
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol., 07 April 2023
Sec. Ethnopharmacology
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1176618
This article is part of the Research TopicHerbal Medicines for Gastrointestinal and Hepatic Diseases - Novel Pharmacological and Toxicological approaches, Volume IIView all 7 articles
 Gul Rehman Elmi1,2,3†
Gul Rehman Elmi1,2,3† Kamil Anum2†
Kamil Anum2† Kalsoom Saleem2†
Kalsoom Saleem2† Rameesha Fareed2†
Rameesha Fareed2† Sobia Noreen4†
Sobia Noreen4† Haiyan Wei1†
Haiyan Wei1† Yongxing Chen1†
Yongxing Chen1† Avirup Chakraborty5†
Avirup Chakraborty5† Masood Ur Rehman2*†
Masood Ur Rehman2*† Shi Liyuan6*†
Shi Liyuan6*† Muhammad Abbas6*†
Muhammad Abbas6*† Yongtao Duan1*†
Yongtao Duan1*†Diabetes mellitus (DM) is a widespread metabolic disorder with a yearly 6.7 million deaths worldwide. Several treatment options are available but with common side effects like weight gain, cardiovascular diseases, neurotoxicity, hepatotoxicity, and nephrotoxicity. Therefore, ethnomedicine is gaining the interest of researchers in the treatment of DM. Ethnomedicine works by preventing intestinal absorption and hepatic production of glucose as well as enhancing glucose uptake in muscles and fatty tissues and increasing insulin secretion. A variety of plants have entered clinical trials but very few have gained approval for use. This current study provides an evaluation of such clinical trials. For this purpose, an extensive literature review was performed from a database using keywords like “ethnomedicine diabetes clinical trial”, “clinical trials”, “clinical trial in diabetes”, “diabetes”, “natural products in diabetes”, “ethno-pharmacological relevance of natural products in diabetes”, etc. Clinical trials of 20 plants and natural products were evaluated based on eligibility criteria. Major limitations associated with these clinical trials were a lack of patient compliance, dose-response relationship, and an evaluation of biomarkers with a small sample size and treatment duration. Measures in terms of strict regulations can be considered to achieve quality clinical trials. A specific goal of this systematic review is to discuss DM treatment through ethnomedicine based on recent clinical trials of the past 7 years.
Diabetes mellitus (DM) is a healthcare challenge prevailing worldwide with 8.75 million individuals affected with type 1 diabetes mellitus (T1DM) alone (using the Markov Model and machine learning techniques) with 1.52 million being children less than 20 years of age. 182,000 deaths were estimated worldwide due to T1DM in 2022 even after allopathic treatment (International Diabetes Federation ATLAS Report, 2022). An overall estimate made by IDF (International Diabetes Federation) in 2021 shows that 537 million adults are living with both types of DM [T1DM and type 2 diabetes (T2DM)], and it is expected to increase to 578 million by 2030 and 700 million by 2045 (International Diabetes Federation ATLAS, 2023).
With such a huge number of individuals affected with DM and its prevalence exacerbating worldwide, a proper treatment plan is the need of the hour. This has drawn the attention of researchers toward natural product strategies, focusing on the appropriate cure for DM. This shift in focus is attributed to complications associated with DM as well as the use of allopathic medicines. For instance, prolonged uncontrolled DM leads to complications such as amputation, vision loss, renal failure, and cardiovascular diseases (Rangaswami et al., 2020; Bhatti et al., 2021; Borderie et al., 2023). According to the World Health Organization (WHO) in 2022, 1 million individuals suffer from diabetes-induced blindness. Patients suffering from DM have a threefold increased risk of stroke and renal failure (World Health Organization, 2023). Furthermore, oral hypoglycemic drugs like biguanides, thiazolidinediones, sulfonylureas, and a-glucosidase inhibitors though play a vital role in DM management but cause significant adverse effects such as hepatorenal disturbances, hypoglycemic coma, etc. (Menne et al., 2019; Joseph, 2021). Therefore, there is a rising concern in ethnomedicine (ethno means ethnicity, cultural group, or people, and ethnomedicine means the study of healing practices or medical systems utilizing traditional medicine and theoretical perspectives of culture (Erickson, 2016)) due to its safety compared to allopathic or chemical anti-diabetic agents (Tran et al., 2020). As a matter of fact, plants have always been a source of medicine and many anti-diabetic agents extracted from plants have received FDA approval, for example, metformin derived from Galega officinalis (Asif et al., 2020).
Several natural products have extensively been used in the cure of DM in the context of pre-clinical and clinical studies (Abdelmagyd et al., 2019; Lu et al., 2019; Venkatakrishnan et al., 2019; Rahman et al., 2022). Outcomes from these clinical trials provide evidence for ethnomedicine in the cure of DM. For instance, zinc and copper have been studied to regulate insulin receptors causing extended insulin activity (Bjørklund et al., 2020), and magnesium supplementation showed beneficial effects on blood pressure in T2DM patients (Asbaghi et al., 2021). Cinnamomum showed beneficial effects in the reduction of the serum levels of glucose (Namazi et al., 2019) and clove (Syzygium aromaticum) possesses an enhanced antioxidant capacity in patients with diabetes (Thomas et al., 2022). Olive oil and garlic (Allium sativum) are useful in preventing dyslipidemia in T2DM patients (Memon et al., 2018), and garlic tablets allicor is useful in maintaining plasma lipid profile in T2DM patients (Sobenin et al., 2008), and so on. Likewise, various plants with distinctive effects such as fenugreek (Trigonella foenum-graecum) used in controlling blood glucose (A. AHMAD et al., 2020), bitter melon (Momordica charantia) used in enhancing insulin sensitivity, repairing damaged pancreas islet β-cells, stimulating insulin secretion (Gao et al., 2021), and lowering blood glucose (Mahwish et al., 2021), star anise (Illcium Verum Hook. F) known for its anti-oxidant properties in DM (Dewajanthi et al., 2020) and multiple others have been studied but are not approved for clinical studies in DM.
