
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol., 06 April 2023
Sec. Ethnopharmacology
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1162036
Codonopsis Radix, a traditional Chinese medicine in China, has great medicinal and scientific value. Moreover, it can also be used as a health product in daily diet. This paper reviews the botany, ethnopharmacology, phytochemistry, analysis method and quality control, processing methods, pharmacological effects, pharmacokinetics and toxicity related to Codonopsis Radix. The information of Codonopsis Radix is obtained from scientific databases (such as Baidu Scholar, CNKI, Google Scholar, PubMed, Science Direct, Web of Science, and SciFinder Scholar), Chinese herbal classics, Chinese Pharmacopoeia, PhD and MSc dissertations, and so on. The chemical components mainly include alkaloids, alkynes and polyacetylenes, flavonoids, lignans, steroids, terpenoids, organic acids, volatile oils, saccharides and other components, which have a wide range of neuroprotective effects, protection of gastrointestinal mucosa and anti-ulcer, regulation of body immunity, anti-tumor, endocrine regulation, improvement of hematopoietic function, cardiovascular protection, anti-aging and antioxidant effects. In conclusion, this paper summarizes in depth the shortcomings of the current research on Codonopsis Radix and proposes corresponding solutions. At the same time, this paper provides theoretical support for further research on the biological function and potential clinical efficacy of Codonopsis Radix.
Codonopsis Radix (CR), the Chinese name is Dangshen. According to Chinese Pharmacopoeia (Edition 2020), CR is the dried root of Codonopsis pilosula (Franch.) Nannf., Codonopsis pilosula Nannf. var.modesta (Nannf.) L. T. Shen and Codonopsis tangshen Oliv., and harvested, washed and dried in autumn.
It belongs to the spleen and lung meridians, and has a sweet taste. In addition, it is effective in strengthening the spleen and benefiting the lung, nourishing the blood and promoting the production of body fluid. It is often used to treat deficiency of spleen and lung qi, eat less and feel tired easily, coughing and deficient panting, deficiency of qi and blood, withered face, palpitation and shortness of breath, thirst with fluid, internal heat and thirst.
In recent years, scholars at home and abroad have conducted more in-depth research on CR. Phytochemical research shows that the main chemical components of CR include alkaloids, alkynes and polyacetylenes, flavonoids, lignans, steroids, terpenoids, organic acids, volatile oils, saccharides and other components. In addition, the extract of CR has extensive pharmacological effects, including neuroprotective effects, protection of gastrointestinal mucosa and anti-ulcer, regulation of body immunity, anti-tumor, endocrine regulation, improvement of hematopoietic function, cardiovascular protection, anti-aging and antioxidant effects (Bian et al., 2022). In clinical application, CR is often used in combination with other traditional Chinese medicines (TCM), it can mainly treat anemia, hypotension, digestive disorders, acute plateau reaction (Liu et al., 2019; Wang et al., 2019). Moreover, there are studies and reports indicating that CR has almost no toxic side effects (Feng and Gao, 2012). Furthermore, CR is also widely used in healthcare medicines, health food, healthcare cosmetics and other fields, such as drinks, tea, biscuits, medicated meals, moisturizing cream, etc., (Lei et al., 2022).
CR is a valuable botanical drug that has received a lot of attention for its unique medicinal value and healthcare effects. In this study, we collected and organized the literature on CR from 1982 to the present. Except for duplicates and irrelevant literature, a total of 192 articles related to the botany, traditional applications, phytochemistry, pharmacology, toxicity, analytical methods and quality control, processing methods, and pharmacokinetics of CR were systematically summarized in this paper, to provide references for further development and utilization of CR and to explore possible research directions and new prospects of CR. In conclusion, this review is of great significance for further research and utilization of CR.
Codonopsis Radix, a perennial botanical drug of Campanulaceae. The stem is usually very short, and most of them have tumor-like stem marks. The roots are often plump, cylindrical, conical, spindle-shaped, block-shaped, oval, spherical or rosary, and are fleshy or woody. Stems erect or twining, climbing, inclined, ascending or procumbent. Leaves alternate, opposite, clustered or pseudowhorled. Flowers are borne singly at the tips of the main stem and lateral branches, opposite the petiole, and less frequently in the axils of the leaves, sometimes in scape form. CR enjoys a mild and cool climate, is cold-tolerant, and its roots can overwinter in the open ground in the soil. It is born at the mountain forest edge and shrub at an altitude of 1560–3100 m. The pictures of CR and its slices are shown in Figure 1.
There are more than 40 species in the whole genus of Codonopsis, distributed in eastern and central Asia, about 39 species in China, 21 species for medicinal purposes and 4 varieties. The Flora of China records that CR is produced in southeastern Tibet, western Sichuan, northwestern Yunnan, eastern Gansu, southern Shaanxi, Ningxia, eastern Qinghai, Henan, Shanxi, Hebei, Inner Mongolia and northeastern China, and is cultivated in large quantities throughout the country. The species of Radix Codonopsis are diverse and widely distributed, and most of them are named after their traits, origin, processing characteristics, etc., the phenomenon of different substance with the same name and different meaning for the same items often occurs (Bian et al., 2022). In addition, some local Codonopsis Radix is better than genuine Codonopsis Radix in certain pharmacological activities. For example, Codonopsis clematidea (Schrenk) C.B.Clarke [Campanulaceae; Xinjiang Dangshen] (CCS) polysaccharides can improve SOD activity and reduce MDA production, and its anti-free radical effect is better than that of Lu Dangshen (Xiong et al., 2000). In some areas, Codonopsis clematidea, Codonopsis nervosa (Chipp) Nannf. [Campanulaceae; Maihua Dangshen]and Codonopsis lanceolata (Sieb. et Zucc.) Trautv. [Campanulaceae; Lunye Dangshen] are also often used as medicine. According to “the ConPhyMP statement”, we sorted out the botanical drugs involved in the article (Rivera et al., 2014; Heinrich et al., 2022). Besides, we compiled the geographical distribution of common medicinal Codonopsis (genuine and local customary) and their identification methods, as shown in Table 1. However, the source of CR is complex, and the quality of different producing areas varies greatly, exists the phenomenon of substitute shoddy goods for good cargo. Therefore, it is necessary for us to establish a scientific, systematic and reasonable quality control standard and evaluation system of CR to provide assurance for safe and reasonable clinical use. In addition, the sources and characteristics of the confusions of CR are shown in Supplementary Table S1.
CR is a commonly used tonic botanical drug in China, through textual research of ancient books, CR has a long medicinal history, and it has been mixed with ginseng for 1,000 years. The name of Dangshen was first recorded in the Qing Dynasty’s “Baicaojing”, and before that there were similar records in the “Bencao”, but the name of Dangshen was not mentioned. In this section, we have compiled a list of famous writers on CR since it was recorded, to facilitate the better exploitation of Codonopsis Radix.
It is recorded in Ben Jing Feng Yuan (Qing Dynasty, 1617–1700) that the Shang Dang Ren Shen, though not capable of nourishing and warming, has the power of calming and clearing the lung, and it is not as cold as the Glehnia littoralis (A.Gray) F. Schmidt ex Miq. [Apiaceae; Glehniae Radix]. According to the New Compilation of Materia Medica (本草从新, AD 1757), it can tonify the stomach and spleen, and eliminate polydipsia. This effect is consistent with the discovery in modern pharmacology that CR protects the gastrointestinal tract and enhances the function of the spleen and stomach. Gang Mu Shi Yi (纲目拾遗, AD 1765) recorded that CR can cure lung deficiency and benefit lung qi. It is recorded in “De Pei Ben Cao” (得配本草, AD 1644–1911) that when Codonopsis pilosula is served and steamed with honey, it can tonify the lungs. According to Ben Cao Zheng Yi (本草正义, AD 1920), CR can nourish the spleen and stomach, moisten the lungs and produce fluid, and the tendons transport qi, which is not far from Panax ginseng C. A. Mey. [Araliaceae; Ginseng Radix Et Rhizoma]. Modern pharmacological studies have proved that the functions of CR in enhancing immunity, nourishing stomach and invigorating spleen are consistent with those in ancient books. In addition, we learned from ancient books that the efficacy of CR and Ginseng Radix Et Rhizoma was similar, and it was once used as a substitute for Ginseng Radix Et Rhizoma in clinical application. In general, for chronic asthenia such as spleen and stomach deficiency, lung qi deficiency, and body fatigue and weakness, CR can be used to treat these diseases instead of Radix Ginseng, and the two have been mixed for thousands of years.
Herb pair compatibility is not the stacking of any two drugs, but rather the pairing of drugs according to their properties, with the drugs cooperating with or restricting each other to achieve a better curative effect. It contains the wisdom and clinical experience of past dynasties of TCM physicians, and is a relatively fixed collocation form of two drugs in clinical TCM compatibility.
CR, under the guidance of Chinese traditional medicine theory, is often used in combination with other drugs. The compatibility of CR and Angelica sinensis (Oliv.) Diels [Apiaceae; Angelicae Sinensis Radix] can be used for patients with spleen and lung deficiency and can significantly enhance the immune function of the body (Wang et al., 2017a; Wang et al., 2017b). CR is combined with Atractylodes macrocephala Koidz. [Asteraceae; Atractylodis Macrocephalae Rhizoma], Poria cocos (Schw.) Wolf [Polyporaceae; Poria], and Glycyrrhiza uralensis Fisch. ex DC. [Fabaceae; Glycyrrhizae Radix Et Rhizoma] to treat spleen and stomach deficiency, anorexia, loose stool, and fatigue due to qi deficiency (Chen S. et al., 2002). Compatibility with Astragalus mongholicus Bunge [Fabaceae; Astragali Radix] can strengthen the effect of invigorating qi and enhancing immunity, and it is the most commonly used tonic traditional Chinese medicine (Zhou and Zhou, 2011; Shen and Jin, 2012). CR is combined with Atractylodis Macrocephalae Rhizoma, Angelicae Sinensis Radix, and Rehmannia glutinosa (Gaertn.) DC. [Orobanchaceae; Rehmanniae Radix Praeparata] to invigorate both qi and blood, and is used for treat sallow complexion, palpitation, and dizziness due to blood deficiency (Feng and Zhang, 2016). CR is combined with Schisandra chinensis (Turcz.) Baill. [Magnoliaceae; Schisandrae Chinensis Fructus] and Ophiopogon japonicus (L. f.) Ker Gawl. [Asparagaceae, Ophiopogonis Radix] for treating lung, kidney, and fluid injury, manifested as asthenia, soreness of waist and spermatorrhea, with the effects of invigorating qi, nourishing yin, and promoting fluid production (Yan et al., 2015).
CR is a traditional botanical drug commonly used in China and one of the most valuable herbs typically used. The commonly used clinical prescriptions include Shengmaiyin (Dangshen Prescription), Shenqi Baizhu Decoction, Shiquan Dabu Pill and so on. Among them, Shengmaiyin is most widely used in clinical practice and has the effects of benefiting qi, nourishing yin and promoting fluid production. In addition, the common dosage forms of CR involved decoction, pill, tablet, granule, capsule, syrup, etc. The auxiliary prescriptions of CR commonly used in clinic summarized from the ancient books of traditional Chinese medicine and China Pharmacopoeia are shown in Table 2.
As we all know, CR is one of the traditional Chinese medicines with homology of medicine and food. In some parts of China, CR is often used as food in daily diet, such as porridge, soup, stew, bubble wine, tea, etc. According to “China Medicated Diet Dictionary”, CR is a commonly used qi-reinforcing drug in medicinal diet for tonifying the middle, replenishing qi, and promoting fluid production, and is often used for the treatment of spleen and stomach deficiency, qi and blood deficiency, fatigue and weakness. In addition, Li Yan and others compiled “Miao Yong Dangshen Zhi Bai Bing”, which selected more than 100 therapeutic prescriptions of CR summarized by physicians of past dynasties. Besides, Codonopsis lanceolata has been listed as edible wild vegetable for a long time in China’s border and North Korea, South Korea, Japan and other regions without any toxic or side effects. In 2019, the National Health and Wellness Commission of China and State Administration for Market Regulationissued the Notice on the pilot work of material management for 9 kinds of substances, such as Codonopsis pilosula, which are traditionally both food and Chinese herbal medicines. Hebei Province, Gansu Province and other places have carried out pilot work of Codonopsis pilosula in combination with local characteristics. In recent years, there are research teams developed Dangshen biscuits, Dangshen Gao, Dangshen Fu, Dangshen nougat, Dangshen Mi-jian, Dangshen-Huang-qi drinks and other products. Therefore, we organized some common recipes of CR and their effects, as shown in Table 3. In addition, studies have also reported the application of CR in beauty and skin care products. Codonopsis oligosaccharides have anti-aging effect and can be used in emollient creams (Zhou, 2021).
CR contains a variety of chemical components, mainly including alkaloids, alkynes and polyacetylenes, flavonoids, lignans, steroids, terpenoids, organic acids, volatile oils, sugars and other components. In addition, CR is also rich in amino acids, inorganic elements and trace elements required by the human body (Zhan et al., 2021). In this section, we summarize the main chemical constituents of CR in Table 4.
Alkaloids are an important class of natural organic compounds, which are cyclic compounds containing negative oxidation state nitrogen ions and present in biological organisms, including pyrroles, piperidines, tropanes, etc. The structures are shown in Supplementary Figure S1.
