- 1Department of Radiation Oncology, Sichuan Clinical Research Center for Cancer, Sichuan Cancer Hospital and Institute, Sichuan Cancer Center, Affiliated Cancer Hospital of University of Electronic Science and Technology of China, Chengdu, China
- 2West China School of Medicine, Sichuan University, Chengdu, China
- 3Department of Cleft Lip and Palate, Plastic Surgery Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Shijingshan, Beijing, China
- 4Department of Pharmacy, Southwest Medical University, Luzhou, China
- 5Department of Pathology, Sichuan Clinical Research Center for Cancer, Sichuan Cancer Hospital and Institute, Sichuan Cancer Center, Affiliated Cancer Hospital of University of Electronic Science and Technology of China, Chengdu, China
- 6Dazhou Quxian People’s Hospital, Dazhou, China
In non-small cell lung cancer (NSCLC), two key genetic alterations, epidermal growth factor receptor (EGFR) mutations and anaplastic lymphoma kinase (ALK) rearrangements, are commonly believed to be mutually exclusive. Studies have reported that concurrent EGFR/ALK co-mutation in non-small cell lung cancer patients is rare, with a prevalence ranging from 0.1% to 1.6%. However, the clinical and pathological characteristics of these patients are not well-defined, and the optimal treatment approach for such cases remains controversial. In this report, we present a case of stage IV lung adenocarcinoma with both epidermal growth factor receptor and anaplastic lymphoma kinase mutations, along with high PD-L1 expression. The patient initially received treatment with epidermal growth factor receptor tyrosine kinase inhibitors (TKIs), but the disease progressed. However, following a switch to ALK-TKI therapy and local radiotherapy, the lesion showed regression. Our report also provides a comprehensive summary of the clinical and pathological features, as well as treatment strategies, for non-small cell lung cancer patients with concurrent epidermal growth factor receptor mutation and anaplastic lymphoma kinase rearrangement.
Introduction
Non-small cell lung cancer (NSCLC) is the most common type of lung cancer, accounting for approximately 80%–85% of cases (Zhou et al., 2021). In recent years, molecular genetics research on lung cancer has made remarkable progress, and the treatment of NSCLC has entered the era of targeted therapy. The most common driver gene mutation in NSCLC is epidermal growth factor receptor (EGFR), which is found in 45% of Asian patients and 20% of Caucasian patients with adenocarcinoma histology (Kim E. et al., 2021a; Kim ES. et al., 2021b). In individuals with sensitizing EGFR mutations, EGFR-tyrosine kinase inhibitors (TKIs) are suggested as first-line treatment. Anaplastic lymphoma kinase (ALK) rearrangement is less common than EGFR mutation, occurring in approximately 5% of NSCLC patients (Jahanzeb et al., 2020). ALK-TKIs are indicated as first-line treatment for individuals with ALK rearrangement. Early studies showed that ALK positivity and EGFR mutation are mutually exclusive and cannot coexist. However, recent studies have shown some cases or studies with coexistence of EGFR and ALK rearrangement, although this proportion of NSCLC is rare. Despite this, little is known about the molecular biology of these two oncogenes or the effect of EGFR-TKIs or ALK inhibitors in concomitant NSCLC patients. In this report, we present a case of NSCLC in a patient who harbored simultaneous EGFR mutation, ALK rearrangement, and high expression of PD-L1.
Case presentation
In February 2022, a 54-year-old male heavy smoker presented to our hospital with persistent coughing and sputum production. A chest computed tomography (CT) scan revealed a mass measuring 4.2 cm × 3.3 cm in the middle and lower lobe of the right lung (Figures 1A, B). The scan also showed multiple enlarged lymph nodes in the bilateral supraclavicular fossa, mediastinum, and right hilar region. Further brain magnetic resonance imaging (MRI) and positron emission computed tomography (PET) scans indicated the presence of multiple metastases in the brain and ribs (Figure 1C). A transthoracic needle biopsy of the right lung mass revealed non-small cell carcinoma-favor adenocarcinoma (Figure 2). Based on these findings, the patient was diagnosed with stage IVB right middle and lower lobe adenocarcinoma, T4N3M1c.
