
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 29 March 2023
Sec. Gastrointestinal and Hepatic Pharmacology
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1180016
This article is part of the Research Topic Advances in Novel Drugs and Targets for Hepatic and Gastrointestinal Diseases View all 33 articles
Background: With the increasing prevalence of obesity and metabolic syndrome, the incidence of non-alcoholic fatty liver disease (NAFLD) is also increasing. In the next decade, NAFLD may become the main cause of liver transplantation. Therefore, the choice of treatment plan is particularly important. The purpose of this study was to compare several interventions in the treatment of NAFLD to provide some reference for clinicians in selecting treatment methods.
Methods: We searched Public Medicine (PubMed), Medline, Excerpta Medica Database (Embase), and Cochrane Library from January 2013 to January 2023 to identify randomized controlled trials (RCTs) published in English. The network meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Forty-three studies accounting for a total of 2,969 patients were included, and alanine aminotransferase (ALT), total cholesterol (TC), and low-density lipoprotein cholesterol (LDL) were selected as outcome measures for analysis and comparison.
Results: We evaluated the results of drug, diet, and lifestyle interventions between the intervention and control groups. Curcumin (CUN) and probiotics (PTC) were selected for medication, the Mediterranean diet (MDED) was selected for special diet (SPD), and various kinds of exercise and lifestyle advice were selected for lifestyle interventions (LFT). The SUCRA was used to rank interventions according to the effect on ALT indicators (SUCRA: PTC 80.3%, SPD 65.2%, LFT 61.4%, PLB 32.8%, CUN 10.2%), TC indicators (SUCRA: PTC 89.4%, SPD 64%, CUN 34%, LFT 36.6%, PLB 17%), and LDL indicators (SUCRA: PTC 84.2%, CUN 69.5%, LFT 51.7%, PLB 30.1%, SPD 14.5%). The pairwise meta-analysis results showed that MDED was significantly better than NT in improving ALT [SMD 1.99, 95% CI (0.38, 3.60)]. In terms of improving TC and LDL, ATS was significantly better than NT [SMD 0.19, 95% CI (0.03, 0.36)] [SMD 0.18, 95% CI (0.01, 0.35)].
Conclusion: Our study showed that PTC is most likely to be the most effective treatment for improving NAFLD indicators. Professional advice on diet or exercise was more effective in treating NAFLD than no intervention.
Non-alcoholic fatty liver disease (NAFLD) has become increasingly common. It is defined as bullous fatty degeneration in ≥5% of liver cells that is not related to alcohol or drug use (Li et al., 2021). The incidence rate is high in patients with central obesity, type 2 diabetes, dyslipidemia and metabolic syndrome, and it is also the main cause of chronic liver disease worldwide (Su et al., 2021; Chen et al., 2022; Wing-Sum Cheu et al., 2023). The progression of NAFLD is mainly from non-alcoholic fatty liver disease (NAFL) to non-alcoholic fatty hepatitis (NASH), fibrosis, and cirrhosis, but the public’s understanding of the disease is still very limited (Blot et al., 2023). Despite the obesity epidemic, public health education has not focused on the complications of cirrhosis and even the development of liver cancer.
The treatment principles of NAFLD include identifying high-risk groups, improving metabolic risk factors, detecting the progression of advanced liver disease, and preventing liver injury caused by alcohol, obesity and other factors (Yamada et al., 2022). One study have found that (Sakane et al., 2021) the autophagy ability in white adipose tissue of mice fed with high-fat diet (HFD) was enhanced, and the liver pathology of NAFLD can be improved through autophagy inhibition. Metabolic changes caused by impaired lipid transfer have been speculated to be involved in the molecular pathogenesis of NAFLD (He et al., 2021), and steatosis is a necessary condition for the development of non-alcoholic steatosis. The oxidation of fatty acids in the damaged liver leads to the imbalance of lipid accumulation and redox, and promotes the development of fatty liver disease and insulin resistance. Sun N et al. (Sun et al., 2021)found that the direct KLF16-PPAR α pathway was closely related to liver lipid homeostasis and redox balance, and its dysfunction promotes insulin resistance and liver steatosis.
In addition, a higher body mass index is a risk factor for steatosis, and excessive intake of high-calorie foods and obesity contribute to the development of NAFLD (Gao et al., 2017). Therefore, weight loss through diet and exercise is the mainstay of treatment for NAFLD. Studies have shown that regular exercise, such as aerobic exercise, resistance exercise and flexibility training, can improve NAFLD, reduce risk factors inducing NAFLD, such as diabetes, hypertension and cardiovascular disease, and improve liver function and markers of intrahepatic fat in NAFLD (Novelle et al., 2021). The substrates of leptin and adiponectin can regulate energy balance and glucose metabolism through melanocortin activity (Liu et al., 2022). A mouse model (Smn2B/mouse) of surviving motor neuron (SMN) depletion developed severe liver steatosis within less than 2 weeks after birth. The rapid onset of Smn2B/mouse fatty liver provides an opportunity to identify molecular markers of NAFLD (Deguise et al., 2021).
However, for some patients who are unable to achieve weight loss through diet or exercise, drug intervention may be considered. There are many conflicting findings on the efficacy and safety of NASH drug therapy, and the recommendations of management guidelines vary. Therefore, the individual characteristics of patients should be considered to select appropriate drug therapies (Yang et al., 2021; Meijnikman et al., 2022).
The role of the intestinal microbiota in metabolic diseases has received increasing attention (Wang et al., 2021). The imbalance of the microorganisms in the intestine is considered a contributing factor of NAFLD (Qiu et al., 2022). The changes in peripheral and intrahepatic immune responses caused by ecological imbalance accelerate the development of NASH. Ecological imbalance destroys the permeability of the intestinal epithelium, and bacterial antigens enter the portal vein circulation and trigger a downstream inflammatory cascade reaction involving Toll-like receptor 4 (TLR-4) and coreceptors in the liver. In addition, it may also change the intestinal short-chain fatty acids (SCFA), resulting in fatty degeneration and NAFLD (Ramos et al., 2021).
Although research reports on intervention measures to treat NAFLD have emerged continuously, no article has compared the effects of various intervention measures to summarize the adaptive population and characteristics of each measure. Therefore, we selected several intervention methods to treat NAFLD for network meta-analysis (NMA). In terms of medicines, we searched for studies involving curcumin (CUN) and probiotics (PTC). For diet, we chose the Mediterranean diet (MDED), and various exercise or exercise recommendations were searched for. Through the comparison of clinical indicators related to NAFLD, we aim to provide some suggestions for clinicians in the process of choosing intervention measures.
The systematic review was conducted strictly in accordance with the PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-analyses (Hutton et al., 2015), and the study protocol was registered with the PROSPERO database of systematic reviews (CRD42023401640).
We searched Public Medicine (PubMed), Medline, Excerpta Medica Database (Embase), and Cochrane Library from January 2013 to January 2023 to identify randomized controlled trials (RCTs) published in English. We conducted the literature search using the following MeSH terms: “Non-alcoholic Fatty Liver Disease” or “NAFLD” or “Non-alcoholic Steatohepatitis” or “Liver, Non-alcoholic Fatty” and “Curcumin Phytosome” or “Curcumin” or “Phytosome, Curcumin” or “Curcumin Phytosome” or “Probiotics” or “Diet” or “Mediterranean Diet” or “Diets, Mediterranean” or “Mediterranean Diets” or “Lifestyle” or “Exercise” or “Physical Activity” or “Exercise Training” or “motion” or “movement” or “Guideline, Health Planning” or “recommendation” or “Health Planning Recommendation”.
