- 1Kidney Research Center and Department of Nephrology, Linkou Chang Gung Memorial Hospital, Taoyuan, Taiwan
- 2College of Medicine, Chang Gung University, Taoyuan, Taiwan
- 3Department of Physical Medicine and Rehabilitation, New Taipei Municipal Tucheng Hospital, Chang Gung Memorial Hospital, New Taipei, Taiwan
- 4Division of Cardiology, Department of Internal Medicine, Linkou Chang Gung Memorial Hospital, Taoyuan, Taiwan
- 5Division of Endocrinology and Metabolism, Department of Internal Medicine, Linkou Chang Gung Memorial Hospital, Taoyuan, Taiwan
- 6Department of Nephrology, New Taipei Municipal Tucheng Hospital, New Taipei, Taiwan
- 7Center for Artificial Intelligence in Medicine, Chang Gung Memorial Hospital at Linkou, Taoyuan, Taiwan
Background: Metabolic acidosis is a common complication in patients with chronic kidney disease (CKD). Oral sodium bicarbonate is often used to treat metabolic acidosis and prevent CKD progression. However, there is limited information about the effect of sodium bicarbonate on major adverse cardiovascular events (MACE) and mortality in patients with pre-dialysis advanced CKD.
Method: 25599 patients with CKD stage V between January 1, 2001 and December 31, 2019 were identified from the Chang Gung Research Database (CGRD), a multi-institutional electronic medical record database in Taiwan. The exposure was defined as receiving sodium bicarbonate or not. Baseline characteristics were balanced using propensity score weighting between two groups. Primary outcomes were dialysis initiation, all-cause mortality, and major adverse cardiovascular events (MACE) (myocardial infarction, heart failure, stroke). The risks of dialysis, MACE, and mortality were compared between two groups using Cox proportional hazards models. In addition, we performed analyzes using Fine and Gray sub-distribution hazard models that considered death as a competing risk.
Result: Among 25599 patients with CKD stage V, 5084 patients (19.9%) were sodium bicarbonate users while 20515 (80.1%) were sodium bicarbonate non-users. The groups had similar risk of dialysis initiation (hazard ratio (HR): 0.98, 95% confidence interval (CI): 0.95-1.02, p < 0.379). However, taking sodium bicarbonate was associated with a significantly lower risks of MACE (HR: 0.95, 95% CI 0.92–0.98, p < 0.001) and hospitalizations for acute pulmonary edema (HR: 0.92, 95% CI 0.88–0.96, p < 0.001) compared with non-users. The mortality risks were significantly lower in sodium bicarbonate users compared with sodium bicarbonate non-users (HR: 0.75, 95% CI 0.74–0.77, p < 0.001).
Conclusion: This cohort study revealed that in real world practice, use of sodium bicarbonate was associated with similar risk of dialysis compared with non-users among patients with advanced CKD stage V. Nonetheless, use of sodium bicarbonate was associated with significantly lower rate of MACE and mortality. Findings reinforce the benefits of sodium bicarbonate therapy in the expanding CKD population. Further prospective studies are needed to confirm these findings.
Background
Metabolic acidosis (MA) is one of the common complications in patients with chronic kidney disease (CKD), especially when the glomerular filtration rate (GFR) is below 30 ml/min per 1.73 m2 (Kraut and Kurtz, 2005). The main pathogenesis of MA in CKD patients is the impaired ability to excrete nonvolatile acid, mainly ammonium, and reduced bicarbonate reabsorption and production. Failure to neutralize the net endogenous acid load will lead to acid retention (Kuczera, 2020). MA is generally defined as serum bicarbonate level falls below 22 mmol/L (Moranne et al., 2009). The GFR threshold for developing MA has not been clearly defined, but its prevalence increases as kidney function declines (Kuczera, 2020). It is estimated to be about 56% in CKD stage V patients (Kuczera, 2020).
Numerous observational studies have shown deleterious effects of MA on patients with CKD. Acidosis contributes to enhanced muscle catabolism (EUSTACE et al., 2004), inducing protein energy wasting (Ballmer et al., 1995), exacerbation of metabolic bone disease (Chen et al., 2015), more rapid progression of CKD (Menon et al., 2010), worse cardiovascular health and increased mortality (Dobre et al., 2013). To prevent these complications, The 2012 Kidney Disease Improving Global Outcomes (KDIGO) guidelines recommend alkali therapy for chronic metabolic acidosis to maintain venous bicarbonate levels between 24 and 26 mEq/L (Kidney Disease: Improving Global Outcomes KDIGO CKD Work Group, 2012). However, it is unclear whether these recommendations should apply unmodified to all CKD population. In addition, physicians may remain concerned about the potential harms of sodium-based alkali therapy in patients with CKD, including edema due to expansion of extracellular fluid, uncontrolled hypertension, and exacerbation of heart failure. MA is most common in pre-dialysis CKD stage V patients, who are more likely to require alkali therapy, but these patients are at the highest risk of developing side effects of sodium-based alkali therapy.
Two recent systematic reviews and meta-analyses of small trials showed that oral alkali supplementation modestly improved estimated GFR, but had an indeterminate effect on progression to dialysis and the effect on body weight, blood pressure and risk of edema was controversial (Hu et al., 2019; Navaneethan et al., 2019; Bi, 2020). The number of trials included in these systemic reviews was limited and these studies had small sample sizes, lacking long-term follow up for analyzing mortality and cardiovascular events. Moreover, the severity of CKD in these trials varied, mainly CKD stage III to IV. To our knowledge, only three intervention studies had examined the effect of oral sodium bicarbonate therapy in CKD stage V patients, but their results had shown different outcomes. In an open-label randomized study, Jeong et al. revealed the preservation of eGFR in CKD stage IV after sodium bicarbonate supplement for MA, but not in pre-dialysis CKD stage V. However, bicarbonate supplement improves nutritional indices in CKD stage V13. Iorio et al. (2019) reported that correction of MA with oral sodium bicarbonate in patients with CKD stage III-V significantly reduces the progression of stage V CKD to dialysis and improves patients’ survival irrespective of baseline renal function. Recently, data from the BiCARB study (Bi, 2020), a multicenter, double-blind, placebo-controlled trial which enrolled patients with aged > = 60 years with CKD stage IV or V, failed to observe any significant treatment effect of sodium bicarbonate on renal outcome including eGFR or risk of dialysis initiation. Moreover, oral sodium bicarbonate did not improve physical function but increase adverse events.
