- 1Molecular Chemistry, Materials and Catalysis Laboratory, Faculty of Sciences and Technologies, Sultan Moulay Slimane University, Beni-Mellal, Morocco
- 2AgroBioSciences, Mohammed VI Polytechnic University (UM6P), Ben Guerir, Morocco
- 3Institute of BioEconomy, IBE, National Research Council, Florence, Italy
Introduction: The Tanacetum genus consists of 160 accepted flowering species thriving throughout temperate regions, mainly in the Mediterranean Basin, Northern America, and southwestern and eastern Asia. Tanacetum species bear a long-standing record of use in the folk medicine of indigenous tribes and communities worldwide, along with multitudinous applications in traditional cuisines, cosmeceuticals, and agricultural fields.
Methods: Up-to-date data related to traditional uses, phytochemistry, biological activities, toxicity and clinical trials of the genus Tanacetum were systematically reviewed from several online scientific engines, including PubMed, Web of Science, Scopus, SciFinder, Wiley Online, Science Direct, and Cochrane library.
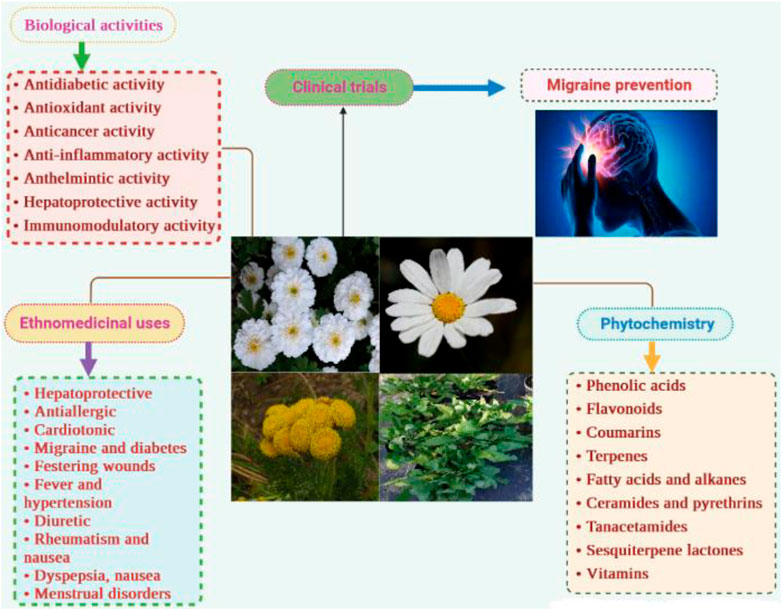
Results and discussion: Over the past three decades, 241 metabolites have been isolated from nearly twenty species, including phenolic acids, flavonoids, coumarins, fatty acids and alkanes, aldehydes, volatile compounds, and naphthoquinones. Some unique metabolites have also been identified, such as the ceramides tanacetamide (A-D) from T. artemisioides, pyrethrins from T. cinerariifolium, and sesquiterpene lactones from several species. However, these secondary metabolites are still poorly studied despite in vitro clues highlighting their colossal pharmacological properties, especially as hypotensive, neuroprotective, anticancer, and antimicrobial agents. Scientific studies have validated some traditional claims of the plant, such as antidiabetic, anticancer, anthelmintic, insecticide, antioxidant, and hepatoprotective activities, as well as against festering wounds, skin ulcers, urinary tract infections, and sexually transmitted diseases. Other ethnomedicinal uses for arthritis, gout, rheumatism, anemia, and as a litholytic, antivenom and diaphoretic have not yet been supported and would constitute the subject of further research.
1 Introduction
Since the dawn of time, our ancestors have relied heavily on nature to meet their daily basic needs, such as shelter, foodstuffs, clothing, and medicines. Consequently, rich indigenous pharmacopeias have evolved through hit-and-miss, handed down, and maintained among healers and members of ethnic tribes and communities across generations. Today, it is estimated that more than 50% of modern therapeutic drugs are derived synthetically from herbal preparations and formulas, making them attractive templates for new drug leads (Heinrich, 2000; Drissi et al., 2022).
Species of the genus Tanacetum from the Asteraceae family bears a long history of traditional uses in various fields, including medicine, cosmetics, agriculture, and cuisines. They have been used ethnopharmacologically to treat many health-related conditions such as diabetes, migraine, cholecystitis, dyspepsia, nausea, diarrhea, hypertension, stomach pain and bloating, ringworms, and sexually transmitted diseases, among others (Molares and Ladio, 2009; Bouhlal et al., 2017; Ullah et al., 2019; Khatib et al., 2021).
A few taxa, mainly T. balsamita (Costmary), are still appreciated in the traditional cuisine of several countries, especially Italy, owing to their spicy odor and minty balsam aroma (Ghirardini et al., 2007; Cornara et al., 2014). For instance, leaves from costmary are used to prepare herbal tea, aromatize salads, omelets, soups, meats, and vegetable pies, and cosmetically to soothe and perfume the skin (Guarrera et al., 2005; Ghirardini et al., 2007). In the agricultural field, pyrethrum, from the dried and blended flowers of T. cinerariifolium, has long been used to repulse flying insects and ward off fleas and body lice, even before the chemistry of active metabolites emerged (Jeran et al., 2021).
Recently, phytochemical investigations have identified more than 240 secondary metabolites from the genus Tanacetum, including volatile compounds, phenolic acids, flavonoids, fatty acids and alkanes, aldehydes, and coumarins (Bagci et al., 2008; Benedec et al., 2016; Rezaei et al., 2017; Savci et al., 2020). Some dietary components such as carbohydrates and vitamins have also been found in the leaves, roots, and whole plants of T. vulgare and T. densum (Polle et al., 2001; Emre, 2021). Moreover, various unique compounds have exclusively been alarmed in the genus Tanacetum, such as the ceramides tanacetamide (A-D) (72-74) from T. artemisioides, pyrethrins (113-118) from T. cinerariifolium, and some sesquiterpene lactones (119-139) (Gonzalez et al., 1990; Hussain et al., 2005; 2005; Jeran et al., 2021). Thus, these metabolites could serve as crucial chemotaxonomic markers of the genus Tanacetum.
On the other hand, crude extracts and isolated metabolites have demonstrated various biological activities such as antidiabetic (Khan et al., 2018), antimicrobial (Kameri et al., 2019), cytotoxic (Coté et al., 2017), anthelmintic (Godinho et al., 2014), antioxidant (Bączek et al., 2017), and immunomodulatory activities (Jannesar et al., 2014), which are attributed to the produced synergetic effect or/and action of a single metabolite.
To our knowledge, this is the first comprehensive review of the genus Tanacetum since 2002 (Gören et al., 2002). Our review collates the fragmented ethnobotanical information on the genus during the last two decades to identify the validated medical applications and unveil the knowledge gaps to be fulfilled by further studies. We have also reviewed and updated the botanical features, phytochemical composition, pharmacological studies, toxicity, and clinical trials. A general discussion was established to link folkloric uses and secondary or/and primary metabolites potentially involved in the claimed uses, while shedding light on their unexplored therapeutic attributes.
2 Methodology
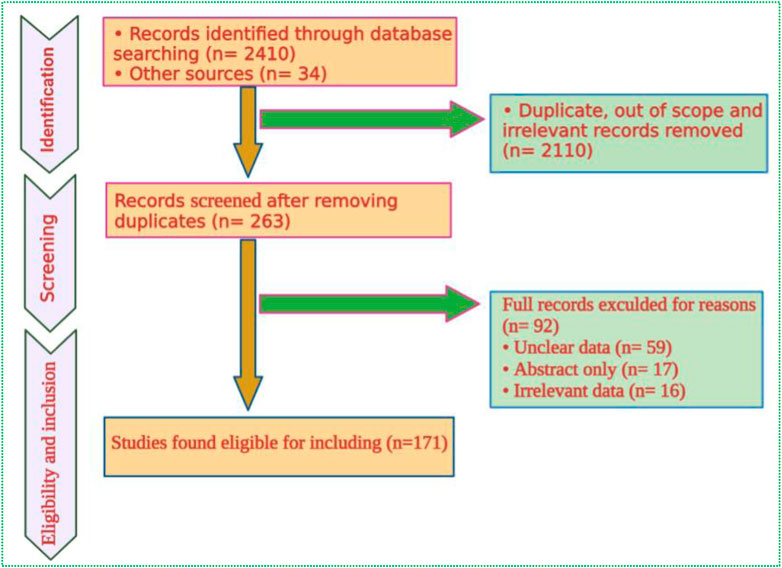
Data were retrieved and systematically reviewed from several online scientific engines, including PubMed, Web of Science, Scopus, SciFinder, Wiley Online, and Science Direct (Figure 1). We have also reviewed the Cochrane Central Register of Controlled Trials to acquire the available evidence regarding randomized controlled trials (www.cochrane library.com). The key search words such as Tanacetum, ethnobotany, ethnoveterinary, geographical distribution, morphological features, phytochemistry, and biological activities, were used during the data search. The botanical names of Tanacetum taxa were validated using the World Flora Online (WFO, www.worldfloraonline.org) database.
3 Taxonomy, geographical distribution and IUCN status of Tanacetum spp
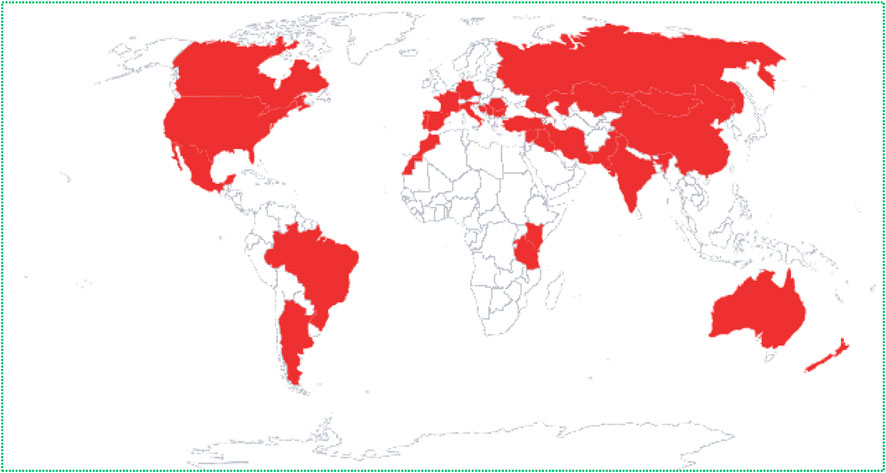
The genus Tanacetum L. from the Asteraceae family is the third largest genus of the chamomile tribe Compositae–Anthemideae, consisting of about 160 species of flowering plants, after the two rich-species genera Artemisia L. (522 species) and Anthemis L. (177 species) (Sonboli et al., 2012; Moradi Behjou et al., 2022). According to The Plant List, 553 names have been granted to Tanacetum spp., including 179 accepted names, 206 synonyms, and 168 unresolved names (The Plant List, accessed on: 13 September 2022), while the worldfloraonline database has included 189 subordinate taxa (http://www.worldfloraonline.org/taxon/wfo-4000037526, accessed on: 13 September 2022) (Table 1). The species of the genus Tanacetum finds habitat throughout temperate regions, especially in the Mediterranean Basin region, some parts of northern America, and Southwestern and Eastern Asia, including Azerbaijan, Armenia, Iran, and Türkiye (Figure 2) (Moradi Behjou et al., 2022).
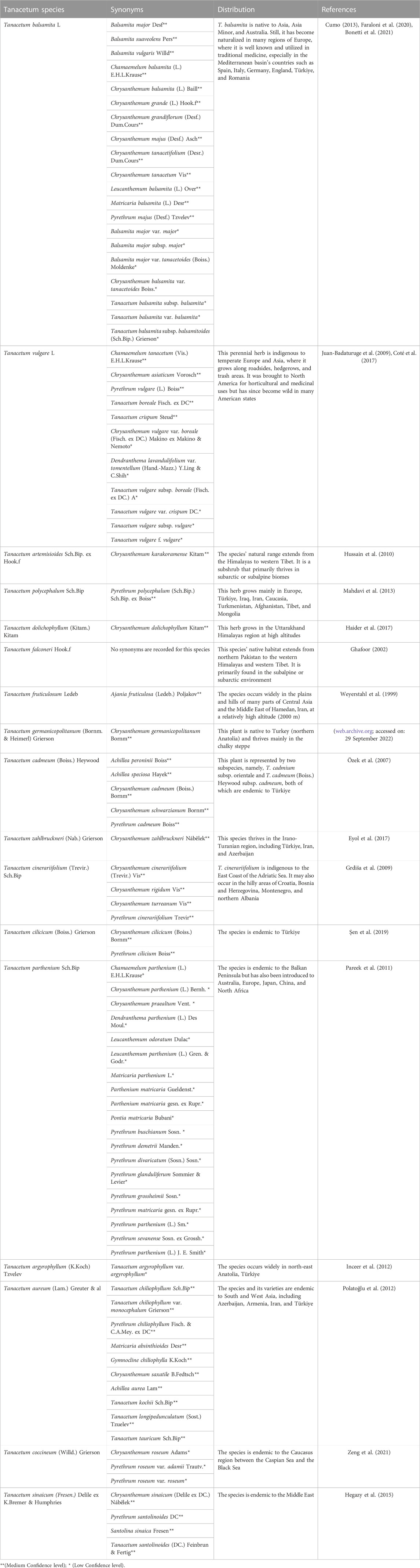
TABLE 1. Synonyms and geographical distribution of Tanacetum species used in the traditional medicine.
It is worth noting that the genus exhibited considerable morphological variations encompassing perennial herbs and subshrubs, with the capitula either solitary or clustered in lax to dense corymbs and are either radiate or disciform-to-discoid (Sonboli et al., 2012). Due to its highly complex taxonomical history, phylogenetic position, and morphological intraspecific diversity, the infrageneric classification of the genus Tanacetum remains controversial within this medium-sized tribe (Sonboli et al., 2012; Moradi Behjou et al., 2022). For instance, Bremer and Humphries (1993) proposed a subtribal classification for the tribe based mostly on morphological traits, which molecular-phylogenetic studies later discovered to be substantially polyphyletic (Bremer, 1993; Oberprieler et al., 2007). Subsequently, molecular-phylogenetic investigations have excluded some species from the genus Tanacetum and were transferred to other circum-Mediterranean Anthemidinae genera, such as Nananthea, Anthemis, Cota, and Tripleurospermum (Sonboli et al., 2012).
According to the IUCN database, three Tanacetum taxa met the B2ab and C2a criteria of endangered species, and have recently been deemed as critically threatened, rendering their preservation and sustainability utterly necessary (www.iucnredlist.org; accessed on: 28 September 2022). These species are T. ptarmiciflorum Sch.Bip., T. oxystegium (Sosn.) Grierson, and T. oshanahanii “Marrero Rodr., Febles & C.Suárez” (www.iucnredlist.org; accessed on: 28 September 2022).
4 Morphological features of Tanacetum spp
In botanical Latin, the generic name Tanacetum came seemingly from the Latin name Athanasia referring to “eternal life and immortality” since tansies were once sown between the grave clothes of the deceased to ward off vermin. Tansies are mostly perennial herbs, but a few can be annuals, evergreen, herbaceous perennials, or sub-shrubs. Tanacetum species vary in height from a few centimeters (5 cm) to 150 cm, with strongly scented, hairy, and occasionally silvery foliage. The leaves are alternate, basal and cauline, petiolate, or sessile, with the blades mostly obovate to spatulate (Figure 3). The flowers have distinct layers of phyllaries encircling their base and range in shape from flat to hemispherical. The fruit is a cypsela with ribs and glands that typically has a pappus at the end (http://www.efloras.org; accessed on: 28 September 2022). Tansies thrive naturally in well-drained sandy or coarse soils, requiring a limited amount of soil nutrients and humidity, and can be propagated by rooting stem cuttings under mist, tissue culture, vegetative splits, and seed propagation (Keskitalo, 1999).

FIGURE 3. A collection of pictures of Tanacetum spp. (A) T. dolichophyllum (Kitam.) Kitam (https://sites.google.com/site/efloraofindia). (B) T. parthenium (L.) Sch.Bip (https://sites.google.com/site/efloraofindia). (C) T. cinerariifolium (Trevir.) Sch.Bip (https://sites.google.com/site/efloraofindia). (D) T. balsamita L., Florence, Italy, 2023©.
5 Traditional and edible uses
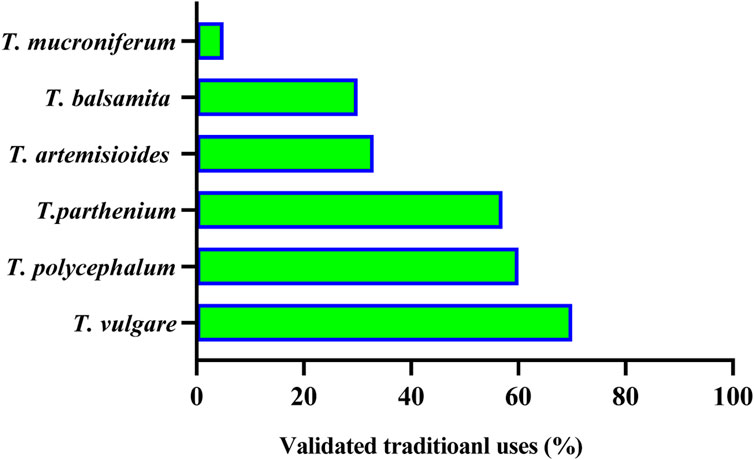
Out of 160 Tanacetum species, ethnobotanical data for only sixteen taxa (10%) are available, while the remaining species have not yet been surveyed. Analysis of more than 50 ethnobotanical studies, undertaken worldwide, revealed that T. vulgare, T. balsamita, and T. parthenium are the major Tanacetum taxa used in ethnomedicinal practices. Meanwhile, the leaves (45.31%), flowers (18.76%), and aerial parts (15.63%) are the predominant parts (Figure 4). In Ayurvedic medicine, mountainous communities drank the juice made from crushed and boiled roots of Pleurospermum and Tanacetum spp. three to four times daily to cure gastritis and stomachache. The underground parts are also cleansed, cut into small pieces, and chewed for arthritis and fever (Abbasi and Bussmann, 2021). The following subsections and Table 2 compiled exhaustive details about the traditional/ethnopharmacological uses of the sixteen taxa, including their vernacular names, used parts, ethno-preparations, and routes of administration.
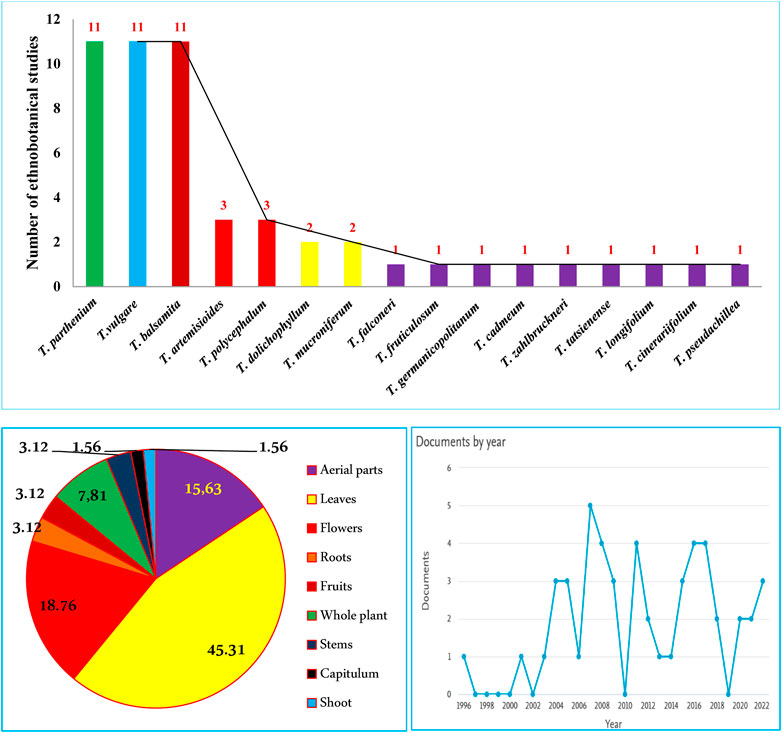
FIGURE 4. Number of ethnobotanical studies per Tanacetum species, main used parts, and publication trends 2023©.
5.1 T. balsamita (costmary)
In Southern Europe, the leaves decoction from costmary had been applied as an insect repellent for cattle and children and as an insecticidal agent. Costmary had also been used to disguise unpleasant odors in houses and to disseminate a pleasant smell in closets (Cumo, 2013). By 1614, the balsamic scent of costmary inspired Fra’ Angiolo Marchissi to create a fragrant preparation in water with Ceylon cinnamon, rosemary, and mint. This concoction was used for coughs, colds, and its relaxing properties. Therefore, this distilled preparation was commonly known as “Anti-hysteric Water” (Nelli and Ena, 2012). Costmary had once been employed as a beer flavoring, but in the 15th century with the extensive usage of hop (Humulus lupulus), it gradually fell into decline for this purpose (Nelli and Ena, 2012).