We reviewed the list of references for all eligible articles, study reports and conference reports and searched unpublished literature on the WHO’s International Clinical Research Trials Registry (ICTRP) to obtain additional studies to avoid omissions. After we used EndNote 20 to exclude duplicates, two researchers (XC and XQ) independently screened the titles and abstracts to identify all potentially relevant studies. Two researchers assessed the articles to determine whether they met the inclusion criteria for the study. Differences were resolved through discussion or negotiation with a third party (CL), and experts were consulted if necessary.
We determined the eligibility criteria based on PICOS (population, interventions, comparisons outcomes, study designs). The studies included in this review met the following criteria: 1) all study designs were RCTS, 2) participants were diagnosed with NAFLD based on clinical and laboratory data and liver biopsy, which are routine evaluations of the Hepatology Service (de Oliveira et al., 2019), 3) intervention measures included CUN or PTC, the Mediterranean diet, or various types of exercise or exercise recommendations, 4) the control group received no other drug intervention, special diet, exercise, care, or advice, 5) the research results included one of the following indicators: alanine aminotransferase (ALT), total cholesterol (TC), or low-density lipoprotein cholesterol (LDL), and 6) the study language was English. The exclusion criteria were as follows: 1) non-RCTs, 2) review articles, 3) the control group was given special treatment, 4) outcome measures were not correlated with NAFLD, and 5) incomplete data.
Different from traditional meta-analysis, our NMA does not extract and analyze the relevant results of each study separately but rather extracts, combines and analyzes the results across RCTs. Outcome indicators included ALT to measure liver injury, TC to verify lipid metabolism function, and LDL to detect lipid cholesterol transport.
The Cochrane Collaboration (Higgins et al., 2011) guidelines were used to evaluate the bias of RCTs included in the analysis, which was mainly evaluated in the following aspects: generation of random sequences, blinding of the outcome data from the implementation, blinding of the subjects and clinical investigators, completeness of outcome data, checking for whether outcomes were present or had been selectively reported, other sources of bias, and whether or not they existed.
Continuous variables of NMA were extracted using Stata 20.0 software, and standardized mean differences (SDs) with 95% confidence intervals (CIs) or odds ratios (ORs) with 95% CIs were generated. The statistical heterogeneity criteria for the application of the fixed-effects model were I2 < 50% and p > 0.01. If these criteria were not met, the random-effects model was used. Heterogeneity among the included studies was quantified by the I2 statistic and assessed by Cochran’s Q statistic. I2 = 0% indicated no heterogeneity, and I2 = 100% indicated maximum heterogeneity. Publication bias and small sample effects were evaluated using funnel plots. Each intervention was ranked using a cumulative ranking curve (SUCRA). Higher SUCRA values indicated a higher likelihood of achieving better therapeutic effects. A matrix was developed to compare all interventions and detect significant SUCRA differences between each pair of interventions. The consistency or inconsistency of these relationships was assessed to enhance the stability of the results. Subgroup analysis of treatment time was performed using Review Manager 5.3 software.
A total of 33,012 studies were identified in the initial literature search, and 296 studies were selected for a detailed review after the titles and abstracts were screened. Among them, 253 studies were excluded, including 51 studies in which the control group received other drugs, 16 studies which were non-RCTs, 13 studies in which no indicators were related to NAFLD, 79 studies in which the control group had other liver diseases, 74 studies which were reviews, and 20 studies which had incomplete data. In the end, 43 studies (Pugh et al., 2013; Pugh et al., 2014; Abenavoli et al., 2015; Hallsworth et al., 2015; Dong et al., 2016; Panahi et al., 2016; Rahmani et al., 2016; Zhang et al., 2016; Abenavoli et al., 2017; Cheng et al., 2017; Houghton et al., 2017; Manzhalii et al., 2017; Misciagna et al., 2017; Navekar et al., 2017; Panahi et al., 2017; Dinu et al., 2018; Katsagoni et al., 2018; Wong et al., 2018; Ahn et al., 2019; Cai et al., 2019; Chashmniam et al., 2019; Ghaffari et al., 2019; Ghetti et al., 2019; Mirhafez et al., 2019; Saadati et al., 2019; Campanella et al., 2020; Cicero et al., 2020; Hariri et al., 2020; Nourian et al., 2020; Saberi-Karimian et al., 2020; Çevik Saldiran et al., 2020; Chong et al., 2021; Mirhafez et al., 2021; Mohamad Nor et al., 2021; Alami et al., 2022; Asghari et al., 2022; Chiurazzi et al., 2022; George et al., 2022; Khodami et al., 2022; Li et al., 2022; Mobasheri et al., 2022; Yurtdaş et al., 2022; Ezpeleta et al., 2023) were included in the NMA. The selection process is shown in Figure 1. A total of 2,969 patients with NAFLD were included in our NMA, and the characteristics of the subjects are shown in Table 1.
The 43 studies included in the NMA were assessed for risk of bias using methods recommended by the Cochrane Collaboration, and they had varying degrees of bias (In Figure 2).
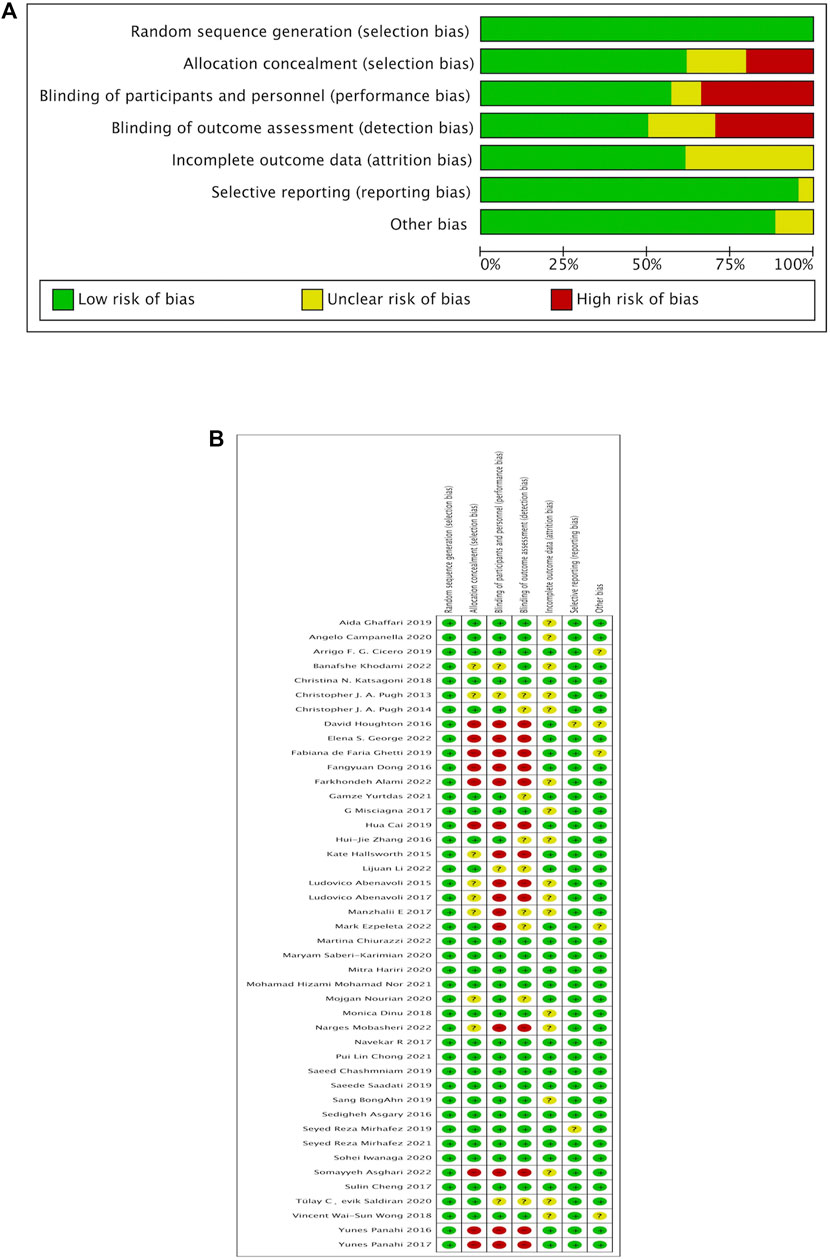
FIGURE 2. Quality assessment (Cochrane risk of bias tool) for included RCTs. RCT, randomized control study, (A) Risk of bias graph, (B) Risk of bias summary.