Alkali therapy in CKD may have beneficial effects, but the effects may differ depending on the stage of CKD. Few studies have focused on clinical outcomes and side effect of alkali therapy in pre-dialysis CKD stage V patients. Therefore, we conducted a retrospective cohort study employing Chang Gung Research Database (CGRD), a multi-institutional electronic medical record database in Taiwan, to determine whether administration of oral sodium bicarbonate can improve renal outcome or reduce risks of mortality and major adverse cardiovascular event (MACE) in patients with pre-dialysis CKD stage V.
Methods
Data source
Patient data were obtained from Chang Gung Research Database (CGRD). CGRD is a de-identified database derived from the electronic medical records (EMR) of Chang Gung Memorial Hospital (CGMH), Taiwan’s largest healthcare provider, which includes four tertiary medical centers and three teaching hospitals across different regions of Taiwan, with a total of 10,050 beds and approximately 280,000 admissions annually (Shao et al., 2019), accounting for approximately 10% of all medical services in Taiwan. Before the data are released to researchers, any information in the CGRD that could identify any particular patient or healthcare provider is scrambled and encrypted to ensure privacy. The need for patient consent was waived and the study protocol was approved by the Institutional Review Board of Chang Gung Medical Foundation, Taiwan (IRB No: 202201897B0).
Patient selection and study design
We designed a retrospective cohort study to evaluate the effect of oral sodium bicarbonate on mortality and cardiovascular outcome in patients with advanced CKD. As illustrated in Figure 1, patients diagnosed with CKD stage V, which is defined as of patients with CKD stage V diagnosis (ICD9:585.5; ICD10:N185) and estimated GFR less than 15 ml/min/1.73 m (Kuczera, 2020) using CKD-EPI equation (Levey and Stevens, 2010) between January 1, 2001, and December 31, 2019 were identified by searching electronic medical records from the CGRD. The index date was defined as 180 days after the date of patient diagnosed with CKD stage V. Patient’s age <20 (N = 278), enrolled for less than 1 year (N = 17460), and death or date of last visit within 90 days of CKD stage V (N = 8429) were excluded from this study. To avoid participants with acute kidney injury receiving temporary hemodialysis, we excluded patients receiving hemodialysis or peritoneal dialysis within 180 days before the index date (N = 5552). Finally, 25599 patients with CKD stage V were included in the study cohort. We used landmark analysis with exposure windows of 180days to define oral sodium bicarbonate exposure (Dafni, 2011; Ravi et al., 2013). Sodium bicarbonate exposure was defined as CKD stage V patients who had received the first prescription for sodium bicarbonate within 180days of index date. Among 25599 patients, there were 5084 sodium bicarbonate users and 20515 sodium bicarbonate non-users.
Covariates and study outcomes
Baseline demographic and clinical characteristics were identified using International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes prior to 2016 or ICD-10-CM codes later. Baseline characteristics in our study included age, sex, Charlson Comorbidity Index (CCI), comorbidities, medications, laboratory values, and follow-up years. The comorbidities in question were hypertension, diabetes mellitus (DM), hyperlipidemia, stroke, coronary artery disease (CAD), myocardial infarction (MI), heart failure, peripheral artery disease, atrial fibrillation, liver cirrhosis, chronic obstructive pulmonary disease (COPD), hepatitis B virus/hepatitis C virus (HBV/HCV), and dementia. Comorbidities were identified based on the presence of more than 2 outpatient visits or at least one inpatient admission within the year preceding the index date. Most of the diagnostic codes used for these comorbidities have been validated in previous national database studies (Wu et al., 2014; Hsieh et al., 2015). Baseline medications were identified based on filling prescriptions at least twice or refilling a prescription for a chronic illness at least once within the year prior to the index date. Baseline laboratory results including the estimated glomerular filtration rate (eGFR), hemoglobin, sodium, potassium, calcium and albumin levels were obtained using the most recent record within 3 months preceding the index date.
The key outcomes were all-cause mortality, dialysis (hemodialysis or peritoneal dialysis), MACE (myocardial infarction, heart failure and stroke) and acute pulmonary edema. The diagnoses of dialysis, MACE and acute pulmonary edema were based on the principal diagnosis in the emergency department or hospitalization. The
Follow-up duration was from the index date until the first occurrence of death, MACE, dialysis, or the end of the follow-up period (December 31, 2019), whichever came first.
Statistical analysis
The baseline characteristics showed substantial differences between the study groups (NaHCO3 vs. non-NaHCO3), which may induce selection bias. Thus, we used
Inverse probability of treatment weighting (IPTW) based on propensity score to balance the baseline differences between the two groups (Xu et al., 2010; Austin, 2014). Propensity score was defined as the probability of a patient receving NaHCO3 treatment, which was calculated by the logistic regression model using age, sex, follow-years, Charlson comorbidity index, comorbidity, medication and laboratory values.
The balance between groups before and after IPTW was assessed using the absolute value of standardized mean differences, and absolute value less than 0.1 indicates non-substantial difference between groups (Austin, 2011). The risk of mortality between the NaHCO3 and non-NaHCO3 groups was compared using Cox proportional hazard model. For other time to event outcomes, we used the Fine and Gray sub-distributional hazards model, which considered all-cause mortality as a competing risk.
Subgroups analyses were conducted on MACE and mortality stratified by age groups, sex, dialysis, diabetes, heart failure, myocardial infarction, stroke, and atrial fibrillation. Analyses were performed using SAS Version 9.4 (SAS Institute, Cary, NC). A two-sided p-value of <0.05 was considered significant.
Results
Subject characteristics
A total of 25599 CKD stage V patients who met the inclusion criteria between January 1, 2001, and December 31, 2019 were extracted from CGRD and divided into NaHCO3 users (n = 5084) and NaHCO3 non-users (n = 20515) groups according to their NaHCO3 exposure. The baseline characteristics of each group are listed in Table 1. The mean age (years) of NaHCO3 users was 67.5.1 ± 13.7 and that of NaHCO3 non-users was 66.3 ± 13.8. Compared with NaHCO3 non-users, NaHCO3 users had shorter follow-up, lower baseline eGFR, calcium, and albumin level. NaHCO3 users are more likely to receive medications including thiazide, oral hypoglycemic agents, and statin than NaHCO3 non-users. After the PS weighting, all ASMD values were less than 0.1, suggestive of well-balanced baseline demographic and clinical characteristics between groups.
