- 1Fang Zheng Center, Institute of Basic Theory for Chinese Medicine, China Academy of Chinese Medical Sciences, Beijing, China
- 2Experimental Research Center, China Academy of Chinese Medical Sciences, Beijing, China
- 3Department of Pharmacy, Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing, China
Revealing the connotation of the compatibility of Chinese medicines (CM) is a requirement for the modernization of traditional Chinese medicine (TCM). However, no consensus exists on the specific mechanism of traditional Chinese medicine compatibility (TCMC). Many studies have shown that the occurrence and development of diseases and the efficacy of CM are closely related to intestinal flora (IF), which may provide a new perspective to understand the theory of TCM. This study aimed to summarize the relationship between the changes in IF before and after the compatibility of different drugs and the synergistic, toxicity reduction, and incompatibility effects of drug pairs from the perspective of the effects of CM on the IF and the regulation of microbial metabolites. These studies showed that the effect of drug pairs on the composition of the IF is not a simple superposition of two single drugs, and that the drug pairs also play a specific role in regulating the production of intestinal bacterial metabolites; therefore, it has a different pharmacodynamic effect, which may provide a perspective to clarify the compatibility mechanism. However, research on the interpretation of the scientific connotations of TCMC from the perspective of the IF is still in its infancy and has limitations. Therefore, this study aimed to summarize previous research experience and proposed to conduct a deep and systematic study from the perspective of drug pair dismantling, IF, intestinal bacteria metabolite, organism, and disease to provide a reference for scientific research on the compatibility mechanism of CM.
1 Introduction
Many Chinese medicines (CM) are effective but toxic to humans. By combining different CM, adjusting the bias, restraining the toxicity, and taking advantage of the strength of the efficacy of the CM, toxicity can be reduced and effectiveness can be increased. This combination has been widely recognized by ancient and modern physicians and is a feature of the clinical application of traditional Chinese medicine (TCM). In the compatibility theory of TCM, the mutual reactions between drugs can be summarized into seven situations, named “Qi Qing,” including “Dan Xing,” “Xiang Xu,” “Xiang Shi,” “Xiang Wei,” “Xiang Sha,” “Xiang Wu,” and “Xiang Fan.” Disclosing the connotation of the TCMC is required to modernize TCM; however, no consensus exists on the specific mechanism of traditional Chinese medicine compatibility (TCMC). The inability to scientifically clarify the connotations of compatibility has somewhat limited the development of TCM.
Intestinal flora (IF) has become a popular research topic in recent years. Imbalance of the IF is not only related to intestinal diseases, but also to hepatic, cardiovascular, and neurological diseases through the intestine-liver, intestine-heart, and intestine-brain axis (Lin Z. et al., 2016; Sampson et al., 2016). With the popularization of gene sequencing technology, several studies have shown that CM can promote beneficial bacteria, inhibit harmful bacteria, regulate the metabolites of bacteria, such as bile acids (Bas) and short-chain fatty acids (SCFAs), and thus exert a regulatory effect on the organism (Xu et al., 2017).
The IF is a key link between efficacy and CM. TCM theory states that a healthy human body needs not only to maintain harmony and unity with the external environment but also to maintain the balance of the internal environment. Maintaining the stability of the IF conforms to the concept of “holism” of TCM, and the IF can provide a new perspective to understand the TCM theory. From the perspective of the effect of CM on the IF and the regulation of microbial metabolites, this study summarized the relationship between the changes in the IF and the effects of synergism and toxicity reduction after CM combination, with a view for providing ideas for future systematic studies of the mechanism of CM compatibility.
2 Current situation of research on the relationship between the synergism effect of TCMC and IF
