- Department of Gynecology and Obstetrics, Southwest Hospital, Third Military Medical University (Army Medical University), Chongqing, China
Objective: To evaluate whether periconceptional or pregnancy exposure of human papillomavirus (HPV) vaccination would increase the risk of adverse pregnancy outcomes.
Methods: The PubMed, Web of Science, Embase, the Cochrane Library of clinical trials were searched from inception to March 2023. We computed relative risk (RR) and 95% confidence intervals (CIs) and prediction intervals (PIs) regarding the association between HPV vaccination in periconceptional period or during pregnancy and the risks of adverse pregnancy outcomes by using R software Version 4.1.2 and STATA Version 12.0. A trial sequential analysis (TSA) was performed with TSA v0.9.5.10 Beta software.
Results: Four randomized controlled trials (RCTs) and eight cohort studies were included in this meta-analysis. Analysis of RCTs showed that HPV vaccination in periconceptional period or during pregnancy did not increase the risks of spontaneous abortion (RR = 1.152, 95% CI: 0.909–1.460, 95% PI: 0.442–3.000), birth defects (RR = 1.171, 95% CI: 0.802–1.709, 95% PI: 0.320–4.342), stillbirth (RR = 1.053, 95% CI: 0.616–1.800, 95% PI: 0.318–3.540), preterm birth (RR = 0.940, 95% CI: 0.670–1.318) and ectopic pregnancy (RR = 0.807, 95% CI: 0.353–1.842, 95% PI: 0.128–5.335). In cohort studies, periconceptional or pregnancy exposures of HPV vaccine were not associated with the increased risk of spontaneous abortion (RR = 0.987, 95% CI: 0.854–1.140, 95% PI: 0.652–1.493), birth defects (RR = 0.960, 95% CI: 0.697–1.322, 95% PI: 0.371–2.480), stillbirth (RR = 1.033, 95% CI: 0.651–1.639, 95% PI: 0.052–21.064), small size for gestational age (SGA) (RR = 0.971, 95% CI: 0.873–1.081, 95% PI: 0.657–1.462) and preterm birth (RR = 0.977, 95% CI: 0.874–1.092, 95% PI: 0.651–1.444).
Conclusion: HPV vaccine exposures in periconceptional period or during pregnancy did not increase the risks of adverse pregnancy outcomes, including spontaneous abortion, birth defects, stillbirth, SGA, preterm birth and ectopic pregnancy.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42023399777.
Introduction
Cervical cancer is the fourth most common cancer of women in the world. An estimated 570,000 new cases and 311,000 deaths were reported worldwide in 2018 (Bray et al., 2018). High-risk human papillomavirus (HPV) persistent infection is the leading cause of cervical cancer (Kjær et al., 2010). HPV vaccine, as the only vaccine to prevent cervical cancer, has been used among 72 million women worldwide since it was first approved in 2006 and has been demonstrated to be effective and safe in preventing the development of high-grade cervical cancer (Markowitz et al., 2007; Medeiros et al., 2009). Three prophylactic HPV vaccines are currently available, including bivalent vaccine (2vHPV), quadrivalent vaccine (4vHPV) and nonavalent vaccine (9vHPV), which target either two or seven high-risk HPV genotypes (Harper and DeMars, 2017). The 2vHPV and 4vHPV vaccines target HPV-16 and HPV-18, which leads to about 70% of cervical cancers worldwide, while the 9vHPV vaccine targets seven high-risk HPV genotypes (HPV-16/18/31/33/45/52/58), which causes approximately 90% of cervical cancer cases in the world (Burger et al., 2021).
The Advisory Committee on Immunization Practice has recommended routine vaccination of HPV vaccine in girls aged 11–12 years with supplementary vaccination for women under 26 years of age (Markowitz et al., 2007). Therefore, large numbers of women at childbearing age may be exposed to HPV vaccination. These include those who may be unintentionally vaccinated in periconceptional period or during pregnancy, especially those who were unplanned or unrecognized pregnant (Finer and Zolna, 2016). It was reported that HPV vaccine exposure occurred during or around the time of 1.5% of pregnancies among female adolescents and young adults aged 13–27 years who received care in seven large health systems from 2007 to 2013 (Lipkind et al., 2017). In view of the lack of well controlled studies in pregnant women, fears of teratogenicity or other potentially adverse pregnancy outcome to the pregnant woman or the unborn child, such as spontaneous abortion, birth defects, preterm birth and stillbirth, have arisen among both recipients and healthcare providers (Canfell, 2015; Bonde et al., 2016; Gidengil et al., 2021). A previous study showed that the incidence of spontaneous abortion and stillbirth among women who received HPV vaccine in periconceptional period or during pregnancy was higher than that of women not vaccinated with HPV vaccine within this specific period (Moreira et al., 2016). The latest analysis suggested the peripregnancy or during-pregnancy HPV vaccine exposure was not associated with an increased risk of spontaneous abortion, preterm births, small size for gestational age (SGA) and birth defects (Kharbanda et al., 2021).
Whether HPV vaccination in the periconceptional period or during pregnancy will increase the risk of adverse maternal or infant outcomes remains largely uncertain. Although an analysis has been conducted to assess the association of periconceptional or pregnancy exposure of HPV vaccination and the risk of spontaneous abortion (Tan et al., 2019). Given the current absence of a comprehensive systematic review of HPV vaccination in the periconceptional period or during pregnancy and adverse maternal or infant outcomes (e.g., birth defects, stillbirth, small size for gestational age, preterm birth and ectopic pregnancy), we conducted a meta-analysis of all relevant clinical research evidence to further explore whether periconceptional or pregnancy exposure of HPV vaccination increased the risk of adverse pregnancy outcomes in randomized controlled trials (RCTs) and cohort studies, respectively.
Materials and methods
This study does not require ethical approval and informed consent because it is a systematic review and meta-analysis of previously published literature and does not address ethics or patient privacy. Our study was analyzed and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Moher et al., 2009). The protocol for this meta-analysis has been registered in the PROSPERO database (CRD42023399777).
Search strategy
We thoroughly searched the PubMed, Web of Science, Embase, and the Cochrane Library of clinical trials for all potential articles from inception to March 2023, using the following search items: (“human papillomavirus virus”, “HPV”, “human papilloma virus”, “vaccine”, “vaccination”, “vaccinated”) AND (“pregnant women,” “pregnancy,” “conception,” “parturient,” “child bearing”) AND (“preterm,” “small for gestational age,” “spontaneous abortion,” “stillbirth,” “birth defect,” “reproductive outcome,” “pregnancy outcome”). The detailed search strategy was provided in Supplementary Material S1. References within the identified articles were manually examined to identify other potentially eligible studies.
Inclusion and exclusion criteria
Studies should meet the following inclusion criteria: 1) clinical trials or cohort studies if they contained primary data regarding pregnant women who received HPV vaccine, 2) describe the association between HPV vaccine exposures in periconceptional period or during pregnancy and the risk of adverse pregnancy outcomes, and 3) report adverse maternal or fetal outcomes (primary outcomes: spontaneous abortion and birth defects, secondary outcomes: stillbirth, small size for gestational age, preterm birth and ectopic pregnancy). Accordingly, the exclusion criteria were as follows: 1) studies with unusable or duplicate outcome data, 2) non-controlled studies, and 3) conference abstracts, reviews, case reports, and meta-analyses.