We indirectly compared the effects of drugs, diet and lifestyle interventions on the NAFLD-related indicators ALT, TC and LDL. The results showed that there were no significant differences in the NAFLD-related indicators among the various interventions.
We used SUCRA to rank interventions according to the effect on ALT indicators, as shown in Table 2(a). The SUCRA of PTC was 80.3%, which represented the best intervention. The next best intervention was the SPD, with a SUCRA of 65.2%. LFT interventions were ranked third, with a SUCRA of 61.4%. PLB and CUN ranked fourth and fifth, respectively, with SUCRA values of 32.8% and 10.2%. The effect on TC indicators is shown in Table 2(b). The SUCRA of PTC was 89.4%, which represented the best intervention. The next highest was the SPD, with a SUCRA of 64%. CUN ranked third, with a SUCRA of 34%; the SUCRA of LFT and PLB were 36.6% and 17%, respectively. The effect on LDL indicators is shown in Table 2(c). The SUCRA of PTC was 84.2%, which represented the best intervention. The next best intervention was CUN, with a SUCRA of 69.5%. LFT ranked third, with a SUCRA of 51.7%, and PLB and SPD ranked fourth and fifth, respectively, with SUCRA values of 30.1% and 14.5%.
We subdivided medication, diet and lifestyle intervention measures and then conducted a pairwise comparison of ALT, TC and LDL. The medication subdivisions were CUN, PTC and PLB, and there were no significant differences in improvements in ALT, TC and LDL levels Figure 3A(a–c), and the network map were shown in Figures 4A(a–c). The diet subdivisions were MDED, low-fat diet (LFD), and no treatment (NT). As shown in Figure 3Ba, MDED was significantly better than NT in improving ALT [SMD 1.99, 95% CI (0.38, 3.60)]. There was no significant difference in improving TC and LDL Figure 3B(b, c), and the network map were shown in Figure 4B(a–c). The lifestyle subdivisions were exercise training (ETN), acceptance of theoretical suggestions (ATS), alternate-day fasting (ADF) and NT. There was no significant difference in improvements to ALT, as shown in Figure 3C(a). In terms of improving TC and LDL, ATS was significantly better than NT [SMD 0.19, 95% CI (0.03, 0.36)] [SMD 0.18, 95% CI (0.01, 0.35)] Figure 3C(b, c), and the network map were shown in Figure 4C(a–c).

FIGURE 3. (A) Forest plot effcacy of medication with placebo (PLB), (a)Forest plot effcacy of curcumin (CUN) and probiotics (PTC) with placebo in improving alanine aminotransferase (ALT), (b)Forest plot effcacy of curcumin (CUN) and probiotics (PTC) with placebo (PLB) in improving total cholesterol (TC), (c) Forest plot effcacy of curcumin (CUN) and probiotics (PTC) with placebo (PLB) in improving low-density lipoprotein cholesterol (LDL). (B) Forest plot effcacy of diet with control, (a) Forest plot effcacy of mediterranean diet (MDED) and low-fat diet (LFD) with not have any type of treatment (NT) in improving alanine aminotransferase (ALT), (b) Forest plot effcacy of mediterranean diet (MDED) and low-fat diet (LFD) with not have any type of treatment (NT) in improving total cholesterol (TC), (c) Forest plot effcacy of mediterranean diet (MDED) and low-fat diet (LFD) with not have any type of treatment (NT) in improving low-density lipoprotein cholesterol (LDL).(C) Forest plot effcacy of lifestyle with control, (a) Forest plot effcacy of exercise training (ETN), accept theoretical suggestions (ATS) and alternate-day fasting (ADF) with not have any type of treatment (NT) in improving alanine aminotransferase (ALT), (b) Forest plot effcacy of exercise training (ETN), accept theoretical suggestions (ATS) and alternate-day fasting (ADF) with not have any type of treatment (NT) in improving total cholesterol (TC), (c) Forest plot effcacy of exercise training (ETN), accept theoretical suggestions (ATS) and alternate-day fasting (ADF) with not have any type of treatment (NT) in improving low-density lipoprotein cholesterol (LDL).
