- 1Department of Integrated Traditional Chinese and Western Medicine, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China
- 2Grade 2019 of Integrated Traditional Chinese and Western Clinical Medicine, Second Clinical School, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China
Backgrounds: The incidence of melanosis coli (MC) has gradually increased annually, attracting significant attention and efforts into this field. A potential risk for MC is the long-term use of anthraquinone laxatives in patients with constipation. Most traditional cathartic drugs are made from herbs containing anthraquinone compounds. This review aims to provide guidance for the application of traditional Chinese herbs containing anthraquinones for physicians and researchers.
Materials and methods: We reviewed risk factors and pathogenesis of MC, and natural anthraquinones isolated from TCM herbs. We searched Pubmed and CNKI databases for literature related to MC with keywords such as“traditional Chinese medicine”, “Chinese herbs”, “anthraquinones”, and “melanosis coli”. The literature is current to January 2023 when the searches were last completed. After the literature retrieval, the TCM herbs containing anthraquinones (including component identification and anthraquinone content determination) applied in clinical were selected. According to the collected evidence, we provide a list of herbs containing anthraquinones that could cause MC.
Results: We identified 20 herbs belonging to 7 families represented by Polygonaceae, Fabaceae, Rhamnaceae, and Rubiaceae, which may play a role in the pathogenesis of MC. Among these, the herbs most commonly used include Dahuang (Rhei Radix et Rhizome), Heshouwu (Radix Polygoni Multiflori), Huzhang (Rhizoma Polygoni Cuspidati), Juemingzi (Semen Cassiae), Luhui (Aloe) and Qiancao (Rubiae Radix et Rhizoma).
Conclusion: Due to a lack of awareness of the chemical composition of TCM herbs, many patients with constipation and even some TCM physicians take cathartic herbal remedies containing abundant anthraquinones to relieve defecation disturbances, resulting in long-term dependence on these herbs, which is potentially associated with most cases of MC. When such treatments are prescribed, TCM physicians should avoid long-term use in large doses to reduce their harm on colonic health. Individuals who take healthcare products containing these herbs should also be under the supervision of a doctor.
1 Melanosis coli
1.1 Introduction to melanosis coli
Melanosis Coli (MC) is a reversible, non-inflammatory, and non-precancerous disease characterized by brownish pigmentation of the colonic mucosa. Billiard noted this colonic pigmentation in 1825 for the first time, and Cruveilhier described it for the first time in 1829. In 1857, Virchow named the condition ‘Melanosis Coli’, which was subsequently defined as a benign condition.
The characteristic image of MC is granular or reticular brownish pigmentation distributed in part or the whole colonic mucosa under endoscope (Grilo et al., 2014). Histological observation of colon pigmentation under the light microscope shows that epithelial cells and different levels of macrophage deposition are distinguished in the mucosa lamina propria. Colonic mucosal biopsies examined by electron microscopy show abnormalities of absorptive epithelial cells, apoptosis of colonic epithelial cells, and phagocytosis of apoptotic bodies by macrophages in the epithelium. On the surface of absorptive cells, the microvilli become less and even disappear. Macrophages migrate towards the lamina propria, where lipofuscin was formed under intracellular degradation of the apoptotic bodies. Apart from pigment-laden macrophages, plasma cells and degenerated nerves are also present in the lamina propria. (Balázs, 1986; Walker et al., 1993).
There are no individual symptoms or physical signs in patients with MC. The most relevant symptom is constipation, which is one of the etiologies of MC. The morbidity of MC is between 0.82% and 1.13%. MC tends to occur in individuals who rely on anthraquinone laxatives to relieve constipation and individuals who use laxatives as weight-reducing drugs, beauty products, and health products. Approximately 95% of MC patients have a medication history of taking anthraquinone laxatives for a prolonged time, especially in females. Studies revealed that males in their 60s and females in their 50s were more likely to suffer from MC. (Yang et al., 2020b). A multicenter study based on 6,090 cases in China concluded that MC detection rates increased with age (Wang et al., 2018). The colonic polyp is the most common concomitant disease of MC in the Chinese population. (Wang et al., 2018).
1.2 Etiology and risk factors of melanosis coli
1.2.1 Anthraquinone laxatives
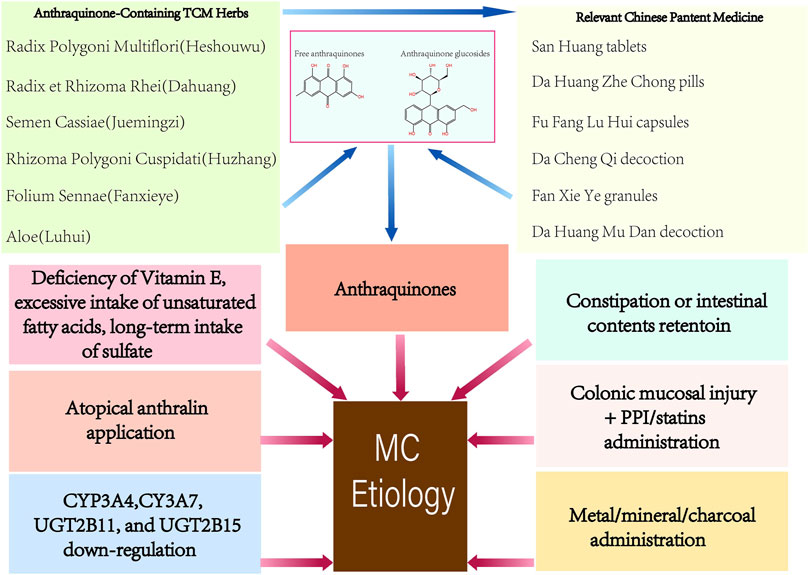
The abuse of anthraquinones was the first identified and the primary cause of MC (Figure 1). As contact cathartics, anthraquinones help relieve constipation by promoting bowel motility and reabsorption of water in the colonic mucosa. However, its side effects and harm to the human body are evident with long-term administration. Scientists have proposed several theories on how anthraquinones contribute to MC (Wan et al., 2008). The initial theory is that the colon epithelium absorbs anthranoid combinations because most research correlates black pigment with anthraquinones metabolites due to their similarities in color. Hoshi et al. assumed that the pigmentation in macrophages resulted from their direct uptake of anthraquinones (Hoshi et al., 1996), (Figure 2). With the help of advanced technology, it was confirmed that absorbed anthranoid free radicals exist in the melanosis tissue (Krbavcic et al., 1998), (Figure 2). Furthermore, some researchers have proposed that anthraquinones could activate enzymes that involve in melanin pigmentation and intestinal dysfunction (Wan et al., 2008).
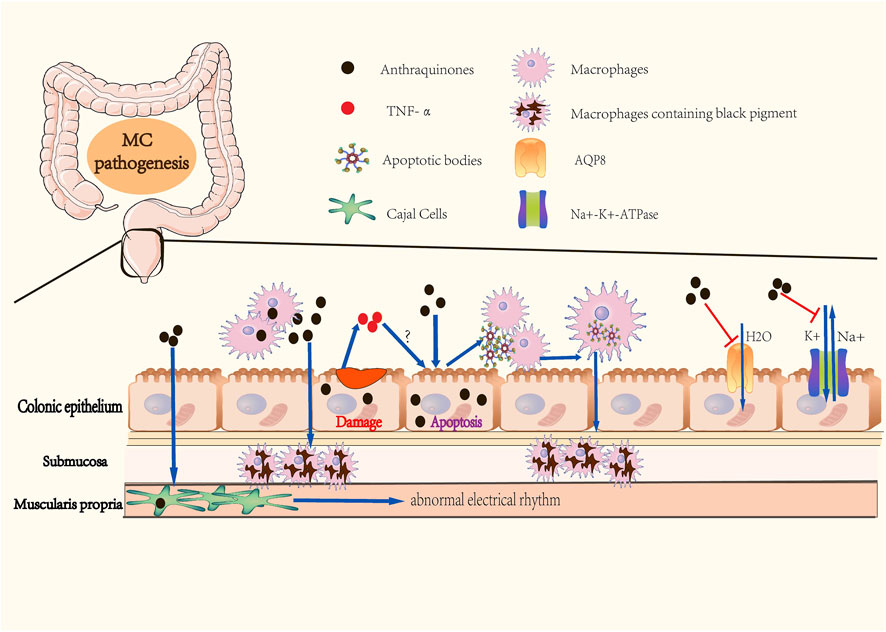
FIGURE 2. Pathogenesis of Melanosis Coli. (A) Colonic epitheliums absorb anthraquinones in the colonic cavity. (B) Macrophages directly intake anthraquinones, then migrate to submucosa. (C) Anthraquinones cause abnormal apoptosis of colonic epithelial cells, followed by macrophages phagocytizing apoptotic bodies. This step may be facilitated by TNF-α release caused by anthraquinones-induced epithelial damage. Macrophages containing apoptotic bodies migrate to submucosa and form black pigments. (D) Anthraquinones retain water in the colonic cavity by inhibiting the activities of Na+-K+-ATPase and AQP8. Anthraquinones also contribute to abnormal electrial rhythm of Cajal cells. These mechanisms promote dependency on anthraquinones in individuals with constipation.
1.2.2 Constipation or retention of intestinal contents
Many researchers have focused on constipation, the most common symptom of MC, to identify a more reasonable explanation of the onset of colonic mucous melanosis (Figure 1). Instead of abandoning the view of the vital role of anthraquinones in MC, it has been proposed that chronic use of anthraquinones masks the underlying history of refractory constipation in patients. There are two main hypotheses about how constipation leads to MC. One theory is that the colon absorbs brownish pigment granules synthesized by intestinal microorganisms; the other is that digestive enzymes decompose proteins originating from incompletely digested food into peptides and amino acids, which are turned into pigment granules by submucosal enzymes, and such granules are absorbed by the colon mucosa eventually (Wan et al., 2008).
