
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pharmacol. , 14 November 2023
Sec. Pharmacology of Anti-Cancer Drugs
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1131703
This article is part of the Research Topic Stevens Johnson Syndrome: Past, Present, and Future Directions View all 11 articles
Background: The treatment paradigm for advanced non-small-cell lung cancer (NSCLC) is rapidly changing. Epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) and anti-programmed death-1 (PD-1) antibodies have increasingly been incorporated into routine care for nearly all patients with NSCLC. Toripalimab was recently approved as the first-line treatment for advanced non-squamous NSCLC in combination with chemotherapy. Stevens–Johnson syndrome (SJS) is a rare but potentially fatal complication of TKI and anti-PD-1 therapy. We reported a case of SJS after sequential use of EGFR-TKIs and toripalimab in an NSCLC patient with EGFR mutations 19 del/T790M/C797S in trans and cis.
Case presentation: A 58-year-old man with stage IV NSCLC received gefitinib because next-generation sequencing (NGS) revealed an EGFR 19del, followed by osimertinib and pemetrexed with the emergence of EGFR T790M. Four EGFR mutations 19 del/T790M/C797S in trans and cis were detected after osimertinib resistance. The combination of toripalimab and docetaxel was administered as a third-line treatment. The patient developed SJS at 21 days, and toripalimab was discontinued. After treatment with methylprednisolone and prednisolone, the skin toxicity of the patient gradually decreased and eventually disappeared. The patient received osimertinib and anlotinib after recovery, and SJS has not recurred. The ongoing treatment is still effective and results in stable disease.
Conclusion: We reported the first case of SJS induced by toripalimab in a patient with lung adenocarcinoma harboring multiple EGFR mutations. The TKI treatment after SJS was well tolerated and effective.
Lung adenocarcinoma is one of the most common types of non-small-cell lung cancer (NSCLC). The treatment paradigm for advanced NSCLC is rapidly changing. Epidermal growth factor receptor (EGFR) is the most common driver genes of lung cancer, and EGFR tyrosine kinase inhibitors (TKIs), such as gefitinib, osimertinib, and anlotinib, dramatically improve the clinical outcomes of EGFR mutant lung cancers (Han et al., 2018; Soria et al., 2018; Hosomi et al., 2020). At the same time, immune checkpoint inhibitors (ICIs), such as anti-programmed death-ligand-1 (PD-(L)1) monoclonal antibodies, have increasingly been incorporated into routine care for nearly all patients with NSCLC. Pembrolizumab was approved as the front-line therapy with or without chemotherapy in patients with metastatic NSCLC (Reck et al., 2016). Nivolumab plus ipilimumab was approved as a first-line treatment for NSCLC patients by the Food and Drug Administration (Hellmann et al., 2019). Toripalimab, an anti-PD-1 antibody, significantly improved both progression-free survival (PFS) and overall survival (OS) with chemotherapy in patients with advanced NSCLC with a manageable safety profile (Wang et al., 2023).
However, cutaneous eruptions are one of the most common immune-related adverse events, including lichenoid reactions, eczema, and vitiligo (Hwang et al., 2016), most of which are mild. Stevens–Johnson syndrome (SJS) is a rare but life-threatening cutaneous adverse reaction, mainly elicited by exposure to certain drugs including EGFR-TKIs and ICIs (Chen et al., 2018). There is growing concern that the combination of PD-(L)1 and EGFR-TKIs may be associated with an increased risk of toxicity. It is reported that PD-(L)1 blockade followed by osimertinib is associated with severe immune-related adverse events (Schoenfeld et al., 2019). There were no reports of SJS in a patient treated with toripalimab and EGFR-TKIs. Here, we reported the first case of SJS induced by toripalimab in a previously EGFR-TKI-treated advanced lung adenocarcinoma patient harboring multiple EGFR mutations.
A 58-year-old male non-smoker presented to our hospital complaining of persistent pain in the lower back in January 2019. He had no existing physical health issues and no special underlying diseases. The family medical history was unremarkable. The enhanced computerized tomography (CT) scan of the chest and lumbar spine revealed multiple nodules in both the lungs and spinal lesions (Figure 1A). A CT-guided percutaneous needle biopsy was performed. The pathological examination showed lung adenocarcinoma. Together, these results suggested the clinical stage was classified as cT4N3M1, stage IV (TNM classification seventh edition). The patient performance status (PS) was 1. Next-generation sequencing (NGS) identified EGFR exon 19 deletion (19 del) with a mutant allele frequency (MAF) of 60.2%. The patient was begun on gefitinib 250 mg once daily in March 2019. Partial response (PR) was achieved with 3 months’ treatment based on the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 (Figure 1B), and the PFS was 16 m. New lesions were seen in the left lower lobe (Figure 1C), and gefitinib was discontinued. Blood-based NGS detected EGFR T790M (MAF 3.4%) and the retention of EGFR 19 del (MAF 5.99%). Osimertinib (oral) and pemetrexed (0.9 g, iv, q3w) were administered in July 2020, and stable disease (SD) was achieved with a PFS of 14 m. The patient complained of pain in the lower back accompanied by numbness in the lower leg in August 2021. The spine magnetic resonance imaging (MRI) revealed more spinal lesions, and the chest CT scan showed the lung nodules were stable (Figure 1D).