Data extraction
Two independent researchers screened the literature and extracted all needed information from the included studies. All disagreements were resolved by discussion with a third investigator. The following information was extracted from each article using the predesigned data-collection form: name of first author, publication year, country or region, study design, study time, sample size, vaccination exposure time and vaccine type of exposure group and control group, age of the study population, duration of follow-up and outcomes.
Risk of bias assessment
Cohort studies were assessed using the Newcastle-Ottawa scale (NOS) (Wells et al., 2014) consisting of three domains: i) selection of subjects, ii) comparability of groups, and iii) assessment of outcome. A score of 0–9 was allocated to each relevant study. While the NOS has no established thresholds, we considered the quality of each study as low (0–3 score), moderate (4–6 score), or high (7–9 score) (Chung et al., 2021). We used the modified Jadad scale to assess the quality of RCTs (Jadad et al., 1996). The evaluation criteria of the modified Jadad scale included four items: randomization, randomization concealment, double blind, and withdrawals and dropouts. The score 0–3 out of 7 was considered a low-quality study and a score of 4–7 was a high-quality study. When inconsistency exists, a third reviewer will make the final decision after verification and discussion.
Statistical analysis
The comparison of adverse pregnancy outcomes between HPV exposure group and control group were estimated by the relative risk (RR) and their 95% confidence intervals (CIs). Heterogeneity was assessed statistically by using the Cochran’s Q test, I2 and Tau2 statistic and 95% prediction interval (PI) (Bowden et al., 2011; IntHout et al., 2016). When I2 ≤ 50% or p > 0.1, the results of the associated studies were considered to have acceptable heterogeneity, and a fixed-effects model was utilized. When I2 > 50% or p ≤ 0.1, it was considered that there was heterogeneity in the results of the included studies, and a random-effects model was selected (Higgins and Thompson, 2002). Sensitivity analysis was conducted to explore the possible sources of heterogeneity. The presence of publication bias was assessed with the Egger’s regression asymmetry test (Sterne and Egger, 2001). Statistical analyses were performed with R software Version 4.1.2 and STATA Version 12.0 (StataCorp, College Station, TX, United States).
Trial sequential analysis
A trial sequential analysis (TSA) was performed to assess if the available evidence is up to the required information size (RIS) for robust conclusion (Wetterslev et al., 2017). For dichotomous outcomes, the trial sequential analysis was performed with TSA v0.9.5.10 Beta software (www.ctu.dk/tsa). We calculated the RIS and built O’ Brien-Fleming α-spending boundaries by using type I error of 5% and type II error of 20%, which were two-side values. If the cumulative Z-curve crossed the trial sequential monitoring boundary or RIS boundary, no further trials were considered to be needed and firm evidence was obtained.
Results
Literature search
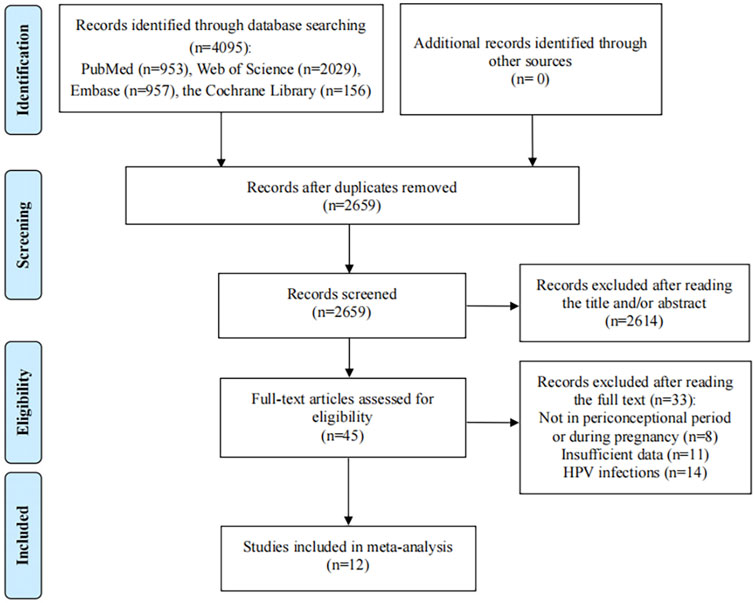
Depending on the search strategy, 4,095 studies were identified. After eliminating the duplicates, 2,659 records remained. Of these, 2,614 studies excluded for their titles or abstracts being not relevant, and 45 full texts were assessed for eligibility. After reading the full text, 33 articles did not meet the inclusion criteria: 11 studies provided insufficient outcome data, 8 articles reported HPV vaccination not in periconceptional period or during pregnancy; 14 articles reported the pregnancy outcomes of HPV infections rather than HPV vaccination. Finally, 12 eligible studies were included in the present meta-analysis (Figure 1) (Garland et al., 2009; Kharbanda et al., 2021; Angelo et al., 2014; Baril et al., 2015; Panagiotou et al., 2015; Scheller et al., 2017; Kharbanda et al., 2018; Lipkind et al., 2017; Moreira et al., 2016; Chen et al., 2019; Faber et al., 2019; Bukowinski et al., 2020).
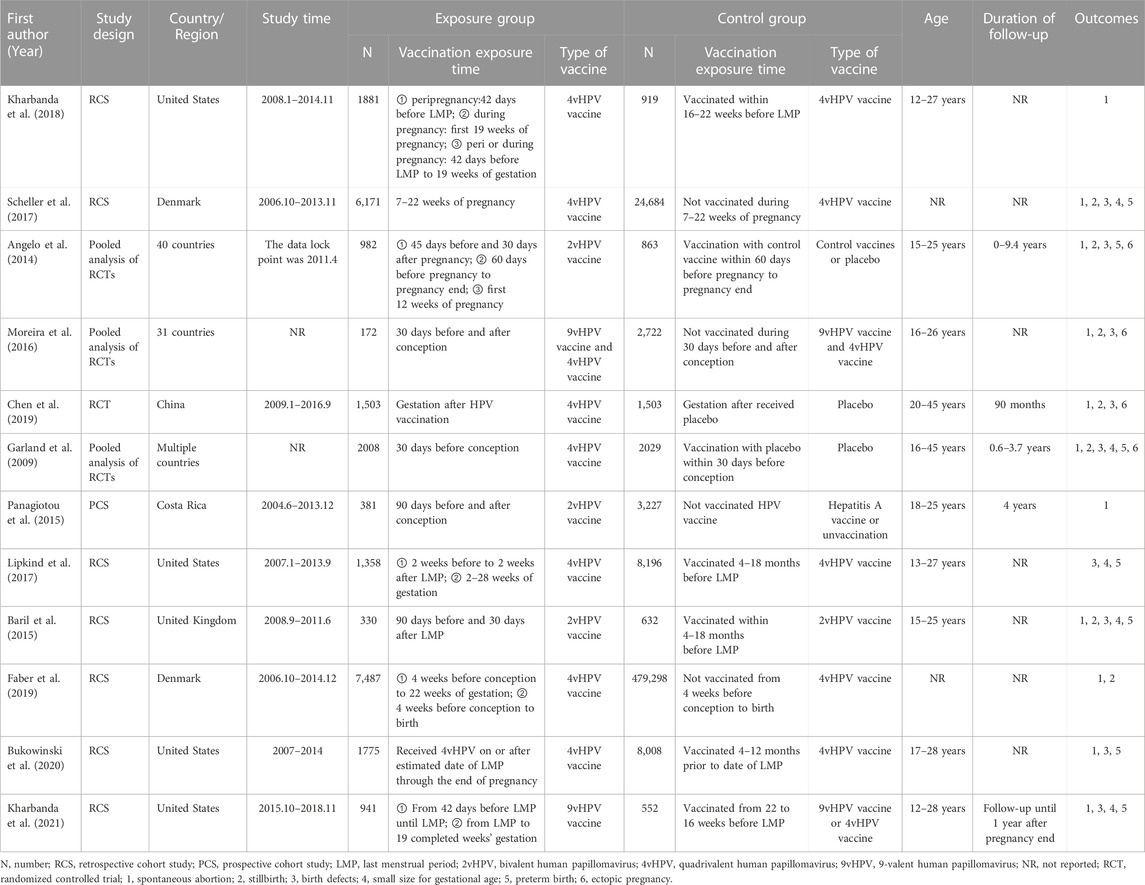
Characteristics and quality assessment of the included studies
The main characteristics of included researches and research participants were summarized in Table 1. All of included studies were RCTs and cohort studies. Three articles reported pooled results, including pooled analysis of 42 (conducted in 40 countries), seven (conducted in 31 countries), and five trials (conducted in multiple countries). There were three studies, eight studies and two studies, focused on the effect of 2vHPV, 4vHPV, and 9vHPV vaccine, respectively. Eligible participants were women who received HPV vaccine in periconceptional period or during pregnancy. Two RCTs were considered as low quality. Eight cohort studies were assessed as high quality, because the study design had been described in detail (Supplementary Material S2).
