
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 13 April 2023
Sec. Ethnopharmacology
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1164150
Acute ischemic stroke (AIS) is one of the leading diseases causing death and disability worldwide, and treatment options remain very limited. Traditional Chinese Medicine (TCM) has been used for thousands of years to treat ischemic stroke and has been proven to have significant efficacy, but its mechanism of action is still unclear. As research related to the brain-gut-microbe axis progresses, there is increasing evidence that the gut microbiota plays an important role during AIS. The interaction between TCM and the gut microbiota has been suggested as a possible key link to the therapeutic effects of TCM. We have compiled and reviewed recent studies on the relationship between AIS, TCM, and gut microbiota, with the expectation of providing more ideas to elucidate the mechanism of action of TCM in the treatment of AIS.
Stroke is an acute focal injury to the central nervous system (CNS) caused by vascular causes and is the leading cause of disability and death worldwide (Sacco et al., 2013), with acute ischemic stroke (AIS) accounting for 71% of all strokes (Campbell et al., 2019). Treatment options for AIS are still very limited. Many of the agents shown in preclinical studies to improve neuronal damage do not have significant real-world efficacy (O’Collins et al., 2006). Currently, intravenous thrombolysis and endovascular thrombectomy remain the treatment of choice for AIS, but many patients are unable to access these treatments in a timely manner due to the underlying disease and time of onset (Emberson et al., 2014; Saver et al., 2016). Ischemia/reperfusion (I/R) injury should not be overlooked, as it is commonly seen in patients following endovascular treatment (Shaik et al., 2021). TCM has a long history of treating AIS. Over thousands of years of practice and heritage, hundreds of herbal formulae have been created and applied in the treatment of stroke. Many Chinese herbs, such as Ligusticum chuanxiong, Astragalus membranaceus, Radix Salvia Miltiorrhizae, Carthamus tinctorius L., Angelica sinensis, etc., have been found to be effective in improving the prognosis of AIS (Zhao et al., 2022). Studies in pharmacology have found that many herbs have active effects, such as antioxidative stress, antiapoptotic, anti-inflammatory, proangiogenic, and neuroprotective effects (Zhu et al., 2021a). Therefore, herbal medicine has great potential for the treatment of AIS and deserves to be explored and studied.
Recently, as the concept of the gut-brain-microbe axis (GBMAx) has become clearer (Figure 1), the importance of the gut microbiota and its metabolites in the course of AIS has been gradually revealed (Rhee et al., 2009; Pluta et al., 2021). In short, the disturbance of the gut environment caused by AIS can lead to a disorder of the gut microbiota and its metabolites, and this breakdown in microbiota diversity can in turn further aggravate AIS (Arya and Hu, 2018). The gut microbiota and its metabolites can influence inflammatory damage in the ischemic brain through direct regulation and indirect intervention (Yamashiro et al., 2017; Chen et al., 2019c; Durgan et al., 2019; Huang and Xia, 2021). Disorders of the gut microbiota can also damage the gut mucosal barrier, leading to the spread of harmful toxins and bacteria into the peripheral organs, causing infections and even sepsis (Crapser et al., 2016; Roubalová et al., 2020). Therefore, regulating the gut environment and maintaining the stability of the gut microbiota and metabolites is considered a new therapeutic direction for AIS. Studies have found that TCM plays crucial roles in promoting health and treating disease by interacting with the gut microbiota (Chen et al., 2016). Chinese herbs have a significant impact on the host gut environment in a variety of ways, including inhibiting pathogenic bacteria, promoting probiotics, regulating key flora metabolites, and maintaining the integrity of the intestinal barrier (Zhang et al., 2012; Zhao et al., 2012; Guo et al., 2015; Liu et al., 2018; Nie et al., 2019). Meanwhile, many herbal compounds effective in treating AIS have been found to require a transformation of the gut microbiota before they can exert their active effects (Gong et al., 2020). Increasing evidence indicates that the gut microbiota and its metabolites may be the key to the efficacy of TCM in the treatment of AIS. We have collated and reviewed recent studies on AIS, TCM, and gut microbiota for the purpose of providing more data on the mechanism of action of Chinese herbs in the treatment of AIS.
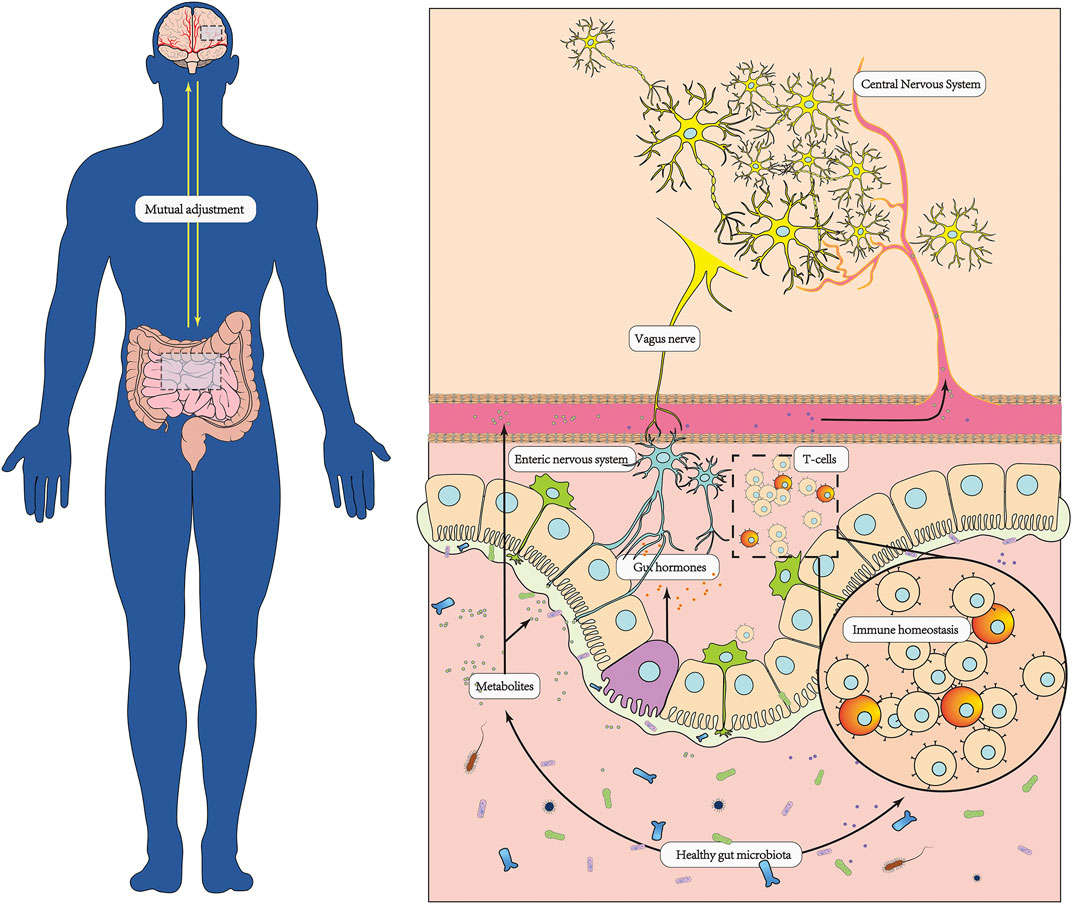
FIGURE 1. In healthy conditions, the brain and the gut microbiota have bidirectional regulation. The brain regulates the function of the gut through multiple neurological, endocrine and immune pathways, providing a stable and healthy environment for the colonization of the gut microbiota. Meanwhile, the gut microbiota plays an important role in facilitating the conversion and absorption of nutrients, maintaining homeostasis of the enteric and organic immune systems, protecting the integrity of the intestinal mucosal barrier, and promoting the development of the nervous system.
Traditional Chinese herbs have the advantages of being multicentric, multitargeted, holistically regulated, and having few side effects in the treatment of AIS (Fan et al., 2020; Li et al., 2022b; Ye et al., 2022) Several meta-analyses and systematic evaluations of proprietary herbal compound preparations have shown significant effects on the degree of neurological deficits, the speed of neurological recovery, and the ability to perform activities of daily living in patients with AIS (Lyu et al., 2020a; 2020b; Liu et al., 2021; Tian et al., 2021; Wang et al., 2022c). Recent pharmacological studies have found that Chinese herbal medicine can treat AIS through several mechanisms, including protecting neurological function, promoting microvascular regeneration, and improving energy metabolism in ischemic cells.
Many herbs have been found to promote neuroprotection and reduce excitotoxicity, oxidative stress, apoptosis, inflammation and autophagy of neurons in ischemic conditions (Zhu et al., 2022). Gastrodin is the main active ingredient of the Chinese herb Gastrodia. Gastrodin can exert antioxidant and anti-inflammatory bioactivities in the treatment of cerebral ischemia and reduce neuroapoptosis in damaged brain tissue by inhibiting the Wnt/β-Catenin pathway (Qiu et al., 2019). Ginsenoside R1 is the most abundant active ingredient in ginseng, and many scholars believe that R1 may have beneficial therapeutic effects as a neuroprotective agent in AIS. Li et al. (2017) showed that ginsenoside Rg1 increased the survival rate of ischemic neural stem cells by reducing oxygen-glucose deprivation (OGD)-mediated oxidative stress and p38/JNK2 phosphorylation. Chu et al. (2019) revealed that R1 inhibits miR-144 activity and activates the Nrf2/ARE pathway to reduce oxidative stress injury in neuronal cells after ischemia‒reperfusion. Zheng et al. (2019) found that R1 attenuates ubiquitinated protein aggregation in ischemia-reperfused brain tissue and reduces the activation of NF-κB and IκBα for neuroprotective effects. Baicalin (BA), the main active component of Scutellaria baicalensis, has been found to have neuroprosthetic effects by stimulating the conversion of astrocytes into reactive astrocytes and secreting more brain-derived neurotrophic factor (BDNF), thereby promoting dendritic growth and branching of nerve cells (Li et al., 2021). Salidroside (Sal) is an active component of Rhodiola rosea L. with anti-inflammatory, antioxidant and anti-apoptotic effects, and recent studies have found that Sal promotes dendritic and synaptic plasticity through the FGF2-mediated cAMP/PKA/CREB pathway (Li et al., 2020b).
Promoting capillary angiogenesis is considered to be an effective way to combat ischemic diseases, and many herbal formulations, with monomeric compounds that have a pro-angiogenic effect, have an ameliorative effect on improving persistent ischemia in brain tissue in acute cerebral infarction (Bu et al., 2020). For example, the famous Chinese herbal formula BUYANGHUANGWU Tang for stroke was found to increase capillary density in brain tissue to improve blood supply to the brain in a rat model of focal cerebral ischemia/reperfusion injury (CI/RI) (Shen et al., 2014; Yang et al., 2015). Angelica Astragali Radix (AR), one of the constituent drugs of Tonic Yang Returning Five Soup, was found to promote angiogenesis in cerebral ischemic rats by activating the p38 MAPK/HIF1α/VEGF-A/vWF signaling pathway in aqueous extract of Angelica (Cheng et al., 2017). Panaxatriol saponins (PTS), an extract of Panax notoginseng, were found to enhance angiogenesis in the ischemic border zone by activating the Shh signaling pathway to upregulate vascular endothelial growth factor (VEGF) and angiopoietin-1 (Ang-1) expression (Hui et al., 2017). Bilobalide B and ginkgolide K were found to activate the Akt/eNOS pathway and JAK2/STAT3 pathway, respectively, to promote angiogenesis in ischemic brain tissue (Chen et al., 2018; Zheng et al., 2018).
Improved intracellular energy metabolism can effectively protect ischemic semidark zone nerve cells. Dan Hong injection (DHI) is a compound Chinese medicine used for the treatment of acute ischemic stroke. Dan Hong injection can improve energy metabolism in ischemic brain cells and reduce nerve damage in the semidark zone by restoring mitochondrial activity and cytoplasmic glycolytic activity (Zeng et al., 2021; Du et al., 2023). Herba leonuri (HL) is an herbal medicine with blood-activating properties and is often used to treat AIS. Leonurine, an alkaloid of HL, was found to reduce mitochondrial swelling in ischemic brain tissue and restore mitochondrial function to some extent (Qi et al., 2010).
