- 1School of Pharmacy, Hunan University of Chinese Medicine, Changsha, China
- 2College of Integrated Traditional Chinese Medicine and Western Medicine, Hunan University of Chinese Medicine, Changsha, China
Ischemic stroke (IS) is the second leading cause of death and disability in the world. Pyroptosis, a form of programmed cell death initiated by caspases, participates in the occurrence and development of IS. Because it can increase cell membrane permeability, mediate the release of inflammatory factors, and aggravate inflammation, inhibiting this process can significantly reduce the pathological injury of IS. The nucleotide binding oligomerization domain-like receptor family pyrin domain protein 3 (NLRP3) is a multiprotein complex whose activation is the core link of pyroptosis. In recent years, studies have reported that traditional Chinese medicine (TCM) could regulate pyroptosis mediated by NLRP3 inflammasome through multi-channel and multi-target networks and thus exert the effect against IS. This article reviews 107 papers published in recent years in PubMed, Chinese National Knowledge Infrastructure (CNKI), and WanFang Data in recent years. It has found that the activation factors of NLRP3 inflammasome include ROS, mitochondrial dysfunction, K+, Ca2+, lysosome rupture, and trans-Golgi breakdown. TLR4/NF-κB/NLRP3, ROS/TXNIP/NLRP3, AMPK/Nrf2/NLRP3, DRP1/NLRP3, TAK1/JNK/NLRP3 signaling pathways regulate the initiation and assembly of the NLRP3 inflammasome, subsequently induce pyroptosis, affecting the occurrence and development of IS. TCM can affect the above signaling pathways and regulate the pyroptosis mediated by NLRP3 inflammasome, so as to play a protective role against IS, which provides a new entry point for discussing the pathological mechanism of IS and a theoretical basis for developing TCM treasure house.
Introduction
An analysis of global disease systems in Lancet Neurology shows that ischemic stroke (IS) is the second leading cause of mortality and disability worldwide, and the economic costs of its treatment and post-stroke care are substantial (GBD, 2016 Stroke Collaborators, 2019). IS is a clinical syndrome of neurological damage caused by cerebral blood supply disorder, brain tissue hypoxia, sugar deficiency, and tissue necrosis (Zhao et al., 2022). In recent years, a new type of programmed cell death called pyroptosis, which is closely related to inflammation, has been discovered (Tang et al., 2019). The 2002 Nobel Prize in Physiology or Medicine and the Nobel Prize in Chemistry in 2004 were awarded to scientists who have made pioneering contributions in the field of pyroptosis. The research indicates that pyroptosis has the characteristics of both apoptosis and necrosis, showing that the nuclear morphology is complete and the cell membrane is broken, leading to the release of cell contents and causing inflammation (Wang et al., 2017). Nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3) inflammasome is the key protein of pyroptosis and closely participates in the process of pyroptosis (Huang et al., 2021). Recent studies have found that pyroptosis mediated by NLRP3 inflammasome participates in the pathological process of IS (Fu et al., 2020; McKenzie et al., 2020). Kind of literature suggests that acupuncture, traditional Chinese medicine monomer, traditional Chinese medicine compound, and Chinese patent medicine can regulate NLRP3 inflammasome-mediated pyroptosis related signaling pathways to play a neuroprotective role in IS (Wang M et al., 2020; Ran et al., 2021; Chen et al., 2022). This article will elucidate the activation mechanism of NLRP3 inflammasome in the central nervous system (CNS), and review the research on the intervention of traditional Chinese medicine on NLRP3 inflammasome.
NLRP3 inflammasome in central nervous system and activation mechanism
The NLRP3 inflammasome is a multi-protein complex composed of NLRP3, ASC and pro-cassase-1, which plays an important role in the classical pyroptosis pathway (Lu et al., 2020). In 2004, Immunity first reported that NLRP3 inflammasomes are the basic molecules related to auto-inflammation (Agostini et al., 2004). In 2018, Nature further reported the activation mechanism of NLRP3 inflammasome, that is, the disbanded trans-Golgi network activated inflammasome by inducing the transport and aggregation of NLRP3 through phospholipid PtdIns4P (Chen and Chen, 2018). The activation of the NLRP3 inflammasome produces caspase-1. Subsequently, caspase-1 cleaves and splits gasdermin D (GSDMD) and pro-interleukin-1β (pro-IL-1β), pro-interleukin-18 (pro-IL-18), forming GSDMD-N and IL-1β, IL-18 (Chen et al., 2018; Alishahi et al., 2019). Then GSDMD-N acts on phospholipid molecules on the cell membrane to form pores, leading to cell osmotic swelling, cell membrane rupture and pyroptosis, simultaneously IL-1β and IL-18 are released out, which expands the local inflammatory response (Chen S et al., 2018). NLRP3 widely exists in neurons, microglia and cerebral vascular endothelial cells. The activation of NLRP3 inflammasome proceeds in two steps. The first step is priming. The second step is the assembly of inflammasome, which is a step of many studies on activation. Multiple upstream signals can induce the formation of the inflammasome by activating the oligomerization of NLRP3 protein.
Priming of NLRP3 inflammasome
Damage-associated molecular patterns (DAMPs) and pathogen-associated molecular pattern ligands (PAMPs) generated by cerebral ischemia and hypoxia cross the blood-brain barrier (BBB) into the CNS and activate the Toll-like receptor 4 (TLR4) on the surface of neurons, microglia, and astrocyte cell membranes (Shi et al., 2019). TLR4 is a transmembrane receptor protein involved in innate immune response (Li L et al., 2021), which can detect the danger signals of the extracellular environment. TLR4 initiates a signaling cascade to produce inflammatory cytokines (TNF, IL-12, IL-12, IL-6, IL-8, IL-1β, etc.). The key signal ligand downstream of TLR4 is myeloid differentiation factor 88 (MyD88). TLR4 and MyD88 promote the activation of tumor necrosis factor receptor-associated factor 6 (TRAF6) and activate the downstream transcriptional regulator nuclear factor κB (NF-κB) (Mitchell et al., 2018). NF-κB activation increases the transcription of NLRP3 inflammasome related protein coding gene (Jin et al., 2019), thereby activating caspase-1 and isolating the N and C ends of GSDMD to induce pyroptosis (Mohamadi et al., 2018) (Figure 1).
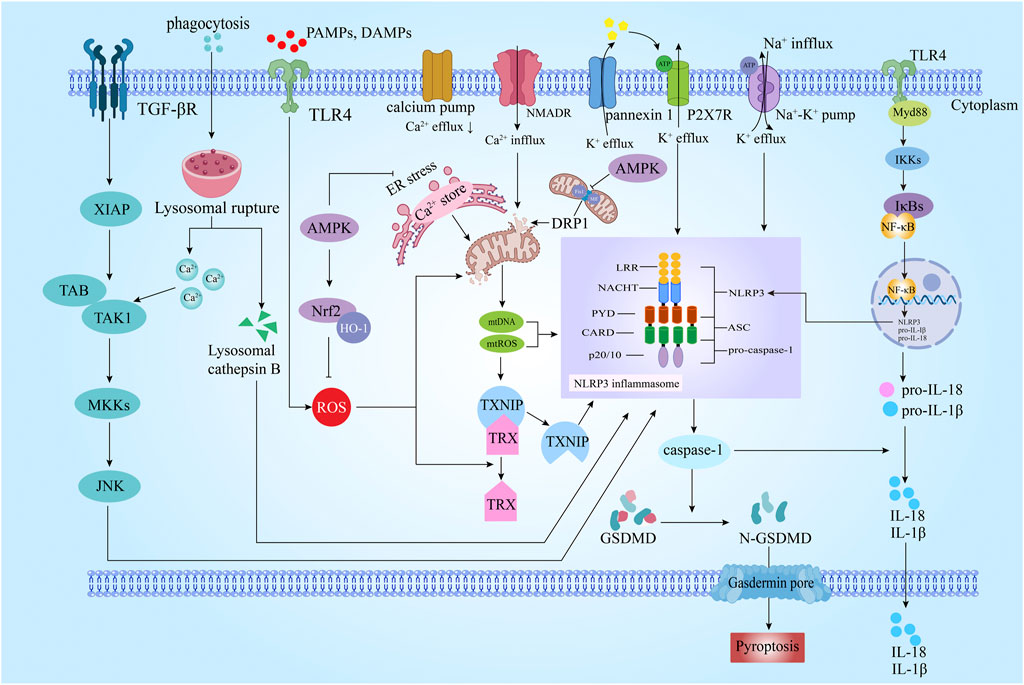
FIGURE 1. Schematic diagram of the molecular mechanism of pyroptosis in ischemic stroke (IS). Pyroptosis is a kind of programmed cell death process mediated by nucleotide-binding oligmerization domain-like receptor protein 3 (NLRP3) inflammasome and activated by Caspase-1) . It has the characteristics of both apoptosis and necrosis NLRP3. The initiation of the NLRP3 inflammasome involves a series of signaling pathways, such as TLR4/NF-κB/NLRP3, ROS/TXNIP/NLRP3, AMPK/NLRP3, DRP1/NLRP3, TAK1/JNK/NLRP3. TGFβ: transforming growth factor-β; XIAP: X-linked inhibitor of apoptosis protein; TAK1: transforming growth factor-beta activated kinase 1; TAB: transforming growth factor-β activated kinase 1 binding protein; MKKs: mitogen-activated protein kinase kinase; JNK: c-JunN-terminalkinase; DAMPs: damage associated molecular pattern; PAMPs: pathogen associated molecular patterns; ROS: Reactive oxygen species; AMPK: AMP-activated protein kinase; Nrf2: nuclear factor erythroid 2-related factor 2; HO-1: Heme Oxygenase-1; ER: endoplasmic reticulum; TXNIP: TRX-interacting protein; TRX: thioredoxin; mtROS: mitochondrial ROS; mtDNA: mitochondrial DNA; DRP1: dynamin-related protein 1; NMDAR: Nmethyl-D-aspartate receptor; ASC: apoptosis-associated speck-like protein containing acaspase recruitment domain; LRR: leucine-rich repeats; NACHT: nucleotidebinding and oligomerization; PYD: pyrin domain; CARD: Caspaseact ivat ing recruitment do main; caspase-1: cysteinyl aspartate specific proteinase-1; TLR4: toll-like receptor 4; MyD88: myeloid differentiation factor 88; IKKs: IkappaB kinases; IκBs: I kappaBs; NF-κB: nuclear factor kappa B; GSDMD: Gasdermin D.
