
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 02 May 2023
Sec. Pharmacology of Anti-Cancer Drugs
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1155163
 Meenakshi Gupta1
Meenakshi Gupta1 Deepti Singh2
Deepti Singh2 Shruti Rastogi1,3
Shruti Rastogi1,3 Hifzur R. Siddique2
Hifzur R. Siddique2 Noura Al-Dayan4
Noura Al-Dayan4 Ajaz Ahmad5
Ajaz Ahmad5 Mohammad Sikander6
Mohammad Sikander6 Maryam Sarwat1*
Maryam Sarwat1*Background: Guggulsterone (pregna-4,17-diene-3,16-dione; C21H28O2) is an effective phytosterol isolated from the gum resin of the tree Commiphora wightii (Family Burseraceae) and is responsible for many of the properties of guggul. This plant is widely used as traditional medicine in Ayurveda and Unani system of medicine. It exhibits several pharmacological activities, such as anti-inflammatory, analgesic, antibacterial, anti-septic and anticancer. In this article, the activities of Guggulsterone against cancerous cells were determined and summarized.
Methods: Using 7 databases (PubMed, PMC, Google Scholar, Science Direct, Scopus, Cochrane and Ctri.gov), the literature search was conducted since conception until June 2021. Extensive literature search yielded 55,280 studies from all the databases. A total of 40 articles were included in the systematic review and of them, 23 articles were included in the meta-analysis.The cancerous cell lines used in the studies were for pancreatic cancer, hepatocellular carcinoma, head and neck squamous cell carcinoma, cholangiocarcinoma, oesophageal adenocarcinoma, prostrate cancer, colon cancer, breast cancer, gut derived adenocarcinoma, gastric cancer, colorectal cancer, bladder cancer, glioblastoma, histiocytic leukemia, acute myeloid leukemia and non-small cell lung cancer. The reliability of the selected studies was assessed using ToxRTool.
Results: Based on this review, guggulsterone significantly affected pancreatic cancer (MiaPaCa-2, Panc-1, PC-Sw, CD18/HPAF, Capan1, PC-3), hepatocellular carcinoma (Hep3B, HepG2, PLC/PRF/5R), head and neck squamous cell carcinoma (SCC4, UM-22b, 1483), cholangiocarcinoma (HuCC-T1, RBE, Sk-ChA-1, Mz-ChA-1) and oesophageal adenocarcinoma (CP-18821, OE19), prostrate cancer (PC-3), colon cancer (HT-29), breast cancer (MCF7/DOX), gut derived adenocarcinoma (Bic-1), gastric cancer (SGC-7901), colorectal cancer (HCT116), bladder cancer (T24, TSGH8301), glioblastoma (A172, U87MG, T98G), histiocytic leukemia (U937), acute myeloid leukemia (HL60, U937) and non-small cell lung cancer (A549, H1975) by inducing apoptotic pathways, inhibiting cell proliferation, and regulating the expression of genes involved in apoptosis. Guggulsterone is known to have therapeutic and preventive effects on various categories of cancers. It can inhibit the progression of tumors and can even reduce their size by inducing apoptosis, exerting anti-angiogenic effects, and modulating various signaling cascades. In vitro studies reveal that Guggulsterone inhibits and suppresses the proliferation of an extensive range of cancer cells by decreasing intrinsic mitochondrial apoptosis, regulating NF-kB/STAT3/β-Catenin/PI3K/Akt/CHOP pathway, modulating the expression of associated genes/proteins, and inhibiting angiogenesis. Furthermore, Guggulsterone reduces the production of inflammatory markers, such as CDX2 and COX-2. The other mechanism of the Guggulsterone activity is the reversal of P-glycoprotein-mediated multidrug resistance. Twenty three studies were selected for meta-analysis following the PRISMA statements. Fixed effect model was used for reporting the odds ratio. The primary endpoint was percentage apoptosis. 11 of 23 studies reported the apoptotic effect at t = 24 h and pooled odds ratio was 3.984 (CI 3.263 to 4.865, p < 0.001). 12 studies used Guggulsterone for t > 24 h and the odds ratio was 11.171 (CI 9.148 to 13.643, 95% CI, p < 0.001). The sub-group analysis based on cancer type, Guggulsterone dose, and treatment effects. Significant alterations in the level of apoptotic markers were reported by Guggulsterone treatment.
Conclusion: This study suggested that Guggulsterone has apoptotic effects against various cancer types. Further investigation of its pharmacological activity and mechanism of action should be conducted. In vivo experiments and clinical trials are required to confirm the anticancer activity.
Cancer is a multi-stage disease characterized by replicative immortality, an aberration in the signaling circuitry, and evasion of the immune system. Risk factors include sex, race, genetics, epigenetics, lifestyle, nutrition, obesity, smoking, and alcohol consumption. Currently, available treatment strategies include radiotherapy, surgical resection, stem cell transplant, hormone therapy, immunotherapy, targeted therapy, and chemotherapy. Management with the current regimen is associated with severe side effects like hair loss, bleeding, bruising, nausea, vomiting, and fatigue and leaves the relevant population without effective therapy. Major limitations of existing strategies are cancer recurrence, poor selectivity towards tumor tissues, and multi-drug resistance against chemotherapeutic agents. These limitations restrain the use of chemotherapeutic drugs and impair the patient’s quality of life (Abdulridha et al., 2020). Provided that the cases of cancer are on the rise and the treatment is expensive and less effective, it is decisive to explore economically viable and potent methods for the patients. The need for the identification of novel and promising anticancer agents having better efficacy and lesser side effects continues.
In recent times, herbal medicines have unraveled pleiotropic effects in the treatment of various diseases and have gained enormous attention. Extraction of these phytoconstituents and gaining detailed information about the performance of these compounds for the management of different types of cancer and various other diseases has become the center of attraction among researchers. Amongst the wide range of medicinal herbs, Guggulsterone (pregna-4,17-diene-3,16-dione; C21H28O2) is an effective phytosterol isolated from the gum resin of the tree Commiphora wightii and is responsible for many of the properties of guggul (Figure 1). Guggulsterone has been proven to be an antagonist ligand for the farnesoid X receptor (FXR) and to suppress the expression of FXR agonist-induced genes (Bhat et al., 2017).
