- 1Department of Pharmaceutical Sciences, Zhuhai Campus, Zunyi Medical University, Zhuhai, China
- 2Department of Pharmacology, Zhuhai Campus, Zunyi Medical University, Zhuhai, China
- 3China Traditional Chinese Medicine Holdings Company Limited, Foshan, China
- 4Key Laboratory of Basic Pharmacology of Ministry of Education and Joint International Research Laboratory of Ethnomedicine of Ministry of Education, Zunyi Medical University, Zunyi, China
- 5Key Laboratory of Basic Pharmacology of Guizhou Province and School of Pharmacy, Zunyi Medical University, Zunyi, China
Ulcerative colitis (UC) is a chronic aspecific gut inflammatory disorder that primarily involves the recta and colons. It mostly presents as a long course of repeated attacks. This disease, characterized by intermittent diarrhoea, fecal blood, stomachache, and tenesmus, severely decreases the living quality of sick persons. UC is difficult to heal, has a high recurrence rate, and is tightly related to the incidence of colon cancer. Although there are a number of drugs available for the suppression of colitis, the conventional therapy possesses certain limitations and severe adverse reactions. Thus, it is extremely required for safe and effective medicines for colitis, and naturally derived flavones exhibited huge prospects. This study focused on the advancement of naturally derived flavones from edible and pharmaceutical plants for treating colitis. The underlying mechanisms of natural-derived flavones in treating UC were closely linked to the regulation of enteric barrier function, immune-inflammatory responses, oxidative stress, gut microflora, and SCFAs production. The prominent effects and safety of natural-derived flavones make them promising candidate drugs for colitis treatment.
Introduction
Ulcerative colitis (UC) is a major type of inflammatory bowel disease, which has been widely prevalent all over the world (Alsoud et al., 2021). Traditionarily, UC is considered to be a common Western disorder (Ge et al., 2021). It was announced that the yearly morbidity of colitis in European countries is as high as 0.0243%, and that in Northern America is up to 0.0192% (Du and Ha, 2020). By comparison, the annual morbidity of UC in Asian nationalities is fairly lower, at less than 0.01% (Wei et al., 2021). Whereas, in recent years, due to the changes in life and environment, the annual incidence rate of UC among Asian nationalities has been rising steadily over time (Hirten and Sands, 2021; Zeng et al., 2022). Consequently, this disease brought great pressure to the financial and medical services of all countries.
UC is a chronic nonspecific inflammatory disorder of the colon and rectum with uncertain aetiology (Sinopoulou et al., 2021). The lesion is confined to the mucosal and submucosal layers of intestinum crassum. Furthermore, the majority of lesions are found in the rectum and sigmoid colon, but they can also extend to the descending colon or the whole colon (Li et al., 2022). UC has a long course and often occurs repeatedly. Moreover, this disease occurs at any age, although it most frequently strikes people between the ages of 20 and 40 (Li C. L. et al., 2021). Although the aetiology of UC is uncertain, it is generally accepted that genes are an important cause of colitis. Psychological factors play an important role in the deterioration of UC. Following colectomy, the original morbid spirit, such as depression or social isolation, is obviously improved. In addition, it is also recognized that colitis is an autoimmune disease (Fiorino et al., 2021).
To date, there are still no satisfactory clinical drugs for colitis in terms of efficacy and safety. Nowadays, the drugs for colitis primarily consist of 5-amino salicylic acids, glucocorticoids and immunosuppressors, which lack specificity and are difficult to cure UC, accompanied by some adverse reactions, such as high recurrence, elevated resistance, etc. In this case, natural flavones raised extensive concerns as their no/low toxicity and excellent anti-inflammatory, antioxidative, antineoplastic, antibacterial, and immune-regulative activities in the prophylaxis and therapy of gastrointestinal diseases, tumors, and cardiovascular problems (Juca et al., 2020; Jeong et al., 2022). Numerous studies have testified that many kinds of flavones (Figure 1) from edible and medicinal plants possessed excellent therapeutic effects and safety in UC by multiple mechanisms involving ameliorating oxidative damage, reducing the inflammatory responses, preserving enteric barrier function, and adjusting gut flora structure, indicating that natural-derived flavones have the potentiality to suppress the progression of UC and put off the clinical course of this disease. Therefore, in this review, our team summarized the acting mechanisms of natural-derived flavones inhibiting colitis, such as apigenin, baicalein, diosmetin, sinensetin, wogonin, etc. These would provide new perspectives to develop effective medications for the management of colitis.
Methods
For the sake of identifying the research connected with the therapeutic role and acting mechanism of natural flavones treating UC, our team conducted an extensive search of relevant articles in several databases, including Web of Science, PubMed, Elsevier, Google Scholar, and CNKI, covering the period from their inception until May 2023. We employed a combination of the following keywords during the literature retrieval: (“flavone” OR “flavonoid”) AND (“colitis” OR “colonitis” OR “UC”). All papers providing abstract will be considered.
After the search, the retrieved studies underwent a rigorous screened. Initially, studies on the therapeutic role and mechanisms of natural flavones treating UC were screened on the basis of titles and abstracts. For studies that were not able to be conclusively identified during the initial screening, their full-text versions were further evaluated. At last, all relevant studies including cell experiments, animal experiments and clinical trials were gathered and imported into EndNote software as support resources for the present review.
Natural flavones against ulcerative colitis
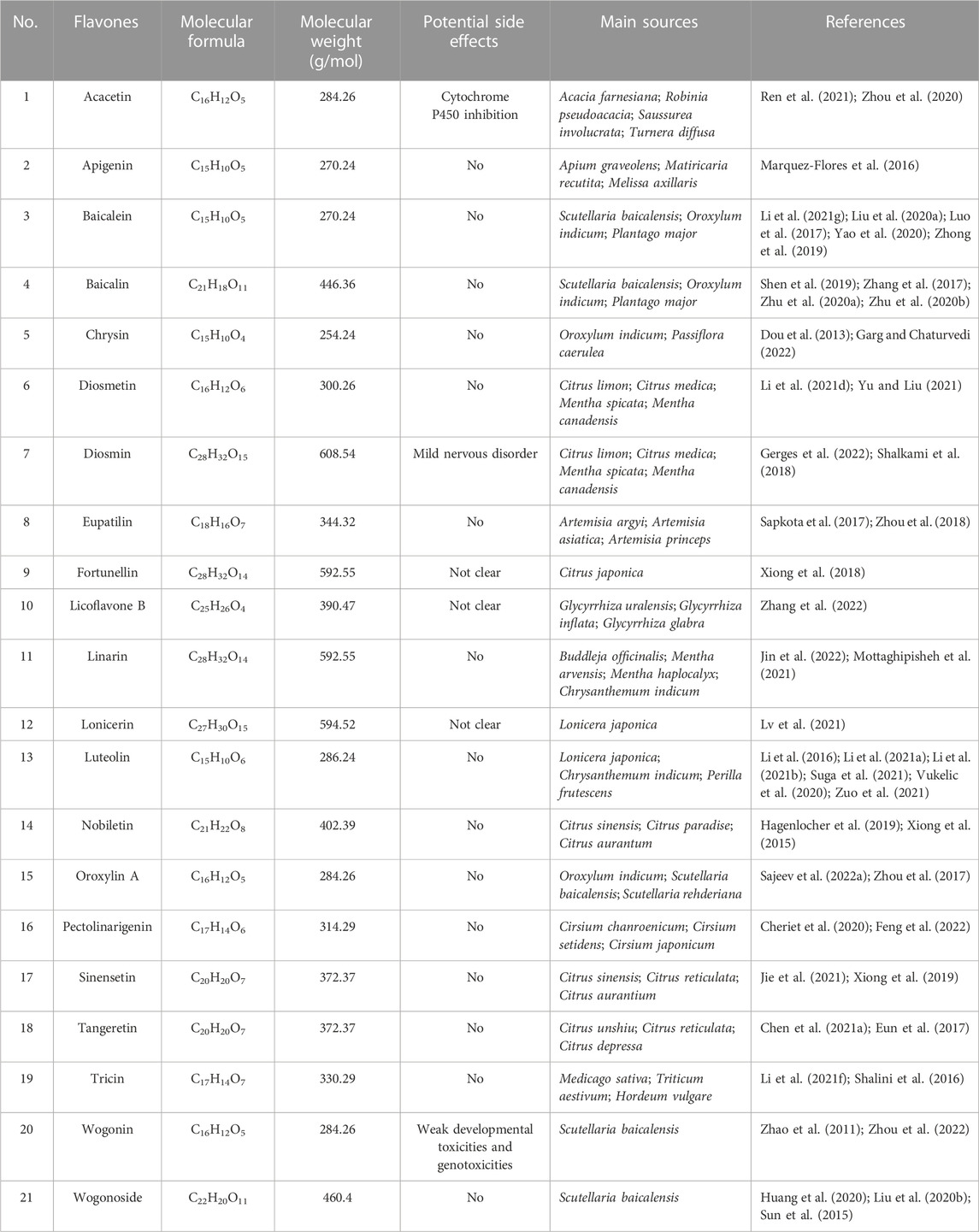
The amount of identified flavonoids has risen to four thousand since the discovery of the first type of flavonoid vitamin P (namely, rutin), the amount of identified flavonoids has been up to four thousand. Of which, some possess powerful pharmacologic action and exhibit great potential in the exploitation of new medicines and their clinic practice. Based on the fundamental structure, currently known flavonoids are separated into seventeen classes, and flavones are the most important class and have received extensive attention. Therefore, the present review focused on natural-derived flavones for the treatment of UC. The general information of the included flavones is given in Table 1, while the chemical structures are presented in Figure 2. Additionally, Table 2 provides the pharmacological information of these flavones.

FIGURE 2. Chemical structures of naturally derived flavones for the treatment of ulcerative colitis.
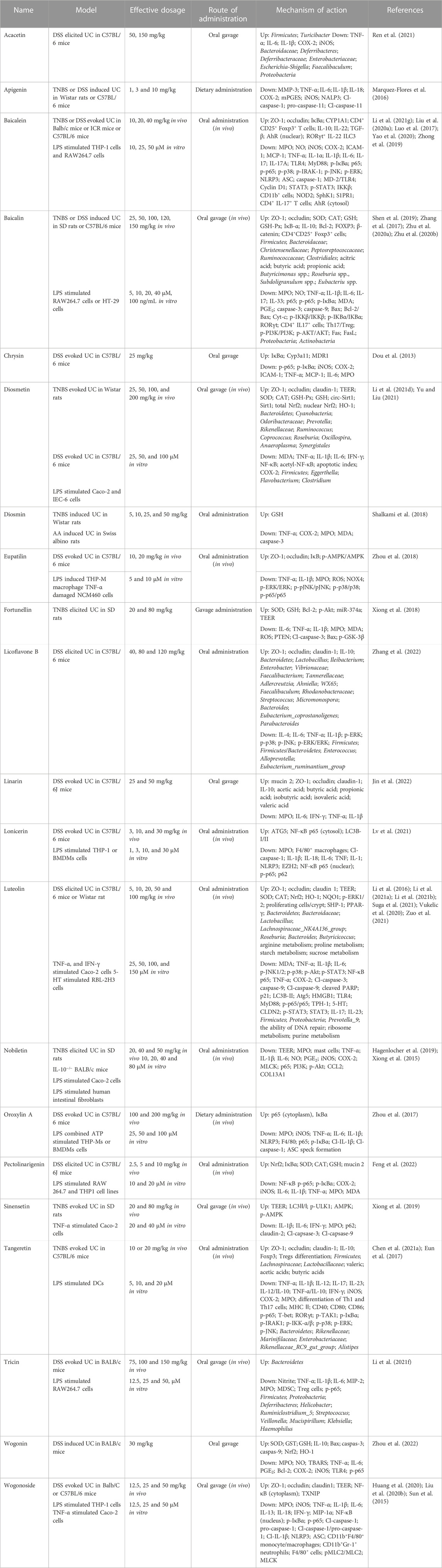
Acacetin is a natural product discovered in many plants, including Acacia farnesiana, Saussurea involucrata, and Turnera diffusa (Wu et al., 2018). Researchers reported that acacetin possesses multiple pharmacologic functions covering anti-cancer, anti-inflammation, anti-obesity, cardioprotection, and neuroprotection (Singh et al., 2020). Ren et al. (2021) probed whether acacetin could improve UC in mice induced by DSS. Results showed that acacetin alleviated the clinic signs of DSS-treated UC, as determined by weight reduction, diarrhoea, colonic shortening, inflammatory infiltration, and histologic damage. Acacetin was found to suppress the in vitro inflammatory response of macrophages as well as the production of inflammation mediators in UC murine models. Moreover, a few characters of the intestinal microflora were disordered in DSS-treated UC mice, as manifested by a prominent decrease in microflora richness and a remarkable alteration in bacteria profiles. Whereas, acacetin administration inhibited this unbalance and recovered intestine microflora to levels consistent with normal group. Collectively, the results manifested that acacetin could mitigate DSS-treated UC in mice, at least in part, by suppressing inflammation and modulating the gut microflora.