Alkynes and polyacetylenes are widely distributed in Campanulaceae plants. What’s more, the Chinese Pharmacopoeia also uses lobetyolin as an index component for the quality evaluation of CR. Studies have shown that in addition to anticancer, antibacterial and anti-inflammatory effects, lobetyolin also have good protective effects against gastric mucosal damage (Gao et al., 2018; Xie and Wang, 2022). The structures of Alkynes, Polyacetylenes and their glycosides are shown in Supplementary Figure S2.
Flavonoids refer to a series of compounds consisting of two benzene rings with phenolic hydroxyl groups interconnected by three central carbon atoms—compounds composed of C6-C3-C6 units. It is widely distributed in the plant kingdom and has a variety of physiological activities. In addition, it has antioxidant, antiviral, hepatoprotective, anticancer, anti-inflammatory and other pharmacological activities, and is an important class of natural organic compounds. The flavonoid components isolated from CR are mainly flavones, flavonols and their glycosides. The structures of Flavonoids compounds are shown in Supplementary Figure S3. At present, there are fewer studies on the flavonoid component of CR, and in future studies, we should pay more attention to this component with multiple pharmacological effects.
Lignans are defined as a class of natural products with a phenyl propane backbone formed by two structures linked by β, β´ or 8, 8´-carbons therein, usually referring to their dimers and, to a lesser extent, trimers and tetramers.
Tangshenoside I-IV are exclusive components of CR, of which tangshenoside I is about ten times more than the other three glycosides (Mizutani et al., 1988). However, there are still few reports on the pharmacological effects of Tangshenoside I. The structures of lignans are summarized in Supplementary Figure S4. In the following research, we should pay more attention to this exclusive ingredient of CR, and expand the scope of its medical use.
Steroids are a naturally widespread class of chemical components, of which there are many types, but all have cyclopentano-perhydrophenanthrene in their structure. The steroids components of CR are sterols, steroidal glycosides and steroids, and the structures of each component are shown in Supplementary Figure S5.
Terpenes are the most abundant class of natural products with a wide distribution, complex skeleton and a variety of biological activities. In addition, terpenes are compounds that have an isoprene unit (C5) as the basic structural unit. The terpene components isolated from CR mainly include tetra- and pentacyclic triterpenes, hemi- and sesquiterpenes, and they mostly exist in the form of glycosides. The sesquiterpene lactone compounds atractylenolide II and atractylenolide III were isolated for the first time from CR by Wang ZT et al. and reported for the first time in Campanulaceae (Wang et al., 1988). Atractylenolide III is one of the main components of CR, which has relatively obvious anti-inflammatory effect. Some researchers believe that atractylenolide III can also be used as an index component in the quality evaluation of CR. In this section, we systematically summarize the chemical composition of CR and its structures are summarized in Supplementary Figure S6.
Organic acids are compounds containing carboxyl groups (excluding amino acids) that are widely found in living things. The most common organic acids are carboxylic acids, whose acidity originates from the carboxyl group (-COOH). In addition, sulfonic acid (R-SO3H), sulfinic acid (RSOOH) and thiocarboxylic acid (RCOSH) are also organic acids. The organic acid composition in CR is summarized in Supplementary Figure S7.
Volatile oil, also known as essential oil, is a generic term for a class of oily droplets with an aromatic odor, which contains a complex composition, usually consisting of tens to hundreds of components. Liao J et al. identified 32 components from the volatile oil of CR, 11 acidic components, mainly brown shackle acid, and 21 neutral components (Liao and Lu, 1987); Li Cong et al. used GC-MS coupling technique to isolate 66 components from the volatile components of CR, and identified 50 chemical components by Wiley and NBS standard spectral library search (Li et al., 1993); Guo QQ used steam distillation and headspace gas chromatography to extract volatile components of CR for GC-MS analysis, and 117 volatile compounds were detected (Guo, 2016). The volatile oil components of CR are mainly alcohols, aldehydes, terpenes, ketones, furans, acids, olefins, alkanes, phenols, sulfides and other compounds. In this paper, we compiled 120 volatile oil components extracted from CR, as shown in Table 5.
Saccharides are widely found in plants, and ginseng is also rich in saccharides, including monosaccharides, oligosaccharides and polysaccharides, etc. Moreover, Codonopsis polysaccharides (CPPs) is one of the main components of CR, mainly composed of five-carbon sugar, six-carbon sugar and its derivatives, which has good central inhibitory effect and anti-ulcer effect (Zhao, 2016). We have systematically summarized the saccharides compounds of CR, the methods of extraction, separation and purification, monosaccharide composition and analysis method, as shown in Table 6.
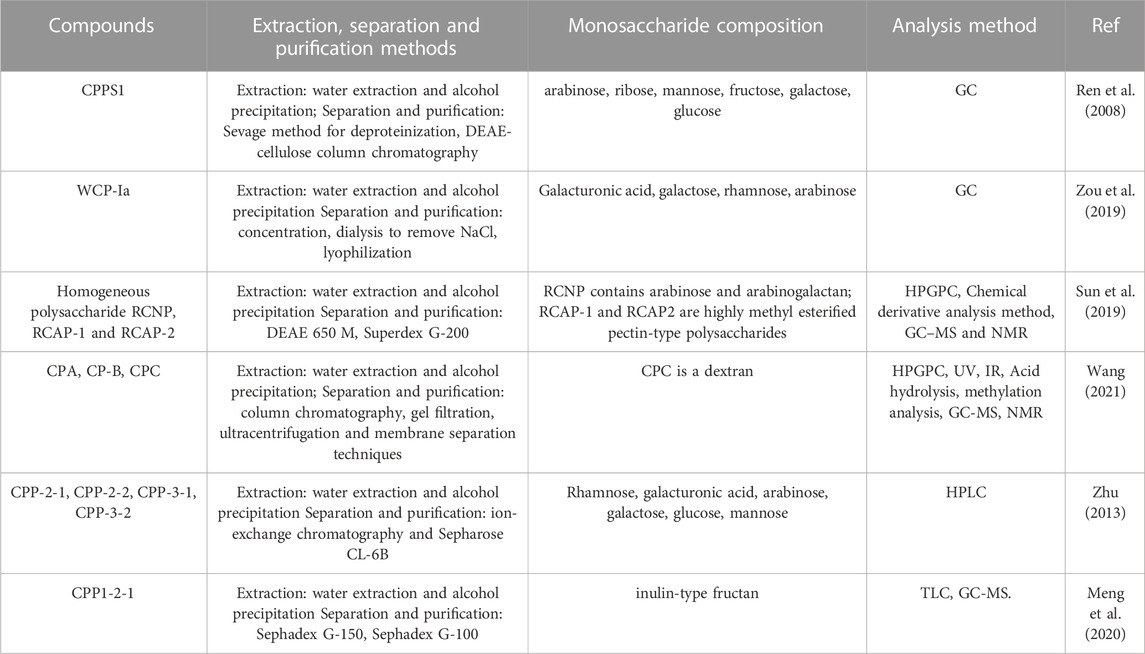
TABLE 6. The saccharides compounds of CR, the methods of extraction, separation and purification, monosaccharide composition and analysis method.
In addition to the above chemical constituents, other chemical compositions of CR are listed in Table 7, and the structures are shown in Supplementary Figure S8. In addition, CR contains many amino acids, such as glutamic acid, glycine, tyrosine, serine and other 15 amino acids, as well as Ca, Mg, Zn, Fe, Cu, Mn and other 33 inorganic elements. Among them, the content of nutrients and beneficial elements (such as P, Ca, Mg, Fe, Zn, etc.) required by human body is high, while the content of harmful elements (such as Pb, Cd, AS, Hg, etc.) is low (Wang et al., 2011; Sun et al., 2015).
In this section, we provide a detailed summary of the chemical constituents obtained from the current extraction and isolation of CR. Currently, more than 400 compounds are obtained from CR, including 120 volatile oil components. In addition, the traditional medicinal part of CR is the root, and the above-ground part is less studied, while the above-ground part contains a variety of chemical components with high utilization value, but its utilization and research are only in the primary stage, and the reasonable and effective exploitation has not yet been carried out, resulting in a serious waste of resources. In the next research, we should study other parts of CR, such as the leaves and stems of it, in order to enhance the utilization of non-traditional medicinal parts of medicinal plants and carry out a deeper comprehensive utilization of resources to avoid wasting resources.
There are many chemical components and various methods of determination of CR. With the development of modern technology, the instruments are also becoming more sensitive, precise and accurate. In this paper, we have compiled the analytical methods of CR in recent years in order to help scholars to better develop and utilize CR and establish quality control standards for CR. Phenol-sulfuric acid method is the main method for determination of polysaccharide content, scholars used UV-300 ultraviolet spectrophotometer, 721 spectrophotometer and phenol-sulfuric acid colorimetric method to analyze the polysaccharides of CR (Wang et al., 1993). Analysis of total saponins of Codonopsis (TSC) and CPPs of CR by ultraviolet spectrophotometry (UV-Vis) by Dou et al. (2015); Dou et al. (2016). Zhen X et al. used UV spectrophotometric method to determine CPPs of Lu Dangshen and other origins of CR and found that the average recovery was 97.56%, and the average value of CPPs of CR was 18.39%, which provided the basis for the quality standard of Lu Dangshen (Zhen et al., 2014). The method is characterized by higher sensitivity, better accuracy, simple instrumentation, easy operation and faster analysis, but lacks selectivity for related substances with similar structures. The volatile oil components are often determined by gas chromatography, Guo analyzed the volatile components of CR by Head space gas chromatography-mass spectrometry (HS-GC-MS) to search for the main volatile components with special aroma in CR (Guo, 2016). The pretreatment of this method is simple and the cost of the instrument is low, but it requires the substance to be measured to have volatility. Therefore, this method has certain limitations and narrow application scope.
At present, the chemical constituents of CR are mostly studied by HPLC, which provides reference for establishing the quality standard of CR. For example, some scholars use HPLC method to analyze the content of lobetyolin in different origins (Song et al., 2008; Shi et al., 2011; Chen H. et al., 2014); Other scholars used HPLC to simultaneously determine the contents of lobetyolin and syringing in CR from different origins (Chen et al., 2016). This method is featured with wide applicability, high separation efficiency, high automation and easy sample recovery. The mobile phase, however, is mostly toxic organic reagent, which will pollute the environment. There are also scholars who evaluate the quality evaluation of Codonopsis Radix and processed products based on the analysis of monosaccharides and oligosaccharides by liquid chromatography coupled with charged aerosol detector, the method is independent of the optical or structural characteristics of the sample and is responsive to samples that do not contain chromophoric groups (Xie et al., 2022). With the enhancement of people’s awareness of environmental protection, supercritical fluid chromatography (UPC2) has come into the view of the majority of scholars. It uses supercritical fluid as the mobile phase (supercritical CO2 is often used), which has the advantages of both gas chromatography and liquid chromatography, and can achieve rapid and effective separation. Using the UPC2 method, Yang Liu et al. screened and quantified the quality markers of Angelica pubescens Maxim. f. biserrata Shan et Yuan [Apiaceae, Angelicae Pubescentis Radix], which is greener and safer compared to the traditional chromatographic method (Yang et al., 2020). This can be used as a reference for relevant analytical work in future research on CR. In addition, the higher sensitivity and better accuracy of chromatography-mass spectrometry tandem technique can also effectively reduce the experimental cost. For example, some scholars have established UPLC-MS/MS method to determine the chemical composition of CR (Ma F.et al., 2014; An et al., 2018; Zheng et al., 2021). Compared with the traditional liquid phase method, this method has the advantages of high sensitivity, short analysis time, good selectivity, low detection line and wide application range. However, the instrumentation of the above methods is high-end and expensive. We believe that with the development of science and technology, more low-cost and efficient instruments as well as green reagents will be available, and in the near future, we can use more efficient, accurate and green methods for the research related to CR.
CR, a traditional Chinese medicine, has complex chemical components and wide drug sources. As a natural product of medicine and food, people’s demand for CR is increasing day by day. However, there are many kinds of CR, and it is difficult to distinguish them. Moreover, fake and inferior products of CR are often found in the Chinese herbal medicine market. In addition, the quality of Chinese medicinal materials is affected by geographical location, climatic environment, cultivation techniques and other aspects. There are some differences in the quality of medicinal materials from different places of origin, and more importantly, the quality of medicinal materials from the same place of origin is unstable. Due to the above factors, currently, the quality and clinical efficacy of CR cannot be effectively controlled. Therefore, it is necessary for us to use modern science and technology to establish quality control standards for the quality of CR and to evaluate its quality. In this paper, we have compiled literature on quality control of CR from a wide range of scholars in recent years, hoping that it can be helpful for the establishment of quality control standards for CR.
Currently, the 2020 edition of Chinese Pharmacopoeia uses thin-layer chromatography to identify CR, and requires that the moisture content should not exceed 16.0%, the total ash content should not exceed 5.0%, and the sulfur dioxide residue should not exceed 400 mg/kg. Using the hot leaching method to determine, with 45% ethanol as the solvent, the leachate should not be less than 55.0%.