Ye et al. (2019) established an adult male C57BL/6J wild-type mouse middle cerebral occlusion/reperfusion (MCAO/R) in vivo. Immunofluorescence staining and western blot showed that the expression of NLRP3 inflammasome and their related proteins in neurons and microglia was activated. Subsequently, an in vitro oxygen-glucose deprivation/reperfusion (OGD/R) model was established in HT22 and BV2 cells. Results showed TLR4/NF-κB was significantly upregulated, NLRP3 inflammasomes were activated and M1 microglia/macrophages were polarized (Ye et al., 2019). Liu et al. (2021) used the TLR4 inhibitor TAK242 to reverse prove that the TLR4/NF-KB/NLRP3 pathway was activated in OGD/R BV2 cells. Wang K et al. (2020) detected pyroptosis in the ischemic cortex by dUTP nick end labeling (TUNEL) measurement and lactate dehydrogenase (LDH) release, and detected NLRP3 inflammasome assembly and inflammatory cytokine secretion by enzyme-linked immunosorbent assay (ELISA) and western blot. Confirming the activation of the TLR4/NF-κB/NLRP3 pathway induced by MCAO. While inhibiting NF-κB/NLRP3 protects neurons from OGD-induced pyroptosis (Kang et al., 2021). The above research shows that pyroptosis mediated by TLR4/NF-κB/NLRP3 pathway has a negative regulatory effect on IS, and targeted inhibition of this pathway plays a protective role.
Assembly of NLRP3 inflammasome
NLRP3 inflammasome assembly induced by reactive oxygen species (ROS)
All known PAMPs and DAMPs trigger the production of ROS, which can then induce the assembly of the NLRP3 inflammasome. High levels of ROS promote the dissociation of the oxidation-reduction sensitive signal complex TXNIP, which is translocated from the nucleus to the cytoplasm (Luo et al., 2022). TXNIP binds to the NLRP3 receptor domain (mainly LRR domain), thus inducing the activation of NLRP3 inflammasome (Chen D et al., 2018). In vivo experiments show that ROS drives TXNIP overexpression in MCAO rats and MCAO/R C57BL/6 mice, then TXNIP aggravates brain damage through redox imbalance, subsequently activates NLRP3 inflammasome, caspase-1, and causes the release of IL-1β (Cao et al., 2016; Zeng et al., 2021; Yao et al., 2022). In vitro experiments show that ROS/TXNIP/NLRP3 signaling pathway activation induces pyroptosis in OGD/R and OGD primary cortical neurons (Yao et al., 2022). Knockdown of TXNIP significantly decreased the expression of NLRP3 in OGD-induced neurons (Liu et al., 2020). The above results suggest that ROS/TXNIP mediated activation of NLRP3 inflammasome is a key factor in IS.
The AMP-activated protein kinase (AMPK)/NF-E2 related factor 2 (Nrf2) pathway suppresses the assembly of NLRP3 inflammasome through anti-ROS. AMPK is phosphorylated and activated in response to an increase in the intracellular AMP/ADP ratio during ischemia and glycogen deprivation (Gu et al., 2018). AMPK directly phosphorylates the Nrf2 with an endogenous neuroprotective effect (Joo et al., 2016; Yu H et al., 2020). The activated Nrf2 translocates from the cytoplasm to the nucleus and binds to the antioxidant response element (ARE) (Fuse and Kobayashi, 2017). Hou et al. (2018) intraperitoneally injected tert-butylhydroquinone (tBHQ) to activate Nrf2. Western blot and qRT-PCR results showed that NLRP3 inflammasome and downstream caspase-1, IL-18, and IL-1β were significantly reduced, whereas Nrf2 knockout produced the opposite result. Transfection of Nrf2 into mice with inflammation had the same effect (Liu et al., 2017). When MCAO rats were treated with AMPK inhibitor dorsomorphin, immunofluorescence and western blot results showed that microglia/macrophages were activated, p-AMPK and Nrf2 were decreased, and NLRP3 was upregulated (Yu J et al., 2020). This is consistent with the result of knocking out Nrf2 (Liu et al., 2017), indicating that Nrf2 could inhibit the expression of NLRP3 (Liu et al., 2017). The downregulation of Nrf2 can also promote the pyroptosis of vascular endothelial cells induced by NLRP3 (Hu et al., 2018). The above experimental results suggest that up-regulating the expression of AMPK or Nrf2 can significantly improve pyroptosis, promote neural function recovery and improve IS (Qiu et al., 2016).
NLRP3 inflammasome assembly induced by mitochondrial dysfunction
Mitochondrial dysfunction is an important feature of IS pathophysiology (Hasan et al., 2021). DRP1 is a protein related to mitochondrion division which was originally mainly distributed in the cytoplasm of cells. It was recruited by some signals and translocated to the outer membrane of mitochondria to form helical oligomers, which caused mitochondrion division and aggravated mitochondrial dysfunction (Duan et al., 2020). Phosphorylation of DRP1 reduces mitochondrial fission by regulating its translocation via AMPK (Hu et al., 2017). After activation, DRP1 combines with Fission1 (Fis1) and mitochondrial fission factor (Mff) to mediate the metabolic disorder of cells and inhibit glutathione in mitochondria to weaken the ability of free radical scavenging, further increase mitochondrial reactive oxygen species (mtROS), thus up-regulating the level of NLRP3 protein and producing IL-1β, cause pyroptosis, and eventually cause ischemic damage to neurons (Park et al., 2018; Kleele et al., 2021). Knockout of DRP1 can improve the function of mitochondria and reduce the level of NLRP3 protein to reduce pyroptosis (Zhong et al., 2022). It was found that in MCAO/R rats and OGD SH-SY5Y cells, DRP1 translocated mitochondria, resulting in the mitochondrial division, mitochondrial dysfunction, and then produced a large number of ROS, activated NLRP3 inflammasome, and induced pyroptosis (Guo et al., 2018; Hu et al., 2020). Inhibition of the DRP1 phosphorylation cannot only protect the integrity of mitochondria, but also reduce the activation of NLRP3 inflammasome and reduce pyroptosis (Zhou et al., 2011; Carneiro et al., 2012). Therefore, downregulation of the DRP1/NLRP3 pathway can effectively improve mitochondrial damage, inhibit pyroptosis and play a neuroprotective role.
NLRP3 inflammasome assembly induced by ionic steady-state imbalance
Intracellular K+ efflux is a key factor in the activation of NLRP3 inflammasome (Muñoz-Planillo et al., 2013). K+ efflux leads to the interaction between the inactive NLRP3 positive motif and the negative PIP on the Golgi membrane, which causes the local accumulation of NLRP3 and provides sufficient conditions for the activation of NLRP3 inflammasomes. The researchers found that NLRP3 inflammasomes could be activated without decreasing the intracellular K+ concentration in the early stage of crystal stimulation, indicating that the activation of NLRP3 activation signal was not dependent on K+ efflux, which denied the necessity of K+ efflux for the activation of NLRP3 inflammasomes (Zhang et al., 2018). Therefore, the reduction of intracellular K+ concentration provides sufficient and unnecessary conditions for the activation of NLRP3 inflammasomes. Both ATP synthesis and Na+- K+- ATPase activity are reduced during IS, leading to an increase in Na+ influx and K+ efflux, thus reducing the intracellular K+ (Zhu et al., 2022). ATP released by necrotic cells binds to the P2X4 receptor, causing the receptor to open, leading to K+ efflux. In addition, the necrotic cells passively release K+, resulting in high extracellular K+. K+ activates the pannexin 1 channel on the cell membrane, further releasing ATP. Previous studies have revealed that P2X7R triggers the second stage of assembly and activation of NLRP3 inflammasomes (Albalawi et al., 2017), which can activate NLRP3 inflammasomes in astrocytes and participate in the pathogenesis of IS (Ye et al., 2017).
Ca2+ influx is another important factor independent of the activation of NLRP3 inflammasomes induced by K+ efflux (Katsnelson et al., 2015). Ca2+ influx is another important factor in NLRP3 inflammasome activation. There are four main ways to cause cytoplasm Ca2+ overload in IS. The first is that the N-methyl-D-aspartate receptor (NMDAR) of the postsynaptic membrane is overexcited, which mediates the opening of its coupled calcium channel, and a large amount of extracellular Ca2+ influx (Ludhiadch et al., 2022). The second is the release of Ca2+ from the endoplasmic reticulum cavity to the cytoplasm caused by endoplasmic reticulum stress (ERS) (Groenendyk et al., 2021). The third is that Ca2+ in lysosomes is released to the cytoplasm through the non-selective cation channel TRPML (Weber and Schilling, 2014). The last one is that the permeability of plasma membrane-related calcium pump is reduced due to the lack of ATP activity, leading to the reduction of Ca2+ transfer to the outside cell (Hu and Song, 2017). At present, there are two opinions about the Ga2+ in the activation of NLRP3 inflammasome. One is that Ca2+ is directly involved in the activation of the NLRP3 inflammasome, because the increase of Ca2+ can promote the interaction between NLRP3 and ASC (Lee et al., 2012). Another opinion is that the increase of the excessive release of Ca2+ from the endoplasmic reticulum (ER) leads to an overload of mitochondrial Ca2+, leading to mitochondrial dysfunction and activation of NLRP3 inflammasome (Murakami et al., 2012). No matter whether Ca2+ directly or indirectly activates the NLRP3 inflammasome, it is clear that Ca2+ overload induces pyroptosis in IS (Wang L et al., 2020). Interestingly, Science published a research article related to the mechanism of Ca2+ activating pyroptosis in 2018. It was found that Ca2+ influx through the GSDMD pore was used as a signal for cells to start membrane repair, and ESCRT complexes required for transport were recruited to repair damaged membrane systems. Since the inhibition of ESCRT-III could significantly improve pyroptosis, this article provides new insight into the endogenous mechanism of pyroptosis (Rühl et al., 2018).