It has been found that the gut microbiota plays an important role in facilitating food conversion and absorption, regulating body metabolism, maintaining homeostasis of the immune system, protecting the integrity of the intestinal mucosal barrier, and preventing pathogenic invasion (Clemente et al., 2012; Bunker et al., 2015; Hamilton et al., 2015; Gomaa, 2020). The gut microbiota and its fermentation/degradation products have been directly or indirectly linked to the development of many diseases (Han et al., 2010; Smith, 2015; Nie et al., 2019). Growing evidence indicates a subtle interaction between the gut flora and the injured brain after AIS (Figure 2). Brain damage can lead to gut dysfunction and microbiota disruption (Camilleri, 2021), and the gut microbiota influences the severity of neurological damage by modulating the host’s immune metabolism in reverse (Pluta et al., 2021).
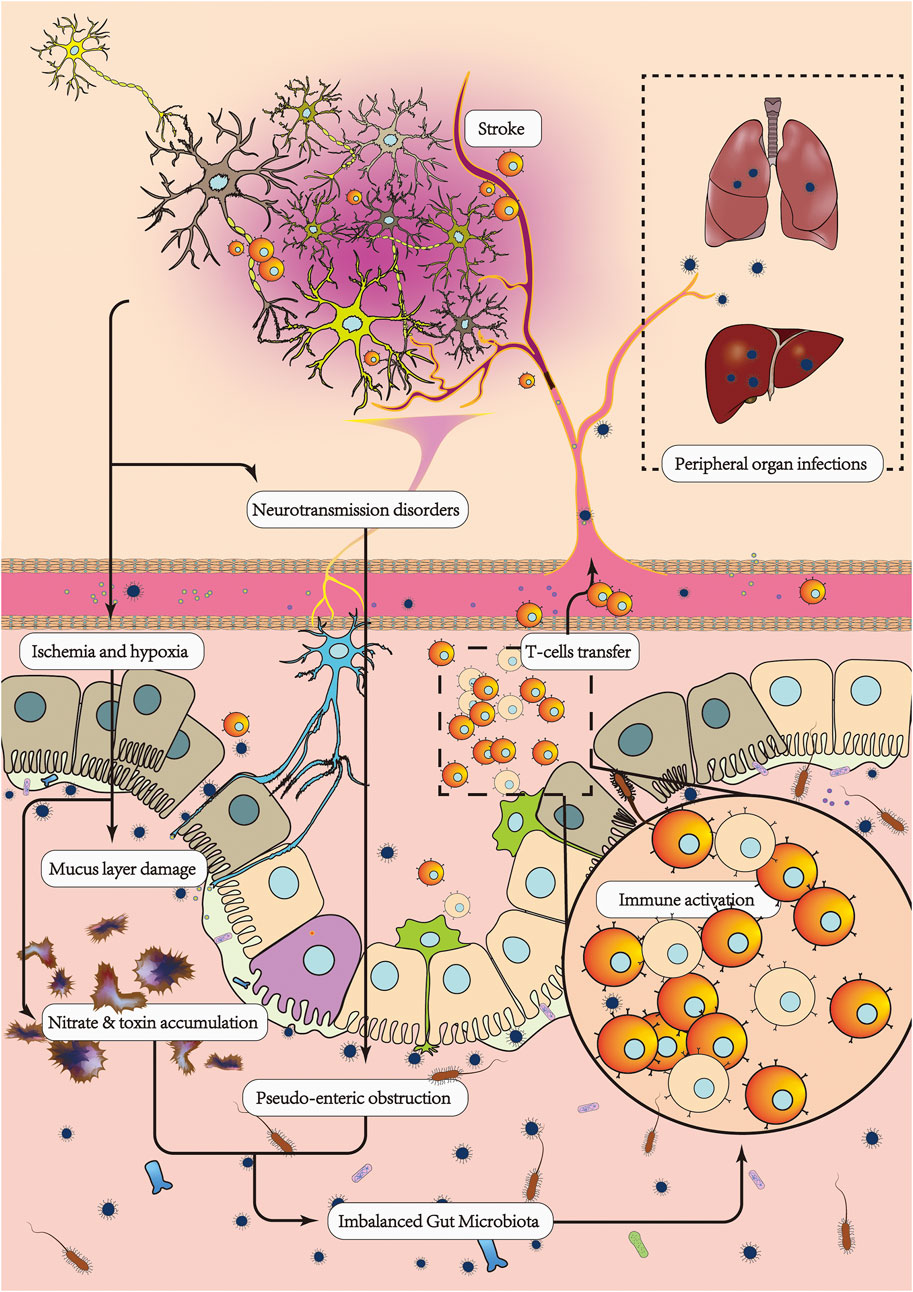
FIGURE 2. After AIS, the brain-gut regulatory mechanism is inhibited, resulting in an imbalance in the intestinal environment, leading to a decrease in probiotic bacteria and an increase in pathogenic bacteria. Enteric nerve conduction disorders cause pseudointestinal obstruction and constipation, which leads to a failure of pathogenic bacteria and harmful substances in the intestinal tract to be excreted in the feces in a timely manner. On the other hand, a disturbed gut microbiota has a serious impact on AIS. Intestinal immune homeostasis is disrupted, the proinflammatory/anti-inflammatory lymphocyte ratio is imbalanced, and more proinflammatory lymphocytes are transferred from the gut to the damaged brain, leading to increased inflammatory damage and apoptosis in the brain. Massive loss of probiotic bacteria and thinning of the intestinal mucus layer leads to increased intestinal barrier permeability, making it easier for pathogenic bacteria to cross the intestinal wall and enter the circulation, causing peripheral organ infections and sepsis. Dysregulated secretion of metabolites from the gut microbiota, such as decreased SCFAs, increased TMAO, increased LPS, increased excitatory neurotransmitters, and decreased inhibitory neurotransmitters, leads to a poorer prognosis for AIS.
Gut microbes are so numerous and able to coevolve with their hosts that their gene count far exceeds that of the host (Sekirov et al., 2010; Loscalzo, 2013). In addition to conventional factors such as diet, age and sex, the gut microbiota is also regulated by the nervous system. Many studies have demonstrated that the brain can regulate gut function and the composition of the gut microbiota through multiple neural, endocrine and immune pathways (Mazmanian et al., 2005; Barugh et al., 2014; Houlden et al., 2016). Therefore, when the brain is injured by AIS, the gut microbiota also suffers a serious impact. It was found that the gut microbiota of stroke patients is clearly different from that of healthy volunteers, with more opportunistic pathogenic bacteria such as Parabacteroides, Oscillospira, Enterobacteriaceae, Megasphaera, Oscillibacter and Desulfovibrio present in the gut of stroke patients compared to healthy individuals whose gut is rich in Prevotella, Roseburia, fecalibacterium, etc., (Yin et al., 2015; Xia et al., 2019). The disruption of the gut microbiota due to AIS may be related to intestinal wall damage and abnormal intestinal function. AIS can cause ischemia and hypoxia in the intestinal wall, leading to a large accumulation of nitrates in the gut, which allows for a decrease in the abundance of beneficial bacteria and an overgrowth of pathogenic bacteria (Xu et al., 2021b). Moreover, abnormalities in intestinal nerve conduction caused by AIS can lead to intestinal disorders such as pseudoenteric obstruction and constipation, which prevent the accumulation of pathogenic bacteria and harmful substances in the intestinal tract from being excreted in time with the feces (Camilleri, 2021). This top-to-bottom (brain-gut-microbiota) disruption was found to persist for up to 12 months after the onset of AIS (Chen et al., 2019c).
Disorders of the gut microbiota have a significant impact on the prognosis of AIS. The accumulation of pathogenic bacteria in the gut can cause an imbalance in intestinal immunity and increased differentiation of pro-inflammatory lymphocytes, with the result that more pro-inflammatory lymphocytes are transferred to the brain and increase inflammatory damage to brain tissue and apoptosis of nerve cells. Without the protection of probiotics, the intestinal barrier becomes weaker, and pathogenic bacteria have a higher chance of crossing the intestinal barrier into the internal environment and causing infections and sepsis. Metabolic imbalances caused by disorders of the gut microbiota are also not to be overlooked, and gut flora metabolism/degradation products can affect the extent of brain damage in several ways.
Studies have found that disorders of the gut microbiota can lead to more proinflammatory T lymphocytes being transferred from the gut to the brain after AIS and exacerbate brain tissue inflammation. Inflammation is the main cause of ongoing brain damage after AIS (Tuttolomondo et al., 2009; Jin et al., 2010; Sorby-Adams et al., 2017), and the transfer of intestinal T lymphocytes to the brain is thought to be a key link in the induction of inflammation in brain tissue (Gelderblom et al., 2009; Liesz et al., 2009; Shichita et al., 2009; Kleinschnitz et al., 2010; Meng et al., 2019; Brea et al., 2021). The gut microbiota is a powerful regulator of the intestinal immune environment, and different species of flora can regulate the differentiation of a variety of T lymphocytes through direct or indirect contact with intestinal dendritic cells (Mazmanian et al., 2005). TH1, TH17 and γδ T cells can exacerbate inflammatory brain injury through the release of proinflammatory factors such as IL-17, TNF-α, γ interferon, perforin, and neutrophil aggregation factor cxcl1 (Shichita et al., 2009; Liesz et al., 2011; Gelderblom et al., 2012). In contrast, Treg cells can attenuate brain injury by suppressing immune responses, which may be associated with the release of IL-10 and inhibition of the differentiation of proinflammatory lymphocytes such as intestinal γδT cells (Liesz et al., 2015; Benakis et al., 2016). Disruption of intestinal immune homeostasis due to disorders of the gut microbiota and upregulation of pathogenic bacteria abundance leads to increased differentiation of pro-inflammatory T lymphocytes and decreased numbers of Treg cells (Benakis et al., 2016), which leads to more pro-inflammatory T lymphocytes transferring to injured brain tissue and exacerbating inflammatory damage (Feng et al., 2019b). Therefore, maintaining a stable gut microbiota is important in reducing inflammatory damage to brain tissue after AIS.
Disorders of the gut microbiota can exacerbate the damage to the intestinal barrier caused by AIS, making it easier for pathogenic bacteria that have accumulated in the gut to cross the intestinal barrier and spread to peripheral organs, leading to infection and sepsis, which is an important cause of increased mortality from AIS (Crapser et al., 2016). A clinical sentinel study found that disorders of the gut microbiota in stroke patients were an independent risk factor for the development of pneumonia (Xia et al., 2021). Studies on stroke animals have also demonstrated that the bacteria causing pneumonia in MACO mice do indeed originate from the gut (Stanley et al., 2016). The integrity of the intestinal barrier function depends on the tight attachment of mucus proteins to the intestinal epithelium (Maria-Ferreira et al., 2018). It has been found that many probiotics are guarantors of the integrity of the intestinal barrier, such as A. muciniphila, a commensal bacterium of the intestinal mucus layer that degrades mucin for energy and forms a protective intestinal barrier (Paone and Cani, 2020), and Lactobacillus, which can prevent pathogenic bacteria from invading the intestinal wall by adhering to and colonizing the intestinal mucosa to increase intestinal epithelial tight junctions (Liu et al., 2011). Disorders of the gut microbiota result in the loss of probiotic bacteria, reduced secretion of intestinal tight junction proteins, decreased mucus levels, and increased intestinal permeability, which makes it easier for pathogenic bacteria to cross the barrier and disseminate to peripheral vital organs (Zhang et al., 2021). Therefore, maintaining a stable gut microbiota after AIS is important to protect the integrity of the intestinal barrier and reduce the incidence of poststroke infections.