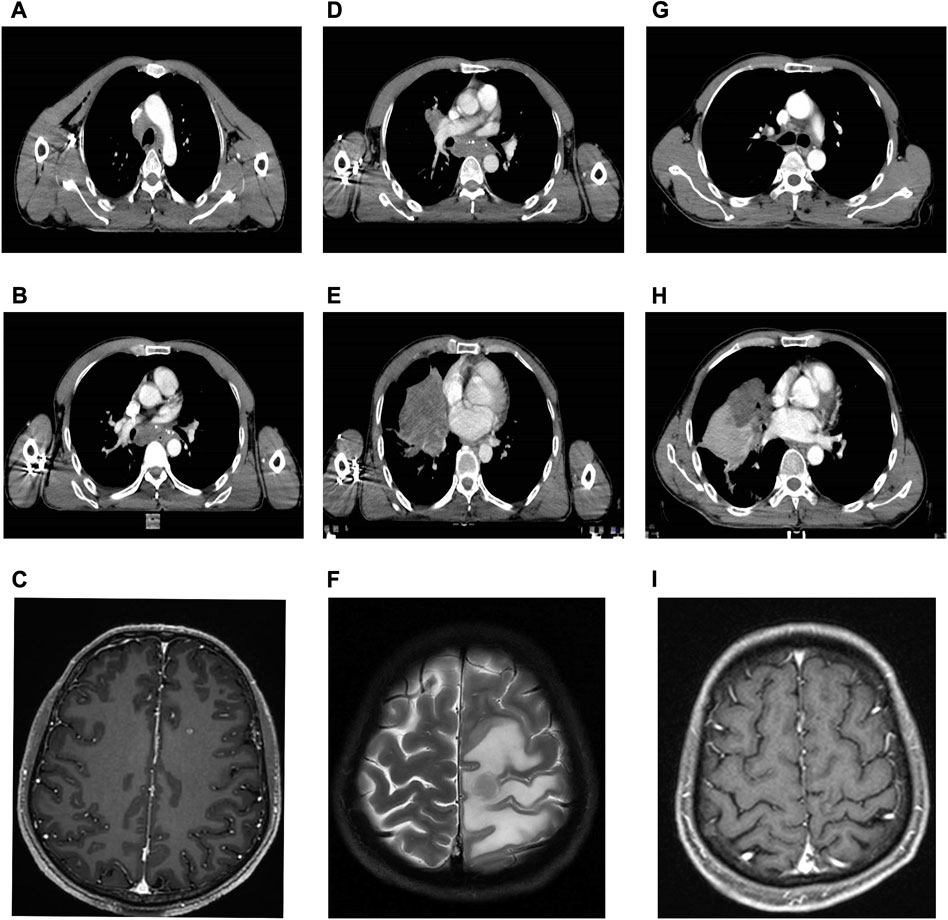
FIGURE 1. Serial CT and MRI of the case. (A,B) Baseline CT at diagnosis of the lung. (C) Baseline CT at diagnosis of the brain metastases. (D,E) CT after 3 months of osimertinib therapy with progressive disease of the lung. (F) MRI after 3 months of osimertinib therapy with progressive disease of the brain metastases with encephaledema. (G,H) CT after 3 months of alectinib therapy with a partial response of the lung. (I) MRI after 3 months of alectinib therapy and radiotherapy with a partial response of the brain metastases.
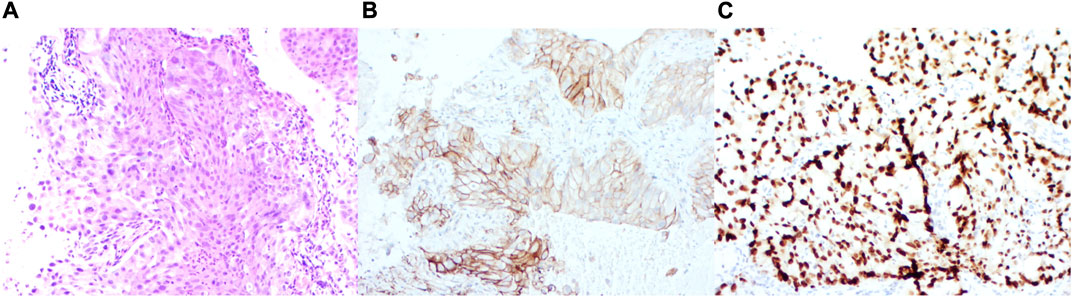
FIGURE 2. Immunohistochemical staining features. (A) Immunohistochemistry of the lung. (B) Expression patterns of PD-L1. (C) Expression patterns of TTF-1. Immunohistochemistry of ALK rearrangement.