Recently, Sõukand and Pieroni, (2016) stated that the aboriginal inhabitants in the Hutsuls of Bukovina area used the alcohol infusion of T. balsamita (costmary) flower buds and leaves topically to treat heart diseases and festering wounds. In some cases, they utilized fresh aerial parts soaked in hot water to cure old and deep wounds and furuncles (Sõukand and Pieroni, 2016).
In the Persian pharmacopeia, the leaves and flowerheads in the form of decoction, infusion, and floral water of costmary have been used as a general tonic, antiallergic, anticancer, hepatoprotective, sedative, flatulent, and cardiotonic, whereas in Serbia, the leaves’ tea aids to ease terrible migraines and female issues during the menopause (Hassanpouraghdam, 2009; Jarić et al., 2015; Hassanpouraghdam et al., 2022). Moreover, the decoction of the leaves and stems was applied topically as a rheumatism ointment, antipyretic, and a menstrual regulator (Güneş and Özhatay, 2011).
In Northern Istria, the indigenous population breathed the ensuing vapors of T. balsamita scorched leaves with rose petals and wormwood on June 21st for their relaxing properties (Pieroni and Giusti, 2008). In Southern Italy, locals ingested an infusion from costmary leaves against bile insufficiency, cholecystitis, and nervous dyspepsia, and for its sedative, antispasmodic, anti-inflammatory, and anti-insomnia properties (Guarrera et al., 2005; Ghirardini et al., 2007; Guarino, 2008; Vitalini et al., 2015). In Turkish folk medicine, two teacups from an infusion of T. balsamita leaves are prescribed thrice daily for three consecutive weeks against diabetes (Dalar, 2018).
Intriguingly, costmary still finds application in the traditional cuisine of central Italy owing to its distinctive bitter taste; the minty-lemony leaves served to aromatize salads, omelets, vegetable pies, liqueurs, and as a component of the filling of Tortelli, especially on Easter Day (Ghirardini et al., 2007; Cornara et al., 2014). They have also been employed to flavor garlic cloves, mallow leaves, cheese, and eggs (Ghirardini et al., 2007).
The macerated water of T. balsamita and Santolina etrusca has been frequently used to make fragrant water on St. John’s evening to soften and perfume the skin (Guarrera et al., 2005). In accordance with tradition, the most appropriate day for harvesting this plant is June 24, St. John’s Day, and for this reason, this medicinal plant is also called St. John’s herb. Perhaps, the tradition follows the findings that this period typically coincide with the highest balsamic period of costmary, featured by an intense aromatic flavor (Pukalskas et al., 2010).
5.2 T. vulgare (tansy)
In Syria, T. vulgare is widely known as “Hasheshet eldood”, referring to its miraculous ability to eradicate internal worms. Thereby, it is used to remove parasitic worms and externally for its wound healing properties. Indigenous villagers also used to swallow an infusion from the aboveground parts to heal neurological and venereal conditions, coughs, gastritis, and respiratory tract infections. It is said to have repellent properties against some kinds of ants owing to its aromatic odors (Khatib et al., 2021).
In Russian folk medicine, tansy has a long-established use against diarrhea and intestinal worms (Enterobius and Ascaris), as well as an antipyretic and diaphoretic agent (Shikov et al., 2014). Interestingly, 10 g of the decocted flowers in 200 mL of water is believed to have anthelmintic and choleretic effects when consumed at a dose of 1 tablespoon daily (Shikov et al., 2014). Externally, the poultice from the whole plant is applied for sprains, swellings, contusions, gout, and some eruptive skin conditions (Abad et al., 1995; Shikov et al., 2014). Moreover, the leaves, flowers, and whole plant infusion are mentioned in preventing and treating rheumatism, anemia, hypercholesterolemia, kidney weakness, migraine, and hysteria (Shikov et al., 2014; Tribess et al., 2015; Chaachouay et al., 2019b).
In the Russian Pharmacopoeia, the dried flowers of tansy are used as a substitute for cinnamon and ginger. They can also be used to preserve meat and add flavor to fish, meat, and beverages. Leaf and flower parts are used as tea substitutes, and in beer as hop substitutes (Shikov et al., 2017).
5.3 T. artemisioides
The geographical restriction of T. artemisioides in Pakistan allowed the emergence and spread of rich beliefs and practical knowledge within the mountains tribes. In the Kurram Valley, the locals call this species “Zawil” and “Zoon in Gilgit”, and they are used to relieve the flu by mixing and consuming powdered flowers with oil and sugar (Hussain et al., 2010; Ali et al., 2019). They also used powdered leaves and fruits to alleviate and treat diabetes, high blood pressure, kidney, headache, fever, hepatitis, abdominal disorders, ringworm, and flatulence (Hussain et al., 2010; 2018; Ullah et al., 2019). Moreover, a glance at the existing data gathered from different geo-cultural areas indicated a typical usage of several Tanacetum species for diabetes management, including T. artemisioides. For instance, ethnic groups from Khyber Pakhtunkhwa, Pakistan, used to deal with diabetes by preparing and consuming 10 g water infusion of T. artemisioides aerial parts (Ullah et al., 2019).
5.4 T. cinerariifolium (dalmatian pyrethrum)
Since the 19th century, Dalmatian pyrethrum has been widely cultivated to repel and control mosquitoes and body lice on both animals and humans before the chemistry of active ingredients (Grdiša et al., 2009). The early 20th century marked the pioneering discovery of the active ingredients in Pyrethrum products by the German chemist Herman Staudinger and the Croatian scientist Lavoslav Ružička (Grdiša et al., 2009). Today, Kenya, Rwanda, and Tanzania are the leading producers of pyrethrum in the world, accounting for nearly 90% of the world’s output and 85% of exports; the ground pyrethrum flowers are manufactured and commercially sold as “Dalmatian Insect Powder” (Hitmi et al., 2000; Grdiša et al., 2009; Grdiša et al., 2022).
In the Mbulu district of Tanzania, agropastoralists call this species “Pareto”, and they grow it to control field pests and veterinary to manage ticks (Qwarse et al., 2018). It is also used by herbalists in central Morocco to control vector-borne diseases (EL-Akhal et al., 2021). Medically, ethnic communities in south India are still using the whole plant as an antidote for poisoning (Kumar et al., 2019). In North-Eastern Morocco, T. cinerariifolium is widely known as عود العطاس)) by local inhabitant, and they orally consume the stem infusion at a dose of one tablespoon daily to treat kidney stones (Bencheikh et al., 2021).
5.5 T. parthenium (feverfew)
In Southern Brazil, this species is known by rural communities as “Rainha-das-ervas” and “Artemisia”. The leaves and flowers decoction is used to jump-start and relieve menstrual pain, stomachache and infections (Tribess et al., 2015). In northwest Greece, this species is widely used against digestive system inflammations, puerperal fever, rheumatism, and arthritis, and as diaphoretic, emmenagogue, tonic, and stimulant (Vokou et al., 1993).
The local people in the Irano-Turanian region call this species “Babune gavi” and “Colous”, and they used the leaves decoction to cure fever and gastric disorders and as a sedative and nerve relaxant (Rajaei and Mohamadi, 2012). To ease toothache, they prepared a decoction of crushed roots and put it on the tooth (Delfan et al., 2014). Feverfew is called “Santamaría" in Mexican folk medicine; high doses from the aerial parts and leaves infusion are orally taken to induce abortion (Andrade-Cetto, 2009). The roots are mixed with honey and vinegar and used as a litholytic for bladder stones (Ahmed et al., 2016).
5.6 T. polycephalum
In Northern Iraq, this species is known as “Borzhan”. The locals consumed one glass of the flowers decoction on an empty stomach for cold and flu (Kawarty et al., 2020). The water decoction of the aerial parts is mixed with the Thymus and Achillea and used for gastroenteritis (Mosaddegh et al., 2012). It is also used as a traditional Iranian remedy for hemorrhoids and inflammation (Ghasemi Pirbalouti et al., 2012).
5.7 T. nubigenum
The available data regarding this species revealed that the local inhabitants in the Indian Himalayas privileged this species for preparing fragrant materials and incense owing to its distinguished pleasant smell (Beauchamp et al., 2001; Khan et al., 2018). They also used whole plant decoction to alleviate and treat fever (Chanotiya et al., 2006).
5.8 T. macrophyllum
The water infusion of T. macrophyllum flowers is reportedly used for earache (Kazancı et al., 2020).
5.9 T. zahlbruckneri
Indigenous villagers from the Eastern Anatolia region of Turkey drank the decoction of the aerial parts for flu and cold (Mükemre et al., 2015).
5.10 T. cadmeum
A single ethnobotanical study reported that some Turkish people chew the above-ground parts of this plant for stomach ulcers (Altınbaşak et al., 2018).
5.11 T. ferulaceum
The only traditional medicinal indication for this species is treating gastric ulcers (Kumar and Tyagi, 2013). Further ethnopharmacological studies are needed to document the traditional medicinal uses related to this species.
5.12 T. corymbosum
The whole plant is mainly used against digestive disorders, gastritis, and parasitic intestinal worms (Ciocarlan et al., 2021; Ivănescu et al., 2021).
5.13 T. sinaicum (pyrethrum santolinoides)
The species is native to the Middle East and has traditionally been used for migraine, fever, stomach disorders, arthritis, and bronchitis (Hegazy et al., 2015).
5.14 T. argyrophyllum
The species has been traditionally used to treat migraine, neuralgia, anorexia, and rheumatism and as an anthelmintic (Akpulat et al., 2005).
6 Ethnoveterinary applications
Ethnoveterinary medicine (EVM) refers to a complex multifaceted system of beliefs, skills, techniques, and practices used to prevent, treat, and promote the health of husbandry livestock and other income-generating animals (McGaw and Eloff, 2008; McGaw and Abdalla, 2020). Even though these practices have steadily been handed down across generations, a myriad of ethnoveterinary surveys stated that the know-how pertaining to livestock healthcare is mainly retained by elderly people (Bartha et al., 2015; Jamil Ahmed and Murtaza, 2015; Eiki et al., 2021; Güler et al., 2021; Khatib et al., 2022b). Thereby, this ancestral medical wealth may be doomed to disappear with the death of their practitioners.
As such, tremendous efforts are poured into preserving and documenting ethnospecies used in the ethnoveterinary practices of several countries to sustain their empirical medical knowledge for posterity. In the Kyrgyz Republic, nomadic herders are used to cure their livestock by preparing an infusion of T. vulgare flowers, which is subsequently orally or topically administered to cattle to treat parasites, scabies, and osteoporosis (Aldayarov et al., 2022). In Spain, people believed that the tisane made from aerial parts of T. vulgare has aphrodisiac effects in sows (Bonet and Vallès, 2007). In the rural areas of Serbia, T. vulgare aerial parts tea is allegedly prescribed as a remedy to cleanse animals with maggot-infested wounds (Jarić et al., 2014).
T. parthenium is frequently combined with other plants as part of herbal formulas and administered to cattle to cure a variety of conditions. For instance, in the traditional medicine system of Canada, equal amounts of the dried aerial parts of T. parthenium (Widely known as feverfew), Filipendula ulmaria (L.) Maxim., Achillea millefolium L., and Salix alba L. bark or leaves are blended to make a poultice fed to feverish horses (Lans et al., 2006). In Spain, the tisane from the flowering aerial parts of T. parthenium mixed with Plantago lanceolata L., Lippia triphylla (L'Hér.) Kuntze and Triticum aestivum L. is orally fed to cows as a postpartum antiseptic (Bonet and Vallès, 2007).
In summary, several Tanacetum taxa have proved their efficacy in preventing, treating, and promoting livestock health. As such, they may constitute a promising alternative for poorer livestock keepers due to their affordability, easy accessibility, and effectiveness. They may also unlock avenues for new antimicrobial agent discovery and remain a choice for rich livestock raisers, especially if the animal’s market value does not meet the cost of veterinary care.
7 Phytochemistry
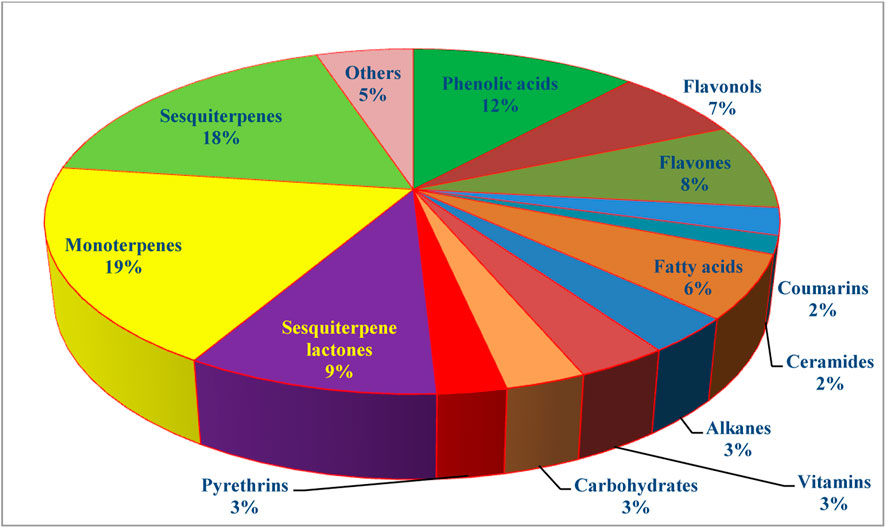
The genus Tanacetum was demonstrated to be a rich source of both secondary and primary metabolites with a broad spectrum of therapeutic merits. Analysis of more than 240 identified metabolites showed that monoterpenes are the preponderant metabolites (19%), followed by sesquiterpenes (18%), flavonoids (15%), phenolic acids (12%), and fatty acids and alkanes (9%) (Figure 5).
7.1 Phenolic acids
Phenolic acids are aromatic acids with a phenolic ring and at least a carboxylic functional group (Kumar and Goel, 2019). They are categorized into two main subclasses, namely, hydroxybenzoic acids and hydroxycinnamic acids (known as phenol carboxylic acids) (Kumar and Goel, 2019). So far, 28 phenolic acids have been identified in the Tanacetum species (1-28), including 22 hydroxycinnamic acids (1-22) and six hydroxybenzoic acids (23-28) (Benedec et al., 2016; Bączek et al., 2017; Devrnja et al., 2017; Rezaei et al., 2017). T. vulgare is the richest source of phenolic acids; eighteen phenolic acids (1, 2, 4, 5, 10-23, and 25) have been successfully found and identified, predominantly from the leaves, flowers, aerial parts, and roots using mainly high-performance liquid chromatography (HPLC). These phenolic compounds are mainly derivatives of p-coumaric acid (6), caffeic acid (2), and ferulic acid (7). Moreover, Eight caffeoylquinic acid derivatives were identified in the aerial parts of two Tanacetum taxa using HPLC fingerprinting analysis, including one in T. balsamita (3) and seven in T. vulgare (12-15, 18, 20, 22) (Yu et al., 2017; Ak et al., 2021).
7.2 Flavonoids
Thirty-five flavonoids (31-65) have been isolated and identified from the aerial parts, leaves, and whole plants of T. vulgare, T. balsamita, T. densum, T. cilicicum, T. parthenium, T. sinaicum, T. parthenifolium, and T. zahlbruckneri. Flavonoids in the genus Tanacetum can be divided into two main subclasses according to their structural variations, namely, flavonols (31-46) and flavones (47-65). The name, species and parts sources, and skeleton types of these metabolites are listed in the (Table 3). Several studies correlated these secondary metabolites with the free radical scavenging capacity. For instance, the hydroethanolic extract from T. balsamita and T. vulgare-air dried whole plant displayed antioxidant capacity at DPPH and FRAP assays (IC50 = 13.59 ± 0.21 µmol Trolox/g extract, IC50 = 13.86 ± 0.19 µmol Trolox/g extract in DPPH assay, respectively, and IC50 = 339.1 ± 17.12 µmol Trolox/g extract, 585.6 ± 2.05 µmol Trolox/g extract in FRAP assay, respectively) (Bączek et al., 2017). The ethanolic extract from flowers and leaves of six Iranian Tanacetum taxa, namely, T. tabrisianum, T. sonboli, T. chiliophyllum, T. hololeucum, T. kotschyi, and T. budjnurdense, displayed in vitro antioxidant activity in the DPPH assay with IC50 values ranging from 59.55 to 157.24 µg/mL (Esmaeili et al., 2010). While these in vitro assays can provide preliminary information on the antioxidant capacity of a compound/extract, in vivo studies are necessary to fully evaluate their pharmacological relevance and explore their safety, efficacy, and potential mechanisms of action.
7.3 Coumarins
Coumarins are naturally occurring phenolic metabolites formed through condensing benzene and β-pyrone rings (Bouhaoui et al., 2021). These secondary compounds are categorized into four basic subgroups; simple coumarins, furanocoumarins, pyranocoumarins, and pyrone-substituted coumarins (4-Hydroxycoumarin, 3-phenylcoumarin, and 3,4-benzocoumarin) (Sarkhail, 2014). To date, 6 simple coumarins (66-71) have already been isolated and identified from the genus Tanacetum using HPLC, TLC, and spectroscopic methods, including NMR, UV, and IR, among others (Table 3). Kisiel and Stojakowski, (1997) have isolated and characterized isofraxidin (66) and 9-epipectachol B (67) from the hexane extract of T. parthenium roots (Kisiel and Stojakowska, 1997). Scopoletin (68) was detected in the aerial parts of four Tanacetum taxa, namely, T. cadmeum, T. ferulaceum, T. parthenium, and T. balsamita (Gonzalez et al., 1990; Susurluk et al., 2007). Scoparone (69) was found in the aerial parts methanolic extract of T. ferulaceum and T. ptarmiciflorum, while 7-hydroxycoumarin (70) was yielded from T. cadmeum and T. mucroniferum (Çalişkan et al., 2004; Triana et al., 2013; Servi̇ and Gören, 2019). Likewise, dimethylfraxetin (71) was reported in the ethanolic extract of T. ferulaceum aboveground parts (Gonzalez et al., 1990).
7.4 Ceramides
Ceramides are bioactive lipids made up of sphingosine and a fatty acid. They are abundantly found throughout chloroplast membranes and are crucial to biological processes, including apoptosis, cell senescence, differentiation, and stresses (Kurz et al., 2019). Indeed, T. artemisioides is almost the only species from the genus Tanacetum that have demonstrated to contain ceramides. Tanacetamides A and B (72, 73) were isolated and structurally elucidated from the chloroform soluble fraction of the whole plant methanolic extract based on 1D and 2D NMR analysis. In the same study, tanacetamides A and B disclosed substantial in vitro acetylcholinesterase inhibitory properties, with IC50 values of 67.1 ± 1.5 and 74.1 ± 5.0 μM, respectively, compared to the standard drug galanthamine (IC50 = 8.5 ± 0.0001 μM) (Ahmad et al., 2004). Likewise, tanacetamides C and D (73, 74), with promising vasorelaxant properties, were isolated and identified from the chloroform fraction of the whole plant methanolic extract (Hussain et al., 2005; Hussain et al., 2010). However, the antihypertensive properties of these ceramides are still poorly understood. Thus, further in vitro and in vivo studies are required to corroborate the vasorelaxant properties of these compounds in line with the traditional usage of T. artemisioides as an antihypertensive agent.
7.5 Fatty acids and alkanes
Phytochemical investigations of three Tanacetum taxa have led to the isolation and identification of fourteen fatty acids. Nine saturated fatty acids (76-78, 80, 83, 85-87, and 89) and five mono-and polyunsaturated fatty acids (79, 81, 82, 84, and 88) were detected in the aerial parts, leaves, and flowers of T. parthenium, T. zahlbruckneri, and T. densum, using GC-MS and HPLC (Table 3) (Caglar et al., 2017; Rezaei et al., 2017; Emre, 2021). On the other hand, Korpinen et al. (2021) identified eight alkanes (90-97) from T. vulgare inflorescences essential oil based on the GC-MS analysis (Korpinen et al., 2021).
7.6 Pyrethrins
In southern Europe, the leaf decoction of several Tanacetum species, such as T. cinerariifolium, T. vulgare and T. balsamita, had been traditionally used as an insect repellent for cattle and children, and as household insecticides to control fleas and body lice (Cumo, 2013; Jeran et al., 2021; Souto et al., 2021). As early as the middle 19th century, the insecticidal properties of Pyrethrum, a natural extract retrieved from T. cinerariifolium flowers, have been widely recognized in the United States and Western Europe. By the early 20th century, Pyrethrum was already used to prevent insect-borne diseases (Malaria, leishmaniasis, and yellow fever, among others) and as an efficient alternative to synthetic pesticides due to its specific effect on target insects, short environmental lifespan (Half-live ranging from 2 h to 2 days), and limited mammalian toxicity (Cumo, 2013; Matsuo, 2019; Lybrand et al., 2020; Souto et al., 2021).