Abnormal metabolite expression due to disorders of the gut microbiota has a significant impact on the prognosis of AIS. Short-chain fatty acids (SCFAs) are organic fatty acids produced by the breakdown of leftover nutrients in the gut microbiota and are considered to be key signaling molecules in the communication between the gut and the brain (Smith and Macfarlane, 1997; Garcia et al., 2008; Kotani et al., 2009; O’Mahony et al., 2015; Yissachar et al., 2017; Jameson and Hsiao, 2018). Lower levels of SCFAs in the feces of AIS patients compared to healthy groups are thought to be related to the loss of SCFA-producing bacteria in the gut (Li et al., 2019; Tan et al., 2021). SCFAs can promote neurological repair by modulating peripheral lymphocytes to influence brain microglial phenotypic differentiation (Sadler et al., 2020). A study found that reduced intestinal acetic acid may lead to a higher risk of 90-day malfunction in AIS patients (Tan et al., 2021). Preclinical studies have found that butyrate supplementation could improve the degree of brain edema and reduce the area of brain damage in MCAO rats (Chen et al., 2019b), which may be related to butyrate activating PI3K/Akt via GPR41/Gbc and attenuating neuronal apoptosis (Zhou et al., 2021). Trimethylamine N-oxide (TMAO) has been thought to be a gut microbial metabolite closely related to AIS (Méjean et al., 1994; Craciun and Balskus, 2012; Koeth et al., 2014; Fu et al., 2020). TMAO could be used as a biological risk predictor for stroke (Koeth et al., 2013; Tang et al., 2013; Zhu et al., 2016; Xu et al., 2021a). Meanwhile, elevated TMAO levels often indicate poor clinical outcomes in patients with AIS (Zhai et al., 2019; Tan et al., 2020). Elevated serum TMAO concentrations in MCAO rats lead to enlarged cerebral infarcts and increased neurological deficits (Zhu et al., 2021b). TMAO mediates the activation of multiple inflammatory signaling pathways, such as nuclear factor-Kappa B (NF-κβ), NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) inflammasomes, and MAPK/JNK in the brain and periphery, which may aggravate AIS (Praveenraj et al., 2022). Gut microbiota can remotely influence central nervous function by synthesizing neurotransmitters (Benakis et al., 2020). After AIS, gut microbiota disorder can lead to abnormalities in gut neurotransmitter metabolism, as evidenced by upregulated expression of excitatory neurotransmitters (e.g., Glu, ASP) and decreased expression of inhibitory neurotransmitters (e.g., GABA), resulting in neuro excitotoxic injury, and this brain injury would be reduced by correcting the neurotransmitter disorder (Gao et al., 2013; Bhuvanendran et al., 2019; Guo et al., 2021b). Lipopolysaccharide (LPS) is an immunogenic endotoxin from the microbiota that can promote neuroinflammation by direct mechanisms and/or by inducing the migration of peripheral immune cells to the brain (Lukiw et al., 2018). Polysaccharide A (PSA) is one of the metabolites of Bacteroides fragilis and has immunosuppressive effects on inflammation (Dasgupta et al., 2014; Wang et al., 2014; Johnson et al., 2015). Therefore, influencing metabolite expression by modulating the gut microbiota may be an effective way to improve AIS prognosis.
In recent years, there has been increasing evidence that the gut microbiota may play a key role in the treatment of AIS with TCM (Figure 3) (Feng et al., 2019a; Lin et al., 2021; Song et al., 2021; Tang et al., 2022). AIS can cause disruption of the gut microbiota, which is an important cause of poor prognosis in AIS, and Traditional Chinese herbs can correct this microbiota disorder. It was found that the use of Chinese herbal preparations (both formulae and compounds) could restore the diversity of the gut microbiota to varying degrees, increase the abundance of probiotic bacteria, reduce the abundance of conditionally pathogenic bacteria, and bring the gut microbiota closer to that of healthy populations after AIS(Cai et al., 2019; Zhang et al., 2020; Fu et al., 2021; Lin et al., 2021; Wang et al., 2022b; Cheng et al., 2022; Xu et al., 2022). To investigate how TCM regulates gut microbiota and how it exerts therapeutic effects, we searched for recently published studies on the regulation of gut microbiota by TCM in the treatment of AIS, including Chinese herbal formulae (Table 1) and herbal compounds (Table 2). In this way, we hope to elucidate the mechanism of action of TCM in treating AIS by modulating the gut microbiota and to provide scientific data for further research.
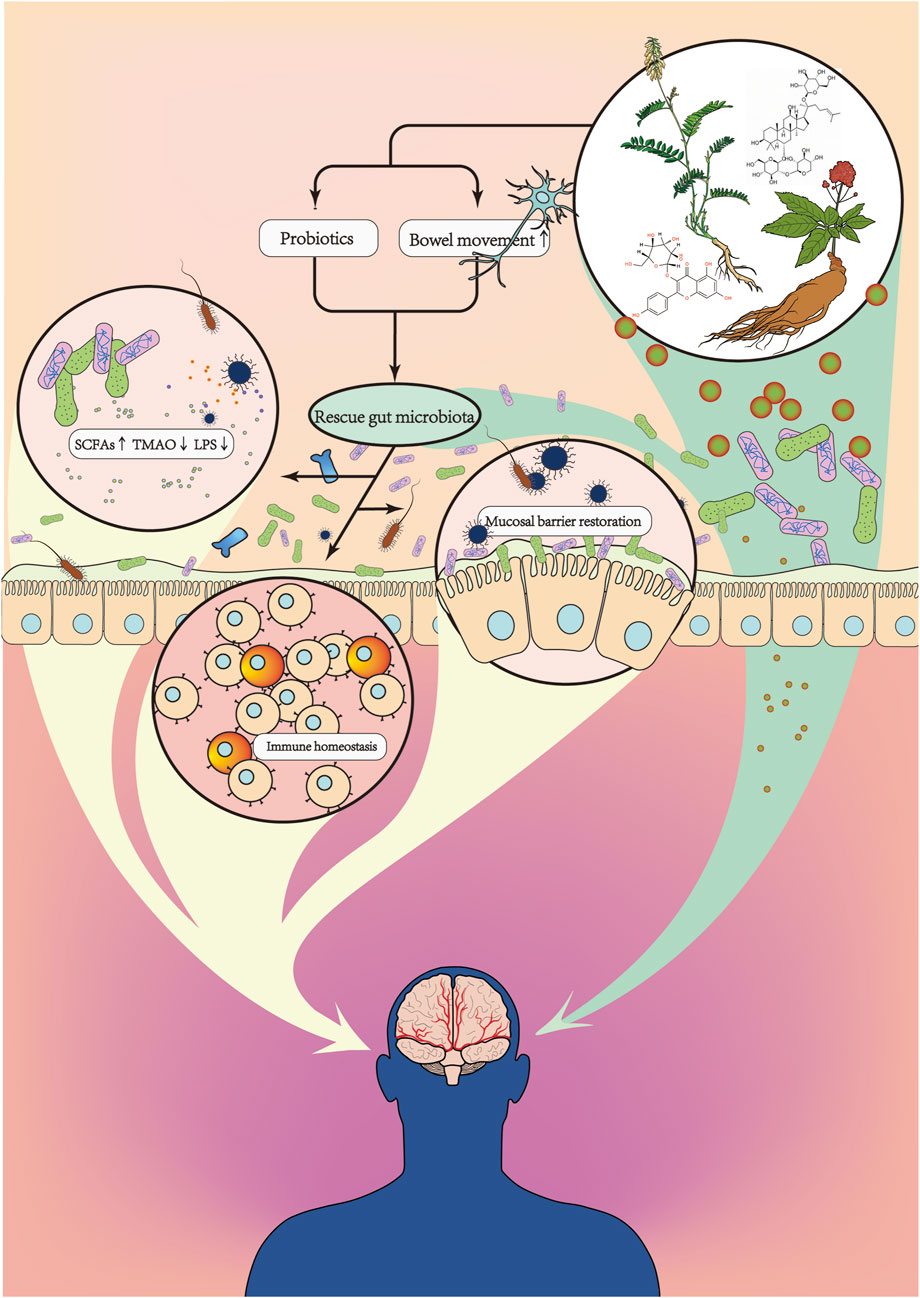
FIGURE 3. The interaction of traditional Chinese medicine with gut microbiota may be one of the mechanisms responsible for its efficacy against AIS. Chinese herbal medicines (including formulae and compounds) can restore gut microbiota diversity to varying degrees, bringing the intestinal flora closer to a healthy population after AIS. At the same time, traditional Chinese medicine can upregulate certain enteric neurotransmitters, increase intestinal motility, and promote the excretion of harmful substances. By correcting gut microbiota disorder, TCM influences intestinal microbial metabolite levels, improves the intestine’s inflammatory environment, attenuates neurohypoptosis, protects the integrity of the intestinal barrier, and reduces the escape of harmful bacteria. Meanwhile, many herbal compounds can be transformed by the action of the gut microbiota into structures with higher activity and bioavailability, which can then be absorbed by the intestine to exert therapeutic effects.
Traditional Chinese herbs regulate the diversity of the flora through direct contact with the gut microbiota. Oral administration of herbal tonics is the traditional method of administration in TCM. Interestingly, this creates enough time and space for the herbal ingredients to interact with the gut microbiota (Zhao et al., 2012). It has been found that there are hundreds of components in herbs, such as alkaloids, polysaccharides, glycosides, tannins, and enzymes (Zhu et al., 2021a). Most of these components are not directly absorbed in the gut but become metabolic substrates for intestinal bacteria (Feng et al., 2019a). Similar to prebiotics, these herbal ingredients have the ability to inhibit the multiplication of pathogenic bacteria, promote the growth of probiotics, and regulate microbial metabolites in the gut (Zhang et al., 2012; Zhao et al., 2012; Guo et al., 2015; Huang et al., 2016; Liu et al., 2018; Nie et al., 2019; Habtemariam, 2020; Wang et al., 2022a). Chinese herbs maintain a healthy gut environment by promoting bowel movement and stimulating bowel movements. For example, Xinglou Chengqi Decoction (XCD) and Tanhuo decoction (THD) are both effective in improving gut dysfunction caused by AIS, reducing symptoms such as bad breath, thick tongue, bloating, dry stools or poor bowel movements (Chen et al., 2020; Wang et al., 2021; Guo et al., 2022). The gut motility-promoting effect of Chinese herbs may be related to their stimulation of the release of intestinal neurotransmitters. Anthraquinone glycosides, one of the main active ingredients of the Chinese medicine rhubarb, are barely absorbed into the intestinal tract as a prototype. Anthraquinone glycosides have been found to improve the imbalance of intestinal neurotransmitter release and catabolism caused by disorder of gut microbiota, increase the levels of 5-HT and 5-HIAA in the gut, and thus promote intestinal motility (Guo et al., 2021b).
By regulating the gut microbiota, Chinese herbs have been found to influence the metabolic levels of intestinal flora metabolites, improve the inflammatory environment in the gut, reduce neuronal apoptosis, and protect the integrity of the intestinal barrier.
Reduced metabolic levels of SCFAs, particularly acetate and butyric acid, showed a positive correlation with the severity of AIS (Chen et al., 2019b; Tan et al., 2021). Several studies have found that Chinese herbs can upregulate SCFA levels to improve the prognosis of AIS by correcting disorders of the gut microbiota. THD makes SCFA-producing flora such as Blautia, Coprococcus, Streptococcus, Dorea, Streptococcus, Bifidobacterium, and Lactobacillus more accessible to healthy people (Guo et al., 2022). XCD can upregulate Verrucomicrobia and Akkermansia abundance to increase the levels of enteric butyric acid (Gao et al., 2021). The PLR&CXR combination increases the abundance of Oscillospira, Lachnospiraceae, and Akkermansia to increase butyrate release (Chen et al., 2019a). In a study of herbal compounds, researchers found that supplementation with puerarin, astragaloside IV, and berberine (BBR) significantly replenished the intestinal flora producing SCFAs and upregulated the relative levels of acetic acid and butyric acid (Wu et al., 2020b; Wu et al., 2020a; Gong et al., 2021). On the other hand, plasma TMAO concentrations were positively associated with the incidence of adverse clinical outcomes in AIS (Zhai et al., 2019; Tan et al., 2020). BBR was found to downregulate the abundance of Bilophila in the gut and reduce the expression of TMAO-related enzymes to decrease the level of TMAO (Wu et al., 2020a). Enema with an aqueous extract of rhubarb inhibits the abundance of pathogens such as Lachnospiraceae and Romboutsia and reduces serum TMAO and trimethylamine (TMA) levels in rats (Ji et al., 2021). Ginkgolide B (GB) inhibits the mRNA and protein expression of FMO3 and reduces the concentrations of TMA and TMAO (Lv et al., 2021). Based on quantitative analysis of predicted genes, THD was found to reduce the biosynthetic genes of trimethylamine (TMA), a precursor of trimethylamine N-oxide (TMAO), and to enhance trimethylamine-nucleotide protein comethyltransferase (mttB)-related genes, which breakdown TMA into methane (Guo et al., 2021a).