The principle of “Qi Qing” in TCM achieves the result of compatibility and synergy. “Xiang Xu” refers to a combination of drugs with similar performance, which have synergistic effects and enhance the original efficacy. “Xiang Shi” refers to a combination of drugs that have certain similarities in performance and efficacy, with one drug as the main drug and one as a supplement to improve the efficacy of the main drug. Both “Xiang Xu” and “Xiang Shi” can play a synergistic role. We believe that the increase in therapeutic efficacy is related to the specific regulation of the IF by the combination of the two drugs. The combination of two drugs may have a stronger regulatory effect on a certain bacterium, resulting in one plus one being greater than two, or it may be that the combination has a specific regulatory effect on a new bacterium and a different effect from that of a single drug. This may be one of the ways to clarify the synergistic effect of compatibility.
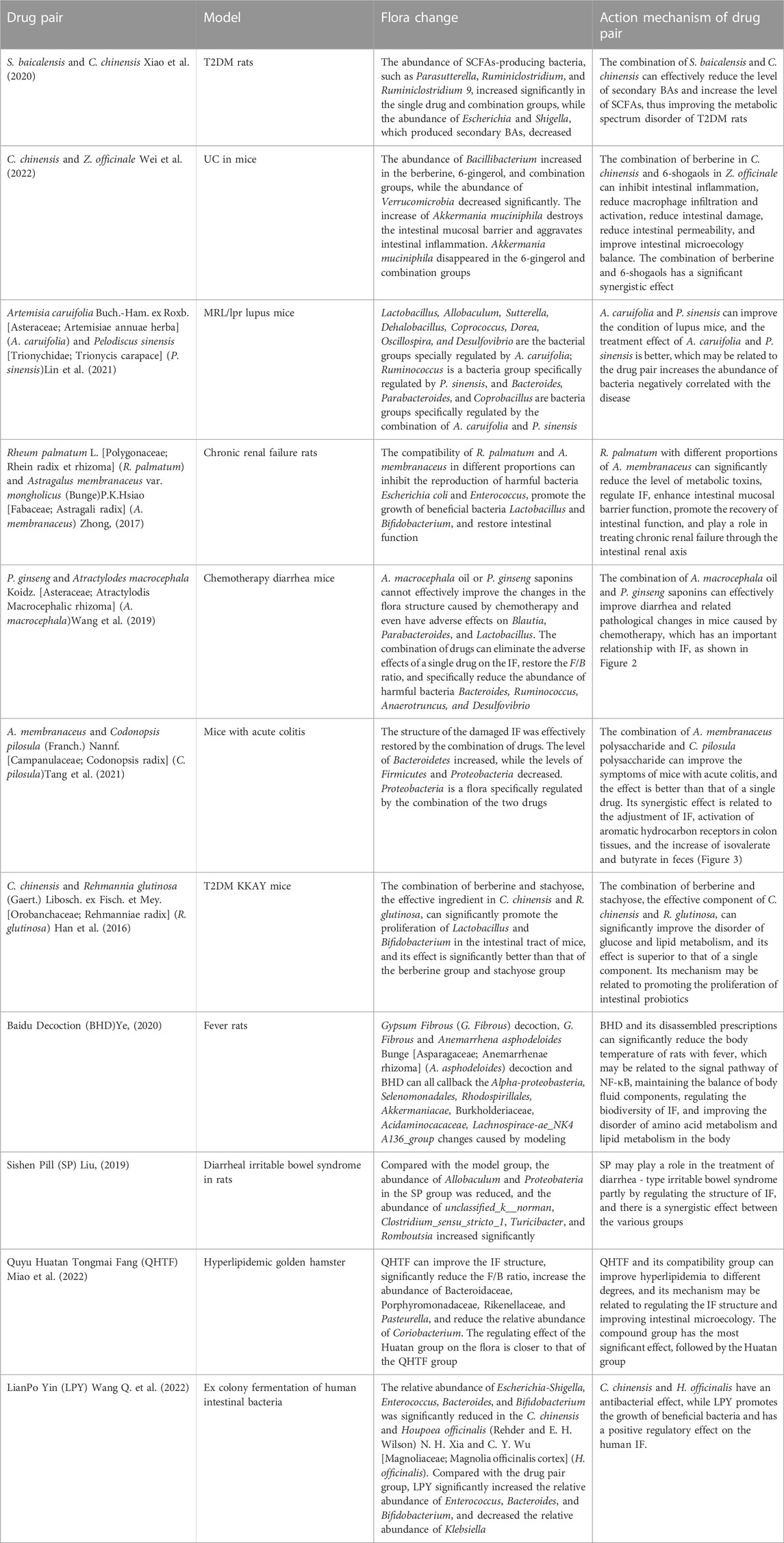
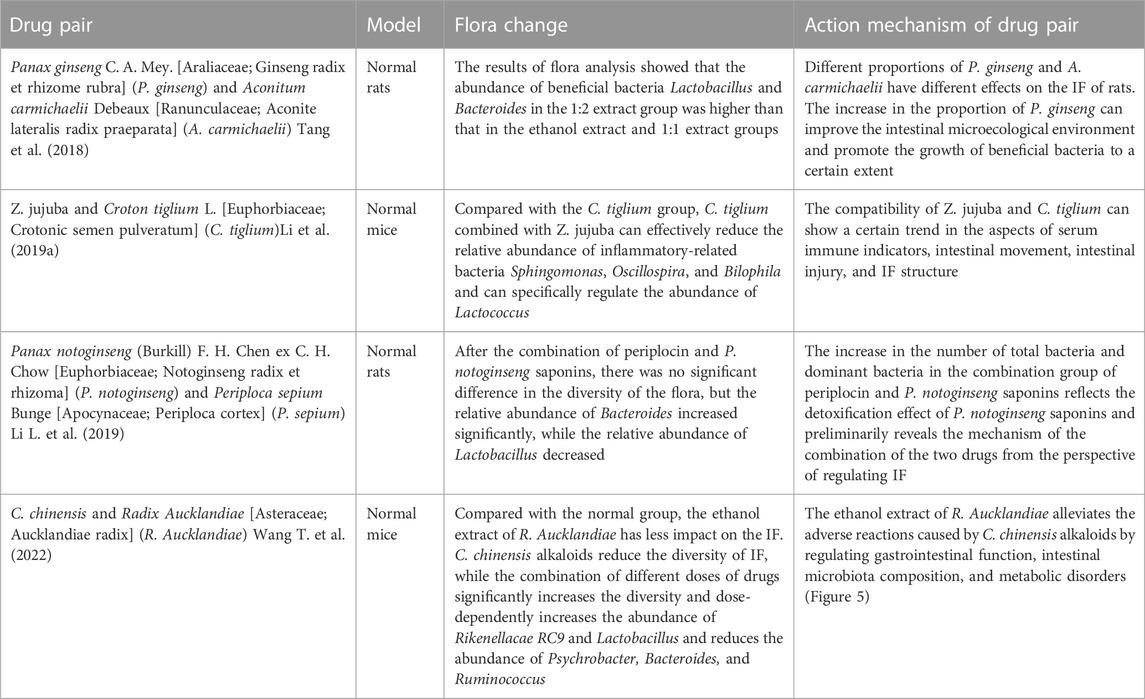
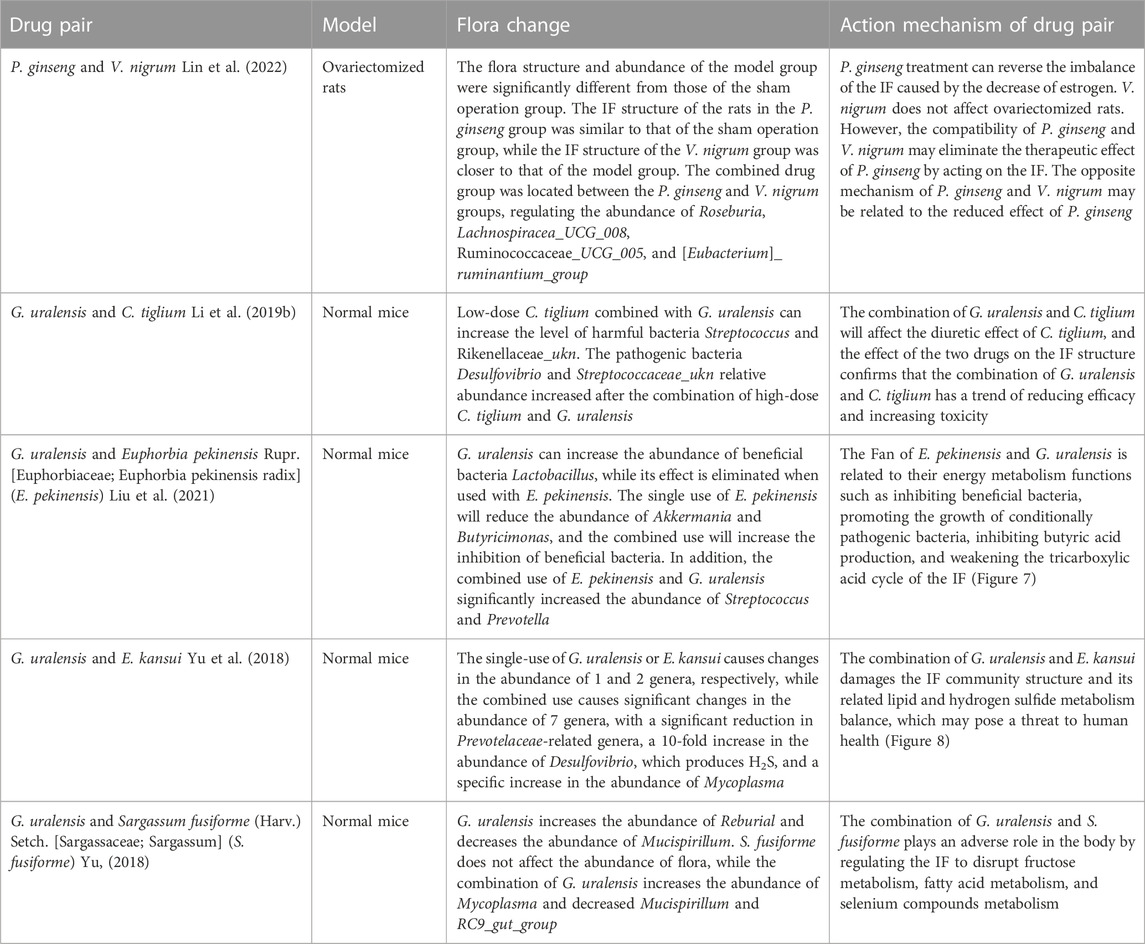
Scutellaria baicalensis Georgi (Lamiaceae; Scutellaria radix) (Scutellaria baicalensis)- Coptis chinensis Franch (Ranunculaceae; Coptidis Rhizoma) (C. chinensis) compose a classical “drug pair” applied in clinical practice to dispel heat, dryness, and dampness. Hyperglycemia, dyslipidemia, inflammation, and insulin resistance in type 2 diabetes mellitus (T2DM) were ameliorated after oral administration of S. baicalensis and C. chinensis, particularly the combined extract. Moreover, the effects of the combined extracts were more remarkable than those of the single-drug treatment (Cui et al., 2018). The unique efficacy of S. baicalensis—C. chinensis may be related to the regulation of glucose and lipid metabolism and improvement of the IF (Ding et al., 2019). In vitro experiments showed that single or combined use of S. baicalensis and C. chinensis can promote the growth of beneficial bacteria Bifidobacteria and Lactobacilli in the intestinal tract of normal and T2DM model rats and inhibit the growth of harmful bacteria Enterococcus and Enterobacter; and the effect of drug pairs is stronger than that of a single drug (Xu, 2014). Acidic metabolites of beneficial intestinal bacteria, such as Bifidobacteria and Lactobacilli, can reduce the local pH of the intestine and produce substances with broad-spectrum antibacterial effects, thereby improving intestinal function by inhibiting the growth of intestinal and conditional pathogens. This indicates that the combination of S. baicalensis and C. chinensis can have a positive effect on IF. Liu (Liu et al., 2022) studied the effects of separate and combined applications of S. baicalensis and C. chinensis on ulcerative colitis (UC) induced by the administration of dextran sulfate sodium (DSS) in mice, as shown in Figure 1. These results revealed that the combined application of S. baicalensis and C. chinensis significantly relieved colon inflammation in mice. Notably, the protective effects of S. baicalensis and C. chinensis against colon inflammation were weakened when the antibiotic mixture was partially consumed by the gut microbiota. A fecal microbial transplantation experiment further proved that the therapeutic effects of S. baicalensis and C. chinensis on UC were closely related to IF. The results of 16S rRNA sequencing showed that the group treated with combined applications of S. baicalensis and C. chinensis exhibited a higher intestinal microbial diversity and the IF composition than those of the separate groups; the abundance of norman_f_Muribaculateae increased relatively, and the abundance of Bacteroides, Akkermania and Lactobacillus also changed, but the difference was not significant. Correlation analysis showed that the bacterial flora regulated by S. baicalensis and C. chinensis was closely related to inflammatory factors in UC treatment. These results indicate that the therapeutic effect of the combination of S. baicalensis and C. chinensis is better than that of a single drug, which is related to the regulation of the IF and inhibition of inflammation.
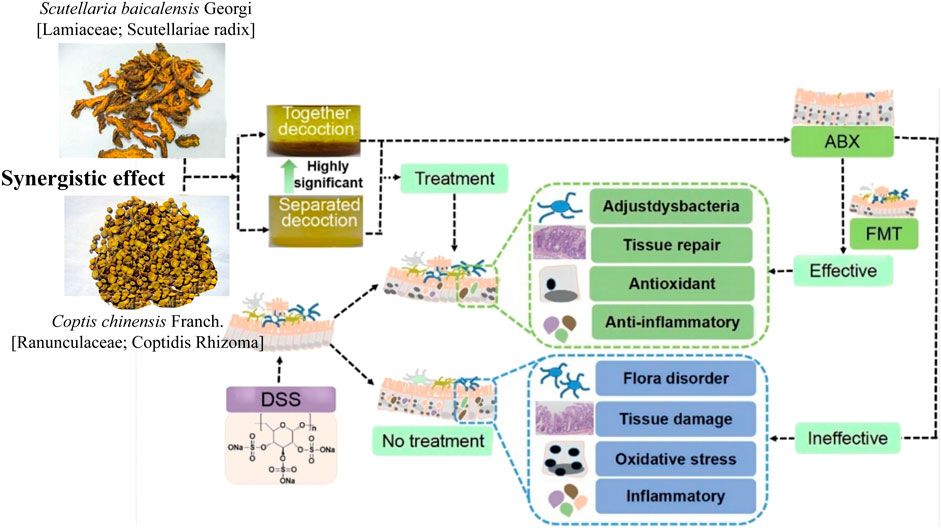
FIGURE 1. Therapeutic effect of the combination of S. baicalensis and C. chinensis on DSS-induced ulcerative colitis in mice by acting on intestinal flora (DSS, dextran sulfate sodium; ABX: Antibiotic interference, FMT: Colony transplantation) (Liu et al., 2022).