However, there is no content determination item in Chinese Pharmacopoeia, but only characterization and identification items. Chinese medicines are featured with multi-component and multi-target, etc. To establish a perfect quality standard research, it is also necessary to clarify the active ingredients or indicator components for Chinese medicines to exert their medicinal effects. Ichikawa et al. simultaneously determined the contents of seven saponins in CR by LC-MS (Ichikawa et al., 2009); Chen J et al. measured the total ash and acid-insoluble ash and determined the content of Atractylenolide III in CR by HPLC (Chen et al., 2012); Kim et al. used HPLC-UV to analyze tangshenoside Ⅰ, lobetyolin and lobetyol to establish the fingerprint profile of CR (Kim et al., 2014); Peng R et al. used HPLC to measure the content of lobetyolin, colorimetric method to determine CPPs and TSC, atomic absorption spectrometry to analyze the contents of As, Pb, Hg, Cd and Cu in CR, gas chromatography to determine BHC and DDT, and the rest of the items were determined by referring to the method included in the Chinese Pharmacopoeia. The results showed that the content of lobetyolin was not less than 0.53 mg g−1, CPPs was not less than 14.7%, and the TSC was not less than 7.3 mg g−1 (Peng et al., 2014).
In addition, the chemical components of traditional Chinese medicine are complex and diverse, and the therapeutic effect is through the whole of multiple components and multiple targets. The components of CR are diverse and have different pharmacological effects. CPPs has the function of enhancing immunity, Atractylenolide III has the effect of anti-inflammation, and lobetyolin has anti-gastric ulcer pharmacological activity. Therefore, there are certain limitations in applying a single component as a quality control standard to effectively keep the quality of CR. The complex and diverse chemical composition and the unclear pharmacological pathways are the key points and difficulties in the quality evaluation of Chinese medicine. More methods should be built on activity-related or quality evaluation based on chemical activity, and the quality standard and evaluation system that can reflect the core efficacy of CR should be worked out to ensure the scientific, rational and safe use of CR (Qi et al., 2022).
Processing is a major feature of TCM medication and an important part in improving clinical efficacy. According to the needs of the disease, the original properties of the drug are traded off through processing, so that the desired effect is highlighted and other effects are diminished to better suit clinical treatment.
The CR has been involved in the mixing of Radix Ginseng for thousands of years. The name of Dangshen and its medicinal method were first recorded in the Qing Dynasty, with a short medicinal history. Further, when used as a medicine, raw Codonopsis Radix is commonly used, and has the effects of replenishing qi and promoting fluid production, which is mostly used for the treatment of spleen and lung qi deficiency, food deficiency and fatigue, as well as qi and blood deficiency. Therefore, its ancient processing method is relatively simple and is occasionally recorded in ancient books.
In terms of purification, the main method used is to “remove the tip” (Qing “Zhi Jin”); “Bamboo knife scraping, violently dried” (Qing dynasty, “Ben Cao Hai Li”); Processing, have “Honey broiling” (Qing dynasty, “Zhi Jin”); Tonifying the lungs, steaming with honey. (Qing Dynasty, “De Pei Ben Cao”); Stir-baking with rice of CR, can cure diarrhea due to deficiency-cold of spleen (Qing Dynasty, “Shi Bing Lun”) (Li et al., 1993; Chen and Fang, 2013). In addition to that above processing methods, there is also the characteristic processing method of CR of Menghe Medical School, including Ginger Juice fried Codonopsis Radix, (Ma Peizhi’s medical case, 马培之医案; He jiheng’s medical case, 贺季衡医案), Agastachis Herba fried Codonopsis Radix (Ma Peizhi’s medical case), Aucklandia Radix fried Codonopsis Radix (Deng Xingbo’s Clinical Medical Collection, 邓星伯临证医集) and so on (Lv et al., 2018; Shen et al., 2023).
In conclusion, the ancient methods of purification of CR include removing the tip and bolting skin, and the methods of processing include honey-roasted CR, steaming with honey, and fried CR with rice.
The modern common processing methods of CR include fried CR with rice (Wang et al., 2020), honey-roasted CR (Tang et al., 2022), soil fried CR, fried CR with bran and CR processed with wine (Liu et al., 2006). The chemical composition changes and pharmacological effects before and after processing by different processing methods are summarized in Table 8.
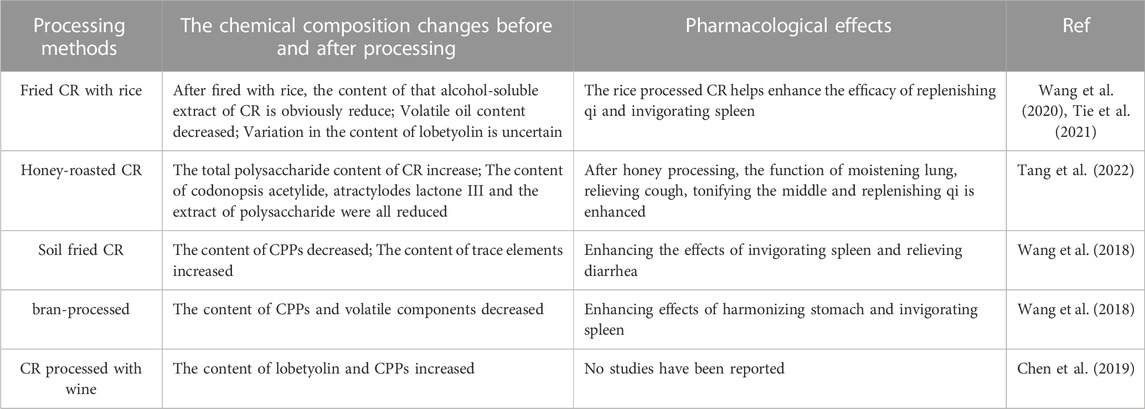
TABLE 8. The chemical composition changes before and after processing and pharmacological effects by different processing methods.
Fried CR with rice is the most commonly used method of processing CR, which is included in China Pharmacopoeia (2020 edition). After fried with rice, the smell is burnt and fragrant, which can enhance the effect of invigorating qi and invigorating spleen. At present, the changes of lobetyolin content after rice frying are inconsistent, which may be due to differences in processing technology. Studies have shown that with the increase of frying time, the content of lobetyolin increases firstly and then decreases (Wang M. et al., 2021). So far, the processing technology of CR has mostly continued the ancient method without specific process parameters, and still relies on empirical judgment and does not quantify indicators such as firepower and temperature, which is not conducive to the large-scale processing and production of modern enterprises. Therefore, in the next study, the processing standard of CR should be established, and the factors affecting the quality of CR such as its processing materials, processing time and fire power should be detailed and digitized, and the quality evaluation standard of CR should be established to facilitate the rational clinical application of CR. In addition, the current research on the changes of chemical composition of CR before and after concoction is only limited to CPPs, lobetyolin and volatile oil. Chinese medicines are characterized by multi-target and multi-component combined effects, so the study of only a few components is limited and unspecific. The study of the changes in chemical composition before and after concoction should be more in-depth and comprehensive.
Modern pharmacological studies have shown that CR has the ability to protect nerve cells (Luo et al., 2017), protect gastrointestinal mucosa and anti-ulcer (Cui et al., 1988), regulate body immunity (Zhang et al., 2003), anti-tumor (Wu et al., 2016), improve hematopoietic function and protect cardiovascular (Li Q. et al., 2019), antibacterial and antiviral (Bai et al., 2015), anti-aging and antioxidant effects (Xu et al., 2006), which have been confirmed by many scholars. These pharmacological effects are summarized in Table 9 and are discussed in detail below.
CPPs had a significant protective effect against sodium thiosulfate injury in neural stem cells (Wu et al., 2008). The study by Zhang Q et al. found that CPPs could activate PP2A to prevent AD-like tau protein hyperphosphorylation, which could rescue neuronal loss and alleviate AD-like cognitive impairment (Zhang et al., 2018). Banqiao Badix Codonopsis Polysaccharide (BCP) could effectively improve cognitive dysfunction induced by elevated GSK-3β activity in rats, and the possible mechanism was related to the downregulation of GSK-3β activity, which in turn inhibited Tau protein hyperphosphorylation and promoted neuronal development, and had some preventive or delaying effects on AD (Luo et al., 2017). Wan L et al. found that CPPs attenuate Aβ pathology in APP/PS1 mice and that downregulation of BACE1 may be a potential mechanism which could serve as a therapeutic target to attenuate cognitive deficits in AD pathology (Wan et al., 2020). Hu Y R et al. suggest that CPPs protect PC12 cells from Aβ1-40-induced injury, indicating that these components of CR may represent an early therapeutic option for AD patients (Hu et al., 2021). However, the therapeutic effect and mechanism of CPPs on early AD model in vivo and in vitro still need further study. CPPs improved memory consolidation impairment in mice induced by cycloheximide, and the mechanism was related to the upregulation of protein expression of CaMKII/CREB signaling pathway (Liu, 2020).
Cui J C et al. found that CPPs had significant protective effects on four rat gastric ulcer models (stress type, anti-inflammatory pain type, acetic acid type, and pylorus ligation type). In addition, it had a significant inhibitory effect on the increase of gastric acid caused by pilocarpine, and the content of PGE2 in gastric juice was increased (Cui et al., 1988). Chen S F et al. observed that CR increased the content of growth inhibitors in the gastric and duodenal mucosa of rabbits, which is beneficial for the treatment of peptic ulcer (Chen S. et al., 2002). Li R L et al. discovered that the water extract of CR could reduce gastric mucosal injury and pathological damage in rats with stress ulcers and increased the content of polyamines (spermidine) in gastric mucosa. And in a subsequent study, it was found that the cell migration-promoting effect of the flavonoid-extracted part of CR was related to the influence of polyamine regulatory signaling pathways (Li et al., 2013; Li L. et al., 2014). Ma F L et al. showed that the CPPs had the effect of promoting small intestinal peristalsis in mice. Moreover, it could also promote the growth and improve the digestive ability of rats (Ma X. et al., 2014). Zhao Xiaofang studied the significant alleviating and antagonistic effects of BCP on TNBS-induced ulcerative colitis in rats, and significantly improved the damage caused by UC on colonic mucosa; The mechanism of therapeutic effect on UC may be through the activation of TLR4-NF-κB pathway to regulate the secretion of cellular inflammatory factors (IL-6, TNF-α and IL-10) and inflammatory mediators (NO), and is also associated with antioxidant and anti-inflammatory effects (Zhao, 2016). Li J K et al. first reported that inulin-type fructan CP-A may be a potential component in CR for the treatment of acute gastric ulcer. CP-A significantly increased SOD and GSH-Px activities in gastric tissues. Besides, MDA and NO content and MPO activity were decreased, and in a dose-dependent manner (p < 0.05) (Li J. et al., 2017). However, this experiment is only based on theory, lacking in vivo verification. In the future research, further animal experiments are needed to verify its anti-ulcer effect. Meng J found that CR can intervene in aging as a whole and protect the gastrointestinal tract of aging mice, OC105243318, Fam132a, RORC and 1200016E24Rik genes may be potential targets for CR to protect the aging gastrointestinal tract, and their interaction network may play an important role in CR protection of the aging gastrointestinal tract (Meng, 2021). Liu et al. revealed that TSC had a significant protective effect on colonic mucosal injury in UC rats, and the mechanism may be related to anti-lipid peroxidation, inhibition of NF-κB signaling pathway and thus regulation of inflammatory factor release (Liu et al., 2021).
The methanol extract of CR has effect in regulating macrophage-mediated immune response, and promote anti-inflammatory activity (Lee et al., 2007). Lu Dangshen Oral Liquid could regulate immunosuppressed mice by promoting the development of immune organs, proliferation of immune cells and increasing the level of immune factors (Tianhui et al., 2019). CPPs enhanced the proliferation of rat splenic lymphocytes and their ability to secrete IL-2 and IL-4, and enhanced the proliferation and phagocytosis of rat macrophages and their ability to secrete TNF-α and IL-6 in vitro. In vivo animal experiments, CPPs can increase the spleen index and thymus index of immunocompromised rats, and increase the levels of IgG, IgM, IgA, C3 and C4. Its molecular mechanism may be related to the inhibition of the expression of Bax and NF-κBp-p65 proteins in immune organs, and the promotion of the expression of Bcl-2 protein in immune organs (Xu, 2018). BAI R B et al. studied that Codonopsis pilosula oligosaccharide (CPO), CPO could increase immune organ index, phagocytic index and immunoglobulin content, stimulate the proliferation of splenic lymphocytes (in synergy with ConA and LPS), enhance ear swelling in DTH response, promote the production of NO and cytokines (IL-2 and IFN-γ), and upregulate the expression of corresponding mRNA (Bai et al., 2020). This study is the first to show that the quality of Codonopsis pilosula is related to its sweet component, oligosaccharides. However, although the sugar content of CPO up to 92.7%, it is still a mixture of glycans, and its specific active ingredients need to be verified. SUN Q L et al. showed that RCAP-1 and RCAP-2 had a significant immune stimulating effect on NO production by RAW264.7 macrophages (Sun et al., 2019).
