- 1Department of Pharmacy, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2School of Pharmacy, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Introduction: Tumor necrosis factor (TNF) inhibitors (adalimumab, infliximab, etanercept, golimumab, and certolizumab pegol) have revolutionized the treatment of severe immune-mediated inflammatory diseases, including rheumatoid arthritis, Crohn’s disease, psoriatic arthritis, ankylosing spondylitis, and ulcerative colitis. This study assessed adverse drug reactions (ADRs) after the use of TNFα inhibitors in VigiAccess of the World Health Organization (WHO) and compared the adverse reaction characteristics of five inhibitors to select the drug with the least risk for individualized patient use.
Methods: The study was a retrospective descriptive analysis method in design. We sorted out five marketed anti-TNFα drugs, and their ADR reports were obtained from WHO-VigiAccess. Data collection included data on the age groups, sex, and regions of patients worldwide covered by ADR reports, as well as data on disease systems and symptoms caused by ADRs recorded in annual ADR reports and reports received by the WHO. By calculating the proportion of adverse reactions reported for each drug, we compared the similarities and differences in adverse reactions for the five drugs.
Results: Overall, 1,403,273 adverse events (AEs) related to the five anti-TNFα agents had been reported in VigiAccess at the time of the search. The results show that the 10 most commonly reported AE manifestations were rash, arthralgia, rheumatoid arthritis, headache, pneumonia, psoriasis, nausea, diarrhea, pruritus, and dyspnea. The top five commonly reported AE types of anti-TNFα drugs were as follows: infections and infestations (184,909, 23.0%), musculoskeletal and connective tissue disorders (704,657, 28.6%), gastrointestinal disorders (122,373, 15.3%), skin and subcutaneous tissue disorders (108,259, 13.5%), and nervous system disorders (88,498, 11.0%). The preferred terms of myelosuppression and acromegaly were obvious in golimumab. Infliximab showed a significantly higher ADR report ratio in the infusion-related reaction compared to the other four inhibitors. The rate of ADR reports for lower respiratory tract infection and other infections was the highest for golimumab.
Conclusion: No causal associations could be established between the TNFα inhibitors and the ADRs. Current comparative observational studies of these inhibitors revealed common and specific adverse reactions in the ADR reports of the WHO received for these drugs. Clinicians should improve the rational use of these high-priced drugs according to the characteristics of ADRs.
Introduction
Tumor necrosis factor (TNF)α is a proinflammatory, multifunctional cytokine that is synthesized by various cells, including activated monocytes, macrophages, and T cells (Darrigade et al., 2017). TNFα has been shown to play an essential role in the pathogenesis of autoimmune diseases such as rheumatoid arthritis (RA), psoriatic arthritis (PSA), and ankylosing spondylitis (AS). Therefore, drugs targeting TNF have been developed to neutralize the effects of these pro-inflammatory cytokines (Jang et al., 2021). Anti-TNFα drugs are usually well-tolerated; however, there have been reports of many potentially serious adverse effects. Long-term use of anti-TNFα agents has been associated with the risk of serious infections, malignancies, skin and soft tissue infections, and tuberculosis (Kroesen et al., 2003; Shivaji et al., 2019). A meta-analysis showed that treatment with anti-TNFα agents increased the risk of serious infections (OR: 1.72, 95% CI: 1.56–1.90, p < 0.00001) and an increase in cancer risk (OR: 1.36, 95% CI: 1.20–1.53, p < 0.00001), whereas the risk of tuberculosis was not significantly different (Li et al., 2021). Although there was no consensus on the risk of infection associated with anti-TNFα treatments in published clinical trials, post-marketing surveillance and retrospective studies have shown an increased risk of tuberculosis and other granulomatous infections (Wallis et al., 2004; Godfrey and Friedman, 2019; Athimni et al., 2022; Doughty et al., 2023). A meta-analysis carried out in 2006 showed an increase in the risk of malignancies and serious infections in patients treated with infliximab and adalimumab, where a higher dose was associated with increased cancer risk (Galloway et al., 2011). Despite the rigor of pre-marketing drug trials, the safety of medicines is not completely understood from pre-authorization clinical trial data as these trials are conducted in controlled settings different from settings of real-world use (Gagliardi et al., 2022). In particular, biological agents such as TNFα inhibitors, which have been on the market for a long time, have a large population base and a wide range of use, so safety research based on a large number of real-world data is more appropriate and valuable. Therefore, adverse drug reactions (ADRs) associated with the TNFα inhibitors need to be further characterized from spontaneous reports in pharmacovigilance databases. More significantly, there are no studies to compare the similarities and differences of ADRs produced by these drugs.
Despite the intrinsic limitations, spontaneous reporting systems represent a valuable source to obtain real-world data about the safety profile of drugs and vaccines, compare therapeutic options, and gain insight into the potential mechanisms of ADRs (Hazell and Shakir, 2006). Spontaneous reporting systems have been the backbone of pharmacovigilance since their introduction in the 1960s. The main aim of spontaneous reporting is the early detection of previously unrecognized ADRs. In addition, spontaneous reporting can also be useful for obtaining information on new aspects of known associations between drugs and ADRs (Srba et al., 2012). The Uppsala Monitoring Center (UMC), on behalf of the World Health Organization (WHO)’s Programme for International Drug Monitoring (PIDM), brings together safety data from all corners of the world. As of the end of 2018, the UMC had received and stored over 20 million ADR reports from more than 170 countries in VigiBase, which is a worldwide voluntary reporting program. Since 2015, data stored in VigiBase can be freely accessed by the public via VigiAccess (Watson et al., 2018; Habarugira and Figueras, 2021). The VigiAccess database supports searching by the trade name of the drug, but the database will identify the active ingredient it contains and display the results of its ADR reports according to the active ingredient. This study searched for five biological TNFα blockers approved by the Food and Drug Administration (FDA): adalimumab, infliximab, etanercept, golimumab, and certolizumab pegol.
The five TNFα inhibitors showed similar efficacy profiles, although recent data show that adalimumab seems to be most effective in geriatric patients, while etanercept is associated with a lower risk of developing tuberculosis (Bonek et al., 2021). Therefore, clinicians are often required to tailor treatment decisions based on the risk of adverse events for the individual patient. To compare the differences in the occurrence of these five anti-TNFα-related adverse reactions, we conducted a descriptive study of spontaneous reported adverse reactions in the VigiAccess database and compared the reported rates of adverse reactions caused by these five drugs.