After being harvested, the plant’s flowers are reduced into a powder and then subjected to extraction with organic solvents, such as hexane and petroleum ether (Jeran et al., 2021). After the solvent removal, the active ingredients are recovered as an orange-colored liquid containing six naturally occurring insecticides called pyrethrins (Isman, 2006; Jeran et al., 2021). These metabolites have been identified/quantified as pyrethrin I and II, cinerin I and II, and jasmolin I and II (113-118) using mainly liquid chromatography coupled to UV or DAD detectors (Nagar et al., 2015; Jeran et al., 2021).
7.7 Dietary components
Polle et al. (2001) analyzed and quantified the polysaccharide contents in the roots, sprouts, and floscules of T. vulgare using aqueous ammonium oxalate extraction. They noted the presence of rhamnose, galactose, galacturonic acid, and arabinose residues as the main constituents, whereas glucose, mannose, apiose,2-O-methylxylose, and xylose residues were found in traces (Table 3) (Polle et al., 2001).
Analysis of fat-soluble vitamin contents in two T. densum subspecies (T. densum subsp. laxum and T. densum subsp. subsp. amani) revealed the presence of two forms of vitamin K, namely, vitamin K1 (1.5 ± 0.22 and 0.75 ± 0.19 μg/g, respectively) and vitamin K2 (traces).In addition, two forms of vitamin D (Vitamin D2 and D3), vitamin E (α-tocopherol and β-tocopherol), and vitamin A (Retinol and Retinol acetate) have also been alarmed in the two subspecies (0.05 ± 0.01, 0.05 ± 0.01; 0.1 ± 0.01, 0.2 ± 0.01; 7.3 ± 0.67, 5 ± 0.57; 0.55 ± 0.1, 0.8 ± 0.14; traces, 0.6 ± 0.1, 0.65 ± 0.26 μg/g, respectively) (Emre, 2021).
7.8 Sesquiterpene lactones
A total of 21 sesquiterpene lactones have been identified in the genus Tanacetum, including 8 germacranolide-type sesquiterpene lactones (119-124, 138, and 139) and 13 eudesmane-type sesquiterpene lactones (125-137) (Table 3). Parthenolide (119) was isolated from the hydroethanolic extract of T. parthenium aerial parts (Tiuman et al., 2005). Moreover, the sesquiterpene lactones (120-128) were detected in the aerial parts ethanolic extract of T. ferulaceum. The metabolites (129-133) were found in a petroleum ether extract of T. vulgare flowers, whereas the sesquiterpenes (133-139) were yielded from flowers’ alcoholic extract of T. cinerariifolium (Gonzalez et al., 1990; Rosselli et al., 2012). These metabolites displayed potent antimicrobial, antioxidant, anticancer, anti-inflammatory, and neuroprotective activities (Fischedick et al., 2012; Rosselli et al., 2012).
7.9 Essential oil
The genus Tanacetum is a well-known source of essential oils (EOs) retrieved from various parts, especially aerial parts such as leaves, stems, and flowers using conventional hydrodistillation techniques such as Clevenger-type apparatus and advanced extraction techniques, including microwave-assisted extraction. The EO yields varied considerably between 0.04%–1.09% (v/w), depending on the species, extracted parts, and abiotic and biotic factors (Başer et al., 2001; El-Shazly et al., 2002; Salamci et al., 2007; Elshamy et al., 2021). The volatile constituents have been analyzed and quantified using GC-MS and GC-FID analyses. As such, a wide variety of chemical compounds belonging to diverse groups have been identified. These metabolites are mainly monoterpene hydrocarbons (140-153), oxygenated monoterpenes (154-189), sesquiterpenes hydrocarbons (190-205), oxygenated sesquiterpenes (206-231), and diterpenes (232, and 233) (Table 3).
7.10 Other metabolites
Two cyclitols (29, 30) were detected in a hydroethanolic extract of the aerial parts of T. vulgare (Ak et al., 2021). Moreover, Kubo and Kubo, (1995) have isolated and identified eight α,β-unsaturated aldehydes (234-241) from an hexanic extract of T. balsamita flowers (Kubo and Kubo, 1995).
8 Biological activities
8.1 Antidiabetic activity
Carbohydrates are the primary constituents of the human diet occurring in panoply of beverages and foods. These hydrocarbons in the form of sucrose, starches, and fibers are broken down into glucose, which is subsequently absorbed, causing spikes in the systemic glycemia (Prasad et al., 2019). The cleavage of these macromolecules is under the control of key enzymes involved in carbohydrate digestion, such as α-glucosidase, β-glucosidase, and α-amylase (Al-Zuhair et al., 2010; Ramzi and Hosseininaveh, 2010; Olvera-Sandoval et al., 2022). Thereby, inhibiting or slowing down the activity of these target enzymes may effectively reduce the postprandial hyperglycemia and, therefore, successfully contribute to the management of diabetes mellitus (Khatib et al., 2022a).
In this sense, Özek (2018) evaluated the in vitro α-amylase inhibitory effects of T. praeteritum ssp. praeteritum aerial parts essential oils using the Caraway Somogyi iodine/potassium iodide (IKI) method and acarbose as the reference drug. The author indicated that the essential oil displayed α-amylase inhibitory features with an IC50 value of 0.89 ± 0.13 mg/mL compared to acarbose 0.08 mg/mL. The author attributed the inhibitory effects to the high amount of oxygenated monoterpenes in the EO (Özek, 2018).
Similarly, T. haussknechtii leaves, stems, and capitula essential oils and extracts (Methanol, water, and ethyl acetate), were in vitro assessed by Yur et al. (2017) for their α-amylase inhibitory capacities using the same method (I/KI). The authors observed that the water extracts had no inhibitory action on α-amylase, while the essential oils, methanol, and ethyl acetate extracts exhibited strong activity, with capitula ethyl acetate extract being the most active (356.9 ± 0.06 mg acarbose equivalent/g extract). The noticeable inhibitory effects of ethyl acetate extract were ascribed to the presence of caffeoylquinic acid derivatives endowed with substantial antidiabetic properties such as 1,3-O-dicaffeoylquinic acid, 3,4-O-dicaffeoylquinic acid, and 4,5-O-dicaffeoylquinic acid (Yur et al., 2017). T. balsamita aerial parts ethyl acetate extract displayed moderate inhibitory activity towards α-glucosidase enzyme with an IC50 value of 0.808 mg/mL. In a recent study, Gevrenova et al. (2023) reported that roots methanolic extract of T. balsamita had good α-glucosidase and α-amylase inhibitory effects with IC50 values of 0.71 ± 0.07 mmol acarbose/g and 0.43 ± 0.02 mmol acarbose/g, respectively (Gevrenova et al., 2023). However, no further in vivo studies have been carried out to assess the antidiabetic activity of T. balsamita extracts.
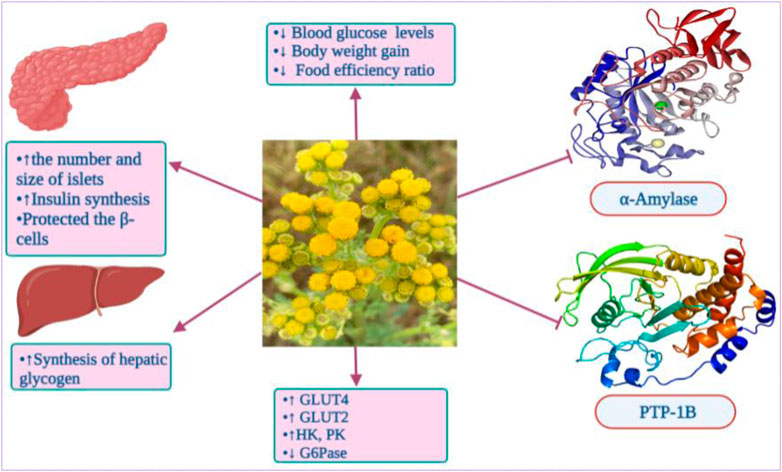
Furthermore, Khan et al. (2018) reported the capacity of T. nubigenum leaves ethanol extract and its butanol fraction at the concentrations of 10 μg/mL and 20 μg/mL to significantly inhibit human recombinant protein tyrosine phosphatase-1B (PTP-1B) up to 63.8%. In the same study, both ethanol and butanol extracts at 10 μg/mL substantially increased the glucose uptake in C2Cl2 cells by 61.2% and 41.2%, respectively (Khan et al., 2018).
On the other hand, the ethanol extract from T. nubigenum leaves at 60 mg/kg of body weight significantly dropped blood glucose level in STZ-induced Sprague-Dawley rats, after 5 h and 24 h by 15.5% and 10.8%, respectively, compared to the standard drug metformin (27.8%, 26.8%, respectively). The butanol fraction from the ethanolic extract showed stronger effects, decreasing the blood glucose levels by 17.9% and 21.3% after 5 h and 24 h, respectively (Khan et al., 2018).
The antidiabetic action of Tanacetum spp. could be attributed to a myriad of active compounds, especially sesquiterpene lactones and phenolic compounds. For instance, parthenolide (119) from T. parthenium suppressed high-glucose stimulating IκBα protein degradation, nuclear factor kappa B (NF-κB) activation, growth factor beta (TGF-β1) and chemoattractant protein-1 (MCP-1) in mesangial cells (MCs) from rats (Jia et al., 2013). Chlorogenic acid, also known as 5-caffeoylquinic acid, has been identified in several Tanacetum leaves and whole plant. Previous clinical trials reported the ability of this phenolic acid to markedly reduce fasting blood glucose when consumed three times a day for 12 weeks at a dose of 400 mg capsules. Chlorogenic acid can improve glucose homeostasis by up-regulating the expression and translocation of glucose transporter type 4 (GLUT-4) in the skeletal muscle of mice models (Figure 6) (Wan et al., 2013). It has also been demonstrated to reduce the expression of serum vascular endothelial growth factor (VEGF)-mediating diabetic retinopathy in mice (Zhou et al., 2016).
However, only four taxa have been evaluated for their in vitro antidiabetic activity, namely, T. praeteritum, T. haussknechtii, T. balsamita, and T. nubigenum. Moreover, T. nubigenum is the only species assessed for its in vivo antidiabetic activity. We also noticed that all the in vitro studies are conducted using the caraway–Somogyi method. Therefore, using the 3,5-dinitrosalicylic acid reagent (DNS) method is recommended due to its ten-time sensitivity compared to the caraway-Somogyi method (Godinho et al., 2014). Likewise, it does not require stoichiometric data and allows comparing both methods. Additionally, further in vivo studies are needed to confirm the in vitro results.
8.2 Antimicrobial activity
Tanacetum species have been widely used for oral hygiene, festering wounds, skin ulcers and contusions, gastroenteritis, and venereal conditions. As demonstrated in (Table 4), the ethnomedicinal application of the plant as a traditional antimicrobial agent have been substantiated by several studies, especially crude extracts, essential oils, and isolated compounds. The antimicrobial potency of ethyl acetate extract (EtOAc) from air-dried aerial parts of T. vulgare was investigated against the caries-inducing fungus C. albicans (ATTC 1023) using the disc diffusion method (Kameri et al., 2019). The EtOAc alone disclosed moderate antifungal activity toward C. albicans after 24 h (IZD = 20 mm) at a dose of 100 mg/mL. The effect was more pronounced when supplementing the extract (100 mg/mL) with 2% chlorhexidine (IZD values ranging from 30 to 32.7 mm, after 5 min, 60 min, and 24 h, suggesting a synergetic effect toward C. albicans (Kameri et al., 2019). Pieces of evidence from a previous study depicted the efficacy of T. balsamita aerial parts EO against dental decay-causing bacteria, including Streptococcus mutans (PTCC 1683), Streptococcus salivarius (PTCC 1448), and Streptococcus sanguinis (PTCC 1449), compared to chlorhexidine and Oral B mouthwashes (Karimzadeh et al., 2021). The previous findings partially support the empirical usage of Tanacetum for dental hygiene, urging that the crude extracts and bioactive constituents could serve as targets for discovering new endodontic therapies.
The hydroethanolic extract (40:60, v/v) and EOs of T. vulgare and T. balsamita exhibited bacteriostatic effects on a broad range of Gram-positive bacteria, displaying MIC values ranging from 1 to 16 mg/mL for crude extracts, and 0.5–8 µg/mL for EOs, except for L. monocytogenes (17/1), which had a relatively higher MIC value (>64 mg/mL) (Table 4). Interestingly, the EO from T. balsamita showed promising bacteriostatic activity toward all the tested pathogenic Gram-negative bacteria, with E. coli (ATCC 25922) and Y. enterocolitica (O3 383/11) being the most prone to the EO (MIC value of 1 µg/mL) (Table 4). The authors suggested that the bacteriostatic activity of crude extracts and EOs could be due to the main volatile and phenolic compounds (camphor, α-thujone, and β-thujone), which mainly act by preventing the synthesis of nucleic acids, disrupting cytoplasmic membrane functions and deregulating bacterial metabolism (Bączek et al., 2017).
The aboveground methanolic extract of eight Serbian endemic medicinal plants, including T. parthenium, was tested against 16 pathogenic bacteria, such as Escherichia coli, Staphylococcus aureus, S. pyogenes, and Pseudomonas aeruginosa, among others, using the micro-well dilution method. Noteworthy, the methanolic extract from T. parthenium aerial parts displayed bactericidal activity against wound swabs-isolated bacteria, namely, S. pyogenes (MIC/MBC = 12.5/12.5 mg/mL) and E. coli (MIC/MBC = 25/50 mg/mL) (Stanković et al., 2016).
Rezazadeh et al. (2014) used the disc diffusion method to assess the antibacterial potency of T. polycephalum air-dried aerial parts EO against three Gram-positive and four Gram-negative bacteria. The essential oil was found to be active against the Gram-positive bacteria; S. epidermidis (ATCC 12228) (IZD = 28 mm), Bacillus subtilis (ATCC 6633) (IZD = 22 mm), and S. aureus subsp. aureus (ATCC 25923) (IZD = 25 mm). Likewise, three Gram-negative bacteria were also susceptible to the EO, namely, E. coli (ATCC 25922) (IZD = 19 mm), Klebsiella pneumonia (ATCC 10031) (IZD = 15 mm), and Salmonella typhi (PTCC 1609) (IZD = 15 mm), while Shigella dysenteriae (PTCC 1188) was relatively resistant (IZD = 5 mm) (Rezazadeh et al., 2014).
The α, β-unsaturated aldehydes (E)-2-decenal (237), (E)-2-undecenal (238), and (E,E)-2,4-decadienal (239) from the hexane extract of T. balsamita flowers, disclosed good antimicrobial activity against the uropathogenic Gram-negative bacteria Proteus vulgaris (MIC values of 12.5, 6.25, and 12.5 µg/mL, respectively). The α, β-unsaturated aldehydes also evidenced important activity toward five yeasts, namely, saccharomyces cerevisiae, Candida utilis, Pityrosporum ovale, Penicillium chrysogenum, and trichophyton mentagrophytes with MIC values within the range 1.56–25 µg/mL (Kubo and Kubo, 1995). The previous results justify the traditional uses of the genus Tanacetum for festering wounds, skin ulcers, and urinary tract infections. The promising MICs and MBCs suggested that the species in the genus warrant further studies to isolate its active components responsible for the bactericidal and fungicidal activities. Further investigations are also needed to screen the unexplored species for their antimicrobial activity.
A petroleum ether soluble fraction (PEE) from T. vulgare rhizome methanolic extract exerted dose-dependent toxic effects toward herpes simplex virus HSV-1 and HSV-2 (IC50 = 256.57 ± 9.27 and 126.29 ± 19.36 µg/mL, respectively) by disrupting the viral adsorption and uncoating. A bio-guided fractionation of PEE has led to the isolation of a spiroketal-enol ether derivative named (E)-2-(2,4-hexadiynyliden)-1,6-dioxaspiro [4.5]dec-3-ene using the thin layer chromatography (TLC) and 1H NMR. Intriguingly, the pure compound demonstrated virucidal activity on HSV-1 and HSV-2 (IC50 = 0.146 ± 0.013 and 0.127 ± 0.009 µg/mL, respectively) compared to the standard acyclovir (IC50 = 0.9 ± 0.01 and 0.7 ± 0.09 µg/mL, respectively). The significant activity of the compound was supposedly related to its capacity to alter viral gene expressions and therefore, the production of viral proteins such as envelope proteins (gG-2) (Álvarez et al., 2011; Álvarez et al., 2015).
The previous results partially validate the ethnomedicinal application of Tanacetum against sexually transmitted diseases, especially those caused by the herpes simplex virus (HSV). However, further investigations are needed to assess the antimicrobial potency of unexplored species on venereal conditions-causing microbes such as gonorrhea, syphilis, and trichomoniasis.
8.3 Anthelmintic activity
Since the 1940s, the overuse of synthetic drugs to boost productivity and control related livestock-infective helminths has led to parasitic resistances, in which pathogenic helminths have evolved elusive ways to circumvent the lethal effects of drug treatment (Dzoyem et al., 2020; McGaw and Abdalla, 2020; Doyle et al., 2022). Several Tanacetum taxa, including T.vulgare, T. balsamita, and T. parthenium, have traditionally been used as a vermifuge to control helminth infections in livestock, especially worms and tapeworms (Table 2).
The hydroethanolic extract and essential oil from T. vulgare aerial parts had significant in vitro schistosomicidal potency against S. mansoni. The crude extract causes 100% mortality of adult worms at doses of 100 and 200 µg/mL by decreasing motor activity and triggering tegumental damage, whereas the EO was only active at 200 µg/mL (Godinho et al., 2014).
A later study showed that the hydroalcoholic extract of T. parthenium aerial parts at a dose of 200 µg/mL killed all the adult parasites of S. mansoni after 48 h. The novelty of the study was the isolation and characterization of apigenin, santin, and parthenolide from the hydroalcoholic extract. Both flavones santin and apigenin were ineffective against S. mansoni adults up to 100 μM, whereas these sesquiterpene lactone parthenolide showed remarkable activity at 12.5 µM, causing 100% mortality, compared to the standard praziquantel (100% of mortality at 5 µM). The significant activity of parthenolide was purportedly related to its ability to reduce motor activity and induce tegumental alterations in schistosomes (de Almeida et al., 2016).
Moreover, the alcoholic extract of T. vulgare leaves and flowers reduced the viability of Echinococcus granulosus in a dose-and-time-dependent manner, causing 97.8% mortality after 30 min at 4 µg/mL (Omer, 2013). The previous findings validate the ethnomedicinal uses of Tanacetum species as a vermifuge, suggesting that T. vulgare could be a potential source for discovering safe and efficacious schistosomicidal compounds. However, further in vivo studies in S. mansoni-infected mice are required to validate the capacity of the plant to treat schistosomiasis.
8.4 Cytotoxic activity
The essential oil retrieved predominantly from the aerial parts of several species, including T. balsamita and T. vulgare, along with its main compounds, showed moderate cytotoxic properties, whereas minor compounds revealed remarkable in vitro cytotoxicity, indicating that the anticancer activity of the EOs may be driven by these constituents or a potential synergetic action of the entire mixture.
Gospodinova et al. (2014) used the MTT assay to examine the cytotoxic effects of the crude aqueous ethanolic extract from T. vulgare overground parts against the human breast cancer cell line (MCF7). The authors witnessed a time- and dose-dependent decrease in the cell viability with an IC50 value of 286.8 μg/mL after 72 h (Gospodinova et al., 2014). Moreover, five sesquiterpene lactones with the eudesmane skeleton from T. vulgare flowers dichloromethane extract were isolated and tested by Rosselli et al. (2012) for their in vitro cytotoxic activities against human lung cancer cells (A549) and hamster lung fibroblast cells (V79379A).Based on the 13C-NMR data, the authors identified these compounds as douglanin (129), ludovicin A (130), ludovicin B (131), 1α-hydroxy-1-deoxoarglanine (132), and 11,13-dehydrosantonin (133). Accordingly, the isolated compounds disclosed significant time- and dose-dependent cytotoxic effects toward A549 with IC50 values ranging from 15.3 ± 0.1 to 59.4 ± 3.9 μM compared to the standard anticancer drug cisplatin 7.7 ± 2.1 μM. The cytotoxic properties of the five sesquiterpene lactones seem to be linked to their ability to induce apoptosis through the mitochondrial pathway. The authors concluded that these compounds could not disappointedly be used as anticancer drugs due to their non-selective nature against V79379A healthy cells (Rosselli et al., 2012). However, synthesizing derivatives of these compounds could be an effective approach to increase their selective distribution to cancer cells, while reducing their adverse effects on healthy normal cells.
Moreover, the essential oils obtained from the aerial parts of T. vulgare exhibited in vitro anticancer properties against both A-549 and healthy fibroblast cell line WS1 with IC50 values exceeding 200 μg/mL. Remarkably, colon adenocarcinoma cell lines DLD-1were the most susceptible to the EOs, with an IC50 value of 105 μg/mL. The authors indicated that the EOs’ preponderant compounds, namely, borneol, camphor, and 1,8-cineole had moderate cytotoxic potencies, while some minor volatile compounds such as β-pinene, caryophyllene oxide, β-caryophyllene, camphene, and γ-terpinene displayed promising activities (IC50 values ranging from 28 to 112 μg/mL) (Coté et al., 2017).
