
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 05 May 2023
Sec. Pharmacology of Anti-Cancer Drugs
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1168566
Herein, we report an efficient and eco-friendly, ultrasound assisted synthetic strategy for the construction of diversified pyrrolobenzodiazepine-triazole hybrids, which are potentially pharmaceutically important scaffolds, via a domino reaction involving intermolecular electrophilic substitution followed by intramolecular Huisgen 1,3-dipolar azide-alkyne cycloaddition. The USP of the reported protocol is the use of benign and inexpensive, recyclable molecular iodine-ionic liquid synergistic catalytic system cum reaction media for achieving the synthesis. The other salient features of this method are the use of mild reaction conditions, high yield and atom economy, operational simplicity, broad substrate scope and easy workup and purification. All the synthesized compounds were evaluated for in vitro anti-proliferative activity against various cancer cell lines. From among the synthesized title compounds, 9,9-dimethyl-8-phenyl-9H-benzo [b]pyrrolo [1,2-d][1,2,3]triazolo[5,1-g][1,4]diazepine (7) was found most to be the most active compound exhibiting IC50 value of 6.60, 5.45, 7.85, 11.21, 12.24, 10.12, and 11.32 µM against MCF-7, MDA-MB-231, HeLa, SKOV-3, A549, HCT-116 and DLD-1 cell lines, respectively. Further the compounds were found to be non-toxic against normal human embryonic kidney (HEK-293) cell line.
The classical methods for the construction of diverse fused heterocyclic compounds entail the sequential formation of the individual bonds in the target molecule. However, a more effective strategy is to form the various bonds in a single operation via domino/cascade or multi-component reactions (MCR), without separating the intermediates, altering the reaction conditions, leading to faster synthesis and low waste production, thus drastically reducing the amount of solvent, reagents, energy and labor used, as well as simplifying the purification process (Tietze, 1996; Lu et al., 2012; Cioc et al., 2014). Due to the mounting awareness for a sustainable future in the last 2 decades the use of eco-friendly techniques and processes, reagents, solvents and catalysts in organic syntheses has become imperative (Li and Trost, 2008; Sheldon, 2008; Tiwari et al., 2018; Rather and Ali, 2021, 2022; Kar et al., 2022; Saquib et al., 2022). In this milieu domino/cascade strategy assumes even greater relevance and has become the one of the strategies of choice for the synthesis of medicinally significant molecules (Masson, 2014; Ahamad et al., 2016a, 2016b, 2018; Xie et al., 2021; Hussain et al., 2022). Conventional volatile organic compound (VOC) based solvents and metal based toxic catalysts are responsible for the major part of waste emanating from chemical processes and industries. Thus, the designing of an effective eco-friendly synthesis cannot be possible without the replacement of conventional VOC based solvents and toxic catalysts with eco-friendly solvents and catalysts (Marafi and Stanislaus, 2008; Menges, 2018).
Molecular iodine has received considerable attention as an inexpensive, non-toxic, readily available catalyst for various organic transformations, affording the corresponding products in excellent yields with high selectivity. The mild Lewis acidity associated with iodine enhanced its usage in organic synthesis to realize several organic transformations using stoichiometric levels to catalytic amounts of iodine. Due to these reasons iodine has been used as a green catalyst for dif-ferent organic reactions (Yusubov and Zhdankin, 2015; Breugst et al., 2016; Marsili et al., 2018; Tran et al., 2019; Velasco et al., 2022; Monika et al., 2023). Likewise, ionic liquids (ILs) are a new and versatile class of compounds with immense utility across many spheres of scientific research. Their unique eco-friendly physical and chemical properties are increasingly attracting chemists to explore their use as a green reaction media for organic synthesis. ILs offer a variety of advantages over conventional organic solvents, for instance product isolation is easier, they can be reused and catalysts can be more easily recovered and recycled, they are usually not flammable, vapor pressure is very low, and very importantly they have good ability to dissolve organic, organometallic, and even many inorganic compounds. Consequently, over the past decade or so, there has been a dramatic increase in the number of publications in the field of ionic liquids. [Bmim]BF4 is an inexpensive and commercially available, efficient, less toxic and recyclable ILs that has been successfully used in a variety of organic transformations. It has been reported to be superior to other commonly used imidazolium based ILs (Earle and Seddon, 2000; Rogers and Seddon, 2003; Ren and Cai, 2010; Banerjee, 2017; Dong et al., 2017; Gupta P. K. et al., 2022).
Cancer is one of the leading causes of mortality worldwide (Siegel et al., 2023). An estimated 19.3 million new cancer cases and more than 10 million deaths due to cancer in were reported 2020. Breast cancer is now the most commonly diagnosed malignancy, with a projected 2.3 million new cases (11.7%), followed by lung (11.4%), colorectal (10.0%), prostate (7.3%), and stomach (5.6%) cancers (Sung et al., 2021). The worrying rise in occurrence of new types of cancer represents a big crisis for public health systems around the world. Although a variety of anti-cancer drugs are currently available (Olivier et al., 2021) their efficacy is limited by toxicity to normal cells and drug resistance (Nurgali et al., 2018; Vasan et al., 2019). Therefore, the development of newer anti-cancer therapeutics having greater effectiveness and lesser toxicity, and with novel mechanisms of action, are urgently required (Hussain et al., 2014b; Saquib et al., 2019; Zhong et al., 2021; Gupta A. et al., 2022). Pyrrole, benzodiazepines and 1,2,3-triazoles are among the most medicinally relevant heterocyclic scaffolds (Ahamad et al., 2018; Mateev et al., 2022; Tolu-Bolaji et al., 2022).
A number of researchers have recently reported the synthesis of scaffolds in which these moieties have been fused together, many of which show good bioactivities including anti-cancer activity (Figure 1) (Ali et al., 1990; Fotso et al., 2009; Kamal et al., 2010, 2011; Antonow and Thurston, 2011; Granger et al., 2011; Banerji et al., 2013; Sudhapriya et al., 2019, 2015; Shafie et al., 2020). This result was unsurprising as it is assumed that if different active cores are integrated onto one platform, using the concept of molecular hybridization, the novel hybrid scaffold that would be obtained is expected to show enhanced medicinal properties (Meunier, 2008; Bosquesi et al., 2011; Tukulula et al., 2013; Shaveta et al., 2016).