Materials and methods
Drug sample
Table 1 shows the five anti-TNFα agents that we have studied that are available for clinical use. Humanized anti-TNF-α mAbs include Humira® (adalimumab), Simponi® (golimumab), and Cimzia® (certolizumab pegol). Remicade® (infliximab) was the first chimeric anti-TNF-α mAb for the treatment of Crohn’s disease. The use of Remicade was then extended to other therapeutic areas, including RA (in combination with methotrexate), ankylosing spondylitis, psoriatic arthritis, ulcerative colitis, pediatric Crohn’s disease, plaque psoriasis, and pediatric ulcerative colitis. The first Ab fusion protein, Enbrel® (etanercept), was approved by the FDA for clinical use in patients with RA in 1998. It comprises the Fc region of Ab conjugated with TNF-receptor 2 (TNFR2). The approval of etanercept opened up the way for the development of several recombinant protein methodologies based on the fusion of various proteins to different antibody regions, including single-chain variable fragments (scFvs), heavy-chain Abs (hcAbs), single-chain Abs (scAbs), and antigen-binding fragments (Fabs) (Leone et al., 2023). It can be seen in Table 1 that their structures are not completely the same, and the sources of synthesis are also different. The earliest launch time of these five drugs is more than 10 years, and they are currently among the world’s best-selling drugs, and all of them are marketed in China.
By December 2022, there were thirteen adalimumab biosimilars, seven infliximab biosimilars, and three etanercept biosimilars (Table 1). There was no increase in adverse events (AEs) in patients treated with adalimumab biosimilars and concomitant methotrexate (MTX) therapy compared to Humira and MTX therapy in patients with RA (Cohen et al., 2017; Fleischmann et al., 2018). Studies have shown that there are no differences in safety, immunogenicity, and pharmacokinetics between infliximab and infliximab biosimilars (Subedi et al., 2019). Only proven similar efficacy and safety in short- and long-term studies allow switching between originator and biosimilar products. The current time available for comparative research is limited (Agca et al., 2017; Kameda et al., 2020).
Data sources
WHO-VigiAccess was searched on 2 January 2022 for all reported adverse events following the introduction of anti-TNFα agents. The login URL is https://www.vigiaccess.org. All study drugs were identified by the generic name. Data were captured among age groups, sex, report year, and continents of the world by WHO-VigiAccess. Descriptive data were calculated using Excel 2019 version.
WHO-VigiAccess is a free-access portal to the PIDM database allowing retrieval of medicinal products’ safety reports received by the UMC. The definition relied on system organ class (SOC) and preferred terms (PTs) by the Medical Dictionary for Regulatory Activities (MedDRA). Thus, records on each anti-TNFα drug were retrieved, and all individual AEs based on MedDRA SOC and PT levels recorded were identified to describe the spectrum of toxicities. Reporting terms used in MedDRA were derived from several dictionaries, including the WHO Adverse Reaction Terminology (WHO-ART), among others (Sultana et al., 2020). A total of 27 items were classified by SOC, of which 20 items directly related to disease symptoms were selected for analysis. In the present study, we focused on the PTs, the level used in the VigiBase database publicly accessible information via WHO-VigiAccess.
To study the outcomes of detected safety signals, we grouped them using outcome code to produce the three severe categories: death, hospitalization, and major events comprising life-threatening events, disability, and congenital anomaly.
Statistical analysis
The study followed a retrospective quantitative study design. Excel descriptive analysis was used to analyze the characteristics of the victims of adverse reactions to the five drugs. The ADR symptom number of each drug divided by the total number of ADR reports was defined as the ADR report rate of the drug. Common ADRs of each drug refer to the symptoms of the top 20 ADR report rate. The rate of reported ADR symptoms for each drug was calculated, and a descriptive comparative analysis was performed. Frequencies and percentages were used to categorize descriptive variables.
Results
Description of the studied cases
The earliest reports of adalimumab, infliximab, etanercept, golimumab, and certolizumab pegol adverse reactions were received in the WHO-VigiAccess database in 2001, 1999, 1999, 2008, and 2003, respectively. By 2021, the WHO had received a total of 591,705, 172,961, 542,647, 38,629, and 57,331 ADR reports for these five drugs, with a total of 1,403,273 reports. The numbers of adverse events covered in these ADR reports were 840,417 cases of adalimumab, 280,811 cases of infliximab, 654,269 cases of etanercept, 4,895 cases of golimumab, and 78,388 cases of certolizumab pegol. Among the 1,403,273 reports related to the five anti-TNFα agents shown in Table 2, except for 73,803 cases in which the sex was unknown, the number of women (916,873) who had ADRs was significantly greater than that of men (412,597), and the female–male ratio was 2.22:1 with a big discrepancy. Excluding the unknown age reports, most of the age groups with the highest reported rates are between 45 and 64 years. Most of the AEs were reported from the Americas (77.94%). Table 2 also lists the reporting years for each of the studied drugs.
Distribution of 20 SOCs of five anti-TNFα drugs
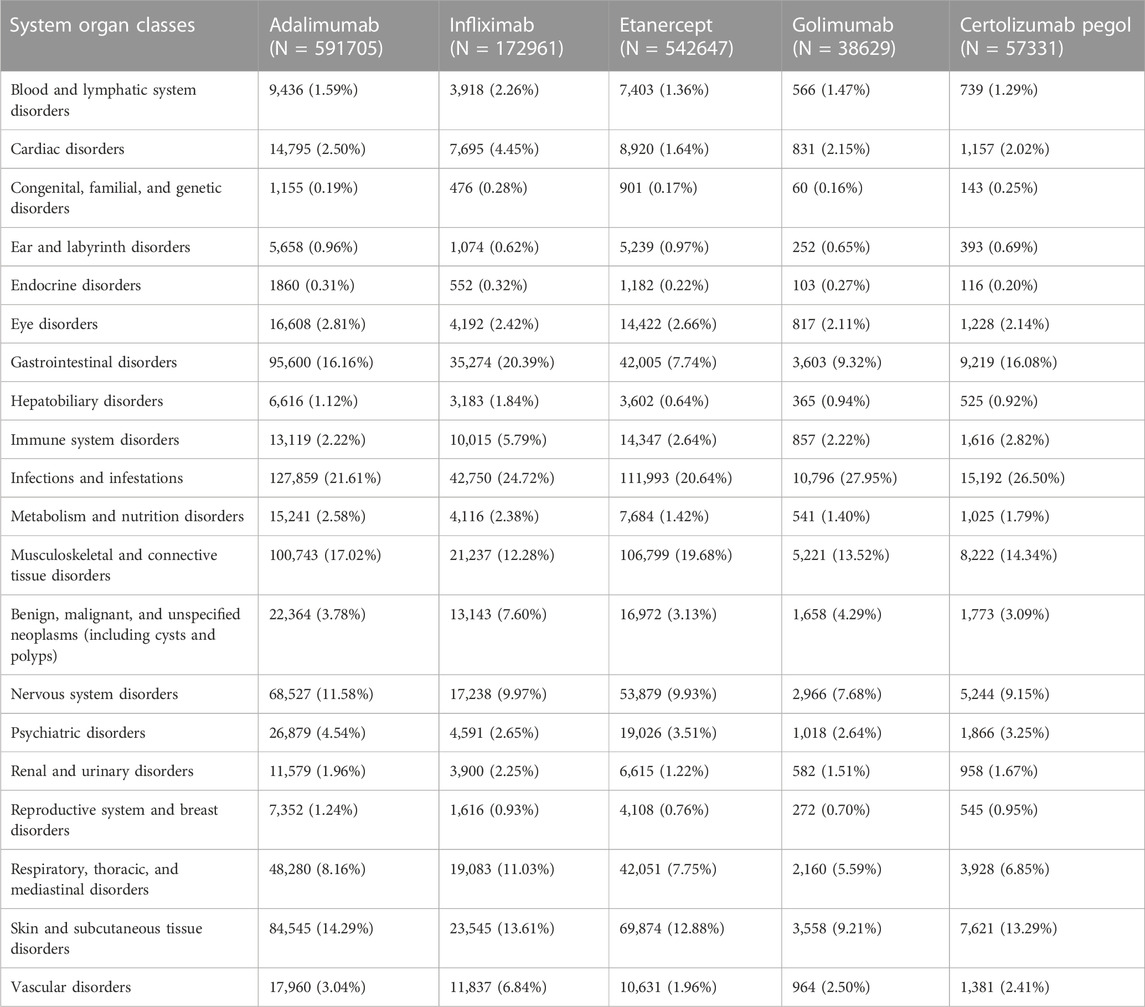
Table 3 shows the report rates of 20 SOCs of five anti-TNFα drugs. Adalimumab-related nervous system disorders and skin and subcutaneous tissue disorders were significantly higher than the disorders of the other four TNFα inhibitors. The rates of ADR reports of infliximab-related gastrointestinal disorders, cardiac disorders, benign, malignant, and unspecified neoplasms, vascular disorders, and respiratory, thoracic, and mediastinal disorders were significantly higher than those of the other four TNFα inhibitors. Higher rates of ADRs were reported for musculoskeletal and connective tissue disorders in etanercept, as well as infections and infestations in golimumab.