Codonoposide 1c is a potent apoptosis inducer that induces apoptosis in HL-60 cells (Lee et al., 2005). The saponins of Codonopsis could inhibit the proliferation of human hepatocellular carcinoma HepG-2 and SMMC-7721 cells. (Li, 2012; Fang et al., 2015).The water-soluble polysaccharides CPP1a and CPP1c induce apoptosis in HepG2 cells by upregulating the Bax/Bcl-2 ratio and activating caspase-3. In addition, it is cytotoxic to cervical cancer Hela cells and gastric cancer MKN45 cells. (Bai et al., 2018). Pectic polysaccharide CPP1b is significantly cytotoxic to human lung adenocarcinoma A549 cells in a dose- and time-dependent manner (Chen W. et al., 2014). More importantly, CR could exert anti-gastric precancerous effects by ameliorating gastritis damage and selectively inhibiting the proliferation of gastric cancer cells but not normal cells (He W. et al., 2022). CPW1 stabilized SeNPs are selectively tumorigenic to Huh-7 and HepG2 cells with strong anti-proliferative and pro-apoptotic activities, but do not inhibit the viability of human normal cells (Hu et al., 2022). Lobetyolin and lobetyol are the main active anti-tumor components of Codonopsis lanceolata, Codonopsis lanceolata Polyacetylenes induces apoptosis in lung cancer cells and ameliorates lung dysfunction by inactivating the Ras/PI3K/AKT pathway (Wang et al., 2022). CPPs inhibit MCF-7 cell proliferation and induce apoptosis through downregulation of CCHE1 expression (He R. et al., 2022).
CR attenuates insulin-like growth factor II receptor pathway damage in cardiomyocytes and reduces cardiomyocyte apoptosis (Tsai et al., 2013). CR restored LV systolic pressure (LVSP) and maximum rate of intraventricular pressure change (±dp/dtmax) to varying degrees (Р < 0.01), reduced LV end-diastolic pressure (LVEDP) (Р < 0.01), inhibited the elevation of MDA, LDH and CK, and enhanced SOD, GSH-Px, Na+, K+-ATP and Ca2+-ATP activity (Ling, 2012). CR intervention improves cardiac systolic and diastolic function to varying degrees, increases peak calcium transients and shortens fallback time (Qun et al., 2017). CPPs can retard X-ray-induced hematopoietic stem cell senescence, and its mechanism of action may be related to p53-p21 signaling pathway, Bax and Bcl-2 apoptosis pathway (Li Y. et al., 2017). The alcoholic extract of CR and potentially active compounds can promote the proliferation of hematopoietic stem cells, maintain the stemness of hematopoietic stem cells, and thus perform hematopoietic improvement functions (Gao, 2020). The extract of CR can promote the cardiogenic differentiation of embryonic stem cells and improve the cardiac function of infarcted heart (Wang J.-N. et al., 2021). Codonopsis lanceolata can significantly increase SOD activity, improve myocardial antioxidant level and reduce MDA expression, while significantly reducing the expression of sICAM-1, sVCAM-1 and TNF-α in rat cardiomyocytes, with certain myocardial protective effects (Zhang Y. et al., 2021).
The ethanolic extract of CR had certain antibacterial effect, while the crude polysaccharide of CR had no obvious antibacterial effect, and only had some antibacterial activity against Salmonella (Bai et al., 2015). In addition, it has been found that the alcoholic extract of CR has not only inhibited Bacillus catharticus, Staphylococcus epidermidis, Streptococcus hemolyticus type A and B, Bacillus subtilis, Bacillus anthracis, Escherichia coli, Staphylococcus aureus and Klebsiella pneumoniae, but also has inhibited B. anthracis and Salmonella typhi (Duan et al., 2012). Phosphorylated Codonopsis pilosula polysaccharide reduces the amount of DHAV and has stronger anti-DHAV activity than CPPs (Ming et al., 2017). In addition, studies have also found that BCP has a good preventive effect against BVDV virus (Xuan, 2019).
It has been found that the water extract of CR can delay aging by antioxidant, but not with the activity of antioxidant enzymes SOD and CAT (Wu, 2021). Moreover, Lu-dangshen has the effect of anti-skin photoaging, and one of the possible mechanisms is that Lu-dangshen can reduce the expression of inflammatory factors in skin tissue of photoaging mice, and directly or indirectly reduce the effect of inflammatory factors and their receptors on the genes and proteins related to MAPK and PI3K signal transduction pathways (Wang, 2020). There are scholars who have used a network pharmacology approach and found that the antioxidant stress effects of lobetyolin, L-tryptophan, and syringin may act through total antioxidant enzymes and the Keap1-Nrf2 pathway (Liao, 2022). However, this experiment does not have data to support its results, which can be discussed in depth by scholars next.
In addition to the above-mentioned pharmacological effects, CR also has a wide range of pharmacological activities in anti-stress (Hou et al., 2013), anti-inflammatory (Zhang J. et al., 2021), antifatigue (Yi et al., 2021) and antihypoxia (Yan et al., 2006; Xie et al., 2020a; Xie et al., 2020b).
In conclusion, there are many common problems in the current research on the pharmacological effects of CR, such as the lack of controlled studies between positive drugs and CR, unclear active components in related pharmacological effects and insufficient research on the mechanism of related pharmacological effects.
First of all, currently, the research on CR and its extract has a wide research prospect. But the extract is complex and its mechanism is difficult to elucidate. Therefore, it is necessary for us to further study the monomer compounds of TCM. However, up to now, studies on the pharmacological activity of CR mainly focus on CPPs, lobetyolin and TSC. The possible reason is that the other components in CR are lower and the efficacy is not obvious. Consequently, the current understanding of the active ingredients in CR is relatively limited. Therefore, scholars can use more sophisticated instrumentation methods to investigate the other components in CR.
Second, although we have made some progress in our current research on the pharmacological effects of CR, we still have a long way to go, such as the lack of research on the optimal extraction process of polysaccharide compounds in CR and the lack of research on the optimal effective dose of CR immunomodulation. Moreover, it is difficult to have an accurate indicator of clinical dosage due to the different content of polysaccharides extracted by different methods. Thus, it is necessary to establish a unified standard for the extraction of polysaccharide compounds in CR for better clinical application.
Third, Cao H F found that total saponins of Codonopsis pilosula nanoemulsion could enhance the regulation of cellular, humoral and non-specific immunity by TSC (Cao and Wang, 2019). In the following research, scholars may also consider developing cheaper, environmentally friendly, safe, and effective materials to replace the traditional codonopsis preparations, and developing more new dosage forms of Chinese medicine for clinical application.
Fourth, TCM has natural advantages that synthetic drugs do not have the anticancer activity of CR has been demonstrated in previous studies. But there is no doubt that the inclusion of CR in the list of anticancer drugs still requires a great deal of research. In the next step, it is necessary to establish suitable models to determine the mechanism of action, safety and dose range, as well as to perform histological and immunohistochemical assays. Besides, the antimicrobial activity of Chinese herbs, which are of wide interest because of their high efficiency, low toxicity and green. However, due to the complexity of the active ingredients of CR, the above experiments were performed only for preliminary antibacterial tests on CR extracts. Further research and studies are needed to investigate the components of the extract that have antibacterial activity and their antibacterial mechanisms.
Last but not least, Flavonoids from CCS have obvious antioxidant and anti-fatigue pharmacological effects (Wang et al., 2012). This also reminds us to strengthen research on non-traditional drug parts and local commonly used products, expand drug sources, and make rational use of drug resources. For example, the development of stems and leaves and local common products into health products or food additives can not only reduce the waste of resources but also extend the industrial chain of CR products, which has important ecological and practical significance.
In a word, we have systematically summarized the pharmacological effects of CR and provided a reference for its development and utilization for future studies. In subsequent studies, researchers can use this as a basis for in-depth studies on CR.
The Chinese botanical drug CR has a complex and diverse composition and can be used for both qi and fluid injuries or qi and blood deficiencies. Moreover, it is necessary for us to study the toxicity of CR in depth to provide a scientific basis for the rational clinical use of CR. In this paper, we reviewed the literature related to the toxicity of CR that we could collect so far, and the results showed that CR has no significant toxic side effects and a wide range of safety. Cheng J L et al. conducted an acute toxicity test on SPF grade KM mice with broken wall powder of CR, and no acute toxic reactions were observed in mice (Cheng et al., 2011). Scholars studied the acute toxicity of CPPs on ordinary-grade mice. The mice were oral administered with the maximum concentration of CPPs 0.5 g/mL (20 g/kg) for three times a day. There was no adverse effect on the body weight, activity, food intake, and external signs of the mice. All the mice survived in good health 7 days after administration (Feng and Gao, 2012). WU J L et al. conducted an acute toxicity test on mice with CPPs and adopted the methods of intravenous administration, intraperitoneal administration and gavage administration, respectively, and obtained the result of low toxicity of CPPs by continuous observation for 2 weeks (Wu et al., 2016); Hou L L et al. conducted a long-term toxicity test on rats with the oral solution of CPPs. No significant toxic effects were observed by analyzing hematology, blood biochemistry, weight, and organ histopathology sections of rats (Hou et al., 2016). There are also researchers conducted acute oral toxicity test, genetic toxicity test and subchronic toxicity test on ICR mice and SD rats with the extract of CR, and the results showed no significant toxicity. Moreover, no harmful effect was observed at the dose of 8.0 g/kg BW (equivalent to 16.0 g/kg BW of CR), which met the requirements of National food safety standards (Hu Z. et al., 2018). Xu X H et al. showed no significant toxic effects in the acute oral toxicity test, three genotoxicity tests (bacterial revertant mutation test, mammalian erythrocyte micronucleus test and mouse spermatocyte chromosome aberration test) and subchronic toxicity test on Wen Dang and Baitiao Dang (Xu et al., 2021; Xu et al., 2022).
In conclusion, there is no acute toxicity, subchronic toxicity, genotoxicity or long-term toxicity in CR and its extracts, and CR has a wide range of safety. Thus, CR has a high edible safety and the efficacy of tonifying the middle and replenishing qi. While worrying about “The medicine has side effects”, we cannot ignore the dual-use of some Chinese medicines as both food and medicine. Currently, CR has been included in the National List of Food and Drug Substances in Japan, Singapore and other countries. On April 24, 2018, the National Health and Wellness Commission of China issued the letter on soliciting opinions on the administration of using 9 substances such as CR as substances that are traditionally considered as both food and Chinese medicinal materials (national health office food letter [2018] No.278), clarifying the legal status of CR as food. At present, CR is not included in the list of medicinal and food products. Therefore, in the future, a large number of animal and clinical experiments should be conducted to verify its safety, so that it can be better used as a medicinal and food product for clinical treatment and healthcare, and to provide information for the development and utilization of CR in the food field.
Currently, there are few reports on the pharmacokinetics of CR extract and its main active components. DOU X et al. et al. demonstrated that the micronization of CR could promote the absorption and bioavailability of lobetyolin in rats (Xia et al., 2016). However, the main active ingredients of CR include CPPs besides lobetyolin. In order to clarify the advantages of ultramicro-powder more scientifically and comprehensively, it is necessary to continue to study the pharmacokinetics of CPPs in vivo. Dong J J et al. established the UPLC method for the determination of lobeyolin in rat plasma and found that, lobeyolin is poorly absorbed or widely metabolized in rats, and there are interactions among the components of CR extract, which leads to differences in pharmacokinetics between lobeyolin and CR extract. The reasons for the low bioavailability of lobetyolin and the methods to improve its oral absorption need to be further studied, so as to provide reference for its preparation (Dong et al., 2021). The concentration of inulin-type fructan CPA in CR was detected by fluorescent labeling method, and the in vivo process of CPA was analyzed. It was found that FCPA could be absorbed through blood circulation after oral administration, with low bioavailability, and its tissue distribution was wide, with long T1/2 and MRT (Guo et al., 2022). Scholars used UHPLC-Q/TOF-MS and UHPLC-MS/MS methods to analyze the metabolic pathways of three polyacetylene compounds from CR: lobetyol, lobetyolin, and lobetyolinin in vivo and in vitro (Xie et al., 2023).
However, at present, there are few studies on pharmacokinetics of CR, and little is known about how the active ingredients of CR are absorbed from outside to inside, how they are distributed, metabolized and excreted in the body. The complexity of ingredients, uncertainty of effective ingredients and diversity of analogues of traditional Chinese medicine make it difficult to develop pharmacokinetics of traditional Chinese medicine. Therefore, we need to strengthen the work in this area, combine the exploration of pharmacokinetics with the theoretical exploration of TCM, and strengthen the study of metabolites kinetics.
Chinese medicine is a natural product that has advantages not found in synthetic drugs. CR, as one of the traditional Chinese medicines, can be used as both medicine and food. In this paper, the compounds extracted and separated from CR and their structural formulas were summarized systematically, which had certain reference value. Moreover, in the pharmacological section, we have summarized in detail its neuroprotective effects, protection of gastrointestinal mucosa and anti-ulcer, regulation of body immunity, anti-tumor, endocrine regulation, improvement of hematopoietic function, cardiovascular protection, anti-aging and antioxidant effects, which can provide reference for the next studies. Such an in-depth and comprehensive summary has never been seen in the previous literature on CR. In addition, in this review, we also discuss in depth the inadequacies in the existing studies on CR, and present our own views and solutions.
