
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 12 May 2023
Sec. Cardiovascular and Smooth Muscle Pharmacology
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1170039
This article is part of the Research Topic Pharmacoepidemiology and pharmacovigilance post-marketing drug safety studies View all 17 articles
Objective: This study aimed to identify the different associations between antiarrhythmic drugs (AADs) and arrhythmias, and to determine whether pharmacokinetic drug interactions involving AADs increase the risk of AAD-related arrhythmias compared to using AADs alone.
Materials and methods: The disproportionality analysis of AAD-associated cardiac arrhythmias, including AAD monotherapies and concomitant use of pharmacokinetic interacting agents involving AADs, was conducted by using reporting odds ratio (ROR) and information component (IC) as detection of potential safety signals based on FAERS data from January 2016 to June 2022. We compared the clinical features of patients reported with AAD–associated arrhythmias between fatal and non-fatal groups, and further investigated the onset time (TTO) following different AAD regimens.
Results: A total of 11754 AAD–associated cardiac arrhythmias reports were identified, which was more likely to occur in the elderly (52.17%). Significant signals were detected between cardiac arrhythmia and all AAD monotherapies, with ROR ranging from 4.86 with mexiletine to 11.07 with flecainide. Regarding four specific arrhythmias in High Level Term (HLT) level, the AAD monotherapies with the highest ROR were flecainide in cardiac conduction disorders (ROR025 = 21.18), propafenone in rate and rhythm disorders (ROR025 = 10.36), dofetilide in supraventricular arrhythmias (ROR025 = 17.61), and ibutilide in ventricular arrhythmias (ROR025 = 4.91). Dofetilide/ibutilide, ibutilide, mexiletine/ibutilide and dronedarone presented no signal in the above four specific arrhythmias respectively. Compared with amiodarone monotherapy, sofosbuvir plus amiodarone detected the most significantly increased ROR in arrhythmias.
Conclusion: The investigation showed the spectrum and risk of AAD–associated cardiac arrhythmias varied among different AAD therapies. The early identification and management of AAD-associated arrhythmias are of great importance in clinical practice.
Antiarrhythmic drugs (AADs) are prescribed to treat symptomatic or life-threatening arrhythmias, such as supraventricular arrhythmias and ventricular arrhythmias (Al-Khatib et al., 2018; January et al., 2019; Viskin et al., 2019; Andrade et al., 2022). Although most AADs used for treating arrhythmia have been available for decades, there is still a significant knowledge gaps in their comparative safety.
The proarrhythmic effect of AADs is a significant concern in using them (Reimold and Reynolds, 2018), which had not been systematically studied and only limited numbers of arrhythmias involving AADs were captured in clinical trials and incidental reports (Hindricks et al., 2021; Wharton et al., 2022). Despite the type of proarrhythmic events reported in previous clinical trials and meta-analyses differed among AAD treatments (Freemantle et al., 2011; Friberg, 2018; Valembois et al., 2019; Hindricks et al., 2021; Wharton et al., 2022; Singh et al., 2023), it is almost impossible to reach definitive conclusions from these studies on whether one AAD is more likely than another to result in a higher incidence of arrhythmias. The American Heart Association released a scientific statement for clinical evaluation of drug-induced arrhythmias (Tisdale et al., 2020), which have not systematically focused on the incidence of many general and specific AAD-induced arrhythmias. Moreover, drug-drug interaction (DDI) has been reported to affect the safety of AAD use, resulting in new or recurrent arrhythmias and other adverse events (Haddad and Anderson, 2002; Rajpurohit et al., 2014; Back and Burger, 2015; Mar et al., 2022). The concomitant use of amiodarone with sofosbuvir had been reported to cause serious cases of bradycardia, which may be due to sofosbuvir-based treatments displacing amiodarone from plasma binding proteins and potentiating the bradycardic effects of amiodarone (Back and Burger, 2015; Mar et al., 2022). Additionally, case reports suggested concomitant administration of flecainide with CYP2D6 inhibitors like venlafaxine and citalopram caused serious arrhythmias (Garcia, 2008; Rajpurohit et al., 2014). It is still unclear whether subsequent alterations in plasma AAD concentrations due to drug-drug interaction (DDI), compared with AAD monotherapies, can increase reporting of arrhythmias. In addition, the overviewed relationship between AADs and arrhythmias, factors related to death, potential signal spectra, as well as clinical information of AAD–related arrhythmias are still unknown.
