- 1Borneo Research on Algesia, Inflammation and Neurodegeneration (BRAIN) Group, Faculty of Medicine and Health Sciences, Universiti Malaysia Sabah, Kota Kinabalu, Sabah, Malaysia
- 2Department of Biomedical Sciences, Faculty of Medicine and Health Sciences, Universiti Putra Malaysia, Serdang, Malaysia
- 3Department of Human Anatomy, Faculty of Medicine and Health Sciences, Universiti Putra Malaysia, Serdang, Malaysia
- 4Centre for Tissue Engineering and Regenerative Medicine, Faculty of Medicine, Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia
- 5Department of Emergency Medicine, Faculty of Medicine, Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia
This study assessed the toxicity of lutein-rich purple sweet potato leaf (PSPL) extract in male Sprague–Dawley rats.
Methods and study design: A total of 54 adult male Sprague–Dawley rats were used. For the acute toxicity study, three rats in the acute control group were fed 2,000 mg/kg of PSPL for 14 days. The subacute toxicity study included six rats each in four groups administered 50, 250, 500, or 1,000 mg/kg for 28 days and observed for further 14 days without treatment in the subacute control and subacute satellite groups. Changes in body weight; blood biochemistry; hematological parameters; relative organ weight; and histological sections of the heart, kidney, liver, pancreas, aorta, and retina were observed for signs of toxicity.
Results: The gradual increase in weekly body weight, normal level full blood count, normal liver and kidney profile, relative organ weight, and histological sections of all stained organ tissue in the treated group compared with the acute, subacute, and satellite control groups demonstrated the absence of signs of toxicity.
Conclusion: Lutein-rich PSPL extract shows no signs of toxicity up to 2,000 mg/kg/day.
1 Introduction
Functional foods have attracted research attention due to safety concerns. Any natural product containing bioactive molecules integrated into food is known as a functional food. In this context, one of the widely used functional food currently being studied is PSPL (Khairani et al., 2022). PSPL, also known as Ipomoea batatas (L.) Lam. is a dicotyledonous plant that belongs to the botanical family of Convolvulaceae. It is widely known for its natural medicinal properties, with the leaf itself containing up to 130 known metabolites (Naomi et al., 2021). This plant usually grows well in tropical and subtropical regions and is widely cultivated in more than 100 countries worldwide (Alam, 2021). In China alone, PSPL cultivation exceeds more than 76% of the world’s production (Nguyen et al., 2021). This is attributed due to the plant’s high drought tolerance and ability to grow in changing climates (Shekhar et al., 2014). Purple sweet potato is the sixth most common crop worldwide, with the highest production in the southeastern parts of India (Alam, 2021). Approximately, 115 million metric tons of sweet potatoes are cultivated annually through vegetative propagation (Mohanraj and Sivasankar, 2014). Most parts of the purple sweet potato can be eaten since they contain nutritional and bioactive compounds. Purple sweet potatoes are significantly rich in lutein, proteins, vitamins, minerals, fibers, and β-carotene compared to other commonly available major greens in Asian countries (Alam, 2021). A recent study showed that PSPL is a strong antioxidant with radical scavenging ability due to the presence of natural bioactive compounds such as anthocyanin (Yuzhi Jiao Studies on antioxidant, 2012). Due to its natural essences, PSPL has been formulated into a probiotic to make its consumption easier and so that more people can experience the maximum benefits (Kim et al., 2015). Some of the other identified minerals in PSPL include sodium, potassium, calcium, magnesium, iron, copper, manganese, and zinc (Sun et al., 2014).
The most well-known health benefit of purple sweet potato is its anti-diabetic effects due to xenobiotic phytochemical constituents (Strugała et al., 2019). Some of the plant’s genotypes are associated with nutraceutical value (Escobar-Puentes et al., 2022). Despite its known functional value, the plant is underutilized, possibly due to safety concerns regarding the consumption of the natural plant and the lack of scientific data on its therapeutic use. In some countries, I. batatas is completely avoided by women due to concerns about unintentional miscarriages. However, no specific study has reported that I. batatas is toxic for consumption or its adverse effects in pregnant subjects (Gunn et al., 2013). A previous study reported that purple sweet potato contains oxalic acid, which has the potential be converted into oxalate stones in the urinary tract. Thus, to avoid unwanted adverse effects, individuals with a history of kidney stones should avoid eating purple sweet potatoes (Noonan and Savage, 1999). However, this remains unconfirmed since no recent data have been reported regarding the safety and efficacy of purple sweet potatoes. Hence, the present study evaluated the toxicological effects of PSPL extract in male Sprague–Dawley rats.
2 Results
2.1 Lutein quantification
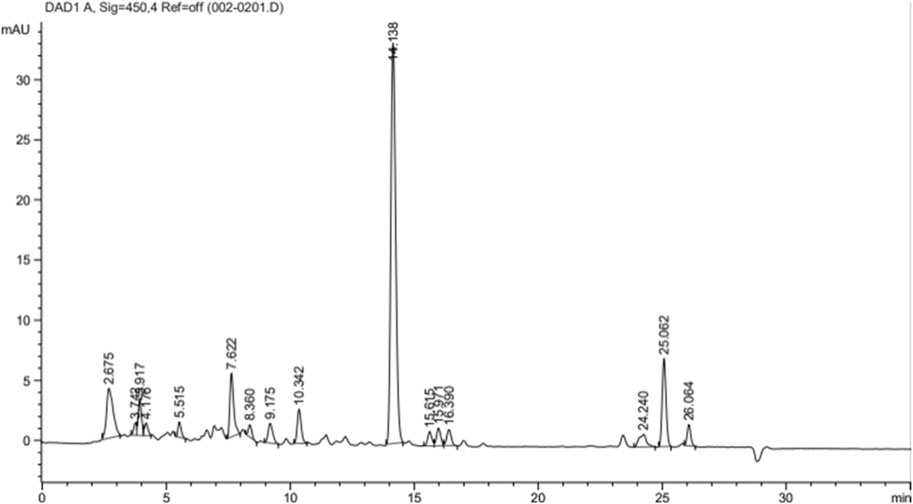
Figure 1 shows the chromatograms of lutein quantification from 1 g of PSPL extract and the peak maxima of the lutein fractions. A variety of peaks have been recorded for the desired lutein compound, with a total quantified lutein content of approximately 20.25% in 1 g of PSPL extract. The maximal elution was recorded at a peak area % of 51.38% at the 14th minute.
2.2 Acute toxicity study
2.2.1 Clinical observations
The oral administration of ethanol-extracted Ipomoea batatas leaf extract/PSPL did not induce any form of abnormalities in behavioral or eating patterns in the male Sprague–Dawley rats. Moreover, no abnormalities were observed in the gross morphology of the liver, kidney, pancreas, retina, aorta, and heart in the rats administered the highest supplement dose of 2,000 mg/kg. The size, shape, and color of all harvested organs in the AT 2000 group were similar to those in the AC group. All rats survived until the end of the experiments.