FXR is a bile acid nuclear receptor that regulates the expression of key genes involved in bile acid and cholesterol homeostasis. FXR inhibits the expression of cholesterol 7-hydroxylase (Cyp7a1), sterol 12-hydroxylase, the Na/taurocholate co-transporting polypeptide, and apolipoprotein A-I, while activating the expression of intestinal bile acid-binding protein (I-BABP), bile salt export pump (BSEP), apolipoprotein C-II, phospholipid transfer protein, and dehydroepiandrosterone sulfotransferase. Guggulsterone also have a significant role in nutritional metabolism by inhibiting cholesterol production in the liver via FXR and bile-acid receptor antagonism (Chiang et al., 1990; Owsley and Chiang, 2003). Guggulsterone effectively lowers low density lipoprotein cholesterol and triglyceride levels in the blood while increasing high density lipoprotein cholesterol levels. The cholesterol homeostasis regulating, anti-hyperlipidemic and nutritional metabolism maintaining properties of guggulsterone are attributable to the inhibition of FXR (Kunnumakkara et al., 2018). Increased FXR expression has been reported to positively influence cancer cell proliferation and tumour development in non-small cell lung cancer (NSCLC), which may include the activation of multiple oncogenes such as cyclin D1. FXR, on the other hand, works as a tumour suppressor in intestinal tumours, and its lack of expression leads to accelerated tumour development. It implies that FXR has a dual role as a proto-oncogene or tumour suppressor gene depending on its tissue function. The FXR protein is also implicated in the regulation of other molecules, including TNF-α, p21, Bcl-2, nuclear factor kappa-B (NF-κB), and other pro-inflammatory cytokines. Guggulsterone is known to have therapeutic and preventive effects on various categories of cancers. It can inhibit the progression of tumors and can even reduce their size by inducing apoptosis, exerting anti-angiogenic effects, and modulating various signaling cascades (Bhat et al., 2017; Kunnumakkara et al., 2018). In vitro studies reveal that Guggulsterone inhibits and suppresses the proliferation of an extensive range of cancer cells by decreasing intrinsic mitochondrial apoptosis, regulating NF-κB/STAT3/β-Catenin/PI3K/Akt/CHOP pathway, modulating the expression of associated genes/proteins, and inhibiting angiogenesis. Furthermore, Guggulsterone reduces the production of inflammatory markers, such as CDX2 and COX-2 (Figure 2). The other mechanism of the Guggulsterone activity is the reversal of P-glycoprotein-mediated multidrug resistance (Bhat et al., 2017; Kunnumakkara et al., 2018).
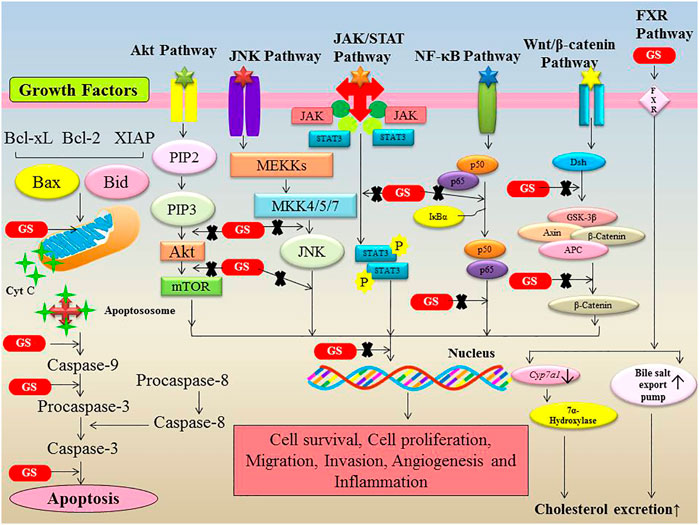
FIGURE 2. Modulation of various signalling pathways involved in cancer by Guggulsterone. Guggulsterone modulates the expression of numerous proteins involved in cell survival, cell proliferation, migration, invasion, angiogenesis and inflammation.
Another nuclear receptor, liver-X-receptor-α (LXR-α), play a role in cholesterol homeostasis, and LXR-α activation has an anti-inflammatory impact by suppressing NF-κB-mediated signalling. Although FXRs respond to bile acids and LXRs to oxysterol molecules within the cellular nucleus, their coordinated ligand-specific activities stimulate transcription and change the expression patterns of numerous genes. Genes, in particular, are in charge of cholesterol, lipid, bile acid, and glucose metabolism, as well as general liver function (Shiragannavar et al., 2023).
Nowadays, the anti-cancer effect of Guggulsterone is a matter of interest in experimental research. However, there is very less information that suggests the use of Guggulsterone for pre-clinical and clinical studies. Systematic reviews and meta-analysis can therefore help clarify whether this molecule could be beneficial in the management of various cancer types and decide if Guggulsterone could be explored further on animal and clinical grounds. To date, no study has systematically synthesized nor critically appraised the original research studies that have explored the impact of Guggulsterone in cancer cell growth and metastasis. Therefore, the present study was constructed to investigate the therapeutic effect of Guggulsterone in the management of various cancers in studies done on various cancer cell lines.
The review followed the recommendations in the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) Statement. The literature search was conducted until June 2021. The following databases were searched: PubMed, PMC, Google Scholar, Science Direct, Scopus, Cochrane and Ctri.gov. For the title or abstract, the following keywords were used: sets “Guggulsterone”, “Commiphora mukul”, and “Commiphora wightii”. The following strings were also used in combination to ensure maximum capture of the literature “HCC”, “Hepatocellular carcinoma”, “Liver cancer”, “Cancer”, “Cirrhosis”, and “Fibrosis”. The bibliographic reference list of eligible studies was also checked. The final search result from each database was limited to the studies published in the English language.
Inclusion and exclusion criteria were identified according to the Population, Intervention, Control, and Outcomes (PICO) principle. We included the in vitro studies where Guggulsterone was tested against a control arm (vehicle). There was no limitation on the type of cancer, dosage of Guggulsterone, or duration of treatment. Based on the title, abstracts, and, full manuscripts, the data extraction, and quality assessment were done by the independent reviewers (MG and DS). The studies were based on the following criteria: i) Guggulsterone used as a major intervention, ii) as an anti-cancer therapeutic, and iii) focusing on the effect on apoptosis and apoptotic pathways. The following studies were excluded: i) papers focused on studies other than cancer; ii) in silico studies; iii) human studies; iv) in vivo studies v) review articles, vi) studies having inadequate information; vii) studies in which Guggulsterone was not used as a major intervention and viii) studies not published in English.
All the experimental groups treated with different doses of Guggulsterone and demonstrating an increase in the percentage of apoptotic cells as compared to control were considered. In addition, the treated group also revealed alteration in the expression of apoptotic markers such as Bcl-2, Bcl-xL, Mcl-1, Bax, Bad, Bak, Bid, Fas, cIap-1/2, Survivin, and Caspase-3/8/9 were considered. If different doses of Guggulsterone were used in the study, the highest dose was chosen for performing a meta-analysis.
