
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 25 April 2023
Sec. Ethnopharmacology
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1153583
This article is part of the Research TopicClinical PhytopharmacologyView all 6 articles
Background: Based on their anti-oxidative and anti-fibrotic properties, Angelica sinensis (Oliv.) Diels roots [Apiaceae; Radix Angelicae sinensis] (Danggui [abbreviated as S in the context]), Astragalus membranaceus (Fisch.) Bunge [Fabaceae; Astragalus membranaceus] (Huangqi [A]), Rheum palmatum L. [Polygonaceae; Rheum palmatum] (Dahuang [R]), and Salvia miltiorrhiza Bunge [Lamiaceae; Salvia miltiorrhiza Bunge radix et rhizoma] (Danshen [D]) are potential renoprotective Chinese herbal medicines (CHMs). Renoprotection using ARD alone for the treatment of chronic kidney disease (CKD) has been documented in pre-clinical, clinical, and meta-analysis research; however, only pre-clinical data are available for the use of S alone. Moreover, with an increasing number of CKD patients taking prescribed CHMs, hyperkalemia risk remains unclear.
Methods: This study retrospectively analyzed national health insurance claims data in 2001–2017. Propensity score matching was used to analyze renal and survival outcomes and the dose-response effects of S without ARD use in 18,348 new S users, 9,174 new ARD users, and 36,696 non-users. Cox proportional hazard regression was used to investigate adjusted hazard ratios (aHRs) for end-stage renal disease (ESRD) in the presence of competing mortality and death. The additive effect of the S herb in single form to compounds was also analyzed. Additionally, to analyze hyperkalemia risk, an exact match on each covariate was used to include 42,265 new CHM users and non-users, while Poisson regression was used to estimate adjusted incidence rate ratios (aIRRs) of hyperkalemia of prescribed CHMs.
Results: S users and ARD users were associated with aHRs of 0.77 (95% confidence interval; 0.69–0.86) and 1.04 (0.91–1.19), respectively, for ESRD and 0.55 (0.53–0.57) and 0.71 (0.67–0.75), respectively, for death. The renal and survival benefits of S use were consistent in several sensitivity analyses. The dose- and time-dependent renoprotection and dose-dependent survival benefits were observed for S use. The top two additive renoprotective collocations of the S herb in compounds were Xue-Fu-Zhu-Yu-Tang and Shen-Tong-Zhu-Yu-Tang, followed by Shu-Jing-Huo-Xue-Tang and Shen-Tong-Zhu-Yu-Tang. Moreover, CHM users were associated with aIRRs of 0.34 (0.31–0.37) for hyperkalemia.
Conclusion: This study suggests dose- and time-dependent renoprotection and dose-dependent survival benefits of the S herb in compounds and no increased hyperkalemia risk of the prescribed CHMs in CKD patients.
Chronic inflammation and subsequent oxidative stress, which are at the heart of chronic kidney disease (CKD) pathophysiology, are involved in CKD development and progression (Impellizzeri et al., 2014; Tinti et al., 2021). Regardless of the CKD etiology, they contribute to ultimate renal fibrosis and account for the increased morbidity and cardiovascular and all-cause mortality (Akchurin and Kaskel, 2015). CKD increases the global burden on health systems due to its insidious onset, progressive and debilitating nature, and increasing incidence worldwide (Khan et al., 2022). Renin–angiotensin–aldosterone system inhibitors are the cornerstone therapy for attenuating the progression of CKD to end-stage renal disease (ESRD) because they aim to lowering the intraglomerular pressure-mediated injury, a well-recognized mechanism for structural damage in CKD progression; however, the effect is moderate (Zhong et al., 2013).
Chinese herbal medicine (CHM) interventions have been proposed as a complementary alternative therapy to mitigate CKD progression as they can multi-target inflammation and oxidative stress related to CKD with subsequent benefits in renal fibrosis, as documented in pre-clinical studies, and confer additive or synergistic renoprotective effects, shown in clinical studies when combined with Western medicine (Peng et al., 2005; Zhong et al., 2013; Zhong et al., 2015). Astragalus membranaceus (Fisch.) Bunge [Fabaceae; Astragalus membranaceus] (Huangqi), Rheum palmatum L. [Polygonaceae; Rheum palmatum] (Dahuang), Salvia miltiorrhiza Bunge [Lamiaceae; Salvia miltiorrhiza Bunge radix et rhizoma] (Danshen), and A. sinensis (Oliv.) Diels roots [Apiaceae; Radix Angelicae sinensis] (Danggui) have been documented to have potential renoprotective effects. A. membranaceus, R. palmatum, and S. miltiorrhiza alone are reported to have renoprotective effects in pre-clinical, clinical, and meta-analysis (Peng et al., 2005; Wang et al., 2012; Zhong et al., 2013; Zhang et al., 2014; Zhong et al., 2015; Ma et al., 2017; Shen et al., 2020) research. A. sinensis and A. membranaceus are commonly used together to confer renoprotection in pre-clinical and clinical studies. However, the current evidence on whether A. sinensis use alone is renoprotective or not is limited to in vitro and in vivo experimental studies (Yeh et al., 2011; Cheng et al., 2012; Mo et al., 2018).
Two national health insurance (NHI)-based retrospective studies analyzed the data from Taiwan’s NHI program and indicated a substantial percentage of CHM usage in patients with CKD (Lin et al., 2015; Guo et al., 2021). However, whether prescribed CHMs could increase hyperkalemia risk in patients with CKD is unclear because the herbs and CKD are risk factors for hyperkalemia (Palmer, 2004). Therefore, this study aims to investigate the overall and dose–response effects of A. sinensis without the use of A. membranaceus, R. palmatum, or S. miltiorrhiza on renal and survival outcomes and the association between prescribed CHMs and hyperkalemia risk in patients with CKD.