Therefore, post-marketing surveillance is important to mine and reflect profiles of arrhythmias caused by different AAD regimens. In this study, we leveraged the Food and Drug Administration Adverse Event Reporting System (FAERS) to comprehensively characterize and investigate arrhythmias associated with AAD monotherapy and combination.
To investigate the association between cardiac arrhythmias and AADs, we used the FAERS database containing spontaneous adverse event reports between 1 January 2016, and 30 July 2022 to perform a disproportionality analysis. The AADs in the study included quinidine, disopyramide, mexiletine, flecainide, propafenone, sotalol, dofetilide, amiodarone, dronedarone, ibutilide, ivabradine, and adenosine. To our knowledge, pharmacokinetic drug interactions involving AADs increased the plasma concentration of AADs (Mar et al., 2022). Thus, the following concomitant use of pharmacokinetic interacting agents involving AADs were also considered in our studies: fluoxetine plus flecainide, duloxetine plus flecainide, paroxetine plus flecainide, amiodarone plus flecainide, citalopram plus propafenone, venlafaxine plus propafenone, sofosbuvir plus amiodarone, verapamil plus dronedarone, diltiazem plus dronedarone, verapamil plus ivabradine, and amiodarone plus ivabradine. Meanwhile, we searched for all adverse event reports related to concomitant use of AAD with pharmacokinetic interacting agents mentioned above. Open Vigil FDA, a pharmacovigilance tool, was adapted to extract FAERS data (Bohm et al., 2021).
Based on Medical Dictionary for Drug Regulatory Activities (MedDRA version 23.0), the high-level group term (HLGT) we researched was “Cardiac arrhythmias (10007521).” The full list of preferred terms (PTs) within considered cardiac arrhythmias was provided in Supplementary Table S1. The above PT level adverse events (AEs) belonged to the following four High Level Terms (HLTs): “Supraventricular arrhythmias (10042600),” “Rate and rhythm disorders NEC (10037908),” “Cardiac conduction disorders (10000032),” and “Ventricular arrhythmias and cardiac arrest (10047283).” Moreover, we collected clinical and demographic features of AE cases when data was available, including drug information (indication, concurrent medications, receipt date, treatment start and end dates), patient characteristics (gender, age, country of origin), and final patient outcomes (symptoms, seriousness). Clinical characteristics of patients with AAD-associated arrhythmias were compared between fatal and non-fatal groups. The fatal group referred to patients whose final outcome was death. The monotherapy of AAD-associated cardiac arrhythmias was defined as AAD as a primary suspected (PS) drug, without another AAD and pharmacokinetic interacting agent listed as concomitant, interacting or second suspected drugs.
Descriptive statistics were utilized to present the clinical characteristics of the cardiac arrhythmias associated with AADs. The chi-square test was used to compare the categorical variables between the fatal and non-fatal group. We used the t-test and non-parametric tests (Kruskal–Wallis tests) to compare the onset time of AAD-related cardiac arrhythmias. Disproportionality analysis was conducted by using reporting odds ratio (ROR) and information component (IC) as detection of potential safety signals for AEs in the FAERS (Noren et al., 2013; Zhai et al., 2019). If there were at least three reports and one algorithm are positive, it was defined as a significant signal. All the data analysis was performed by SPSS 24.0 (SPSS Inc., Chicago, IL, United States), and p values <0.05 were considered significant.
The FAERS database recorded 70,100 AAD-associated adverse events (AEs) and 177,896 reports related to cardiac arrhythmias between January 2016 and June 2022. We identified 11754 cases of AAD-related arrhythmias and described the clinical features of reports in Table 1. The AAD-related cardiac arrhythmia AE records were mainly from the North America (5264, 44.78%) and Europe (5252, 44.68%). Regarding cardiac arrhythmia AEs, the proportion of males is greater than that of females (47.35% vs. 44.58%). Amiodarone monotherapy generated the highest number of cases related with arrhythmias (5657, 48.13%), followed by flecainide monotherapy (1675, 14.25%), and sotalol (1179, 10.03%).
