for gastric lesions.
Previous studies have shown that simethicone use can improve gastric image quality by removing air bubbles (Chang et al., 2014; Zhu et al., 2018), and repetitive positional changes may improve gastric cleanliness (Wang et al., 2019). Nevertheless, no systematic evaluation of factors influencing the quality of gastric preparation for MCE has been conducted. In this prospective observational study, our objective is to explore these factors and provide valuable insights into pre-examination preparation for MCE in clinical practice.
This prospective observational study was approved by the Ethics Committee of Qilu Hospital, Shandong University and registered at ClinicalTrials.gov (NCT04933643). We included patients aged 18–75 years who required MCE at the endoscopy center of Qilu Hospital, Shandong University, from June 2021 to July 2022. All patients provided written informed consent.
Patients with the following were excluded: 1) severe physical diseases who were unable to adhere to the examination requirements; 2) dysphagia, known or suspected gastrointestinal fistula, stenosis or obstruction; 3) known active upper gastrointestinal bleeding; 4) altered gastrointestinal anatomy due to previous surgery; 5) exclusion criteria for magnetic resonance imaging examinations, including patients with implanted electronic medical instruments and magnetic metal devices; 6) pregnancy; and 7) claustrophobia or other mental disorders.
Patients were instructed to follow a soft diet the day before the examination and fast overnight (>8 h). Colored drinks were not permitted after 8 p.m. To reduce the impact of foam on the visual field, an appropriate bubble-removing agent (10 mL of simethicone dissolved in 50 mL of water) was administered 40 min before the examination. Before undergoing MCE, the patients were instructed to drink 500–1,000 mL of water to fill the stomach and provide the airwater interface for capsule sailing (Chinese Digestive Endoscopist Committee et al., 2017; Zhu et al., 2018; Capsule Endoscopy Group of the Chinese Society of Digestive Endoscopy, 2021).
For the MCE procedure, the patient ingested the capsule with 100 mL of water and was instructed to lay on the examination bed in the supine position. The operator then adjusted the endoscopic capsule using the magnetic control system. In order to observe the cardia, fundus, body, angulus, antrum, and pylorus in sequence, the patient position was adjusted as necessary. The patient position alternated between the left lateral, supine, and right lateral positions. At least two examinations of each gastric area were performed. The patient continuously consumed water when the stomach was underfilled. The gastric examination time for MCE was recorded.
The content of the questionnaire, with the aim to record patient information and evaluate gastric cleanliness, was discussed and formulated jointly by the research team. Considerations included the relevant literature and available information, including patients’ demographic and related clinical data, such as patient sources, history of basic diseases (e.g., diabetes, hypertension), family history of gastric cancer, H. pylori infection, drug use, diet before examination, fasting time, and premedication time after administering simethicone. Premedication time after administering simethicone was defined as the time from administering simethicone to swallowing capsule.
The primary outcome was to identify factors associated with inadequate gastric preparation. The gastric preparation quality was expressed as the gastric cleanliness score (GCS). We evaluated the six primary anatomical landmarks of the stomach (cardia, fundus, body, angulus, antrum, and pylorus). A 4-point grading scale was used to objectively assess the cleanliness of each landmark as either excellent (only traces of adherent mucus or foam present: score 4), good (small amount of mucus or foam present but no interference of the examination: score 3), fair (considerable amount of mucus or foam present preventing a completely reliable examination: score 2), or poor (large amount of mucus, foam, or chyme present that seriously impede observation: score 1) (Liao et al., 2016; Zhu et al., 2018). Since the gastric lumen is not sufficiently extended in the fasting state, quality was assessed on the basis of images obtained from each site sufficiently distended by water. The GCS was calculated as the total score of all six landmarks, ranging from 6 (totally inadequate) to 24 (perfect). A GCS ≥18 was considered adequate gastric preparation (Wang et al., 2019) (Figure 1), and these patients were defined as the adequate preparation group, while the remaining patients were defined as the inadequate gastric preparation group.
Using the scoring criteria, two endoscopists (with at least 3 years of reading experience) independently evaluated the cleanliness of the endoscopic images obtained during MCE. When the grading scores varied between the two endoscopists, the final GCS was determined by a senior endoscopist (with over 5 years of reading experience) who was in charge of quality control.
