- 1State Key Laboratory of Component-based Chinese Medicine, Tianjin University of Traditional Chinese Medicine, Tianjin, China
- 2Tianjin Key Laboratory of Phytochemistry and Pharmaceutical Analysis, Tianjin University of Traditional Chinese Medicine, Tianjin, China
- 3Haihe Laboratory of Modern Chinese Medicine, Tianjin, China
As a Traditional Chinese Medicine prescription, Qingjin Yiqi Granules (QJYQ) provides an effective treatment for patients recovering from COVID-19. However, the pharmacokinetics characteristics of the main components of QJYQ in vivo are still unknown. An efficacious ultra-performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS) was developed and validated for the simultaneous determination of 33 components in rat plasma after oral administration of QJYQ. The plasma samples were precipitated with 400 µL methanol/acetonitrile (1/1, v/v) and analyzed in scheduled multiple reaction monitoring mode. The linear relationship of the 33 components was good (r > 0.9928). The lower limit of quantification for 33 components ranged from 0.4–60.5 ng/mL. The average recoveries and matrix effects of the analytes ranged from 72.9% to 115.0% with RSD of 1.4%–15.0%. All inter-day and intra-day RSDs were within 15.0%. After oral administration (3.15 g/kg), the validated approach was effectively applied to the pharmacokinetics of main components of QJYQ. Finally, fifteen main constituents of QJYQ with large plasma exposure were obtained, including baicalin, wogonoside, wogonin, apigenin-7-O-glucuronide, verbenalin, isoferulic acid, hesperidin, liquiritin, harpagide, protocatechuic acid, p-Coumaric acid, ferulic acid, sinapic acid, liquiritin apioside and glycyrrhizic acid. The present research lays a foundation for clarifying the therapeutic material basis of QJYQ and provides a reference for further scientific research and clinical application of QJYQ.
1 Introduction
Traditional Chinese medicines (TCMs) have been used clinically for thousands of years as natural healing agents. TCMs treatment has the advantages of fewer side effects and low toxicity. It can play a more comprehensive role in the treatment of diseases through its unique multi-target (Xiang, et al., 2021; Duya, et al., 2022; Li, et al., 2023). It has a special function in treating tough and complex disorders in particular, and it is indispensable in chemical medicine (Luo, et al., 2020; Zhang, et al., 2020; Liao, et al., 2022).
Pharmacokinetic study of TCMs components is an important bridge between the study of chemical composition and active components of TCMs, mainly to elucidate the absorption, distribution, metabolism and excretion characteristics of various major chemical components in vivo (Lu et al., 2008; Huang, et al., 2022). Pharmacokinetic study can identify components that have significant systemic exposure in the systemic blood after administration (Liu et al., 2009). This provides the key research object for the material basis research of the curative effect of TCMs.
Due to the complexity and huge difference in content of the ingredients contained in TCMs, it is difficult to analyze and determine multiple components in vivo. Therefore, the development of sensitive and reliable biological sample analysis for simultaneous quantitative determination of multiple components in vivo is a focus of the study of pharmacokinetics of multiple components in TCMs (Zou, et al., 2019). Ultra-performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS) is often used for the analysis of complex components of TCMs. The schedule multiple reaction monitoring mode (sMRM) can solve the problem that the peak is too thin and the scanning points are not enough, compared with MRM mode. This acquisition mode greatly improves the efficiency of quantitative analysis (Li et al., 2021).
Qingjin Yiqi Granules (QJYQ) was developed by Academician Zhang Boli, which is used to treat the body damage and immune system adjustment for patients recovering from COVID-19. QJYQ consists of Ginseng radix et rhizoma, Ophiopogonis radix, Schisandrae chinensis fructus of principle medicine, Poria, Pinelliae rhizoma praeparatum cum alumine, bran stir-baked Atractylodis Rhizoma, Citri reticulatae pericarpium, Coicis semen of minister medicines, Scrophulariae radix, Cimicifugae rhizoma, Bupleuri radix, Scutellariae radix, Phragmitis rhizoma, Lophatheri herba of assistant medicine, verbenae herba, Glycyrrhizae radix et rhizoma of envoy medicines. The detailed components of QJYQ are presented in the literature (Yang, et al., 2023). These 16 Chinese medicines mostly contain phenolic acids, flavonoids, iridoids and triterpenoid saponins (Tarnawski et al., 2006; Lara-Issasi et al., 2019). At present, QJYQ has been widely used as a rehabilitation drug for discharged patients with COVID-19 in Hebei and Tianjin of China. QJYQ can effectively treat low fever during the recovery period of COVID-19 (Wang, et al., 2021; Tian, et al., 2022). In addition, QJYQ can improve symptoms of breathlessness and fatigue in convalescent patients (Pang, et al., 2022). However, there are no publications about the pharmacokinetic of multiple components in rat plasma after oral administration of QJYQ.
In this study, an UHPLC-sMRM method was established for simultaneous determination of thirty-three compounds in rat plasma to explore the main absorbed compounds of QJYQ. Moreover, a total of 15 main compounds with large plasma exposure in rat plasma were detected, including baicalin, wogonoside, wogonin, apigenin-7-O-glucuronide, verbenalin, isoferulic acid, sinapic acid, hesperidin, p-Coumaric acid, glycyrrhizic acid, liquiritin, ferulic acid, harpagide, protocatechuic acid and liquiritin apioside. This study provides comprehensive insights into the pharmacokinetic of QJYQ, and would be valuable for future clinical development and utilization of QJYQ.
2 Materials and methods
2.1 Chemicals and reagents
Acetonitrile and methanol were HPLC-grade from Fisher Scientific (Pittsburg, PA, United States). All other reagents were of analytical grade and obtained by Anaqua™ Chemicals Supply (Wilmington, DE, United States). Ultrapure water is prepared by the Millipore Ultra-Pure Water System. Harpagide, protocatechuic acid, atractyloside A, verbenalin, paeoniflorin, p-Coumaric acid, sinapic acid, vitexin, liquiritin, liquiritin apioside, isoliquiritin apioside, cimifugin, scutellarin, apigenin-7-O-glucuronide, ononin, isoliquiritin, naringenin, glycyrrhizic acid, baicalein, icariin (IS, internal standards), isopimpinellin (IS), astragaloside II (IS) were purchased from Chengdu Desite Bio-Technology Co., Ltd (Chengdu, China). Quercitrin were purchased from Shanghai yuanye Bio-Technology Co., Ltd. (Shanghai, China). Catechin, cryptochlorogenin acid, hyperoside, wogonoside, chlorogenic acid, ginsenosides Rf, ginsenosides Rh1 were purchased from Chengdu Must Bio-Technology Co., Ltd. (Chengdu, China). Isoferulic acid, hesperidin, baicalin, harpagoside, wogonin, ferulic acid were purchased from National Institutes for Food and Drug Control (Beijing, China). Their purity was higher than 98%. QJYQ were made by the laboratory of Tianjin University of Traditional Chinese Medicine, and the batch numbers in this study was 210601.