S. baicalensis and Sophora japonica Linn (Fabaceae; Sophora Flos) (S. japonica) were originally obtained from Renzhai Zhizhi and are clinically applicable to hypertensive patients with hyperactive liver fires. Guan (Guan et al., 2021) established spontaneously hypertensive models to explore the renal protective effects of a combination of S. baicalensis and S. japonica against chronic kidney disease. The results showed that the combination of S. baicalensis and S. japonica significantly ameliorated the severity of renal injury induced by hypertension compared with effectiveness of single drugs. The antihypertensive effect and renal protection of S. baicalensis and S. japonica are affected after the bacterial flora is disturbed by antibiotics, which indicates that the combination of S. baicalensis and S. japonica plays a therapeutic role by acting on IF. The regulation of the intestinal microecological balance may be a mechanism of action of S. baicalensis and S. japonica in the treatment of hypertension and renal damage. The regulatory effect of the combination of S. baicalensis and S. japonica on the IF was different from that of the single drugs. Compared with the model group, the diversity of the IF in the combination group increased, and the ratio of Firmicutes/Bacteroidetes (F/B) decreased. Compared with model group, the relative abundances of Prevotella-9 and Akkermansia were higher in the S. baicalensis group, whereas those of Corynebacterium and Prevotella-9 were increased in the S. japonica group. The relative abundance of Lactobacillus increased, and that of Clostridiales decreased in the S. baicalensis and S. japonica group. Prevotella-9, Lactobacillaceae, and Bifidobacteriaceae are beneficial bacteria. Lactobacillus can reduce the serum cholesterol level of hyperlipidemic rat models by improving the balance between intestinal microorganisms and increasing the intestinal transit time (Xie et al., 2011), which is closely associated with metabolic diseases. Clostridiaceae, an indole-positive bacterium, is positively correlated with indole, which has negative effects on the kidney (Niwa, 2013). With an increase in the abundance of dominant bacteria, the intestinal barrier improves, and the change in dominant bacteria reduces indole accumulation, further inhibiting oxidative stress activation in the kidneys. Olfr78 regulates renin secretion and increases blood pressure. Activated GPR41 relaxes blood vessels and lowers blood pressure. S. baicalensis and S. japonica increased SCFA production, inhibited the release of inflammatory factors, and regulated blood pressure by decreasing the expression of Olfr78 and increasing GPR41 expression, thereby alleviating kidney damage. These results indicate that the hypotensive effects of S. baicalensis and S. japonica in rats may be related to the regulation of IF, thereby increasing SCFA levels (Pluznick, 2014).
Gegen Qinlian Decoction (GQD), derived from the Treatise on Febrile Diseases, is a typical prescription for the clinical treatment of acute enteritis which is composed of Pueraria montana var. Lobata (Willdenow) Maesen and S. M. Almeida ex Sanjappa & Predeep [Fabaceae; Pueraria Lobata Radix] (P. montana), S. baicalensis, C. chinensis, and Glycyrrhiza uralensis Fisch (Fabaceae; Glycyrrhizae radix et rhizoma) (G. uralensis). The study found that GQD can restore the diversity of IF and significantly increase the relative abundance of bacteria that generate SCFAs, thus increasing the concentrations of acetic acid, propionic acid, and butyric acid in feces. Increased SCFAs can inhibit the HADC and NF-κB pathways to alleviate inflammatory reactions in the intestinal mucosa. GQD treatment of diarrhea may modulate the gut microbiota and increase SCFA levels (Liu et al., 2019). Chen (Chen et al., 2020) found that GQD and its different compatibilities had different therapeutic effects on acute enteritis, and GQD and the whole prescription without G. uralensis had more obvious anti-inflammatory and mucosal reconstruction and ulcer repair effects on colon tissue. Based on this difference, Chen analyzed the diversity of IF. Alpha and beta diversity showed that the IF composition in each group was significantly different. Compared with the model group, GQD and its different compatibilities significantly reduced the relative abundance of Clostridium_sensu_stricto_1 which is associated with intestinal inflammatory diseases. It can be seen from the results of the group of GQD without S. baicalensis and C. chinensis that the combination of P. montana and G. uralensis increases the abundance of Bacteroidales_S24-7_ukn, which is a beneficial bacterium, and Allobaculum, which is an SCFA-producing bacterium, while the abundance of pathogenic bacteria Parabacteroides decreases, but at the same time, the abundance of Desulfovibrio, which is toxic to colon cells, increases. The genomes of Bacteroidales_S24-7_ukn and Akkermania both encode the ability to produce propionate, and the increase of propionate is closely related to the stability of intestinal inflammation (Borton et al., 2017); Allobaculum can rapidly ferment glucose to produce lactic acid and butyric acid; Parabacteroides, as a pathogen in infectious diseases, can induce inflammation and immune disorder (Larsen, 2017); Desulfovibrio can damage the intestinal barrier by producing lipopolysaccharides (Beerens and Romond, 1977). These results indicated that the combination of P. montana and G. uralensis can inhibit the occurrence of inflammation and metabolic disorders. The results showed that the combination of S. baicalensis and C. chinensis increased the relative abundance of the beneficial bacterium Akkermannia and decreased that of the pathogenic bacterium Parabacteroides, indicating that the combination of S. baicalensis and C. chinensis plays an important role in regulating IF, and this compatibility could play a positive role in acute enteritis. Simultaneously, Allobaculum abundance decreased in the S. baicalensis and C. chinensis group. Combined with the results of the GQD group, the GQD without the G. uralensis group and the GQD without P. montana, shows that the compatibility of G. uralensis and P. montana also plays a key role in the regulation of IF. Therefore, it was concluded that S. baicalensis and C. chinensis are the key components in GQD that regulate the balance of IF, and the compatibility of G. uralensis and P. montana enhances the regulation of IF. It can be found that there is a complex network relationship between disease, flora and drugs. The differences at the gene level between different administration groups and model groups may be the biological basis for the different compatibilities of GQD to produce different effects.
Banxia Xiexin Decoction (BXD), derived from the Treatise on Febrile Diseases, is widely used to treat digestive system diseases, such as gastritis, enteritis, gastric ulcer, and gastrointestinal dysfunction. The whole prescription can be divided into the “Xinkai” compatibility unit of the combination of Pinellia ternata (Thunb.) Ten. ex Breitenb (Araceae; Pinelliae rhizoma) (P. ternata) and Zingiber officinale Roscoe (Zingiberaceae; Zingiberis rhizoma) (Z. officinale), the “Kujiang” compatibility unit of the combination of S. baicalensis and C. chinensis,, and the “Ganbu” compatibility unit of the combination of Panax ginseng C. A. Mey (Araliaceae; Ginseng radix et rhizoma) (P. ginseng), Ziziphus jujuba Mill (Rhamnaceae; Jujube fructus) (Z. jujuba), and G. uralensis. Previous studies have shown that BXD can reduce intestinal inflammation and treat ulcerative colitis by improving IF imbalance (Chen et al., 2021). Studies have shown that the coordination between IF, tight junction proteins, and the intestinal mucosal barrier plays an important role in maintaining the steady state of the intestinal barrier (Fan et al., 2019). Therefore, Zhang (Zhang et al., 2021; Dai et al., 2022) believed that antibiotic exposure leads to IF disorder in young rats, thus damaging the intestinal mucosal barrier, and that BXD and different disassembled prescriptions can regulate the IF structure, protect the intestinal mucosal barrier from pathological damage caused by antibiotic exposure, and improve the immune response. After antibiotic interference, the IF of young rats changed significantly. After treatment, the difference in the IF between the BXD group and blank group was significantly reduced, and the recovery effect of the BXD group was the best. By studying the flora composition at the genus level, it can be found that, compared with the model group, the BXD group and the different disassembled formula groups can significantly reverse the increase of Klebsiella and Enterobacter abundance caused by modeling, and the effect of “Xinkai” group is the most significant. At the same time, the abundance of Bacteroides and Lactobacillus increased in each treatment group, and the increase in Lactobacillus abundance in the BXD group was the most significant. The abundance of Bacteroides in “Xinkai” group and “Ganbu” group was the highest. Enterobacter is a common pathogenic bacterium that can be colonized by host inflammatory reactions to further increase the severity of intestinal inflammation (Li et al., 2020). Klebsiella is a conditional pathogen that causes respiratory and digestive tract infections (Li J. et al., 2019). Bacteroides play important roles in intestinal mucosal angiogenesis, intestinal microecological balance, and host immunity. Lactobacillus has beneficial effects on intestinal inflammation, oxidative stress, and symbiosis of microbiota (El-Baz et al., 2020). In summary, BXD and different decoctions can adjust the IF structure of antibiotic-exposed young rats. Among them, the “Ganbu” and “Xinkai” decoctions play a central role. The “Xinkai” group can effectively reduce the abundance of pathogenic bacteria, and has more advantages in regulating the balance of flora, while the “Ganbu” group can effectively increase the abundance of probiotics. Liang (Liang et al., 2021) studied the effect of BXD and its compatibility with gastrointestinal bacteria using in vitro antibacterial and bacteriostatic activity tests. Helicobacter pylori infection is closely associated with chronic gastritis and gastric mucosal damage. The research results show that the whole formula group has good bacteriostatic and bactericidal effects on H. pylori, followed by “Kujiang” group. The BXD and different compatibilities also have inhibitory effects on two harmful intestinal bacteria, Escherichia cloacae and Enterococcus faecalis, to varying degrees and are dose-dependent within a certain concentration range. The antibacterial effect of the BXD group and “Kujiang” group is the strongest. Therefore, it was speculated that the material basis of BXD against harmful bacteria is mainly composed of Z. officinale, S. baicalensis, and C. chinensis. When observing the effect of BXD on beneficial bacteria, it was found that the growth of beneficial bacteria was inhibited in “Kujiang” group, while the growth of Bifidobacteria adolescentis and Lactobacillus acidophilus was promoted in the whole recipe group, “Ganbu” group and “Xinkai” group within a certain concentration range. Thus, it is speculated that the “Kujiang” group in BXD can effectively inhibit the growth of pathogenic bacteria in vitro, while the “Ganbu” group can promote the proliferation of beneficial bacteria.