The top five commonly reported AE types of anti-TNFα drugs were as follows: infections and infestations (184,909, 23.0%), musculoskeletal and connective tissue disorders (704,657, 28.6%), gastrointestinal disorders (122,373, 15.3%), skin and subcutaneous tissue disorders (108,259, 13.5%), and nervous system disorders (88,498, 11.0%). The rate of ADRs reported more than 10% in the SOC, and there were four in adalimumab, five in infliximab, three in etanercept, two in golimumab, and four in certolizumab pegol.
Most common ADRs of five anti-TNFα drugs
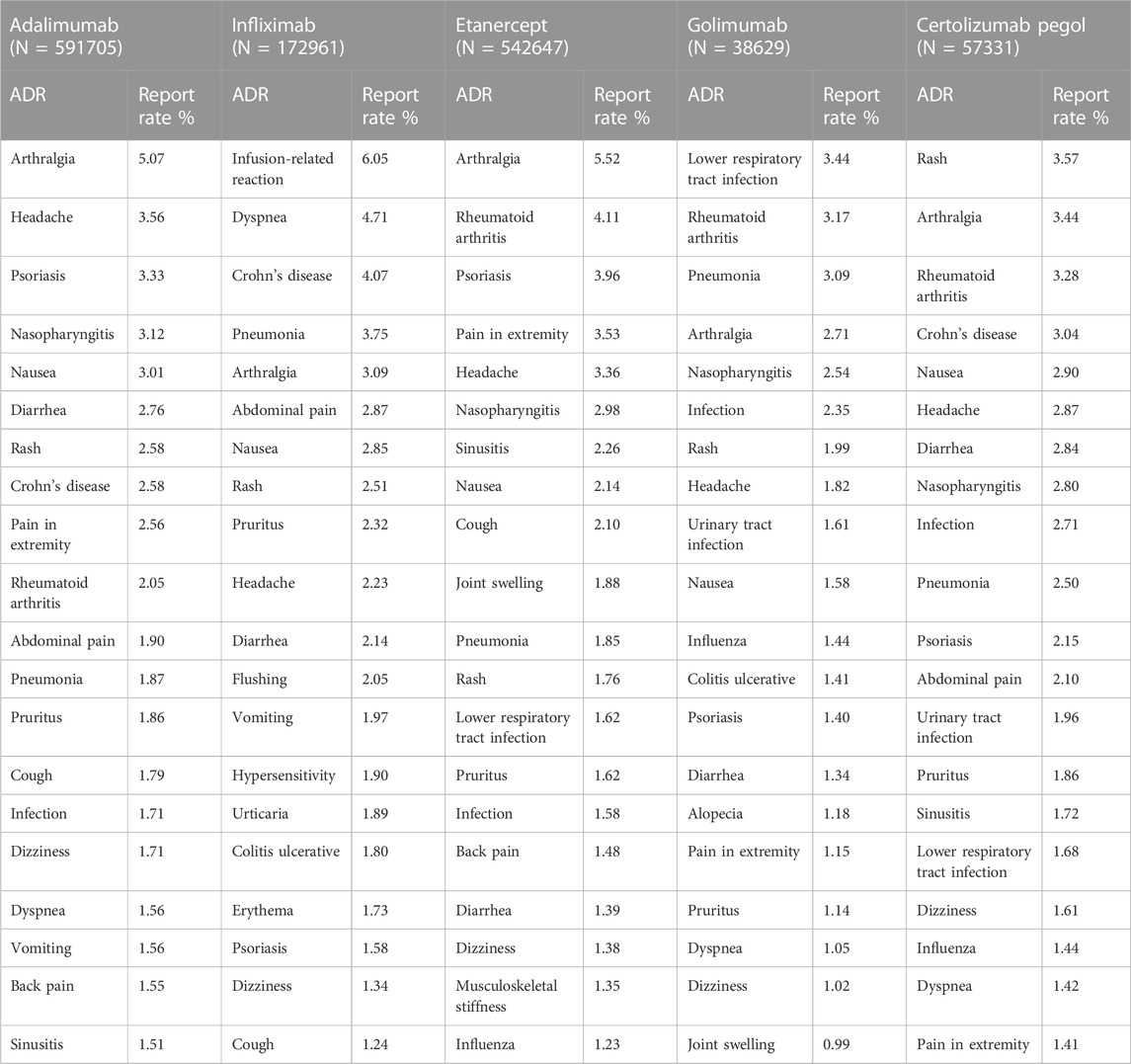
The 20 most commonly reported ADRs of the five drugs are presented in Table 4, and the manifestations listed were preferred terms from within the SOC. The common ADRs of all five TNFα inhibitors were rash, arthralgia, rheumatoid arthritis, headache, pneumonia, psoriasis, nausea, diarrhea, and pruritus. Infliximab showed a significantly higher ADR report rate in the infusion-related reaction compared to the other four inhibitors. The rate of ADR reports of lower respiratory tract infection and other infections in golimumab ranks at the top. Most of the AEs in the top 20 commonly reported were minor events that are self-limiting. However, there were some noteworthy events, and these were Crohn’s disease, rheumatoid arthritis, and all infections (pneumonia, urinary tract infection, and nasopharyngitis).
Serious AEs of five anti-TNFɑ drugs
Through WHO-VigiAccess, we can also find major adverse events of anti-TNFα drugs, including life-threatening events, disability, and congenital malformations. The proportion of serious adverse reactions that occurred for certolizumab pegol, infliximab, golimumab, adalimumab, and etanercept was 1.79%, 1.51%, 1.17%, 1.16%, and 1.01%, respectively (Figure 1).