CHM services have been covered by a single-payer compulsory Taiwan’s NHI program since 1995, which allows long-term tracking, and reimbursed by the NHI Administration as dosage forms of scientific Chinese medicine powders. All reimbursed CHMs must be produced by pharmaceutical factories possessing the Good Manufacturing Practice certification from Taiwan’s Food and Drug Administration, recommended by Taiwan’s Committee of Chinese Medicine and Pharmacy and prescribed by licensed Chinese medicine physicians. This study retrospectively analyzed a NHI dataset from Taiwan’s 2005 Longitudinal Generation Tracking Database (LGTD 2005), which randomly sampled 2 million beneficiaries from all beneficiaries in the 2015 Taiwan’s NHI program and recorded patient medical information between 2000 and 2017. The database is managed by the Health and Welfare Data Science Center (HWDC) of the Taiwan Ministry of Health and Welfare. The HWDC has validated the representativeness of LGTD 2005, which has been described in detail in previous studies (Chen et al., 2022a; Chen et al., 2022b). The HWDC only provides de-identified data for research. Therefore, patient consent is not required to access LGTD 2005. Patient informed consent and full review were exempted by the Institutional Review Board of the Dalin Tzu Chi Hospital (B10804001). LGTD 2005 adopts ICD-9-CM (before 2016) and ICD-10-CM (after 2016) diagnosis codes to define diseases (Hsieh et al., 2019) and anatomical therapeutic chemical codes to capture drugs.
This study identified 480,062 patients with a claim-based diagnosis of CKD from LGTD 2005 between 2000 and 2017 (Figure 1) and selected 429,855 patients with incident CKD between 2001 and 2017. Overall, 128,964 patients with CKD who were aged <18 years, had taken any CHMs in 3 months, had a renal transplant, experienced ESRD, or died before the CKD inception date were excluded, and an initial CKD cohort of 300,891 patients was obtained.

FIGURE 1. Flow diagram of three study cohorts. Abbreviations: CKD, chronic kidney disease; ESRD, end-stage renal disease; CHM, Chinese herbal medicine; ARD, A: Astragalus membranaceus (Fisch.) Bunge, R: Rheum palmatum L., and D: Danshen (Salvia miltiorrhiza Bunge); S, Angelica sinensis (Oliv.) Diels roots.
The covariates included age, sex, baseline comorbidities (including diabetes defined by ICD-9/10-CM codes or anti-diabetic agents, hypertension defined by ICD-9/10-CM codes or anti-hypertensive drugs, coronary heart disease defined by ICD-9/10-CM codes, hyperlipidemia defined by ICD-9/10-CM codes or anti-lipidemic drugs, and chronic liver disease defined by ICD-9/10-CM codes), number of medical visits, Charlson Comorbidity Index in 1 year before the CKD inception date, and two confounding drugs (angiotensin-converting enzyme inhibitors/angiotensin-II receptor antagonists and non-steroid anti-inflammatory drugs).
Based on the prescribed CHMs, 300,891 patients with CKD were divided into CHM users (n = 146,540) and non-users (n = 154,351) after CKD onset. CHM users were divided into five cohorts during the study period based on the new use of A. sinensis (abbreviated as S in the context), new use of A. membranaceus (abbreviated as A in the context), R. palmatum (abbreviated as R in the context), or S. miltiorrhiza (Danshen, abbreviated as D in the context) (Supplementary Table S1) (Chen et al., 2022a); and new use of other CHMs, except for A. sinensis, A. membranaceus, R. palmatum, or S. miltiorrhiza. Cohort 1 was the control cohort (no use of any CHM; n = 154,351; 51.3%). Cohort 2 comprised patients on the new use of A. sinensis but never the use of A. membranaceus, R. palmatum, or S. miltiorrhiza, which was abbreviated as S users in the S cohort (n = 29,203; 9.7%). Cohort 3 comprised patients on the new use of any A. membranaceus, R. palmatum, or S. miltiorrhiza but never the use of A. sinensis, which was abbreviated as ARD users in the ARD cohort (n = 9,676; 3.2%). Cohort 4 (n = 91,119; 30.3%) comprised patients on the new use of A. sinensis, A. membranaceus, R. palmatum, or S. miltiorrhiza. Cohort 5 (n = 16,542; 5.5%) comprised patients on the new use of other CHMs, except for A. sinensis, A. membranaceus, R. palmatum, or S. miltiorrhiza. To analyze the effect of S vs. ARD use on ESRD and death, cohorts 4 and 5 were excluded from propensity score matching. A new user (exposed to A. sinensis, A. membranaceus, R. palmatum, and S. miltiorrhiza) design was used with the follow-up for each ARD user beginning on the date of the first prescription of A. membranaceus, R. palmatum, or S. miltiorrhiza to prevent an immortal bias (Shariff et al., 2008; Chen et al., 2022b). Additionally, each qualified propensity-matched S user and control must have been alive at the time when ARD was commenced. Each new ARD user was matched with two new S users, who were in the same median interval (2.4 years) from the CKD onset date to the first prescription date (index date) of S and ARD. Furthermore, each new ARD user was matched with four non-users at the ARD commencement date, and the index date of the non-user was the corresponding date of the ARD user. The propensity score was calculated using logistic regression built on all study covariates to adjust for baseline differences between ARD users, S users, and non-users. The average standardized mean difference was 0.208 (S vs. non-user)/0.137 (ARD vs. non-user) and 0.015 (S vs. non-user)/0.013 (ARD vs. non-user) before and after propensity score matching, respectively. Thus, propensity score matching in this study was well-balanced in the three study cohorts. Finally, 64,218 patients with CKD, comprising 18,348 S users, 9,174 ARD users, and 36,696 non-users, were included in the final analysis.
Moreover, an exact match was used on each covariate among 146,540 CHM users and 154,351 non-users, and 42,265 CHM users and 42,265 non-users were obtained for an additional analysis of hyperkalemia risk of the prescribed CHMs in patients with CKD.
For the main analysis, all study participants were followed from their index date to the occurrence of ESRD, death, or the end of the study (31 December 2017), whichever occurred first; the latter two were considered as censoring observations. Death before reaching ESRD was considered a competing risk event when estimating the cumulative incidence and risk of ESRD (Hsu et al., 2014a). ESRD was confirmed in the Registry for Catastrophic Illness Patient Database (Hsu et al., 2014b; Chen et al., 2022a), a subset of LGTD 2005. Death was defined as the participant’s withdrawal from the NHI program (Hsu et al., 2014a). For additional analyses, hyperkalemia was defined by the ICD-9/10-CM code, use of potassium-lowering agents, or the procedure code for immediate hemodialysis in the presence of the ICD-9/10-CM code for hyperkalemia.