Data from the included studies were extracted independently by two authors (MG and DS) and cross-checked to avoid any discrepancies with a third author (SR). Other authors verified the same and gave the final confirmation. Studies that assess the therapeutic effects of Guggulsterone against various cancer types were included in the review. The following data were extracted: author’s name, year of publication, cancer type, study design, the cell line used, type of intervention, dosage, duration, genes upregulated or downregulated, and the pathway of action. To ensure consistency, all the data was summarized in a structured table.
Two independent reviewers (MG and DS) performed the quality assessment of studies using the in-vitro ToxRTool. This tool provides a detailed and transparent evaluation of the ecotoxicity data. Individual quality items were examined using the 5 criteria groups: 1) test substance identification, (2) test system characterization, (3) study design description, (4) study results documentation, and (5) plausibility of study design and data. A score of 0 (‘no-not met’) or 1 (‘yes-met’) was assigned to the sub-criteria by two reviewers (MG and DS) while evaluating the study. A combined score was calculated for each rater and each study, by summing the scores of the above-mentioned criteria of each selected study. The following approach was used to grade the quality of each study as category 1 (Score15-18; reliable without restrictions), category 2 (Score11-14; reliable with restrictions), and category 3 (score <11; not reliable).
For inferential purposes, a fixed-effect model was used. Standardized mean difference (SMD) was chosen for consolidating the statistical data. The data of each study was analyzed using the odds ratio (OR) and 95% confidence interval. Forest plots and the I2 index were calculated to detect heterogeneity. Thresholds of I2 were in line with Cochrane recommendations: 0%–40% (“might not be important”), 30%–60% (“may represent moderate heterogeneity”), 50%–90% (“may represent substantial heterogeneity”), and 75%–100% (“considerable heterogeneity”). The importance of the I2 value was interpreted alongside the p-value from the Chi-squared test. Outcome analysis was performed based on the exposure duration (24 h and >24 h). A funnel plot was used to assess the presence of publication bias for small-study effects. The statistical significance (p < 0.05) is based on the data provided in the original publications. MedCalc Version 19.6.1 software was used to perform all the computations.
Search results were based on the different combinations of keywords (“Guggulsterone”, “Commiphora mukul”, and “Commiphora wightii” in combination with “HCC”, “Hepatocellular carcinoma”, “Liver cancer”, “Cancer”, “Cirrhosis”, and “Fibrosis”). The search results fetched 55,280 records from seven different databases viz. 1675 from Pubmed, 8487 from PMC, 38 from Cochrane library, 4 from ctri.gov.in, 40185 from Google Scholar, 3486 from Science Direct and 1405 from Scopus.
Out of the 55,280 articles, the 716 articles obtained from the keyword “Guggulsterone and Cancer” were used further for the study, and the remaining articles were excluded. A manual bibliographic search was also done and 138 articles were screened through it. The resulting 854 articles (716 + 138) were later subjected to duplicate exclusion. 4 duplicates were found and excluded and the remaining 850 articles were subjected to title and abstract screening. Out of the 850 articles, 725 articles were found to be irrelevant to the subject in focus and did not fulfill the inclusion criteria and were thus, excluded. The remaining 125 articles were assessed for eligibility, and 87 of them were found to be eligible for analysis. From a pool of 87 articles, 9 articles reported in vivo studies (Supplementary Table S1), 38 studies were based on the use of Guggulsterone only as an FXR antagonist, and 40 studies were based on the therapeutic implications of Guggulsterone in various cancer cells lines. The in vivo studies and studies using Guggulsterone as an FXR antagonist were excluded. Out of the 40 remaining studies, 17 studies were again excluded as they differ from the objective and only 23 articles were included in the study as they met the following inclusion criteria: i) apoptosis induced by Guggulsterone tested in vitro and/or ii) Guggulsterone-induced cell cycle arrest. The articles providing a detailed description of the experimental design and findings were finally included in the quantitative analysis.
All of the articles reported the protective role of Guggulsterone against various cancer types in vitro at different doses and durations. The PRISMA flow diagram describing the search strategy and study selection process is available in Figure 3.
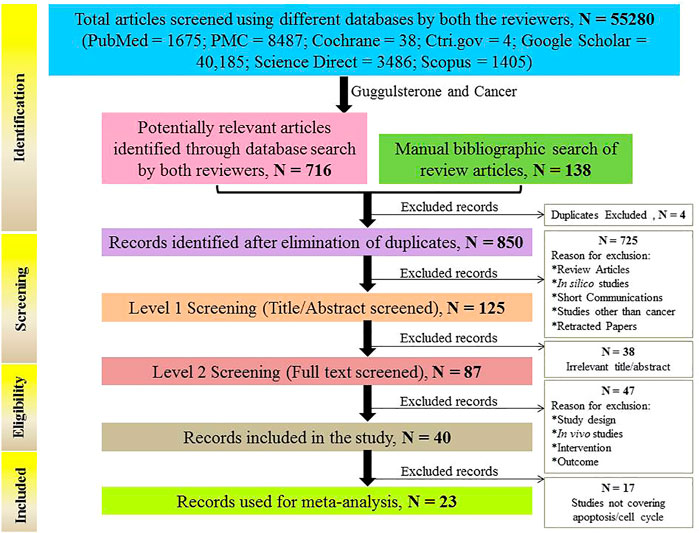
FIGURE 3. Flow chart explaining the selection process of studies (in vitro) included in the meta-analysis.
The selected studies were classified based on the i) type of cancer, ii) cell lines used iii) dose of Guggulsterone used in the study and duration of its treatment, iv) type of assay conducted to determine the apoptotic effect v) outcomes and pathway of drug action. The main characteristics of the studies included in the meta-analysis are summarised in Table 1. All the selected studies reported the apoptotic effect of Guggulsterone in various cancer cells at different time points and doses.
