
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 11 May 2023
Sec. Ethnopharmacology
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1159906
 Yue Dong1
Yue Dong1 Lin Liu1
Lin Liu1 Xiaowen Zhang1,2,3
Xiaowen Zhang1,2,3 Yijia Gong1
Yijia Gong1 Shiyan Yan4
Shiyan Yan4 Wei Li5
Wei Li5 Shunping Li6
Shunping Li6 Hongguo Rong1,2,3*
Hongguo Rong1,2,3* Jianping Liu1,2,3
Jianping Liu1,2,3Objectives: Patient-reported outcomes (PROs) provide a global perspective of patient health status which plays an enormous role in evaluating clinical efficacy. However, the application of PROs in traditional Chinese medicine (TCM) was still insufficiently studied in mainland China.
Methods: This cross-sectional study was performed based on interventional clinical trials of TCM that were conducted in mainland China from 1 January 2010, to 15 July 2022. Data was retrieved from the ClinicalTrials.gov and Chinese Clinical Trial Registry. We included interventional clinical trials of TCM for which the country of the primary sponsors or recruitment settings in mainland China. For each included trial, data including clinical trial phases, study settings, participant’s age, sex, diseases, and the patient-reported outcome measures (PROMs) were extracted. Trials were categorized into four categories according to 1) listed PROs as primary endpoints, 2) listed PROs as secondary endpoints, 3) listed PROs as coprimary outcomes (both primary and secondary endpoints), and 4) did not mention any PROMs.
Results: Among a total of 3,797 trials, 680 (17.9%) trials listed PROs as primary endpoints, 692 (18.2%) trials listed PROs as secondary endpoints, and 760 (20.0%) trials listed PROs as coprimary endpoints. Among 675,787 participants included in the registered trials, 448,359 (66.3%) patients’ data were scientifically collected by PRO instruments. Neurological diseases (11.8%), musculoskeletal symptoms (11.5%), mental health conditions (9.1%) were the most common conditions evaluated by PROMs. Disease-specific symptoms related concepts were used most frequently (51.3%), followed by health-related quality of life concepts. Visual analog scale, 36-item Short-Form Health Questionnaire, and TCM symptom score were the most common PROMs in these trials.
Conclusion: In this cross-sectional study, the use of PROs increased in the past decades according to clinical trials of TCM conducted in mainland China. Considering that the application of PROs in clinical trials of TCM has some existing issues including uneven distribution and lack of normalized PROs of TCM, further study should be focused on the standardization and normalization of TCM-specific scales.
Patient-reported outcomes (PROs) are any report of the status of a patient’s health condition that directly comes from the patient, without interpretation of the patient’s response by a clinician or anyone else (Patrick et al., 2007). Patient-reported outcome measures (PROMs) were the standardized questionnaires that collect information on health outcomes directly from patients (Churruca et al., 2021). PROs and PROMs were widely used in evaluating health-related quality of life, physical capacity, mental and cognitive changes, functional status, symptoms, and overall wellbeing (Casey, 2022). Over the past decades, healthcare systems have increasingly perceived patients' opinions as the fundamental condition to ensure that a high-quality, equitable, and safe service was delivered (Marshall et al., 2006). PROs provide evidence for supporting clinical decision making, prioritizing the surgical procedures of the patients, comparing outcomes among healthcare providers, promoting quality of treatment, and evaluating practices and policies (Dawson et al., 2010; Black, 2013; Chen et al., 2013; Chan et al., 2016; Price et al., 2019).
Traditional Chinese medicine (TCM) originated in mainland China with a history of more than 2,000 years and has been widely used in clinical practice. TCM plays an important role in global major public health emergencies, especially during the COVID-19 pandemic (Dai et al., 2021). The development of TCM Basic Quality of Life (ChQOL) demonstrates an evidence-based approach that can evaluate the therapeutic effectiveness of TCM through PROs or health-related quality of life measurements. With PROs becoming increasingly important in medicine, an increasing number of clinical trials of TCM used PROs as primary or secondary outcomes (Kochar et al., 2018; Santosa et al., 2018; Kotecha et al., 2020; Jia et al., 2021). TCM practitioners intend to focus on the patient as a unified person instead of individual symptoms, as Western medicine does (Zhang and Chor, 2023). PROs share a similar concept with the TCM approach (Jiang et al., 2010), which often represents the effect of diseases on all aspects of health and functioning (U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health, 2006). Using PROs, which are accepted by Western doctors, to evaluate the TCM clinical effect could form the basis for further testing and applications of TCM in Western countries (Leung et al., 2005; Zhao and Chan, 2005). It is widely believed among TCM practitioners that the current quality of life instruments may not be sufficiently sensitive to detect the changes in body status/symptoms that are deemed crucial in TCM treatment (Jiang et al., 2010).The patients' input and subjective experience were important elements to assess TCM treatment outcomes, which was consistent with the core concept of PROs (Zhang et al., 2017). Over the past several years, PROs have come into widespread use in the world, including China. However, China still faces many challenges in the development and implementation of PROs involving the large population, socio-economic status, geographical location, healthcare labor supply and many other variables. A cross-sectional study of clinical trials conducted in China indicated that only 29.7% of the selected eligible trials including PROs precisely listed PROMs as outcomes (Zhou et al., 2022). Meanwhile, lack of comprehensive evaluations assessing the application of PROs in clinical trials of TCM in mainland China.
Over the past several years, the number of clinical trials of TCM conducted in mainland China increased consistently. It is necessary to investigate the application of PROs in clinical trials of TCM to understand how PROs were being used and provide suggestions for conducting high-quality clinical trials of TCM. Based on the registration information of randomized clinical trials of TCM conducted in China, this study was aimed at reviewing and evaluating the use of PROs and providing potential study directions for future clinical practice of TCM.
This cross-sectional study analyzed the data from clinical trials of TCM conducted in mainland China with PROMs to evaluate primary and/or secondary outcomes from 1 January 2010 to 15 July 2022. Data was collected from ClinicalTrials.gov and Chinese Clinical Trial Registry. Only intervention studies in the two databases were retrieved (search strategy in the Supplementary Method S1). In light of these data, this study focused on these PROMs which were continually utilized in the conditions. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline was followed in this study.