Furthermore, many studies have reported on the relationship between the synergistic effects of TCMC and IF, as shown in Table 1.

FIGURE 2. Combined use of A. macrocephala oil or P. ginseng saponins decreases chemotherapy-induced diarrhea in mice by affecting intestinal flora (Wang et al., 2019).
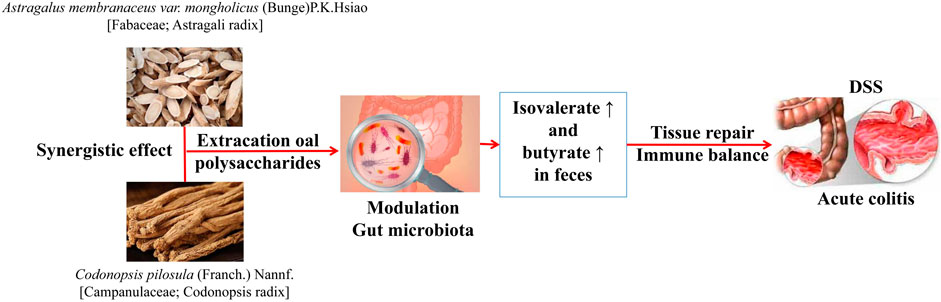
FIGURE 3. Therapeutic effect of A. membranaceus polysaccharide combined with C. pilosula polysaccharide on acute colitis mice by acting on intestinal flora (Tang et al., 2021).
3 Current situation of research on the relationship between the attenuation effect of TCMC and IF
Reasonable compatibility reduces drug toxicity and expands the scope of clinical applications. Although the mechanism of CM toxicity is very complex, current research shows that the IF is also an important factor affecting the toxicity of CM. The principle of “Xiang Wei” and “Xiang Sha” in “Qi Qing” achieves the result of toxicity reduction. “Xiang Wei” refers to the toxicity or side effects of one drug can be eliminated by another drug, and “Xiang Sha” refers to one drug can alleviate or eliminate the toxicity or side effects of another drug. “Xiang Wei” and “Xiang Sha” illustrate the same problem from two perspectives. We believe that the elimination or alleviation of toxic effects is related to the specific regulation of the IF by the combination of the two drugs. CM with toxicity or side effects may affect the structure of the IF, reduce the abundance of beneficial bacteria, and increase the abundance of harmful bacteria. After compatibility, the negative effects of CM with toxicity or side effects on the IF are eliminated, which has a positive effect on the body.
The combination of Glycine max (Linn.) Merr (Fabaceae; Sojae Semen Praeparatum) (G. max) and Gardenia jasminoides J. Ellis (Rubiaceae; Gardenia fructus) (G. jasminoides) is from the Zhizi Chi Decoction (ZCD) in Zhongjing Zhang’s Treatise on Febrile Diseases which is a classic prescription for treating insomnia caused by heat stagnation chest diaphragm (Shi et al., 2012). The combination of these two drugs reduced the liver toxicity of G. jasminoides. Luo (Luo et al., 2021) suggested that the improvement of G. max in G. jasminoides -induced liver injury was related to the IF. At the same dose, the hepatotoxicity of ZCD was significantly lower than that of the G. jasminoides. The IF analysis revealed that G. jasminoides affected the IF composition of mice, reduced the abundance of Lactobacillus and Enterococcus, and increased the abundance of Parasutterella. However, the abundance of the beneficial bacteria Akkermania and Prevotella increased significantly after G. jasminoides was combined with G. max. Prevotella can promote glycogen storage in the mouse liver and maintain glucose homeostasis in the host (Purushe et al., 2010). In addition, G. jasminoides reduced the level of butyrate in feces, which was reversed after combination with G. max. When the level of butyrate increases, it plays a protective role in the liver by improving the integrity of the colon and promoting the activation of Nrf2. The combination of G. max and G. jasminoides cured G. jasminoides—induced liver injury by regulating the microbiota and promoting butyrate production (Figure 4). Chen (Chen L. et al., 2019) found that ZCD can maintain the relative balance of the IF better than G. max or G. jasminoides can, via an in vitro study. Therefore, G. jasminoides has a negative impact on the IF, and the compatibility of G. max and G. jasminoides can not only benefit the IF but also positively reverse the disorder of the IF caused by G. jasminoides.
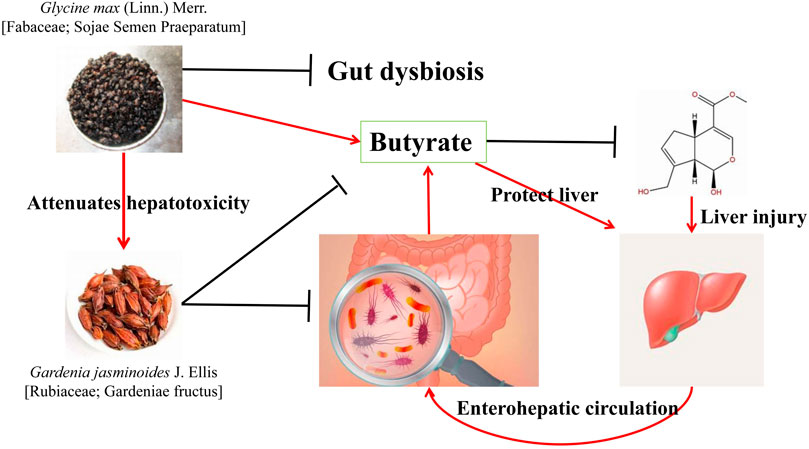
FIGURE 4. Combination of G. max and G. jasminoides reduces the toxicity of G. max by affecting the intestinal flora (Luo et al., 2021).
Realgar is a mineral and heavy-metal CM with significant therapeutic effects in the treatment of leukemia and various solid tumors. However, there are several adverse reactions, including intestinal, cardiac, and liver toxicities. The compatibility of Realgar and S. miltiorrhiza Bunge (Lamiaceae; Salvias miltiorrhizae radix et rhizoma) (Salvia miltiorrhiza) was derived from the Compound Huangdai Tablet, which was formulated by Professor Shilin Huang. Clinical practice has confirmed that the treatment for acute promyelocytic leukemia is effective, with a high cure rate and mild adverse reactions (Zhu et al., 2018). Experiments have shown that the combination of Realgar and S. miltiorrhiza can effectively alleviate adverse reactions caused by Realgar, such as those involving the heart and liver (Wang et al., 2008). Sun (Sun, 2020) found that Realgar affects the IF composition of normal mice in a dose-dependent manner, reduces the abundance of Firmicutes and Bacteroidetes, and increases the abundance of Proteobacteria. Disturbances in the IF make Realgar more toxic and cause higher mortality. After transplanting normal mouse flora into a model of flora disorder, the toxicity of Realgar could be alleviated by improving the IF disorder. This indicates that the IF is a key factor in the toxicity of Realgar. In an acute promyelocytic leukemia (APL) mouse model, Realgar increased the intestinal permeability of APL mice. When combined with S. miltiorrhiza, S. miltiorrhiza can reversed the adverse effects of Realgar. Akkermansia_muciniphila is a specific flora regulated by S. miltiorrhiza combined with Realgar, and is related to the occurrence of colitis. Therefore, Realgar can disturb the IF of APL mice and improve intestinal permeability in APL mice. S. miltiorrhiza reduces intestinal permeability and alleviates Realgar toxicity by repairing the intestinal mucosal barrier, which may be associated with IF.
Moreover, many studies have investigated the relationship between the attenuation effect of TCMC and the IF, as shown in Table 2.
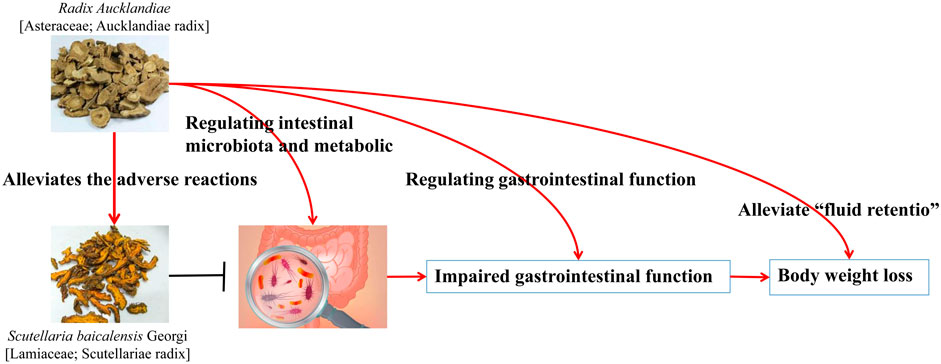
FIGURE 5. Adverse reactions to the ethanol extract of R. Aucklandiae caused by Coptidis alkaloids by regulating the composition of intestinal microflora (Wang T. et al., 2022).