Various clinical trials regarding the use of ethnomedicine in DM have been conducted and published. Post-prandial and fasting blood glucose levels, insulin sensitivity, HbAlc, glycemic exposure and variability, and hypo- and hyperglycemia were amongst the few parameters that were measured in these trials to assess the effects of natural products in improving the quality of life (R. Ahmad et al., 2021) and general health of the patients (Fagherazzi and Ravaud, 2019). This study is designed to collect, analyze, and document primary ethnomedicine for DM and provide an update on the current understanding of ethnopharmacological studies for DM. An elucidation of this will facilitate the identification of plant species/natural products that can be used for the effective treatment of DM patients. The clinical trials discussed in this systematic review were primarily based on studies from 2015 to 2023.
A systemic review of interventional and observational studies was conducted that highlighted the efficacy and safety facts of clinical trials conducted on ethnomedicine for diabetes. This included qualitative and quantitative studies which help in enriching the knowledge based on the impacts of ethnomedicine on DM patients and in developing possibilities for ethnomedicine in complementing clinical practices.
The eligibility criteria presented in Table 1 were developed according to the qualification standards of the PICO (Population, Intervention, Comparison, and Outcome) approach adhering to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) rules. Briefly, the current review includes only those studies that fulfilled any parameter of the eligibility criteria: plants that are believed to be anti-diabetic, assessing outcome measures related to DM such as increasing insulin sensitivity or lowering blood glucose levels among patients/participants in clinical studies, and clinical studies that have been completed (Table A1).
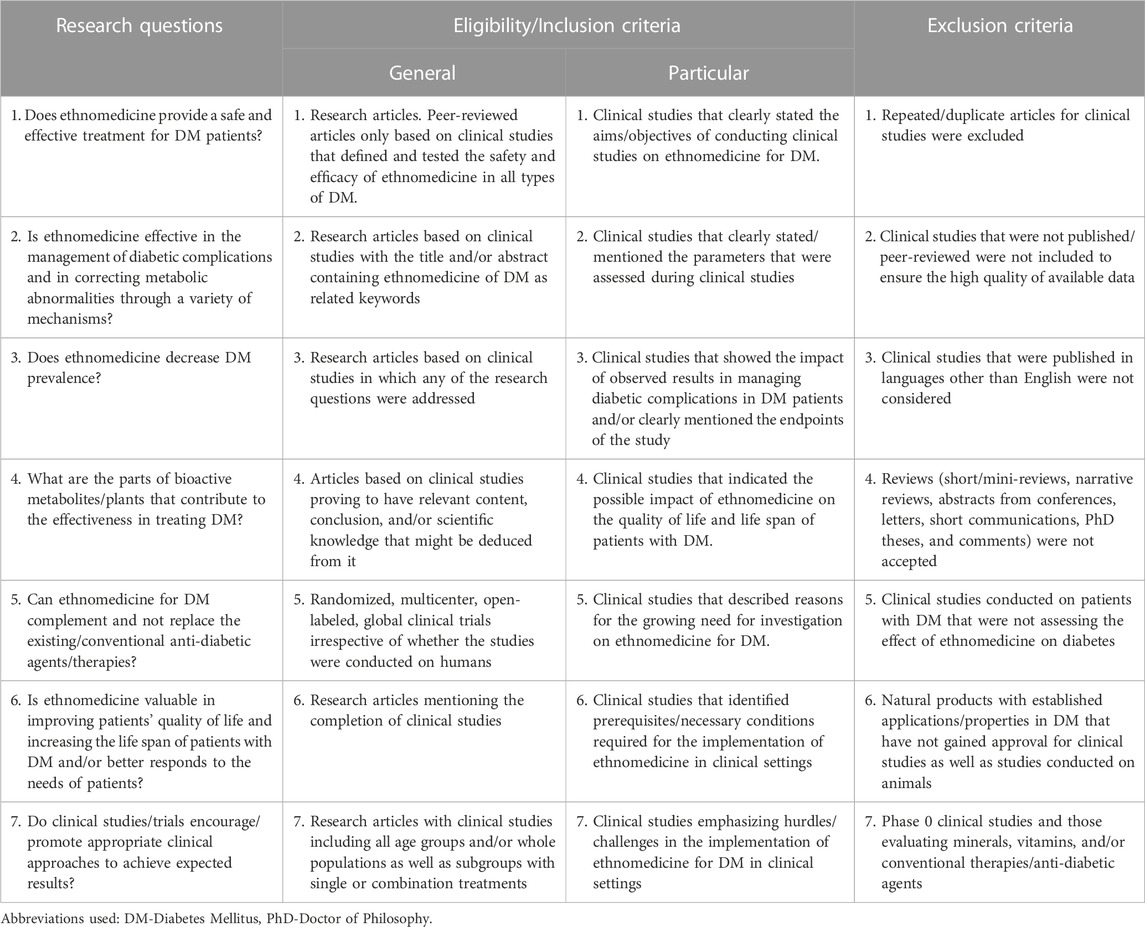
TABLE 1. Research questions and eligibility and exclusion criteria for systemic review based on clinical studies on ethnomedicines for Diabetes Mellitus.
The study population comprised all types of patients with DM (T1DM, T2DM, gestational diabetes, and juvenile diabetes) in any geographical area and animals involved in clinical studies for ethnomedicine for DM.
