
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 05 June 2023
Sec. Pharmacology of Infectious Diseases
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1146938
Objectives: This study reviewed factors influencing the length of hospital stay in adult inpatients with confirmed Coronavirus disease (COVID-19) who were treated with Nirmatrelvir/Ritonavir.
Methods: We did a retrospective analysis of data from a cohort of inpatients with confirmed diagnosis of Omicron variant of SARS-CoV-2 infection who were treated with Nirmatrelvir/Ritonavir. We included patients who were treated from 13th March 2022 to 6th May 2022 in various in-patient treatment units in Quanzhou, Fujian Province, China. The primary study outcome was the length of hospital stay. Secondary study outcome was viral elimination defined as negative for ORF1ab and N genes [cycle threshold (Ct) value ≥35 in real-time PCR], according to local guidelines. Hazard ratios (HR) of event outcomes were analyzed using Multivariate Cox regression models.
Results: We studied 31 inpatients with high risk for severe COVID-19 who were treated with Nirmatrelvir/Ritonavir. We found that inpatients with shorter length of hospital stay (≤17 days) were mostly females with lower body mass index (BMI) and Charlson Comorbidity Index (CCI) index. Their treatment regimen with Nirmatrelvir/Ritonavir was started within 5 days of diagnosis (p < 0.05). Multivariate Cox regression indicated that inpatients starting treatment of Nirmatrelvir/Ritonavir within 5 days had a shorter length of hospital stay (HR 3.573, p = 0.004) and had a faster clearance of viral load (HR 2.755, p = 0.043).
Conclusion: This study assumes relevance during the Omicron BA.2 epidemic as our findings suggest that early treatment with Nirmatrelvir/Ritonavir within 5 days of diagnosis (≤5 days) was highly effective in shortening the length of hospital stay and faster viral load clearance.
The Coronavirus disease 2019 (COVID-19) epidemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has spread worldwide and emerged as a serious public health problem. More than 500 million confirmed cases and 6.3 million deaths were reported worldwide as of 15th May 2022 (Worldometer, 2022). The Omicron BA.2 variant has higher infectivity, stronger vaccine breakthrough ability, and higher antibody drug resistance (Walls et al., 2020; Dejnirattisai et al., 2022a; Dejnirattisai et al., 2022b; Kawaoka et al., 2022; Liu et al., 2022; Meng et al., 2022; Migueres et al., 2022; Mohapatra et al., 2022).
Earlier studies have reported treating COVID-19 with multiple antiviral agents, monoclonal antibodies, and immunomodulators, but none of these treatments have shown significant clinical efficacy for BA.2 strain (Drożdżal et al., 2021; Weinreich et al., 2021; Ohashi et al., 2022; VanBlargan et al., 2022). Nirmatrelvir/Ritonavir targets 3CLpro inhibitor (Nirmatrelvir) and cytochrome CYP3A4, thereby increasing Nirmatrelvir serum levels (ritonavir) (Hammond et al., 2022). Results from the Phase III clinical trial of Nirmatrelvir/Ritonavir (EPIC-HR) showed reduced risk of hospitalization and mortality caused by the Delta variant of COVID-19 in outpatients with high risk. There is limited evidence of Nirmatrelvir/Ritonavir’s efficacy on Omicron variant infections in high risk inpatients. On 11th February 2022, the National Medical Products Administration (NMPA) of China approved Nirmatrelvir/Ritonavir as the standard treatment for adult patients with mild to moderate COVID-19, who had risk factors for progression to severe disease. The first case of Omicron BA.2 variant of COVID-19 was diagnosed in Quanzhou City, China on 13th March 2022. Since then, 32 inpatients with high viral load were treated with Nirmatrelvir/Ritonavir.
Hence, we decided to conduct a small retrospective cohort study of COVID-19 inpatients to verify the effectiveness of Nirmatrelvir/Ritonavir in shortening length of hospital stay and hastening clearance of viral load.
The study subjects were adult inpatients with confirmed Omicron BA.2 variant of COVID-19 who were admitted in makeshift/mobile field hospitals, isolation wards, and intensive care units in Quanzhou City, Fujian Province. The study period was from 13th March 2022 to 6th May 2022. This study was reviewed and approved by the Ethics Committee of Quanzhou 1st Hospital Affiliated to the Fujian Medical University [Quan Yilun (2022) 162].
According to the National Health Commission of the People’s Republic of China diagnosis and treatment protocol for COVID-19 (trial version 9) (National Health Commission of the People’s Republic of China, 2022), there are four groups of clinical classification of cases: mild, moderate, severe, and critical. The details are as follows:
Mild: Mild clinical symptoms, no pneumonia manifested in imaging.