2.2.2 Body weight changes
Table 1 shows the changes in body weight in the AT 2000 and AC groups. The body weight gradually increased from day 0, week 1, and week 2 in both the AC and AT 2000 groups. However, the body weight (p > 0.05) of the AT 2000 group did not change significantly compared to that of the AC group on day 0, week 1, and week 2.

TABLE 1. Changes in body weight in the acute toxicity study. Values are presented as means ± SEM. AC, acute control group; AT 2000, 2,000 mg/kg of PSPL.
2.2.3 Calorie and water intake
Table 2 presents the total calorie and water intake for the PSPL acute toxicity study in male Sprague–Dawley rats. The total calorie and water intake did not differ significantly (p > 0.05) between the AT 2000 and AC groups.
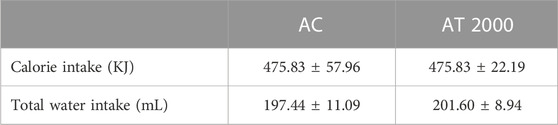
TABLE 2. Total calorie and water intake in the acute toxicity study. Values are presented as means ± SEM. AC, acute control group; AT 2000, 2,000 mg/kg of PSPL.
2.2.4 Relative organ weight
Table 3 shows the relative organ weights in the AC and AT 2000 groups. The relative organ weight (g) and standardized organ weight (%) did not differ significantly between the AT 2000 and AC groups (p > 0.05).
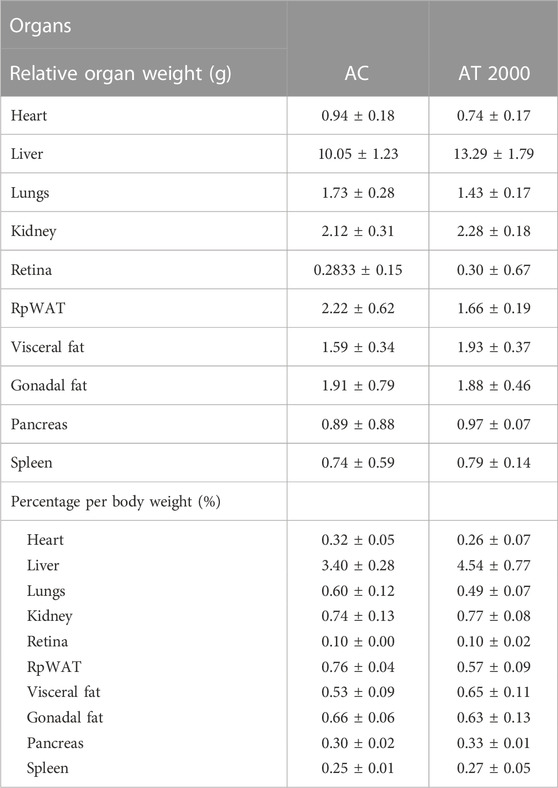
TABLE 3. Relative organ weights of SD male rats in the acute toxicity study. Values are expressed as means ± SEM. RpWAT, retroperitoneal; AC, acute control group; AT 2000, 2,000 mg/kg of PSPL.
2.2.5 Hematological analysis
Table 4 presents the results of the hematological analysis for the PSPL acute toxicity study in male SD rats. The oral administration of 2,000 mg/kg of PSPL extract led to a significant increase (p < 0.05) in MCV and MCHC in the AT 2000 group compared to the AC group. The other measured blood parameters did not differ significantly between the AT 2000 and AC groups (p > 0.05).

TABLE 4. Effects of PSPL extract on hematological parameters in the acute toxicity study. Values are expressed as means ± SEM. The letters indicate significant differences (p < 0.05). Hb, hemoglobin; RBC, red blood cell; RDW, red cell distribution width; PCV, packed cell volume; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; WBC, white blood cell; AC, acute control group; AT 2000, 2,000 mg/kg of PSPL.
2.2.6 Blood biochemistry analysis
Table 5 presents the results of the blood biochemistry analysis in the PSPL acute toxicity study in male Sprague–Dawley rats. The data showed no significant difference (p > 0.05) in the renal and liver profiles between the AT 2000 and AC groups.
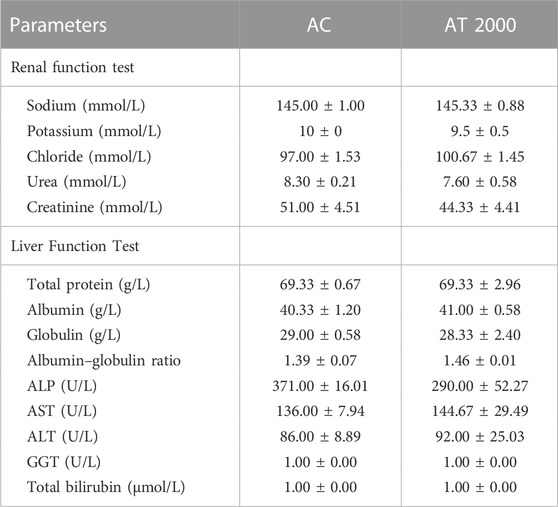
TABLE 5. Effect of PSPL extracts on blood biochemistry parameters in the SD male acute toxicity study. Values are expressed as means ± SEM. ALP, alkaline phosphatase; AST, aspartate transaminase; ALT, alanine transaminase; GGT, gamma-glutamyl transferase; AC, acute control group; AT 2000, 2,000 mg/kg of PSPL.
2.2.7 Histopathological analysis
Figure 2 shows the histological sections of the retina, kidney, pancreas, liver, heart, and aorta. No pathological abnormalities were observed, and the AT 2000 group showed similar morphological and histological features compared to those in the AC group. The retina showed normal features of the ganglion cell layer (GCL), inner plexiform layer (IPL), inner nuclear layer (INL), outer plexiform layer (OPL), outer nuclear layer (ONL), photoreceptor layer (PL), and retinal pigmented epithelium monolayer (RPE). The kidney histology showed the presence of Bowman’s capsule (BC) and glomerulus (GLO). The normal histology features of the islet of Langerhans (ioL) and Acini were observed in the pancreas, and normal strands of the hepatic portal triad (HPL) were visible in liver histology. The histological sections showed the presence of the aorta tunica intima (TI), tunica media (TM), and tunica adventitia (TA). The heart structure shows the nucleus (N) and myocardium (M).