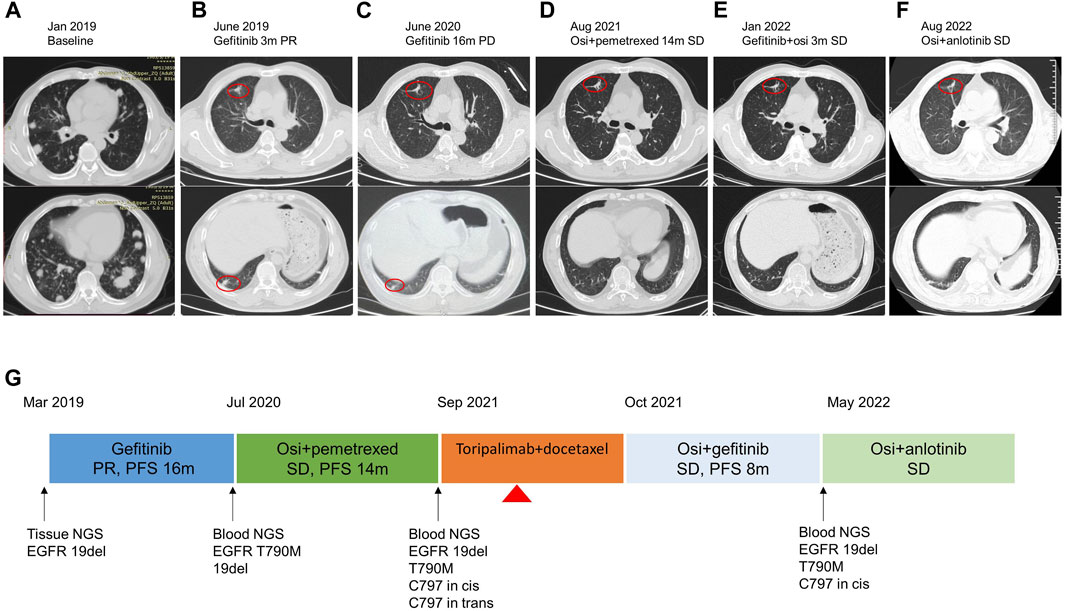
FIGURE 1. Treatment timeline of the patient. (A–F) The chest CT images at each time point. The red circle indicated the tumor lesion. (G) Flow diagram of the clinical course of the patient. Osi, osimertinib; PR, partial response; PD, progressive disease; SD, stable disease; PFS, progression-free survival. The red triangle indicates the SJS onset on day 12 since treatment with toripalimab plus docetaxel.
NGS targeting eight core lung cancer driver genes (Lung Cure, Burning Rock Biotech, Guangzhou, China) was performed on blood samples. EGFR mutations 19 del/T790M/C797S in trans and cis were detected, with MAF of 1.7%, 0.44%, 0.16%, and 0.14%, respectively. In a phase-II trial, toripalimab plus chemotherapy showed promising anti-tumor activity as the second-line setting in patients with EGFR-mutant NSCLC (Jiang et al., 2021). In September 2021, the patient received toripalimab 240 mg and docetaxel 120 mg as the third-line therapy.
The patient began to develop oral ulcers and scattered rash on the 12th day since treatment with toripalimab plus docetaxel, and the rash gradually worsened. He had not received treatment for the skin reactions before visiting our hospital on day 24. He presented with multiple macules and vesicles, and detachment of the epidermis on the mucous membranes of the mouth, face, and body trunk (Figure 2A). He had no fever, and the PS was 1. Routine blood examinations were normal. Bacterial cultures from blood, urine, and sputum revealed no evidence of bacterial infection. Skin biopsy showed a sub-epidermal cell poor blister and perivascular infiltrate of lymphocytes (Figure 2B). Throughout the course of the disease, there were no other organ function abnormalities. He had normal levels of alanine aminotransferase, aspartate aminotransferase, creatinine, urea nitrogen, cardiac enzymes, or brain natriuretic peptide. The patient was diagnosed as SJS with a severity-of-illness score for toxic epidermal necrolysis (SCORTEN) as 4 (Supplementary Table S1). In terms of pharmacogenetic assessments, we performed human leukocyte antigen (HLA) typing using NGS, which revealed HLA-A*24:02, HLA-A*11:01, HLA-B*40:01, HLA-B*15:01, HLA-C*04:01, and HLA-C*03:04.
