- Department of Foot and Ankle Surgery, Honghui Hospital of Xi’an Jiaotong University, Xi’an, China
Charcot neuroarthropathy (CN) is a chronic, destructive, and painless damage of the skeletal system that affects the life quality of patients. CN, with an unclear mechanism, is characterized with invasive destruction of bones and a serious abnormality of bone metabolism. Unfortunately, development of an effective prevention and treatment strategy for CN is still a great challenge. Of note, recent studies providing an insight into the molecular mechanisms of bone metabolism and homeostasis have propelled development of novel CN therapeutic strategies. Therefore, this review aims to shed light on the pathogenesis, diagnosis, and treatment of CN. In particular, we highlight the eminent role of the osteoprotegerin (OPG)-receptor activator of nuclear factor-κB (RANK)-RANK ligand (RANKL) system in the development of CN. Furthermore, we summarize and discuss the diagnostic biomarkers of CN as well as the potential pharmacological mechanisms of current treatment regimens from the perspective of bone metabolism. We believe that this review will enhance the current state of knowledge on the diagnosis, prevention, and therapeutic efficacy of CN.
1 Introduction
The incidence of diabetes has steadily surged among the aging population all over the world (Ahmad et al., 2022). Chronic and uncontrolled blood glucose disorder can result in a series of harmful effects on the body. One of the most serious complications of diabetes is neuropathy, which is reported to affect up to 60% patients diagnosed with diabetes (Zakin et al., 2019). A considerable percentage of patients with symmetrical distal neuropathy is affected with a long-term and destructive disease called Charcot neuroarthropathy (CN) (Dardari, 2020). CN is a chronic, destructive, and painless damage of the skeletal system that affects the life quality of patients. While the mechanism underlying its pathogenesis is yet unclear, CN is characterized with invasive destruction of bones and a serious abnormality of bone metabolism (Pitocco et al., 2019). Unfortunately, effective prevention and treatment for CN is still a great challenge.
CN was first described in a Spanish article by Temesio et al. (1963), while its pathogenesis was first explained by (Bruckner and Kendall, 1969). Over the past couple of decades, several research studies have explored the potential molecular mechanism of CN (Dodd and Daniels, 2018; Botek et al., 2019). To our knowledge, the definitive mechanisms of CN are elusive, and there are several representative theories related to CN pathogenesis (Shapiro et al., 1998). Given the crucial role of the osteoprotegerin (OPG)-receptor activator of nuclear factor-κB (RANK)-RANK ligand (RANKL) system in the development of CN (Bruhn-Olszewska et al., 2017), bone metabolism was considered to be actively involved in the pathological alteration of CN. Since then, numerous studies have been conducted to understand CN and its pathogenesis, as exemplified in Figure 1.
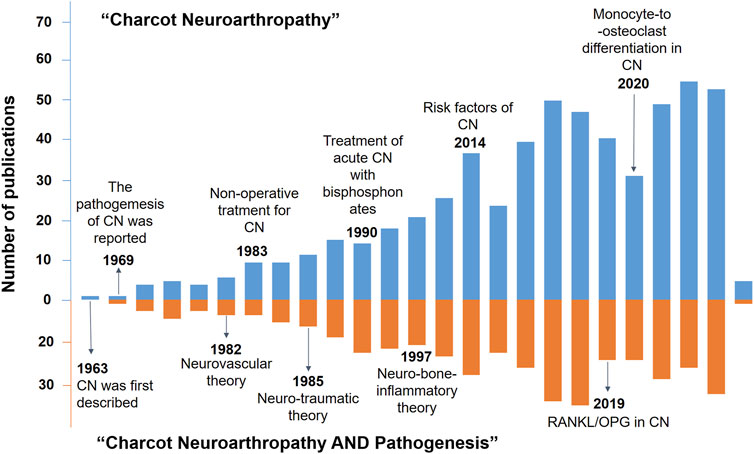
FIGURE 1. Timeline of the key development events in the knowledge (blue histogram) and pathogenesis of CN (orange histogram).
The studies published so far have improved our understanding of CN pathogenesis as well as the progress in its diagnosis and treatment. In fact, recent advances in the knowledge of the molecular mechanisms of bone metabolism and homeostasis have provided a promising direction for scheduling new CN therapeutic strategies. However, the underlying mechanisms and the actual efficacy of current therapeutic strategies for CN are yet to be elucidated. During the novelty retrieval via Web of Science search engine, we set “Charcot Neuroarthropathy” and “Charcot Neuroarthropathy AND Pathogenesis AND Bone metabolism” as search topics, which led to a dramatic decrease in the number of publications from 650 to 28 (Table 1). Thus, the correlation between CN pathogenesis and bone metabolism has not been extensively investigated.