Apigenin is a usual dietary flavone which is broadly existent in some fruits, vegetables and pharmaceutical plants. It has various bioactivities involving anti-cancer, anti-oxidation, anti-inflammation, anti-bacteria, and anti-virus (Ginwala et al., 2019; Salehi et al., 2019). Marquez-Flores et al. (2016) elucidated the protective effect and mechanism of dietary apigenin enrichment in DSS-evoked colitis murine models. Apigenin supplementation reduced the macroscopic symptoms and histopathological injury of UC, according to the findings. Moreover, it reduced the expression of mPGES, COX-2 and iNOS in colon tissues and decreased the serum MMP-3 level. Likewise, apigenin diet decreased TNF-α and IL-1β secretion in LPS-activated splenocytes. In addition, apigenin’s anti-inflammatory effect was linked to the suppression of both canonical and non-canonical NLRP3 inflammasome paths via modulating cleaved caspase-1 and caspase-11 enzymes to reduce IL-1β and IL-18 expressions. In conclusion, an apigenin supplement may offer a basis for formulating a novel dietary method for preventing and treating UC.
Baicalein, a main active constituent in the roots of Scutellaria baicalensis, was testified to possess multifarious effects involving anti-cancer, anti-inflammation, anti-oxidation, anti-hepatotoxicity, as well as neuroprotection (Dinda et al., 2017; Song et al., 2021). Luo et al. (2017) probed the function and mechanism of baicalein against UC in TNBS-evoked UC model. Experimental results revealed that baicalein relieved the seriousness of TNBS-treated UC in mice through reducing MPO activity and pro-infammatory factors expression. The downregulation of NF-κB and p38 MAPK was related to the reduced expression of TLR4 and its adaptor MyD88 in mucosa. In vitro, baicalein suppressed the TLR4/MyD88 signal cascades (NF-κB and MAPKs) in LPS-activated macrophages. Besides, baicalein could bind to the hydrophobic domain of the MD-2 pocket and restrain the formation of LPS-evoked MD-2/TLR4 complex. Moreover, baicalein decreased NLRP3 infammasome excitation and downriver IL-1β expression in a dosage-dependent mode. Therefore, these results indicated that baicalein might ameliorate TNBS-treated UC by the suppression of TLR4/MyD88 signal cascade and desactivation of NLRP3 infammasome. Zhong et al. (2019) found that the protection of baicalein on UC was correlated with inhibiting NF-κB and STAT3 signal pathways. Yao et al. (2020) discovered that baicalein administration alleviated UC in mice via suppressing S1P-STAT3 signal pathway. In the experiment of Liu C. et al. (2020), they proved that baicalein could prevent DSS-evoked UC, and the therapeutical mechanism may be relevant with the modulation of Th17/Treg differentiation by AhR excitation. In another research, Li Y. Y. et al. (2021) demonstrated that baicalein ameliorated UC through improving the enteric epithelia barrier by AhR/IL-22 pathway in ILC3s. Taken together, baicalein may be beneficial in the therapy of UC.
Baicalin is a biologically active flavone glycoside separated from the dry roots of S. baicalensis and possesses diversified effects covering anti-virus, anti-inflammation, anti-bacteria, hepatoprotection, and cardioprotection (Guo et al., 2019; Jin et al., 2019). The role and mechanism of baicalin in the treatment of colitis was investigated by Zhu et al. (2020a). Experimental data showed that baicalin observably alleviated TNBS-elicited UC through decreasing the levels of pro-inflammatory factor (TNF-α, IL-6 and IL-1β), elevating the content of anti-inflammatory mediator IL-10, and enhancing the expression of TJ proteins ZO-1 and β-catenin, which may be attained by blocking the PI3K/AKT signal path. Furthermore, baicalin obviously restrained the disequilibrium between pro- and anti-inflammatory cytokines and markedly reduced apoptosis through blocking the PI3K/AKT signal path in LPS-treated HT-29 cells, which was consistently in line with the in vivo finding. Therefore, these results illustrated that baicalin could treat colitis by the inhibition of PI3K/AKT signal pathway. Zhu et al. (2020b) further found that baicalin probably protected mice against UC via maintaining Th17/Treg balance and modulating both gut microflora and SCFAs. Among the research from Zhang et al. (2017), they proved that baicalin could attenuate DSS-evoked UC via restraining IL-33 expression and NF-κB excitation. Shen et al. (2019) also discovered that baicalin exerted a regulatory role on the IKK/IKB/NF-κB signal cascade and apoptosis-associated proteins in murine models of colitis. Collectively, baicalin might be a perspective therapy candidate for colitis.
Chrysin, a natural flavone in many plants, including Oroxylum indicum and Passiflora caerulea. Studies proved that chrysin has anticancerous, antidiabetic, antidepressive, immunoregulatory, and neuroprotective activities (Kasala et al., 2015; Garg and Chaturvedi, 2022). Dou et al. (2013) evaluated the efficacy of chrysin as a putative murine PXR activator in suppressing UC. Chrysin administration alleviated inflammatory signs in DSS or TNBS induced murine models and caused a decrease in NF-κB target genes (e.g., iNOS, COX-2, ICAM-1, and MCP-1) in the colonic tissues. Chrysin restrained the phosphorylation/degradation of IκBα, resulting in lower colonic of MPO, TNF-α and IL-6 levels. Consistent with the in vivo findings, chrysin prevented LPS-activated migration of NF-κB p65 into the nucleus of RAW264.7 cells. Moreover, chrysin dosage-dependently stimulated human/murinse PXR in reporter gene experiments and up-modulated xenobiotic detoxification genes in the colonic mucosa, but not in the liver. RNA interference-mediated silencing of PXR demonstrated the crucial role that PXR plays in chrysin’s induction of xenobiotic detoxification genes and excitation of NF-κB. In vitro PXR transduction experiments confirmed that chrysin suppresses NF-κB transcriptional activity. The results suggested that chrysin’s role in UC inhibition is primarily mediated by the PXR/NF-κB pathway. In conclusion, chrysin has the potential to be developed as gut-specific PXR agonists.
Diosmetin, a flavone commonly found in citrus plants, exhibits a wide range of effects covering anti-inflammation, anti-tumor, anti-apoptosis, and anti-bacteria (Zhang et al., 2012; Patel et al., 2013). A study conducted by Yu and Liu (2021) investigated the mechanism and efficacy of diosmetin in treating UC using TNBS-induced colitis rats as a model. Experimental results exhibited that diosmetin notably reduced colon anabrosis and inflammatory symptoms in the colon mucosa. TNBS-induced decrease of SOD and CAT was observably inhibited, but MDA content in the colon tissues was reduced. After diosmetin administration, the concentrations of TNF-α, IL-6 and NF-κB and the quantity of apoptotic cells were markedly decreased. In another research, Li H. L. et al. (2021) investigated the function and mechanism of diosmetin against UC in DSS-evoked UC mice. Results demonstrated that diosmetin exerted therapeutical roles in DSS-treated UC by several paths, involving the decrease of proinflammatory cytokines and oxidant stress, the elevated expression of TJ proteins (ZO-1, claudin-1 and occludin), and the regulation of intestinal microflora. The circ-Sirt1/Sirt1 axis partially mediates the therapeutic effects of diosmetin on UC. However, further studies are needed to determine whether diosmetin modulates the composition of intestinal microflora through the Sirt1 pathway. Therefore, diosmetin could be effectively adopted to prevent and treat UC.
Diosmin is a dietary flavone glycoside abundantly existed in citrus plants. Numerous studies have proven that diosmin possesses anti-inflammatory, antioxidative, anticancerous, hepatoprotective, neuroprotective, and renoprotective effects (Zheng et al., 2020; Gerges et al., 2022). Shalkami et al. (2018) investigated the efficacy of diosmin on colitis. Evidences manifested that acetic acid led to a rise of DAI and colonic injury index scores. The indexes of inflammation (MPO, TNF-α and COX-2) and oxidant stress (MDA and decreased GSH) were notably increased. These variations were related to raise in colonic caspase-3 expression. Diosmin treatment dosage-dependently decreased the DAI and colonic injury index scores. Moreover, diosmin caused a prominent decline of inflammatory and oxidant stress markers in addition to decreasing caspase-3 level. Collectively, diosmin treatment reduced colitis progression, deciding by its capacity to suppress inflammation, oxidant stress, and apoptosis in the colons of rats.
Eupatilin is a natural flavone mainly existed in Artemisia plants, such as Artemisia argyi, Artemisia asiatica and Artemisia princeps (Nageen et al., 2020). Numerous studies indicated that eupatilin possesses multiple bioactivities ranging from anti-ulcer, anti-inflammation, and anti-cancer (Cho et al., 2011; Sapkota et al., 2017). Zhou et al. (2018) investigated the activity of eupatilin against UC and illustrated the mechanism. Experimental results elucidated that eupatilin significantly mitigated inflammatory reactions in LPS-provoked macrophages. Eupatilin notably safeguarded colon epithelia through reducing over expression of TJs and NOX4, and improving AMPK excitation in TNF-α excited NCM460 cells. Moreover, in vivo research proved that eupatilin therapy markedly alleviated the symptoms and pathological variations of UC murines. Administration of an AMPK pharmacologic suppressant in mice resulted in a decrease in the therapeutic effects of eupatilin. In brief, eupatilin could ameliorate DSS-treated murine colitis via restraining the inflammatory response and keeping the integrality of the intestine epithelia barrier by AMPK excitation.
Fortunellin, a natural dietary flavone mainly existed in Citrus japonica, has multifarious bioactivities covering anti-tumor, anti-oxidation, and anti-inflammation (Zhao et al., 2017; Panagiotopoulos et al., 2021). Xiong et al. (2018) explored the function and mechanism of fortunellin in treating UC using TNBS-induced colitis rats as a model. Fortunellin alleviated the clinical signs of UC, involving excessive inflammatory symptoms and oxidative stress. Fortunellin reduced the apoptosis of epithelial cells in UC by suppressing PTEN expression. Fortunellin-caused decrease of PTEN could be neutralized by miR-374a decline. Furthermore, miR-374a knockdown in vivo partially restrained the effect of fortunellin on UC model. Together, PTEN suppression facilitates the reinforced effect of fortunellin on UC. Fortunellin targeting miR-374a is a negative modulator of PTEN. This research offers new ideas into the pathologic mechanism and therapy alternatives of UC.
Licoflavone B is a minor flavone in licorice, which is a kind of medicinal and edible Chinese herbal medicine (de Carvalho et al., 2015; Liu et al., 2022). Zhang et al. (2022) examined the efficacy and mechanism of licoflavone B against UC in DSS-exposed C57BL/6 murines. Experimental result showed that licoflavone B notably restrained DSS induced weight reduction, DAI rise, histologic injury, and colon inflammation, manifesting that licoflavone B possesses a beneficial effect on UC. It was discovered that licoflavone B maintained the integrality of the colon barrier through suppressing the apoptosis of colon cells and increasing the levels of ZO-1, occludin, and claudin-1. Furthermore, licoflavone B remodeled the microbiota composition via restraining detrimental bacterium and promoting beneficial microbes. Besides, licoflavone B exhibited an anti-colitis effect via the blockage of the MAPK path. In conclusion, the results provided worthy information for the exploration of new anti-colitis drugs.