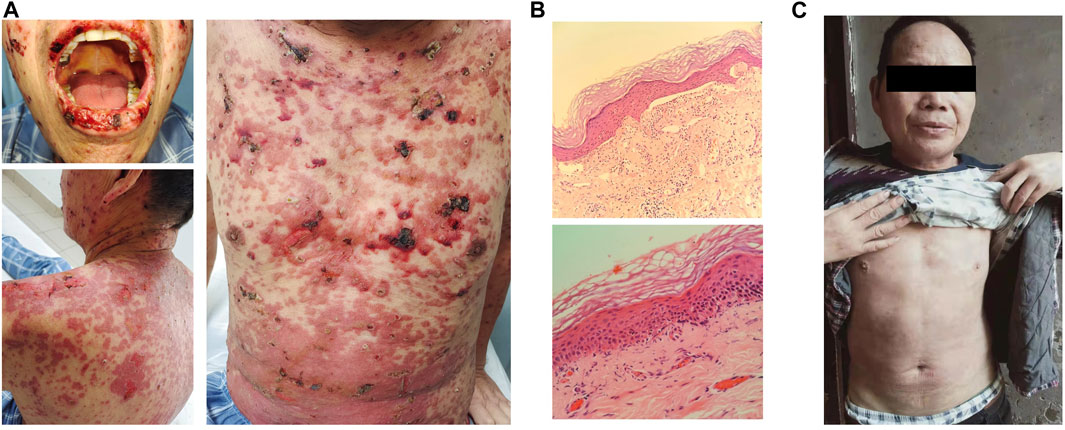
FIGURE 2. Diagnosis and treatment of Stevens–Johnson syndrome. (A) Erosions were seen on the mouth, face, and body trunk after 24 days of toripalimab treatment. (B) Hematoxylin and eosin staining of skin biopsy. Original magnifications, the upper panel × 40 and the lower panel × 100. (C) Reduction in diffuse erythema at 6 weeks of steroid therapy.
Toripalimab and docetaxel were discontinued immediately. A dermatologist was consulted for the diagnosis of skin symptoms. The patient presented with diffuse erythema, and vesicles and ulcerations on extremities, the trunk, oral cavity, throat, nose, eyelids, and genitalia, which were accompanied by skin detachment and tissue necrosis. These manifestations were consistent with SJS. We also checked serum levels of several autoantibodies, including anti-BM antibody, anti-AD antibody, and anti-EC antibody, all of which were negative. Taking into account the clinical and pathological manifestations and the medication history, we arrived at the diagnosis of SJS. The patient was treated with 100 mg/day of methylprednisolone on 10 October 2021 for 1 week, followed by 90 mg/day of prednisolone for 3 weeks. Prednisolone was tapered off and eventually discontinued after 2 months. The patient recovered from SJS after steroid therapy (Figure 2C). During this period, the patient took medicine at home, and the local broken surface was disinfected with iodine and covered with dry gauze to keep the wound dry. He did not experience any concurrent infection.
Afterward, the patient received spine stereotactic body radiotherapy (SBRT) to treat spinal metastases. The combination of gefitinib and osimertinib was administered between October 2021 and May 2022, and SD was achieved with a PFS of 8 m (Figure 1E). A follow-up CT scan revealed stable lung nodules but more spinal lesions, which led to pathological bone fracture and paraplegia. NGS targeting 520 cancer-related genes (OncoScreen Plus, Burning Rock Biotech, Guangzhou, China) was performed on blood samples in May 2022 and revealed mutations of EGFR 19 del/T790M/C797S in cis, with MAF of 18.9%, 3.53%, and 3.06%. The patient received osimertinib 80 mg and anlotinib 12 mg in May 2022. A CT scan showed the shrinkage of the lung tumors (Figure 1F) and stable lesions of the spine after 2 months. The patient declined the surgery for spinal metastasis because of financial concerns. He is still treated with osimertinib and anlotinib, and SJS has not recurred. The clinical course is shown in Figure 1G.