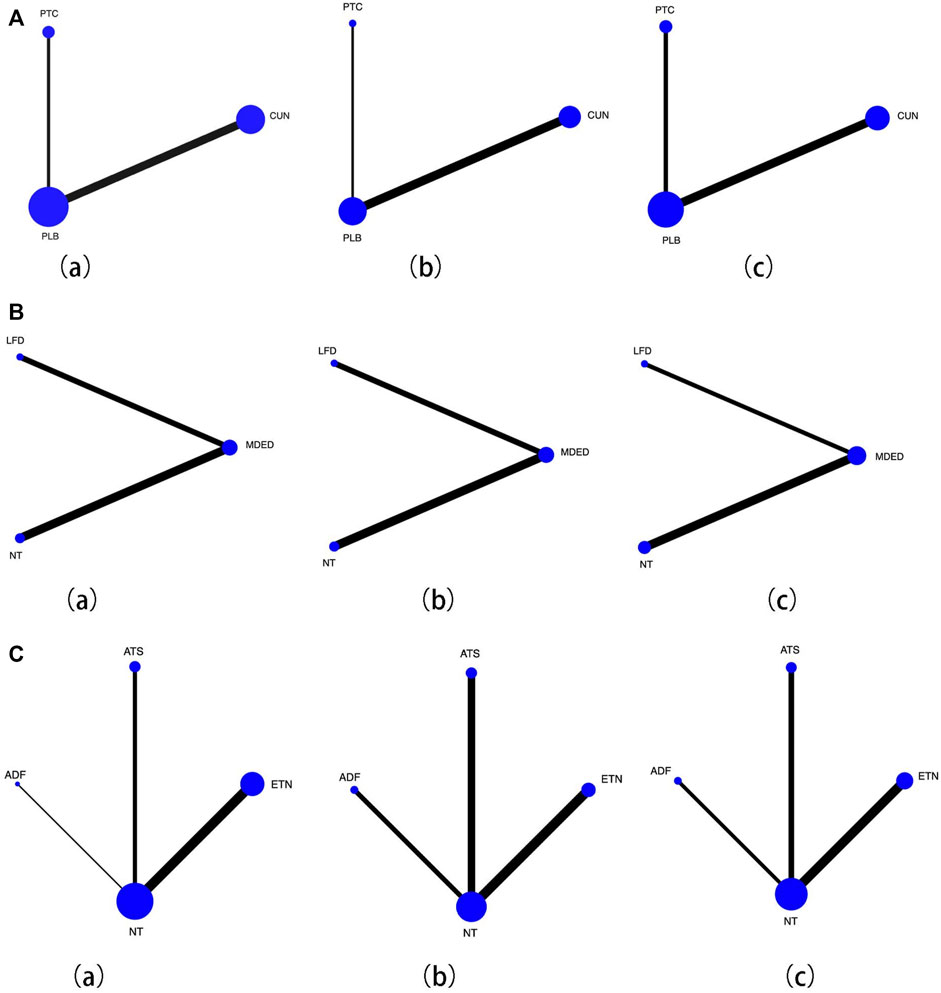
FIGURE 4. (A) Network map for effcacy of medication with placebo (PLB), (a) Forest plot effcacy of curcumin (CUN) and probiotics (PTC) with placebo in improving alanine aminotransferase (ALT), (b) Forest plot effcacy of curcumin (CUN) and probiotics (PTC) with placebo (PLB) in improving total cholesterol (TC), (c) Forest plot effcacy of curcumin (CUN) and probiotics (PTC) with placebo (PLB) in improving low-density lipoprotein cholesterol (LDL). Lines connect the interventions that have been studied in head-to-head comparisons in eligible RCTs. The width of the lines represents the total number of RCTs for each pairwise comparison. The size of each node is proportional to the number of randomized participants. (B) Network map for effcacy of diet with control, (a) Forest plot effcacy of mediterranean diet (MDED) and low-fat diet (LFD) with not have any type of treatment (NT) in improving alanine aminotransferase (ALT), (b) Forest plot effcacy of mediterranean diet (MDED) and low-fat diet (LFD) with not have any type of treatment (NT) in improving total cholesterol (TC), (c) Forest plot effcacy of mediterranean diet (MDED) and low-fat diet (LFD) with not have any type of treatment (NT) in improving low-density lipoprotein cholesterol (LDL). Lines connect the interventions that have been studied in head-to-head comparisons in eligible RCTs. The width of the lines represents the total number of RCTs for each pairwise comparison. The size of each node is proportional to the number of randomized participants. (C) Network map for effcacy of lifestyle with control, (a) Forest plot effcacy of exercise training (ETN), accept theoretical suggestions (ATS) and alternate-day fasting (ADF) with not have any type of treatment (NT) in improving alanine aminotransferase (ALT), (b) Forest plot effcacy of exercise training (ETN), accept theoretical suggestions (ATS) and alternate-day fasting (ADF) with not have any type of treatment (NT) in improving total cholesterol (TC), (c) Forest plot effcacy of exercise training (ETN), accept theoretical suggestions (ATS) and alternate-day fasting (ADF) with not have any type of treatment (NT) in improving low-density lipoprotein cholesterol (LDL). Lines connect the interventions that have been studied in head-to-head comparisons in eligible RCTs. The width of the lines represents the total number of RCTs for each pairwise comparison. The size of each node is proportional to the number of randomized participants.
The sensitivity analysis showed that any single study or cluster study with certain characteristics had little effect on the change in SMD and its corresponding 95% CI. Significant publication bias was not reported by Egger’s regression test or Begg’s adjusted rank correlation test.
To the best of our knowledge, this study was the first to evaluate various interventions for NAFLD using pairwise comparisons and network meta-analyses to explore the effects of different types of interventions on liver cell damage in NAFLD, the impact on lipid metabolism and the relative efficacy of reducing the content of blood lipids and cholesterol. The most important finding of this study was that compared with diet and lifestyle, the use of probiotics had the best effect on improving NAFLD indicators, while MDED and ATS were superior to no treatment.
CUN, a natural polyphenol compound extracted from turmeric, exhibits oxidative activity by removing ROS, helps to prevent and resolve liver damage, and has a protective effect on the liver (Yarru et al., 2009). CUN inhibits adipocyte differentiation by inhibiting PPAR-γ and increasing adenosine monophosphate-activated protein kinase, thereby reducing body fat mass and leading to increased lipolysis (Bradford, 2013). In our NMA, there was no significant difference in the impact of CUN on ALT, TC and LDL compared with that of PLB and PTC. However, SUCRA ranked CUN as performing better than the placebo group in terms of improving TC and LDL. Several previous studies have reported conflicting effects of CUN on body composition in NAFLD patients (Song et al., 2022). One study reported (Chen et al., 2021) that CUN had no significant effect on body mass index (BMI), body weight, or waist circumference (WC). Another study (Mousavi et al., 2020) showed that the use of different doses of CUN and the long duration of the trial could affect BMI and WC in patients with NAFLD. Therefore, we found that due to the difference in sample sizes of the included studies, different dosages and durations of medication resulted in heterogeneity.
PTCs are acquired symbiotic microorganisms that are beneficial to host health when consumed in sufficient quantities (Pan et al., 2022). PTC can treat NAFLD by regulating the composition of the intestinal flora and the production of antibacterial factors, changing the permeability and function of intestinal epithelial cells, modifying endotoxemia, inhibiting inflammation, and regulating the immune system. (Hecht et al., 2016). At the time of our analysis of the included studies, PTC was not significantly different from CUN and PLB, but SUCRA indicated that PTC was the best intervention for improving ALT, TC, and LDL. Adverse effects of probiotics are rare, except for changes in flatulence or bowel habits, but one study (Borriello et al., 2003) suggested that additional use should be carefully evaluated in immunocompromised or critically ill patients to limit the risk of endocarditis or sepsis. In addition, Lactobacillus is considered an emerging pathogen due to its clinically important resistance to the antibiotic vancomycin (Sponton et al., 2020). The PTCs used to treat NAFLD mainly include Lactobacillus acidophilus, Lactobacillus rhamnosus, Streptococcus paralactis, Lactobacillus pentosus, Lactobacillus lactis and Lactobacillus brevis. A new type of called “NGPs” has also been applied, including the mucobacter Akkermansia A. muciniphila, faecalis F. praussnitzii and Bacteroides B. fragilis (Sáez-Lara et al., 2016).
The mediterranean diet (MDED), a healthy eating pattern with a diet low in saturated fats and animal protein, high in antioxidants, adequate fiber, monounsaturated fatty acids, and a balance of omega-3 and omega-6 fats, generally consists of a high consumption of whole grains, fruits, legumes, vegetables, nuts, moderate amounts of dairy products, and a moderate consumption of red wine (Cheng et al., 2020). Studies have shown that MDED can reduce the development risk of NAFLD through the nutritional effects of its bioactive compounds and the antioxidant and anti-inflammatory effects of the phytochemicals it contains (Sato and Sassone-Corsi, 2022). MDED not only positively improves inflammatory biomarkers but also improves clinical parameters such as body weight, waist circumference, liver fat accumulation, blood drug level transaminase, glutamyltransferase, triglycerides, cholesterol, insulin, and insulin resistance (Hunter, 2019). Our NMA shows that MDED can significantly improve the indicators of NAFLD compared with no intervention. MDED is appropriate for individuals who are not fit for exercise and patients who have gastrointestinal intolerance to drugs.