NLRP3 inflammasome assembly induced by lysosome rupture
The lysosomal membrane loses its stability, integrity and permeability, and lysosome membrane permeabilization (LMP) occurs after IS, leading to the release of cathepsin B and Ca2+ (Bruchard et al., 2013; Repnik et al., 2014). Various cathepsins play a role in pro-synthesis and NLRP3 activation (Orlowski et al., 2015). It is reported that the release of lysosomal cathepsin B is IL-1β release required in Nature (Duewell et al., 2010). Pharmacological inhibition of cathepsin activity or genomic deletion of various cathepsin can significantly reduce the signal transduction of NLRP3 inflammasome. For example, cathepsin B inhibitor CA-074-Me could partially inhibit the activation of NLRP3 inflammasome (Bruchard et al., 2013). However, there are also studies showing that gene deletion or knockdown of cathepsin B has little effect on NLRP3 inflammasome activation (Orlowski et al., 2015). Therefore, whether lysosomal cathepsin B is a sufficient and necessary condition for the activation of NLRP3 inflammasome is controversial. Ca2+ released by lysosome rupture is activated by NLRP3 inflammasomes via transforming growth factor-beta activated kinase 1 (TAK1)/c-Jun NH2 terminal kinase (JNK) pathway. Ga2+-CaMKII promotes the binding of TAK to TAK 1 binding protein (transforming growth factor-β activated kinase 1 binding protein, TAB), so that phosphorylation of TAK1 activates downstream MAPK kinase (MAPK kinase, MAPKK) MKK4/7, and subsequently MKK4/7 specifically activates JNK (Hirata et al., 2017). JNK promotes the activation of the NLRP3 inflammasome by regulating ASC oligomerization (Hara et al., 2013). Zhang and colleagues confirmed that TAK1/JNK pathway is involved in the pathogenesis of IS in MCAO/R rats and OGD/R primary cortical neurons (Zhang Z et al., 2022). CNS extracellular pathological signal molecule lysophosphatidic acid (LPA) induced PC12 pyroptosis is related to JNK (Choi and Chun, 2013; Li and Zhang, 2020). In addition, lysosome rupture will also trigger a significant reduction of cytoplasmic K+ before NLRP3 inflammasome assembly and caspase-1 production, and is not related to NLRP3 inflammasome assembly and accumulation (Muñoz-Planillo et al., 2013). This indicates that lysosome rupture activates plasma membrane cation channel, which is a key early signal of NLRP3 inflammasome assembly (Katsnelson et al., 2015). Some studies have also shown that under certain experimental conditions, the effect of lysosome rupture on activating NLRP3 inflammasome signal may be very low. Therefore, lysosome rupture determines whether to activate or inhibit NLRP3 inflammasomes according to different cell environments.
The intervention of traditional Chinese medicine on NLRP3 inflammasome-mediated pyroptosis in ischemic stroke
Modern pharmacological studies have found that most of the traditional Chinese medicine aims to prevent and treat IS by inhibiting the activation of NLRP3 inflammasomes and caspase-1, and by regulating its upstream related signal pathways, such as TLR4/NF-κB, ROS/TXNIP, AMPK/Nrf2 or DRP1/NLRP3 to inhibit the occurrence of pyroptosis. At the same time, it is also found that the same monomer or compound prescription can regulate and control the above multiple pathways to improve NLRP3-mediated pyroptosis. Thus, the influence of traditional Chinese medicine on pyroptosis anti-IS is characterized by multiple pathways.
Acupuncture
Acupuncture is recommended by the World Health Organization (WHO) as an alternative strategy for IS treatment and nursing. Acupuncture can effectively improve IS by promoting nerve regeneration, improving blood flow in the infarct area, fighting apoptosis, and regulating neurochemicals (Chavez et al., 2017; Xu et al., 2018). In recent years, studies have found that acupuncture plays a neuroprotective role by inhibiting NLRP3 inflammasome-mediated pyroptosis (Zhang T et al., 2021; Chen et al., 2022). For example, acupuncture can protect MCAO rats by up-regulating the expression of SIRT1, inhibiting the activation of NLRP3 inflammasome and down-regulating the expression of IL-18 (Zhou et al., 2022). Xu et al. (2020) treated the MCAO/R rats with electroacupuncture at the four points of Zusanli, Sanyinjiao, Chize, and Hegu, and found that the infarction volume of the electroacupuncture group was reduced compared with the model group, and caspase-1 mRNA was lower. Liu et al. (2019) intervened in MCAO/R rats with eye acupuncture. Intervention of MCAO/R rats with eye acupuncture has no significant difference from that of the NLRP3 inflammasome inhibitor glibenclamide group. Both of them can inhibit the expression of P2X7R, NLRP3, pro-capase-1, ASC, caspase-1 protein in rats, indicating that eye acupuncture can inhibit the occurrence of pyroptosis. The results show that eye-acupuncture could inhibit the expression of P2X7R, NLRP3, pro-caspase-1, ASC, and caspase-1 proteins in rats, which is consistent with the trend of the group using NLRP3 inflammasome inhibitor glibenclamide, indicating that eye acupuncture could inhibit the occurrence of pyroptosis. miRNA-mediated pyroptosis is also involved in the development of IS. For example, the level of miR-223 in the cortex around the infarction of MCAO rats treated with electroacupuncture increased, while the level of NLRP3, caspase-1, IL-1β and IL-18 decreased (Sha et al., 2019). To sum up, acupuncture can regulate IS with multiple targets, which may become a new treatment tragedy for IS. However, a minority of the above studies have not carried out specific signal pathway studies, so the protective mechanism of acupuncture on neuronal damage after IS still needs to be confirmed by further research (Table 1).
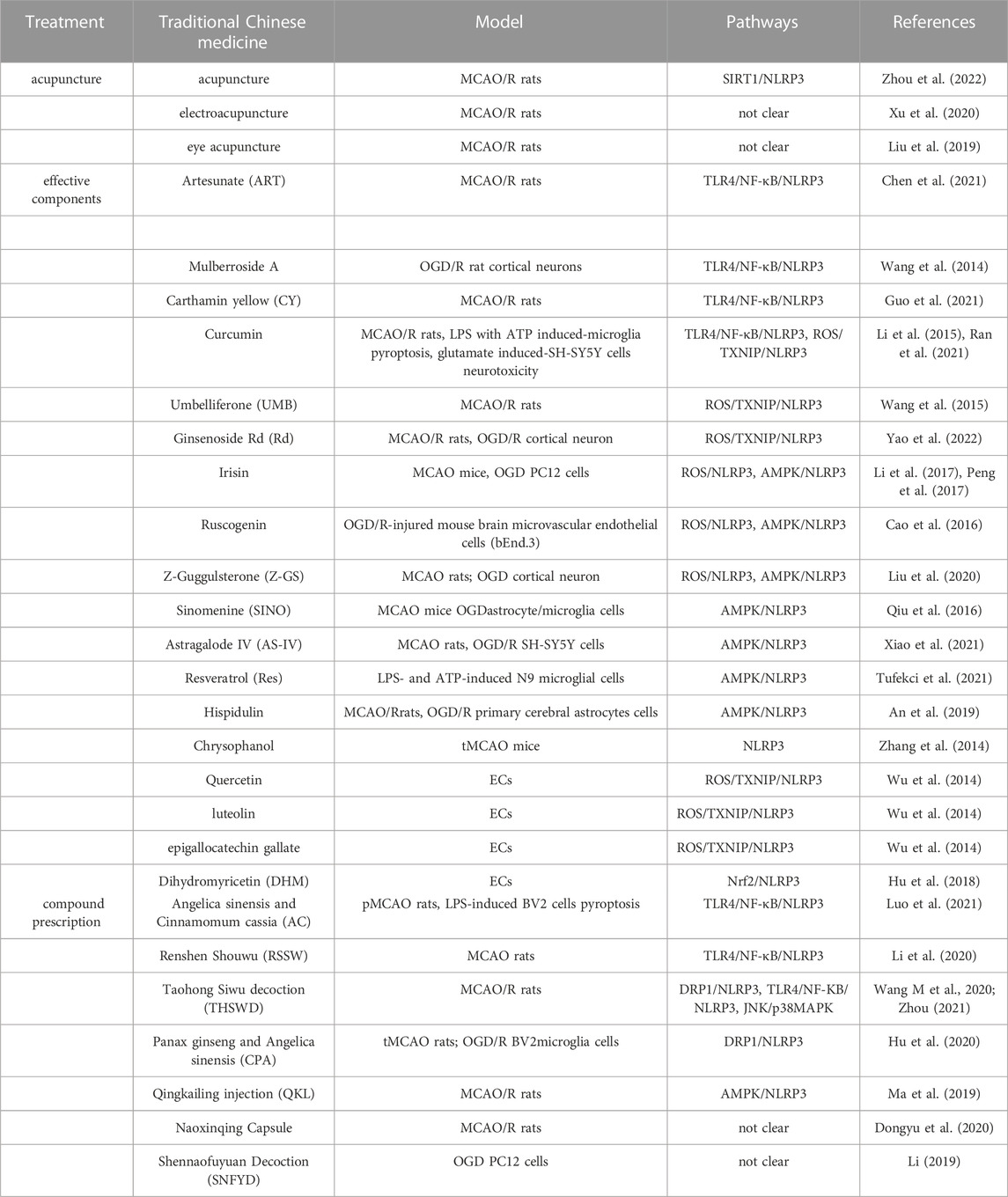
TABLE 1. Advance in treatment of traditional Chinese medicine against IS by drug intervention pyroptosis.