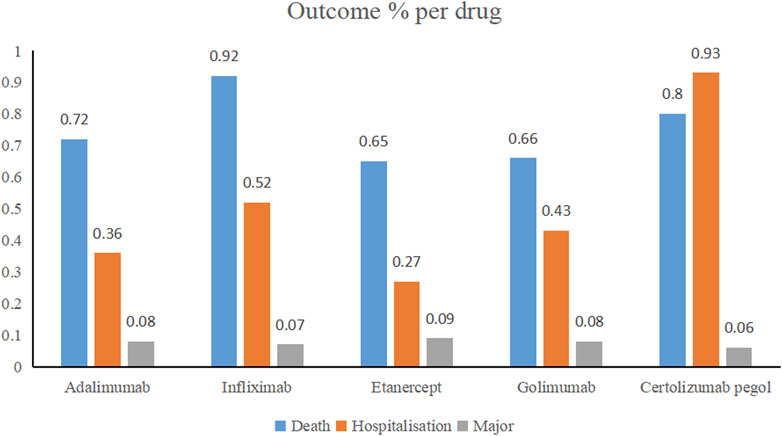
FIGURE 1. Outcomes for serious adverse events associated with anti-TNFα drugs at the level of preferred terms (major events comprising life-threatening events, disability, and congenital anomaly).
The same and different points of common ADRs of five anti-TNFɑ drugs
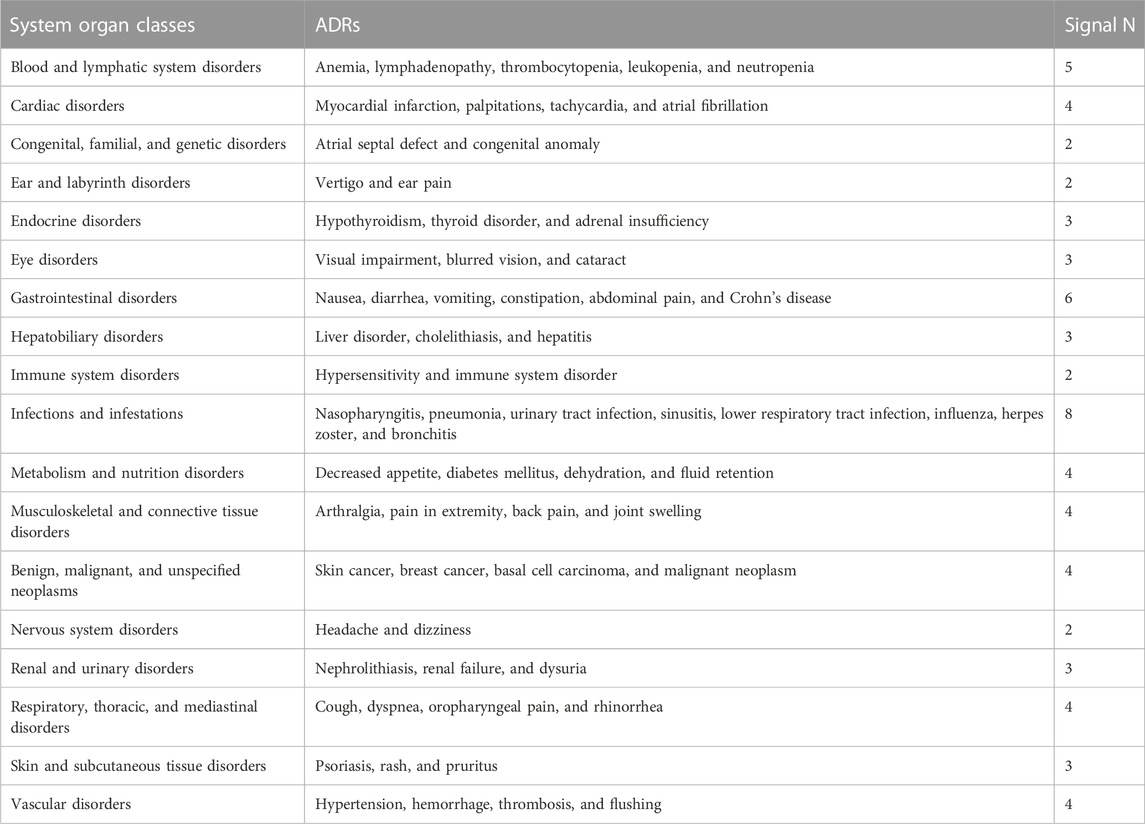
By comparing the top 20 ADRs reported by each anti-TNFα drug in the SOCs, a total of 66 same signals were found at PTs for the five inhibitors. All common signals were sorted into Table 5. The SOC that contained the most adverse signals was infections and infestations, and the top five reports were nasopharyngitis, pneumonia, urinary tract infection, sinusitis, and lower respiratory tract infection. The second was gastrointestinal disorders, and the top five reports were nausea, diarrhea, vomiting, constipation, and Crohn’s disease.
When comparing the top 20 ADRs reported for each anti-TNFα drug in the SOCs, all the five TNFα inhibitors had different PTs of ADR in congenital, familial, and genetic disorders (Table 6). The number of distinctive symptoms for adalimumab, infliximab, etanercept, golimumab, and certolizumab pegol was three, six, five, four, and seven, respectively.
Discussion
The SRS has been utilized in pharmacovigilance for safety assessment of suspected AEs due to inherent limitations of clinical trials, such as stringent trial design, strict enrollment criteria, relatively small sample size, and limited follow-up duration. Furthermore, study data from clinical trials may not fit the real world where patients and comorbidities are heterogenous. The SRS plays a major part in signal identification (Lindquist et al., 2000). At present, research on the safety signals of most drugs mainly comes from three main databases: the EudraVigilance Data Analysis System (EVDAS), Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS), and WHO-VigiBase® (Vogel et al., 2020). WHO-VigiAccess was launched by the WHO in 2015 to provide public access to information in VigiBase®, the WHO global database of reported potential side effects of medicinal products. Data mining of the WHO-VigiAccess database would provide previously unknown drug–AE associations and some well-established clinical ones (Yamoah et al., 2022). The present study was conducted to assess the post-market adverse events associated with TNFɑ inhibitors in the WHO-VigiAccess database.
Data from WHO-VigiAccess show that 70% of AEs associated with the five TNFα inhibitors are reported from the Americas, followed by Europe. Poor reporting of AEs from the African and Oceania continents had been commonly observed in other studies (Alawadhi et al., 2012; Gidudu et al., 2020). In South Africa, the lack of medical knowledge of biologic medicines among health professionals, coupled with high costs and complex procurement processes, increased barriers to the use of these drugs (Hajjaj-Hassouni et al., 2012; Martelli et al., 2017; Kvamme et al., 2020). Although the use of TNFα inhibitors to treat rheumatoid arthritis is more common among the South African doctors surveyed, accounting for about half of the prescriptions, it can be seen from this study that the number of reported adverse events in Africa is still very low (Mocke-Richter et al., 2021). This may be due to poor quality social mobilization, inadequate accessibility of adverse reaction reporting systems, and low information system coverage.