Linarin, a naturalflavone existed in the plants of Buddleja, Mentha, and Cirsium, possesses various activities covering anti-inflammation, anti-allergen, as well as hepatoprotection (Han et al., 2018; Mottaghipisheh et al., 2021). Jin et al. (2022) evaluated the protection of linarin against DSS-evoked colitis in C57BL/6J murines and explored the possible mechanisms. Experimental results displayed that linarin administration relieved the DSS-evoked histopathologic injury, and strengthened the mucosa layer and enteric barrier function. Significantly, linarin markedly lowered MPO vitality and the levels of pro-inflammatory factors, whereas increased the mRNA expression of anti-inflammatory factor in colonal tissues. Furthermore, linarin recovered the intestinal flora injured by DSS. Linarin also partially enhanced the relative amounts of SCFAs-generating bacterium and the levels of SCFAs. The findings of the study suggest that linarin may be a promising nutritional intervention for the treatment of UC.
Lonicerin is a flavone glycoside extracted from Lonicera japonica, the flower buds of which are often employed for treating inflammatory and infectious disorders (Xu et al., 2019). Lv et al. (2021) reported the therapeutical role of lonicerin on intestine inflammation via binding directly to EZH2 histone methyltransferase. The modification of H3K27me3 by EZH2 was found to promote ATG5 expression, which in turn results in elevatory autophagy and expedites autolysosome-mediated degradation of NLRP3. The dynamic simulation study shows that the mutation of EZH2 residues (His1 and Arg685) greatly reduces the protective role of lonicerin. Moreover, in vivo researches verified that lonicerin treatment disturbs the assembly of NLRP3-ASC-pro-caspase-1 complex and relieves UC in a dose-dependent manner, an effect that is attenuated via administering an EZH2-overexpressing plasmid. Therefore, the results suggest that lonicerin may be a potential anti-inflammatory epigenetic agent, and the EZH2/ATG5/NLRP3 axis could be a novel therapeutic target for the treatment of UC and other inflammatory ailments.
Luteolin is a common dietary flavone in some edible plants, including Chrysanthemum indicum, L. japonica, and Perilla frutescens (Ganai et al., 2021). Previous studies have demonstrated that possess numerous beneficial effects, including anti-carcinogenic, anti-oxidative, anti-inflammatory, anti-allergic, and antimicrobial effects (Nabavi et al., 2015; Aziz et al., 2018). Li et al. (2016) probed the function and mechanism of luteolin against UC in DSS-evoked colitis murines. The results displayed that luteolin prominently decreased DAI scores, and restrained colonal shortening and histologic injury. Furthermore, luteolin effectually lowered the levels of inflammatory factors, covering iNOS, TNF-α and IL-6. Administration of luteolin was found to increase the levels of colon contents of SOD and CAT, as well as the expression of Nrf2 and its downstream targets, such as HO-1 and NQO1. These findings suggested that luteolin may restrain UC by activation of the Nrf2 signal path. Among the study from Vukelic et al. (2020), the researchers discovered that luteolin has the potential to attenuate experimental UC by exerting anti-inflammatory, anti-apoptotic, and anti-autophagic effects. These effects are mediated by the inhibition of JNK1/2, p38, PI3K/Akt, NF-κB, and STAT3 signal paths, along with the induction of ERK1/2. In the research of Suga et al. (2021), they discovered that luteolin may possess the potential to relieve inflammation responses through reducing excessive 5-HT by inhibition of TPH-1 in RBL-2H3 cells. Zuo et al. (2021) proved that luteolin notably mitigated DSS-elicited UC, and the mechanism was associated with the modulation of enteric HMGB1-TLR-NF-κB signal path. In two other studies, Li et al. (2021a), Li et al. (2021b) indicated that luteolin could maintain intestine epithelia barrier function by suppressing STAT3 signaling and alleviate inflammatory reactions by regulating intestinal microflora in the treatment of colitis. Altogether, luteolin may be a prospective therapeutic drug for UC.
Nobiletin is a natural dietary flavone prevailingly found in a few citrus plants composed of citrus sinensis, citrus paradise, and citrus aurantum (Ashrafizadeh et al., 2020). Modern studies proved that nobiletin possesses multiple pharmacological actions, including anti-cancer, anti-inflammation, anti-oxidation, anti-dementia, and neuroprotection (Braidy et al., 2017). Hagenlocher et al. (2019) explored the function of nobiletin on inflammation and fibrosis in IL-10−/− colitis. The results showed that nobiletin administration caused a decline of clinic signs and a longer life expectancy. Moreover, histologic scores of UC were decreased in comparation with normal groups. Administration of nobiletin in IL-10−/− mice was found to decrease the number of mast cells and reduce their degranulation, which was positively correlated with the DAI. Besides, nobiletin administration also led to a decrease in fibrotic marker collagen deposition. In LPS stimulated human intestinal fibroblasts, the levels of collagen and pro-inflammatory factors COL13A1, IL-6, TNF, and CCL2 was down-modulated after nobiletin administration. Therefore, nobiletin administration was found to reduce signs and markers of inflammation, as well as the deposition and expression of fibrotic collagen in UC murines. Among another test, Xiong et al. (2015) investigated the effects of nobiletin on the exaggerated inflammatory reaction and weakened barrier function in UC rats. Experimental data manifested that nobiletin played anti-inflammatory roles in TNBS-treated UC by reducing iNOS and COX-2 levels. Nobiletin recovered the barrier function destroyed following TNBS treatment via the control of the Akt-NF-κB-MLCK signalling. In conclusion, nobiletin might be a prospective candidate for the treatment of colitis.
Oroxylin A, a main ingredient in the roots of O. indicum and S. baicalensis, possessed a wide range of beneficial bioactivities, including anti-inflammatory, antineoplastic, anticoagulative, cytoprotective, and neuroprotective bioactivities (Sajeev et al., 2022a; Sajeev et al., 2022b). Zhou et al. (2017) investigated the role of oroxylin A on DSS-treated mice UC by targeting NLRP3 inflammasome. The results displayed that oroxylin A alleviated UC, characterized by inhibiting weight reduction, colonic length shortening and inflammatory infiltration. The levels of TNF-α, IL-1β, and IL-6 in colons were also notably lowered by oroxylin A. Moreover, oroxylin A prominently inhibited the NLRP3 level in gut mucosa tissues. Besides, NLRP3 gene knockout mice had an obvious protective effect on UC treated by DSS, and oroxylin A administration exhibited no roles on relieving inflammatory symptoms in NLRP3−/− murine. Further research discovered that oroxylin A dosage-dependently suppressed the excitation of NLRP3 inflammasome in BMDMs and THP-Ms, leading to a decrease in cleaved caspase-1 and cleaved IL-1β. Moreover, luteolin A mitigated the expression of NLRP3 protein depending on the suppression of p65 and nuclear transsituation. In addition, oroxylin A specifically inhibited the formation of ASC specks and the assembly of inflammasomes, both of which contributed to the blockade of the NLRP3 inflammasome. The above evidences indicated that oroxylin A suppressed NLRP3 inflammasome excitation and might be potentially employed to treat colitis.
Pectolinarigenin, a natural flavone existed in Cirsium and Citrus species, possesses different bioactivities including anti-inflammatory, antidiabetic, as well as anticancer properties (Lee et al., 2018; Cheriet et al., 2020). Feng et al. (2022) probed the possible protective effects of pectolinarigenin on LPS-treated macrophage cells and DSS-evoked UC mice. The results displayed that pectolinarigenin suppressed the LPS-evoked NF-κB excitation through disturbing IκB-α degradation. Subsequently, elevated Nrf2 protein expression was found on pectolinarigenin administrated RAW 264.7 and THP1 cells. Besides, they uncovered that pectolinarigenin mediated the NF-κB/Nrf2 path modulation, subsequently restrained the levels of iNOS, COX-2, IL-6, IL-1β, and TNF-α in RAW 264.7 and THP1 cells. Moreover, pectolinarigenin dosage-dependently mitigated colonic inflammation through adjusting NF-κB/Nrf2 signal path and improving MPO vitality and redox modulators in DSS-evoked UC mice. Likewise, we found the minimal pathologic injuries in the pectolinarigenin-treated mice colons, and the rise of goblet cell population and mucin-2 generation. Collectively, the results manifested that pectolinarigenin alleviated the DSS-evoked UC in murine through modulating the NF-κB/Nrf2 path. Therefore, pectolinarigenin may hold potential as a therapeutic agent for the treatment of UC.
Sinensetin, a dietary flavone mainly existed in citrus plants such as Citrus sinensis, was discovered to possess anticancerous, antioxidative, anti-inflammatory as well as antibacterial effects (Kim et al., 2020; Jie et al., 2021). Xiong et al. (2019) probed the therapeutic potential and underlying mechanism of sinensetin against UC in TNBS and DSS evoked UC rats. Sinensetin reversed colitis-related rise in intestine penetrability, notably facilitated epithelia cells autophagy, reduced epithelia cells apoptosis, and lowered mucosa claudin-2. Sinenstetin relieved the clinical signs in UC rats and mice. Knockdown of AMPK changed the encouragement of epithelia autophagy by sinensetin. Collectively, sinensetin markedly relieved intestine barrier dysfunction in UC through boosting epithelia cells autophagy, and further suppressing apoptosis and claudin-2 expression. Thus, these findings indicated the new promising benefit of sinensetin in UC.
Tangeretin is a polymethoxylated dietary flavone existed broadly in citrus plants, involving Citrus unshiu, Citrus reticulata, and Citrus depressa. Studies have proved that tangeretin possesses diversified biological activities, such as anti-inflammation, anti-cancer, and neuroprotection (Alhamad et al., 2021; Arafa et al., 2021). Eun et al. (2017) studied the therapeutic potential and underlying mechanism of tangeretin in treating UC. The results showed that tangeretin restrained TNF-α, IL-12, and IL-23 levels and NF-κB excitation in LPS-activated dendritic cells, but not affected IL-10 level. Moreover, tangeretin restrained the excitation and translocation of p65 into the nucleus in vitro through suppressing LPS binding to dendritic cells. Tangeretin treatment inhibited the inflammatory reactions, involving NF-κB and MAPK excitation and MPO vitality, in the colons of murine with TNBS evoked UC. Tangeretin elevated TNBS induced low expression of TJs including occludin, claudin-1, and ZO-1. Tangeretin also suppressed TNBS-evoked differentiation of Th1 and Th17 cells and the levels of T-bet, RORγt, interferon-γ, IL-12, IL-17, and TNF-α. Whereas, tangeretin elevated TNBS-restrained differentiation of regulatory T cells and the levels of Foxp3 and IL-10. The results suggested that tangeretin might ameliorate UC through repressing IL-12 and TNF-α productions and NF-κB excitation via the suppression of LPS bond on immunocytes. In another research of Chen B. et al. (2021), they also probed the therapeutic potential and underlying mechanism of tangeretin against UC in DSS-evoked colitis mice. Experimental results indicated that dietary tangeretin could relieve UC through restraining inflammatory reactions, recovering intestine barrier function, and regulating intestine microflora.
Tricin is a dietary flavone monomer broadly distributed in grains, and has antineoplastic, anti-inflammatory, and antiangiogenic properties (Shalini et al., 2016; Jiang et al., 2020). Li X. X. et al. (2021) studied the potential protective mechanism of tricin on LPS-stimulated RAW264.7 cells and probed the effect of tricin on UC mice treated by 4.5% DSS for 7 days. The result displayed that tricin observably decreased NO level in LPS stimulated RAW264.7 cells and the anti-inflammation role of tricin was proved to inhibit the NF-κB pathway. Moreover, tricin administration (150 mg/kg) markedly mitigated colonic length decline, decreased MPO vitality and DAI scores, and recovered the elevatory myeloid-derived suppressor cells in acute UC mice. The effect of DSS on intestinal flora, including the incremental population of Proteobacteria and Ruminococcaceae, was indicated to be alleviated by tricin therapy. Therefore, tricin could improve acute colitis through relieving colon inflammation and regulating intestinal microflora profile.