As first-line treatment measures, health education and physical activity can reduce the level of liver enzymes, improve fatty liver, and reduce the content of triglycerides in the liver and markers of liver cell injury in patients with NAFLD, with the benefits of low cost and high return (Hackstein and Klenerman, 2020). High-intensity interval training and aerobic exercise can significantly reduce ALT, AST, triglycerides and other indicators in patients with NAFLD (Wang et al., 2019). One study showed that patients who receive dietary and exercise advice from a nutritionist and exercise physiologist, respectively, and were encouraged to follow the advice, including controlling total calorie intake, changing sedentary lifestyles, and choosing appropriate exercise for their preferences, could effectively improve their symptoms and indicators of NAFLD (Umpierre et al., 2011). In our study, patients who received health education had significantly different results than those who received no intervention, suggesting an important role for advice and intervention in the lifestyle of NAFLD patients. However, the difference in exercise mode and intensity may be a source of the greater heterogeneity.
First, there was substantial heterogeneity in this NMA due to the large number of studies included in our study, patients with different dosages and durations of medication, exercise methods, exercise times and exercise. Although we conducted subgroup analyses, the studies did not mention individual age, gender, exercise intensity, exercise time, exercise facilities and other contents, which severely limited the availability of data. Second, the drugs we included were only natural extracts and probiotics, which cannot represent the efficacy and safety of all drugs in terms of treatment. Finally, only English articles were selected, which may limit the comprehensiveness of the data.
The network meta-analysis integrated the available evidence from previous studies, summarized several measures to improve NAFLD in terms of drugs, lifestyle and diet, and provided some suggestions for clinicians to choose some treatment methods. Our study shows that probiotics are most likely to be the most effective treatment for improving NAFLD indicators. Professional advice on diet or exercise is more effective in treating NAFLD than no intervention. However, considering the possible limitations of our meta-analysis, the results should be interpreted with caution. More multigroup randomized controlled trials should be conducted in the future to provide more direct evidence of the relative efficacy of different interventions.
XW and XJ finished the data analysis. XW, KL, and XC wrote and revised the manuscript. XW and XZ conceived and designed the study. HL and CC acquired funding supports and approved the final manuscript as submitted.
This work was supported by National Natural Science Foundation of China (82004246). This study was supported by the School of Food and Pharmaceutical Engineering, Zhaoqing University.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abenavoli, L., Greco, M., Milic, N., Accattato, F., Foti, D., Gulletta, E., et al. (2017). Effect of mediterranean diet and antioxidant formulation in non-alcoholic fatty liver disease: A randomized study. Nutrients 9 (8), 870. doi:10.3390/nu9080870
Abenavoli, L., Greco, M., Nazionale, I., Peta, V., Milic, N., Accattato, F., et al. (2015). Effects of Mediterranean diet supplemented with silybin-vitamin E-phospholipid complex in overweight patients with non-alcoholic fatty liver disease. Expert Rev. gastroenterology hepatology 9 (4), 519–527. doi:10.1586/17474124.2015.1004312
Ahn, S. B., Jun, D. W., Kang, B. K., Lim, J. H., Lim, S., and Chung, M. J. (2019). Randomized, double-blind, placebo-controlled study of a multispecies probiotic mixture in nonalcoholic fatty liver disease. Sci. Rep. 9 (1), 5688. doi:10.1038/s41598-019-42059-3
Alami, F., Alizadeh, M., and Shateri, K. (2022). The effect of a fruit-rich diet on liver biomarkers, insulin resistance, and lipid profile in patients with non-alcoholic fatty liver disease: A randomized clinical trial. Scand. J. gastroenterology 57 (10), 1238–1249. doi:10.1080/00365521.2022.2071109
Asghari, S., Rezaei, M., Rafraf, M., Taghizadeh, M., Asghari-Jafarabadi, M., and Ebadi, M. (2022). Effects of calorie restricted diet on oxidative/antioxidative status biomarkers and serum fibroblast growth factor 21 levels in nonalcoholic fatty liver disease patients: A randomized, controlled clinical trial. Nutrients 14 (12), 2509. doi:10.3390/nu14122509
Blot, F., Marchix, J., Ejarque, M., Jimenez, S., Meunier, A., Keime, C., et al. (2023). Gut microbiota remodeling and intestinal adaptation to lipid malabsorption after enteroendocrine cell loss in adult mice. Cell. Mol. gastroenterology hepatology S2352-345X (23), 00032–2. doi:10.1016/j.jcmgh.2023.02.013
Borriello, S. P., Hammes, W. P., Holzapfel, W., Marteau, P., Schrezenmeir, J., Vaara, M., et al. (2003). Safety of probiotics that contain lactobacilli or bifidobacteria. Clin. Infect. Dis. 36 (6), 775–780. doi:10.1086/368080
Cai, H., Qin, Y. L., Shi, Z. Y., Chen, J. H., Zeng, M. J., Zhou, W., et al. (2019). Effects of alternate-day fasting on body weight and dyslipidaemia in patients with non-alcoholic fatty liver disease: A randomised controlled trial. BMC Gastroenterol. 19 (1), 219. doi:10.1186/s12876-019-1132-8
Campanella, A., Iacovazzi, P. A., Misciagna, G., Bonfiglio, C., Mirizzi, A., Franco, I., et al. (2020). The effect of three mediterranean diets on remnant cholesterol and non-alcoholic fatty liver disease: A secondary analysis. Nutrients 12 (6), 1674. doi:10.3390/nu12061674
Çevik Saldiran, T., Mutluay, F. K., Yağci, İ., and Yilmaz, Y. (2020). Impact of aerobic training with and without whole-body vibration training on metabolic features and quality of life in non-alcoholic fatty liver disease patients. Ann. d'endocrinologie 81 (5), 493–499. doi:10.1016/j.ando.2020.05.003
Chashmniam, S., Mirhafez, S. R., Dehabeh, M., Hariri, M., Azimi Nezhad, M., and Nobakht M Gh, B. F. (2019). A pilot study of the effect of phospholipid curcumin on serum metabolomic profile in patients with non-alcoholic fatty liver disease: A randomized, double-blind, placebo-controlled trial. Eur. J. Clin. Nutr. 73 (9), 1224–1235. doi:10.1038/s41430-018-0386-5