1.2.3 Other causes
Apart from anthraquinones, anthralin for topical application (Lestina, 2001), bisacodyl (Mennecier and Vergeau, 2004), proton-pump inhibitor (PPI) (Dore et al., 2014), statins (Dore et al., 2014), ipecac (Johnson et al., 1991), and bamboo leaf extract (Iseki et al., 1998) have been reported to cause MC lesions (Figure 1). Deficiency of vitamin E, excessive intake of unsaturated fatty acids, oxidative damage (Wilberts et al., 2015), and drinking sulfate-contaminated water (Rodríguez-Gómez et al., 2021) for a prolonged time have also been reported as possible factors associated with MC (Figure 1). Chronic oral administration or respiratory inhalation of metallic elements (Cha et al., 2009) or mineral substances (Kim et al., 2011) is considered to be responsible for intestinal melanosis (Figure 1). Progress in genetic polymorphisms research shows that the cytochrome P450-related genes CYP3A4, CYP3A7, UGT2B11, and UGT2B15 were significantly downregulated in MC (Li et al., 2015), (Figure 1).
1.3 Pathogenesis of melanosis coli
In 1975, Steer and Colin-Jones determined that the lysosomal activity and the number of lysosomes in macrophages, Schwann cells, and neurons of the submucosal plexus of the colonic mucosa increased in MC patients with abuse of anthraquinones (Steer and Colin-Jones, 1975). In 1988, Walker et al. observed that daily oral administration of anthraquinones in MC guinea pigs induced transient dose-related apoptosis in colonic epithelial cells (Walker et al., 1988). Research in MC patients by Walker et al. revealed the involvement of apoptotic bodies in the pathogenesis of MC (Walker et al., 1993). They deduced that macrophages possessing phagocytosed apoptotic bodies could cross the fenestrae in the basal lamina, which is similar to those present in the small intestine and skin. Due to existing limitations in both anthraquinone purgatives- and constipation-related theories of the pathogenesis of MC, some scholars changed their focus and developed a theory whereby the increased apoptosis of colonic epithelial cells is the root cause of MC. Byers et al. measured the colonic epithelial apoptosis of 38 MC patients, and proposed that MC is simply a nonspecific manifestation of increased apoptosis of colonic epithelial cells (Byers et al., 1997). In other words, any elements that facilitates the apoptosis of colonic epithelial cells can underlie the etiology of MC. Chen et al. found that rhubarb increased TNF-α levels both in the serum and colon of guinea pigs with MC (Chen J. Y. et al., 2009). Their follow-up research in MC guinea pigs also revealed that there was a positive correlation in the severity of induced MC with the rate of cell apoptosis and serum TNF-α levels (Chen et al., 2011). Based on these findings, the researchers summarized a possible molecular mechanism for the formation of apoptotic bodies. Anthraquinone destroys the colon mucosal barrier and facilitates the release of pro-inflammatory TNF-α, which causes apoptosis of colonic epithelial cells followed by the production of apoptotic bodies, which ultimately results in the formation of phagocytizing apoptotic bodies by macrophages in the mucosal membrane (Figure 2). Regitnig and Denk determined that apoptotic fragments of epithelial cells remained in the epithelium of adenomas, accompanied by a reduction in macrophage levels and an increased expression of Ki-67 and Bcl-2 in adenoma tissue without melanosis (Regitnig and Denk, 2000). The study suggested that low-grade adenomas or tumors might be related to the absence of melanosis in proliferative lesions, and supported the role of apoptosis in the pathogenesis of MC.
1.4 Treatment and prevention of melanosis coli
Patients with MC are reported to have an increased risk of colonic hyperplastic polyps (Katsumata et al., 2021), low-grade adenomas (Kassim et al., 2020) and nonspecific ulceration of the distal ileum (Liu et al., 2017). A retrospective study including 718 MC patients and 2,154 healthy people showed that MC is not related to increased diagnosis of colorectal cancer but enhanced polyp detection due to “chromo-endoscopy-like effect” (Abu Baker et al., 2018). A systemic review aiming to determine the effects of anthraquinone laxatives on colorectal cancer has concluded that a history of anthraquinone laxatives use brought an increased trend in colorectal cancer development, although not showing any significance (Lombardi et al., 2020; Lombardi et al., 2022). Therefore, medications or therapeutic interventions for MC are required. The primary therapeutic objective is to normalize the colon mucosa. Anthraquinones should be discontinued immediately after MC diagnosis (Roerig et al., 2010). Remission of MC is reported to occur in 6 months to 1 year after stopping treatment with anthraquinones (Balázs, 1986). It is suggested that any application period of anthraquinones laxatives longer than 1–2 weeks require physician supervision (Younes et al., 2018). Patients with cosmetic or dietary demands should achieve their goals preferentially by modifying their lifestyle rather than by taking medication. Further, patients with constipation should change their lifestyle and switch to alternative cathartics, such as bulk cathartics, prokinetic agents, osmotic laxatives, and lubricating laxatives. Related food supplement business operators should label the recommended doses on their products; medicinal laxatives containing anthraquinones should be only short-term applied in adults, elderly, and adolescents over 12 years (Younes et al., 2018); some licensed drugs applied in other diseases except for constipation should be labeled the existence of the risk of inducing MC under the condition of long-term use.
2 Anthraquinones
2.1 Introduction to anthraquinones
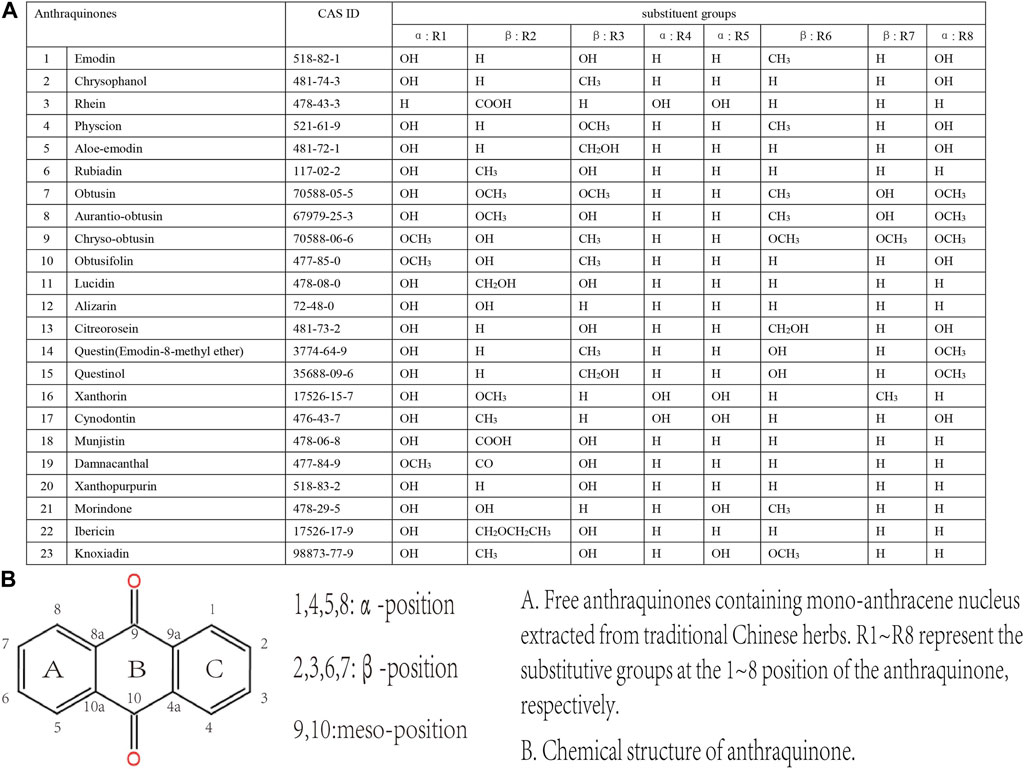
Anthraquinones belong to the quinones family characterized by the cyclic diketone structure. Depending on whether the mono-anthracene nucleus is combined with other kinds of compounds, they are classified into two main classes, the free anthraquinones and the combined anthraquinones. The primary members of free anthraquinones are anthracene and various reduction products of anthraquinones, such as anthranol and anthranone; the most significant part of combined anthraquinones are anthraquinone glycosides, whose aglycones are free anthraquinones (Malik and Müller, 2016).
Natural anthraquinones are mainly applied as irritant laxatives, whose pharmacokinetic characteristics have been widely studied. The absorption of anthraquinones mainly occurs in the intestine rather than in the stomach, which is attributed to the longer retention time of anthraquinones in the intestinal tract and the increase in anthraquinones ionization associated with pH level (Malik and Müller, 2016). Most anthraquinones are absorbed by intestinal villous epithelial cells via passive diffusion. Therefore, the absorption of anthraquinones typically depends on their physicochemical properties, especially on the chemical structures and lipid solubilities (Malik and Müller, 2016). After absorption, anthraquinones are widely distributed throughout the body, mainly in organs and tissues with a rich blood supply, such as intestines, stomach, liver, lung, kidney, and fat (Wang D. et al., 2021).
Anthraquinone biotransformation occurs primarily in the liver. Some studies (Malik and Müller, 2016) have shown that oral anthraquinone biotransformation also occurs in the intestine, mainly by the intestinal microorganism such as bifidobacterium, peptostreptococcus, and clostridium. The metabolic pathways of anthraquinones include mainly hydrolysis, glucuronidation, sulfation, methylation/demethylation, hydroxylation/dehydroxylation, oxidation/reduction, acetylation, and esterification, among which hydrolysis, glucuronidation, and sulfation are dominant (Malik and Müller, 2016). It should be noted that anthraquinones are excreted mainly with the urine, bile or feces in the form of its prototype or metabolites. Anthraquinones excreted with bile can be reabsorbed in the intestines and utilized to form hepato-enteric circulation and maintain a relatively constant blood concentration (Wang X. X. et al., 2021).
Anthraquinones laxatives inhibit the absorption of water and sodium in the colon by inhibiting the activities of Na+-K+-ATPase of colonic epithelial cells (Malik and Müller, 2016), (Figure 2). Another mechanism involves anthracenes-induced downregulation of AQP8 expression in the colon epithelium, thus retaining water in the colonic cavity (Liu et al., 2011), (Figure 2). In addition to increasing the water content of the stool to soften it and make it easier to discharge, anthraquinones can directly stimulate the plexus of the colonic myenteric nerve and facilitate colon peristalsis activity. Research has reported that emodin can disturb the peristaltic rhythm of the colon, which can be attributed to cell membrane damage and energy metabolism disorder resulting from inhibition of the activities of Na+-K+-ATPase and Ca2+-ATPase and over-activation of IP3 and cAMP in Cajal interstitial cells (Peng et al., 2009), (Figure 2).