Wogonin, a natural flavone in S. baicalensis, has multifold functions, including anti-oxidation, anti-inflammation, anti-cancer, anti-virus, and neuroprotection (Wu et al., 2016; Huynh et al., 2020). Zhou et al. (2022) investigated the role and mechanism of wogonin against UC in DSS-treated colitis mice. Results exhibited that DSS strikingly reduced weight and colonic length, and elevated inflammatory symptoms in the colon. Wogonin effectively restrained colon ulcer, neutrophil infiltration and histologic variations elicited by DSS. The increase of pro-inflammatory factors (e.g., TNF-α, IL-6, COX-2, iNOS) and decreased activities of antioxidative enzymes (e.g., SOD, GST and GSH) were also notably regulated after wogonin treatment. In addition, wogonin activated apoptosis by suppressing Bcl-2 and elevating the contents of Bax, caspase-3, and -9. Further investigation revealed that wogonin’s protective role against DSS-induced UC was closely linked to the modulation of the Nrf2/TLR4/NF-B signaling.
Wogonoside, one of the main flavones from S. baicalensis, was discovered to possess multifarious pharmacologic actions including anti-inflammation, anti-cancer, and anti-oxidation (Chen et al., 2013; Liu Q. et al., 2020). Sun et al. (2015) assessed the action and mechanism of wogonoside against UC in DSS-elicited murine colitis. Experimental data certified that wogonoside dosage-dependently alleviated DSS-evoked weight reduction and colonic length shortening. Furthermore, wogonoside reversed DSS-evoked colon pathologic injury, notably restrained inflammation cell infiltration and reduced MPO and iNOS vitalities. The contents of pro-inflammatory mediators in serums and colons were also markedly suppressed by wogonoside. Besides, wogonoside significantly reduced the generation of IL-1β, TNF-α and IL-6 and restrained mRNA levels of pro-IL-1β and NLRP3 in PMA-differentiated monocytic THP-1 cells by preventing the activization of NF-κB and NLRP3 inflammasome. Therefore, these evidences verified that wogonoside exhibited an anti-inflammatory function via control of NF-κB and NLRP3 inflammasome. Among the experiment of Huang et al. (2020), the researchers explored whether wogonoside regulates intestine barrier function. The results manifested that wogonoside mitigated gut inflammation in UC and exerted a protective action on gut epithelia barrier function both in vivo and in vitro. Meanwhile, it was proven that wogonoside regulated gut epithelia TJs primarily via suppressing the MLCK/pMLC2 signal pathway. Additionally, Liu Q. et al. (2020) also explored the tissular distribution of wogonoside and its therapeutical effect on UC and the probable mechanism. Results testified that wogonoside could be absorbed by the colon and relieve inflammation reactions by suppressing NLRP3 inflammasome formation and excitation, which was associated with an inhibiting role on the TXNIP-dependent NF-κB signal pathway. In summary, wogonoside shows promise as a new medication for treating UC.
Discussion
UC is a multifactorial persistent inflammatory bowel disorder. The destruction of the gut barrier is associated with UC and can result in pathogenetic antigen intrusion (Lee et al., 2020). Therefore, novel means of restoring the epithelial and mucus barrier, facilitating mucosa healing, and decreasing mucosa penetrability are regarded as promising methods for treating UC (Li J. J. et al., 2021). TJ forms a paracellular osmotic barrier to limit the traverse of ions, small solutes, and water (Han et al., 2023). Among various proteins that make up the TJ, ZO are the main tightly linked cytoskeletal proteins related to epithelia integrality, occludin is a crucial protein in maintaining barrier function and TJ stabilization, while claudin-1 is a membrane-spanning protein that forms part of the TJ strands (Atsugi et al., 2020; Kuo et al., 2021). The formation and disruption of the multiprotein complex made up of ZO-1, occludin and claudin-1 in intestinal epithelia cells could observably influence the intestine epithelia barrier function (Lan et al., 2021; Liu and Zhu, 2022). In the present review, natural flavones involving diosmetin, licoflavone B, luteolin, tangeretin, and wogonoside were found to notably reduce paracellular permeability and increase TEER value which was concomitant with increased levels of TJ proteins (ZO-1, occludin, and claudin-1). These evidences indicated that flavones could suppress the breakdown of intestinal barrier in UC models evoked by DSS, TNBS and LPS in vivo and in vitro.
Oxidant stress exerts a crucial action in pathophysiology of UC (Wojcik-Grzybek et al., 2022). Studies have revealed that increased production of ROS destroys the cellular macromolecules (DNA, lipids and proteins) and breaks epithelia cell integrality (Juan et al., 2021). Superoxide anion, continuously produced through endogenous processes and exogenous sources, is the main free radical to induce oxidative injury, which can be restrained by the first-line defense enzyme systems like SOD and CAT (Qiu et al., 2021). SOD converts the superoxide anion into H2O2, a metabolite that is easy to diffuse and stable, and then CAT further neutralizes H2O2 into water (Yu et al., 2023). CAT could enhance the oxidative resistibility and preserve the low steady state level of ROS. GSH, as a capital hydrophilic and intracellular nonenzymatic antioxidant, exerts an important effect in relieving tissue injury through eliminating reactive oxygen and nitrogen species (Ighodaro and Akinloye, 2018; Wu et al., 2021). Early studies showed that GSH concentration was found to be evidently decreased in colonic tissues when antioxidants were neutralized by released oxygen derived free radicals (Hu et al., 2021). The reduction of GSH can further cause a rise in MDA level, a final product of lipid peroxidation that finally brings about oxidative injury. As demonstrated in the present review, baicalin, diosmetin, fortunellin, luteolin, and wogonin could notably elevate the levels of the antioxidative enzymes SOD, GSH, GST, and CAT and suppress ROS and MDA activity induced by AA, DSS or TNBS treatment in the colitis tissue. These data indicated that natural flavones can effectively alleviate colitis by reducing oxidative stress.
Hyperactive inflammation cells, particularly neutrophils and macrophages, generate some pro-inflammatory factors, ROS, MPO and nitrogen metabolites, which are related to the pathogenic mechanism of colitis (Saraiva et al., 2022). Moreover, COX-2 and iNOS are pro-inflammatory enzymes that can be induced in inflamed tissues, leading to the generation of NO and PGE2 (Zhao et al., 2022). Elevated production of inflammatory cytokines, such as TNF-α, IL-1β, and IL-6, could injure gut epithelia cells and exacerbate colitis (Ahmad et al., 2021). IL-10 takes part in a Th2-mediated immunological response, and suppresses TNF-α, IL-1β, and IL-6 levels, exerting an anti-inflammatory action in the intestine mucosa immunity system. Thus, modulating the balance between pro- and anti-inflammatory factors is regarded as a necessary therapy approach for colitis (Yin et al., 2020; Zhuang et al., 2021). In this review, we firstly revealed that all natural-derived anti-colitis flavones could remarkably modulate the balance of pro- and anti-inflammatory cytokines in colonic tissues of colitis murine via attenuating pro-inflammatory agents and elevating anti-inflammatory factors.
The intrinsic immunity system identifies the existence of specific bacteria antigens through a broad family of pattern-recognition receptor (PRR). TLR4 belongs to the PRR that can recognize LPS (endotoxin), a main ingredient of gram-negative bacteria’s outer membrane, and stimulate the generation of pro-inflammatory factors, causing an inflammatory reaction (Dai et al., 2022). Moreover, TLR4 level was elevated in colon tissues and gut epithelia cells of UC animals and patients (Fajstova et al., 2020). Convincing proofs proves the therapeutic role of restraining TLR4/MyD88 signal molecules, subsequently resulting in the devitalization of NF-κB and MAPKs, and the suppression of pro-inflammatory factors (Ma et al., 2020). In this review, we found that natural-derived flavones, including baicalein, luteolin and wogonin observably restrained the enhancement of TLR4 and MyD88 in TNBS or DSS-treated UC murine and in LPS-treated macrophages or intestinal epithelial cells. Subsequently, the TNBS or LPS-treated excitation of NF-κB and MAPKs pathways and their downstream regulatory genes, including pro-inflammatory mediators (e.g., TNF-α, IL-6, and IL-1β), adhesion molecules (e.g., ICAM-1), NO and PGE2 were suppressed. These results showed that downregulation of TLR4/MyD88 signal cascades was associated with the anti-inflammatory roles of the above natural-derived anti-colitis flavones in UC models in vivo and in vitro.
Besides, the generation of pro-inflammatory factors is also modulated by a multiprotein complex known as inflammasome, which mainly consists of 3 ingredients including NLR, ASC and caspase-1. Among a variety of inflammasome, NLRP3 inflammasome composed of NLRP3, ASC and pro-caspase-1 is the most widely investigated (Mangan et al., 2018). The NLRP3 inflammasome plays a vital role in regulating the inflammatory response in the intestines and maintaining homeostasis by mediating multiple pro-inflammatory signals (Busch et al., 2022; Martinez-Lopez et al., 2022). After stimulation, NLRP3 recruits ASC adaptor to accelerate the recruitment of pro-caspase-1. Pro-caspase-1 then aggregates and automatically splits to produce active caspase-1. Activating caspase-1 is needed to transform pro-IL-1β and pro-IL-18 into their mature active forms IL-1β and IL-18 (Paik et al., 2021). Subsequently, IL-1β and IL-18 are secreted outside of the cell, trigger the “waterfall” cascade of downstream signaling, and participate in the development of numerous inflammatory illnesses, including UC (Chen C. et al., 2021). Chen et al. discovered that the serum levels of NLRP3, caspase-1, HMGB1 and IL-1β were significantly increased in UC patients (Chen et al., 2020). In congruence with previous investigations, in this review, we discovered that natural-derived anti-colitis flavones, such as baicalein, oroxylin A, lonicerin and wogonoside notably preserved IL-1β and IL-18 production, which was linked to the suppression of NLRP3, ASC and caspase-1 in a dosage-dependent mode both in TNBS- or DSS-induced UC murine and LPS-treated THP-1 or RAW264.7 cells.
Furthermore, research personnel obtained crucial insight into gut microflora constitution during gut inflammation and have uncovered novel directions for colitis therapy (Li et al., 2020). Some studies have emphasized that the prominent variations in intestinal microflora constitution in colitis sufferers can bring about intestine inflammation (Wang et al., 2022). Serious imbalance is primarily presented in the intestine of colitis sufferers, where there is a decrease in Firmicutes and Bacteroidetes, and an increase in Proteobacteria. The elevated endotoxin generated by Proteobacteria may cause damage to the gut’s penetrability, resulting in adhesion and invasion of gut epithelia cells, destruction of the host’s defenses, excitation of the inflammatory reaction, alterations in the composition of intestinal microflora, and eventually facilitating the occurrence of colitis (Jang et al., 2019). Moreover, Enterobacteriaceae, facultative anaerobic bacteria, are associated with the pathogenesis of colitis through aggravating intestinal inflammation and barrier injury (Kim et al., 2021; Qin et al., 2021). Escherichia-Shigella, gram-negative bacteria with an outer LPS membrane, can intrude into the colon epithelia and cause intestinal inflammation (Dai et al., 2021). Roseburia and Lactobacillus are probiotic strains with vital roles in maintaining intestinal homeostasis, inducing host’s colonic goblet cells, modulating immune system and promoting SCFAs productions (Zhang et al., 2020; Wang et al., 2021). In this review, the levels of Proteobacteria, Enterobacteriaceae and Escherichia-Shigella were reduced, while those of Bacteroidetes, Roseburia and Lactobacillus were elevated after acacetin, baicalin, diosmetin, luteolin, licoflavone B, and tangeretin administration in DSS treated murine UC. Surprisingly, some of them significantly increased overall richness and diversity while also regulating the composition of gut microbiota in a manner similar to the control group.
SCFAs, such as acetate, butyrate and propionate, are important energy sources and metabolites of intestinal microorganisms and colon epithelia cells, which exert remarkable action in preserving intestine homeostasis and strengthening gut barrier function (Feng et al., 2018; Venegas et al., 2019). Consistent with former researches, this research indicated that enteric SCFAs were reduced after DSS exposure, and baicalin and tangeretin administration could reverse these alterations through elevating the acetate, propionate, valeric and butyrate contents. Moreover, baicalin enriched certain specific bacteria populations that could motivate the generation of SCFAs, such as Butyricimonas spp., and Lactobacillus spp., manifesting that their protective action against DSS-evoked UC was mechanistically connected with elevating SCFAs generation mediated by their remodeling roles on the intestinal flora.