We included interventional randomized clinical trials of TCM where the country of the primary sponsors or recruitment settings in mainland China, and recruited participants who were older than 18 years (Figure 1). In cases where the age of participants was “unclear,” we determine whether the trial involves children based on a comprehensive evaluation of the trial’s overall characteristics, such as the trial introduction, target diseases, and other relevant details. We excluded trials with duplicate registration numbers (retain ClinicalTrials.gov). Information collected to assess the conditions and characteristics of trials included 1) basic information, such as registration number, date of registration, official title, country, and uniform resource locator (URL), 2) key information, such as outcomes (including PROs), target disease, participant age and gender, inventions, and 3) feature information, such as primary sponsor, primary sponsor’s address, countries of recruitment, study settings, and clinical trial phases.
Eligible trials were classified into four categories according to the outcomes reported: 1) trial registration listed PROs as primary endpoints, 2) listed PROs as secondary outcome. 3) listed PROs as coprimary outcome (both primary and secondary endpoints), and 4) did not mention any PROMs (trial registration did not mention the use of PROs).
The data from included trials were extracted independently by two authors (D.Y. and L.L.), using predesigned data extraction tables. Among them, the clinical trial phase, study setting, participant age and gender, region of the primary sponsor, center, and interventions of TCM were shown in Table 1. Owing to the varied categories and wide variation of target diseases, we classified similar target diseases as the same groups according to the International Classification of Diseases-11 (Supplementary Table S1 in the Supplement). Based on our categorization of conditions, our study summarized the PRO instruments used in each trial to calculate the most used measurements. We include only items that list the names of PRO tools in statistical analysis for quantitative analysis to understand which evaluation tools were used. Descriptive statistics were performed with Stata version 14.0 (StataCorp).
The general characteristics of the included trials were presented in Table 1. We identified 3,797 interventional studies conducted in mainland China, including 2,607 from the Chicrt.org.cn and 1,190 from ClinicalTrials.gov. Our study excluded 883 trials, including 14 duplicates, 532 clinical trials with participants younger than 18 years old, 331 trials not conducted in mainland China, and 6 trials with incomplete reporting - whose outcome measures/outcomes were not posted on ClinicalTrials.gov or the Chinese registries (Figure 1). Among the 3,797 included trials, 2,132 (56.1%) trials used PRO instruments as their primary and/or secondary endpoints, 782 (20.6%) trials did not mention the use of PROMs.
Of the all clinical trials, early-stage trials [846/2914 (29.0%)] were the most common, followed by phase-4 (12.5%). Of the 2132 trials including PROs, early-stage trials [615/2132 (28.8%)] were still the most common, same followed by phase 4 (11.6%) trials (Table 1). Nearly 90% [2606/2914 (89.4%)] of the trials were conducted in hospitals, and less than 1% (0.7%) were performed in the community. Most primary sponsors were seated in eastern mainland China, followed by northern, southern, and southwestern mainland China; the other regions, including central, northeastern and northwestern mainland China, accounted for 1%–5%. There were similar findings considering only trials including PROs, with nearly 90% [1919/2,132 (90.0%)] of primary sponsors coming from the eastern, northern, southern, and southwest areas of mainland China; less than 10% of primary sponsors were from the central, northeastern, and northwestern mainland China (Table 1). There were considerable differences in the proportion of primary sponsors of trials including PROs among the different provinces. The percentage of primary sponsors of trials including PROs in Chinese provinces is shown in Figure 2.
We counted the number of implementation centers of trials included. 78.1% (n = 2,276) trials were performed at single center, and 21.2% (n = 618) trials were conducted at multiple centers. For interventions of TCM used in clinical trials, 40% (1,288/72,914) trials used Chinese herbal medicines as interventions, followed by acupuncture (33.0%), and exercises (11.2%). There were similar findings when considering only the trials including PROs.
Figure 3 illustrates the increase in the number of TCM clinical registration trials from 2010 to 2022, and demonstrates the proportion of trials that list PRO as the outcome among the TCM trials. In the 2,132 trials that used PRO instruments, neurological (11.8%), musculoskeletal (11.5%), mental health conditions (9.1%), cardiovascular (8.5%), and gynecology diseases (8.0%) were the top five conditions which applied PRO measures as outcomes (Figure 4). Although the instruments used were varied by disease type, we found the most frequently used PROMs in these trials were visual analog scale (VAS), 36-item Short-Form Health Questionnaire (SF-36), and TCMSS (Supplementary Table S2 in the Supplement).
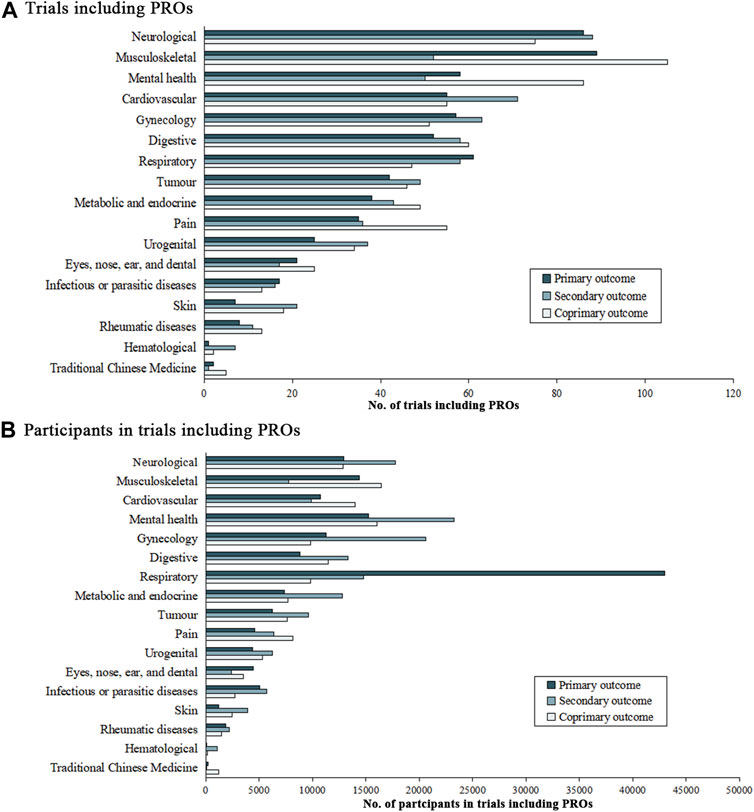
FIGURE 4. Number of trials and participants with patient-reported outcomes. No. of trials including PROs for (A), and No. of participants in trials including PROs for (B).