Recently, several comprehensive reviews have summarized and discussed the excellent stride of bone metabolism in CN development (Bruhn-Olszewska et al., 2012; Yates et al., 2020). However, it remains a lack of comprehensive summarization of BN’s pathogenesis specially with a bone metabolism perspective. Considering this issue and the potential involvement of bone metabolism in the development of CN, we believe that it is necessary and timely to present a review on this topic to shed light on the pathogenesis, diagnosis, and treatment of CN. Herein, we highlight the eminent role of the OPG-RANKL-RANK system in the development of CN. Furthermore, we summarize and discuss the diagnostic biomarkers of CN as well as the potential pharmacological mechanism action of current treatment regimens from the prospective of bone metabolism. We believe that this review will improve the current state of knowledge on the diagnosis, prevention, and treatment of CN.
2 Pathophysiology of CN
While numerous studies in the past decades have focused on the pathogenesis of CN, the definitive molecular mechanism remains unknown. Increasing evidence indicates multifactorial events that contribute to this pathogenic condition, among which diabetic neuropathy and angiopathy, mechanical stress and injuries, excessive and persistent inflammation, and disturbance in bone metabolism are thought to play predominant roles (Trieb, 2016). In particular, the impairment of proprioception caused by peripheral neuropathy is believed to aggravate joint instability and, therefore, markedly increase the risk of injury from the small mechanical stress (Botek et al., 2019). The consequent transformation in the physical biomechanics of the foot is known to induce abnormal plantar pressure and add to subsequent localized injuries (Botek et al., 2019). Under physiological conditions, there is a relative balance in bone metabolism maintained by appropriate regulation between osteoclasts that drive bone resorption and osteoblasts responsible for bone formation. However, overexpression and secretion of inflammatory cytokines, including interleukin (IL)-1β and IL-6, under CN pathogenic condition induce the over-activation of the RANKL-RANK system and consequently impair the balance of bone metabolism. This phenomenon can lead to excessive bone resorption and joint destruction (Lee et al., 2003). Osteoclast activation is initiated by an increase in RANKL level in the OPG- RANK-RANKL system. Under diabetic condition, hyperglycemia enhances the production and accumulation of advanced glycation end products (AGEs), which induce bone resorption by promoting osteoblast apoptosis and osteoclast differentiation (Pitocco et al.,2019). In addition, micro-trauma and fracture perpetuate inflammation via increased expression of cytokines and further aggravate bone resorption (Figure 2).
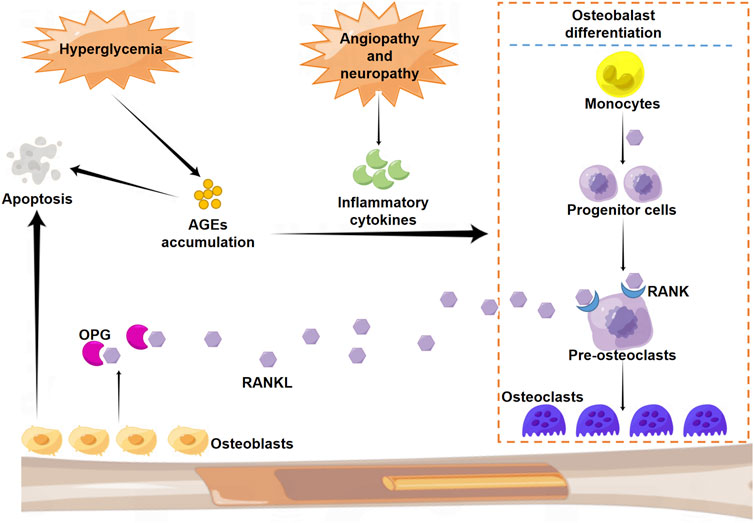
FIGURE 2. Schematic illustration of the molecular mechanism of osteoclast differentiation. RANK: nuclear factor-κB; RANKL: RANK ligand.
3 Regulatory factors in the development of CN
3.1 OPG-RANKL-RANK system associated with bone metabolism
The dysregulation in the balance between inflammatory and anti-inflammatory activities is highly associated with the development of CN. As a classical signaling pathway involved in the regulation of bone metabolism, the OPG-RANK-RANKL system can be significantly upregulated by the excessive release of inflammatory cytokines (Hou et al., 2012). In the bone matrix, RANKL is associated with differentiation and maturation of osteoclast precursor cells (Yang et al., 2020). In general, mature osteoclasts and several stem cells express RANK receptors that bind to RANKL and consequently exert multiple biological functions (Tyrovola, 2017). The activation of the signal cascade following binding between RANK and RANKL leads to the recruitment of tumor necrosis factor receptor-associated factors (TRAFs) to the cytoplasmic domain of RANK and the consequent maturation of preosteoclasts (Ma et al., 2012). Of note, OPG serves as a soluble decoy receptor for RANKL and ameliorates bone absorption by blocking the binding of RANKL to RANK (Wang et al., 2022). Although the crosstalk between RANKL and OPG drives bone metabolism, other factors such as AGEs, reactive oxygen species (ROS), and inflammatory cytokines are also involved in the regulation of this process and contribute to the progression of CN. For instance, a prior study reported that inflammation can be aggravated by the accumulation of AGEs, and the persistent if a crucial stimulation for the development of CN (Hockenbury et al., 2007).