Secondary outcomes include upper and lower GCS and positive lesions. The senior endoscopist described and recorded any positive lesions observed during MCE. In this study, positive lesions were defined as focal lesions of the stomach, including focal erosion, polyps, ulcers, gastric varices, submucosal tumors, etc. Diffuse lesions, including superficial or atrophic gastritis, were regarded as negative. The number of lesions per patient (NLPP) was recorded to evaluate the total detection in the population.
Continuous variables are reported as means and standard deviation (SD), or medians and interquartile range (IQR) when not normally distributed, while categorical variables are expressed as percentages. Variables were compared using the chi-square or Kruskal–Wallis tests, when appropriate. Multivariable logistic regression analysis was performed to identify variables independently associated with inadequate gastric preparation quality, using estimated odds ratios (ORs) and 95% confidence intervals (95% CIs). Statistical significance was defined as a two-sided p-value of <0.05.
The sample size was calculated using events per variable (EPV), which is widely recognized as an effective means of determining sample size when the aim is to develop logistic regression prediction models (Wynants et al., 2015). According to the previous study (Wang et al., 2019), the proportion of the primary outcome (inadequate gastric preparation rate) was approximately 30% when the EPV was set at ten. Thus, to ensure reliability, at least 200 patients had to be enrolled in this study. In the cases where the univariate analysis showed results of p <0.10, multivariate analysis was performed. All statistical data were analyzed using R Statistics software (version 4.1.0, R Foundation for Statistical Computing, Vienna, Austria).
Overall, 214 patients underwent gastric preparation and MCE from June 2021 to July 2022. Three patients were excluded due to upper gastrointestinal bleeding or missing data. Ultimately, 211 patients were enrolled in this study. The patient characteristics, clinical indications for MCE, and comorbidities are shown in Table 1.
High inter-observer consistency was found between the endoscopists in assessing the quality of gastric preparation. The intra-group correlation coefficient for the GCS was 0.89 (p <0.001) and the consistency for assessing the eligibility of gastric preparation between the assessors was ranked as “excellent” (Kappa = 0.914, p <0.001).
Inadequate gastric preparation was reported in 54% (114/211) of the patients and the GCS was 17.01 ± 2.82 for the total population. Univariate analysis revealed that proton pump inhibitor (PPI) use (OR 4.45; 95% CI 2.18–9.08; p <0.001), nausea or acid reflux as an indication of MCE (OR 6.00; 95% CI 1.56–23.06; p = 0.01), hypertension (OR 2.04; 95% CI 1.03–4.06; p = 0.04), and premedication time after administering simethicone <30 min (OR 2.86; 95%CI 1.10–7.39; p = 0.03) were associated with inadequate gastric preparation.
In the multivariable analysis, PPI use (OR 3.57; 95% CI 1.69–7.95; p <0.01) and premedication time after administering simethicone <30 min (OR 2.86; 95%CI 1.10–7.39; p = 0.03) were independent risk factors for inadequate gastric preparation in patients requiring MCE (Table 2).