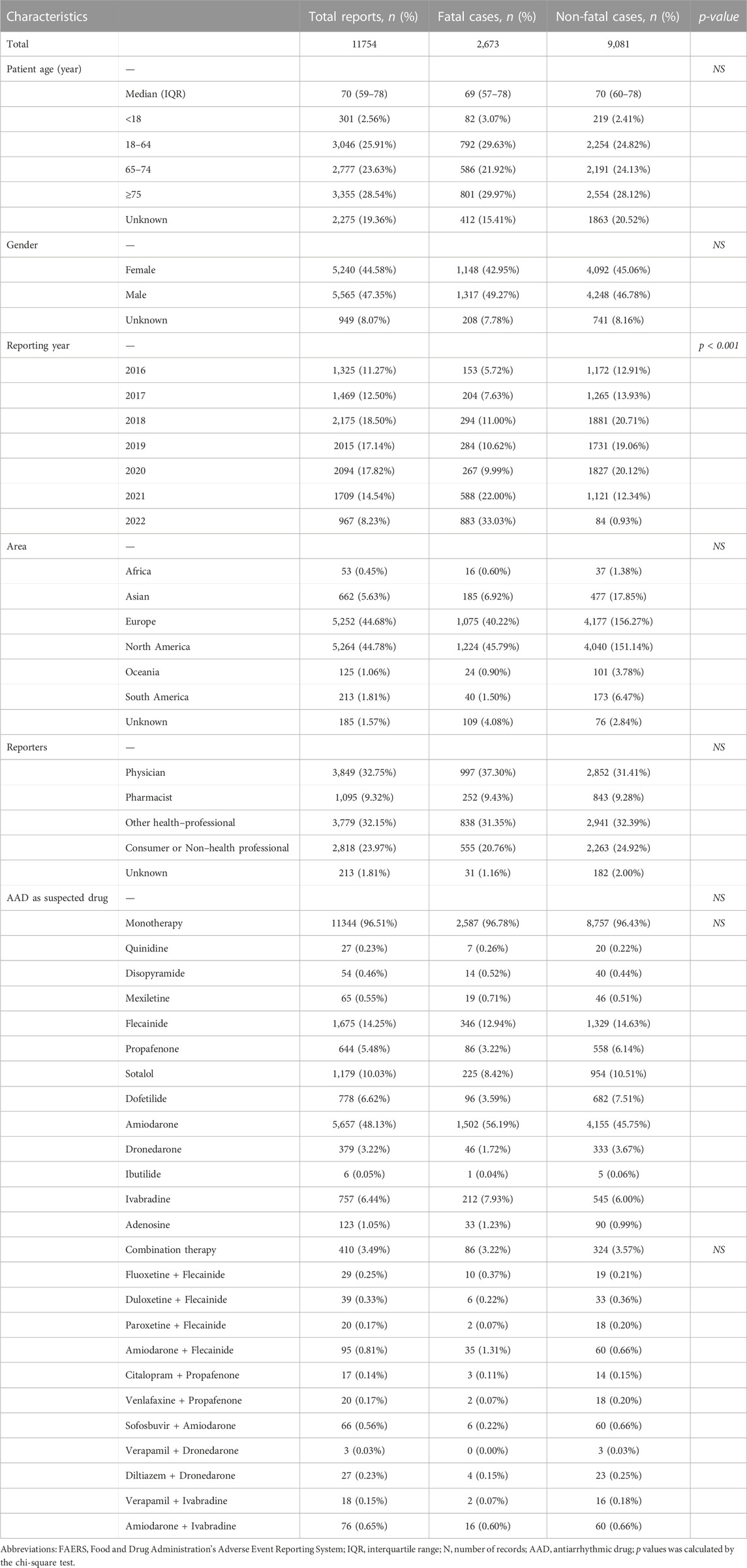
TABLE 1. Characteristics of patients with AAD-associated cardiac arrhythmias sourced from the FAERS database (January 2016 to June 2022).
As shown in Table 1, no significant difference was found in patient gender, age, area, reporter and AAD regimen for fatal vs. non-fatal reports.
The signal values and the association between AADs and arrhythmias were shown in Table 2. All studied AAD monotherapies were significantly correlated with the reporting frequency of cardiac arrhythmia (HLGT), with ROR ranging from 4.86 with mexiletine to 11.07 with flecainide (Table 2). Regarding four specific arrhythmias in HLT level, the AAD monotherapies with the highest ROR were flecainide in cardiac conduction disorders (ROR025 = 23.22), propafenone in rate and rhythm disorders (ROR025 = 11.32), dofetilide in supraventricular arrhythmias (ROR025 = 18.85), and ibutilide in ventricular arrhythmias (ROR025 = 11.47). Dofetilide/ibutilide, ibutilide, mexiletine/ibutilide and dronedarone presented no signal in the above four specific arrhythmias respectively. Compared with amiodarone monotherapy, sofosbuvir plus amiodarone detected the most significantly increased ROR in arrhythmias. Four in eleven different class-specific AAD combination therapy (amiodarone plus flecainide, sofosbuvir plus amiodarone, verapamil plus ivabradine, and amiodarone plus ivabradine) were detected with pharmacovigilance signals of cardiac arrhythmias (HLGT) compared with AAD monotherapy.