Baseline characteristics between S, ARD, and control cohorts were compared using chi-squared and ANOVA tests for categorical and continuous variables, respectively. The modified Kaplan–Meier and Gray’s methods (Gray, 1988) were used to calculate and compare the cumulative incidences in data with competing risks. The modified Cox proportional hazard model was applied to examine the association of S and ARD use with ESRD and Cox regression for death, with adjustments for all study covariates after confirming the assumption of proportional hazards by plotting the graph of the log (−log(survival)) versus the log of survival time. The CHM prescription day was assessed to address the association between the duration of exposure to S and ARD herbs and the risk of study outcomes among participants having at least a >1 year of follow-up. The duration was measured in cumulative days of use and categorized as 1–30, 31–60, and ≥61 days, with non-use as the reference. The mean dosage of A. sinensis prescribed by a licensed CHM practitioner in Taiwan is 1.5 g/d for a single herb and 4–6 g/d for compounds (Chen et al., 2019). The additive effect of A. sinensis in one single form to 72 compounds on ESRD risk was also analyzed. Poisson regression was used to estimate the adjusted incident rate ratio of hyperkalemia in association with the prescribed CHM use throughout the study. Recurrent episodes of hyperkalemia were considered separate events if they occurred at least 28 days apart and were considered a long event if they occurred less than 28 days apart (Chen et al., 2022b). In addition, for the main analysis, four sensitivity analyses were conducted to validate the main finding. First, multivariate stratified analyses were conducted on different subgroups. Second, patients with CKD who died or developed ESRD within 30, 60, and 90 days after the index date were excluded to reappraise the risk of study outcomes. Third, S and ARD usage groups were redefined based on the cumulative days of use as >30 days and >60 days. Fourth, three potentially renoprotective drugs sodium-glucose co-transporter 2 inhibitors (Din et al., 2022), glucagon-like peptide-1 agonists (Vitale et al., 2020), and Ketosteril (Wang et al., 2020) were added to the regression model to reappraise the risk of the study outcomes. For additional analyses of hyperkalemia risk, two sensitivity analyses were conducted according to the different exact match models. All data were analyzed using SAS (version 9.4; SAS Institute, Inc., Cary, N.C.). Missing data were not imputed. The statistical significance was set at a two-sided p-value less than 0.05.
The average age of the three groups was 54 years, and 52% of them were male (Table 1). No significant difference was observed in the sex, age, comorbidities, Charlson Comorbidity Index, number of medical visits, and confounding drugs among the three groups.
During the follow-up period, 14.4%, 16.4%, and 18.3% of the S, ARD, and control cohorts, respectively, died before developing ESRD (p< 0.0001) (Table 2), and 1,666 (2.6%) patients developed ESRD. The 15-year cumulative incidences of ESRD and overall mortality were significantly the lowest in the S cohort [5.1% and 95% confidence interval (CI): 4.5%–5.7%; 38% and 95% CI: 36%–39%], followed by ARD (6.4% and 95% CI: 5.4%–7.5%; 43% and 95% CI: 40%–45%) and control cohorts (7.0% and 95% CI: 6.3%–7.8%; 56% and 95% CI: 54%–58%) (all p< 0.0001).
Compared with non-use, S use was associated with an adjusted hazard ratio (aHR) of 0.77 (95% CI: 0.69–0.86; p< 0.0001) for ESRD and 0.55 (95% CI: 0.53–0.57; p< 0.0001) for overall mortality; ARD use was associated with an aHR of 1.04 (95% CI: 0.91–1.19; p = 0.59) for ESRD and 0.71 (95% CI: 0.67–0.75; p< 0.0001) for overall mortality (Table 3). The benefits of the S herb on renal and survival outcomes remained consistent and statistically significant when adding three potentially renoprotective drugs, sodium-glucose co-transporter 2 inhibitors, glucagon-like peptide-1 agonists, and Ketosteril (Supplementary Table S5).
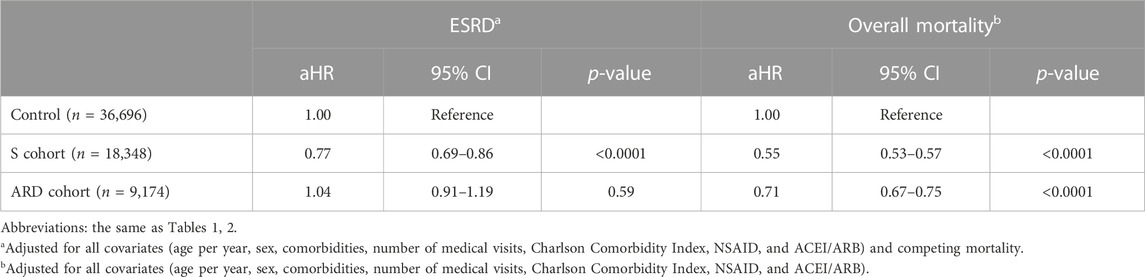
TABLE 3. Adjusted hazard ratios for end-stage renal disease and the overall mortality in the three cohorts.
Compared with non-use, a graded association was observed between the cumulative exposure duration of S, not ARD, and renoprotection among those exposed to 1–30 (aHR, 0.76; 95% CI: 0.66–0.86; p< 0.0001), 31–60 (0.52; 95% CI: 0.31–0.89; p = 0.017), and ≥61 (0.46; 95% CI: 0.22–0.98; p = 0.045) days (Table 4). However, no graded association was observed between the cumulative exposure duration to S and ARD use and the overall mortality among those exposed for 1–30, 31–60, and ≥61 days.