AEs were more commonly reported in females than males. Except for infliximab, the 45–64 age group had the most adverse reactions after anti-TNFα treatment. The reason for this finding is that the inhibitors of TNFα, such as infliximab, etanercept, and adalimumab, are now second line to methotrexate in RA (Papadopoulos et al., 2019). Golimumab and certolizumab pegol can be combined with methotrexate in the treatment of moderate-to-severe active RA with poor efficacy of disease-modifying anti-rheumatic drugs (DMARDs, including methotrexate) (Smolen et al., 2023). Real-world surveys based on the FAERS database found that more than half of anti-TNF therapy was used to treat rheumatoid arthritis. RA affects at least twice as many women as men, and although it can occur at any age, the peak incidence is at the age of 50 years (Deepak et al., 2013).
An AE with a reporting rate of ≥1% is usually considered common (Chen et al., 2019). Therefore, the serious adverse events, including life-threatening events, disabling, and congenital malformations, of the five anti-TNF α drugs are not common. The most common ADRs for all five TNFα inhibitors were rash, arthralgia, rheumatoid arthritis, headache, pneumonia, psoriasis, nausea, diarrhea, and pruritus.
Both patients with RA and patients with IBD have a higher incidence and severity of infectious diseases compared to the general population. The increased risk of infections in these patients seems to be attributed mainly to immunosuppressive therapies (Calvet et al., 2021). Treatment with systemic corticosteroids is associated with a very high risk of serious infection. The risk of infection also significantly increases when anti-TNFα therapies are combined (Beaugerie and Kirchgesner, 2019; Zabana et al., 2019; Singh et al., 2020). Through a review of the English literature over the last 30 years, experts noted an increased risk of bacterial and mycobacterial infections in patients with arthritis treated with TNF inhibitors compared to non-biologic agents (Chiu and Chen, 2020).
As of November 2016, the FAERS database showed that the top five adverse reactions related to etanercept infections were nasopharyngitis, sinusitis, bronchitis, pneumonia, and influenza, accounting for 13.98%, 10.22%, 6.90%, 6.07%, and 5.54%, respectively. The top five adverse reactions of adalimumab-related infections were nasopharyngitis (15.50%), sinusitis (7.83%), pneumonia (6.23%), bronchitis (5.60%), and influenza (4.45%). The adverse reactions of infliximab-related infections were pneumonia (7.69%), tuberculosis (6.15%), herpes zoster (4.72%), pulmonary tuberculosis (2.53%), and sepsis (2.49%) (Chen et al., 2019). However, through the VigiAccess database, we found that the proportion of infection-related adverse reactions in etanercept was nasopharyngitis- 2.98%, sinusitis- 2.26%, pneumonia- 1.85%, lower respiratory tract infection- 1.62%, and infection- 1.60%. The proportion of infection-related adverse reactions in adalimumab was nasopharyngitis- 3.12%, pneumonia- 1.86%, infection- 1.71%, sinusitis- 1.51%, and influenza- 1.27%. Infliximab-related adverse reactions were pneumonia (3.75%), lower respiratory tract infection (1.12%), infection (1.09%), cellulitis (0.91%), and tuberculosis (0.84%). WHO-VigiAccess and FAERS, as the databases to evaluate post-marketing drug vigilance, showed differences in the types and incidence of infection-related adverse reactions caused by anti-TNFα agents. Due to the voluntary reporting of adverse events, the passive-monitoring FAERS database and WHO-VigiAccess database do not represent a complete and comprehensive count of adverse events and may lack information about reported events. In comparison, the FAERS database can display specific reports of each adverse reaction in most cases and screen eligible case reports more accurately, but professional personnel and intelligent analysis software are needed (Mikami et al., 2021). This may require WHO-VigiAccess to further provide more reporting information to the public to screen for potential links between drugs and adverse reactions in order to avoid incorrect guidance.
Another notable adverse event of anti-TNFɑ therapy is an increased risk of cancer. An initial meta-analysis showed that the treatment of RA with TNFi was associated with 3.3 times greater odds of cancer when compared to placebo (Bongartz et al., 2006). One published series described 48 cases of malignancy reported to the FDA in children on a TNF inhibitor, half of which were lymphomas (Diak et al., 2010). An analysis of TNFɑ-inhibitor-treated patients with inflammatory bowel disease (IBD) in the French National Health Insurance database also showed a higher rate of lymphoma (HR 2.41, 95% CI 1.60–3.64) compared with patients with IBD who had no TNF inhibitor exposure (Lemaitre et al., 2017). Furthermore, a study of patients with juvenile idiopathic arthritis, IBD, or psoriasis that used a Medicaid database hinted at a similar, albeit non-significant, increase in the risk of lymphoma in patients receiving TNFα inhibitor treatment (adjusted HR 2.64, 95% CI 0.93–7.51) (Beukelman et al., 2018). However, many register-based cohort studies and systematic reviews of randomized trials had not identified such an increased risk of overall cancer with the use of anti-TNFα, with the exception of an increased risk of squamous cell skin cancer risk in patients treated with abatacept (Xie et al., 2020; Chen et al., 2021; Li et al., 2021). Our results showed that the 10 most common adverse reactions reported were rash, arthralgia, rheumatoid arthritis, headache, pneumonia, psoriasis, nausea, diarrhea, pruritus, and dyspnea, and cancer-related adverse reactions were not included. Based on the definition of an AE, these may be self-limiting or temporal and are, therefore, not a cause for alarm. We also found that common cancer-related adverse reactions of five TNFα inhibitors include skin cancer, breast cancer, and basal cell carcinoma.
We found that the five TNFα inhibitors had different PTs of ADR in congenital, familial, and genetic disorders. It is worth noting that of all the biopharmaceuticals used to treat RA, only TNFα is approved for use during pregnancy and lactation. Prenatal exposure to TNFα has been shown to have no effect on T- or B-cell development (Förger et al., 2019; Kristjansdottir et al., 2019). Yet, there are some safety concerns regarding the risk of developing serious infections, such as tuberculosis, due to detectable anti-TNF antibodies in infants’ sera (Romanowska-Próchnicka et al., 2021). Certolizumab pegol and etanercept are approved by the European League Against Rheumatism (EULAR) for use during pregnancy and breastfeeding. Because the concentration of etanercept in breast milk is low and not detectable in neonatal serum, the European registry considers etanercept to be safe, although the level of evidence for etanercept is lower than that for certolizumab pegol (Clowse et al., 2018; Emery et al., 2020). Data on the teratogenic effects of TNFα are limited, and there is no strong evidence of potentially harmful effects if used in the preconception period (Beltagy et al., 2021).
There is increasing data on ocular adverse events associated with anti-TNFα therapy (Nicolela and Pavesio, 2020). Our study found that the most common ocular adverse reactions caused by the five TNFα inhibitors were visual impairment and blurred vision, and the swelling of the eyelid and blepharospasm caused by certolizumab pegol was prominent.