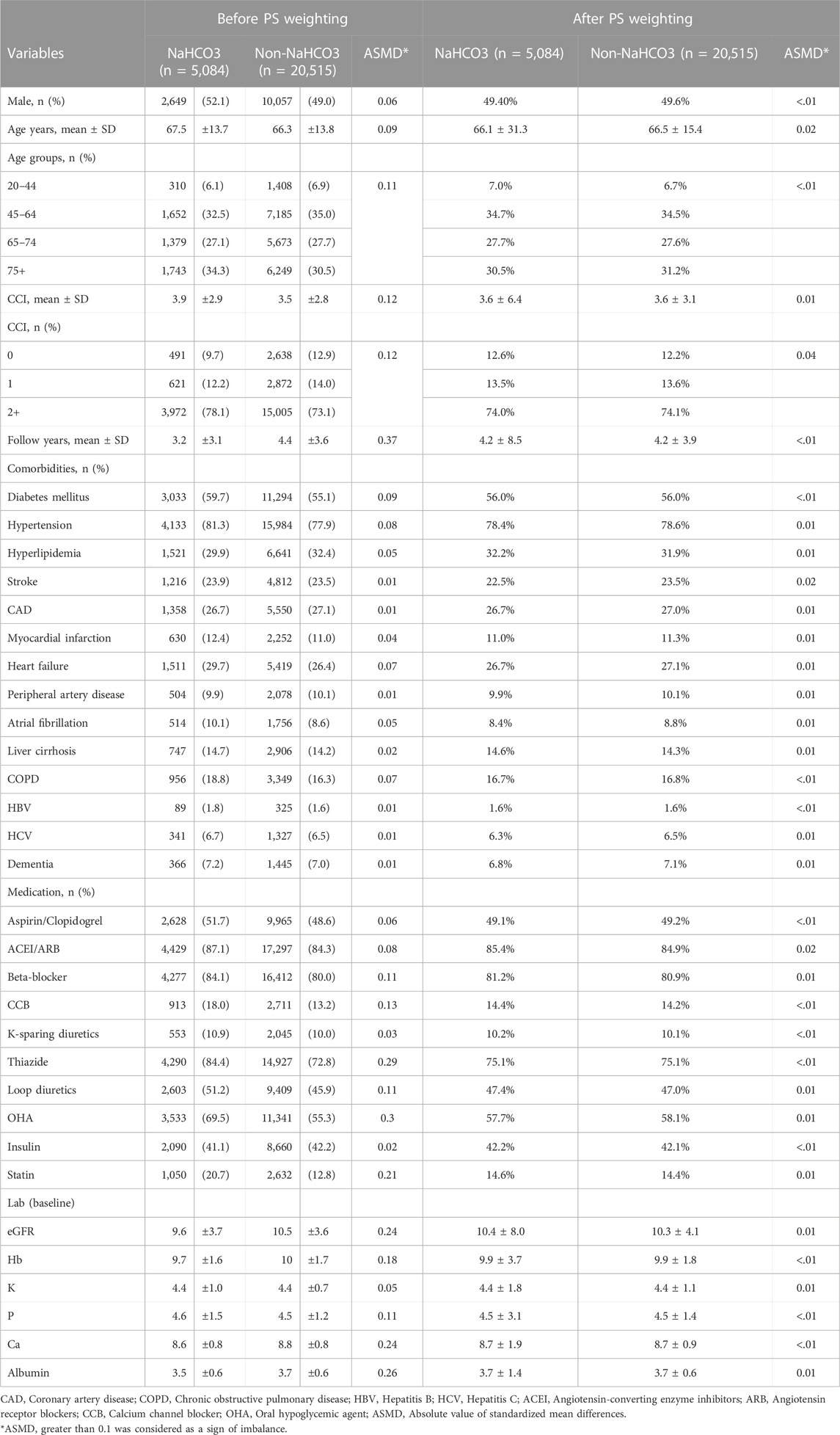
TABLE 1. Demographic characteristic of study population before and after propensity scores matching.
Effect of oral sodium bicarbonate on dialysis and MACE in patients with advanced CKD
Table 2 shows the risk of dialysis, MACE, and mortality in NaHCO3 users compared to NaHCO3 non-users after IPTW. The Cox proportional hazards model with competing risk analysis showed no difference in dialysis risks (HR: 0.98, 95% CI 0.95–1.02, p = 0.379) between the two groups. Those using NaHCO3 demonstrated a lower risk of MACE (HR: 0.95, 95% CI 0.86–0.97, p < 0.001) compared to NaHCO3 non-users. Regarding the components of MACE, NaHCO3 users had lower risk of myocardial infarction (HR: 0.91, 95% CI 0.86–0.97, p = 0.002) and stroke (HR: 0.92, 95% CI 0.88–0.96, p < 0.001) than NaHCO3 non-users.