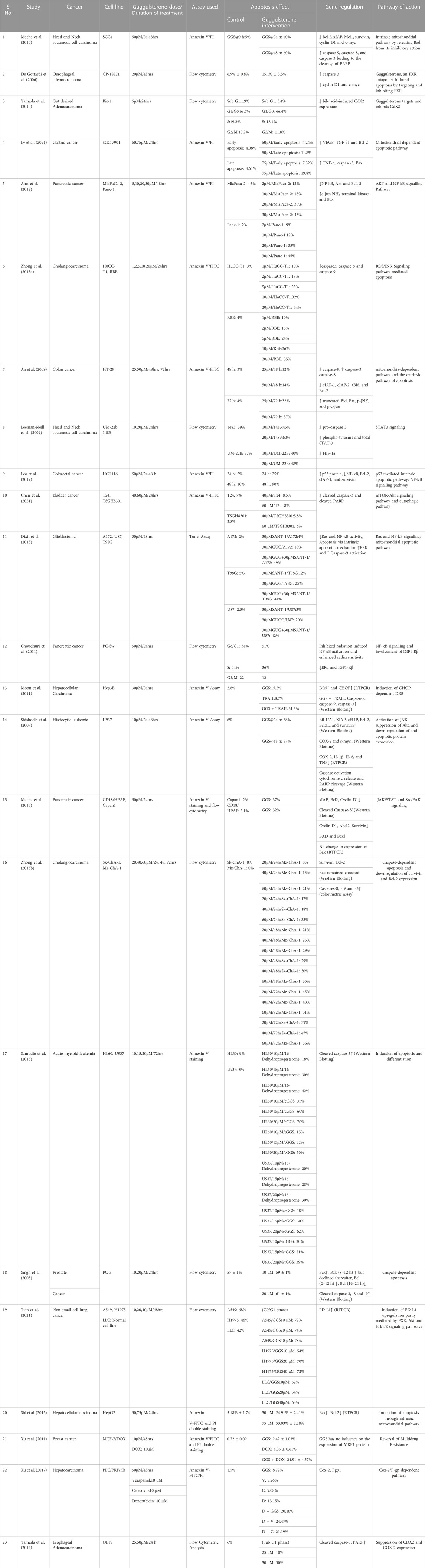
TABLE 1. Main characteristics of the 23 studies included in the meta-analysis related to the role of Guggulsterone and apoptosis induction in cancer cells.
The selected 23 studies reporting the apoptotic effect of Guggulsterone in various cancer cell lines were eligible for meta-analysis. The percentage apoptosis compared to control was used as the primary outcome measure. The studies were grouped into 2 sub-sets based on the time point viz. 24 h and >24 h. In the first subset, the studies with the therapeutic role of Guggulsterone in the induction of apoptosis in cells treated for 24 h duration as compared to untreated cells were considered. Among the 23 studies, 11 studies reported the apoptotic effect of Guggulsterone at 24 h (Singh et al., 2005; Leeman-Neill et al., 2009; Yamada et al., 2010; Choudhuri et al., 2011; Moon et al., 2011; Macha et al., 2013; Yamada et al., 2014; Shi et al., 2015; Zhong et al., 2015a; Chen et al., 2021; Lv et al., 2021). The second subset included the Guggulsterone-induced apoptosis in the treated cancer cells for >24 h. 7 of 23 studies reported the apoptotic effect of Guggulsterone at t > 24 h i.e. either at 48 h or 72 h. 6 of these 7 studies reported the effect of Guggulsterone at 48 h (De Gottardi et al., 2006; Xu et al., 2011; Ahn et al., 2012; Dixit et al., 2013; Xu et al., 2017; Tian et al., 2021) and 1 study used the treatment for 72 h (Samudio et al., 2015). 5 of 23 studies reported the apoptotic effect at multiple time points i.e. at 24 and 48 h (Shishodia et al., 2007; Macha et al., 2010; Leo et al., 2019), at 48 and 72 h (An et al., 2009), and at 24, 48 and 72 h (Zhong et al., 2015b).
Among the 23 studies, there were 11 studies for this outcome. 4 of the 11 studies reported the apoptotic effect in more than 1 cell line (Leeman-Neill et al., 2009; Macha et al., 2013; Zhong et al., 2015a; Chen et al., 2021). The pooled OR for the fixed model effect was 3.984 (CI 3.263 to 4.865, p < 0.001). Figure 4 describes the results from the fixed-effects model combining the OR for the apoptotic effect of Guggulsterone exposed for 24 h treatment.
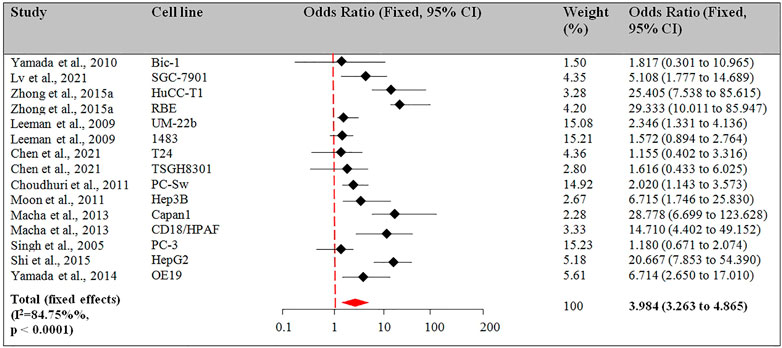
FIGURE 4. Guggulsterone (t = 24 h) versus control odds ratio in cancer cells. Fixed effects meta-analysis was performed for the outcomes to compare data for Guggulsterone (t = 24 h) versus control and the odds ratio was determined.
Figure 5 describes the results from the fixed-effects model combining the OR for the apoptotic effect of Guggulsterone exposed for a time >24 h. There were 12 studies for this outcome. Among these 12 studies, 5 studies used multiple cell lines to report the apoptotic effect of Guggulsterone (Ahn et al., 2012; Dixit et al., 2013; Samudio et al., 2015; Zhong et al., 2015b; Tian et al., 2021). The OR for the association varied from 9.148 to 13.643 across studies. Overall, the combined OR showed significant apoptosis in the cancer cells treated with Guggulsterone as compared to the control (OR: 11.171, 95% CI, p < 0.001).
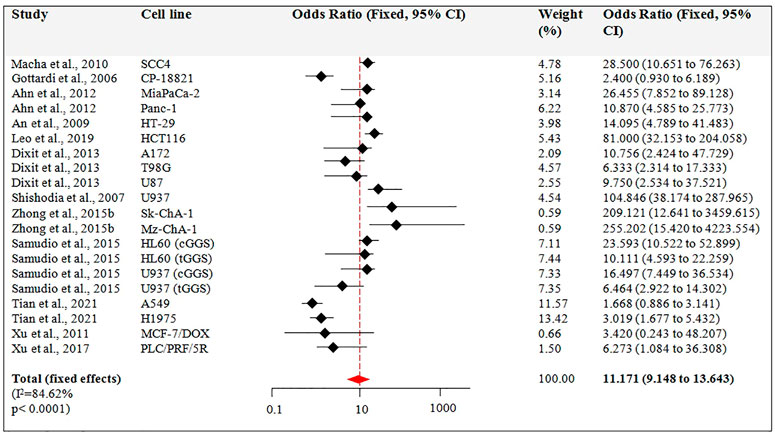
FIGURE 5. Guggulsterone (t > 24 h) versus control odds ratio in cancer cells. Fixed effects meta-analysis was performed for the outcomes to compare data for Guggulsterone (t > 24 h) versus control and the odds ratio was determined.