Common type: With the above clinical manifestations, pneumonia can be seen on imaging.
Severe: 1) Shortness of breath, RR ≥ 30 times/min; 2) In a resting state, oxygen saturation ≤ when inhaling air 93%; 3) Arterial blood oxygen partial pressure (PaO2)/inhaled oxygen concentration (FiO2) ≤ 300 mmHg (1 mmHg = 0.133 kPa), high altitude (over 1,000 m above sea level) areas should correct PaO2/FiO2 according to the following formula: PaO2/FiO2 × [760/atmospheric pressure (mmHg)]; 4) The clinical symptoms are progressively aggravated, and lung imaging shows that the lesion progresses >50% within 24–48 h.
Critical: 1) Respiratory failure occurs and requires mechanical ventilation; 2) Shock occurs; 3) Combining with other organ failure requires ICU monitoring and treatment.
Admission criteria were as follows: Once the patient is diagnosed, he should be transferred immediately (within 2 h) to a designated hospital for treatment or release the cabin hospital for centralized isolation and treatment, so all confirmed patients are admitted to the hospital. Discharge criteria were as follows: 1) Normal body temperature for more than 3 days; 2) Significantly recovered respiratory symptoms; 3) Lung CT imaging shows obvious absorption and recovery; 4) Ct value ≥35 of N gene and ORF gene as detected by real-time PCR of COVID-19 (National Health Commission of the People’s Republic of China, 2022). The principles of respiratory support treatment were as follows: 1) Severe patients diagnosed as PaO2/FiO2 lower than 300 mmHg are given oxygen therapy; 2) PaO2/FiO2 lower than 200 mmHg are given nasal high-flow oxygen therapy (HFNC) or non-invasive ventilation (NIV); 3) PaO2/FiO2 If FiO2 is lower than 150 mmHg, especially for patients with significantly increased inspiratory effort, endotracheal intubation and invasive mechanical ventilation should be performed.
Inclusion criteria: 1) Patients who fulfilled the diagnostic criteria; 2) Age ≥18 years; 3) Treated with a full course of Nirmatrelvir/Ritonavir antiviral therapy during hospitalization as follows: Patients with normal renal function and mild renal dysfunction [estimated glomerular filtration rate (eGFR) 60–90 mL/min/1,73 m2 (Kawaoka et al., 2022)] were administered 300 mg Nirmatrelvir (150 mg/tablet) and 100 mg Ritonavir (100 mg/tablet). Three tablets were orally administered twice daily for 5 days. In patients with moderate renal impairment [estimated e GFR30 ∼ 60 mL/min/1,73 m2 (Kawaoka et al., 2022)], the dosage was adjusted to 150 mg Nirmatrelvir and 100 mg Ritonavir were orally administered twice daily for 5 days.
We excluded patients who were younger than 18 years, those who were pregnant, had contraindications, and those who did not complete the oral 5-day course of Nirmatrelvir/Ritonavir.
We used the Haitai Medical Records System (Nanjing Haitai Medical Information System Co., Ltd.) to collect clinical data. Details collected included gender, age, BMI, underlying diseases, complications, smoking history, number of vaccinations, disease classification, type of hospitalization, basic parameters at the time of admission (whole blood lymphocyte count, plasma Interleukin 6 (IL-6), plasma C-reactive protein (CRP)), the time of initiating Nirmatrelvir/Ritonavir treatment, Ct value before treatment, liver and kidney functions before and after treatment, coagulation function before and after treatment, routine blood tests results before and after treatment, the time taken to reach Ct value ≥35 of N gene and ORF gene, and the length of hospital stay.
The primary outcome was defined as the length of the hospital (compared with a median number of days in the cohort), and the secondary outcome was the time taken to reach a Ct value ≥35 of the N gene and ORF gene. The survival time is the time from the patient’s admission to the occurrence of the positive event. In this study, positive events were defined as the primary outcome, “patient discharged from hospital,” and the secondary outcome, “nucleic acid CT value > 35”. Therefore, the follow-up duration was defined as the time from the patient’s admission to discharge from the hospital. Moreover, all 31 patients were discharged cured after the diagnosis of novel coronavirus infection was confirmed and admitted within 24 h, and all met the discharge criteria of the cited “Novel Coronavirus Pneumonia Treatment Protocol (Trial Version 9)", with no censoring events and a 100% positive event rate.
We analyzed the following variables.
1) Underlying diseases, which included cardiovascular and cerebrovascular diseases, chronic lung diseases, diabetes, chronic liver and kidney diseases, tumors, immunodeficiency, and so on.