Conclusion
This paper first generalizes the effective protection and potential mechanisms of natural flavones against UC in vivo and in vitro models. Their therapeutic roles manifested as the alleviation of clinical symptoms, relief of colonic mucosal injury, and inhibition of inflammatory responses. The potential mechanism of these effects mainly involves the blockage of various signalling pathways, including TLR4/MyD88/NF-κB, MAPKs, PI3K/AKT, and NLRP3 inflammasome, which regulate the intestinal microflora and Treg/Th17 balance, decreasing inflammatory responses and oxidative stress, and increasing TJ expression (Figure 3). The present review highlights the effects of flavones derived from natural sources in the treatment of UC by promoting gut homeostasis, improving intestinal barrier function, reducing oxidative stress, and regulating the immuno-inflammatory response. These findings revealed that these naturally derived flavones are effective and prospective drug candidates for UC and other inflammatory diseases. Whereas, the latent mechanism or toxicology of most natural flavones remains in-depth research, and their actual effect on colitis sufferers has not been verified in clinical experiments. Thus, more efforts should be focused to illuminating the molecular mechanisms and long-range effect and security in clinically relevant experiments to accelerate the perspective utilization of these flavones as colitis remedies in the near future.
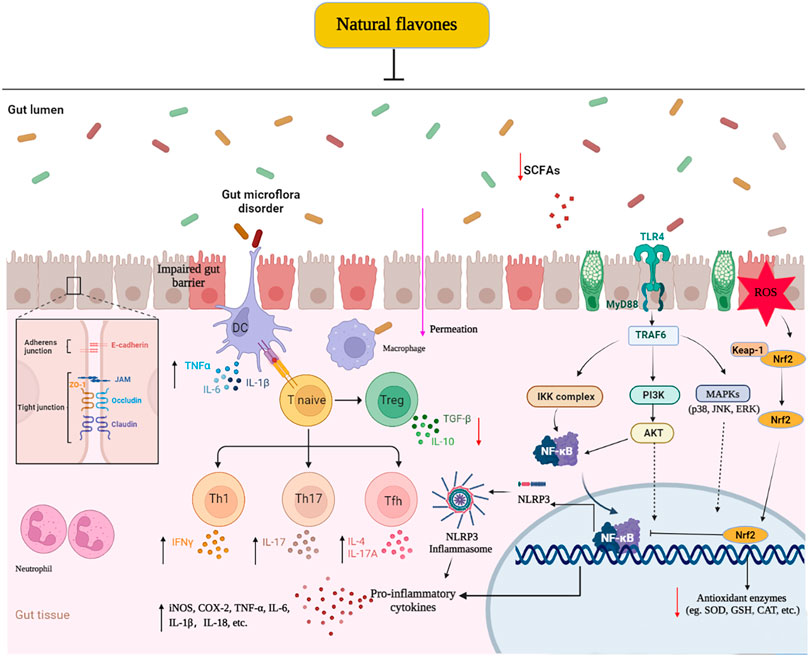
FIGURE 3. Molecular mechanisms of naturally derived flavones in the remedy of ulcerative colitis. ↓: Decrease, ↑: Increase: Inhibit.
Author contributions
CL proceeded the design of this study. QL, CL, and YX conducted the literature search. QL, CL, and JL constructed the figures and tables. QL wrote the manuscript. CL and QG made revisions to the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was financially supported by the National Natural Science Foundation of China (Nos 82003771 and 82160785), Science and Technology Foundation of Guizhou Province [No. QKHJC-ZK (2021) YB525], The future “science and technology elite” project of Zunyi Medical University (ZYSE-2022-01).
Conflict of interest
Author JL was employed by the company China Traditional Chinese Medicine Holdings Company Limited.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest..
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmad, A., Ansari, M. M., Mishra, R. K., Kumar, A., Vyawahare, A., Verma, R. K., et al. (2021). Enteric-coated gelatin nanoparticles mediated oral delivery of 5-aminosalicylic acid alleviates severity of DSS-induced ulcerative colitis. Mat. Sci. Eng. C Mat. Biol. Appl. 119, 111582. doi:10.1016/j.msec.2020.111582
Alhamad, D. W., Elgendy, S. M., Al-Tel, T. H., and Omar, H. A. (2021). Tangeretin as an adjuvant and chemotherapeutic sensitizer against various types of cancers: A comparative overview. J. Pharm. Pharmacol. 73 (5), 601–610. doi:10.1093/jpp/rgab013
Alsoud, D., Verstockt, B., Fiocchi, C., and Vermeire, S. (2021). Breaking the therapeutic ceiling in drug development in ulcerative colitis. Lancet Gastroenterol. Hepatol. 6 (7), 589–595. doi:10.1016/S2468-1253(21)00065-0
Arafa, E. A., Shurrab, N. T., and Buabeid, M. A. (2021). Therapeutic implications of a polymethoxylated flavone, tangeretin, in the management of cancer via modulation of different molecular pathways. Adv. Pharmacol. Pharm. Sci. 2021, 4709818. doi:10.1155/2021/4709818
Ashrafizadeh, M., Zarrabi, A., Saberifar, S., Hashemi, F., Hushmandi, K., Hashemi, F., et al. (2020). Nobiletin in cancer therapy: How this plant derived-natural compound targets various oncogene and onco-suppressor pathways. Biomedicines 8 (5), 110. doi:10.3390/biomedicines8050110
Atsugi, T., Yokouchi, M., Hirano, T., Hirabayashi, A., Nagai, T., Ohyama, M., et al. (2020). Holocrine secretion occurs outside the tight junction barrier in multicellular glands: Lessons from Claudin-1-deficient mice. J. Invest. Dermatol. 140 (2), 298–308. doi:10.1016/j.jid.2019.06.150
Aziz, N., Kim, M. Y., and Cho, J. Y. (2018). Anti-inflammatory effects of luteolin: A review of in vitro, in vivo, and in silico studies. J. Ethnopharmacol. 225, 342–358. doi:10.1016/j.jep.2018.05.019
Braidy, N., Behzad, S., Habtemariam, S., Ahmed, T., Daglia, M., Nabavi, S. M., et al. (2017). Neuroprotective effects of citrus fruit-derived flavonoids, nobiletin and tangeretin in alzheimer's and Parkinson's disease. CNS Neurol. Disord. Drug Targets 16 (4), 387–397. doi:10.2174/1871527316666170328113309
Busch, M., Ramachandran, H., Wahle, T., Rossi, A., and Schins, R. P. F. (2022). Investigating the role of the NLRP3 inflammasome pathway in acute intestinal inflammation: Use of THP-1 knockout cell lines in an advanced triple culture model. Front. Immunol. 13, 898039. doi:10.3389/fimmu.2022.898039
Chen, B., Luo, J. K., Han, Y. H., Du, H. J., Liu, J., He, W., et al. (2021a). Dietary tangeretin alleviated dextran sulfate sodium-induced colitis in mice via inhibiting inflammatory response, restoring intestinal barrier function, and modulating gut microbiota. J. Agric. Food Chem. 69 (27), 7663–7674. doi:10.1021/acs.jafc.1c03046
Chen, C., Liu, X. Q., Gong, L. J., Zhu, T. Y., Zhou, W. X., Kong, L. Y., et al. (2021b). Identification of Tubocapsanolide A as a novel NLRP3 inhibitor for potential treatment of colitis. Biochem. Pharmacol. 190, 114645. doi:10.1016/j.bcp.2021.114645
Chen, Y., Hui, H., Yang, H., Zhao, K., Qin, Y. S., Gu, C., et al. (2013). Wogonoside induces cell cycle arrest and differentiation by affecting expression and subcellular localization of PLSCR1 in AML cells. Blood 121 (18), 3682–3691. doi:10.1182/blood-2012-11-466219
Chen, Y. M., Wu, D., and Sun, L. J. (2020). Clinical significance of high-mobility group box 1 protein (HMGB1) and nod-like receptor protein 3 (NLRP3) in patients with ulcerative colitis. Med. Sci. Monit. 26, e919530. doi:10.12659/MSM.919530
Cheriet, T., Ben-Bachir, B., Thamri, O., Seghiri, R., and Mancini, I. (2020). Isolation and biological properties of the natural flavonoids pectolinarin and pectolinarigenin-A Review. Antibiot. Basel 9 (7), 417. doi:10.3390/antibiotics9070417
Cho, J. H., Lee, J. G., Yang, Y. I., Kim, J. H., Ahn, J. H., Baek, N. I., et al. (2011). Eupatilin, a dietary flavonoid, induces G2/M cell cycle arrest in human endometrial cancer cells. Food Chem. Toxicol. 49 (8), 1737–1744. doi:10.1016/j.fct.2011.04.019
Dai, W. B., Long, L. H., Wang, X. Q., Li, S., and Xu, H. P. (2022). Phytochemicals targeting Toll-like receptors 4 (TLR4) in inflammatory bowel disease. Chin. Med. 17 (1), 53. doi:10.1186/s13020-022-00611-w
Dai, Z. F., Ma, X. Y., Yang, R. L., Wang, H. C., Xu, D. D., Yang, J. N., et al. (2021). Intestinal flora alterations in patients with ulcerative colitis and their association with inflammation. Exp. Ther. Med. 22 (5), 1322. doi:10.3892/etm.2021.10757
de Carvalho, L. S. A., Geraldo, R. B., de Moraes, J., Pinto, P. L. S., Pinto, P. D., Pereira, O. D., et al. (2015). Schistosomicidal activity and docking of Schistosoma mansoni ATPDase 1 with licoflavone B isolated from Glycyrrhiza inflata (Fabaceae). Exp. Parasitol. 159, 207–214. doi:10.1016/j.exppara.2015.09.015
Dinda, B., Dinda, S., DasSharma, S., Banik, R., Chakraborty, A., and Dinda, M. (2017). Therapeutic potentials of baicalin and its aglycone, baicalein against inflammatory disorders. Eur. J. Med. Chem. 131, 68–80. doi:10.1016/j.ejmech.2017.03.004
Dou, W., Zhang, J. J., Zhang, E. Y., Sun, A. N., Ding, L. L., Chou, G. X., et al. (2013). Chrysin ameliorates chemically induced colitis in the mouse through modulation of a PXR/NF-kappa B signaling pathway. J. Pharmacol. Exp. Ther. 345 (3), 473–482. doi:10.1124/jpet.112.201863
Du, L. L., and Ha, C. (2020). Epidemiology and pathogenesis of ulcerative colitis. Gastroenterol. Clin. North Am. 49 (4), 643–654. doi:10.1016/j.gtc.2020.07.005
Eun, S. H., Woo, J. T., and Kim, D. H. (2017). Tangeretin inhibits IL-12 expression and NF-kappa B activation in dendritic cells and attenuates colitis in mice. Planta Med. 83 (6), 527–533. doi:10.1055/s-0042-119074
Fajstova, A., Galanova, N., Coufal, S., Malkova, J., Kostovcik, M., Cermakova, M., et al. (2020). Diet rich in simple sugars promotes pro-inflammatory response via gut microbiota alteration and TLR4 signaling. Cells 9 (12), 2701. doi:10.3390/cells9122701
Feng, Y. H., Wang, Y., Wang, P., Huang, Y. L., and Wang, F. J. (2018). Short-chain fatty acids manifest stimulative and protective effects on intestinal barrier function through the inhibition of NLRP3 inflammasome and autophagy. Cell Physiol. biochem. 49 (1), 190–205. doi:10.1159/000492853
Feng, Y. L., Bhandari, R., Li, C. M., Shu, P. F., and Shaikh, I. I. (2022). Pectolinarigenin suppresses LPS-induced inflammatory response in macrophages and attenuates DSS-induced colitis by modulating the NF-kappa B/Nrf2 signaling pathway. Inflammation 45 (6), 2529–2543. doi:10.1007/s10753-022-01710-4
Fiorino, G., Danese, S., Giacobazzi, G., and Spinelli, A. (2021). Medical therapy versus surgery in moderate-to-severe ulcerative colitis. Dig. Liver Dis. 53 (4), 403–408. doi:10.1016/j.dld.2020.09.022
Ganai, S. A., Sheikh, F. A., Baba, Z. A., Mir, M. A., Mantoo, M. A., and Yatoo, M. A. (2021). Anticancer activity of the plant flavonoid luteolin against preclinical models of various cancers and insights on different signalling mechanisms modulated. Phytother. Res. 35 (7), 3509–3532. doi:10.1002/ptr.7044