The included studies involved the effect of Guggulsterone in the treatment of various cancers. Three studies were on pancreatic cancer (MiaPaCa-2, Panc-1, PC-Sw, CD18/HPAF, Capan1, PC-3) (Choudhuri et al., 2011; Ahn et al., 2012; Macha et al., 2013), three on hepatocellular carcinoma (Hep3B, HepG2, PLC/PRF/5R) (Moon et al., 2011; Xu et al., 2011; Shi et al., 2015), two studies each on head and neck squamous cell carcinoma (SCC4, UM-22b, 1483) (Leeman-Neill et al., 2009; Macha et al., 2010), cholangiocarcinoma (HuCC-T1, RBE, Sk-ChA-1, Mz-ChA-1) (Zhong et al., 2015a; Zhong et al., 2015b) and oesophageal adenocarcinoma (CP-18821, OE19) (De Gottardi et al., 2006; Yamada et al., 2010) and one study each on prostrate cancer (PC-3) (Singh et al., 2005), colon cancer (HT-29) (An et al., 2009), breast cancer (MCF7/DOX) (Xu et al., 2011), gut derived adenocarcinoma (Bic-1) (Yamada et al., 2010), gastric cancer (SGC-7901) (Lv et al., 2021), colorectal cancer (HCT116) (Leo et al., 2019), bladder cancer (T24, TSGH8301) (Chen et al., 2021), glioblastoma (A172, U87MG, T98G) (Dixit et al., 2013), histiocytic leukemia (U937) (Shishodia et al., 2007), acute myeloid leukemia (HL60, U937) (Samudio et al., 2015) and non-small cell lung cancer (A549, H1975) (Tian et al., 2021). All the studies reported that Guggulsterone exerts its anticancer effects by inducing apoptotic pathways, inhibiting cell proliferation, and regulating the expression of genes involved in apoptosis. Figure 6 shows the graphical representation of this data.
8 out of 23 studies used Guggulsterone at a dose greater than 20 μM (Singh et al., 2005; De Gottardi et al., 2006; Shishodia et al., 2007; Leeman-Neill et al., 2009; Yamada et al., 2010; Xu et al., 2011; Samudio et al., 2015; Zhong et al., 2015b). The highest doses used were 75 μM (n = 2) (Shi et al., 2015; Lv et al., 2021), 60 μM (n = 2) (Zhong et al., 2015b; Chen et al., 2021), 50 μM (n = 7) (An et al., 2009; Macha et al., 2010; Choudhuri et al., 2011; Macha et al., 2013; Yamada et al., 2014; Xu et al., 2017; Leo et al., 2019), 40 μM (n = 1) (Tian et al., 2021) and 30 μM (n = 3) (Moon et al., 2011; Dixit et al., 2013; Tian et al., 2021). 20μM concentration of Guggulsterone was used in 5 studies (Singh et al., 2005; De Gottardi et al., 2006; Leeman-Neill et al., 2009; Zhong et al., 2015a; Samudio et al., 2015) and 3 studies used the intervention at a dose less than 20 μM (Shishodia et al., 2007; Macha et al., 2010; Yamada et al., 2010). The doses used were 10 μM (n = 2) (Shishodia et al., 2007; Macha et al., 2010) and 5 μM (n = 1) (Yamada et al., 2010). The data has been graphically presented in Figure 7A.
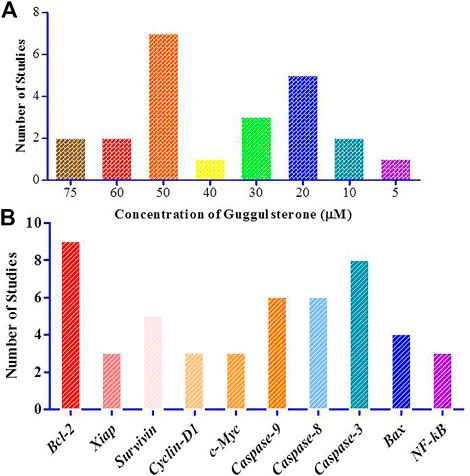
FIGURE 7. Graphical representation of the study characteristics and the observations (A). Concentration of Guggulsterone used for the treatment of the various cancer in vitro in selected 23 studies; (B). Number of studies that have shown the modulation of these major genes.
Treatment of these cell lines with Guggulsterone brought alterations in the status of cancer-critical genes. Significant upregulation in the level of caspase-9 was discussed in 7 studies (Shishodia et al., 2007; An et al., 2009; Macha et al., 2010; Moon et al., 2011; Dixit et al., 2013; Zhong et al., 2015a; Zhong et al., 2015b). Further, 5 studies reported the upregulation of caspase 8 (Shishodia et al., 2007; Macha et al., 2010; Moon et al., 2011; Zhong et al., 2015a; Zhong et al., 2015b) and 8 studies discussed the increased expression of caspase-3 (De Gottardi et al., 2006; Shishodia et al., 2007; Macha et al., 2010; Moon et al., 2011; Samudio et al., 2015; Zhong et al., 2015a; Zhong et al., 2015b; Lv et al., 2021). 4 studies reported the upregulation of Bax as observed and analyzed by qRTPCR and western blotting (Singh et al., 2005; Ahn et al., 2012; Shi et al., 2015; Lv et al., 2021). In cancer cells, Guggulsterone decreased the expression of Bcl-2 (n = 9) (Singh et al., 2005; Shishodia et al., 2007; An et al., 2009; Yamada et al., 2010; Ahn et al., 2012; Macha et al., 2013; Zhong et al., 2015b; Shi et al., 2015; Leo et al., 2019), xiAP (n = 3) (Shishodia et al., 2007; Macha et al., 2010; Macha et al., 2013), survivin (n = 5) (Shishodia et al., 2007; Macha et al., 2010; Macha et al., 2013; Leo et al., 2019; Zhong et al., 2015b), cyclin D (n = 3) (De Gottardi et al., 2006; Yamada et al., 2010; Macha et al., 2013), c-myc (n = 3) (De Gottardi et al., 2006; Yamada et al., 2010; Macha et al., 2013) and NF-κβ (n = 3) (Ahn et al., 2012; Dixit et al., 2013; Leo et al., 2019). The levels of JNK, ciAP-1, cleaved caspase 3, and cox-2 were also altered when subjected to Guggulsterone treatment (Figure 7B).