Clinical studies pertaining to assessing the effect/impact of ethnomedicine on different parameters in DM, evaluating the efficacy, safety, and clinical implementations and/or large-scale applications of clinical studies from 2015 to 2023.
Ethical approval was not mandatory as this review did not include any animal or human subjects.
This systemic review was written to assess whether ethnomedicines for DM have an impact on the quality of life of all types of patients with DM. To carry out this review, included clinical studies were assessed for at least one of the parameters viz: i) its provision of safe and effective treatment for patients with DM, ii) its effectiveness in the management of diabetic complications and alleviation of metabolic abnormalities via multiple mechanisms, iii) decreased DM prevalence, iv) parts of bioactive metabolites/phytoconstituents/plants contributing to the effectiveness in treating DM, v) its effectiveness in complementing conventional anti-diabetic medicines, vi) its significance in enhancing the quality of life and increasing the life span of patients with DM, and vii) its ability to promote/encourage appropriate strategies in achieving expected results.
The method used followed the PICO approach (Figure 1). The hunt for articles on clinical studies published in English was conducted through online databases (PubMed or Medline, Cochrane, ScienceDirect, and Clinicaltrials.gov). The following keywords were used and retained in query databases: ethnomedicine diabetes clinical trial, ethnomedicine diabetes randomized clinical trial, Aloe vera, diabetes clinical trial, Aloe barbadensis, Aloe chinensis, Aloe elongate, American ginseng, Panax quinquefolius, Bilberry, Vaccinium myrtillus, Cinnamon, diabetes Cinnamomum aromaticum, Fenugreek, Trigonella foenum-graecum, Garlic, Gymnema, diabetes Jambolan seeds, Bitter melon, Maitake, Neem, Nopal, Onion, Psyllium, Siberian Turmeric (Table A2).
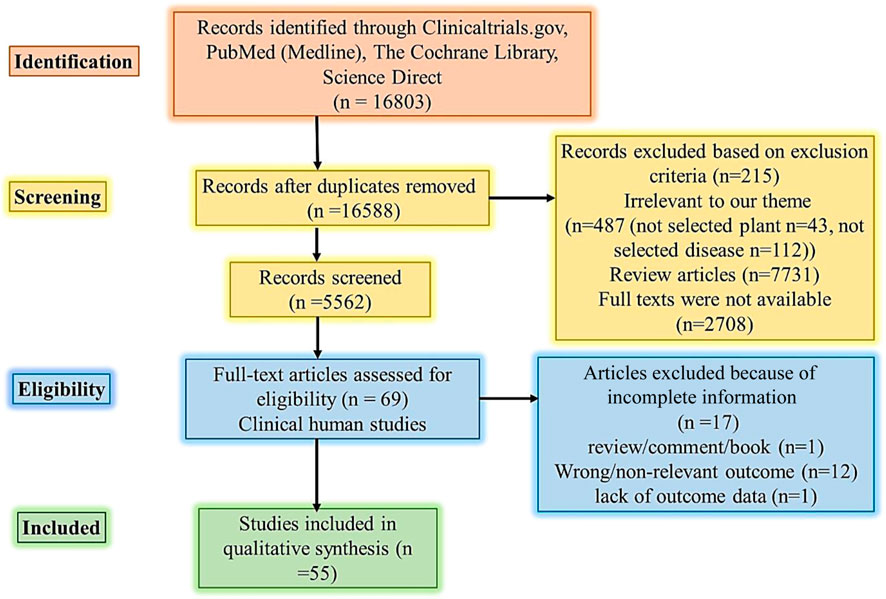
FIGURE 1. PRISMA flow diagram literature search and selection. Abbreviations: PICO Population, Intervention, Comparison, and Outcome.
The selection and addition of research articles were done stepwise. Firstly, titles and abstracts were scrutinized for eligibility to avoid the addition of duplicate articles. Later, the full text of articles that were found relevant after scrutiny were retrieved and checked against the checklist/eligibility criteria (Table 1). Information mined was the year of study, country/continent/geographical area, the year of publication, clinical study design, intervention, aims of studies, and outcome measures. Data mining was done by two authors (GRE and KS).
Two authors (KS and GRE) executed a methodological quality assessment based on the ROB (Cochrane Collaboration Risk of Bias) tool and the Newcastle Ottawa scale (NOS) for clinical studies. Negative bias impacts on clinical studies, and to prevent this, methodologies were placed within the research. Quality assessment relates to the inclusion of methodological quality within research, while ROB relates to the implication of such methodology for study results (Furuya-Kanamori et al., 2021). The ROB tools comprise seven domains, i.e., i) sequence creation, ii) allocation concealment, iii) blinding, iv) imperfect outcome measure data, v) selective outcome reporting, and vi) other sources of bias. The NOS comprises i) the basis of selection of clinical study, ii) comparability of groups, iii) ascertainment of exposure, and iv) evaluation of outcome measures. The quality of this study was rated with scores from zero (lowest score/grade) to nine (highest score/grade) and those studies with the lowest/low operational quality were excluded in order to avoid misleading outcomes (Côté et al., 2020).
DM is a life-threatening disease throughout the world caused by metabolic disorders mainly due to high blood glucose because of insulin inadequate synthesis by the pancreas or insufficient insulin activity. In addition to high blood glucose levels, the abnormal insulin level also alters lipid metabolism thereby raising the risk of vascular diseases (Nugroho et al., 2022). The major types of DM are T1DM, T2DM, and gestational diabetes. Being a global threat, 463 million people suffered from DM in 2019, that was 9.3%, and the prevalence can reach 10.2% in 2030% and 10.9% in 2045. This is because the prevalence of the prediabetes stage is 20.2% globally, which can lead to numerous diabetic complications involving neuropathy, nephropathy, and retinopathy (Fareed et al., 2017; Saeedi et al., 2019).