2.2 UHPLC-sMRM conditions
Exion LC AD tandem Blueline 3500 triple quadrupole mass spectrometer (AB SCIEX, Framingham, MA, United States) was used to thirty-three compounds in rat plasma. The conditions of liquid chromatography and mass spectrometry are the same as those for the quantitative determination of 50 components in vitro by UHPLC-sMRM (Yang, et al., 2023). Briefly, quantitation was operated using sMRM of the transitions of m/z 362.9→138.9 for harpagide at 2.10 min, m/z 152.9→109.0 for protocatechuic acid at 2.40 min, m/z 433.0→225.0 for verbenalin at 5.56 min, m/z 163.0→119.0 for p-Coumaric acid at 6.32 min, m/z 192.9→134.0 for ferulic acid at 7.08 min, m/z 222.9→164.0 for sinapic acid at 7.26 min, m/z 417.0→134.8 for liquiritin at 7.32 min, m/z 549.1→255.0 for liquiritin apioside at 7.43 min, m/z 193.0→133.0 for isoferulic acid at 7.48 min, m/z 609.0→301.0 for hesperidin at 9.12 min, m/z 445.1→268.8 for apigenin-7-O-glucuronide at 9.25 min, m/z 445.0→268.9 for baicalin at 10.90 min, m/z 459.0→268.0 for wogonoside at 13.62 min, m/z 821.1→351.1 for glycyrrhizic acid at 17.56 min and m/z 282.8→267.9 for wogonin at 17.91 min. The whole detailed parameters of sMRM were obtained in Supplementary Table S1.
2.3 Preparation of standard and quality control (QC) samples
Baicalin and wogonoside were accurately weighed and dissolved in methanol to obtain the standard stock solutions at the concentration of 2 mg/mL. The other 31 compounds were prepared into 1 mg/mL in methanol. The ISs (icariin, isopimpinellin and astragaloside II) were configured in the same way as 1 μg/mL. The mixed stock solution was stepwise diluted to desired concentrations with methanol for plotting standard curves. Each mixed standard solution was accurately taken and diluted with methanol to prepare 4 different concentrations of lower limit of quantification (LLOQ), low, medium and high as QC reserve solution. All solutions are stored in the refrigerator at 4°C for future use.
2.4 Preparation of plasma sample
Rat plasma (100 μL) was mixed with 10% formic acid (10 μL) and vortexed for 1 min at room temperature. ISs (10 μL) and methanol/acetonitrile (1/1, v/v) (400 μL) were added and vortexed for 5 min, then centrifuged for 10 min at 14,000 rpm and 4°C. The transported supernatant was condensed to dryness under the flow of nitrogen gas. The dried residue was reconstituted with 70% methanol (100 μL) by vortex-mixing for 5 min and centrifuging at 14,000 rpm for 10 min. Last, 2 μL of the solution were injected into the UHPLC-sMRM system.
2.5 Method validation
The UHPLC-sMRM bioanalytical method was validated for specificity, LLOQ, linearity, accuracy and precision, extraction recovery, matrix effects and stability. The results should comply with the currently recognized U.S. Food and Drug Administration (FDA) bioanalytical method validation guidelines.
2.5.1 Selectivity
The selectivity was determined by comparing the chromatograms of six separate batches of blank rat plasma samples, blank plasma spiked with corresponding mixed standards and ISs, and real plasma samples obtained following oral administration of QJYQ.
2.5.2 Linearity and LLOQ
Linearity investigation was conducted by adding a mixed solution of reference substances with 8 concentration levels into blank plasma, processing the samples according to the preparation method of plasma samples, and sampling analysis. The ratio of the analyte peak area to the ISs peak area was taken as the vertical coordinate (y), and the concentration of analyte was taken as the horizontal coordinate (x). The weighted (1/X, 1/X2) least squares linear regression was used to establish the linear relationship. The LLOQ was determined by analyzing blank plasma spiked with mixed standards at a signal to noise ratio of approximately 10.
2.5.3 Precision and accuracy
QC at four concentrations were added to blank plasma (n = 6), and the samples were treated according to the plasma sample preparation method. The intra-day precision and accuracy were evaluated by analyzing samples within the same day. The inter-day precision and accuracy were verified by repeating the same procedure for three consecutive days and applying the accompanying standard curve.
2.5.4 The recovery and matrix effect
The extraction recovery and matrix effect of six replicate groups were determined at 4 QC levels. The recovery is determined by comparing the peak area of the analyte in the pre-extraction spiked plasma samples with the spiked solutions in the post-extraction blank plasma. The matrix effect is tested by comparing the peak area of the post-extracted spiked samples with that of the standard solution of four different concentrations in six replicates of QC samples. A single concentration of ISs was also determined by the above method.
2.5.5 Stability
The stability of plasma samples needs to be evaluated based on processing and storage conditions. The stability of analytes in plasma was evaluated by analyzing 4 QC samples (n = 6). The stability was included auto-sampler stability (keeping the sample in auto-sampler for 24 h), three times of freeze-thaw cycles stability (freezing cycle at −80°C, thawing cycle at room temperature), room temperature stability (storing samples at room temperature for 24 h), long-term stability (storing samples at −80°C for 1 month). In addition, the stability of the working solution needs to be determined (the working solution is stored at 4°C for 1 month). Accompanying curve was established when testing the sample, calculate and compare the ratio of the actual measured concentration to the theoretical concentration, expressed in accuracy and RSD.