The scale of heterogeneity not imputable to the sampling error was determined by the I2 value. When the cells were exposed to the treatment for 24 h, the total amount of heterogeneity was considerable (I2 = 84.75%, p < 0.0001). Treatment with Guggulsterone for time >24 h also showed considerable heterogeneity (I2 = 84.62%, p < 0.0001). Visual inspection of the funnel plot showed some asymmetry in both cases (Supplementary Figure S1A and S1B). Supplementary Table S2 summarizes the ToxR reliability assessment score for the individual in vitro toxicity studies. Of the 23 evaluated studies, all the studies were found to be “Reliable Without Restriction”. Even, checking the weighted scores (red scores) did not revise the results.
Guggulsterone is a plant sterol present in the gum resin of the Commiphora species and is known for its pleiotropic effects in the treatment of multiple human diseases (Owsley and Chiang, 2003; Bhat et al., 2017; Kunnumakkara et al., 2018). It has been found to induce apoptosis in many cancer types via the modulation of apoptotic proteins and survival signaling pathways (Lv et al., 2021). In the present study, we summarized all (until June 2021) in vitro experiments investigating the apoptotic effect of Guggulsterone on cancer cells. In total, 23 in vitro studies were of interest and were thoroughly analyzed. Of these 23 studies, 8 studies used “guggulsterone” and 12 studies used “z-guggulsterone” for their research. However, the remaining 3 studies used both the forms of guggulsterone: one study used both compounds in all the tests conducted (Samudio et al., 2015), one used an even mixture (Leeman-Neill et al., 2009), and in the third study, both form were initially used for comparsion and based upon the results, “e” form was selected for further research (Chen et al., 2021). 23 studies included multiple cancer types, cell lines, the dose of Guggulsterone and time points, regulation of various genes, and different pathways of action thereby enhancing the relevance of these results.
Our meta-analysis confirms and strengthens previous evidence that Guggulsterone (5–75 μM) is effective in inducing apoptosis in cancer cell lines. In particular, cancer cells treated with Guggulsterone for 24 h showed an odds ratio of 3.984 (CI 3.263 to 4.865, p < 0.001) compared to the control. When cells were exposed to different concentrations of Guggulsterone for t˃24h, the odds ratio reported was 11.171 (CI 9.148 to 13.643, p < 0.001). This shows that Guggulsterone induces apoptosis in a time-dependent manner and the results are consistent with the previous reports (Shishodia et al., 2007; An et al., 2009; Zhong et al., 2015b).
Bioactive compounds have several action mechanisms involved in the induction of cancer cell apoptosis. In tumor cells, they function via modulating the extrinsic and intrinsic pathways of apoptosis, channeling important cell signaling pathways such as mTOR-AKT, Ras/NF-κβ, ROS/JNK, JAK/STAT, and Src/Fak and regulating gene expression (Huang et al., 2018; Gupta et al., 2021; Gupta et al., 2022a; Gupta et al., 2022b; Gupta et al., 2023). Treatment with Guggulsterone also altered the expression of different gene families which collaborate in the induction of apoptosis. For instance, in the present study, the levels of the members of the caspase family, the conserved cysteine aspartic-specific proteases which are considered crucial in apoptosis induction (Brentnall et al., 2013), were found to be increased. Significant upregulation in the level of caspase-9, the initiator caspase important in the formation of apoptosome complex in the mitochondrial pathway of apoptosis (Li et al., 2017), was observed. Further, caspase-8 and caspase-3 were also found to be upregulated in the studies. Mechanistically, caspase 8 either directly induces the apoptotic pathway or activates caspase-3. Another pro-apoptotic protein Bax (a BCL-2 family protein) was found to be upregulated in the analyzed studies. Bax is involved in the mitochondrial pathway of apoptosis and stimulates the release of cytochrome c from the mitochondria thereby promoting the apoptosis of cancer cells (Shi et al., 2015; Lv et al., 2021). Further, in the cancer cells, Guggulsterone decreased the expression of anti-apoptotic protein thereby leading to increased apoptosis viz. Bcl-2; xiAP (apoptotic inhibitor). Survivin, another identified mammalian inhibitor of apoptosis, was found to be decreased in a few studies. A reduction in the expression of genes and proteins involved in cancer cell proliferation was also observed in various studies (cyclin D1, c-myc, NF-κβ). The levels of other genes involved in the apoptotic pathways viz. JNK, ciAP-1, cleaved caspase 3, and cox-2 were also altered when subjected to Guggulsterone treatment.
Our study, being the first of its kind, is characterized by strengths like extensive literature search and meta-analysis, and highlighting promising results for Guggulsterone, it is plagued with shortcomings as well. Above all is the high heterogeneity between the studies, which is probably due to a limited number of studies (leading to multiple cancer types and cells, different apoptotic assays, and different study designs). Although all the databases are screened expansively and carefully, still the studies on a particular cancer type are very less. Therefore, a generalizable interpretation was affected by high study variability. In general, to overcome heterogeneity, it is advised to explore the heterogeneity by conducting subgroup analyses, changing the effect measure, and excluding studies from the meta-analysis based on their results. Next, the funnel plot revealed publication bias, which is also due to the limited number of articles and therefore, suggests more quality research papers on this molecule.
Meta-analysis and systematic review of basic research offer collective and critical insights into the current state of knowledge in the respective field. In this manuscript, we have used a meta-analytic approach on in vitro studies to evaluate the apoptotic effects of Guggulsterone against various cancer types and to the best of our knowledge, it is the first meta-analysis of this kind. Our current findings are promising and highlight the importance of Guggulsterone in cancer management, which may direct future research. However, data is still insufficient to establish the effect of Guggulsterone on various cancer types. The statistical evaluation shows considerable heterogeneity and publication bias among the studies, which, however, does not invalidate the efficacy of Guggulsterone as there is a significant difference in the treated and control groups. Existing studies establish the role of Guggulsterone as a potential candidate; alongside, provide a rationale for further pre-clinical and clinical evaluation to develop this molecule as an anticancer agent. Additionally, the isolation and chemical synthesis of guggulsterone is an exorbitant process, which might be the reason that a limited number of studies are utilizing it. Therefore, more research should be done for the economical chemical synthesis of this very important molecule.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Conceptualization and methodology MG, SR, NAD, and MSa; literature search, data extraction and study selection: MG and DS; data analysis MG and DS; writing—original draft preparation, MG and DS; writing—review and editing SR, HS, DS, NAD, AA, MSi, and MSa. All authors have read and agreed to the published version of the manuscript.
