- 1Beijing Key Laboratory of Pharmacology of Chinese Materia Region, Xiyuan Hospital, Institute of Basic Medical Sciences, National Clinical Research Center of Cardiovascular Disease of Traditional Chinese Medicine, China Academy of Chinese Medical Sciences, Beijing, China
- 2Institute of Crop Sciences, Chinese Academy of Agricultural Sciences, Beijing, China
- 3Dongfang Hospital, Beijing University of Chinese Medicine, Beijing, China
- 4Institute of Materia Medica, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China
Objective: Astragali Radix (AR, Huangqi in Chinese) has a neuroprotective effect on cerebral infarction (CI). In order to explore the biological basis and therapeutic mechanism of AR in CI, a double-blind randomized controlled trial was established in this study, and proteomics analysis was carried out on serum samples of patients.
Methods: The patients were divided into the AR group (n = 35) and the control group (n = 30). The curative effect was evaluated by the traditional Chinese medicine (TCM) syndrome score and clinical indicators, and the serum of the two groups was analyzed by proteomics. Based on bioinformatics analysis methods, the changes in differential proteins between two groups of samples were explored, and the key proteins were validated through enzyme-linked immunosorbent assay (ELISA).
Results: The results of this study showed that the scores of deficiency of vital energy (DVE), blood stasis (BS), and NIH Stroke Scale (NIHSS) decreased significantly (p < 0.05), while the scores of the Barthel Index (BI) increased, indicating that AR could significantly improve the symptoms of CI patients. In addition, we found that compared with the control group, AR upregulated 43 proteins and downregulated 20 proteins, especially focusing on anti-atherosclerosis and neuroprotective effects. Moreover, ELISA indicated the levels of IL-6, TNF-α, VCAM-1, MCP-1, and ICAM-1 were significantly decreased in the serum of the AR group (p < 0.05, p < 0.01).
Conclusion: This study found that AR can significantly recover the clinical symptoms of CI. Serum proteomics research results show that AR may act on IL-6, TNF-α, VCAM-1, MCP-1, and ICAM-1, and play anti-atherosclerosis and neuroprotective roles.
Clinical Trial Registration: [clinicaltrials.gov], identifier [NCT02846207]
Introduction
Clinically, cerebral infarction (CI) or ischemic stroke is linked to cell death induced by ischemia and hypoxia of local brain tissue caused by vasospasm, stenosis, or occlusion due to local lesions of the vascular wall (Fan et al., 2020; Hui et al., 2021; Wang et al., 2020). It is reported that CI is one of the most common causes of death in the world. According to the most recent prevalence statistics from the American Heart Association, it is estimated that 5,400,000 people have experienced a stroke (Zhou et al., 2019). With the global aging population, an estimated 23 million people suffer from stroke and 7.8 million people will die from 2002 to 2030. About 3/4 of the survivors are incapacitated to varying degrees, and the severe disability rate will be 40% or higher (Kleindorfer et al., 2021; Mathers and Loncar, 2006). Cohort studies have shown that with the improvement of stroke treatment strategies, mortality has dropped. However, the prevalence of recurrent stroke still deserves attention, and effective means are needed to prevent and alleviate secondary stroke (Boot et al., 2020; Ma et al., 2021; Shen et al., 2022). Therefore, it is vital to effectively improve the quality of life and long-term prognosis of CI, and to prevent and alleviate secondary stroke.
In cerebral ischemia, cerebrovascular obstruction deprives brain cells of essential nutrients and oxygen, leading to severe neurological deficits. Ischemic injury may lead to irreversible injury or death of ischemic core neurons. Ischemic stroke involves many mechanisms, such as neurotoxicity, mitochondrial dysfunction, oxidative stress, inflammation, and apoptosis (Chen et al., 2020). These pathophysiological mechanisms overlap and correlate with the development of ischemic stroke and are potential pharmacological targets for treating ischemic stroke.
Chinese herbal medicine has been applied in the treatment of cerebral ischemia for thousands of years and has accumulated much clinical experience (Li et al., 2016; Seto et al., 2016). Astragali Radix (AR, Huangqi in Chinese), the dried root of Astragalus membranaceus (Fisch.) Bge. var. mongholicus (Bge.) Hsiao or A. membranaceus (Fisch.) Bge., which was first recorded in the Shennong Materia Medica (220–280 AD) and was used for ischemic stroke since Tang Dynasty (Gong et al., 2018). Pharmacological studies have found that AR contains a variety of bioactive substances, including saponins, polysaccharides, flavonoids, amino acids, and trace elements (Fu et al., 2014).
Proteomics technology has been widely used in discovering potential protein molecular markers and drug targets of diseases, and exploring protein action mechanisms of action (Tewari et al., 2018). Numerous studies have shown that proteomics technology can be used to explore the proteins related to diseases and reveal their pathogenesis (Chen et al., 2021; Yang et al., 2021; Zhang et al., 2021). So what is AR’s mechanism in treating stroke and what essential proteins will be regulated to play a therapeutic role?
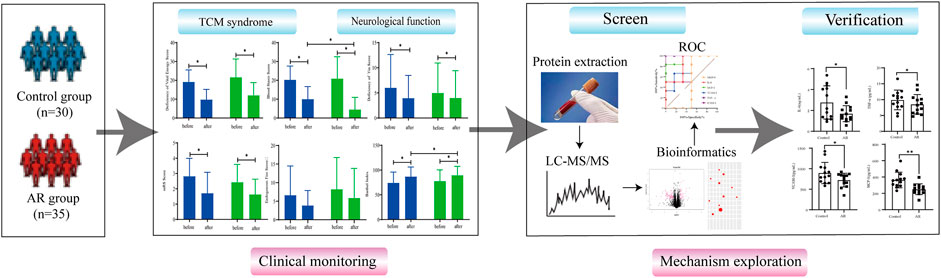
In this study, a randomized, double-blind, placebo-controlled clinical trial was conducted to evaluate the effectiveness of AR in treating CI. Then, the difference in the serum protein content was analyzed using proteomics technology to explore further the neuroprotective effect and potential target protein of AR on ischemic stroke (See Figure 1 for the flowchart of this study).