FIGURE 2. Histology section of the AT 2000 and AC groups. No abnormalities in the histological sections of the retina, kidney, liver, pancreas, heart, and aorta of the AT 2000-treated group were observed compared to the AC group. GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; PL, photoreceptor layer; RPE, retinal pigmented epithelium monolayer; BC, Bowman’s capsule; GLO, glomerulus; iOL, islet of Langerhans; HPL, hepatic portal triad; TI, tunica intima; TM, tunica media; TA, tunica adventitia; N, nucleus; M, myocardium; AC, acute control group; AT 2000, 2,000 mg/kg of PSPL.
2.3 Subacute toxicity study (28-day repeated dose study)
2.3.1 Clinical observation
The oral administration of PSPL extract at various concentrations (ST 50, ST 250, ST 500, and ST 1000) for subacute toxicity study for 28 days and SST 1000 for 28 days of treatment followed by an additional 14-day observation without any treatment did not induce any abnormalities in behavior patterns or physical condition. All rats showed normal responses to touch and in salivation, hair coat, eye color, sleeping pattern, and grip strength. No locomotor dysfunction, convulsions, or tremors were recorded in the rats. All rats survived until the end of the experiment.
2.3.2 Body weight changes
Table 6 shows the changes in body weight in the subacute toxicity groups recorded for 28 days, and the satellite group recorded for 42 consecutive days. The body weights did not differ significantly between the ST 50, ST 250, ST 500, ST 1000, and SC groups at day 0 and weeks 1, 2, 3, and 4 (p > 0.05). The satellite group analysis showed no significant differences in body weight between the SST and SSC groups on day 0 and weeks 1, 2, 3, 4, 5, and 6 (p > 0.05).
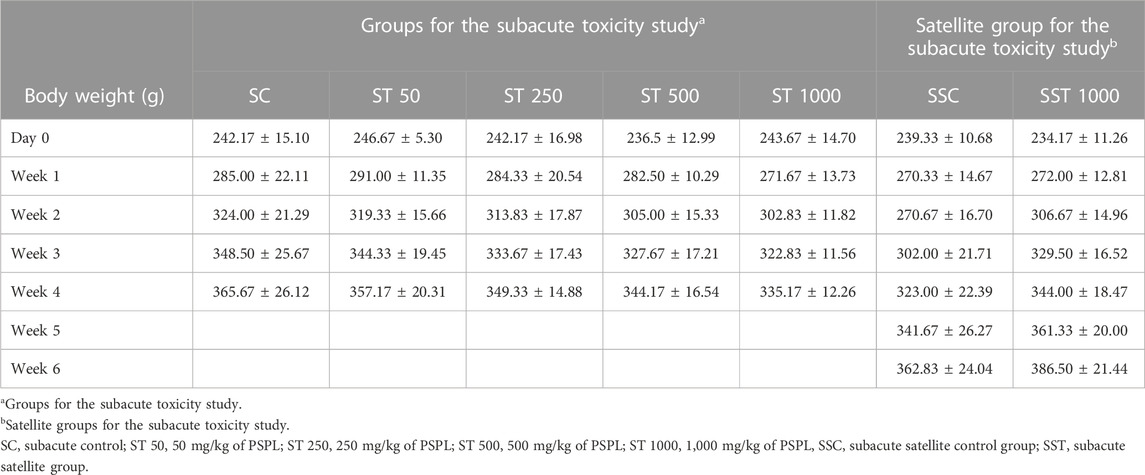
TABLE 6. Weekly body weight changes in the subacute toxicity study. Values are expressed as means ± SEM.
2.3.3 Total calorie and water intake
Table 7 presents the weekly calorie and 24-h water intakes for the subacute toxicity and satellite groups. The calorie and water intake did not differ significantly between the ST 50, ST 250, ST 500, ST 1000, and SC groups (p > 0.05). The satellite group analysis showed no significant differences in calorie and water intake between the SST 1000 and SSC groups (p > 0.05).
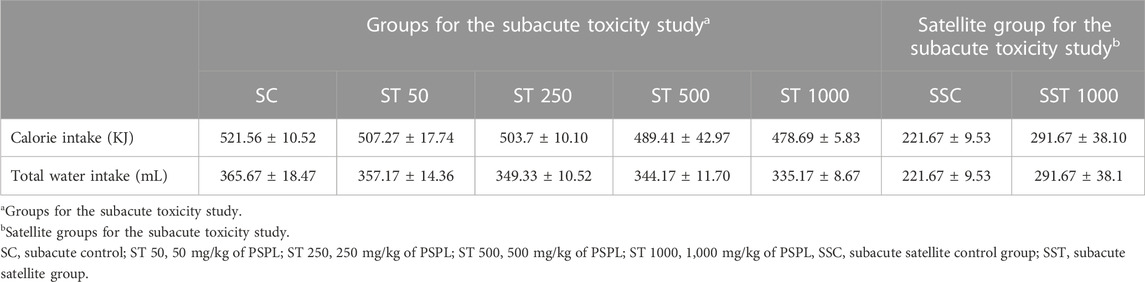
TABLE 7. Calorie and water intake in the subacute toxicity study. Values are presented as means ± SEM.
2.3.4 Relative organ weights
Table 8 shows the relative organ weights in the subacute toxicity and satellite groups. The relative and standardized organ weights (%) did not differ significantly between the ST 50, ST 250, ST 500, ST 1000, and SC groups (p > 0.05). The satellite group analysis showed no significant difference in relative and standardized organ weights (%) in the SST 1000 group compared to the SSC group (p > 0.05).