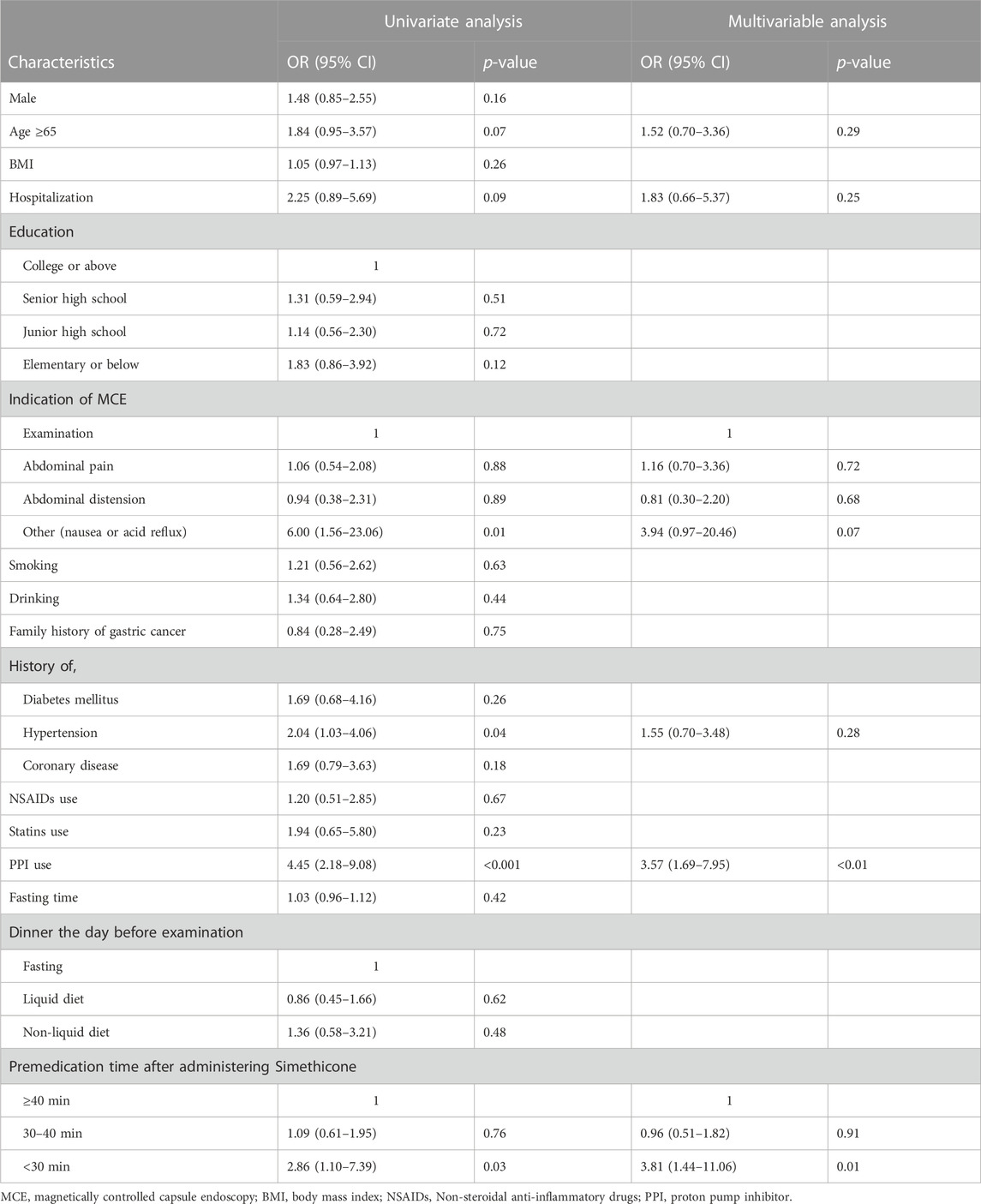
TABLE 2. Univariate and multivariable analysis of risk factors for inadequate gastric preparation of MCE.
Regarding the GCS of each site, the score of the lower stomach (the angulus, antrum, and pylorus) was higher than that of the upper (the cardia, fundus, and body), and the difference was statistically significant (p <0.001, Figure 2). Therefore, we opted to analyze the relationship between gastric preparation factors and upper gastric cleanliness. The multivariable analysis revealed PPI use (OR 4.01; 95% CI 1.91–8.88; p <0.001) and premedication time after administering simethicone <30 min (OR 3.60; 95% CI 1.37–10.41; p = 0.012) were associated with inadequate gastric cleanliness of the upper gastric site (Table 3).
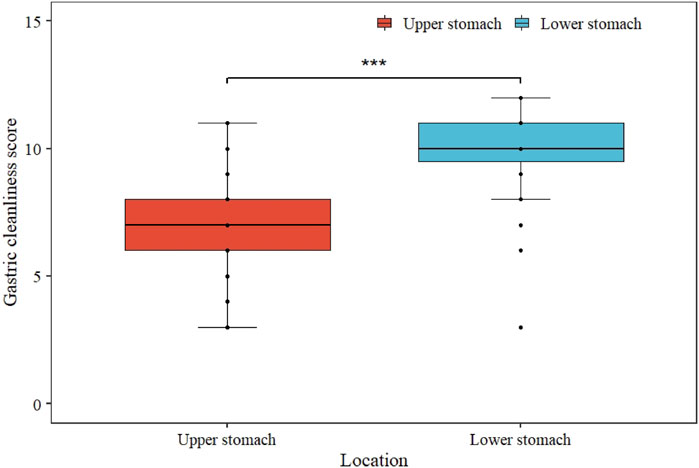
FIGURE 2. Comparison of upper and lower gastric cleanliness score. The median GCS of lower stomach was significantly higher than that of upper stomach (*** represents p <0.001).