Pooled effect of adverse pregnancy outcomes in RCTs
Four RCTs examined the association between HPV vaccination and spontaneous abortion. The random-effects pooled estimate showed no significant association between HPV vaccination and spontaneous abortion (RR = 1.152, 95% CI: 0.909–1.460, 95% PI: 0.442–3.000), with significant heterogeneity (I2 = 65.2%, Tau2 = 0.0349) (Table 2; Figure 2A). Four RCTs compared birth defects between periconceptional or pregnancy women vaccinated HPV vaccine and those who were not. The difference in birth defects following HPV vaccination was not statistically significant (RR = 1.171, 95% CI: 0.802–1.709; 95% PI: 0.320–4.342, I2 = 17.9%, Tau2 = 0.0396) (Table 2; Figure 2B).
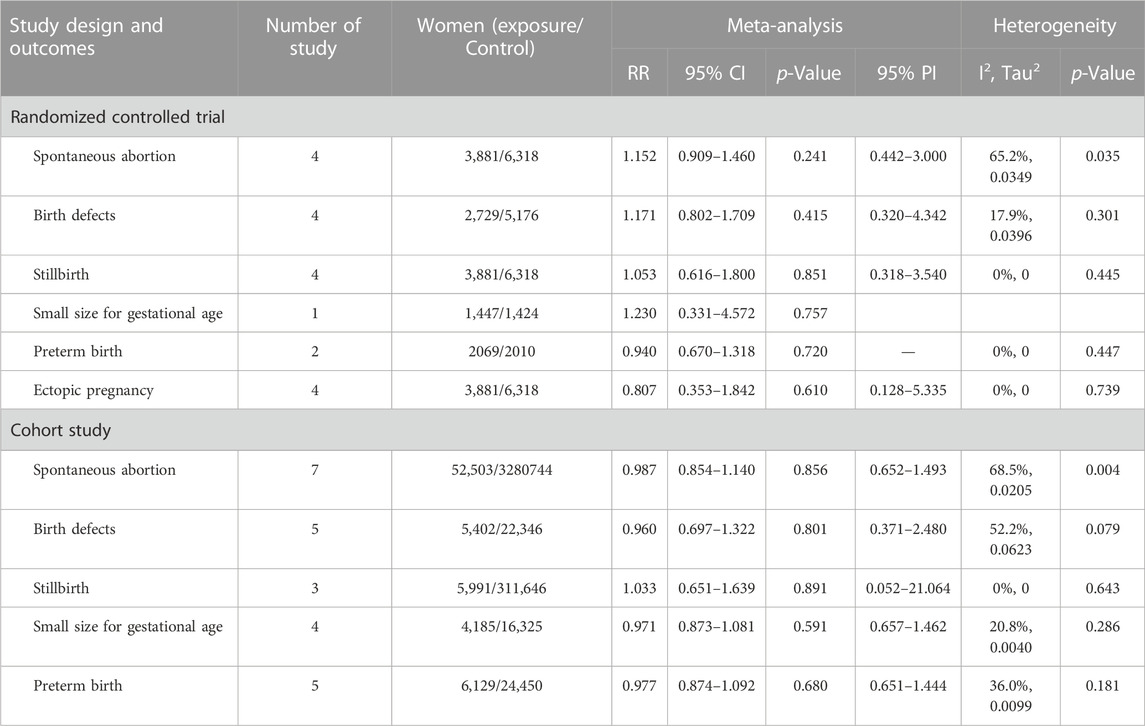
TABLE 2. Pooled effect of adverse pregnancy outcomes in randomized controlled trials and cohort studies.
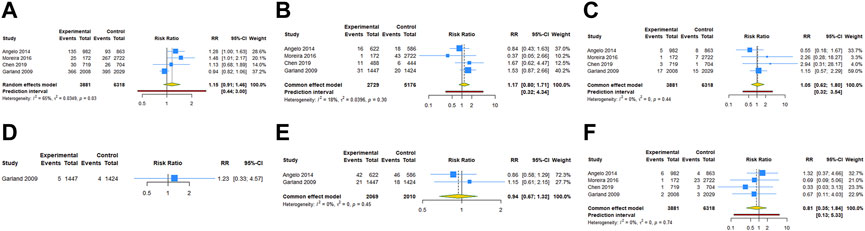
FIGURE 2. Forest plot of adverse pregnancy outcomes in RCTs. (A) Spontaneous abortion. (B) Birth defects. (C) Stillbirth. (D) Small size for gestational age. (E) Preterm birth. (F) Ectopic pregnancy.
Stillbirth was evaluated in four studies. HPV vaccination in periconceptional period or during pregnancy was not associated with an elevated risk of stillbirth (RR = 1.053, 95% CI: 0.616–1.800, 95% PI: 0.318–3.540; I2 = 0, Tau2 = 0) (Table 2; Figure 2C). Only one study reported the association between HPV vaccination and small size for gestational age (SGA). In the study, the RR of HPV vaccination for SGA was 1.230 (95% CI = 0.331–4.572) (Table 2; Figure 2D). Two RCTs assessed preterm birth in HPV vaccine vaccinated/unvaccinated pregnancies. Compared with the unexposed pregnancies, HPV vaccination pregnancies were not associated with higher risk for preterm birth (RR = 0.940, 95% CI: 0.670–1.318; I2 = 0, Tau2 = 0) (Table 2; Figure 2E). Four studies reported the association between HPV vaccination and ectopic pregnancy. The results showed HPV vaccination in periconceptional period or during pregnancy seem to decrease the risk of ectopic pregnancy, but without statistical significance (RR = 0.807, 95% CI: 0.353–1.842, 95% PI: 0.128–5.335; I2 = 0, and Tau2 = 0) (Table 2; Figure 2F).