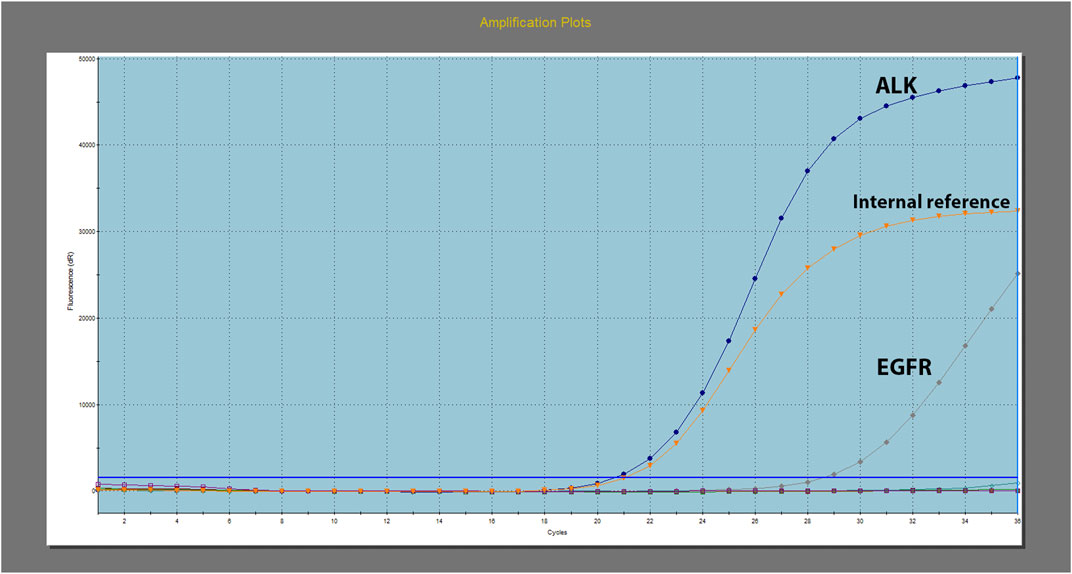
Further molecular screening using real-time polymerase chain reaction (PCR) revealed the presence of EGFR 19 exon deletion and echinoderm microtubule-associated protein-like 4-anaplastic lymphoma kinase (EML4-ALK) fusion (Figure 3). The test was conducted using the Human EGFR/ALK/ROS1 Gene Mutation Combination Test Kit (Fluorescent PCR method) by Xiamen Aide, and the detection instrument used was Agilent MX3000P. Additionally, EML4-ALK was retested using immunohistochemistry, which showed strong positive expression of PD-L1 (>90%) (Figure 2).
Due to economic constraints, combined treatment with EGFR-TKIs and ALK-TKIs was not pursued. Instead, the patient began a daily dose of 80 mg of osimertinib (EGFR-TKI) in March 2022. However, after 2 months, a follow-up chest CT scan showed that the irregular mass in the right lung had increased in size compared to previous measurements. Multiple lymph node metastases had also grown larger, measuring up to 3 cm × 2 cm (Figure 1D-e). A subsequent brain MRI revealed multiple scattered nodules in both brain hemispheres, which had increased in both size and quantity since the last examination (Figure 1F). The therapeutic response was determined to be progressive disease (PD), resulting in an initial progression-free survival (PFS) of 2 months.
Subsequently, the patient received twice daily administration of 600 mg alectinib (ALK-TKI) due to the presence of the EML4-ALK fusion gene. Stereotactic body radiotherapy (SBRT) was employed eight times, with a cumulative dose of 5 Gy/F, to address brain metastases. Additionally, bevacizumab (200 mg/q3w) was administered to mitigate brain edema. After 2 weeks of treatment, follow-up chest CT and brain MRI scans showed a noticeable reduction in the size of the mass in the right lung, enlarged lymph nodes, and brain metastasis (Figure 1H-j). Furthermore, subsequent therapy with alectinib resulted in a partial response (PR) thus far. The sum of the maximum diameter of the tumor target lesions was reduced by ≥ 30% and maintained for at least 4 weeks. Alectinib therapy will continue, and the decision regarding chest radiotherapy will be based on subsequent examinations.
Discussion
In this report, we describe a patient with a rare molecular subtype of NSCLC harboring concurrent EGFR mutations and ALK rearrangement. To our knowledge, there is little information available on the therapeutic efficacy of EGFR-TKIs and ALK-TKIs for these patients, with the exception of a small number of studies that reveal inconsistent findings regarding the response to the two TKIs (Won et al., 2015; Zhou et al., 2015; Lo Russo et al., 2017; Schmid et al., 2017). Additionally, choosing the optimum targeted medication in this circumstance is challenging, since there is no clinical guideline that specifies the order in which EGFR-TKIs and ALK-TKIs should be used.