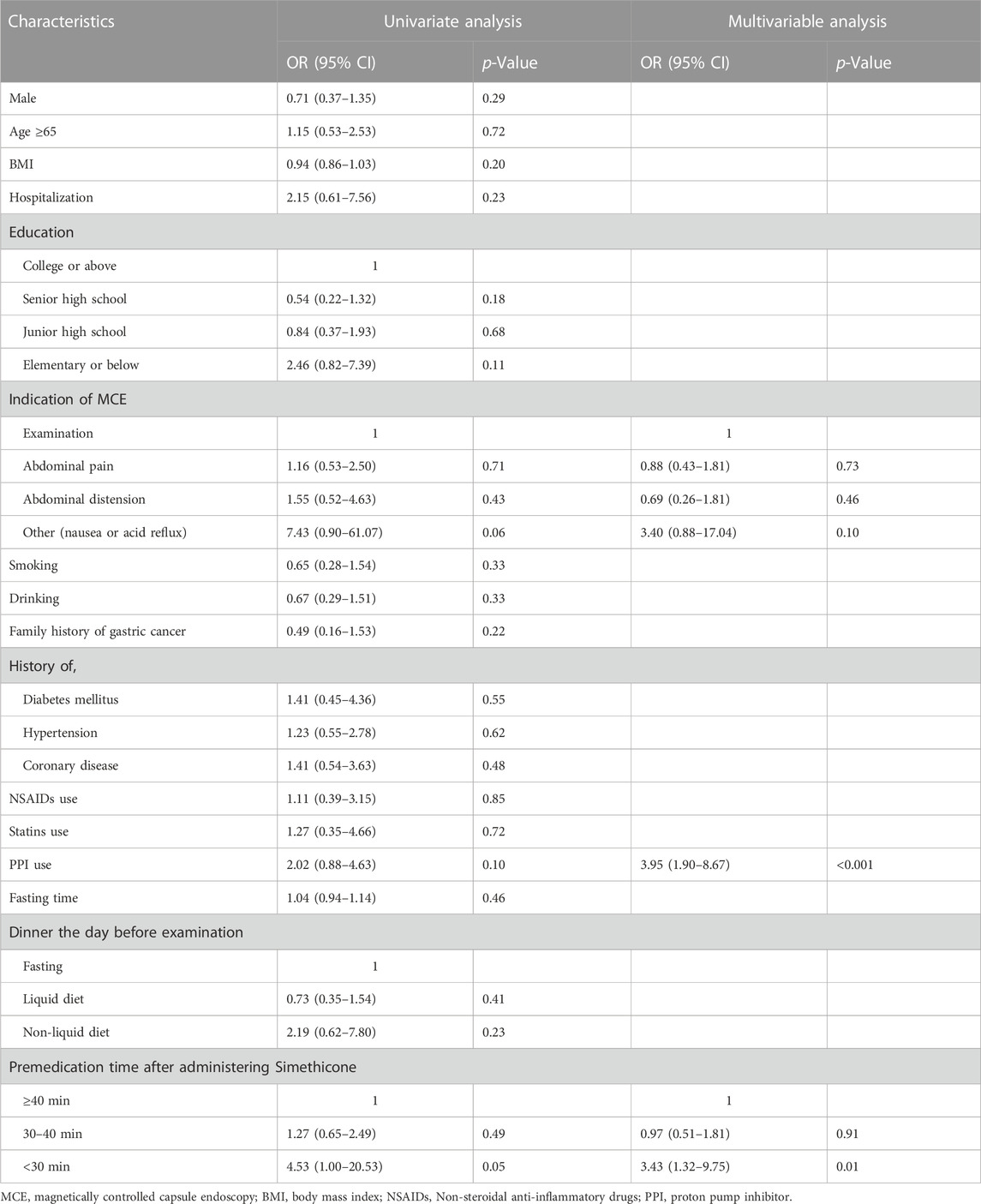
TABLE 3. Univariate and multivariable analysis of risk factors for inadequate upper gastric preparation of MCE.