Pooled effect of adverse pregnancy outcomes in cohort studies
Seven cohort studies examined the association between HPV vaccination and spontaneous abortion. The results with a random-effect model showed that HPV vaccination in periconceptional period or during pregnancy seem to reduce the risk of spontaneous abortion, but without statistical significance (RR = 0.987, 95% CI: 0.854–1.140, 95% PI: 0.652–1.493; I2 = 68.5%, Tau2 = 0.0205) (Table 2; Figure 3A). Five studies reported the association between HPV vaccine exposure and birth defects. The result suggested that HPV vaccination in periconceptional period or during pregnancy did not increase the risk of birth defects with pooled RR of 0.960 (95% CI: 0.697–1.322, 95% PI: 0.371–2.480; I2 = 52.2%, Tau2 = 0.0623) (Table 2; Figure 3B).
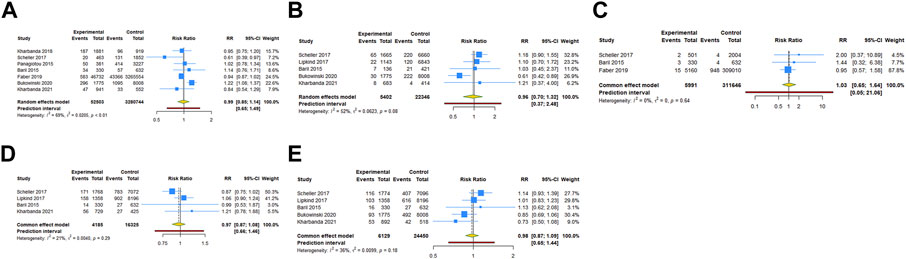
FIGURE 3. Forest plot of adverse pregnancy outcomes in cohort studies. (A) Spontaneous abortion. (B) Birth defects. (C) Stillbirth. (D) Small size for gestational age. (E) Preterm birth.
Three cohort studies assessed stillbirth in HPV vaccine exposed/unexposed pregnancies. The pooled RR was 1.033 (95% CI: 0.651–1.639, 95% PI: 0.052–21.064; I2 = 0, Tau2 = 0), indicating HPV vaccine exposed pregnancies were associated with no higher risk for stillbirth (Table 2; Figure 3C). Four studies reported the association between HPV vaccination and SGA. The result showed HPV vaccination in periconceptional period or during pregnancy seem to decrease the risk of SGA, but without statistical significance (RR = 0.971, 95% CI: 0.873–1.081, 95% PI: 0.657–1.462; I2 = 20.8%, Tau2 = 0.0040) (Table 2; Figure 3D). Five cohort studies compared preterm birth between periconceptional or pregnancy women vaccinated HPV vaccine and those who were not. Among women with HPV vaccination as opposed to those who were not vaccinated, a nonsignificant decrease in preterm birth was demonstrated (RR = 0.977, 95% CI: 0.874–1.092, 95% PI: 0.651–1.444; I2 = 36.0%, Tau2 = 0.0099) (Table 2; Figure 3E).
Subgroup analysis of adverse pregnancy outcomes in RCTs and cohort studies
For the subgroups with ≥2 studies included, we conducted a subgroup analysis by the type of vaccine. The results of RCTs showed that 4vHPV vaccination in periconceptional period or during pregnancy did not increase the risk of spontaneous abortion (RR = 0.948, 95% CI: 0.838–1.074; I2 = 0, Tau2 = 0), birth defects (RR = 1.559, 95% CI: 0.960–2.533; I2 = 0, Tau2 = 0), stillbirth (RR = 1.259, 95% CI: 0.654–2.425; I2 = 0, Tau2 = 0), and ectopic pregnancy (RR = 0.499, 95% CI: 0.125–1.988; I2 = 0, Tau2 = 0) (Table 3; Figures 4A–D). Subgroup analysis of cohort studies suggested that 2vHPV (RR = 1.060, 95% CI: 0.845–1.329; I2 = 0, Tau2 = 0) or 4vHPV vaccine (RR = 0.970, 95% CI: 0.794–1.184, 95% PI: 0.410–2.292; I2 = 83.3%, Tau2 = 0.0296) exposure was not associated with the increased risk of spontaneous abortion (Table 3; Figures 5A, B). In cohort studies, 4vHPV vaccination in periconceptional period or during pregnancy did not increase the risk of birth defects (RR = 0.930, 95% CI: 0.609–1.421, 95% PI: 0.007–131.804; I2 = 75.7%, Tau2 = 0.1052), stillbirth (RR = 0.999, 95% CI: 0.614–1.624; I2 = 0, Tau2 = 0), SGA (RR = 0.961, 95% CI: 0.797–1.158; I2 = 64.3%, Tau2 = 0.0117), and preterm birth (RR = 0.996, 95% CI: 0.885–1.121, 95% PI: 0.195–5.116; I2 = 46.9%, Tau2 = 0.0097) (Table 3; Figures 5C–F).
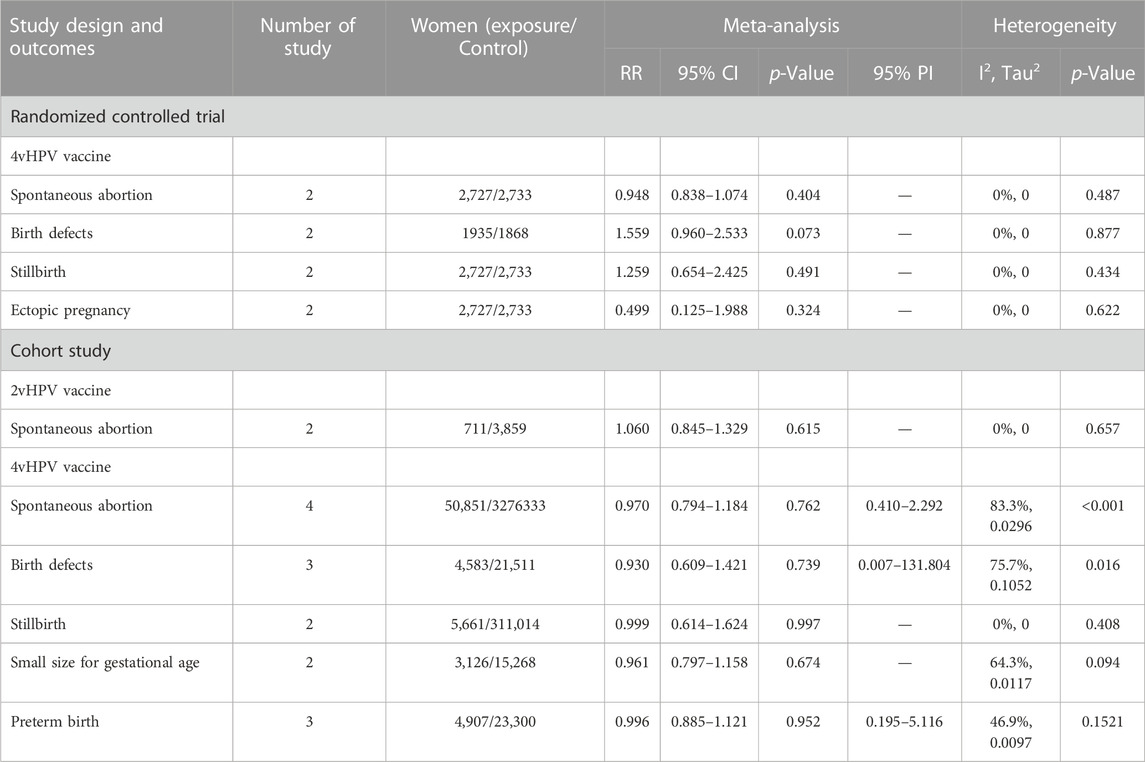
TABLE 3. Subgroup analysis of adverse pregnancy outcomes in randomized controlled trials and cohort studies.