For patients with constipation and individuals relied on anthraquinone-containing healthcare products, the most noteworthy item is the intestinal toxicity of anthraquinones (Lombardi et al., 2020). Anthraquinone laxatives have been demonstrated to have tumor-inducing potential in animal studies. Exposing to an anthraquinone-containing diet for 480 days induced 25 colorectal cancer in rats; another animal study showed that using anthraquinone laxatives for 112 days developed 4 adenocarcinomas and 5 adenomas in the large intestine of rats (Mohammed et al., 2021). However, a prospective case control study demonstrated that the long-term use of anthraquinone laxatives was not associated with increased risk for colorectal adenoma or colorectal cancer (Nusko et al., 2000). Considering the potential oncogenicity of anthraquinones, the Panel on Food Additives and Nutrient Sources added to Food (ANS) proposed some application suggestions for the use of anthraquinone-containing food supplements, healthcare products, ethicals and herbs. The daily dose of short-term application of anthraquinone laxative in adults to relieve constipation should be controlled within 30 mg/d and 2–3 times per week (Younes et al., 2018). In addition, products containing anthraquinones should be marked with special symbols, to make medical workers and purchasers realize that the using term, recommended dose, intestinal toxicity, contraindications are several key items for regular use of anthraquinones. Especially for people with other risk factors of colorectal cancer, careful use of anthraquinones might help mitigate the risks of developing colorectal cancer. Either healthcare supplement producers or medical pharmaceutical companies, they all should perform safety experiments of anthraquinone-containing production and submit their maximum recommended doses to food and drug administration. It is a pity that TCM herbal products on the market in China are rarely marked with potential toxicity or contraindications. Therefore, users need to carefully check whether the compositions of these drugs or food supplements include any of the herbs listed here in order to use them with caution. There is still a plenty room for improvement in the food and drug administration.
2.2 Common anthraquinones present in TCM herbs
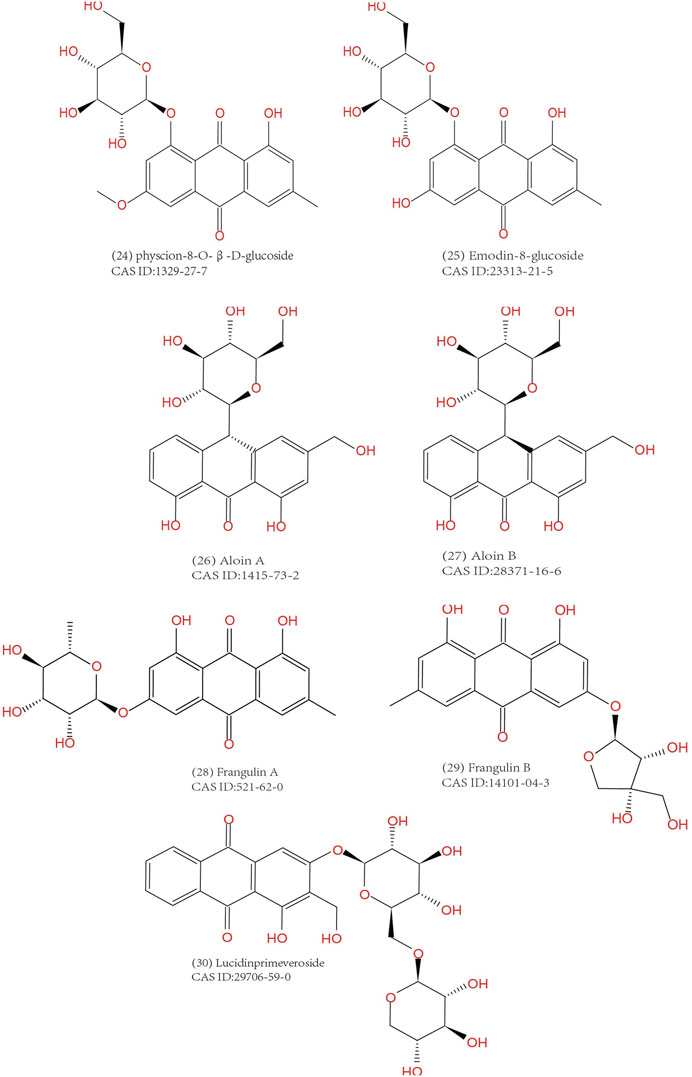
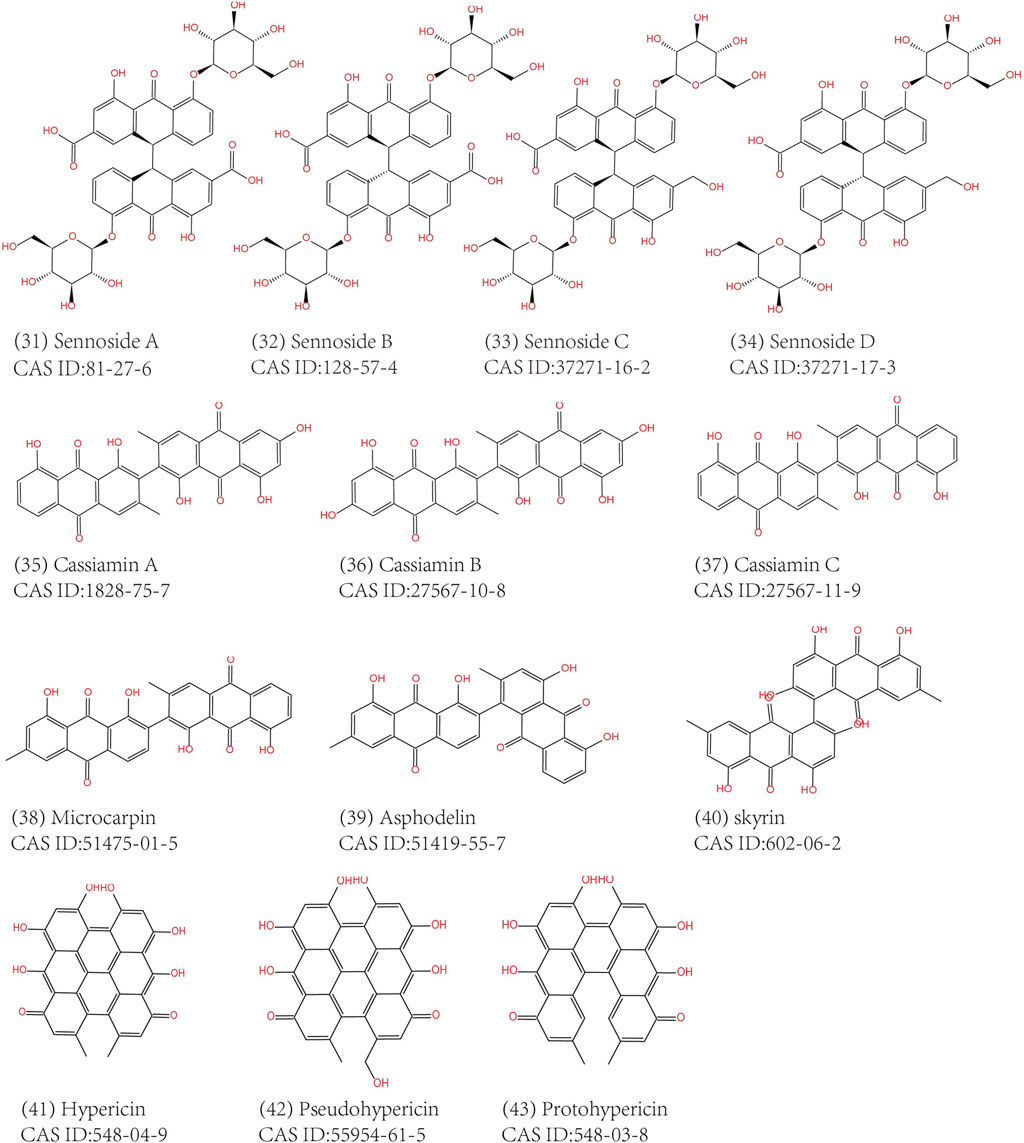
Anthraquinones are widely distributed in Chinese medicine, but the types and contents differ. For Dahuang (Rhei Radix Et Rhizoma), Huzhang (Rhizoma Polygoni Cuspidati), Heshowu (Radix Polygoni Multiflori) and Juemingzi (Semen Cassiae), anthraquinones are the main pharmacological compounds in them. However, anthraquinones in most other herbs are discovered unexpectedly, for that anthraquinones is not a compelling reason to explain their traditional use. Currently, emodin, aloe-emodin and aloin are the most thoroughly studied natural anthraquinone. Dahuang was the first anthraquinone-containing Chinese herb attracting widespread attention because it has been widely used in TCM in all ages, and its effective cathartic function and antimicrobial activity corresponds to its conventional use. Therefore, its main active composition, emodin and related derivatives, have also raised most attention of scholars (Yang et al., 2020a). Till now, research on medicine leading to MC has always been placed emphasis on emodin or aloe emodin, as well as related prescriptions and herbal extracts. Nevertheless, we cannot indiscreetly draw a conclusion that emodin is the most abundant anthraquinone extracted from TCM herbs and it has the most potential to cause MC. In order to provide a reference for rational herbal use in clinic, we briefly summarized the anthraquinones extracted from TCM herbs. It needs to be clarified that the MC lesions are caused by the combined effects of these anthraquinone compounds rather than single one.
Common anthraquinones isolated from anthraquinone-containing TCM herbs related to MC are shown below (Figures 3–5).