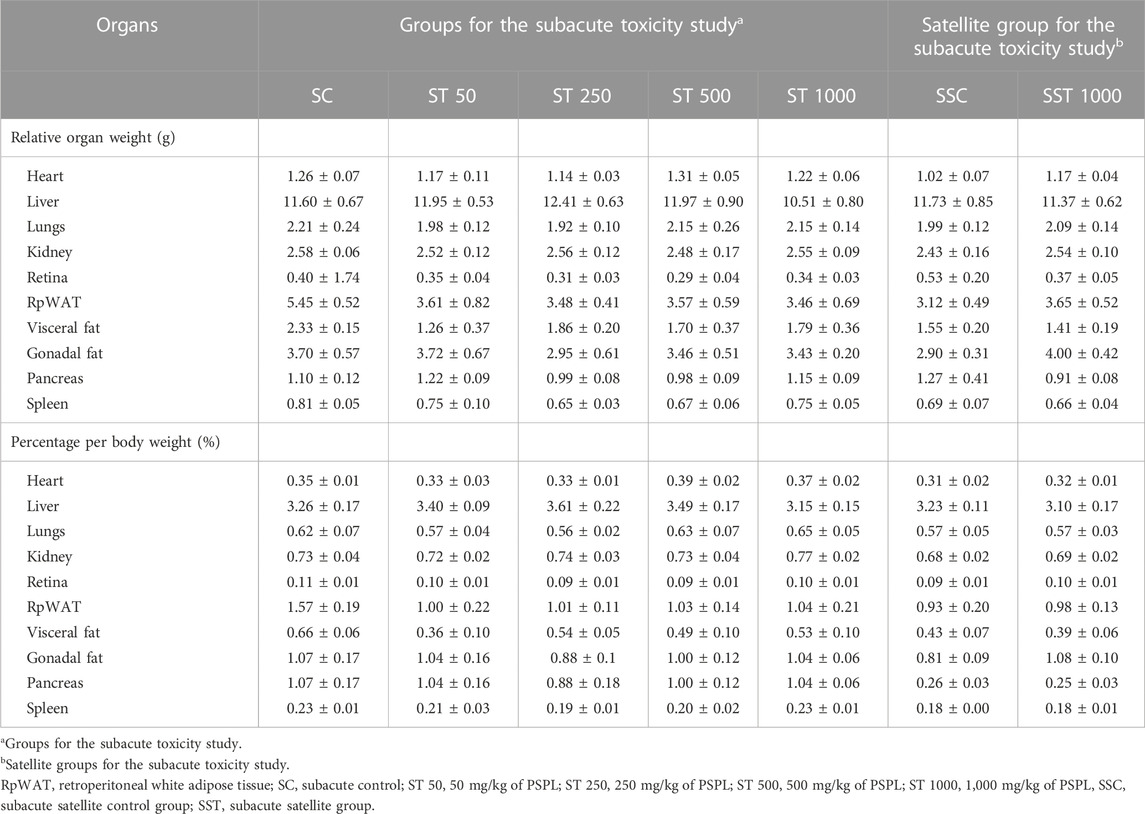
TABLE 8. Relative organ weights of rats treated with PSPL extract in the subacute toxicity study. Values are expressed as means ± SEM.
2.3.5 Hematological analysis
Table 9 presents the hematological parameters in the subacute toxicity and satellite groups. The hematological parameters did not differ significantly between the ST 50, ST 250, ST 500, ST 1000, and SC groups (p > 0.05). The satellite group analysis showed no significant differences in hematological parameters between the SST 1000 and SSC groups (p > 0.05).
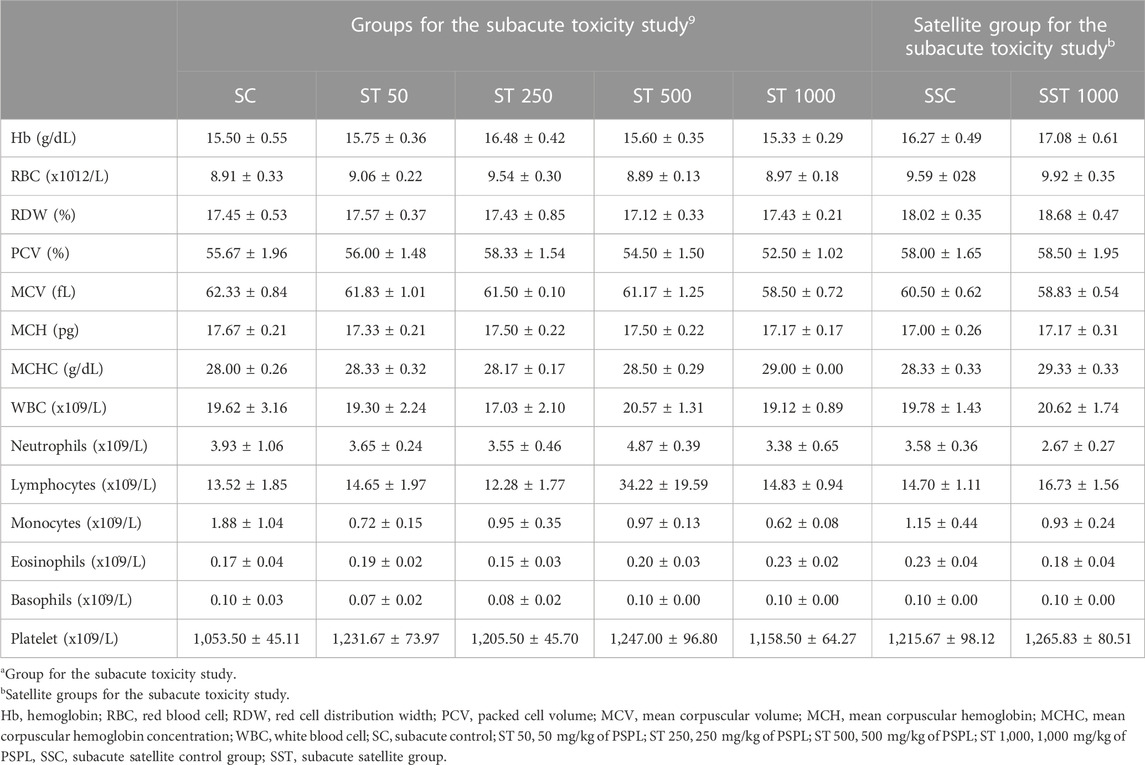
TABLE 9. Hematological effects of different treatment doses of PSPL extract on male Sprague–Dawley rats in the subacute toxicity study. Values are expressed as means ± SEM.
2.3.6 Blood biochemistry analysis
Table 10 shows the blood biochemistry data for the subacute toxicity and satellite groups. The blood biochemical parameters did not differ significantly between the ST 50, ST 250, ST 500, ST 1000, and SC groups (p > 0.05). The satellite group analysis showed no significant differences in blood biochemical parameters between the SST 1000 and SSC groups (p > 0.05).
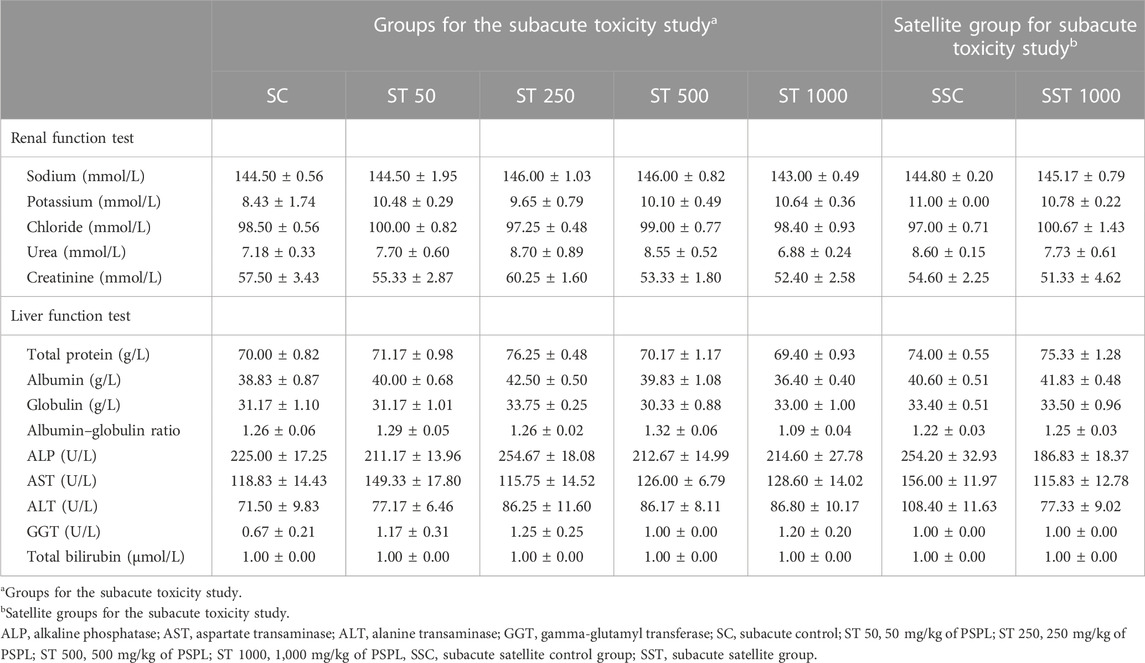
TABLE 10. Effects of PSPL extract on biochemistry analysis in the subacute toxicity studies. Values are expressed as means ± SEM.