The number of conditions and participants in trials that included PROMs were shown in Figure 4. In the 1,440 trials that listed PROs as the primary endpoints, musculoskeletal diseases (13.5%), neurological diseases (11.2%), and mental health condition (10.0%) were considered as the most common conditions, followed by cardiovascular symptoms (7.6%), digestive (7.8%), respiratory (7.5%), and gynecology (7.5%) conditions. Pain, tumor, metabolic and endocrine, urogenital and eyes, nose, ear, and dental conditions accounted for 3%–7% of these trials. Infectious or parasitic disease conditions (2.1%), skin diseases (1.7%), rheumatic diseases (1.5%), TCM symptoms (0.5%), and hematological (0.2%) were the least common conditions considered.
Of the 448,359 individuals in trials that included PROs data, 15.1% (n = 67,605) were diagnosed with respiratory diseases, 12.2% (n = 54,499) were experiencing mental health condition and 9.7% (n = 43,471) had neurological conditions. Participants with gynecology [41,616 (9.3%)], musculoskeletal [38,576 (8.6%)], cardiovascular [34,637 (7.7%)], digestive [33,565 (7.5%)], metabolic and endocrine [27,790 (6.2%)] and tumor [23,392 (5.2%)] conditions were also well represented (>20,000 participants in all cases). More than 10,000 participants in these trials had pain [19,081 (4.3%)], urogenital [15,959 (3.6%)], infectious or parasitic diseases [13,474 (3.0%)] and eyes, nose, ears, and dental [10,294 (2.3%)] conditions. Less than 10,000 participants in this group had skin diseases [7,502 (1.7%)], rheumatic diseases [5575 (1.2%)], traditional Chinese medicine symptoms [1,522 (0.3%)], and hematological diseases [1,288 (0.3%)].
Consistent with previous systematic reviews in this setting (Gnanasakthy et al., 2022), trial outcomes were classified into four categories: symptoms, function, health-related quality of life (HRQOL), and others. Outcomes related to the kind of clinical manifestations (e.g., hot flash, pain, and bellyache) were classified as symptoms; the category function included concepts such as physical functioning, activity limitation, and emotional function were classified as function; changes in a patient’s quality of life, HRQOL, and perceived wellbeing were classified as HRQOL; outcomes related to patients’ satisfaction with treatment or feasibility were classified as other. Pre-specified concepts of PRO-related outcomes were italicized.
Table 2 shows the classification based on the scale content. Symptoms were reported in 1,947 trials, of which 596 were primary outcomes, 591 were secondary outcomes and 760 were coprimary outcomes. 582 reported trials focused predominantly on function. Function was used to support primary outcomes in 169 trials, secondary outcomes in 126 trials and coprimary outcomes in 287 trials. Of the 942 HRQOL trials, 425 (24.9%) were based on coprimary outcomes related to HRQOL.
To determine the accurate application of different PROMs in trials including PROs, we categorized similar target diseases into 15 conditions according to ICD-11 (Tables 3–5). The VAS was used in 17.4% of neurological condition trials and 31.5% of musculoskeletal condition trials to assess primary outcomes. Of 181 identified trials with cardiovascular disease trials and 191 gynecology condition trials, TCMSS was the most-used PROMs.
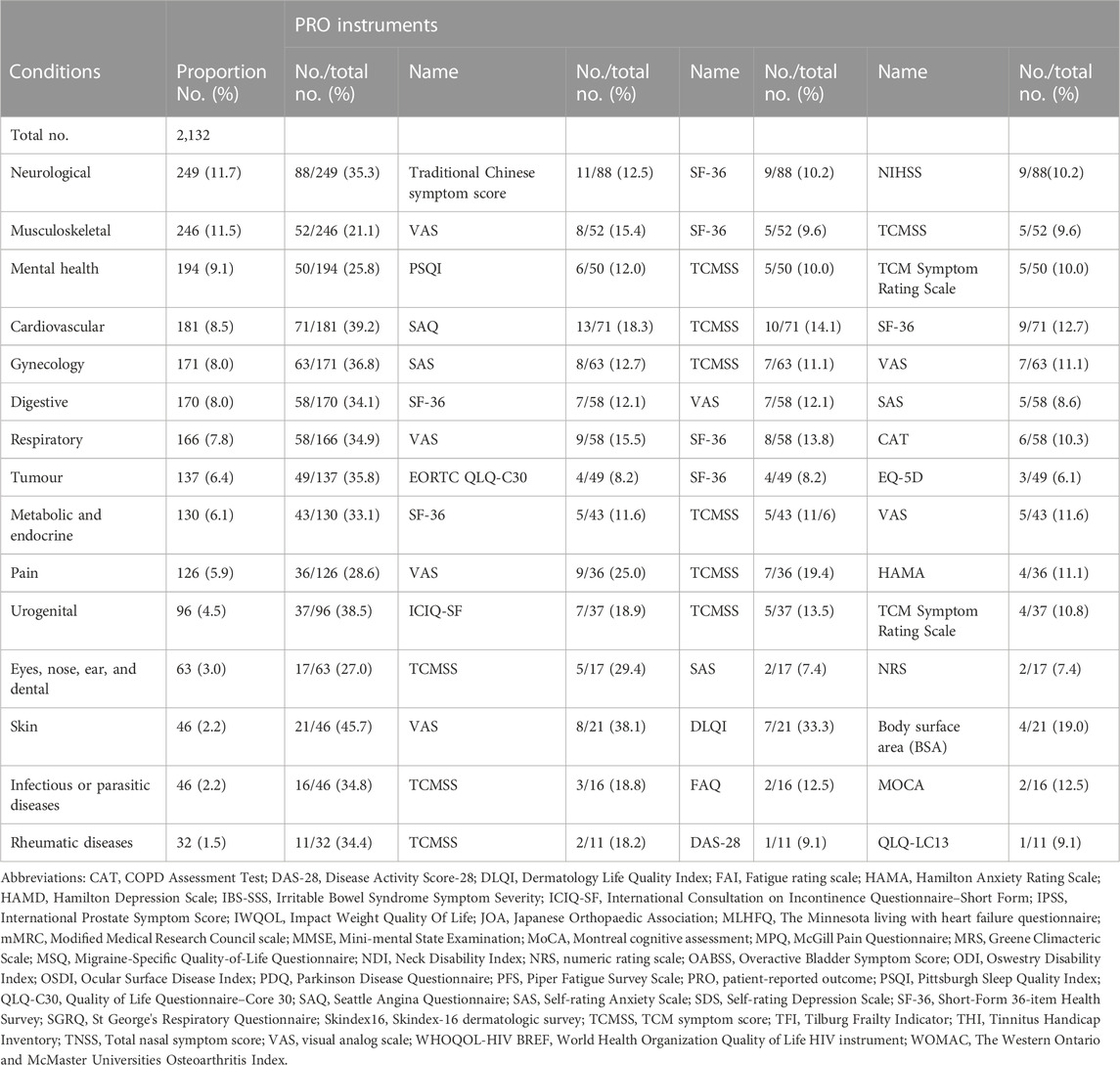
TABLE 3. Frequency of the use of PROMs as primary outcome in different classification of trials of TCM by condition.