To date, ≥107 patients with a concurrent EGFR mutation and ALK rearrangement have been documented, and their clinical features and treatment outcomes are listed in Table 1 ((5-7), ()). Inferring from the data in Table 1, we discovered that 86 patients had received an EGFR-TKI, 2 had achieved complete response (CR), 25 partial response (PR), 19 objective response (OR, OR = CR + PR), 15 stable disease (SD), and 25 progressive disease (PD). The objective response rate (ORR) was 53.5% (46 of 86), while the disease control rate (DCR) was 70.9% (61 of 86). In addition, ALK-TKI was administered to 50 patients, of whom 1 experienced CR, 22 PR, 8 OR, 7 SD, and 12 PD. The ORR was 62.0% (31 of 50), and the DCR was 76% (38 of 50). Compared with previous reports (Zhao et al., 2015), the most recent data, which encompassed a large number of cases, revealed a decrease in the overall response rate (ORR) of EGFR-TKIs, while patients treated with ALK-TKIs had an increased ORR. These results align with our own observations. Furthermore, the data showed that cases with EGFR and ALK concomitant mutations were more frequently reported in East Asian populations, which was consistent with the higher EGFR mutation rate in East Asian patients than in Caucasians (Paez et al., 2004).
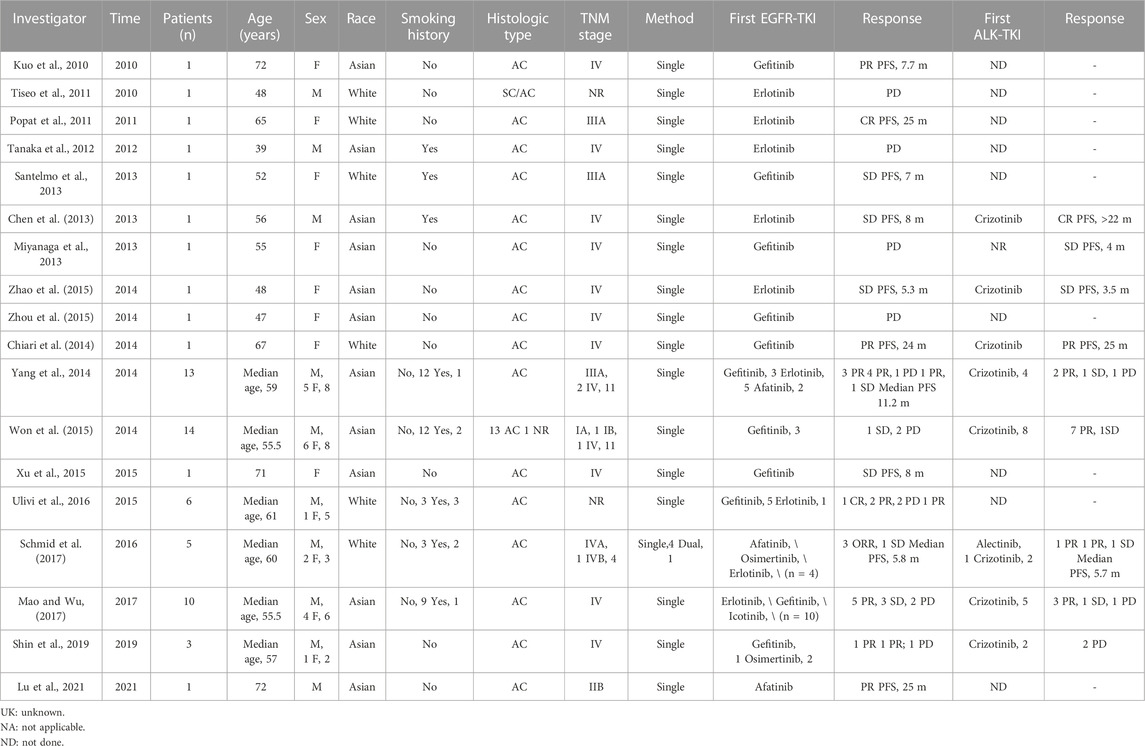
TABLE 1. A total of 107 patients with a concurrent EGFR mutation and ALK rearrangement have been documented, and their clinical features and treatment outcomes are listed.