Several chemical drugs presently utilized as first-line treatment options that are administered orally to control the blood glucose level include thiazolidinediones (pioglitazone), biguanide (metformin), sodium-glucose cotransporter inhibitor (empagliflozin), GLP-1 agonists (exenatide, dulaglutide), dipeptidyl peptidase 4 inhibitors (sitagliptin, vildagliptin), meglitinides (nateglinide and repaglinide), and sulfonylureas (glipizide, glyburide) (Chaudhury et al., 2017; Kalsi et al., 2017). These drugs are linked with several side effects like weight gain, hepatotoxicity, nephrotoxicity, and risk of cardiovascular illnesses (But et al., 2014; Krass et al., 2017). Therefore, plant-based therapy is recommended as an alternative treatment option due to comparable safety and sustainability. Over 1,200 plants are used for the traditional treatment of diabetes and are preferred to chemical agents due to their lesser side effects and economic burden (Asgary et al., 2016; Ullah et al., 2019). Flavonoids, alkaloids, phenolics, and tannins are the most common active ingredients in plants used for diabetes treatment (Mamun et al., 2014). Hypoglycemic plants work through several mechanisms including the prevention of glucose absorption and production in intestines and liver cells, respectively, enhancing glucose absorption into muscle and fat tissues and increasing insulin secretion (Hegazy et al., 2013).
Literature reviews showed that the majority of plants have proven hypoglycemic effects in animal models. Afterward, their clinical trials had also been conducted. We have compiled the recent clinical trials of plants reported in the last 7 years from 2015 to 2023. During clinical trials, the extracts of antidiabetic plants were chiefly administered orally in combination with normal lifestyle modifications and/or other antidiabetic and anti-hyperlipidemic drugs for a specified treatment period compared to the placebo-controlled groups. The patient’s lifestyle and other parameters remained constant during the treatment period. The main parameters evaluated for hypoglycemic efficacy in all clinical trials were HbA1c and fasting blood and 2 hours postprandial blood sugar. The risk parameters for cardiovascular complications were serum lipids like cholesterol and triglyceride, and biomarkers of vascular inflammation, endothelial function, and oxidative stress. As shown in Tables 2, 3, plants had considerable hypoglycemic efficacy in clinical trials with safety and minimized risk of cardiovascular complications via hypoglycemic, hypolipidemic, and antioxidant properties.
Despite the good pharmacological effects of plants, several limitations were observed in clinical trials in terms of the heterogeneity of the study population, clinical protocols, and design and outcome evaluation parameters. Regarding the small sample size, an important consideration was that the number of patients initiating treatment was higher than those completing treatment which may be attributable to compliance and financial issues. The recruitment of healthy people for a clinical trial always remains a difficult task for researchers. Furthermore, the short study duration was a common limitation in all clinical trials (Zarrintan et al., 2016; Ranasinghe et al., 2017a; Asadi et al., 2019; Soltanian and Janghorbani, 2019; Gaytán Martínez et al., 2021). However, population size and duration of treatment must be considered because a larger study population with a longer treatment duration will depict more reliable safety and efficacy results. Another important limitation was the burden of polypharmacy and compliance of patients that were affected due to large size dosage form, i.e., capsule or tablet, with frequent dosing each day (Talaei et al., 2017; Tavakoly et al., 2018; Jovanovski et al., 2021). Moreover, clinical trials did not portray the dose-response relationship in patients, instead, even phase 1 clinical trials were based on single-dose utilization that cannot depict a complete safety profile (Sengsuk et al., 2016; Asadi et al., 2019; Jovanovski et al., 2021). The inclusion criteria were a crucial reason why some clinical trials failed to produce effective glycemic control. The inclusion criteria must specify that patients with diabetes with a history of a number of years with increased HbA1c levels can enter the study and/or set the exclusion criteria for patients with diabetes with high HbA1c levels or having diabetes for a long period of time (Zarrintan et al., 2016; Zarvandi et al., 2017). A lack of control groups was observed in some clinical trials which must not be ignored before drawing conclusions (Banerji and Banerjee, 2016; Ranasinghe et al., 2017b). Also, none of the clinical trials addressed if there was a difference between male and female patients with diabetes. Most of the population in the studies were women which may be due to a low willingness of men to participate in the study.
Evaluating biomarkers for therapeutic and toxicological effects can support positive outcomes of the clinical trials being conducted. Most importantly, before initiating a clinical trial, the quantity and quality of plant phytochemicals must be verified to prevent differences due to geographical origins and environmental factors.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
This project received funding from Henan Provincial Key Laboratory of Pediatric Hematology, Children’s Genetics and Metabolic Diseases, Children’s Hospital Affiliated to Zhengzhou University, Zhengzhou University, Zhengzhou, Henan, China.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdelmagyd, H. A. E., Shetty, S. R., and Al-Ahmari, M. M. M. (2019). Herbal medicine as adjunct in periodontal therapies-A review of clinical trials in past decade. J. Oral Biol. Craniofacial Res. 9 (3), 212–217. doi:10.1016/j.jobcr.2019.05.001
Adab, Z., Eghtesadi, S., Vafa, M. R., Heydari, I., Shojaii, A., Haqqani, H., et al. (2019). Effect of turmeric on glycemic status, lipid profile, hs-CRP, and total antioxidant capacity in hyperlipidemic type 2 diabetes mellitus patients. Phytotherapy Res. 33 (4), 1173–1181. doi:10.1002/ptr.6312
Ahmad, A., Amir, R. M., Ameer, K., Ali, S. W., Siddique, F., Hayat, I., et al. (2020). Ameliorative effects of fenugreek (Trigonella foenum-graecum) seed on type 2 diabetes. Food Sci. Technol. 41 (5), 349–354.