Garg, A., and Chaturvedi, S. (2022). A comprehensive review on chrysin: Emphasis on molecular targets, pharmacological actions and bio-pharmaceutical aspects. Curr. Drug Targets 23 (4), 420–436. doi:10.2174/1389450122666210824141044
Ge, H. F., Cai, Z. A. Z., Chai, J. L., Liu, J. Y., Liu, B. Q., Yu, Y. D., et al. (2021). Egg white peptides ameliorate dextran sulfate sodium-induced acute colitis symptoms by inhibiting the production of pro-inflammatory cytokines and modulation of gut microbiota composition. Food Chem. 360, 129981. doi:10.1016/j.foodchem.2021.129981
Gerges, S. H., Wahdan, S. A., Elsherbiny, D. A., and El-Demerdash, E. (2022). Pharmacology of diosmin, a citrus flavone glycoside: An updated review. Eur. J. Drug Metab. Pharmacokinet. 47 (1), 1–18. doi:10.1007/s13318-021-00731-y
Ginwala, R., Bhavsar, R., Chigbu, D. I., Jain, P., and Khan, Z. K. (2019). Potential role of flavonoids in treating chronic inflammatory diseases with a special focus on the anti-inflammatory activity of apigenin. Antioxidants 8 (2), 35. doi:10.3390/antiox8020035
Guo, L. T., Wang, S. Q., Su, J., Xu, L. X., Ji, Z. Y., Zhang, R. Y., et al. (2019). Baicalin ameliorates neuroinflammation-induced depressive-like behavior through inhibition of toll-like receptor 4 expression via the PI3K/AKT/FoxO1 pathway. J. Neuroinflamm. 16, 95. doi:10.1186/s12974-019-1474-8
Hagenlocher, Y., Gommeringer, S., Held, A., Feilhauer, K., Koninger, J., Bischoff, S. C., et al. (2019). Nobiletin acts anti-inflammatory on murine IL-10-/- colitis and human intestinal fibroblasts. Eur. J. Nutr. 58 (4), 1391–1401. doi:10.1007/s00394-018-1661-x
Han, H., You, Y., Cha, S., Kim, T. R., Sohn, M., and Park, J. (2023). Multi-species probiotic strain mixture enhances intestinal barrier function by regulating inflammation and tight junctions in lipopolysaccharides stimulated caco-2 cells. Microorganisms 11 (3), 656. doi:10.3390/microorganisms11030656
Han, X., Wu, Y. C., Meng, M., Sun, Q. S., Gao, S. M., and Sun, H. (2018). Linarin prevents LPS-induced acute lung injury by suppressing oxidative stress and inflammation via inhibition of TXNIP/NLRP3 and NF-kappa B pathways. Int. J. Mol. Med. 42 (3), 1460–1472. doi:10.3892/ijmm.2018.3710
Hirten, R. P., and Sands, B. E. (2021). New therapeutics for ulcerative colitis. Annu. Rev. Med. 72, 199–213. doi:10.1146/annurev-med-052919-120048
Hu, T. T., Fan, Y., Long, X. Y., Pan, Y. N., Mu, J. F., Tan, F., et al. (2021). Protective effect of Lactobacillus plantarum YS3 on dextran sulfate sodium-induced colitis in C57BL/6J mice. J. Food Biochem. 45 (2), e13632. doi:10.1111/jfbc.13632
Huang, S. W., Fu, Y. J., Xu, B., Liu, C., Wang, Q., Luo, S., et al. (2020). Wogonoside alleviates colitis by improving intestinal epithelial barrier function via the MLCK/pMLC2 pathway. Phytomedicine 68, 153179. doi:10.1016/j.phymed.2020.153179
Huynh, D. L., Ngau, T. H., Nguyen, N. H., Tran, G. B., and Nguyen, C. T. (2020). Potential therapeutic and pharmacological effects of wogonin: An updated review. Mol. Biol. Rep. 47 (12), 9779–9789. doi:10.1007/s11033-020-05972-9
Ighodaro, O. M., and Akinloye, O. A. (2018). First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 54 (4), 287–293. doi:10.1016/j.ajme.2017.09.001
Jang, H. M., Park, K. T., Noh, H. D., Lee, S. H., and Kim, D. H. (2019). Kakkalide and irisolidone alleviate 2,4,6-trinitrobenzenesulfonic acid-induced colitis in mice by inhibiting lipopolysaccharide binding to toll-like receptor-4 and proteobacteria population. Int. Immunopharmacol. 73, 246–253. doi:10.1016/j.intimp.2019.05.008
Jeong, S. H., Kim, H. H., Ha, S. E., Park, M. Y., Bhosale, P. B., Abusaliya, A., et al. (2022). Flavones: Six selected flavones and their related signaling pathways that induce apoptosis in cancer. Int. J. Mol. Sci. 23 (18), 10965. doi:10.3390/ijms231810965
Jiang, B., Song, J., and Jin, Y. (2020). A flavonoid monomer tricin in gramineous plants: Metabolism, bio/chemosynthesis, biological properties, and toxicology. Food Chem. 320, 126617. doi:10.1016/j.foodchem.2020.126617
Jie, L. H., Jantan, I., Yusoff, S. D., Jalil, J., and Husain, K. (2021). Sinensetin: An insight on its pharmacological activities, mechanisms of action and toxicity. Front. Pharmacol. 11, 553404. doi:10.3389/fphar.2020.553404
Jin, C. N., Liu, J. Y., Jin, R. Y., Yao, Y. P., He, S. L., Lei, M., et al. (2022). Linarin ameliorates dextran sulfate sodium-induced colitis in C57BL/6J mice via the improvement of intestinal barrier, suppression of inflammatory responses and modulation of gut microbiota. Food Funct. 13 (20), 10574–10586. doi:10.1039/d2fo02128e
Jin, X., Liu, M. Y., Zhang, D. F., Zhong, X., Du, K., Qian, P., et al. (2019). Baicalin mitigates cognitive impairment and protects neurons from microglia-mediated neuroinflammation via suppressing NLRP3 inflammasomes and TLR4/NF-kappa B signaling pathway. CNS Neurosci. Ther. 25 (5), 575–590. doi:10.1111/cns.13086
Juan, C. A., de la Lastra, J. M. P., Plou, F. J., and Perez-Lebena, E. (2021). The chemistry of reactive oxygen species (ROS) revisited: Outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci. 22 (9), 4642. doi:10.3390/ijms22094642
Juca, M. M., Cysne, F. M. S., de Almeida, J. C., Mesquita, D. D., Barriga, J. R. D., Ferreira, K. C. D., et al. (2020). Flavonoids: Biological activities and therapeutic potential. Nat. Prod. Res. 34 (5), 692–705. doi:10.1080/14786419.2018.1493588
Kasala, E. R., Bodduluru, L. N., Madana, R. M., Athira, K. V., Gogoi, R., and Barua, C. C. (2015). Chemopreventive and therapeutic potential of chrysin in cancer: Mechanistic perspectives. Toxicol. Lett. 233 (2), 214–225. doi:10.1016/j.toxlet.2015.01.008
Kim, J. W., Kim, C. Y., Kim, J. H., Jeong, J. S., Lim, J. O., Ko, J. W., et al. (2021). Prophylactic catechin-rich green tea extract treatment ameliorates pathogenic enterotoxic escherichia coli-induced colitis. Pathogens 10 (12), 1573. doi:10.3390/pathogens10121573
Kim, S. M., Ha, S. E., Lee, H. J., Rampogu, S., Vetrivel, P., Kim, H. H., et al. (2020). Sinensetin induces autophagic cell death through p53-related AMPK/mTOR signaling in hepatocellular carcinoma HepG2 cells. Nutrients 12 (8), 2462. doi:10.3390/nu12082462
Kuo, W. T., Zuo, L., Odenwald, M. A., Madha, S., Singh, G., Gurniak, C. B., et al. (2021). The tight junction protein ZO-1 is dispensable for barrier function but critical for effective mucosal repair. Gastroenterology 161 (6), 1924–1939. doi:10.1053/j.gastro.2021.08.047
Lan, H., Zhang, L. Y., He, W., Li, W. Y., Zeng, Z., Qian, B., et al. (2021). Sinapic acid alleviated inflammation-induced intestinal epithelial barrier dysfunction in lipopolysaccharide- (LPS-) treated caco-2 cells. Mediat. Inflamm. 2021, 5514075. doi:10.1155/2021/5514075
Lee, H. J., Saralamma, V. V. G., Kim, S. M., Ha, S. E., Raha, S., Lee, W. S., et al. (2018). Pectolinarigenin induced cell cycle arrest, autophagy, and apoptosis in gastric cancer cell via PI3K/AKT/mTOR signaling pathway. Nutrients 10 (8), 1043. doi:10.3390/nu10081043
Lee, Y., Sugihara, K., Gillilland, M. G., Jon, S., Kamada, N., and Moon, J. J. (2020). Hyaluronic acid-bilirubin nanomedicine for targeted modulation of dysregulated intestinal barrier, microbiome and immune responses in colitis. Nat. Mat. 19 (1), 118–126. doi:10.1038/s41563-019-0462-9
Li, B. L., Du, P. L., Du, Y., Zhao, D. Y., Cai, Y. R., Yang, Q., et al. (2021a). Luteolin alleviates inflammation and modulates gut microbiota in ulcerative colitis rats. Life Sci. 269, 119008. doi:10.1016/j.lfs.2020.119008
Li, B. L., Zhao, D. Y., Du, P. L., Wang, X. T., Yang, Q., and Cai, Y. R. (2021b). Luteolin alleviates ulcerative colitis through SHP-1/STAT3 pathway. Inflamm. Res. 70 (6), 705–717. doi:10.1007/s00011-021-01468-9
Li, C. L., Ai, G. X., Wang, Y. F., Lu, Q., Luo, C. D., Tan, L. H., et al. (2020). Oxyberberine, a novel gut microbiota-mediated metabolite of berberine, possesses superior anti-colitis effect: Impact on intestinal epithelial barrier, gut microbiota profile and TLR4-MyD88-NF-kappa B pathway. Pharmacol. Res. 152, 104603. doi:10.1016/j.phrs.2019.104603
Li, C. L., Dong, N., Wu, B. W., Mo, Z. M., Xie, J. H., and Lu, Q. (2021c). Dihydroberberine, an isoquinoline alkaloid, exhibits protective effect against dextran sulfate sodium-induced ulcerative colitis in mice. Phytomedicine 90, 153631. doi:10.1016/j.phymed.2021.153631
Li, C. L., Wang, J. H., Ma, R. F., Li, L. H., Wu, W. F., Cai, D. K., et al. (2022). Natural-derived alkaloids exhibit great potential in the treatment of ulcerative colitis. Pharmacol. Res. 175, 105972. doi:10.1016/j.phrs.2021.105972
Li, H. L., Wei, Y. Y., Li, X. H., Zhang, S. S., Zhang, R. T., Li, J. H., et al. (2021d). Diosmetin has therapeutic efficacy in colitis regulating gut microbiota, inflammation, and oxidative stress via the circ-sirt1/sirt1 axis. Acta Pharmacol. Sin. 43 (4), 919–932. doi:10.1038/s41401-021-00726-0
Li, J. J., Zhang, L., Wu, T., Li, Y. F., Zhou, X. J., and Ruan, Z. (2021e). Indole-3-propionic acid improved the intestinal barrier by enhancing epithelial barrier and mucus barrier. J. Agr. Food Chem. 69 (5), 1487–1495. doi:10.1021/acs.jafc.0c05205
Li, X. X., Chen, S. G., Yue, G. L., Kwok, H. F., Lau, B. S., Zheng, T., et al. (2021f). Natural flavone tricin exerted anti-inflammatory activity in macrophage via NF-κB pathway and ameliorated acute colitis in mice. Phytomedicine 90, 153625. doi:10.1016/j.phymed.2021.153625
Li, Y., Shen, L., and Luo, H. S. (2016). Luteolin ameliorates dextran sulfate sodium-induced colitis in mice possibly through activation of the Nrf2 signaling pathway. Int. Immunopharmacol. 40, 24–31. doi:10.1016/j.intimp.2016.08.020