EGFR mutation and ALK rearrangement were previously believed to be mutually exclusive (Horn and Pao, 2009; Shaw et al., 2009) and to be mutual causes of resistance to EGFR-TKIs or ALK-TKIs (Sasaki et al., 2011; Liang et al., 2016). In contrast, studies have shown that it is possible for patients harboring concurrent EGFR and ALK mutations to respond to both EGFR-TKIs and ALK-TKIs (Chen et al., 2013; Chiari et al., 2014; Schmid et al., 2017). This implied that these individuals may not be resistant to both EGFR-TKIs and ALK-TKIs but could instead obtain different responses with one of these medications. The degree of the relevant gene modifications may determine which medication is more effective if the tumor is caused by two different driver genes (Zhao et al., 2015). In addition, as the degree of signaling pathway activation is correlated with the level of phosphorylation of downstream proteins, the detection of the abundance of EGFR mutations and ALK rearrangements and the levels of phosphorylation of downstream proteins is essential to optimize the selection of TKIs in clinical practice (Lou et al., 2016).
Furthermore, it is also important to determine when EGFR/ALK coalterations occur and the subtype of ALK rearrangement. An earlier study discovered that patients with EML4-ALK/EGFR coalterations frequently had a significantly shorter median PFS than patients with non-EML4-ALK/EGFR coalterations after receiving EGFR-TKI therapy (Liu et al., 2019). According to the author, non-EML4-ALK coalterations may be an acquired resistance mechanism brought on by EGFR-TKIs, while EML4-ALK coalterations were likely to be a de novo change. Additionally, there are also two possibilities for the case where both genetic alterations exist from the beginning of tumor proliferation. The two biomolecular alterations may coexist in different cellular clones, which represents the expression of the heterogeneity of tumors or the same tumor cell. All mechanisms are possible and may emerge in the same patient at different times over the course of the illness (Lo Russo et al., 2017). Since the two alterations may coexist ab initio, the combination of EGFR-TKIs and ALK-TKIs may exert satisfactory efficacy, and further study is needed (Noronha et al., 2022).
In our case, due to economic constraints, the patient did not opt for combination therapy with EGFR-TKI and ALK-TKI. As there is no consensus on treatment for patients with EGFR/ALK coalterations, we selected EGFR-TKI as the first-line treatment for the patient, as recommended by some studies. The efficacy of alectinib and lack of efficacy of osimertinib may be due to the higher abundance of ALK rearrangement and relatively higher activation of ALK than the EGFR signaling pathway (Lou et al., 2016). However, a limitation of this case report is the lack of measurement of mutation abundance and downstream pathway activation. Additionally, the overexpression of PD-L1 (>90%) in our patient may potentially negatively impact the efficacy of TKIs and further complicate treatment options (He et al., 2022). Patients with ALK mutations have a significantly higher incidence of high PD-L1 expression (≥50%). Previous research has shown that NSCLCs with ALK rearrangements have limited objective response rates and a short median progression-free survival (PFS) when treated with PD-1/PD-L1 inhibitors. Moreover, PD-L1 expression was not found to be a crucial biomarker for immune checkpoint inhibitor (ICI) therapy in patients with genetic mutations (Kris et al., 2011; Barlesi et al., 2013). For patients harboring dual mutations and exhibiting high PD-L1 expression, further clinical trials are imperative to validate the efficacy of combining immunosuppressive drugs and assess the manageability of potential side effects.
As part of the initial diagnosis, EGFR, ALK, KRAS, and other gene mutations must be detected before treatment. Oncologists must take into account the presence of dual or multiple oncogenes when selecting the most suitable therapeutic strategies, which may include combination or sequential treatment methods. However, more research is required to gain a deeper understanding of therapeutic approaches in patients with both EGFR mutation and ALK rearrangement.
Conclusion
In conclusion, we presented a case of a patient with both EGFR mutation and ALK rearrangement, who experienced disease progression (PD) after osimertinib treatment. Subsequent therapy with alectinib resulted in a partial response (PR) so far. Recent data, including more cases, have suggested that ALK-TKIs may have better efficacy than EGFR-TKIs, which is consistent with our case. Moreover, it is essential to evaluate the abundance of gene mutations and levels of downstream protein phosphorylation to optimize treatment outcomes. Further research is required to gain a better understanding of the molecular mechanisms underlying responsiveness and resistance to EGFR-TKIs and ALK-TKIs in this specific subgroup of patients with coalterations. Additionally, exploring potential combination or sequential therapy strategies is necessary.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individuals for the publication of any potentially identifiable images or data included in this article.