Ahmad, R., AlLehaibi, L. H., AlSuwaidan, H. N., Alghiryafi, A. F., Almubarak, L. S., AlKhalifah, K. N., et al. (2021). Evaluation of clinical trials for natural products used in diabetes: An evidence-based systemic literature review. Medicine 100 (16), 25641–25654. doi:10.1097/md.0000000000025641
Asadi, S., Gholami, M. S., Siassi, F., Qorbani, M., Khamoshian, K., and Sotoudeh, G. (2019). Nano curcumin supplementation reduced the severity of diabetic sensorimotor polyneuropathy in patients with type 2 diabetes mellitus: A randomized double-blind placebo-controlled clinical trial. Complementary Ther. Med. 43 (1), 253–260. doi:10.1016/j.ctim.2019.02.014
Asbaghi, O., Hosseini, R., Boozari, B., Ghaedi, E., Kashkooli, S., and Moradi, S. (2021). The effects of magnesium supplementation on blood pressure and obesity measure among type 2 diabetes patient: A systematic review and meta-analysis of randomized controlled trials. Biol. Trace Elem. Res. 199 (2), 413–424. doi:10.1007/s12011-020-02157-0
Asgary, S., RafieianKopaei, M., Sahebkar, A., Shamsi, F., and Goli-malekabadi, N. (2016). Anti-hyperglycemic and anti-hyperlipidemic effects of Vaccinium myrtillus fruit in experimentally induced diabetes (antidiabetic effect of Vaccinium myrtillus fruit). J. Sci. Food Agric. 96 (3), 764–768. doi:10.1002/jsfa.7144
Asif, M., Acharya, M., and Imran, M. (2020). Metformin: A review on its ethnobotanical source and versatile uses. Egypt. Pharm. J. 19 (2), 81–93. doi:10.4103/epj.epj_50_19
Atkin, M., Laight, D., and Cummings, M. H. (2016). The effects of garlic extract upon endothelial function, vascular inflammation, oxidative stress and insulin resistance in adults with type 2 diabetes at high cardiovascular risk. A pilot double blind randomized placebo controlled trial. J. Diabetes its Complicat. 30 (4), 723–727. doi:10.1016/j.jdiacomp.2016.01.003
Banerji, S., and Banerjee, S. (2016). A formulation of grape seed, Indian gooseberry, turmeric and fenugreek helps controlling type 2 diabetes mellitus in advanced-stage patients. Eur. J. Integr. Med. 8 (5), 645–653. doi:10.1016/j.eujim.2016.06.012
Bhatti, M. I., Memon, A. R., Laghari, M. J., Memon, R. A., Laghari, S., Memon, F. R., et al. (2021). Role of glucose 6 phosphate dehydrogenase (G6PD) in diabetic cataract. J. Pharm. Res. Int. 33 (60), 1646–1651. doi:10.9734/jpri/2021/v33i60b34789
Bjørklund, G., Dadar, M., Pivina, L., Doşa, M. D., Semenova, Y., and Aaseth, J. (2020). The role of zinc and copper in insulin resistance and diabetes mellitus. Curr. Med. Chem. 27 (39), 6643–6657. doi:10.2174/0929867326666190902122155
Borderie, G., Foussard, N., Larroumet, A., Blanco, L., Domenge, F., Mohammedi, K., et al. (2023). Albuminuric diabetic kidney disease predicts foot ulcers in type 2 diabetes. J. Diabetes its Complicat. 37 (2), 108403–108415. doi:10.1016/j.jdiacomp.2023.108403
But, A., Wang, H., Männistö, S., Pukkala, E., and Haukka, J. (2014). Assessing the effect of treatment duration on the association between anti-diabetic medication and cancer risk. PLoS One 9 (11), 113162–113174. doi:10.1371/journal.pone.0113162
Chaudhury, A., Duvoor, C., Reddy Dendi, V. S., Kraleti, S., Chada, A., Ravilla, R., et al. (2017). Clinical review of antidiabetic drugs: Implications for type 2 diabetes mellitus management. Front. Endocrinol. 8 (1), 6–14. doi:10.3389/fendo.2017.00006
Clarita, C., and Fidelia, A. (2020). Hypoglicemic and antioxidant activities in extracts star anise (illcium Verum Hook.f) on wistar rat (Rattus norvegicus) diabetes, on wistar rat (Rattus norvegicus) diabetes. Indonesian J. Biotechnol. Biodivers. 4 (1), 34–43. doi:10.47007/ijobb.v4i1.53
Cortez, N., MarisolMartinez Abundis, E., Perez Rubio, K. G., Gonzalez-Ortiz, M., and Méndez-del Villar, M. (2018). Momordica charantia administration improves insulin secretion in type 2 diabetes mellitus. J. Med. Food 21 (7), 672–677. doi:10.1089/jmf.2017.0114
Côté, A., Beogo, I., Abasse, K. S., Laberge, M., Dogba, M. J., and Dallaire, C. (2020). The clinical microsystems approach: Does it really work? A systematic review of organizational theories of health care practices. J. Am. Pharm. Assoc. 60 (6), e388–e410. doi:10.1016/j.japh.2020.06.013
DeMello, V. D., Lankinen, M. A., Lindström, J., Puupponen-Pimiä, R., Laaksonen, D. E., Pihlajamäki, J., et al. (2017). Fasting serum hippuric acid is elevated after bilberry (Vaccinium myrtillus) consumption and associates with improvement of fasting glucose levels and insulin secretion in persons at high risk of developing type 2 diabetes. Mol. Nutr. Food Res. 61 (9), 1700019–1700028. doi:10.1002/mnfr.201700019
Dewajanthi, A. M., Limanto, A., Clarita, C., and Fidelia, A. (2020). Hypoglicemic and antioxidant activities in extracts star anise. Illcium Verum Hook.