3.2 The role of the OPG-RANKL-RANK system in CN development
Any dysfunction in the OPG/RANKL/RANK system can result in unexpected bone absorption and a series of events, including CN (Ndip et al., 2011). In a pioneering study, Alexander group reported that the activation of the OPG/RANKL/RANK system can aggravate the development of CN by mediating arterial calcification. This study provided a promising direction for anti-CN therapies through targeting of the OPG/RANKL/RANK system (Ndip et al., 2011). Similarly, Shanahan et al. reported inflammatory cytokine accumulation and RANKL gene expression in the area of bone destruction among CN patients, and highlighted the close correlation between the OPG/RANKL/RANK system and CN pathogenesis (Petrova and Shanahan, 2014).
Several studies have demonstrated the critical involvement of OPG and RANKL variants in bone destruction observed among CN patients (Roshandel et al., 2010; Wang et al., 2012). For instance, OPG gene single nucleotide polymorphism (SNP) was found to be highly involved in the development of diabetic CN. In particular, there was a positive correlation with G alleles for both the OPG 1181G>C and 245T>G variants in CN patients as compared to patients with diabetic neuropathy and healthy controls (Pitocco et al., 2009). Clinical data revealed much higher RANKL and OPG protein levels in the serum of CN patients than in healthy controls, consistent with a high RANKL/OPG ratio among CN patients (Jansen et al., 2018). Evidence suggests that elevated serum levels of RANKL in CN patients can enhance osteogenic differentiation and mineralization of vascular smooth muscle cells (Ndip et al., 2011), which can eventually aggravate the ischemic necrotic lesion of the limb. Together, all these findings reveal the dominant role of the OPG-RANKL-RANK system in the development of CN pathogenesis.
3.3 Neuro-bone-inflammatory axis in CN
While the molecular mechanism underlying CN pathogenesis remains elusive, studies have speculated a critical role of the neuro-bone-inflammatory axis in the pathogenic development of CN (O'Connor et al., 1985). CN patients were found to have lower bone density in the affected limbs than other neuropathic participants (Young et al., 1995). In particular, previous studies on bone metabolism revealed enhanced osteoclastic activity, instead of osteoblastic activity, in acute and chronic CN patients (Jeffcoate, 2005). Furthermore, inflammatory cytokines such as IL-1β and IL-6 are found to be highly involved in the pathogenesis of CN through induction of nuclear factor kappa B (NF-κB) and RANKL activities (Connors et al., 2018). Although some studies reported that there was no significant difference in the ratio of RANKL/OPG between CN patients and participants without CN (Connors et al., 2018), in vitro and in vivo results have proposed the importance of this system in the development of CN (Geusens et al., 2006). Together, these studies suggest a “neuro-bone-inflammatory theory” in CN that might explain the correlation between the intercellular communication of neural cells and bone cells as well as the crucial role of inflammatory signaling in CN regulation.
4 Pharmacological therapies of CN
4.1 Therapeutic agents targeting bone metabolism
The typical radiographic characteristics of Charcot joint involve excessive bone resorption and joint destruction, which indicate the imbalance in bone metabolism. A wide array of studies have shown abnormal upregulation in the expression of multiple bone resorptive markers among CN patients (Yates, et al., 2020). It is well-documented that therapeutic strategies targeting inhibition of excessive bone absorption can be promising for CN therapy (Schulze et al., 2022). The most widely used treatment is the application of bisphosphonates (BPs), which were first synthesized in 1865 and have been used in medicine since 1968. BPs exhibit a robust anti-resorptive activity and alleviate bone turnover (Jude et al., 2001).
Mechanistically, BPs have high affinity for bone minerals and can be deposited in the mineralized bone matrix, wherein they exert pharmacological functions before bone resorption (Dissanayake et al., 2012). Their high affinity for bone minerals and selective uptake by osteoclasts under the resorptive condition ensures specific toxicity only to osteoclasts. Non-nitrogen-containing BPs are first-generation agents that function as cytotoxic adenosine triphosphate analogues in osteoclasts. The deposition of these toxic adenosine triphosphate analogues results in the apoptosis of osteoclasts (Rastogi et al., 2021). Second- and third-generation BPs such as alendronate, ibandronate, and zoledronate have a nitrogen side chain bound to the central carbon that enhances their potency. Mechanistically, upon internalization, nitrogen-containing BPs suppress the activity of farnesyl pyrophosphate (FPP) synthase, which is responsible for production of cholesterol and isoprenoid lipids (Kavanagh et al., 2006). This phenomenon leads to suppression of isoprenylation of guanosine triphosphate-binding proteins such as Ras, Rho, and Rac, which are closely associated with cell proliferation, maturation, and differentiation (Li et al., 2021). The suppression of Ras signaling pathway in osteoclasts leads to defective intracellular vesicle transportation and failure of ruffled border formation, which results in amelioration of bone resorption (Cremers et al., 2019). In addition, FPP synthase inhibition can also prevent bone resorption through induction of osteoclast apoptosis (Naylor et al., 2016).