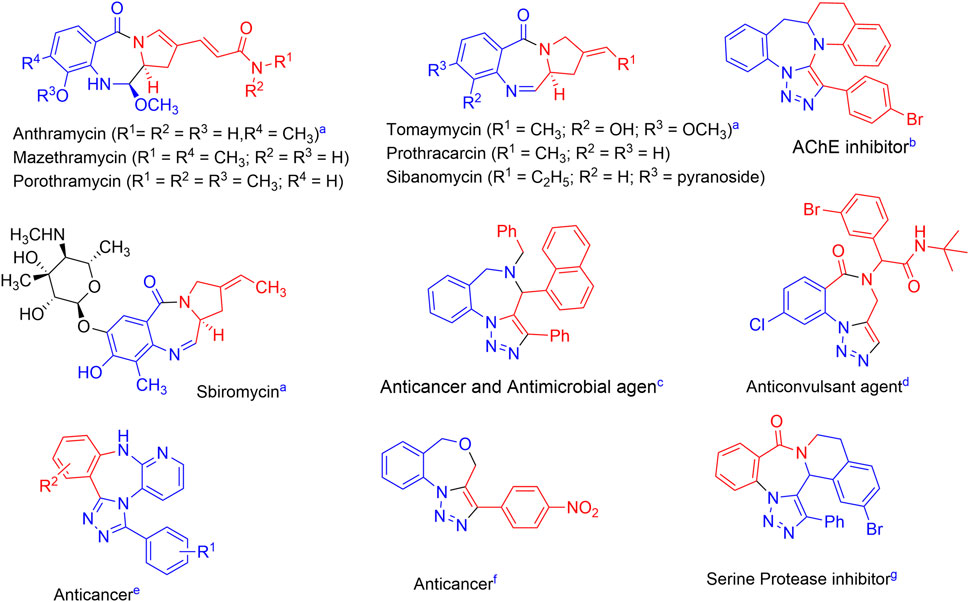
FIGURE 1. Selected medicinally active pyrrolobenzodiazepine-triazoles hybrids and related mole-cules. a(Fotso, S. et al., 2009; Antonow and Thurston, 2011); b(Sudhapriya et al., 2019); c(Shafie et al., 2020); d(Ali et al., 1990); e(Banerji et al., 2013); f(Granger et al., 2011); g(Kamal et al., 2011).
Working on this hypothesis we recently reported the development of a new method for the synthesis of the title compounds using scandium triflate in acetonitrile (MeCN) (Figure 2) (Hussain et al., 2014a). However, with the current emphasis on green synthesis the real challenge is the development of an eco-friendly, efficient approach for the construction of the title pyrrolobenzodiazepine-triazoles fused hybrids. In continuation of our earlier work on the design and synthesis of new hybrid scaffolds as potential bioactive agents (Ansari et al., 2015; Tiwari et al., 2017; Saquib et al., 2021) we herein report an efficient and atom-economic eco-friendly domino approach to highly diversified pyrrolobenzodiazepine-triazoles fused hybrids using a synergistic iodine-[Bmim]BF4 catalytic system, using easily accessible substrates, in the presence of ultrasonic radiation, and the in vitro evaluation of their anti-cancer activity against various cancer cell lines.
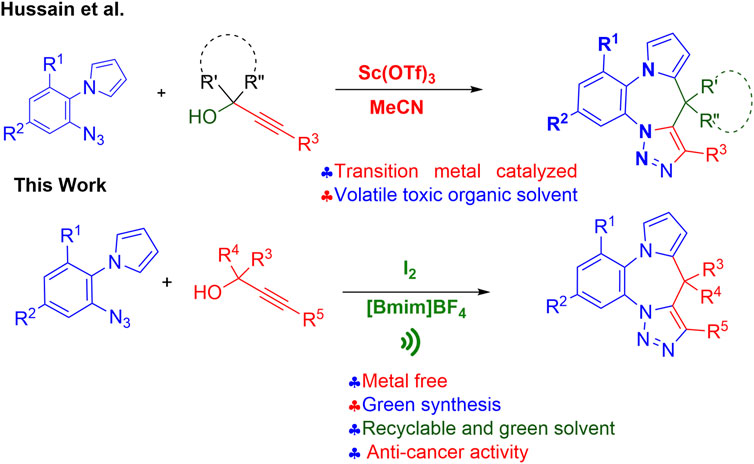
FIGURE 2. Previous and present methods for the synthesis of pyrrolobenzodiazepine-triazoles hybrids.
The required precursors (Figure 3), 1-(2-azidoaryl)-1H-pyrroles (1a and 1b) were obtained from 2-azidoanilines through Paal-Knorr pyrrole synthesis while propargyl alcohols (2–5) were synthesized from aldehydes and terminal alkynes (Hussain et al., 2014a). With the precursors in hand, we investigated the intermolecular propargylation followed by intramolecular 1,3-dipolar cycloaddition reaction by using a variety of Bronsted and Lewis acid catalysts (Table 1). We attempted the reaction of 1-(2-azidophenyl)-1H-pyrrole 1a and 4-(4-methoxyphenyl)-2-methylbut-3-yn-2-ol 2a under the influence of PTSA, oxalic acid, acetic acid and triflic acid (TfOH) at temperatures ranging from RT to 80°C. However, no reaction was observed with any of these catalysts (Table 1, entries 1–4).