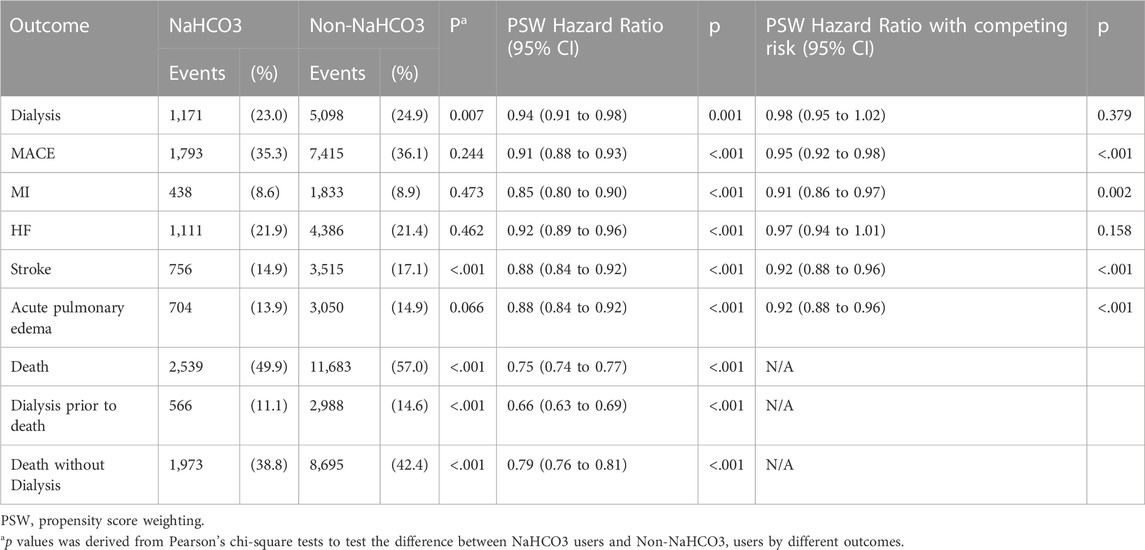
TABLE 2. Frequency and Propensity Scores Weighting (PSW) Hazard Ratio Hazard Ratios with/without Competing Risk for Interested Outcomes by NaHCO3 Exposure.
Effect of oral sodium bicarbonate on acute pulmonary edema and mortality in patients with advanced CKD.
The Cox proportional hazards model with competing risk analysis revealed that NaHCO3 users had a lower risk of acute pulmonary edema (HR: 0.92, 95% CI 0.88–0.96, p < 0.001) than NaHCO3 non-users. Regardless of patients entering dialysis prior to death or died without dialysis, NaHCO3 users had a lower risk of mortality (HR: 0.75, 95% CI 0.74–0.77, p < 0.001) compared with NaHCO3 non-users.
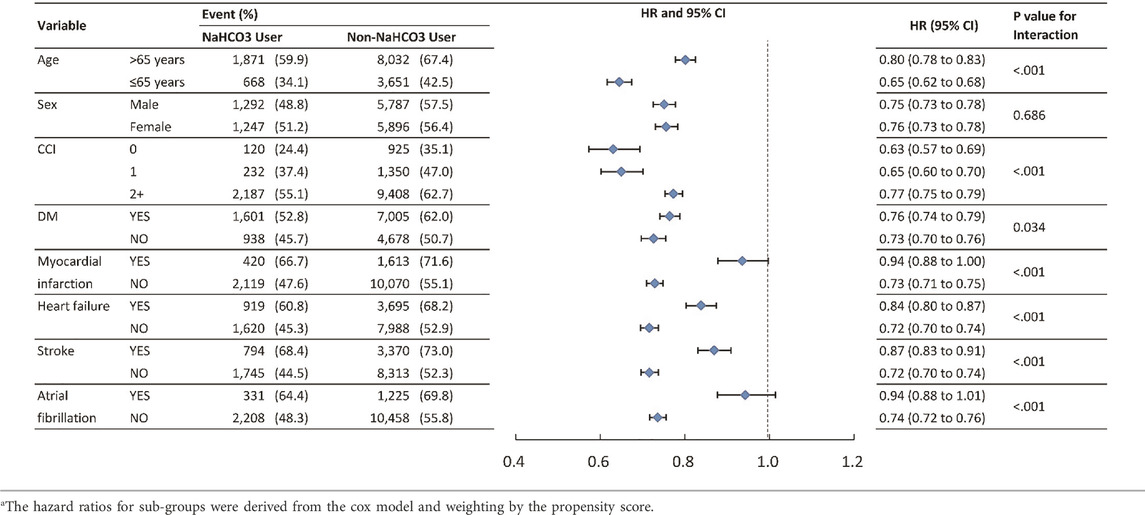
Subgroup analysis
To verify whether clinical conditions modified the association between the use of NaHCO3 and primary outcomes, we performed subgroup analyses for outcomes of MACE and mortality (Tables 3, 4). The results were generally consistent in favor of NaHCO3 users except for atrial fibrillation and stroke in MACE, and atrial fibrillation in mortality. Patients without atrial fibrillation and stroke benefited more from the protective effect of NaHCO3 in MACE.
Discussion
The key findings of this retrospective multi-institutional observational study are: 1) use of oral sodium bicarbonate in patients with advanced CKD reduces the risk of MACE, mainly in reducing the events of myocardial infarction and stroke; 2) patients with advanced CKD treated with sodium bicarbonate have a lower risk of death and acute pulmonary edema during clinical follow-up compared with sodium bicarbonate non-users; and 3) nevertheless, sodium bicarbonate treatment fails to decrease the risk of dialysis in patients with pre-dialysis CKD stage V. An intriguing result of this research provides real-world outcome and additional clinical evidence for a better understanding of the pathophysiology and long-term complication in the advanced CKD population.
Effect of sodium bicarbonate on renal progression in advanced CKD
Previous studies proposed possible mechanisms linking MA to rapid progression of CKD, which included activation of alternative complement cascade driven by compensatory increases in renal ammonia production in residual nephrons (Nath et al., 1985), triggering the production of endothelin and proinflammatory cytokines (Wesson et al., 2007; Phisitkul et al., 2008), and stimulation of the renin-angiotensin system (Wesson and Simoni, 2010; Ng et al., 2011; Wesson et al., 2015), each of which promotes acute acid excretion but chronically contributes to tubulointerstitial damage and fibrosis. An observational cohort study with participants with CKD stage II-IV showed that low serum bicarbonate level was an independent risk factor for kidney disease progression, however, it is also important to note that this association was stronger for participants with eGFR above 45 ml/min/1.73 m2 (Paul Chubb et al., 2016). A number of potential protective mechanisms of sodium bicarbonate supplementation have been identified (Mannon and O'Connor, 2020). One possible mediating mechanism is that alkali corrects the renal compensatory response to an acidic milieu, including lowering interstitial NH4+ concentration, resulting in decreased complement activation, reduced interstitial acidosis, and thus reduced local production of endothelin-1 and angiotensin II, as well as reducing H+ secretion and thereby preventing tubular cast formation. Another extrarenal regulation mechanism is to reduce renal inflammation by activating cholinergic anti-inflammatory pathway (Ray et al., 2018) and to improve glycemic control and metabolic status by correcting acidosis (Bellasi et al., 2016). Alkali supplementation may provide protection on renal long-term outcomes and may also influence other disease states in CKD population through two regulatory mechanisms. However, not all interventional studies enrolling advanced CKD population have established the benefit of using sodium bicarbonate in delaying renal disease progression to ESRD (de Brito-Ashurst et al., 2009; Jeong et al., 2014; Iorio et al., 2019; Bi, 2020). In this study, we were unable to demonstrate that oral sodium bicarbonate successfully delayed the progression of advanced CKD to dialysis in pre-dialysis CKD stage V patients. This may be explained by the competing risk of death in this vulnerable population, short treatment duration from CKD stage V to dialysis and complex uremic environment causing rapid renal progression. These results may not necessarily conclude that sodium bicarbonate is not effective in halting renal progression in this population. Further studies are warranted to confirm these findings.