4.2 Anti-inflammatory therapies
The pathogenic characteristics such as excessive and persistent inflammation play a significant role in the development of CN. Emerging findings have uncovered the roles of several signaling pathways, wherein excessive inflammation is known to contribute to osteoclast over-activation in CN. This observation can provide clues for development of promising therapeutic options for CN (Sinacore et al., 2017).
A variety of cytokines and hormones are involved in regulation of OPG/RANK, and can thereby mediate osteoclast maturation and differentiation (Zaidi et al., 2003). Stimulants of osteoclastogenesis, including IL-1, IL-6, tumor necrosis factor (TNF)-alpha, parathyroid hormone, and calcitonin, play an eminent role in osteoclast function and bone resorption. For example, a clinical randomized control study suggested that teriparatide (recombinant human parathyroid hormone) can enhance bone remodeling in CN patients by mediating an osteoanabolic effect and can increase the mineral density of foot bones (Rastogi et al., 2019). Similarly, indicated that denosumab could provide a beneficial effect on prevention of bone and joint destruction, together with a metabolic effect in CN treatment (Carves et al., 2021). Evidence suggests a link between the inflammatory responses and bone metabolism abnormalities in CN (Pitocco et al., 2008). In particular, excessive inflammation can be initiated by fracture or other micro-trauma issues, which lead to production and release of several proinflammatory cytokines that can stimulate RANKL overexpression and osteoclast maturation. Abnormally upregulated TNF-α, IL-1, and IL-6 levels have been demonstrated as critical prognostic markers for CN (Baumhauer et al., 2006).
Therefore, it was assumed that TNF-α inhibitors and high-dose corticosteroids (that suppress NF-κB expression) could serve as beneficial agents for the treatment of CN. However, clinical data and verification of efficacy of these agents are highly desired. In addition, certain anti-inflammatory therapies, including inhibitors of RANKL, NF-κB, and IL-1β, have already been applied in animal experiments to ameliorate inflammation in arthritis (Molines et al., 2010).
4.3 Nutritional and pharmacological agents
The interaction between diabetes, neuropathy, and excessive inflammatory response is one of the main causes for CN development that can lead to bone absorption and joint deformity (Rader and Ruter, 2022). Oxidative stress (OS) can initiate activation of inflammation and eventually lead to bone destruction (Rizzo et al., 2017). AGEs are over produced in response to OS and can mediate apoptosis of osteoblasts (Rizzo et al., 2017). The receptor binding to AGEs (RAGE) is known to be closely associated with the pathogenesis of CN (Korzon-Burakowska et al., 2012). RAGE was previously shown to increase the activity of RANKL and contribute to osteoclastogenesis of the bone (Yaturu, 2009). Furthermore, RAGE expression was found to be upregulated in CN and was associated with the development of atherosclerotic lesions and vascular calcification through an increase in the expression of bone morphogenetic protein 4 in arteries (Witzke et al., 2011).
Diabetes is associated with an increase rate of lipoperoxidation, while vascular calcification (VC) is linked with upregulation in oxidized low-density lipoprotein expression (Thomsen et al., 2010). In a prior study, antioxidants such as 4-hydroxy-tempol, alpha-lipoic acid, and apocynin were shown to prevent calcification in the femoral artery, but only apocynin significantly alleviated femoral artery calcification (Brodeur et al., 2014). Furthermore, alpha-lipoic acid was found to markedly decrease aortic calcification in diabetic mice by inhibiting apoptosis of endothelial cells and restoring the mitochondrial function (Kim et al., 2012). As a commonly used nutritional agent, vitamin D has been demonstrated to protect pancreatic beta cells from OS through activation of endogenous antioxidant pathways (Wei et al., 2018). Taken together, nutritional and pharmacological agents can be used as potential therapeutic options for CN treatment.
5 Challenges and perspectives
Considering the tremendous surge in its incidence and prevalence, diabetes has become the most common cause of CN affecting the foot and ankle. From the perspective of bone metabolism, the main goal of pharmacological intervention involves suppression of excessive inflammation and bone resorption. Therapeutic strategies targeting inhibition of osteoclast function and pro-inflammatory signaling can provide a promising direction for CN treatment. Anti-resorptive treatments, especially with BPs, have been used in animal models and randomized clinical trials. Although there is no evident verification of an ideal dosage regime and true long-term validity, clinical trial results suggest improved symptom control, a more rapid decrease in the foot temperature, and a marked decline in bone resorption marker levels without any serious side-effects. While evidence suggests the direct involvement of monocytes in the pathogenesis of CN, further research focused on the monocyte-to-osteoclast differentiation process may give us a better insight into early prevention strategies for this pathogenic condition. Early diagnosis is still the best strategies for the management of patients with CN. In patients with diabetes and lower extremity neuropathy, any delicate injury deserves necessary observation due to the tendency of the limb to proceed to a Charcot’s process (Galhoum et al., 2021). In summary, the accumulation of knowledge about the molecular pathways underlying the pathogenesis of bone metabolism in CN is of great importance to facilitate advances in pharmacological treatments.