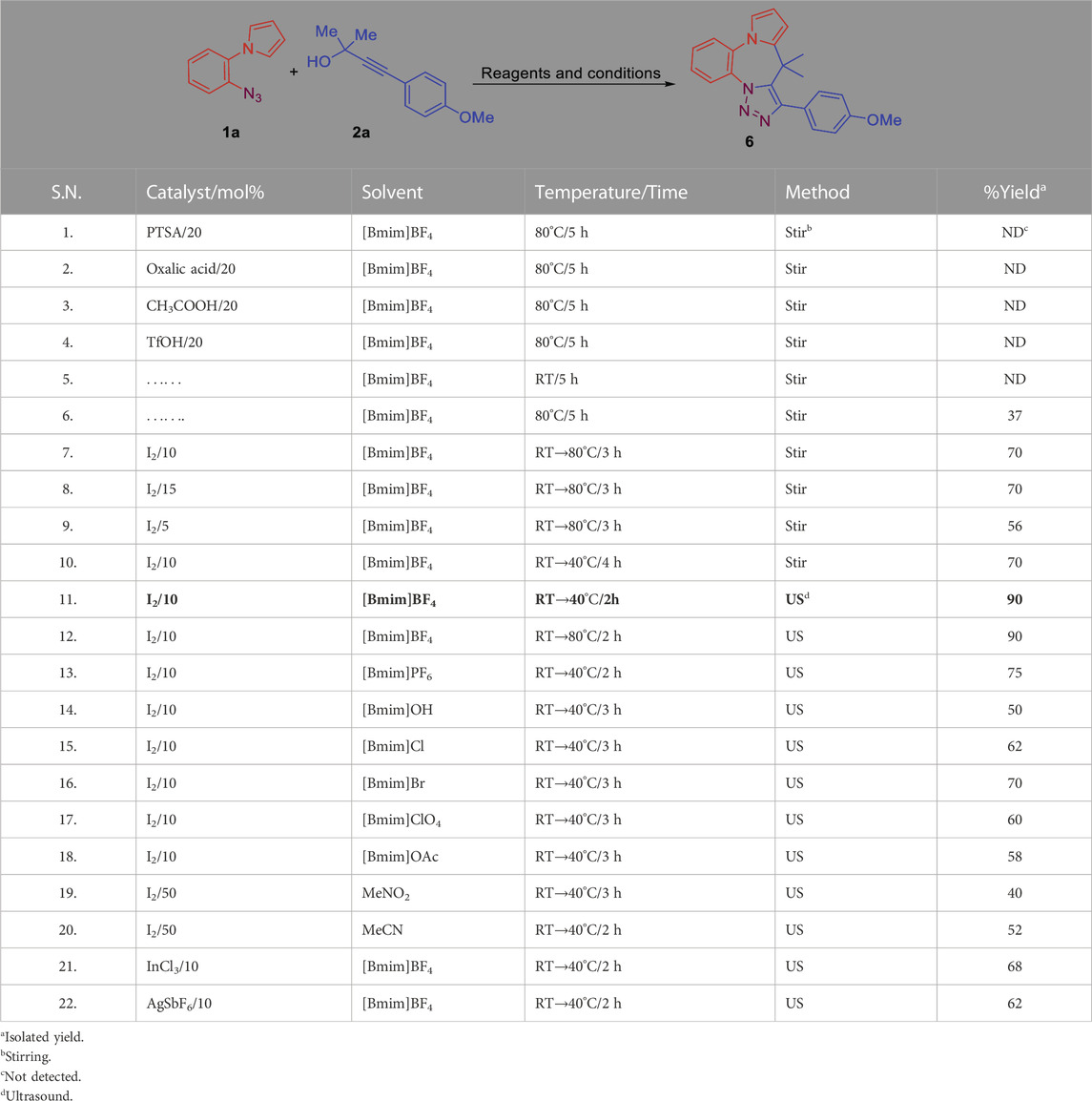
TABLE 1. Screening of various catalysts and solvents under normal stirring and under ultrasonic irradiation.
When this reaction was carried at room temperature (RT) in [bmim][BF4], no reaction was observed (TLC) (Table 1, entry 6). However, when the experiment was repeated at 80°C, a new spot was observed on TLC after 5 h of stirring. The compound was isolated in about 37% yield and identified as the desired pyrrolobenzodiazepine-triazoles hybrid, 8-(4-methoxyphenyl)-9,9-dimethyl-9H-benzo [b]pyrrolo [1,2-d][1,2,3]triazolo[5,1-g][1,4]diazepine (6). Encouraged by this positive result we increased the reaction temperature (90°C) and the reaction time but a better result was not observed. We now decided to use molecular iodine as a catalyst in the reaction, Initially, 10 mol% iodine was used. Addition of iodine was done at RT and the reaction temperature was then increased to 80°C. After 3 h, the desired product was obtained in 70% yield (Table 1, entry 7). Stirring for a longer time did not result in any improvement in the yield. To optimize the yield of reaction we increased the catalyst loading to 15 mol%; however, no increase in the yield was observed (Table 1, entry 8). On the other hand, decreasing the catalyst loading to 5 mol% caused a substantial decrease in yield (56%) (Table 1, entry 9). In our effort to further increase the yield of the desired compound 6 we decided to carry out the reaction in presence of ultrasonic irradiation. Consequently, when the reaction was carried out in the presence of 10 mol% iodine, followed by use of ultrasonic irradiation a large enhancement in yield (90%) and faster reaction time was observed (Table 1, entry 11).
We also investigated the effect of different ionic liquids such as [Bmim]PF6, [Bmim]OH, [Bmim]Cl, [Bmim]Br, [Bmim]ClO4 and [Bmim]OAc on the yield and rate of the reaction. However, none of the other ionic liquids screened were found to be as effective as [Bmim]BF4. The effectiveness of these ionic liquids for the above reaction was observed to be in the order [bmim][BF4] > [Bmim]PF6 > [Bmim]Br >[Bmim]Cl > [Bmim]ClO4 > [Bmim]OAc > [Bmim]OH (Table 1, entries 13–18; Figure 4). The yield of the reaction was also found to be very low in VOC based solvents such as MeNO2 and MeCN (Table 1, entries 19, 20). Other Lewis acid catalysts such as InCl3 and AgSbF6 were also used but were found to be less efficient as compared to iodine under the optimized reaction conditions (Table 1, entries 21, 22). From the above set of experiments the use of 10 mol% iodine, using [Bmim]BF4 as the reaction medium at 40°C followed by ultrasonic irradiation of the reaction mixture was identified as the best condition for carrying out this reaction (Table 1, entry 11).
To investigate the scope of this protocol, two 1-(2-azidoaryl)-1H-pyrroles (1a and 1b) anilines and a variety of substituted propargyl alcohols (2–5) were used as substrates to obtain the corresponding pyrrolobenzodiazepine-fused triazole hybrids. The corresponding 9H-benzo [b]pyrrolo [1,2-d][1,2,3]triazolo[5,1-g][1,4]diazepines were obtained in good to excellent yields. The disclosed protocol was also found to work well with both aliphatic propargyl alcohols and aromatic ring bearing propargyl alcohols having electron donating and electron withdrawing functionalities (Scheme 3). Use of propargyl alcohols with electron releasing groups at para position of the phenyl ring attached to alkynyl carbon gave better yields (Compounds 11, 17, and 18) as compared to propargyl alcohols having electron withdrawing groups (9–10). Presence of electron releasing group at para position of phenyl ring attached to C-1 carbon of propargyl alcohols also greatly assisted in the product formation leading to enhanced yield of the products (Compounds 14–16). Furan ring at 1-position of propargyl alcohols also enhanced the yield of the product (Compounds 21–22). Presence of methyl substituents at 2 and 3 position of 1-(2-azidoaryl)-1H-pyrroles led to slightly lower yields (Compounds 8, 10, 11, and 13) (Figure 5).