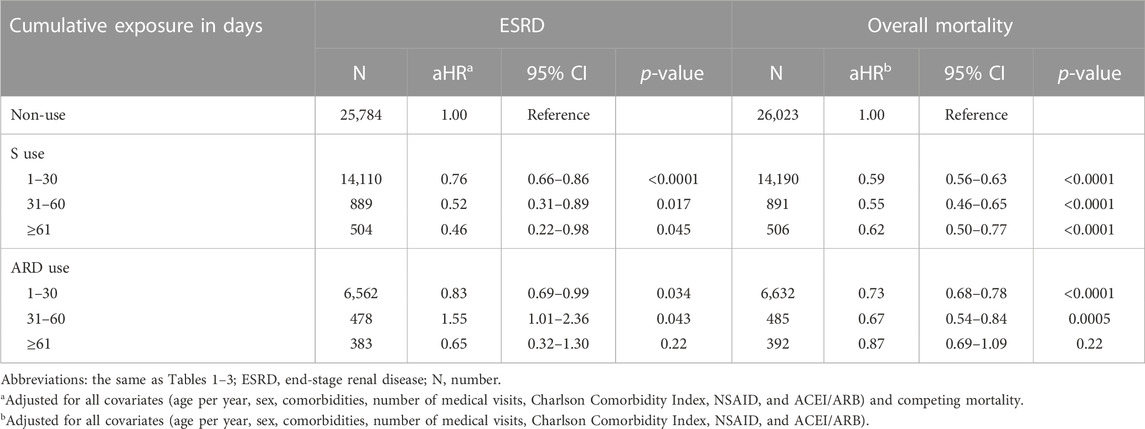
TABLE 4. Cumulative exposure duration of S and ARD use and the risk of the study outcomes of at least >1 year of follow-up.
A dose–response relationship of S use with risks of ESRD and overall mortality was observed when the cumulative S dose was divided into four isometric levels of ≤4.9, 5–9.9, 10–14.9, and ≥ 15 g (Table 5).
The top four orders of additive renoprotection were the use of Xue-Fu-Zhu-Yu-Tang and Shen-Tong-Zhu-Yu-Tang, followed by the use of Shu-Jing-Huo-Xue-Tang and Shen-Tong-Zhu-Yu-Tang, the use of Shen-Tong-Zhu-Yu-Tang, and the use of Shu-Jing-Huo-Xue-Tang (Table 6).
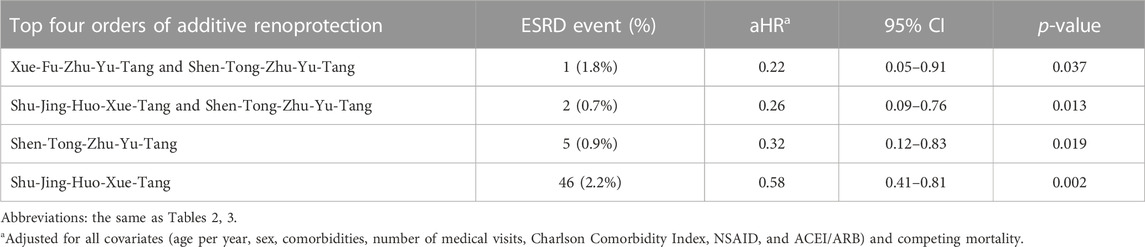
TABLE 6. Additive effect of one single form for 72 compounds of S use on end-stage renal disease risk.
Compared with non-use, the prescribed CHM (adjusted incidence rate ratio, 0.34; 95% CI: 0.31–0.37) usage in patients with CKD was not associated with the increased hyperkalemia risk during the follow-up period (Supplementary Table S2). The results remained consistent despite the different exact match models. S and ARD users also had no increased risk of hyperkalemia during the follow-up period (data not shown).
Four sensitivity analyses were conducted to support the reliability of our findings. The association between S use and lower risks of ESRD and overall mortality, and between ARD use and the lower overall mortality remained consistent in three sensitivity analyses, including multivariate stratified analyses (Figure 2), using different definitions of S and ARD use (Supplementary Table S3), and the exclusion of patients with CKD who died or developed ESRD within 30, 60, and 90 days after the index date (Supplementary Table S4).
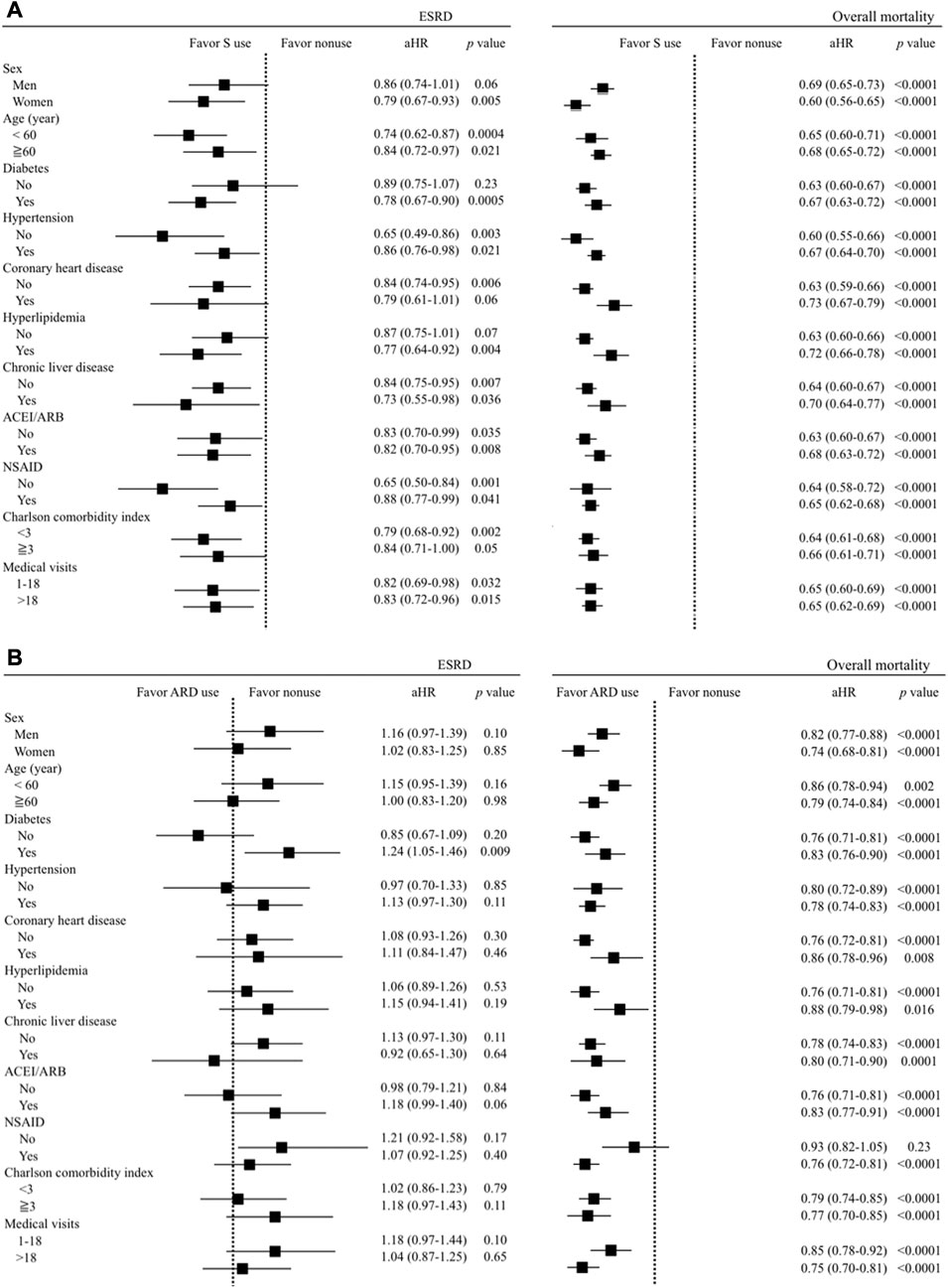
FIGURE 2. Multivariate stratified analyses for the association between (A) S use (vs. non-use) and (B) ARD use (vs. non-use) and the risks of ESRD and overall mortality.