3 Traditional Chinese herbs associated with melanosis coli
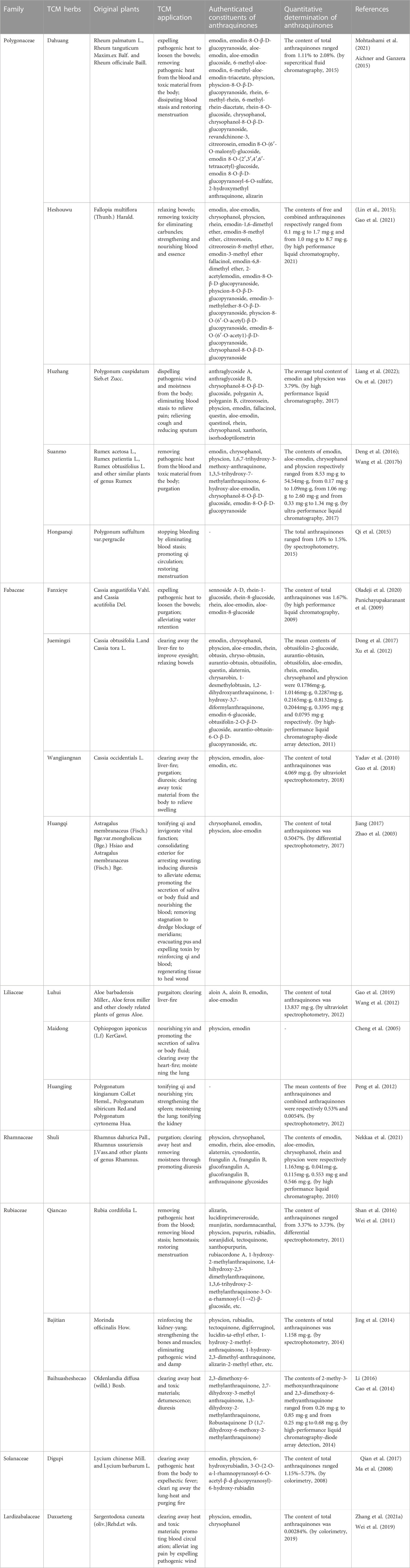
To get the picture of the general situation of anthraquinone-containing TCM herbs in connection with MC, we performed related information retrieval in CNKI and Pubmed. Our retrieved results showed that there were many MC cases diagnosed by gastrointestinal endoscopy after long-term administration of Chinese herbs like Dahuang, Fanxieye, Shuli (Kew and Chakravarthi, 2013; Bechara et al., 2016; Iwamuro et al., 2020). However, Dahuang and Maidong (Radix Ophiopogonis) are the only two herbs which have been verified to cause MC in animal models. The reason that Shuli (Rhammus) and Fanxieye are considered to be associated with MC is that their similar laxative functions and rich contents of anthraquinones. Since the specific mechanism of MC caused by the administration of anthraquinone-containing herbs is still unclear, all the retrieved herbs with isolated anthraquinones or with anthraquinones content determination are summarized in Table 1 to avoid omissions. At the same time, it should not be ignored that long-term or dependent use of anthraquinones might play a critical role in MC pathogenesis, so we made a simple classification of these herbs based on whether they are administrated for a long time or not (Table 1). In the future, it will be necessary to perfect the isolation, identification and determination of anthraquinone compositions from TCM herbs above. We are looking forward to more studies to reveal underlying molecular mechanisms of MC and explore the MC pathogenesis related to these herbs.
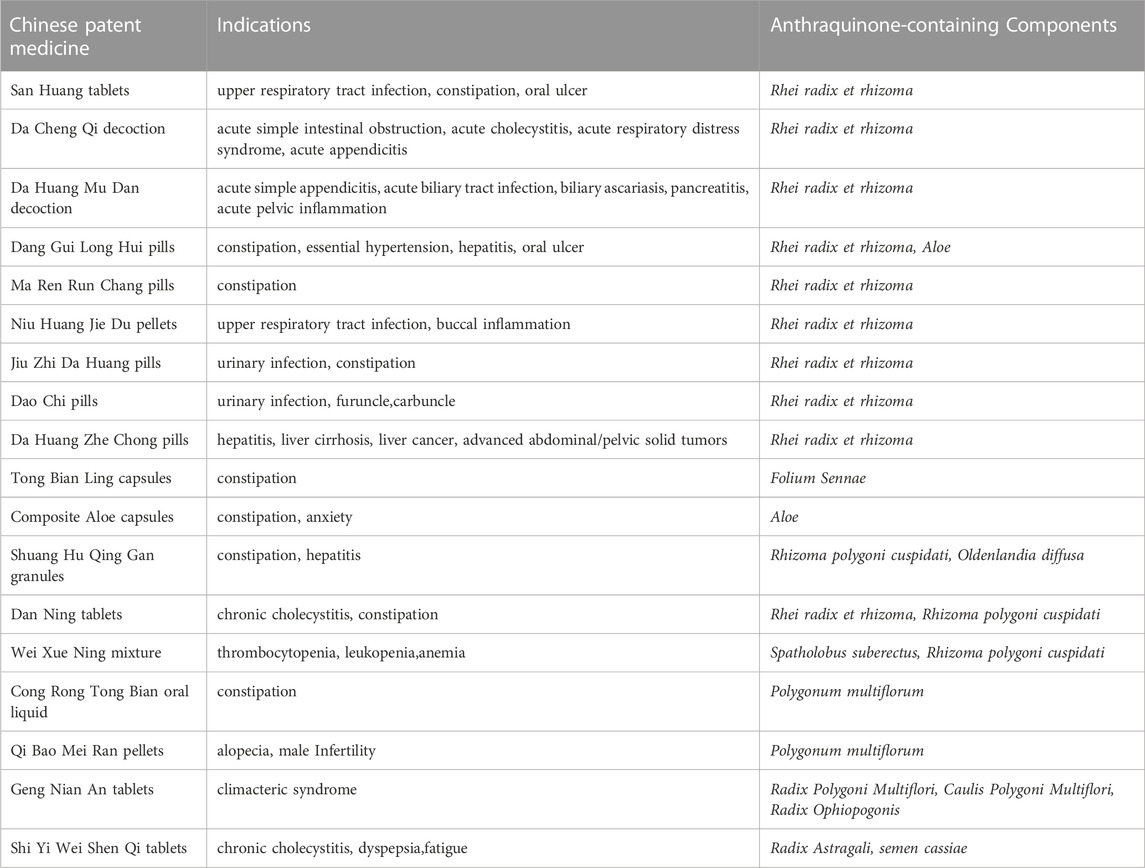
We are inclined to think that not only TCM herbs containing anthraquinones but also long-term oral administration is an etiological factor. Below, we provide a detailed introduction of herbs that meet the above conditions. Table 1 shows a partial list of TCM herbs containing anthraquinones. We review their use and application in TCM. In this section, we described the herbs of the same genera together, for the reason that the herbs of same genera may contain anthaquinones with similar classes and contains, and this approach also systematically convey the information of original plants of Chinese herbs to readers lacking knowledge of TCM. Moreover, A short list of Chinese patent medicines is presented Table 2.
3.1 Polygonaceae
3.1.1 Rhei Radix et Rhizoma (Dahuang)
Rhei Radix et Rhizoma, also called Dahuang in China, derives from the roots and rhizome of Rheum palmatum L., Rheum tanguticum Maxim. ex Balf., and Rheum officinale Baill. Dahuang has been used in the TCM field for thousands of years to treat coagulated heat by purgation and clearing heat-fire. The potency of Dahuang is distinguished by the different processing method. The toxicity and side effects of Cooked Dahuang and Dahuang Parched in Wine are less than Dahuang.
The main anthraquinone chemical components of Dahuang include rhein (3), chrysophanol (2), emodin (1), aloe-emodin (5), physcin (4), and sennoside A–D (31–34)). Aichner and Ganzera determined that the free anthraquinone content in Dahuang ranged from 0.32% to 0.73%, and the anthraquinone content after hydrolysis in Dahuang extract rose to 2.1% (Aichner and Ganzera, 2015). The content of combined anthraquinones in Dahuang will be reduced after being processed at a high temperature because of the poor water solubility of free anthraquinones in this condition. Dahuang is the main composition of several commonly used Chinese medicinal prescriptions, such as the Da Cheng Qi decoction and its derivative decoction, the Da Huang Mu Dan decoction and the Da Huang Fu Zi Xi Xin decoction. Dahuang is commonly used as a laxative herb in both traditional and modern medicine. Some Chinese patent medicines present on the market, like San Huang tablets, Dang Gui Long Hui pills, and Ma Ren Run Chang pills, have been abused for the treatment of constipation.
It was demonstrated that low-dose (1 g/kg) and high-dose (4 g/kg) extract of Dahuang cause inflammatory changes such as neutrephil infiltration, and exfoliation of mucosal epithelial cells in colonic tissue (Zhang, 2013). Mitochondrial swelling, lymphocyte/leukocyte invasion induced by low-dose extract of Dahuang could be observed under the electron microscope (Zhang, 2013). High-dose extract of Dahuang significantly increased the expression of Bax and the apoptosis index of colon of guinea pigs (Zhang, 2013). Another study showed that extract of Dahuang with a dosage of 3–24 g/kg for 60 days induced MC in a dose-dependent fashion in guinea pigs, and the degree of induced MC was positively correlated with the apoptosis rate and TNF-α level in colonic epithelium (Chen and Gang, 2011).
3.1.2 Radix Polygoni Multiflori (Heshouwu) and Caulis Polygoni Multiflori (Yejiaoteng)
Heshouwu and Yejiaoteng come from the same plant called Fallopia multiflora (Thunb.) Harald. or Polygonum multiflorum Thunb., a perennial twining vine of the polygonum family. Heshouwu refers to the underground portion, and Yejiaoteng refers to the aboveground portions.
The predominant anthraquinones in Heshouwu are emodin-type anthraquinones, such as emodin, aloe-emodin, chrysophanol, physcion rhein, 1,6-dimethyl ether-emodin,emodin-8-methyl ether (14), citreorosein (13), citreorosein-8-methyl ether (Lin et al., 2015). Gao et al. (Gao et al., 2021) measured the content of anthraquinones in Heshouwu from various producing areas. The authors concluded that the contents of free and combined anthraquinones ranged from 0.1 mg/g to 1.7 mg/g and 1.0 mg/g to 8.7 mg/g, respectively. Moreover, they found that processing increased the emodin content in Heshouwu, but the reasons remain uncertain.
Heshouwu is a traditional Chinese herb with potency to nourish the liver and kidney, replenish essence and blood, blacken the beard and hair, strengthen muscles and bones, detoxify and relax the bowels. It is a common herb present in most tonic Chinese patent medicines, mainly available as over-the-counter (OTC) preparations in China, such as Qi Bao Mei Ran pellets, Geng Nian An tablets, Shi Yi Wei Shen Qi tablets, and Ren Shen Zai Zao pills. There are also some preparations with the effects of strengthening the body containing Heshowu Although Yejiaoteng and Heshouwu are from the same source, their Chinese medical pharmacology differs slightly from each other. Yejiaoteng belongs to wind-damp-dispelling medicine, and due to its tonic effect, it is often used to treat wind syndrome caused by blood deficiency. These two herbs are also used to relieve constipation caused by the deficiency of Yin and blood in traditional Chinese theories.