2) Complications, which included gastrointestinal bleeding, infection with other pathogens, abnormal liver function, coagulation dysfunction, allergic dermatitis, and so on.
3) Charlson Comorbidity Index (CCI), which provides an integrated evaluation of the patient’s comorbidities (Charlson et al., 1994).
4) Risk classification criteria of inpatients.
5) The Ct value before Nirmatrelvir/Ritonavir was administered.
6) The time taken to reach of Ct value ≥35.
7) Time of administering Nirmatrelvir/Ritonavir
We used SPSS 25.0 statistical software (IBM, New York) for the statistical analysis of data. The Kolmogorov-Smirnov test was used to assess the normality of the distribution of continuous variables. Continuous variables measurement conforming to the normal distribution were expressed as mean ± standard deviation (–x ± s), or as median (interquartile range) [M (Q1, Q3)]. Categorical variables were presented as frequencies (percentages). Independent sample t-test, Wilcoxon rank-sum test, Fisher’s exact probability test, and the Chi-square test were used for comparing the two groups (the short hospitalization group and the long hospitalization group). Schoenfeld residuals method was used to examine whether each factor met the PH hypothesis test. Univariate Cox regression analysis was used to estimate the hazard ratios (HRs) and 95% confidence interval (CI) of the effect of each variable on the outcome for factors included in the proportional hazards (PH) assumption. Log-rank test was used to compare the survival distribution. Tests of significance were done using R version 4.2.0 (University of Auckland, Auckland) and the Survminer R package was used to plot the survival curve. We used Multivariate Cox regression models to analyze HRs of event outcomes. p < 0.05 was considered statistically significant.
Initially, 32 inpatients were included in the study. Of them, one patient did not complete the 5-day Nirmatrelvir/Ritonavir treatment due to personal reasons. The final cohort consisted of 31 inpatients whose data we collected. There were 17 males and 14 females, with a median age of 42 years, median time of reaching Ct value ≥35 was 12 days, and median length of hospital stay was 17 days.
Sixteen inpatients belonged to the short hospitalization group (≤17 d) and 15 inpatients belonged to the long hospitalization group (>17 d).
There were no statistical differences in age, gender composition ratio, completion rate of 3 doses of vaccine, BMI >25 kg/m2, laboratory tests (lymphocyte count, IL-6, CRP), risk classification criteria, and nucleic acid CT value before treatment between the two groups (p > 0.05). There was no significant difference in the underlying diseases and complications between the two groups (p > 0.05), but there was a significant difference in the Charlson comorbidity index (CCI) ≥ 1 between the two groups (p = 0.043). Population characteristics and group comparisons are detailed in Table 1.
ICU admission rate was higher in the long hospitalization group (4 cases, 26.7%) than the short hospitalization group (0 cases, 0.0%), and the difference was statistically significant (p = 0.043).
Nirmatrelvir/Ritonavir initiation time ≤5 days was significantly different in the short hospital group when compared with the long hospital group (93.8% vs. 33.3%, respectively) (p = 0.001). The treatment course characteristics and group comparisons are detailed in Table 1.
Univariate analysis for factors influencing the PH hypothesis test: male sex (HR 0.358, 95% CI 0.159–0.803, p = 0.013) and CCI ≥1 (HR 0.443, 95% CI 0.203–0.965, p = 0.040); BMI >25 kg/m2 (Kawaoka et al., 2022) (HR 3.819, 95% CI 1.422–10.260, p = 0.008), Nirmatrelvir/Ritonavir initiation time ≤ 5 d (HR 4.403, 95% CI 1.842–10.53, p = 0.001) (Table 2).
Gender, BMI, CCI category, and Nirmatrelvir/Ritonavir initiation time were selected to draw the Kaplan-Meier (K-M) survival curves in Figures 1A–D.