2.6 Application for pharmacokinetic study
The pharmacokinetic study was approved by the Animal Ethics Committee of Tianjin University of Traditional Chinese Medicine (TCM-LAEC2021228). Ten male Sprague-Dawley rats (weight 220–240 g) was kept at the animal center of Tianjin University of Traditional Chinese Medicine (Tianjin, China). Rats were fasted for 12 h and drank water freely before administration. According to the clinical dose, the dose in rat is 3.15 g/kg. Blood samples (about 100 μL) were collected before dosing and at 0.083, 0.17, 0.25, 0.33, 0.5,0.75, 1, 2, 3, 4, 6, 8, 10, 12, 24, 36 h after administration of QJYQ from vein of the eye sockets into heparinized tubes. The sample was transferred right away following a 10 min period of centrifugation at 7000 rpm and 4°C. All plasma samples were frozen and stored at −80°C.
2.7 Data analysis for pharmacokinetic study
All the pharmacokinetic parameters were calculated using the DAS 1.0 software (Drug and Statistics 1.0, Medical College of Wannan, China). Pharmacokinetic parameters include the maximum drug concentration in plasma (Cmax), the time to achieve maximum drug concentration (Tmax), the area under the plasma concentration-time curve (AUC), elimination half-life (T1/2), and mean residence time (MRT). The pharmacokinetic parameters were computed using the non-compartmental model. All data are expressed as mean ± standard deviation (SD). GraphPad Prism 8.0 (GraphPad Software Inc., La Jolla, CA, United States) software was used to draw the drug-time curve.
3 Results
3.1 Optimization of UHPLC-sMRM conditions
The liquid chromatography conditions were optimized to obtain better separation in a short time. Different mobile phases (acetonitrile-water, methanol-water), concentrations of additive (0.05%, 0.1%, and 0.2% formic acid), column temperatures (30, 35°C and 40°C) and flow rates (0.2, 0.3 and 0.4 mL/min) were optimized. The results show that a good separation effect and stable and high response value can be achieved under the following conditions: acetonitrile-0.1% formic acid in water as mobile phases, flow rate at 0.3 mL/min and column temperature at 40°C (Supplementary Figure S1).
3.2 Optimization of sample preparation
Two methods were used to optimize the treatment of plasma samples, namely, protein precipitation method and liquid-liquid extraction method. The optimal extraction recovery and matrix effect of each analyte were 85.0%–115.0%. It was found that the extraction recovery of each analyte was very small when ethyl acetate was used for liquid-liquid extraction, while the extraction recovery was higher when methanol, acetonitrile and methanol-acetonitrile mixed solution was used as the extraction solvent. Further optimization showed that the average recovery rate of methanol/acetonitrile (1/1, v/v) was more than 85%, which was suitable for the determination of biological samples. On this basis, vortex time (1 min, 3 min, 5 min) and resolution solvent (methanol, 70% methanol, 50% methanol) were optimized. The results showed that the extraction recovery and matrix effect were the best when 70% methanol was resolution and vortex time was 5 min. In addition, it has been reported that baicalin and wogonoside are not stable under alkaline and light and heat conditions (Wang et al., 2008; Feng et al., 2017). When a certain amount of formic acid was added into methanol-acetonitrile protein precipitation method, the extraction recovery of each analyte was improved obviously. Altogether, 10% (v/v) formic acid, methanol/acetonitrile (1/1, v/v), vortex time (5 min) and reconstitution solvent (70% methanol) were selected as the best processing conditions for plasma samples (Supplementary Figure S2). The extraction recovery rate and matrix effect of each analyte were in line with the determination requirements of biological samples.
3.3 Method validation
3.3.1 Specificity
According to the typical chromatograms of the blank sample, blank plasma spiked with mixed standards and ISs and plasma sample, it can be seen that the analytes were well separated without interference from endogenous substances or metabolites (Figure 1).
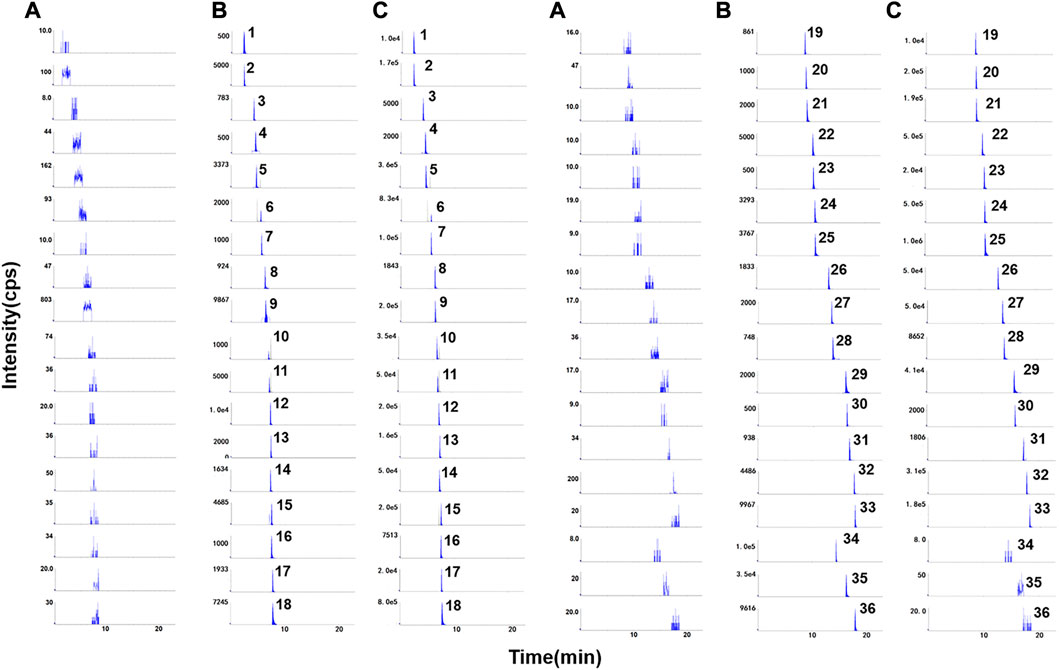
FIGURE 1. Representative chromatograms of (A) blank rat plasma, (B) blank rat plasma spiked with mixed standard compounds at LLOQs and ISs, (C) real samples after administration of QJYQ. 1–36 were harpagide, protocatechuic acid, atractyloside A, catechin, chlorogenic acid, cryptochlorogenic acid, verbenalin, paeoniflorin, p-Coumaric acid, ferulic acid, sinapic acid, vitexin, liquiritin, liquiritin apioside, isoferulic acid, hyperoside, cimifugin, scutellarin, quercitrin, hesperidin, apigenin-7-O-glucuronide, isoliquiritin apioside, ononin, baicalin, isoliquiritin, harpagoside, wogonoside, naringenin, baicalein, ginsenoside Rf, ginsenoside Rh1, glycyrrhizic acid, wogonin, icariin (IS), isopimpinellin (IS) and astragaloside II (IS), respectively.