The key finding of this nationwide cohort study, including 64,218 patients with CKD, is the association between A. sinensis in the absence of any A. membranaceus, R. palmatum, or S. miltiorrhiza aid and a 23% reduced ESRD risk. This benefit is dose- and time-dependent, and a higher dose and longer use of A. sinensis lower ESRD risks by 52% and 54%, respectively. These findings are reinforced by the experimental model results that suggest biologically plausible mechanisms for renal benefit (Yeh et al., 2011; Cheng et al., 2012; Mo et al., 2018) and are consistent across subgroups and robust in several sensitivity analyses. The top two additive renoprotective collocations are the use of Xue-Fu-Zhu-Yu-Tang and Shen-Tong-Zhu-Yu-Tang and the use of Shu-Jing-Huo-Xue-Tang and Shen-Tong-Zhu-Yu-Tang. In addition, this study adds two novel findings to the existing literature. A dose–response relationship exists between A. sinensis and overall mortality. Overall, the prescribed CHMs are not associated with an increased hyperkalemia risk in patients with CKD.
A. sinensis, A. membranaceus, R. palmatum, and S. miltiorrhiza are potential renoprotective herbs because of their multi-functional properties of anti-oxidation, anti-inflammation, and anti-fibrosis (Peng et al., 2005; Chao and Lin, 2011; Zhong et al., 2013; Zhong et al., 2015; Xu et al., 2016). The use of A. membranaceus, R. palmatum, and S. miltiorrhiza alone can confer renoprotection, which has been documented in pre-clinical and clinical studies, and meta-analyses (Peng et al., 2005; Wang et al., 2012; Zhong et al., 2013; Zhang et al., 2014; Zhong et al., 2015; Ma et al., 2017; Shen et al., 2020). In contrast, the reported renoprotection of A. sinensis alone was only documented in pre-clinical research (Yeh et al., 2011; Cheng et al., 2012; Mo et al., 2018). This study is the first to document the dose- and time-dependent renoprotection of A. sinensis without the aid of A. membranaceus, R. palmatum, and S. miltiorrhiza in patients with CKD, which concurred with the previous observations (Yeh et al., 2011; Cheng et al., 2012; Mo et al., 2018). Ferulic acid and ligustilide are considered major bioactive components of A. sinensis (Chao and Lin, 2011). In mice with a D-galactose-induced renal injury (Mo et al., 2018), A. sinensis treatment, with ligustilide as the major component for 8 weeks, improved the renal function by dose-dependent attenuation of the NF-kB pathway activation and inflammatory cytokine expression and the dose-dependent increase of anti-oxidative enzyme activities and gene expressions, thereby ameliorating renal histological deterioration. In streptozotocin-induced diabetic rats (Yeh et al., 2011; Lv et al., 2018), A. sinensis improved the renal function by inhibiting the transforming growth factor (TGF)-β1 expression and collagen IV (Lv et al., 2018) or directly increasing their expression more effectively in a 4-week treatment than 1-week treatment of the renal endogenous bone morphogenetic protein-7 (Yeh et al., 2011). In a mouse model of membranous nephropathy (Cheng et al., 2012), ferulic acid extracted from A. sinensis decreased proteinuria dose-dependently and delayed renal progression by reducing oxidative stress. In high glucose-exposed rat mesangial cells (Yeh et al., 2011), the S herb directly scavenged free radicals in a concentration-dependent manner. Furthermore, A. sinensis demonstrated a renoprotective potential toward cisplatin-mediated tubulotoxicity (Bunel et al., 2015a) since in addition to being a potent anti-oxidant, ferulic acid has a dose–effect relationship for alleviating cisplatin-induced cell death and apoptosis. This study found that the top two additive renoprotective collocations of A. sinensis in compounds were the use of Xue-Fu-Zhu-Yu-Tang and Shen-Tong-Zhu-Yu-Tang and the use of Shu-Jing-Huo-Xue-Tang and Shen-Tong-Zhu-Yu-Tang. These findings are consistent with previous studies (Guo et al., 2013; Wang et al., 2023). The main bioactive compound of Xue-Fu-Zhu-Yu-Tang, Shu-Jing-Huo-Xue-Tang, and Shen-Tong-Zhu-Yu-Tang is Tao Ren [semen of Prunus persica (L.) Batsch], which is a potent anti-fibrotic agent that may have a therapeutic potential in patients with fibrotic kidney disease, as it can attenuate renal fibroblast activation and rat renal interstitial fibrosis (Guo et al., 2013). Another common bioactive compound of Xue-Fu-Zhu-Yu-Tang and Shen-Tong-Zhu-Yu-Tang is Hong Hua (Carthamus tinctorius L.), which also has therapeutic effects on renal fibrosis (Wang et al., 2023). Given that oxidative stress, inflammation, and fibrosis are three well-documented mechanistic processes implicated in CKD pathogenesis (Khan et al., 2022), A. sinensis alone can confer renoprotection as the main effect in clinical practice.