First, the chemical composition, especially the material basis of the medicinal effect of CR and its constitutive relationship should be studied in depth. In addition, non-traditional medicinal parts of CR, such as stems and leaves, also contain a variety of chemical components. However, relatively little research has been conducted on it. In the next step, the research on non-traditional medicinal parts of CR should be strengthened to make full use of herbal resources and avoid waste. More importantly, there are many varieties of CR. As mentioned above, the local products, Codonopsis clematidea, has stronger anti-free radical ability than Lu Dangshen, but it is still less exploited. Besides, in addition to traditional Chinese medicine, Dangshen is also widely used in Mongolian medicine and Tibetan doctor. For example, Weihe granule II has good effect on chronic gastritis (Li L. et al., 2014); Ganshen granules is used for deficiency of both qi and blood, dizziness, insomnia, fatigue and soreness of waist and legs (Cheng et al., 2019); ShanHuQiShiWei Pill are mainly used for cerebral thrombosis, coronary heart disease, epilepsy and various neuritis; Eighteen Flavor Dangshen Pills in the treatment of Pyocutaneous Disease, diminishing inflammation and relieving painand (Li et al., 2021). Therefore, our research on Codonopsis should not be limited to traditional Chinese medicine, but also combined with Mongolian medicine, Tibetan medicine and other clinical applications of Codonopsis, in order to facilitate a more comprehensive development and utilization of Codonopsis. In the future, we should strengthen the research on local products and establish a number of breeding bases in the concentrated distribution areas of medicinal wild resources, such as Xinjiang, Tibet and Sichuan, for artificial introduction and cultivation, so as to better develop and utilize the medicinal resources of CR.
Second, as a traditional Chinese medicine commonly used in China, CR has the characteristics of multi-component and multi-target combined effect. Currently, the indexes of quality evaluation of CR are only limited to CPPs, Atractylenolide III and lobetyolin, which still have certain shortcomings. Whether the quality evaluation of several components can represent the overall clinical efficacy of CR remains to be studied in depth. In addition, CPPs is the main active component of CR. However, polysaccharides are a series of components, and the precise isolation and preparation of polysaccharides are still difficult, the mechanism of pharmacological effect still needs to be studied in depth, and it is still challenging to obtain polysaccharide components that can represent the characteristics of CR. We need to establish scientific and reasonable quality control standards to contribute to the safe and rational use of CR. In the future, scholars can use low-cost and environmentally friendly UPLC-QDA and UPC2 methods to screen quality markers by spectral-effect relationships or network pharmacology.
Thirdly, most of the processing methods of CR have been extended to the ancient methods without specific process parameters, which is not conducive to mass industrial production. In addition, the different processing methods had different effects on the content of the components in CR. Therefore, in the next study, scholars should focus on establishing a unified and scientific process standard, quantifying and specifying the processing ingredients, temperature, time and other factors that he would affect the quality of the product of CR.
Fourthly, CR is a natural medicine that can be used as both food and medicine, with low toxicity and high edibility. However, CR is not listed in the table of contents of homology of medicine and food in China. In subsequent studies, it is necessary to establish more animal and clinical trials to verify its safety. In addition, researchers should strive to develop more edible products using CR so that it can be better used as a health product in the daily life of people.
In conclusion, this paper reviews the research results on the botany, ethnopharmacology, phytochemistry, analysis method and quality control, processing methods, pharmacological effects, pharmacokinetics and toxicity of CR in recent years, and puts forward some opinions and suggestions to provide new ideas for further development of the plant resources of CR.
JD, AH, and SZ conceived of and designed the review; YN, HY, and SZ searched the literature and downloaded the documents and made classification; YN wrote the paper; LY and WL provided a holistic idea, checking the chemical structures and formula and contributed comments for version of the manuscript. All authors read and approved the final manuscript.
This work was financially supported by the National Natural Science Foundation of China (Grant Numbers 81960771); National Key Research and Development Program of China (2017YFC1703901, 2017YFC1703902).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1162036/full#supplementary-material
An, T., Chen, X., Zhang, M., and Zhang, Y. (2018). Rapid analysis on chemical constituents in roots of Codonopsis tangshen by UPLC coupled with Q-Exactive quadrupole-orbitrap mass spectrometry. Chin. Traditional Herb. Drugs 49 (07), 1533–1542.
A, X. B. E., Li, H., Chen, J., and Li, P. (2012). Chemical constituents from the aerial parts of Codonopsis nervosa. Chin. J. Nat. Med. 10 (05), 366–369.
Bai, R., Li, W., Li, Y., Ma, M., Wang, Y., Zhang, J., et al. (2018). Cytotoxicity of two water-soluble polysaccharides from Codonopsis pilosula Nannf. var. modesta (Nannf.) L.T.Shen against human hepatocellular carcinoma HepG2 cells and its mechanism. Int. J. Biol. Macromol. 120, 1544–1550. doi:10.1016/j.ijbiomac.2018.09.123
Bai, R., Zhang, Y., Fan, J., Jia, X., Li, D., Wang, Y., et al. (2020). Immune-enhancement effects of oligosaccharides from Codonopsis pilosula on cyclophosphamide induced immunosuppression in mice. Food Funct. 11 (4), 3306–3315. doi:10.1039/c9fo02969a
Bai, Z., Wang, H., and Long, Y. (2015). Study on antibacterial activity of extracts of codonopsis pilosula. Liaoning J. Traditional Chin. Med. 42 (07), 1290–1291. doi:10.13192/j.issn.1000-1719.2015.07.056
Bian, H., Wu, X., Xia, P., Duan, W., Yang, F., Li, J., et al. (2022). Research progress of Codonopsis Radix and predictive analysis on quality markers. West China J. Pharm. Sci. 37 (03), 337–344. doi:10.13375/j.cnki.wcjps.2022.03.023
Cao, F., and Wang, Y. (2019). Effect of total saponins of Codonopsis pilosula nanoemulsion on immunologic function of mice. J. Northwest A F Univ. 47, 125–131. doi:10.13207/j.cnki.jnwafu.2019.05.016
Cao, Y. (2012). Studies on Ouality evaluation of Radix codonopsis. Master. Guangzhou, China: Guangzhou University of Chinese Medicine.
Chao, C., Juang, S., Chan, H., Shen, D., Liao, Y., Shih, H., et al. (2015). UV-guided isolation of polyynes and polyenes from the roots of Codonopsis pilosula. RSC Adv. 5 (52), 41324–41331. doi:10.1039/c5ra02765a
Chen, H., Niu, W., Li, S., Zhang, Y., Zhao, H., and Zheng, H. (2014a). Determination of lobetyolin in codonopsis radix from different Habitats by HPLC. Food Drug 16 (01), 37–39.
Chen, H., Wang, R., Dong, J., Xing, H., Jia, M., and Chen, X. (2019). Effects of different processing methods on chemical constituents and product quality otCodonopsis pilosula. Chin. Tradit. Pat. Med. 41 (07), 1631–1634.
Chen, H., Wang, Y., Han, C., and Cai, D. (1985). Studies on the chemical constituents of Codonopsis pilosula (III). Chin. Traditional Herb. Drugs 16 (07), 7–8.
Chen, J., Shu, X., and Yu, N. (2012). Study on quality control of codonopsis pilosula. Chin. Med. Mod. Distance Educ. China 10 (14), 154–155.
Chen, L., and Fang, Y. (2013). Historical evolution and research progres of processing method of radix codonopsis. J. Mod. Med. Health 29 (14), 2143–2144.
Chen, Q., Deng, X., and Zhu, H. (2016). Determination of lobetyolin and syringin in Codonopsis Radix from different places by HPLC. Sci. Technol. Food Industry 37 (06), 64–67. doi:10.13386/j.issn1002-0306.2016.06.004
Chen, W., Chen, J., Wu, H., Gou, Y., Hu, F., Liu, L., et al. (2014b). Optimization of selenylation conditions for a pectic polysaccharide and its structural characteristic. Int. J. Biol. Macromol. 69, 244–251. doi:10.1016/j.ijbiomac.2014.05.046
Chen, W., Zhang, Z., Wang, J., Shen, X., Han, L., Zhou, L., et al. (2002). Effects of extracts from Codonopsis pilosula and Atractylodes macrocephalaon growth and differentiation of IEC-6 cells. Chin. Pharmacol. Bull. 4, 444–447.
Cheng, J., Deng, W., Huang, P., Li, J., and Wu, J., and (2011). Experimental study on anti-ulcer effect and acute toxicity of wall-broken powder of codonopsis pilosula. Northwest Pharm. J. 26 (02), 120–122.
Cheng, J., Zhang, P., Bao, B., Liang, Y., Tang, Y., and Liu, D. (2019). Study Preparation,Quality Stand. Fingerpr. Ganshen Granules China Pharm. 30 (21), 2913–2919.
Cheng, P. (2010). Studies on that chemical composition of Tibetan medicine codonopsis pilosula. Chin. Tradit. Pat. Med. 32 (07), 1248–1249.
Chen., S., He, L., Zhou, Z., Li, Y., and Li, Y. (2002). Effects of Codonapsis pilosula on Gastrin and Somatostatin of Gastroduodenal mucosa in rabbits. J. China Med. Univ. 3, 6–7.
Cui, J., Wei, Z., Yanqiu, Z., and Yajuan, X. (1988). Anti-ulcer effect of codonopsis radix polysaccharide. Chin. Traditional Herb. Drugs 19 (08), 21–23.
Dong, J., Cheng, M., Xue, R., Deng, C., Liu, H., Zhang, T., et al. (2021). Comparative pharmacokinetic and bioavailability study of lobetyolin in rats after administration of lobetyolin and Codonopsis pilosula extract by ultra-performance LC–tandem mass spectrometry. Biomed. Chromatogr. 35 (8), e5125. doi:10.1002/bmc.5125
Dou, W., Cui, Z., Hou, J., Wang, M., and Yang, F. (2016). Determination of polysaccharide content in codonopsis pilosula from different places in Gansu province. J. Traditional Chin. Veterinary Med. 35 (03), 55–59. doi:10.13823/j.cnki.jtcvm.2016.03.017
Dou, W., Cui, Z., Hou, J., Wang, M., and Yang, F. (2015). Determination of total saponins in codonopsis pilosula from different places in Gansu province. J. Traditional Chin. Veterinary Med. 34 (06), 59–62. doi:10.13823/j.cnki.jtcvm.2015.06.019
Duan, Q., Liang, Z., Yang, D., and Liu, W. (2012). Chemical constituents from the aerial parts of Codonopsis nervosa. Chin. J. Nat. Med. 10 (05), 366–369. doi:10.1016/s1875-5364(12)60073-9
Fan, Q. (2011). Study on chemical constituents of codonopsis Nervoa Chipp Nannf. Master. Chengdu, Sichuan, China: Southwest Jiaotong University.
Fan, Q., Zhang, Y., Zhou, X., Lang, G. S. S., and Huang, C. (2011). Study on chemical constituents of codonopsis nervoa Chipp Nannf. Chin. Pharm. J. 46 (04), 256–259.
Fang, Z., Li, Y., Yang, Y., and Leng, J. (2015). Lnhibitory effects and mechanism of total saponins from codonopsis pilosula on HumanHepatocellular carcinoma SMMC-7721 cells. China Pharm. 26 (10), 1356–1359.
Feng, B., and Zhang, J. (2016). Compatibility of codonopsis radix with different herbs. Chin. J. Exp. Traditional Med. Formulae 22 (19), 194–198. doi:10.13422/j.cnki.syfjx.2016190194
Feng, H., and Gao, J. S. J. T. C. M. (2012). Study of polysaccharides in Codonopsis pilosula on antineoplastic activity in vivo and the acute toxicity testing. Shanxi J. Traditional Chin. Med. 28, 49–50.
Feng, Y., Wang, X., Zhuang, P., Zhang, D., Gao, L., Chen, J., et al. (2017). Study on chemical constituents of Codonopsis pilosula. China J. Chin. Materia Medica 42 (01), 135–139. doi:10.19540/j.cnki.cjcmm.20161222.046
Gao, S. (2020). Quality evaluation and effective substances onImmune regulation and HematopoieticImprovement of codonopsis radix. Doctor, Peking union medical College.
Gao, S., Liu, J., Wang, M., Cao, T., Qi, Y., Zhang, B., et al. (2018). Traditional uses, phytochemistry, pharmacology and toxicology of codonopsis: A review. J. Ethnopharmacol. 219, 50–70. doi:10.1016/j.jep.2018.02.039
Guo, Q. (2016). Analysis of volatile components and special aroma in freshroots of Codonopsises. Master. Shanxi, China: Shanxi Medical University.
Guo, Y., Shao, Y., Zhao, Y., Zhang, X., Chang, Z., Sun, Y., et al. (2022). Pharmacokinetics, distribution and excretion of inulin-type fructan CPA after oral or intravenous administration to mice. Food Funct. 13 (7), 4130–4141. doi:10.1039/d1fo04327g
He, J., Zhu, S., Goda, Y., Cai, S., and Komatsu, K. (2014). Quality evaluation of medicinally-used Codonopsis species and Codonopsis Radix based on the contents of pyrrolidine alkaloids, phenylpropanoid and polyacetylenes. J. Nat. Med. 68, 326–339. doi:10.1007/s11418-013-0801-0
He, Q., Enyuan, Z., Zhengtao, W., Guixin, C., Luoshan, X., and Zhibi, H. (2006). Study on chemical Constitutes of codonopsis pilosula. Chin. Pharm. J. 1, 10–12.