The intestinal immune environment has an important role in regulating inflammatory damage to brain tissue after AIS. Increasingly, studies have shown that by regulating gut microbiota, Chinese herbs can effectively improve the intestinal immune environment and reduce brain and intestinal inflammatory responses. At the cellular level, the HQ-HH combination reduces the number of TH17 cells in the gut, increases the number of Treg cells, and reduces inflammation in the gut and brain tissue (Wang et al., 2022b). Tongqiao Huoxue decoction (TQHXD) reduces the number of γδ T cells in the gut and brain, increases the number of Treg cells and attenuates AIS-induced gut-brain inflammatory injury (Zhang et al., 2020). BBR can regulate the Treg/Th17 balance by improving the gut microbiota (Cui et al., 2018). At the molecular level, XCD promotes the release of anti-inflammatory factors such as IL-10 while downregulating proinflammatory factors such as TNF-α, IL-17A and IL-22 (Gao et al., 2021). Qishiwei Zhenzhu pills (QSW) can regulate the relative abundance of Firmicutes and Proteobacteria in the gut, downregulate p38-MAPK and TNF-α and inhibit inflammatory factors such as IL-6 and IL-1β (Fu et al., 2021). Salvia polysaccharides act directly on the gut microbiota to restore the balance between the expression of enteric proinflammatory factors (IL-6 and IL23) and anti-inflammatory factors (IL2, IL10 and TGF-β) (Li et al., 2022a). Lipopolysaccharide (LPS) has been found to promote neuroinflammation in the brain through direct and indirect pathways (Lukiw et al., 2018). THD significantly reduces the number of LPS-producing intestinal flora, such as Mycobacterium avium, in the gut, thereby reducing the level of LPS biosynthesis (Guo et al., 2022). It was found that the reduction in AIS-induced neuronal apoptosis by Chinese herbs may be related to the regulation of the gut microbiota. AS-IV reverses the disruption of the gut microbiota caused by AIS and downregulates the expression of the autophagic markers Beclin-1, Atg12, and LC3 II, which is thought to be one of the mechanisms by which AS-IV protects against ischemic brain apoptosis (Xu et al., 2018).
An intact gut barrier is essential to reduce peripheral organ infections caused by the spread of enteric pathogens after AIS (Crapser et al., 2016). Studies have shown that many Chinese herbal medicines, including herbal formulae and compounds, have a reparative effect on intestinal microvillus damage, which enhances the integrity of the intestinal barrier and reduces pathogenic bacterial transfer and endotoxin leakage (Peng et al., 2013; Zhang et al., 2020; Fu et al., 2021; Li et al., 2022a). The protective effect of Chinese herbs on the intestinal barrier is thought to be related to the regulation of key probiotics such as A. muciniphila and Lactobacillus (Liu et al., 2011; Paone and Cani, 2020). Oral administration of Korean red ginseng (KRG) restores gut microbial diversity and increases the level of Lactobacillus (Lee et al., 2022). BBR was found to promote A. muciniphila reproduction by stimulating intestinal mucus protein secretion, which forms a dense protective intestinal layer (Dong et al., 2021), and puerarin has been found to have the same effect (Wang et al., 2019). In addition, the tight junctions of intestinal cells are the most important mechanical barriers that maintain the structure, function, and permeability of the gut (Hohman and Osborne, 2022). It was found that Chinese herbs could increase the protein levels of enteric claudins, occludin, and ZO-1 by regulating gut microbiota to contribute to the stability of the intestinal barrier (Chen et al., 2019a; Hu et al., 2022; Wang et al., 2022b; Yan et al., 2022). Bile acids play an important role in maintaining the stability of the gut microbiota and the balance of the immune system of the intestinal mucosa (Sun et al., 2021). It was found that HQ-HH could affect the expression of FXR, a bile acid regulator in the small intestine, by regulating gut microbiota to reduce enteric bile acid levels, thereby reducing bile acid-mediated gut immune injury and hence barrier homeostasis disruption (Goodwin et al., 2000; Wang et al., 2022b).
It has been found that the gut microbiota interacts with Chinese herbs and that many herbal compounds need to be transformed into more active and bioavailable molecular structures with the cooperation of gut microbiota (Gong et al., 2020; Qu et al., 2021; Zhang et al., 2022). The rich and numerous microbiota secrete a wide range of enzymes, which may have a significant impact on the stability of the molecular structure of herbal compounds (Wang et al., 2011). For example, anthraquinone aglycones, a poorly absorbed ingredient of rhubarb, can be converted into anthraquinone condensates (anthraquinone aglycones) by the gut microbiota, which will be easily absorbed and exert an anti-ischemic effect (Li et al., 2020a). BBR can be broken down by the gut microbiota into DhBBR and enter the intestinal tissues, where DhBBR will be reduced again to BBR and enter the circulation, and this microbiota-dependent absorption process can significantly improve the bioavailability of BBR (Wang et al. 2022a). Ginsenosides Rb1, Rb2, and Rc can be converted by the gut microbiota to the more biologically active compound K [20-O-beta-D-glucopyranosyl-20(S)-propanediol] (Qi et al., 2011). Fermented ginsenosides were found to have higher anti-inflammatory effects, reducing the LPS-induced increase in the pro-inflammatory cytokines IL-6, TNF-α, and IL-1β and alleviating the intestinal inflammatory response through the TLR4/MAPK signaling pathway (Fan et al., 2021). The malic acid in Pueraria lobata can be fermented to lactic acid by Bifidobacterium breve, which may contribute to the enrichment of certain microorganisms with anti-inflammatory properties, such as Lactococcus and Ruminococcus (Choi et al., 2020). In the presence of gut microbiota, tanshinones and salvianolic acids can undergo metabolic transformations such as methylation, dehydrogenation, and glucuronidation, resulting in higher biological activity and absorption (Cai et al., 2019).
The gut microbiota has an important impact on AIS prognosis, while the microbiota’s significance to gut health should not be overlooked. One study found that depletion of gut microbes by antibiotics alleviated brain injury in MCAO mice; however, the mice that lost their gut microbes invariably developed severe acute colitis after discontinuation of antibiotics (Winek et al., 2016). Traditional Chinese herbs can increase the relative abundance of beneficial flora and decrease the relative abundance of pathogenic flora in the gut, bringing the gut microbiota of AIS patients closer to that of healthy populations. This helps the gut microbiota to play a greater role in positively regulating AIS rather than aggravating it. The study of both Chinese herbal formulae and herbal compounds has its own special significance. For example, herbal compounds can better reveal the mechanisms of interaction between TCM and the gut microbiota, while in the study of herbal formulae, we can see a wider range of synergistic regulatory mechanisms and applications (Ma et al., 2021). Therefore, combining the two seems to be a future direction for continued research into the regulation of gut microbiota by TCM for the treatment of AIS. On the other hand, we found some differences between the available reports, especially for the diversity of gut microbiota, which may be related to differences in host species, feeding environment, and diet. We should consider a potential reason that the dominant microbial species in different segments of the gut are different in the same individual.
Most notably, the study of the regulatory effects of Chinese herbal medicine on the gut microbiota could shed more light on the principles of TCM theory. For example, the same treatment for different diseases is a very important and classical theory in TCM. In short, when different kinds of diseases have the same pattern, they can be grouped together as the same Chinese medicine syndrome and treated with the same therapeutic methods (Zhai et al., 2020). Looking back at stroke, hemorrhagic stroke and ischemic stroke are completely different diseases, but they will be treated in the same way under the guidance of TCM theory (Chang et al., 2016). To investigate the reasons, apart from the currently known antioxidative stress, antiapoptotic, anti-inflammatory, and antiangiogenic effects (Zhu et al., 2021a; Duan et al., 2022), the regulatory effect of Chinese herbs on the gut microbiota may be a potential mechanism. It has been found that both ischemic and hemorrhagic strokes lead to intestinal dysfunction and dysbiosis of the gut microbiota, which can further aggravate neurological injury and induce serious complications (Xu et al., 2021b; Zhang et al., 2021). Traditional Chinese herbs can improve the prognosis of stroke by correcting disorders of the gut microbiota, regulating the expression of microbial metabolites, maintaining intestinal immune homeostasis, and repairing gut barrier damage. Therefore, an in-depth study of the interaction between gut microbiota and Chinese herbs can be a complement to the “same treatment for different diseases” theory of TCM.
In summary, TCM has the advantages of multipathway, multitarget, and synergistic treatment for AIS. Exploring the interaction between Traditional Chinese herbs and gut microbiota is a novel idea that is important for elucidating the mechanism of Traditional Chinese herbs in treating AIS. Meanwhile, this study can help bring the clinical value of TCM into play to a greater extent and has broad research potential.
XZ, YZ, and LG contributed to the conception and design of the study. YS and XX organized the database. LG wrote the first draft of the manuscript. XX, HZ, WJ, and LG wrote sections of the manuscript. All authors contributed to the manuscript revision, read, and approved the submitted version.