3.1.3 Rhizoma Polygoni Cuspidati (Huzhang)
In China, Rhizoma Polygoni Cuspidati, called Huzhang, is obtained from dried roots and the rhizome of Polygonum cuspidatum Sieb. et Zucc. Huzhang effectively relieves jaundice by expelling moistness and removing pathogenic heat and toxicity. It is often prescribed externally for bruises, bruising, swelling, and pain, and orally for jaundice, hepatitis, and cholelithiasis in the Li Dan Pai Du decoction and the Bie Jia decoction. It can also be used to treat constipation with dry stools. The content of anthraquinones in Huzhang by HPLC (High Performance Liquid Chromatography) is approximately 3.79%.
3.1.4 Rumex (Suanmo)
Called Suanmo in China, plants of the Rumex genus are used not only in TCM but also as a vegetable with the functions of removing pathogenic heat and toxic materials. Due to its purgative effect, it is commonly used to relieve constipation. Wang et al. (Wang J. F. et al., 2017) measured the contents of several kinds of anthraquinones in the Suanmo by Ultra Performance Liquid Chromatography. Their results showed that the contents of emodin, aloe-emodin, chrysophanol, and physcion were 8.53 mg/g to 54.54 mg/g, 0.17mg to 1.09 mg/g, 1.06 mg/g to 2.60 mg/g, and 0.33 mg/g to 1.34 mg/g, respectively. As a widely used herb, the side effects caused by its anthraquinones constituents also deserve greater attention.
3.1.5 Radix Polygonum Suffultum (Hongsanqi)
Hongsanqi, from the dried rhizome of Polygonum suffultum var. Hongsanqi has been used to stop bleeding, regulate menstruation, and relieve traumatic swelling and pain in TCM. The constituents content measurement in a pharmacological study in Hongsanqi showed that the total anthraquinones content was 1.0%–1.5% (Qi et al., 2015). When used for treating trauma, it is usually ground into a powder and then soaked in wine and taken as an oral medicine. In this preparation, the extraction ratio of anthraquinones will be higher than that in water solution because alcohol is an organic solvent.
3.2 Fabaceae
3.2.1 Folium Sennae (Fanxieye)
Folium Sennae, called Fanxieye in China, with the familiar name Senna, are dry leaflets of Cassia angustifolia Vahl. and Cassia acutifolia Del. Fanxieye is one of the most commonly used herbs for constipation. In recent years, driven by economic benefit, this herb has been marketed and touted as a remedy for weight loss, detoxification, and beauty for those who want to lose weight and improve their appearance. In addition, it even appears as a kind of flavor vegetable in some middle Eastern and Mediterranean countries. There is no such abuse of Fanxieye in China, and Fanxieye is prescribed more frequently by professional traditional Chinese physicians.
The chemical compositions in the extracts of Fanxieye are mainly anthraquinones, such as sennoside A–D, rhein, aloe-emodin, and chrysophanol. Panichayupakaranant et al. reported that the content of anthraquinones in Fanxieye is 1.67% by HPLC (Panichayupakaranant et al., 2009). Abuse of Fanxieye can not relieve constipation but may exacerbate abdominal pain, bloating, and irregular bowel movements and form a vicious circle.
A prospective study showed that child patients taking sennosides to treat functional constipation had a higher morbidity of MC, with an adjusted odds ratio 13.88 (Chen et al., 2022). It was reported that intragastric administration of 100 mg/kg per day sennoside for 12 weeks induced lipofuscin deposition and colonic melanosis (Shen, 2017). Long-term administration of Sennoside A facilitates apoptosis and inflammation of colon epithelial cells through increasing the mRNA level of IL-1β,IL-6,IL-10, and TNF-α, and decreasing the expression of Ki67(Shen, 2017). It is worth noting that the effect of Sennoside A is dependent on the concentration of intestinal butyric acid and the abundance of intestinal butyricogenic bacteria (Shen, 2017). van Gorkom et al. demonstrated that Sennosides induced colonic epithelial cell apoptosis through p53 and p21/WAF signaling pathway, which may be a potential target for suppressing apoptosis in MC (van Gorkom et al., 2001).
3.2.2 Semen Cassiae and Cassia mimosoides (Juemingzi or Shanbiandou)
Semen Cassiae, with the Chinese name “Juemingzi,” is from the mature seeds of Cassia obtusifolia L. or Cassia tora L. Shanbiandou are dry aerial parts of Cassia mimosoides L. and are also obtained from the genus Cassiae.
Recent studies have shown that its purgative effect is due to its abundant anthraquinones as a stimulant and oil lubricant. Xu et al. discovered the content of seven free anthraquinones and one combined anthraquinones in Juemingzi by HPLC-diode array detection (HPLC-DAD) (Xu et al., 2012). Their results showed that the total contents of eight anthraquinones were 3.075 mg/g.
Juemingzi has been used in East Asian countries (China, Japan, Korea.) for thousands of years to ‘clear liver fire’, which means relieving some symptoms of cardiovascular and cerebrovascular diseases and improving complications caused by hyperglycemia and hyperlipidemia. Moreover, with research on its pharmacology increasingly perfected, Juemingzi has become the favorite choice as a healthy tea beverage in recent years. Many people consistently ignore the side effects of chronic administration of Juemingzi extracts. This risk factor is exacerbated by a lack of medical knowledge or the deliberate concealment of the side effects of Juemingzi by many ‘Jue Ming Zi tea’ beverage producers. In recent years, MC caused by the long-term administration of Juemingzi has become more and more common. However, Shanbiandou is not so much abused, which is used mainly as a diuretic drug to alleviate water retention in patients with nephritis edema.
Wangjiangnan, mature seeds of Cassia Occidentials L., with similar appearance and TCM effects to Juemingzi, is used as a substitute for Juemingzi to relieve constipation. However, more frequent and significant side effects and toxicity have been reported, for which its application is limited. Modern pharmacological studies have revealed that emodin, aloe-emodin, and physcion are the main effective constituents of Wangjiangnan (Yadav et al., 2010). Quantitative determination of total anthraquinones by ultraviolet spectrophotometry in Wangjiangnan is 4.096 mg/g (Guo et al., 2018).
3.2.3 Radix Astragali (Huangqi)
Radix Astragali, called Huangqi in China, is made from dried roots of Astragalus membranaceus (Fisch.) Bge. var. mongholicus (Bge.) Hsiao and Astragalus membranaceus (Fisch.) Bge. Many researchers have not linked Huangqi with anthraquinones because Huangqi is a well-known qi-invigorating herb with the power to boost immunity, and the capacity to protect liver and kidney. Due to its potency to tonify the body and strengthen health, Huangqi is usually present in many daily healthcare products. There have been reported to be several types of anthraquinones with a concentration of 0.5047% in Huangqi (Zhao et al., 2003; Jiang, 2017).
3.3 Liliaceae
3.3.1 Aloe (Luhui)
Aloe, called Luhui in China, native to South Africa and South America, is derived from the leaves of Aloe barbadensis Miller., Aloe ferox Miller, and other closely related plants of the genus Aloe. In TCM, Luhui refers to the dried and concentrated sap of Aloe leaves. Luhui is mainly used to promote defecation and clear liver fire. The sap of Luhui is also an externally applied agent for anaphylaxis, microbial infections, and inflammation of local skin and promotes wound healing and skincare.
Anthraquinones are the primary composition in the extract of Luhui, including aloin A (26), aloin B (27), aloe-emodin, emodin, aloin, and Aloesaponol I, II, III, IV (Gao et al., 2019). Wang et al. reported that the content of total anthraquinones in Luhui was 13.837 mg/g. Moreover, the content of combined anthraquinones in different prepared Luhui are directly drying, stir-frying, stir-frying with wine, stir-frying with salt, stir-frying with vinegar, and steaming with vinegar from the highest to the lowest (Wang et al., 2012).
Luhui extract often appears on the ingredient lists of many cosmetics, skincare, and beauty products. Some companies add Luhui to dairy products, tea-based beverages, and desserts to provide detoxifying, beautifying, and weight loss functions. Some combine Luhui, Fanxieye and Juemingzi to make teabags possessing the effects of excreting fat. Furthermore, these applications expose individuals to higher risk of Laxative Abuse Syndrome and MC. Therefore, Luhui is not a suitable or completely safe herb for chronic oral administration. Case reports of MC with the habit of oral administration of Aloe are not uncommon (Iwamuro et al., 2020).
3.3.2 Rhizoma Polygonati (Huangjing)
Huangjing, or Rhizoma Polygonati, is a Yin-tonifying herb obtained from dried roots and the rhizome of Polygonatum kingianum Coll. et Hemsl., Polygonatum sibiricum Red. and Polygonatum cyrtonema Hua. With the effects of stimulating qi and nourishing yin, strengthening the spleen, moistening the lung and tonifying the kidney, Huangjing is a good remedy choice in TCM for fatigue, hemoptysis tussiculation, and weakness of the waist and knees. Peng et al. showed that the average contents of free anthraquinones and combined anthraquinones of Huangjing are 0.53% and 0.0054%, respectively (Peng et al., 2012).
3.3.3 Radix Ophiopogonis (Maidong)
Radix Ophiopogonis (Maidong) is prescribed in the Chinese medical diet for clearing heat and arresting coughing. The extract of Maidong with a dosage of 1.5 g/kg∼6 g/kg led to colonic mucosal inflammation and excessive apoptosis in guinea pigs (Zhang, 2013). Although the concentration of emodin in Maidong is less than Dahuang, the extract of Maidong with a dosage of 1.5 g/kg was also able to induce a marked increase in the expression of Bcl-2 in colonic epithelium in guinea pigs (Zhang, 2013).