3.3.2 Linearity and LLOQ
The curve of 33 analytes was fitted using a weighted (1/X or 1/X2) least squares linear regression approach, and a satisfactory correlation coefficient (r > 0.9928) was achieved. The LLOQs for 33 components ranged from 0.4–60.5 ng/mL (Supplementary Table S2).
3.3.3 Precision and accuracy
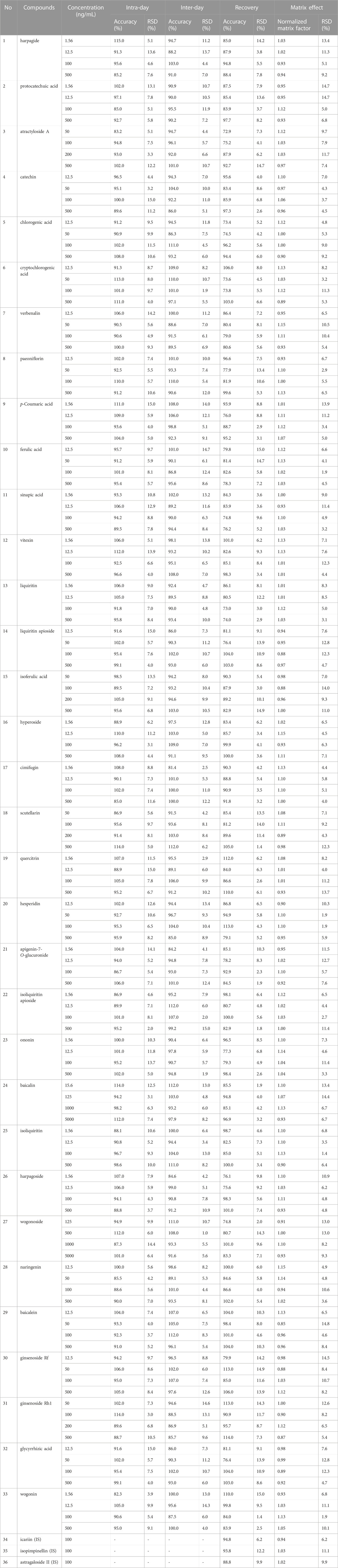
The accuracy varied from 81.4% to 115.0%, and the RSDs for both intra-day and inter-day was under 15.0%. The outcomes showed that the developed method was reliable for determination of the 33 compounds in rat plasma (Table 1).
3.3.4 Recovery and matrix effect
The extraction recoveries and matrix effect of all analytes ranged from 72.9% to 115.0% in four levels of QC samples. The RSDs were less than 15.0%. The results show that this plasma sample pre-treatment method can meet the needs of multi-component determination in vivo and pharmacokinetic studies (Table 1).
3.3.5 Stability
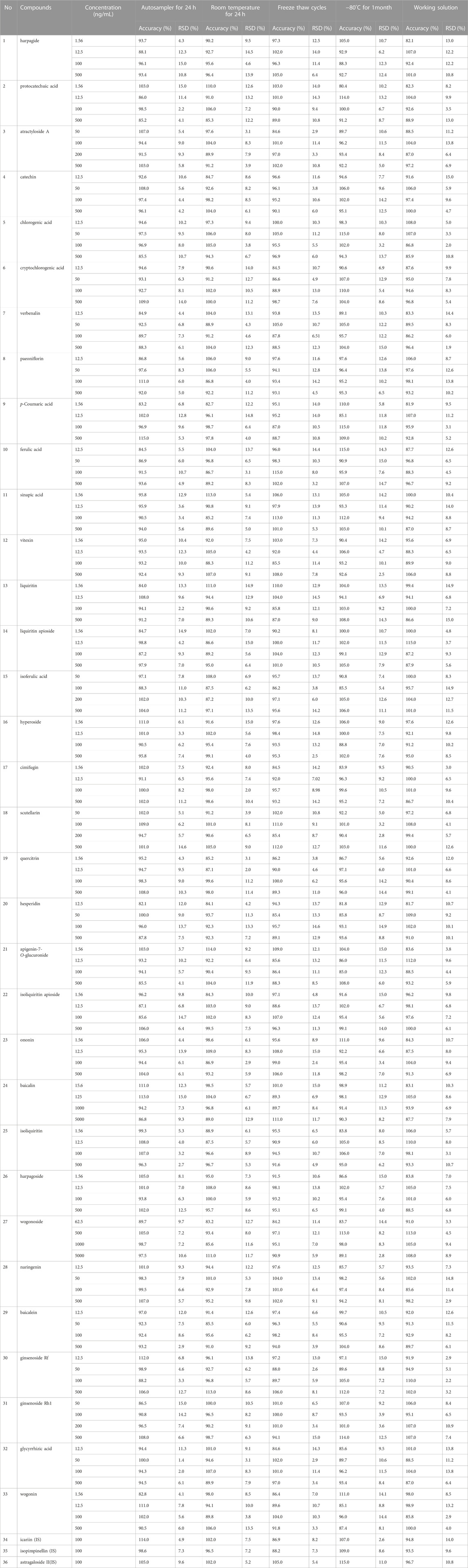
The 33 compounds were stable in rat plasma under the following conditions (room temperature for 24 h, auto-sampler for 24 h, three freeze-thaw cycles and stored at −80°C for 1 month). The stability of the working solution was relatively stable for all the target analytes. The results indicated that the method could be used to simultaneous determine the 33 compounds in rat plasma (Table 2).