Effect of sodium bicarbonate on MACE in advanced CKD
To the best of our knowledge, this is the first cohort study to evaluate the effect of oral sodium bicarbonate therapy on MACE in the advanced CKD population. The association of serum bicarbonate with atherosclerotic cardiovascular disease and heart failure has been poorly understood, and the results are inconsistent across different study population. The Chronic Renal Insufficiency Cohort (CRIC) study investigating patients with CKD stages II–IV illustrated an increased incidence of heart failure with serum bicarbonate levels above 24–26 mEq/L, but there is no significant association with atherosclerotic events, including coronary artery disease. (Dobre et al., 2013; Dobre et al., 2015). However, it is important to note that the CRIC study excluded patients with New York Heart Association class III and IV heart failure. In the cohort of all patients with type 2 DM with varying degrees of kidney function, serum bicarbonate level was independently and inversely associated with incident coronary heart disease, but not with heart failure (Paul Chubb et al., 2016). The data from patients with diabetic nephropathy who enrolled in the Reduction of End points in Non-insulin-dependent diabetes with the Angiotensin II Antagonist Losartan (RENAAL) trial or the Irbesartan Diabetic Nephropathy Trial (IDNT) showed no relationship between bicarbonate level and cardiovascular events, heart failure (Schutte et al., 2015). The RENAAL/IDNT study also exclude patients who had a history of heart failure. In contrast, another current large analysis of hypertensive individuals without diabetes showed that serum bicarbonate level less than 22 mEq/L increase the risk of fatal and nonfatal cardiovascular events (Dobre et al., 2020).
The detailed mediating mechanisms linking serum bicarbonate to heart failure and the discrepancy across different cohort phenotypes require advanced research. Our study demonstrated that the use of sodium bicarbonate dose not increase the risk of heart failure, thereby reducing the doubts and concerns about the clinical use of this drug in pre-dialysis CKD stage V patients. The results echo previous experiences that sodium bicarbonate administration did not significantly affect blood pressure, edema, or weight gain, and it was well tolerated (de Brito-Ashurst et al., 2009; Iorio et al., 2019; Dubey et al., 2020).
Moreover, for the first time, we found that oral sodium bicarbonate therapy reduce the risk of MACE, including myocardial infarction and stroke in CKD population. Cardiovascular events are the leading cause of death in patients with chronic kidney disease. In addition to traditional cardiovascular risk factors such as hypertension and dyslipidemia, metabolic acidosis has been postulated as one of the potential mechanisms for progression of CKD. Acidosis stimulates the expression of multiple inflammatory genes and upregulates endothelial cell adhesion in endothelial cells (Chen et al., 2011; Dong et al., 2013; Dobre et al., 2020). This leads to recruitment and activation of leukocytes and plasma leakage, which in turn cause tissue damage. The persisting low-grade systemic inflammation impairs endothelial function and predisposes to accelerated atherosclerosis (Recio-Mayoral et al., 2011). Thus, the link between metabolic acidosis and atherosclerotic heart disease may be mediated through endothelial inflammatory processes (Dobre et al., 2020). Furthermore, acidosis is associated with the activation of the renin-angiotensin-aldosterone system, and elevated aldosterone levels may contribute to increase the incidence of cardiovascular and atherosclerotic disease (Hillege et al., 2000). Recently, Kendrick et al. performed a pilot randomized crossover study showing that a 6-week supply of sodium bicarbonate has favorable effects on vascular endothelial function, as assessed by flow-mediated dilation, in patients with stage IIIb and IV CKD (Kendrick, 2018). Although the research have not been entirely conclusive, it is plausible that taking sodium bicarbonate to correct acidosis and suppress chronic inflammation reduces the risk of MACE in advanced CKD population.
Effect of sodium bicarbonate on mortality in advanced CKD
Serum bicarbonate tends to display a U-shaped relationship with mortality from prior observational studies in moderate and advanced CKD (Kovesdy et al., 2009). Patients with serum bicarbonate level <22 mmol/L and > 29 mmol/L were at increased risk of death (Navaneethan et al., 2011). Our findings demonstrate that sodium bicarbonate supplementation reduces risk of death in pre-dialysis CKD stage V patients. This result is consistent with a recent randomized clinical trial study, the UBI study, which reported a survival benefit in patients with CKD stages III-V, who achieve a target serum bicarbonate level of 24–28 mmol/L by taking sodium bicarbonate (Iorio et al., 2019). We also observed a protective effect of sodium bicarbonate in reducing the risk of death both prior to and after entering dialysis. Although the mechanisms are uncertain, this may be due in part to the protective effect of alkali therapy on reducing the risk of MACE. Moreover, malnutrition could be reversed with sodium bicarbonate treatment, which may also partially explain our finding of reduced mortality. Approximately 40% of patients at the start of dialysis suffer from protein-energy wasting, and malnutrition is thought to be one of the major risks of increased morbidity and death in CKD population (Ikizler et al., 1995). Extensive data from short-term metabolic studies indicate that chronic metabolic acidosis in CKD results in decreased protein synthesis, enhanced muscles proteolysis, endocrine abnormalities (e.g., insulin resistance), ultimately the development of protein-energy wasting (Ballmer et al., 1995; EUSTACE et al., 2004). And available interventional evidence confirms oral bicarbonate supplementation increases serum albumin and has positive nutritional benefits among patients with CKD and dialysis patients (de Brito-Ashurst et al., 2009; Jeong et al., 2014; Dubey et al., 2020). Attenuating malnutrition and inflammation by treating metabolic acidosis may contribute to reduce mortality. One potential clinical concern issue is that excess administration of exogenous alkali therapy may result in metabolic alkalosis and associated side effects. However, according to previous studies, serum bicarbonate level did not exceed normal values when administrated for 1 year at a daily dose of oral sodium bicarbonate at 1 mEq/kg body weight/d (5.9 g/d for 70 kg body weight) in CKD stage IV patients with MA (Goraya et al., 2013). Therefore, sodium bicarbonate at doses (2–3 g/d), which are primarily used in patients with CKD, are unlikely to cause this complication (Goraya and Wesson, 2017).