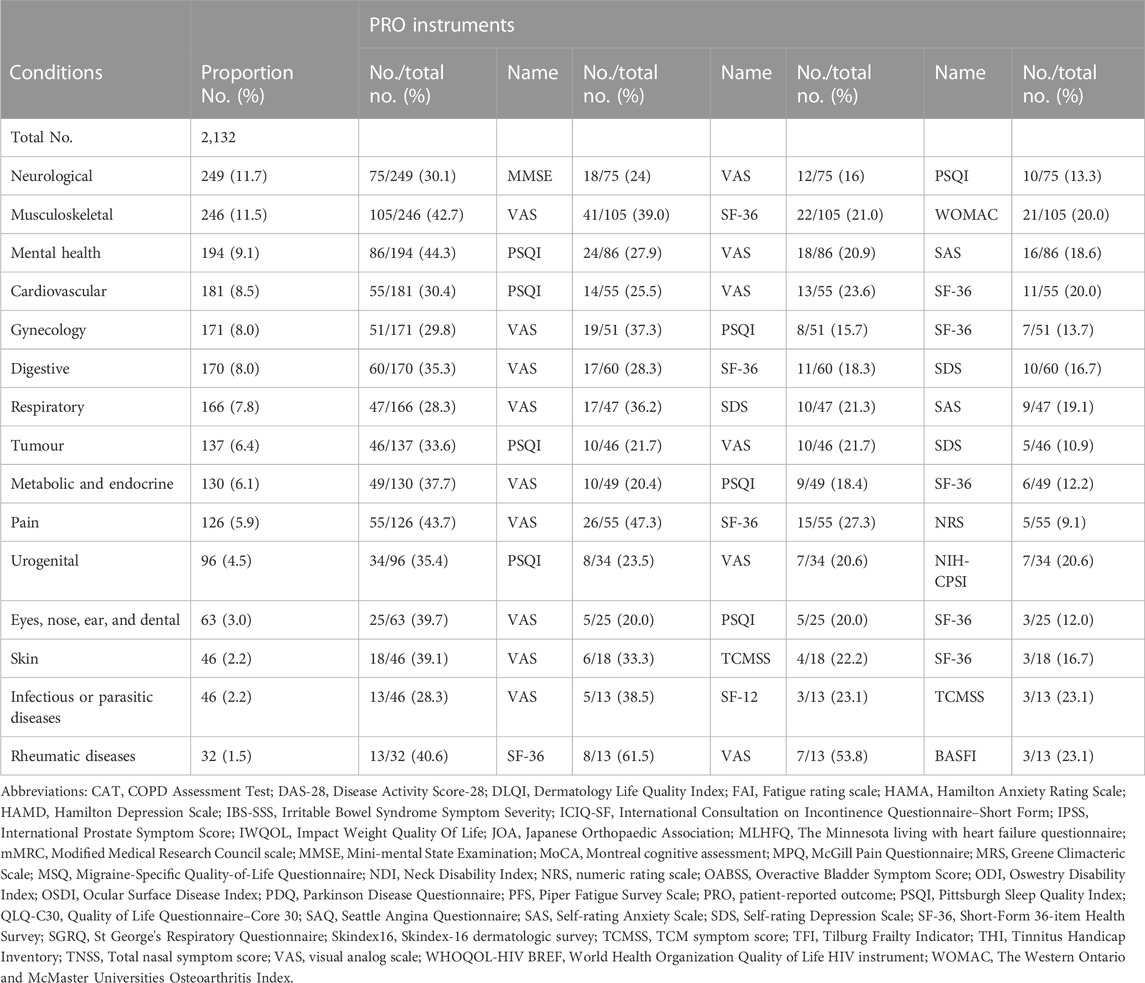
TABLE 4. Frequency of the use of PROMs as coprimary outcome in different classification of trials of TCM by condition.
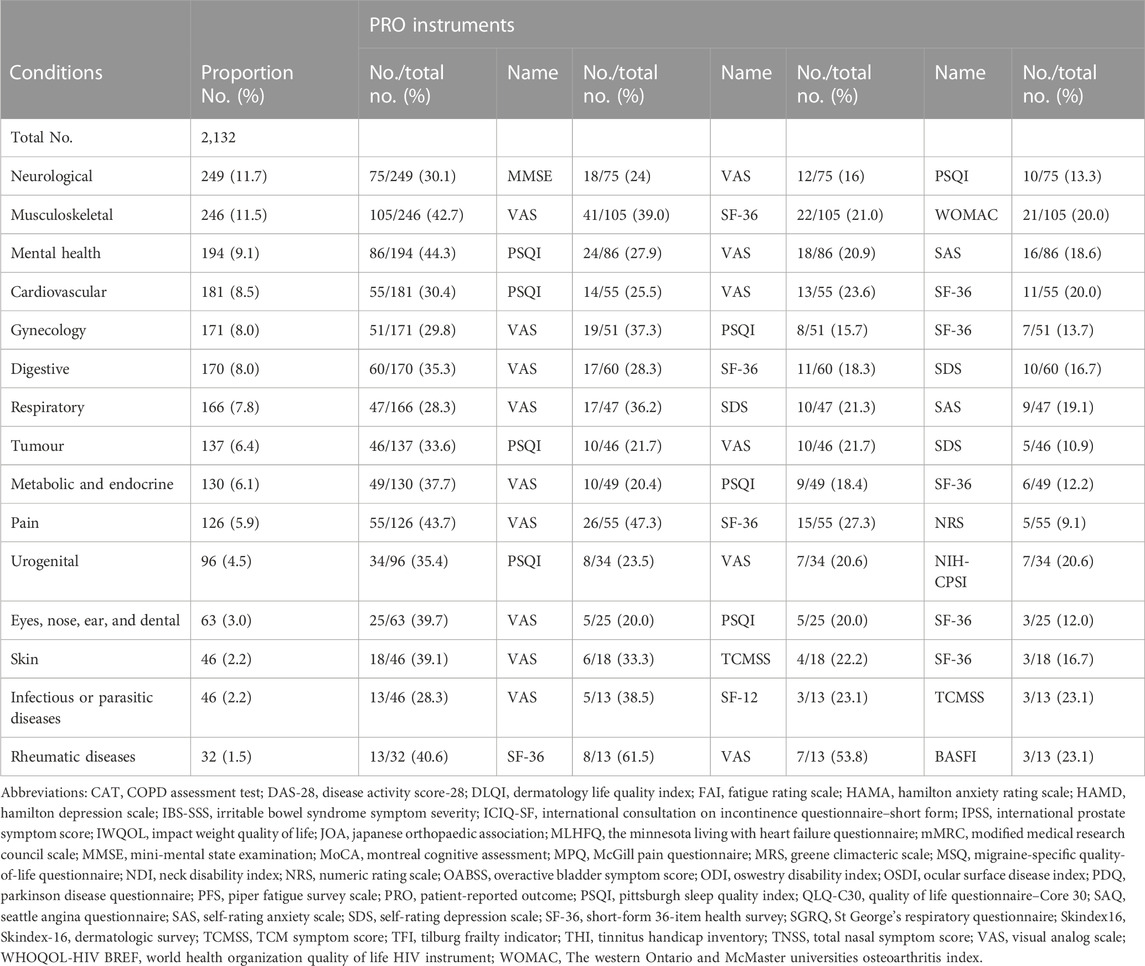
TABLE 5. Frequency of the use of PROMs as secondary outcome in different classification of trials of TCM by condition.