Positive lesions detected during MCE are shown in Table 4. There was a significant difference in the detection rate of positive findings between the two groups (p = 0.001). The NLPP in the adequate preparation group (0.77 ± 0.86) was significantly higher than that of the inadequate group (0.38 ± 0.83) (p <0.001). The NLPP for the lower stomach in the adequate preparation group (0.35 ± 0.50) was also higher than that of the inadequate group (0.14 ± 0.37) (p = 0.001), while there was no significant difference in the upper stomach comparison (p = 0.084).
Gastric cleanliness greatly affects the quality of MCE, and the presence of gastric mucus and foam reduces clinical observation ability (Rahman et al., 2016; Zhu et al., 2018). In able to ensure accurate MCE, it is crucial to systematically explore the factors that affect the quality of gastric preparation. We prospectively collected and analyzed factors associated with the quality of MCE gastric preparation. Logistic regression analysis indicated that PPI use and inadequate premedication time after administering simethicone affected gastric preparation quality.
PPI use as an independent risk factor for reduced gastric mucosal cleanliness may be associated with its impact on gastric emptying (Anjiki et al., 2005; Takahashi et al., 2006; Sanaka et al., 2007; Sanaka et al., 2010; Li et al., 2020). The “acid-pepsin maldigestion hypothesis” proposes that PPI use reduces pepsin activity and hinders the hydrolysis process by elevating the gastric pH, and thus prolonging the persistence of undigested large particles in the stomach. Ota et al. (2021) suggested that suppression of gastric acid secretion delayed gastric fluid emptying, which may be related to elevated levels of ghrelin and abnormal gastric peristalsis (Parkman et al., 1998; Sanaka et al., 2010).
As for the drugs used for gastric preparation, previous studies have shown that simethicone, an antifoaming substance, can effectively improve the visibility of the gastric mucosa (Chang et al., 2014; Elvas et al., 2017; Zhu et al., 2018). Regarding upper gastrointestinal endoscopy, Chang et al. (2014) suggested that 5 mL of simethicone suspension administered >30 min before the exam provides clear endoscopic visibility, while Woo et al. (2013) recommended a premedication time of 10–30 min. However, the optimal premedication time for MCE remains unknown. In some studies the medication was administered 50 min or 1 h before the examination (Keller et al., 2011; Rey et al., 2012), while 40 min is recommended by the consensus (Chinese Digestive Endoscopist Committee et al., 2017; Capsule Endoscopy Group of the Chinese Society of Digestive Endoscopy, 2021). In our study, premedication time <30 min after administering the simethicone resulted in substandard gastric mucosal cleanliness, while there was no differential gastric preparation quality between the 30–40 min and >40 min intervals. Simethicone dissolved in 50 mL of water will be emptied after antifoaming sufficiently. However, during MCE, the patient is required to consume a large amount of water before swallowing the capsule to provide an airwater interface. If the interval between medication administration and the initiation of water consumption is too short, gastric emptying and simethicone removal can be accelerated, and the contact and action time of simethicone and gastric mucosa will be insufficient, resulting in inadequate gastric cleanliness.
Similar to previous studies (Zou et al., 2015; Rahman et al., 2016; Wang et al., 2019), we found that the mucosal cleanliness of the upper stomach was worse than that of the lower stomach. Due to the effects of gravity and the left lateral and supine positions used during MCE, mucus accumulates more easily at the gastric fundus than at the antrum and pylorus. The effects of gravity also decrease the mucosa-detergent contact time in these regions, which again reduce the quality of gastric preparation. Further studies are warranted to explore potential drugs or methods specifically targeting upper gastric cleanliness.