Materials and methods
Patient population
Overall, a randomized, double-blind, placebo-controlled, parallel-group clinical study was conducted in China from January 2016 to December 2018. This study recruited 65 patients who met the inclusion and exclusion criteria, according to the 2013 Guidelines for the Early Management of Patients with Acute Ischemic Stroke from the American Heart Association/American Stroke Association and the Chinese guidelines for diagnosis and treatment of acute ischemic stroke 2014. In brief, diagnostic inclusion criteria include 1) age 40 to 85; 2) Qi deficiency and blood stasis (BS) syndrome (Ren et al., 2012); 3) accord with the diagnostic criteria of atherosclerotic thrombotic CI; carotid artery color Doppler ultrasound showed evidence of atherosclerotic plaque, and CT/MRI showed definite infarct; 4) NIHSS scores range from 3 to 22; 5) the course of the disease was in the recovery period (2 weeks–6 months); and 6) submitted informed consent. (See Supplementary Material S1 for specific inclusion and exclusion criteria).
Interventions
The composition of AR granules was 60 g of Astragalus membranaceus (Fisch.) Bge. var. mongholicus (Bge.) Hsiao, which was provided by China Resources Sanjiu Pharmaceutical Co., Ltd., and the batch number was 1812002C. By using the method of liquid chromatography tandem mass spectrometry (LC-MS/MS), we identified eight metabolites from the AR (Please see Supplementary Material S2 for more information). Patients with CI were randomly divided into the Astragali Radix group (AR) and the control group (Con). Both groups received primary treatment in the convalescent stage of CI (refer to Chinese guidelines for diagnosing and treating acute ischemic stroke 2014). Based on the standard treatment of Western medicine, the AR and placebo were added. The placebo group was given a traditional Chinese medicine (TCM) simulator. The patient takes AR orally twice a day, with one pack administered each time. The entire course of the treatment lasts for 12 weeks. All patients were suspended from using TCM related to the treatment of stroke treatment 1 week in advance. The randomization procedure was designed by the Data Manager at Xiyuan Hospital of China Academy of Chinese Medical Sciences using SAS statistical software.
Outcome assessments
The primary efficacy endpoint included TCM syndrome and neurological function scores, including the NIH Stroke Scale (NIHSS) and Barthel Index (BI). The efficacy criteria were developed according to the principles of clinical research. The secondary efficacy endpoint includes total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C). The safety endpoints include blood, liver, and kidney function tests, and record the aforementioned endpoints once. The adverse event (AE)/adverse drug reaction (ADR) record form was truthfully completed during the trial.
Preparation of serum samples
The subjects’ whole blood was collected and centrifuged at 1,500 g for 15 min to obtain the serum. The upper serum was carefully sucked out and stored at −80°C. In each group, a total of six biological replicates for each of the groups were used for proteomics analysis, including three men and three women. The serum was thawed, and 50 μl was taken as a sample for subsequent processing. Albumin and IgG of the serum were depleted using a ProteoExtract Albumin/IgG Removal Kit (Merck, Cat. No. 122642). The serum was transferred to a 10-kDa ultrafiltration tube, and dithiothreitol (DTT, Thermo Fisher, Cat. No.3483-12-3) was added to a final concentration of 50 mmol/L and placed at 37 °C for 30 min. Subsequently, 50 mmol/L urea (Affymetrix, 57–13–6) was replaced thrice. The final concentration of iodoacetamide (IAA, GE, Cat. No.I1149) was 100 mmol/L and placed in the dark at 37°C for 30 min, and then replaced with 50 mmol/L urea and ammonium bicarbonate (NH4CO3, Amresco, Cat. No.1066-33-7) solution three times. The proteins were then digested with trypsin (Promega, Cat. No. V5111, enzyme/protein ratio of 1:25 w/w) at 37°C for rocking overnight. Furthermore, 0.1% formic acid (FA, Thermo Fisher, Cat. No.214241) was added to terminate the reaction, and the receiver tube was replaced and centrifuged at 12,000 g for 20 min. The centrifuged solution could be used for mass spectrometry analysis (Zhu et al., 2022).
LC-MS/MS analysis
The qualitative proteomics was analyzed by Easy-nLC 1,000 nl liquid chromatography (Thermo Fisher Scientific, Waltham, United States). The flow phase A was 0.1% FA-water, and the flow phase B was 0.1% FA-ACN, gradient elution: 0–25 min, 5%–9% B; 25–65 min, 9%–23% B; 65–75 min, 23%–32% B; 75–76 min, 32%–95% B; and 76–90 min, 95%–95% B. The flow rate is 400 nl min-1. Mass spectrometry was performed on a QE Plus mass spectrometer in the data-dependent acquisition mode, and specific details were according to our previous research (Ma et al., 2018).
Protein identification and quantitation
The raw data obtained by mass spectrometry were imported into Proteome Discoverer software (version 2.5) for protein identification. The qualitative analysis of proteins was performed using MaxQuant software (version 1.5.6.5) with Uniprot Data (http://www.uniprot.org/, contains 20,359 proteins) (Cox and Mann, 2008). The parameters were set as follows: maximum of two missing protein sites; fixed modification: cysteine and iodide acetalization; variable modification: methionine oxidation and N-acetylation; mass deviation of fragment ions: 0.02 Da; and minimum detection value of peptide: seven amino acids. A maximum false positive rate of 1% was allowed. The analysis was developed according to the previous research (Wang et al., 2021).
Bioinformatics analysis
The false-positive rate Q < 0.01, fold change>1.2, and p-value < 0.05 were regarded as differentially expressed proteins. After obtaining quantitative information, bioinformatics software analyzed the differential proteins to find the relevant biological pathways and network interaction information. The biological function of the protein was analyzed by using the gene ontology analysis database GeneOntology (http://geneontology.org/) and UniProt (HYPERLINK "https://www.uniprot.org/" \o "https://www.uniprot.org/"https://www.uniprot.org/) database, and the biological function enrichment analysis was carried out based on the DAVID (https://david.ncifcrf.gov), Kyoto Encyclopedia of Genes and Genomes (KEGG) (www.genome.jp/kegg/), and STRING (http://www.string-db.org/) databases.
Enzyme-linked immunosorbent assay
The levels of relative proteins were detected by enzyme-linked immunosorbent assay (ELISA). The levels of IL6 and MCP-1 were determined by employing the ELISA kit (Thermo Fisher Scientific, Waltham, United States). The levels of TNF-α, ICAM-1, and VCAM-1 were measured using an ELISA kit (Nanjing Jiancheng Bioengineering Institute). All operations shall be carried out according to the manufacturer’s protocol.
Statistical analysis
SPSS22.0 statistical software was used for statistical processing, and the measurement data were expressed by x ± s. One-way analysis of variance was used to analyze the data, and a t-test was used for group comparison. Means and standard deviations (SDs) were used to present the findings. p < 0.05 was considered to be statistically significant. The ROC curve was established by GraphPad Prism 8.