Considering PROs were secondary outcomes, PROMs were used as follows. TCMSS and SF-36 were widely used in neurological conditions. Trials focused on urogenital conditions tended to use International Consultation on Incontinence Questionnaire-Short Form (ICIQ-SF) (18.9%) to assess urinary incontinence.
In trials reporting PROs as coprimary outcomes, Mini-mental State Examination (MMSE) was used as chief instrument to access the neurological condition. Besides, the preliminary data suggested trials for mental health, cardiovascular, tumor, and urogenital conditions had a tendency to use Pittsburgh Sleep Quality Index (PSQI) to evaluate their coprimary outcomes. The VAS was reported as coprimary outcome measure in all trials. However, SF-36 was frequently focused on in trials of musculoskeletal and digestive conditions. For the 2,315 trials included PROs, the VAS (25.7%), TCMSS (14.6%), SF-36 (11.8%), PSQI (11.1%), and Self-Rating Anxiety Scale (SAS) (9.4%) were the top five measurements used.
This cross-sectional study analyzed the application and characteristics of the PROs in randomized clinical trials of TCM conducted in mainland China from 2010 to 2022. Our study found that 20% of the eligible trials used PRO instruments as coprimary outcomes to assess the subjective perception of patients. We proposed that the other 80% of the trials neglected subjective evaluations of patients. Our results also indicated that the standard and widely used PRO instruments for TCM were not enough, which was inconsistent with our anticipation. Introducing PROs could benefit TCM assessment in clinical practice. It is significant to introduce PROs to TCM evaluation, which can improve the comprehensiveness, accuracy, and degree of individuation of the evaluation, while also contributing to the monitoring and adjustment of the treatment effect of TCM (Rubenstein et al., 1989; Roter, 2000; Gilbody et al., 2003; Cleland et al., 2009; Jiang et al., 2012; Calvert et al., 2018).
It is not surprising that neurological trials were the most common in clinical trials of TCM. Many TCM unique therapies have confirmed the beneficial effect on neurological diseases (Guo et al., 2014; Liu et al., 2019), and the patients' opinions were the key factor for the modernization of TCM (Lu et al., 2020). Meanwhile, the long-term consequences of the stroke event would be reduced during routine healthcare (Lynch et al., 2008). PROs could capture more subtle changes in the life of the stroke patient (Roberts and Counsell, 1998; Kaplan, 2003), which filled the gaps in the routine healthcare for the stroke patient.
Our study suggested that most PROMs were employed for evaluating the symptoms in clinical trials of TCM. TCM symptoms were basic units of TCM treatment, in addition to being key factors for the modernization of TCM (Lu et al., 2020). This should not be ignored that TCM has its unique advantages to treat complex diseases with holistic concept (Huang et al., 2020). To treat patients based on the holistic consideration of individuals’ health, more retinue health data are required. Therefore, PROs on function and HRQOL also deserve enough attention (Reich et al., 2012; Myles et al., 2017; Alghadir et al., 2018; Chiarotto et al., 2019).
Both the primary and secondary outcomes placed a strong emphasis on VAS, which was used to quickly and easily evaluate patients' status, especially symptoms. The reason for wide application of VAS could be that VAS assesses subjective indicators such as pain and discomfort (Kelly, 2001; Karcioglu et al., 2018), which were highly compatible with the assessment indicators commonly used in Western medical clinical research, facilitating the comparison and comprehensive analysis of research results. There was sufficient information on irregular and inadequate PROMs in the included trials. Our results suggested that the Traditional Chinese symptom score and TCM Symptom Rating Scale were widely used, but syndrome-specific scales, such as TCM-SDS, were still underutilized. Our study revealed that PROs in trials mainly concentrated on evaluating symptoms, functioning, and quality of life, while neglecting the assessment of patients' treatment expectations and satisfaction. We found some researchers had begun to independently design new PROMs, which were correctly targeted for TCM syndrome and more widespread in clinical trials (Zhang et al., 2012; Sun et al., 2021a; Jiang et al., 2021). The development of these scales suggested that efforts should be directed towards the development of standardized PROMs tailored to quantifying the various components of TCM consultations and enabling patients to evaluate their symptoms using a standardized methodology.
Early-stage clinical trials accounted for the largest proportion in all included trials, which suggested that patients’ participation was important for the evaluation of the effectiveness and safety of drugs (Haslam et al., 2020). The early stages of clinical trials were followed by Phase 4. Phase 4 clinical trials provided a better reflection of effectiveness, tolerability, and safety in the real world by evaluating TCM intervention among diverse, large and heterogeneous patient populations, thus informing regulatory and reimbursement decisions and contributing to health policy-making (Maruszczyk et al., 2022). The large percentage of Phase 4 indicated that patients’ subjective feelings should be more emphasized when the trial did not mainly concentrate on marketing.