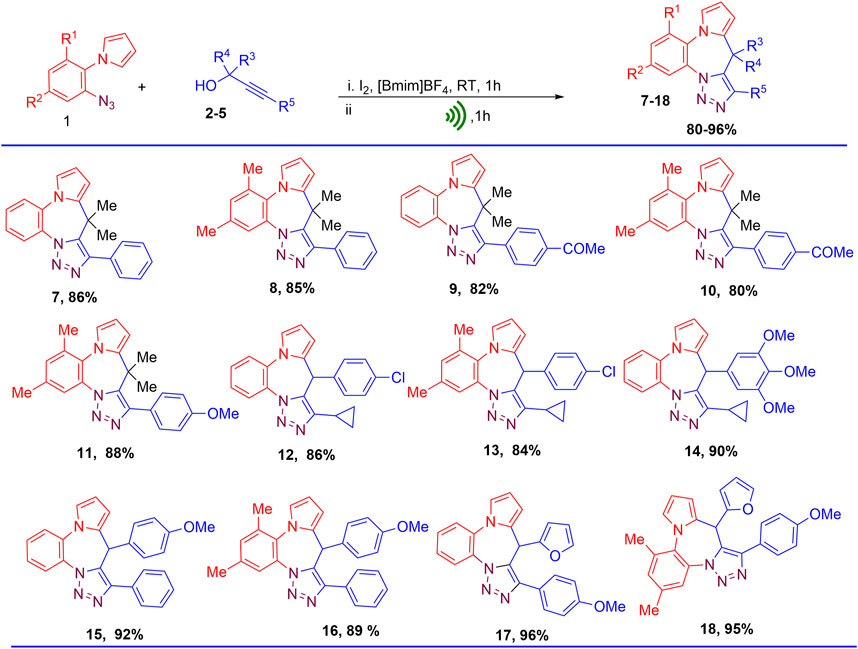
FIGURE 5. Substrate scope for the synthesis of pyrrolobenzodiazepine-triazoles hybrids (9H-benzo[b]pyrrolo[1,2-d][1,2,3]triazolo[5,1-g][1,4]diazepines).
The reaction is assumed to initiate through the activation of alcohol by iodine and Bmim+ by forming an interaction with the oxygen atom of the propargyl alcohol A. In the presence of strong π-nucleophile pyrrole B, an electrophilic substitution reaction then takes place at the C-2 position of the pyrrole ring (the most nucleophilic center on the pyrrole nucleus) to form an intermediate species C, which undergoes re-aromatization to form C-2 propargylated pyrrole D. Ultrasonic irradiation is thought to assist the intramolecular azide−alkyne 1,3-dipolar cycloaddition reaction in intermediate D furnishing the target molecule E (Figure 6).
We also investigated the recyclability potential of [Bmim]BF4 for the formation of 8-(4-methoxyphenyl)-9,9-dimethyl-9H-benzo [b]pyrrolo [1,2-d][1,2,3]triazolo[5,1-g][1,4]diazepine 6 using the reaction of 1-(2-azidophenyl)-1H-pyrrole 1a with 4-(4-methoxyphenyl)-2-methylbut-3-yn-2-ol 2a. After completion of the reaction, the reaction mixture was extracted thrice with ethyl acetate. The ionic liquid residue was washed with hexane and dried in vacuum to obtain pure [bmim][BF4] which was used for the next cycle. The recycled Bmim][BF4] so obtained could be used for the reaction up to four cycles with only a small drop in yield (Figure 7).
Taking into consideration the potential medicinal significance of the title molecules we ex-amined the scale-up potential of the present protocol. Two representative reactions were performed on a gram-scale (Table 2). In both the instances the scale-up reaction was fully successful leading to the desired target molecules 6 and 7 in 90% and 86% yields, respectively (Table 2). The successful scale-up of the reaction clearly demonstrates the suitableness of this protocol on a gram-scale for further anti-cancer studies.
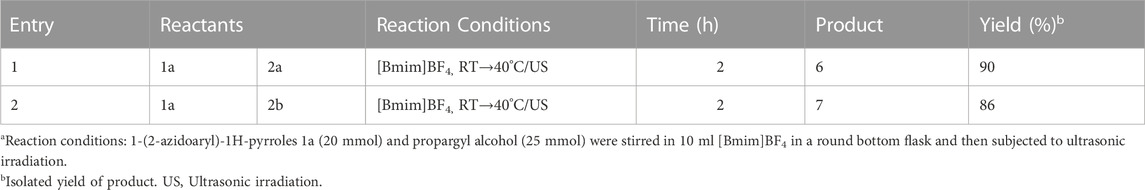
TABLE 2. Scale-up potentiala.
We evaluated all the molecules of the synthesized library against various cancer cell lines such as MCF-7 (ER +ve breast cancer), MDA-MB-231 (ER -ve breast cancer), HeLa (cervical cancer), SKOV-3 (ovarian cancer), A549 (lung cancer), HCT-116 and DLD-1 (colon cancer) in order to test their anti-proliferative potential (Table 3; Figure 8). Toxicity of the molecules was also evaluated against normal human embryonic kidney (HEK-293) cell line through MTT assay [35–39]. Among the evaluated molecules, compound 7 exhibited excellent activity against various cancer cells and was found to be non-toxic against HEK-293 cells. Compound 7 showed maximum anti-proliferative activity against MDA-MB-231 (IC50 = 5.45 µM). Compound 7 also exerted significant anti-proliferative effect against MCF-7 and Hela cell line with IC50 values of 6.60 and 7.85 µM respectively. Compound 7 also exhibited good anti-cancer activity against SKOV-3, A549, HCT-116 and DLD-1 cell lines with IC50 of 11.21, 12.24, 10.12 and 11.32 µM respectively. Compound 6 exhibited moderate anti-proliferative against MCF-7, MDA-MB-231 and HeLa cell lines with IC50 of 12.58, 15.72, and 18.45 µM, respectively, and was found to be inactive against SKOV-3, A549, HCT-116 and DLD-1 cell lines. Compound 8 showed activity against SKOV-3 cell line (IC50 = 12.86 µM). Compound 8 also exhibited mild activity against MCF-7 (IC50 = 19.28 µM), MDA-MB-231 (IC50 = 14.48 µM), HeLa (IC50 = 18.30 µM), A549 (IC50 = 15.86 µM) and HCT-116 (IC50 = 16.72 µM) cell lines. Compound 12 was found inactive against DLD-1 cell line. Compounds 9, 10, 12, 15, and 16 showed good to mild activity against MCF-7, MDA-MB-231, HeLa and SKOV-3 cell lines in an IC50 range of 12.80–19.10 µM. These compounds were found inactive against A549, HCT-116 and DLD-1 cell lines. Compounds 13 and 14 exhibited mild activity against MCF-7 and SKOV-3 cell lines. Compound 14 exhibited good activity against HCT-116 and DLD-1 cell lines with the IC50 value of 12.68 and 10.82 µM. Compound 14 also showed moderate inhibition against HeLa cell line (IC50 = 15.10 µM) and was found inactive against MDA-MB-231 and A549 cell lines. Compound 11 exhibited good activity against MDA-MB-231(IC50 = 12.48 µM), mild activity against HeLa (IC50 = 12.48 µM) and weak activity against A549 (IC50 = 18.44 µM) cell lines. Compounds 17 and 18 were found inactive against HeLa and SKOV-3 cell lines while in other cell lines these compounds exhibited mild to weak inhibition. From the above discussion it is clear that compound 7 having un-substituted phenyl ring attached to the triazole moiety exhibited the highest anti-cancer activity. Introduction of methyl substituents on the fused benzene ring greatly reduced the anti-cancer activity (compound 7 and 8, Table 3, entries 3 and 4). Further introduction of electron withdrawing group (compound 9 and 10) and electron releasing groups (compounds 11, 17, and 18) also decrease the activity against cancer cell lines. Introduction of cyclopropyl ring at the triazole moiety did not yield molecules with good bioactivity (compounds 12–14). Introduction of aryl ring on diazepine moiety by replacing the gem-dimethyl substituents was also found ineffective as these compound exhibited mild activity against the various cancer cell lines (compounds 12–18).