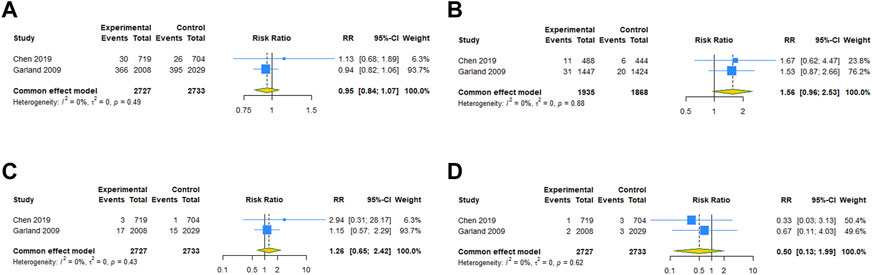
FIGURE 4. Subgroup analysis of adverse pregnancy outcomes after 4vHPV vaccination in RCTs. (A) Spontaneous abortion. (B) Birth defects. (C) Stillbirth. (D) Ectopic pregnancy.
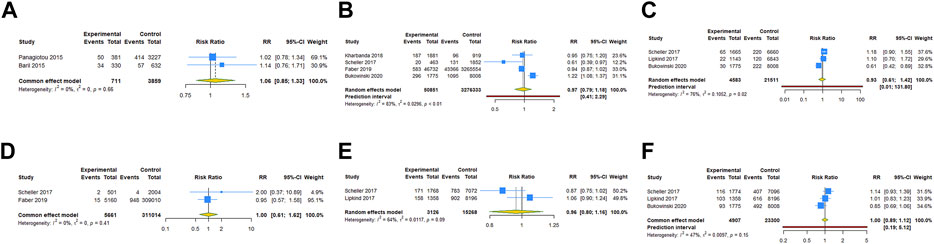
FIGURE 5. Subgroup analysis of adverse pregnancy outcomes after 2vHPV or 4vHPV vaccination in cohort studies. (A) Spontaneous abortion after 2vHPV vaccination. (B) Spontaneous abortion, (C) birth defects, (D) stillbirth, (E) small size for gestational age, and (F) preterm birth after 4vHPV vaccination.
Trial sequential analysis results
In trial sequential analysis of RCTs, we observed that all the cumulative Z-curves did not cross the trial sequential monitoring boundary and RIS boundary, suggesting that we cannot draw a definitive conclusion about spontaneous abortion, birth defects, stillbirth, preterm birth and ectopic pregnancy in RCTs due to the presence of false positive (Figure 6). For cohort studies, the cumulative Z-curve significantly crossed the RIS boundary, but did not cross the trial sequential monitoring boundary, suggesting that a relatively definite conclusion of spontaneous abortion, birth defects, stillbirth, SGA and preterm birth can be obtained in cohort studies (Figure 7).
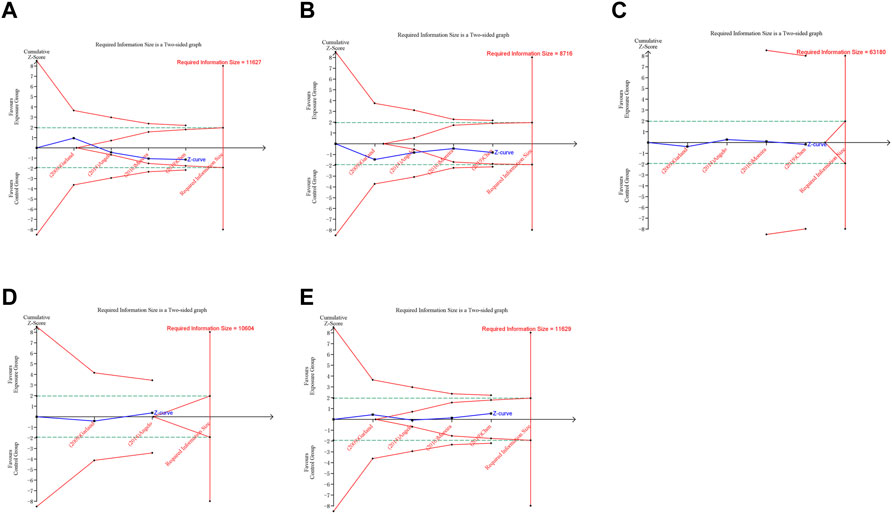
FIGURE 6. Trial sequential analysis (TSA) of adverse pregnancy outcomes in RCTs. (A) Spontaneous abortion. (B) Birth defects. (C) Stillbirth. (D) Preterm birth. (E) Ectopic pregnancy. Uppermost and lowermost red curves represent trial sequential monitoring boundary lines for benefit and harm, respectively. Horizontal green lines represent the conventional boundaries for statistical significance. Inner red lines represent the futility boundary.
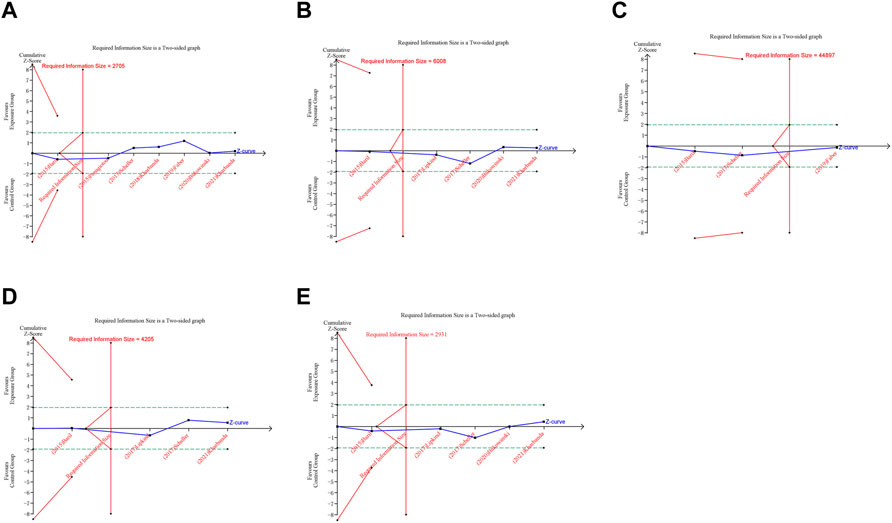
FIGURE 7. Trial sequential analysis (TSA) of adverse pregnancy outcomes in cohort studies. (A) Spontaneous abortion. (B) Birth defects. (C) Stillbirth. (D) Small size for gestational age. (E) Preterm birth. Uppermost and lowermost red curves represent trial sequential monitoring boundary lines for benefit and harm, respectively. Horizontal green lines represent the conventional boundaries for statistical significance. Inner red lines represent the futility boundary.
Publication bias and sensitivity analysis
We conducted publication bias test and sensitivity analysis for the pooled result of spontaneous abortion which included seven studies. Begg’s test and Egger’s test were performed to evaluate the publication bias and the results indicated that no significant publication bias existed in cohort studies (Begg’s test: p = 0.764, Egger’s test: p = 0.784). The funnel plot was shown in Supplementary Figure S1 (Supplementary Material S3). Sensitivity analysis was performed by calculating the pooled RRs and the corresponding 95% CIs after individual studies were omitted to assess whether the pooled results were affected by a single study. The sensitivity analysis indicated that Bukowinski’s study may be the cause of high heterogeneity (Supplementary Figure S1, Supplemental Material S3).