Author contributions
LL and QW wrote the manuscript; YZ, JL, and PL reviewed some papers and participated in the preparation of the manuscript; HZ provided the idea and supervised this study.
Funding
This work was supported by the Free exploration project of Xi’an Jiaotong University (No. xzy012022130); Scientific research project of Xi’an Health Commission (2021ms07).
Acknowledgments
The authors would like to thank MJEditor (www.mjeditor.com) for its linguistic assistance during the preparation of this manuscript, and the Figdraw (https://www.figdraw.com) for the assistance in preparation of the figures.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmad, E., Lim, S., Lamptey, R., Webb, D. R., and Davies, M. J., 2022. Type 2 diabetes. Lancet. Nov. 19;400:1803-1820. doi:10.1016/s0140-6736(22)01655-5
Baumhauer, J. F., O'Keefe, R. J., Schon, L. C., and Pinzur, M. S. (2006). Cytokine-induced osteoclastic bone resorption in charcot arthropathy: An immunohistochemical study. Foot Ankle Int. Oct. 27, 797–800. Epub 2006/10/24. doi:10.1177/107110070602701007
Botek, G., Figas, S., and Narra, S., 2019. Charcot neuroarthropathy advances: Understanding pathogenesis and medical and surgical management. Clin. Podiatr. Med. Surg., ;36:663–684. doi:10.1016/j.cpm.2019.07.002
Brodeur, M. R., Bouvet, C., Bouchard, S., Moreau, S., Leblond, J., Deblois, D., et al. (2014). Reduction of advanced-glycation end products levels and inhibition of RAGE signaling decreases rat vascular calcification induced by diabetes. PLoS One 9, e85922. Epub 2014/01/28. doi:10.1371/journal.pone.0085922
Bruckner, F. E., and Kendall, B. E. (1969). Neuroarthropathy in charcot-marie-tooth disease. Ann. Rheum. Dis. Nov. 28, 577–583. Epub 1969/11/01. doi:10.1136/ard.28.6.577
Bruhn-Olszewska, B., Korzon-Burakowska, A., Gabig-Ciminska, M., Olszewski, P., Wegrzyn, A., and Jakobkiewicz-Banecka, J. (2012). Molecular factors involved in the development of diabetic foot syndrome. Acta Biochim. Pol. 59, 507–513. Epub 2012/12/20. doi:10.18388/abp.2012_2085
Bruhn-Olszewska, B., Korzon-Burakowska, A., Wegrzyn, G., and Jakobkiewicz-Banecka, J. (2017). Prevalence of polymorphisms in OPG, RANKL and RANK as potential markers for Charcot arthropathy development. Sci. Rep. Mar. 29 (7), 501. Epub 2017/03/31. doi:10.1038/s41598-017-00563-4
Carves, S., Bourgeon-Ghittori, M., Henry, J., Belkhir, R., Besson, F. L., Levante, S., et al. (2021). Denosumab in active Charcot neuro-osteoarthropathy of the foot. Jt. Bone Spine. Dec 88, 105241. Epub 2021/06/20. doi:10.1016/j.jbspin.2021.105241
Connors, J. C., Hardy, M. A., Kishman, L. L., Botek, G. G., Verdin, C. J., Rao, N. M., et al. (2018). Charcot pathogenesis: A study of in vivo gene expression. J. Foot Ankle Surg. Nov-Dec 57, 1067–1072. Epub 2018/10/29. doi:10.1053/j.jfas.2018.03.023
Cremers, S., Drake, M. T., Ebetino, F. H., Bilezikian, J. P., and Russell, R. G. G. (2019). Pharmacology of bisphosphonates. Br. J. Clin. Pharmacol. Jun 85, 1052–1062. Epub 2019/01/17. doi:10.1111/bcp.13867
Dardari, D. (2020). An overview of Charcot's neuroarthropathy. J. Clin. Transl. Endocrinol. Dec 22, 100239. Epub 2020/12/01. doi:10.1016/j.jcte.2020.100239
Dissanayake, S. U., Bowling, F. L., and Jude, E. B. (2012). The diabetic Charcot foot. Curr. Diabetes Rev. May 8, 191–194. Epub 2012/03/21. doi:10.2174/157339912800563981
Dodd, A., and Daniels, T. R., 2018. Charcot neuroarthropathy of the foot and ankle. J. Bone Jt. Surg. Am. Apr 18;100:696-711. doi:10.2106/jbjs.17.00785
Galhoum, A. E., Trivedi, V., Askar, M., Tejero, S., Herrera-Perez, M., AlRashidi, Y., et al. (2021). Management of ankle charcot neuroarthropathy: A systematic review. J. Clin. Med., 10. Epub 2021/12/25. doi:10.3390/jcm10245923