The arrhythmia signal spectra of different AAD therapies were shown in Table 3. Amiodarone presented a broadest spectrum of cardiac arrhythmias AEs, with 42 PTs detected as positive signals, ranging from cardiac flutter (IC 025 = 0.72) to torsade de pointes (TdP) (IC 025 = 4.93). There were 38 PTs as signals associated with flecainide, with signal values ranging from IC 025 = 1.08 (long QT syndrome) to IC 025 = 4.88 (atrioventricular block first degree). However, the drug with the least PTs was ibutilide, with only one signal detected, followed by quinidine, with five signals detected. Ventricular tachycardia, ventricular fibrillation and atrial fibrillation were three overlapping PTs, all of which were found significantly associated with disopyramide, flecainide, propafenone, sotalol, dofetilide, amiodarone, ivabradine, and adenosine. Torsade de pointes were detected as the strongest signal in amiodarone (IC 025 = 4.93).
A total of 3742 AAD–associated cardiac arrhythmias reported the time to onset (TTO), as shown in Table 4. (There were no or few known data on quinidine and ibutilide, which was not shown in Table 4). According to all AADs, the median onset time is 45 days, and the interquartile range is 3–331 days. Among AAD monotherapies, we found significant differences in the reported TTO of arrhythmias (p < 0.001). The median TTO was 46 days for amiodarone (IQR 5-330), 47 days for flecainide (IQR 4-349), 112 days for propafenone (IQR 3-433), 165 days for dronedarone (IQR 14-565), 64 days for sotalol (IQR 3-351), 13 days for disopyramide (IQR 0-84), 0 days for ibutilide (IQR 0-0), 14 days for ivabradine (IQR 0-132), 65 days for adenosine (IQR 0-366), 43 days for dofetilide (IQR 2-332), and 11 days for mexiletine (IQR 1-139), respectively. Moreover, there was no significant difference in the TTO between AAD monoregimen and combinationtherapy (flecainide vs. fluoxetine/duloxetine/paroxetine/amiodarone plus flecainide, p = 0.117; propafenone vs. citalopram/venlafaxine plus propafenone, p = 0.525; amiodarone vs. sofosbuvir plus amiodarone, p = 0.061; dronedarone vs. verapamil/diltiazem plus dronedarone, p = 0.411; dronedarone vs. verapamil/diltiazem plus dronedarone, p = 0.525; ivabradine vs. verapamil/amiodarone plus ivabradine, p = 0.444).
This study comprehensively evaluated the adverse events of AAD-induced cardiac arrhythmias based on the FAERS database. By employing the FAERS database, we analyzed the clinical characteristics, spectrum, TTO, and outcomes of AAD-induced arrhythmia AEs.
To assess the proarrhythmic effects of AADs, our research detected significant signals between cardiac arrhythmia and all AAD monotherapies, with ROR ranging from 4.86 with mexiletine to 11.07 with flecainide. In the disproportionate analysis of arrhythmias at HLT level, ibutilide monotherapy presented no signal in three specific arrhythmias except for ventricular arrhythmias and cardiac arrest, while mexiletine, dofetilide and dronedarone monotherapy demonstrated negative signal in supraventricular arrhythmias, cardiac conduction disorders, and ventricular arrhythmias and cardiac arrest, respectively. Notably, the risk of ventricular arrhythmia/TdP of dronedarone varied in different literatures, some of which showed a lower risk of dronedarone (Lafuente-Lafuente et al., 2012; Friberg, 2018; Tisdale et al., 2020), while others showed the opposite (Kao et al., 2012; Wu et al., 2022). Previous study reported 138 cases of ventricular arrhythmia associated with dronedarone between July 2009 and June 2011 (Kao et al., 2012), while our research identified only 19 reports during January 2016–June 2022. Additionally, the FAERS database recorded 61 cases of TdP related to dronedarone from the first quarter of 2009 to the fourth quarter of 2015 but only 2 reports between January 2016 and June 2022, resulting in the positive signal of TdP in dronedarone after incorporating data before 2016 (Kao et al., 2012; Wu et al., 2022). The higher reports of ventricular arrhythmia/TdP before 2016 and the lower cases after 2016 may be related to the early non-standard use of dronedarone, as it clearly worsens outcomes in patients with decompensated heart failure (Kober et al., 2008) and permanent atrial fibrillation (Rosenstein and Woods, 2012). The negative signal of dronedarone in ventricular arrhythmia/TdP shown in our study is updated and more consistent with clinical research and meta-analysis (Hohnloser et al., 2009; Freemantle et al., 2011; Lafuente-Lafuente et al., 2012; Friberg, 2018; Reimold and Reynolds, 2018; Valembois et al., 2019; Tisdale et al., 2020; Wharton et al., 2022), and will provide more accurate reference for the selection of AAD in clinical practice.