Traditional Chinese medicine monomer
Traditional Chinese medicine monomer is an active ingredient extracted from traditional Chinese medicine. These traditional Chinese medicine monomers are mainly extracted by alcohol, supplemented by ultrasonic cracking (Wang et al., 2014; Yue. et al., 2016). Some TCM monomers have been approved by the China Medical Products Administration (NMPA) for the treatment of IS. In recent years, it has been found that many traditional Chinese medicine monomer can play an anti-pyroptosis role by regulating the signal pathway upstream of NLRP3 inflammasome. Mulberroside A is considered to be the main active ingredient of Cortex Mori, which shows extensive benefits in the routine oral water administration route (Wang et al., 2011). Mulberroside A can inhibit TLR4/NF-κB induced by OGD/R cortical neurons (Wang CP et al., 2014). Artesunate (ART), a derivative of artemisinin (Chen et al., 2021), can reduce the score of neurological deficits induced by MCAO/R rats, improve brain edema, and reduce the volume of cerebral infarction. Its mechanism is related to down-regulating TLR4/NF-κB/NLRP3 pathway (Ran et al., 2021). Carthamin yellow (CY) is a flavonoid compound isolated from safflower, it is considered that CY improves blood circulation and alleviates pain. Thus, CY is used for the treatment of cerebrovascular disease (Lu QY et al., 2019). Its protection mechanism is the same as that of ART (Guo et al., 2021). Curcumin is a natural polyphenolic compound in Curcuma longa. Curcumin can reduce NF-κB/NLRP3 signal pathway to inhibit LPS-ATP-induced primary microglial pyroptosis (Ran et al., 2021). In addition to affecting NLRP3 inflammasomes through TLR4/NF-κB pathway, traditional Chinese medicine monomer can also regulate ROS/TXNIP/NLRP3 pathway to prevent pyroptosis. Curcumin (10 μmol L-1) inhibits the activation of the TXNIP/NLRP3 pathway by up-regulating AMPK activity in human neuroblastoma SH-SY5Y cells of human neuroblastoma and reducing ROS produced by endoplasmic reticulum stress (Li et al., 2015). Umbelliferone (UMB), a natural antioxidant belonging to coumarin derivatives, is able to cross the blood-brain barrier and protect neuronal cells from death. UMB (15 and 30 mg/kg) pretreatment for 7 days significantly upregulates peroxisome proliferator-activated receptor (PPAR-γ) with an antioxidant effect in MCAO/R rats, and inhibits ROS, thereby reducing the expression of TXNIP to inhibit NLRP3 inflammasome (Wang X et al., 2015). Ginsenoside Rd (Rd), a monomer component of Panax ginseng and Panax notoginseng, is reported to confer neuroprotection in brain injury models. Rd activates the Nrf2 pathway by up-regulating miR-139-5p, and downregulates ROS/TXNIP/NLRP3 induced by MCAO rats and OGD cortical neurons (Yao et al., 2022). Ruscogenin, an important steroid sapogenin derived from Ophiopogon japonicus, has been shown to inhibit cerebral ischemic injury. In the mouse brain microvascular endothelial cells (bEnd.3) and OGD/R primary cortical neuron, ROS was significantly inhibited by the administration of Ruscogenin (Cao et al., 2016). Z-Guggulsterone (Z-GS), an active component derived from myrrh. MCAO rats were treated with different doses of Z-GS. Morphological results showed that Z-GS significantly alleviated neurological deficits, infarct volume and histopathological damage in MCAO rats. It was related to TXNIP and NLRP3 by immunohistochemistry and immunofluorescence staining. The author conducted in vitro experiments to verify the mechanism, it was found that Z-GS treatment scarcely changed the expressions of NLRP3 in siRNA-TXNIP pretreated cells compared with the siRNA-TXNIP alone treatment group, suggesting that the neuroprotective effect of Z-GS was dependent on TXNIP-NLRP3 axis (Liu et al., 2020). Traditional Chinese medicine monomer can also upregulate AMPK/Nrf2 and inhibit NLRP3 inflammasome. In vitro and in vivo experimental studies showed that AMPK/Nrf2 pathway could be downregulated and NLPR3 inflammasome activated in MCAO/R rats or mice, OGD/R or LPS-ATP SH-SY5Y cells, microglial, and astrocytes. When intervening with an alkaloid extracted from Sinomenium acutum, the expression of AMPK was upregulated, and NLRP3 inflammasome was suppressed (Qiu et al., 2016). Astragaloside IV (AS-IV) is the main effective component of Astragalus membranaceus, and is widely used in the prevention and treatment of cerebrovascular diseases (Li S et al., 2021). Xiao et al. (2021) established MCAO/R rat in vivo, OGD/R SH-SY5Y cell in vitro, they enhanced NLRP3, caspase-1, IL-1β, GSDMD and GSDMD-N protein levels, indicating that NLRP3/caspase-1/GSDMD pathway is activated to promote pyroptosis. AS-IV increased the protein levels of Nrf2 and promoted the transfer of Nrf2 to the nucleus, accelerating Nrf2 activation. It shows that AS-IV inhibits NLRP3-mediated pyroptosis by activating Nrf2 (Xiao et al., 2021). Resveratrol (Res), which is a natural polyphenolic compound, inhibits LPS- and ATP-activated NLRP3 inflammasome and protects microglial cells upon pyroptosis. Mechanismly, it inhibits NF-κB and activates AMPK/Sirt1 pathways (Tufekci et al., 2021). Hispidulin is a flavonoid present in many Chinese herbal medicines (Kut et al., 2022). When MCAO rats were treated with different doses of hispidulin, hispidulin exerted its neuroprotective effects in vivo and in vitro by suppressing NLRP3-mediated pyroptosis by modulating the AMPK/GSK3β signaling pathway (An et al., 2019). Particularly revealing was that the AMPK inhibitor Compound C (An et al., 2019) or the Nrf2 inhibitor ML385 (Xiao et al., 2021), some of the effects of the drugs are offset, indicating that the traditional Chinese medicine monomers play an anti-pyroptosis effect through AMPK/Nrf2/NLRP3 pathway. Chrysophanol, the main active ingredient from Dahuang (Radix Et Rhizoma Rhei), Heshouwu (Radix Polygoni Multiflori) and Huzhang (Rhizoma Et Radix Polygoni Cuspidwi), can inhibit the activation of NLRP3 induced by transient MCAO (tMCAO) in mice, but the specific pathway mediated by it is not clear at present, which needs to be further explored (Zhang et al., 2014).
Moreover, endothelial cells (ECs) pyroptosis plays an important role in IS. Wang et al. (2021) confirmed that IS can induce pyroptosis of microvascular endothelial cells (ECs) and aggravate the ischemia-reperfusion injury. When MCC950, a specific drug targeting NLRP3, is used to interfere with ECs in OGD, the results show that endothelial NLRP3 is inhibited, indicating that endothelial NLRP3 inflammasome-mediated pyroptosis is also an effective target (Bellut et al., 2021). Some TCM monomers can target ECs pyroptosis. For example, quercetin, luteolin, and epigallocatechin gallate inhibit TXNIP/NLRP3 by reducing ROS in ECs (Wu et al., 2014). Dihydromyricetin (DHM) pretreated vascular ECs, reduced the release of IL-1β related to pyroptosis, significantly decreased the levels of intracellular ROS, and promoted the activation of Nrf2. When the knockdown of Nrf2 by siRNA, the inhibitory effect of DHM on ECs pyroptosis was counteracted. Therefore, DHM plays an anti-pyroptosis role by activating the Nrf2/NLRP3 pathway of vascular ECs (Hu Q et al., 2018) (Table 1).
Compound prescription and Chinese patent medicine
Due to the occurrence and development of IS is a multi-path and multi-target collaborative process. In the process of treatment, the most effective way is to inhibit or block all the relevant pathways corresponding to the onset of IS. Compound prescription and Chinese patent medicine contain many kinds of effective ingredients of traditional Chinese medicine, which are the most widely used forms of traditional Chinese medicine in clinical practice. At present, studies have confirmed that they can exert neuroprotective effects by affecting pyroptosis. For example, Naoxinqing Capsule, a traditional Chinese patent drug, can effectively inhibit the protein expression of NLRP3, ASC, caspase-1, IL-18 and IL-1β in MCAO/R rats and protect the cerebrovascular function when administered continuously for 21 days at the dose of 100 mg/(kg · d). When OGD PC12 cells were cultured with Shennaofuyuan Decoction (SNFYD) drug-containing serum, the expression level of IL-18 and IL-1β were significantly decreased, suggesting that SNFYD can inhibit neuronal pyroptosis (Li, 2019). Luo et al. (2021) showed for the first time that the expression of TLR4, NLRP3, ASC and caspase-1 was downregulated in a dose-dependent manner after three doses of Angelica Cinnamomum (AC) extract were given to pMCAO rats for 7 days. In LPS-induced BV2 cells, cerebrospinal fluid containing AC extract inhibited the secretion of pro-inflammatory cytokines, and its intervention effect was similar to that of TLR4 siRNA treatment. It suggests that AC may play a neuroprotective role by inhibiting the formation of NLRP3 inflammasome through TLR4/NF-κB. Renshen Shouwu (RSSW), composed of Ginseng (Root of Panax ginseng C.A. Mey) and fleece flower root (Polygonum multiflorum Thunb.), is a patented Traditional Chinese Medicine included in Chinese Pharmacopoeia. RSSW (50 mg/kg, 100 mg/kg) was administered to MCAO rats. Western blot showed that RSSW significantly downregulated TLR4/NF-κB/NLRP3 signaling pathway (Li. et al., 2020). Taohong Siwu Decoction (THSWD) can also regulate TLR4/NF-κB/NLRP3 (Wang M et al., 2020). THSWD originated from the traditional Chinese medicine book Yizong Jinjian of the Qing Dynasty, which is composed of Prunus persica (L.) Batsch, Carthamus tinctorius L, Angelica sinensis (Oliv.) Diels, Rehmannia glutinosa (Gaertn.) DC., Ligusticum chuanxiong Hort, Paeonia lactiflora Pall. Also, previous report has investigated the major constituents of THSWD by UPLCQ-TOF-MS. A total of 95 components have been identifified, including aromatic acids, flflavonoids, polysaccharides, volatile oils, monoterpene glycosides, aromatic cyanoglycosides (Dong et al., 2019). They are the basis of THSWD inhibitors of pyroptosis (Ye et al., 2020; Yin et al., 2020). Unlike RSSW, THSWD can reduce the expression of TXNIP, p38MAPK and JNK. In addition, THSWD has been found to inhibit MCAO/R rats pyroptosis via DRP1/NLRP3 pathway (Zhou, 2021). These again illustrate the characteristics of multi-component and multi-target action of traditional Chinese medicine. Panax ginseng and Angelica sinensis (CPA) treatment ameliorated MCAO-induced cerebral damage and neurological dysfunction by inhibiting NLRP3 inflammasome activation and microglial pyroptosis. Its inhibitory effect was comparable to that of MCC950, a well-known inhibitor of NLRP3 inflammasome. Further in vitro study revealed that the key active ingredients of Panax ginseng and Angelica sinensis inhibited OGD/R-induced NLRP3 inflammasome activation and pyroptosis by inhibiting DRP1-mediated mitochondrial fission (Hu et al., 2020). Qingkailing (QKL) injection, a patented Chinese medicine approved by the China Food and Drug Administration, has been widely used in clinical practice to treat cerebral ischemia in China. Rats in the QKL group received intraperitoneal injections of 3 mL/kg QKL, QKL relieved IS and suppressed the inflammatory response by inhibiting AMPK-mediated activation of the NLRP3 inflammasome (Ma et al., 2019) (Table 1).