Author contributions
YW conceived the case report, and HH, ST, and MX collected and analyzed data and wrote the manuscript. QY and KZ were directly involved in the treatment of the patient and collected data. PG and QL worked for the figures. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors thank the patient for his understanding and cooperation with the treatment.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Barlesi, F., Blons, H., Beau-Faller, M., Rouquette, I., Ouafik, L. H., Mosser, J., et al. (2013). Biomarkers (BM) France: results of routine EGFR, HER2, KRAS, BRAF, PI3KCA mutations detection and EML4-ALK gene fusion assessment on the first 10,000 non-small cell lung cancer (NSCLC) patients (pts). J. Clin. Oncol. 31, 8000. 15_suppl. doi:10.1200/jco.2013.31.15_suppl.8000
Chen, X., Zhang, J., Hu, Q., Li, X., and Zhou, C. (2013). A case of lung adenocarcinoma harboring exon 19 EGFR deletion and EML4-ALK fusion gene. Lung cancer (Amsterdam, Neth). 81 (2), 308–310. doi:10.1016/j.lungcan.2013.05.003
Chiari, R., Duranti, S., Ludovini, V., Bellezza, G., Pireddu, A., Minotti, V., et al. (2014). Long-term response to gefitinib and crizotinib in lung adenocarcinoma harboring both epidermal growth factor receptor mutation and EML4-ALK fusion gene. J. Clin. Oncol. official J. Am. Soc. Clin. Oncol. 32 (9), e30–e32. doi:10.1200/JCO.2012.47.7141
He, H., Yang, W., Huang, Y., Zhang, X., Peng, Y., and Yang, Z. (2022). Successful management of lung adenocarcinoma with ALK/EGFR coalterations and PD-L1 overexpression by bevacizumab combined with chemotherapy. Angiogenesis 25 (1), 5–8. doi:10.1007/s10456-021-09811-8
Horn, L., and Pao, W. (2009). EML4-ALK: honing in on a new target in non-small cell lung cancer. J. Clin. Oncol. official J. Am. Soc. Clin. Oncol. 27 (26), 4232–4235. doi:10.1200/JCO.2009.23.6661
Jahanzeb, M., Lin, H., Pan, X., Yin, Y., Wu, Y., Nordstrom, B., et al. (2020). Real-world treatment patterns and progression-free survival associated with anaplastic lymphoma kinase (ALK) tyrosine kinase inhibitor therapies for ALK+ non-small cell lung cancer. Oncol. 25 (10), 867–877. doi:10.1634/theoncologist.2020-0011
Kim, E., Melosky, B., Park, K., Yamamoto, N., and Yang, J. (2021a). EGFR tyrosine kinase inhibitors for EGFR mutation-positive non-small-cell lung cancer: outcomes in asian populations. Future Oncol. Lond. Engl. 17 (18), 2395–2408. doi:10.2217/fon-2021-0195
Kim, E. S., Melosky, B., Park, K., Yamamoto, N., and Yang, J. C. (2021b). EGFR tyrosine kinase inhibitors for EGFR mutation-positive non-small cell lung cancer: outcomes in asian populations. Future Oncol. 17 (18), 2395–2408. doi:10.2217/fon-2021-0195
Kris, M. G., Johnson, B. E., Kwiatkowski, D. J., Iafrate, A. J., Wistuba, , Aronson, S. L., et al. (2011). Identification of driver mutations in tumor specimens from 1,000 patients with lung adenocarcinoma: the NCI’s Lung Cancer Mutation Consortium (LCMC). J. Clin. Oncol. 29. 18_suppl (CRA7506-CRA). doi:10.1200/jco.2011.29.18_suppl.cra7506
Kuo, Y., Wu, S., Ho, C., and Shih, J. (2010). Good response to gefitinib in lung adenocarcinoma harboring coexisting EML4-ALK fusion gene and EGFR mutation. J. Thorac. Oncol. official Publ. Int. Assoc. Study Lung Cancer 5 (12), 2039–2040. doi:10.1097/JTO.0b013e3181f43274
Liang, W., He, Q., Chen, Y., Chuai, S., Yin, W., Wang, W., et al. (2016). Metastatic EML4-ALK fusion detected by circulating DNA genotyping in an EGFR-mutated NSCLC patient and successful management by adding ALK inhibitors: a case report. BMC cancer 16, 62. doi:10.1186/s12885-016-2088-5