In our study, the obvious regional difference in the use of PROs was found. PROs were more frequently adopted in eastern, northern, and southern mainland China, especially in Shanghai, Beijing, Sichuan, and Guangdong; while rarely in northwestern, and northeastern, especially in Qinghai and Tibet. This may be related to the level of political, economic, and medical development level of corresponding regions and provinces (Liniker et al., 2013). Remarkably, in trials conducted in remote regions such as Tibet and Qinghai, PROs may not be able to implement or need to be simplified, which may influence the selection of PRO instruments (Zhou et al., 2022).
PROs can provide a reference for the effectiveness and safety of interventions, while serving as the vital basis for labeling claims on noninnovative drug trials, such as bioequivalence studies (Dougados et al., 2013; Kyte et al., 2016; Rivera et al., 2019). Our study indicated that Chinese herbal medicine was the most commonly used in TCM intervention. This may be due to Chinese herbal medicine being licensed and widely recognized in mainland China, and they were therapeutic modalities that were frequently used in integrative medicine (Sun et al., 2021b; Weiwei, 2022). Previous studies suggested that Chinese herbal medicine has been used to treat a broad range of neurological conditions, including stroke, Alzheimer’s disease, and Parkinson’s disease (Yao et al., 2017).
The Chinese government released Guidelines for the Application of Patient-Reported Outcomes in Drug Clinical Research in 2021, and thought highly of the clinical advantages of PROs. However, the current PROs may lack the necessary sensitivity to capture essential information relevant to TCM (Jiang et al., 2010). Investigators should join their efforts in conducting high-quality trials to improve the well-established protocols of PRO in TCM trials and enrich PRO instruments based on the current status of trials of TCM including PROs. Given the unique and esoteric terminology used in TCM practices, such as “Shen Xu” and “Na Dai,” it is important to consider the promotion of PROMs that are accessible and comprehensible to individuals without a background in TCM education in clinical settings (Sun et al., 2021a).
Our study has several limitations. Firstly, considering young children may not express their feelings accurately, and parents reported outcomes could be influenced by multi-factors, we did not include studies of children to avoid the potential bias of the results. Secondly, only trials conducted in mainland China were included in our study. Thus, our results may be limited to generalize due to cultural and regional factors. Finally, during data extraction, we found that some registration information of trials was not updated. For instance, a number of trials that were registered a few years ago were still shown as being in the recruiting phase, which may cause bias in the sample size.
In this cross-sectional study, the use of PROs increased in clinical trials of TCM conducted in mainland China in the past decades. Considering the application of PROs in clinical trials of TCM has some existing issues including uneven distribution and lack of standardized PROMs of TCM, further study should be focused on the development of standardized PROMs tailored to quantifying the various components of TCM consultations. It should be noticed that disease-specific versus generic scales for TCM trials need to be strongly developed.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
HR and JL conceived of the presented idea. YD and LL coordinated the data collection and analysis. YD, LL, XW, and YG did the data extraction. YD wrote a first draft of the paper and SY, SL, and WL inputted into subsequent drafts. JL and HR provided comments related to the presentation of the findings and critically reviewed the manuscript. All authors read and approved the final manuscript.
This work was supported by Scientific Research Supporting Funding of School of Traditional Chinese Medicine, Beijing University of Chinese Medicine (No. SRSFTCM-202301) and Fundamental Research Funds for the Central Universities (No. 2022-JYB-PY-013).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1159906/full#supplementary-material
Alghadir, A. H., Anwer, S., Iqbal, A., and Iqbal, Z. A. (2018). Test-retest reliability, validity, and minimum detectable change of visual analog, numerical rating, and verbal rating scales for measurement of osteoarthritic knee pain. J. Pain Res. 11, 851–856. doi:10.2147/JPR.S158847
Black, N. (2013). Patient reported outcome measures could help transform healthcare. Bmj 346, f167. doi:10.1136/bmj.f167
Calvert, M., Kyte, D., Mercieca-Bebber, R., Slade, A., Chan, A. W., King, M. T., et al. (2018). Guidelines for inclusion of patient-reported outcomes in clinical trial protocols the SPIRIT-PRO extension. Jama-Journal Am. Med. Assoc. 319 (5), 483–494. doi:10.1001/jama.2017.21903
Casey, D. E. (2022). Patient-reported outcome measures-challenges and opportunities for China. JAMA Netw. Open 5 (5), e2211652. doi:10.1001/jamanetworkopen.2022.11652
Chan, G., Bezuidenhout, L., Walker, L., and Rowan, R. (2016). The impact on life questionnaire: Validation for elective surgery prioritisation in New Zealand prioritisation criteria in orthopaedic surgery. N. Z. Med. J. 129 (1432), 26–32.