3.4 Rhamnaceae
Shuli is derived from mature fruits, leaves, or bark of Rhamnus dahurica Pall., Rhamnus ussuriensis J. Vass. and other plants of the genus Rhamnus. In the theory of TCM, it is used to remove intestinal heat and activate detoxicification. Another use of Shuli is to relieve constipation, which is the most common application of Shuli in traditional medicine from other countries, except in China. Rich in anthraquinones with a classical structure, Shuli is an irritant laxative (Nekkaa et al., 2021). MC patients with chronic use of Rhamnus bark and leaves have been widely reported. MC of patients with medication history of Shuli are mainly detected by routine digestive endoscopy (Bechara et al., 2016). Interestingly, as long as these laxatives are abandoned, MC will spontaneous relive to normal appearance (Kew and Chakravarthi, 2013).
3.5 Rubiaceae
3.5.1 Rubiae Radix et rhizoma (Qiancao)
Rubiae Radix et Rhizoma, called Qiancao in China, is from the dried roots and rhizome of Rubia cordifolia L. As a hemostatic herbs, Qiancao is used to treat hemorrhage caused by blood heat with blood stasis in TCM. After being stir-fried, its drug property will be milder and has become one of the most effective herbs for regulating menstruation and stopping bleeding for female patients. Oil and wine containing Qiancao for medical use are effective external agents for pain in joints and bones caused by blood stasis. Wei et al. determined that the content of total anthraquinones in Qiancao ranged from 3.37% to 3.73% (Wei et al., 2011). Zheng et al. treated normal rats with a sequential dosage of 70% ethanol extract of Qiancao (5/10/30 g/kg) for 60 days, which was respectively equivalent to 6/12/36 times the prescribed dosage of human. Their results showed that even though the dosage of Qiancao had reached 30 g/kg, it still could not cause MC in rats (Zheng, 2017). More dose-ranging studies are needed to clarify whether Qiancao can cause MC. Even so, Patients who use Chinese patent medicines and herbal prescriptions containing Qiancao to treat hemorrhagic disorders should be mindful, especially for those with long medication cycles to treat gynecological diseases.
Xiaohongshen, from Rubia yunnanensis Diels, whose usage is similar to Qiancao, can replace Qiancao in prescriptions. 1,3,6-trihydroxy-2-methyl anthraquinone, munjistin (18), lucidin (11), 1-hydroxy-2-methyl anthraquinone, 1,3-dihydroxy-2-methyl anthraquinone, xanthopurpurin (19) and 1,4-dihydroxy-2-hydroxymethylanthraquinone have been identfied in its preparations (Wang, 2012).
3.5.2 Radix Morindae Officinalis (Bajitian)
Bajitian or Radix Morindae Officinalis, a Yang-tonifying herb, is the dried root of Morinda officinalis How. Bajitian possesses the TCM effects of recharging the kidney, strengthening bones and muscles, and dispelling wind and moistness. As an adjuvant therapy, the prescriptions containing Bajitian is helpful in the treatment of impotence, erectile dysfunction, male infertility, and oligospermia. The content of anthraquinones in Bajitian was reported to be 1.518 mg/g. Processing technologies can reduce the content of combined anthraquinones and increase the content of free anthraquinones (Jing et al., 2014). From the standpoint of preventing MC, processed Bajitian is safer than crude Bajitian.
Morinda umbellata (Yangjiaoteng), as a succedaneum of Bajitian, can effectively alleviate symptoms of rheumatic diseases, such as rheumatoid arthritis, gouty arthritis, and rheumatic low back pain. Li et al. extracted 1,6-dihydroxy-2-methoxymethylanthraquinone, and several other anthraquinones with similar chemical structures from Yangjiaoteng (Li et al., 2019).
3.5.3 Oldenlandia diffusa (Baihuasheshecao)
The entire dried plant of Oldenlandia diffusa (willd.) Boxb. is called baihuasheshecao in China. It is a detoxifying herb applied in the traditional medical treatment for insect bites, snake bites, and various inflammatory diseases. Its anti-tumor effects has been discovered in recent years, so its application in the neoplastic diseases is becoming more common. Several anthraquinones, such as 2,3-dimethoxy-6-methyl anthraquinone and 2,7-dihydroxy-3-methyl anthraquinone, have been identified in this herb (Li, 2016). The contents of 2-methyl-3-methoxy anthraquinone and 2,3-dimethoxy-6-methyl anthraquinone range from 0.26 mg/g to 0.85 mg/g and from 0.25 mg/g to 0.68 mg/g, respectively (Cao et al., 2014).
3.6 Solanaceae
Digupi, or Cortex Lycii, is the dried root bark of Lycium chinense Mill. or Lycium barbarum L. Digupi has a dual effect on heat removal and Yin tonification, so in TCM, it is used to treat fever, night sweats, diabetes, heart disease, gynecological diseases, and neurasthenia. Ma et al. determined that the content of total anthraquinones in Digupi ranged from 1.15% to 5.73% (Ma et al., 2008). In addition, the older the cultivation of Digupi is, the higher content of anthraquinones Digupi contains. As a key ingredient, Digupi is present in some Chinese patent medicines with the effects of resisting fatigue and aging, strengthening tendons and bones, and improving reproductive function. These drugs are likely to be taken over the long-term by middle-aged and elder patients.
3.7 Lardizabalaceae
Daxueteng, or Caulis Sargentodoxae, is derived from vines and stems of Sargentodoxa cuneata (oliv.) Rehd. et Wils., which is widely distributed in the subtropical region of China. As a heat-clearing drug in TCM, it is empirically applied in treating amenorrhea caused by blood-heat, infectious skin diseases, appendicitis, and wind-heat type rheumatic arthralgia. Isolated anthraquinone were mainly divided into emodin, chrysophanol and physcion (Zhang W. et al., 2021). The total anthraquinones content in Daxueteng measured by colorimetry was 0.00284% (Wei et al., 2019).
4 Conclusion and future perspectives
This review summarizes risk factors and etiologies of MC and some traditional Chinese herbs that may be associated with the onset and development of MC. The most acceptable views accounting for MC development are anthraquinones abuse and constipation. The pathogenesis is mainly attributed to increased colonic epithelial apoptosis. Currently, studies supporting the apoptosis hypothesis are more advanced than others, but the underlying molecular mechanisms corresponding to each herb still require further studies. This review provides an overview of anthraquinones-contained Chinese herbs that cause MC. Dahuang (Rhei Radix et Rhizome), Heshouwu (Radix Polygoni Multiflori), Huzhang (Rhizoma Polygoni Cuspidati), Juemingzi (Semen Cassiae), Luhui (Aloe), and Qiancao (Rubiae Radix et Rhizoma) are herbs with the most abundant anthraquinones known to date. Unfortunately, the isolation, identification and content determination of anthraquinones for most TCM herbs need to be perfected. Another limitation is that the specific molecular mechanism by which each herb cause MC is still an undeveloped territory. Our advice on using these herbs with caution is mainly based on the fact that they contain anthraquinones and are commonly used in TCM. Traditional Chinese physicians should avoid heavily using those herbs or Chinese patent drugs to alleviate chronic symptoms for too long. The daily dose of short-term application of anthraquinones in adults to relieve constipation should be restrained within 30 mg/d and 2–3 times per week (Younes et al., 2018), and any use time of anthraquinones laxatives longer than 1–2 weeks requires medical supervision. Under the condition of related producers have given a clear indication of anthraquinones contents and suggestive doses, users can regulate their daily doses of administration according to this information. Lifestyle modification, dietary fiber supplementation, pelvic muscle exercise, and fecal microbiota transplantation are optional and harmless non-pharmaceutical therapies for constipation. It is necessary to strengthen education on the rational use of natural laxatives among medical workers. Anthraquinone-containing TCM herbs, prescription drugs, food supplement and healthcare products need a more strict production and application management. Finally, further studies on toxicity-structure relationship between anthraquinone compounds and MC need to be carried out to further elucidate the pathogenesis of MC.
Author contributions
RD designed the study; RZ and CH wrote the manuscript; GC, FW, KF, SJ, and YZ reviewed and edited the manuscript. All authors have read and approved the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82004254).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abu Baker, F., Mari, A., Feldman, D., Suki, M., Gal, O., and Kopelman, Y. (2018). Melanosis coli: A helpful contrast effect or a harmful pigmentation? Clin. Med. Insights Gastroenterol. 11, 1179552218817321. doi:10.1177/1179552218817321
Aichner, D., and Ganzera, M. (2015). Analysis of anthraquinones in rhubarb (Rheum palmatum and Rheum officinale) by supercritical fluid chromatography. Talanta 144, 1239–1244. doi:10.1016/j.talanta.2015.08.011
Balázs, M. (1986). Melanosis coli. Ultrastructural study of 45 patients. Dis. Colon Rectum 29 (12), 839–844. doi:10.1007/bf02555359
Bechara, R., Marcon, N., and Streutker, C. J. (2016). Melanosis coli: A disappearing act. Gastrointest. Endosc. 83 (6), 1296–1298. doi:10.1016/j.gie.2015.10.050
Byers, R. J., Marsh, P., Parkinson, D., and Haboubi, N. Y. (1997). Melanosis coli is associated with an increase in colonic epithelial apoptosis and not with laxative use. Histopathology 30 (2), 160–164. doi:10.1046/j.1365-2559.1997.d01-574.x
Cao, G. S., Yang, P. M., Zhang, J. Y., Li, F., Ni, J., and Gao, P. (2014). Determination of two anthraquinones in hedyotidis herba by HPLC-DAD. Chin. J. Exp. Tradit. Med. Formulae 20 (20), 54–56. doi:10.13422/j.cnki.syfjx.2014200054
Cha, J. M., Lee, J. I., Joo, K. R., Jung, S. W., and Shin, H. P. (2009). Melanosis ilei associated with chronic ingestion of oral iron. Gut Liver 3 (4), 315–317. doi:10.5009/gnl.2009.3.4.315
Chen, J. J., Kitzia Colliard, R. N., Nurko, S., and Rodriguez, L. (2022). Melanosis coli is not associated with colonic dysmotility nor severity of pediatric functional constipation. Dig. Dis. Sci. 67 (8), 3922–3928. doi:10.1007/s10620-021-07191-z
Chen, J. Y., Pan, F., and Zhang, T. (2009). Rhubarb induced change of tumor necrosis factor-alpha level in Guinea pig model of melanosis coli and its significance. Chin. J. Integr. Tradit. West. Med. Chin. Ed.) 29 (2), 140–142.