This study has several limitations that should be acknowledged. First, to assess the effects of sodium bicarbonate in CKD stage V, we defined sodium bicarbonate exposure as a prescription within 180 days from the date of a diagnosis of CKD stage V. Patients who were prescribed sodium bicarbonate during early CKD were excluded. The influence of different drug regimens and dosages was beyond the scope of this study. Full prescription and refill information was not available as patients are not obliged to take their medications at our health care facilities. There was also incomplete information on medication adherence. Second, even though PSW analysis was adopted to minimize relevant confounders that we could identify, the observational nature of this study made complete elimination of other residual biases impossible and may have inherent limitations. Third, our study is a multi-center study which did not account for site specific environmental factors as urban versus rural settings, academic hospitals versus community hospitals, the reproducibility and generalizability of this report will need further validation.
In conclusion, our study revealed that oral sodium bicarbonate therapy might reduce the risk of MACE and mortality in patients with pre-dialysis CKD stage V without increasing the incidence of heart failure and acute pulmonary edema. This inexpensive and simple strategy is consistent with current renal care recommendations and has the potential to yield significant economic, quality of life and clinical outcome benefits in the expanding CKD population (Fine and Gray, 1999).
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Review Board Chang Gung Medical Foundation. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
Research idea and study design: Y-LC, S-CH, M-YH, and C-CH; Data Acquisition: M-YH, W-CS, P-YF, and Y-RL; Statistical analysis: J-SC and CL; Data interpretation: Y-LC, C-LY, P-YF, and K-HC; Writing: Y-LC, C-CH, and S-CH; Supervision/mentorship: C-CH and CL. All authors read and approved the final manuscript. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication. All authors contributed to the article and approved the submitted version.
Acknowledgments
We thank all the clinicians and patients enrolled in this study. The authors thank the statistical assistance and wish to acknowledge the support of the Ministry of Science and Technology of Taiwan (grant number MOST 111-2410-H-182A-001 -) and Chang Gung Memorial Hospital (grant number CLRPG3H0014, CORPG3M0231) for study design and monitor, data analysis and interpretation.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Austin, P. C. (2011). An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar. Behav. Res. 46, 399–424. doi:10.1080/00273171.2011.568786
Austin, P. C. (2014). The use of propensity score methods with survival or time-to-event outcomes: Reporting measures of effect similar to those used in randomized experiments. Stat. Med. 33, 1242–1258. doi:10.1002/sim.5984
Ballmer, P. E., McNurlan, M. A., Hulter, H. N., Anderson, S. E., Garlick, P. J., and Krapf, R. (1995). Chronic metabolic acidosis decreases albumin synthesis and induces negative nitrogen balance in humans. J. Clin. Invest. 95, 39–45. doi:10.1172/JCI117668
Bellasi, A., Di Micco, L., Santoro, D., Marzocco, S., De Simone, E., Cozzolino, M., et al. (2016). Correction of metabolic acidosis improves insulin resistance in chronic kidney disease. BMC Nephrol. 17, 158. doi:10.1186/s12882-016-0372-x
Bi, C. S. G. (2020). Clinical and cost-effectiveness of oral sodium bicarbonate therapy for older patients with chronic kidney disease and low-grade acidosis (BiCARB): A pragmatic randomised, double-blind, placebo-controlled trial. BMC Med. 18, 91. doi:10.1186/s12916-020-01542-9
Chen, A., Dong, L., Leffler, N. R., Asch, A. S., Witte, O. N., and Yang, L. V. (2011). Activation of GPR4 by acidosis increases endothelial cell adhesion through the cAMP/epac pathway. PLoS ONE 6, e27586. doi:10.1371/journal.pone.0027586
Chen, W., Melamed, M. L., and Abramowitz, M. K. (2015). Serum bicarbonate and bone mineral density in US adults. Am. J. Kidney Dis. 65, 240–248. doi:10.1053/j.ajkd.2014.07.007
Dafni, U. (2011). Landmark analysis at the 25-year landmark point. Circ. Cardiovasc Qual. Outcomes 4, 363–371. doi:10.1161/CIRCOUTCOMES.110.957951
de Brito-Ashurst, I., Varagunam, M., Raftery, M. J., and Yaqoob, M. M. (2009). Bicarbonate supplementation slows progression of CKD and improves nutritional status. J. Am. Soc. Nephrol. 20, 2075–2084. doi:10.1681/ASN.2008111205
Dobre, M., Pajewski, N. M., Beddhu, S., Chonchol, M., Hostetter, T. H., Li, P., et al. (2020). Serum bicarbonate and cardiovascular events in hypertensive adults: Results from the systolic blood pressure intervention trial. Nephrol. Dial. Transpl. 35, 1377–1384. doi:10.1093/ndt/gfz149
Dobre, M., Yang, W., Chen, J., Drawz, P., Hamm, L. L., Horwitz, E., et al. (2013). Association of serum bicarbonate with risk of renal and cardiovascular outcomes in CKD: A report from the chronic renal insufficiency cohort (CRIC) study. Am. J. Kidney Dis. 62, 670–678. doi:10.1053/j.ajkd.2013.01.017