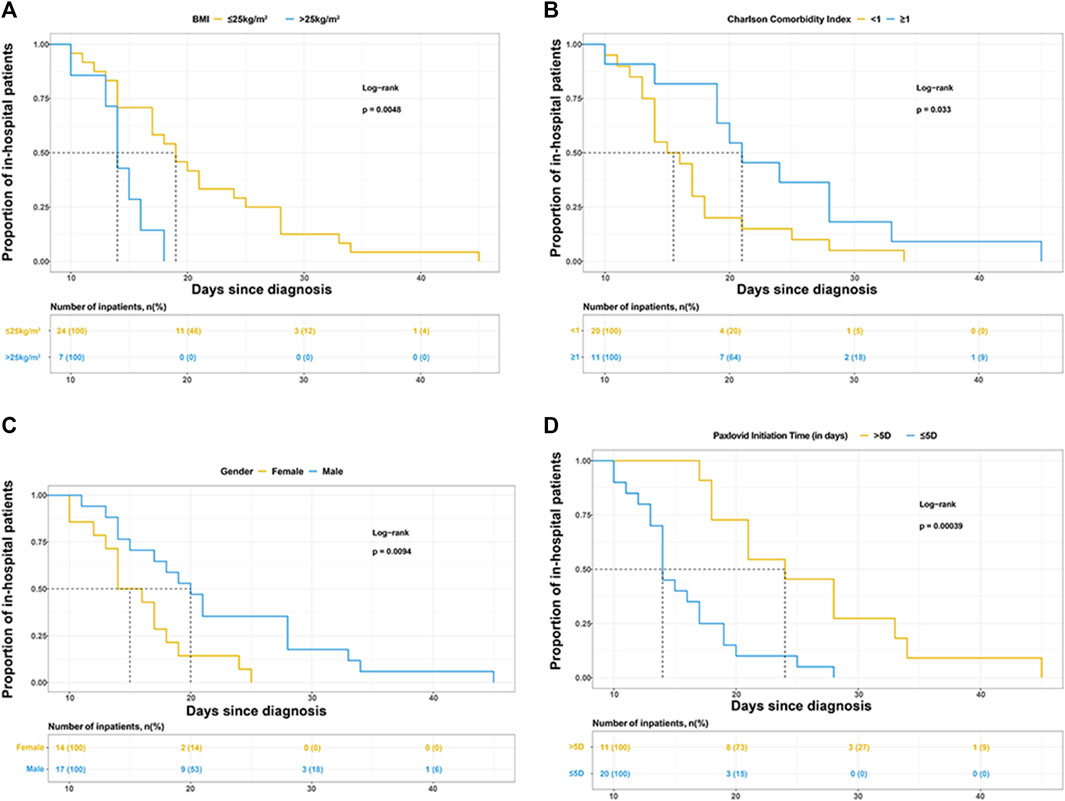
FIGURE 1. Kaplan-Meier curves for the proportion of in-hospital patients infected with SARS-COV-2 in different groups. The median (interquartile range) length of hospital stay was (A) 19 days (14–26.5 days) in patients with BMI ≤25 kg/m2 and 14 days (13–16 days) in patients with BMI>25 kg/m2, (B) 15.5 days (13.5–18 days) in patients with Charlson comorbidity index (CCI) < 1 and 21 days (19–28 days) in patients with CCI >1, (C) 20 days (15–28 days) in male and 15 days (13–18 days) in female, (D) 14 days (13–18 days) in patients treated with Paxlovid within 5 days since diagnosis and 24 days (18–33 days) in patients treated with Paxlovid beyond 5 days since diagnosis.
Multivariate Cox regression model was performed using independent variables: basic information (gender, BMI, age ≥60 years), underlying disease information (CCI ≥1), treatment and prevention (Nirmatrelvir/Ritonavir initiation time, completion of 3 vaccinations), and disease condition (severity classification). We found that Nirmatrelvir/Ritonavir initiation time ≤5 days was conducive to facilitate patient discharge (HR 3.835, 95% CI 1.330–11.062, p = 0 .013), as shown in Table 3.
Univariate Cox regression analysis showed that CCI ≥1 (HR 0.440, 95% CI 0.200–0.968, p = 0.042); BMI >25 kg/m2 (Kawaoka et al., 2022) (HR 4.772, 95% CI 1.782–12.78, p = 0.002), Nirmatrelvir/Ritonavir initiation time ≤5 days (HR 3.573, 95% CI 1.518–8.407, p = 0 .004) (see Table 4).