Conclusion and perspectives
Pyroptosis is a form of death characterized by cell swelling, membrane rupture, and inflammatory cytokine release. It can be caused by NLRP3 inflammasome that assembly after activation of secreted IL-1β and IL-18. NLRP3 inflammasome play an important role in the development of IS. Most in vitro and in vivo studies in our review have confirmed that inhibition of NLRP3 inflammasome can improve neural function and reduce the volume of cerebral infarction to a large extent. However, a study from Stroke showing that the injury degree of IS has nothing to do with NLRP3 inflammasome (Lemarchand et al., 2019). We speculate that this may be related to experimental modeling, because in this report, C57BL/6 mice were modeled with FeCl3, which is different from the MCAO and OGD models we summarized previously. A variety of signal molecules lead to early pathological changes through corresponding signaling pathways after IS, subsequently activating NLRP3 inflammasome-mediated pyroptosis. These signal-mediated pathological changes include ROS damage, mitochondrial dysfunction, ion imbalance, lysosomal rupture, and trans-Golgi disintegration. The activation of NLRP3 inflammasome consists of two steps, namely, the activation and assembly of NLRP3 inflammasome. The signaling pathway involved in the activation step is TLR4/NF-κB/NLRP3. The signaling pathways involved in the assembly include ROS/TXNIP/NLRP3, AMPK/Nrf2/NLRP3, DRP1/NLRP3, and TAK1/JNK/NLRP3. Current pharmacological studies show that traditional Chinese medicine can inhibit NLRP3 inflammasome by regulating the above signal pathways, thus inhibiting pyroptosis and achieving the purpose of alleviating the process of IS, showing the characteristics of multi-target and multi-channel treatment of traditional Chinese medicine. This provides a positive signal for exploring the role of traditional Chinese medicine in pyroptosis (Table 1).
We believe that there are still some challenges in the research of anti-IS effect of traditional Chinese medicine based on pyroptosis. 1) Strengthen the research of compound prescription and Chinese patent medicine. Our previous summaries found that compared with traditional Chinese medicine monomer, the compound prescription and Chinese patent medicine, as a common clinical form of traditional Chinese medicine, has less research reports, which is not conducive to providing scientific basis for the clinical application of the compound. In the future, bioinformatics methods can be used to identify the potential effective components and target of action of the compound prescription and Chinese patent medicine, structural pharmacology methods such as molecular docking technology can be used to further reveal the binding sites of monomer components, and methods such as gene knockout or inhibition of key proteins can be used to verify the target of action of monomer components. Adopt modern pharmacological methods to carry out simultaneous component research and comparative study with compound prescription, so as to clarify the material basis of drug function. 2) The IS body model is diversified. Most of the models summarized in this review are MACO or MCAO/R models. However, studies have shown that the selection of models may have an impact on the role of NLRP3-mediated pyroptosis in IS (Lemarchand et al., 2019). Therefore, different models can be used for comparative study in future studies, and mutual verification can fully illustrate the empirical conclusions. 3) To explore the potential of non-classical pyroptosis in the study of IS pathological mechanism. Previous studies have shown that the expression of caspase-11 protein upregulated in OGD microglial cells, which indicates that there is activation of non-classical pyrolytic pathway in the process of IS (Fradejas et al., 2010). However, there is no in vivo experiment to show the relationship between non-classical pyrolytic pathway and IS. Moreover, there is little research on non-classical pyroptosis pathway in traditional Chinese medicine.
To sum up, NLPR3 inflammasome may be the switch molecular target of the upstream channel of the mechanism of pyroptosis in IS. Deeply exploring the relevant molecular mechanism of NLRP3 inflammaasome affecting pyroptosis, summarizing the relationship between NLRP3 mediated-pyroptosis signal pathway and IS, and the research status of TCM prevention and treatment, clarifying the specific mechanism of TCM, provide new ideas for TCM treatment of IS, and provide theoretical basis for TCM to effectively and economically serve human health.
Author contributions
J-XL and M-ZT contributed equally to this manuscript. J-XL designed the review. J-XL and M-ZT were in charge of searching all the relative papers. H-HY, HD, FL, and KD gave their valuable and professional suggestions and guide in organizing and drafting this manuscript. X-YC and KD revised the manuscript.
Funding
This work was supported by State Key Laboratory Open Program of China (GTZK201711), Hunan Provincial Natural Science Foundation of China (2020JJ9050), Double-class Discipline Pharmacy Open Fund of China (2018YX03), Hunan Provincial Department of Education Postgraduate Innovation and Entrepreneurship Training Program Project of China (CX20220794), Innovation and Entrepreneurship Training Program for College Students of Hunan Provincial Department of Education of China (S202210541074), First-class undergraduate courses in Hunan Province of China (2021(28)-371) and Research project of educational reform in universities in Hunan Province of China (HNJG-2022-0134).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agostini, L., Martinon, F., Burns, K., McDermott, M. F., Hawkins, P. N., and Tschopp, J. (2004). NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity 20 (3), 319–325. doi:10.1016/s1074-7613(04)00046-9
Albalawi, F., Lu, W., Beckel, J. M., Lim, J. C., McCaughey, S. A., and Mitchell, C. H. (2017). The P2X7 receptor primes IL-1β and the NLRP3 inflammasome in astrocytes exposed to mechanical strain. Front. Cell. Neurosci. 11, 227. doi:10.3389/fncel.2017.00227
Alishahi, M., Farzaneh, M., Ghaedrahmati, F., Nejabatdoust, A., Sarkaki, A., and Khoshnam, S. E. (2019). NLRP3 inflammasome in ischemic stroke: As possible therapeutic target. Int. J. Stroke 14 (6), 574–591. doi:10.1177/1747493019841242
An, P., Xie, J., Qiu, S., Liu, Y., Wang, J., Xiu, X., et al. (2019). Hispidulin exhibits neuroprotective activities against cerebral ischemia reperfusion injury through suppressing NLRP3-mediated pyroptosis. Life Sci. 232, 116599–599. doi:10.1016/j.lfs.2019.116599
Bellut, M., Papp, L., Bieber, M., Kraft, P., Stoll, G., and Schuhmann, M. K. (2021). NLPR3 inflammasome inhibition alleviates hypoxic endothelial cell death in vitro and protects blood-brain barrier integrity in murine stroke. Cell Death Dis. 13 (1), 20. doi:10.1038/s41419-021-04379-z
Bruchard, M., Mignot, G., Derangère, V., Chalmin, F., Chevriaux, A., Végran, F., et al. (2013). Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat. Med. 19 (1), 57–64. doi:10.1038/nm.2999
Cao, G., Jiang, N., Hu, Y., Zhang, Y., Wang, G., Yin, M., et al. (2016). Ruscogenin attenuates cerebral ischemia-induced blood-brain barrier dysfunction by suppressing TXNIP/NLRP3 inflammasome activation and the MAPK pathway. Int. J. Mol. Sci. 17 (9), 1418. doi:10.3390/ijms17091418
Carneiro, L., Allard, C., Guissard, C., Fioramonti, X., Tourrel-Cuzin, C., Bailbé, D., et al. (2012). Importance of mitochondrial dynamin-related protein 1 in hypothalamic glucose sensitivity in rats. Antioxid. Redox Signal 17 (3), 433–444. doi:10.1089/ars.2011.4254
Chavez, L. M., Huang, S. S., MacDonald, I., Lin, J. G., Lee, Y. C., and Chen, Y. H. (2017). Mechanisms of acupuncture therapy in ischemic stroke rehabilitation: A literature review of basic studies. Int. J. Mol. Sci. 18 (11), 2270. doi:10.3390/ijms18112270
Chen, D., Dixon, B. J., Doycheva, D. M., Li, B., Zhang, Y., Hu, Q., et al. (2018). IRE1α inhibition decreased TXNIP/NLRP3 inflammasome activation through miR-17-5p after neonatal hypoxic-ischemic brain injury in rats. J. Neuroinflammation 15 (1), 32. doi:10.1186/s12974-018-1077-9
Chen, J., and Chen, Z. J. (2018). PtdIns4P on dispersed trans-Golgi network mediates NLRP3 inflammasome activation. Nature 564 (7734), 71–76. doi:10.1038/s41586-018-0761-3
Chen S, S., Mei, S., Luo, Y., Wu, H., Zhang, J., and Zhu, J. (2018). Gasdermin family: A promising therapeutic target for stroke. Transl. stroke Res. 9 (6), 555–563. doi:10.1007/s12975-018-0666-3
Chen, Y., Hao, C., Chen, W., Cheng, W., Li, P., Shen, J., et al. (2022). Anti-depressant effects of acupuncture: The insights from NLRP3 mediated pyroptosis and inflammation. Neurosci. Lett. 785, 136787. doi:10.1016/j.neulet.2022.136787
Chen, Y., Wu, J., Zhu, J., Yang, G., Tian, J., Zhao, Y., et al. (2021). Artesunate provides neuroprotection against cerebral ischemia-reperfusion injury via the TLR-4/NF-κB pathway in rats. Biol. Pharm. Bull. 44 (3), 350–356. doi:10.1248/bpb.b20-00604
Choi, J. W., and Chun, J. (2013). Lysophospholipids and their receptors in the central nervous system. Biochim. Biophys. Acta 1831 (1), 20–32. doi:10.1016/j.bbalip.2012.07.015
Dong, W., Zhu, Q., Yang, B., Qin, Q., Wang, Y., Xia, X., et al. (2019). Polychlorinated biphenyl quinone induces caspase 1-mediated pyroptosis through induction of pro-inflammatory HMGB1-TLR4-NLRP3-GSDMD signal Axis. Chem. Res. Toxicol. 32 (6), 1051–1057. doi:10.1021/acs.chemrestox.8b00376
Dongyu, M., Li, H., Guan, L., Jiang, C., Zhang, H., Cui, X., et al. (2020). Protective mechanism of Naoxinqing Capsule in rat models of cerebral ischemia/reperfusion injury. Chin. J. Tissue Eng. Res. 24 (2), 215–222.