Results
Population characteristics
A total of 65 patients were recruited for this study, and 56 completed the 12-week treatment. A total of nine subjects fell off during the trial, with a fall-off rate of 13.8%. The baseline characteristics of the subjects are summarized in Table 1. In this study, the age and sex of the two groups were matched, and there were more male patients than female patients. There was no significant difference in clinical indicators and disease history at recruitment. More than 36.67% of the patients had hypertension, and a few had coronary heart disease and cerebrovascular disease history. The commonly used indicators in blood detection showed no significant difference between the two groups (p > 0.05).
Outcomes of Astragali Radix
After 12 weeks of treatment, the scores of deficiency of vital energy (DVE), blood stasis (BS), NIHSS, and mRS in the AR and control groups were significantly decreased (p < 0.05; Figure 2), and the endogenous fire score was decreased, whereas the BI score was increased (p < 0.05). Furthermore, the study found that compared with the Con, BS in the AR group was significantly decreased with a sharp increase in BI (p < 0.05). The scores indicated that AR could effectively alleviate the symptoms of patients with CI and protect the white matter to a certain extent, which may mainly improve the neurological function of the patients.
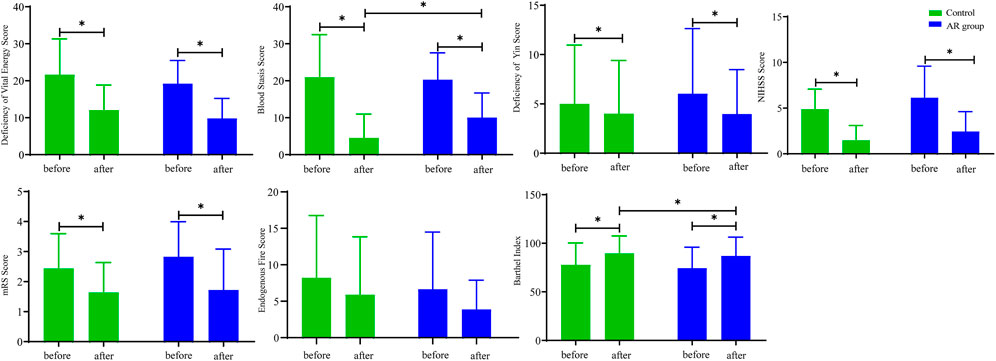
FIGURE 2. Indicators of symptom scores in the AR and control groups. AR: n = 30; control: n = 26. Before: represents the level of the patient before treatment; after: represents the level of the patient after 12 weeks of AR. Data are presented as mean ± SD. *p < 0.05.
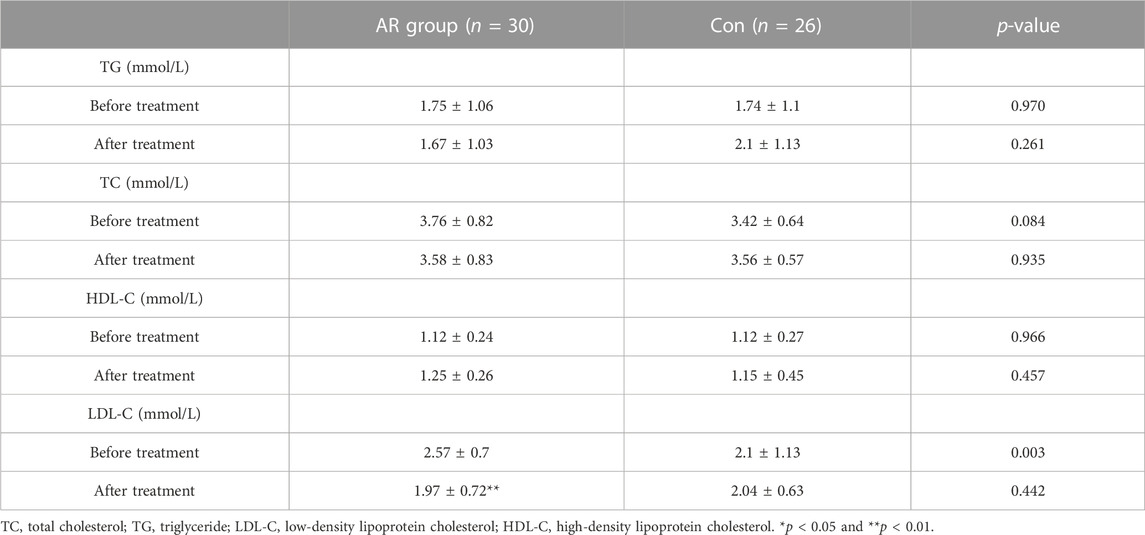
As shown in Table 2, there was no significant difference in TG, TC, and HDL-C between the two groups before and after treatment (p > 0.05); compared with the same group before treatment, AR treatment significantly reduced LDL-C as the control group (p<0.01).
Safety assessment
Laboratory indexes were tested in this study, and there was no significant difference between the AR and the control groups (p > 0.05; see Supplementary Material S3). In addition, no significant adverse reactions were reported in both groups during the treatment period. See Supplementary Material S3 for details.
Results of proteomics
Data quality control of quantitative proteomics
As shown in Figure 3, the standard deviation of the same sample is very small, indicating that the data on the same sample have good repeatability. The analysis of the AR group and the Con discovered significant differences in the coverage of 30%–40% and 60%–70%. It can be seen from the signal intensity distribution diagram that there are significant differences between the AR group and the Con in different signal intensities of the two groups (such as <1×108 and <1×1010). The Pearson correlation results illustrate a difference between the AR group and the Con. Interestingly, specific differences are also found among the six samples of each group, which the contrasts between males and females may cause. Details of the LC-MS data can be found in Supplementary Material S4.
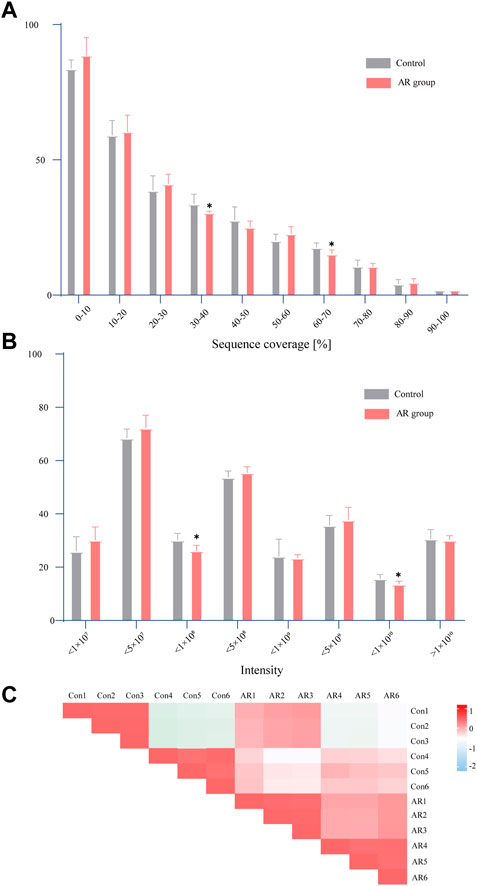
FIGURE 3. Statistical analysis of proteome data. (A) Distribution of sequence coverage of proteome data on different samples. (B) Signal intensity distribution of proteome data on different samples. (C) Pearson correlation analysis (the comparison between the two groups was conducted using an independent sample t-test, n = 6 per group, *p < 0.05).