2.3.7 Histopathological analysis
Figure 3 shows histological sections of the retina, kidney, pancreas, liver, heart, and aorta. No pathological abnormalities were observed in the ST 50, ST 250, ST 500, and ST 1000 groups, which showed similar morphological and histological features as the SC group. Similarly, the satellite group showed no histological abnormalities in the SST 1000 and SST 1000 groups, with similar features as the SSC group (Figure 4). Histological analysis of the retina showed normal features of the ganglion cell layer (GCL), inner plexiform layer (IPL), inner nuclear layer (INL), outer plexiform layer (OPL), outer nuclear layer (ONL), and retinal pigmented epithelium monolayer (RPE). The kidney histology showed the presence of Bowman’s capsule (BC) and glomerulus (GLO). Normal histological features of the islet of Langerhans (ioL) and Acini were seen in the pancreas, while normal strands of the hepatic portal triad (HPL) were visible in liver histology. The histological sections showed the aorta tunica intima (TI), tunica media (TM), and tunica adventitia (TA). The heart structure showed the nucleus (N) and myocardium (M).
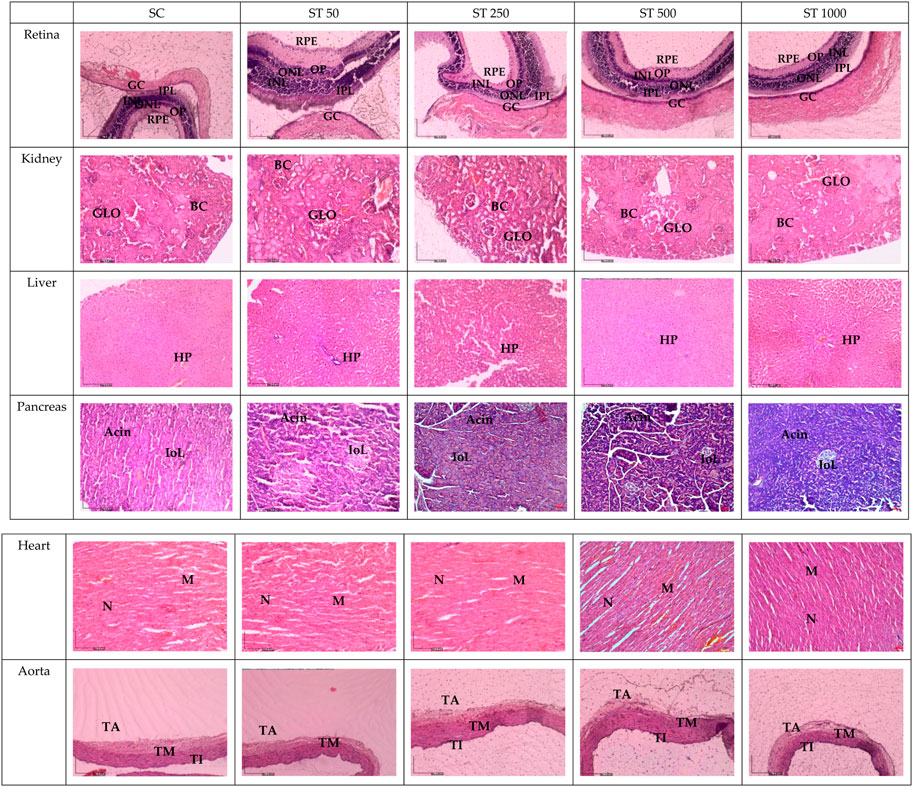
FIGURE 3. Histology sections in the subacute studies. No abnormalities in the histological sections of the retina, kidney, liver, pancreas, heart, and aorta of the ST 50, ST 250, ST 500, and ST 1000-treated groups were observed compared to the SC group. GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; PL, photoreceptor layer; RPE, retinal pigmented epithelium monolayer; BC, Bowman’s capsule; GLO, glomerulus; iOL, islet of Langerhans; HPL, hepatic portal triad; TI, tunica intima; TM, tunica media; TA, tunica adventitia; N, nucleus; M, myocardium; SC, subacute control; ST 50, 50 mg/kg of PSPL; ST 250, 250 mg/kg of PSPL; ST 500, 500 mg/kg of PSPL; ST 1000, 1,000 mg/kg of PSPL.
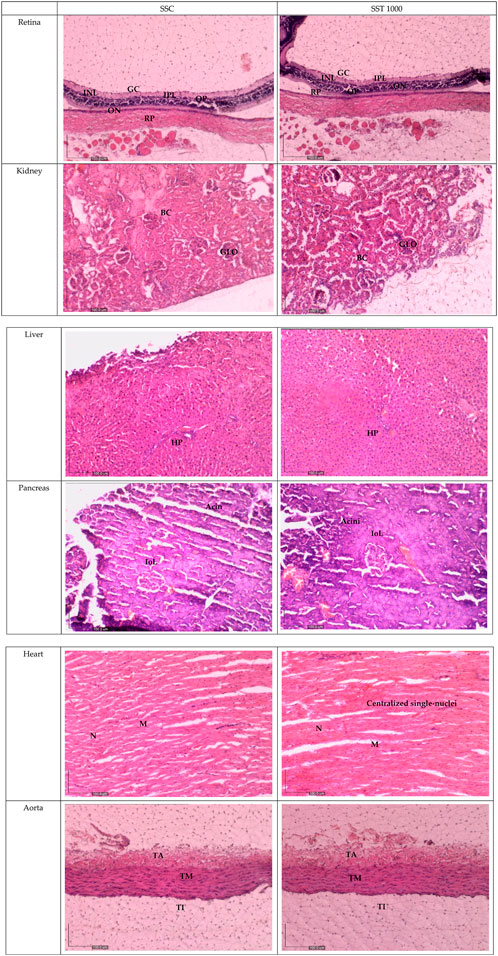
FIGURE 4. Histology sections for the satellite groups in the subacute studies. No abnormalities in the histological sections of the retina, kidney, liver, pancreas, heart, and aorta of the SST 1000-treated group were observed compared to the SSC group. GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; PL, photoreceptor layer; RPE, retinal pigmented epithelium monolayer; BC, Bowman’s capsule; GLO, glomerulus; iOL, islet of Langerhans; HPL, hepatic portal triad; TI, tunica intima; TM, tunica media; TA, tunica adventitia; N, nucleus; M, myocardium; SSC, subacute satellite control group; SST, subacute satellite group.