This study had some limitations. The data were derived from a single center and may have some bias in patient characteristics. Further validation in multicenter studies is desirable. Second, PPI use was found to potentially reduce the quality of gastric preparation for MCE. However, the type, dose, duration of medication, and discontinuation time of PPIs were not collected and analyzed because of the limited sample size, and future studies might consider including this data. Other types of gastric acid secretion inhibitor should also be studied in future. Although pronase and other mucolytics have been reported to be effective in conventional gastroscopy (Bhandari et al., 2010; Liu et al., 2018), their use did not show significant improvement in MCE compared to the use of simethicone alone (Chang et al., 2014; Zhu et al., 2018). Therefore, pronase was not used in our protocol. Further studies are needed to explore the effect of pronase for MCE and determine the optimum dose required to maximize the mucolytic action of pronase. In addition, H. pylori infection was not included in the final analysis due to the restriction of urea breath test in some patients taking PPIs (Malfertheiner et al., 2022). Further methods (e.g., gastroscopy) could be used in prospective studies to assess the effect of H. pylori infection on gastric cleanliness.
In conclusion, our study showed that PPI use may reduce the quality of gastric preparation for MCE, whereas adequate premedication time (≥30 min) after administering simethicone may improve the cleanliness of the gastric mucosa. The type, dose, duration of medication, and discontinuation time of PPIs was well worth further exploration. Improving the type and time of premedication may be the key to improving the overall gastric cleanliness. Better control of these factors in the future will improve gastric cleanliness and a higher lesion detection rate is expected, which will be conducive to promoting the application of MCE in gastric cancer screening.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the Medical Ethics Committee of Qilu Hospital of Shandong University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Q-ZK and CP: coordinated the study, designed and recorded the case report form, recruited and followed up the patients, analyzed the data, and wrote the manuscript. B-LT performed MCE examination. ZL and Y-YL involved in data collection and analysis. X-LZ and Y-QL obtained funding, designed the protocol, and coordinated the study. F-XC designed the protocol and planned the study, and critically reviewed the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by National Key R&D Program of China (66010189395113), and Shandong Provincial Key Research and Development Program (Major Scientific and Technological Innovation Project) (2021CXGC010506).
We thank Editage for the help in language polishing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Anjiki, H., Sanaka, M., and Kuyama, Y. (2005). Dual effects of rabeprazole on solid-phase gastric emptying assessed by the 13C-octanoate breath test. Digestion 72, 189–194. doi:10.1159/000088465
Bhandari, P., Green, S., Hamanaka, H., Nakajima, T., Matsuda, T., Saito, Y., et al. (2010). Use of gascon and pronase either as a pre-endoscopic drink or as targeted endoscopic flushes to improve visibility during gastroscopy: A prospective, randomized, controlled, blinded trial. Scand. J. Gastroenterol. 45, 357–361. doi:10.3109/00365520903483643
Capsule Endoscopy Group of the Chinese Society of Digestive Endoscopy (2021). Capsule Endoscopy Collaborative Group of Chinese Society of D, et al. Chinese guideline on magnetically controlled capsule gastroscopy (2021, Shanghai). Chin. J. Dig. Endosc. 38, 949–963. doi:10.1111/1751-2980.13173