3.4 Pharmacokinetic analysis
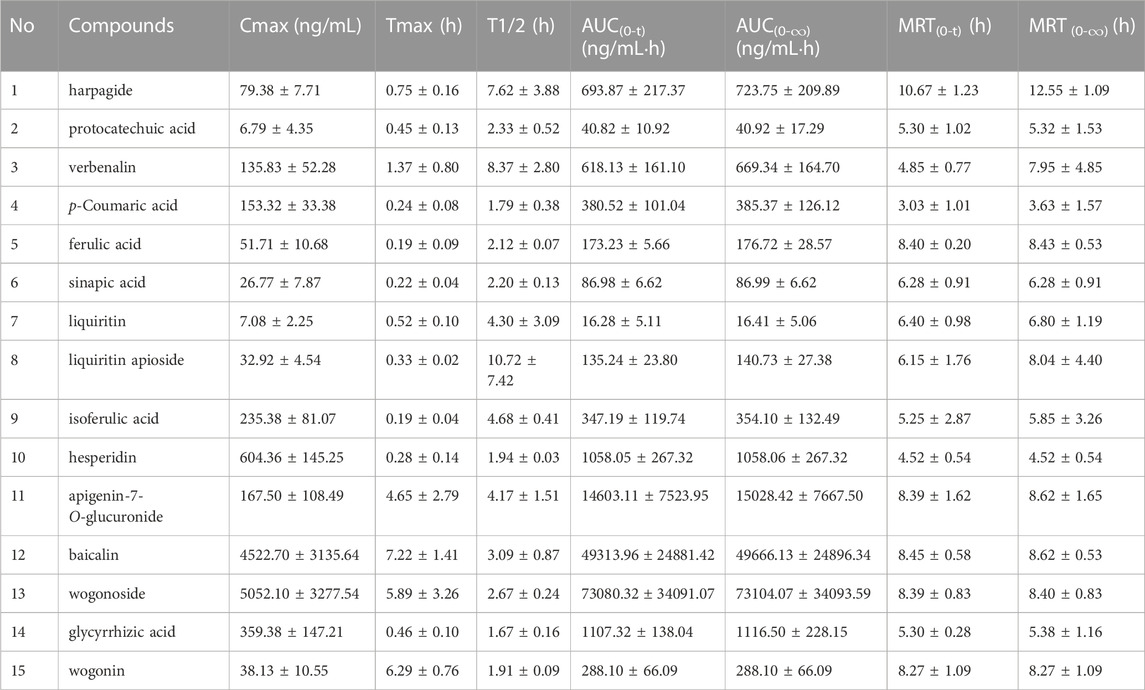
Although TCMs composition was complex, only some components with certain concentration could play the corresponding curative effect when components were absorbed into the blood. Therefore, components that have absorbed into the blood with large plasma exposure could be used as indicators for evaluating their quality standards (He et al., 2018; Zhang et al., 2018). In this research, a total of 15 major constituents of QJYQ with large plasma exposure were obtained. The results showed the mean plasma concentration-time profiles and the major pharmacokinetic parameters of the 15 analytes (n = 8) (Figure 2; Table 3).
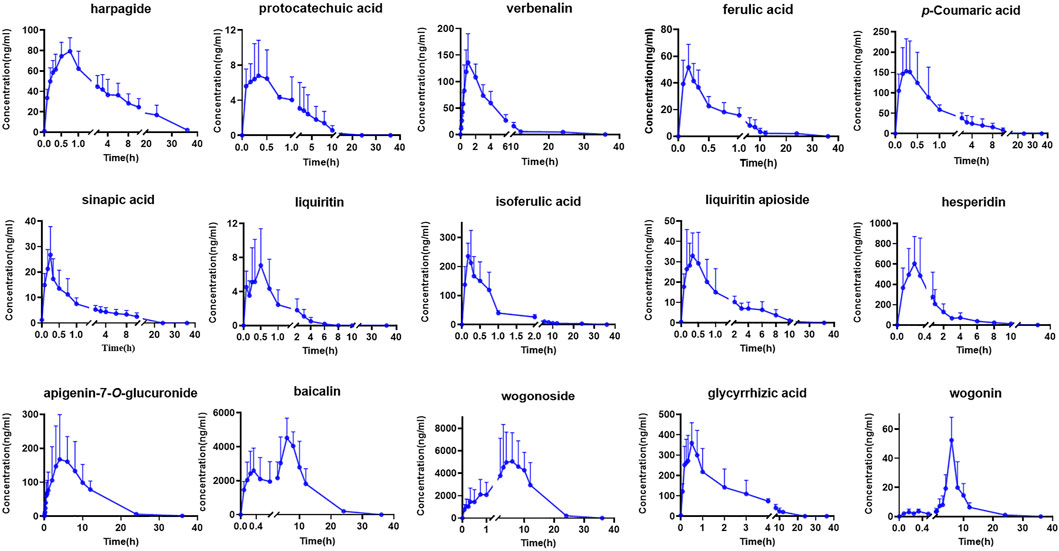
FIGURE 2. The mean plasma concentration-time of 15 compounds in rats after oral administration of QJYQ (n = 8, Mean ± SD).
The Tmax of isoferulic acid, ferulic acid, sinapic acid, p-Coumaric acid, glycyrrhizic acid, liquiritin apioside, protocatechuic acid and hesperidin is less than 0.5 h, suggesting that these compounds could be rapidly absorbed into blood circulation system. The Tmax of baicalin is 7.22 h ± 1.41, which was the longest.
Baicalin, wogonoside and hesperidin have large maximum plasma concentrations, which are 4522.70 ± 3135.64, 5052.10 ± 3277.54, 604.36 ± 145.25 ng/mL, respectively, and they are also the main components of QJYQ (Yang, et al., 2023). Baicalin, wogonoside and apigenin-7-O-glucuronide have greater plasma exposure. The MRT of harpagide, ferulic acid, apigenin-7-O-glucuronide, wogonin, wogonoside and baicalin are all greater than 8 h, suggesting that they take a long time to be eliminated in vivo.
Baicalin has a higher AUC(0-∞), which is 49666.13 ± 24896.34 ng/L·h. It may be attributed to the high content of the compound and its interaction with other components (Yang, et al., 2023). The drug-time curves of baicalin showed double peaks, suggesting that baicalin can be reabsorbed in vivo through the enterohepatic circulation (Huang et al., 2019). This will undoubtedly increase the blood concentration of baicalin and maintain a high level, which will be beneficial to the therapeutic effect of QJYQ.