As compared to studied AAD monotherapy, seven pharmacokinetic drug interactions involving AADs were associated with a higher risk of reports of cardiac arrhythmias at HLGT or HLT level, which provided evidence for and endorsed the warnings included in the prescribing information of these drugs (Gareri et al., 2008; Back and Burger, 2015; McDonald et al., 2015; Tisdale, 2016; Mar et al., 2022). Four in eleven different specific AAD combination therapies (paroxetine plus flecainide vs. flecainide, diltiazem/verapamil plus dronedarone vs. dronedarone, citalopram plus propafenone vs. propafenone) were detected with no signal of cardiac arrhythmias at HLGT and HLT level compared with monotherapy, which was not affected by an increase in ADD concentrations demonstrated in previous studies (Garcia, 2008; Tisdale, 2016; Mar et al., 2022). Owing to the lack of studies on arrhythmias associated with AAD combination therapy, the rationale for no increased signal for the above four combination need to be further elucidated and explored.
Atrial fibrillation (AF) induced by disopyramide, adenosine and ivabradine was over-reported, but the signal intensity was weak; quinidine, mexiletine and ibutilide did not present a significant signal value. Ivabradine presented weak association with over-reporting frequency of AF in our study, consistent with the increased AF incidence with ivabradine found in previous clinical trials (Fox et al., 2008; Swedberg et al., 2010; Tendera et al., 2011; Fox et al., 2014; Bohm et al., 2015; Fox et al., 2015; Koruth et al., 2017). Prior studies showed that patients in the ivabradine group were more likely to develop new-onset AF (Fox et al., 2015; Koruth et al., 2017), and were associated with increased risk of AF in a previous meta-analysis (Martin et al., 2014). Moreover, the evidence concerning effect of ivabradine on AF in preclinical and clinical studies was conflicting, which provided modest evidence for ivabradine to reduce the incidence of AF in animal models (Li et al., 2015; Wang et al., 2019), but provided strong evidence for increased incidence of AF in human models by ivabradine (Fox et al., 2008; Swedberg et al., 2010; Tendera et al., 2011; Fox et al., 2014; Bohm et al., 2015); however, there is a concept that ivabradine in combination with beta-blockers could successfully control heart rate in AF, which is currently being investigated in a placebo-controlled clinical trials (RCT) (Fontenla et al., 2020). Although at risk of inducing atrial fibrillation, according to the 2021 update to the 2017 ACC expert consensus decision pathway for optimization of heart failure treatment, a history of paroxysmal AF is not a contraindication to ivabradine (Writing et al., 2021). Further studies are needed to establish the role of ivabradine in AF.
The time interval between the initial of AAD therapy to the onset of arrhythmia varies greatly. There was significant difference in the distribution of TTO among AAD mono-regimens (p < 0.001). According to all AADs, the median onset time is 45 days, with a interquartile range of 3–331 days, suggesting the significance of cardiac monitoring during the higher-risk time window of 45 days and individualized cardiac monitoring after AAD administration. Moreover, there was no significant difference in the TTO between AAD mono regimen and combination therapy.
Our study has certain limitations inherent to pharmacovigilance databases. Firstly, the true incidence of AE is unclear owing to the voluntary nature of FAERS reporting, including missing information, misspelled drug names, under-reporting and over-reporting, all of which are common in databases. Secondly, a slight increase of ROR only provided safety signals, not real risks of AE in clinical practice, which may be relevant and need further confirmation. Thirdly, due to the lack of denominator, we can neither calculate the incidence rate nor quantify the adverse reaction signals for AAD-related arrhythmias.