Liu, J., Mu, Z., Liu, L., Li, K., Jiang, R., Chen, P., et al. (2019). Frequency, clinical features and differential response to therapy of concurrent ALK/EGFR alterations in Chinese lung cancer patients. Drug Des. Dev. Ther. 13, 1809–1817. doi:10.2147/DDDT.S196189
Lo Russo, G., Imbimbo, M., Corrao, G., Proto, C., Signorelli, D., Vitali, M., et al. (2017). Concomitant EML4-ALK rearrangement and EGFR mutation in non-small cell lung cancer patients: a literature review of 100 cases. Oncotarget 8 (35), 59889–59900. doi:10.18632/oncotarget.17431
Lou, N., Zhang, X., Chen, H., Zhou, Q., Yan, L., Xie, Z., et al. (2016). Clinical outcomes of advanced non-small cell lung cancer patients with EGFR mutation, ALK rearrangement and EGFR/ALK coalterations. Oncotarget 7 (40), 65185–65195. doi:10.18632/oncotarget.11218
Lu, Z., Wang, X., Luo, Y., Wei, J., Zeng, Z., Xiong, Q., et al. (2021). Egfr (p. G719A+L747V)/EML4-ALK coalterations in lung adenocarcinoma with leptomeningeal metastasis responding to Afatinib treatment: a case report. OncoTargets Ther. 14, 2823–2828. doi:10.2147/OTT.S294635
Mao, Y., and Wu, S. (2017). ALK and ROS1 concurrent with EGFR mutation in patients with lung adenocarcinoma. OncoTargets Ther. 10, 3399–3404. doi:10.2147/OTT.S133349
Miyanaga, A., Shimizu, K., Noro, R., Seike, M., Kitamura, K., Kosaihira, S., et al. (2013). Activity of EGFR-tyrosine kinase and ALK inhibitors for EML4-ALK-rearranged non-small cell lung cancer harbored coexisting EGFR mutation. BMC cancer 13, 262. doi:10.1186/1471-2407-13-262
Noronha, V., Chougule, A., Chandrani, P., Kaushal, R., Patil, V., Menon, N., et al. (2022). EGFRLung cancer with dual and driver alterations at baseline: a retrospective observational cohort study. Acta Oncol. Stockh. Swed., 1–5.
Paez, J., Jänne, P., Lee, J., Tracy, S., Greulich, H., Gabriel, S., et al. (2004). EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Sci. (New York, NY) 304 (5676), 1497–1500. doi:10.1126/science.1099314
Popat, S., Vieira de Araújo, A., Min, T., Swansbury, J., Dainton, M., Wotherspoon, A., et al. (2011). Lung adenocarcinoma with concurrent exon 19 EGFR mutation and ALK rearrangement responding to erlotinib. J. Thorac. Oncol. official Publ. Int. Assoc. Study Lung Cancer 6 (11), 1962–1963. doi:10.1097/JTO.0b013e31822eec5e
Santelmo, C., Ravaioli, A., Barzotti, E., Papi, M., Poggi, B., Drudi, F., et al. (2013). Coexistence of EGFR mutation and ALK translocation in NSCLC: literature review and case report of response to gefitinib. Lung cancer (Amsterdam, Neth. 81 (2), 294–296. doi:10.1016/j.lungcan.2013.04.009
Sasaki, T., Koivunen, J., Ogino, A., Yanagita, M., Nikiforow, S., Zheng, W., et al. (2011). A novel ALK secondary mutation and EGFR signaling cause resistance to ALK kinase inhibitors. Cancer Res. 71 (18), 6051–6060. doi:10.1158/0008-5472.CAN-11-1340
Schmid, S., Gautschi, O., Rothschild, S., Mark, M., Froesch, P., Klingbiel, D., et al. (2017). Clinical outcome of ALK-positive non-small cell lung cancer (NSCLC) patients with de novo EGFR or KRAS Co-mutations receiving tyrosine kinase inhibitors (TKIs). J. Thorac. Oncol. official Publ. Int. Assoc. Study Lung Cancer 12 (4), 681–688. doi:10.1016/j.jtho.2016.12.003
Shaw, A., Yeap, B., Mino-Kenudson, M., Digumarthy, S., Costa, D., Heist, R., et al. (2009). Clinical features and outcome of patients with non-small cell lung cancer who harbor EML4-ALK. J. Clin. Oncol. official J. Am. Soc. Clin. Oncol. 27 (26), 4247–4253. doi:10.1200/JCO.2009.22.6993