In another study, the cytotoxic effects of T. vulgar, T. macrophyllum, and T. corymbosum aerial parts crude chloroform extracts were investigated against human melanoma cells (A375), human cervical cancer cells (Hela), and Chinese hamster lung fibroblast cells (V79). The MTT test was performed to assess any decline in the cell viability of the tested cell lines. The authors recorded a dose-dependent reduction in cell viability, with HeLa cells being the most prone to the extracts displaying an inhibition rate ranging from 69.87% to 93.71% at the highest dosage of 200 μg/mL. The authors also reported the capacity of T. vulgare chloroform extract to induce apoptosis through the mitochondrial pathway, trigger DNA damage, and disrupt the cell cycle progression of V79 and A375 cells at the G2/M phase (Figure 7). In this study, the pronounced cytotoxic activity of T. vulgare was mainly associated with the presence of two trimethoxyflavone compounds, namely, eupatorin (41.92 μg/g dry weight of the plant) and eupatilin (0.31 μg/g dw plant) (Ivănescu et al., 2021).
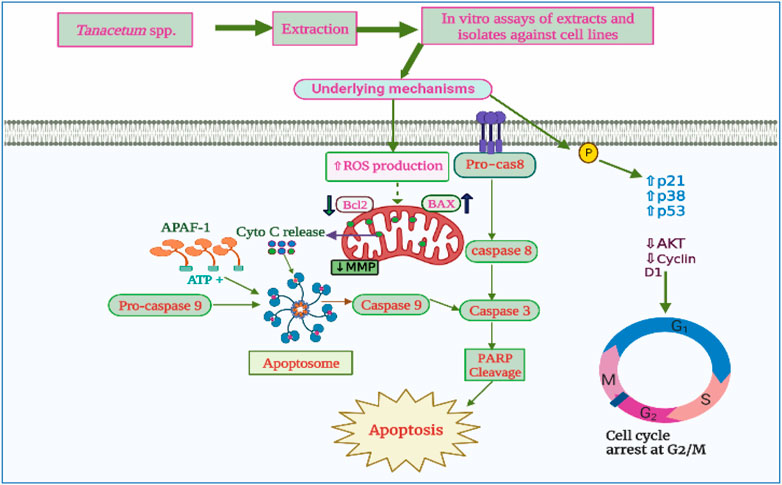
FIGURE 7. Anticancer activity of Tanacetum spp. extracts and isolates 2023©. The crude extracts and isolated compounds from Tanacetum triggered the intrinsic pathway of apoptosis in various cancer cells by increasing ROS production, Bax/Bcl2 ratio, cytochrome C release, and activating caspase cascade pathway. They also induced cell cycle block at the G2/M phase.
On the other hand, nanoparticles (NP)-based drug delivery systems have proved several benefits, including accurate targeting of cancer cells, substantial drop in adverse effects and multi-drug resistance. In a recent in vitro study, Alipanah et al. (2021) stated that carvone (160) and EOs from T. balsamita exhibited weak cytotoxic effects toward breast cancer cells (MDA-MB468) and human melanoma (A375) cell lines with IC50 values of (3657.4, 6038.0 μg/mL) and (1312.1, 2323.6 μg/mL), respectively. Statistical analysis revealed that the cytotoxic activity of both carvone and EOs on A375 cells was not significantly different (p > 0.05), whereas EOs was slightly more efficient than carvone toward MDA-MB468 cells (p > 0.05). Subsequently, chitosan nanoparticles containing carvone and EOs of T. balsamita were prepared to improve their cytotoxic efficacies. Accordingly, chitosan nanoparticles containing T. balsamita EOs showed the best activity against both cell lines (A375 and MDA-MB468), with IC50 values of 85.3 and 240.1 µg/mL, respectively (Alipanah et al., 2021).
8.5 Antioxidant and hepatoprotective activities
The genus Tanacetum has been traditionally used to manage several oxidative stress-related diseases such as diabetes, hypercholesterolemia, and nerve system-related conditions. Various in vitro and in vivo studies have corroborated the ethnopharmacological uses of Tanacetum spp. as a traditional antioxidant remedy.
Pretreatment with the ethanolic extract from flowers and leaves of six Iranian Tanacetum taxa, namely, T. tabrisianum, T. sonboli, T. chiliophyllum, T. hololeucum, T. kotschyi, and T. budjnurdense at doses ranging from 10 to 100 µg/mL suppressed oxidative stress in hydrogen peroxide (H2O2)-treated K562 cells by increasing the intracellular glutathione (GSH), decreasing reactive oxygen species (ROS), glutathione peroxidase (GPx), and glutathione reductase (GR) activities (Esmaeili et al., 2010).
In another study, the pre-treatment and post-treatment of 70% methanolic extract of T. parthenium at doses of 80 and 120 mg/kg exhibited hepatoprotective effects in CCl4-induced liver damage in rats by substantially dropping LDL levels, total cholesterol, triglyceride, and glucose levels, compared to non-treated groups. The extract also increased HDL and albumin levels and brought antioxidant enzymes to near-normal ranges (AST, ALT, SOD, and GPx), indicating its capacity to prevent enzyme leakage and stabilize the cell membranes. The hepatoprotective effects were associated with tannins and flavonoids-rich methanolic extract (Mahmoodzadeh et al., 2017).
Elven guaianolides, germacranolides, and eudesmanolides sesquiterpene lactones from ethanolic extract of T. parthenium aerial parts, including parthenolide, 11,13dihydroparthenolide, 3-hydroxyparthenolide, santamarine, artemorin, and reynosin with α-methylene-γ-lactone moiety, were able to activate the nuclear factor E2-related factor 2 (Nrf2) through binding to antioxidant response element (ARE) in the genes’ promoter of mouse primary cortical neurons (Fischedick et al., 2012). Therefore, sesquiterpene lactones, tannins, and flavonoids from T. parthenium could be used as a template for developing new neurodegenerative and hepatoprotective drugs.
8.6 Antispasmodic activity
Ahmadnejad-Asl-Gavgani et al. (2022) investigated the anti-spasmodic properties of T. balsamita EO and its major component (carvone) on spasmogen-induced contractions in bovine ileum smooth muscle obtained from slaughtered bulls by adding nine cumulative concentrations from 0.10 to 1000 μg/mL to the tissue samples. Results showed that EO and its major constituent carvone remarkably reduced the in vitro spontaneous and spasmogen-induced contractions in ileum circular smooth muscle through inhibiting Ca++ channels in smooth muscle. The authors recommended T. balsamita as a strong candidate for treating hypermobility and intestinal spasms (Ahmadnejad-Asl-Gavgani et al., 2022).
8.7 Immunomodulatory activity
Polysaccharide-rich fractions from T. vulgare florets at doses 200–1600 μg/mL improved the immunomodulatory functions in murine J774.A1 macrophages by activating and increasing nitric oxide (NO) and reactive oxygen species (ROS) production, and tumor necrosis factor α (TNF-α).The polysaccharide fractions dose-dependently prevented erythrocyte hemolysis due to their ability to fix complement (serum proteins) compared to heparin, a complement fixing agent (Xie et al., 2007).
A flavonoid-rich extract from T. parthenium pollen grains at doses of 50 and 70 mg/kg significantly increased delayed-type hypersensitivity (DHR) and lymphocyte immune response in male Balb/C mice compared to non-treated animals (Jannesar et al., 2014). Previous studies suggest that Tansy polysaccharides can be used as a scaffold for new immunotherapeutic adjuvants.
8.8 Anti-inflammatory and antinociceptive activities
To bolster the ethnomedicinal claims of T. balsamita as a traditional anti-inflammatory remedy, Sharif et al. (2020) evaluated the in vivo acute anti-inflammatory activity of the aerial parts EOs in carrageenan-induced paw edema in a rat model at dosages of 100, 150, and 250 mg/kg. Mefenamic acid at 30 mg/kg served as the standard drug. Findings showed that the EO at 100 and 150 mg/kg failed to reduce carrageenan-induced paw edema compared to the reference drug. However, at a concentration of 250 mg/kg, the EO drastically (p < 0.05) lowers the carrageenan-induced rat paw edema production (54.91%), especially during the third hour of the test. The authors attributed the anti-inflammatory effects to the oxygenated monoterpene carvone (Sharif et al., 2020).
In a similar in vitro study, Karaca et al. (2009) stated that diethyl ether extract of T. balsamita above-ground parts at doses of 25, 50, and 100 mg/kg substantially suppressed carrageenan-induced paw edema formation in rats. The anti-inflammatory activity was linked to the presence of considerable amounts of flavonoids and their inhibitory action on inflammation mediators such as inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) (Karaca et al., 2009).
Furthermore, a water extract containing 0.5% parthenolide from T. parthenium was investigated by Recinella et al. (2021) for its in vitro neuromodulatory and anti-inflammatory effects. The extract effectively reduced the release of prostaglandin (PGE2), extracellular dopamine, and IL-1β gene expression in hypothalamic Hypo-E22 cells, while increased dopamine transporter (DAT), IL-1β, IL-10 and brain-derived neurotrophic factor (BDNF) genes expression. The authors concluded that targeting dopaminergic pathways could be an effective therapeutic approach in preventing and managing migraine attacks (Recinella et al., 2021).
Moreover, extracts of T. parthenium (ethanol, acetone and a mixture of water, acetone, and alcohol) depleted of the sesquiterpene lactone parthenolide (a skin sensitizer) inhibited pro-inflammatory enzymes such as phosphodiesterase-3 and 4 and 5-lipoxygenase in murine macrophages. Extracts also showed the ability to inhibit pro-inflammatory mediators, including TNF-α, nitric oxide, PGE2, IFN-γ, IL-2, and IL-4. According to the authors, extracts depleted of parthenolide were effective in alleviating inflammation without stimulating the immune system (Sur et al., 2009).
On the other hand, several straightforward assays, including the writhing test, tail-flick test, formalin test, and hot-plate test, have been used to validate the antinociceptive properties of T. balsamita as a traditional painkiller remedy. Sharif et al. (2020) performed the hot plate test to assess the in vivo anti-nociceptive activity of T. balsamita aerial parts EOs at the dosages of 50, 100, 200, and 400 mg/kg. The authors stated that the EOs at 25 and 50 mg/kg did not affect the reaction time to the heat source compared to the conventional drug morphine. However, at a dose of 100 mg/kg, the EO displayed significant anti-nociceptive activity by delaying the response time to the thermal stimulant. The authors reported that the antinociceptive mechanism of the essential oil at this concentration was most likely non-opioid (Sharif et al., 2020).
8.9 Insecticidal, larvicidal, and repellent activities
Several studies have validated the traditional uses of Tanacetum spp. as insecticidal, larvicidal, and repellent agents. Attighi Lorestani et al. (2013) investigated the fumigant toxicity of T. balsamita essential oil against eggs and adults of Callosobruchus maculatus F. (Cowpea weevil) at doses ranging from 5.3 to 17.4 μL/Lair for eggs, and 5.12–20.24 μL/Lair for adults. The authors found that the essential oil had dose- and time-dependent fumigant toxicity, with the treated adults being generally more vulnerable than eggs. The lowest concentration of 5.12 μL/lair caused a mortality rate of 59.82% in adults, while a mortality rate of 22.21% was recorded in eggs at the lowest concentration of 5.3 μL/Lair after 72 h of exposure. The authors attributed the significant fumigant toxicity to the major constituents of T. balsamita, such as camphor, bornyl acetate, pinocarvone, and terpinolene (Attighi Lorestani et al., 2013).
Similarly, Gokturk et al. (2017) revealed that T. balsamita EO at the dosage of 20 μL/Petri causes a mortality rate of 34.4% in Hyphantria cunea (Drury) (White Butterfly) after 96 h of exposure (Gokturk et al., 2017). In another study, Grulova et_al., 2017 tested the repellency effects of the EOs of six medical plants, including T. balsamita toward Rhopalosiphum padi L, a major pest in cereal crops, using doses ranging from 0.1% to 1%. Costmary EO disclosed a dose-dependent repellency effect, which was more pronounced at the highest concentration of 1.0% (Grulova et al., 2017).
Moreover, CO2 essential oil and extract of T. parthenium aerial parts exhibited antifeedant and growth inhibition on Spodoptera littoralis (Boisduval) larvae with LD50 values of 0.05 and 0.11 µL/g, respectively (Pavela et al., 2010). Additionally, the 80% ethanolic extract of powdered T. parthenium was tested by Erdoğan and Yıldırım (2016) on the green peach aphid Myzus persicae Sulzer using leaf-dipping and spraying methods. The extract showed aphidicidal activity against nymphs and adults when diluted at 6% and 12%. Mortality rates of 82% and 88% were observed against nymphs, and 75% and 88% against adults, respectively. In the spraying method, the extract at 6% and 12% caused 70% and 87% of mortality among adults of M. persicae Sulzer (Erdoğan and Yıldırım, 2016).
8.10 Anticholinesterase activity
The acetonitrile extracts of leaf and flowers of several Tanacetum species were investigated for cholinesterase inhibitory activity at 100 µg/mL. The extracts significantly inhibited acetylcholinesterase (AChE) with T. argenteum subsp. flabellifolium having the highest inhibition (96.68% ± 0.35%), whereas a moderate activity was observed against butyrylcholinesterase (BChE) (Orhan et al., 2015).
9 Clinical evidence
According to the Cochrane library database, there are 36 documents dealing with clinical studies of the genus Tanacetum, especially T. parthenium (feverfew).The first report about the prophylactic properties of feverfew surfaced in British Health Magazine in 1978, documenting the case of a patient suffering from migraine attacks since the age of 16. At the age of 68, she commenced taking three feverfew leaves daily for 10 months, and her terrible headache entirely ceased (Pareek et al., 2011). Afterward, several double-blind, randomized controlled trials (RCTs) have been enrolled to examine the safety and the clinical effectiveness of feverfew-based nutraceutical formulations for episodic migraines without aura (as a symptomatic treatment) and as a prophylactic therapy for migraine with aura. For instance, a double-blind, placebo-controlled clinical study (n = 57) was undertaken by Palevitch et al. (1997) to explore the efficacy of feverfew leaves as a prophylactic measure towards migraine attacks and their commonly associated symptoms such as nausea, vomiting, and light sensitivity. Results revealed that feverfew substantially reduced the severity of pain by 4.27 scale points compared with the placebo. Additionally, the authors reported a noticeable decrease in the intensity of the typical migraine symptoms, including nausea, vomiting, and sensitivity to light and sound (Palevitch et al., 1997). However, some patients claimed incapacitating headaches as a result of the quick withdrawal of feverfew after switching to the placebo medication.
In a recent study, oral administration of a fixed dose of Partena (2 tablets per day), consisting of riboflavin, magnesium, CQ10, and T. parthenium, significantly dropped headache frequencies (50%) among pediatric patients having tension-type headaches (TTH) (n = 91) after 16 weeks. However, 4.4% of the patients claimed to have gastrointestinal symptoms and interrupted the treatment (Moscano et al., 2019).
A double-blind, placebo-controlled, multicenter open-label randomized controlled trial (RCT) was conducted by Diener et al. (2005) to assess the prophylactic effects of feverfew CO2-extract in patients (n = 218) diagnosed with migraine with or without aura according to the IHS criteria. Data from 170 patients showed that extract at a dose of 6.25 mg significantly decreased the monthly migraine frequency attacks from 4.8 to 2.9 (p = 0.0456) compared to a placebo (4.8–3.5) between weeks 5 and 12 (Diener et al., 2005).
Evidence from studies showed that the anti-migraine properties of feverfew are likely associated with the stimulation of cytokines, and the suppression of nitric oxide production, serotonin release from platelets, nuclear factor-kappa B(NF-κB), and CGRP (calcitonin gene-related peptide) from the trigeminovascular system (Moscano et al., 2019).
However, we noticed that the studies were relatively small in size (ranging from 17 to 218 participants). Therefore, their statistical analyses could be biased, due to the hazards of random chance, which can make small sample sizes prone to overestimation. Therefore, long-term clinical trials with relatively larger sizes and rigorous methodologies are required to validate the efficacy and the safety of feverfew in preventing and treating migraine attacks.
10 Toxicity
Tanacetum spp. extracts have been evaluated by several research groups for acute or/and chronic toxicity and safety. Yousefzadi et al. (2009) evaluated the cytotoxic effect of T. balsamita aerial parts EOs towards monkey kidney (Vero) and human fetal skin fibroblast (HFSF) cell lines using the MTT assay. Accordingly, a weak cytotoxic effect has been noticed against both cell lines with IC50 values of 2500 and 1250 μg/mL, respectively. The results from this study may indicate the safe use of the plant’s EOs. However, further in vivo acute and chronic toxicity studies are required to validate the plant’ safety (Yousefzadi et al., 2009).
Lahlou et al. (2008) stated that oral and intraperitoneal administration of single doses (0–13 g/kg and 0–4.5 g/kg, respectively) of an aqueous extract from T. vulgare leaves for 90 days had insignificant acute and chronic toxicity in rodents due to relatively no-observed adverse effect levels (NOAEL) values (9.0 g/kg and 1.5 g/kg, respectively) and absence of noticeable effects on rats’ hematological and biological parameters after 90 days in rats (Lahlou et al., 2008). The potential acute and chronic toxicities of extracts and isolated compounds, especially sesquiterpene lactones, should be further investigated.
11 Potential use of the genus Tanacetum as natural food preservative
The widespread distrust towards synthetic additives puts increasing pressure to seek natural and health-beneficial substitutes for chemical additives (Karimi et al., 2021). The use of plant-based extracts and essential oils as natural antimicrobial and antioxidants has been corroborated by a plethora of studies, which constitute a renewable supply of active agents for eco-friendly food packaging (Carocho et al., 2014; Karimi et al., 2021).
In this sense, Khodayari et al. (2019) investigated the effect of the poly (lactic acid) composite film containing 2% T. balsamita EO (TBE), 1% cellulose nanocrystals composite (CNC), and 2% propolis ethanolic extract (PEE) on vacuum-packed sausages shelf life. Results revealed that the prepared film disclosed potent antimicrobial capacity and significantly prolonged cooked sausages’ shelf life by 50 days of refrigerated storage. They also showed that the active film was especially active against the Gram-positive bacteria, with B. cereus being the most susceptible. In the same study, the authors witnessed a synergic effect between T. balsamita EO (TBE) and propolis ethanolic extract and reported that TBE operated as a plasticizer on the blend (Khodayari et al., 2019). In another study, Nobakht and Moghaddam, (2013) reported the capacity of 1.5% and 2% of costmary added to laying hens’ diets to improve their overall performance, blood biochemical parameters, and egg characteristics (Nobakht and Moghaddam, 2013).
Altogether, the genus Tanacetum might be a valuable repository of chemical compounds that could be exploited as natural food additives and as a platform for biodegradable active packaging development in the food industries.
12 General discussion
Tanacetum species carry a long history of traditional uses in various fields, including medicine, cosmetics, agriculture, and cuisines. Overall, various ethnomedicinal applications have been recently supported through in vitro and in vivo pharmacological studies. For instance, the use of the several Tanacetum species, including T. vulgare, T. balsamita, and T. parthenium for festering wounds, skin ulcers, urinary tract infections, and gastrointestinal and venereal conditions is evident from their antibacterial, antiviral, and antispasmodic activities. The use of T. vulgare for oral hygiene has been validated by the in vitro inhibition of cariogenic oral bacteria, mainly Streptococcus mutans. The use of T. vulgare and T. parthenium as a vermifuge has been confirmed by in vitro anthelmintic studies. In addition, the use of T. vulgare, T. balsamita, and T. parthenium against inflammation, pain, and fever was backed up by their anti-inflammatory and antinociceptive activities, mainly through inhibiting pro-inflammatory mediators’ release, such as nitric oxide, TNF-α, PGE2, IL-2, IL-4, and IFN-γ. Ethnopharmacological studies have also documented the use of several Tanacetum species for liver disorders. Pharmacological investigations have confirmed this usage by establishing its hepatorestorative action against hepatotoxicity induced by various substances, such as CCl4, in animal studies. The use of T. vulgar, T. balsamita, T. macrophyllum, and T. corymbosum for cancer treatment has been supported by cytotoxic studies against various cancer cell lines. The ethnomedicinal use of Tanacetum spp. for diabetes management has been proven by its α-glucosidase, α-amylase, and protein tyrosine phosphatase-1B (PTP-1B) activity, as well as in a STZ-induced Sprague-Dawley rat’s model. However, the use of the Tanacetum spp. for bile acid deficiency, arthritis, gout, rheumatism, anemia, and as a litholytic, diaphoretic, and antivenom has not yet been checked. Table 5 and Figure 8 include further details about the validated traditional uses to establish a basis for future studies and help to fulfill the research gaps. The following paragraphs provide additional insights into the validated traditional uses and research gaps.