Identification of differentially expressed proteins
The difference in the protein expression level was more than 1.2 times a significant difference, and the differentially expressed proteins among the three groups were analyzed (Figure 4). In the volcanic map, green dots represent downregulated proteins, red dots represent upregulated proteins, and blue dots represent proteins with no significant difference. The horizontal axis is represented by log 2 (fold change), and the proteins with significant differences are distributed at both ends. The ordinate is represented by log10 (p-value). The larger the value, the clearer is the difference. Compared with the control, AR upregulated 43 proteins and downregulated 20 proteins. The heat map in Figure 5 shows the distribution of all the different proteins in the serum samples, and the colors in different degrees represent the protein content. It can be seen that the proteins show evident clustering in the two groups. The heat map results are similar to those of the volcano map. Compared with Con, more differential proteins in the AR group were upregulated.
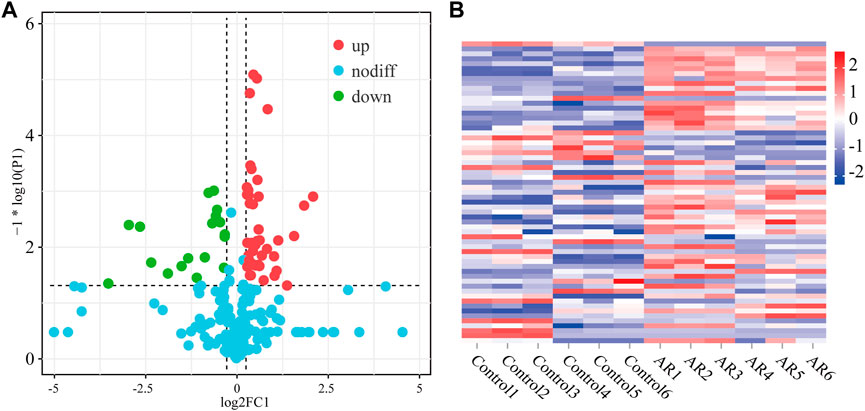
FIGURE 4. Differentially expressed proteins of the AR and control groups. (A) Volcano map of differentially expressed proteins of AR/Con. (B) Heatmap analysis of serum samples of the AR and control groups (n = 6 per group).
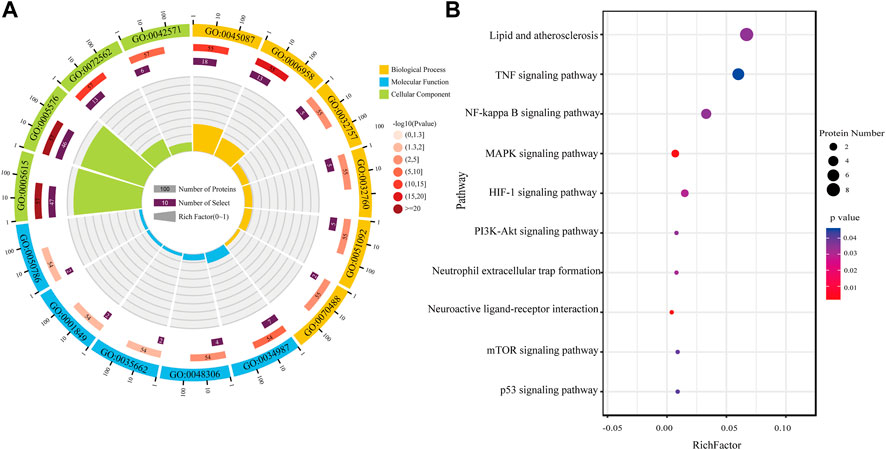
FIGURE 5. Functional enrichment analysis. (A) GO analysis of differential proteins in AR/Con. (B) KEGG analysis of differential proteins in AR/Con.
Functional classification of differentially expressed proteins
To understand the functional categories of differential proteins, the Gene Ontology and STRING databases were used for Gene Ontology (GO) analysis. The results were expressed using an enrichment circle map (Figure 5). The first circle from the outside to the inside is the enrichment classification. Different colors represent different classifications, including biological process (BP), molecular function (MF), and cellular component (CC). The scale with the number of proteins is outside the circle. The second circle is the number of background proteins and the p-value. The more the number of proteins, the longer the bar. The redder the color, the smaller the p-value. The third circle is the total number of proteins in each classification. The fourth circle represents the rich factor of each classification (Qie et al., 2020).
After the AR exerts efficacy in the organism of patients with CI, it may play a role in molecular function, including the immune system and energy metabolism-related functions such as immunoglobulin receptor binding (GO:0034987), Toll-like receptor four bindings (GO:0035662), complement component C1q binding (GO:0001849), and calcium-dependent protein binding (GO:0048306), through biological processes such as complement activation, classical pathway (GO:0006958), innate immune response (GO:0045087), positive regulation of tumor necrosis factor production (GO:0032760), and neutrophil aggregation (GO:0070488). All the differentially expressed proteins were at the extracellular space (GO:0005615), extracellular region (GO:0005576), and blood microparticle (GO:0072562).
Cluster analysis of differentially expressed proteins
Based on the aforementioned ontology analysis, the biological functions of differential proteins were analyzed by KEGG. The rich factor indicated the ratio of differential proteins enriched to the number of all proteins in a pathway. The higher the rich factor, the higher the degree of enrichment. According to the value of the enrichment factor, the top 10 enrichment paths were selected for visualization. As the results indicate, the proteins regulated after treatment with AR in patients with CI were mainly related to biological processes such as the TNF-α signaling pathway, NF-κB signaling pathway, HIF-1 signaling pathway, neuroactive ligand–receptor interaction, and p53 signaling pathway. The study showed that AR might play a neuroprotective role in CI through anti-inflammatory and antioxidant properties, promoting neurogenesis and angiogenesis.