Geusens, P. P., Landewe, R. B., Garnero, P., Chen, D., Dunstan, C. R., Lems, W. F., et al. (2006). The ratio of circulating osteoprotegerin to RANKL in early rheumatoid arthritis predicts later joint destruction. Arthritis Rheum. Jun 54, 1772–1777. Epub 2006/06/01. doi:10.1002/art.21896
Hockenbury, R. T., Gruttadauria, M., and McKinney, I. (2007). Use of implantable bone growth stimulation in Charcot ankle arthrodesis. Foot Ankle Int. Sep. 28, 971–976. Epub 2007/09/21. doi:10.3113/FAI.2007.0971
Hou, J. M., Xue, Y., and Lin, Q. M. (2012). Bovine lactoferrin improves bone mass and microstructure in ovariectomized rats via OPG/RANKL/RANK pathway. Acta Pharmacol. Sin. Oct. 33, 1277–1284. Epub 2012/08/21. doi:10.1038/aps.2012.83
Jansen, R. B., Christensen, T. M., Bulow, J., Rordam, L., Holstein, P. E., Jorgensen, N. R., et al. (2018). Bone mineral density and markers of bone turnover and inflammation in diabetes patients with or without a Charcot foot: An 8.5-year prospective case-control study. J. Diabetes Complicat. Feb 32, 164–170. Epub 2017/12/03. doi:10.1016/j.jdiacomp.2017.11.004
Jeffcoate, W. J. (2005). Theories concerning the pathogenesis of the acute charcot foot suggest future therapy. Curr. Diab Rep. Dec 5, 430–435. Epub 2005/12/01. doi:10.1007/s11892-005-0050-z
Jude, E. B., Selby, P. L., Burgess, J., Lilleystone, P., Mawer, E. B., Page, S. R., et al. (2001). Bisphosphonates in the treatment of charcot neuroarthropathy: A double-blind randomised controlled trial. Diabetol. Nov. 44, 2032–2037. Epub 2001/11/24. doi:10.1007/s001250100008
Kavanagh, K. L., Guo, K., Dunford, J. E., Wu, X., Knapp, S., Ebetino, F. H., et al. 2006. The molecular mechanism of nitrogen-containing bisphosphonates as antiosteoporosis drugs. Proc. Natl. Acad. Sci. U. S. A.;103:7829–7834. doi:10.1073/pnas.0601643103
Kim, H., Kim, H. J., Lee, K., Kim, J. M., Kim, H. S., Kim, J. R., et al. (2012). α-Lipoic acid attenuates vascular calcification via reversal of mitochondrial function and restoration of Gas6/Axl/Akt survival pathway. J. Cell Mol. Med. Feb 16, 273–286. Epub 2011/03/03. doi:10.1111/j.1582-4934.2011.01294.x
Korzon-Burakowska, A., Jakobkiewicz-Banecka, J., Fiedosiuk, A., Petrova, N., Koblik, T., Gabig-Ciminska, M., et al. (2012). Osteoprotegerin gene polymorphism in diabetic Charcot neuroarthropathy. Diabet. Med. Jun 29, 771–775. Epub 2011/09/15. doi:10.1111/j.1464-5491.2011.03442.x
Lee, L., Blume, P. A., and Sumpio, B. (2003). Charcot joint disease in diabetes mellitus. Ann. Vasc. Surg. Sep. 17, 571–580. Epub 2003/09/26. doi:10.1007/s10016-003-0039-5
Li, J., Yang, G., Luo, X. Q., Mo, L. H., Qiu, S. Y., Yang, L. T., et al. (2021). Interaction between Ras and Bcl2L12 in B cells suppresses IL-10 expression. Clin. Immunol. Aug 229, 108775. Epub 2021/06/12. doi:10.1016/j.clim.2021.108775
Ma, B., Zhang, Q., Wu, D., Wang, Y. L., Hu, Y. Y., Cheng, Y. P., et al. (2012). Strontium fructose 1,6-diphosphate prevents bone loss in a rat model of postmenopausal osteoporosis via the OPG/RANKL/RANK pathway. Acta Pharmacol. Sin. Apr 33, 479–489. Epub 2012/03/20. doi:10.1038/aps.2011.177
Molines, L., Darmon, P., and Raccah, D. (2010). Charcot's foot: Newest findings on its pathophysiology, diagnosis and treatment. Diabetes Metab. Sep. 36, 251–255. Epub 2010/06/24. doi:10.1016/j.diabet.2010.04.002
Naylor, K. E., Jacques, R. M., Paggiosi, M., Gossiel, F., Peel, N. F., McCloskey, E. V., et al. (2016). Response of bone turnover markers to three oral bisphosphonate therapies in postmenopausal osteoporosis: The TRIO study. Osteoporos. Int. Jan. 27, 21–31. Epub 2015/05/21. doi:10.1007/s00198-015-3145-7
Ndip, A., Williams, A., Jude, E. B., Serracino-Inglott, F., Richardson, S., Smyth, J. V., et al. (2011). The RANKL/RANK/OPG signaling pathway mediates medial arterial calcification in diabetic Charcot neuroarthropathy. Diabetes. Aug 60, 2187–2196. Epub 2011/06/11. doi:10.2337/db10-1220