He, R., Ma, R., Jin, Z., Zhu, Y., Yang, F., Hu, F., et al. (2022). Proteomics and Metabolomics Unveil Codonopsis pilosula (Franch.) Nannf. Ameliorates gastric precancerous Lesions via regulating energy metabolism. Front. Pharmacol. 13, 933096. doi:10.3389/fphar.2022.933096
He, W., Li, L., Yuan, Y., and Cheng, X. (2022) Codonopsis pilosula polysaccharide influences the proliferation and apoptosis of breastcancer cell MCF-7 by regulating LncRNA CCHE1. J. Toxicol. 36(02), 147–151. doi:10.16421/j.cnki.1002-3127.2022.02.002
Heinrich, M., Jalil, B., Abdel-Tawab, M., Echeverria, J., Kulić, Ž., McGaw, L. J., et al. (2022). Best practice in the chemical characterisation of extracts used in pharmacological and toxicological research—the ConPhyMP—Guidelines. Front. Pharmacol. 13, 953205. doi:10.3389/fphar.2022.953205
Hou, L., Han, L., Gong, Z., and Yan, Y. (2016). Effects of codonopsis pilosula polysaccharide oral liquids on the long-term toxicity of rats. Chin. J. Veterinary Drug 50 (12), 40–44.
Hou, L., Yan, Y., Cai, M., and Fang, X. (2013). The anti-stress ability and anti-inflammation effect of codonopsis pilosula polysaccharide OraLiquids. Chin. J. Veterinary Drug 47 (11), 37–39.
Hu, N., Gao, Z., Cao, P., Song, H., Hu, J., Qiu, Z., et al. (2022). Uniform and disperse selenium nanoparticles stabilized by inulin fructans from Codonopsis pilosula and their anti-hepatoma activities. Int. J. Biol. Macromol. 203, 105–115. doi:10.1016/j.ijbiomac.2022.01.140
Hu, Q., Li, X., Huang, H., Mu, H., Tu, P., and Li, G. (2012a). New benzofuranylpropanoids from the roots of Codonopsis lanceolata. Helvetica Chim. Acta 95 (2), 349–352. doi:10.1002/hlca.201100339
Hu, Q., Li, X., Huang, H., Mu, H., Tu, P., and Li, G. (2012b). Phenylpropanoids from the roots of codonopsis cordifolioidea and their biological activities. Bull. Korean Chem. Soc. 33 (1), 278–280. doi:10.5012/bkcs.2012.33.1.278
Hu, X., Qin, F., Lu, X., Zhang, L., and Cheng, Y. (2018). Three new polyynes from Codonopsis pilosula and their activities on lipid metabolism. Molecules 23 (4), 887. doi:10.3390/molecules23040887
Hu, Y. R., Xing, S. L., Chen, C., Shen, D. Z., and Chen, J. L. (2021). Codonopsis pilosula polysaccharides alleviate Aβ1-40-induced PC12 cells energy dysmetabolism via CD38/NAD+ signaling pathway. Curr. Alzheimer Res. 18 (3), 208–221. doi:10.2174/1567205018666210608103831
Hu, Z., Zhao, J., Chai, J., Liu, Z., Liu, D., and Wang, Y. (2018). Toxicological study of Codonopsis pilosula. Chin. J. Health Laboratory Technol. 28 (19), 2325–2329.
Huang, Y., Zhang, Y., Kang, L., Yu, Y., and Guo, L. (2018). Research progress on chemical constituents and their pharmacological activities of plant from Codonopsis. Chin. Traditional Herb. Drugs 49 (01), 239–250.
Ichikawa, M., Ohta, S., Komoto, N., Ushijima, M., Kodera, Y., Hayama, M., et al. (2008). Rapid identification of triterpenoid saponins in the roots of Codonopsis lanceolata by liquid chromatography–mass spectrometry. J. Nat. Med. 62 (4), 423–429. doi:10.1007/s11418-008-0270-z
Ichikawa, M., Ohta, S., Komoto, N., Ushijima, M., Kodera, Y., Hayama, M., et al. (2009). Simultaneous determination of seven saponins in the roots of Codonopsis lanceolata by liquid chromatography-mass spectrometry. Simultaneous Determ. seven saponins roots Codonopsis lanceolata by Liq. Chromatogr. Spectrom. 63 (1), 52–57. doi:10.1007/s11418-008-0294-4
Ishida, S., Okasaka, M., Ramos, F., Kashiwada, Y., Takaishi, Y., Kodzhimatov, O. K., et al. (2008). New alkaloid from the aerial parts of Codonopsis clematidea. J. Nat. Med. 62 (2), 236–238. doi:10.1007/s11418-007-0219-7
Jiang, K., and Xu, L. (2018). Analvsis of volatile oil in different Picking time of dried/Fresh Daozhen Luolong Codonopsisby GC-MS. Nat. Prod. Res. Dev. 30 (12), 2110–2119+2186. doi:10.16333/j.1001-6880.2018.12.012
Jiang, Y., Liu, Y., Guo, Q., Jiang, Z., Xu, C., Zhu, C., et al. (2015a). C₁₄-polyacetylene glucosides from Codonopsis pilosula. J. Asian Nat. Prod. Res. 17 (6), 601–614. doi:10.1080/10286020.2015.1041932
Jiang, Y., Liu, Y., Guo, Q., and Shi, J. (2015b). C14-polyacetylenol glycosides from the roots of Codonopsis pilosula. J. Asian Nat. Prod. Res. 17 (12), 1166–1179. doi:10.1080/10286020.2015.1112797
Jiang, Y., Liu, Y., Guo, Q., Xu, C., Zhu, C., and Shi, J. (2016). Sesquiterpene glycosides from the roots of Codonopsis pilosula. Acta Pharm. Sin. B 6 (01), 46–54. doi:10.1016/j.apsb.2015.09.007
Jing, J., Jifa, Z., Hui, L., Shuai, H., Lianhai, S., and Xianli, Z. (2013). Study on chemical constituents of codonopsis thalictrifolia wall. Var. mollis (Chipp) L. T. Shen. Lishizhen Med. Materia Medica Res. 24 (10), 2340–2342.
Kan, Y. (2009). Phenolic compounds and antioxidant activity in cell wall materials from Deodeok (Codonopsis lanceolata). Korean J. Food Sci. Technol. 41 (3), 345–349.
Kim, E. Y., Kim, J. A., Jeon, H. J., Kim, S., Kim, Y. H., Kim, H. Y., et al. (2014). Chemical fingerprinting of Codonopsis pilosula and simultaneous analysis of its major components by HPLC-UV. Arch. Pharm. Res. 37 (9), 1148–1158. doi:10.1007/s12272-014-0335-3
Lee, K., Choi, J., Jung, W., Nam, J., Jung, H., and Park, H. (2002). Structure of a new echinocystic acid bisdesmoside isolated from Codonopsis lanceolata roots and the cytotoxic activity of prosapogenins. J. Agric. Food Chem. 50 (15), 4190–4193. doi:10.1021/jf011647l
Lee, K., Jung, H., Park, H., Kim, D., Lee, J., and Lee, K. (2005). Beta-D-xylopyranosyl-(1->3)-beta-D-glucuronopyranosyl echinocystic acid isolated from the roots of Codonopsis lanceolata induces caspase-dependent apoptosis in human acute promyelocytic leukemia HL-60 cells. Biol. Pharm. Bull. 28 (5), 854–859. doi:10.1248/bpb.28.854
Lee, Y., Kim, J., Lee, J., Byeon, S., Hong, E., Lee, J., et al. (2007). Regulatory effects of Codonopsis lanceolata on macrophage-mediated immune responses. J. Ethnopharmacol. 112 (1), 180–188. doi:10.1016/j.jep.2007.02.026
Lei, W., Wang, L., Zhu, R., Du, H., Zhang, L., Jingjing, D., et al. (2022). Experimental study and clinical observation on immune regulation of Codonopsis pilosulablack tea. Chin. Nurs. Res. 36 (13), 2386–2389.
Li, C., Xu, H., Han, Q., and Wu, T. (2009). Quality assessment of Radix Codonopsis by quantitative nuclear magnetic resonance. J. Chromatogr. A 1216 (11), 2124–2129. doi:10.1016/j.chroma.2008.10.080
Li, C., Zhang, H., Peng, L., Xiang, J., Yang, M., Dong, W., et al. (1993). Study on volatile chemical constituents of Codonopsis pilosula. J. Yunnan Univ. S2, 86–90.
Li, F., Li, H., Zhang, Y., Lian, Y., Liu, X., Jiang, G., et al. (2021). Herbal textual research on local varieties of codonopsis radix. Chin. J. Exp. Traditional Med. Formulae 27 (15), 132–138. doi:10.13422/j.cnki.syfjx.20211016
Li, J. (2012). The research of the extraction and isolation of chemical compounds from Codonopsis Lanceolata and the anti effect of the hepg-2 cell line. Master. Jilin, China,: Yanbian University.
Li, L., Ma, C., and Yang, X. (2014). The effect of Weihe granule ll on the expression level of EGF and EGFR in CAG rats. World Chin. Med. 9 (01), 81–83.
Li, Q., Tang, Q., Hou, Y., Wang, X., Zhao, J., and Mao, J. (2019). Advances in cardiovascular pharmacology of codonopsis pilosula. Chin. J. Integr. Med. Cardio-Cerebrovascular Dis. 17 (17), 2604–2606.
Li, R., Nian, L., Zhao, S., Wen, P., Sui, J., Tao, Y., et al. (2013). Effect of radix codonopsis on stress Ulcer,Cell migration and polyamine content in rats. J. Guangzhou Univ. Traditional Chin. Med. 30 (04), 519–524+604. doi:10.13359/j.cnki.gzxbtcm.2013.04.026
Li, R., Zeng, D., Wen, P., Tao, Y., Zhao, S., Chen, W., et al. (2014). Effect of radix codonopsis flavonoids on intestinal Endothelial cells 6 migration and polyamines content. Traditional Chin. Drug Res. Clin. Pharmacol. 25 (05), 523–526.
Li, X., Cheng, L., Yan, Y., Liu, B., and Cheng, Y. (2019). SIRT1 inhibitory compounds from the roots of Codonopsis pilosula. J. Asian Nat. Prod. Res. 21 (1), 25–32. doi:10.1080/10286020.2017.1422491
Li, Y., Yang, B., Hou, Q., Xie, R., and Zhang, F. (2017). Effect of codonopsis Polysaceharide on protein expression of p53 p21 Bax and Bcl-2Associated with aging of hematopoietic stem cells in mice. Pharm. J. Chin. People's Liberation Army 33 (02), 120–124.
Liang, Z., Lin, J., and Yuan, Z. (2007). Researchs on chemical constituents of codonopsis lanceolata. China journal of Chinese Materia Medica (13), 1363–1364.
Liao, J. (2022). Study on active components of codonopsis pilosula based on antioxidant stress. Master. Chongqing, China: Chongqing Medical University.
Liao, J., and Lu, Q. (1987). Studies on the components of volatile oil from codonopsis pilosula V. Chin. Traditional Herb. Drugs 18 (09), 2–4.
Li., J., Wang, T., Zhu, Z., Yang, F., Cao, L., and Gao, J. (2017). Structure features and anti-gastric ulcer effects of inulin-type fructan CP-A from the roots of codonopsis pilosula (Franch.) Nannf. molecules 22 (12), 2258. doi:10.3390/molecules22122258
Lin, L., Tsai, T., and Kuo, C. (2013). Chemical constituents comparison of Codonopsis tangshenCodonopsis pilosula var. modesta and Codonopsis pilosula. Nat. Prod. Res. 27 (19), 1812–1815. doi:10.1080/14786419.2013.778849
Ling, Z. (2012). Protective of radix codonopsis on hemodynamics and myocardial enzyme after myocardiaischemia reperfusion injury. Chin. J. Gerontology 32 (05), 966–968.
Liu, H., Guo, X., Wang, Y., and Wang, X. (2006). A Comparative study on the volatile constituents of stir-baked with rice and bran CodonopsisPilosula. J. Cap. Normal Univ. 3, 41–44+36. doi:10.19789/j.1004-9398.2006.03.011
Liu, M. (2020). Study about the brain protective effect and its mechanism of cpps on cycloheximide induced memory consolidation disorder in mice. Master. Hebei: Hebei North University.
Liu, X., Qiao, J., Gao, J., Chen, Z., and Liu, X. (2021). Protective effects of total saponins of Codonopsis on ulcerative colitis induced by TNBS in ratsand its mechanism. Chin. J. Appl. Physiology 37 (04), 397–401. doi:10.12047/j.cjap.6051.2021.032+406
Liu, X., Weiwei, P., Jin, M., and Piao, C. (2019). Exploration about the clinical application and dosage of codonopsis pilosula. Jilin J. Chin. Med. 39 (04), 453–456. doi:10.13463/j.cnki.jlzyy.2019.04.010
Luo, H., Liu, X., Mou, N., Chen, W., Shasha, F., Wenzhi, X., et al. (2017). Banqiao Codonopisis Pilosula improves cognitive dysfunction induced by high GSK-3B activityand its possible mechanism. Chin. Pharmacol. Bull. 33 (08), 1060–1067.