Protein–protein interaction analysis of differentially expressed proteins
Proteins in the body usually interact with each other to complete a series of biological processes. Furthermore, the STRING database (http://string-db.org) was used to predict the interactions of enriched proteins in the pathway to identify key nodes in the development of AR therapy for CI. Aiming to improve the reliability of the PPI analysis, the confidence score was set at high confidence (≥0.700). Figure 6A demonstrates their interaction. In total, 32 proteins were detected and showed complex interactions with other proteins between the AR and the control. In Figure 6A, two protein clusters were demonstrated to be highly related to CI, and the red cluster contained more proteins; the interactions were complex and formed a network that represents the target proteins that AR may act on. Combined with the proteins in the top 10 KEGG pathways, IL-6, TNF-α, MMP-9, ICAM-1, VCAM-1, and MCP-1 are the central proteins of the PPI network, indicating that they have multiple interactions with other proteins and may be potential targets for AR.
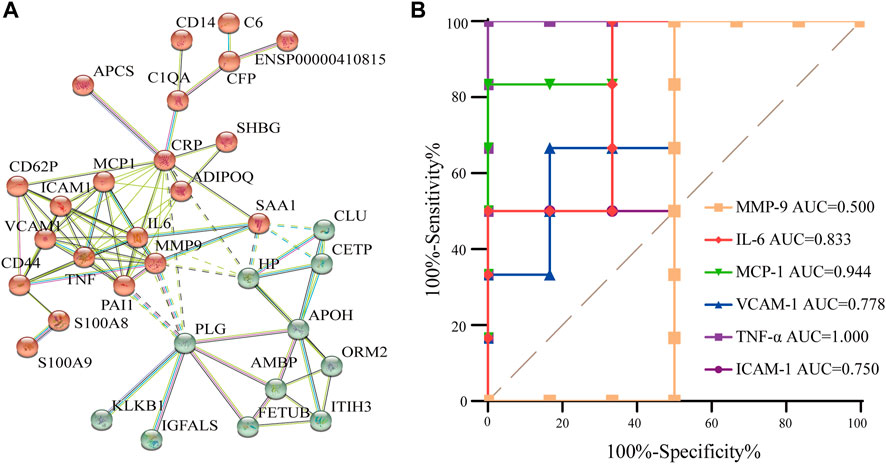
FIGURE 6. PPI analysis and the ROC curve of differentially expressed proteins. (A) PPI analysis of differential protein in AR/Con. (B) ROC curve of six potential proteins.
Origin prediction by the ROC curve
To verify the accuracy and specificity of the screening of potential target proteins, an ROC curve was established to judge the feasibility of prediction. As shown in Figure 6B, the area under curve (AUC) of IL-6, TNF- α, ICAM-1, VCAM-1, and MCP-1 is greater than 0.7, and the AUC of MMP-9 is less than 0.7, indicating that IL-6, TNF- α, ICAM-1, VCAM-1, and MCP-1 have good accuracy in the diagnosis of AR, and the five screened proteins have diagnostic abilities.
Validation of the five candidate proteins
The aforementioned analysis results showed that IL-6, TNF-α, ICAM-1, VCAM-1, and MCP-1 may be potential candidate proteins for the AR treatment of CI. ELISA quantified the levels of these proteins in the serum, and the results are shown in Figure 7. Compared with the control, the levels of IL-6, TNF-α, VCAM-1, MCP-1, and ICAM-1 were significantly decreased in the serum of the AR group (p < 0.05, p < 0.01). IL-6, TNF-α, VCAM-1, MCP-1, and ICAM-1 might be potential target proteins for the AR to treat CI.
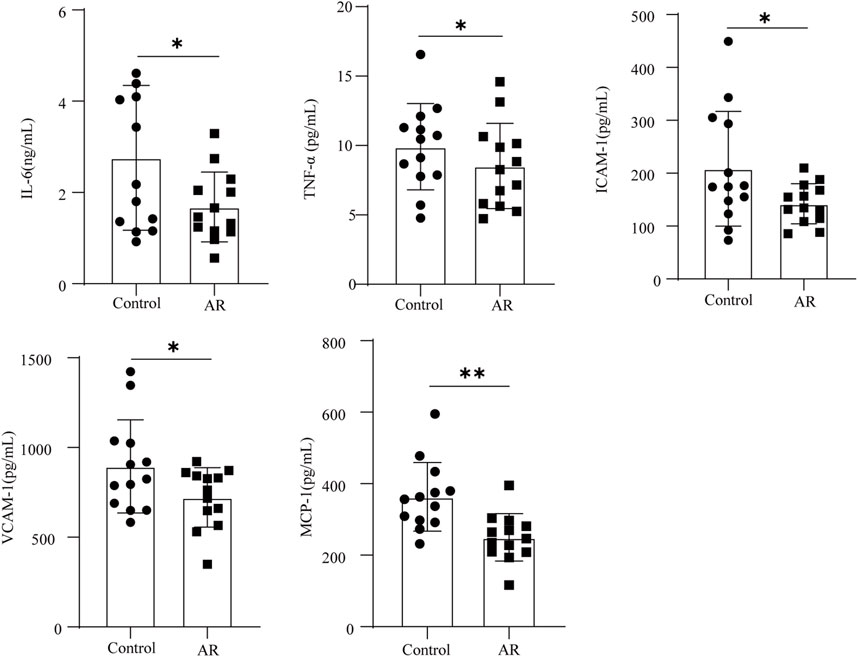
FIGURE 7. Protein content of the AR and control groups was determined by ELISA (n = 13 in each group). Data are presented as mean ± SD. *p < 0.05; **p < 0.01.
Discussion
In this study, we assessed the effectiveness and safety of AR in treating CI for the first time through a randomized controlled trial. The combined results showed that the AR significantly improved the clinical symptoms and cardiac function of patients with CI, ameliorated energy metabolism, and regulated coagulation and neurological function. The results also showed that the scores of DVE, BS, NIHSS, mRS, and LDL-C significantly decreased with the treatment of the AR (p < 0.05), yet that of BI increased (p < 0.05). We inferred the therapeutic mechanism of AR may be related to the regulation of blood glucose and blood lipid levels, the improvement of neurological function and anticoagulant activity, and the reduction of the risk of thrombosis.