Duan, C., Kuang, L., Xiang, X., Zhang, J., Zhu, Y., Wu, Y., et al. (2020). Drp1 regulates mitochondrial dysfunction and dysregulated metabolism in ischemic injury via Clec16a-BAX-and GSH- pathways. Cell Death Dis. 11 (4), 251. doi:10.1038/s41419-020-2461-9
Duewell, P., Kono, H., Rayner, K. J., Sirois, C. M., Vladimer, G., Bauernfeind, F. G., et al. (2010). NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464 (7293), 1357–1361. doi:10.1038/nature08938
Fradejas, N., Pastor, M. D., Burgos, M., Beyaert, R., Tranque, P., and Calvo, S. (2010). Caspase-11 mediates ischemia-induced astrocyte death: Involvement of endoplasmic reticulum stress and C/EBP homologous protein. J. Neurosci. Res. 88 (5), 1094–1105. doi:10.1002/jnr.22280
Fu, C., Zhang, X., Lu, Y., Wang, F., Xu, Z., Liu, S., et al. (2020). Geniposide inhibits NLRP3 inflammasome activation via autophagy in BV-2 microglial cells exposed to oxygen-glucose deprivation/reoxygenation. Int. Immunopharmacol. 84, 106547. doi:10.1016/j.intimp.2020.106547
Fuse, Y., and Kobayashi, M. (2017). Conservation of the keap1-nrf2 system: An evolutionary journey through stressful space and time. Molecules 22 (3), 436. doi:10.3390/molecules22030436
GBD 2016 Stroke Collaborators (2019). Global, regional, and national burden of stroke, 1990-2016: A systematic analysis for the global burden of disease study 2016. Lancet Neurol. 18 (5), 439–458. doi:10.1016/s1474-4422(19)30034-1
Groenendyk, J., Agellon, L. B., and Michalak, M. (2021). Calcium signaling and endoplasmic reticulum stress. Int. Rev. Cell Mol. Biol. 363, 1–20. doi:10.1016/bs.ircmb.2021.03.003
Gu, C., Li, T., Jiang, S., Yang, Z., Lv, J., Yi, W., et al. (2018). AMP-activated protein kinase sparks the fire of cardioprotection against myocardial ischemia and cardiac ageing. Ageing Res. Rev. 47, 168–175. doi:10.1016/j.arr.2018.08.002
Guo, H., Zhu, L., Tang, P., Chen, D., Li, Y., Li, J., et al. (2021). Carthamin yellow improves cerebral ischemia-reperfusion injury by attenuating inflammation and ferroptosis in rats. Int. J. Mol. Med. 47 (4), 52. doi:10.3892/ijmm.2021.4885
Guo, M., Wang, X., Zhao, Y., Yang, Q., Ding, H., Dong, Q., et al. (2018). Ketogenic diet improves brain ischemic tolerance and inhibits NLRP3 inflammasome activation by preventing drp1-mediated mitochondrial fission and endoplasmic reticulum stress. Front. Mol. Neurosci. 11, 86. doi:10.3389/fnmol.2018.00086
Hara, H., Tsuchiya, K., Kawamura, I., Fang, R., Hernandez-Cuellar, E., Shen, Y., et al. (2013). Phosphorylation of the adaptor ASC acts as a molecular switch that controls the formation of speck-like aggregates and inflammasome activity. Nat. Immunol. 14 (12), 1247–1255. doi:10.1038/ni.2749
Hasan, T. F., Hasan, H., and Kelley, R. E. (2021). Overview of acute ischemic stroke evaluation and management. Biomedicines 9 (10), 1486. doi:10.3390/biomedicines9101486
Hirata, Y., Takahashi, M., Morishita, T., Noguchi, T., and Matsuzawa, A. (2017). Post-translational modifications of the TAK1-TAB complex. Int. J. Mol. Sci. 18 (1), 205. doi:10.3390/ijms18010205
Hou, Y., Wang, Y., He, Q., Li, L., Xie, H., Zhao, Y., et al. (2018). Nrf2 inhibits NLRP3 inflammasome activation through regulating Trx1/TXNIP complex in cerebral ischemia reperfusion injury. Behav. Brain Res. 336, 32–39. doi:10.1016/j.bbr.2017.06.027
Hu, C., Huang, Y., and Li, L. (2017). Drp1-Dependent mitochondrial fission plays critical roles in physiological and pathological progresses in mammals. Int. J. Mol. Sci. 18 (1), 144. doi:10.3390/ijms18010144
Hu, H. J., and Song, M. (2017). Disrupted ionic homeostasis in ischemic stroke and new therapeutic targets. J. Stroke Cerebrovasc. Dis. 26 (12), 2706–2719. doi:10.1016/j.jstrokecerebrovasdis.2017.09.011
Hu, J., Zeng, C., Wei, J., Duan, F., Liu, S., Zhao, Y., et al. (2020). The combination of Panax ginseng and Angelica sinensis alleviates ischemia brain injury by suppressing NLRP3 inflammasome activation and microglial pyroptosis. Phytomedicine 76, 153251. doi:10.1016/j.phymed.2020.153251
Hu, Q., Zhang, T., Yi, L., Zhou, X., and Mi, M. (2018). Dihydromyricetin inhibits NLRP3 inflammasome-dependent pyroptosis by activating the Nrf2 signaling pathway in vascular endothelial cells. Biofactors 44 (2), 123–136. doi:10.1002/biof.1395
Huang, Y., Xu, W., and Zhou, R. (2021). NLRP3 inflammasome activation and cell death. Cell Mol. Immunol. 18 (9), 2114–2127. doi:10.1038/s41423-021-00740-6
Jin, X., Liu, M. Y., Zhang, D. F., Zhong, X., Du, K., Qian, P., et al. (2019). Baicalin mitigates cognitive impairment and protects neurons from microglia-mediated neuroinflammation via suppressing NLRP3 inflammasomes and TLR4/NF-κB signaling pathway. CNS Neurosci. Ther. 25 (5), 575–590. doi:10.1111/cns.13086
Joo, M. S., Kim, W. D., Lee, K. Y., Kim, J. H., Koo, J. H., and Kim, S. G. (2016). AMPK facilitates nuclear accumulation of Nrf2 by phosphorylating at serine 550. Mol. Cell Biol. 36 (14), 1931–1942. doi:10.1128/mcb.00118-16
Kang, C., Lu, Z., Zhu, G., Chen, Y., and Wu, Y. (2021). Knockdown of TRIM22 relieves oxygen-glucose deprivation/reoxygenation-induced apoptosis and inflammation through inhibition of NF-κB/NLRP3 Axis. Cell Mol. Neurobiol. 41 (2), 341–351. doi:10.1007/s10571-020-00855-w
Katsnelson, M. A., Rucker, L. G., Russo, H. M., and Dubyak, G. R. (2015). K+ efflux agonists induce NLRP3 inflammasome activation independently of Ca2+ signaling. J. Immunol. 194 (8), 3937–3952. doi:10.4049/jimmunol.1402658
Kleele, T., Rey, T., Winter, J., Zaganelli, S., Mahecic, D., Perreten Lambert, H., et al. (2021). Distinct fission signatures predict mitochondrial degradation or biogenesis. Nature 593 (7859), 435–439. doi:10.1038/s41586-021-03510-6
Kut, K., Bartosz, G., Soszyński, M., and Sadowska-Bartosz, I. (2022). Antioxidant properties of hispidulin. Nat. Prod. Res. 36 (24), 6401–6404. doi:10.1080/14786419.2022.2032050
Lee, G. S., Subramanian, N., Kim, A. I., Aksentijevich, I., Goldbach-Mansky, R., Sacks, D. B., et al. (2012). The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature 492 (7427), 123–127. doi:10.1038/nature11588
Lemarchand, E., Barrington, J., Chenery, A., Haley, M., Coutts, G., Allen, J. E., et al. (2019). Extent of ischemic brain injury after thrombotic stroke is independent of the NLRP3 (NACHT, LRR and PYD domains-containing protein 3) inflammasome. Stroke 50 (5), 1232–1239. doi:10.1161/strokeaha.118.023620
Li, L., Acioglu, C., Heary, R. F., and Elkabes, S. (2021). Role of astroglial toll-like receptors (TLRs) in central nervous system infections, injury and neurodegenerative diseases. Brain Behav. Immun. 91, 740–755. doi:10.1016/j.bbi.2020.10.007
Li, S., Dou, B., Shu, S., Wei, L., Zhu, S., Ke, Z., et al. (2021). Suppressing NK cells by astragaloside IV protects against acute ischemic stroke in mice via inhibiting STAT3. Front. Pharmacol. 12, 802047. doi:10.3389/fphar.2021.802047
Li, X. (2019). The protective effect of shennaofuyuan decoction on PC12 cells in hypoxia and glucose deprivation based on the mechanism of pyroptosis. Changsha (hunan Province in China). Hunan: Hunan University of Chinese Medicine.