4 Current situation of research on the relationship between the incompatibility effect of CM and IF
“Xiang Wu” and “Xiang Fan” are both contraindicated combination of TCM. “Xiang Wu” refers to one drug acting in combination with another, resulting in reduced or even loss of efficacy. For example, the effect of P. ginseng on promoting energy metabolism, regulating immune and antioxidation in the spleen qi deficiency rats were decreased after the compatibility of P. ginseng and V. nigrum L (Melanthiaceae; Veratrum nigrum) (Veratrum nigrum) (Lin H. et al., 2016). “Xiang Fan” refers to the occurrence of severe toxic reactions or side effects when two drugs are combined. Chen (Chen Y.Y. et al., 2019) conducted a contraindication evaluation on the compatibility of D. genkwa Siebold & Zucc (Thymelaeaceae; Genk flos) (Daphne genkwa) and G. uralensis, and found that the combination of D. genkwa s and G. uralensis showed severe liver, kidney, and reproductive organ toxicity in rats. Euphorbia kansui T. N. Liou ex S. B. Ho (Euphorbiaceae; Kansui radix) (E. kansui) alone has no obvious toxicity, but it can show toxicity when combined with G. uralensis, and the toxicity increases with the increase of the proportion of G. uralensis (Juan et al., 2015). The “Shiba Fan” obtained by summarizing the rule is one of the most representative theories of TCM contraindicated combination. Although the “Shiba Fan” of TCM has existed for millennia, and there are many studies about the mechanism in recent decades, and even the Pharmacopoeia stipulates that “Shiba Fan” cannot be used together in the form of law, the specific mechanism of “Fan” has not yet been proved. After summarizing previous studies, we believe that “Xiang Wu” or “Xiang Fan” of the two drugs is also related to the regulation of IF. We speculated that a drug plays a better therapeutic role by increasing the abundance of beneficial bacteria and decreasing the abundance of harmful bacteria. However, when combined with another drug, the structure of the IF changes, resulting in reduced efficacy or even loss of efficacy. This may be a possible mechanism for the effect of “Xiang Wu.” The possible mechanism of “Xiang Fan” may be that the compatibility of the two drugs specifically increases the abundance of harmful bacteria and decreases the abundance of beneficial bacteria, so it manifests as a toxic reaction or side effect.
The “Fan” drug combination of G. uralensis and D. genkwa is the representative combination in the “Shiba Fan”. Yu (Yu et al., 2019) found that, compared with the use of G. uralensis or D. genkwa alone, the combination of G. uralensis and D. genkwa significantly changed the IF structure in mice. G. uralensis or D. genkwa use alone caused the abundance of 3 and 2 genera to change, respectively, whereas combined use caused the abundance of 13 genera to change significantly. Among them, the combination of G. uralensis or D. genkwa specifically increased the abundance of Bacillus and increased the abundance of Desulfovibrio, which produced H2S nine times, indicating that the combination of G. uralensis and D. genkwa greatly enhanced their ability to regulate the IF community structure. Macrogenomic prediction analysis showed that hydrogen sulfide metabolism-related genes appeared in the first 20 differential chemical reactions caused by G. uralensis or D. genkwa, and the abundance of these 10 genes further increased in the combined G. uralensis and D. genkwa group. Moreover, through the detection of hydrogen sulfide levels in mouse feces and serum, it was found that the combination of G. uralensis and D. genkwa significantly increased the content of hydrogen sulfide in mouse feces and significantly reduced the concentration of hydrogen sulfide in mouse serum, indicating that the combination of G. uralensis and D. genkwa could disrupt the metabolic balance of hydrogen sulfide in the mouse intestine. The combination of G. uralensis and D. genkwa showed obvious negative effects in regulating the IF community structure and hydrogen sulfide metabolism, which may be related to “increasing toxicity” (Figure 6).
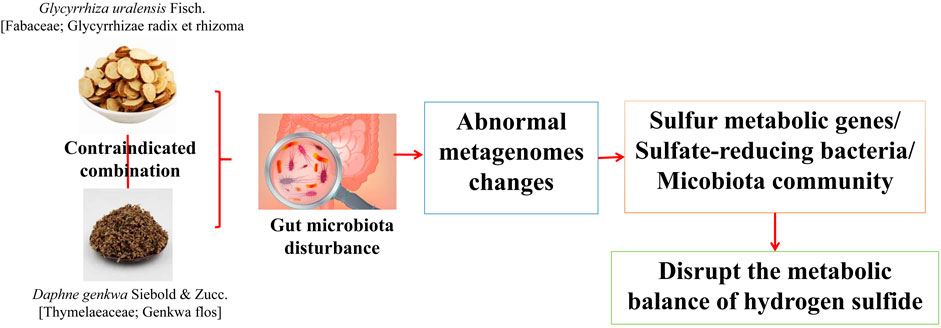
FIGURE 6. Combination of G. uralensis and D. genkwa produces toxic and side effects by affecting intestinal flora (Yu et al., 2019).
Tao (Tao et al., 2018) studied the toxicity and side effects of a combination of Euphorbia lathyris L (Euphorbiaceae; Euphorbia Semen) (E. lathyris) and G. uralensis in normal mice and found that G. uralensis had no significant impact on the gastrointestinal tract. E. lathyris damages the intestinal mucosa, thus damaging the intestinal barrier function and weakening gastrointestinal motility regulation. The combination of G. uralensis and E. lathyris significantly enhanced E. lathyris damage to the intestinal mucosa. The results of the intestinal microbial analysis showed that G. uralensis, E. lathyris, and their combination caused changes in the IF structure. The levels of beneficial bacteria, Lactobacillus, were significantly reduced after E. lathyris administration, reflecting the intestinal toxicity of E. lathyris. The characteristic differences caused by the combination of G. uralensis and E. lathyris included Enterococcus, S24_ 7_ ukn, Candidatus arthromitus, Roseburia, and Erysipelotrichaceae_incertae_se-dis. Different bacterial populations with increased abundance were associated with toxicity and side effects to varying degrees. Enterococcus is a common opportunistic pathogen and S24_ 7_ Un is one of the main lipopolysaccharide synthesizers in animal intestines. The increase in this bacterium will lead to an increase in intestinal endotoxin production, thus disrupting intestinal immune function or damaging intestinal mucosa (Kang et al., 2017); Erysipelothrichaceae is involved in the pathogenesis of chronic heart failure, and this flora is one of the core bacteria missing in patients with chronic heart failure (Luedde et al., 2017). According to the IF analysis, the combination of G. uralensis and E. lathyris probably aggravates intestinal injury through the abnormal regulation of the IF and its function. The results of the metagenomic analysis showed that the combination of G. uralensis and E. lathyris increased the content of genes related to aromatic amino acid degradation and mucus degradation functions, which was significantly different from the single-use group. This indicated that the combination of G. uralensis and E. lathyris changed the regulatory effect of a single drug, resulting in new and harmful regulatory effects, and then increased the production of intestinal urinary toxins and other toxic substances, causing or aggravating the risk of disease.
Further, many studies have been conducted on the relationship between the incompatibility effect of TCMC and IF, as shown in Table 3.
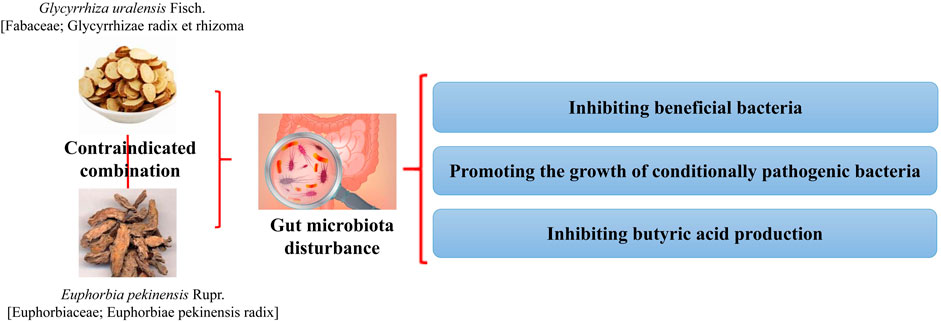
FIGURE 7. Combination of G. uralensis and E. pekinensis produces toxic and side effects by inhibiting beneficial bacteria and promoting the growth of conditional pathogenic bacteria (Liu et al., 2021).
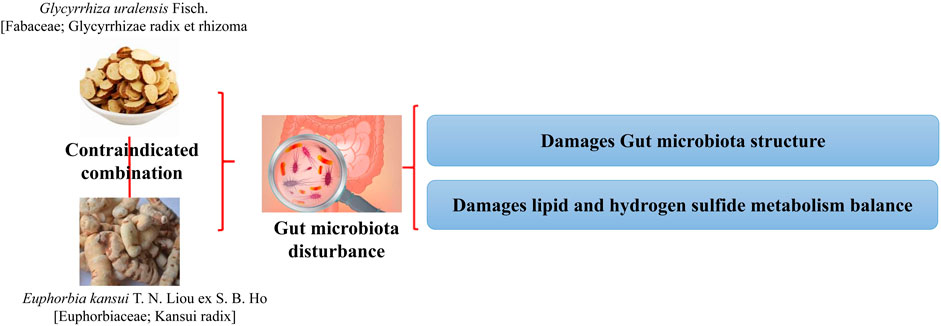
FIGURE 8. Combination of G. uralensis and E. kansui produces toxic and side effects by disrupting the structure of the intestinal flora and the associated metabolic balance (Yu et al., 2018).
5 Relationship between CM, the IF, and the Metabolites of the IF
CM can regulate the abundance of beneficial and harmful bacteria in the IF. For example, polysaccharides are a high proportion of components in CM, which can not only change the growth environment of the IF but can also be used as a substrate by beneficial bacteria to promote their growth of beneficial bacteria (Li et al., 2017). The organic acids, in the effect as pH buffers, can maintain the stability of the intestinal pH and provide a suitable environment for the proliferation of beneficial bacteria. In addition, the metabolites produced by beneficial bacteria can indirectly inhibit the growth of harmful bacteria. Some CM can directly inhibit the growth of pathogenic microorganisms, thereby regulating the intestinal microecological balance. Heat-clearing CM has a significant inhibitory effect on harmful bacteria (Xiao et al., 2019). Toxic CM, such as Tripterygium wilfordii, can effectively reduce the number of harmful bacteria, including Enterobacteriaceae, Enterococcus, and Bacteroides, in the intestines of UC mice and rats with IgA nephropathy (Ren et al., 2020; Wu et al., 2020). Therefore, CM can alter the metabolic products of the IF by adjusting the overall structure of the flora.