Chang, W. K., Yeh, M. K., Hsu, H. C., Chen, H. W., and Hu, M. K. (2014). Efficacy of simethicone and N-acetylcysteine as premedication in improving visibility during upper endoscopy. J. Gastroenterol. Hepatol. 29, 769–774. doi:10.1111/jgh.12487
Chinese Digestive Endoscopist Committee, Chinese Endoscopist Association, the Health Management and Physical Examination Committee of Digestive Endoscopy, Capsule Endoscopy Collaboration Group of Chinese Society of Digestive Endoscopy, Chinese Anti-Cancer Association, the Society of Oncological Endoscopy, Chinese Society of Health Management (2017). The China expert consensus of clinical practice for magnetically controlled capsule gastroscopy(2017, Shanghai). Zhonghua Nei Ke Za Zhi 56, 876–884. doi:10.3760/cma.j.issn.0578-1426.2017.11.023
Eliakim, R. (2017). Where do I see minimally invasive endoscopy in 2020: clock is ticking. Ann. Transl. Med. 5, 202. doi:10.21037/atm.2017.04.17
Elvas, L., Areia, M., Brito, D., Alves, S., Saraiva, S., and Cadime, A. T. (2017). Premedication with simethicone and N-acetylcysteine in improving visibility during upper endoscopy: A double-blind randomized trial. Endoscopy 49, 139–145. doi:10.1055/s-0042-119034
Geropoulos, G., Aquilina, J., Kakos, C., Anestiadou, E., and Giannis, D. (2021). Magnetically controlled capsule endoscopy versus conventional gastroscopy: A systematic review and meta-analysis. J. Clin. Gastroenterol. 55, 577–585. doi:10.1097/MCG.0000000000001540
Jiang, X., Qiu, X. O., Li, Z., Pan, J., Peng, C., Zuo, X. L., et al. (2022). Small-sized versus standard magnetic capsule endoscopy in adults: A two-center, double-blinded randomized controlled trial. Endoscopy 55, 52–57. doi:10.1055/a-1881-4369
Keller, J., Fibbe, C., Volke, F., Gerber, J., Mosse, A. C., Reimann-Zawadzki, M., et al. (2011). Inspection of the human stomach using remote-controlled capsule endoscopy: A feasibility study in healthy volunteers (with videos). Gastrointest. Endosc. 73, 22–28. doi:10.1016/j.gie.2010.08.053
Kim, H., Hwang, Y., Sung, H., Jang, J., Ahn, C., Kim, S. G., et al. (2018). Effectiveness of gastric cancer screening on gastric cancer incidence and mortality in a community-based prospective cohort. Cancer Res. Treat. 50, 582–589. doi:10.4143/crt.2017.048
Li, J., Li, L., and Fu, Z. (2020). The effect of proton pump inhibitor on the image quality of magnetically controlled capsule endoscopy in aging patients. Gastroenterology 158, S565. doi:10.1016/S0016-5085(20)32115-6
Li, Z., Liu, J., Ji, C. R., Chen, F. X., and Liu, F. G. (2021). Screening for upper gastrointestinal cancers with magnetically controlled capsule gastroscopy: A feasibility study. Endoscopy 53, 914–919. doi:10.1055/a-1333-2120
Liao, Z., Duan, X. D., Xin, L., Bo, L. M., Wang, X. H., Xiao, G. H., et al. (2012). Feasibility and safety of magnetic-controlled capsule endoscopy system in examination of human stomach: A pilot study in healthy volunteers. J. Interv. Gastroenterol. 2, 155–160. doi:10.4161/jig.23751
Liao, Z., Hou, X., Lin-Hu, E. Q., Sheng, J. Q., Ge, Z. Z., Jiang, B., et al. (2016). Accuracy of magnetically controlled capsule endoscopy, compared with conventional gastroscopy, in detection of gastric diseases. Clin. Gastroenterol. Hepatol. 14, 1266–1273. doi:10.1016/j.cgh.2016.05.013
Liu, X., Guan, C. T., Xue, L. Y., He, S., Zhang, Y. M., Zhao, D. L., et al. (2018). Effect of premedication on lesion detection rate and visualization of the mucosa during upper gastrointestinal endoscopy: A multicenter large sample randomized controlled double-blind study. Surg. Endosc. 32, 3548–3556. doi:10.1007/s00464-018-6077-4
Malfertheiner, P., Megraud, F., Rokkas, T., Gisbert, J. P., and Liou, J. M. (2022). Management of Helicobacter pylori infection: the maastricht VI/florence consensus report. Gut 71, 1724–1762. doi:10.1136/gutjnl-2022-327745