The use of a spontaneous reporting system database has some important implicit limitations because reporting is influenced by factors such as notoriety bias, selection bias, and under-reporting (Faillie, 2019). Missing data, as observed in the results of the current study where some AEs were reported, can neither be attributed to males nor females as well as age groups. In addition, since the VigiAccess database of the WHO is cumulative data, the ADRs of every year cannot be obtained. When drugs are put on the market at different times, the number of ADRs collected is quite different, and the signal difference of all target inhibitors cannot be compared at the same time. Therefore, further data mining will be not possible. In this study, the number of ADRs over the past years and the number of PTs were collected, and the rate of ADR reports of different drugs was compared to avoid the influence of the time of drug marketing. The study results were limited to the relative results of the five TNFα inhibitors.
Conclusion
Anti-TNFα biological agents are an important part of autoimmune diseases. The study showed that WHO-VigiAccess reported more than 1 million adverse reactions to anti-TNF antibody treatment. The ADR of these drugs was mainly concentrated in infections and infestations, gastrointestinal disorders, and blood and lymphatic system disorders. The ADR symptoms of infection and gastrointestinal disorders had been confirmed again. In addition, infusion-related reactions caused by infliximab and lower respiratory tract infections caused by golimumab were very prominent. Even though most of the ADRs were minor and self-limiting, there were some serious ADRs that could lead to hospitalization and even death. Countries should also actively conduct safety studies on biological agents, such as cohort event monitoring, to determine the causal relationship between adverse reactions and drugs. These findings could also be stored in open-access repositories for the general public to enhance their knowledge of AEs associated with biotech drugs.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Author contributions
ML performed statistical analysis of the data from WHO-VigiAccess and wrote the manuscript. RY and YS collected the database from WHO-VigiAccess. HZ contributed to the review and editing of the manuscript. SG planned the study and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (Grant number 82104489) and Natural Science Foundation of Hubei Province (Grant number 2018CFB488). This study was performed using the VigiAccess database that was provided by the WHO. The information, results, or interpretation of the current study do not represent any opinion of the WHO.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1169327/full#supplementary-material
References
Agca, R., Heslinga, S. C., Rollefstad, S., Heslinga, M., Mcinnes, I. B., Peters, M. J., et al. (2017). EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann. Rheum. Dis. 76 (1), 17–28. doi:10.1136/annrheumdis-2016-209775
Alawadhi, A., Alawneh, K., and Alzahrani, Z. A. (2012). The effect of neutralizing antibodies on the sustainable efficacy of biologic therapies: what's in it for african and middle eastern rheumatologists. Clin. Rheumatol. 31 (9), 1281–1287. doi:10.1007/s10067-012-2040-2
Athimni, S., Slouma, M., Dhahri, R., Gharsallah, I., Metoui, L., and Louzir, B. (2022). Tuberculosis infection under anti-TNF alpha treatment. Curr. Drug Saf. 17 (3), 235–240. doi:10.2174/1574886316666211109092354
Beaugerie, L., and Kirchgesner, J. (2019). Balancing benefit vs risk of immunosuppressive therapy for individual patients with inflammatory bowel diseases. Clin. Gastroenterol. Hepatol. 17 (3), 370–379. doi:10.1016/j.cgh.2018.07.013
Beltagy, A., Aghamajidi, A., Trespidi, L., Ossola, W., and Meroni, P. L. (2021). Biologics during pregnancy and breastfeeding among women with rheumatic diseases: Safety clinical evidence on the road. Front. Pharmacol. 12, 621247. doi:10.3389/fphar.2021.621247
Beukelman, T., Xie, F., Chen, L., Horton, D. B., Lewis, J. D., Mamtani, R., et al. (2018). Risk of malignancy associated with paediatric use of tumour necrosis factor inhibitors. Ann. Rheum. Dis. 77 (7), 1012–1016. doi:10.1136/annrheumdis-2017-212613
Bonek, K., Roszkowski, L., Massalska, M., Maslinski, W., and Ciechomska, M. (2021). Biologic drugs for rheumatoid arthritis in the context of biosimilars, genetics, epigenetics and COVID-19 treatment. Cells 10 (2), 323. doi:10.3390/cells10020323
Bongartz, T., Sutton, A. J., Sweeting, M. J., Buchan, I., Matteson, E. L., and Montori, V. (2006). Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: Systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA 295 (19), 2275–2285. doi:10.1001/jama.295.19.2275
Calvet, X., Carpio, D., Rodríguez-Lago, I., García-Vicuña, R., Barreiro-De-Acosta, M., Juanola, X., et al. (2021). Risk of infection associated with Janus Kinase (JAK) inhibitors and biological therapies in inflammatory intestinal disease and rheumatoid arthritis. Prevention strategies. Gastroenterol. Hepatol. (English Ed. 44 (8), 587–598. doi:10.1016/j.gastrohep.2021.01.007
Chen, A. Y., Wolchok, J. D., and Bass, A. R. (2021). TNF in the era of immune checkpoint inhibitors: Friend or foe? Nat. Rev. Rheumatol. 17 (4), 213–223. doi:10.1038/s41584-021-00584-4
Chen, C., Borrego, M. E., Roberts, M. H., and Raisch, D. W. (2019). Comparison of post-marketing surveillance approaches regarding infections related to tumor necrosis factor inhibitors (TNFi's) used in treatment of autoimmune diseases. Expert Opin. Drug Saf. 18 (8), 733–744. doi:10.1080/14740338.2019.1630063
Chiu, Y. M., and Chen, D. Y. (2020). Infection risk in patients undergoing treatment for inflammatory arthritis: Non-biologics versus biologics. Expert Rev. Clin. Immunol. 16 (2), 207–228. doi:10.1080/1744666X.2019.1705785
Clowse, M., Scheuerle, A. E., Chambers, C., Afzali, A., Kimball, A. B., Cush, J. J., et al. (2018). Pregnancy outcomes after exposure to certolizumab pegol: Updated results from a pharmacovigilance safety database. Arthritis Rheumatol. 70 (9), 1399–1407. doi:10.1002/art.40508