This work was supported by a grant from the Central Council for Research in Unani Medicine (CCRUM), New Delhi, India (Grant No. 3–31/2014-ccrum/Tech). The authors would like to acknowledge Deanship of scientific research Grant (No. 3411/03/2015), Prince Sattam Bin Abdulaziz University, Al-Kharj, Saudi Arabia.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1155163/full#supplementary-material
Abdulridha, M. K., Al-Marzoqi, A. H., Al-Awsi, G. R., Mubarak, S. M., Heidarifard, M., and Ghasemian, A. (2020). Anticancer effects of herbal medicine compounds and novel formulations: A literature review. J. Gastrointest. Cancer 5, 765–773. doi:10.1007/s12029-020-00385-0
Ahn, D. W., Seo, J. K., Lee, S. H., Hwang, J. H., Lee, J. K., Ryu, J. K., et al. (2012). Enhanced antitumor effect of combination therapy with gemcitabine and guggulsterone in pancreatic cancer. Pancreas 41, 1048–1057. doi:10.1097/MPA.0b013e318249d62e
An, M. J., Cheon, J. H., Kim, S. W., Kim, E. S., Kim, T. I., and Kim, W. H. (2009). Guggulsterone induces apoptosis in colon cancer cells and inhibits tumor growth in murine colorectal cancer xenografts. Cancer Lett. 279, 93–100. doi:10.1016/j.canlet.2009.01.026
Bhat, A. A., Prabhu, K. S., Kuttikrishnan, S., Krishnankutty, R., Babu, J., Mohammad, R. M., et al. (2017). Potential therapeutic targets of Guggulsterone in cancer. Nutr. Metab. (Lond) 14, 23–11. doi:10.1186/s12986-017-0180-8
Brentnall, M., Rodriguez-Menocal, L., De Guevara, R. L., Cepero, E., and Boise, L. H. (2013). Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 14, 32–39. doi:10.1186/1471-2121-14-32
Chen, Y., Wang, H. H., Chang, H. H., Huang, Y. H., Wang, J. R., Changchien, C. Y., et al. (2021). Guggulsterone induces apoptosis and inhibits lysosomal-dependent migration in human bladder cancer cells. Phytomedicine 87, 153587. doi:10.1016/j.phymed.2021.153587
Chiang, J. Y., Miller, W. F., and Lin, G. M. (1990). Regulation of cholesterol 7 alpha-hydroxylase in the liver. Purification of cholesterol 7 alpha-hydroxylase and the immunochemical evidence for the induction of cholesterol 7 alpha-hydroxylase by cholestyramine and circadian rhythm. J. Biol. Chem. 265, 3889–3897. doi:10.1016/S0021-9258(19)39677-2
Choudhuri, R., DeGraff, W., Gamson, J., Mitchell, J. B., and Cook, J. A. (2011). Guggulsterone-mediated enhancement of radiosensitivity in human tumor cell lines. Front. Oncol. 1, 19. doi:10.3389/fonc.2011.00019
De Gottardi, A., Dumonceau, J. M., Bruttin, F., Vonlaufen, A., Morard, I., Spahr, L., et al. (2006). Expression of the bile acid receptor FXR in Barrett's esophagus and enhancement of apoptosis by guggulsterone in vitro. Mol. Cancer 5, 48–10. doi:10.1186/1476-4598-5-48
Dixit, D., Ghildiyal, R., Anto, N. P., Ghosh, S., Sharma, V., and Sen, E. (2013). Guggulsterone sensitizes glioblastoma cells to Sonic hedgehog inhibitor SANT-1 induced apoptosis in a Ras/NFκB dependent manner. Cancer Lett. 336, 347–358. doi:10.1016/j.canlet.2013.03.025
Gupta, M., Yadav, V., and Sarwat, M. (2021). “Pharmacological importance of the active molecule ‘guggulsterone’ in overall human health,” in Herbal medicines. Editors M. Sarwat, and H. Siddique (Academic Press: Elsevier), 191–206.
Gupta, M., Chandan, K., and Sarwat, M. (2022a). Natural products and their derivatives as immune check point inhibitors: Targeting cytokine/chemokine signalling in cancer. Semin. Cancer Biol. 86, 214–232. Academic Press. doi:10.1016/j.semcancer.2022.06.009
Gupta, M., Ghufran, M. S., Kausar, T., Ali, R., Biswas, S., Nayeem, S., et al. (2022b). Z-guggulsterone is a potential lead molecule of dawa-ul-kurkum against hepatocellular carcinoma. Molecules 27, 5104. doi:10.3390/molecules27165104
Gupta, M., Sumaiya, S., Ali, S., Naved, T., Sharma, A., Ahmad, A., et al. (2023). Pharmacognostical and phytochemical evaluation of a Unani polyherbal formulation: Dawa ul kurkum by HPTLC. Separations 10, 89. doi:10.3390/separations10020089
Huang, Y., Zhang, J., Wang, G., Chen, X., Zhang, R., Liu, H., et al. (2018). Oxymatrine exhibits anti-tumor activity in gastric cancer through inhibition of IL-21R-mediated JAK2/STAT3 pathway. Int. J. Immunopathol. Pharmacol. 32, 2058738418781634. doi:10.1177/2058738418781634
Kunnumakkara, A. B., Banik, K., Bordoloi, D., Harsha, C., Sailo, B. L., Padmavathi, G., et al. (2018). Googling the guggul (Commiphora and boswellia) for prevention of chronic diseases. Front. Pharmacol. 9, 686. doi:10.3389/fphar.2018.00686
Leeman-Neill, R. J., Wheeler, S. E., Singh, S. V., Thomas, S. M., Seethala, R. R., Neill, D. B., et al. (2009). Guggulsterone enhances head and neck cancer therapies via inhibition of signal transducer and activator of transcription-3. Carcinogenesis 30, 1848–1856. doi:10.1093/carcin/bgp211
Leo, R., Therachiyil, L., Siveen, S. K., Uddin, S., Kulinski, M., Buddenkotte, J., et al. (2019). Protein expression profiling identifies key proteins and pathways involved in growth inhibitory effects exerted by guggulsterone in human colorectal cancer cells. Cancers 11, 1478. doi:10.3390/cancers11101478
Li, P., Zhou, L., Zhao, T., Liu, X., Zhang, P., Liu, Y., et al. (2017). Caspase-9: Structure, mechanisms and clinical application. Oncotarget 8, 23996–24008. doi:10.18632/oncotarget.15098
Lv, R., Zhu, M., Chen, K., Xie, H., Bai, H., and Chen, Q. (2021). Z-guggulsterone induces apoptosis in gastric cancer cells through the intrinsic mitochondria-dependent pathway. Sci. World J. 2021, 3152304. doi:10.1155/2021/3152304