YZ and XZ was supported by the Sichuan Provincial Administration of Traditional Chinese Medicine (grant numbers 2021MS106 and 2020ZD003).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Arya, A. K., and Hu, B. (2018). Brain-gut axis after stroke. Brain Circ. 4, 165–173. doi:10.4103/bc.bc_32_18
Barugh, A. J., Gray, P., Shenkin, S. D., MacLullich, A. M. J., and Mead, G. E. (2014). Cortisol levels and the severity and outcomes of acute stroke: A systematic review. J. Neurol. 261, 533–545. doi:10.1007/s00415-013-7231-5
Benakis, C., Brea, D., Caballero, S., Faraco, G., Moore, J., Murphy, M., et al. (2016). Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nat. Med. 22, 516–523. doi:10.1038/nm.4068
Benakis, C., Martin-Gallausiaux, C., Trezzi, J.-P., Melton, P., Liesz, A., and Wilmes, P. (2020). The microbiome-gut-brain axis in acute and chronic brain diseases. Curr. Opin. Neurobiol. 61, 1–9. doi:10.1016/j.conb.2019.11.009
Bhuvanendran, S., Bakar, S. N. S., Kumari, Y., Othman, I., Shaikh, M. F., and Hassan, Z. (2019). Embelin improves the spatial memory and hippocampal long-term potentiation in a rat model of chronic cerebral hypoperfusion. Sci. Rep. 9, 14507. doi:10.1038/s41598-019-50954-y
Brea, D., Poon, C., Benakis, C., Lubitz, G., Murphy, M., Iadecola, C., et al. (2021). Stroke affects intestinal immune cell trafficking to the central nervous system. Brain. Behav. Immun. 96, 295–302. doi:10.1016/j.bbi.2021.05.008
Bu, L., Dai, O., Zhou, F., Liu, F., Chen, J.-F., Peng, C., et al. (2020). Traditional Chinese medicine formulas, extracts, and compounds promote angiogenesis. Biomed. Pharmacother. 132, 110855. doi:10.1016/j.biopha.2020.110855
Bunker, J. J., Flynn, T. M., Koval, J. C., Shaw, D. G., Meisel, M., McDonald, B. D., et al. (2015). Innate and adaptive humoral responses coat distinct commensal bacteria with immunoglobulin A. Immunity 43, 541–553. doi:10.1016/j.immuni.2015.08.007
Cai, H., Su, S., Li, Y., Zhu, Z., Guo, J., Zhu, Y., et al. (2019). Danshen can interact with intestinal bacteria from normal and chronic renal failure rats. Biomed. Pharmacother. 109, 1758–1771. doi:10.1016/j.biopha.2018.11.047
Camilleri, M. (2021). Gastrointestinal motility disorders in neurologic disease. J. Clin. Invest. 131, 143771. doi:10.1172/JCI143771
Campbell, B. C. V., De Silva, D. A., Macleod, M. R., Coutts, S. B., Schwamm, L. H., Davis, S. M., et al. (2019). Ischaemic stroke. Nat. Rev. Dis. Primer 5, 70. doi:10.1038/s41572-019-0118-8
Chang, C.-C., Lee, Y.-C., Lin, C.-C., Chang, C.-H., Chiu, C.-D., Chou, L.-W., et al. (2016). Characteristics of traditional Chinese medicine usage in patients with stroke in taiwan: A nationwide population-based study. J. Ethnopharmacol. 186, 311–321. doi:10.1016/j.jep.2016.04.018
Chen, F., Wen, Q., Jiang, J., Li, H.-L., Tan, Y.-F., Li, Y.-H., et al. (2016). Could the gut microbiota reconcile the oral bioavailability conundrum of traditional herbs? J. Ethnopharmacol. 179, 253–264. doi:10.1016/j.jep.2015.12.031
Chen, M., Zou, W., Chen, M., Cao, L., Ding, J., Xiao, W., et al. (2018). Ginkgolide K promotes angiogenesis in a middle cerebral artery occlusion mouse model via activating JAK2/STAT3 pathway. Eur. J. Pharmacol. 833, 221–229. doi:10.1016/j.ejphar.2018.06.012
Chen, R., Wu, P., Cai, Z., Fang, Y., Zhou, H., Lasanajak, Y., et al. (2019a). Puerariae Lobatae Radix with chuanxiong Rhizoma for treatment of cerebral ischemic stroke by remodeling gut microbiota to regulate the brain-gut barriers. J. Nutr. Biochem. 65, 101–114. doi:10.1016/j.jnutbio.2018.12.004
Chen, R., Xu, Y., Wu, P., Zhou, H., Lasanajak, Y., Fang, Y., et al. (2019b). Transplantation of fecal microbiota rich in short chain fatty acids and butyric acid treat cerebral ischemic stroke by regulating gut microbiota. Pharmacol. Res. 148, 104403. doi:10.1016/j.phrs.2019.104403
Chen, Y., Liang, J., Ouyang, F., Chen, X., Lu, T., Jiang, Z., et al. (2019). Persistence of gut microbiota dysbiosis and chronic systemic inflammation after cerebral infarction in cynomolgus monkeys. Front. Neurol. 10, 661. doi:10.3389/fneur.2019.00661
Chen, P., Jiang, L., Geng, H., Han, X., Xu, Y., Chen, Y., et al. (2020). Effectiveness of Xinglou Chengqi decoction on constipation in patients with acute ischemic stroke: A randomized controlled trial. J. Tradit. Chin. Med. Chung Tsa Chih Ying Wen Pan 40, 112–120.
Cheng, C.-Y., Ho, T.-Y., Hsiang, C.-Y., Tang, N.-Y., Hsieh, C.-L., Kao, S.-T., et al. (2017). Angelica sinensis exerts angiogenic and anti-apoptotic effects against cerebral ischemia-reperfusion injury by activating p38MAPK/HIF-1[Formula: See text]/VEGF-A signaling in rats. Am. J. Chin. Med. 45, 1683–1708. doi:10.1142/S0192415X17500914
Cheng, H., Liu, J., Zhang, D., Wang, J., Tan, Y., Feng, W., et al. (2022). Ginsenoside Rg1 alleviates acute ulcerative colitis by modulating gut microbiota and microbial tryptophan metabolism. Front. Immunol. 13, 817600. doi:10.3389/fimmu.2022.817600
Choi, Y., Bose, S., Shin, N. R., Song, E.-J., Nam, Y.-D., and Kim, H. (2020). Lactate-fortified puerariae Radix fermented by Bifidobacterium breve improved diet-induced metabolic dysregulation via alteration of gut microbial communities. Nutrients 12, E276. doi:10.3390/nu12020276
Chu, S.-F., Zhang, Z., Zhou, X., He, W.-B., Chen, C., Luo, P., et al. (2019). Ginsenoside Rg1 protects against ischemic/reperfusion-induced neuronal injury through miR-144/Nrf2/ARE pathway. Acta Pharmacol. Sin. 40, 13–25. doi:10.1038/s41401-018-0154-z
Clemente, J. C., Ursell, L. K., Parfrey, L. W., and Knight, R. (2012). The impact of the gut microbiota on human health: An integrative view. Cell 148, 1258–1270. doi:10.1016/j.cell.2012.01.035
Craciun, S., and Balskus, E. P. (2012). Microbial conversion of choline to trimethylamine requires a glycyl radical enzyme. Proc. Natl. Acad. Sci. U. S. A. 109, 21307–21312. doi:10.1073/pnas.1215689109
Crapser, J., Ritzel, R., Verma, R., Venna, V. R., Liu, F., Chauhan, A., et al. (2016). Ischemic stroke induces gut permeability and enhances bacterial translocation leading to sepsis in aged mice. Aging 8, 1049–1063. doi:10.18632/aging.100952
Cui, H., Cai, Y., Wang, L., Jia, B., Li, J., Zhao, S., et al. (2018). Berberine regulates Treg/Th17 balance to treat ulcerative colitis through modulating the gut microbiota in the colon. Front. Pharmacol. 9, 571. doi:10.3389/fphar.2018.00571
Dasgupta, S., Erturk-Hasdemir, D., Ochoa-Reparaz, J., Reinecker, H.-C., and Kasper, D. L. (2014). Plasmacytoid dendritic cells mediate anti-inflammatory responses to a gut commensal molecule via both innate and adaptive mechanisms. Cell Host Microbe 15, 413–423. doi:10.1016/j.chom.2014.03.006
Dong, C., Yu, J., Yang, Y., Zhang, F., Su, W., Fan, Q., et al. (2021). Berberine, a potential prebiotic to indirectly promote Akkermansia growth through stimulating gut mucin secretion. Biomed. Pharmacother. 139, 111595. doi:10.1016/j.biopha.2021.111595
Du, H., He, Y., Zhu, J., Zhou, H., Shao, C., Yang, J., et al. (2023). Danhong injection alleviates cerebral ischemia-reperfusion injury by inhibiting mitochondria-dependent apoptosis pathway and improving mitochondrial function in hyperlipidemia rats. Biomed. Pharmacother. 157, 114075. doi:10.1016/j.biopha.2022.114075
Duan, T., Li, L., Yu, Y., Li, T., Han, R., Sun, X., et al. (2022). Traditional Chinese medicine use in the pathophysiological processes of intracerebral hemorrhage and comparison with conventional therapy. Pharmacol. Res. 179, 106200. doi:10.1016/j.phrs.2022.106200
Durgan, D. J., Lee, J., McCullough, L. D., and Bryan, R. M. (2019). Examining the role of the microbiota-gut-brain Axis in stroke. Stroke 50, 2270–2277. doi:10.1161/STROKEAHA.119.025140
Emberson, J., Lees, K. R., Lyden, P., Blackwell, L., Albers, G., Bluhmki, E., et al. (2014). Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: A meta-analysis of individual patient data from randomised trials. Lancet lond. Engl. 384, 1929–1935. doi:10.1016/S0140-6736(14)60584-5
Fan, F., Yang, L., Li, R., Zou, X., Li, N., Meng, X., et al. (2020). Salidroside as a potential neuroprotective agent for ischemic stroke: A review of sources, pharmacokinetics, mechanism and safety. Biomed. Pharmacother. 129, 110458. doi:10.1016/j.biopha.2020.110458
Fan, J., Liu, S., Ai, Z., Chen, Y., Wang, Y., Li, Y., et al. (2021). Fermented ginseng attenuates lipopolysaccharide-induced inflammatory responses by activating the TLR4/MAPK signaling pathway and remediating gut barrier. Food Funct. 12, 852–861. doi:10.1039/d0fo02404j
Feng, W., Ao, H., Peng, C., and Yan, D. (2019). Gut microbiota, a new frontier to understand traditional Chinese medicines. Pharmacol. Res. 142, 176–191. doi:10.1016/j.phrs.2019.02.024
Feng, Y., He, X., Luo, S., Chen, X., Long, S., Liang, F., et al. (2019). Chronic colitis induces meninges traffic of gut-derived T cells, unbalances M1 and M2 microglia/macrophage and increases ischemic brain injury in mice. Brain Res. 1707, 8–17. doi:10.1016/j.brainres.2018.11.019
Fu, B. C., Hullar, M. A. J., Randolph, T. W., Franke, A. A., Monroe, K. R., Cheng, I., et al. (2020). Associations of plasma trimethylamine N-oxide, choline, carnitine, and betaine with inflammatory and cardiometabolic risk biomarkers and the fecal microbiome in the Multiethnic Cohort Adiposity Phenotype Study. Am. J. Clin. Nutr. 111, 1226–1234. doi:10.1093/ajcn/nqaa015
Fu, K., Zhang, D., Song, Y., Xu, M., Wu, R., Xiong, X., et al. (2021). Tibetan medicine qishiwei zhenzhu pills can reduce cerebral ischemia-reperfusion injury by regulating gut microbiota and inhibiting inflammation. Evid.-Based Complement. Altern. Med. ECAM 2021, 2251679. doi:10.1155/2021/2251679
Gao, J., Yang, H., Chen, J., Fang, J., Chen, C., Liang, R., et al. (2013). Analysis of serum metabolites for the discovery of amino acid biomarkers and the effect of galangin on cerebral ischemia. Mol. Biosyst. 9, 2311–2321. doi:10.1039/c3mb70040b
Gao, Q., Han, Z.-Y., Tian, D.-F., Liu, G.-L., Wang, Z.-Y., Lin, J.-F., et al. (2021). Xinglou Chengqi Decoction improves neurological function in experimental stroke mice as evidenced by gut microbiota analysis and network pharmacology. Chin. J. Nat. Med. 19, 881–899. doi:10.1016/S1875-5364(21)60079-1
Garcia, A., Olmo, B., Lopez-Gonzalvez, A., Cornejo, L., Rupérez, F. J., and Barbas, C. (2008). Capillary electrophoresis for short chain organic acids in faeces Reference values in a Mediterranean elderly population. J. Pharm. Biomed. Anal. 46, 356–361. doi:10.1016/j.jpba.2007.10.026