Li, Y., Li, J., Li, S., Li, Y., Wang, X., Liu, B., et al. (2015). Curcumin attenuates glutamate neurotoxicity in the hippocampus by suppression of ER stress-associated TXNIP/NLRP3 inflammasome activation in a manner dependent on AMPK. Toxicol. Appl. Pharmacol. 286 (1), 53–63. doi:10.1016/j.taap.2015.03.010
Li, Y., Liang, W., Guo, C., Chen, X., Huang, Y., Wang, H., et al. (2020). Renshen Shouwu extract enhances neurogenesis and angiogenesis via inhibition of TLR4/NF-κB/NLRP3 signaling pathway following ischemic stroke in rats. J. Ethnopharmacol. 253, 112616. doi:10.1016/j.jep.2020.112616
Li, Y., and Zhang, Z. (2020). LPA induced pyroptosis of PC12 cells by JNK phosphorylation. Stroke Nerv. Dis. 27 (3), 283–292. (in Chinese).
Liu, J., Ma, W., Zang, C. H., Wang, G. D., Zhang, S. J., Wu, H. J., et al. (2021). Salidroside inhibits NLRP3 inflammasome activation and apoptosis in microglia induced by cerebral ischemia/reperfusion injury by inhibiting the TLR4/NF-κB signaling pathway. Ann. Transl. Med. 9 (22), 1694. doi:10.21037/atm-21-5752
Liu, T., Wang, W., Liu, M., Ma, Y., Mu, F., Feng, X., et al. (2020). Z-Guggulsterone alleviated oxidative stress and inflammation through inhibiting the TXNIP/NLRP3 axis in ischemic stroke. Int. Immunopharmacol. 89, 107094. doi:10.1016/j.intimp.2020.107094
Liu, X., Zhang, X., Ding, Y., Zhou, W., Tao, L., Lu, P., et al. (2017). Nuclear factor E2-related factor-2 negatively regulates NLRP3 inflammasome activity by inhibiting reactive oxygen species-induced NLRP3 priming. Antioxid. Redox Signal 26 (1), 28–43. doi:10.1089/ars.2015.6615
Liu, Y. L., Ma, X. D., and Song, C. Q. (2019). Study on mechanism of anti-pyroptosis effect of eye on CIRI rats. Chin. J. Immunol. 35 (24), 2964–2970. (in Chinese).
Lu, F., Lan, Z., Xin, Z., He, C., Guo, Z., Xia, X., et al. (2020). Emerging insights into molecular mechanisms underlying pyroptosis and functions of inflammasomes in diseases. J. Cell Physiol. 235 (4), 3207–3221. doi:10.1002/jcp.29268
Lu, Q. Y., Ma, J. Q., Duan, Y. Y., Sun, Y., Yu, S., Li, B., et al. (2019). Carthamin yellow protects the heart against ischemia/reperfusion injury with reduced reactive oxygen species release and inflammatory response. J. Cardiovasc Pharmacol. 74 (3), 228–234. doi:10.1097/fjc.0000000000000710
Ludhiadch, A., Sharma, R., Muriki, A., and Munshi, A. (2022). Role of calcium homeostasis in ischemic stroke: A review. CNS Neurol. Disord. Drug Targets. 21 (1), 52–61. doi:10.2174/1871527320666210212141232
Luo, C., Chen, Q., Liu, B., Wang, S., Yu, H., Guan, X., et al. (2021). The extracts of Angelica sinensis and Cinnamomum cassia from oriental medicinal foods regulate inflammatory and autophagic pathways against neural injury after ischemic stroke. Oxid. Med. Cell Longev. 2021, 9663208. doi:10.1155/2021/9663208
Luo, T., Zhou, X., Qin, M., Lin, Y., Lin, J., Chen, G., et al. (2022). Corilagin restrains NLRP3 inflammasome activation and pyroptosis through the ROS/TXNIP/NLRP3 pathway to prevent inflammation. Oxid. Med. Cell Longev. 2022, 1652244. doi:10.1155/2022/1652244
Ma, C., Wang, X., Xu, T., Yu, X., Zhang, S., Liu, S., et al. (2019). Qingkailing injection ameliorates cerebral ischemia-reperfusion injury and modulates the AMPK/NLRP3 Inflammasome Signalling pathway. BMC Complement. Altern. Med. 19 (1), 320. doi:10.1186/s12906-019-2703-5
McKenzie, B. A., Dixit, V. M., and Power, C. (2020). Fiery cell death: Pyroptosis in the central nervous system. Trends Neurosci. 43 (1), 55–73. doi:10.1016/j.tins.2019.11.005
Mitchell, J., Kim, S. J., Seelmann, A., Veit, B., Shepard, B., Im, E., et al. (2018). Src family kinase tyrosine phosphorylates Toll-like receptor 4 to dissociate MyD88 and Mal/Tirap, suppressing LPS-induced inflammatory responses. Biochem. Pharmacol. 147, 119–127. doi:10.1016/j.bcp.2017.11.015
Mohamadi, Y., Mousavi, M., Khanbabaei, H., Salarinia, R., Javankiani, S., Hassanzadeh, G., et al. (2018). The role of inflammasome complex in ischemia-reperfusion injury. J. Cell Biochem. 2018, 27368. doi:10.1002/jcb.27368
Muñoz-Planillo, R., Kuffa, P., Martínez-Colón, G., Smith, B. L., Rajendiran, T. M., and Núñez, G. (2013). K⁺ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38 (6), 1142–1153. doi:10.1016/j.immuni.2013.05.016
Murakami, T., Ockinger, J., Yu, J., Byles, V., McColl, A., Hofer, A. M., et al. (2012). Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc. Natl. Acad. Sci. U. S. A. 109 (28), 11282–11287. doi:10.1073/pnas.1117765109
Orlowski, G. M., Colbert, J. D., Sharma, S., Bogyo, M., Robertson, S. A., and Rock, K. L. (2015). Multiple cathepsins promote pro-IL-1β synthesis and NLRP3-mediated IL-1β activation. J. Immunol. 195 (4), 1685–1697. doi:10.4049/jimmunol.1500509
Park, H. S., Liu, G., Liu, Q., and Zhou, Y. (2018). Swine influenza virus induces RIPK1/DRP1-mediated interleukin-1 beta production. Viruses 10 (8), 419. doi:10.3390/v10080419
Qiu, J., Wang, M., Zhang, J., Cai, Q., Lu, D., Li, Y., et al. (2016). The neuroprotection of Sinomenine against ischemic stroke in mice by suppressing NLRP3 inflammasome via AMPK signaling. Int. Immunopharmacol. 40, 492–500. doi:10.1016/j.intimp.2016.09.024
Ran, Y., Su, W., Gao, F., Ding, Z., Yang, S., Ye, L., et al. (2021). Curcumin ameliorates white matter injury after ischemic stroke by inhibiting microglia/macrophage pyroptosis through NF-κB suppression and NLRP3 inflammasome inhibition. Oxid. Med. Cell Longev. 2021, 1552127. doi:10.1155/2021/1552127
Repnik, U., Hafner Česen, M., and Turk, B. (2014). Lysosomal membrane permeabilization in cell death: Concepts and challenges. Mitochondrion 19, 49–57. doi:10.1016/j.mito.2014.06.006
Rühl, S., Shkarina, K., Demarco, B., Heilig, R., Santos, J. C., and Broz, P. (2018). ESCRT-dependent membrane repair negatively regulates pyroptosis downstream of GSDMD activation. Science 362 (6417), 956–960. doi:10.1126/science.aar7607
Sha, R., Zhang, B., Han, X., Peng, J., Zheng, C., Zhang, F., et al. (2019). Electroacupuncture alleviates ischemic brain injury by inhibiting the miR-223/NLRP3 pathway. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 25, 4723–4733. doi:10.12659/msm.917213
Shi, K., Tian, D. C., Li, Z. G., Ducruet, A. F., Lawton, M. T., and Shi, F. D. (2019). Global brain inflammation in stroke. Lancet Neurol. 18 (11), 1058–1066. doi:10.1016/s1474-4422(19)30078-x
Tang, D., Kang, R., Berghe, T. V., Vandenabeele, P., and Kroemer, G. (2019). The molecular machinery of regulated cell death. Cell Res. 29 (5), 347–364. doi:10.1038/s41422-019-0164-5
Tufekci, K. U., Eltutan, B. I., Isci, K. B., and Genc, S. (2021). Resveratrol inhibits NLRP3 inflammasome-induced pyroptosis and miR-155 expression in microglia through Sirt1/AMPK pathway. Neurotox. Res. 39 (6), 1812–1829. doi:10.1007/s12640-021-00435-w
Wang, C. P., Wang, Y., Wang, X., Zhang, X., Ye, J. F., Hu, L. S., et al. (2011). Mulberroside a possesses potent uricosuric and nephroprotective effects in hyperuricemic mice. Planta Med. 77 (8), 786–794. doi:10.1055/s-0030-1250599
Wang, C. P., Zhang, L. Z., Li, G. C., Shi, Y. W., Li, J. L., Zhang, X. C., et al. (2014). Mulberroside A protects against ischemic impairment in primary culture of rat cortical neurons after oxygen-glucose deprivation followed by reperfusion. J. Neurosci. Res. 92 (7), 944–954. doi:10.1002/jnr.23374
Wang, K., Ru, J., Zhang, H., Chen, J., Lin, X., Lin, Z., et al. (2020). Melatonin enhances the therapeutic effect of plasma exosomes against cerebral ischemia-induced pyroptosis through the TLR4/NF-κB pathway. Front. Neurosci. 14, 848. doi:10.3389/fnins.2020.00848
Wang, L., Negro, R., and Wu, H. (2020). TRPM2, linking oxidative stress and Ca2+ permeation to NLRP3 inflammasome activation. Curr. Opin. Immunol. 62, 131–135. doi:10.1016/j.coi.2020.01.005
Wang, M., Liu, Z., Hu, S., Duan, X., Zhang, Y., Peng, C., et al. (2020). Taohong siwu decoction ameliorates ischemic stroke injury via suppressing pyroptosis. Front. Pharmacol. 11, 590453. doi:10.3389/fphar.2020.590453
Wang, X., Li, R., Wang, X., Fu, Q., and Ma, S. (2015). Umbelliferone ameliorates cerebral ischemia-reperfusion injury via upregulating the PPAR gamma expression and suppressing TXNIP/NLRP3 inflammasome. Neurosci. Lett. 600, 182–187. doi:10.1016/j.neulet.2015.06.016
Wang, Y., Gao, W., Shi, X., Ding, J., Liu, W., He, H., et al. (2017). Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 547 (7661), 99–103. doi:10.1038/nature22393
Wang, Y., Guan, X., Gao, C. L., Ruan, W., Zhao, S., Kai, G., et al. (2021). Medioresinol as a novel PGC-1α activator prevents pyroptosis of endothelial cells in ischemic stroke through PPARα-GOT1 axis. Pharmacol. Res. 169, 105640. doi:10.1016/j.phrs.2021.105640
Weber, K., and Schilling, J. D. (2014). Lysosomes integrate metabolic-inflammatory cross-talk in primary macrophage inflammasome activation. J. Biol. Chem. 289 (13), 9158–9171. doi:10.1074/jbc.M113.531202
Wu, J., Xu, X., Li, Y., Kou, J., Huang, F., Liu, B., et al. (2014). Quercetin, luteolin and epigallocatechin gallate alleviate TXNIP and NLRP3-mediated inflammation and apoptosis with regulation of AMPK in endothelial cells. Eur. J. Pharmacol. 745, 59–68. doi:10.1016/j.ejphar.2014.09.046
Xiao, L., Dai, Z., Tang, W., Liu, C., and Tang, B. (2021). Astragaloside IV alleviates cerebral ischemia-reperfusion injury through NLRP3 inflammasome-mediated pyroptosis inhibition via activating Nrf2. Oxid. Med. Cell Longev. 2021, 9925561. doi:10.1155/2021/9925561
Xu, H. X., Zhou, G. P., Dong, M. M., Li, Y. L., Yang, L., and Zhan, Z. L. (2020). Effect of electroacupuncture on caspase-1 in hippocampus of rats with cerebral ischemia-reperfusion injury. Mod. Traditional Chin. Med. Materia Materia-World Sci. Technol. 22 (2), 446–451.