Chen, T., Dalton, G., Oh, S. H., Maeso-Diaz, R., Du, K., Meyers, R. A., et al. (2022). Hepatocyte smoothened activity controls susceptibility to insulin resistance and nonalcoholic fatty liver disease. Cell. Mol. gastroenterology hepatology 15 (4), 949–970. doi:10.1016/j.jcmgh.2022.12.008
Chen, Y., Wu, R., Chen, W., Liu, Y., Liao, X., Zeng, B., et al. (2021). Curcumin prevents obesity by targeting TRAF4-induced ubiquitylation in m6 A-dependent manner. EMBO Rep. 22 (5), e52146. doi:10.15252/embr.202052146
Cheng, B., Gao, W., Wu, X., Zheng, M., Yu, Y., Song, C., et al. (2020). Ginsenoside Rg2 ameliorates high-fat diet-induced metabolic disease through SIRT1. J. Agric. food Chem. 68 (14), 4215–4226. doi:10.1021/acs.jafc.0c00833
Cheng, S., Ge, J., Zhao, C., Le, S., Yang, Y., Ke, D., et al. (2017). Effect of aerobic exercise and diet on liver fat in pre-diabetic patients with non-alcoholic-fatty-liver-disease: A randomized controlled trial. Sci. Rep. 7 (1), 15952. doi:10.1038/s41598-017-16159-x
Chiurazzi, M., Cacciapuoti, N., Di Lauro, M., Nasti, G., Ceparano, M., Salomone, E., et al. (2022). The synergic effect of a nutraceutical supplementation associated to a mediterranean hypocaloric diet in a population of overweight/obese adults with NAFLD. Nutrients 14 (22), 4750. doi:10.3390/nu14224750
Chong, P. L., Laight, D., Aspinall, R. J., Higginson, A., and Cummings, M. H. (2021). A randomised placebo controlled trial of VSL#3® probiotic on biomarkers of cardiovascular risk and liver injury in non-alcoholic fatty liver disease. BMC Gastroenterol. 21 (1), 144. doi:10.1186/s12876-021-01660-5
Cicero, A. F. G., Sahebkar, A., Fogacci, F., Bove, M., Giovannini, M., and Borghi, C. (2020). Effects of phytosomal curcumin on anthropometric parameters, insulin resistance, cortisolemia and non-alcoholic fatty liver disease indices: A double-blind, placebo-controlled clinical trial. Eur. J. Nutr. 59 (2), 477–483. doi:10.1007/s00394-019-01916-7
de Oliveira, D. G., de Faria Ghetti, F., Moreira, A. P. B., Hermsdorff, H. H. M., de Oliveira, J. M., and de Castro Ferreira, L. E. V. V. (2019). Association between dietary total antioxidant capacity and hepatocellular ballooning in nonalcoholic steatohepatitis: A cross-sectional study. Eur. J. Nutr. 58 (6), 2263–2270. doi:10.1007/s00394-018-1776-0
Deguise, M. O., Pileggi, C., De Repentigny, Y., Beauvais, A., Tierney, A., Chehade, L., et al. (2021). SMN depleted mice offer a robust and rapid onset model of nonalcoholic fatty liver disease. Cell. Mol. gastroenterology hepatology 12 (1), 354–377.e3. doi:10.1016/j.jcmgh.2021.01.019
Dinu, M., Whittaker, A., Pagliai, G., Giangrandi, I., Colombini, B., Gori, A. M., et al. (2018). A khorasan wheat-based replacement diet improves risk profile of patients with nonalcoholic fatty liver disease (NAFLD): A randomized clinical trial. J. Am. Coll. Nutr. 37 (6), 508–514. doi:10.1080/07315724.2018.1445047
Dong, F., Zhang, Y., Huang, Y., Wang, Y., Zhang, G., Hu, X., et al. (2016). Long-term lifestyle interventions in middle-aged and elderly men with nonalcoholic fatty liver disease: A randomized controlled trial. Sci. Rep. 6, 36783. doi:10.1038/srep36783
Ezpeleta, M., Gabel, K., Cienfuegos, S., Kalam, F., Lin, S., Pavlou, V., et al. (2023). Effect of alternate day fasting combined with aerobic exercise on non-alcoholic fatty liver disease: A randomized controlled trial. Cell metab. 35 (1), 56–70.e3. doi:10.1016/j.cmet.2022.12.001
Gao, Y., Yao, T., Deng, Z., Sohn, J. W., Sun, J., Huang, Y., et al. (2017). TrpC5 mediates acute leptin and serotonin effects via pomc neurons. Cell Rep. 18 (3), 583–592. doi:10.1016/j.celrep.2016.12.072
George, E. S., Reddy, A., Nicoll, A. J., Ryan, M. C., Itsiopoulos, C., Abbott, G., et al. (2022). Impact of a Mediterranean diet on hepatic and metabolic outcomes in non-alcoholic fatty liver disease: The MEDINA randomised controlled trial. Liver Int. 42 (6), 1308–1322. doi:10.1111/liv.15264
Ghaffari, A., Rafraf, M., Navekar, R., Sepehri, B., Asghari-Jafarabadi, M., and Ghavami, S. M. (2019). Turmeric and chicory seed have beneficial effects on obesity markers and lipid profile in non-alcoholic fatty liver disease (NAFLD). J. Int. de vitaminologie de Nutr. 89 (5-6), 293–302. doi:10.1024/0300-9831/a000568
Ghetti, F. F., De Oliveira, D. G., De Oliveira, J. M., Ferreira, L. E. V. V. C., Cesar, D. E., and Moreira, A. P. B. (2019). Effects of dietary intervention on gut microbiota and metabolic-nutritional profile of outpatients with non-alcoholic steatohepatitis: A randomized clinical trial. J. Gastrointest. liver Dis. 28 (3), 279–287. doi:10.15403/jgld-197
Hackstein, C. P., and Klenerman, P. (2020). Swimming against the current: MAIT cell function is preserved in the peritoneum of advanced liver disease patients. Cell. Mol. gastroenterology hepatology 9 (4), 709–710. doi:10.1016/j.jcmgh.2020.02.002
Hallsworth, K., Thoma, C., Hollingsworth, K. G., Cassidy, S., Anstee, Q. M., Day, C. P., et al. (2015). Modified high-intensity interval training reduces liver fat and improves cardiac function in non-alcoholic fatty liver disease: A randomized controlled trial. Clin. Sci. 129 (12), 1097–1110. doi:10.1042/CS20150308
Hariri, M., Gholami, A., Mirhafez, S. R., Bidkhori, M., and Sahebkar, A. (2020). A pilot study of the effect of curcumin on epigenetic changes and dna damage among patients with non-alcoholic fatty liver disease: A randomized, double-blind, placebo-controlled, clinical trial. Complementary Ther. Med. 51, 102447. doi:10.1016/j.ctim.2020.102447
He, L., Yan, R., Yang, Z., Zhang, Y., Liu, X., Yang, J., et al. (2021). SCFJFK is functionally linked to obesity and metabolic syndrome. EMBO Rep. 22 (7), e52036. doi:10.15252/embr.202052036
Hecht, A. L., Casterline, B. W., Earley, Z. M., Goo, Y. A., Goodlett, D. R., and Bubeck Wardenburg, J. (2016). Strain competition restricts colonization of an enteric pathogen and prevents colitis. EMBO Rep. 17 (9), 1281–1291. doi:10.15252/embr.201642282
Higgins, J. P., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 343, d5928. doi:10.1136/bmj.d5928
Houghton, D., Thoma, C., Hallsworth, K., Cassidy, S., Hardy, T., Burt, A. D., et al. (2017). Exercise reduces liver lipids and visceral adiposity in patients with nonalcoholic steatohepatitis in a randomized controlled trial. Clin. gastroenterology hepatology 15 (1), 96–102.e3. doi:10.1016/j.cgh.2016.07.031
Hunter, P. (2019). Diet and exercise: Clinical studies and molecular biology show that diet and other lifestyle changes have significant potential for treating metabolic diseases. EMBO Rep. 20 (4), e47966. doi:10.15252/embr.201947966