Gelderblom, M., Leypoldt, F., Steinbach, K., Behrens, D., Choe, C.-U., Siler, D. A., et al. (2009). Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke 40, 1849–1857. doi:10.1161/STROKEAHA.108.534503
Gelderblom, M., Weymar, A., Bernreuther, C., Velden, J., Arunachalam, P., Steinbach, K., et al. (2012). Neutralization of the IL-17 axis diminishes neutrophil invasion and protects from ischemic stroke. Blood 120, 3793–3802. doi:10.1182/blood-2012-02-412726
Gomaa, E. Z. (2020). Human gut microbiota/microbiome in health and diseases: A review. Ant. Van Leeuwenhoek 113, 2019–2040. doi:10.1007/s10482-020-01474-7
Gong, X., Li, X., Bo, A., Shi, R.-Y., Li, Q.-Y., Lei, L.-J., et al. (2020). The interactions between gut microbiota and bioactive ingredients of traditional Chinese medicines: A review. Pharmacol. Res. 157, 104824. doi:10.1016/j.phrs.2020.104824
Gong, P., Xiao, X., Wang, S., Shi, F., Liu, N., Chen, X., et al. (2021). Hypoglycemic effect of astragaloside IV via modulating gut microbiota and regulating AMPK/SIRT1 and PI3K/AKT pathway. J. Ethnopharmacol. 281, 114558. doi:10.1016/j.jep.2021.114558
Goodwin, B., Jones, S. A., Price, R. R., Watson, M. A., McKee, D. D., Moore, L. B., et al. (2000). A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol. Cell 6, 517–526. doi:10.1016/s1097-2765(00)00051-4
Guo, M., Ding, S., Zhao, C., Gu, X., He, X., Huang, K., et al. (2015). Red Ginseng and Semen Coicis can improve the structure of gut microbiota and relieve the symptoms of ulcerative colitis. J. Ethnopharmacol. 162, 7–13. doi:10.1016/j.jep.2014.12.029
Guo, Q., Jiang, X., Ni, C., Li, L., Chen, L., Wang, Y., et al. (2021a). Gut microbiota-related effects of Tanhuo decoction in acute ischemic stroke. Oxid. Med. Cell. Longev. 2021, 5596924. doi:10.1155/2021/5596924
Guo, Y., Li, Q., Yu, X., and Liang, Y. (2021b). Rhubarb anthraquinone glycosides protect against cerebral ischemia-reperfusion injury in rats by regulating brain-gut neurotransmitters. Biomed. Chromatogr. BMC 35, e5058. doi:10.1002/bmc.5058
Guo, Q., Ni, C., Li, L., Li, M., Jiang, X., Gao, L., et al. (2022). Integrated traditional Chinese medicine improves functional outcome in acute ischemic stroke: From clinic to mechanism exploration with gut microbiota. Front. Cell. Infect. Microbiol. 12, 827129. doi:10.3389/fcimb.2022.827129
Habtemariam, S. (2020). Berberine pharmacology and the gut microbiota: A hidden therapeutic link. Pharmacol. Res. 155, 104722. doi:10.1016/j.phrs.2020.104722
Hamilton, M. K., Boudry, G., Lemay, D. G., and Raybould, H. E. (2015). Changes in intestinal barrier function and gut microbiota in high-fat diet-fed rats are dynamic and region dependent. Am. J. Physiol. Gastrointest. Liver Physiol. 308, G840–G851. doi:10.1152/ajpgi.00029.2015
Han, J., Antunes, L. C. M., Finlay, B. B., and Borchers, C. H. (2010). Metabolomics: Towards understanding host-microbe interactions. Future Microbiol. 5, 153–161. doi:10.2217/fmb.09.132
Hohman, L. S., and Osborne, L. C. (2022). A gut-centric view of aging: Do intestinal epithelial cells contribute to age-associated microbiota changes, inflammaging, and immunosenescence? Aging Cell 21, e13700. doi:10.1111/acel.13700
Houlden, A., Goldrick, M., Brough, D., Vizi, E. S., Lénárt, N., Martinecz, B., et al. (2016). Brain injury induces specific changes in the caecal microbiota of mice via altered autonomic activity and mucoprotein production. Brain. Behav. Immun. 57, 10–20. doi:10.1016/j.bbi.2016.04.003
Hu, Q., Wu, C., Yu, J., Luo, J., and Peng, X. (2022). Angelica sinensis polysaccharide improves rheumatoid arthritis by modifying the expression of intestinal Cldn5, Slit3 and Rgs18 through gut microbiota. Int. J. Biol. Macromol. 209, 153–161. doi:10.1016/j.ijbiomac.2022.03.090
Huang, Q., and Xia, J. (2021). Influence of the gut microbiome on inflammatory and immune response after stroke. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 42, 4937–4951. doi:10.1007/s10072-021-05603-6
Huang, J., Chen, L., Xue, B., Liu, Q., Ou, S., Wang, Y., et al. (2016). Different flavonoids can shape unique gut microbiota profile in vitro. J. Food Sci. 81, H2273–H2279. doi:10.1111/1750-3841.13411
Hui, Z., Sha, D.-J., Wang, S.-L., Li, C.-S., Qian, J., Wang, J.-Q., et al. (2017). Panaxatriol saponins promotes angiogenesis and enhances cerebral perfusion after ischemic stroke in rats. BMC Complement. Altern. Med. 17, 70. doi:10.1186/s12906-017-1579-5
Jameson, K. G., and Hsiao, E. Y. (2018). Linking the gut microbiota to a brain neurotransmitter. Trends Neurosci. 41, 413–414. doi:10.1016/j.tins.2018.04.001
Ji, C., Li, Y., Mo, Y., Lu, Z., Lu, F., Lin, Q., et al. (2021). Rhubarb enema decreases circulating trimethylamine N-oxide level and improves renal fibrosis accompanied with gut microbiota change in chronic kidney disease rats. Front. Pharmacol. 12, 780924. doi:10.3389/fphar.2021.780924
Jin, R., Yang, G., and Li, G. (2010). Inflammatory mechanisms in ischemic stroke: Role of inflammatory cells. J. Leukoc. Biol. 87, 779–789. doi:10.1189/jlb.1109766
Johnson, J. L., Jones, M. B., and Cobb, B. A. (2015). Polysaccharide A from the capsule of Bacteroides fragilis induces clonal CD4+ T cell expansion. J. Biol. Chem. 290, 5007–5014. doi:10.1074/jbc.M114.621771
Kleinschnitz, C., Schwab, N., Kraft, P., Hagedorn, I., Dreykluft, A., Schwarz, T., et al. (2010). Early detrimental T-cell effects in experimental cerebral ischemia are neither related to adaptive immunity nor thrombus formation. Blood 115, 3835–3842. doi:10.1182/blood-2009-10-249078
Koeth, R. A., Wang, Z., Levison, B. S., Buffa, J. A., Org, E., Sheehy, B. T., et al. (2013). Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 19, 576–585. doi:10.1038/nm.3145
Koeth, R. A., Levison, B. S., Culley, M. K., Buffa, J. A., Wang, Z., Gregory, J. C., et al. (2014). γ-Butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of L-carnitine to TMAO. Cell Metab. 20, 799–812. doi:10.1016/j.cmet.2014.10.006
Kotani, A., Miyaguchi, Y., Kohama, M., Ohtsuka, T., Shiratori, T., and Kusu, F. (2009). Determination of short-chain fatty acids in rat and human feces by high-performance liquid chromatography with electrochemical detection. Anal. Sci. Int. J. Jpn. Soc. Anal. Chem. 25, 1007–1011. doi:10.2116/analsci.25.1007
Lee, M., Lee, S.-H., Kim, M.-S., Ahn, K.-S., and Kim, M. (2022). Effect of Lactobacillus dominance modified by Korean Red Ginseng on the improvement of Alzheimer’s disease in mice. J. Ginseng Res. 46, 464–472. doi:10.1016/j.jgr.2021.11.001
Li, Y., Suo, L., Liu, Y., Li, H., and Xue, W. (2017). Protective effects of ginsenoside Rg1 against oxygen-glucose-deprivation-induced apoptosis in neural stem cells. J. Neurol. Sci. 373, 107–112. doi:10.1016/j.jns.2016.12.036
Li, N., Wang, X., Sun, C., Wu, X., Lu, M., Si, Y., et al. (2019). Change of intestinal microbiota in cerebral ischemic stroke patients. BMC Microbiol. 19, 191. doi:10.1186/s12866-019-1552-1
Li, Q., Guo, Y., Yu, X., Liu, W., and Zhou, L. (2020). Protective mechanism of rhubarb anthraquinone glycosides in rats with cerebral ischaemia-reperfusion injury: Interactions between medicine and intestinal flora. Chin. Med. 15, 60. doi:10.1186/s13020-020-00341-x
Li, S., Lu, Y., Ding, D., Ma, Z., Xing, X., Hua, X., et al. (2020). Fibroblast growth factor 2 contributes to the effect of salidroside on dendritic and synaptic plasticity after cerebral ischemia/reperfusion injury. Aging 12, 10951–10968. doi:10.18632/aging.103308
Li, C., Sui, C., Wang, W., Yan, J., Deng, N., Du, X., et al. (2021). Baicalin attenuates oxygen-glucose deprivation/reoxygenation-induced injury by modulating the BDNF-TrkB/PI3K/Akt and MAPK/Erk1/2 signaling axes in neuron-astrocyte cocultures. Front. Pharmacol. 12, 599543. doi:10.3389/fphar.2021.599543
Li, L., Lan, X., Peng, X., Shi, S., Zhao, Y., Liu, W., et al. (2022). Polysaccharide from Salviae miltiorrhizae Radix et Rhizoma Attenuates the Progress of Obesity-Induced Non-Alcoholic Fatty Liver Disease through Modulating Intestinal Microbiota-Related Gut-Liver Axis. Int. J. Mol. Sci. 23, 10620. doi:10.3390/ijms231810620
Li, X.-H., Yin, F.-T., Zhou, X.-H., Zhang, A.-H., Sun, H., Yan, G.-L., et al. (2022). The signaling pathways and targets of natural compounds from traditional Chinese medicine in treating ischemic stroke. Mol. Basel Switz. 27, 3099. doi:10.3390/molecules27103099
Liesz, A., Suri-Payer, E., Veltkamp, C., Doerr, H., Sommer, C., Rivest, S., et al. (2009). Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat. Med. 15, 192–199. doi:10.1038/nm.1927
Liesz, A., Zhou, W., Mracskó, É., Karcher, S., Bauer, H., Schwarting, S., et al. (2011). Inhibition of lymphocyte trafficking shields the brain against deleterious neuroinflammation after stroke. Brain J. Neurol. 134, 704–720. doi:10.1093/brain/awr008
Liesz, A., Hu, X., Kleinschnitz, C., and Offner, H. (2015). Functional role of regulatory lymphocytes in stroke: Facts and controversies. Stroke 46, 1422–1430. doi:10.1161/STROKEAHA.114.008608
Lin, H., Chen, S., Shen, L., Hu, T., Cai, J., Zhan, S., et al. (2021). Integrated analysis of the cecal microbiome and plasma metabolomics to explore NaoMaiTong and its potential role in changing the intestinal flora and their metabolites in ischemic stroke. Front. Pharmacol. 12, 773722. doi:10.3389/fphar.2021.773722
Liu, Z., Ma, Y., and Qin, H. (2011). Potential prevention and treatment of intestinal barrier dysfunction using active components of Lactobacillus. Ann. Surg. 254, 832–833; author reply 833. doi:10.1097/SLA.0b013e318235dd56
Liu, D., Zhang, Y., Liu, Y., Hou, L., Li, S., Tian, H., et al. (2018). Berberine modulates gut microbiota and reduces insulin resistance via the TLR4 signaling pathway. Exp. Clin. Endocrinol. Diabetes Off. J. Ger. Soc. Endocrinol. Ger. Diabetes Assoc. 126, 513–520. doi:10.1055/s-0043-125066
Liu, M., Pu, Y., Gu, J., He, Q., Liu, Y., Zeng, Y., et al. (2021). Evaluation of zhilong huoxue tongyu capsule in the treatment of acute cerebral infarction: A systematic review and meta-analysis of randomized controlled trials. Phytomed. Int. J. Phytother. Phytopharm. 86, 153566. doi:10.1016/j.phymed.2021.153566
Liu, Y., Cai, C., and Qin, X. (2022). Regulation of gut microbiota of Astragali Radix in treating for chronic atrophic gastritis rats based on metabolomics coupled with 16S rRNA gene sequencing. Chem. Biol. Interact. 365, 110063. doi:10.1016/j.cbi.2022.110063