Discussion
The number of women in the world who were inadvertently vaccinated with HPV in periconceptional period or during pregnancy was enormous. If HPV vaccination during periconceptional period or during pregnancy increases the risk of adverse pregnancy outcomes, even if the risk is very small, we should be vigilant. Although several meta-analyses have reported the relationship between HPV vaccination in the periconceptional period or during pregnancy and adverse pregnancy outcomes (Tan et al., 2019; Wang et al., 2020), our meta-analysis included four recent studies to update previous results, and further performed meta-analysis of RCT and retrospective cohort studies, respectively. The results indicated that HPV vaccination in periconceptional period or during pregnancy did not increase the risks of adverse pregnancy outcomes, including spontaneous abortion, birth defects, stillbirth, SGA, preterm birth and ectopic pregnancy.
Previous studies on the safety of HPV exposure around conception or during pregnancy included combined analyses of clinical trials, large observational cohort studies and post-marketing surveillance pregnancy registries. Among these various research methods and populations, there were no indications of increased risks for spontaneous abortion (Goss et al., 2015), birth defects (Moro et al., 2015), preterm birth (Forinash et al., 2011) or SGA (Scheller et al., 2017) after exposure to HPV vaccines during pregnancy. However, the majority of prior studies have only reported the relationship between exposure of 4vHPV vaccine during pregnancy and adverse pregnancy outcomes, the evidence of the association between 2vHPV vaccination and adverse pregnancy outcomes was limited. There were only one RCT and two cohort studies, focused on the effect of 2vHPV in our analysis. No association was found between 2vHPV exposure around conception and spontaneous abortion. There is no established pathophysiological mechanism by which 2vHPV vaccination would affect the risk of spontaneous abortion (Panagiotou et al., 2015). A theoretical debate involves alterations in the maternal immune system during early gestation caused by ASO4 adjuvant, which is composed of aluminum phosphate and monophosphoryl lipid A (Goldhaber and Fireman, 1991). Nevertheless, other ASO4 based vaccines were not associated with the risk of spontaneous abortion (Tavares et al., 2013). More generally, the evidence of a causal effect of autoimmunity itself on the risk of spontaneous abortion is weak (Larsen et al., 2013). Also, the vaccine was not associated with autoimmune conditions related to abortion (e.g., antiphospholipid syndrome and thyroid autoimmunity) (Panagiotou et al., 2015).
Policymakers have published reassuring reports on the safety of 2vHPV and 4vHPV vaccines, both overall and for pregnancy related outcomes specifically. A review of the latest evidence used in the recommendations of the Advisory Committee on Immunization Practice concluded that the public health benefits of HPV vaccination outweigh the potential harms (Oshman and Davis, 2020). Our pooled analysis suggested that 4vHPV vaccination in periconceptional period or during pregnancy was not associated with the increased risks of spontaneous abortion, birth defects, stillbirth, ectopic pregnancy, SGA and preterm birth. A previous analysis of pregnancy outcomes in women with 4vHPV vaccination in a global clinical program found similar incidence of adverse pregnancy outcomes in the 4vHPV vaccine and placebo groups without evidence of a negative effect of vaccination on pregnancy outcomes (Garland et al., 2009). Post-marketing registry data indicated that 4vHPV vaccine exposure around conception or during pregnancy was not associated with an increased risk of adverse pregnancy outcomes, such as spontaneous abortion or birth defects (Goss et al., 2015; Sy et al., 2018). Given the large amount of evidence that suggests accidental HPV vaccination during pregnancy, including 4vHPV, does not cause a risk to the pregnancy or developing fetus, it was suggested that HPV vaccination be included in routine prenatal care, as this is a time when women regularly encounter the healthcare system (Berenson et al., 2014). Although HPV vaccination before initiation of sexual activity is most effective, study has showed that HPV vaccination can provide protection against HPV-related dysplasia even among women who have previously been exposed to and/or infected with HPV (Bukowinski et al., 2020).
2vHPV, 4vHPV and 9vHPV vaccines are all recombinant, contain virus-like particles, and are enhanced by adjuvants that trigger higher immune responses than natural infections (Bonde et al., 2016). Despite these HPV vaccines are noninfectious recombinant vaccines, excipients need to be considered in addition to the types of recombinant HPV when determining maternal and fetal safety (Forinash et al., 2011). Both 4vHPV and 9vHPV vaccines contain an amorphous aluminum hydroxyphosphate sulfate adjuvant which is contained in other products made by Merck, such as Haemophilus influenzae B conjugate vaccine, hepatitis A vaccine and hepatitis B vaccine. These products are appropriate for use in pregnancy (Forinash et al., 2011). Given this fact, 4vHPV and 9vHPV vaccines appear to be relatively safe from the excipient standpoint. Due to the limited number of included studies, the pooled results of the association between 9vHPV vaccination and adverse pregnancy outcomes both in RCTs and cohort studies cannot be obtained. 9vHPV vaccine was generally well tolerated in clinical trials, with adverse event profile similar to that of 4vHPV vaccine. Discontinuations due to adverse events and serious vaccine-related adverse events were rare (Moreira et al., 2016). We believe that this meta-analysis supports the current recommendations of the Advisory Committee on Immunization Practices that although 9vHPV vaccine is not recommended to be used during pregnancy, it can be administered to women of childbearing age without routine pregnancy testing (Kharbanda et al., 2021).
Although there has been ample evidence of vaccine safety from post-marketing surveillance studies and clinical trials, public misperceptions and concerns about the safety of vaccines may hinder the implementation of HPV vaccination programs (Chen et al., 2019). Immunization-related anxiety reactions have occurred in some regions, adversely affecting HPV vaccination programs and leaving young female individuals vulnerable to preventable HPV-related diseases (Hanley et al., 2015; Tanaka et al., 2016). However, concerns regarding inadvertent HPV vaccination in periconceptional period or during pregnancy may further decrease, given the increasing intensity of HPV vaccination at the recommended age of 11–12 years (Cullen et al., 2014). Preparing, facilitating communication and enhancing vaccine infrastructure can ensure the implementation of high coverage and sustainable vaccination schedules (Chen et al., 2019).
The present study leaded to some meaningful implications, it yet has some limitations. Firstly, some of these studies included pregnancies with small sample sizes, and there were no or limited adjustments for factors affecting pregnancy outcomes and malformations, which may bias the conclusions. Secondly, the majority of the studies included in present meta-analysis did not clearly divide fetal development stages at which HPV vaccine administered or doses of HPV vaccination. This information should be reported and analyzed to further evaluate the safety of HPV vaccine exposure around conception or during pregnancy. Thirdly, the safety profiles from included studies were based primarily on 2vHPV or 4vHPV vaccination, and very few studies evaluated the 9vHPV vaccine exposures in periconceptional period or during pregnancy. Fourthly, most of the included studies reported the association between HPV vaccination in periconceptional period (including before and after conception) and adverse pregnancy outcomes, and it was unclear whether HPV vaccines was administered before or after conception in the majority of studies. Only two RCTs explicitly reported the association between HPV vaccination before conception and adverse pregnancy outcomes, and the results showed that HPV vaccination before conception did not increase the risks of spontaneous abortion, birth defects, stillbirth and ectopic pregnancy. More studies are need to be included for further subgroup analysis by vaccination exposure time.