The double-peak or even triple-peak phenomenon of wogonoside may be due to the transformation of wogonoside and wogonin in vivo (Dai et al., 2015). The AUC(0-∞) of wogonoside is 73104.07 ± 34093.59 ng/L·h, which is the largest among the tested ingredients. It has been found that the Caco-2 cell membrane permeability of wogonoside is greater than baicalin, indicating that the absorption of wogonoside is better than baicalin. AUC(0-t) and AUC(0-∞) confirmed the result (Cai et al., 2016). The MRT(0-t) of wogonoside and wogonin are both larger, which may be related to the local circulation of intestinal cells.
It has been reported that chlorogenic acid may be metabolized to ferulic acid and isoferulic acid after entering the body, resulting in increased levels of ferulic acid and isoferulic acid in vivo (Wang, et al., 2018). In this study, chlorogenic acid was not detected, which may have been converted to ferulic acid and isoferulic acid. At the same time, ferulic acid and isoferulic acid had shorter peak time and higher content in this study, which may be attributed to the conversion of chlorogenic acid. Studies have shown that glycyrrhizic acid consists of one molecule of glycyrrhetinic acid and two molecules of glucuronic acid, which are easily lost in vivo by the hydrolysis of two molecules of glucuronic acid to form glycyrrhetinic acid (Du, et al., 2019). In this study, the peak time and residence time of glycyrrhizic acid are short, so the research on its metabolites in vivo needs further exploration.
In this experiment, we detected 33 compounds in rat plasma. Among them, 18 compounds were not detected, probably due to their low concentrations, low oral availability in vivo, or metabolization into other products, which need further study. In summary, a total of 15 major constituents of QJYQ with large plasma exposure were obtained, including baicalin, wogonoside, wogonin, apigenin-7-O-glucuronide, verbenalin, isoferulic acid, hesperidin, glycyrrhizic acid, liquiritin, sinapic acid, ferulic acid, p-Coumaric acid, protocatechuic acid, harpagide and liquiritin apioside. The pharmacokinetic results indicated that these compounds of QJYQ had high absorption concentration, large plasma exposure in vivo, which was beneficial for QJYQ to exert its efficacy.
4 Discussions
The pharmacokinetics of multiple components in plasma after oral administration of QJYQ in rats have not been investigated. In the present study, the pharmacokinetics of 33 components in plasma after oral administration of QJYQ were investigated. Compounds selection principles are as follows: firstly, based on the previous in vitro quantitative study of QJYQ, we found 50 quantifiable components, among which 33 components with high content were selected as indicators (Yang, et al., 2023). Secondly, it has been reported that these compounds can be absorbed into the blood and have high plasma exposure. For example, studies have shown that baicalin, baicalein, wogonoside and wogonin can be well absorbed into blood in the pharmacokinetics study of Scutellariae radix (He, et al., 2018). Some researchers have studied the pharmacokinetics of verbenae herba, showing that verbenalin has a large plasma exposure (Liu, et al., 2019). Finally, those compounds have potential activity, which is helpful to the further pharmacological studies of QJYQ (Yang et al., 2017; Yuan et al., 2018). Based on the above principles, we selected these 33 compounds to explore their processes in vivo.
Most of the reported methods for pharmacokinetic determination of TCMs compound preparations are HPLC or LC-MS/MS (Si, et al., 2008; Wang, et al., 2019). In this study, the established UHPLC-sMRM method has the advantages of low injection volume, short running time and wider linear range compared with the published method (Xing, et al., 2013; Guan, et al., 2017; Zhao, et al., 2019). Compared with the MRM scan mode, sMRM mode can analyze targeted ion pairs in a specific time window, improving the sensitivity of detection (Zhang, et al., 2015; Chen, et al., 2019). All in all, the established UHPLC-sMRM method is more rapid, simple, high selective and sensitive, which is conducive to the accurate quantitative analysis of multiple targets with different concentrations in the complex matrix of TCMs (Si, et al., 2008; Cai, et al., 2016; Wang, et al., 2019; Wu, et al., 2019) (Supplementary Table S3).
5 Conclusion
A rapid and sensitive UHPLC-sMRM was successfully established and validated for 33 components in rat plasma of QJYQ, showing its excellent precision, stability and recovery. Fifteen major constituents including wogonin, baicalin, sinapic acid, ferulic acid, hesperidin, wogonoside, apigenin-7-O-glucuronide, verbenalin, isoferulic acid, liquiritin, glycyrrhizic acid, liquiritin apioside, harpagide, p-Coumaric acid and protocatechuic acid of QJYQ with large plasma exposure were obtained. This experiment preliminarily provides reference for elucidating the pharmacodynamic substance basis, further study of human pharmacokinetics and design of rational drug regimen.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was reviewed and approved by Animal Ethics Committee of Tianjin University of Traditional Chinese Medicine (TCM-LAEC2021228).
Author contributions
XY: Data curation. Investigation, writing-original draft. SC: Data curation. Investigation. KD: Writing—original draft, formal analysis. YS: Software, validation. SF: Writing—review and editing. JL: Writing—review and editing. HZ: Conceptualization, Writing—review and editing. YC: Conceptualization, Funding acquisition, Project administration, Writing—review and editing.