Loscalzo, J. (2013). Gut microbiota, the genome, and diet in atherogenesis. N. Engl. J. Med. 368, 1647–1649. doi:10.1056/NEJMe1302154
Lukiw, W. J., Cong, L., Jaber, V., and Zhao, Y. (2018). Microbiome-derived lipopolysaccharide (LPS) selectively inhibits neurofilament light chain (NF-L) gene expression in human neuronal-glial (HNG) cells in primary culture. Front. Neurosci. 12, 896. doi:10.3389/fnins.2018.00896
Lv, Z., Shan, X., Tu, Q., Wang, J., Chen, J., and Yang, Y. (2021). Ginkgolide B treatment regulated intestinal flora to improve high-fat diet induced atherosclerosis in ApoE-/- mice. Biomed. Pharmacother. 134, 111100. doi:10.1016/j.biopha.2020.111100
Lyu, J., Xie, Y., Sun, M., and Zhang, L. (2020a). Clinical evidence and GRADE assessment for breviscapine injection (DengZhanHuaSu) in patients with acute cerebral infarction. J. Ethnopharmacol. 262, 113137. doi:10.1016/j.jep.2020.113137
Lyu, J., Xie, Y., Sun, M., and Zhang, L. (2020b). Efficacy and safety of xueshuantong injection on acute cerebral infarction: Clinical evidence and GRADE assessment. Front. Pharmacol. 11, 822. doi:10.3389/fphar.2020.00822
Ma, P., Peng, Y., Zhao, L., Liu, F., and Li, X. (2021). Differential effect of polysaccharide and nonpolysaccharide components in Sijunzi decoction on spleen deficiency syndrome and their mechanisms. Phytomed. Int. J. Phytother. Phytopharm. 93, 153790. doi:10.1016/j.phymed.2021.153790
Maria-Ferreira, D., Nascimento, A. M., Cipriani, T. R., Santana-Filho, A. P., Watanabe, P. da S., Sant Ana, D. de M. G., et al. (2018). Rhamnogalacturonan, a chemically-defined polysaccharide, improves intestinal barrier function in DSS-induced colitis in mice and human Caco-2 cells. Sci. Rep. 8, 12261. doi:10.1038/s41598-018-30526-2
Mazmanian, S. K., Liu, C. H., Tzianabos, A. O., and Kasper, D. L. (2005). An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 122, 107–118. doi:10.1016/j.cell.2005.05.007
Méjean, V., Iobbi-Nivol, C., Lepelletier, M., Giordano, G., Chippaux, M., and Pascal, M. C. (1994). TMAO anaerobic respiration in Escherichia coli: Involvement of the tor operon. Mol. Microbiol. 11, 1169–1179. doi:10.1111/j.1365-2958.1994.tb00393.x
Meng, H., Zhao, H., Cao, X., Hao, J., Zhang, H., Liu, Y., et al. (2019). Double-negative T cells remarkably promote neuroinflammation after ischemic stroke. Proc. Natl. Acad. Sci. U. S. A. 116, 5558–5563. doi:10.1073/pnas.1814394116
Nie, Q., Chen, H., Hu, J., Fan, S., and Nie, S. (2019). Dietary compounds and traditional Chinese medicine ameliorate type 2 diabetes by modulating gut microbiota. Crit. Rev. Food Sci. Nutr. 59, 848–863. doi:10.1080/10408398.2018.1536646
O’Collins, V. E., Macleod, M. R., Donnan, G. A., Horky, L. L., van der Worp, B. H., and Howells, D. W. (2006). 1,026 experimental treatments in acute stroke. Ann. Neurol. 59, 467–477. doi:10.1002/ana.20741
O’Mahony, S. M., Clarke, G., Borre, Y. E., Dinan, T. G., and Cryan, J. F. (2015). Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 277, 32–48. doi:10.1016/j.bbr.2014.07.027
Paone, P., and Cani, P. D. (2020). Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 69, 2232–2243. doi:10.1136/gutjnl-2020-322260
Peng, J.-H., Cui, T., Huang, F., Chen, L., Zhao, Y., Xu, L., et al. (2013). Puerarin ameliorates experimental alcoholic liver injury by inhibition of endotoxin gut leakage, Kupffer cell activation, and endotoxin receptors expression. J. Pharmacol. Exp. Ther. 344, 646–654. doi:10.1124/jpet.112.201137
Pluta, R., Januszewski, S., and Czuczwar, S. J. (2021). The role of gut microbiota in an ischemic stroke. Int. J. Mol. Sci. 22, E915. doi:10.3390/ijms22020915
Praveenraj, S. S., Sonali, S., Anand, N., Tousif, H. A., Vichitra, C., Kalyan, M., et al. (2022). The role of a gut microbial-derived metabolite, trimethylamine N-oxide (TMAO), in neurological disorders. Mol. Neurobiol. 59, 6684–6700. doi:10.1007/s12035-022-02990-5
Qi, J., Hong, Z. Y., Xin, H., and Zhu, Y. Z. (2010). Neuroprotective effects of leonurine on ischemia/reperfusion-induced mitochondrial dysfunctions in rat cerebral cortex. Biol. Pharm. Bull. 33, 1958–1964. doi:10.1248/bpb.33.1958
Qi, L.-W., Wang, C.-Z., Du, G.-J., Zhang, Z.-Y., Calway, T., and Yuan, C.-S. (2011). Metabolism of ginseng and its interactions with drugs. Curr. Drug Metab. 12, 818–822. doi:10.2174/138920011797470128
Qiu, C.-W., Liu, Z.-Y., Zhang, F.-L., Zhang, L., Li, F., Liu, S.-Y., et al. (2019). Post-stroke gastrodin treatment ameliorates ischemic injury and increases neurogenesis and restores the Wnt/β-Catenin signaling in focal cerebral ischemia in mice. Brain Res. 1712, 7–15. doi:10.1016/j.brainres.2019.01.043
Qu, Q., Yang, F., Zhao, C., Liu, X., Yang, P., Li, Z., et al. (2021). Effects of fermented ginseng on the gut microbiota and immunity of rats with antibiotic-associated diarrhea. J. Ethnopharmacol. 267, 113594. doi:10.1016/j.jep.2020.113594
Rhee, S. H., Pothoulakis, C., and Mayer, E. A. (2009). Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat. Rev. Gastroenterol. Hepatol. 6, 306–314. doi:10.1038/nrgastro.2009.35
Roubalová, R., Procházková, P., Papežová, H., Smitka, K., Bilej, M., and Tlaskalová-Hogenová, H. (2020). Anorexia nervosa: Gut microbiota-immune-brain interactions. Clin. Nutr. Edinb. Scotl. 39, 676–684. doi:10.1016/j.clnu.2019.03.023
Sacco, R. L., Kasner, S. E., Broderick, J. P., Caplan, L. R., Connors, J. J. B., Culebras, A., et al. (2013). An updated definition of stroke for the 21st century: A statement for healthcare professionals from the American heart association/American stroke association. Stroke 44, 2064–2089. doi:10.1161/STR.0b013e318296aeca
Sadler, R., Cramer, J. V., Heindl, S., Kostidis, S., Betz, D., Zuurbier, K. R., et al. (2020). Short-chain fatty acids improve poststroke recovery via immunological mechanisms. J. Neurosci. Off. J. Soc. Neurosci. 40, 1162–1173. doi:10.1523/JNEUROSCI.1359-19.2019
Saver, J. L., Goyal, M., van der Lugt, A., Menon, B. K., Majoie, C. B. L. M., Dippel, D. W., et al. (2016). Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: A meta-analysis. JAMA 316, 1279–1288. doi:10.1001/jama.2016.13647
Sekirov, I., Russell, S. L., Antunes, L. C. M., and Finlay, B. B. (2010). Gut microbiota in health and disease. Physiol. Rev. 90, 859–904. doi:10.1152/physrev.00045.2009
Shaik, N. F., Regan, R. F., and Naik, U. P. (2021). Platelets as drivers of ischemia/reperfusion injury after stroke. Blood Adv. 5, 1576–1584. doi:10.1182/bloodadvances.2020002888
Shen, J., Zhu, Y., Yu, H., Fan, Z., Xiao, F., Wu, P., et al. (2014). Buyang Huanwu decoction increases angiopoietin-1 expression and promotes angiogenesis and functional outcome after focal cerebral ischemia. J. Zhejiang Univ. Sci. B 15, 272–280. doi:10.1631/jzus.B1300166
Shichita, T., Sugiyama, Y., Ooboshi, H., Sugimori, H., Nakagawa, R., Takada, I., et al. (2009). Pivotal role of cerebral interleukin-17-producing gammadeltaT cells in the delayed phase of ischemic brain injury. Nat. Med. 15, 946–950. doi:10.1038/nm.1999
Smith, E. A., and Macfarlane, G. T. (1997). Dissimilatory amino Acid metabolism in human colonic bacteria. Anaerobe 3, 327–337. doi:10.1006/anae.1997.0121
Smith, P. A. (2015). The tantalizing links between gut microbes and the brain. Nature 526, 312–314. doi:10.1038/526312a
Song, J., Chen, X., Lyu, Y., Zhuang, W., Zhang, J., Gao, L., et al. (2019). Sanhuang Xiexin decoction promotes good functional outcome in acute ischemic stroke. Brain Behav. 9, e01185. doi:10.1002/brb3.1185
Song, Y., Fu, K., Zhang, D., Xu, M., Wu, R., Xiong, X., et al. (2021). The absorption, distribution, and excretion of 18 elements of Tibetan medicine qishiwei zhenzhu pills in rats with cerebral ischemia. Evid.-Based Complement. Altern. Med. ECAM 2021, 4508533. doi:10.1155/2021/4508533
Sorby-Adams, A. J., Marcoionni, A. M., Dempsey, E. R., Woenig, J. A., and Turner, R. J. (2017). The role of neurogenic inflammation in blood-brain barrier disruption and development of cerebral oedema following acute central nervous system (CNS) injury. Int. J. Mol. Sci. 18, E1788. doi:10.3390/ijms18081788
Stanley, D., Mason, L. J., Mackin, K. E., Srikhanta, Y. N., Lyras, D., Prakash, M. D., et al. (2016). Translocation and dissemination of commensal bacteria in post-stroke infection. Nat. Med. 22, 1277–1284. doi:10.1038/nm.4194
Sun, R., Xu, C., Feng, B., Gao, X., and Liu, Z. (2021). Critical roles of bile acids in regulating intestinal mucosal immune responses. Ther. Adv. Gastroenterol. 14, 17562848211018098. doi:10.1177/17562848211018098
Tan, C., Wang, H., Gao, X., Xu, R., Zeng, X., Cui, Z., et al. (2020). Dynamic changes and prognostic value of gut microbiota-dependent trimethylamine-N-oxide in acute ischemic stroke. Front. Neurol. 11, 29. doi:10.3389/fneur.2020.00029
Tan, C., Wu, Q., Wang, H., Gao, X., Xu, R., Cui, Z., et al. (2021). Dysbiosis of gut microbiota and short-chain fatty acids in acute ischemic stroke and the subsequent risk for poor functional outcomes. JPEN J. Parenter. Enter. Nutr. 45, 518–529. doi:10.1002/jpen.1861
Tang, W. H. W., Wang, Z., Levison, B. S., Koeth, R. A., Britt, E. B., Fu, X., et al. (2013). Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 368, 1575–1584. doi:10.1056/NEJMoa1109400
Tang, R., Yi, J., Lu, S., Chen, B., and Liu, B. (2022). Therapeutic effect of buyang huanwu decoction on the gut microbiota and hippocampal metabolism in a rat model of cerebral ischemia. Front. Cell. Infect. Microbiol. 12, 873096. doi:10.3389/fcimb.2022.873096
Tian, Z.-Y., Feng, L.-D., Xie, Y., Xu, D.-H., Zhang, C.-Y., Kong, L.-B., et al. (2021). Chinese herbal medicine xingnaojing injection for acute ischemic stroke: An overview of systematic reviews and meta-analyses. Front. Pharmacol. 12, 659408. doi:10.3389/fphar.2021.659408
Tuttolomondo, A., Di Sciacca, R., Di Raimondo, D., Renda, C., Pinto, A., and Licata, G. (2009). Inflammation as a therapeutic target in acute ischemic stroke treatment. Curr. Top. Med. Chem. 9, 1240–1260. doi:10.2174/156802609789869619
Wang, H.-Y., Qi, L.-W., Wang, C.-Z., and Li, P. (2011). Bioactivity enhancement of herbal supplements by intestinal microbiota focusing on ginsenosides. Am. J. Chin. Med. 39, 1103–1115. doi:10.1142/S0192415X11009433