3 Discussion
PSPL is traditionally known for its beneficial therapeutic effects (Akhtar et al., 2018). However, it remains underutilized due to safety concerns. The results of the present study showed that the PSPL extract contained the highest % of lutein compound, approximately 20.25%, compared to other leafy vegetables (Sahli et al., 2015). The study is divided into two phases. The first phase focused on the phytochemical analysis of lutein percentage, while the second phase focused on the toxicological evaluation of PSPL extract in male Sprague–Dawley rats. The safety analysis of PSPL extracts in SD male rats in the first phase was further categorized into acute or subacute studies. In the acute study, a single maximum dose of 2,000 mg/kg per body weight did not induce any side effects or behavioral or sleep abnormalities. All rats survived until necropsy. Similar findings were observed in the subacute study, including satellite groups comprising four different doses (ST 50, ST 250, ST 500, and ST 1000) of PSPL. Neither the acute nor the subacute studies revealed any drastic or significant changes in body weight, organ weight, calorie intake, or water consumption from the beginning to the end of the assessment period.
Due to their roles in metabolism and excretion, the liver and kidneys are the most important organs to rule out toxicity (Peter et al., 2018). Gross examination and histopathological changes indicate toxicity (Zakaria et al., 2011). However, the similar histological features of the kidney and liver between the AT 2000 and AC groups; between the ST 50, ST 250, ST 500, and ST 1000 groups and the SC group; and between the SST 1000 and SSC groups further proves the safety of up to 2,000 mg/kg of PSPL extract. The gross examinations of the liver and kidney revealed no changes in the treated groups compared to the control group. In addition, H&E staining of the retina, pancreas, heart, and aorta to assess any abnormalities showed that the treated group was indistinguishable from the control groups in both acute and subacute studies. Moreover, the liver and kidney profile assessments of the blood biochemistry appeared within normal ranges for all assessed parameters, specifically ALP, AST, and ALP, which are also biomarkers of liver toxicity (Meunier and Larrey, 2019), thus further proving that the PSPL extract was safe for consumption up to a dose of 2,000 mg/kg.
Changes in hematological parameters are an indicator of the emergence of toxicity (Kamisan et al., 2012). However, the administration of 2,000 mg/kg of PSPL extract caused a significant increase in MCV and MCHC compared to AC. Increased MCV suggests macrocytic effects, while increased MCHC is associated with hyperchromic effects. Such conditions are usually associated with increased RBC rigidity (Tempelhoff et al., 2016). However, given the normal levels of other blood parameters (RBC, WBC, HB, platelet, RDW, and MCH), the possibilities of macrocytic and hyperchromic conditions were ruled out. The slight increase in MCV and MCHC in the AT 2000 group could be due to incidental instead of treatment-related effects. Moreover, MCV and MCHC usually increase with age (Tempelhoff et al., 2016). Thus, slight increases in MCH and MCHC are not concerning. Hence, the findings regarding the changes in body weight, organ weight, calorie intake, water consumption, blood biochemistry assessment, hematological analysis, and histopathological assessments in the acute and subacute studies demonstrated that the administration of up to 2,000 mg/kg/day of PSPL extract is a tolerable dosage.
4 Conclusion
In the present study, the PSPL extract showed the highest concentration of lutein of approximately 20.25%, which is the highest among natural roots. The therapeutic effects of the extract in biomedical applications were further analyzed. No signs of toxicity in the blood components or serum biochemical measures were observed in either the acute and subacute studies. The toxicological evaluation showed no abnormalities in the tissue histology nor gross morphology of the retina, kidney, liver, pancreas, aorta, and heart, indicating that the administration of up to 2,000 mg/kg/day of PSPL was well-tolerated. However, further assessments of the genotoxicity, compound toxicity, and subchronic and chronic toxicity are needed before proceeding with clinical trials, and it is highly recommended to test the compound in various age groups before PSPL use in clinical prescriptions.
5 Materials and methods
5.1 Plant species collection and confirmation
Fresh PSPL was obtained from a commercial sweet potato farm located at Sungai Pelek, Sepang, Selangor, Malaysia. The leaves were cleaned and sent to Herbarium Biodiversity Unit, Universiti Putra Malaysia for confirmation (voucher code: MFI 0188/20).
5.2 Ethanol extraction of Ipomoea batatas leaves
The PSPL leaves were cleaned with running tap water to remove any foreign material. About 20 g of PSPL leaves was soaked in 200 mL of 80% ethanol in a conical flask. The mixture was then placed in an orbital incubator shaker for 24 h at 150 rpm at room temperature. The supernatant was then collected and filtered using Whatman N°1 paper. The process was repeated three times to obtain the maximum yield. The filtrates from each extraction were then combined and evaporated using a rotary evaporator at 48°C. The obtained crude extract was then mixed with maltodextrin in a 1:1 ratio and oven-dried overnight (Fu et al., 2016). The final extracted powder was then weighed and stored at −20°C until further use.
5.3 HPLC quantification of the active compounds
High-performance liquid chromatography (HPLC) estimation of the lutein compound was performed in an Agilent 1200 Infinity instrument (Agilent Technology, United States) equipped with a DAD detector, an autosampler, a column heater, and a Welchrom® C18 (4.67 mm ID, 250 mm, 5 μm particle size) column. The mobile phase consisted of two solvents: (A) 0.140 g of anhydrous potassium dihydrogen orthophosphate (KH2PO) dissolved in 900 mL HPLC-grade water with the addition of 0.5 mL orthophosphoric acid, made up to 1,000 mL with water and filtered through a 0.45-μ membrane filter, and degassed in a sonicator for 3 min and (B) acetonitrile (HPLC-grade). With a flow rate of 1.5 mL/min, gradient elution was performed using the two solvents at a detection wavelength of 348 nm. A 20 μL sample volume was injected into the system by the autosampler. About 10.97 mg of the isolated esculetin was dissolved in 10 mL of ethanol, while 6.26 mg PSPL extract was dissolved in 10 mL of ethanol. HPLC was then performed for both the standard and sample (Makwana and Pandya, 2020).