Funding
This research was supported by Science and Technology Program of Tianjin (21ZYJDJC00080), Tianjin Research Innovation Project for Postgraduate Students in China (2021YJSS181) and Postgraduate Research Innovation Program of Tianjin University of Traditional Chinese Medicine in China (YJSKC-20212002).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1155973/full#supplementary-material
References
Cai, Y., Li, S., Li, T., Zhou, R., Wai, A. T., and Yan, R. (2016). Oral pharmacokinetics of baicalin, wogonoside, oroxylin A 7-O-beta-d-glucuronide and their aglycones from an aqueous extract of Scutellariae Radix in the rat. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1026, 124–133. doi:10.1016/j.jchromb.2015.11.049
Chen, Y. Y., Cao, Y. J., Tang, Y. P., Yue, S. J., and Duan, J. A. (2019). Comparative pharmacodynamic, pharmacokinetic and tissue distribution of Dahuang-Gancao decoction in normal and experimental constipation mice. Chin. J. Nat. Med. 17 (11), 871–880. doi:10.1016/S1875-5364(19)30104-9
Dai, P., Zhu, L., Luo, F., Lu, L., Li, Q., Wang, L., et al. (2015). Triple recycling processes impact systemic and local bioavailability of orally administered flavonoids. AAPS J. 17 (3), 723–736. doi:10.1208/s12248-015-9732-x
Du, T., Sun, R., Du, S., Gao, S., Hu, M., Zhang, Y., et al. (2019). Metabolic profiles of Xiao Chai Hu Tang in mouse plasma, bile and urine by the UHPLC-ESI-Q-TOF/MS technique. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1128, 121767. doi:10.1016/j.jchromb.2019.121767
Duya, P. A., Chen, Y., Bai, L., Li, Z., Li, J., Chai, R., et al. (2022). Nature products of traditional Chinese medicine provide new ideas in γδT cell for tumor immunotherapy. Acupunct. Herb. Med. 2 (2), 78–83. doi:10.1097/hm9.0000000000000032
Feng, Z., Zhou, J., Shang, X., Kuang, G., Han, J., Lu, L., et al. (2017). Comparative research on stability of baicalin and baicalein administrated in monomer and total flavonoid fraction form of Radix scutellariae in biological fluids in vitro. Pharm. Biol. 55 (1), 1177–1184. doi:10.1080/13880209.2017.1285321
Guan, H., Wang, X., Wang, S., He, Y., Yue, J., Liao, S., et al. (2017). Comparative intestinal bacteria-associated pharmacokinetics of 16 components of Shengjiang Xiexin decoction between normal rats and rats with irinotecan hydrochloride (CPT-11)-induced gastrointestinal toxicity in vitro using salting-out sample preparation and LC-MS/MS. RSC Adv. 7 (69), 43621–43635. doi:10.1039/c7ra03521g
He, J., Feng, X., Wang, K., Liu, C., and Qiu, F. (2018). Discovery and identification of quality markers of Chinese medicine based on pharmacokinetic analysis. Phytomedicine 44, 182–186. doi:10.1016/j.phymed.2018.02.008
Huang, T., Liu, Y., and Zhang, C. (2019). Pharmacokinetics and bioavailability enhancement of baicalin: A review. Eur. J. Drug Metab. Pharmacokinet. 44 (2), 159–168. doi:10.1007/s13318-018-0509-3
Huang, Y., Xu, L., Zhang, F., Liu, Y., Wang, Y., Meng, F., et al. (2022). Preparation and pharmacokinetics in vivo of linarin solid dispersion and liposome. Chin. Herb. Med. 14 (2), 310–316. doi:10.1016/j.chmed.2021.12.004
Lara-Issasi, G., Salgado, C., Pedraza-Chaverri, J., Medina-Campos, O. N., Morales, A., Aguila, M. A., et al. (2019). Antimicrobial, antioxidant activities, and HPLC determination of the major components of verbena carolina (verbenaceae). Molecules 24 (10), 1970. doi:10.3390/molecules24101970
Li, T., Zhao, M., Zhu, M., Zhang, S., He, J., Pan, H., et al. (2023). Xuanfei baidu decoction, a Chinese herbal medicine for coronavirus disease 2019 (COVID-19): A randomized clinical trial. Acupunct. Herb. Med. 258, 56, doi:10.1097/hm9.0000000000000056
Li, Z., Zhang, X., Liao, J., Fan, X., and Cheng, Y. (2021). An ultra-robust fingerprinting method for quality assessment of traditional Chinese medicine using multiple reaction monitoring mass spectrometry. J. Pharm. Anal. 11 (1), 88–95. doi:10.1016/j.jpha.2020.01.003
Liao, K., Gong, L., Yang, Y., He, Y., Wang, F., Huang, Y., et al. (2022). A comprehensive review of research progress in Chinese medicines for primary liver cancer treatment. Tradit. Med. Res. 7 (2), 10. doi:10.53388/tmr20220207263
Liu, H., Yang, J., Du, F., Gao, X., Ma, X., Huang, Y., et al. (2009). Absorption and disposition of ginsenosides after oral administration of Panax notoginseng extract to rats. Drug. Metab. Dispos. 37 (12), 2290–2298. doi:10.1124/dmd.109.029819
Liu, X., Zhang, H., Xu, J., Gong, S., Han, Y., Zhang, T., et al. (2019). Identification of absorbed components and their metabolites in rat plasma after oral administration of Shufeng Jiedu capsule using ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 33 (19), 1494–1501. doi:10.1002/rcm.8498
Lu, T., Yang, J., Gao, X., Chen, P., Du, F., Sun, Y., et al. (2008). Plasma and urinary tanshinol from Salvia miltiorrhiza (Danshen) can be used as pharmacokinetic markers for cardiotonic pills, a cardiovascular herbal medicine. Drug Metab. Dispos. 36 (8), 1578–1586. doi:10.1124/dmd.108.021592
Luo, C. H., Ma, L. L., Liu, H. M., Liao, W., Xu, R. C., Ci, Z. M., et al. (2020). Research progress on main symptoms of novel coronavirus pneumonia improved by traditional Chinese medicine. Front. Pharmacol. 11, 556885. doi:10.3389/fphar.2020.556885
Pang, W., Yang, F., Zhao, Y., Dai, E., Feng, J., Huang, Y., et al. (2022). Qingjin Yiqi granules for post-COVID-19 condition: A randomized clinical trial. J. Evid. Based Med. 15 (1), 30–38. doi:10.1111/jebm.12465
Si, D., Sun, X., Qi, D., Chen, X., and Bi, K. (2008). Determination and pharmacokinetics of isoferulic acid in rat plasma by high-performance liquid chromatography after oral administration of isoferulic acid and Rhizoma Cimicifugae extract. J. Pharm. Biomed. Anal. 47 (1), 140–145. doi:10.1016/j.jpba.2007.12.044
Tarnawski, M., Depta, K., Grejciun, D., and Szelepin, B. (2006). HPLC determination of phenolic acids and antioxidant activity in concentrated peat extract--a natural immunomodulator. J. Pharm. Biomed. Anal. 41 (1), 182–188. doi:10.1016/j.jpba.2005.11.012
Tian, Y., Zhang, S., Zheng, W., Li, B., Xue, X., Shan, Z., et al. (2022). Qingjin Yiqi granule in the treatment of low fever in convalescence of COVID-19(omicron): A case report. Tianjin J. Tradit. Chin. Med. 39 (06), 692–696.