Hutton, B., Salanti, G., Caldwell, D. M., Chaimani, A., Schmid, C. H., Cameron, C., et al. (2015). The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 162 (11), 777–784. doi:10.7326/M14-2385
Katsagoni, C. N., Papatheodoridis, G. V., Ioannidou, P., Deutsch, M., Alexopoulou, A., Papadopoulos, N., et al. (2018). Improvements in clinical characteristics of patients with non-alcoholic fatty liver disease, after an intervention based on the mediterranean lifestyle: A randomised controlled clinical trial. Br. J. Nutr. 120 (2), 164–175. doi:10.1017/S000711451800137X
Khodami, B., Hatami, B., Yari, Z., Alavian, S. M., Sadeghi, A., Varkaneh, H. K., et al. (2022). Effects of a low free sugar diet on the management of nonalcoholic fatty liver disease: A randomized clinical trial. Eur. J. Clin. Nutr. 76 (7), 987–994. doi:10.1038/s41430-022-01081-x
Li, L., Hou, K., Yuan, M., Zhang, Y., and Zhang, Y. (2022). Change lifestyle modification plan/transtheoretical model in non-alcoholic simple fatty liver disease: A pilot randomized study. BMC Gastroenterol. 22 (1), 483. doi:10.1186/s12876-022-02506-4
Li, Y., Chen, L., Li, L., Sottas, C., Petrillo, S. K., Lazaris, A., et al. (2021). Cholesterol-binding translocator protein TSPO regulates steatosis and bile acid synthesis in nonalcoholic fatty liver disease. iScience 24 (5), 102457. doi:10.1016/j.isci.2021.102457
Liu, C., Pan, Z., Wu, Z., Tang, K., Zhong, Y., Chen, Y., et al. (2022). Hepatic SIRT6 modulates transcriptional activities of FXR to alleviate acetaminophen-induced hepatotoxicity. Cell. Mol. gastroenterology hepatology 14 (2), 271–293. doi:10.1016/j.jcmgh.2022.04.011
Manzhalii, E., Virchenko, O., Falalyeyeva, T., Beregova, T., and Stremmel, W. (2017). Treatment efficacy of a probiotic preparation for non-alcoholic steatohepatitis: A pilot trial. J. Dig. Dis. 18 (12), 698–703. doi:10.1111/1751-2980.12561
Meijnikman, A. S., Lappa, D., Herrema, H., Aydin, O., Krautkramer, K. A., Tremaroli, V., et al. (2022). A systems biology approach to study non-alcoholic fatty liver (NAFL) in women with obesity. iScience 25 (8), 104828. doi:10.1016/j.isci.2022.104828
Mirhafez, S. R., Azimi-Nezhad, M., Dehabeh, M., Hariri, M., Naderan, R. D., Movahedi, A., et al. (2021). The effect of curcumin phytosome on the treatment of patients with non-alcoholic fatty liver disease: A double-blind, randomized, placebo-controlled trial. Adv. Exp. Med. Biol. 1308, 25–35. doi:10.1007/978-3-030-64872-5_3
Mirhafez, S. R., Farimani, A. R., Gholami, A., Hooshmand, E., Tavallaie, S., and Nobakht M Gh, B. F. (2019). The effect of curcumin with piperine supplementation on pro-oxidant and antioxidant balance in patients with non-alcoholic fatty liver disease: A randomized, double-blind, placebo-controlled trial. Drug metabolism personalized Ther. 34 (2). doi:10.1515/dmpt-2018-0040)
Misciagna, G., Del Pilar Díaz, M., Caramia, D. V., Bonfiglio, C., Franco, I., Noviello, M. R., et al. (2017). Effect of a low glycemic index mediterranean diet on non-alcoholic fatty liver disease. A randomized controlled clinici trial. J. Nutr. health aging 21 (4), 404–412. doi:10.1007/s12603-016-0809-8
Mobasheri, N., Ghahremani, L., Fallahzadeh Abarghooee, E., and Hassanzadeh, J. (2022). Lifestyle intervention for patients with nonalcoholic fatty liver disease: A randomized clinical trial based on the theory of planned behavior. BioMed Res. Int. 2022, 3465980. doi:10.1155/2022/3465980
Mohamad Nor, M. H., Ayob, N., Mokhtar, N. M., Raja Ali, R. A., Tan, G. C., Wong, Z., et al. (2021). The effect of probiotics (MCP® BCMC® strains) on hepatic steatosis, small intestinal mucosal immune function, and intestinal barrier in patients with non-alcoholic fatty liver disease. Nutrients 13 (9), 3192. doi:10.3390/nu13093192
Mousavi, S. M., Milajerdi, A., Varkaneh, H. K., Gorjipour, M. M., and Esmaillzadeh, A. (2020). The effects of curcumin supplementation on body weight, body mass index and waist circumference: A systematic review and dose-response meta-analysis of randomized controlled trials. Crit. Rev. food Sci. Nutr. 60 (1), 171–180. doi:10.1080/10408398.2018.1517724
Navekar, R., Rafraf, M., Ghaffari, A., Asghari-Jafarabadi, M., and Khoshbaten, M. (2017). Turmeric supplementation improves serum glucose indices and leptin levels in patients with nonalcoholic fatty liver diseases. J. Am. Coll. Nutr. 36 (4), 261–267. doi:10.1080/07315724.2016.1267597
Nourian, M., Askari, G., Golshiri, P., Miraghajani, M., Shokri, S., and Arab, A. (2020). Effect of lifestyle modification education based on health belief model in overweight/obese patients with non-alcoholic fatty liver disease: A parallel randomized controlled clinical trial. Clin. Nutr. 38, 236–241. doi:10.1016/j.clnesp.2020.04.004
Novelle, M. G., Bravo, S. B., Deshons, M., Iglesias, C., García-Vence, M., Annells, R., et al. (2021). Impact of liver-specific GLUT8 silencing on fructose-induced inflammation and omega oxidation. iScience 24 (2), 102071. doi:10.1016/j.isci.2021.102071
Pan, Z., Guo, J., Tang, K., Chen, Y., Gong, X., Chen, Y., et al. (2022). Ginsenoside rc modulates SIRT6-NRF2 interaction to alleviate alcoholic liver disease. J. Agric. food Chem. 70 (44), 14220–14234. doi:10.1021/acs.jafc.2c06146
Panahi, Y., Kianpour, P., Mohtashami, R., Jafari, R., Simental-Mendía, L. E., and Sahebkar, A. (2016). Curcumin lowers serum lipids and uric acid in subjects with nonalcoholic fatty liver disease: A randomized controlled trial. J. Cardiovasc. Pharmacol. 68 (3), 223–229. doi:10.1097/FJC.0000000000000406
Panahi, Y., Kianpour, P., Mohtashami, R., Jafari, R., Simental-Mendía, L. E., and Sahebkar, A. (2017). Efficacy and safety of phytosomal curcumin in non-alcoholic fatty liver disease: A randomized controlled trial. Drug Res. 67 (4), 244–251. doi:10.1055/s-0043-100019
Pugh, C. J., Cuthbertson, D. J., Sprung, V. S., Kemp, G. J., Richardson, P., Umpleby, A. M., et al. (2013). Exercise training improves cutaneous microvascular function in nonalcoholic fatty liver disease. Am. J. physiology. Endocrinol. metabolism 305 (1), E50–E58. doi:10.1152/ajpendo.00055.2013
Pugh, C. J., Spring, V. S., Kemp, G. J., Richardson, P., Shojaee-Moradie, F., Umpleby, A. M., et al. (2014). Exercise training reverses endothelial dysfunction in nonalcoholic fatty liver disease. Heart circulatory physiology 307 (9), H1298–H1306. doi:10.1152/ajpheart.00306.2014