Chen, J., Ou, L., and Hollis, S. J. (2013). A systematic review of the impact of routine collection of patient reported outcome measures on patients, providers and health organisations in an oncologic setting. BMC Health Serv. Res. 13, 211. doi:10.1186/1472-6963-13-211
Chiarotto, A., Maxwell, L. J., Ostelo, R. W., Boers, M., Tugwell, P., and Terwee, C. B. (2019). Measurement properties of visual analogue scale, numeric rating scale, and pain severity subscale of the brief pain inventory in patients with low back pain: A systematic review. J. Pain 20 (3), 245–263. doi:10.1016/j.jpain.2018.07.009
Churruca, K., Pomare, C., Ellis, L. A., Long, J. C., Henderson, S. B., Murphy, L. E. D., et al. (2021). Patient-reported outcome measures (PROMs): A review of generic and condition-specific measures and a discussion of trends and issues. Health Expect. 24 (4), 1015–1024. doi:10.1111/hex.13254
Cleland, J. G. F., Calvert, M. J., Verboven, Y., and Freemantle, N. (2009). Effects of cardiac resynchronization therapy on long-term quality of life: An analysis from the CArdiac Resynchronisation-Heart Failure (CARE-HF) study. Am. Heart J. 157 (3), 457–466. doi:10.1016/j.ahj.2008.11.006
Dai, T. Y., Zhang, L., Dai, X., Zhang, X., Lu, B., Zheng, Y., et al. (2021). Multimode participation of traditional Chinese medicine in the treatment of COVID-19. Integr. Med. Res. 10, 100781. doi:10.1016/j.imr.2021.100781
Dawson, J., Doll, H., Fitzpatrick, R., Jenkinson, C., and Carr, A. J. (2010). The routine use of patient reported outcome measures in healthcare settings. Bmj 340, c186. doi:10.1136/bmj.c186
Dougados, M., Nataf, H., Steinberg, G., Rouanet, S., and Falissard, B. (2013). Relative importance of doctor-reported outcomes vs patient-reported outcomes in DMARD intensification for rheumatoid arthritis: The DUO study. Rheumatol. Oxf. 52 (2), 391–399. doi:10.1093/rheumatology/kes285
Gilbody, S., Whitty, P., Grimshaw, J., and Thomas, R. (2003). Educational and organizational interventions to improve the management of depression in primary care: A systematic review. Jama 289 (23), 3145–3151. doi:10.1001/jama.289.23.3145
Gnanasakthy, A., Norcross, L., DeMuro Romano, C., and Carson, R. T. (2022). A review of patient-reported outcome labeling of FDA-approved new drugs (2016-2020): Counts, categories, and comprehensibility. Value Health 25 (4), 647–655. doi:10.1016/j.jval.2021.10.006
Guo, Y., Yan, S., Xu, L., Zhu, G., Yu, X., and Tong, X. (2014). Use of angong niuhuang in treating central nervous system diseases and related research. Evidence-Based Complementary Altern. Med. 2014, 346918–346919. doi:10.1155/2014/346918
Haslam, A., Herrera-Perez, D., Gill, J., and Prasad, V. (2020). Patient experience captured by quality-of-life measurement in oncology clinical trials. JAMA Netw. Open 3 (3), e200363. doi:10.1001/jamanetworkopen.2020.0363
Huang, S. J., Mu, F., Li, F., Wang, W. J., Zhang, W., Lei, L., et al. (2020). Systematic elucidation of the potential mechanism of erzhi pill against drug-induced liver injury via network Pharmacology approach. Evid. Based Complement. Altern. Med. 2020, 6219432. doi:10.1155/2020/6219432
Jia, Y. X., Wen, J., Qureshi, R., Ehrhardt, S., Celentano, D. D., Wei, X., et al. (2021). Effect of redundant clinical trials from mainland China evaluating statins in patients with coronary artery disease: Cross sectional study. Bmj-British Med. J. 372, n48. doi:10.1136/bmj.n48
Jiang, M. A., Yang, J., Zhang, C., Liu, B., Chan, K., Cao, H., et al. (2010). Clinical studies with traditional Chinese medicine in the past decade and future research and development. Planta Medica 76 (17), 2048–2064. doi:10.1055/s-0030-1250456
Jiang, M., Lou, X. E., Zhang, X., Nan, Q., Gao, X., and Liu, H. (2021). Development and validation of the diagnostic scale of traditional Chinese medicine syndrome elements for diabetic kidney disease. Ann. Palliat. Med. 10 (12), 12291–12299. doi:10.21037/apm-21-3147
Jiang, M., Lu, C., Zhang, C., Yang, J., Tan, Y., Lu, A., et al. (2012). Syndrome differentiation in modern research of traditional Chinese medicine. J. Ethnopharmacol. 140 (3), 634–642. doi:10.1016/j.jep.2012.01.033
Kaplan, R. M. (2003). The significance of quality of life in health care. Qual. Life Res. 12, 3–16. doi:10.1023/a:1023547632545
Karcioglu, O., Topacoglu, H., Dikme, O., and Dikme, O. (2018). A systematic review of the pain scales in adults: Which to use? Am. J. Emerg. Med. 36 (4), 707–714. doi:10.1016/j.ajem.2018.01.008
Kelly, A. M. (2001). The minimum clinically significant difference in visual analogue scale pain score does not differ with severity of pain. Emerg. Med. J. 18 (3), 205–207. doi:10.1136/emj.18.3.205
Kochar, B., Martin, C. F., Kappelman, M. D., Spiegel, B. M., Chen, W., Sandler, R. S., et al. (2018). Evaluation of gastrointestinal patient reported outcomes measurement information system (GI-PROMIS) symptom scales in subjects with inflammatory Bowel diseases. Am. J. Gastroenterology 113 (1), 72–79. doi:10.1038/ajg.2017.240
Kotecha, D., Bunting, K. V., Gill, S. K., Mehta, S., Stanbury, M., Jones, J. C., et al. (2020). Effect of digoxin vs bisoprolol for heart rate control in atrial fibrillation on patient-reported quality of life: The RATE-AF randomized clinical trial. Jama-Journal Am. Med. Assoc. 324 (24), 2497–2508. doi:10.1001/jama.2020.23138
Kyte, D., Ives, J., Draper, H., and Calvert, M. (2016). Management of patient-reported outcome (PRO) alerts in clinical trials: A cross sectional survey. PLoS One 11 (1), e0144658. doi:10.1371/journal.pone.0144658
Leung, K. F., Liu, F. b., Zhao, L., Fang, J. q., Chan, K., and Lin, L. z. (2005). Development and validation of the Chinese quality of life instrument. Health Qual. Life Outcomes 3, 26. doi:10.1186/1477-7525-3-26
Liniker, E., Harrison, M., Weaver, J. M. J., Agrawal, N., Chhabra, A., Kingshott, V., et al. (2013). Treatment costs associated with interventional cancer clinical trials conducted at a single UK institution over 2 years (2009-2010). Br. J. Cancer 109 (8), 2051–2057. doi:10.1038/bjc.2013.495
Liu, H., Yan, Y., Pang, P., Mao, J., Hu, X., Li, D., et al. (2019). Angong niuhuang pill as adjuvant therapy for treating acute cerebral infarction and intracerebral hemorrhage: A meta-analysis of randomized controlled trials. J. Ethnopharmacol. 237, 307–313. doi:10.1016/j.jep.2019.03.043