As a bridge between the IF and the body, the metabolites of the IF are mainly SCFAs. SCFAs are composed of 1–6 carbon atoms and are products of fermentation by IF. The SCFAs include acetic, propionic, and butyric acids. The production and consumption of SCFAs are dynamic processes, and their content reflects the activity of bacteria and the number of bacterial populations. SCFAs also affect energy metabolism, mucosal growth, and cell differentiation. SCFAs are not only anti-inflammatory but also reduce the pH in the intestine to inhibit harmful bacteria and balance the IF, and can maintain the balance of water and electrolytes and stimulate the secretion of hormones in the gastrointestinal tract. Therefore, SCFAs are closely associated with many diseases, including ulcerative colitis, obesity, diabetes, nonalcoholic fatty liver disease, autism, airway allergic inflammation, and hypertension (Shao et al., 2019). The IF is also involved in BA metabolism. In the liver, cholesterol is converted to primary free Bas through a multistage enzymatic reaction. Primary free Bas bind to taurine and glycine in the liver, convert them into conjugated Bas, and pass them through the biliary tract for discharge into the intestinal tract. Under the conjugate action of IF, taurine or glycine is removed, and the conjugated Bas become secondary Bas. Secondary Bas return to the liver through the portal system to continue binding. This is known as the enterohepatic cycle. Various Bas form Bas pools in different proportions and act on the host through Bas receptors such as the farcesoid X receptor and G-protein-coupled bile acid receptor, thereby affecting host metabolism, glycolipid metabolism, and energy homeostasis (Guo et al., 2022).
6 Problems and suggestions of the study on the connotation of the IF and CM
The occurrence and development of diseases and the efficacy of CM are closely related to IF. In summary, we found that the effect of a single drug on the regulation of the IF was different from its compatibility. The composition of the IF regulated by CM combinations is not a simple superposition of the effects of two individual drugs; the compatibility of drugs also plays a specific role in regulating intestinal metabolites, thus producing a different pharmacodynamic effect. This may be the angle from which the compatibility mechanism can be clarified. At present, the research on intestinal microbiota in TCM is still in its infancy. By summarizing previous research results, we provide suggestions for research on intestinal microbiota in terms of compatibility.
First, when studying the relationship between compatibility and IF, most of the research objects are drug pairs or whole prescriptions, but did not involve the comparison of changes in the IF before and after the treatments. Such a line of research cannot show that the changes in efficacy produced by the combination are related to the IF and cannot reflect where the characteristics of the combination lie. Therefore, we suggest that when studying the relationship between compatibility and flora, drug pairs or groupings should be studied by splitting the prescriptions. By comparing the composition and abundance changes of IF, we can find the specific flora regulated by the drug pair, and on this basis, we can further analyze the role played by the IF in the treatment of diseases by the drug pair.
Second, some studies only observed changes in the IF after drug compatibility treatment, which only showed a correlation between the compatibility of drugs, flora, and diseases, lacking verification of the causal relationship, and were unable to draw the conclusion that drugs play a therapeutic role through the action of flora, which is relatively less reliable. Therefore, we suggest that pseudo-sterile animal models of broad-spectrum antibiotic interference and fecal transplants can be used to study the role of the intestinal flora in the efficacy of drug pairs.
Third, 16s rDNA gene sequencing technology is the most widely used in IF research at present. Although this method overcomes the limitations of traditional culture methods and can provide relative abundance from the phylum to the genus level, this sequencing technology cannot identify specific changes in the IF at the species level; therefore, it is unable to identify the strains and related metabolites specifically regulated by the compatibility of drugs. It was impossible to further verify the relationship between the flora and compatibility. Therefore, we suggest the use of macrogenome sequencing. This method can not only clearly provide species-level composition information of IF, but also provide information on gene function, and on this basis, verify the role of flora in the body through specific flora colonization.
Therefore, when studying the relationship between the compatibility mechanism of CM and IF, we should systematically conduct in-depth research from the perspective of CM, IF, intestinal metabolite, and disease.
First, the prescription was decomposed into different parts, and an appropriate disease model was established. The effectiveness of compatibility was verified by comparing the efficacy of each drug and prescription. High-throughput sequencing technology was used to compare the composition and abundance of each drug and prescription in the IF of model animals, and specific bacteria regulated by the drug were identified. Second, the correlation between the changes in efficacy and flora specificity after compatibility was studied. Sterile or pseudosterile animals treated with antibiotics were used to observe the correlation between the IF and the occurrence and development of diseases. Flora transplantation is used to verify the therapeutic effect of specific flora on diseases and to study whether the therapeutic effect of compatible drugs can be transmitted through feces. Finally, the modes of action of the specific bacteria and their bodies were studied. However, IF may play a therapeutic role by directly acting on intestinal tissues (Mai and Draganov, 2009). In contrast, the IF affects body balance by regulating metabolites. SCFAs formed by the IF can affect energy metabolism, mucosal growth, cell differentiation, and other activities (Shao et al., 2019). Intestinal bacteria also affect Bas metabolism and regulate host metabolism, glucose metabolism, lipid metabolism, and energy homeostasis (Thomas et al., 2008). By studying the regulatory effect of compatible drugs on various metabolites after they act on IF, we can observe the influence of the drugs on the body to clarify the mechanism of drug compatibility.
7 Summary
The research on intestinal microorganisms is developing rapidly. Research on intestinal microorganisms provides a new perspective for us to understand the occurrence law of diseases and the mechanism of drug efficacy, as well as a new angle to clarify the theory of compatibility of CM, which is worthy of in-depth study. This paper summarizes the relationship between changes in the IF and its metabolites after compatibility with CM and the synergism, toxicity reduction, and toxicity enhancement after compatibility with CM. These studies show that the special effects of CM compatibility are related to the specific regulation of the IF and its metabolites by the drug; however, the current research still has some limitations. Therefore, this study suggests conducting in-depth research from the perspective of drug pair prescription–IF–intestinal metabolite–organism–disease to provide help for scientific research on the compatibility mechanism of CM in the future.
Author contributions
YG collected essays and wrote original draft preparation, YG, NZ, DB, and XW revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by: The Fundamental Research Funds for the Central Public Welfare Research Institutes (82204974 and ZZ2018013); The Science and Technology Innovation Project of the China Academy of Chinese Medical Sciences (CI2021A0O108); Independent topic selection research project of the Institute of Basic Theory of Chinese Medicine, China Academy of Chinese Medical Sciences (YZ-202109, YZ-202048, and YZ-202219).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Beerens, H., and Romond, C. (1977). Sulfate-reducing anaerobic bacteria in human feces. Am. J. Clin. Nutr. 30 (11), 1770–1776. doi:10.1093/ajcn/30.11.1770
Borton, M. A., Sabag-Daigle, A., Wu, J., Solden, L. M., O'Banion, B. S., Daly, R. A., et al. (2017). Chemical and pathogen-induced inflammation disrupt the murine intestinal microbiome. Microbiome 5 (1), 47. doi:10.1186/s40168-017-0264-8
Chen, J., Zhang, L. K., Gu, W. C., Xin sheng, Z., Ling, L., Tao, H., et al. (2021). Effect of Bangia Xiexin Decoction on intestinal flora of mice with ulcerative colitis induced by dextran sodium sulfate. China J. Chin. Materia Medica 46 (11), 2871–2880. doi:10.19540/j.cnki.cjcmm.20210119.401
Chen, L., Zhang, L., Guan, X. l., Fang, Z. R., and Wang, W. M. (2019). The effect of Zizia-Chi decoction and its decomposed recipe on six gut bacteria. Chin. J. Microecology 31 (1), 8–11+16. doi:10.13381/j.cnki.cjm.201901002
Chen, Y., Lu, J., Zhu, S. M., Wang, T. T., Fan, Y. H., Ji, W. l., et al. (2020). Effect of Geogen Qilian Decoction and it's different compatibility groups on gut microbiota in rats with acute enteritis based on high-throughput sequencing. China J. Chin. Materia Medica 45 (06), 1406–1417. doi:10.19540/j.cnki.cjcmm.20191112.401
Chen, Y. Y., Tang, Y. P., Shang, E. X., Zhu, Z. H., Tao, W. W., Yu, J. G., et al. (2019). Incompatibility assessment of Genkwa Flos and Glycyrrhizae Radix et Rhizoma with biochemical, histopathological and metabonomic approach. J. Ethnopharmacol. 229, 222–232. doi:10.1016/j.jep.2018.10.014
Cui, X., Qian, D. W., Jiang, S., Shang, E. X., Zhu, Z. H., and Duan, J. A. (2018). Scutellaria radix and coptidis rhizoma improve glucose and lipid metabolism in T2DM rats via regulation of the metabolic profiling and MAPK/PI3K/akt signaling pathway. Int. J. Mol. Sci. 19 (11), 3634. doi:10.3390/ijms19113634
Dai, L., Chen, Q. m., Liu, X. p., Lin, Y. y., Li, K., Yue, J., et al. (2022). Bangia xiexintang and its disassembled prescriptions regulate colonic mucosal immunity in young rats with flora disorder. Chin. J. Exp. Traditional Med. Formulae 28 (11), 42–50. doi:10.13422/j.cnki.syfjx.20221038
Ding, M., Dong, H. l., Jiang, G. r., Yan, S., and Zong, Y. (2019). Overview of research on the compatibility of scutellaria baicalensis and coptis chinensis in the treatment of type 2 diabetes. China Pharm. 30 (17), 2440–2444. doi:10.6039/j.issn.1001-0408.2019.17.27
El-Baz, A. M., Khodir, A. E., Adel, E.-S. M. M., and Shata, A. (2020). The protective effect of Lactobacillus versus 5-aminosalicylic acid in ulcerative colitis model by modulation of gut microbiota and Nrf2/Ho-1 pathway. Life Sci. 256, 117927. doi:10.1016/j.lfs.2020.117927
Fan, H., Wang, A., Wang, Y., Sun, Y., Han, J., Chen, W., et al. (2019). Innate lymphoid cells: Regulators of gut barrier function and immune homeostasis. J. Immunol. Res. 2019, 2525984. doi:10.1155/2019/2525984
Guan, Y., Chen, K., Quan, D., Kang, L., Yang, D., Wu, H., et al. (2021). The combination of scutellaria baicalensis Georgi and Sophora japonica L. Ameliorate renal function by regulating gut microbiota in spontaneously hypertensive rats. Front. Pharmacol. 11, 575294. doi:10.3389/fphar.2020.575294
Guo, X., Okpara, E. S., Hu, W., Yan, C., Wang, Y., Liang, Q., et al. (2022). Interactive relationships between intestinal flora and bile acids. Int. J. Mol. Sci. 23 (15), 8343. doi:10.3390/ijms23158343
Han, Y., Li, C. N., Huan, Y., Sun, S. j., Mu, Y. z., and Shen, Z. f. (2016). Effects of berberine compatible with stachyose on glycolipid metabolism and gut microbiota in diabetic mice. Chin. J. Clin. Pharmacol. 32 (12), 1121–1124. doi:10.13699/j.cnki.1001-6821.2016.12.021
Juan, S., Yu-ping, T., Shu-jiao, L., Wei-wei, T., Shu-an, S., Da-wei, Q., et al. (2015). Factor analysis on the dosage-toxicity relationship of kansui-licorice. China J. Traditional Chin. Med. Pharm. 30, 1531–1537.