Macha, M. A., Matta, A., Chauhan, S. S., Siu, K. M., and Ralhan, R. (2010). 14-3-3 zeta is a molecular target in guggulsterone induced apoptosis in head and neck cancer cells. BMC cancer 10, 655–662. doi:10.1186/1471-2407-10-655
Macha, M. A., Rachagani, S., Gupta, S., Pai, P., Ponnusamy, M. P., Batra, S. K., et al. (2013). Guggulsterone decreases proliferation and metastatic behavior of pancreatic cancer cells by modulating JAK/STAT and Src/FAK signaling. Cancer Lett. 341, 166–177. doi:10.1016/j.canlet.2013.07.037
Moon, D. O., Park, S. Y., Choi, Y. H., Ahn, J. S., and Kim, G. Y. (2011). Guggulsterone sensitizes hepatoma cells to TRAIL-induced apoptosis through the induction of CHOP-dependent DR5: Involvement of ROS-dependent ER-stress. Biochem. Pharmacol. 82, 1641–1650. doi:10.1016/j.bcp.2011.08.019
Owsley, E., and Chiang, J. Y. (2003). Guggulsterone antagonizes farnesoid X receptor induction of bile salt export pump but activates pregnane X receptor to inhibit cholesterol 7alpha-hydroxylase gene. Biochem. Biophys. Res. Commun. 304, 191–195. doi:10.1016/S0006-291X(03)00551-5
Samudio, I., Konopleva, M., Safe, S., McQueen, T., and Andreeff, M. (2015). Guggulsterones induce apoptosis and differentiation in acute myeloid leukemia: Identification of isomer-specific antileukemic activities of the pregnadienedione structure. Mol. CancerTher 4, 1982–1992. doi:10.1158/1535-7163.MCT-05-0247
Shi, J. J., Jia, X. L., Li, M., Yang, N., Li, Y. P., Zhang, X., et al. (2015). Guggulsterone induces apoptosis of human hepatocellular carcinoma cells through intrinsic mitochondrial pathway. World J. Gastroenterol. 21, 13277–13287. doi:10.3748/wjg.v21.i47.13277
Shiragannavar, V. D., Sannappa Gowda, N. G., Puttahanumantharayappa, L. D., Karunakara, S. H., Bhat, S., Prasad, S. K., et al. (2023). The ameliorating effect of withaferin A on high-fat diet-induced non-alcoholic fatty liver disease by acting as an LXR/FXR dual receptor activator. Front. Pharmacol. 14, 1135952. doi:10.3389/fphar.2023.1135952
Shishodia, S., Sethi, G., Ahn, K. S., and Aggarwal, B. B. (2007). Guggulsterone inhibits tumor cell proliferation, induces S-phase arrest, and promotes apoptosis through activation of c-Jun N-terminal kinase, suppression of Akt pathway, and downregulation of antiapoptotic gene products. Biochem. Pharmacol. 74, 118–130. doi:10.1016/j.bcp.2007.03.026
Singh, S. V., Zeng, Y., Xiao, D., Vogel, V. G., Nelson, J. B., Dhir, R., et al. (2005). Caspase-dependent apoptosis induction by guggulsterone, a constituent of Ayurvedic medicinal plant Commiphora mukul, in PC-3 human prostate cancer cells is mediated by Bax and Bak. Mol. Cancer Ther. 4, 1747–1754. doi:10.1158/1535-7163.MCT-05-0223
Tian, H., Gui, Y., Wei, Y., Shang, B., Sun, J., Ma, S., et al. (2021). Z-guggulsterone induces PD-L1 upregulation partly mediated by FXR, Akt and Erk1/2 signaling pathways in non-small cell lung cancer. Int. Immunopharmacol. 93, 107395. doi:10.1016/j.intimp.2021.107395
Xu, H. B., Li, L., and Liu, G. Q. (2011). Reversal of multidrug resistance by guggulsterone in drug-resistant MCF-7 cell lines. Chemotherapy 57, 62–70. doi:10.1159/000321484
Xu, H. B., Fu, J., Huang, F., and Yu, J. (2017). Guggulsterone sensitized drug-resistant human hepatocarcinoma cells to doxorubicin through a Cox-2/P-gp dependent pathway. Eur. J. Pharmacol. 803, 57–64. doi:10.1016/j.ejphar.2017.03.045
Yamada, T., Osawa, S., Hamaya, Y., Furuta, T., Hishida, A., Kajimura, M., et al. (2010). Guggulsterone suppresses bile acid-induced and constitutive caudal-related homeobox 2 expression in gut-derived adenocarcinoma cells. Anticancer Res. 30, 1953–1960.
Yamada, T., Osawa, S., Ikuma, M., Kajimura, M., Sugimoto, M., Furuta, T., et al. (2014). Guggulsterone, a plant-derived inhibitor of NF-TB, suppresses CDX2 and COX-2 expression and reduces the viability of esophageal adenocarcinoma cells. Digestion 90, 208–217. doi:10.1159/000365750
Zhong, F., Tong, Z. T., Fan, L. L., Zha, L. X., Wang, F., Yao, M. Q., et al. (2015a). Guggulsterone-induced apoptosis in cholangiocarcinoma cells through ROS/JNK signaling pathway. Am. J. Cancer Res. 6, 226–237.
Zhong, F., Yang, J., Tong, Z. T., Chen, L. L., Fan, L. L., Wang, F., et al. (2015b). Guggulsterone inhibits human cholangiocarcinoma Sk-ChA-1 and Mz-ChA-1 cell growth by inducing caspase-dependent apoptosis and downregulation of survivin and Bcl-2 expression. Oncol. Lett. 10, 1416–1422. doi:10.3892/ol.2015.3391
Keywords: guggulsterone, meta-analysis, systematic literature review, apoptosis, anti-cancer
Citation: Gupta M, Singh D, Rastogi S, Siddique HR, Al-Dayan N, Ahmad A, Sikander M and Sarwat M (2023) Anti-cancer activity of guggulsterone by modulating apoptotic markers: a systematic review and meta-analysis. Front. Pharmacol. 14:1155163. doi: 10.3389/fphar.2023.1155163
Received: 31 January 2023; Accepted: 12 April 2023;
Published: 02 May 2023.
Edited by:
Carmela Spagnuolo, National Research Council (CNR), ItalyReviewed by:
Prasanna K. Santhekadur, JSS Academy of Higher Education and Research, IndiaCopyright © 2023 Gupta, Singh, Rastogi, Siddique, Al-Dayan, Ahmad, Sikander and Sarwat. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maryam Sarwat, bXNhcndhdEBhbWl0eS5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.