First of all, most of the reported antimicrobial studies focused on crude extracts rather than isolated compounds. Therefore, it is likely that the reported antimicrobial activity is due to a synergistic effect of the active metabolites present in the plant extracts. Accordingly, species in the genus warrant further investigations to isolate potentially active compounds that could be involved in the bactericidal, virucidal and fungicidal properties. Moreover, most of these studies have tended to use disc diffusion assay, which is unreliable for measuring antimicrobial activity since the compounds’ polarity impact how effectively they diffuse into the polar agar medium and consequently alter the inhibition zone size. In contrast, agar dilution and broth microdilution methods enable precise quantitative conclusions by determining MIC values for antimicrobials (Khatib et al., 2022b). Therefore, they are highly recommended in future studies for regular antimicrobial susceptibility testing. In line with the traditional uses of the genus Tanacetum against venereal conditions, the anti-infective activities of crude extracts and isolated compounds may potentially consider microbial threatening diseases, including the resistant strains of gonorrhea Neisseria gonorrhoeae and bacteria causing sexually transmitted infections (STI) such as Chlamydia Trachomatis and Mycoplasma genitalium, and so forth.
Several taxa from the genus, especially T. balsamita and T. vulgare, are still appreciated in the traditional cuisine of several countries, including Italy and Russia, owing to their pleasant aroma and bitter taste. Perhaps their usage could also be justified by their richness in minerals, vitamins (A, D, E, K), mono- and polysaccharides (rhamnose, galactose, glucose, mannose, apiose, and xylose), and other crucial elements of a balanced diet. Previous studies highlighted the capacity of these metabolites to protect many target tissues against oxidative stress-induced diseases (e.g., neurological, cardiovascular, and liver diseases, among others) (Uberti et al., 2014; Zeng et al., 2017). Hence, ROS and free radicals scavenging, ferric reducing capacity, as well as the rise in physiological antioxidants, including SOD, AST, ALT, HDL, and GPx, could be attributed to the antioxidant vitamins and polyphenolic content.
Ethnopharmacological studies reported that T. artemisioides whole plant is used indigenously for high blood pressure and neurological conditions. Though scientific reports on T. artemisioides’ neuroprotective potency are still scarce or even missing, Ahmad et al. (2004) reported the isolation of two newly identified ceramides called tanacetamide A and B from a methanolic extract of T. artemisioides. The newly identified compounds exhibited remarkable in vitro acetylcholinesterase inhibitory properties, with IC50 values of 67.1 ± 1.5 and 74.1 ± 5.0 μM, respectively, compared to the standard galanthamine (IC50 = 8.5 ± 0.0001 μM) (Ahmad et al., 2004).
Indeed, several hypotheses have been put forward to explain the pathogenesis of Alzheimer’s disease. One of them is known as the Cholinergic Hypothesis, describing the inhibition of Cholinesterase (ChE) enzyme family (Kumar et al., 2022). Thus, these metabolites may lead to a breakthrough in disease treatment. However, further studies are required to evaluate the potency of these ceramides to interfere with the amyloid-β (Aβ) pathway. In addition, drug-drug interactions, particularly those involving anticoagulants and antiplatelet drugs, should be carefully examined, as well as the risk-to-benefit ratio for isolated compounds can be established through long-term multicenter trials with large sample sizes and rigorous methodologies. Besides, the structure-activity relationship tool (SAR) can be used to modify and optimize these compounds to compete with current market drugs.
Hypertension is a global health problem involving the interaction of genetic and environmental factors. Indeed, it is associated with an increased risk of stroke, cardiovascular and kidney diseases (Donfack et al., 2021). Ethnobotanical studies highlighted the extensive usage of T. artemisioides for high blood pressure. Although the available data revealed a critical shortage on Tanacetum’ antihypertensive activity, a study showed that the sphingosine-type tanacetamide isolated from an aqueous extract of Vitex cienkowskii stem bark displayed potent vasorelaxant activity through increasing the endothelial production of nitric oxide (NO) and the activation of vascular smooth muscle soluble guanylate cyclase (sGC) (Dongmo et al., 2011). Therefore, the ethnomedicinal usage of T. artemisioides as a hypotensive agent could be attributed to the presence of tanacetamide (A-D). For this reason, in vivo and in vitro studies on the antihypertensive potency of T. artemisioides crude extracts and isolated tanacetamide (A-D) are of utmost necessity, especially against the angiotensin-converting enzyme (ACE).
T. vulgar, T. macrophyllum, and T. corymbosum extracts and isolated compounds displayed promising in vitro cytotoxic activity against various cancer cell lines through triggering the intrinsic pathway of apoptosis, increasing ROS production, Bax/Bcl2 ratio, cytochrome C release, and activating caspase cascade pathway. They also showed the capacity to induce cell cycle block at the G2/M phase. Sesquiterpene lactones with the eudesmane skeleton, including douglanin (129), ludovicin A (130), ludovicin B (131), 1α-hydroxy-1-deoxoarglanine (132) have been correlated with the cytotoxic activity of Tanacetum spp. suggesting, therefore, that these metabolites could be behind the significant cytotoxic activity of these species, as well as solid leads of anticancer compounds.
A prominent hepatoprotective activity of Tanacetum spp. has been noticed by restoring antioxidant enzymes, biochemical factors, lipid peroxidation, and liver enzymes in animal models. In fact, a careful examination showed that high doses have been used in the reported studies (400 mg/kg). When used in such doses, it may cause harmful or severe adverse effects on humans. Thus, further studies with tolerable doses to be used in human subjects are required. The hepatoprotective tests may also consider noting the dose and range utilized the animal type, number, sex, the drug vehicle, and the method of anesthesia and/or killing (appropriate).
In conclusion, Tanacetum spp. and their isolated compounds showed broad and significant therapeutic merits both in vitro and in vivo. However, numerous studies had some gaps to be addressed by more studies. The current review help-build a foundation for further research.
13 Conclusion and recommendations
The genus Tanacetum has been ethnopharmacologically used to treat numerous diseases such as arthritis and fever, hypertension, nausea, kidney problems, dyspepsia, stomach pain and bloating, diabetes, festering wounds, flu and cold, and migraine. Several pharmacological studies have supported enormous traditional uses such as anthelmintic, antidiabetic, anticancer, antioxidant, insecticide, and hepatoprotective activities as well as against skin ulcers, festering wounds, urinary tract infections, and sexually transmitted diseases. An extensive literature search using various online search engines showed that ethnobotanical data for only 16 taxa (10%) out of 160 accepted were available. Hence, further ethnobotanical surveys should be undertaken to document and preserve the folkloric knowledge of the remaining species.
Moreover, several species are reportedly under critical threat of extinction by the International Union for Conservation of Nature (IUCN), especially T. ptarmiciflorum, T. oxystegium, and T. oshanahanii, and were included on the critically endangered species red list. Also, there were only eight species (5%) out of 160 accepted taxa from the genus evaluated by the International Union for Conservation of Nature (IUCN) for their statuses. Therefore, a large-scale risk assessment and ex-situ and in-situ measures are necessary to ensure the sustainability of the genus and prevent its extinction.
Ceramides such as tanacetamide A-D (72-75), pyrethrins I and II (113, 114), cinerin I and II (115, 116), and jasmolin I and II (117, 118)) could serve as chemotaxonomic markers of the genus Tanacetum due to their restricted occurrence within the genus Tanacetum. They may serve along with DNA barcoding methods as crucial tools to resolve the controversial infrageneric classification of the genus Tanacetum and ensure its quality control. Despite their relatively toxic nature, these compounds exhibit numerous interesting pharmacological properties such as anti-acetylcholinesterase, antihypertensive, antimicrobial, neuroprotective and cytotoxic activities. Thus, further investigations should be done to investigate the unexplored biological activities of these compounds in line with traditional uses of Tanacetum species.
The anticancer activity of crude extracts and isolated metabolites, especially sesquiterpene lactones, are based on preliminary cytotoxic studies. While providing valuable insight into the cellular mechanisms underlying the anticancer effects, as well as initial data on their toxicity and selectivity, they do not fully capture the complexity of the in vivo environment, especially the intercellular and tissue interactions, which can influence drug metabolism, distribution, and toxicity. Thus, in vivo studies are needed to validate the in vitro studies and determine the pharmacological relevance of these metabolites, while providing insight into their efficacy, safety, and pharmacokinetics, as well as their potential impact on the host organism. Similarly, the antidiabetic studies are mostly based on in vitro models. Therefore, further in vivo studies on animal models are needed to identify potential therapeutic targets and evaluate the effectiveness and safety of extracts and isolated metabolites from the genus species.
Finally, some studies, especially those evaluating the anti-inflammatory, antimicrobial, and insecticidal activities of Tanacetum spp. are poorly reported due to the lack of positive control and high doses used, which make their findings less reliable.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
LB and SK designed the study, drafted the manuscript, collected and arranged the references; LB, CF, and MS analyzed the data, reviewed and edited the manuscript; LB and MS supervised the final version of the paper. All authors have read and agreed to the submitted version of the manuscript.
Acknowledgments
We would like to thank Sultan Moulay Slimane University, Beni-Mellal, Mohammed VI Polytechnic University, Benguerir, Morocco, and Istituto per la BioEconomia, IBE, CNR Florence, Italy for the partial support of this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
Ace, Acetone; ALT, Alanine transaminase; AmOx, Ammonium oxalate; AST, Aspartate aminotransferase; Bax, Bcl-2 Associated X-protein; Bcl2, B-cell lymphoma 2; CC, Column chromatography; CH2Cl2, Dichloromethane; DPPH, 1,1-diphenyl-2-picrylhydrazyl; Dw, Dry weight; EO, Essential oil; EtOAc, Ethyl acetate; FRAP, Ferric ion reducing antioxidant power; HDL, High-density lipoprotein; HPLC, High-Performance Liquid Chromatography; IHS, The official International Headache Society; i-PrOH, Isopropanol; IUCN, The International Union for Conservation of Nature; IZD, Inhibition zone diameter; LDL, Low-density lipoprotein; MCP-1, Chemoattractant protein-1; MeOH, Methanol; MFC, Minimum fungicidal concentration; NF-κB, Nuclear factor kappa B; PEE, Petroleum ether; SFE, Supercritical fluid extraction; SOD, Superoxide dismutase; TGF-β1, Growth factor beta; TLC, Thin Layer Chromatography.
References
Abad, M. J., Bermejo, P., and Villar, A. (1995). An approach to the genus Tanacetum L. (Compositae): Phytochemical and pharmacological review. Phytother. Res. 9, 79–92. doi:10.1002/ptr.2650090202
A. M. Abbasi, and R. W. Bussmann eds. (2021). Ethnobiology of mountain communities in Asia. Springer Nature Switzerland AG. Cham, Switzerland: Springer.
Abbaszadeh, S., Teimouri, H. T., and Farzan, B. (2019). Ethno-botanical study of sedative medicinal plants in Shahrekord. Egypt. J. Vet. Sci. 50, 99–105. doi:10.21608/ejvs.2019.12613.1078
Agelet, A., Bonet, M. À., and Vallès, J. (2000). Homegardens and their role as a main source of medicinal plants in mountain regions of Catalonia (Iberian Peninsula). Econ. Bot. 54, 295–309. doi:10.1007/bf02864783
Ahmad, V. U., Hussain, J., Hussain, H., Farooq, U., Akber, E., Nawaz, S. A., et al. (2004). Two ceramides from Tanacetum artemesioides. Z. Für Naturforsch. B 59, 329–333. doi:10.1515/znb-2004-0316
Ahmadnejad-Asl-Gavgani, M., Maham, M., and Dalair-Naghadeh, B. (2022). In vitro effects of essential oils of Tanacetum balsamita and carvone on the contractility of bovine ileum smooth muscles. Vet. Res. Forum Int. Q. J. 13, 29–37. doi:10.30466/vrf.2021.521204.3118
Ahmed, S., Hasan, M., and Mahmood, Z. (2016). Urolithiasis management and treatment: Exploring historical vistas of Greco-Arabic contribution. J. Pharmacogn. Phytochem. 5, 167–178.
Ak, G., Gevrenova, R., Sinan, K. I., Zengin, G., Zheleva, D., Mahomoodally, M. F., et al. (2021). Tanacetum vulgare L. (Tansy) as an effective bioresource with promising pharmacological effects from natural arsenal. Food Chem. Toxicol. 153, 112268. doi:10.1016/j.fct.2021.112268
Akpulat, H. A., Tepe, B., Sokmen, A., Daferera, D., and Polissiou, M. (2005). Composition of the essential oils of Tanacetum argyrophyllum (C. Koch) Tvzel. Var. argyrophyllum and Tanacetum parthenium (L.) Schultz Bip. (Asteraceae) from Turkey. Biochem. Syst. Ecol. 33, 511–516. doi:10.1016/j.bse.2004.10.006
Al-Zuhair, S., Dowaidar, A., and Kamal, H. (2010). Inhibitory effect of dates-extract on α-Amylase and α-glucosidase enzymes relevant to non-insulin dependent diabetes mellitus. J. Biochem. Tech. 2, 158–160.
Aldayarov, N., Tulobaev, A., Salykov, R., Jumabekova, J., Kydyralieva, B., Omurzakova, N., et al. (2022). An ethnoveterinary study of wild medicinal plants used by the Kyrgyz farmers. J. Ethnopharmacol. 285, 114842. doi:10.1016/j.jep.2021.114842
Ali, M., Aldosari, A., Tng, D. Y. P., Ullah, M., Hussain, W., Ahmad, M., et al. (2019). Traditional Uses of plants by indigenous communities for veterinary practices at Kurram district, Pakistan. Ethnobot. Res. Appl. 18, 1–19. doi:10.32859/era.18.24.1-19
Alipanah, H., Rasti, F., Zarenezhad, E., Dehghan, A., Sahebnazar, B., and Osanloo, M. (2021). Comparison of anticancer effects of carvone, carvone-rich essential oils, and chitosan nanoparticles containing Each of them. Biointerface Res. Appl. Chem. 12, 5716–5726. doi:10.33263/BRIAC124.57165726
Altınbaşak, O., Anıl, S., Melikoğlu, G., and Kültür, Ş. (2018). Türkiye de mide ülserinde kullanılan tıbbi bitkiler. Marmara Pharm. J. 22, 1–14. doi:10.12991/mpj.2018.35
Altundag, E., and Ozturk, M. (2011). Ethnomedicinal studies on the plant resources of east Anatolia, Turkey. Procedia - Soc. Behav. Sci. 19, 756–777. doi:10.1016/j.sbspro.2011.05.195
Álvarez, Á. L., Habtemariam, S., Abdel Moneim, A. E., Melón, S., Dalton, K. P., and Parra, F. (2015). A spiroketal-enol ether derivative from Tanacetum vulgare selectively inhibits HSV-1 and HSV-2 glycoprotein accumulation in Vero cells. Antivir. Res. 119, 8–18. doi:10.1016/j.antiviral.2015.04.004
Álvarez, Á. L., Habtemariam, S., Juan-Badaturuge, M., Jackson, C., and Parra, F. (2011). In vitro anti HSV-1 and HSV-2 activity of Tanacetum vulgare extracts and isolated compounds: An approach to their mechanisms of action. Phytother. Res. 25, 296–301. doi:10.1002/ptr.3382
Andrade-Cetto, A. (2009). Ethnobotanical study of the medicinal plants from Tlanchinol, Hidalgo, México. J. Ethnopharmacol. 122, 163–171. doi:10.1016/j.jep.2008.12.008
Attighi Lorestani, F., Khashaveh, A., and Attighi Lorestani, R. (2013). Fumigant toxicity of essential oil from Tanacetum balsamita L. (Compositae) against adults and eggs of Callosobruchus maculatus F. (Coleoptera: Bruchidae). Arch. Phytopathol. Plant Prot. 46, 2080–2086. doi:10.1080/03235408.2013.785112
Azizi, V., Allahyari, F., Rezaali, F., and Hosseini, A. (2020). The Anxiolytic and Antidepressant effect of tanacetum polycephalum in the Pentylenetetrazole Kindled rats. Res. J. Pharmacogn. 7, 13–20. doi:10.22127/rjp.2020.211810.1541
Bączek, K. B., Kosakowska, O., Przybył, J. L., Pióro-Jabrucka, E., Costa, R., Mondello, L., et al. (2017). Antibacterial and antioxidant activity of essential oils and extracts from costmary (Tanacetum balsamita L.) and tansy (Tanacetum vulgare L.). Ind. Crops Prod. 102, 154–163. doi:10.1016/j.indcrop.2017.03.009
Bagci, E., Kursat, M., Kocak, A., and Gur, S. (2008). Composition and Antimicrobial Activity of the Essential Oils of Tanacetum balsamita L. subsp. balsamita and T. chiliophyllum (Fisch. et Mey.) Schultz Bip. var. chiliophyllum (Asteraceae) from Turkey. J. Essent. Oil Bear. Plants 11, 476–484. doi:10.1080/0972060X.2008.10643656
Bartha, S. G., Quave, C. L., Balogh, L., and Papp, N. (2015). Ethnoveterinary practices of Covasna county, Transylvania, Romania. J. Ethnobiol. Ethnomedicine 11, 35. doi:10.1186/s13002-015-0020-8
Basati, G., Abbaszadeh, S., Zebardast, A., and Teimouri, H. (2019). Analgesic medicinal plants in Shahrekord, southwest of Iran: An ethnobotanical study. Galen. Med. J. 8, 1593. doi:10.31661/gmj.v8i0.1593
Başer, K. H. C., Demirci, B., Tabanca, N., Özek, T., and Gören, N. (2001). Composition of the essential oils of Tanacetum armenum (DC.) Schultz Bip., T. balsamita L., T. chiliophyllum (Fisch. & Mey.) Schultz Bip. var. chiliophyllum and T. haradjani (Rech. fil.) Grierson and the enantiomeric distribution of camphor and carvone†. Flavour Fragr. J. 16, 195–200. doi:10.1002/ffj.977
Beauchamp, P., Dev, V., Kashyap, T., Melkani, A., Mathela, C., and Bottini, A. T. (2001). Composition of the essential oil of Tanacetum nubigenum Wallich ex DC. J. Essent. Oil Res. 13, 319–323. doi:10.1080/10412905.2001.9712223
Bencheikh, N., Elbouzidi, A., Kharchoufa, L., Ouassou, H., Alami Merrouni, I., Mechchate, H., et al. (2021). Inventory of medicinal plants used traditionally to manage kidney diseases in North-Eastern Morocco: Ethnobotanical Fieldwork and pharmacological evidence. Plants Basel Switz. 10, 1966. doi:10.3390/plants10091966
Benedec, D., Filip, L., Vlase, L., Bele, C., Sevastre, B., Raita, O., et al. (2016). In vitro study of antioxidant activity and phenolic content of Chrysanthemum balsamita varieties. Pak. J. Pharm. Sci. 29, 1359–1364.
Bonet, M. A., and Vallès, J. (2007). Ethnobotany of Montseny biosphere reserve (Catalonia, Iberian Peninsula): Plants used in veterinary medicine. J. Ethnopharmacol. 110, 130–147. doi:10.1016/j.jep.2006.09.016
Bonetti, A., Faraloni, C., Venturini, S., Baini, G., Miraldi, E., and Biagi, M. (2021). Characterization of phenolic profile and antioxidant activity of the leaves of the forgotten medicinal plant Balsamita major grown in Tuscany, Italy, during the growth cycle. Plant Biosyst. - Int. J. deal. Asp. Plant Biol. 155, 908–913. doi:10.1080/11263504.2020.1810805
Bouhaoui, A., Eddahmi, M., Dib, M., Khouili, M., Aires, A., Catto, M., et al. (2021). Synthesis and biological properties of coumarin derivatives. A review. A Rev. Chem. 6, 5848–5870. doi:10.1002/slct.202101346
Bouhlal, T., Loukili, K., Zidane, L., and Fadli, M. (2017). Plants used in traditional medicines by the human population of the Gharb plain (Morocco). J. Med. Plants Stud. 5, 170–174.
Bremer, K. (1993). Generic monograph of the asteraceae-Anthemideae. Bull. Nat. Hist. Mus. Bot. Ser. 23, 71–177.
Bremer, K., and Humphries, C. J. (1993). Generic monograph of the Asteraceae-Anthemideae. Bull. Nat. Hist. Mus. Bot. Ser. 23, 71–177.
Bukhari, I. A., Khan, R. A., Gilani, A. H., Shah, A. J., Hussain, J., and Ahmad, V. U. (2007). The analgesic, anti-inflammatory and calcium antagonist potential of Tanacetum artemisioides. Arch. Pharm. Res. 30, 303–312. doi:10.1007/BF02977610
Caglar, P., Sarikahya, N., Karakoç, Ö., Gökçe, A., Demirci, F., Kirmizigul, S., et al. (2017). Fatty acid composition and biological activities of Tanacetum zahlbruckneri (Náb.) Grierson growing in Turkey. Rec. Nat. Prod. 11, 401–405.