Lu, Y., Qi, Y., Yan, Y., Yao, D., Deng, H., Deng, J., et al. (2020). Analysis of microRNA expression in peripheral blood monocytes of three Traditional Chinese Medicine (TCM) syndrome types in psoriasis patients. Chin. Med. 15, 39. doi:10.1186/s13020-020-00308-y
Lynch, E., Butt, Z., Heinemann, A., Victorson, D., Nowinski, C., Perez, L., et al. (2008). A qualitative study of quality of life after stroke: The importance of social relationships. J. Rehabilitation Med. 40 (7), 518–523. doi:10.2340/16501977-0203
Marshall, S., Haywood, K., and Fitzpatrick, R. (2006). Impact of patient-reported outcome measures on routine practice: A structured review. J. Eval. Clin. Pract. 12 (5), 559–568. doi:10.1111/j.1365-2753.2006.00650.x
Maruszczyk, K., Aiyegbusi, O. L., Cardoso, V. R., Gkoutos, G. V., Slater, L. T., Collis, P., et al. (2022). Implementation of patient-reported outcome measures in real-world evidence studies: Analysis of ClinicalTrials.gov records (1999-2021). Contemp. Clin. Trials 120, 106882. doi:10.1016/j.cct.2022.106882
Myles, P. S., Myles, D. B., Galagher, W., Boyd, D., Chew, C., MacDonald, N., et al. (2017). Measuring acute postoperative pain using the visual analog scale: The minimal clinically important difference and patient acceptable symptom state. Br. J. Anaesth. 118 (3), 424–429. doi:10.1093/bja/aew466
Patrick, D. L., Burke, L. B., Powers, J. H., Scott, J. A., Rock, E. P., Dawisha, S., et al. (2007). Patient-reported outcomes to support medical product labeling claims: FDA perspective. Value Health 10 (2), S125–S137. doi:10.1111/j.1524-4733.2007.00275.x
Price, A., Smith, J., Dakin, H., Kang, S., Eibich, P., Cook, J., et al. (2019). The arthroplasty candidacy help engine tool to select candidates for hip and knee replacement surgery: Development and economic modelling. Health Technol. Assess. 23 (32), 1–216. doi:10.3310/hta23320
Reich, A., Heisig, M., Phan, N. Q., Taneda, K., Takamori, K., Takeuchi, S., et al. (2012). Visual analogue scale: Evaluation of the instrument for the assessment of pruritus. Acta Dermato-Venereologica 92 (5), 497–501. doi:10.2340/00015555-1265
Rivera, S. C., Kyte, D. G., Aiyegbusi, O. L., Slade, A. L., McMullan, C., and Calvert, M. J. (2019). The impact of patient-reported outcome (PRO) data from clinical trials: A systematic review and critical analysis. Health Qual. Life Outcomes 17 (1), 156. doi:10.1186/s12955-019-1220-z
Roberts, L., and Counsell, C. (1998). Assessment of clinical outcomes in acute stroke trials. Stroke 29 (5), 986–991. doi:10.1161/01.str.29.5.986
Roter, D. (2000). The enduring and evolving nature of the patient-physician relationship. Patient Educ. Couns. 39 (1), 5–15. doi:10.1016/s0738-3991(99)00086-5
Rubenstein, L. V., Calkins, D. R., Young, R. T., Cleary, P. D., Fink, A., Kosecoff, J., et al. (1989). Improving patient function: A randomized trial of functional disability screening. Ann. Intern Med. 111 (10), 836–842. doi:10.7326/0003-4819-111-10-836
Santosa, K. B., Qi, J., Kim, H. M., Hamill, J. B., Wilkins, E. G., and Pusic, A. L. (2018). Long-term patient-reported outcomes in postmastectomy breast reconstruction. Jama Surg. 153 (10), 891–899. doi:10.1001/jamasurg.2018.1677
Sun, L., Mao, J. J., Yan, Y., Xu, Y., and Yang, Y. (2021). Patient reported traditional Chinese medicine spleen deficiency syndrome (TCM-SDS) scale for colorectal cancer: Development and validation in China. Integr. Cancer Ther. 20, 15347354211020105. doi:10.1177/15347354211020105
Sun, X., Li, L., Liu, Y., Wang, W., Yao, M., Tan, J., et al. (2021). Assessing clinical effects of traditional Chinese medicine interventions: Moving beyond randomized controlled trials. Front. Pharmacol. 12, 713071. doi:10.3389/fphar.2021.713071
U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health (2006). Guidance for industry: Patient-reported outcome measures: Use in medical product development to support labeling claims: Draft guidance. Health Qual. Life Outcomes 4, 79. doi:10.1186/1477-7525-4-79
Weiwei, J. (2022). Analysis of real word study of tradtrional Chinese medcine based on Chinese clinical trial registry. World Chin. Med. 2022, 1–20.
Yao, Y. (2017). “Effect of Chinese herbal medicine on molecular imaging of neurological disorders,” in Neurobiology of Chinese herb medicine. Editors B. Y. Zeng, and K. Zhao (San Diego: Elsevier Academic Press Inc), 181–196.
Zhang, C. J., and Chor, W. (2023). Realizing holism in traditional Chinese medicine (TCM) consultations through the voice of TCM (VOTCM): An interactional analytical approach. Health Commun. 38 (2), 275–284. doi:10.1080/10410236.2021.1950292
Zhang, H., Lv, H., Huang, P. X., Lin, Y., Hu, X. C., and Liu, P. (2012). Comparative study of TCM syndrome scale for liver disease and chronic liver disease questionnaire based on assessment of posthepatitic cirrhosis. Evidence-Based Complementary Altern. Med. 2012, 496575. doi:10.1155/2012/496575
Zhang, Y. H., Lv, J., Gao, W., Li, J., Fang, J. Q., He, L. Y., et al. (2017). Practitioners' perspectives on evaluating treatment outcomes in traditional Chinese medicine. BMC Complement. Altern. Med. 17 (1), 269. doi:10.1186/s12906-017-1746-8
Zhao, L., and Chan, K. (2005). Building a bridge for integrating Chinese medicine into conventional healthcare: Observations drawn from the development of the Chinese quality of life instrument. Am. J. Chin. Med. 33 (6), 897–902. doi:10.1142/S0192415X05003533
Keywords: traditional Chinese medicine, clinical trial, patient-reported outcomes, secondary outcomes, primary outcomes
Citation: Dong Y, Liu L, Zhang X, Gong Y, Yan S, Li W, Li S, Rong H and Liu J (2023) A cross-sectional study on the application of patient-reported outcome measurements in clinical trials of traditional Chinese medicine in mainland China. Front. Pharmacol. 14:1159906. doi: 10.3389/fphar.2023.1159906
Received: 06 February 2023; Accepted: 19 April 2023;
Published: 11 May 2023.
Edited by:
Luca Rastrelli, University of Salerno, ItalyReviewed by:
Dalinda Isabel Sánchez-Vidaña, Hong Kong Polytechnic University, Hong Kong SAR, ChinaCopyright © 2023 Dong, Liu, Zhang, Gong, Yan, Li, Li, Rong and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongguo Rong, aGdyb25nQGhzYy5wa3UuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.