Chen, J. Y., Pan, F., Zhang, T., Xia, J., and Li, Y. J. (2011). Experimental study on the molecular mechanism of anthraquinone cathartics in inducing melanosis coli. Chin. J. Integr. Med. 17 (7), 525–530. doi:10.1007/s11655-011-0786-z
Chen, Z. F., and Gang, A. S. (2011). Content of active component in wild medicinal plant polygonum ciliinerve determined by HPLC and their relationship with tuber size. J. Hebei Agric. Sci. 15 (01), 165–167. doi:10.16318/j.cnki.hbnykx.2011.01.059
Cheng, Z. H., Wu, T., Li, L. Z., Liu, N., Yu, B. Y., and Xu, L. S. (2005). in Studies on the liposoluble components from tuber of Ophiopogon japonicusChin. Pharm. J. (Beijing, China), 20–24.
Deng, Y. H., Sun, Y. N., Guo, D. D., and Ni, Y. (2016). Advances in active ingredients and indicative components of Chinese herbs from Rumex. Chin. J. Ethnomed. Ethnopharm. 25 (17), 41–44.
Dong, X., Fu, J., Yin, X., Yang, C., Zhang, X., Wang, W., et al. (2017). Cassiae semen: A review of its phytochemistry and pharmacology (review). Mol. Med. Rep. 16 (3), 2331–2346. doi:10.3892/mmr.2017.6880
Dore, M. P., Villanacci, V., Manca, A., Soro, S., Schiavo-Lena, M., Sabatino, G., et al. (2014). Cherry-tree colon: Colonoscopic appearance suggesting drug-induced mucosal injury. Intern. Emerg. Med. 9 (4), 405–409. doi:10.1007/s11739-013-0930-1
Gao, H. Y., Yang, J. B., Sun, H., Song, Y. F., Cheng, X. L., Wang, X. T., et al. (2021). Determination of anthraquinones in Polygoni Multiflori Radix from different origins and processed differently by HPLC. Chin. J. Pharmacovigil., 1–11.
Gao, Y., Kuok, K. I., Jin, Y., and Wang, R. (2019). Biomedical applications of Aloe vera. Crit. Rev. Food Sci. Nutr. 59 (1), S244–s256. doi:10.1080/10408398.2018.1496320
Grilo, I., Torres-Gómez, J., and Gómez-Regife, L. (2014). Atypical melanosis coli resembling the appearance of cheetah skin. Endoscopy 46 (1), E437–E438. UCTN. doi:10.1055/s-0034-1377427
Guo, Y., Li, J., Xu, J., and Zhuang, T. (2018). Optimization of extraction process of anthraquinone from Cassia occidentalis L. And DPPH free radical scavenging experiment. Chin. Arch. Tradit. Chin. Med. 36 (02), 428–431. doi:10.13193/j.issn.1673-7717.2018.02.045
Hoshi, O., Iwanaga, T., and Fujino, M. A. (1996). Selective uptake of intraluminal dextran sulfate sodium and senna by macrophages in the cecal mucosa of the Guinea pig. J. Gastroenterol. 31 (2), 189–198. doi:10.1007/bf02389517
Iseki, K., Ishikawa, H., Suzuki, T., Murakami, T., Otani, T., and Ishiguro, S. (1998). Melanosis coli associated with ingestion of bamboo leaf extract. Gastrointest. Endosc. 47 (3), 305–307. doi:10.1016/s0016-5107(98)70333-5
Iwamuro, M., Tanaka, T., and Okada, H. (2020). Melanosis coli due to aloe vera consumption. Intern Med. 59 (20), 2633–2634. doi:10.2169/internalmedicine.5183-20
Jiang, P. (2017). Determination of total anthraquinone content in Radix Astragalus. Heilongjiang Med. J. 30 (02), 243–246. doi:10.14035/j.cnki.hljyy.2017.02.003
Jing, H. Y., Cui, N., Shi, J., and Jia, T. Z. (2014). Comparation in content of anthraquinones in Morinda officinalis and other different processing products. Asia-Pac. Tradit. Med. 10 (01), 21–23.
Johnson, J. E., Carpenter, B. L., Benton, J., Cross, R., Eaton, L. A., and Rhoads, J. M. (1991). Hemorrhagic colitis and pseudomelanosis coli in ipecac ingestion by proxy. J. Pediatr. Gastroenterol. Nutr. 12 (4), 501–506. doi:10.1097/00005176-199105000-00015
Kassim, S. A., Abbas, M., Tang, W., Wu, S., Meng, Q., Zhang, C., et al. (2020). Retrospective study on melanosis coli as risk factor of colorectal neoplasm: A 3-year colonoscopic finding in zhuhai hospital, China. Int. J. Colorectal Dis. 35 (2), 213–222. doi:10.1007/s00384-019-03435-7
Katsumata, R., Manabe, N., Fujita, M., Ayaki, M., Sunago, A., Kamada, T., et al. (2021). Colorectal neoplasms in melanosis coli: A survey in Japan and a worldwide meta-analysis. Int. J. Colorectal Dis. 36 (10), 2177–2188. doi:10.1007/s00384-021-03970-2
Kew, S. T., and Chakravarthi, S. (2013). Images in clinical medicine: Melanosis coli. N. Engl. J. Med. 368 (24), 2303. doi:10.1056/NEJMicm1204882
Kim, G. M., Jun, E. J., Kim, Y. C., Park, J. M., Hong, S. I., Cheung, D. Y., et al. (2011). Melanosis ilei induced by prolonged charcoal ingestion. J. Korean Surg. Soc. 81 (1), 66–69. doi:10.4174/jkss.2011.81.1.66
Krbavcic, A., Pecar, S., Schara, M., Müller, K., and Wiegrebe, W. (1998). Anthranoid free radicals found in pseudomelanosis coli. Pharmazie 53 (5), 336–338.
Lestina, L. S. (2001). An unusual case of melanosis coli. Gastrointest. Endosc. 54 (1), 119–121. doi:10.1067/mge.2001.115323
Li, B. (2016). Advances in constituents and pharmacology of hedyotis diffusa. Tianjin Pharm. 28 (5), 75–78. doi:10.3969/j.issn.1006-5687.2016.05.027
Li, C., Su, X., Li, F., Fu, J., Wang, H., Li, B., et al. (2019). Cytotoxic quinones from the aerial parts of Morinda umbellata L. Phytochemistry 167, 112096. doi:10.1016/j.phytochem.2019.112096
Li, X., Zhou, Y., Zhou, S., Liu, H. R., Xu, J. M., Gao, L., et al. (2015). Histopathology of melanosis coli and determination of its associated genes by comparative analysis of expression microarrays. Mol. Med. Rep. 12 (4), 5807–5815. doi:10.3892/mmr.2015.4126
Liang, C. X., Wang, S. S., Chen, S. J., Wang, Y., Li, J., and Chang, Y. X. (2022). Research development on chemical composition and pharmacology of Polygoni Cuspidati Rhizoma et Radix. Chin. Tradit. Herb. Drugs 53 (04), 1264–1276.
Lin, L., Ni, B., Lin, H., Zhang, M., Li, X., Yin, X., et al. (2015). Traditional usages, botany, phytochemistry, pharmacology and toxicology of polygonum multiflorum Thunb.: A review. J. Ethnopharmacol. 159, 158–183. doi:10.1016/j.jep.2014.11.009
Liu, J., Tian, D. A., Wang, J. P., Zhang, S. Z., Feng, J., Zhao, Z. Z., et al. (2011). Expression of aquaporin 8 and its relationship with melanosis coli. Chin. Med. J. 124 (19), 3061–3065.
Liu, Z. H., Foo, D. C. C., Law, W. L., Chan, F. S. Y., Fan, J. K. M., and Peng, J. S. (2017). Melanosis coli: Harmless pigmentation? A case-control retrospective study of 657 cases. PLoS One 12 (10), e0186668. doi:10.1371/journal.pone.0186668
Lombardi, N., Bettiol, A., Crescioli, G., Maggini, V., Gallo, E., Sivelli, F., et al. (2020). Association between anthraquinone laxatives and colorectal cancer: Protocol for a systematic review and meta-analysis. Syst. Rev. 9 (1), 19. doi:10.1186/s13643-020-1280-5
Lombardi, N., Crescioli, G., Maggini, V., Bellezza, R., Landi, I., Bettiol, A., et al. (2022). Anthraquinone laxatives use and colorectal cancer: A systematic review and meta-analysis of observational studies. Phytother. Res. 36 (3), 1093–1102. doi:10.1002/ptr.7373
Ma, X. Q., Huang, Q., Fu, X. Y., and Dong, L. (2008). Comparison of the content of anthraquinone in Lycium barbarum L. From different regions and different years of ningxia. Lishizhen Med. Mat. Med. Res. (03), 636–637.
Malik, E. M., and Müller, C. E. (2016). Anthraquinones as pharmacological tools and drugs. Med. Res. Rev. 36 (4), 705–748. doi:10.1002/med.21391
Mennecier, D., and Vergeau, B. (2004). Melanosis coli? N. Engl. J. Med. 350 (2), 197; author reply 197. author reply 197. doi:10.1056/nejm200401083500223
Mohammed, A., Paranji, N., Singh, A., and Sanaka, M. R. (2021). Pseudomelanosis coli, its relation to laxative use and association with colorectal neoplasms: A comprehensive review. JGH Open 5 (6), 643–646. doi:10.1002/jgh3.12546
Mohtashami, L., Amiri, M. S., Ayati, Z., Ramezani, M., Jamialahmadi, T., and Emami, S. A. (2021). Ethnobotanical uses, phytochemistry and pharmacology of different rheum species (Polygonaceae): a review. Adv. Exp. Med. Biol. 1308, 309–352. doi:10.1007/978-3-030-64872-5_22
Nekkaa, A., Benaissa, A., Mutelet, F., and Canabady-Rochelle, L. (2021). Rhamnusalaternus plant: Extraction of bioactive fractions and evaluation of their pharmacological and phytochemical properties. Antioxidants 10 (2), 300. doi:10.3390/antiox10020300
Nusko, G., Schneider, B., Schneider, I., Wittekind, C., and Hahn, E. G. (2000). Anthranoid laxative use is not a risk factor for colorectal neoplasia: Results of a prospective case control study. Gut 46 (5), 651–655. doi:10.1136/gut.46.5.651
Oladeji, O. S., Adelowo, F. E., Oluyori, A. P., and Bankole, D. T. (2020). Ethnobotanical description and biological activities of senna alata. Evid.-based Complement. Altern. Med. 2580259. doi:10.1155/2020/2580259
Ou, S. P., Ren, L., Wang, S., Chen, L., and Wang, Y. H. (2017). Research on preparation and determination of Polygonum cuspidatum extract. J. Pharm. Res. 36 (10), 567–570+608. doi:10.13506/j.cnki.jpr.2017.10.003
Panichayupakaranant, P., Sakunpak, A., and Sakunphueak, A. (2009). Quantitative HPLC determination and extraction of anthraquinones in Senna alata leaves. J. Chromatogr. Sci. 47 (3), 197–200. doi:10.1093/chromsci/47.3.197
Peng, C., Wang, L., Wang, Y. H., Li, Y. X., and Pan, Y. (2009). The toxicity of aconitine, emodin on ICC cell and the antagonist effect of the compatibility. Eur. J. Drug Metabol. Pharmacokinet. 34 (3-4), 213–220. doi:10.1007/bf03191176
Peng, X. B., Wang, H., and Yang, T. (2012). Determination of anthraquinones content in rhizoma polygonati. J. Med. Pharm. Chin. Minor. 18 (8), 72–73. doi:10.3969/j.issn.1006-6810.2012.08.037
Qi, L., Jiang, H., and Niu, X. F. (2015). Study on the pharmacognosy of Polygonum suffultum var. pergracile. China J. Tradit. Chin. Med. Pharm. 5(17), 54–56+92.