Ota, K., Takeuchi, T., Kojima, Y., Kawaguchi, S., and Iwatsubo, T. (2021). Administration of a standard dose of vonoprazan fumarate delays gastric emptying in Japanese healthy adults: A prospective clinical trial. J. Gastroenterol. 56, 722–731. doi:10.1007/s00535-021-01801-3
Parkman, H. P., Urbain, J. L., Knight, L. C., Brown, K. L., Trate, D. M., Miller, M. A., et al. (1998). Effect of gastric acid suppressants on human gastric motility. Gut 42, 243–250. doi:10.1136/gut.42.2.243
Rahman, I., Pioche, M., Shim, C. S., Sung, I. K., and Saurin, J. C. (2016). Magnetic-assisted capsule endoscopy in the upper GI tract by using a novel navigation system (with video). Gastrointest. Endosc. 83, 889–895. doi:10.1016/j.gie.2015.09.015
Rey, J. F., Ogata, H., Hosoe, N., Ohtsuka, K., Ogata, N., Ikeda, K., et al. (2012). Blinded nonrandomized comparative study of gastric examination with a magnetically guided capsule endoscope and standard videoendoscope. Gastrointest. Endosc. 75, 373–381. doi:10.1016/j.gie.2011.09.030
Sanaka, M., Anjiki, H., Yamamoto, T., and Kuyama, Y. (2007). Rabeprazole delays gastric emptying of a nutrient liquid. J. Gastroenterol. Hepatol. 22, 1806–1809. doi:10.1111/j.1440-1746.2006.04763.x
Sanaka, M., Yamamoto, T., and Kuyama, Y. (2010). Effects of proton pump inhibitors on gastric emptying: A systematic review. Dig. Dis. Sci. 55, 2431–2440. doi:10.1007/s10620-009-1076-x
Takahashi, Y., Amano, Y., Yuki, T., Ose, T., Miyake, T., Kushiyama, Y., et al. (2006). Influence of acid suppressants on gastric emptying: cross-over analysis in healthy volunteers. J. Gastroenterol. Hepatol. 21, 1664–1668. doi:10.1111/j.1440-1746.2006.04270.x
Van Cutsem, E., Sagaert, X., Topal, B., Haustermans, K., and Prenen, H. (2016). Gastric cancer. Lancet 388, 2654–2664. doi:10.1016/S0140-6736(16)30354-3
Wang, Y. C., Pan, J., Jiang, X., Su, X. J., Zhou, W., Zou, W. B., et al. (2019). Repetitive position change improves gastric cleanliness for magnetically controlled capsule gastroscopy. Dig. Dis. Sci. 64, 1297–1304. doi:10.1007/s10620-018-5415-7
Woo, J. G., Kim, T. O., Kim, H. J., Shin, B. C., Seo, E. H., Heo, N. Y., et al. (2013). Determination of the optimal time for premedication with pronase, dimethylpolysiloxane, and sodium bicarbonate for upper gastrointestinal endoscopy. J. Clin. Gastroenterol. 47, 389–392. doi:10.1097/MCG.0b013e3182758944
Wynants, L., Bouwmeester, W., Moons, K. G., Moerbeek, M., Timmerman, D., Van Huffel, S., et al. (2015). A simulation study of sample size demonstrated the importance of the number of events per variable to develop prediction models in clustered data. J. Clin. Epidemiol. 68, 1406–1414. doi:10.1016/j.jclinepi.2015.02.002
Zhang, X., Li, M., Chen, S., Hu, J., Guo, Q., Liu, R., et al. (2018). Endoscopic screening in asian countries is associated with reduced gastric cancer mortality: A meta-analysis and systematic review. Gastroenterology 155, 347–354. doi:10.1053/j.gastro.2018.04.026
Zhu, S. G., Qian, Y. Y., Tang, X. Y., Zhu, Q. Q., Zhou, W., Du, H., et al. (2018). Gastric preparation for magnetically controlled capsule endoscopy: A prospective, randomized single-blinded controlled trial. Dig. Liver Dis. 50, 42–47. doi:10.1016/j.dld.2017.09.129
Keywords: magnetically controlled capsule endoscopy, gastric disease, preparation, proton pump inhibitor, simethicone
Citation: Kong Q-Z, Peng C, Li Z, Tian B-L, Li Y-Y, Chen F-X, Zuo X-L and Li Y-Q (2023) Inadequate gastric preparation and its associated factors for magnetically controlled capsule endoscopy. Front. Pharmacol. 14:1184754. doi: 10.3389/fphar.2023.1184754
Received: 12 March 2023; Accepted: 14 August 2023;
Published: 28 August 2023.
Edited by:
Liqun Yang, Shanghai Jiao Tong University, ChinaReviewed by:
Wen-Bin Zou, Second Military Medical University, ChinaCopyright © 2023 Kong, Peng, Li, Tian, Li, Chen, Zuo and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fei-Xue Chen, Y2hlbmZlaXh1ZUBlbWFpbC5zZHUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.