Shin, H., Kho, B., Kim, M., Park, H., Kim, T., Kim, Y., et al. (2019). Coalteration of EGFR mutation and ALK rearrangement in non-small cell lung cancer: case series. Medicine 98 (9), e14699. doi:10.1097/MD.0000000000014699
Tanaka, H., Hayashi, A., Morimoto, T., Taima, K., Tanaka, Y., Shimada, M., et al. (2012). A case of lung adenocarcinoma harboring EGFR mutation and EML4-ALK fusion gene. BMC cancer 12, 558. doi:10.1186/1471-2407-12-558
Tiseo, M., Gelsomino, F., Boggiani, D., Bortesi, B., Bartolotti, M., Bozzetti, C., et al. (2011). EGFR and EML4-ALK gene mutations in NSCLC: a case report of erlotinib-resistant patient with both concomitant mutations. Lung cancer (Amsterdam, Neth. 71 (2), 241–243. doi:10.1016/j.lungcan.2010.11.014
Ulivi, P., Chiadini, E., Dazzi, C., Dubini, A., Costantini, M., Medri, L., et al. (2016). Nonsquamous, non-small cell lung cancer patients who Carry a Double mutation of EGFR, EML4-ALK or KRAS: frequency, clinical-pathological characteristics, and response to therapy. Clin. lung cancer 17 (5), 384–390. doi:10.1016/j.cllc.2015.11.004
Won, J., Keam, B., Koh, J., Cho, H., Jeon, Y., Kim, T., et al. (2015). Concomitant ALK translocation and EGFR mutation in lung cancer: a comparison of direct sequencing and sensitive assays and the impact on responsiveness to tyrosine kinase inhibitor. Ann. Oncol. official J. Eur. Soc. Med. Oncol. 26 (2), 348–354. doi:10.1093/annonc/mdu530
Xu, C., Cai, X., Shao, Y., Li, Y., Shi, M., Zhang, L., et al. (2015). A case of lung adenocarcinoma with a concurrent EGFR mutation and ALK rearrangement: a case report and literature review. Mol. Med. Rep. 12 (3), 4370–4375. doi:10.3892/mmr.2015.4001
Yang, J., Zhang, X., Su, J., Xu, C., Zhou, Q., Tian, H., et al. (2014). Lung cancers with concomitant EGFR mutations and ALK rearrangements: diverse responses to EGFR-TKI and crizotinib in relation to diverse receptors phosphorylation. Clin. cancer Res. official J. Am. Assoc. Cancer Res. 20 (5), 1383–1392. doi:10.1158/1078-0432.CCR-13-0699
Zhao, N., Zheng, S., Yang, J., Zhang, X., Xie, Z., Xie, B., et al. (2015). Lung adenocarcinoma harboring concomitant EGFR mutation and EML4-ALK fusion that benefits from three kinds of tyrosine kinase inhibitors: a case report and literature review. Clin. lung cancer 16 (2), e5–e9. doi:10.1016/j.cllc.2014.11.001
Zhou, J., Zheng, J., Zhao, J., Sheng, Y., Ding, W., and Zhou, J. (2015). Poor response to gefitinib in lung adenocarcinoma with concomitant epidermal growth factor receptor mutation and anaplastic lymphoma kinase rearrangement. Thorac. cancer 6 (2), 216–219. doi:10.1111/1759-7714.12146
Keywords: ALK rearrangement, EGFR mutation, lung adenocarcinoma, targeted therapy, case report
Citation: Hu H, Tan S, Xie M, Guo P, Yu Q, Xiao J, Zhao K, Liao Q and Wang Y (2023) Case report: Concomitant EGFR mutation and ALK rearrangement in non-small cell lung cancer. Front. Pharmacol. 14:1167959. doi: 10.3389/fphar.2023.1167959
Received: 17 February 2023; Accepted: 01 August 2023;
Published: 29 August 2023.
Edited by:
Francesco Pepe, University of Naples Federico II, ItalyReviewed by:
Songxiao Xu, University of Chinese Academy of Sciences, ChinaAnurag Mehta, Rajiv Gandhi Cancer Institute and Research Centre, India
Copyright © 2023 Hu, Tan, Xie, Guo, Yu, Xiao, Zhao, Liao and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiong Liao, bmljb2xnQDE2My5jb20=; Yi Wang, d2FuZ2xlb0AxMjYuY29t
†These authors have contributed equally to this work and share first authorship
 Haoyue Hu
Haoyue Hu Songtao Tan
Songtao Tan Meng Xie
Meng Xie Peng Guo5
Peng Guo5 Yi Wang
Yi Wang