Conclusion
This meta-analysis demonstrated that HPV vaccine exposures in periconceptional period or during pregnancy did not increase the risks of adverse pregnancy outcomes, such as spontaneous abortion, birth defects, stillbirth, SGA, preterm birth and ectopic pregnancy. Moreover, periconceptional or pregnancy exposure of 2vHPV vaccine was not associated with the increased risk of spontaneous abortion, and 4vHPV vaccination around conception or during pregnancy was not associated with the increased risk of spontaneous abortion, birth defects, stillbirth, SGA, preterm birth and ectopic pregnancy. Despite the limited studies included in present analysis, our meta-analysis revealed no association between 9vHPV vaccination in periconceptional period or during pregnancy and spontaneous abortion, birth defects, SGA and preterm birth.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
DW and PY conceived and designed the study. BS, GH, and QC searched the literature, selected papers for inclusion, and collected the data. XY and HL analyzed the data. XY and HL prepared the manuscript. DW and PY edited the manuscript. All authors contributed to data analysis and manuscript revision, and approved publication of the final version.
Funding
This work was supported by the Key Program of Technological Innovation and Application Development of Chongqing (CSTB2022TIAD-KPX0173) and the National Natural Fund Project (81771619).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1181919/full#supplementary-material
References
Angelo, M. G., David, M. P., Zima, J., Baril, L., Dubin, G., Arellano, F., et al. (2014). Pooled analysis of large and long-term safety data from the human papillomavirus-16/18-AS04-adjuvanted vaccine clinical trial programme. Pharmacoepidemiol Drug Saf. 23 (5), 466–479. doi:10.1002/pds.3554
Baril, L., Rosillon, D., Willame, C., Angelo, M. G., Zima, J., van den Bosch, J. H., et al. (2015). Risk of spontaneous abortion and other pregnancy outcomes in 15-25 year old women exposed to human papillomavirus-16/18 AS04-adjuvanted vaccine in the United Kingdom. Vaccine 33 (48), 6884–6891. doi:10.1016/j.vaccine.2015.07.024
Berenson, A. B., Patel, P. R., and Barrett, A. D. (2014). Is administration of the HPV vaccine during pregnancy feasible in the future? Expert Rev. Vaccines 13 (2), 213–219. doi:10.1586/14760584.2014.867236
Bonde, U., Joergensen, J. S., Lamont, R. F., and Mogensen, O. (2016). Is HPV vaccination in pregnancy safe? Hum. Vaccin Immunother. 12 (8), 1960–1964. doi:10.1080/21645515.2016.1160178
Bowden, J., Tierney, J. F., Copas, A. J., and Burdett, S. (2011). Quantifying, displaying and accounting for heterogeneity in the meta-analysis of RCTs using standard and generalised Q statistics. BMC Med. Res. Methodol. 11, 41. doi:10.1186/1471-2288-11-41
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., and Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68 (6), 394–424. doi:10.3322/caac.21492
Bukowinski, A. T., Hall, C., Chang, R. N., Gumbs, G. R., and Marie, S. C. A. (2020). Maternal and infant outcomes following exposure to quadrivalent human papillomavirus vaccine during pregnancy. Vaccine 38 (37), 5933–5939. doi:10.1016/j.vaccine.2020.06.073
Burger, E. A., Portnoy, A., Campos, N. G., Sy, S., Regan, C., and Kim, J. J. (2021). Choosing the optimal HPV vaccine: The health impact and economic value of the nonavalent and bivalent HPV vaccines in 48 Gavi-eligible countries. Int. J. Cancer 148 (4), 932–940. doi:10.1002/ijc.33233
Chen, W., Zhao, Y., Xie, X., Liu, J., Li, J., Zhao, C., et al. (2019). Safety of a quadrivalent human papillomavirus vaccine in a Phase 3, randomized, double-blind, placebo-controlled clinical trial among Chinese women during 90 months of follow-up. Vaccine 37 (6), 889–897. doi:10.1016/j.vaccine.2018.12.030
Chung, S. M., Moon, J. S., and Chang, M. C. (2021). Prevalence of sarcopenia and its association with diabetes: A meta-analysis of community-dwelling asian population. Front. Med. 8, 681232. doi:10.3389/fmed.2021.681232
Cullen, K. A., Stokley, S., and Markowitz, L. E. (2014). Uptake of human papillomavirus vaccine among adolescent males and females: Immunization Information System sentinel sites, 2009-2012. Acad. Pediatr. 14 (5), 497–504. doi:10.1016/j.acap.2014.03.005
Faber, M. T., Duun-Henriksen, A. K., Dehlendorff, C., Tatla, M. K., Munk, C., and Kjaer, S. K. (2019). Adverse pregnancy outcomes and infant mortality after quadrivalent HPV vaccination during pregnancy. Vaccine 37 (2), 265–271. doi:10.1016/j.vaccine.2018.11.030
Finer, L. B., and Zolna, M. R. (2016). Declines in unintended pregnancy in the United States, 2008-2011. N. Engl. J. Med. 374 (9), 843–852. doi:10.1056/NEJMsa1506575
Forinash, A. B., Yancey, A. M., Pitlick, J. M., and Myles, T. D. (2011). Safety of the HPV bivalent and quadrivalent vaccines during pregnancy. Ann. Pharmacother. 45 (2), 258–262. doi:10.1345/aph.1P396
Garland, S. M., Ault, K. A., Gall, S. A., Paavonen, J., Sings, H. L., Ciprero, K. L., et al. (2009). Pregnancy and infant outcomes in the clinical trials of a human papillomavirus type 6/11/16/18 vaccine: A combined analysis of five randomized controlled trials. Obstet. Gynecol. 114 (6), 1179–1188. doi:10.1097/AOG.0b013e3181c2ca21
Gidengil, C., Goetz, M. B., Newberry, S., Maglione, M., Hall, O., Larkin, J., et al. (2021). Safety of vaccines used for routine immunization in the United States: An updated systematic review and meta-analysis. Vaccine 39 (28), 3696–3716. doi:10.1016/j.vaccine.2021.03.079
Goldhaber, M. K., and Fireman, B. H. (1991). The fetal life table revisited: Spontaneous abortion rates in three kaiser permanente cohorts. Epidemiology 2 (1), 33–39. doi:10.1097/00001648-199101000-00006
Goss, M. A., Lievano, F., Buchanan, K. M., Seminack, M. M., Cunningham, M. L., and Dana, A. (2015). Final report on exposure during pregnancy from a pregnancy registry for quadrivalent human papillomavirus vaccine. Vaccine 33 (29), 3422–3428. doi:10.1016/j.vaccine.2015.04.014
Hanley, S. J., Yoshioka, E., Ito, Y., and Kishi, R. (2015). HPV vaccination crisis in Japan. Lancet 385 (9987), 2571. doi:10.1016/s0140-6736(15)61152-7
Harper, D. M., and DeMars, L. R. (2017). HPV vaccines - a review of the first decade. Gynecol. Oncol. 146 (1), 196–204. doi:10.1016/j.ygyno.2017.04.004