Kang, C., Wang, B., Kaliannan, K., Wang, X., Lang, H., Hui, S., et al. (2017). Erratum for Kang et al., "Gut Microbiota Mediates the Protective Effects of Dietary Capsaicin against Chronic Low-Grade Inflammation and Associated Obesity Induced by High-Fat Diet". mBio 8 (3), e00900–e00917. doi:10.1128/mBio.00900-17
Larsen, J. M. (2017). The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology 151 (4), 363–374. doi:10.1111/imm.12760
Li, C., Wang, Y. Y., Li, J. P., Yu meng, W., Sen, Z., Jin'ao, D., et al. (2020). Analysis of the strategy to intervene the progress of inflammatory bowel disease by targeting intestinal bacterial respiration and energy metabolism. Acta Pharm. Sin. 55 (9), 2008–2018. doi:10.16438/J.0513-4870.2020-0715
Li, J., Huang, Z. Y., Yu, T., Tao, X. Y., Hu, Y. M., Wang, H. C., et al. (2019). Isolation and characterization of a sequence type 25 carbapenem-resistant hypervirulent Klebsiella pneumoniae from the mid-south region of China. BMC Microbiol. 19 (1), 219. doi:10.1186/s12866-019-1593-5
Li, L., Ma, W. J., Jia, Q., Ou Yang, H. Z., Chang, Y. X., and He, J. (2019). Compatibility of periplocin and Panax notoginseng saponins on intestinal flora in SD rats by Real-time fluorescence quantitative PCR and High-throughput sequencing. Drug Eval. Res. 42 (03), 432–436. doi:10.7501/j.issn.1674-6376.2019.03.009
Li, M.-z., Qiu, F.-C., Qian, C., Ruo-tong, L., Yu, J.-y., Sun, S., et al. (2017). Research progress of Chinese medicine polysaccharides in regulating intestinal flora. Food Nutr. China 23 (12), 13–16. doi:10.3969/j.issn.1006-9577.2017.12.003
Li, Y., Guo, S., Tao, W. W., Yu, J. G., Su, S. l., and Duan, J. A. (2019a). Detoxification mechanism of Jujube Fructus and Crotonic Semen Pulveratum based on the toxicity of gastrointestinal system and diuretic effect in mice. Acta Pharm. Sin. 54 (1), 95–103.
Li, Y., Guo, S., Tao, W. W., Yu, J. G., Su, S. l., and Duan, J. A. (2019b). Incompatibility mechanism of Crotonis Semen Pulveratum and Glycyrrhizae Radix et Rhizoma based on diuretic effect and intestinal flora structure. China J. Chin. Materia Medica 44 (3), 518–525. doi:10.19540/j.cnki.cjcmm.20181012.002
Liang, K., Fan, Y. h., Xu, Y. w., Yang, G. z., An, R., and Wang, X. h. (2021). Interaction between Banxia Xiexin Decoction with its different compatibility and gastrointestinal bacteria. China J. Traditional Chin. Med. Pharm. 36 (10), 5887–5893.
Lin, H., Liu, Z., Liu, Z., and Lin, Z. (2022). Incompatible effects of Panax ginseng and Veratrum nigrum on estrogen decline in rats using metabolomics and gut microbiota. J. Pharm. Biomed. Analysis 208, 114442. doi:10.1016/j.jpba.2021.114442
Lin, H., Pi, Z. F., Men, L. H., Chen, W. J., Liu, Z. Q., and Liu, Z. Y. (2016). Untargeted metabonomics study of compatibility of Panax ginseng and Veratrum nigrum in spleen qi deficiency rat. Chin. J. Anal. Chem. 44 (11), 1755–1762. doi:10.11895/j.issn.0253-3820.160224
Lin, X.-Y., Yu, Y. R., Liu, Q. P., He, Z. X., Li, H. C., and Wen, C. P. (2021). Regulating effect of Qinghao-Biejia on gut microbiota in the treatment of MRL/lpr mice. China J. Traditional Chin. Med. Pharm. 36 (11), 6743–6746.
Lin, Z., Zu, X. p., Xie, H. s., Jin, H. z., Yang, N., Liu, X. r., et al. (2016). Research progress in mechanism of intestinal microorganisms in human diseases. Acta Pharm. Sin. 51 (6), 843–852. doi:10.16438/j.0513-4870.2015-0803
Liu, C. S., Liang, X., Wei, X. H., Jin, Z., Chen, F. L., Tang, Q. F., et al. (2019). Geogen qinlian decoction treats diarrhea in piglets by modulating gut microbiota and short-chain fatty acids. Front. Microbiol. 10, 825. doi:10.3389/fmicb.2019.00825
Liu, D., Zhao, R., Wu, Y., Wang, Y., Yang, R., and Ke, X. (2022). Variation in the efficacy of anti-ulcerative colitis treatments reveals the conflict between precipitating compatibility of traditional Chinese medicine and modern technology: A case of scutellaria-coptis. Front. Pharmacol. 13, 819851. doi:10.3389/fphar.2022.819851
Liu, J.-x. (2019). “Study on the compatibility of Swishen Wan which is based on the pharmacodynamics of diarrhea-predominant irritable bowel syndrome,”. Master (Guiyang, China: Guizhou University).
Liu, S., Qiao, S., Wang, S., Tao, Z., Wang, J., Tao, J., et al. (2021). Intestinal bacteria are involved in radix Glycyrrhizae and radix Euphorbia pekinensis incompatibility. J. Ethnopharmacol. 273, 113839. doi:10.1016/j.jep.2021.113839
Luedde, M., Winkler, T., Heinsen, F. A., Ruhlemann, M. C., Spehlmann, M. E., Bajrovic, A., et al. (2017). Heart failure is associated with depletion of core intestinal microbiota. Esc. Heart Fail 4 (3), 282–290. doi:10.1002/ehf2.12155
Luo, Y., Zhang, X., Zhang, W., Yang, Q., You, W., Wen, J., et al. (2021). Compatibility with Semen Sojae Praeparatum attenuates hepatotoxicity of Gardenia Fructus by regulating the microbiota, promoting butyrate production and activating antioxidant response. Phytomedicine 90, 153656. doi:10.1016/j.phymed.2021.153656
Mai, V., and Draganov, P. V. (2009). Recent advances and remaining gaps in our knowledge of associations between gut microbiota and human health. World J. Gastroenterol. 15 (1), 81–85. doi:10.3748/wjg.15.81
Miao, L., Peng, Q., Sun, M. Q., Zhang, Y. H., Zhang, Y., Ren, C. Y., et al. (2022). Regulatory effect of quyu huatan tonga prescription on intestinal microflora in golden hamster with hyperlipidemia. Chin. J. Exp. Traditional Med. Formulae 28 (1), 109–120. doi:10.13422/j.cnki.syfjx.20212403
Niwa, T. (2013). Targeting protein-bound uremic toxins in chronic kidney disease. Expert Opin. Ther. Targets 17 (11), 1287–1301. doi:10.1517/14728222.2013.829456
Pluznick, J. (2014). A novel SCFA receptor, the microbiota, and blood pressure regulation. Gut Microbes 5 (2), 202–207. doi:10.4161/gmic.27492
Purushe, J., Fouts, D. E., Morrison, M., White, B. A., Mackie, R. I., Coutinho, P. M., et al. (2010). Comparative genome analysis of Prevotella ruminicola and Prevotella bryantii: Insights into their environmental niche. Microb. Ecol. 60 (4), 721–729. doi:10.1007/s00248-010-9692-8
Ren, R.-Y., Han, X., and Song, C. D. (2020). Effect of Tripterygium wilfordii multiglucoside on intestinal flora and immune function in lgA nephropathy rats based on C1GALT1/Cosmc pathway. Chin. J. Pathophysiol. 36 (11), 2050–2055. doi:10.3969/j.issn.1000-4718.2020.11.018
Sampson, T. R., Debelius, J. W., Thron, T., Janssen, S., Shastri, G. G., Ilhan, Z. E., et al. (2016). Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson's disease. Cell. 167 (6), 1469–1480.e12. doi:10.1016/j.cell.2016.11.018
Shao, M., Tan, W., and Luo, H. S. (2019). Progress of short chain fatty acids in the pathogenesis of various diseases. Chin. J. Gastroenterology Hepatology 28 (8), 951–954. doi:10.3969/j.issn.1006-5709.2019.08.026
Shi, J., Zhang, Y. l., Zhang, X., and Li, H. Q. (2012). Observation on the therapeutic effect of Zizia Chi Decoction on 44 patients with depression syndrome. Chin. J. Exp. Traditional Med. Formulae 18 (18), 316–318.