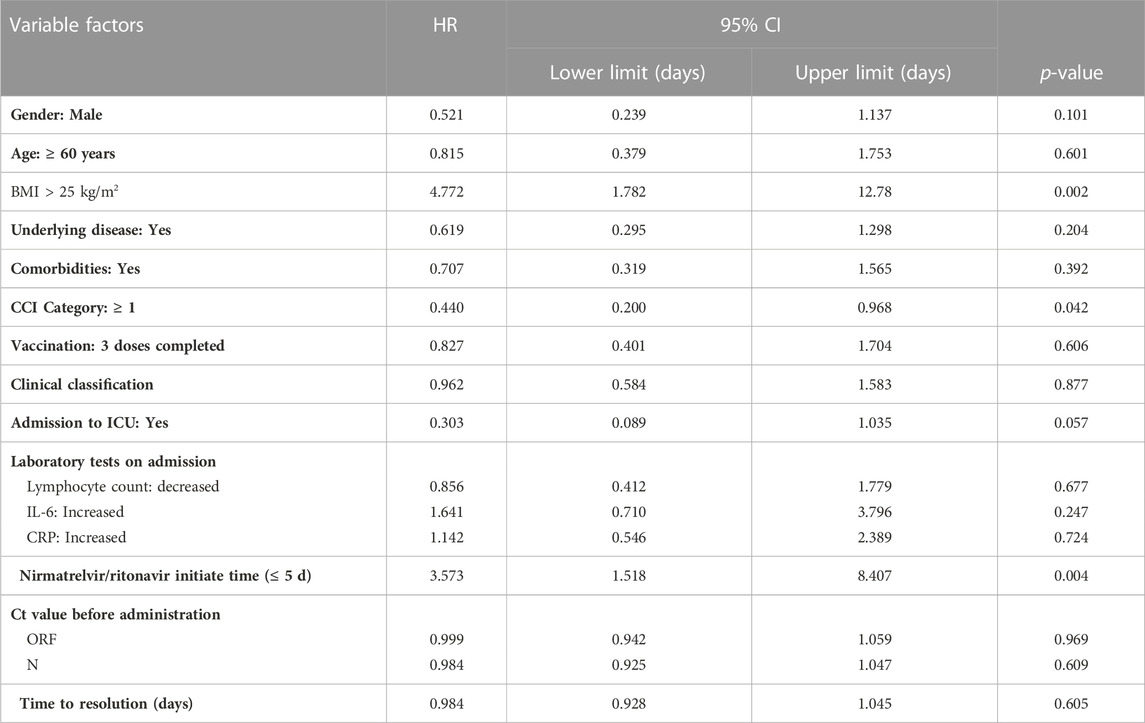
TABLE 4. Univariate Cox regression analysis of time to nucleic acid conversion for variables complying with PH hypothesis test.
BMI, CCI category, and Nirmatrelvir/Ritonavir initiation time were selected to draw the K-M survival curve, as shown in Figures 2A–C.
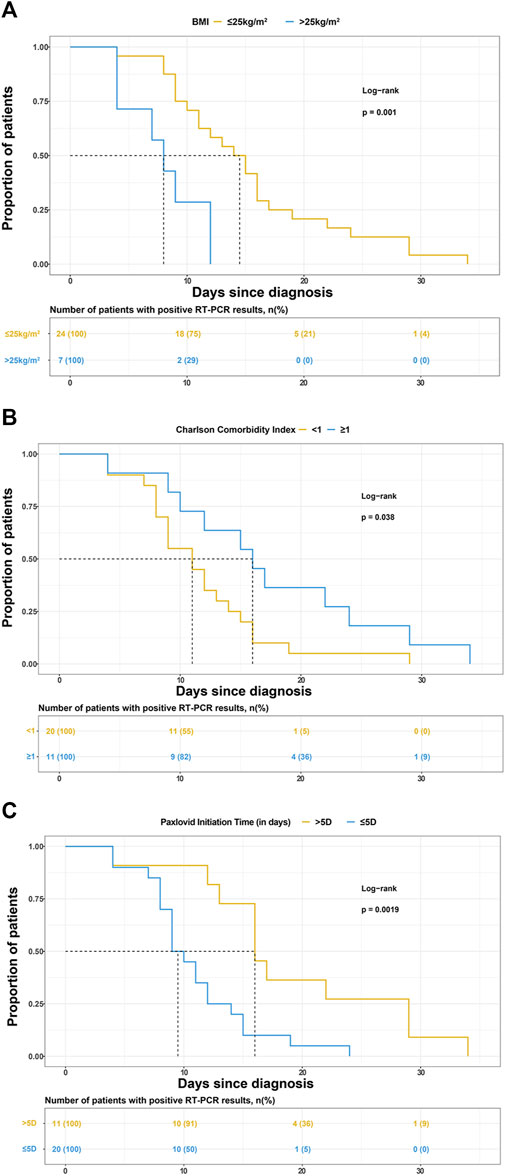
FIGURE 2. Kaplan-Meier curves for the proportion of patients with positive reverse transcription-polymerase chain reaction (RT-PCR) results for SARS-COV-2 infection in different groups. The median (interquartile range) time for the positive RT-PCR results converted to negative was (A) 14.5 days (9.5–18 days) in patients with BMI ≤25 kg/m2 and 8.5 days (4–12 days) in patients with BMI>25 kg/m2, (B) 11 days (8–14.5 days) in patients with Charlson comorbidity index (CCI) < 1 and 16 days (10–24 days) in patients with CCI>1, (C) 9.5 days (8–13 days) in patients treated with Paxlovid within 5 days since diagnosis and 16 days (13–29 days) in patients treated with Paxlovid beyond 5 days since diagnosis.