The findings from this study demonstrated a significant survival benefit of A. sinensis, A. membranaceus, R. palmatum, and S. miltiorrhiza in patients with CKD, consistent with two prior NHI-based cohort studies. Another randomized clinical trial (Zhang et al., 2020) of 426 patients with acute coronary syndrome and mild-to-moderate renal insufficiency also demonstrated that A. sinensis combined with Western medicine improved cardiovascular outcomes in 1 year of follow-up compared with only the use of Western medicine. Cumulative evidence (Tang et al., 2021) has explored vasodilation in vitro and anti-hypertensive properties in vivo of A. sinensis, A. membranaceus, R. palmatum, and S. miltiorrhiza, which are mainly attributed to the regulation of the endothelium-dependent mechanism or vascular smooth muscle cell-mediated mechanism and are effective in treating cardiovascular diseases, such as atherosclerosis, hypertension, and acute coronary syndrome. This study also discovered that A. sinensis exhibited a better survival rate than A. membranaceus, R. palmatum, and S. miltiorrhiza, consistent with a prior NHI-based cohort study (Hsieh et al., 2017). This study is the first to document the dose–response effect of A. sinensis on overall mortality in patients with CKD. This is consistent with the previous studies that showed high (vs. low) doses of A. sinensis treatment enhanced cell survival in cisplatin-induced proximal tubule cell toxicity (Bunel et al., 2015a; Bunel et al., 2015b). Furthermore, A. sinensis exerted a dose-dependent cardioprotective effect on myocardial ischemia rats by regulating the PI3K/Akt pathway (Cao et al., 2020) and on cardiomyoblast cells by inhibiting angiotensin II-induced apoptosis (Huang et al., 2014). Complementary therapy with A. sinensis, A. membranaceus, R. palmatum, and S. miltiorrhiza may offer a considerable potential since patients with CKD are at a high risk for cardiovascular and overall mortality, which is largely driven by cardiovascular deaths (Akchurin and Kaskel, 2015).
CHMs containing aristolochic acid have been withdrawn from the NHI program since November 2003, and Taiwan’s Ministry of Health and Welfare went through a traditional Chinese medicine-enhanced CKD outpatient care program in 2019; however, no consensus has been reached on this issue among nephrologists regarding the suitability of the integrating prescribed CHMs into Western medicine in patients with CKD. Several cohort studies retrospectively analyzed the NHI claims data and documented the renal (Lin et al., 2015; Guo et al., 2021) and survival (Lin et al., 2015; Hsieh et al., 2017; Guo et al., 2021) benefits of the overall prescribed CHMs in patients with CKD, but they did not address if a higher risk of hyperkalemia ensued. Consistent with these two studies, the current study’s CKD population between 2001 and 2017 had 48.7% of prescribed CHM usage after CKD inception, regardless of the indications. Therefore, addressing this concern is crucial. This study is the first to document no increased hyperkalemia risk after a 16-year follow-up in patients with CKD taking prescribed CHMs. This finding was consistent with our previous work (Chen et al., 2022a), documenting no increased hyperkalemia risk in advanced CKD patients taking prescribed A. sinensis, A. membranaceus, R. palmatum, and S. miltiorrhiza. Several manufacturing processes, including extraction, decoration, concentration, and granulation, of scientific Chinese medicine powders from raw herb materials may account for the substantially low potassium content of the raw herbs.
This study undertook several methods for minimizing the bias and potential confounding factors, including a relatively large sample size from the universal coverage of a nationwide population and long-term tracking of all study outcomes. First, the enrolled participants were matched with the propensity scores to optimize the comparability among study cohorts. Second, the time when patients received A. sinensis, A. membranaceus, R. palmatum, and S. miltiorrhiza was set as the baseline for matching and observation entry to preclude the possibility of an immortal time bias. Third, considering competing mortality prevents an overestimation of non-fatal outcomes in the control cohort (Hsu et al., 2014b).
The study also had several limitations. First, self-paid or non-prescribed CHM is not included in LGTD 2005. Second, the indications of A. sinensis, A. membranaceus, R. palmatum, and S. miltiorrhiza could not be determined because of the study’s retrospective design. Thus, a causal association between a drug of interest and the risk of study outcomes cannot be inferred based on an observational study. Furthermore, Taiwan’s Committee of Chinese Medicine and Pharmacy provided A. sinensis, A. membranaceus, R. palmatum, and S. miltiorrhiza formulas. However, caution is, thus, recommended before directly applying our results in the West. Third, the adherence to the prescribed CHMs, herb–drug interactions between the prescribed CHM and Western medicine, and pulse-taking diagnoses were unavailable in LGTD 2005. Fourth, LGTD 2005 lacked the exact main etiology of CKD (Hsu et al., 2014a; Chen et al., 2022b), laboratory data, family history, and lifestyle information, which might impact the study outcomes. Fifth, as in any observational study, unmeasured confounders may still exist.
This nationwide analysis suggests that A. sinensis in compounds is associated with dose- and time-dependent renoprotection and dose-dependent survival benefits. Furthermore, prescribed CHMs are not associated with hyperkalemia risk in patients with CKD. The causal relationship mechanisms underlying this association warrant further prospective research.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Institutional Review Board of the Buddhist Dalin Tzu Chi Hospital (B10804001). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Y-CC and H-TC designed the research; Y-CC and B-HY performed the statistical analysis; Y-CC and B-HY wrote the manuscript; H-TC, B-HY, M-HY, S-KH, and Y-CC analyzed the data; Y-CC supervised the study. All authors have read and agreed to the submitted version of the manuscript.
This study was funded by the Buddhist Dalin Tzu Chi Hospital (DTCRD109-I-24).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1153583/full#supplementary-material
CKD, chronic kidney disease; ESRD, end-stage renal disease; LGTD 2005, 2005 Longitudinal Generation Tracking Database; NHI, National Health Insurance; S, Angelica sinensis (Oliv.) Diels roots; A, Astragalus membranaceus (Fisch.) Bunge; R, Rheum palmatum L; D, Danshen (Salvia miltiorrhiza Bunge).