O'Connor, B. L., Palmoski, M. J., and Brandt, K. D. (1985). Neurogenic acceleration of degenerative joint lesions. J. Bone Jt. Surg. Am. Apr 67, 562–572. Epub 1985/04/01. doi:10.2106/00004623-198567040-00010
Petrova, N. L., and Shanahan, C. M. (2014). Neuropathy and the vascular-bone axis in diabetes: Lessons from charcot osteoarthropathy. Osteoporos. Int. Apr 25, 1197–1207. Epub 2013/10/05. doi:10.1007/s00198-013-2511-6
Pitocco, D., Collina, M. C., Musella, T., Ruotolo, V., Caputo, S., Manto, A., et al. (2008). Interaction between IGF-1, inflammation, and neuropathy in the pathogenesis of acute charcot neuroarthropathy: Lessons from alendronate therapy and future perspectives of medical therapy. Horm. Metab. Res. Mar. 40, 163–164. Epub 2008/02/08. doi:10.1055/s-2008-1042425
Pitocco, D., Scavone, G., Di Leo, M., Vitiello, R., Rizzi, A., Tartaglione, L., et al. (2019). Charcot neuroarthropathy: From the laboratory to the bedside. Curr. Diabetes Rev. 16, 62–72. Epub 2019/05/07. doi:10.2174/1573399815666190502121945
Pitocco, D., Zelano, G., Gioffre, G., Di Stasio, E., Zaccardi, F., Martini, F., et al. 2009. Association between osteoprotegerin G1181C and T245G polymorphisms and diabetic charcot neuroarthropathy: A case-control study. Diabetes Care;32:1694–1697. doi:10.2337/dc09-0243
Rader, A. J., and Ruter, A. (2022). The association of olfactory impairment with charcot neuroarthropathy and possible links to causation. J. Am. Podiatr. Med. Assoc. Sep-Oct 112, 21-100. Epub 2022/10/18. doi:10.7547/21-100
Rastogi, A., Bhansali, A., and Jude, E. B., 2021. Efficacy of medical treatment for charcot neuroarthropathy: A systematic review and meta-analysis of randomized controlled trials. Acta Diabetol.;58:687–696. doi:10.1007/s00592-020-01664-9
Rastogi, A., Hajela, A., Prakash, M., Khandelwal, N., Kumar, R., Bhattacharya, A., et al. (2019). Teriparatide (recombinant human parathyroid hormone [1-34]) increases foot bone remodeling in diabetic chronic charcot neuroarthropathy: A randomized double-blind placebo-controlled study. J. Diabetes. Sep. 11, 703–710. Epub 2019/01/12. doi:10.1111/1753-0407.12902
Rizzo, P., Pitocco, D., Zaccardi, F., Di Stasio, E., Strollo, R., Rizzi, A., et al. (2017). Autoantibodies to post-translationally modified type I and II collagen in Charcot neuroarthropathy in subjects with type 2 diabetes mellitus. Diabetes Metab. Res. Rev. Feb 33, e2839. Epub 2016/07/28. doi:10.1002/dmrr.2839
Roshandel, D., Holliday, K. L., Pye, S. R., Boonen, S., Borghs, H., Vanderschueren, D., et al. (2010). Genetic variation in the RANKL/RANK/OPG signaling pathway is associated with bone turnover and bone mineral density in men. J. Bone Min. Res. Aug 25, 1830–1838. Epub 2010/03/06. doi:10.1002/jbmr.78
Schulze, S., Neuber, C., Moller, S., Hempel, U., Hofbauer, L. C., Schaser, K. D., et al. (2022). Impact of sulfated hyaluronan on bone metabolism in diabetic charcot neuroarthropathy and degenerative arthritis. Int. J. Mol. Sci. Dec 2, 23. Epub 2022/12/12.
Shapiro, S. A., Stansberry, K. B., Hill, M. A., Meyer, M. D., McNitt, P. M., Bhatt, B. A., et al. (1998). Normal blood flow response and vasomotion in the diabetic Charcot foot. J. Diabetes Complicat. 12, 147–153. Epub 1998/06/09. doi:10.1016/s1056-8727(97)00080-9
Sinacore, D. R., Bohnert, K. L., Smith, K. E., Hastings, M. K., Commean, P. K., Gutekunst, D. J., et al. (2017). Persistent inflammation with pedal osteolysis 1year after Charcot neuropathic osteoarthropathy. J. Diabetes Complicat. 31, 1014–1020. Epub 2017/03/04. doi:10.1016/j.jdiacomp.2017.02.005
Temesio, P., Crespodi, B, and Gomensoro, J. B. (1963). Diabetic (Charcot type) neuroarthropathy. Sem. Med. Feb 28 (122), 273–280. Epub 1963/02/28.