We reviewed arrhythmia AEs related with AADs from the FAERS database, as well as assessing whether pharmacokinetic drug interactions involving AADs increased the risk of arrhythmias compared to using AADs alone. Our research is practical for clinicians to understand the safety profile of AADs for arrhythmia and optimize their use among individual patients.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
The manuscript was designed and written by FW and XW. The data acquisition, statistical analysis and revising were performed by FW, HS, and BZ. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1170039/full#supplementary-material
Al-Khatib, S. M., Stevenson, W. G., Ackerman, M. J., Bryant, W. J., Callans, D. J., Curtis, A. B., et al. (2018). 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Executive summary: A report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society. Heart rhythm. 15 (10), e190–e252. doi:10.1016/j.hrthm.2017.10.035
Andrade, J. G., Turgeon, R. D., Macle, L., and Deyell, M. W. (2022). Cryoablation or drug therapy for initial treatment of atrial fibrillation. Eur. Cardiol. 17, e10. doi:10.15420/ecr.2021.38
Back, D. J., and Burger, D. M. (2015). Interaction between amiodarone and sofosbuvir-based treatment for hepatitis C virus infection: Potential mechanisms and lessons to be learned. Gastroenterology 149 (6), 1315–1317. doi:10.1053/j.gastro.2015.09.031
Bohm, M., Borer, J. S., Camm, J., Ford, I., Lloyd, S. M., Komajda, M., et al. (2015). Twenty-four-hour heart rate lowering with ivabradine in chronic heart failure: Insights from the SHIFT holter substudy. Eur. J. Heart Fail 17 (5), 518–526. doi:10.1002/ejhf.258
Bohm, R., Bulin, C., Waetzig, V., Cascorbi, I., Klein, H. J., and Herdegen, T. (2021). Pharmacovigilance-based drug repurposing: The search for inverse signals via OpenVigil identifies putative drugs against viral respiratory infections. Br. J. Clin. Pharmacol. 87 (11), 4421–4431. doi:10.1111/bcp.14868
Fontenla, A., Lopez-Gil, M., Tamargo-Menendez, J., Matia-Frances, R., Salgado-Aranda, R., Rey-Blas, J. R., et al. (2020). Ivabradine for chronic heart rate control in persistent atrial fibrillation. Design of the BRAKE-AF project. Rev. Esp. Cardiol. Engl. Ed. 73 (5), 368–375. doi:10.1016/j.rec.2019.09.004
Fox, K., Ford, I., Steg, P. G., Tardif, J. C., Tendera, M., Ferrari, R., et al. (2015). Bradycardia and atrial fibrillation in patients with stable coronary artery disease treated with ivabradine: An analysis from the SIGNIFY study. Eur. Heart J. 36 (46), 3291–3296. doi:10.1093/eurheartj/ehv451
Fox, K., Ford, I., Steg, P. G., Tardif, J. C., Tendera, M., Ferrari, R., et al. (2014). Ivabradine in stable coronary artery disease without clinical heart failure. N. Engl. J. Med. 371 (12), 1091–1099. doi:10.1056/NEJMoa1406430
Fox, K., Ford, I., Steg, P. G., Tendera, M., Ferrari, R., and Investigators, B. (2008). Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): A randomised, double-blind, placebo-controlled trial. Lancet 372 (9641), 807–816. doi:10.1016/S0140-6736(08)61170-8
Freemantle, N., Lafuente-Lafuente, C., Mitchell, S., Eckert, L., and Reynolds, M. (2011). Mixed treatment comparison of dronedarone, amiodarone, sotalol, flecainide, and propafenone, for the management of atrial fibrillation. Europace 13 (3), 329–345. doi:10.1093/europace/euq450
Friberg, L. (2018). Ventricular arrhythmia and death among atrial fibrillation patients using anti-arrhythmic drugs. Am. Heart J. 205, 118–127. doi:10.1016/j.ahj.2018.06.018