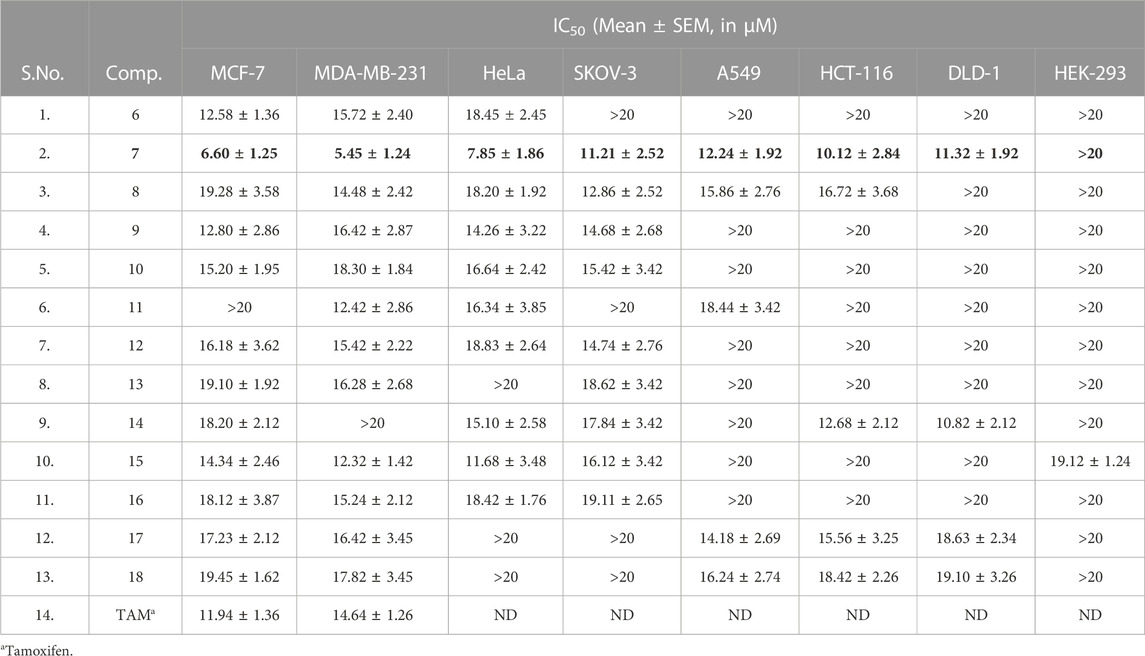
TABLE 3. In vitro antiproliferative activity of pyrrolobenzodiazepine-triazoles (6–18), against various cancer cell lines.
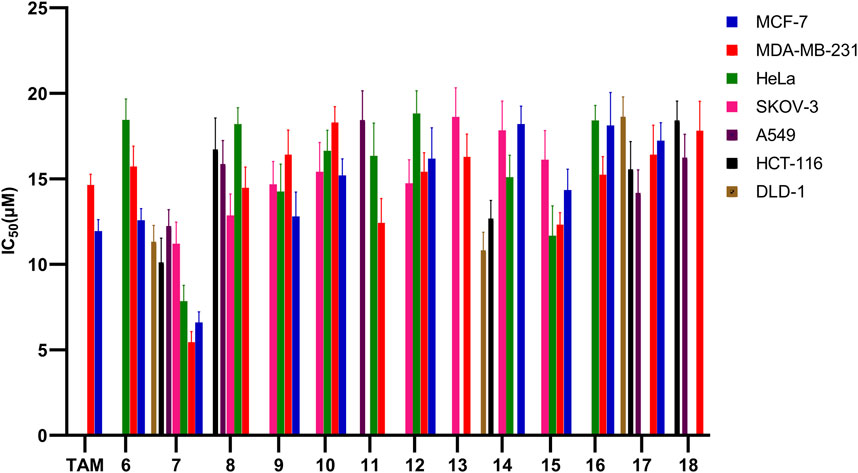
FIGURE 8. Graphical representation of calculated IC50 values (<19 µM) for compounds 6 to 18 against a panel of eight cancer cell-lines.
To a mixture of 1-(2-azidoaryl)-1H-pyrroles 1 (2 mmol) and propargyl alcohol 2–5 (2.5 mmol) and in [bmim][BF4] (4 ml) in a round bottom flask was added a catalytic amount of iodine (10 mol%) at room temperature, and the reaction temperature was allowed to rise to 40°C. The reaction was stirred at this temperature for the next 1 h and then it was subjected to ultrasound irradiation till completion of the reaction. The reaction mixture was now quenched with addition of water and extracted thrice with 10 ml of ethyl acetate. The combined organic extracts were treated with an aqueous solution of Na2S2O3 (1 M), washed with saturated solution of NaHCO3 and dried in vacuo to afford the crude product mixture, which was purified through column chromatography (EtOAc/hexane). The ionic liquid residue was washed with hexane and dried in vacuum to recover the [bmim][BF4] which was used in the next cycle.
The anti-cancer activity of the synthesized pyrrolobenzodiazepine-triazoles hybrids against various cancer cell lines was evaluated using MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) reduction assay (Ansari et al., 2015; Saquib et al., 2021). 2.5 × 103 cells per well were seeded in a 100 µl Dulbecco’s modified eagle’s medium (DMEM) mixed with 10% fetal bovine serum (FBS), in each well of 96-well microculture plates and incubated at 37°C for 24 h in carbon dioxide (CO2) incubator. The compounds were then diluted to the desired concentrations in the culture medium. After 48 h, the media was removed and 100 µl MTT (0.5 mg/ml) solution was added to each well, and the plates were further incubated for another 3 h. Supernatant from each well was removed, formazan crystals were dissolved in 100 µl of dimethyl sulfoxide (DMSO) and absorbance was recorded at 540 nm wavelength using a spectrophotometer.