Furthermore, quantitative proteomics was used to further elucidate the mechanism of the AR. Comparison analysis revealed a significant quantitative alteration in protein with differential abundance between the two groups, resulting in 63 differentially expressed proteins in the AR group. Moreover, the bioinformatics analyses and validations revealed that the AR exerted the effects by acting on lipid and atherosclerosis, the TNF-α signaling pathway, NF-kappa B signaling pathway, and neuroactive ligand–receptor interaction. A further comprehensive discussion was conducted on the research results of the protein–protein interaction functional network, combined with the proteins in the top 10 KEGG pathways, and we discovered that IL-6, TNF-α, MMP-9, ICAM-1, VCAM-1, and MCP-1 were located in the center of the network with the treatment of the AR recipe, serving as a hub to interact with other proteins. The proteins mainly participated in biological processes of the immune system, including the TNF-α signaling pathway and NF-kappa B signaling pathway. The ROC curve and ELISA indicated that IL-6, TNF- α, ICAM-1, VCAM-1, and MCP-1 have good accuracy in the diagnosis of AR.
The formation of atherosclerotic lesions is a complex process, which often proceeds over decades. Several different factors contribute to the formation of atherosclerotic lesions. Atherosclerosis is the predecessor of stroke, which belongs to chronic vascular inflammatory reactions. Tumor necrosis factor α (TNF-α) is the main pro-inflammatory cytokine that contributes to the occurrence and maintenance of vascular inflammation. TNF-α induces the production of reactive oxygen species (ROS), thereby driving the redox reaction that constitutes “ROS signaling.” However, these ROS may also cause oxidative stress, leading to vascular dysfunction (Lamb et al., 2020). Atherosclerosis may be related to the increase in intracellular IL-6 levels. During the aging process, IL-6 signaling in bone marrow adipocytes increases, which may lead to the differentiation of hematopoietic stem cells toward bone marrow cells and increase the risk of gene mutations encoding transcription regulatory factors (such as TET2), which may lead to positive selection and expansion of hematopoietic cell clones. Clone results of bone marrow cells with TET2 mutation showed IL-6 and IL-1 β, which may lead to accelerated atherosclerosis (Tyrrell and Goldstein, 2021). Activation of the NF-kappa B signaling pathway may be associated with the aggravation of ischemic cardiocerebrovascular disease (Li et al., 2018). Previous studies have also shown that prescriptions of TCM including AR can inhibit the NF-kappa B signaling pathway and adjust blood lipid levels to treat atherosclerosis (Liu et al., 2020).
The TNF-α signaling and NF-kappa B signal pathways are closely related to inflammation and neuronal apoptosis, which are essential nuclear transcription factors involved in regulating cell differentiation and apoptosis in organisms and controlling inflammatory and immune responses. Inflammatory signaling is activated and completed by blood-derived leukocytes, which penetrate the brain during ischemia and produce inflammatory cytokines and proinflammatory mediators, including TNF-α, IL-6, and ICAM-1 (Xu et al., 2018). TNF-α is a common cytokine that promotes inflammation, induces the release of inflammatory cytokines, and promotes cell necrosis (Chen et al., 2019). Compared with the control, the level of TNF-α in the AR group significantly decreased after treatment (p < 0.05), which suggested that the neuroprotective effects of AR may be related to the inhibition of neurogenic inflammation caused by cerebral ischemia. As for ICAM-1, other studies defined the protein’s crucial role in the development of atherosclerosis by participating in the processes of inflammation and apoptosis (Patel et al., 2020). ICAM-1 is present in atherosclerotic lesions and is involved in their progress. In addition to its well-known role in white cell migration, ICAM-1 has now been proven to be able to transmit intracellular signals, leading to the rearrangement of actin to the skeleton and the activation of pro-inflammatory cascade reactions, thus making the inflammatory response continuous (Lawson and Wolf, 2009). In addition, AR can reduce the expression of ICAM-1 and VCAM-1, thereby reducing the activation of NF-kappa B in human endothelial cells. Over the years, MCP-1 has emerged as an important chemokine, playing a pivotal role in many CNS disorders (Joly et al., 2020; Singh et al., 2021). MCP-1 has been recommended as an early predictor of ischemic stroke (Bonifacic et al., 2016; Li et al., 2020). AR downregulates MCP-1 significantly, which may play a role by reducing the neurological impairments, inflammatory response, and ischemic infarct area. The aforementioned results suggest that AR may play a neuroprotective role in CI by reducing proinflammatory substances, thereby inhibiting the activation of the TNF-α signaling and NF-kappa B signaling pathways.
Our research still has some limitations and shortcomings. First, we only tested the serum proteins of the patient after treatment; so, we need to further examine the changes in serum proteomics and other time points throughout the entire process. In addition, more patients and more experiments may be needed to prove the aforementioned conclusion.
Conclusion
In summary, this study found that AR can significantly improve the clinical symptoms of CI. In addition, based on the results of serum proteomics research of clinical patients with CI, we found that compared with control, AR upregulated 43 proteins and downregulated 20 proteins. Through analysis of protein functional enrichment, it was found that AR may act on IL-6, TNF-α, ICAM-1, VCAM-1, and MCP-1 and play anti-atherosclerosis and neuroprotective roles. This study explores the biological mechanism of the AR formula in treating CI at the protein level, providing a molecular basis for the clinical treatment of CI and related research of TCM.
Data availability statement
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the iProX partner repository (Ma et al., 2018) with the dataset identifier PXD029388.
Ethics statement
The studies involving human participants were reviewed and approved by Dongfang Hospital affiliated with the Beijing University of Traditional Chinese Medicine (JDF-IRB-2016030101). The patients/participants provided their written informed consent to participate in this study.
Author contributions
YL: writing—original draft, methodology, validation, software, and data curation. DW: conceptualization, writing—original draft, and software. BM: conceptualization and methodology. LM: data compilation and formal analysis. MS: writing—review and editing. RG: conceptualization, methodology, and resources. LH: conceptualization, methodology, and writing—review and editing. LL: conceptualization, methodology, and writing—review and editing. YP: conceptualization, methodology, and writing—review and editing. RG: conceptualization, methodology, and resources. JL: conceptualization, methodology, resources, and funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the National Natural Science Foundation of China (Grant No. 82030124), the National Key Basic Research Special Foundation of China (Grant No. 2015CB554400), the Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (Grant No. ZYYCXTD-C-202007), and the Science and Technology Innovation Project of China Academy of Chinese Medical Sciences (Grant No. CI 2021A04508).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1162134/full#supplementary-material
Abbreviations
AR, Astragali Radix; AUC, area under curve; BP, biological process; BI, Barthel Index; BS, blood stasis; CC, cellular component; CI, cerebral infarction; DEPs, differentially expressed proteins; DTT, dithiothreitol; DY, deficiency of Yin; DVE, deficiency of vital energy; ELISA, enzyme-linked immunosorbent assay; FA, formic acid; GO, Gene Ontology; Con, control group; HDL-C, high-density lipoprotein cholesterol; ICAM-1, intercellular adhesion molecule 1; IAA, iodoacetamide; IL-6, interleukin-6; KEGG, Kyoto Encyclopedia of Genes and Genomes; LDL-C, low-density lipoprotein cholesterol; MMP-9, matrix metalloproteinase-9; MF, molecular function; MCP-1, monocyte chemoattractant protein-1; NIHSS, NIH Stroke Scale; PPI, protein–protein interaction; TG, triglyceride; TC, total cholesterol; TNF-α, tumor necrosis factor-alpha; VCAM-1, vascular cell adhesion protein 1.