Erickson, P. I. (2016). The healing lessons of ethnomedicine, 188. Understanding Applying Med Anthropol.
Fagherazzi, G., and Ravaud, P. (2019). Digital diabetes: Perspectives for diabetes prevention, management and research. Diabetes and Metabolism 45 (4), 322–329. doi:10.1016/j.diabet.2018.08.012
Fareed, M., Salam, N., Khoja, A. T., Mahmoud, M. A., and Ahamed, M. (2017). Life style related risk factors of type 2 diabetes mellitus and its increased prevalence in Saudi arabia: A brief review. Int. J. Med. Res. Health Sci. 6 (3), 125–132.
Furuya-Kanamori, L., Xu, C., Hasan, S. S., and Doi, S. A. (2021). Quality versus Risk-of-Bias assessment in clinical research. J. Clin. Epidemiol. 129 (4), 172–175. doi:10.1016/j.jclinepi.2020.09.044
Gao, Y., Li, X., Huang, Y., Chen, J., and Qiu, M. (2021). Bitter melon and diabetes mellitus. Food Rev. Int. 2 (1), 1–21. doi:10.1080/87559129.2021.1923733
Gaytán Martínez, L. A., Sánchez-Ruiz, L. A., Zuñiga, L. Y., González-Ortiz, M., and Martínez-Abundis, E. (2021). Effect of Gymnema sylvestre administration on glycemic control, insulin secretion, and insulin sensitivity in patients with impaired glucose tolerance. J. Med. Food 24 (1), 28–32. doi:10.1089/jmf.2020.0024
Gaytán, M., Luis, A., Sánchez-Ruiz, L. A., Zuñiga, L. Y., González-Ortiz, M., and Martínez-Abundis, E. (2021). Effect of Gymnema sylvestre administration on glycemic control, insulin secretion, and insulin sensitivity in patients with impaired glucose tolerance. J. Med. food 24 (1), 28–32. doi:10.1089/jmf.2020.0024
Ghafarzadegan, R., Javaheri, J., Asgari, M., Golitaleb, M., Maraki, F., Ghafarzadegan, R., et al. (2021). The effect of combined herbal capsule on glycemic indices and lipid profile in patients with type 2 diabetes mellitus: A randomized controlled clinical trial. Jundishapur J. Nat. Pharm. Prod. 16 (4), 1–12. doi:10.5812/jjnpp.109488
Hegazy, G. A., Alnoury, A. M., and Gad, H. G. (2013). The role of Acacia Arabica extract as an antidiabetic, antihyperlipidemic, and antioxidant in streptozotocin-induced diabetic rats. Saudi Med. J. 34 (7), 727–733.
International Diabetes Federation ATLAS. (2023). IDF diabetes atlas Retrieved from Available at: https://diabetesatlas.org/
International Diabetes Federation ATLAS Report (2022). IDF diabetes atlas. Retrieved from Available at: https://diabetesatlas.org/idfawp/resource-files/2022/12/IDF-T1D-Index-Report.pdf.
Jenkins, A. L., Morgan, L. M., Bishop, J., Jovanovski, E., Jenkins, D. J., and Vuksan, V. (2018). Co-Administration of a konjac-based fibre blend and American ginseng (Panax quinquefolius L.) on glycaemic control and serum lipids in type 2 diabetes: A randomized controlled, cross-over clinical trial. Eur. J. Nutr. 57 (2), 2217–2225. doi:10.1007/s00394-017-1496-x
Joseph, C. M. (2021). Symptomatic hypoglycemia during treatment with a therapeutic dose of metformin. Am. J. Case Rep. 22 (3), 931311–931321. doi:10.12659/ajcr.931311
Jovanovski, E., Smircic-Duvnjak, L., Komishon, A., Au-Yeung, F. R., Sievenpiper, J. L., Zurbau, A., et al. (2021). Effect of coadministration of enriched Korean red ginseng (Panax ginseng) and American ginseng (Panax quinquefolius L) on cardiometabolic outcomes in type-2 diabetes: A randomized controlled trial. J. Ginseng Res. 45 (5), 546–554. doi:10.1016/j.jgr.2019.11.005
Kalsi, A., Singh, S., Taneja, N., Kukal, S., and Mani, S. (2017). Current treatments for Type 2 diabetes, their side effects and possible complementary treatments. Int. J. 10 (3), 13–18.
Krass, I., Carter, R., Mitchell, B., Mohebbi, M., Shih, S. T., Trinder, P., et al. (2017). Pharmacy diabetes screening trial: Protocol for a pragmatic cluster-randomised controlled trial to compare three screening methods for undiagnosed type 2 diabetes in Australian community pharmacy. BMJ Open 7 (12), e017725–e017732. doi:10.1136/bmjopen-2017-017725
Lu, Z., Zhong, Y., Liu, W., Xiang, L., and Deng, Y. (2019). The efficacy and mechanism of Chinese herbal medicine on diabetic kidney disease. J. Diabetes Res. 2 (1), 1–14. doi:10.1155/2019/2697672
MahwishSaeed, F., Sultan, M. T., Riaz, A., Ahmed, S., Bigiu, N., Amarowicz, R., et al. (2021). Bitter melon (momordica charantia L.) fruit bioactives charantin and vicine potential for diabetes prophylaxis and treatment. Plants 10 (4), 730–740. doi:10.3390/plants10040730
Mamun, o.-R., Anmhossain, M. S., Hassan, N., Dash, B. K., Sapon, M. A., and Sen, M. K. (2014). A review on medicinal plants with antidiabetic activity. J. Pharmacogn. Phytochemistry 3 (4), 149–159.