In conclusion, a green and efficient domino synthetic strategy for the C-2 selective propargylation of pyrroles followed by intramolecular azide-alkyne Huisgen 1,3-dipolar cycloaddition leading to the construction of diversified pyrrolobenzodiazepine-fused triazole hybrids in high yields is reported. To the best of our information this is the first eco-friendly method for the synthesis of these biologically relevant hybrid scaffolds, and only the second synthesis of these molecules overall. The driving force for the present method is a simple and inexpensive, green, iodine-[bmim][BF4] synergistic catalytic system cum reaction medium under ultrasound irradiation. The disclosed protocol proceeds at ambient temperature, under ligand-, metal-, and base-free conditions, is operationally simple, atom economic and the ionic liquid reaction media can be recycled, further enhancing its green credentials. The synthesized molecules exhibited good in vitro anti-proliferative activity against different cell lines cancer cell lines in an IC50 range of 5.45–19.10 µM. Further, the evaluated molecules were found to be non-toxic against normal human embryonic kidney (HEK-293) cell line. Compound 7 exhibited the best anti-cancer activity with IC50 values of 6.60, 5.45, 7.85, 11.21, 12.24, 10.12, and 11.32 µM against MCF-7, MDA-MB-231, HeLa, SKOV-3, A549, HCT-116 and DLD-1 cell lines, respectively, and can be considered as a new anti-cancer lead molecule.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
MS: Conceived and designed the synthesis, helped with performing the reactions, performed the analysis and wrote the paper; SA and MFK performed the analysis, MIK and MKH: Conceived and designed the synthesis, helped with performing the reactions, performed the analysis and wrote the paper.
This research work was funded by Institutional Fund Projects under grant no. (IFPIP: 710-130-1443) Ministry of Education and King Abdulaziz University, DSR, Jeddah, Saudi Arabia.
The authors are thankful their respective institutions for technical and administrative support. The authors gratefully acknowledge technical and financial support provided by the Ministry of Education and King Abdulaziz University, DSR, Jeddah, Saudi Arabia.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1168566/full#supplementary-material
Ahamad, S., Kant, R., and Mohanan, K. (2016a). Metal-free three-component domino approach to phosphonylated triazolines and triazoles. Org. Lett. 18, 280–283. doi:10.1021/acs.orglett.5b03437
Ahamad, S., Kant, R., and Mohanan, K. (2016b). Three-component domino HWE olefination/1,3-dipolar cycloaddition/oxidation strategy for the rapid synthesis of trisubstituted pyrazoles. ChemistrySelect 1, 5276–5280. doi:10.1002/slct.201601113
Ahamad, S., Kumar, A., Kant, R., and Mohanan, K. (2018). Metal-free three-component assembly of fully substituted 1,2,3-triazoles. Asian J. Org. Chem. 7, 1698–1703. doi:10.1002/ajoc.201800340
Ali, S. M., Ashwell, M. A., Kelleher, E., Koerner, S., Lapierre, J.-M., and Link, J. S. (1990). Substituted benzo-pyrido-triazolo-diazepine compounds. Available at: https://patents.google.com/patent/US8541407B2/en.
Ansari, M. I., Hussain, M. K., Arun, A., Chakravarti, B., Konwar, R., and Hajela, K. (2015). Synthesis of targeted dibenzo[b,f]thiepines and dibenzo[b,f]oxepines as potential lead molecules with promising anti-breast cancer activity. Eur. J. Med. Chem. 99, 113–124. doi:10.1016/j.ejmech.2015.05.035
Antonow, D., and Thurston, D. E. (2011). Synthesis of DNA-Interactive Pyrrolo[2,1- c ] [1,4]benzodiazepines (PBDs). Chem. Rev. 111, 2815–2864. doi:10.1021/cr100120f
Banerjee, B. (2017). [Bmim]BF 4: A versatile ionic liquid for the synthesis of diverse bioactive heterocycles. ChemistrySelect 2, 8362–8376. doi:10.1002/slct.201701700
Banerji, B., Pramanik, S. K., Sanphui, P., Nikhar, S., and Biswas, S. C. (2013). Synthesis and cytotoxicity studies of novel triazolo-benzoxazepine as new anticancer agents. Chem. Biol. Drug Des. 82, 401–409. doi:10.1111/cbdd.12164
Bosquesi, P. L., Melo, T. R. F., Vizioli, E. O., Santos, J. L. dos, and Chung, M. C. (2011). Anti-inflammatory drug design sing a molecular hybridization approach. Pharmaceuticals 4, 1450–1474. doi:10.3390/ph4111450
Breugst, M., Detmar, E., and von der Heiden, D. (2016). Origin of the catalytic effects of molecular iodine: A computational analysis. ACS Catal. 6, 3203–3212. doi:10.1021/acscatal.6b00447
Cioc, R. C., Ruijter, E., and Orru, R. V. A. (2014). Multicomponent reactions: Advanced tools for sustainable organic synthesis. Green Chem. 16, 2958–2975. doi:10.1039/C4GC00013G
Dong, K., Liu, X., Dong, H., Zhang, X., and Zhang, S. (2017). Multiscale studies on ionic liquids. Chem. Rev. 117, 6636–6695. doi:10.1021/acs.chemrev.6b00776
Earle, M. J., and Seddon, K. R. (2000). Ionic liquids. Green solvents for the future. Pure Appl. Chem. 72, 1391–1398. doi:10.1351/pac200072071391
Fotso, S., Zabriskie, T. M., Proteau, P. J., Flatt, P. M., Santosa, D. A., Sulastri, , et al. (2009). Limazepines A−F, pyrrolo[1,4]benzodiazepine antibiotics from an Indonesian micrococcus sp. J. Nat. Prod. 72, 690–695. doi:10.1021/np800827w
Granger, B. A., Kaneda, K., and Martin, S. F. (2011). Multicomponent assembly strategies for the synthesis of diverse tetrahydroisoquinoline scaffolds. Org. Lett. 13, 4542–4545. doi:10.1021/ol201739u
Gupta, A., Iqbal, S., RoohiHussain, M. K., Zaheer, M. R., and Shankar, K. (2022a). Visible light-promoted green and sustainable approach for one-pot synthesis of 4,4’-(arylmethylene)bis(1H-pyrazol-5-ols), in vitro anticancer activity, and molecular docking with covid-19 M pro. ACS Omega 7, 34583–34598. doi:10.1021/acsomega.2c04506
Gupta, P. K., Azzam, M. A., Saquib, M., and Hussain, M. K. (2022b). A highly efficient and eco-friendly synthesis of disubstituted imidazoles in ionic liquid from gem -dibromo vinylarenes and amidines. Polycycl. Aromat. Compd., 1–10. doi:10.1080/10406638.2022.2061532
Hussain, M. K., Ansari, M. I., Kant, R., and Hajela, K. (2014a). Tandem C-2 functionalization–intramolecular azide–alkyne 1,3-dipolar cycloaddition reaction: A convenient route to highly diversified 9 H -benzo [b]pyrrolo[1,2-g] [1,2,3]triazolo[1,5-d] [1,4]diazepines. Org. Lett. 16, 560–563. doi:10.1021/ol403420z
Hussain, M. K., Ansari, M. I., Yadav, N., Gupta, P. K., Gupta, A. K., Saxena, R., et al. (2014b). Design and synthesis of ERα/ERβ selective coumarin and chromene derivatives as potential anti-breast cancer and anti-osteoporotic agents. RSC Adv. 4, 8828–8845. doi:10.1039/C3RA45749D
Hussain, Y., TamannaSharma, M., Kumar, A., and Chauhan, P. (2022). Recent development in asymmetric organocatalytic domino reactions involving 1,6-addition as a key step. Org. Chem. Front. 9, 572–592. doi:10.1039/D1QO01561C
Jones, G. B., Davey, C. L., Jenkins, T. C., Kamal, A., Kneale, G. G., Neidle, S., et al. (1990). The non-covalent interaction of pyrrolo[2, 1-c] [1, 4]benzodiazepine-5, 11-diones with DNA. Anticancer. Drug Des. 5, 249–264. Available at: http://www.ncbi.nlm.nih.gov/pubmed/2400538.