Çalişkan, Z., Gören, N., and Watson, W. H. (2004). Isolation and structures of eudesmanolides from Tanacetum cadmeum ssp. Cadmeum. J. Chem. Crystallogr. 34, 307–310. doi:10.1023/B:JOCC.0000026275.39368.fc
Carocho, M., Barreiro, M. F., Morales, P., and Ferreira, I. C. F. R. (2014). Adding molecules to food, pros and Cons: A review on synthetic and natural food additives. Compr. Rev. Food Sci. Food Saf. 13, 377–399. doi:10.1111/1541-4337.12065
Chaachouay, N., Benkhnigue, O., el Ibaoui, H., Ayadi, R., and Zidane, L. (2019a). Medicinal plants used for diabetic problems in the Rif, Morocco. Ethnobot. Res. Appl. 18. doi:10.32859/era.18.21.1-29
Chaachouay, N., Benkhnigue, O., Fadli, M., El Ibaoui, H., and Zidane, L. (2019b). Ethnobotanical and ethnopharmacological studies of medicinal and aromatic plants used in the treatment of metabolic diseases in the Moroccan Rif. Heliyon 5, e02191. doi:10.1016/j.heliyon.2019.e02191
Chagas Nogueira, M. V., Bezerra de Lima Castro, S. A., Amorim, A. M. de, Maia, R. M., and SartiniPaulillo, L. C. M. (2016). Ethnobotanical survey of plants from the Caatinga with Possible therapeutic Uses. Int. J. Curr. Microbiol. Appl. Sci. 5, 767–772. doi:10.20546/ijcmas.2016.506.083
Chanotiya, C. S., Sammal, S. S., and Mathela, C. S. (2006). Composition of a new Chemotype of tanacetum nubigenum. Indian J. Chem. 37. doi:10.1002/chin.200603194
Cheng, Z., Hu, X., Lu, X., Fang, Q., Meng, Y., and Long, C. (2022). Medicinal plants and Fungi traditionally used by Dulong people in northwest Yunnan, China. Front. Pharmacol. 13, 895129. doi:10.3389/fphar.2022.895129
Ciocarlan, A., Lupascu, L., Aricu, A., Dragalin, I., Ciocarlan, N., Zinicovscaia, I., et al. (2021). Chemical composition of the essential oil and antimicrobial properties of crude extract from tanacetum corymbosum (L.) Shi. Bip. Chem. J. Mold. 16, 83–90. doi:10.19261/cjm.2021.877
Cornara, L., La Rocca, A., Terrizzano, L., Dente, F., and Mariotti, M. G. (2014). Ethnobotanical and phytomedical knowledge in the north-Western Ligurian Alps. J. Ethnopharmacol. 155, 463–484. doi:10.1016/j.jep.2014.05.046
Coté, H., Boucher, M.-A., Pichette, A., and Legault, J. (2017). Anti-inflammatory, antioxidant, Antibiotic, and cytotoxic activities of tanacetum vulgare L. Essential oil and its constituents. Medicines 4, 34. doi:10.3390/medicines4020034
Cumo, C. (2013). Encyclopedia of cultivated plants: From Acacia to Zinnia [3 volumes]. 3, 1307. Available at: https://publisher.abc-clio.com/9781598847758 (Accessed June 12, 2022).
Dalar, A. (2018). Plant taxa used in the treatment of diabetes in van province, Turkey. Int. J. Second. Metab. 5, 170–184. doi:10.21448/ijsm.430703
Daneshmand, P., Saliminejad, K., Dehghan Shasaltaneh, M., Kamali, K., Riazi, G. H., Nazari, R., et al. (2016). Neuroprotective effects of herbal extract (Rosa canina, tanacetum vulgare and Urtica dioica) on rat model of Sporadic Alzheimer’s disease. Avicenna J. Med. Biotechnol. 8, 120–125.
Danna, C., Poggio, L., Smeriglio, A., Mariotti, M., and Cornara, L. (2022). Ethnomedicinal and ethnobotanical survey in the Aosta valley Side of the gran Paradiso National Park (Western Alps, Italy). Plants 11, 170. doi:10.3390/plants11020170
Davis, K., Guimarães, D. de O., Davis, T., and Do, C. A. B. (2021). Ethnobotanical study of anti-malarials among communities in the municipal of Portel-PA, Brazil. Rev. Fitos 15, 166–177. doi:10.32712/2446-4775.2021.1079
de Almeida, L. M. S., Carvalho, L. S. A. de, Gazolla, M. C., Silva Pinto, P. L., Silva, M. P. N. da, de Moraes, J., et al. (2016). Flavonoids and sesquiterpene lactones from Artemisia absinthium and Tanacetum parthenium against Schistosoma mansoni worms. Evid. Based Complement. Altern. Med. 2016, e9521349. doi:10.1155/2016/9521349
Delfan, B., Bahmani, M., Rafieian-Kopaei, M., Delfan, M., and Saki, K. (2014). A review study on ethnobotanical study of medicinal plants used in relief of toothache in Lorestan Province, Iran. Asian pac. J. Trop. Dis. 4, S879–S884. doi:10.1016/S2222-1808(14)60751-9
Devrnja, N., Anđelković, B., Aranđelović, S., Radulović, S., Soković, M., Krstić-Milošević, D., et al. (2017). Comparative studies on the antimicrobial and cytotoxic activities of Tanacetum vulgare L. essential oil and methanol extracts. South Afr. J. Bot. 111, 212–221. doi:10.1016/j.sajb.2017.03.028
Diener, H. C., Pfaffenrath, V., Schnitker, J., Friede, M., and Henneicke-von Zepelin, H.-H. (2005). Efficacy and safety of 6.25 mg t.i.d. feverfew CO2-extract (MIG-99) in migraine prevention--a randomized, double-blind, multicentre, placebo-controlled study. Cephalalgia Int. J. Headache 25, 1031–1041. doi:10.1111/j.1468-2982.2005.00950.x
Donfack, M. F. M., Atsamo, A. D., Guemmogne, R. J. T., Kenfack, O. B. N., Dongmo, A. B., and Dimo, T. (2021). Antihypertensive effects of the Vitex cienkowskii (Verbenaceae) stem-bark extract on L-NAME-induced hypertensive rats. Evid.-Based Complement. Altern. Med. ECAM 2021, 6668919. doi:10.1155/2021/6668919
Dongmo, A. B., Azebaze, A. G. B., Donfack, F. M., Dimo, T., Nkeng-Efouet, P. A., Devkota, K. P., et al. (2011). Pentacyclic triterpenoids and ceramide mediate the vasorelaxant activity of Vitex cienkowskii via involvement of NO/cGMP pathway in isolated rat aortic rings. J. Ethnopharmacol. 133, 204–212. doi:10.1016/j.jep.2010.09.033
Doskotch, R. W., El-Feraly, F. S., and Hufford, C. D. (1971). Sesquiterpene lactones from pyrethrum flowers. Can. J. Chem. 49, 2103–2110. doi:10.1139/v71-341
Doyle, S. R., Laing, R., Bartley, D., Morrison, A., Holroyd, N., Maitland, K., et al. (2022). Genomic landscape of drug response reveals mediators of anthelmintic resistance. Cell Rep. 41, 111522. doi:10.1016/j.celrep.2022.111522
Drissi, B., Mahdi, I., Yassir, M., Ben Bakrim, W., Bouissane, L., and Sobeh, M. (2022). Cubeb (Piper cubeba L.f.): A comprehensive review of its botany, phytochemistry, traditional uses, and pharmacological properties. Front. Nutr. 9, 1048520. Available at: https://www.frontiersin.org/articles/10.3389/fnut.2022.1048520 (Accessed November 29, 2022). doi:10.3389/fnut.2022.1048520
Dutt, H. C., Bhagat, N., and Pandita, S. (2015). Oral traditional knowledge on medicinal plants in jeopardy among Gaddi shepherds in hills of northwestern Himalaya, J&K, India. J. Ethnopharmacol. 168, 337–348. doi:10.1016/j.jep.2015.03.076
Dzoyem, J., Tchuenguem, R., Ibrahim, M., Nkeza, A., Roland, A., Njouendou, J., et al. (2020). “Ethnoveterinary medicine and medicinal plants used in the treatment of livestock diseases in Cameroon,” in Ethnoveterinary medicine (Cham: Springer), 175–209. doi:10.1007/978-3-030-32270-0_9
Ebadi, M., and Eftekharian, R. (2019). Ethnobotanical study of medicinal plants used in Ahar-Arasbaran (protected area in East Azerbaijan Province of Iran). Mediterr. Bot. 40, 209–214. doi:10.5209/mbot.62985
Eblaghi, M., Khajehie, N., Golmakani, M.-T., and Eskandari, M. H. (2016). Investigating the effects of microwave-assisted hydrodistillation on antioxidant and antifungal activities of Tanacetum polycephalum and Artemisia chamaemelifolia essential oils. J. Essent. Oil Res. 28, 528–539. doi:10.1080/10412905.2016.1175977
Eiki, N., Sebola, N. A., Sakong, B. M., and Mabelebele, M. (2021). Review on ethnoveterinary practices in Sub-Saharan Africa. Vet. Sci. 8, 99. doi:10.3390/vetsci8060099
EL-Akhal, F., Guemmouh, R., Zoubi, Y. E., Fadil, M., and Lalami, A. E. O. (2021). Survey on plants used by the population of Fez City (central Morocco) as bioinsecticides in the control of insects responsible for vector-borne diseases. J. Appl. Pharm. Sci. 11, 106–113. doi:10.7324/JAPS.2021.110214
El-Shazly, A., Dorai, G., and Wink, M. (2002). Composition and antimicrobial activity of essential oil and hexane-ether extract of Tanacetum santolinoides (dc.) Feinbr. and Fertig. Z. Für Naturforsch. C 57, 620–623. doi:10.1515/znc-2002-7-812
Elshamy, A., Abd-ElGawad, A., Mohamed, T., El Gendy, A. E., Abd El Aty, A. A., Saleh, I., et al. (2021). Extraction development for antimicrobial and phytotoxic essential oils from asteraceae species: Achillea fragrantissima, Artemisia judaica and Tanacetum sinaicum. Flavour Fragr. J. 36, 352–364. doi:10.1002/ffj.3647
Emre, İ. (2021). The biochemical content and antioxidant capacities of endemic Tanacetum densum (Lab.) Schultz Bip. subsp. laxum, and Tanacetum densum (Lab.) Schultz Bip. subsp. amani Heywood growing in Turkey. Braz. J. Biol. Rev. Brasleira Biol. 81, 1106–1114. doi:10.1590/1519-6984.239020
Erdoğan, P., and Yıldırım, A. (2016). Insecticidal activity of three different plant extracts on the green peach aphid [(Myzus persicae Sulzer) (Hemiptera: Aphididae)]. J. Entomol. Res. 18 (1), 27–35. Available at: https://www.entomol.org/journal/index.php/JERS/article/view/890 (Accessed November 29, 2022).
Eruçar, F., Tan, N., and Mi̇ski̇, M. (2022). Ethnobotanical records of medicinal plants of Turkey effective on stress management Complied with the literature survey in their chemical content and activities. Rec. Nat. Prod. 1, 1–92. doi:10.25135/rnp.324.2203.2372
Esmaeili, M. A., Sonboli, A., and Ayyari Noushabadi, M. (2010). Antioxidant and protective properties of six tanacetum species against hydrogen peroxide-induced oxidative stress in K562 cell line: A comparative study. Food Chem. 121, 148–155. doi:10.1016/j.foodchem.2009.12.022
Eyol, P. C., Sarikahya, N. B., Karakoc, O. C., Gokce, A., Demirci, F., Kirmizigul, S., et al. (2017). Fatty acid composition and biological activities of tanacetum zahlbruckneri (Náb.) Grierson growing in Turkey. Rec. Nat. Prod. 11, 5.
Faraloni, C., Bonetti, A., and Leva, A. (2020). Long–term micropropagation of Balsamita major. as a promising in vitro strategy for producing phenolic compounds with antioxidant capacity. Plant Biosyst. - Int. J. deal. Asp. Plant Biol. 155, 336–343. doi:10.1080/11263504.2020.1747562
Farzadfar, S., Zarinkamar, F., and Hojati, M. (2017). Magnesium and manganese affect photosynthesis, essential oil composition and phenolic compounds of Tanacetum parthenium. Plant Physiol. biochem. 112, 207–217. doi:10.1016/j.plaphy.2017.01.002
Fischedick, J. T., Standiford, M., Johnson, D. A., De Vos, R. C. H., Todorović, S., Banjanac, T., et al. (2012). Activation of antioxidant response element in mouse primary cortical cultures with sesquiterpene lactones isolated from Tanacetum parthenium. Planta Med. 78, 1725–1730. doi:10.1055/s-0032-1315241
Gairola, S., Sharma, J., and Bedi, Y. S. (2014). A cross-cultural analysis of Jammu, Kashmir and Ladakh (India) medicinal plant use. J. Ethnopharmacol. 155, 925–986. doi:10.1016/j.jep.2014.06.029
Gecibesler, I. H., Kocak, A., and Demirtas, I. (2016). Biological activities, phenolic profiles and essential oil components of Tanacetum cilicicum (BOISS.) GRIERSON. Nat. Prod. Res. 30, 2850–2855. doi:10.1080/14786419.2016.1163692
Gevrenova, R., Zengin, G., Sinan, K. I., Zheleva-Dimitrova, D., Balabanova, V., Kolmayer, M., et al. (2023). An in-Depth study of metabolite profile and biological potential of Tanacetum balsamita L. (Costmary). Plants 12, 22. doi:10.3390/plants12010022
Ghafoor, A. (2002). Flora of Pakistan: Asteraceae (I) - Anthemideae. N° 207. Karachi, Pakistham: Department of Botany, University of Karachi.
Ghasemi Pirbalouti, A., Momeni, M., and Bahmani, M. (2012). Ethnobotanical study of medicinal plants used by Kurd tribe in Dehloran and Abdanan districts, Ilam province, Iran. Afr. J. Tradit. Complement. Altern. Med. 10, 368–385. doi:10.4314/ajtcam.v10i2.24
Ghirardini, M. P., Carli, M., del Vecchio, N., Rovati, A., Cova, O., Valigi, F., et al. (2007). The importance of a taste. A comparative study on wild food plant consumption in twenty-one local communities in Italy. J. Ethnobiol. Ethnomedicine 3, 22. doi:10.1186/1746-4269-3-22
Gholami, S., Azadbakht, M., Ziaei Hezarjaribi, H., and Rahimi-Esboei, B. (2014). Anti-giardial activity of chloroformic extract of Tanacetum parthenium and Artemisia annua in vitro. Res. Mol. Med. 2, 46–51. doi:10.18869/acadpub.rmm.2.1.46
Godinho, L. S., Aleixo de Carvalho, L. S., Barbosa de Castro, C. C., Dias, M. M., Pinto, P. de F., Crotti, A. E. M., et al. (2014). Anthelmintic activity of crude extract and essential oil of Tanacetum vulgare (asteraceae) against adult worms of Schistosoma mansoni. Sci. World J. 2014, e460342. doi:10.1155/2014/460342
Gokturk, T., Kordali, S., and Bozhuyuk, A. U. (2017). Insecticidal effect of essential oils against Fall Webworm (Hypantria cunea Drury (Lepidoptera: Arctiidae)). Nat. Prod. Commun. 12, 1934578X1701201. doi:10.1177/1934578X1701201034
Gonzalez, A. G., barrera, J. B., Mendez, J. T., Sanchez, M. L., and Eiroa Martinez, J. L. (1990). Sesquiterpene lactones from Tanacetum ferulaceum. Phytochemistry 29, 2339–2341. doi:10.1016/0031-9422(90)83068-C
Gören, N., Arda, N., and Caliskan, Z. (2002). Chemical characterization and biological activities of the genus Tanacetum (Compositae). Stud. Nat. Prod. Chem. 27, 547–658. doi:10.1016/S1572-5995(02)80044-8
Gören, N., Demirci, B., and Başer, K. H. C. (2001). Composition of the essential oils of tanacetum spp. from Turkey†: Essential oils of TANACETUM SPP. Flavour Fragr. J. 16, 191–194. doi:10.1002/ffj.976
Gospodinova, Z., Antov, G., Angelova, S., and Krasteva, M. (2014). In vitro antitumor potential of Bulgarian Tanacetum vulgare L. on human breast adenocarcinoma cells. Results Pharma Sci. 4, 468–472.
Grdiša, M., Carović-Stanko, K., Kolak, I., and Šatović, Z. (2009). Morphological and biochemical diversity of Dalmatian pyrethrum (Tanacetum cinerariifolium (Trevir.) Sch. Bip.). Agric. Conspec. Sci. 74, 8.
Grdiša, M., Jeran, N., Varga, F., Klepo, T., Ninčević, T., and Šatović, Z. (2022). Accumulation Patterns of six pyrethrin compounds across the flower developmental stages—comparative analysis in six natural Dalmatian pyrethrum populations. Agronomy 12, 252. doi:10.3390/agronomy12020252
Grulova, D., Hyblerova, S., Jeliazkov (Zheljazkov), V., Salamon, I., and Rondon, S. (2017). Effect of plant essential oils against Rophalosiphum padi on Wheat and Barley. Nat. Prod. Commun. 12, 1934578X1701200–1528. doi:10.1177/1934578X1701200933
Guarino, C. (2008). Ethnobotanical study of the Sannio area, Campania, southern Italy. Ethnobot. Res. Appl. 6, 255. doi:10.17348/era.6.0.255-317
Guarrera, P. M., Forti, G., and Marignoli, S. (2005). Ethnobotanical and ethnomedicinal uses of plants in the district of Acquapendente (Latium, Central Italy). J. Ethnopharmacol. 96, 429–444. doi:10.1016/j.jep.2004.09.014
Güler, O., Polat, R., Karaköse, M., Çakılcıoğlu, U., and Akbulut, S. (2021). An ethnoveterinary study on plants used for the treatment of livestock diseases in the province of Giresun (Turkey). South Afr. J. Bot. 142, 53–62. doi:10.1016/j.sajb.2021.06.003
Güneş, F., and Özhatay, N. (2011). An ethnobotanical study from Kars Eastern Turkey. Biyolojik Çeşitlilik Ve Koruma 4, 30–41.
Habibi, Z., Biniyaz, T., Ghodrati, T., Masoudi, S., and Rustaiyan, A. (2007). Volatile Constituents of Tanacetum paradoxum Bornm. and Tanacetum tabrisianum (Boiss.) Sosn. et Takht., from Iran. J. Essent. Oil Res. 19, 11–13. doi:10.1080/10412905.2007.9699216
Haider, S. Z., Mohan, M., Pandey, A. K., and Singh, P. (2017). Use of Tanacetum tomentosum and Ta. dolichophyllum essential oils as botanical repellents and insecticidal agents against storage pest Tribolium castaneum (Coleoptera: Tenebrionidae). Entomol. Res. 47, 318–327. doi:10.1111/1748-5967.12228
Hassanpouraghdam, M. B. (2009). Flowerhead volatile oil composition of soilless culture-grown Chrysanthemum balsamita L. Nat. Prod. Res. 23, 672–677. doi:10.1080/14786410802591182
Hassanpouraghdam, M. B., Vojodi Mehrabani, L., Kheiri, M., Chrysargyris, A., and Tzortzakis, N. (2022). Physiological and biochemical responses of Tanacetum balsamita L. to the foliar application of Dobogen biostimulant, glucose and KNO3 under salinity stress. Sci. Rep. 12, 9320. doi:10.1038/s41598-022-13150-z
Hegazy, M.-E. F., Hamed, A. R., Mohamed, T. A., Debbab, A., Nakamura, S., Matsuda, H., et al. (2015). Anti-inflammatory sesquiterpenes from the medicinal herb Tanacetum sinaicum. RSC Adv. 5, 44895–44901. doi:10.1039/C5RA07511D
Heinrich, M. (2000). Ethnobotany and its role in drug development. Phytother. Res. PTR 14, 479–488. doi:10.1002/1099-1573(200011)14:7<479:aid-ptr958>3.0.co;2-2
Hitmi, A., Coudret, A., and Barthomeuf, C. (2000). The production of pyrethrins by plant cell and tissue cultures of Chrysanthemum cinerariaefolium and Tagetes species. Crit. Rev. Biochem. Mol. Biol. 35, 317–337. doi:10.1080/10409230091169230
Holetz, F. B., Pessini, G. L., Sanches, N. R., Cortez, D. A. G., Nakamura, C. V., and Dias Filho, B. P. (2002). Screening of some plants used in the Brazilian folk medicine for the treatment of infectious diseases. Mem. Inst. Oswaldo Cruz 97, 1027–1031. doi:10.1590/S0074-02762002000700017
Hussain, J., Ahmad, V., Hussain, H., Hassan, Z., Khan, A., and Farooq, U. (2005). Tanacetamide C: One new ceramide from Tanacetum artemisioides. Cheminform 36. doi:10.1002/chin.200547198
Hussain, J., Munir, M., Hassan, Z., Bano, N., Arshad, S., and Ahmad, V. U. (2010). Tanacetamide D: A new ceramide from Tanacetum artemisioides. Helv. Chim. Acta 93, 350–353. doi:10.1002/hlca.200900204
Hussain, W., Badshah, L., Ullah, M., Ali, M., Ali, A., and Hussain, F. (2018). Quantitative study of medicinal plants used by the communities residing in Koh-e-Safaid Range, northern Pakistani-Afghan borders. J. Ethnobiol. Ethnomedicine 14, 30. doi:10.1186/s13002-018-0229-4
Ibrar, M., and Hussain, F. (2010). An ethnobotanical study on the usage of wild medicinal herbs from MALANA HILLS, PARACHINAR KURRAM VALLEY. Int. J. Biol. Biotechnol. 7, 267–271.