Higgins, J. P., and Thompson, S. G. (2002). Quantifying heterogeneity in a meta-analysis. Stat. Med. 21 (11), 1539–1558. doi:10.1002/sim.1186
IntHout, J., Ioannidis, J. P., Rovers, M. M., and Goeman, J. J. (2016). Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open 6 (7), e010247. doi:10.1136/bmjopen-2015-010247
Jadad, A. R., Moore, R. A., Carroll, D., Jenkinson, C., Reynolds, D. J., Gavaghan, D. J., et al. (1996). Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin. Trials 17 (1), 1–12. doi:10.1016/0197-2456(95)00134-4
Kharbanda, E. O., Vazquez-Benitez, G., DeSilva, M. B., Naleway, A. L., Klein, N. P., Hechter, R. C., et al. (2021). Association of inadvertent 9-valent human papillomavirus vaccine in pregnancy with spontaneous abortion and adverse birth outcomes. JAMA Netw. Open 4 (4), e214340. doi:10.1001/jamanetworkopen.2021.4340
Kharbanda, E. O., Vazquez-Benitez, G., Lipkind, H. S., Sheth, S. S., Zhu, J., Naleway, A. L., et al. (2018). Risk of spontaneous abortion after inadvertent human papillomavirus vaccination in pregnancy. Obstet. Gynecol. 132 (1), 35–44. doi:10.1097/aog.0000000000002694
Kjær, S. K., Frederiksen, K., Munk, C., and Iftner, T. (2010). Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: Role of persistence. J. Natl. Cancer Inst. 102 (19), 1478–1488. doi:10.1093/jnci/djq356
Larsen, E. C., Christiansen, O. B., Kolte, A. M., and Macklon, N. (2013). New insights into mechanisms behind miscarriage. BMC Med. 11, 154. doi:10.1186/1741-7015-11-154
Lipkind, H. S., Vazquez-Benitez, G., Nordin, J. D., Romitti, P. A., Naleway, A. L., Klein, N. P., et al. (2017). Maternal and infant outcomes after human papillomavirus vaccination in the periconceptional period or during pregnancy. Obstet. Gynecol. 130 (3), 599–608. doi:10.1097/aog.0000000000002191
Markowitz, L. E., Dunne, E. F., Saraiya, M., Lawson, H. W., Chesson, H., Unger, E. R., et al. (2007). Quadrivalent human papillomavirus vaccine: Recommendations of the advisory committee on immunization Practices (ACIP). MMWR Recomm. Rep. 56 (2), 1–24.
Medeiros, L. R., Rosa, D. D., da Rosa, M. I., Bozzetti, M. C., and Zanini, R. R. (2009). Efficacy of human papillomavirus vaccines: A systematic quantitative review. Int. J. Gynecol. Cancer 19 (7), 1166–1176. doi:10.1111/IGC.0b013e3181a3d100
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 339, b2535. doi:10.1136/bmj.b2535
Moreira, E. D., Block, S. L., Ferris, D., Giuliano, A. R., Iversen, O. E., Joura, E. A., et al. (2016). Safety profile of the 9-valent HPV vaccine: A combined analysis of 7 phase III clinical trials. Pediatrics 138 (2), e20154387. doi:10.1542/peds.2015-4387
Moro, P. L., Zheteyeva, Y., Lewis, P., Shi, J., Yue, X., Museru, O. I., et al. (2015). Safety of quadrivalent human papillomavirus vaccine (gardasil) in pregnancy: Adverse events among non-manufacturer reports in the vaccine adverse event reporting system. Vaccine. 33 (4), 519–522. doi:10.1016/j.vaccine.2014.11.047
Oshman, L. D., and Davis, A. M. (2020). Human papillomavirus vaccination for adults: Updated recommendations of the advisory committee on immunization Practices (ACIP). JAMA 323 (5), 468–469. doi:10.1001/jama.2019.18411
Panagiotou, O. A., Befano, B. L., Gonzalez, P., Rodríguez, A. C., Herrero, R., Schiller, J. T., et al. (2015). Effect of bivalent human papillomavirus vaccination on pregnancy outcomes: Long term observational follow-up in the Costa Rica HPV vaccine trial. BMJ 351, h4358. doi:10.1136/bmj.h4358
Scheller, N. M., Pasternak, B., Mølgaard-Nielsen, D., Svanström, H., and Hviid, A. (2017). Quadrivalent HPV vaccination and the risk of adverse pregnancy outcomes. N. Engl. J. Med. 376 (13), 1223–1233. doi:10.1056/NEJMoa1612296
Sterne, J. A., and Egger, M. (2001). Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J. Clin. Epidemiol. 54 (10), 1046–1055. doi:10.1016/s0895-4356(01)00377-8
Sy, L. S., Meyer, K. I., Klein, N. P., Chao, C., Velicer, C., Cheetham, T. C., et al. (2018). Postlicensure safety surveillance of congenital anomaly and miscarriage among pregnancies exposed to quadrivalent human papillomavirus vaccine. Hum. Vaccin Immunother. 14 (2), 412–419. doi:10.1080/21645515.2017.1403702
Tan, J., Xiong, Y. Q., He, Q., Liu, Y. M., Wang, W., Chen, M., et al. (2019). Peri-conceptional or pregnancy exposure of HPV vaccination and the risk of spontaneous abortion: A systematic review and meta-analysis. BMC Pregnancy Childbirth 19 (1), 302. doi:10.1186/s12884-019-2425-1
Tanaka, Y., Ueda, Y., Egawa-Takata, T., Yagi, A., Yoshino, K., and Kimura, T. (2016). Outcomes for girls without HPV vaccination in Japan. Lancet Oncol. 17 (7), 868–869. doi:10.1016/s1470-2045(16)00147-9
Tavares, F., Cheuvart, B., Heineman, T., Arellano, F., and Dubin, G. (2013). Meta-analysis of pregnancy outcomes in pooled randomized trials on a prophylactic adjuvanted glycoprotein D subunit herpes simplex virus vaccine. Vaccine 31 (13), 1759–1764. doi:10.1016/j.vaccine.2013.01.002
Wang, A., Liu, C., Wang, Y., Yin, A., Wu, J., Zhang, C., et al. (2020). Pregnancy outcomes after human papillomavirus vaccination in periconceptional period or during pregnancy: A systematic review and meta-analysis. Hum. Vaccin Immunother. 16 (3), 581–589. doi:10.1080/21645515.2019.1662363
Wells, G., Shea, B., and O'Connell, J. (2014). The newcastle-ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Health Research Institute Web site. Available at: (https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp).
Keywords: human papillomavirus, vaccine, pregnancy, adverse pregnancy outcomes, meta-analysis
Citation: Yan X, Li H, Song B, Huang G, Chang Q, Wang D and Yan P (2023) Association of periconceptional or pregnancy exposure of HPV vaccination and adverse pregnancy outcomes: a systematic review and meta-analysis with trial sequential analysis. Front. Pharmacol. 14:1181919. doi: 10.3389/fphar.2023.1181919
Received: 08 March 2023; Accepted: 19 April 2023;
Published: 09 May 2023.
Edited by:
Catherine M. T. Sherwin, Wright State University, United StatesReviewed by:
Jessian Munoz, Texas Children’s Hospital, United StatesPalanisamy Amirthalingam, University of Tabuk, Saudi Arabia
Copyright © 2023 Yan, Li, Song, Huang, Chang, Wang and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dan Wang, c3doX3dhbmdAMTYzLmNvbQ==; Ping Yan, eXAyMzEyODUzQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Xiaoli Yan†
Xiaoli Yan† Dan Wang
Dan Wang