Li, Y. Y., Wang, X. J., Su, Y. L., Wang, Q., Huang, S. W., Pan, Z. F., et al. (2021g). Baicalein ameliorates ulcerative colitis by improving intestinal epithelial barrier via AhR/IL-22 pathway in ILC3s. Acta Pharmacol. Sin. 43 (6), 1495–1507. doi:10.1038/s41401-021-00781-7
Liu, C., Li, Y. Y., Chen, Y. P., Huang, S. W., Wang, X. J., Luo, S., et al. (2020a). Baicalein restores the balance of Th17/Treg cells via aryl hydrocarbon receptor to attenuate colitis. Mediat. Inflamm. 2020, 5918587. doi:10.1155/2020/5918587
Liu, L. L., Geng, X. J., Zhang, J. Y., Li, S. H., and Gao, J. (2022). Structure-based discovery of Licoflavone B and Ginkgetin targeting c-Myc G-quadruplex to suppress c-Myc transcription and myeloma growth. Chem. Biol. Drug Des. 100 (4), 525–533. doi:10.1111/cbdd.14064
Liu, Q., Zuo, R., Wang, K., Nong, F. F., Fu, Y. J., Huang, S. W., et al. (2020b). Oroxindin inhibits macrophage NLRP3 inflammasome activation in DSS-induced ulcerative colitis in mice via suppressing TXNIP-dependent NF-kappa B pathway. Acta Pharmacol. Sin. 41 (6), 771–781. doi:10.1038/s41401-019-0335-4
Liu, X. F., and Zhu, H. Q. (2022). Curcumin improved intestinal epithelial barrier integrity by up-regulating ZO-1/Occludin/Claudin-1 in septic rats. Evid. Based Compl. Alt. Med. 2022, 2884522. doi:10.1155/2022/2884522
Luo, X. P., Yu, Z. L., Deng, C., Zhang, J. J., Ren, G. Y., Sun, A. N., et al. (2017). Baicalein ameliorates TNBS-induced colitis by suppressing TLR4/MyD88 signaling cascade and NLRP3 inflammasome activation in mice. Sci. Rep. 7, 16374. doi:10.1038/s41598-017-12562-6
Lv, Q., Xing, Y., Liu, J., Dong, D., Liu, Y., Qiao, H. Z., et al. (2021). Lonicerin targets EZH2 to alleviate ulcerative colitis by autophagy-mediated NLRP3 inflammasome inactivation. Acta Pharm. Sin. B 11 (9), 2880–2899. doi:10.1016/j.apsb.2021.03.011
Ma, H. M., Zhou, M. J., Duan, W. B., Chen, L. Y., Wang, L. L., and Liu, P. (2020). Anemoside B4 prevents acute ulcerative colitis through inhibiting of TLR4/NF-kappa B/MAPK signaling pathway. Int. Immunopharmacol. 87, 106794. doi:10.1016/j.intimp.2020.106794
Mangan, M. S. J., Olhava, E. J., Roush, W. R., Seidel, H. M., Glick, G. D., and Latz, E. (2018). Targeting the NLRP3 inflammasome in inflammatory diseases. Nat. Rev. Drug Discov. 17 (8), 588–606. doi:10.1038/nrd.2018.97
Marquez-Flores, Y. K., Villegas, I., Cardeno, A., Rosillo, M. A., and Alarcon-de-la-Lastra, C. (2016). Apigenin supplementation protects the development of dextran sulfate sodium induced murine experimental colitis by inhibiting canonical and non-canonical inflammasome signaling pathways. J. Nutr. Biochem. 30, 143–152. doi:10.1016/j.jnutbio.2015.12.002
Martinez-Lopez, A., Rivero-Pino, F., Villanueva, A., Toscano, R., Grao-Cruces, E., Marquez-Paradas, E., et al. (2022). Kiwicha (Amaranthus caudatus L) protein hydrolysates reduce intestinal inflammation by modulating the NLRP3 inflammasome pathway. Food Funct. 13 (22), 11604–11614. doi:10.1039/d2fo02177c
Mottaghipisheh, J., Taghrir, H., Dehsheikh, A. B., Zomorodian, K., Irajie, C., Sourestani, M. M., et al. (2021). Linarin, a glycosylated flavonoid, with potential therapeutic attributes: A comprehensive review. Pharmaceuticals 14 (11), 1104. doi:10.3390/ph14111104
Nabavi, S. F., Braidy, N., Gortzi, O., Sobarzo-Sanchez, E., Daglia, M., Skalicka-Wozniak, K., et al. (2015). Luteolin as an anti-inflammatory and neuroprotective agent: A brief review. Brain Res. Bull. 119 (1-11), 1–11. doi:10.1016/j.brainresbull.2015.09.002
Nageen, B., Sarfraz, I., Rasul, A., Hussain, G., Rukhsar, F., Irshad, S., et al. (2020). Eupatilin: A natural pharmacologically active flavone compound with its wide range applications. J. Asian. Nat. Prod. Res. 22 (1), 1–16. doi:10.1080/10286020.2018.1492565
Paik, S., Kim, J. K., Silwal, P., Sasakawa, C., and Jo, E. K. (2021). An update on the regulatory mechanisms of NLRP3 inflammasome activation. Cell. Mol. Immunol. 18 (5), 1141–1160. doi:10.1038/s41423-021-00670-3
Panagiotopoulos, A. A., Karakasiliotis, I., Kotzampasi, D. M., Dimitriou, M., Sourvinos, G., Kampa, M., et al. (2021). Natural polyphenols inhibit the dimerization of the SARS-CoV-2 main protease: The case of fortunellin and its structural analogs. Molecules 26 (19), 6068. doi:10.3390/molecules26196068
Patel, K., Gadewar, M., Tahilyani, V., and Patel, D. K. (2013). A review on pharmacological and analytical aspects of diosmetin: A concise report. Chin. J. Integr. Med. 19 (10), 792–800. doi:10.1007/s11655-013-1595-3
Qin, Y. T., Zhao, R. F., Qin, H., Chen, L., Chen, H. Q., Zhao, Y. L., et al. (2021). Colonic mucus-accumulating tungsten oxide nanoparticles improve the colitis therapy by targeting Enterobacteriaceae. Nano Today 39, 101234. doi:10.1016/j.nantod.2021.101234
Qiu, J. M., Qin, C. F., Wu, S. G., Ji, T. Y., Tang, G. T., Lei, X. Y., et al. (2021). A novel salvianolic acid A analog with resveratrol structure and its antioxidant activities in vitro and in vivo. Drug Dev. Res. 82 (1), 108–114. doi:10.1002/ddr.21734
Ren, J., Yue, B., Wang, H., Zhang, B., Dou, W., Yu, Z., et al. (2021). Acacetin ameliorates experimental colitis in mice via inhibiting macrophage inflammatory response and regulating the composition of gut microbiota. Front. Physiol. 11, 577237. doi:10.3389/fphys.2020.577237
Sajeev, A., Hegde, M., Daimary, U. D., Kumar, A., Girisa, S., Sethi, G., et al. (2022a). Modulation of diverse oncogenic signaling pathways by oroxylin A: An important strategy for both cancer prevention and treatment. Phytomedicine 105, 154369. doi:10.1016/j.phymed.2022.154369
Sajeev, A., Hegde, M., Girisa, S., Devanarayanan, T. N., Alqahtani, M. S., Abbas, M., et al. (2022b). Oroxylin A: A promising flavonoid for prevention and treatment of chronic diseases. Biomolecules 12 (9), 1185. doi:10.3390/biom12091185
Salehi, B., Venditti, A., Sharifi-Rad, M., Kregiel, D., Sharifi-Rad, J., Durazzo, A., et al. (2019). The therapeutic potential of apigenin. Int. J. Mol. Sci. 20 (6), 1305. doi:10.3390/ijms20061305
Sapkota, A., Gaire, B. P., Cho, K. S., Jeon, S. J., Kwon, O. W., Jang, D. S., et al. (2017). Eupatilin exerts neuroprotective effects in mice with transient focal cerebral ischemia by reducing microglial activation. PloS One 12 (2), e0171479. doi:10.1371/journal.pone.0171479
Saraiva, A. L., Vieira, T. N., Notario, A. F. O., Luiz, J. P. M., Silva, C. R., Goulart, L. R., et al. (2022). CdSe magic-sized quantum dots attenuate reactive oxygen species generated by neutrophils and macrophages with implications in experimental arthritis. Nanomed. Nanotechnol. Biol. Med. 42, 102539. doi:10.1016/j.nano.2022.102539
Shalini, V., Pushpan, C. K., Sindhu, G., Jayalekshmy, A., and Helen, A. (2016). Tricin, flavonoid from Njavara reduces inflammatory responses in hPBMCs by modulating the p38MAPK and PI3K/Akt pathways and prevents inflammation associated endothelial dysfunction in HUVECs. Immunobiology 221 (2), 137–144. doi:10.1016/j.imbio.2015.09.016
Shalkami, A. S., Hassan, M., and Bakr, A. G. (2018). Anti-inflammatory, antioxidant and anti-apoptotic activity of diosmin in acetic acid-induced ulcerative colitis. Hum. Exp. Toxicol. 37 (1), 78–86. doi:10.1177/0960327117694075
Shen, J., Cheng, J. Z., Zhu, S. G., Zhao, J., Ye, Q. Y., Xu, Y. Y., et al. (2019). Regulating effect of baicalin on IKK/IKB/NF-kB signaling pathway and apoptosis-related proteins in rats with ulcerative colitis. Int. Immunopharmacol. 73, 193–200. doi:10.1016/j.intimp.2019.04.052
Singh, S., Gupta, P., Meena, A., and Luqman, S. (2020). Acacetin, a flavone with diverse therapeutic potential in cancer, inflammation, infections and other metabolic disorders. Food Chem. Toxicol. 145, 111708. doi:10.1016/j.fct.2020.111708
Sinopoulou, V., Gordon, M., Dovey, T. M., and Akobeng, A. K. (2021). Interventions for the management of abdominal pain in ulcerative colitis. Cochrane Database Syst. Rev. 7, CD013589. doi:10.1002/14651858.CD013589.pub2
Song, J. K., Zhang, L., Xu, Y. F., Yang, D. Z., Zhang, L., Yang, S. Y., et al. (2021). The comprehensive study on the therapeutic effects of baicalein for the treatment of COVID-19 in vivo and in vitro. Biochem. Pharmacol. 183, 114302. doi:10.1016/j.bcp.2020.114302
Suga, N., Murakami, A., Arimitsu, H., Nakamura, T., Nakamura, Y., and Kato, Y. (2021). Luteolin suppresses 5-hydroxytryptamine elevation in stimulated RBL-2H3 cells and experimental colitis mice. J. Clin. Biochem. Nutr. 69 (1), 20–27. doi:10.3164/jcbn.20-192
Sun, Y., Zhao, Y., Yao, J., Zhao, L., Wu, Z. Q., Wang, Y., et al. (2015). Wogonoside protects against dextran sulfate sodium-induced experimental colitis in mice by inhibiting NF-kappa B and NLRP3 inflammasome activation. Biochem. Pharmacol. 94 (2), 142–154. doi:10.1016/j.bcp.2015.02.002
Venegas, D. P., De la Fuente, M. K., Landskron, G., Gonzalez, M. J., Quera, R., Dijkstra, G., et al. (2019). Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 10, 277. doi:10.3389/fimmu.2019.00277
Vukelic, I., Detel, D., Baticic, L., Potocnjak, I., and Domitrovic, R. (2020). Luteolin ameliorates experimental colitis in mice through ERK-mediated suppression of inflammation, apoptosis and autophagy. Food Chem. Toxicol. 145, 111680. doi:10.1016/j.fct.2020.111680
Wang, C., Wei, S. Y., Liu, B. J., Wang, F. Q., Lu, Z. Q., Jin, M. L., et al. (2022). Maternal consumption of a fermented diet protects offspring against intestinal inflammation by regulating the gut microbiota. Gut Microbes 14 (1), 2057779. doi:10.1080/19490976.2022.2057779