Inceer, H., Hayirlioglu-Ayaz, S., Guler, H. S., Aksu, N., and Ozcan, M. (2012). Karyological studies of some representatives of Tanacetum L. (Anthemideae-Asteraceae) from north-east Anatolia. Plant Syst. Evol. 298, 827–834. doi:10.1007/s00606-012-0594-8
Isman, M. B. (2006). Botanical insecticides deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 51, 45–66. doi:10.1146/annurev.ento.51.110104.151146
Ivănescu, B., Pop, C. E., Vlase, L., Corciovă, A., Gherghel, D., Vochița, G., et al. (2021). Cytotoxic effect of chlorofrom extracts from Tanacetum vulgare, T. macrophyllum and T. coryombosum on Hela, A375 and V79 cell lines. Farmacia 69, 12–20. doi:10.31925/farmacia.2021.1.2
Jain, N. K., and Kulkarni, S. K. (1999). Antinociceptive and anti-inflammatory effects of Tanacetum parthenium L. extract in mice and rats. J. Ethnopharmacol. 68, 251–259. doi:10.1016/S0378-8741(99)00115-4
Jamil Ahmed, M., and Murtaza, G. (2015). A study of medicinal plants used as ethnoveterinary: Harnessing potential phytotherapy in Bheri, District Muzaffarabad (Pakistan). J. Ethnopharmacol. 159, 209–214. doi:10.1016/j.jep.2014.11.016
Jannesar, M., Majd, A., Sharif Shoushtari, M., and Oraei, M. (2014). Effect of Total flavonoid extract of Tanacetum parthenium L. (Feverfew) pollen grains on immune system responses in Balb/C mice. Int. J. Biosci. IJB 5, 72–78. doi:10.12692/ijb/5.12.72-78
Jarić, S., Mačukanović-Jocić, M., Djurdjević, L., Mitrović, M., Kostić, O., Karadžić, B., et al. (2015). An ethnobotanical survey of traditionally used plants on Suva planina mountain (south-eastern Serbia). J. Ethnopharmacol. 175, 93–108. doi:10.1016/j.jep.2015.09.002
Jarić, S., Mitrović, M., and Pavlović, P. (2014). “An ethnobotanical and ethnomedicinal study on the Use of wild medicinal plants in rural areas of Serbia,” in Ethnobotany and Biocultural Diversities in the Balkans. Editors A. Pieroni, and C. L. Quave (New York, NY: Springer New York), 87–112. doi:10.1007/978-1-4939-1492-0_6
Jeran, N., Grdiša, M., Varga, F., Šatović, Z., Liber, Z., Dabić, D., et al. (2021). Pyrethrin from Dalmatian pyrethrum (Tanacetum cinerariifolium (Trevir.) Sch. Bip.): Biosynthesis, biological activity, methods of extraction and determination. Phytochem. Rev. 20, 875–905. doi:10.1007/s11101-020-09724-2
Jia, Q.-Q., Wang, J.-C., Long, J., Zhao, Y., Chen, S.-J., Zhai, J.-D., et al. (2013). Sesquiterpene lactones and their derivatives inhibit high glucose-induced NF-κB activation and MCP-1 and TGF-β1 expression in rat mesangial cells. Mol. Basel Switz. 18, 13061–13077. doi:10.3390/molecules181013061
Juan-Badaturuge, M., Habtemariam, S., Jackson, C., and Thomas, M. J. K. (2009). Antioxidant principles of Tanacetum vulgare L. aerial parts. Nat. Prod. Commun. 4, 1934578X0900401–1564. doi:10.1177/1934578x0900401121
Kameri, A., Koçani, F., Hashani, Z., Kurteshi, K., Kamberi, B., Kurti, A., et al. (2019). Antifungal and synergistic effects of the ethyl acetate extract of tanacetum vulgare (L.) against Candida albicans. Med. Sci. Monit. Basic Res. 25, 179–186. doi:10.12659/msmbr.917394
Karaca, M., Ozbek, H., Akkan, H. A., Tutuncu, M., Ozgokce, F., Him, A., et al. (2009). Anti-inflammatory activities of diethyl-ether extracts of Helichrysum plicatum DC. And Tanacetum balsamita L. In rats. Asian J. Anim. Vet. Adv. 4, 320–325. doi:10.3923/ajava.2009.320.325
Karaköse, M. (2022). An ethnobotanical study of medicinal plants in Güce district, north-eastern Turkey. Plant divers. 44, 577–597. doi:10.1016/j.pld.2022.03.005
Karimi, A., Kazemi, M., Samani, S. A., and Simal-Gandara, J. (2021). Bioactive compounds from by-products of eggplant: Functional properties, potential applications and advances in valorization methods. Trends Food Sci. Technol. 112, 518–531. doi:10.1016/j.tifs.2021.04.027
Karimi, M., Mardani, M., and Mahmoodnia, L. (2017). Colic phytotherapy in Iranian ethnobotany: An overview of the ffectiveness of the most important native medicinal plants of Iran on olic disease. Int. J. Pharm. Clin. Res. 9, 60–62. doi:10.25258/ijpcr.v9i1.8265
Karimian, H., Fadaeinasab, M., Moghadamtousi, S. Z., Hajrezaei, M., Zahedifard, M., Razavi, M., et al. (2015). The Chemopreventive effect of tanacetum polycephalum against LA7-induced breast cancer in rats and the Apoptotic effect of a cytotoxic sesquiterpene lactone in MCF7 cells: A bioassay-guided approach. Cell. Physiol. biochem. 36, 988–1003. doi:10.1159/000430273
Karimzadeh, M., Sahar Khademnejad2, S., and Aghazadeh, Z. (2021). Antimicrobial effects of Tanacetum balsamita L essential oil Streptococcus mutants, Streptococcus sanguis and Streptococcus salivarius and its comparison with common mouthwashes. J. Res. Dent. Sci. 18, 5–14. doi:10.52547/jrds.18.1.5
Karpavičienė, B. (2022). Traditional Uses of medicinal plants in south-Western part of Lithuania. Plants 11, 2093. doi:10.3390/plants11162093
Kasaj, D., Rieder, A., Krenn, L., and Kopp, B. (1999). Separation and quantitative analysis of natural pyrethrins by high-performance liquid chromatography. Chromatographia 50, 607–610. doi:10.1007/BF02493668
Kawarty, A. M., Behçet, L., and Çakılcıoğlu, U. (2020). An ethnobotanical survey of medicinal plants in Ballakayati (Erbil, North Iraq). Turk. J. Bot. 44, 345–357. doi:10.3906/bot-1910-39
Kazancı, C., Oruç, S., and Mosulishvili, M. (2020). Medicinal ethnobotany of wild plants: A cross-cultural comparison around Georgia-Turkey border, the Western lesser Caucasus. J. Ethnobiol. Ethnomedicine 16, 71. doi:10.1186/s13002-020-00415-y
Keskitalo, M. (1999). Exploring Biodiversity to Enhance Bioactivity in the genus tanacetum through Protoplast Fusion. Available at: https://helda.helsinki.fi/handle/10138/20731 (Accessed November 16, 2022).
Khan, M. F., Rawat, A. K., Khatoon, S., Hussain, M. K., Mishra, A., and Negi, D. S. (2018). In vitro and in vivo antidiabetic effect of extracts of Melia azedarach, Zanthoxylum alatum, and Tanacetum nubigenum. Integr. Med. Res. 7, 176–183. doi:10.1016/j.imr.2018.03.004
Khan, S. W., and Khatoon, S. (2008). Ethnobotanical studies on some Useful herbs of Haramosh and Bugrote Valleys in Gilgit, northern areas of Pakistan. Pak. J. Bot. 40, 43–58.
Khatib, C., Nattouf, A., and Agha, M. I. H. (2021). “Ethnobotanical survey of medicinal herbs in the Western region in Syria (Latakia and Tartus),” in Review. doi:10.21203/rs.3.rs-355008/v1
Khatib, S., Faraloni, C., and Bouissane, L. (2022a). Exploring the Use of Iris species: Antioxidant properties, phytochemistry, medicinal and industrial applications. Antioxidants 11, 526. doi:10.3390/antiox11030526
Khatib, S., Sobeh, M., and Bouissane, L. (2022b). Tetraclinis articulata (vahl) masters: An insight into its ethnobotany, phytochemistry, toxicity, biocide and therapeutic merits. Front. Pharmacol. 13, 977726. Available at: https://www.frontiersin.org/articles/10.3389/fphar.2022.977726 (Accessed November 17, 2022). doi:10.3389/fphar.2022.977726
Khodayari, M., Basti, A. A., Khanjari, A., Misaghi, A., Kamkar, A., Shotorbani, P. M., et al. (2019). Effect of poly(lactic acid) films incorporated with different concentrations of Tanacetum balsamita essential oil, propolis ethanolic extract and cellulose nanocrystals on shelf life extension of vacuum-packed cooked sausages. Food packag. Shelf Life 19, 200–209. doi:10.1016/j.fpsl.2018.11.009
Khojimatov, O. K., Khamraeva, D. T., and Bussmann, R. W. (2020). An overview of Ethnomedicinal plants of Uzbekistan. Ethnobot. Res. Appl. 20, 1–19. doi:10.32859/era.20.08.1-19
Kisiel, W., and Stojakowska, A. (1997). A sesquiterpene coumarin ether from transformed roots of Tanacetum parthenium. Phytochemistry 46, 515–516. doi:10.1016/S0031-9422(97)87091-4
Korpinen, R. I., Välimaa, A.-L., Liimatainen, J., and Kunnas, S. (2021). Essential oils and supercritical CO2 extracts of Arctic Angelica (Angelica archangelica L.), Marsh Labrador tea (Rhododendron tomentosum) and common tansy (tanacetum vulgare)-chemical compositions and antimicrobial activities. Mol. Basel Switz. 26, 7121. doi:10.3390/molecules26237121
Kubo, A., and Kubo, I. (1995). Antimicrobial agents from tanacetum balsamita. J. Nat. Prod. 58, 1565–1569. doi:10.1021/np50124a013
Kujawska, M., and Schmeda-Hirschmann, G. (2022). The use of medicinal plants by Paraguayan migrants in the Atlantic Forest of Misiones, Argentina, is based on Guaraní tradition, colonial and current plant knowledge. J. Ethnopharmacol. 283, 114702. doi:10.1016/j.jep.2021.114702
Kumar, J. U. S., Kc, M. J., and Semotiuk, A. J. (2019). Indigenous knowledge of medicinal plants used by ethnic communities of South India. Ethnobot. Res. Appl. 18, 1–112. doi:10.32859/era.18.4.1-112
Kumar, M., Paul, Y., and Anand, V. K. (2009). An ethnobotanical study of medicinal plants used by the locals in Kishtwar, Jammu and Kashmir, India. Ethnobot. Leafl. 13, 1240–1256.
Kumar, N., and Goel, N. (2019). Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 24, e00370. doi:10.1016/j.btre.2019.e00370
Kumar, N., Kumar, V., Anand, P., Kumar, V., Ranjan Dwivedi, A., and Kumar, V. (2022). Advancements in the development of multi-target directed ligands for the treatment of Alzheimer’s disease. Bioorg. Med. Chem. 61, 116742. doi:10.1016/j.bmc.2022.116742
Kumar, V., and Tyagi, D. (2013). Chemical composition and biological activities of essential oils of genus tanacetum - a review. J. Pharmacogn. Phytochem. 2, 159–163.
Kurz, J., Parnham, M. J., Geisslinger, G., and Schiffmann, S. (2019). Ceramides as novel disease Biomarkers. Trends Mol. Med. 25, 20–32. doi:10.1016/j.molmed.2018.10.009
Lahlou, S., Israili, Z. H., and Lyoussi, B. (2008). Acute and chronic toxicity of a lyophilised aqueous extract of Tanacetum vulgare leaves in rodents. J. Ethnopharmacol. 117, 221–227. doi:10.1016/j.jep.2008.01.024
Lans, C., Turner, N., Brauer, G., Lourenco, G., and Georges, K. (2006). Ethnoveterinary medicines used for horses in Trinidad and in British Columbia, Canada. J. Ethnobiol. Ethnomedicine 2, 31. doi:10.1186/1746-4269-2-31
Long, C., Sauleau, P., David, B., Lavaud, C., Cassabois, V., Ausseil, F., et al. (2003). Bioactive flavonoids of Tanacetum parthenium revisited. Phytochemistry 64, 567–569. doi:10.1016/s0031-9422(03)00208-5
Lybrand, D. B., Xu, H., Last, R. L., and Pichersky, E. (2020). How plants synthesize pyrethrins: Safe and biodegradable insecticides. Trends Plant Sci. 25, 1240–1251. doi:10.1016/j.tplants.2020.06.012
Mahdavi, M., Jouri, M. H., Mahmoudi, J., Rezazadeh, F., and Mahzooni-Kachapi, S. S. (2013). Investigating the altitude effect on the quantity and quality of the essential oil in Tanacetum polycephalum Sch.-Bip. polycephalum in the Baladeh region of Nour, Iran. Chin. J. Nat. Med. 11, 553–559. doi:10.1016/S1875-5364(13)60100-4
Mahmoodzadeh, Y., Mazani, M., and Rezagholizadeh, L. (2017). Hepatoprotective effect of methanolic Tanacetum parthenium extract on CCl4-induced liver damage in rats. Toxicol. Rep. 4, 455–462. doi:10.1016/j.toxrep.2017.08.003
Mannelli, L. D.-C., Tenci, B., Zanardelli, M., Maidecchi, A., Lugli, A., Mattoli, L., et al. (2015). Widespread pain reliever profile of a flower extract of Tanacetum parthenium. Phytomedicine 22, 752–758. doi:10.1016/j.phymed.2015.05.006
Marzouk, M. M., Mohamed, T. A., Elkhateeb, A., El-toumy, S. A., and Hegazy, M. E. F. (2016). Phenolics from tanacetum sinaicum (fresen.) delile ex Bremer & Humphries (asteraceae). Biochem. Syst. Ecol. 65, 143–146. doi:10.1016/j.bse.2016.02.016
Matsuo, N. (2019). Discovery and development of pyrethroid insecticides. Proc. Jpn. Acad. Ser. B 95, 378–400. doi:10.2183/pjab.95.027
McGaw, L. J., and Eloff, J. N. (2008). Ethnoveterinary use of southern African plants and scientific evaluation of their medicinal properties. J. Ethnopharmacol. 119, 559–574. doi:10.1016/j.jep.2008.06.013
L. J. McGaw, and M. A. Abdalla (Editors) (2020). Ethnoveterinary medicine: Present and future Concepts (Cham: Springer International Publishing). doi:10.1007/978-3-030-32270-0
Mohseni-Salehi-Monfared, S. S., Habibollahzadeh, E., Sadeghi, H., Baeeri, M., and Abdollahi, M. (2010). Efficacy of Setarud (IMODTM), a novel electromagnetically-treated multi-herbal compound, in mouse immunogenic type-1 diabetes. Arch. Med. Sci. AMS 6, 663–669. doi:10.5114/aoms.2010.17078
Mohsenzadeh, F., Chehregani, A., and Amiri, H. (2011). Chemical composition, antibacterial activity and cytotoxicity of essential oils of Tanacetum parthenium in different developmental stages. Pharm. Biol. 49, 920–926. doi:10.3109/13880209.2011.556650
Molares, S., and Ladio, A. (2009). Chemosensory perception and medicinal plants for digestive ailments in a Mapuche community in NW Patagonia, Argentina. J. Ethnopharmacol. 123, 397–406. doi:10.1016/j.jep.2009.03.033
Monfared, A., Davarani, S. S. H., Rustaiyan, A., and Masoudi, S. (2002). Composition of the essential oil of tanacetum balsamita L. ssp. balsamitoides (Schultz Bip.) Grierson from Iran. J. Essent. Oil Res. 14, 1–2. doi:10.1080/10412905.2002.9699741
Moradi Behjou, A., Sonboli, A., Naderifar, M., and Olanj, N. (2022). A taxonomic revision of Tanacetum polycephalum (Asteraceae, Anthemideae) species complex from Iran. Iran. J. Bot. 28, 21–35. doi:10.22092/ijb.2022.126797
Mosaddegh, M., Naghibi, F., Moazzeni, H., Pirani, A., and Esmaeili, S. (2012). Ethnobotanical survey of herbal remedies traditionally used in Kohghiluyeh va Boyer Ahmad province of Iran. J. Ethnopharmacol. 141, 80–95. doi:10.1016/j.jep.2012.02.004
Moscano, F., Guiducci, M., Maltoni, L., Striano, P., Ledda, M. G., Zoroddu, F., et al. (2019). An observational study of fixed-dose Tanacetum parthenium nutraceutical preparation for prophylaxis of pediatric headache. Ital. J. Pediatr. 45, 36. doi:10.1186/s13052-019-0624-z
Mükemre, M., Behçet, L., and Çakılcıoğlu, U. (2015). Ethnobotanical study on medicinal plants in villages of Çatak (Van-Turkey). J. Ethnopharmacol. 166, 361–374. doi:10.1016/j.jep.2015.03.040
Nagar, A., Chatterjee, A., Ur Rehman, L., Ahmad, A., and Tandon, S. (2015). Comparative extraction and enrichment techniques for pyrethrins from flowers of Chrysanthemum cinerariaefolium. Ind. Crops Prod. 76, 955–960. doi:10.1016/j.indcrop.2015.07.043
Nedelcheva, A. (2012). Medicinal plants from an old Bulgarian medical book. J. Med. Plants Researc 6, 2324–2339. doi:10.5897/JMPR11.831
Nelli, M., and Ena, A. (2012). Ritorno alla natura: Balsamita la forza degli antiossidanti fra tradizione ed era moderna/Alba Ena, Manuela Nelli. Roma: Aracne Editrice.
Nobakht, A., and Moghaddam, M. (2013). The effects of different levels of costmary (tanacetum balsamita) medicinal plant onPerformance, egg traits and blood biochemical Parametersof laying hens. Iran. J. Appl. Anim. Sci. 3, 307–312.
Nori-Shargh, D., Norouzi-Arasi, H., Mirza, M., Jaimand, K., and Mohammadi, S. (1999). Chemical composition of the essential oil ofTanacetum polycephalum (Schultz Bip. ssp.heterophyllum). Flavour Fragr. J. 14, 105–106. doi:10.1002/(SICI)1099-1026(199903/04)14:2<105:AID-FFJ791>3.0.CO;2–7
Oberprieler, C., Himmelreich, S., and Vogt, R. (2007). A new subtribal classification of the tribe Anthemideae (Compositae). Willdenowia 37, 89–114. doi:10.3372/wi.37.37104
Olvera-Sandoval, C., Fabela-Illescas, H. E., Fernández-Martínez, E., Ortiz-Rodríguez, M. A., Cariño-Cortés, R., Ariza-Ortega, J. A., et al. (2022). Potential mechanisms of the Improvement of glucose homeostasis in type 2 diabetes by Pomegranate juice. Antioxidants 11, 553. doi:10.3390/antiox11030553
Omer, O. l. (2013). The anthelmintic effect of Urtica dioica and Tanacetum vulgare L. on Protoscoleces of Echinococcus granulosus. Int. J. Sci. Basic Appl. Res. IJSBAR 11, 84–89.