We analyzed basic information (gender, BMI, age ≥60 years), underlying disease information (CCI ≥1), treatment and prevention (Nirmatrelvir/Ritonavir initiation time, completion of 3 vaccinations), and disease condition (severity classification). Among these variables, BMI >25 kg/m2 (HR 4.582, 95% CI 1.356–15.486, p = 0.014), and Nirmatrelvir/Ritonavir initiation time ≤ 5 d (HR 2.755, 95% CI 1.033–7.345, p = 0.043) were influencing factors, shown in Table 5. It was shown that BMI > 25 kg/m2 and Nirmatrelfir/ritonavir initiation time ≤5 days shortened time to Ct value ≥35 but CCI ≥1 prolonged time to Ct value ≥35.
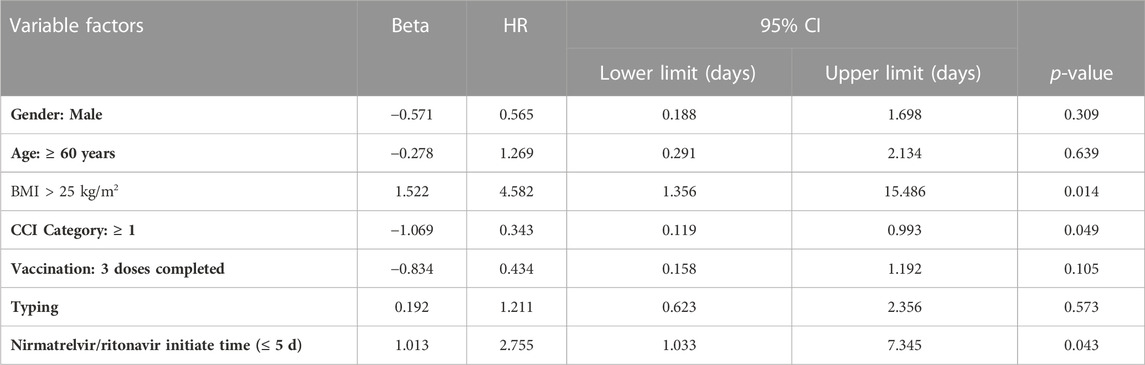
TABLE 5. Multivariate Cox regression model for the secondary outcome (time to nucleic acid conversion).
In this study, we found that initiation of treatment with Nirmatrelvir/Ritonavir within 5 days after the diagnosis of micron BA.2 variant of COVID-19 infection significantly shortened the length of hospital stay and the time for reaching Ct value ≥35, and these findings are consistent with the EPIC-HR (Evaluation of Protease Inhibition for COVID-19 in High-Risk Patients) study results. There was no death associated with COVID-19 in all patients who were administered Nirmatrelvir/Ritonavir. We also found that the Charlson comorbidity index (CCI) and BMI of patients were important factors affecting the time taken to reach Ct value ≥35.
In this study, all patients were infected with Omicron BA.2 variant of COVID-19 We found that timely treatment with Nirmatrelvir/Ritonavir helped inpatients shorten hospitalization stay and reduced their viral load (based on time taken to reach Ct value ≥35). This confirmed the effectiveness of Nirmatrelvir/Ritonavir in treating the Omicron BA.2 variant of COVID-19. These findings are similar to the study results of Najar-Debbinyand Sun et al. (Najjar-Debbiny et al., 2022; Sun et al., 2022) This also confirmed that 3CLpro inhibitors were effective against SARS-CoV-2mutant variants.
We found that that age, classification, and CCI ≥1 factors were not statistically significant in relation to the length of hospital stay. This is contrary to several published studies which indicate that older patients with COVID-19 are at higher risk and more prone to a prolonged hospital stay (da Costa Sousa et al., 2022; Xu et al., 2022; Zheng et al., 2020). In our opinion, one probable reason for the difference may be related to the small sample size of the present study. Meanwhile, more aggressive use of Paxlovid antiviral therapy in patients with high-risk factors, including advanced age and having high risk, may also lead to selection bias and thus outcome differences. This is similar to the study by Sun et al. which noted that immunosuppressed patients were treated with Nirmatrelvir/Ritonavir (da Costa Sousa et al., 2022).
CCI is a comorbidity scoring system used to measure comorbidities based on the severity of disease. The study by Sun et al. showed that the time taken to reach Ct value ≥35 was longer in patients with neurological or cardiovascular disease comorbidities and immunocompromised patients (Sun et al., 2022). In this study, the secondary outcome (the time taken to reach Ct value ≥35) suggests that patients with CCI ≥1 can prolong the time of Ct value ≥35.