Wang, Y., Begum-Haque, S., Telesford, K. M., Ochoa-Repáraz, J., Christy, M., Kasper, E. J., et al. (2014). A commensal bacterial product elicits and modulates migratory capacity of CD39(+) CD4 T regulatory subsets in the suppression of neuroinflammation. Gut Microbes 5, 552–561. doi:10.4161/gmic.29797
Wang, L., Wu, Y., Zhuang, L., Chen, X., Min, H., Song, S., et al. (2019). Puerarin prevents high-fat diet-induced obesity by enriching Akkermansia muciniphila in the gut microbiota of mice. PloS One 14, e0218490. doi:10.1371/journal.pone.0218490
Wang, P., Wang, S., Chen, H., Deng, X., Zhang, L., Xu, H., et al. (2021). TCMIP v2.0 powers the identification of chemical constituents available in Xinglou Chengqi decoction and the exploration of pharmacological mechanisms acting on stroke complicated with tanre fushi syndrome. Front. Pharmacol. 12, 598200. doi:10.3389/fphar.2021.598200
Wang, H., Zhang, H., Gao, Z., Zhang, Q., and Gu, C. (2022). The mechanism of berberine alleviating metabolic disorder based on gut microbiome. Front. Cell. Infect. Microbiol. 12, 854885. doi:10.3389/fcimb.2022.854885
Wang, K., Chen, Y., Cao, J., Liang, R., Qiao, Y., Ding, L., et al. (2022). Mechanism of Huangqi-Honghua combination regulating the gut microbiota to affect bile acid metabolism towards preventing cerebral ischaemia-reperfusion injury in rats. Pharm. Biol. 60, 2189–2199. doi:10.1080/13880209.2022.2136209
Wang, L., Fan, X., Chen, Y., Liang, X., Shen, W., and Zhang, Y. (2022). Efficacy and safety of xingnaojing injection for emergency treatment of acute ischemic stroke: A systematic review and meta-analysis. Front. Pharmacol. 13, 839305. doi:10.3389/fphar.2022.839305
Winek, K., Engel, O., Koduah, P., Heimesaat, M. M., Fischer, A., Bereswill, S., et al. (2016). Depletion of cultivatable gut microbiota by broad-spectrum antibiotic pretreatment worsens outcome after murine stroke. Stroke 47, 1354–1363. doi:10.1161/STROKEAHA.115.011800
Wu, M., Yang, S., Wang, S., Cao, Y., Zhao, R., Li, X., et al. (2020). Effect of berberine on atherosclerosis and gut microbiota modulation and their correlation in high-fat diet-fed ApoE-/- mice. Front. Pharmacol. 11, 223. doi:10.3389/fphar.2020.00223
Wu, Y., Li, Y., Ruan, Z., Li, J., Zhang, L., Lu, H., et al. (2020). Puerarin rebuilding the mucus layer and regulating mucin-utilizing bacteria to relieve ulcerative colitis. J. Agric. Food Chem. 68, 11402–11411. doi:10.1021/acs.jafc.0c04119
Xia, G.-H., You, C., Gao, X.-X., Zeng, X.-L., Zhu, J.-J., Xu, K.-Y., et al. (2019). Stroke dysbiosis index (SDI) in gut microbiome are associated with brain injury and prognosis of stroke. Front. Neurol. 10, 397. doi:10.3389/fneur.2019.00397
Xia, G.-H., Zhang, M.-S., Wu, Q.-H., Wang, H.-D., Zhou, H.-W., He, Y., et al. (2021). Dysbiosis of gut microbiota is an independent risk factor of stroke-associated pneumonia: A Chinese pilot study. Front. Cell. Infect. Microbiol. 11, 715475. doi:10.3389/fcimb.2021.715475
Xu, N., Kan, P., Yao, X., Yang, P., Wang, J., Xiang, L., et al. (2018). Astragaloside IV reversed the autophagy and oxidative stress induced by the intestinal microbiota of AIS in mice. J. Microbiol. Seoul. Korea 56, 838–846. doi:10.1007/s12275-018-8327-5
Xu, D.-J., Wang, K.-C., Yuan, L.-B., Li, H.-F., Xu, Y.-Y., Wei, L.-Y., et al. (2021). Compositional and functional alterations of gut microbiota in patients with stroke. Nutr. Metab. Cardiovasc. Dis. NMCD 31, 3434–3448. doi:10.1016/j.numecd.2021.08.045
Xu, K., Gao, X., Xia, G., Chen, M., Zeng, N., Wang, S., et al. (2021). Rapid gut dysbiosis induced by stroke exacerbates brain infarction in turn. Gut 70, 1486–1494. doi:10.1136/gutjnl-2020-323263
Xu, X., Guo, Y., Chen, S., Ma, W., Xu, X., Hu, S., et al. (2022). The positive influence of polyphenols extracted from Pueraria lobata root on the gut microbiota and its antioxidant capability. Front. Nutr. 9, 868188. doi:10.3389/fnut.2022.868188
Yamashiro, K., Tanaka, R., Urabe, T., Ueno, Y., Yamashiro, Y., Nomoto, K., et al. (2017). Gut dysbiosis is associated with metabolism and systemic inflammation in patients with ischemic stroke. PloS One 12, e0171521. doi:10.1371/journal.pone.0171521
Yan, B.-F., Chen, X., Chen, Y.-F., Liu, S.-J., Xu, C.-X., Chen, L., et al. (2022). Aqueous extract of Paeoniae Radix Alba (Paeonia lactiflora Pall.) ameliorates DSS-induced colitis in mice by tunning the intestinal physical barrier, immune responses, and microbiota. J. Ethnopharmacol. 294, 115365. doi:10.1016/j.jep.2022.115365
Yang, J., Gao, F., Zhang, Y., Liu, Y., and Zhang, D. (2015). Buyang huanwu decoction (BYHWD) enhances angiogenic effect of mesenchymal stem cell by upregulating VEGF expression after focal cerebral ischemia. J. Mol. Neurosci. 56, 898–906. doi:10.1007/s12031-015-0539-0
Ye, Y., Zhu, Y.-T., Xin, X.-Y., Zhang, J.-C., Zhang, H.-L., and Li, D. (2022). Efficacy of Chinese herbal medicine for tPA thrombolysis in experimental stroke: A systematic review and meta-analysis. Phytomedicine Int. J. Phytother. Phytopharm. 100, 154072. doi:10.1016/j.phymed.2022.154072
Yin, J., Liao, S.-X., He, Y., Wang, S., Xia, G.-H., Liu, F.-T., et al. (2015). Dysbiosis of gut microbiota with reduced trimethylamine-N-oxide level in patients with large-artery atherosclerotic stroke or transient ischemic attack. J. Am. Heart Assoc. 4, e002699. doi:10.1161/JAHA.115.002699
Yissachar, N., Zhou, Y., Ung, L., Lai, N. Y., Mohan, J. F., Ehrlicher, A., et al. (2017). An intestinal organ culture system uncovers a role for the nervous system in microbe-immune crosstalk. Cell 168, 1135–1148.e12. doi:10.1016/j.cell.2017.02.009
Zeng, M., Zhou, H., He, Y., Wang, Z., Shao, C., Yin, J., et al. (2021). Danhong injection alleviates cerebral ischemia/reperfusion injury by improving intracellular energy metabolism coupling in the ischemic penumbra. Biomed. Pharmacother. 140, 111771. doi:10.1016/j.biopha.2021.111771
Zhai, Q., Wang, X., Chen, C., Tang, Y., Wang, Y., Tian, J., et al. (2019). Prognostic value of plasma trimethylamine N-oxide levels in patients with acute ischemic stroke. Cell. Mol. Neurobiol. 39, 1201–1206. doi:10.1007/s10571-019-00714-3
Zhai, X., Wang, X., Wang, L., Xiu, L., Wang, W., and Pang, X. (2020). Treating different diseases with the same method-A traditional Chinese medicine concept analyzed for its biological basis. Front. Pharmacol. 11, 946. doi:10.3389/fphar.2020.00946
Zhang, X., Zhao, Y., Zhang, M., Pang, X., Xu, J., Kang, C., et al. (2012). Structural changes of gut microbiota during berberine-mediated prevention of obesity and insulin resistance in high-fat diet-fed rats. PloS One 7, e42529. doi:10.1371/journal.pone.0042529
Zhang, F., Zhai, M., Wu, Q., Jia, X., Wang, Y., and Wang, N. (2020). Protective effect of tong-qiao-huo-xue decoction on inflammatory injury caused by intestinal microbial disorders in stroke rats. Biol. Pharm. Bull. 43, 788–800. doi:10.1248/bpb.b19-00847
Zhang, H., Huang, Y., Li, X., Han, X., Hu, J., Wang, B., et al. (2021). Dynamic process of secondary pulmonary infection in mice with intracerebral hemorrhage. Front. Immunol. 12, 767155. doi:10.3389/fimmu.2021.767155
Zhang, S., Luo, H., Sun, S., Zhang, Y., Ma, J., Lin, Y., et al. (2022). Salvia miltiorrhiza bge. (Danshen) for inflammatory bowel disease: Clinical evidence and network pharmacology-based strategy for developing supplementary medical application. Front. Pharmacol. 12, 741871. doi:10.3389/fphar.2021.741871
Zhao, L., Nicholson, J. K., Lu, A., Wang, Z., Tang, H., Holmes, E., et al. (2012). Targeting the human genome-microbiome axis for drug discovery: Inspirations from global systems biology and traditional Chinese medicine. J. Proteome Res. 11, 3509–3519. doi:10.1021/pr3001628
Zhao, X., He, Y., Zhang, Y., Wan, H., Wan, H., and Yang, J. (2022). Inhibition of oxidative stress: An important molecular mechanism of Chinese herbal medicine (Astragalus membranaceus, Carthamus tinctorius L., Radix Salvia miltiorrhizae, etc.) in the treatment of ischemic stroke by regulating the antioxidant system. Oxid. Med. Cell. Longev. 2022, 1425369. doi:10.1155/2022/1425369
Zheng, Y., Wu, Z., Yi, F., Orange, M., Yao, M., Yang, B., et al. (2018). By activating akt/eNOS bilobalide B inhibits autophagy and promotes angiogenesis following focal cerebral ischemia reperfusion. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. biochem. Pharmacol. 47, 604–616. doi:10.1159/000490016
Zheng, T., Jiang, H., Jin, R., Zhao, Y., Bai, Y., Xu, H., et al. (2019). Ginsenoside Rg1 attenuates protein aggregation and inflammatory response following cerebral ischemia and reperfusion injury. Eur. J. Pharmacol. 853, 65–73. doi:10.1016/j.ejphar.2019.02.018
Zhou, Z., Xu, N., Matei, N., McBride, D. W., Ding, Y., Liang, H., et al. (2021). Sodium butyrate attenuated neuronal apoptosis via GPR41/Gβγ/PI3K/Akt pathway after MCAO in rats. J. Cereb. Blood Flow. Metab. Off. J. Int. Soc. Cereb. Blood Flow. Metab. 41, 267–281. doi:10.1177/0271678X20910533
Zhu, W., Gregory, J. C., Org, E., Buffa, J. A., Gupta, N., Wang, Z., et al. (2016). Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell 165, 111–124. doi:10.1016/j.cell.2016.02.011
Zhu, T., Wang, L., Feng, Y., Sun, G., and Sun, X. (2021). Classical active ingredients and extracts of Chinese herbal medicines: Pharmacokinetics, pharmacodynamics, and molecular mechanisms for ischemic stroke. Oxid. Med. Cell. Longev. 2021, 8868941. doi:10.1155/2021/8868941
Zhu, W., Romano, K. A., Li, L., Buffa, J. A., Sangwan, N., Prakash, P., et al. (2021). Gut microbes impact stroke severity via the trimethylamine N-oxide pathway. Cell Host Microbe 29, 1199–1208.e5. doi:10.1016/j.chom.2021.05.002
Keywords: acute ischemic stroke (AIS), traditional Chinese medicine (TCM), gut microbiota, Chinese herbs, brain-gut-microbe axis (BGMAs), immunology and inflammation, intestinal barrier, microbial metabolites
Citation: Gao L, Xia X, Shuai Y, Zhang H, Jin W, Zhang X and Zhang Y (2023) Gut microbiota, a hidden protagonist of traditional Chinese medicine for acute ischemic stroke. Front. Pharmacol. 14:1164150. doi: 10.3389/fphar.2023.1164150
Received: 12 February 2023; Accepted: 04 April 2023;
Published: 13 April 2023.
Edited by:
Chiou-Feng Lin, Taipei Medical University, TaiwanReviewed by:
Shixin Xu, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, ChinaCopyright © 2023 Gao, Xia, Shuai, Zhang, Jin, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Zhang, emhhbmcxOTgweWlAMTI2LmNvbQ==; Xiaoyun Zhang, emhhbmd4eTE5ODBAMTI2LmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.