Cohen, S., Genovese, M. C., Choy, E., Perez-Ruiz, F., Matsumoto, A., Pavelka, K., et al. (2017). Efficacy and safety of the biosimilar ABP 501 compared with adalimumab in patients with moderate to severe rheumatoid arthritis: A randomised, double-blind, phase III equivalence study. Ann. Rheum. Dis. 76 (10), 1679–1687. doi:10.1136/annrheumdis-2016-210459
Darrigade, A. S., Milpied, B., Truchetet, M. E., Schaeverbeke, T., Laharie, D., Zerbib, F., et al. (2017). Pattern and severity of psoriasiform eruptions in patients with inflammatory bowel diseases, arthritis or skin inflammatory disorders treated with TNF-alpha inhibitors. Acta Derm. Venereol. 97 (6), 731–734. doi:10.2340/00015555-2636
Deepak, P., Stobaugh, D. J., Sherid, M., Sifuentes, H., and Ehrenpreis, E. D. (2013). Neurological events with tumour necrosis factor alpha inhibitors reported to the Food and drug administration adverse event reporting system. Aliment. Pharmacol. Ther. 38 (4), 388–396. doi:10.1111/apt.12385
Diak, P., Siegel, J., La Grenade, L., Choi, L., Lemery, S., and Mcmahon, A. (2010). Tumor necrosis factor alpha blockers and malignancy in children: Forty-eight cases reported to the Food and drug administration. Arthritis Rheum. 62 (8), 2517–2524. doi:10.1002/art.27511
Doughty, H., Scripture, A., Carter, J. B., Sriharan, A., Yan, S., Lansigan, F., et al. (2023). Adnexotropic and granulomatous mycosis fungoides following TNF-α inhibitor treatment. J. Cutan. Pathol. 50, 611–616. doi:10.1111/cup.14441
Emery, P., Vlahos, B., Szczypa, P., Thakur, M., Jones, H. E., Woolcott, J., et al. (2020). Longterm drug survival of tumor necrosis factor inhibitors in patients with rheumatoid arthritis. J. Rheumatol. 47 (4), 493–501. doi:10.3899/jrheum.181398
Faillie, J. L. (2019). Case-non-case studies: Principle, methods, bias and interpretation. Therapie 74 (2), 225–232. doi:10.1016/j.therap.2019.01.006
Fleischmann, R. M., Alten, R., Pileckyte, M., Lobello, K., Hua, S. Y., Cronenberger, C., et al. (2018). A comparative clinical study of PF-06410293, a candidate adalimumab biosimilar, and adalimumab reference product (Humira®) in the treatment of active rheumatoid arthritis. Arthritis Res. Ther. 20 (1), 178. doi:10.1186/s13075-018-1676-y
Förger, F., Bandoli, G., Luo, Y., Robinson, L., Johnson, D. L., and Chambers, C. D. (2019). No association of discontinuing tumor necrosis factor inhibitors before gestational week twenty in well-controlled rheumatoid arthritis and juvenile idiopathic arthritis with a disease worsening in late pregnancy. Arthritis Rheumatol. 71 (6), 901–907. doi:10.1002/art.40821
Gagliardi, A., Iaquinta, F. S., Grembiale, R. D., De Sarro, C., Fabiano, A., Fraija, D., et al. (2022). Real-world safety profile of biologics used in rheumatology: A six-year observational pharmacovigilance study in the calabria region. Pharmaceutics 14 (11), 2328. doi:10.3390/pharmaceutics14112328
Galloway, J. B., Hyrich, K. L., Mercer, L. K., Dixon, W. G., Fu, B., Ustianowski, A. P., et al. (2011). Anti-TNF therapy is associated with an increased risk of serious infections in patients with rheumatoid arthritis especially in the first 6 months of treatment: Updated results from the British society for rheumatology biologics register with special emphasis on risks in the elderly. Rheumatol. Oxf. 50 (1), 124–131. doi:10.1093/rheumatology/keq242
Gidudu, J. F., Shaum, A., Dodoo, A., Bosomprah, S., Bonsu, G., Amponsa-Achiano, K., et al. (2020). Barriers to healthcare workers reporting adverse events following immunization in four regions of Ghana. Vaccine 38 (5), 1009–1014. doi:10.1016/j.vaccine.2019.11.050
Godfrey, M. S., and Friedman, L. N. (2019). Tuberculosis and biologic therapies: Anti-tumor necrosis factor-α and beyond. Clin. Chest Med. 40 (4), 721–739. doi:10.1016/j.ccm.2019.07.003
Habarugira, J., and Figueras, A. (2021). Pharmacovigilance network as an additional tool for the surveillance of antimicrobial resistance. Pharmacoepidemiol Drug Saf. 30 (8), 1123–1131. doi:10.1002/pds.5249
Hajjaj-Hassouni, N., Al-Badi, M., Al-Heresh, A., Al-Emadi, S., El, B. A., El, G. A., et al. (2012). The practical value of biologics registries in Africa and Middle East: Challenges and opportunities. Clin. Rheumatol. 31 (3), 407–416. doi:10.1007/s10067-011-1918-8
Hazell, L., and Shakir, S. A. (2006). Under-reporting of adverse drug reactions: A systematic review. Drug Saf. 29 (5), 385–396. doi:10.2165/00002018-200629050-00003
Jang, D. I., Lee, A. H., Shin, H. Y., Song, H. R., Park, J. H., Kang, T. B., et al. (2021). The role of tumor necrosis factor alpha (TNF-α) in autoimmune disease and current TNF-α inhibitors in therapeutics. Int. J. Mol. Sci. 22 (5), 2719. doi:10.3390/ijms22052719
Kameda, H., Uechi, E., Atsumi, T., Abud-Mendoza, C., Kamei, K., Matsumoto, T., et al. (2020). A comparative study of PF-06438179/GP1111 (an infliximab biosimilar) and reference infliximab in patients with moderate to severe active rheumatoid arthritis: A subgroup analysis. Int. J. Rheum. Dis. 23 (7), 876–881. doi:10.1111/1756-185X.13846
Kristjansdottir, S. R., Steingrimsdottir, T., Grondal, G., Bjarnadóttir, R. I., Einarsdottir, K., and Gudbjornsson, B. (2019). Fæðingarsaga kvenna með alvarlega liðbólgusjúkdóma. Laeknabladid 105 (6), 267–275. doi:10.17992/lbl.2019.06.235
Kroesen, S., Widmer, A. F., Tyndall, A., and Hasler, P. (2003). Serious bacterial infections in patients with rheumatoid arthritis under anti-TNF-alpha therapy. Rheumatol. Oxf. 42 (5), 617–621. doi:10.1093/rheumatology/keg263
Kvamme, M. K., Lie, E., Uhlig, T., Moger, T. A., Kvien, T. K., and Kristiansen, I. S. (2020). Cost-effectiveness of TNF inhibitors vs synthetic disease-modifying antirheumatic drugs in patients with rheumatoid arthritis: A markov model study based on two longitudinal observational studies. Rheumatol. Oxf. 59 (4), 917. doi:10.1093/rheumatology/kez609
Lemaitre, M., Kirchgesner, J., Rudnichi, A., Carrat, F., Zureik, M., Carbonnel, F., et al. (2017). Association between use of thiopurines or tumor necrosis factor antagonists alone or in combination and risk of lymphoma in patients with inflammatory bowel disease. JAMA 318 (17), 1679–1686. doi:10.1001/jama.2017.16071