Memon, A. R., Ghanghro, A. B., Shaikh, I. A., Qazi, N., Ghanghro, I. H., and Shaikh, U. (2018). Effects of olive oil and garlic on serum cholesterol and triglycerides levels in the patients of type–II diabetes mellitus. J. Liaquat Univ. Med. Health Sci. 17 (02), 101–105.
Menne, J., Dumann, E., Haller, H., and Schmidt, B. M. (2019). Acute kidney injury and adverse renal events in patients receiving SGLT2-inhibitors: A systematic review and meta-analysis. PLoS Med. 16 (12), 1002983–1002994. doi:10.1371/journal.pmed.1002983
Namazi, N., Khodamoradi, K., Khamechi, S. P., Heshmati, J., Ayati, M. H., and Larijani, B. (2019). The impact of cinnamon on anthropometric indices and glycemic status in patients with type 2 diabetes: A systematic review and meta-analysis of clinical trials. Complementary Ther. Med. 43 (4), 92–101. doi:10.1016/j.ctim.2019.01.002
Noureddin, S., Mohsen, J., and Payman, A. (2018). Effects of psyllium vs. placebo on constipation, weight, glycemia, and lipids: A randomized trial in patients with type 2 diabetes and chronic constipation. Complementary Ther. Med. 40 (1), 1–7. doi:10.1016/j.ctim.2018.07.004
Nugroho, G. A., Wediasari, F., Fadhilah, Z., Elya, B., Setiawan, H., and Elfahmi E, E. (2022). Potency of antidiabetic effects of the combination of syzygium cumini and andrographis paniculata in rats with high-fat dietand streptozotocin-induced diabetes. Pharmacogn. J. 14 (2), 406–412. doi:10.5530/pj.2022.14.52
Rahman, M. M., Islam, M. R., Shohag, S., Hossain, M. E., Rahaman, M. S., Islam, F., et al. (2022). The multifunctional role of herbal products in the management of diabetes and obesity: A comprehensive review. Molecules 27 (5), 1713–1727. doi:10.3390/molecules27051713
Ranasinghe, P., Jayawardena, R., Pigera, S., Wathurapatha, W. S., Weeratunga, H. D., Premakumara, G. S., et al. (2017a). Evaluation of pharmacodynamic properties and safety of Cinnamomum zeylanicum (ceylon cinnamon) in healthy adults: a phase I clinical trial. BMC Complementary Altern. Med. 17 (1), 550–559. doi:10.1186/s12906-017-2067-7
Ranasinghe, P., Jayawardena, R., Pigera, S., Wathurapatha, W. S., Weeratunga, H. D., Premakumara, G. S., et al. (2017b). Evaluation of pharmacodynamic properties and safety of Cinnamomum zeylanicum (ceylon cinnamon) in healthy adults: a phase I clinical trial. BMC Complementary Altern. Med. 17 (3), 550–559. doi:10.1186/s12906-017-2067-7
Rangaswami, J., Bhalla, V., de Boer, I. H., Staruschenko, A., Sharp, J. A., Singh, R. R., et al. (2020). Cardiorenal protection with the newer antidiabetic agents in patients with diabetes and chronic kidney disease: A scientific statement from the American heart association. Circulation 142 (17), 265–286. doi:10.1161/CIR.0000000000000920
Saeedi, P., Petersohn, I., Salpea, P., Malanda, B., Karuranga, S., Unwin, N., et al. (2019). Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the international diabetes federation diabetes atlas, 9th edition. Diabetes Res. Clin. Pract. 157 (1), 107843–107856. doi:10.1016/j.diabres.2019.107843
Sengsuk, C., Sanguanwong, S., Tangvarasittichai, O., and Tangvarasittichai, S. (2016). Effect of cinnamon supplementation on glucose, lipids levels, glomerular filtration rate, and blood pressure of subjects with type 2 diabetes mellitus. Diabetol. Int. 7 (1), 124–132. doi:10.1007/s13340-015-0218-y
Shoaib, M., Bhargava, M., Devpura, G., and Fiaz, S. (2022). A study assessing efficacy of aloe vera as an adjuvant to conventional therapy in patients of type 2 diabetes mellitus with hyperlipidaemia. Int. J. Food Nutr. Sci. 11 (1), 1–9.