5.4 Experimental animals for the toxicity studies
All animal-related protocols for the toxicity studies were performed according to the guideline of the Institutional Animal Care and Use Committee (IACUC) of UPM (ethical code UPM/IACUC/AUP-R070/2020). A total of 54 adult male Sprague–Dawley rats weighing 150–200 g were used. All rats were subjected to acclimatization for the first week at a controlled temperature room (23°C–25°C) and humidity (55%–60%) and with a 12/12 h light/dark cycle. All rats were fed standard rat pellets ad libitum. Changes in body weight and 24-h water intake were measured weekly (Bahari et al., 2020).
5.5 Acute toxicity study
An acute toxicity study was carried out according to the protocol described in OECD 423. For acute toxicity assessment, the rats were divided into two groups (n = 3). The acute control group (AC) was administered normal saline through bottle feeding, while the treatment group was administered 2,000 mg/kg of PSPL (AT 2000) extract orally for 2 weeks. All rats were under constant observation for the entire treatment duration. The rats were observed for any abnormalities until euthanasia. All rats were euthanized with carbon dioxide (CO2) overdose, and their blood was collected in an EDTA tube for biochemical analysis. Organs such as the liver, kidney, pancreas, retina, aorta, and heart were harvested, weighed, and kept in 10% neutral buffered formalin (NBF) for hematoxylin and eosin (H&E) staining.
5.6 Subacute toxicity study (repeated dose)
A subacute toxicity study was performed according to the protocol described in OECD 407 for oral toxicity study in rodents (repeated dose for 28 days). For the subacute study, the rats were divided into five groups (n = 6), and PSPL extract was administered orally at 50 (ST 50), 250 (ST 250), 500 (ST 500), and 1,000 mg/kg (ST 1000), once daily. The subacute control (SC) group received normal saline for 28 days. The subacute satellite control group (SSC) received normal saline, while the subacute satellite group (SST 1000) was orally administered 1,000 mg/kg of PSPL extract for 28 days. Both the SSC and SST 1,000 mg/kg fed groups were observed for any behavioral abnormalities and treatment-related effects for an additional 14 days without any treatment. All rats were under constant observation for the whole treatment duration. The rats were observed for any abnormalities until euthanasia. All rats were euthanized with CO2 overdose, and their blood was collected in an EDTA tube for biochemical analysis. Organs such as the liver, kidney, pancreas, retina, aorta, and heart were harvested, weighed, and kept in 10% NBF for H&E staining.
5.7 Full blood count and plasma biochemistry
Blood collected in the EDTA tube was subjected to centrifugation at 25,000 rpm for 15 min to obtain serum. Automated BC-2800 VET/Mindray machine was used to analyze plasma biochemistry levels such as red blood cell count (RBC), hemoglobin concentration (Hb), hematocrit (HCt), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), platelets (Pt), and white blood cell count (WBC). Alere Cholestech LDX® Analyzer (Alere, United Kingdom) was used to measure the liver profile (aspartate transaminase [AST], alanine transaminase [ALT], alkaline phosphatase [ALP], total proteins, albumin, gamma-glutamyl transferase [GGT], and total and direct bilirubin) and renal profile (ions including sodium [Na], potassium [K], and chloride [CI−], urea, and creatinine).
5.8 Histopathological analysis
All organs (liver, kidney, pancreas, retina, aorta, and heart) fixed in 10% of NBF were sectioned to obtain paraffin ribbons, followed by fishing of the ribbon from the water bath (40°C–45°C) and fixation to a glass slide. The slides were then stained with H&E as described elsewhere and observed by microscopy (Balan et al., 2021). All images were captured and verified by a certified pathologist from UPM.
5.9 Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics for Windows, version 26.0, and all results were expressed as means ± standard error of the mean (SEM) for body weight, food consumption, and calorie intake, while normality tests were run for all data. One-way ANOVA and post hoc Tukey tests were used to analyze the significant difference among groups. P < 0.05 was defined as a statistically significant result.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was reviewed and approved and all animal-related protocols for the toxicity study were performed according to the guidelines of the Institutional Animal Care and Use Committee (IACUC) of UPM (ethical code of UPM/IACUC/AUP-R070/2020).
Author contributions
Conceptualization, AH, KB, and RN; methodology, HB and MY; software, FO; validation, HE, HB, RN, and MH; formal analysis, AH; investigation, AH, and KB; resources, ZZ; data curation, HB; writing—original draft preparation, AH, KB, and RN; writing—review and editing, AH, KB, and RN; visualization, HE; supervision, HB, MY, ZZ, and MH; project administration, AH, SH, and KB; funding acquisition, MH, and ZZ. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Higher Education, Malaysia, under Fundamental Research Grant Scheme with the code project FRGS/1/2019/SKK10/UPM/02/2 and reference number 04-01-19-2133FR under Universiti Putra Malaysia.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Akhtar, N., Akram, M., Daniyal, M., and Ahmad, S. (2018). Evaluation of antidiabetic activity of Ipomoea batatas L. extract in alloxan-induced diabetic rats. Int. J. Immunopathol. Pharmacol. 32, 2058738418814678. doi:10.1177/2058738418814678
Alam, M. K. (2021). A comprehensive review of sweet potato (Ipomoea batatas [L.] Lam): Revisiting the associated health benefits. Trends Food Sci. Technol. 115, 512–529. doi:10.1016/J.TIFS.2021.07.001
Bahari, H., Abidin, A. Z., Balan, S. S., Perumal, K. V., Rosli, N. S., Lotafi, A. H. A., et al. (2020). The effects of Elateriospermum tapos against obese maternal rat in mitigating obesity development among their adult female offspring. Pharmacogn. Mag. 16, 706–712. doi:10.4103/pm.pm_142_20
Balan, S. S., Abidin, A. Z., Perumal, K. V., Shafie, N. H., Abdullah, M. A., Jasni, A. S., et al. (2021). Transgenerational evaluation of Elateriospermum tapos extracts on the male offspring of obesity-induced sprague dawley rats. Sains Malays. 50, 3045–3057. doi:10.17576/jsm-2021-5010-17
Escobar-Puentes, A. A., Palomo, I., Rodríguez, L., Fuentes, E., Villegas-Ochoa, M. A., González-Aguilar, G. A., et al. (2022). Sweet potato (Ipomoea batatas L.) phenotypes: From agroindustry to health effects. Foods 11, 1058. doi:10.3390/foods11071058
Fu, Z. F., Tu, Z. C., Zhang, L., Wang, H., Wen, Q. H., and Huang, T. (2016). Antioxidant activities and polyphenols of sweet potato (Ipomoea batatas L.) leaves extracted with solvents of various polarities. Food Biosci. 15, 11–18. doi:10.1016/j.fbio.2016.04.004
Gunn, J., Che, C. T., and Farnsworth, N. (2013). in Diabetes and natural products. Bioactive Food as dietary Interventions for diabetes. Editors R. R. Watson, and V. R. Preedy (Chicago, IL, USA: Academic Press), 381–394. 978-0-12-397153-1.