References
Bonifacic, D., Toplak, A., Benjak, I., Tokmadzic, V. S., Lekic, A., and Kucic, N. (2016). Monocytes and monocyte chemoattractant protein 1 (MCP-1) as early predictors of disease outcome in patients with cerebral ischemic stroke. Wien Klin. Wochenschr 128, 20–27. doi:10.1007/s00508-015-0878-4
Boot, E., Ekker, M. S., Putaala, J., Kittner, S., De, L. F., and Tuladhar, A. M. (2020). Ischaemic stroke in young adults: A global perspective. J. Neurol. Neurosurg. Psychiatry 91 (4), 411–417. doi:10.1136/jnnp-2019-322424
Chen, A. Q., Fang, Z., Chen, X. L., Yang, S., Zhou, Y. F., Mao, L., et al. (2019). Microglia-derived TNF-α mediates endothelial necroptosis aggravating blood brain-barrier disruption after ischemic stroke. Cell Death Dis. 10, 487. doi:10.1038/s41419-019-1716-9
Chen, H. S., He, Y. C., Chen, S., Qi, S. H., and Shen, J. G. (2020). Therapeutic targets of oxidative/nitrosative stress and neuroinflammation in ischemic stroke: Applications for natural product efficacy with omics and systemic biology. Pharmacol. Res. 158, 104877–106618. doi:10.1016/j.phrs.2020.104877
Chen, S. J., Wang, Y., Liang, C. X., Li, J., Li, Y. H., Wu, Q., et al. (2021). Buyang Huanwu Decoction ameliorates atherosclerosis by regulating TGF-β/Smad2 pathway to promote the differentiation of regulatory T cells. J. Ethnopharmacol. 269, 113724. doi:10.1016/j.jep.2020.113724
Cox, J., and Mann, M. (2008). MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26, 1367–1372. doi:10.1038/nbt.1511
Fan, J., Saft, M., Sadanandan, N., Gonzales, B., Park, Y. J., Sanberg, P. R., et al. (2020). LncRNAs stand as potent biomarkers and therapeutic targets for stroke. Front. Aging Neurosci. 12, 594571. doi:10.3389/fnagi.2020.594571
Fu, J., Wang, Z., Huang, L., Zheng, S., Wang, D., Chen, S., et al. (2014). Review of the botanical characteristics, phytochemistry, and pharmacology of Astragalus membranaceus (Huangqi). Phytother. Res. 28 (9), 1275–1283. doi:10.1002/ptr.5188
Gong, A. G., Duan, R., Wang, H. Y., Kong, X. P., Dong, T. T., Tsim, K. W., et al. (2018). Evaluation of the pharmaceutical properties and value of Astragali Radix. Medicine 5 (2), 46. doi:10.3390/medicines5020046
Hui, Z., Wang, S. L., Li, J. X., Wang, J. Q., and Zhang, Z. N. (2021). Compound Tongluo Decoction inhibits endoplasmic reticulum stress-induced ferroptosis and promoted angiogenesis by activating the Sonic Hedgehog pathway in cerebral infarction. J. Ethnopharmacol. 2021, 114634.
Joly, A. A., Hunter, J., Quadri, Z., Zamudio, F., Rocha, P. V., Chan, D., et al. (2020). CCL2 overexpression in the brain promotes glial activation and accelerates tau pathology in a mouse model of tauopathy. Front. Immunol. 11, 997. doi:10.3389/fimmu.2020.00997
Kleindorfer, D. O., Towfighi, A., Chaturvedi, S., Cockroft, K. M., Gutierrez, J., Lombardi-Hill, D., et al. (2021). 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: A guideline from the American heart association/American stroke association. Stroke 52 (7), e364–e467. doi:10.1161/STR.0000000000000375
Lamb, F. S., Choi, H., Miller, M. R., and Stark, R. J. (2020). TNFα and reactive oxygen signaling in vascular smooth muscle cells in hypertension and atherosclerosis. Am. J. Hypertens. 33 (10), 902–913. doi:10.1093/ajh/hpaa089
Lawson, C., and Wolf, S. (2009). ICAM-1 signaling in endothelial cells. Pharmacol. Rep. 61 (1), 22–32. doi:10.1016/s1734-1140(09)70004-0
Li, J. H., Chen, Z. X., Zhang, X. G., Li, Y., Yang, W. T., Zheng, X. W., et al. (2016). Bioactive components of Chinese herbal medicine enhance endogenous neurogenesis in animal models of ischemic stroke: A systematic analysis. Medicine 95 (40), e4904. doi:10.1097/MD.0000000000004904
Li, L., Hao, J., Jiang, X., Li, P., and Sen, H. (2018). Cardioprotective effects of ulinastatin against isoproterenol-induced chronic heart failure through the PI3K-akt, P-38 MAPK and NF-?b- P-athways. Mol. Med. Rep. 17 (1), 1354–1360. doi:10.3892/mmr.2017.7934
Li, L., Lou, W., Li, H., Zhu, Y., and Huang, X. (2020). Upregulated C-C Motif chemokine ligand 2 promotes ischemic stroke via chemokine signaling pathway. Ann. Vasc. Surg. 68, 476–486. doi:10.1016/j.avsg.2020.04.047
Liu, B., Song, Z., Yu, J., Li, P., Tang, Y., and Ge, J. (2020). The atherosclerosis ameliorating effects and molecular mechanisms of BuYangHuanWu decoction. Biomed. Pharmacother. 123, 109664. doi:10.1016/j.biopha.2019.109664
Ma, B., Chen, J. C., Mu, Y. Y., Xue, B. J., Zhao, A. M., Wang, D. P., et al. (2018). Proteomic analysis of rat serum revealed the effects of chronic sleep deprivation on metabolic, cardiovascular and nervous system. Plos One 13 (9), e0199237. doi:10.1371/journal.pone.0199237
Ma, R., Xie, Q., Li, H. Y., Guo, X. Q., Wang, J., Li, Y., et al. (2021). l-Borneol exerted the neuroprotective effect by promoting angiogenesis coupled with neurogenesis via ang1-VEGF-BDNF pathway. Front. Pharmacol. 12, 641894. doi:10.3389/fphar.2021.641894
Mathers, C. D., and Loncar, D. (2006). Projections of global mortality and burden of disease from 2002 to 2030. Plos Med. 3 (11), e442. doi:10.1371/journal.pmed.0030442