Dobre, M., Yang, W., Pan, Q., Appel, L., Bellovich, K., Chen, J., et al. (2015). Persistent high serum bicarbonate and the risk of heart failure in patients with chronic kidney disease (CKD): A report from the chronic renal insufficiency cohort (CRIC) study. J. Am. Heart Assoc. 4, e001599. doi:10.1161/JAHA.114.001599
Dong, L., Li, Z., Leffler, N. R., Asch, A. S., Chi, J. T., and Yang, L. V. (2013). Acidosis activation of the proton-sensing GPR4 receptor stimulates vascular endothelial cell inflammatory responses revealed by transcriptome analysis. PLoS ONE 8, e61991. doi:10.1371/journal.pone.0061991
Dubey, A. K., Sahoo, J., Vairappan, B., Haridasan, S., Parameswaran, S., and Priyamvada, P. S. (2020). Correction of metabolic acidosis improves muscle mass and renal function in chronic kidney disease stages 3 and 4: A randomized controlled trial. Nephrol. Dial. Transpl. 35, 121–129. doi:10.1093/ndt/gfy214
Eustace, J. A., Astor, B., Muntner, P. M., Ikizler, T. A., and Coresh, J. (2004). Prevalence of acidosis and inflammation and their association with low serum albumin in chronic kidney disease. Kidney Int. 65, 1031–1040. doi:10.1111/j.1523-1755.2004.00481.x
Fine, J. P., and Gray, R. J. (1999). A proportional hazards model for the subdistribution of a competing risk. J. Am. Stat. Assoc. 94, 496–509. doi:10.1080/01621459.1999.10474144
Goraya, N., Simoni, J., Jo, C. H., and Wesson, D. E. (2013). A comparison of treating metabolic acidosis in CKD stage 4 hypertensive kidney disease with fruits and vegetables or sodium bicarbonate. Clin. J. Am. Soc. Nephrol. 8, 371–381. doi:10.2215/CJN.02430312
Goraya, N., and Wesson, D. E. (2017). Management of the metabolic acidosis of chronic kidney disease. Adv. Chronic Kidney Dis. 24, 298–304. doi:10.1053/j.ackd.2017.06.006
Hillege, H. L., Girbes, A. R., de Kam, P. J., Boomsma, F., de Zeeuw, D., Charlesworth, A., et al. (2000). Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation 102, 203–210. doi:10.1161/01.cir.102.2.203
Hsieh, C. Y., Chen, C. H., Li, C. Y., and Lai, M. L. (2015). Validating the diagnosis of acute ischemic stroke in a National Health Insurance claims database. J. Formos. Med. Assoc. 114, 254–259. doi:10.1016/j.jfma.2013.09.009
Hu, M. K., Witham, M. D., and Soiza, R. L. (2019). Oral bicarbonate therapy in non-haemodialysis dependent chronic kidney disease patients: A systematic review and meta-analysis of randomised controlled trials. J. Clin. Med. 8, 208. doi:10.3390/jcm8020208
Ikizler, T. A., Greene, J. H., Wingard, R. L., Parker, R. A., and Hakim, R. M. (1995). Spontaneous dietary protein intake during progression of chronic renal failure. J. Am. Soc. Nephrol. 6, 1386–1391. doi:10.1681/ASN.V651386
Iorio, B. R. D., Bellasi, A., Raphael, K. L., Santoro, D., Aucella, F., Garofano, L., et al. (2019). Treatment of metabolic acidosis with sodium bicarbonate delays progression of chronic kidney disease: The UBI study. J. Nephrol. 32, 989–1001. doi:10.1007/s40620-019-00656-5
Jeong, J., Kwon, S. K., and Kim, H. Y. (2014). Effect of bicarbonate supplementation on renal function and nutritional indices in predialysis advanced chronic kidney disease. Electrolyte Blood Press 12, 80–87. doi:10.5049/EBP.2014.12.2.80
Kendrick, J. (2018). Effect of treatment of metabolic acidosis on vascular endothelial function in patients with CKD A pilot randomized cross-over study. Clin. J. Am. Soc. Nephrol. 13. doi:10.2215/CJN.00380118
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group (2012). KDIGO clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 3, 1–150.
Kovesdy, C. P., Anderson, J. E., and Kalantar-Zadeh, K. (2009). Association of serum bicarbonate levels with mortality in patients with non-dialysis-dependent CKD. Nephrol. Dial. Transpl. 24, 1232–1237. doi:10.1093/ndt/gfn633
Kraut, J. A., and Kurtz, I. (2005). Metabolic acidosis of CKD: Diagnosis, clinical characteristics, and treatment. Am. J. Kidney Dis. 45, 978–993. doi:10.1053/j.ajkd.2005.03.003
Kuczera, P. (2020). The prevalence of metabolic acidosis in patients with different stages of chronic kidney disease: Single-centre study. Kidney Blood Press Res. 45, 863–872. doi:10.1159/000508980
Levey, A. S., and Stevens, L. A. (2010). Estimating GFR using the CKD epidemiology collaboration (CKD-EPI) creatinine equation: More accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am. J. Kidney Dis. 55, 622–627. doi:10.1053/j.ajkd.2010.02.337
Mannon, E. C., and O'Connor, P. M. (2020). Alkali supplementation as a therapeutic in chronic kidney disease: What mediates protection? Am. J. Physiol. Ren. Physiol. 319, F1090–F1104. doi:10.1152/ajprenal.00343.2020
Menon, V., Tighiouart, H., Vaughn, N. S., Beck, G. J., Kusek, J. W., Collins, A. J., et al. (2010). Serum bicarbonate and long-term outcomes in CKD. Am. J. Kidney Dis. 56, 907–914. doi:10.1053/j.ajkd.2010.03.023
Moranne, O., Froissart, M., Rossert, J., Gauci, C., Boffa, J. J., Haymann, J. P., et al. (2009). Timing of onset of CKD-related metabolic complications. J. Am. Soc. Nephrol. 20, 164–171. doi:10.1681/ASN.2008020159
Nath, K. A., Hostetter, M. K., and Hostetter, T. H. (1985). Pathophysiology of chronic tubulo-interstitial disease in rats. Interactions of dietary acid load, ammonia, and complement component C3. J. Clin. Invest. 76, 667–675. doi:10.1172/JCI112020