Kamal, A., Dastagiri, D., Ramaiah, M. J., Bharathi, E. V., Reddy, J. S., Balakishan, G., et al. (2010). Synthesis, anticancer activity and mitochondrial mediated apoptosis inducing ability of 2,5-diaryloxadiazole–pyrrolobenzodiazepine conjugates. Bioorg. Med. Chem. 18, 6666–6677. doi:10.1016/j.bmc.2010.07.067
Kamal, A., Prabhakar, S., Janaki Ramaiah, M., Venkat Reddy, P., Ratna Reddy, C., Mallareddy, A., et al. (2011). Synthesis and anticancer activity of chalcone-pyrrolobenzodiazepine conjugates linked via 1,2,3-triazole ring side-armed with alkane spacers. Eur. J. Med. Chem. 46, 3820–3831. doi:10.1016/j.ejmech.2011.05.050
Kar, S., Sanderson, H., Roy, K., Benfenati, E., and Leszczynski, J. (2022). Green chemistry in the synthesis of pharmaceuticals. Chem. Rev. 122, 3637–3710. doi:10.1021/acs.chemrev.1c00631
Li, C.-J., and Trost, B. M. (2008). Green chemistry for chemical synthesis. Proc. Natl. Acad. Sci. 105, 13197–13202. doi:10.1073/pnas.0804348105
Lu, L.-Q., Chen, J.-R., and Xiao, W.-J. (2012). Development of cascade reactions for the concise construction of diverse heterocyclic architectures. Acc. Chem. Res. 45, 1278–1293. doi:10.1021/ar200338s
Marafi, M., and Stanislaus, A. (2008). Spent catalyst waste management: A review. Resour. Conserv. Recycl. 52, 859–873. doi:10.1016/j.resconrec.2008.02.004
Marsili, L. A., Pergomet, J. L., Gandon, V., and Riveira, M. J. (2018). Iodine-catalyzed iso-nazarov cyclization of conjugated dienals for the synthesis of 2-cyclopentenones. Org. Lett. 20, 7298–7303. doi:10.1021/acs.orglett.8b03229
Masson, G. (2014). Catalytic cascade reactions. Edited by peng-fei xu and wei wang. Angew. Chem. Int. Ed. 53, 13656–13657. doi:10.1002/anie.201409867
Mateev, E., Georgieva, M., and Zlatkov, A. (2022). Pyrrole as an important scaffold of anticancer drugs: Recent advances. J. Pharm. Pharm. Sci. 25, 24–40. doi:10.18433/jpps32417
Menges, N. (2018). “The role of green solvents and catalysts at the future of drug design and of synthesis,” in Green Chemistry (InTech). doi:10.5772/intechopen.71018
Meunier, B. (2008). Hybrid molecules with a dual mode of action: Dream or reality?. Acc. Chem. Res. 41, 69–77. doi:10.1021/ar7000843
Monika, C., Ram, S., and Sharma, P. K. (2023). A review on molecular iodine catalyzed/mediated multicomponent reactions. Asian J. Org. Chem. 12. doi:10.1002/ajoc.202200616
Nurgali, K., Jagoe, R. T., and Abalo, R. (2018). Editorial: Adverse effects of cancer chemotherapy: Anything new to improve tolerance and reduce sequelae?. Front. Pharmacol. 9, 245. doi:10.3389/fphar.2018.00245
Olivier, T., Haslam, A., and Prasad, V. (2021). Anticancer drugs approved by the US food and drug administration from 2009 to 2020 according to their mechanism of action. JAMA Netw. Open 4, e2138793. doi:10.1001/jamanetworkopen.2021.38793
Rather, I. A., and Ali, R. (2021). A catalytic and solvent-free approach for the synthesis of diverse functionalized dipyrromethanes (DPMs) and calix[4]pyrroles (C4Ps). Green Chem. 23, 5849–5855. doi:10.1039/D1GC01515J
Rather, I. A., and Ali, R. (2022). An efficient and versatile deep eutectic solvent-mediated green method for the synthesis of functionalized coumarins. ACS Omega 7, 10649–10659. doi:10.1021/acsomega.2c00293
Ren, Y., and Cai, C. (2010). Molecular iodine in ionic liquid: A green catalytic system for esterification and transesterification. Synth. Commun. 40, 1670–1676. doi:10.1080/00397910903161660
Rogers, R. D., and Seddon, K. R. (2003). Ionic liquids--solvents of the future?. Science 302, 792–793. doi:10.1126/science.1090313
Saquib, M., Ansari, M. I., Johnson, C. R., Khatoon, S., Kamil Hussain, M., and Coop, A. (2019). Recent advances in the targeting of human DNA ligase I as a potential new strategy for cancer treatment. Eur. J. Med. Chem. 182, 111657. doi:10.1016/j.ejmech.2019.111657
Saquib, M., Baig, M. H., Khan, M. F., Azmi, S., Khatoon, S., Rawat, A. K., et al. (2021). Design and synthesis of bioinspired benzocoumarin-chalcones chimeras as potential anti-breast cancer agents. ChemistrySelect 6, 8754–8765. doi:10.1002/slct.202101853
Saquib, M., Khan, M. F., Singh, J., Khan, B., PritiKumar, P., et al. (2022). Operationally simple, scalable synthesis of aryloxy propanolamines using glycerol as a green promoting media: Practical eco-friendly access to propranolol and atenolol. Sustain. Chem. Pharm. 30, 100860. doi:10.1016/j.scp.2022.100860