Sun, Y.-t. (2020). “The study of realgar's intestinal toxicity and its mechanism of detoxicity by compatibility of Salvia miltiorrhiza,”. Master (Hefei, China: Anhui Medical University).
Tang, S., Liu, W., Zhao, Q., Li, K., Zhu, J., Yao, W., et al. (2021). Combination of polysaccharides from Astragalus membranaceus and Codonopsis pilosula ameliorated mice colitis and underlying mechanisms. J. Ethnopharmacol. 264, 113280. doi:10.1016/j.jep.2020.113280
Tang, Z., Wei, J., Ou Yang, H. Z., and He, J. (2018). Effect of aconiti lateralis radix praeparata and ginseng radix et rhizoma rubra in different compounding ratio on gut microbiota in SD rats evaluated by high-throughput sequencing. Drug Eval. Res. 41 (10), 1781–1785. doi:10.7501/j.issn.1674-6376.2018.10.006
Tao, W., Yu, J. G., Chen, Y. Y., Xiao, D., Guo, J. M., Liu, P., et al. (2018). Incompatible mechanism of compatibility of Chinese medicines based on Qianjinzi and Gancao effect on intestinal flora/barrier system. China J. Chin. Materia Medica 43 (02), 369–371. doi:10.19540/j.cnki.cjcmm.20171027.021
Thomas, C., Pellicciari, R., Pruzanski, M., Auwerx, J., and Schoonjans, K. (2008). Targeting bile-acid signalling for metabolic diseases. Nat. Rev. Drug Discov. 7 (8), 678–693. doi:10.1038/nrd2619
Wang, J., Feng, W., Zhang, S., Chen, L., Sheng, Y., Tang, F., et al. (2019). Ameliorative effect of Atractylodes macrocephala essential oil combined with Panax ginseng total saponins on 5-fluorouracil induced diarrhea is associated with gut microbial modulation. J. Ethnopharmacol. 238, 111887. doi:10.1016/j.jep.2019.111887
Wang, L., Zhou, G. B., Liu, P., Song, J. H., Liang, Y., Yan, X. J., et al. (2008). Dissection of mechanisms of Chinese medicinal formula Realgar-Indigo naturalis as an effective treatment for promyelocytic leukemia. Proc. Natl. Acad. Sci. U. S. A. 105 (12), 4826–4831. doi:10.1073/pnas.0712365105
Wang, Q., Mao, N. f., Zhang, Z. g., Lu, L., and Yin, M. z. (2022). Study on the effect of Lianpu Drink and Coptidis-Magnolia officinalis on human intestinal flora based on in vitro fermentation model. Lishizhen Med. Materia Medica Res. 33, 1–3. doi:10.3969/j.issn.1008-0805.2022.12.22
Wang, T., Zhang, C., Li, H., Zhou, R., Ye, X., Yang, Y., et al. (2022). The underlying rationality of Chinese medicine herb pair Coptis chinensis and Dolomiaea souliei: From the perspective of metabolomics and intestinal function. J. Ethnopharmacol. 289, 115065. doi:10.1016/j.jep.2022.115065
Wei, H.-L., Li, J. T., Chen, Z. G., and Shu guang, Y. (2022). Experimental study on effects of berberine combined with 6-shogaols on intestinal inflammation and flora in mice with ulcerative colitis. China J. Chin. Materia Medica 47 (16), 4418–4427. doi:10.19540/j.cnki.cjcmm.20220413.401
Wu, H., Yu, X. H., Wang, H. J., Ma, G. C., Zhang, C. E., and Ma, Z. J. (2020). Effect of Tripterygium wilfordii on intestinal flora in mice with ulcerative colitis induced by dextran sodium sulfate. Chin. Traditional Herb. Drugs 51 (02), 387–396. doi:10.7501/j.issn.0253-2670.2020.02.015
Xiao, D., Chen, Y. Z., and Mo, X. l. (2019). Study on antibacterial activities of 4 Chinese herbal medicine commonly used in guangdong. Pharm. Today 29 (3), 166–169. doi:10.12048/j.issn.1674-229X.2019.03.002
Xiao, S., Liu, C., Chen, M., Zou, J., Zhang, Z., Cui, X., et al. (2020). Scutellaria radix and coptidis rhizoma ameliorate glycolipid metabolism of type 2 diabetic rats by modulating gut microbiota and its metabolites. Appl. Microbiol. Biotechnol. 104 (1), 303–317. doi:10.1007/s00253-019-10174-w
Xie, N., Cui, Y., Yin, Y. N., Zhao, X., Yang, J. W., Wang, Z. G., et al. (2011). Effects of two Lactobacillus strains on lipid metabolism and intestinal microflora in rats fed a high-cholesterol diet. BMC Complement. Altern. Med. 11, 53. doi:10.1186/1472-6882-11-53
Xu, J., Chen, H. B., and Li, S. L. (2017). Understanding the molecular mechanisms of the interplay between herbal medicines and gut microbiota. Med. Res. Rev. 37 (5), 1140–1185. doi:10.1002/med.21431
Xu, J. (2014). “Study on the interaction between scutellaria-coptis herb couple and intestinal bacteria,”. Master (Nanjing, China: Nanjing University Of Chinese Medicine).
Ye, H.-B. (2020). “Study on material basis and mechanism of antipyretic effect of gypsum and its compatibility,”. Doctor (Changchun: Changchun University Of Chinese Medicine).
Yu, J.-G. (2018). “Basic research of TCM incompatibility of "zao ji sui yuan ju zhan cao" -biological mechanisms based on gut-gut microbiota and aquaporin proteins,”. Doctor (Nanjing: Nanjing University Of Chinese Medicine).
Yu, J., Guo, J., Tao, W., Liu, P., Shang, E., Zhu, Z., et al. (2018). Gancao-Gansui combination impacts gut microbiota diversity and related metabolic functions. J. Ethnopharmacol. 214, 71–82. doi:10.1016/j.jep.2017.11.031
Yu, J., Liu, Y., Guo, J., Tao, W., Chen, Y., Fan, X., et al. (2019). Health risk of Licorice-Yuanhua combination through induction of colonic H2S metabolism. J. Ethnopharmacol. 236, 136–146. doi:10.1016/j.jep.2019.01.042
Zhang, Y., Dai, L. R., Chen, Q. M., Liu, X. P., Yue, J., Shi, L. J., et al. (2021). Effects of banxia Xiexin decoction and its different disassembled prescriptions on colonic mucosal barrier in juvenile mice with antibiotic-induced microbiota dysbiosis. Chin. J. Inf. Traditional Chin. Med. 29, 1–7. doi:10.19879/j.cnki.1005-5304.202110186
Zhong, Y. (2017). “The study of rhubarb, radix astragali on chronic renal failure rat intestinal flora and the influence of metabolic toxins,”. Master (Guangzhou: Guangzhou University Of Chinese Medicine).
Zhu, H. H., Wu, D. P., Du, X., Zhang, X., Liu, L., Ma, J., et al. (2018). Oral arsenic plus retinoic acid versus intravenous arsenic plus retinoic acid for non-high-risk acute promyelocytic leukaemia: A non-inferiority, randomised phase 3 trial. Lancet Oncol. 19 (7), 871–879. doi:10.1016/S1470-2045(18)30295-X
Glossary
Keywords: Chinese medicine, traditional Chinese medicine, compatibility, synergy and attenuation, contraindicated combination, intestinal flora
Citation: Gao Y, Wu X, Zhao N and Bai D (2023) Scientific connotation of the compatibility of traditional Chinese medicine from the perspective of the intestinal flora. Front. Pharmacol. 14:1152858. doi: 10.3389/fphar.2023.1152858
Received: 28 January 2023; Accepted: 26 June 2023;
Published: 19 July 2023.
Edited by:
Abdul Rohman, Gadjah Mada University, IndonesiaReviewed by:
Miao Mingsan, National Research Council (CNR), ItalyXianju Huang, South-Central Minzu University, China
Copyright © 2023 Gao, Wu, Zhao and Bai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoxia Wu, eGlhb3hpYTIzMTFAc2luYS5jb20= Dong Bai, YmFpZG9uZzIwMDBAMTI2LmNvbQ==
 Yuan Gao
Yuan Gao Xiaoxia Wu2*
Xiaoxia Wu2* Ning Zhao
Ning Zhao Dong Bai
Dong Bai