Wang, F., Shang, Z., Xu, L., Wang, Z., Zhao, W., Mei, X., et al. (2018). Profiling and identification of chlorogenic acid metabolites in rats by ultra-high-performance liquid chromatography coupled with linear ion trap-Orbitrap mass spectrometer. Xenobiotica 48 (6), 605–617. doi:10.1080/00498254.2017.1343963
Wang, L., Shen, X., Mi, L., Jing, J., Gai, S., Liu, X., et al. (2019). Simultaneous determinations of four major bioactive components in Acacia catechu (L.f.) Willd and Scutellaria baicalensis Georgi extracts by LC-MS/MS: Application to its herb-herb interactions based on pharmacokinetic, tissue distribution and excretion studies in rats. Phytomedicine 56, 64–73. doi:10.1016/j.phymed.2018.09.239
Wang, Q. (2021). Hebei has issued a COVID-19 recovery plan that integrates traditional Chinese and Western medicine. J. Tradit. Chin. Med. Manage. 29 (03), 50.
Wang, Q., Zhang, Y. J., Li, W. F., Yang, J., Jiang, H., and Sun, W. J. (2008). Study on stability of baicalin and baicalein in Rat plasma by HPLC-ECD. Zhongguo Zhong Yao Za Zhi 33 (22), 2675–2678.
Wu, Y., Wang, P., Yang, H., and Sui, F. (2019). UPLC-Q-TOF-MS and UPLC-MS/MS methods for metabolism profiles and pharmacokinetics of major compounds in Xuanmai Ganjie Granules. Biomed. Chromatogr. 33 (3), 4449. doi:10.1002/bmc.4449
Xiang, K., He, Q., Chen, Y., Yang, D., Duan, Y., Li, H., et al. (2021). Chemical constituents isolated from the aerial parts of Swertia pseudochinensis and their potential neuroprotective effects. Acupunct. Herb. Med. 1 (1), 59–64. doi:10.1097/hm9.0000000000000010
Xing, Z. h., Peng, W. J., Huang, W., Huang, X., and Liu, W. p. (2013). Analysis of major constituents in fructus aurantii-magnolia Bark decoction by UPLC-PDA. J. Chromatogr. Sci. 52 (8), 826–830. doi:10.1093/chromsci/bmt122
Yang, R., Yuan, B. C., Ma, Y. S., Zhou, S., and Liu, Y. (2017). The anti-inflammatory activity of licorice, a widely used Chinese herb. Pharm. Biol. 55 (1), 5–18. doi:10.1080/13880209.2016.1225775
Yang, X., Wang, S., Qi, L., Chen, S., Du, K., Shang, Y., et al. (2023). An efficient method for qualitation and quantitation of multi-components of the herbal medicine Qingjin Yiqi Granules. J. Pharm. Biomed. Anal. 227, 115288. doi:10.1016/j.jpba.2023.115288
Yuan, Y., Liao, Q., Xue, M., Shi, Y., Rong, L., Song, Z., et al. (2018). Shufeng jiedu capsules alleviate lipopolysaccharide-induced acute lung inflammatory injury via activation of GPR18 by verbenalin. Cell. Physiol. biochem. 50 (2), 629–639. doi:10.1159/000494184
Zhang, F., Zhang, Y., Li, X., Zhang, S., Zhu, M., Du, W., et al. (2018). Research on Q-markers of Qiliqiangxin capsule for chronic heart failure treatment based on pharmacokinetics and pharmacodynamics association. Phytomedicine 44, 220–230. doi:10.1016/j.phymed.2018.03.003
Zhang, Y., Li, Y., Wang, X., Qu, R., Li, J., Li, T., et al. (2020). Herbal plants coordinate COVID-19 in multiple dimensions - an insight analysis for clinically applied remedies. Int. J. Med. Sci. 17 (18), 3125–3145. doi:10.7150/ijms.50260
Zhang, Y., Qian, D., Pan, Y., Zhu, Z., Huang, J., Xi, J., et al. (2015). Comparisons of the pharmacokinetic profile of four bioactive components after oral administration of gan-sui-ban-xia decoction plus-minus gansui and gancao drug combination in normal rats. Molecules 20 (5), 9295–9308. doi:10.3390/molecules20059295
Zhao, X., Yu, L., Chen, Y., Wang, Y., Wan, H., and Yang, J. (2019). Comparative pharmacokinetics of hydrophilic components in Salvia miltiorrhiza Bge And Carthamus tinctorius L In rats that underwent cerebral ischemia reperfusion using an HPLC-DAD method. Front. Pharmacol. 10, 1598. doi:10.3389/fphar.2019.01598
Zou, S., Ge, Y., Chen, X., Li, J., Yang, X., Wang, H., et al. (2019). Simultaneous determination of five alkaloids by HPLC-MS/MS combined with micro-SPE in rat plasma and its application to pharmacokinetics after oral administration of Lotus leaf extract. Front. Pharmacol. 10, 1252. doi:10.3389/fphar.2019.01252
Keywords: Qingjin Yiqi Granules, Pharmacokinetics, COVID-19, UHPLC-sMRM, Traditional Chinese Medicine
Citation: Yang X, Chen S, Du K, Shang Y, Fang S, Li J, Zhang H and Chang Y (2023) Simultaneous determination of multiple components in rat plasma by UHPLC-sMRM for pharmacokinetic studies after oral administration of Qingjin Yiqi Granules. Front. Pharmacol. 14:1155973. doi: 10.3389/fphar.2023.1155973
Received: 01 February 2023; Accepted: 03 April 2023;
Published: 13 April 2023.
Edited by:
Xiaowei Shi, Hebei Medical University, ChinaReviewed by:
Chang-Jiang-Sheng Lai, China Academy of Chinese Medical Sciences, ChinaAn Kang, Nanjing University of Chinese Medicine, China
Copyright © 2023 Yang, Chen, Du, Shang, Fang, Li, Zhang and Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Han Zhang, emhhbmdoYW4wMDIzQDEyNi5jb20=; Yanxu Chang, dGNtY3l4QDEyNi5jb20=
 Xiaohua Yang1,2
Xiaohua Yang1,2 Shiming Fang
Shiming Fang Han Zhang
Han Zhang Yanxu Chang
Yanxu Chang