Akchurin, O. M., and Kaskel, F. (2015). Update on inflammation in chronic kidney disease. Blood. Purif. 39 (1-3), 84–92. doi:10.1159/000368940
Bunel, V., Antoine, M. H., Nortier, J., Duez, P., and Stevigny, C. (2015a). Nephroprotective effects of ferulic acid, Z-ligustilide and E-ligustilide isolated from Angelica sinensis against cisplatin toxicity in vitro. Toxicol. Vitro. 29 (3), 458–467. doi:10.1016/j.tiv.2014.12.017
Bunel, V., Antoine, M. H., Nortier, J., Duez, P., and Stévigny, C. (2015b). Potential nephroprotective effects of the Chinese herb Angelica sinensis against cisplatin tubulotoxicity. Pharm. Biol. 53 (7), 985–994. doi:10.3109/13880209.2014.951726
Cao, Y., Liang, X., Li, C., Chen, T., Li, Z., Li, W., et al. (2020). Experimental study on the effect of aconite and Angelica sinensis on myocardial ischemia rats with yang deficiency and blood stasis. Evid. Based. Complement. Altern. Med. 7027391, 7027391. doi:10.1155/2020/7027391
Chao, W. W., and Lin, B. F. (2011). Bioactivities of major constituents isolated from Angelica sinensis (Danggui). Chin. Med. 6, 29. doi:10.1186/1749-8546-6-29
Chen, J. Y., Wang, Y. H., Hidajah, A. C., and Li, C. Y. (2019). A population-based case-control study on the association of Angelica sinensis exposure with risk of breast cancer. J. Tradit. Complement. Med. 10 (5), 454–459. doi:10.1016/j.jtcme.2019.10.003
Chen, Y. C., Chen, H. T., Yeh, C. C., Hung, S. K., and Yu, B. H. (2022a). Four prescribed Chinese herbal medicines provide renoprotection and survival benefit without hyperkalemia risk in patients with advanced chronic kidney disease: A nationwide cohort study. Phytomedicine 95, 153873. doi:10.1016/j.phymed.2021.153873
Chen, Y. C., Chen, Y. C., Chiou, W. Y., and Yu, B. H. (2022b). Impact of acid suppression therapy on renal and survival outcomes in patients with chronic kidney disease: A Taiwanese nationwide cohort study. J. Clin. Med. 11, 5612. doi:10.3390/jcm11195612
Cheng, C. W., Chang, W. L., Chang, L. C., Wu, C. C., Lin, Y. F., and Chen, J. S. (2012). Ferulic acid, an Angelica sinensis-derived polyphenol, slows the progression of membranous nephropathy in a mouse model. Evid. Based. Complement. Altern. Med. 161235, 161235. doi:10.1155/2012/161235
Din, U. A., Salem, M. M., and Abdulazim, D. O. (2022). Sodium-glucose cotransporter 2 inhibitors as the first universal treatment of chronic kidney disease. Nefrol. Engl. Ed. 42, 390–403. doi:10.1016/j.nefroe.2022.08.001
Gray, R. J. (1988). A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann. Stat. 16 (3), 1141–1154.
Guo, J. C., Pan, H. C., Yeh, B. Y., Lu, Y. C., Chen, J. L., Yang, C. W., et al. (2021). Associations between using Chinese herbal medicine and long-term outcome among pre-dialysis diabetic nephropathy patients: A retrospective population-based cohort study. Front. Pharmacol. 12, 616522. doi:10.3389/fphar.2021.616522
Guo, J., Wu, W., Sheng, M., Yang, S., and Tan, J. (2013). Amygdalin inhibits renal fibrosis in chronic kidney disease. Mol. Med. Rep. 7, 1453–1457. doi:10.3892/mmr.2013.1391
Hsieh, C. F., Chang, H. C., Huang, S. L., Chen, C. L., Chen, W. T., and Yang, C. C. (2017). Prescribed renoprotective Chinese herbal medicines were associated with a lower risk of all-cause and disease-specific mortality among patients with chronic kidney disease: A population-based follow-up study in taiwan. Evid. Based. Complement. Altern. Med. 5632195, 5632195. doi:10.1155/2017/5632195
Hsieh, C. Y., Su, C. C., Shao, S. C., Sung, S. F., Lin, S. J., Kao Yang, Y. H., et al. (2019). Taiwan's national health insurance research database: Past and future. Clin. Epidemiol. 11, 349–358. doi:10.2147/CLEP.S196293
Hsu, T. W., Liu, J. S., Hung, S. C., Kuo, K. L., Chang, Y. K., Chen, Y. C., et al. (2014a). Renoprotective effect of renin-angiotensin-aldosterone system blockade in patients with predialysis advanced chronic kidney disease, hypertension, and anemia. JAMA Intern Med. 174, 347–354. doi:10.1001/jamainternmed.2013.12700
Hsu, Y. C., Lin, J. T., Ho, H. J., Kao, Y. H., Huang, Y. T., Hsiao, N. W., et al. (2014b). Antiviral treatment for hepatitis C virus infection is associated with improved renal and cardiovascular outcomes in diabetic patients. Hepatology 59 (4), 1293–1302. doi:10.1002/hep.26892
Huang, C. Y., Kuo, W. W., Kuo, C. H., Tsai, F. J., Liu, P. Y., and Hsieh, D. J. (2014). Protective effect of Danggui (Radix Angelicae Sinensis) on angiotensin II-induced apoptosis in H9c2 cardiomyoblast cells. Bmc. Complement. Altern. Med. 14, 358. doi:10.1186/1472-6882-14-358
Impellizzeri, D., Esposito, E., Attley, J., and Cuzzocrea, S. (2014). Targeting inflammation: New therapeutic approaches in chronic kidney disease (CKD). Pharmacol. Res. 81, 91–102. doi:10.1016/j.phrs.2014.02.007
Khan, M. A., Kassianos, A. J., Hoy, W. E., Alam, A. K., Healy, H. G., and Gobe, G. C. (2022). Promoting plant-based therapies for chronic kidney disease. J. Evid. Based. Integr. Med. 27, 2515690X221079688. doi:10.1177/2515690X221079688
Lin, M. Y., Chiu, Y. W., Chang, J. S., Lin, H. L., Lee, C. T., Chiu, G. F., et al. (2015). Association of prescribed Chinese herbal medicine use with risk of end-stage renal disease in patients with chronic kidney disease. Kidney. Int. 88 (6), 1365–1373. doi:10.1038/ki.2015.226
Lv, W., Booz, G. W., Fan, F., Wang, Y., and Roman, R. J. (2018). Oxidative stress and renal fibrosis: Recent insights for the development of novel therapeutic strategies. Front. Physiol. 9, 105. doi:10.3389/fphys.2018.00105
Ma, Z. G., Xia, H. Q., Cui, S. L., and Yu, J. (2017). Attenuation of renal ischemic reperfusion injury by salvianolic acid B via suppressing oxidative stress and inflammation through PI3K/Akt signaling pathway. Braz. J. Med. Biol. Res. 50 (6), e5954. doi:10.1590/1414-431X20175954
Mo, Z. Z., Lin, Z. X., Su, Z. R., Zheng, L., Li, H. L., Xie, J. H., et al. (2018). Angelica sinensis supercritical fluid CO(2) extract attenuates D-galactose-induced liver and kidney impairment in mice by suppressing oxidative stress and inflammation. J. Med. Food. 21 (9), 887–898. doi:10.1089/jmf.2017.4061
Palmer, B. F. (2004). Managing hyperkalemia caused by inhibitors of the renin-angiotensin-aldosterone system. N. Engl. J. Med. 351 (6), 585–592. doi:10.1056/NEJMra035279
Peng, A., Gu, Y., and Lin, S. Y. (2005). Herbal treatment for renal diseases. Ann. Acad. Med. Singap. 34 (1), 44–51.