Garcia, A. (2008). Adverse effects of propafenone after long-term therapy with the addition of citalopram. Am. J. Geriatr. Pharmacother. 6 (2), 96–99. doi:10.1016/j.amjopharm.2008.05.001
Gareri, P., De Fazio, P., Gallelli, L., De Fazio, S., Davoli, A., Seminara, G., et al. (2008). Venlafaxine-propafenone interaction resulting in hallucinations and psychomotor agitation. Ann. Pharmacother. 42 (3), 434–438. doi:10.1345/aph.1K405
Haddad, P. M., and Anderson, I. M. (2002). Antipsychotic-related QTc prolongation, torsade de pointes and sudden death. Drugs 62 (11), 1649–1671. doi:10.2165/00003495-200262110-00006
Hindricks, G., Potpara, T., Dagres, N., Arbelo, E., Bax, J. J., Blomstrom-Lundqvist, C., et al. (2021). 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 42 (5), 373–498. doi:10.1093/eurheartj/ehaa612
Hohnloser, S. H., Crijns, H. J., van Eickels, M., Gaudin, C., Page, R. L., Torp-Pedersen, C., et al. (2009). Effect of dronedarone on cardiovascular events in atrial fibrillation. N. Engl. J. Med. 360 (7), 668–678. doi:10.1056/NEJMoa0803778
January, C. T., Wann, L. S., Calkins, H., Chen, L. Y., Cigarroa, J. E., Cleveland, J. C., et al. (2019). 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society. J. Am. Coll. Cardiol. 74 (1), 104–132. doi:10.1016/j.jacc.2019.01.011
Kao, D. P., Hiatt, W. R., and Krantz, M. J. (2012). Proarrhythmic potential of dronedarone: Emerging evidence from spontaneous adverse event reporting. Pharmacotherapy 32 (8), 767–771. doi:10.1002/j.1875-9114.2012.01118.x
Kober, L., Torp-Pedersen, C., McMurray, J. J., Gotzsche, O., Levy, S., Crijns, H., et al. (2008). Increased mortality after dronedarone therapy for severe heart failure. N. Engl. J. Med. 358 (25), 2678–2687. doi:10.1056/NEJMoa0800456
Koruth, J. S., Lala, A., Pinney, S., Reddy, V. Y., and Dukkipati, S. R. (2017). The clinical use of ivabradine. J. Am. Coll. Cardiol. 70 (14), 1777–1784. doi:10.1016/j.jacc.2017.08.038
Lafuente-Lafuente, C., Longas-Tejero, M. A., Bergmann, J. F., and Belmin, J. (2012). Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst. Rev. 5, CD005049. doi:10.1002/14651858.CD005049.pub3
Li, Y. D., Ji, Y. T., Zhou, X. H., Jiang, T., Hong, Y. F., Li, J. X., et al. (2015). Effects of ivabradine on cardiac electrophysiology in dogs with age-related atrial fibrillation. Med. Sci. Monit. 21, 1414–1420. doi:10.12659/MSM.894320
Mar, P. L., Horbal, P., Chung, M. K., Dukes, J. W., Ezekowitz, M., Lakkireddy, D., et al. (2022). Drug interactions affecting antiarrhythmic drug use. Circ. Arrhythm. Electrophysiol. 15 (5), e007955. doi:10.1161/CIRCEP.121.007955
Martin, R. I., Pogoryelova, O., Koref, M. S., Bourke, J. P., Teare, M. D., and Keavney, B. D. (2014). Atrial fibrillation associated with ivabradine treatment: meta-analysis of randomised controlled trials. Heart 100 (19), 1506–1510. doi:10.1136/heartjnl-2014-305482
McDonald, M. G., Au, N. T., and Rettie, A. E. (2015). P450-Based drug-drug interactions of amiodarone and its metabolites: Diversity of inhibitory mechanisms. Drug Metab. Dispos. 43 (11), 1661–1669. doi:10.1124/dmd.115.065623
Noren, G. N., Hopstadius, J., and Bate, A. (2013). Shrinkage observed-to-expected ratios for robust and transparent large-scale pattern discovery. Stat. Methods Med. Res. 22 (1), 57–69. doi:10.1177/0962280211403604
Rajpurohit, N., Aryal, S. R., Khan, M. A., Stys, A. T., and Stys, T. P. (2014). Propafenone associated severe central nervous system and cardiovascular toxicity due to mirtazapine: A case of severe drug interaction. S. D. Med. 67 (4), 137–139.