Lv, C., Wang, B., He, M., Meng, X., and Zhang, S. (2018). Research progress in processing techniques and chemical composition changes of dangshen. Mod. Traditional Chin. Med. Materia Medica-World Sci. Technol. 20 (02), 309–312.
Ma, F., Shen, X., and Shi, J. (2014). Effect of codonopsis polysaccharide on gastrointestinal tract of experimental rats and mice. Anhui Med. Pharm. J. 18 (09), 1626–1630.
Ma, X., Leung, A. K. M., Chan, C. L., Su, T., Li, W., Li, S., et al. (2014). UHPLC UHD Q-TOF MS/MS analysis of the impact of sulfur fumigation on the chemical profile of Codonopsis Radix (Dangshen). Analyst 139 (2), 505–516. doi:10.1039/c3an01561k
Mao, S., Sang, S., Lao, Lao, A., and Chen, Z. (2000). Studies on the chemical constituents of Codonopisis lanceolata Benth. et Hook. Nat. Prod. Res. Dev. 12 (1), 1–3.
Mei, R., Lu, Q., Hu, Y., Liu, H., Bao, F., Zhang, Y., et al. (2008). Three new polyyne (= polyacetylene) glucosides from the edible roots of Codonopsis cordifolioidea. Helvetica Chim. Acta 91 (1), 90–96. doi:10.1002/hlca.200890018
Meng, J. (2021). Protection effect of Codonopsis pilosula on gastrointestinal tract of aging mice was studied based on lncRNA and mRNA expression profile. Master. Gansu: Gansu University Of Chinese Medicine.
Meng, Y., Sun, S., and Su, Y. (2003). Determination of ferulic acid in Codonopsis pilosula by HPLC. J. Chengde Med. Univ. 2, 140–141. doi:10.15921/j.cnki.cyxb.2003.02.037
Meng, Y., Xu, Y., Chang, C., Qiu, Z., Hu, J., Wu, Y., et al. (2020). Extraction, characterization and anti-inflammatory activities of an inulin-type fructan from Codonopsis pilosula. Int. J. Biol. Macromol. 163, 1677–1686. doi:10.1016/j.ijbiomac.2020.09.117
Ming, K., Chen, Y., Yao, F., Shi, J., Yang, J., Du, H., et al. (2017). Phosphorylated Codonopsis pilosula polysaccharide could inhibit the virulence of duck hepatitis A virus compared with Codonopsis pilosula polysaccharide. Int. J. Biol. Macromol. 94, 28–35. doi:10.1016/j.ijbiomac.2016.10.002
Mizutani, K., Yuda, M., Tanaka, O., Saruwatari, Y.-i., Jia, M.-R., Ling, Y.-K., et al. (1988). Tangshenosides I and II from chuan-dangshen, the root of Codonopsis tangshen OLIV. Chem. Pharm. Bull. 36 (7), 2726–2729. doi:10.1248/cpb.36.2726
Peng, R., Ma, P., Mo, R., Tan, J., and Li, L. (2014). Study on quality standard of codonopsis tangshen Oliv. Mod. Traditional Chin. Med. Materia Medica-World Sci. Technol. 16 (03), 578–581.
Pi, L., Han, F., Zhao, X., Li, Y., Wang, X., and Deng, L. (2013). Analysis of Fat-soluble components of traditional Tibetan MedicineSphallerocarpus gracilis. Chin. J. Spectrosc. Laboratory 30 (05), 2708–2711.
Qi, H., Wang, R., Liu, Y., and Shi, Y. (2011). Studies on the chemical constituents of codonopsis pilosula. J. Chin. Med. Mater. 34 (04), 546–548. doi:10.13863/j.issn1001-4454.2011.04.020
Qi, J., Liang, J., Hu, Y., Chen, H., Wei, J., Huang, D., et al. (2022). Quality control status and predictive analysis of quality markers of dangshen(RadixCodonopsis). Chin. Archives Traditional Chin. Med. 40 (05), 255–258. doi:10.13193/j.issn.1673-7717.2022.05.060
Qi, X., Xuemei, C., Fangdi, H., and Changhong, W. (2020). Research advance on chemical constituents, pharmacological action and quality control ofRadix Codonopsis. Shanghai J. Traditional Chin. Med. 54 (08), 94–104. doi:10.16305/j.1007-1334.2020.08.013
Qin, F., Cheng, L., Yan, Y., Liu, B., and Cheng, Y. (2018a). Choushenosides A-C, three dimeric catechin glucosides from Codonopsis pilosula collected in Yunnan province, China. Phytochemistry 153, 53–57. doi:10.1016/j.phytochem.2018.05.012
Qin, F., Cheng, L., Yan, Y., Liu, B., and Cheng, Y. (2018b). Two novel proline-containing catechin glucoside from water-soluble extract of Codonopsis pilosula. Molecules 23 (1), 180. doi:10.3390/molecules23010180
Qun, G., Si-nai, L., and Qian, L. (2017). Effects of Huanggi [Astragali radix, 黄芪] and dangshen [codonopsis radix. 党参] CalciumTransients Myocard. Cells Heart Fail. Model Mice J. Traditional Chin. Med. 58 (16), 1408–1411. doi:10.13288/j.11-2166/r.2017.16.015
Ren, J., Lin, Z., and Yuan, Z. (2013). Tangshenosides from Codonopsis lanceolata roots. Phytochem. Lett. 6 (4), 567–569. doi:10.1016/j.phytol.2013.07.008
Ren, L., Zhang, J., Liu, Z., and Sun, R. (2008). Isolation, purification and structure of polysaccharide from codonopsis pilosula. Chin. Traditional Herb. Drugs 7, 986–989.
Rivera, D., Allkin, R., Obón, C., Alcaraz, F., Verpoorte, R., and Heinrich, M. (2014). What is in a name? The need for accurate scientific nomenclature for plants. J. Ethnopharmacol. 152 (3), 393–402. doi:10.1016/j.jep.2013.12.022
Shen, H., Fan, H., Fang, H., and Liu, C. (2023). Variety ldentification of codonopsis radix used in Menghe medical School's characteristic processed products. Chin. J. Ethnomedicine Ethnopharmacy 32 (01), 24–27.
Shen, X., and Jin, H. (2012). Astragalus codonopsis harmacological action and clinical application of meridian. J. Pract. Traditional Chin. Intern. Med. 26 (07), 7–5+77.
Shi, J., Ma, F., Liao, H., and Liang, P. (2011). Determination of lobetyolin in Radix Codonopsis from different origins by HPLC. J. Guangdong Pharm. Univ. 27 (1), 51–53.
Song, D., Cheng, X., Li, L., Zhong, G., and Wang, Z. (2008). Determination of lobetvolin in root of Codonopsis tangshen from various cultivation areas by high-performance liquid chromatography. China J. Chin. Materia Medica 17, 2133–2135.
Su, J. (2018). Study on chemical constituents of codonopsis pilosula (Franch.)Nannffrom Yunnan. Master. Chengdu, China: Chengdu University of Traditional Chinese Medicine.
Su, J., Qin, F., Liu, Y., and Zhang, Y. (2021). Four new polyynes from Codonopsis pilosula collected in Yunnan province, China. Nat. Prod. Res. 35 (21), 3548–3555. doi:10.1080/14786419.2020.1712390
Sun, J., Wang, L., Wang, M., Wang, Z., and Li, F. (2016). Two new polyacetylene glycosides from the roots of Codonopsis tangshen Oliv. Nat. Prod. Res. 30 (20), 2338–2343. doi:10.1080/14786419.2016.1174230
Sun, Q., Li, Y.-u., Cui, Y., Jiang, S., Dong, C., and Du, J. (2019). Structural characterization of three polysaccharides from the roots of Codonopsis pilosula and their immunomodulatory effects on RAW264. 7 macrophages. Int. J. Biol. Macromol. 130, 556–563. doi:10.1016/j.ijbiomac.2019.02.165
Sun, Z., He, S., and Huang, M. (2007). Determination of lobetyolin in radix codonopsis Tubulosae of Guizhou. Lishizhen Med. Mater Med. Res. 18, 1931–1932.
Sun, Z., Shao, J., and Guo, M. (2015). Research progress of codonopsis pilosula chemical component and pharmacological effects. J. Anhui Agric. Sci. 43 (33), 174–176. doi:10.13989/j.cnki.0517-6611.2015.33.057
Tang, Y., Han, X., Liu, Z., Bao, L., Wang, H., Liu, K., et al. (2022). Influence of processing factors on honey-fried Codonopsis Radix. China J. Chin. Materia Medica 47 (11), 2975–2981. doi:10.19540/j.cnki.cjcmm.20211119.302
Tianhui, H., Chao, L., Yuanyuan, X., and Weihong, G. (2019). Lmmune regulation effects of Ludangshen oral liquid on lmmunosuppressed mice. Pharm. Clin. Res. 27 (03), 171–174+186. doi:10.13664/j.cnki.pcr.2019.03.003
Tie, X., Feng, Y., Zhang, Y., Yang, X., Li, Y., and Hu, F. (2021). Determination of alcohol-soluble extract and lobetyolin of radix codonopsis before and after rice stir-frying and comparison of their effects of invigorating spleen and relieving diarrhea. Chin. Tradit. Pat. Med. 43 (07), 1941–1946.
Tsai, K., Lee, N., Chen, G., Hu, W., Tsai, C., Chang, M., et al. (2013). Dung-shen (Codonopsis pilosula) attenuated the cardiac-impaired insulin-like growth factor II receptor pathway on myocardial cells. Food Chem. 138, 1856–1867. doi:10.1016/j.foodchem.2012.11.056
Tsai, T., and Lin, L. (2008). Phenolic glycosides and pyrrolidine alkaloids from Codonopsis tangshen. Chem. Pharm. Bull. 56 (11), 1546–1550. doi:10.1248/cpb.56.1546
Ushijima, M., Komoto, N., Sugizono, Y., Mizuno, I., Sumihiro, M., Ichikawa, M., et al. (2008). Triterpene glycosides from the roots of Codonopsis lanceolata. Chem. Pharm. Bull. 56 (3), 308–314. doi:10.1248/cpb.56.308
Wakana, D., Kawahara, N., and Goda, Y. (2011). Three new triterpenyl esters, codonopilates A–C, isolated from Codonopsis pilosula. J. Nat. Med. 65 (1), 18–23. doi:10.1007/s11418-010-0447-0
Wan, L., Zhang, Q., Luo, H., Xu, Z., Huang, S., Yang, F., et al. (2020). Codonopsis pilosula polysaccharide attenuates Aβ toxicity and cognitive defects in APP/PS1 mice. Aging 12 (13), 13422–13436. doi:10.18632/aging.103445
Wang, H., He, K., and Mao, Q. (1991). Studies on the chemical constituents of Codonopsis pilosula II, isolation and structure determination of Codonolactone and Codopiloic acid. Chin. Traditional Herb. Drugs 22 (05), 195–197+239.
Wang, H., Lin, H., Tan, J., Dong, Q., Wu, F., Li, P., et al. (2019). Research progress on codonopsis pilosula (Franch) Nannf. Of the pharmacological effects andTheir clinical application. World Latest Med. Inf. 19 (07), 21–22+24. doi:10.19613/j.cnki.1671-3141.2019.07.010
Wang, J.-N., Kan, C.-D., Lee, L.-T., Huang, L. L., Hsiao, Y.-L., Chang, A. H., et al. (2021a). Herbal extract from Codonopsis pilosula (Franch.) Nannf. enhances cardiogenic differentiation and improves the function of infarcted rat hearts. Life 11 (5), 422. doi:10.3390/life11050422
Wang, J., Deng, C., Shi, L., and Deng, M. (2011). Modern research progress of Codonopsis pilosula. Guide China Med. 9 (31), 279–281. doi:10.15912/j.cnki.gocm.2011.31.068
Wang, J., and Wang, F. (1996). A study on the chemical composition of Codonopsis pilosula. Nat. Prod. Res. Dev. 2, 8–12. doi:10.16333/j.1001-6880.1996.02.002
Wang, J., Yuan, H., and Li, X. (2012). Antioxidant and antifatigue activities of flavonoid from codonopsis clematidea(Schrenk). Nat. Prod. Res. Dev. 24 (08), 1035–1039. doi:10.16333/j.1001-6880.2012.08.006
Wang, M. (2020). Mechanism of codonopsis pilosula on regulation of skin inflammation in photoaging mice via IL-15 and its receptor. Doctor. Liaoning, China: Liaoning University Of Traditional Chinese Medicine.
Wang, M., Jing, R., Wang, Y., Feng, M., Zhang, W., Xuliang, H., et al. (2020). Historical evolution of radix codonopsis stir-fried with rice and research progress on its modern processing technology, chemical composition and pharmacological action. China Pharm. 31 (14), 1788–1792.