Thomsen, S. B., Rathcke, C. N., Zerahn, B., and Vestergaard, H., 2010. Increased levels of the calcification marker matrix Gla Protein and the inflammatory markers YKL-40 and CRP in patients with type 2 diabetes and ischemic heart disease. Cardiovasc Diabetol., 9, 9:doi:10.1186/1475-2840-9-86
Trieb, K. (2016). The charcot foot: Pathophysiology, diagnosis and classification. Bone Jt. J. Sep. 98-B, 1155–1159. Epub 2016/09/03. doi:10.1302/0301-620X.98B9.37038
Tyrovola, J. B. (2017). The "mechanostat" principle and the osteoprotegerin-OPG/RANKL/RANK system PART II. The role of the hypothalamic-pituitary Axis. J. Cell Biochem. May 118, 962–966. Epub 2016/11/20. doi:10.1002/jcb.25793
Wang, C., Zhang, Z., Zhang, H., He, J. W., Gu, J. M., Hu, W. W., et al. (2012). Susceptibility genes for osteoporotic fracture in postmenopausal Chinese women. J. Bone Min. Res. Dec 27, 2582–2591. Epub 2012/07/19. doi:10.1002/jbmr.1711
Wang, F., Yang, G., Li, Y., Tang, Z., Du, J., Song, H., et al. (2022). A peptide from wheat germ abolishes the senile osteoporosis by regulating OPG/RANKL/RANK/TRAF6 signaling pathway. Phytomedicine. Sep. 104, 154304. Epub 2022/07/07. doi:10.1016/j.phymed.2022.154304
Wei, Z., Yoshihara, E., He, N., Hah, N., Fan, W., Pinto, A. F. M., et al. 2018. Vitamin D switches BAF complexes to protect beta cells. Cell. ;173:1135–1149. doi:10.1016/j.cell.2018.04.013
Witzke, K. A., Vinik, A. I., Grant, L. M., Grant, W. P., Parson, H. K., Pittenger, G. L., et al. 2011. Loss of RAGE defense: A cause of charcot neuroarthropathy? Diabetes Care. ;34:1617–1621. doi:10.2337/dc10-2315
Yang, B., Li, S., Chen, Z., Feng, F., He, L., Liu, B., et al. (2020). Amyloid β peptide promotes bone formation by regulating Wnt/β-catenin signaling and the OPG/RANKL/RANK system. FASEB J. Mar. 34, 3583–3593. Epub 2020/01/17. doi:10.1096/fj.201901550R
Yates, T. H., Cooperman, S. R., Shofler, D., and Agrawal, D. K. (2020). Current concepts underlying the pathophysiology of acute Charcot neuroarthropathy in the diabetic foot and ankle. Expert Rev. Clin. Immunol. Aug 16, 839–845. Epub 2020/08/01. doi:10.1080/1744666X.2020.1804869
Yaturu, S. (2009). Diabetes and skeletal health. J. Diabetes. Dec 1, 246–254. Epub 2010/10/07. doi:10.1111/j.1753-0407.2009.00049.x
Young, M. J., Marshall, A., Adams, J. E., Selby, P. L., and Boulton, A. J. (1995). Osteopenia, neurological dysfunction, and the development of Charcot neuroarthropathy. Diabetes Care. Jan. 18, 34–38. Epub 1995/01/01. doi:10.2337/diacare.18.1.34
Zaidi, M., Blair, H. C., Moonga, B. S., Abe, E., and Huang, C. L. (2003). Osteoclastogenesis, bone resorption, and osteoclast-based therapeutics. J. Bone Min. Res. Apr 18, 599–609. Epub 2003/04/04. doi:10.1359/jbmr.2003.18.4.599
Keywords: bone metabolism, Charcot neuroarthropathy, pharmacology, RANKL, therapeutics
Citation: Liu L, Wang Q, Zhang Y, Liang J, Liu P and Zhao H (2023) Therapeutics of Charcot neuroarthropathy and pharmacological mechanisms: A bone metabolism perspective. Front. Pharmacol. 14:1160278. doi: 10.3389/fphar.2023.1160278
Received: 07 February 2023; Accepted: 28 March 2023;
Published: 12 April 2023.
Edited by:
Yong Xu, Tongji University, ChinaReviewed by:
Zhijie Xu, Central South University, ChinaZeming Liu, Huazhong University of Science and Technology, China
Copyright © 2023 Liu, Wang, Zhang, Liang, Liu and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongmou Zhao, emhhb2hvbmdtb3VAeGl5aS5lZHUuY24=
†These authors have contributed equally to this work
 Liang Liu
Liang Liu Qiong Wang
Qiong Wang Yan Zhang
Yan Zhang Jingqi Liang
Jingqi Liang Peilong Liu
Peilong Liu Hongmou Zhao
Hongmou Zhao