We also found in this study that BMI >25 kg/m2 shortened the time required to achieve a Ct value ≥35 in patients, and these results are different from that of Mufarrih et al. (2022). This difference may be explained by the different CCI in patients with BMI ≤25 kg/m2 and BMI >25 kg/m2. In our study, we found that CCI ≥1 was a factor affecting the time taken to reach Ct value ≥35, and seven patients (29.2%) with BMI ≤25 kg/m2 had a CCI ≥3, while patients with BMI >25 kg/m2 did not have CCI ≥3. The relationship between BMI and the time taken to reach Ct value ≥35 remains to be explored in large samples in future studies.
There are several limitations in this study: First, this is a retrospective study with a small sample size, and this can cause sample selection bias. Second, according to the “Novel Coronavirus Pneumonia Treatment Protocol (Trial Version 9)" and the instructions for use of Paxlovid in China, the indication for the use of Paxlovid is “for adults with mild to moderate COVID-19 with high-risk factors for progression to severe disease as soon as possible within 5 days of diagnosis”. However, in this study, some patients with infections older than 5 days and patients with severe disease were also treated with Paxlovid. Third, this study lacks a control group of COVID-19 inpatients who did not receive NiR, which is not conducive to the comparison of results.
In conclusion, in this study, we found that during the Omicron BA.2 epidemic, administering Nirmatrelvir/Ritonavir early (≤5 days) was highly effective in shortening the length of hospital stay and faster clearance of viral load (measured in terms of the time taken to reach Ct value ≥35).
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Ethics Committee of Quanzhou First Hospital Affiliated to Fujian Medical University. The patients/participants provided their written informed consent to participate in this study.
Conception and design of the research: JZ, CZ, YS, and DH Acquisition of data: JZ, WH, CZ, and HY. Analysis and interpretation of the data: WH, CZ, and HY. Statistical analysis: JZ, DH, and YS Writing of the manuscript: JZ, CZ, YS, and DH. Critical revision of the manuscript for intellectual content: JZ, CZ, YS, and DH. All authors read and approved the final draft. All authors contributed to the article and approved the submitted version.
Startup Fund for scientificresearch, Fujian Medical University (2021QH1245).
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Charlson, M., Szatrowski, T. P., Peterson, J., and Gold, J. (1994). Validation of a combined comorbidity index. J. Clin. Epidemiol. 47 (11), 1245–1251. doi:10.1016/0895-4356(94)90129-5
da Costa Sousa, V., da Silva, M. C., de Mello, M. P., Guimarães, J. A. M., and Perini, J. A. (2022). Factors associated with mortality, length of hospital stay and diagnosis of COVID-19: Data from a field hospital. J. Infect. Public Health 15 (7), 800–805. doi:10.1016/j.jiph.2022.06.010
Dejnirattisai, W., Huo, J., Zhou, D., Zahradník, J., Supasa, P., Liu, C., et al. (2022). SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell 185 (3), 467–484.e15. doi:10.1016/j.cell.2021.12.046
Dejnirattisai, W., Shaw, R. H., Supasa, P., Liu, C., Stuart, A. S., Pollard, A. J., et al. (2022). Reduced neutralisation of SARS-CoV-2 omicron B.1.1.529 variant by post-immunisation serum. Lancet 399 (10321), 234–236. doi:10.1016/S0140-6736(21)02844-0
Drożdżal, S., Rosik, J., Lechowicz, K., Machaj, F., Szostak, B., Przybyciński, J., et al. (2021). An update on drugs with therapeutic potential for SARS-CoV-2 (COVID-19) treatment. Drug Resist Updat 59, 100794. doi:10.1016/j.drup.2021.100794
Hammond, J., Leister-Tebbe, H., Gardner, A., Abreu, P., Bao, W., Wisemandle, W., et al. (2022). Oral Nirmatrelvir for high-risk, nonhospitalized adults with covid-19. N. Engl. J. Med. 386 (15), 1397–1408. doi:10.1056/NEJMoa2118542
Kawaoka, Y., Uraki, R., Kiso, M., Iida, S., Imai, M., Takashita, E., et al. (2022). Characterization and antiviral susceptibility of SARS-CoV-2 Omicron/BA.2. Res. Sq. 3, 1375091. doi:10.21203/rs.3.rs-1375091/v1