Leone, G. M., Mangano, K., Petralia, M. C., Nicoletti, F., and Fagone, P. (2023). Past, present and (foreseeable) future of biological anti-TNF alpha therapy. J. Clin. Med. 12 (4), 1630. doi:10.3390/jcm12041630
Li, J., Zhang, Z., Wu, X., Zhou, J., Meng, D., and Zhu, P. (2021). Risk of adverse events after anti-TNF treatment for inflammatory rheumatological disease. A meta-analysis. Front. Pharmacol. 12, 746396. doi:10.3389/fphar.2021.746396
Lindquist, M., Ståhl, M., Bate, A., Edwards, I. R., and Meyboom, R. H. (2000). A retrospective evaluation of a data mining approach to aid finding new adverse drug reaction signals in the WHO international database. Drug Saf. 23 (6), 533–542. doi:10.2165/00002018-200023060-00004
Martelli, L., Olivera, P., Roblin, X., Attar, A., and Peyrin-Biroulet, L. (2017). Cost-effectiveness of drug monitoring of anti-TNF therapy in inflammatory bowel disease and rheumatoid arthritis: A systematic review. J. Gastroenterol. 52 (1), 19–25. doi:10.1007/s00535-016-1266-1
Mikami, T., Liaw, B., Asada, M., Niimura, T., Zamami, Y., Green-Laroche, D., et al. (2021). Neuroimmunological adverse events associated with immune checkpoint inhibitor: A retrospective, pharmacovigilance study using FAERS database. J. Neurooncol 152 (1), 135–144. doi:10.1007/s11060-020-03687-2
Mocke-Richter, M., Walubo, A., and van Rooyen, C. (2021). A framework for the use of biological medicines in the free state province, South Africa. Pharmacoepidemiol Drug Saf. 30 (11), 1601–1610. doi:10.1002/pds.5332
Nicolela, S. F., and Pavesio, C. (2020). A review of ocular adverse events of biological anti-TNF drugs. J. Ophthalmic Inflamm. Infect. 10 (1), 11. doi:10.1186/s12348-020-00202-6
Papadopoulos, C. G., Gartzonikas, I. K., Pappa, T. K., Markatseli, T. E., Migkos, M. P., Voulgari, P. V., et al. (2019). Eight-year survival study of first-line tumour necrosis factor α inhibitors in rheumatoid arthritis: Real-world data from a University centre registry. Rheumatol. Adv. Pract. 3 (1), rkz007. rkz007. doi:10.1093/rap/rkz007
Romanowska-Próchnicka, K., Felis-Giemza, A., Olesińska, M., Wojdasiewicz, P., Paradowska-Gorycka, A., and Szukiewicz, D. (2021). The role of TNF-α and anti-TNF-α agents during preconception, pregnancy, and breastfeeding. Int. J. Mol. Sci. 22 (6), 2922. doi:10.3390/ijms22062922
Shivaji, U. N., Sharratt, C. L., Thomas, T., Smith, S., Iacucci, M., Moran, G. W., et al. (2019). Review article: Managing the adverse events caused by anti-TNF therapy in inflammatory bowel disease. Aliment. Pharmacol. Ther. 49 (6), 664–680. doi:10.1111/apt.15097
Singh, S., Facciorusso, A., Dulai, P. S., Jairath, V., and Sandborn, W. J. (2020). Comparative risk of serious infections with biologic and/or immunosuppressive therapy in patients with inflammatory bowel diseases: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 18 (1), 69–81.e3. doi:10.1016/j.cgh.2019.02.044
Smolen, J. S., Landewe, R., Bergstra, S. A., Kerschbaumer, A., Sepriano, A., Aletaha, D., et al. (2023). EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann. Rheum. Dis. 82 (1), 3–18. doi:10.1136/ard-2022-223356
Srba, J., Descikova, V., and Vlcek, J. (2012). Adverse drug reactions: Analysis of spontaneous reporting system in europe in 2007-2009. Eur. J. Clin. Pharmacol. 68 (7), 1057–1063. doi:10.1007/s00228-012-1219-4
Subedi, S., Gong, Y., Chen, Y., and Shi, Y. (2019). Infliximab and biosimilar infliximab in psoriasis: Efficacy, loss of efficacy, and adverse events. Drug Des. Devel Ther. 13, 2491–2502. doi:10.2147/DDDT.S200147
Sultana, J., Scondotto, G., Cutroneo, P. M., Morgante, F., and Trifirò, G. (2020). Intravitreal anti-VEGF drugs and signals of dementia and Parkinson-like events: Analysis of the VigiBase database of spontaneous reports. Front. Pharmacol. 11, 315. doi:10.3389/fphar.2020.00315
Vogel, U., van Stekelenborg, J., Dreyfus, B., Garg, A., Habib, M., Hosain, R., et al. (2020). Investigating overlap in signals from EVDAS, FAERS, and VigiBase®. Drug Saf. 43 (4), 351–362. doi:10.1007/s40264-019-00899-y
Wallis, R. S., Broder, M. S., Wong, J. Y., Hanson, M. E., and Beenhouwer, D. O. (2004). Granulomatous infectious diseases associated with tumor necrosis factor antagonists. Clin. Infect. Dis. 38 (9), 1261–1265. doi:10.1086/383317
Watson, S., Chandler, R. E., Taavola, H., Härmark, L., Grundmark, B., Zekarias, A., et al. (2018). Safety concerns reported by patients identified in a collaborative signal detection workshop using VigiBase: Results and reflections from lareb and uppsala monitoring centre. Drug Saf. 41 (2), 203–212. doi:10.1007/s40264-017-0594-2
Xie, W., Xiao, S., Huang, Y., Sun, X., Gao, D., Ji, L., et al. (2020). A meta-analysis of biologic therapies on risk of new or recurrent cancer in patients with rheumatoid arthritis and a prior malignancy. Rheumatol. Oxf. 59 (5), 930–939. doi:10.1093/rheumatology/kez475
Yamoah, P., Mensah, K. B., Attakorah, J., Padayachee, N., Oosthuizen, F., and Bangalee, V. (2022). Adverse events following immunization associated with coronavirus disease 2019 (COVID-19) vaccines: A descriptive analysis from VigiAccess. Hum. Vaccin Immunother. 18 (6), 2109365. doi:10.1080/21645515.2022.2109365
Zabana, Y., Rodríguez, L., Lobatón, T., Gordillo, J., Montserrat, A., Mena, R., et al. (2019). Relevant infections in inflammatory bowel disease, and their relationship with immunosuppressive therapy and their effects on disease mortality. J. Crohns Colitis. 13 (7), 828–837. doi:10.1093/ecco-jcc/jjz013
Keywords: adverse drug reaction, TNFα inhibitors, pharmacovigilance, spontaneous reporting, VigiAccess of the WHO
Citation: Li M, You R, Su Y, Zhou H and Gong S (2023) Characteristic analysis of adverse reactions of five anti-TNFɑ agents: a descriptive analysis from WHO-VigiAccess. Front. Pharmacol. 14:1169327. doi: 10.3389/fphar.2023.1169327
Received: 19 February 2023; Accepted: 10 July 2023;
Published: 24 July 2023.
Edited by:
Zhenyu Pan, Xi’an Children’s Hospital, ChinaReviewed by:
Daniele Mengato, University Hospital of Padua, ItalyLi Wenjing, Wuhan University, China
Copyright © 2023 Li, You, Su, Zhou and Gong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingming Li, bGltaW5nbWluZ3ljQDEyNi5jb20=; Shiwei Gong, c2h3Z29uZ0BodXN0LmVkdS5jbg==
 Mingming Li
Mingming Li Ruxu You1
Ruxu You1 Shiwei Gong
Shiwei Gong