Sobenin, I. A., Nedosugova, L. V., Filatova, L. V., Balabolkin, M. I., Gorchakova, T. V., and Orekhov, A. N. (2008). Metabolic effects of time-released garlic powder tablets in type 2 diabetes mellitus: The results of double-blinded placebo-controlled study. Acta Diabetol. 45 (3), 1–6. doi:10.1007/s00592-007-0011-x
Soltanian, N., and Janghorbani, M. (2019). Effect of flaxseed or psyllium vs. placebo on management of constipation, weight, glycemia, and lipids: A randomized trial in constipated patients with type 2 diabetes. Clin. Nutr. ESPEN 29 (2), 41–48. doi:10.1016/j.clnesp.2018.11.002
Talaei, B., Amouzegar, A., Sahranavard, S., Hedayati, M., Mirmiran, P., and Azizi, F. (2017). Effects of cinnamon consumption on glycemic indicators, advanced glycation end products, and antioxidant status in type 2 diabetic patients. Nutrients 9 (9), 991–1002. doi:10.3390/nu9090991
Tanzidi, R., OmidJafari, F., AkbariRad, M., Asili, J., and Elyasi, S. (2023). Evaluation of a new herbal formulation (Viabet®) efficacy in patients with type 2 diabetes as an adjuvant to metformin: A randomized, triple-blind, placebo-controlled clinical trial. J. Herb. Med. 37 (1), 100617–100626. doi:10.1016/j.hermed.2022.100617
Tavakoly, R., Maracy, M. R., Karimifar, M., and Entezari, M. H. (2018). Does fenugreek (Trigonella foenum-graecum) seed improve inflammation, and oxidative stress in patients with type 2 diabetes mellitus? A parallel group randomized clinical trial. Eur. J. Integr. Med. 18 (1), 13–17. doi:10.1016/j.eujim.2018.01.005
Thomas, J., Patel, A., Sivadasan, S. D., Sreevallabhan, S., Madhavamenon, K. I., and Mohanan, R. (2022). Clove bud (syzygium aromaticum L.) polyphenol helps to mitigate metabolic syndrome by establishing intracellular redox homeostasis and glucose metabolism: A randomized, double-blinded, active-controlled comparative study. J. Funct. Foods 98 (3), 105273–105283. doi:10.1016/j.jff.2022.105273
Tran, N., Pham, B., and Le, L. (2020). Bioactive compounds in anti-diabetic plants: From herbal medicine to modern drug discovery. Biology 9 (9), 252–263. doi:10.3390/biology9090252
Ullah, M., Mehmood, S., Ali, M., Bussmann, R. W., Aldosari, A., Khan, R. A., et al. (2019). An ethnopharmacological study of plants used for treatment of diabetes in the Southern and Tribal regions of Khyber Pakhtunkhwa province, Pakistan. Ethnobot. Res. Appl. 18 (1), 1–20. doi:10.32859/era.18.8.1-20
Venkatakrishnan, K., Chiu, H.-F., and Wang, C.-K. (2019). Popular functional foods and herbs for the management of type-2-diabetes mellitus: A comprehensive review with special reference to clinical trials and its proposed mechanism. J. Funct. Foods 57 (3), 425–438. doi:10.1016/j.jff.2019.04.039
Verma, N., Usman, K., Patel, N., Jain, A., Dhakre, S., Swaroop, A., et al. (2016). A multicenter clinical study to determine the efficacy of a novel fenugreek seed (Trigonella foenum-graecum) extract (Fenfuro™) in patients with type 2 diabetes. Food and Nutr. Res. 60 (1), 32382–32395. doi:10.3402/fnr.v60.32382
Vuksan, V., Xu, Z. Z., Jovanovski, E., Jenkins, A. L., Beljan-Zdravkovic, U., Sievenpiper, J. L., et al. (2019). Efficacy and safety of American ginseng (Panax quinquefolius L.) extract on glycemic control and cardiovascular risk factors in individuals with type 2 diabetes: A double-blind, randomized, cross-over clinical trial. Eur. J. Nutr. 58 (2), 1237–1245. doi:10.1007/s00394-018-1642-0
World Health Organization (2023). Diabetes. Retrieved from https://www.who.int/news-room/fact-sheets/detail/diabetes.
Zarezadeh, M., Saedisomeolia, A., Khorshidi, M., Varkane, H. K., Arzati, M. M., Abdollahi, M., et al. (2019). Asymmetric dimethylarginine and soluble inter-cellular adhesion molecule-1 serum levels alteration following ginger supplementation in patients with type 2 diabetes: A randomized double-blind, placebo-controlled clinical trial. J. Complementary Integr. Med. 16 (2), 1–14. doi:10.1515/jcim-2018-0019
Zarrintan, A., Mobasseri, M., Zarrintan, A., and Ostadrahimi, A. (2016). Effects of Aloe vera supplements on blood glucose level and lipid profile markers in type 2 diabetic patients–a randomized clinical trial. Pharm. Sci. 21 (2), 65–71. doi:10.15171/PS.2015.19
Zarvandi, M., Rakhshandeh, H., Abazari, M., Shafiee-Nick, R., and Ghorbani, A. (2017). Safety and efficacy of a polyherbal formulation for the management of dyslipidemia and hyperglycemia in patients with advanced-stage of type-2 diabetes. Biomed. Pharmacother. 89 (6), 69–75. doi:10.1016/j.biopha.2017.02.016
Keywords: ethnomedicine, diabetes, treatment, clinical trail, outcome, systematic review
Citation: Elmi GR, Anum K, Saleem K, Fareed R, Noreen S, Wei H, Chen Y, Chakraborty A, Rehman MU, Liyuan S, Abbas M and Duan Y (2023) Evaluation of clinical trials of ethnomedicine used for the treatment of diabetes: A systematic review. Front. Pharmacol. 14:1176618. doi: 10.3389/fphar.2023.1176618
Received: 28 February 2023; Accepted: 16 March 2023;
Published: 07 April 2023.
Edited by:
Muhammad Hasnat, University of Veterinary and Animal Sciences, PakistanReviewed by:
Muhammad Amjad Chaudhary, Pakistan Institute of Medical Sciences (PIMS), PakistanCopyright © 2023 Elmi, Anum, Saleem, Fareed, Noreen, Wei, Chen, Chakraborty, Rehman, Liyuan, Abbas and Duan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Masood Ur Rehman, TWFzb29kLnJlaG1hbkByaXBoYWguZWR1LnBr Shi Liyuan, c2hpX2xpeXVuQG5qdWNtLmVkdS5jbg==; Muhammad Abbas, dGFub2xpYWJiYXM3QHlhaG9vLmNvbQ==; Yongtao Duan, ZHVhbnlvbmd0YW84NjA0MDlAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.