Kamisan, F. H., Yahya, F., Ismail, N. A., Din, S. S., Mamat, S. S., Zabidi, Z., et al. (2012). Hepatoprotective activity of methanol extract of Melastoma malabathricum leaf in rats. J. Acupunct. Meridian Stud. 6, 52–55. doi:10.1016/J.JAMS.2012.08.002
Khairani, A. F., Pamela, Y., Oktavia, N., Achadiyani, A., Yusuf Adipraja, M., Zhafira, P. Y., et al. (2022). Acute and sub-chronic oral toxicity study of purple sweet potato (Ipomoea batatas [L.] Lam) yogurt in mice (Mus musculus). Vet. World 15, 789–796. doi:10.14202/vetworld.2022.789-796
Kim, D. C., Won, S. I., and In, M. J. (2015). Substitution effect of enzymatically hydrolyzed purple sweet potato powder on skim milk in yogurt preparation. J. Appl. Biol. Chem. 58, 311–316. doi:10.3839/JABC.2015.049
Makwana, H., and Pandya, D. (2020). Bioactivity-guided isolation, characterization, and estimation of esculetin – a Potential marker from Launaea pinnatifida cass. Pharmacogn. Mag. 16, 713. doi:10.4103/PM.PM_264_20
Meunier, L., and Larrey, D. (2019). Drug-induced liver injury: Biomarkers, requirements, candidates, and validation. Front. Pharmacol. 0, 1482. doi:10.3389/FPHAR.2019.01482
Mohanraj, R., and Sivasankar, S. (2014). Sweet potato (Ipomoea batatas [L.] Lam) - a valuable medicinal food: A review. J. Med. Food 17, 733–741. doi:10.1089/jmf.2013.2818
Naomi, R., Bahari, H., Yazid, M. D., Othman, F., Zakaria, Z. A., and Hussain, M. K. (2021). Potential effects of sweet potato (Ipomoea batatas) in hyperglycemia and dyslipidemia—a systematic review in diabetic retinopathy context. Int. J. Mol. Sci. 22, 10816. doi:10.3390/IJMS221910816
Nguyen, H. C., Chen, C. C., Lin, K. H., Chao, P. Y., Lin, H. H., and Huang, M. Y. (2021). Bioactive compounds, antioxidants, and health benefits of sweet potato leaves. Molecules 26, 1820–1913. doi:10.3390/molecules26071820
Noonan, S. C., and Savage, G. P. (1999). Oxalate content of foods and its effect on humans. Asia Pac. J. Clin. Nutr. 8, 64–74. doi:10.1046/j.1440-6047.1999.00038.x
Peter, A. I., Naidu, E. C. S., Akang, E., Ogedengbe, O. O., Offor, U., Rambharose, S., et al. (2018). Investigating organ toxicity profile of tenofovir and tenofovir nanoparticle on the liver and kidney: Experimental animal study. Toxicol. Res. 34, 221–229. doi:10.5487/TR.2018.34.3.221
Sahli, M. W., Mares, J. A., Meyers, K. J., Klein, R., Brady, W. E., Klein, B. E. K., et al. (2015). Dietary intake of lutein and diabetic retinopathy in the atherosclerosis risk in communities study (ARIC). Ophthalmic Epidemiol. 23, 99–108. doi:10.3109/09286586.2015.1129426
Shekhar, S., Mishra, D., Buragohain, A. K., Chakraborty, S., and Chakraborty, N. (2014). Comparative analysis of phytochemicals and nutrient availability in two contrasting cultivars of sweet potato (Ipomoea batatas L.). Food Chem. 173, 957–965. doi:10.1016/J.FOODCHEM.2014.09.172
Strugała, P., Dzydzan, O., Brodyak, I., Kucharska, A. Z., Kuropka, P., Liuta, M., et al. (2019). Antidiabetic and antioxidative potential of the blue Congo variety of purple potato extract in streptozotocin-induced diabetic rats. Molecules 24, 3126. doi:10.3390/MOLECULES24173126
Sun, H., Mu, T., Xi, L., Zhang, M., and Chen, J. (2014). Sweet potato (Ipomoea batatas L.) leaves as nutritional and functional foods. Food Chem. 156, 380–389. doi:10.1016/j.foodchem.2014.01.079
Tempelhoff, G. F. Von., Schelkunov, O., Demirhan, A., Tsikouras, P., Rath, W., Velten, E., et al. (2016). Correlation between blood rheological properties and red blood cell indices (MCH, MCV, MCHC) in healthy women. Clin. Hemorheol. Microcirc. 62, 45–54. doi:10.3233/CH-151944
Yuzhi Jiao Studies on antioxidant capacity of anthocyanin extract from purple sweet potato (Ipomoea batatas L.). Afr. J. Biotechnol. 2012, 11, 7046–7054. doi:10.5897/ajb11.3859
Keywords: medicinal plant, purple sweet potato, plant extract, phytochemicals, bioactive compounds, toxicity, animal study
Citation: Hisamuddin AS-dB, Naomi R, Bin Manan KA, Bahari H, Yazid MD, Othman F, Embong H, Hadizah Jumidil S, Hussain MK and Zakaria ZA (2023) Phytochemical component and toxicological evaluation of purple sweet potato leaf extract in male Sprague–Dawley rats. Front. Pharmacol. 14:1132087. doi: 10.3389/fphar.2023.1132087
Received: 26 December 2022; Accepted: 03 March 2023;
Published: 03 April 2023.
Edited by:
Swaran J. S. Flora, National Institute of Pharmaceutical Education and Research, IndiaReviewed by:
Ravindra M. Samartha, Bhopal Memorial Hospital and Research Centre, IndiaArmando Caceres, Galileo University, Guatemala
Copyright © 2023 Hisamuddin, Naomi, Bin Manan, Bahari, Yazid, Othman, Embong, Hadizah Jumidil, Hussain and Zakaria. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohd Khairi Hussain, a2hhaXJpQHVwbS5lZHUubXk=; Zainul Amiruddin Zakaria, emF6QHVtcy5lZHUubXk=
 Ahmad Safiyyu’d-din Bin Hisamuddin1,2
Ahmad Safiyyu’d-din Bin Hisamuddin1,2 Hasnah Bahari
Hasnah Bahari Muhammad Dain Yazid
Muhammad Dain Yazid Hashim Embong
Hashim Embong Zainul Amiruddin Zakaria
Zainul Amiruddin Zakaria