Shafie, A., Mohammadi-Khanaposhtani, M., Asadi, M., Rahimi, N., Ranjbar, P. R., Ghasemi, J. B., et al. (2020). Novel fused 1,2,3-triazolo-benzodiazepine derivatives as potent anticonvulsant agents: Design, synthesis, in vivo, and in silico evaluations. Mol. Divers. 24, 179–189. doi:10.1007/s11030-019-09940-9
Shaveta, , Mishra, S., and Singh, P. (2016). Hybrid molecules: The privileged scaffolds for various pharmaceuticals. Eur. J. Med. Chem. 124, 500–536. doi:10.1016/j.ejmech.2016.08.039
Sheldon, R. A. (2008). Why green chemistry and sustainability of resources are essential to our future. J. Environ. Monit. 10, 406–407. doi:10.1039/b801651h
Siegel, R. L., Miller, K. D., Wagle, N. S., and Jemal, A. (2023). Cancer statistics, 2023. Ca. Cancer J. Clin. 73, 17–48. doi:10.3322/caac.21763
Sudhapriya, N., Manikandan, A., Kumar, M. R., and Perumal, P. T. (2019). Cu-mediated synthesis of differentially substituted diazepines as AChE inhibitors; validation through molecular docking and Lipinski’s filter to develop novel anti-neurodegenerative drugs. Bioorg. Med. Chem. Lett. 29, 1308–1312. doi:10.1016/j.bmcl.2019.04.002
Sudhapriya, N., Nandakumar, A., Arun, Y., Perumal, P. T., Balachandran, C., and Emi, N. (2015). An expedient route to highly diversified [1,2,3]triazolo[1,5-a] [1,4]benzodiazepines and their evaluation for antimicrobial, antiproliferative and in silico studies. RSC Adv. 5, 66260–66270. doi:10.1039/C5RA12497B
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca. Cancer J. Clin. 71, 209–249. doi:10.3322/caac.21660
Tietze, L. F. (1996). Domino reactions in organic synthesis. Chem. Rev. 96, 115–136. doi:10.1021/cr950027e
Tiwari, J., Saquib, M., Singh, S., Tufail, F., Singh, J., and Singh, J. (2017). Catalyst-free glycerol-mediated green synthesis of 5′-thioxospiro[indoline-3,3′-[1,2,4]triazolidin]-2-ones/spiro[indoline-3,3′-[1,2,4]triazolidine]-2,5′-diones. Synth. Commun. 47, 1999–2006. doi:10.1080/00397911.2017.1359844
Tiwari, J., Singh, S., Saquib, M., Tufail, F., Sharma, A. K., Singh, S., et al. (2018). Organocatalytic mediated green approach: A versatile new L-valine promoted synthesis of diverse and densely functionalized 2-amino-3-cyano-4H-pyrans. Synth. Commun. 48, 188–196. doi:10.1080/00397911.2017.1393087
Tolu-Bolaji, O. O., Sojinu, S. O., Okedere, A. P., and Ajani, O. O. (2022). A review on the chemistry and pharmacological properties of benzodiazepine motifs in drug design. Arab. J. Basic Appl. Sci. 29, 287–306. doi:10.1080/25765299.2022.2117677
Tran, U. P. N., Oss, G., Breugst, M., Detmar, E., Pace, D. P., Liyanto, K., et al. (2019). Carbonyl–olefin metathesis catalyzed by molecular iodine. ACS Catal. 9, 912–919. doi:10.1021/acscatal.8b03769
Tukulula, M., Sharma, R.-K., Meurillon, M., Mahajan, A., Naran, K., Warner, D., et al. (2013). Synthesis and antiplasmodial and antimycobacterial evaluation of new nitroimidazole and nitroimidazooxazine derivatives. ACS Med. Chem. Lett. 4, 128–131. doi:10.1021/ml300362a
Vasan, N., Baselga, J., and Hyman, D. M. (2019). A view on drug resistance in cancer. Nature 575, 299–309. doi:10.1038/s41586-019-1730-1
Velasco, M., Romero-Ceronio, N., Torralba, R., Hernández Abreu, O., Vilchis-Reyes, M. A., Alarcón-Matus, E., et al. (2022). Piperidine-iodine as efficient dual catalyst for the one-pot, three-component synthesis of coumarin-3-carboxamides. Molecules 27, 4659. doi:10.3390/molecules27144659
Xie, F., Sun, Y., Song, H., Zhao, J., Zhang, Z., Duan, Y., et al. (2021). Cascade Reaction of 2-Naphthols and Azirines: One-Pot Synthesis of C-3 Naphthol-Substituted Benzo[e]indoles. J. Org. Chem. 86, 15631–15639. doi:10.1021/acs.joc.1c02164
Yusubov, M. S., and Zhdankin, V. V. (2015). Iodine catalysis: A green alternative to transition metals in organic chemistry and technology. Resour. Technol. 1, 49–67. doi:10.1016/j.reffit.2015.06.001
Keywords: pyrrolobenzodiazepine-triazole hybrids, ionic liquid, iodine, green synthesis, anti-cancer agents
Citation: Saquib M, Ahamad S, Khan MF, Khan MI and Hussain MK (2023) An ultrasound assisted, ionic liquid-molecular iodine synergy driven efficient green synthesis of pyrrolobenzodiazepine-triazole hybrids as potential anticancer agents. Front. Pharmacol. 14:1168566. doi: 10.3389/fphar.2023.1168566
Received: 17 February 2023; Accepted: 17 April 2023;
Published: 05 May 2023.
Edited by:
Husain Yar Khan, Wayne State University, United StatesReviewed by:
Hasan Küçükbay, İnönü University, TürkiyeCopyright © 2023 Saquib, Ahamad, Khan, Khan and Hussain. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammad Imran Khan, bWlraGFuQGthdS5lZHUuc2E=; Mohd Kamil Hussain, bWtoY2RyaUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.