Patel, R. B., Colangelo, L. A., Reiner, A. P., Gross, M. D., Jacobs, D. R., Launer, L. J., et al. (2020). Cellular adhesion molecules in young adulthood and cardiac function in later life. J. Am. Coll. Cardiol. 75 (17), 2156–2165. doi:10.1016/j.jacc.2020.02.060
Qie, C. X., Jiang, J. W., Liu, W. M., Hu, X. L., Chen, W. T., Xie, X. X., et al. (2020). Single-cell RNA-Seq reveals the transcriptional landscape and heterogeneity of skin macrophages in Vsir murine psoriasis. Theranostics 10 (23), 10483–10497. doi:10.7150/thno.45614
Ren, Y., Zhang, M., Chen, K., You, S., Li, J., Guo, L., et al. (2012). Clinical and epidemiological investigation of TCM syndromes of patients with coronary heart disease in China. Evid. Based Compl Alt. 2012, 714517. doi:10.1155/2012/714517
Seto, S. W., Chang, D., Jenkins, A., Bensoussan, A., and Kiat, H. (2016). Angiogenesis in ischemic stroke and angiogenic effects of Chinese herbal medicine. J. Clin. Med. 5 (6), 56. doi:10.3390/jcm5060056
Shen, Y., Yao, M. J., Su, Y. X., Xu, D. S., Wang, J., Wang, G. R., et al. (2022). Histochemistry of microinfarcts in the mouse brain after injection of fluorescent microspheres into the common carotid artery. Neural Regen. Res. 17 (4), 832–837. doi:10.4103/1673-5374.322470
Singh, S., Anshita, D., and Ravichandiran, V. (2021). MCP-1: Function, regulation, and involvement in disease. Int. Immunopharmacol. 101, 107598. doi:10.1016/j.intimp.2021.107598
Tewari, S. J., Renney, G., Brewin, J., Gardner, K., Kirkham, F., Inusa, B. B., et al. (2018). Proteomic analysis of plasma from children with sickle cell anemia and silent cerebral infarction. Haematologica 103 (7), 1136–1142. doi:10.3324/haematol.2018.187815
Tyrrell, D. J., and Goldstein, D. R. (2021). Ageing and atherosclerosis: Vascular intrinsic and extrinsic factors and potential role of IL-6. Nat. Rev. Cardiol. 18 (1), 58–68. doi:10.1038/s41569-020-0431-7
Wang, A. A., Li, W. J., Xie, X., Huang, W. Q., Pan, Y. J., and Sun, J. (2020). Clinical efficacy of integrated traditional Chinese and Western medicine in the treatment of qi deficiency and blood stasis syndrome in the convalescent stage of cerebral infarction. Chin. J. Integr. Med. Cardio Cerebrovasc. Dis. 18 (4), 661–663.
Wang, D. P., Mu, Y. Y., Hu, X. J., Ma, B., Wang, Z. B., Zhu, L., et al. (2021). Comparative proteomic analysis reveals that the Heterosis of two maize hybrids is related to enhancement of stress response and photosynthesis respectively. BMC Plant Biol. 21 (1), 34. doi:10.1186/s12870-020-02806-5
Xu, H., Qin, W., Hu, X., Mu, S., Zhu, J., Lu, W., et al. (2018). Lentivirus-mediated overexpression of OTULIN ameliorates microglia activation and neuroinflammation by depressing the activation of the NF-κB signaling pathway in cerebral ischemia/reperfusion rats. J. Neuroinflamm 15, 83. doi:10.1186/s12974-018-1117-5
Yang, C., Farias, F. H., Ibanez, L., Suhy, A., Sadler, B., Fernandez, M. V., et al. (2021). Genomic atlas of the proteome from brain, CSF and plasma prioritizes proteins implicated in neurological disorders. Nat. Neurosci. 24 (9), 1302–1312. doi:10.1038/s41593-021-00886-6
Zhang, J., Guo, F., Zhou, R., Xiang, C., Zhang, Y., Gao, J., et al. (2021). Proteomics and transcriptome reveal the key transcription factors mediating the protection of Panax notoginseng saponins (PNS) against cerebral ischemia/reperfusion injury. Phytomedicine 92, 153613. doi:10.1016/j.phymed.2021.153613
Zhou, M., Wang, H., Zeng, X., Yin, P., Zhu, J., Zhu, W., et al. (2019). Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: A systematic analysis for the global burden of disease study. Lancet 394, 1145–1158. doi:10.1016/S0140-6736(19)30427-1
Zhu, M., Wei, J., Li, Y., Wang, Y., Ren, J., Li, B., et al. (2022). Efficacy and mechanism of buyang huanwu decoction in patients with ischemic heart failure: A randomized, double-blind, placebo controlled trial combined with proteomic analysis. Front. Pharmacol. 13, 831208. doi:10.3389/fphar.2022.831208
Keywords: cerebral infarction, proteomics, neuroprotective, mechanism, Astragali Radix
Citation: Li Y, Wang D, Guo R, Ma B, Miao L, Sun M, He L, Lin L, Pan Y, Ren J and Liu J (2023) Neuroprotective effect of Astragali Radix on cerebral infarction based on proteomics. Front. Pharmacol. 14:1162134. doi: 10.3389/fphar.2023.1162134
Received: 16 February 2023; Accepted: 30 May 2023;
Published: 09 June 2023.
Edited by:
Karl Tsim, Hong Kong University of Science and Technology, Hong Kong SAR, ChinaReviewed by:
Liu Qing-Shan, Minzu University of China, ChinaQian Hua, Beijing University of Chinese Medicine, China
Copyright © 2023 Li, Wang, Guo, Ma, Miao, Sun, He, Lin, Pan, Ren and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yinghong Pan, cGFueWluZ2hvbmdAY2Fhcy5jbg==; Junguo Ren, cmVlazIwMDNAMTYzLmNvbQ==; Jianxun Liu, bGl1angwMzI0QHNpbmEuY29t
 Ying Li
Ying Li Daoping Wang
Daoping Wang Rongjuan Guo
Rongjuan Guo Bo Ma
Bo Ma Lan Miao
Lan Miao Mingqian Sun
Mingqian Sun Lijuan He3
Lijuan He3 Yinghong Pan
Yinghong Pan Junguo Ren
Junguo Ren Jianxun Liu
Jianxun Liu