Qiu, H., Song, E., Hu, Y., Li, T., Ku, K. C., Wang, C., et al. (2022). Hepatocyte-secreted autotaxin exacerbates nonalcoholic fatty liver disease through autocrine inhibition of the pparα/FGF21 Axis. Cell. Mol. gastroenterology hepatology 14 (5), 1003–1023. doi:10.1016/j.jcmgh.2022.07.012
Rahmani, S., Asgary, S., Askari, G., Keshvari, M., Hatamipour, M., Feizi, A., et al. (2016). Treatment of non-alcoholic fatty liver disease with curcumin: A randomized placebo-controlled trial. Phytotherapy Res. 30 (9), 1540–1548. doi:10.1002/ptr.5659
Ramos, M. J., Bandiera, L., Menolascina, F., and Fallowfield, J. A. (2021). In vitro models for non-alcoholic fatty liver disease: Emerging platforms and their applications. iScience 25 (1), 103549. doi:10.1016/j.isci.2021.103549
Saadati, S., Sadeghi, A., Mansour, A., Yari, Z., Poustchi, H., Hedayati, M., et al. (2019). Curcumin and inflammation in non-alcoholic fatty liver disease: A randomized, placebo controlled clinical trial. BMC Gastroenterol. 19 (1), 133. doi:10.1186/s12876-019-1055-4
Saberi-Karimian, M., Keshvari, M., Ghayour-Mobarhan, M., Salehizadeh, L., Rahmani, S., Behnam, B., et al. (2020). Effects of curcuminoids on inflammatory status in patients with non-alcoholic fatty liver disease: A randomized controlled trial. Complementary Ther. Med. 49, 102322. doi:10.1016/j.ctim.2020.102322
Sáez-Lara, M. J., Robles-Sanchez, C., Ruiz-Ojeda, F. J., Plaza-Diaz, J., and Gil, A. (2016). Effects of probiotics and synbiotics on obesity, insulin resistance syndrome, type 2 diabetes and non-alcoholic fatty liver disease: A review of human clinical trials. Int. J. Mol. Sci. 17 (6), 928. doi:10.3390/ijms17060928
Sakane, S., Hikita, H., Shirai, K., Myojin, Y., Sasaki, Y., Kudo, S., et al. (2021). White adipose tissue autophagy and adipose-liver crosstalk exacerbate nonalcoholic fatty liver disease in mice. Cell. Mol. gastroenterology hepatology 12 (5), 1683–1699. doi:10.1016/j.jcmgh.2021.07.008
Sato, T., and Sassone-Corsi, P. (2022). Nutrition, metabolism, and epigenetics: Pathways of circadian reprogramming. EMBO Rep. 23 (5), e52412. doi:10.15252/embr.202152412
Song, M., Chen, Z., Qiu, R., Zhi, T., Xie, W., Zhou, Y., et al. (2022). Inhibition of NLRP3-mediated crosstalk between hepatocytes and liver macrophages by geniposidic acid alleviates cholestatic liver inflammatory injury. Redox Biol. 55, 102404. doi:10.1016/j.redox.2022.102404
Sponton, C. H., Hosono, T., Taura, J., Jedrychowski, M. P., Yoneshiro, T., Wang, Q., et al. (2020). The regulation of glucose and lipid homeostasis via PLTP as a mediator of BAT-liver communication. EMBO Rep. 21 (9), e49828. doi:10.15252/embr.201949828
Su, Q., Kim, S. Y., Adewale, F., Zhou, Y., Aldler, C., Ni, M., et al. (2021). Single-cell RNA transcriptome landscape of hepatocytes and non-parenchymal cells in healthy and NAFLD mouse liver. iScience 24 (11), 103233. doi:10.1016/j.isci.2021.103233
Sun, N., Shen, C., Zhang, L., Wu, X., Yu, Y., Yang, X., et al. (2021). Hepatic Krüppel-like factor 16 (KLF16) targets PPARα to improve steatohepatitis and insulin resistance. GUT 70 (11), 2183–2195. doi:10.1136/gutjnl-2020-321774
Umpierre, D., Ribeiro, P. A., Kramer, C. K., Leitão, C. B., Zucatti, A. T., Azevedo, M. J., et al. (2011). Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: A systematic review and meta-analysis. JAMA 305 (17), 1790–1799. doi:10.1001/jama.2011.576
Wang, P., Ni, M., Tian, Y., Wang, H., Qiu, J., You, W., et al. (2021). Myeloid Nrf2 deficiency aggravates non-alcoholic steatohepatitis progression by regulating YAP-mediated NLRP3 inflammasome signaling. iScience 24 (5), 102427. doi:10.1016/j.isci.2021.102427
Wang, T., Xu, Y. Q., Yuan, Y. X., Xu, P. W., Zhang, C., Li, F., et al. (2019). Succinate induces skeletal muscle fiber remodeling via SUNCR1 signaling. EMBO Rep. 20 (9), e47892. doi:10.15252/embr.201947892
Wing-Sum Cheu, J., Lee, D., Li, Q., Goh, C. C., Hao-Ran Bao, M., Wai-Hin Yuen, V., et al. (2023). Ferroptosis suppressor protein 1 inhibition promotes tumor ferroptosis and anti-tumor immune responses in liver cancer. Cell. Mol. gastroenterology hepatology S2352 (23), 00038–43. doi:10.1016/j.jcmgh.2023.03.001
Wong, V. W., Wong, G. L., Chan, R. S., Shu, S. S., Cheung, B. H., Li, L. S., et al. (2018). Beneficial effects of lifestyle intervention in non-obese patients with non-alcoholic fatty liver disease. J. hepatology 69 (6), 1349–1356. doi:10.1016/j.jhep.2018.08.011
Yamada, T., Murata, D., Kleiner, D. E., Anders, R., Rosenberg, A. Z., Kaplan, J., et al. (2022). Prevention and regression of megamitochondria and steatosis by blocking mitochondrial fusion in the liver. iScience 25 (4), 103996. doi:10.1016/j.isci.2022.103996
Yang, H., Arif, M., Yuan, M., Li, X., Shong, K., Türkez, H., et al. (2021). A network-based approach reveals the dysregulated transcriptional regulation in non-alcoholic fatty liver disease. iScience 24 (11), 103222. doi:10.1016/j.isci.2021.103222
Yarru, L. P., Settivari, R. S., Gowda, N. K., Antoniou, E., Ledoux, D. R., and Rottinghaus, G. E. (2009). Effects of turmeric (Curcuma longa) on the expression of hepatic genes associated with biotransformation, antioxidant, and immune systems in broiler chicks fed aflatoxin. Poult. Sci. 88 (12), 2620–2627. doi:10.3382/ps.2009-00204
Yurtdaş, G., Akbulut, G., Baran, M., and Yılmaz, C. (2022). The effects of mediterranean diet on hepatic steatosis, oxidative stress, and inflammation in adolescents with non-alcoholic fatty liver disease: A randomized controlled trial. Pediatr. Obes. 17 (4), e12872. doi:10.1111/ijpo.12872
Keywords: non-alcoholic fatty liver disease, non-alcoholic fatty liver, treatment interventions, systematic review, network meta-analysis
Citation: Wang X, Jin X, Li H, Zhang X, Chen X, Lu K and Chu C (2023) Effects of various interventions on non-alcoholic fatty liver disease (NAFLD): A systematic review and network meta-analysis. Front. Pharmacol. 14:1180016. doi: 10.3389/fphar.2023.1180016
Received: 05 March 2023; Accepted: 20 March 2023;
Published: 29 March 2023.
Edited by:
Yong Gao, Guangzhou University of Chinese Medicine, ChinaReviewed by:
Zhouxi Lei, Guangzhou Baiyunshan Chenliji Pharmaceutical Co., Ltd., ChinaCopyright © 2023 Wang, Jin, Li, Zhang, Chen, Lu and Chu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chenliang Chu, MjAxNzAxMDAzMkB6cXUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.