Shariff, S. Z., Cuerden, M. S., Jain, A. K., and Garg, A. X. (2008). The secret of immortal time bias in epidemiologic studies. J. Am. Soc. Nephrol. 19 (5), 841–843. doi:10.1681/ASN.2007121354
Shen, Y., Wang, S., Liu, Y., Ge, L., Xia, L., Zhang, X., et al. (2020). The effects of salvianolate combined with western medicine on diabetic nephropathy: A systematic review and meta-analysis. Front. Pharmacol. 11, 851. doi:10.3389/fphar.2020.00851
Tang, F., Yan, H. L., Wang, L. X., Xu, J. F., Peng, C., Ao, H., et al. (2021). Review of natural resources with vasodilation: Traditional medicinal plants, natural products, and their mechanism and clinical efficacy. Front. Pharmacol. 12, 627458. doi:10.3389/fphar.2021.627458
Tinti, F., Lai, S., Noce, A., Rotondi, S., Marrone, G., Mazzaferro, S., et al. (2021). Chronic kidney disease as a systemic inflammatory syndrome: Update on mechanisms involved and potential treatment. Life (Basel) 11 (5), 419. doi:10.3390/life11050419
Vitale, M., Haxhi, J., Cirrito, T., and Pugliese, G. (2020). Renal protection with glucagon-like peptide-1 receptor agonists. Curr. Opin. Pharmacol. 54, 91–101. doi:10.1016/j.coph.2020.08.018
Wang, H., Song, H., Yue, J., Li, J., Hou, Y. B., and Deng, J. L. (2012). Rheum officinale (a traditional Chinese medicine) for chronic kidney disease. Cochrane. Database. Syst. Rev. 7, CD008000. doi:10.1002/14651858.CD008000.pub2
Wang, J., Li, X., Chang, H., and Si, N. (2023). Network pharmacology and bioinformatics study on the treatment of renal fibrosis with persicae semen-carthami flos drug pair. Med. Baltim. 102, e32946. doi:10.1097/MD.0000000000032946
Wang, Y. C., Juan, S. H., Chou, C. L., Hsieh, T. C., Wu, J. L., and Fang, T. C. (2020). Long-term effects of ketoanalogues on mortality and renal outcomes in advanced chronic kidney disease patients receiving a low-protein diet. Nutrients 12, 2708. doi:10.3390/nu12092708
Xu, L., Shen, P., Bi, Y., Chen, J., Xiao, Z., Zhang, X., et al. (2016). Danshen injection ameliorates STZ-induced diabetic nephropathy in association with suppression of oxidative stress, pro-inflammatory factors and fibrosis. Int. Immunopharmacol. 38, 385–394. doi:10.1016/j.intimp.2016.06.024
Yeh, C. H., Chang, C. K., Cheng, K. C., Li, Y. X., Zhang, Y. W., and Cheng, J. T. (2011). Role of bone morphogenetic proteins-7 (BMP-7) in the renal improvement effect of DangGui (Angelica sinensis) in type-1 diabetic rats. Evid. Based. Complement. Altern. Med. 796723, 796723. doi:10.1093/ecam/nep167
Zhang, D. W., Wang, S. L., Wang, P. L., Du, J. P., Gao, Z. Y., Wang, C. L., et al. (2020). The efficacy of Chinese herbal medicines on acute coronary syndrome with renal insufficiency after percutaneous coronary intervention. J. Ethnopharmacol. 248, 112354. doi:10.1016/j.jep.2019.112354
Zhang, H. W., Lin, Z. X., Xu, C., Leung, C., and Chan, L. S. (2014). Astragalus (a traditional Chinese medicine) for treating chronic kidney disease. Cochrane. Database. Syst. Rev. 10, CD008369. doi:10.1002/14651858.CD008369.pub2
Zhong, Y., Deng, Y., Chen, Y., Chuang, P. Y., and He, J. C. (2013). Therapeutic use of traditional Chinese herbal medications for chronic kidney diseases. Kidney. Int. 84 (6), 1108–1118. doi:10.1038/ki.2013.276
Keywords: Angelica sinensis, ESRD, CKD, death, dose–response relationship, hyperkalemia risk, renoprotection
Citation: Chen H-T, Yu B-H, Yeh M-H, Hung S-K and Chen Y-C (2023) Dose- and time-dependent renoprotection of Angelica sinensis in patients with chronic kidney disease: A longitudinal cohort study. Front. Pharmacol. 14:1153583. doi: 10.3389/fphar.2023.1153583
Received: 29 January 2023; Accepted: 05 April 2023;
Published: 25 April 2023.
Edited by:
Michael Heinrich, University College London, United KingdomReviewed by:
Yiyi Ma, Shanghai Changzheng Hospital, ChinaCopyright © 2023 Chen, Yu, Yeh, Hung and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi-Chun Chen, YWxpbmV5Y2NAZ21haWwuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.