Navaneethan, S. D., Schold, J. D., Arrigain, S., Jolly, S. E., Wehbe, E., Raina, R., et al. (2011). Serum bicarbonate and mortality in stage 3 and stage 4 chronic kidney disease. Clin. J. Am. Soc. Nephrol. 6, 2395–2402. doi:10.2215/CJN.03730411
Navaneethan, S. D., Shao, J., Buysse, J., and Bushinsky, D. A. (2019). Effects of treatment of metabolic acidosis in CKD: A systematic review and meta-analysis. Clin. J. Am. Soc. Nephrol. 14, 1011–1020. doi:10.2215/CJN.13091118
Ng, H. Y., Chen, H. C., Tsai, Y. C., Yang, Y. K., and Lee, C. T. (2011). Activation of intrarenal renin-angiotensin system during metabolic acidosis. Am. J. Nephrol. 34, 55–63. doi:10.1159/000328742
Paul Chubb, S. A., Davis, W. A., Peters, K. E., and Davis, T. M. E. (2016). Serum bicarbonate concentration and the risk of cardiovascular disease and death in type 2 diabetes: The fremantle diabetes study. Cardiovasc Diabetol. 15, 143. doi:10.1186/s12933-016-0462-x
Phisitkul, S., Hacker, C., Simoni, J., Tran, R. M., and Wesson, D. E. (2008). Dietary protein causes a decline in the glomerular filtration rate of the remnant kidney mediated by metabolic acidosis and endothelin receptors. Kidney Int. 73, 192–199. doi:10.1038/sj.ki.5002647
Ravi, B., Croxford, R., Austin, P. C., Lipscombe, L., Bierman, A. S., Harvey, P. J., et al. (2013). The relation between total joint arthroplasty and risk for serious cardiovascular events in patients with moderate-severe osteoarthritis: Propensity score matched landmark analysis. BMJ 347, f6187. doi:10.1136/bmj.f6187
Ray, S. C., Baban, B., Tucker, M. A., Seaton, A. J., Chang, K. C., Mannon, E. C., et al. (2018). Oral NaHCO(3) activates a splenic anti-inflammatory pathway: Evidence that cholinergic signals are transmitted via mesothelial cells. J. Immunol. 200, 3568–3586. doi:10.4049/jimmunol.1701605
Recio-Mayoral, A., Banerjee, D., Streather, C., and Kaski, J. C. (2011). Endothelial dysfunction, inflammation and atherosclerosis in chronic kidney disease-a cross-sectional study of predialysis, dialysis and kidney-transplantation patients. Atherosclerosis 216, 446–451. doi:10.1016/j.atherosclerosis.2011.02.017
Schutte, E., Lambers Heerspink, H. J., Lutgers, H. L., Bakker, S. J. L., Vart, P., Wolffenbuttel, B. H. R., et al. (2015). Serum bicarbonate and kidney disease progression and cardiovascular outcome in patients with diabetic nephropathy: A Post hoc analysis of the RENAAL (reduction of end points in non–insulin-dependent diabetes with the angiotensin II antagonist losartan) study and IDNT (irbesartan diabetic nephropathy trial). Am. J. Kidney Dis. 66, 450–458. doi:10.53/j.ajkd.2015.03.032
Shao, S. C., Chan, Y. Y., Kao Yang, Y. H., Lin, S. J., Hung, M. J., Chien, R. N., et al. (2019). The Chang Gung Research Database-A multi-institutional electronic medical records database for real-world epidemiological studies in Taiwan. Pharmacoepidemiol Drug Saf. 28, 593–600. doi:10.1002/pds.4713
Wesson, D. E., Jo, C. H., and Simoni, J. (2015). Angiotensin II-mediated GFR decline in subtotal nephrectomy is due to acid retention associated with reduced GFR. Nephrol. Dial. Transpl. 30, 762–770. doi:10.1093/ndt/gfu388
Wesson, D. E., Nathan, T., Rose, T., Simoni, J., and Tran, R. M. (2007). Dietary protein induces endothelin-mediated kidney injury through enhanced intrinsic acid production. Kidney Int. 71, 210–217. doi:10.1038/sj.ki.5002036
Wesson, D. E., and Simoni, J. (2010). Acid retention during kidney failure induces endothelin and aldosterone production which lead to progressive GFR decline, a situation ameliorated by alkali diet. Kidney Int. 78, 1128–1135. doi:10.1038/ki.2010.348
Wu, C. S., Lai, M. S., Gau, S. S., Wang, S. C., and Tsai, H. J. (2014). Concordance between patient self-reports and claims data on clinical diagnoses, medication use, and health system utilization in Taiwan. PLoS One 9, e112257. doi:10.1371/journal.pone.0112257
Keywords: sodium bicarbonate, advanced chronic kidney disease, cardiovascular outcome, mortality, dialysis
Citation: Cheng Y-L, Huang S-C, Ho M-Y, Li Y-R, Yen C-L, Chen K-H, Sun W-C, Fan P-Y, Chen J-S, Lin C and Hsiao C-C (2023) Effect of sodium bicarbonate on cardiovascular outcome and mortality in patients with advanced chronic kidney disease. Front. Pharmacol. 14:1146668. doi: 10.3389/fphar.2023.1146668
Received: 17 January 2023; Accepted: 20 April 2023;
Published: 11 May 2023.
Edited by:
Narayan Prasad, Sanjay Gandhi Post Graduate Institute of Medical Sciences (SGPGI), IndiaReviewed by:
Yuan Gui, University of Connecticut, United StatesElaheh Malakan Rad, Tehran University of Medical Sciences, Iran
Copyright © 2023 Cheng, Huang, Ho, Li, Yen, Chen, Sun, Fan, Chen, Lin and Hsiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ching-Chung Hsiao, Y29saW5odWEwMTIzQGdtYWlsLmNvbQ==; Chihung Lin, bGluMzAzQGNnbWgub3JnLnR3
 Ya-Lien Cheng
Ya-Lien Cheng Shu-Chun Huang
Shu-Chun Huang Ming-Yun Ho
Ming-Yun Ho Yan-Rong Li
Yan-Rong Li Chieh-Li Yen1,2
Chieh-Li Yen1,2 Kuan-Hsing Chen
Kuan-Hsing Chen Jung-Sheng Chen
Jung-Sheng Chen Ching-Chung Hsiao
Ching-Chung Hsiao