Liu, L., Iketani, S., Guo, Y., Chan, J. F. W., and Wang, M. (2022). Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature 602 (7898), 676–681. doi:10.1038/s41586-021-04388-0
Meng, B., Abdullahi, A., Ferreira, I. A. T. M., Goonawardane, N., Saito, A., Kimura, I., et al. (2022). Altered TMPRSS2 usage by SARS-CoV-2 Omicron impacts infectivity and fusogenicity. Nature 603 (7902), 706–714. doi:10.1038/s41586-022-04474-x
Migueres, M., Dimeglio, C., Trémeaux, P., Abravanel, F., Raymond, S., Lhomme, S., et al. (2022). Influence of immune escape and nasopharyngeal virus load on the spread of SARS-CoV-2 Omicron variant. J. Infect. 84 (4), e7–e9. doi:10.1016/j.jinf.2022.01.036
Mohapatra, R. K., Kandi, V., Verma, S., and Dhama, K. (2022). Challenges of the omicron (B.1.1.529) variant and its lineages: A global perspective. Chembiochem 23 (9), e202200059. doi:10.1002/cbic.202200059
Mufarrih, S. H., Qureshi, N. Q., Yunus, R., Ngo, D., Katz, D., Krakower, D., et al. (2022). Influence of increasing age and body mass index of gender in COVID-19 patients. J. Womens Health (Larchmt). 31 (6), 779–786. doi:10.1089/jwh.2021.0615
Najjar-Debbiny, R., Gronich, N., Weber, G., Khoury, J., Amar, M., Stein, N., et al. (2022). Effectiveness of Paxlovid in reducing severe coronavirus disease 2019 and mortality in high-risk patients. Clin. Infect. Dis. 76, e342–e349. doi:10.1093/cid/ciac443
National Health Commission of the People’s Republic of China (2022). Diagnosis and treatment plan for COVID-19 (trial version 9). Int. J. Epidemiol. Infect. Dis. 49, 73–80.
Ohashi, H., Hishiki, T., Akazawa, D., Kim, K. S., Woo, J., Shionoya, K., et al. (2022). Different efficacies of neutralizing antibodies and antiviral drugs on SARS-CoV-2 Omicron subvariants, BA.1 and BA.2. Antivir. Res. 205, 105372. doi:10.1016/j.antiviral.2022.105372
Sun, F., Lin, Y., Wang, X., Gao, Y., and Ye, S. (2022). Paxlovid in patients who are immunocompromised and hospitalised with SARS-CoV-2 infection. Lancet Infect. Dis. 22 (9), 1279. doi:10.1016/S1473-3099(22)00430-3
VanBlargan, L. A., Errico, J. M., Halfmann, P. J., Zost, S. J., Crowe, J. E., Purcell, L. A., et al. (2022). An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies. Nat. Med. 28 (3), 490–495. doi:10.1038/s41591-021-01678-y
Walls, A. C., Park, Y. J., Tortorici, M. A., Wall, A., McGuire, A. T., and Veesler, D. (2020). Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 181 (2), 281–292. doi:10.1016/j.cell.2020.02.058
Weinreich, D. M., Sivapalasingam, S., Norton, T., Ali, S., Gao, H., Bhore, R., et al. (2021). REGN-COV2, a neutralizing antibody cocktail, in outpatients with covid-19. N. Engl. J. Med. 384 (3), 238–251. doi:10.1056/NEJMoa2035002
Worldometer (2022). COVID-19 coronavirus pandemic. Available at: https://www.worldometers.info/coronavirus/#countries.
Xu, X., Sun, D., Cao, M., Zhang, W., Pu, Y., Chen, C., et al. (2022). Analysis of clinical characteristics and prognosis of 4 264 patients with asymptomatic and mild novel coronavirus infections in Shanghai. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 34 (5), 449–453. doi:10.3760/cma.j.cn121430-20220516-00490
Keywords: coronavirus disease 2019 (COVID-19), length of hospital stay, Nirmatrelvir/Ritonavir, Omicron BA.2, retrospective analysis
Citation: Zheng J, Hong W, Zhou C, Hong D, Yan H and Shen Y (2023) A retrospective analysis of factors associated with the length of hospital stay in COVID-19 patients treated with Nirmatrelvir / Ritonavir. Front. Pharmacol. 14:1146938. doi: 10.3389/fphar.2023.1146938
Received: 18 January 2023; Accepted: 03 May 2023;
Published: 05 June 2023.
Edited by:
Giuseppe Castaldo, University of Naples Federico II, ItalyReviewed by:
Lavanya Visvabharathy, Northwestern University, United StatesCopyright © 2023 Zheng, Hong, Zhou, Hong, Yan, Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanghui Shen, c2hlbnlhbmdodWkyMDA0QDEyNi5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.