Wang, M., Wang, Y., Wu, Y., Li, N., Guo, F., Lan, Z., et al. (2021b). Study on dynamic changes of lobetyolin, 5-HMF and Codonopsis pilosula polysaccharidescontent in rice fried Codonopsis pilosula during processing. J. Food Saf. Qual. 12 (07), 2765–2772. doi:10.19812/j.cnki.jfsq11-5956/ts.2021.07.040
Wang, M., Wu, Y., Yu, W., Yu, B., and Ying, H. (2022). Polyacetylenes from Codonopsis lanceolata root induced apoptosis of human lung adenocarcinoma cells and improved lung Dysbiosis. BioMed Res. Int. 2022, 7713355. doi:10.1155/2022/7713355
Wang, Q., Wang, Y., Zhang, X., Wang, G., Ma, Y., Lei, Z., et al. (2018). Research progress of processing in production place and processing of codonopsis radix. Chin. J. Exp. Traditional Med. Formulae 24 (22), 206–214. doi:10.13422/j.cnki.syfjx.20181601
Wang, X., Liu, Y., Zhang, L., and Li, Y. (2017a). “Research on traditional Chinese medicine Professor Niu Jianzhao's medication Rule in prescriptions with Climacteric Syndrome based on data Mining,” in World science technology-Modernization of traditional Chinese medicine, 1680–1686.
Wang, X., Zhuang, P., Chen, J., Yang, Y., Lin, X., and Zhang, D. (2017b). Study on chemical constituents of Codonopsis pilosula. Chin. Traditional Herb. Drugs 48 (09), 1719–1723.
Wang, Y. (2021). Structural characterization and immunomodulatory activity of Codonopsis polysaccharide CPC. Master. Jinzhong, China: Shanxi Medical University.
Wang, Y., Cai, D., and Zhao, W. (1982). Study on the chemical composition of Radix Codonopsis pilosulae (first report). Chin. Traditional Herb. Drugs 13 (01), 1–3.
Wang, Y., Han, C., Li, F., He, Q., and Cai, D. (1986). Study on chemical components of Codonopsis pilosula(Ⅳ). Chin. Traditional Herb. Drugs 17 (05), 41.
Wang, Z., Xu, G., Xiong, F. B. Z., and Xiong, N. B. H. (1993). Determination of Codonopsis pilosula polysaccharide by colorimetry. J. Plant Resour. Environ. 1, 62–64.
Wang, Z., Xu, G., Xiong, F. B. Z., and Xiong, N. B. H. (1988). Isolation and identification of chemical constituents of Codonopsis pilosula. J. China Pharm. Univ. 1, 61.
Wu, B., Yang, J., Xie, H., and Yang, X. (2008). Protective effect of polysaccharides from codonopsis pilosula on neural stem cell InjuryInduced by Na2S2O3. Lishizhen Med. Materia Medica Res. 2, 280–281.
Wu, C. (2021). Anti-aging effect of aqueous extract from Codonopsispilosula and its mechanismon natural senescence Drosophila melanogaster. Master. Taiyuan, China: Shanxi University.
Wu, J., Xu, Q., Xie, Q., Bai, Z., Han, J., Li, X., et al. (2016). Study on Antitwnor effects of dangshen. West. J. Traditional Chin. Med. 29 (08), 18–21.
Xia, D., Zi-ming, J., and Xiao-long, Z. (2016). Comparative pharmacokinetics of ultramicro-powder and fine powder of Radix Codonopsis inrats. Chin. J. Hosp. Pharm. 36 (20), 1749–1752. doi:10.13286/j.cnki.chinhosppharmacyj.2016.20.06
Xie, J., Zhang, Z., and Gu, Y. (2000). Study on the volatile chemical constituents of Codonopsis pillosula. Chinese Pharmaceutical Journal (09), 7–10.
Xie, M., Yan, Z., Ren, X., Li, X., and Qin, B. (2017). Codonopilate A, a triterpenyl ester as main autotoxin in cultivated soil of Codonopsis pilosula (Franch.) Nannf. J. Agric. Food Chem. 65 (10), 2032–2038. doi:10.1021/acs.jafc.6b04320
Xie, Q., Sun, Y., Cao, L., Chen, L., Chen, J., Cheng, X., et al. (2020a). Antifatigue and antihypoxia activities of oligosaccharides and polysaccharides from Codonopsis pilosula in mice. Food Funct. 11 (7), 6352–6362. doi:10.1039/d0fo00468e
Xie, Q., Tian, H., Huan, X., Cao, L., Wang, Y., Cheng, X., et al. (2022). Quality evaluation of Codonopsis Radix and processed products based on the analysis of monosaccharides and oligosaccharides by liquid chromatography coupled with charged aerosol detector. Phytochem. Anal. 33 (2), 262–271. doi:10.1002/pca.3085
Xie, Q., and Wang, C. (2022). Polyacetylenes in herbal medicine: A comprehensive review of its occurrence, pharmacology, toxicology, and pharmacokinetics (2014–2021). Phytochemistry, 113288.
Xie, Q., Wang, H., Guan, H., Xu, N., Zhao, X., Cheng, X., et al. (2023). The in vitro/in vivo metabolic pathways analysis of lobetyol, lobetyolin, and lobetyolinin, three polyacetylenes from Codonopsis Radix, by UHPLC-Q/TOF-MS and UHPLC-MS/MS. J. Pharm. Biomed. Analysis 223, 115140. doi:10.1016/j.jpba.2022.115140
Xie, Q., Xuemei, C., Hu, F., and Wang, C. (2020b). Research advance on chemical constituents, pharmacological action and quality control of Radix Codonopsis. Shanghai J. Traditional Chin. Med. 54 (08), 94–104. doi:10.16305/j.1007-1334.2020.08.013
Xiong, Y., Chen, M., Li, X., Shen, S., and Mu, L. (2000). Effects of codonopsis clematidea polysaccharide on SOD and MDA. Xinjiang J. Traditional Chin. Med., 13.
Xu, A., Zhang, Z., Ge, B., and Pu, J. (2006). Study effect and its mechanism on resisting senility of PCPN. Chinese journal of modern applied pharmacy (S2), 729–731. doi:10.13748/j.cnki.issn1007-7693.2006.s2.001
Xu, L., Wang, H., and Yuan, Z. (2008). Triterpenoid saponins with anti-inflammatory activity from Codonopsis lanceolata. Planta medica. 74 (11), 1412–1415. doi:10.1055/s-2008-1081318
Xu, P. (2018). Studying the immunoregulatory activity of Codonopsispilosula Polysaccharide in SD rats. Master. Zunyi, China: Zunyi Medical University.
Xu, X., Wang, J., Zhang, D., Feng, W., and Song, W. (2021). Safety toxicological evaluation of wen radix codonopsis. J. Hyg. Res. 50 (06), 1012–1018. doi:10.19813/j.cnki.weishengyanjiu.2021.06.023
Xu, X., Wang, J., Zhang, D., Feng, W., Song, W., and Sun, J. (2022). Safety toxicological evaluation of Baitiao dangshen. J. Toxicol. 36 (01), 91–95. doi:10.16421/j.cnki.1002-3127.2022.01.004
Xuan, Z. (2019). Optimization of the extraction process and anti-BVDV mechanism of polysaccharide from Baitiao-codonopsis pilosula. Master. Lanzhou, China: Lanzhou University of Technology.
Yan, J., Chen, C., Chu, C., Li, X., and Wu, Q. (2015). Separation and source of 5-hydroxymethylfurfural in Shengmaiyin(Dangshenfang). J. Zhejiang Univ. Technol. 43, 379–382.
Yan, Y., Zhang, Z., Wei, Y., Niu, F., Cui, W., Wang, S., et al. (2006). Preventive effect of total saponin of Radix Codonopsis on Ast lesion induced by hypoxia andhypoglycemia reoxygenation in rats. J. Beijing Univ. Traditional Chin. Med. 12, 826–829+871.
Yang, D., Li, Z., Wang, X., Yang, Y., Peng, W., Liu, K., et al. (2015). Chemical constituents from roots of Campanumoea javanica and their antiangiogeneic activities. Chin. Traditional Herb. Drugs 46 (04), 470–475.
Yang, L., Hou, A., Sun, Y., Wang, S., Zhang, J., and Jiang, H. (2020). Screening and quantifying the quality markers of DuHuo by fingerprint modeling. J. Liq. Chromatogr. Relat. Technol. 43 (15-16), 604–614. doi:10.1080/10826076.2020.1772287
Yao, Z. (2013). Empirical identification method of codonopsis pilosula and its fake products. Guide China Med. 11 (22), 650–651. doi:10.15912/j.cnki.gocm.2013.22.084
Yi, C., Yan-Yun, Z., Ting, H., and Yi-qi, L. (2021). Effect of codonopsis polysaccharide on fatigue of exhaustion of motion mice. Chin. J. Gerontology 41 (16), 3498–3501.
Zhan, M., Li, G., and Guo, X. (2021). Advances in studies on chemical constituents and pharmacological activities of codonopsis. Shandong Chem. Ind. 50 (19), 79–82. doi:10.19319/j.cnki.issn.1008-021x.2021.19.029+84
Zhang, J., Li, J., Wang, Y., Ji, J., and Gao, J. (2021a). Steroids constituents from Codonopsis pilosula and their anti-inflammatory activities. Chin. Tradit. Pat. Med. 43 (01), 92–97.
Zhang, Q., Xia, Y., Luo, H., Huang, S., Wang, Y., Shentu, Y., et al. (2018). Codonopsis pilosula polysaccharide attenuates tau hyperphosphorylation and cognitive impairments in hTau infected mice. Front. Mol. Neurosci. 11, 437. doi:10.3389/fnmol.2018.00437
Zhang, X., Zhu, C., Hu, L., Lai, X., and Mo, J. (2003). Pharmacological action of polysaccharides from radix codonopsis on immune function and Hematopoiesis in mice. Traditional Chin. Drug Res. Clin. Pharmacol. 3, 174–176. doi:10.19378/j.issn.1003-9783.2003.03.012
Zhang, Y., Wang, X., and Zhi, X. (2021b). Effect of total saponins of Codonopsis lanceolata on the expression of MDA, TNF-a, sICAM-1and sVCAM-1 in myocardium of diabetic rats. Mod. Med. J. China 23 (07), 19–21.
Zhao, X. (2016). The study on prevention and mechanism of BDP againstthe ulcerative colitis on rats. Master. Hubei, China: Hubei University of Chinese Medicine.
Zhen, X., Gao, J., and Cao, L. (2014). Determination of contents of polysaccharide in Lu dangshen. Chin. Archives Traditional Chin. Med. 32 (03), 498–500. doi:10.13193/j.issn.1673-7717.2014.03.016
Zheng, D., Chen, X., Bai, C., and Li, H. (2021). Simultaneous determination of four components in Shenzhu powder by UPLC-MS/MS. Fujian J. Traditional Chin. Med. 52 (07), 37–40. doi:10.13260/j.cnki.jfjtcm.012310
Zheng, T., Cheng, L., Yan, Y., Liu, B., Qin, F., Xu, F., et al. (2018). Two new triterpenoids from the roots of Codonopsis pilosula. Molecules 23(2).
Zhong, Y., and Liang, Z. (2006). A new triterpenoid saponin from codonopsis lanceolata. Chin. Chem. Lett. 17, 1460–1462.
Zhou, J. (2021). Study on anti-aging effect and Developmentof a moisturizer of codonopsis pilosula oligosaccharides. Master. Lanzhou, China: Lanzhou University.
Zhou, P., and Zhou, Y. (2011). Clinical applied experience of Astragalus membranaceus Matched with different herbs ofTraditional Chinese medicine. Chin. J. Exp. Traditional Med. Formulae 17 (10), 275–277. doi:10.13422/j.cnki.syfjx.2011.10.080
Zhou, W., Xiang, L., Lu, H., Chen, Z., Gong, Q., Ren, L., et al. (2016). Radix codonopsi polysaccharide against 5-Fluorouracil-induced gastrointestinal mucositis inMice model. Liaoning J. Traditional Chin. Med. 43 (07), 1495–1498. doi:10.13192/j.issn.1000-1719.2016.07.051+1492
Zhou, X., Fan, Q., Huang, S., Wang, C. j., and Wang, Y. s. Wang, C.-j., and Wang, Y.-s (2012). Identification of a new flavone glycoside from Codonopsis nervosa. Chem. Nat. Compd. 47(6), 888–890. doi:10.1007/s10600-012-0095-6
Zhu, E.-Y., He, Q., Wang, Z.-T., Xu, L.-S., and Xu, G.-J. (2001). Chemical study on the root of Codonopsis pilosnla. J. China Pharm. Univ. 32, 94–95.
Zhu, R. (2013). Analyses and anti-tumor Activitives of the Polvsaccharides from codonopsis pillosula. Master. Changchun, China: Northeast Normal University.
Keywords: codonopsis radix, ethnopharmacology, phytochemistry, analysis method and quality control, processing methods, pharmacological effects
Citation: Dong J, Na Y, Hou A, Zhang S, Yu H, Zheng S, Lan W and Yang L (2023) A review of the botany, ethnopharmacology, phytochemistry, analysis method and quality control, processing methods, pharmacological effects, pharmacokinetics and toxicity of codonopsis radix. Front. Pharmacol. 14:1162036. doi: 10.3389/fphar.2023.1162036
Received: 09 February 2023; Accepted: 23 March 2023;
Published: 06 April 2023.
Edited by:
Wei Peng, Chengdu University of Traditional Chinese Medicine, ChinaReviewed by:
Wuliji Ao, The Research Institute of Traditional Mongolian Medicine Engineering Technolog, ChinaCopyright © 2023 Dong, Na, Hou, Zhang, Yu, Zheng, Lan and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Lan, bGFud2VpNTE2QHNpbmEuY29t; Liu Yang, aHhrX3lsQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.