Xu, Y., Lin, S., Jiang, C., Ye, X., Tao, J., Wilfried, S., et al. (2018). Synergistic effect of acupuncture and mirror therapy on post-stroke upper limb dysfunction: A study protocol for a randomized controlled trial. Trials 19 (1), 303. doi:10.1186/s13063-018-2585-8
Yao, Y., Hu, S., Zhang, C., Zhou, Q., Wang, H., Yang, Y., et al. (2022). Ginsenoside Rd attenuates cerebral ischemia/reperfusion injury by exerting an anti-pyroptotic effect via the miR-139-5p/FoxO1/Keap1/Nrf2 axis. Int. Immunopharmacol. 105, 108582. doi:10.1016/j.intimp.2022.108582
Ye, J. X., Wang, M., Wang, R. Y., Liu, H. T., Qi, Y. D., Fu, J. H., et al. (2020). Hydroxysafflor yellow A inhibits hypoxia/reoxygenation-induced cardiomyocyte injury via regulating the AMPK/NLRP3 inflammasome pathway. Int. Immunopharmacol. 82, 106316. doi:10.1016/j.intimp.2020.106316
Ye, X., Shen, T., Hu, J., Zhang, L., Zhang, Y., Bao, L., et al. (2017). Purinergic 2X7 receptor/NLRP3 pathway triggers neuronal apoptosis after ischemic stroke in the mouse. Exp. Neurol. 292, 46–55. doi:10.1016/j.expneurol.2017.03.002
Ye, Y., Jin, T., Zhang, X., Zeng, Z., Ye, B., Wang, J., et al. (2019). Meisoindigo protects against focal cerebral ischemia-reperfusion injury by inhibiting NLRP3 inflammasome activation and regulating microglia/macrophage polarization via TLR4/NF-κB signaling pathway. Front. Cell. Neurosci. 13, 553. doi:10.3389/fncel.2019.00553
Yin, N., Gao, Q., Tao, W., Chen, J., Bi, J., Ding, F., et al. (2020). Paeoniflorin relieves LPS-induced inflammatory pain in mice by inhibiting NLRP3 inflammasome activation via transient receptor potential vanilloid 1. J. Leukoc. Biol. 108 (1), 229–241. doi:10.1002/jlb.3ma0220-355r
Yu, H., Wu, Z., Wang, X., Gao, C., Liu, R., Kang, F., et al. (2020). Protective effects of combined treatment with mild hypothermia and edaravone against cerebral ischemia/reperfusion injury via oxidative stress and Nrf2 pathway regulation. Int. J. Oncol. 57 (2), 500–508. doi:10.3892/ijo.2020.5077
Yu, J., Wang, W. N., Matei, N., Li, X., Pang, J. W., Mo, J., et al. (2020). Ezetimibe attenuates oxidative stress and neuroinflammation via the AMPK/Nrf2/TXNIP pathway after MCAO in rats. Oxid. Med. Cell Longev. 2020, 4717258. doi:10.1155/2020/4717258
Yue, G. G-L., Jiang, L., Kwok, H-F., Lee, J. K-M., Chan, K-M., Fung, K-P., et al. (2016). Turmeric ethanolic extract possesses stronger inhibitory activities on colon tumour growth than curcumin – the importance of turmerones. J. Funct. Foods 22, 565–577. doi:10.1016/j.jff.2016.02.011
Zeng, C., Liu, Z., Chen, Y., Shang, X., Xia, Z., and He, Q. (2021). The effect of asiaticoside on Nrf2/TXNIP/NLRP3 pathway and microglia activation in rats with cerebral ischemia-reperfusion injury. Stroke Nerv. Dis. 28, 288–294.
Zhang, N., Zhang, X., Liu, X., Wang, H., Xue, J., Yu, J., et al. (2014). Chrysophanol inhibits NALP3 inflammasome activation and ameliorates cerebral ischemia/reperfusion in mice. Mediat. Inflamm. 2014, 370530. doi:10.1155/2014/370530
Zhang, T., Guan, B., Tan, S., Zhu, H., Ren, D., Li, R., et al. (2021). Bushen huoxue acupuncture inhibits NLRP1 inflammasome-mediated neuronal pyroptosis in SAMP8 mouse model of alzheimer's disease. Neuropsychiatr. Dis. Treat. 17, 339–346. doi:10.2147/ndt.S279304
Zhang, Y., Rong, H., Zhang, F. X., Wu, K., Mu, L., Meng, J., et al. (2018). A membrane potential- and calpain-dependent reversal of caspase-1 inhibition regulates canonical NLRP3 inflammasome. Cell Rep. 24 (9), 2356–2369. doi:10.1016/j.celrep.2018.07.098
Zhang, Z., Ma, T., Fu, Z., Feng, Y., Wang, Z., Tian, S., et al. (2022). TBC1Domain Family Member 25 deficiency aggravates cerebral ischemia-reperfusion injury via TAK1-JNK/p38 pathway. J. Neurochem. 160 (3), 392–411. doi:10.1111/jnc.15546
Zhao, Y., Zhang, X., Chen, X., and Wei, Y. (2022). Neuronal injuries in cerebral infarction and ischemic stroke: From mechanisms to treatment (Review). Int. J. Mol. Med. 49 (2), 15. doi:10.3892/ijmm.2021.5070
Zhong, L., Ren, X., Ai, Y., and Liu, Z. (2022). SS-31 improves cognitive function in sepsis-associated encephalopathy by inhibiting the drp1-NLRP3 inflammasome activation. Neuromolecular Med. 2022, 8730. doi:10.1007/s12017-022-08730-1
Zhou, D., Zhang, L., Mao, L., Cao, J., and Gao, J. (2022). Biological mechanism on SIRT1/NLRP3/IL-18 signaling pathway of acupuncture for treatment of ischemic stroke with center poststroke pain. Comput. Intell. Neurosci. 2022, 8958742. doi:10.1155/2022/8958742
Zhou, R., Yazdi, A. S., Menu, P., and Tschopp, J. (2011). A role for mitochondria in NLRP3 inflammasome activation. Nature 469 (7329), 221–225. doi:10.1038/nature09663
Zhou, Y. (2021). Study on the effect and mechanism of Taohong Siwu decoction on pyroptosis after acute cerebral infarction based on DRP1/NLRP3 pathway. Beijing(China): Beijing University of Chinese Medicine.
Keywords: ischemic stroke (IS), pyroptosis, NLRP3, pathways, traditional Chinese medicine (TCM)
Citation: Long J-X, Tian M-Z, Chen X-Y, Yu H-H, Ding H, Liu F and Du K (2023) The role of NLRP3 inflammasome-mediated pyroptosis in ischemic stroke and the intervention of traditional Chinese medicine. Front. Pharmacol. 14:1151196. doi: 10.3389/fphar.2023.1151196
Received: 25 January 2023; Accepted: 07 April 2023;
Published: 21 April 2023.
Edited by:
Jianxun Liu, China Academy of Chinese Medical Sciences, ChinaReviewed by:
Yuxiang Fei, China Pharmaceutical University, ChinaMacarena Hernández, Complutense University of Madrid, Spain
Copyright © 2023 Long, Tian, Chen, Yu, Ding, Liu and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ke Du, ZGtrdHp0Z0AxNjMuY29t
†These authors have contributed equally to this work
 Jia-Xin Long
Jia-Xin Long Meng-Zhi Tian
Meng-Zhi Tian Xiao-Yi Chen1
Xiao-Yi Chen1 Ke Du
Ke Du