Reimold, F. R., and Reynolds, M. R. (2018). Proarrhythmia and death with antiarrhythmic drugs for atrial fibrillation, and the unfulfilled promise of comparative effectiveness research. Am. Heart J. 205, 128–130. doi:10.1016/j.ahj.2018.08.011
Rosenstein, R., and Woods, D. (2012). Dronedarone in high-risk permanent atrial fibrillation. N. Engl. J. Med. 366 (12), 1160. doi:10.1056/NEJMc1200742
Singh, J. P., Blomstrom-Lundqvist, C., Turakhia, M. P., Camm, A. J., Fazeli, M. S., Kreidieh, B., et al. (2023). Dronedarone versus sotalol in patients with atrial fibrillation: A systematic literature review and network meta-analysis. Clin. Cardiol. 2023, 24011. doi:10.1002/clc.24011
Swedberg, K., Komajda, M., Bohm, M., Borer, J. S., Ford, I., Dubost-Brama, A., et al. (2010). Ivabradine and outcomes in chronic heart failure (SHIFT): A randomised placebo-controlled study. Lancet 376 (9744), 875–885. doi:10.1016/S0140-6736(10)61198-1
Tendera, M., Talajic, M., Robertson, M., Tardif, J. C., Ferrari, R., Ford, I., et al. (2011). Safety of ivabradine in patients with coronary artery disease and left ventricular systolic dysfunction (from the BEAUTIFUL Holter Substudy). Am. J. Cardiol. 107 (6), 805–811. doi:10.1016/j.amjcard.2010.10.065
Tisdale, J. E., Chung, M. K., Campbell, K. B., Hammadah, M., Joglar, J. A., Leclerc, J., et al. (2020). Drug-induced arrhythmias: A scientific statement from the American heart association. Circulation 142 (15), e214–e233. doi:10.1161/CIR.0000000000000905
Tisdale, J. E. (2016). Drug-induced QT interval prolongation and torsades de pointes: Role of the pharmacist in risk assessment, prevention and management. Can. Pharm. J. (Ott) 149 (3), 139–152. doi:10.1177/1715163516641136
Valembois, L., Audureau, E., Takeda, A., Jarzebowski, W., Belmin, J., and Lafuente-Lafuente, C. (2019). Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst. Rev. 9 (9), CD005049. doi:10.1002/14651858.CD005049.pub5
Viskin, S., Chorin, E., Viskin, D., Hochstadt, A., Halkin, A., Tovia-Brodie, O., et al. (2019). Quinidine-responsive polymorphic ventricular tachycardia in patients with coronary heart disease. Circulation 139 (20), 2304–2314. doi:10.1161/CIRCULATIONAHA.118.038036
Wang, J., Yang, Y. M., Li, Y., Zhu, J., Lian, H., Shao, X. H., et al. (2019). Long-term treatment with ivabradine in transgenic atrial fibrillation mice counteracts hyperpolarization-activated cyclic nucleotide gated channel overexpression. J. Cardiovasc Electrophysiol. 30 (2), 242–252. doi:10.1111/jce.13772
Wharton, J. M., Piccini, J. P., Koren, A., Huse, S., and Ronk, C. J. (2022). Comparative safety and effectiveness of sotalol versus dronedarone after catheter ablation for atrial fibrillation. J. Am. Heart Assoc. 11 (3), e020506. doi:10.1161/JAHA.120.020506
Writing, C., Maddox, T. M., Januzzi, J. L., Allen, L. A., Breathett, K., Butler, J., et al. (2021). 2021 update to the 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: Answers to 10 pivotal issues about heart failure with reduced ejection fraction: A report of the American college of cardiology solution set oversight committee. J. Am. Coll. Cardiol. 77 (6), 772–810. doi:10.1016/j.jacc.2020.11.022
Wu, Z., Zhou, P., He, N., and Zhai, S. (2022). Drug-induced torsades de pointes: Disproportionality analysis of the United States Food and Drug Administration adverse event reporting system. Front. Cardiovasc Med. 9, 966331. doi:10.3389/fcvm.2022.966331
Keywords: antiarrhythmic drugs, arrhythmia, adverse event reporting system, AAD, ventricular arrhythmia
Citation: Wang F, Zhou B, Sun H and Wu X (2023) Proarrhythmia associated with antiarrhythmic drugs: a comprehensive disproportionality analysis of the FDA adverse event reporting system. Front. Pharmacol. 14:1170039. doi: 10.3389/fphar.2023.1170039
Received: 20 February 2023; Accepted: 04 May 2023;
Published: 12 May 2023.
Edited by:
Yusuf Karatas, Çukurova University, TürkiyeReviewed by:
Faiz Ullah Khan, Xi’an Jiaotong University, ChinaCopyright © 2023 Wang, Zhou, Sun and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinan Wu, d3V4aW5hbkBib2UuY29tLmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.