Wang, T., Wang, P. P., Ge, W. P., Shi, C., Xiao, G. N., Wang, X., et al. (2021). Protective effect of a multi-strain probiotics mixture on azoxymethane/dextran sulfate sodium-induced colon carcinogenesis. Food Biosci. 44, 101346. doi:10.1016/j.fbio.2021.101346
Wei, S. C., Sollano, J., Hui, Y. T., Yu, W., Estrella, P. V. S., Llamado, L. J. Q., et al. (2021). Epidemiology, burden of disease, and unmet needs in the treatment of ulcerative colitis in Asia. Expert Rev. Gastroenterol. Hepatol. 15 (3), 275–289. doi:10.1080/17474124.2021.1840976
Wojcik-Grzybek, D., Hubalewska-Mazgaj, M., Surmiak, M., Sliwowski, Z., Dobrut, A., Mlodzinska, A., et al. (2022). The combination of intestinal alkaline phosphatase treatment with moderate physical activity alleviates the severity of experimental colitis in obese mice via modulation of gut microbiota, attenuation of proinflammatory cytokines, oxidative stress biomarkers and DNA oxidative damage in colonic mucosa. Int. J. Mol. Sci. 23 (6), 2964. doi:10.3390/ijms23062964
Wu, S. M., Wang, P., Qin, J. W., Pei, Y. B., and Wang, Y. L. (2021). GSH-depleted nanozymes with dual-radicals enzyme activities for tumor synergic therapy. Adv. Funct. Mat. 31 (31), 2102160. doi:10.1002/adfm.202102160
Wu, W. Y., Li, Y. D., Cui, Y. K., Wu, C., Hong, Y. X., Li, G., et al. (2018). The natural flavone acacetin confers cardiomyocyte protection against hypoxia/reoxygenation injury via AMPK-mediated activation of Nrf2 signaling pathway. Front. Pharmacol. 9, 497. doi:10.3389/fphar.2018.00497
Wu, X., Zhang, H. J., Salmani, J. M. M., Fu, R., and Chen, B. A. (2016). Advances of wogonin, an extract from Scutellaria baicalensis, for the treatment of multiple tumors. OncoTargets Ther. 9, 2935–2943. doi:10.2147/OTT.S105586
Xiong, Y. J., Chen, D. P., Yu, C. C., Lv, B. C., Peng, J. Y., Wang, J. Y., et al. (2015). Citrus nobiletin ameliorates experimental colitis by reducing inflammation and restoring impaired intestinal barrier function. Mol. Nutr. Food Res. 59 (5), 829–842. doi:10.1002/mnfr.201400614
Xiong, Y. J., Deng, Z. B., Liu, J. N., Qiu, J. J., Guo, L., Feng, P. P., et al. (2019). Enhancement of epithelial cell autophagy induced by sinensetin alleviates epithelial barrier dysfunction in colitis. Pharmacol. Res. 148, 104461. doi:10.1016/j.phrs.2019.104461
Xiong, Y. J., Qiu, J. J., Li, C. Y., Qiu, Y., Guo, L., Liu, Y. J., et al. (2018). Fortunellin-induced modulation of phosphatase and tensin homolog by microRNA-374a decreases inflammation and maintains intestinal barrier function in colitis. Front. Immunol. 9, 83. doi:10.3389/fimmu.2018.00083
Xu, Z. R., Li, K., Pan, T. W., Liu, J., Li, B., Li, C. X., et al. (2019). Lonicerin, an anti-algE flavonoid against Pseudomonas aeruginosa virulence screened from Shuanghuanglian formula by molecule docking based strategy. J. Ethnopharmacol. 239, 111909. doi:10.1016/j.jep.2019.111909
Yao, J., Liu, T., Chen, R. J., Liang, J., Li, J., and Wang, C. G. (2020). Sphingosine-1-phosphate signal transducer and activator of transcription 3 signaling pathway contributes to baicalein-mediated inhibition of dextran sulfate sodium-induced experimental colitis in mice. Chin. Med. J. 133 (3), 292–300. doi:10.1097/CM9.0000000000000627
Yin, S. J., Yang, H. F., Tao, Y., Wei, S. M., Li, L. H., Liu, M. J., et al. (2020). Artesunate ameliorates DSS-induced ulcerative colitis by protecting intestinal barrier and inhibiting inflammatory response. Inflammation 43 (2), 765–776. doi:10.1007/s10753-019-01164-1
Yu, C. G., Lv, Y. J., Li, X. D., Bao, H. Y., Cao, X. L., Huang, J., et al. (2023). SOD-Functionalized gold nanoparticles as ROS scavenger and CT contrast agent for protection and imaging tracking of mesenchymal stem cells in Idiopathic pulmonary fibrosis treatment. Chem. Eng. J. 459, 141603. doi:10.1016/j.cej.2023.141603
Yu, X. Y., and Liu, Y. (2021). Diosmetin attenuate experimental ulcerative colitis in rats via suppression of NF-kappa B, TNF-alpha and IL-6 signalling pathways correlated with down-regulation of apoptotic events. Eur. J. Inflamm. 19, 205873922110672. doi:10.1177/20587392211067292
Zeng, J., Wang, Z., and Yang, X. J. (2022). Factors predicting clinical and endoscopic remission with placebo therapy in east asian patients with ulcerative colitis: A systematic review and meta-analysis. Eur. J. Clin. Pharmacol. 78 (7), 1069–1077. doi:10.1007/s00228-022-03312-3
Zhang, C. L., Zhang, S., He, W. X., Lu, J. L., Xu, Y. J., Yang, J. Y., et al. (2017). Baicalin may alleviate inflammatory infiltration in dextran sodium sulfate-induced chronic ulcerative colitis via inhibiting IL-33 expression. Life Sci. 186, 125–132. doi:10.1016/j.lfs.2017.08.010
Zhang, G. W., Wang, L., and Pan, J. H. (2012). Probing the binding of the flavonoid diosmetin to human serum albumin by multispectroscopic techniques. J. Agr. Food Chem. 60 (10), 2721–2729. doi:10.1021/jf205260g
Zhang, J., Xu, X. Q., Li, N., Cao, L., Sun, Y., Wang, J. C., et al. (2022). Licoflavone B, an isoprene flavonoid derived from licorice residue, relieves dextran sodium sulfate-induced ulcerative colitis by rebuilding the gut barrier and regulating intestinal microflora. Eur. J. Pharmacol. 916, 174730. doi:10.1016/j.ejphar.2021.174730
Zhang, M., Hao, X. N., Aziz, T., Zhang, J., and Yang, Z. N. (2020). Exopolysaccharides from Lactobacillus plantarum YW11 improve immune response and ameliorate inflammatory bowel disease symptoms. Acta Biochim. Pol. 67 (4), 485–493. doi:10.18388/abp.2020_5171
Zhao, C. H., Zhang, Y., Liu, H. Y., Li, P., Zhang, H., and Cheng, G. C. (2017). Fortunellin protects against high fructose-induced diabetic heart injury in mice by suppressing inflammation and oxidative stress via AMPK/Nrf-2 pathway regulation. Biochem. Biophys. Res. Commun. 490 (2), 552–559. doi:10.1016/j.bbrc.2017.06.076
Zhao, J. M., Xu, J. X., Zhang, Z. Q., Shao, Z. T., and Meng, D. L. (2022). Barrigenol-like triterpenoid saponins from the husks of Xanthoceras sorbifolia bunge and their anti-inflammatory activity by inhibiting COX-2 and iNOS expression. Phytochemistry 204, 113430. doi:10.1016/j.phytochem.2022.113430
Zhao, L., Chen, Z., Zhao, Q., Wang, D. D., Guo, Q. L., You, Q., et al. (2011). Developmental toxicity and genotoxicity studies of wogonin. Regul. Toxicol. Pharmacol. 60 (2), 212–217. doi:10.1016/j.yrtph.2011.03.008
Zheng, Y., Zhang, R., Shi, W., Li, L., Wu, L., Chen, Z., et al. (2020). Metabolism and pharmacological activities of the natural health-benefiting compound diosmin. Food Funct. 11 (10), 8472–8492. doi:10.1039/d0fo01598a
Zhong, X. C., Surh, Y. J., Do, S. G., Shin, E., Shim, K. S., Lee, C. K., et al. (2019). Baicalein inhibits dextran sulfate sodium-induced mouse colitis. J. Cancer Prev. 24 (2), 129–138. doi:10.15430/JCP.2019.24.2.129
Zhou, K., Cheng, R., Liu, B., Wang, L., Xie, H. F., and Zhang, C. F. (2018). Eupatilin ameliorates dextran sulphate sodium-induced colitis in mice partly through promoting AMPK activation. Phytomedicine 46, 46–56. doi:10.1016/j.phymed.2018.04.033
Zhou, W., Liu, X. T., Zhang, X., Tang, J. J., Li, Z. Y., Wang, Q., et al. (2017). Oroxylin A inhibits colitis by inactivating NLRP3 inflammasome. Oncotarget 8 (35), 58903–58917. doi:10.18632/oncotarget.19440
Zhou, Y. D., Dou, F. F., Song, H. W., and Liu, T. (2022). Anti-ulcerative effects of wogonin on ulcerative colitis induced by dextran sulfate sodium via Nrf2/TLR4/NF-kappa B signaling pathway in BALB/c mice. Environ. Toxicol. 37 (4), 954–963. doi:10.1002/tox.23457
Zhou, Y. F., Tu, Y. Y., Zhou, Q., Hua, A. L., Geng, P. W., Chen, F. F., et al. (2020). Evaluation of acacetin inhibition potential against cytochrome P450 in vitro and in vivo. Chem. Biol. Interact. 329, 109147. doi:10.1016/j.cbi.2020.109147
Zhu, L., Shen, H., Gu, P. Q., Liu, Y. J., Zhang, L., and Cheng, J. F. (2020a). Baicalin alleviates TNBS-induced colitis by inhibiting PI3K/AKT pathway activation. Exp. Ther. Med. 20 (1), 581–590. doi:10.3892/etm.2020.8718
Zhu, L., Xu, L. Z., Zhao, S., Shen, Z. F., Shen, H., and Zhan, L. B. (2020b). Protective effect of baicalin on the regulation of Treg/Th17 balance, gut microbiota and short-chain fatty acids in rats with ulcerative colitis. Appl. Microbiol. Biotechnol. 104 (12), 5449–5460. doi:10.1007/s00253-020-10527-w
Zhuang, H. D., Lv, Q., Zhong, C., Cui, Y. R., He, L. L., Zhang, C., et al. (2021). Tiliroside ameliorates ulcerative colitis by restoring the M1/M2 macrophage balance via the HIF-1α/glycolysis pathway. Front. Immunol. 12, 649463. doi:10.3389/fimmu.2021.649463
Zuo, T., Yue, Y. Z., Wang, X. H., Li, H., and Yan, S. A. (2021). Luteolin relieved DSS-induced colitis in mice via HMGB1-TLR-NF-kappa B signaling pathway. Inflammation 44 (2), 570–579. doi:10.1007/s10753-020-01354-2
Glossary
Keywords: ulcerative colitis, natural flavones, edible and medicinal plants, therapeutic effect, mechanism
Citation: Lu Q, Xie Y, Luo J, Gong Q and Li C (2023) Natural flavones from edible and medicinal plants exhibit enormous potential to treat ulcerative colitis. Front. Pharmacol. 14:1168990. doi: 10.3389/fphar.2023.1168990
Received: 18 February 2023; Accepted: 24 May 2023;
Published: 01 June 2023.
Edited by:
Marta Chagas Monteiro, Federal University of Pará, BrazilReviewed by:
Paula Coutinho, Instituto Politécnico da Guarda, PortugalGan Zhao, University of Pennsylvania, United States
Copyright © 2023 Lu, Xie, Luo, Gong and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cailan Li, licailan@zmu.edu.cn
 Qiang Lu
Qiang Lu