Qian, D., Zhao, Y., Yang, G., and Huang, L. (2017). Systematic review of chemical constituents in the genus Lycium (solanaceae). Molecules 22 (6), 911. doi:10.3390/molecules22060911
Regitnig, P., and Denk, H. (2000). Lack of Pseudomelanosis coli in colonic adenomas suggests different pathways of apoptotic bodies in normal and neoplastic colonic mucosa. Virchows Arch. 436 (6), 588–594. doi:10.1007/s004289900178
Rodríguez-Gómez, I. M., Gómez-Laguna, J., Ruedas-Torres, I., Sánchez-Carvajal, J. M., Garrido-Medina Á, V., Roger-García, G., et al. (2021). Melanosis coli in pigs coincides with high sulfate content in drinking water. Vet. Pathol. 58 (3), 574–577. doi:10.1177/0300985821991565
Roerig, J. L., Steffen, K. J., Mitchell, J. E., and Zunker, C. (2010). Laxative abuse: Epidemiology, diagnosis and management. Drugs 70 (12), 1487–1503. doi:10.2165/11898640-000000000-00000
Shan, M., Yu, S., Yan, H., Chen, P., Zhang, L., and Ding, A. (2016). A review of the botany, phytochemistry, pharmacology and toxicology of rubiae radix et rhizoma. Molecules 21 (12). doi:10.3390/molecules21121747
Shen, Y. (2017). Sennoside A increases the risk of melanosis coli by inhibiting clostridia. Master. Nanjing Univ. Traditional Chin. Med.
Steer, H. W., and Colin-Jones, D. G. (1975). Melanosis coli: Studies of the toxic effects of irritant purgatives. J. Pathol. 115 (4), 199–205. doi:10.1002/path.1711150403
van Gorkom, B. A., Karrenbeld, A., van der Sluis, T., Zwart, N., de Vries, E. G., and Kleibeuker, J. H. (2001). Apoptosis induction by sennoside laxatives in man; escape from a protective mechanism during chronic sennoside use? J. Pathol. 194 (4), 493–499. doi:10.1002/path.914
Walker, N. I., Bennett, R. E., and Axelsen, R. A. (1988). Melanosis coli. A consequence of anthraquinone-induced apoptosis of colonic epithelial cells. Am. J. Pathol. 131 (3), 465–476.
Walker, N. I., Smith, M. M., and Smithers, B. M. (1993). Ultrastructure of human melanosis coli with reference to its pathogenesis. Pathology 25 (2), 120–123. doi:10.3109/00313029309084783
Wan, J. Z., Xu, X. J., Guo, H. X., Fang, W., and Liu, Y. B. (2008). The researches on the coloring matter and the causes of the Melanosis coli and the relationship between Melanosis coli, Aloe and Rhizome. Int. J. Intern. Med. (04), 227–229+249.
Wang, D., Wang, X. H., Yu, X., Cao, F., Cai, X., Chen, P., et al. (2021a). Pharmacokinetics of anthraquinones from medicinal plants. Front. Pharmacol. 12, 638993. doi:10.3389/fphar.2021.638993
Wang, J. (2012). Advances in the studies of Radix rubiae yunnanensis. Inn. Mong. J. Tradit. Chin. Med. 31 (23), 129–132. doi:10.16040/j.cnki.cn15-1101.2012.23.053
Wang, J. F., Wang, J. X., Teng, Y., Wang, S. S., and Liu, Y. (2017a). Determination of anthraquinones in Rumex acetosa L. J. Southwest Univ. Nat. Sci. Ed. 39 (04), 199–204. doi:10.13718/j.cnki.xdzk.2017.04.030
Wang, K., Gui, M. Y., Zhao, P. F., and Li, J. Y. (2012). Impact of different processing methods on anthraquinones in aloe. Jiangsu Agric. Sci. 40 (8), 247–248. doi:10.3969/j.issn.1002-1302.2012.08.098
Wang, S., Wang, Z., Peng, L., Zhang, X., Li, J., Yang, Y., et al. (2018). Gender, age, and concomitant diseases of melanosis coli in China: A multicenter study of 6,090 cases. PeerJ 6, e4483. doi:10.7717/peerj.4483
Wang, X. X., Wang, C. J., and Li, Z. K. (2021b). Research progress on chemical composition, pharmacological activity of paederia scandens (lour.) merr. World Chin. Med. 16 (05), 826–830.
Wang, Y., Huang, L. L., Wang, D. H., Fang, F., Lai, J. X., Xing, C. C., et al. (2017b). Research progress on chemical composition and function of plants of hemerocallis. Res. Pract. Chin. Med. 31 (01), 79–86. doi:10.13728/j.1673-6427.2017.01.022
Wei, H. Y., Xie, H., Liao, Y., and Dai, P. (2019). Determination of total anthraquinone in saqgentodoxae Caulis and its antioxidant activity in vitro. Acta Med. Sin. 32 (04), 27–31. doi:10.19296/j.cnki.1008-2409.2019-04-008
Wei, Y. L., Liang, F., and Tang, H. M. (2011). Determination of total anthraquinones content in Rubia cordifolia. Guizhou Agric. Sci. 39 (08), 51–53.
Wilberts, B. L., Schwartz, K. J., Gauger, P. C., Wang, C., and Burrough, E. R. (2015). Evidence of oxidative injury in pigs with melanosis coli. Vet. Pathol. 52 (4), 663–667. doi:10.1177/0300985814559403
Xu, L., Chan, C. O., Lau, C. C., Yu, Z., Mok, D. K., and Chen, S. (2012). Simultaneous determination of eight anthraquinones in Semen Cassiae by HPLC-DAD. Phytochem. Anal. 23 (2), 110–116. doi:10.1002/pca.1331
Yadav, J. P., Arya, V., Yadav, S., Panghal, M., Kumar, S., and Dhankhar, S. (2010). Cassia occidentalis L.: A review on its ethnobotany, phytochemical and pharmacological profile. Fitoterapia 81 (4), 223–230. doi:10.1016/j.fitote.2009.09.008
Yang, N., Ruan, M., and Jin, S. (2020a). Melanosis coli: A comprehensive review. Gastroenterol. Hepatol. 43 (5), 266–272. doi:10.1016/j.gastrohep.2020.01.002
Yang, N., Ruan, M., and Jin, S. (2020b). Melanosis coli: A comprehensive review. Gastroenterol. Hepatol. 43 (5), 266–272. doi:10.1016/j.gastrohep.2020.01.002
Younes, M., Aggett, P., Aguilar, F., Crebelli, R., Filipič, M., Frutos, M. J., et al. (2018). Safety of hydroxyanthracene derivatives for use in food. Efsa J. 16 (1), e05090. doi:10.2903/j.efsa.2018.5090
Zhang, C. H., Yang, X., Wei, J. R., Chen, N. M., Xu, J. P., Bi, Y. Q., et al. (2021a). Ethnopharmacology, phytochemistry, pharmacology, toxicology and clinical applications of Radix Astragali. Chin. J. Integr. Med. 27 (3), 229–240. doi:10.1007/s11655-019-3032-8
Zhang, W., Sun, C., Zhou, S., Zhao, W., Wang, L., Sheng, L., et al. (2021b). Recent advances in chemistry and bioactivity of Sargentodoxa cuneata. J. Ethnopharmacol. 270, 113840. doi:10.1016/j.jep.2021.113840
Zhang, Y. (2013). The study of effects of anthraquinone herbs on melanosis coli. Master. Fourth Mil. Med. Univ.
Zhao, M., Duan, J. A., Huang, W. Z., Zhou, R. H., and Che, Z. T. (2003). Steroids and anthraquinones from Astragalus hoantchy. J. China Pharm. Univ. (03), 22–25.
Keywords: melanosis coli, anthraquinones, traditional Chinese herbs, constipation, laxative
Citation: Zhang R, Huang C, Wu F, Fang K, Jiang S, Zhao Y, Chen G and Dong R (2023) Review on melanosis coli and anthraquinone-containing traditional Chinese herbs that cause melanosis coli. Front. Pharmacol. 14:1160480. doi: 10.3389/fphar.2023.1160480
Received: 07 February 2023; Accepted: 18 April 2023;
Published: 04 May 2023.
Edited by:
Muthu Thiruvengadam, Konkuk University, Republic of KoreaReviewed by:
Giada Crescioli, University of Florence, ItalyNiccolò Lombardi, University of Florence, Italy
Copyright © 2023 Zhang, Huang, Wu, Fang, Jiang, Zhao, Chen and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruolan Dong, eWFucm9uZzA3MjdAdG9tLmNvbQ==
 Ruiyuan Zhang
Ruiyuan Zhang Cai Huang
Cai Huang Fan Wu
Fan Wu Ke Fang1
Ke Fang1 Shujun Jiang
Shujun Jiang Guang Chen
Guang Chen Ruolan Dong
Ruolan Dong