Orhan, I. E., Tosun, F., Gülpınar, A. R., Kartal, M., Duran, A., Mihoglugil, F., et al. (2015). LC–MS quantification of parthenolide and cholinesterase inhibitory potential of selected Tanacetum L. (Emend. Briq.) taxa. Phytochem. Lett. 11, 347–352. doi:10.1016/j.phytol.2014.10.003
Özek, G. (2018). Chemical diversity and biological potential of tanacetum praeteritum subsp. Praeteritum essential oils. J. Turk. Chem. Soc. A Chem. 5, 493–510. doi:10.18596/jotcsa.389075
Özek, G., Özek, T., Işcan, G., Başer, K. H. C., Hamzaoglu, E., and Duran, A. (2007). Composition and antimicrobial activity of the essential oil of Tanacetum cadmeum (Boiss.) Heywood subsp. orientale Grierson. J. Essent. Oil Res. 19, 392–395. doi:10.1080/10412905.2007.9699313
Palevitch, D., Earon, G., and Carasso, R. (1997). Feverfew (tanacetum parthenium) as a prophylactic treatment for migraine: A double-blind placebo-controlled study. Phytother. Res. 11, 508–511. doi:10.1002/(SICI)1099-1573(199711)11:7<508:AID-PTR153>3.0.CO;2-H
Pareek, A., Suthar, M., Rathore, G. S., and Bansal, V. (2011). Feverfew (tanacetum parthenium L.): A systematic review. Pharmacogn. Rev. 5, 103–110. doi:10.4103/0973-7847.79105
Pavela, R., Sajfrtová, M., Sovová, H., Bárnet, M., and Karban, J. (2010). The insecticidal activity of Tanacetum parthenium (L.) Schultz Bip. extracts obtained by supercritical fluid extraction and hydrodistillation. Ind. Crops Prod. 31, 449–454. doi:10.1016/j.indcrop.2010.01.003
Petrov, N. M. (2016). Antiviral activity оf plant extract from tanacetum vulgare against Cucumber Mosaic virus and Potato virus Y. J. Biosci. Biotechnol. 5, 189–194.
Pieroni, A., and Giusti, M. E. (2009). Alpine ethnobotany in Italy: Traditional knowledge of gastronomic and medicinal plants among the Occitans of the upper Varaita valley, Piedmont. J. Ethnobiol. Ethnomedicine 5, 32. doi:10.1186/1746-4269-5-32
Pieroni, A., and Giusti, M. E. (2008). The remedies of the folk medicine of the Croatians living in Cićarija, northern Istria. Coll. Antropol. 32, 623–627.
Pieroni, A., Quave, C. L., Villanelli, M. L., Mangino, P., Sabbatini, G., Santini, L., et al. (2004). Ethnopharmacognostic survey on the natural ingredients used in folk cosmetics, cosmeceuticals and remedies for healing skin diseases in the inland Marches, Central-Eastern Italy. J. Ethnopharmacol. 91, 331–344. doi:10.1016/j.jep.2004.01.015
Polatoğlu, K., Demirci, B., Demirci, F., Gören, N., and Başer, K. H. C. (2012). Biological activity and essential oil composition of two new Tanacetum chiliophyllum (Fisch. & Mey.) Schultz Bip. var. chiliophyllum chemotypes from Turkey. Ind. Crops Prod. 39, 97–105. doi:10.1016/j.indcrop.2012.02.005
Polatoglu, K., Demirci, B., Goren, N., and Baser, K. H. C. (2011). Essential oil composition of Tanacetum kotschyi from Turkey. Chem. Nat. Compd. 47, 297–299. doi:10.1007/s10600-011-9912-6
Polatoglu, K., Demirci, F., Demirci, B., Gören, N., and Başer, K. H. C. (2010). Antibacterial activity and the variation of Tanacetum parthenium (L.) Schultz Bip. essential oils from Turkey. J. Oleo Sci. 59, 177–184. doi:10.5650/jos.59.177
Polatoğlu, K., Gören, N., Başer, K. H. C., and Demirci, B. (2009). The essential oil composition of tanacetum densum (Labill.) Heywood ssp. sivasicum Hub.-Mor. & Grierson from Turkey. J. Essent. Oil Res. 21, 200–202. doi:10.1080/10412905.2009.9700148
Polatoğlu, K., Karakoç, Ö. C., Demirci, B., Gören, N., and Can Başer, K. H. (2015). Sitophilus granarius L. (Coleoptera) toxicity and biological activities of the essential oils of tanacetum macrophyllum (Waldst. & Kit.) Schultz Bip. J. Oleo Sci. 64, 881–893. doi:10.5650/jos.ess15078
Polatoğlu, K., Karakoç, O. C., Demirci, F., Gökçe, A., and Gören, N. (2013). Chemistry and biological activities of Tanacetum chiliophyllum var. oligocephalum extracts. J. AOAC Int. 96, 1222–1227. doi:10.5740/jaoacint.sgepolatoglu
Polle, A. Y., Ovodova, R. G., Shashkov, A. S., and Ovodov, Yu. S. (2001). Isolation and general characterization of polysaccharides from tansy tanacetum vulgare L. Russ. J. Bioorg. Chem. 27, 45–49. doi:10.1023/A:1009531219169
Pranskuniene, Z., Ratkeviciute, K., Simaitiene, Z., Pranskunas, A., and Bernatoniene, J. (2019). Ethnobotanical study of cultivated plants in Kaišiadorys district, Lithuania: Possible trends for new herbal based medicines. Evid. Based Complement. Altern. Med. 2019, 3940397. doi:10.1155/2019/3940397
Prasad, B. J., Sharavanan, P. S., and Sivaraj, R. (2019). Efficiency of Oryza punctata extract on glucose regulation: Inhibition of α-amylase and α-glucosidase activities. Grain Oil Sci. Technol. 2, 44–48. doi:10.1016/j.gaost.2019.04.007
Pukalskas, A., Venskutonis, P. R., Dijkgraaf, I., and van Beek, T. A. (2010). Isolation, identification and activity of natural antioxidants from costmary (Chrysanthemum balsamita) cultivated in Lithuania. Food Chem. 122, 804–811. doi:10.1016/j.foodchem.2010.03.064
Qwarse, M., Mihale, M. J., Mugoyela, V., and Sunghwa, F. (2018). Ethnobotanical survey of medicinal and pesticidal plants used by Agro-pastoral communities in Mbulu district, Tanzania. Tanzan. J. Sci. Technol. 1, 22–35.
Rajaei, P., and Mohamadi, N. (2012). Ethnobotanical study of medicinal plants of Hezar mountain Allocated in south east of Iran. Iran. J. Pharm. Sci. 11, 1153–1167.
Ramzi, S., and Hosseininaveh, V. (2010). Biochemical characterization of digestive α-amylase, α-glucosidase and β-glucosidase in pistachio green stink bug, Brachynema germari Kolenati (Hemiptera: Pentatomidae). J. Asia-Pac. Entomol. 13, 215–219. doi:10.1016/j.aspen.2010.03.009
Recinella, L., Chiavaroli, A., di Giacomo, V., Antolini, M. D., Acquaviva, A., Leone, S., et al. (2021). Anti-inflammatory and neuromodulatory effects induced by tanacetum parthenium water extract: Results from in Silico, in vitro and ex vivo studies. Molecules 26, 22. doi:10.3390/molecules26010022
Rezaei, F., Jamei, R., and Heidari, R. (2017). Evaluation of the phytochemical and antioxidant potential of aerial parts of Iranian tanacetum parthenium. Pharm. Sci. 23, 136–142. doi:10.15171/PS.2017.20
Rezazadeh, F., Mahdavi, M., Motavalizadehkakhky, A., Mehrzad, J., Abedi, F., Roozbeh-nasira’ei, L., et al. (2014). Essential oil composition of three population of Tanacetum polycephalum from Iran and their antimicrobial activity. J. Essent. Oil Bear. Plants 17, 317–330. doi:10.1080/0972060X.2013.813260
Rosselli, S., Bruno, M., Raimondo, F. M., Spadaro, V., Varol, M., Koparal, A. T., et al. (2012). Cytotoxic effect of eudesmanolides isolated from flowers of tanacetum vulgare ssp. siculum. Molecules 17, 8186–8195. doi:10.3390/molecules17078186
Rusu, M. A., Tamas, M., Puica, C., Roman, I., and Sabadas, M. (2005). The hepatoprotective action of ten herbal extracts in CCl4 intoxicated liver. Phytother. Res. PTR 19, 744–749. doi:10.1002/ptr.1625
Safa, O., Soltanipoor, M. A., Rastegar, S., Kazemi, M., Nourbakhsh Dehkordi, K., and Ghannadi, A. (2013). An ethnobotanical survey on hormozgan province, Iran. Avicenna J. Phytomedicine 3, 64–81.
Salamci, E., Kordali, S., Kotan, R., Cakir, A., and Kaya, Y. (2007). Chemical compositions, antimicrobial and herbicidal effects of essential oils isolated from Turkish Tanacetum aucheranum and Tanacetum chiliophyllum var. chiliophyllum. Biochem. Syst. Ecol. 35, 569–581. doi:10.1016/j.bse.2007.03.012
Sarkhail, P. (2014). Traditional uses, phytochemistry and pharmacological properties of the genus Peucedanum: A review. J. Ethnopharmacol. 156, 235–270. doi:10.1016/j.jep.2014.08.034
Savci, A., koçpınar, E., Alan, Y., and Kurşat, M. (2020). Antioxidant, antimicrobial, and DNA protection activities of some Tanacetum species and phenolic richness in their ethanolic extracts. Int. Food Res. J. 27, 160–170.
Schinella, G. R., Giner, R.-M., Recio, M. D. C., de Buschiazzo, P. M., Ríos, J., and Máñez, S. (1998). Anti-inflammatory effects of south American tanacetum vulgare. J. Pharm. Pharmacol. 50, 1069–1074. doi:10.1111/j.2042-7158.1998.tb06924.x
Şen, A., Yıldırım, A., Bitis, L., and Doğan, A. (2019). Antioxidant and anti-inflammatory activity of capitula, leaf and stem extracts of Tanacetum cilicicum (Boiss.) Grierson. Int. J. Second. Metab. 6, 211–222. doi:10.21448/ijsm.510316
Servi, H., Goren, N., Sen, A., and Servi, E. Y. (2021). A new eudesmanolide from Tanacetum balsamita L. and biological activities of extracts. Nat. Prod. Res. 37, 1338–1348. doi:10.1080/14786419.2021.2005594
Servi̇, H., and Gören, N. (2019). Chemistry of endemicTanacetum mucroniferumHub.-Mor. & Grierson extractsand three new sesquiterpene lactones. Turk. J. Chem. 43, 352–358. doi:10.3906/kim-1808-26
Sezik, E., Yelada, E., Tabata, M., Honda, G., Takaishi, Y., Fujita, T., et al. (1997). Traditional medicine in Turkey VIII. Folk medicine in East Anatolia; Erzurum, Erzncan, Aǧ ri, Kars, Iǧ dir provinces. Econ. Bot. 51, 195–211.
Shamkhani, H., Nasiri, N., Aliahmadi, A., and Sonboli, A. (2016). Essential oil composition and antibacterial activity of tanacetum hololeucum from Iran. Rec. Nat. Prod. 10, 6.
Sharif, M., Najafizadeh, P., Asgarpanah, J., and Mousavi, Z. (2020). In vivo analgesic and anti-inflammatory effects of the essential oil from Tanacetum balsamita L. Braz. J. Pharm. Sci. 56, e18357. doi:10.1590/s2175-97902019000418357
Shikov, A. N., Pozharitskaya, O. N., Makarov, V. G., Wagner, H., Verpoorte, R., and Heinrich, M. (2014). Medicinal Plants of the Russian Pharmacopoeia; their history and applications. J. Ethnopharmacol. 154, 481–536. doi:10.1016/j.jep.2014.04.007
Shikov, A. N., Tsitsilin, A. N., Pozharitskaya, O. N., Makarov, V. G., and Heinrich, M. (2017). Traditional and current food Use of wild plants listed in the Russian Pharmacopoeia. Front. Pharmacol. 8, 841. doi:10.3389/fphar.2017.00841
Singhal, V. K., Tantray, Y. R., and Gupta, R. C. (2016). Structural Heterozygosity for Reciprocal translocation in Tanacetum artemisioides Sch. Bip. Ex Hook. F. from Ladakh Division of Jammu and Kashmir. Cytol. (Tokyo) 81, 319–322. doi:10.1508/cytologia.81.319
Sonboli, A., Stroka, K., Kazempour Osaloo, S., and Oberprieler, C. (2012). Molecular phylogeny and taxonomy of Tanacetum L. (Compositae, Anthemideae) inferred from nrDNA ITS and cpDNA trnH–psbA sequence variation. Plant Syst. Evol. 298, 431–444. doi:10.1007/s00606-011-0556-6
Sõukand, R., and Pieroni, A. (2016). The importance of a border: Medical, veterinary, and wild food ethnobotany of the Hutsuls living on the Romanian and Ukrainian sides of Bukovina. J. Ethnopharmacol. 185, 17–40. doi:10.1016/j.jep.2016.03.009
Souto, A. L., Sylvestre, M., Tölke, E. D., Tavares, J. F., Barbosa-Filho, J. M., and Cebrián-Torrejón, G. (2021). Plant-derived pesticides as an alternative to pest management and sustainable agricultural production: Prospects, applications and Challenges. Molecules 26, 4835. doi:10.3390/molecules26164835
Stanković, N., Mihajilov-Krstev, T., Zlatković, B., Stankov-Jovanović, V., Mitić, V., Jović, J., et al. (2016). Antibacterial and antioxidant activity of traditional medicinal plants from the Balkan Peninsula. NJAS Wagening. J. Life Sci. 78, 21–28. doi:10.1016/j.njas.2015.12.006
Sur, R., Martin, K., Liebel, F., Lyte, P., Shapiro, S., and Southall, M. (2009). Anti-inflammatory activity of parthenolide-depleted feverfew (tanacetum parthenium). Inflammopharmacology 17, 42–49. doi:10.1007/s10787-008-8040-9
Susurluk, H., Çalışkan, Z., Gürkan, O., Kırmızıgül, S., and Gören, N. (2007). Antifeedant activity of some Tanacetum species and bioassay guided isolation of the secondary metabolites of Tanacetum cadmeum ssp. cadmeum (Compositae). Ind. Crops Prod. 26, 220–228. doi:10.1016/j.indcrop.2007.04.002
Tiuman, T. S., Ueda-Nakamura, T., Garcia Cortez, D. A., Dias Filho, B. P., Morgado-Díaz, J. A., de Souza, W., et al. (2005). Antileishmanial activity of parthenolide, a sesquiterpene lactone isolated from tanacetum parthenium. Antimicrob. Agents Chemother. 49, 176–182. doi:10.1128/AAC.49.11.176-182.2005
Triana, J., Eiroa, J. L., Morales, M., Pérez, F. J., Brouard, I., Marrero, M. T., et al. (2013). A chemotaxonomic study of endemic species of genus Tanacetum from the Canary Islands. Phytochemistry 92, 87–104. doi:10.1016/j.phytochem.2013.04.015
Tribess, B., Pintarelli, G. M., Bini, L. A., Camargo, A., Funez, L. A., de Gasper, A. L., et al. (2015). Ethnobotanical study of plants used for therapeutic purposes in the Atlantic Forest region, Southern Brazil. J. Ethnopharmacol. 164, 136–146. doi:10.1016/j.jep.2015.02.005
Türker, S. (2018). I. International Congress on medicinal and aromatic plants: “Natural and healthy life. Konya, Türkiye: Necmettin Erbakan Üniversitesi Kültür Yayınları.
Uberti, F., Lattuada, D., Morsanuto, V., Nava, U., Bolis, G., Vacca, G., et al. (2014). Vitamin D protects human endothelial cells from oxidative stress through the Autophagic and Survival pathways. J. Clin. Endocrinol. Metab. 99, 1367–1374. doi:10.1210/jc.2013-2103
Uehara, A., Akiyama, S., and Iwashina, T. (2015). Foliar flavonoids from Tanacetum vulgare var. boreale and their geographical variation. Nat. Prod. Commun. 10, 1934578X1501000–405. doi:10.1177/1934578x1501000307
Ullah, M., Mehmood, S., Ali, M., Bussmann, R. W., Aldosari, A., Khan, R. A., et al. (2019). An ethnopharmacological study of plants used for treatment of diabetes in the Southern and Tribal regions of Khyber Pakhtunkhwa province, Pakistan. Ethnobot. Res. Appl. 18, 1–20. doi:10.32859/era.18.8.1-20
Van Wyk, B.-E., and Gorelik, B. (2017). The history and ethnobotany of Cape herbal teas. South Afr. J. Bot. 110, 18–38. doi:10.1016/j.sajb.2016.11.011
Vitalini, S., Puricelli, C., Mikerezi, I., and Iriti, M. (2015). Plants, people and traditions: Ethnobotanical survey in the Lombard Stelvio National Park and neighbouring areas (central Alps, Italy). J. Ethnopharmacol. 173, 435–458. doi:10.1016/j.jep.2015.05.036
Vokou, D., Katradi, K., and Kokkini, S. (1993). Ethnobotanical survey of Zagori (Epirus, Greece), a renowned centre of folk medicine in the past. J. Ethnopharmacol. 39, 187–196. doi:10.1016/0378-8741(93)90035-4
Vukic, M. D., Vukovic, N. L., Obradovic, A. D., Galovičová, L., Čmiková, N., Kačániová, M., et al. (2022). Chemical composition and biological activity of tanacetum balsamita essential oils obtained from different plant Organs. Plants 11, 3474. doi:10.3390/plants11243474
Wan, C.-W., Wong, C. N.-Y., Pin, W.-K., Wong, M. H.-Y., Kwok, C.-Y., Chan, R. Y.-K., et al. (2013). Chlorogenic acid exhibits cholesterol lowering and fatty liver attenuating properties by up-regulating the gene expression of PPAR-α in hypercholesterolemic rats induced with a high-cholesterol diet. Phytother. Res. PTR 27, 545–551. doi:10.1002/ptr.4751
Weyerstahl, P., Marschall, H., Thefeld, K., and Rustaiyan, A. (1999). Constituents of the essential oil of Tanacetum (syn. Chrysanthemum) fruticulosum Ledeb. from Iran. Flavour Fragr. J. 14, 112–120. doi:10.1002/(SICI)1099-1026(199903/04)14:2<112:AID-FFJ786>3.0.CO;2–1
Williams, C. A., Harborne, J. B., and Eagles, J. (1999). Variations in lipophilic and polar flavonoids in the genus Tanacetum. Phytochemistry 52, 1301–1306. doi:10.1016/S0031-9422(99)00425-2
Xie, G., Schepetkin, I. A., and Quinn, M. T. (2007). Immunomodulatory activity of acidic polysaccharides isolated from Tanacetum vulgare L. Int. Immunopharmacol. 7, 1639–1650. doi:10.1016/j.intimp.2007.08.013
Yousefzadi, M., Ebrahimi, S. N., Sonboli, A., Miraghasi, F., Ghiasi, S., Arman, M., et al. (2009). Cytotoxicity, antimicrobial activity and composition of essential oil from tanacetum balsamita L. Subsp. balsamita. Nat. Prod. Commun. 4, 1934578X0900400. doi:10.1177/1934578X0900400126
Yu, Z. M., Tm, H., Ah, K., Tv, I., and Ov, K. (2017). Study of dry extract of tansy (tanacetum vulgare) using the method of high-performance liquid chromatography. Pharma Chem. 9, 1–4.
Yur, S., Tekin, M., Göger, F., Başer, K. H. C., Özek, T., and Özek, G. (2017). Composition and potential of Tanacetum haussknechtii Bornm. Grierson as antioxidant and inhibitor of acetylcholinesterase, tyrosinase, and α-amylase enzymes. Int. J. Food Prop. 20, S2359–S2378. doi:10.1080/10942912.2017.1370600
Zeng, Q., Ko, C.-H., Siu, W.-S., Li, L.-F., Han, X.-Q., Yang, L., et al. (2017). Polysaccharides of Dendrobium officinale Kimura & Migo protect gastric mucosal cell against oxidative damage-induced apoptosis in vitro and in vivo. J. Ethnopharmacol. 208, 214–224. doi:10.1016/j.jep.2017.07.006
Zeng, T., Li, J.-W., Zhou, L., Xu, Z.-Z., Li, J.-J., Hu, H., et al. (2021). Transcriptional responses and GCMS analysis for the biosynthesis of pyrethrins and volatile Terpenes in tanacetum coccineum. Int. J. Mol. Sci. 22, 13005. doi:10.3390/ijms222313005
Keywords: Tanacetum, ethnopharmacology, ceramides, sesquiterpene lactones, pharmacology, toxicity, clinical evidence
Citation: Khatib S, Sobeh M, Faraloni C and Bouissane L (2023) Tanacetum species: Bridging empirical knowledge, phytochemistry, nutritional value, health benefits and clinical evidence. Front. Pharmacol. 14:1169629. doi: 10.3389/fphar.2023.1169629
Received: 19 February 2023; Accepted: 29 March 2023;
Published: 20 April 2023.
Edited by:
Daniela Rigano, University of Naples Federico II, ItalyReviewed by:
Irem I. Tatli, Hacettepe University, TürkiyeValeria D'Angelo, University of Messina, Italy
Copyright © 2023 Khatib, Sobeh, Faraloni and Bouissane. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Latifa Bouissane, bC5ib3Vpc3NhbmVAdXNtcy5tYQ==; Mansour Sobeh, bWFuc291ci5zb2JlaEB1bTZwLm1h
†ORCID Latifa Bouissane, orcid.org/0000-0002-2231-1956
 Sohaib Khatib
Sohaib Khatib Mansour Sobeh
Mansour Sobeh Cecilia Faraloni3
Cecilia Faraloni3 Latifa Bouissane
Latifa Bouissane