Therapeutic anti-PD-1/PD-L1 monoclonal antibodies, such as toripalimab, are important in treatments for patients with advanced NSCLC (Wang et al., 2023). We found that the sequential use of toripalimab and osimertinib was associated with SJS. Importantly, the toxicity appeared associated with toripalimab, given the fact that SJS has not recurred after osimertinib was rechallenged. The patient recovered after steroid treatment and benefited from the EGFR-TKI treatment that was followed. His OS was more than 44 months at the time of preparation the manuscript.
Skin reaction is one of the common adverse reactions of immune checkpoint inhibitors, and once it occurs, it needs to be discontinued permanently. However, with prednisone pre-treatment before using docetaxel, the probability of severe skin adverse reactions is very low, and delayed skin reaction after 1 week of drug use is rare. Based on previous clinical experience and other case reports, it is considered that the patient’s SJS is an adverse reaction to immune checkpoint inhibitors rather than to docetaxel. The incidence of SJS was low, but the lethality was extremely high. Although uncommon, SJS related to anti-PD1 in NSCLC has also been reported, such as pembrolizumab (Saw et al., 2017), atezolizumab (Chirasuthat and Chayavichitsilp, 2018), and ipilimumab (Dika et al., 2017). It has been reported that tumor tissues in NSCLC and skin shared similar antigens; thus, in patients treated with PD-1/PD-L1 antibodies, activated T cells may attack skin tissues as well, causing skin-related immune-related adverse events (irAEs) (Berner et al., 2019). The underlying mechanism of Stevens–Johnson syndrome/toxic epidermal necrolysis (TEN) associated with PD-1/PD-L1 and other drugs may be different. It is hypothesized that small-molecule drugs may bind to proteins in the serum, forming a complex that is recognized by certain HLA molecules and presented to T cells to generate an immune response (Frantz et al., 2021). However, in patients treated with PD-1/PD-L1 antibodies, the immune response is enhanced by the blockade of PD-1 and PD-L1 interaction instead of directly presenting PD-1/PD-L1 antibodies to T cells.
SJS could occur from 1 week to 5 months after the initiation of ICIs, which was usually 1–2 cycles of treatment (Chen et al., 2018). In our case, SJS started to manifest on day 12 since the start of toripalimab plus docetaxel administration. NGS detected HLA-A*24:02, HLA-A*11:01, HLA-B*40:01, HLA-B*15:01, HLA-C*04:01, and HLA-C*03:04 in this case. Because associations between SJS/TEN and certain human leukocyte antigen (HLA) variants have been identified, molecular diagnosis can help to confirm the diagnosis of SJS/TEN. A meta-analysis of Chinese, Korean, and Thai populations found HLA-A*24:02 associated with the susceptibility to SJS/TEN or mild maculopapular eruptions as lamotrigine-induced cutaneous adverse drug reactions (Deng et al., 2018). A study in the Japanese population also identified significant associations between HLA-A*24:02:01 and susceptibility to cold medicine-related SJS/TEN with severe ocular complications (Nakatani et al., 2019). It is possible that the HLA-A*24:02 allele in our patient conferred susceptibility to SJS upon treatment with toripalimab combined with docetaxel.
Toripalimab is a humanized monoclonal antibody and the first domestically approved anti-PD-1 monoclonal antibody in China. The pharmacokinetic (PK) characteristics of toripalimab within the dose range of 1–10 mg/kg showed that Cmax exhibited generally linear PK characteristics, and the increase in area under the curve was slightly greater than the increase in the dosage. The mean clearance rate of toripalimab was 0.18 mL/h/kg (co-efficient of variation%: 37%), and the geometric mean elimination half-life (t1/2) was 12.6 days (co-efficient of variation %: 29%). Toripalimab was degraded through non-specific pathways, and its metabolism was independent of clearance. As monoclonal antibodies are not metabolized by cytochrome P450 enzymes or other drug-metabolizing enzymes, the inhibition or induction of these enzymes by concomitant drugs is not expected to affect the PK of toripalimab. Docetaxel is a taxane that can form a stable, non-functional microtubule bundle by strengthening microtubule polymerization and inhibiting microtubule depolymerization, thereby breaking down tumor cell mitosis to achieve an antitumor effect. Clinical pharmacologic studies have confirmed that docetaxel’s antitumor activity is stronger than that of paclitaxel, and there is no cross-resistance with paclitaxel. It is used for the treatment of advanced or metastatic NSCLC after first-line chemotherapy failure. Chemotherapy in combination with immunotherapy has become one of the standard treatment regimens for lung cancer. Many phase III clinical studies of immunotherapy checkpoint inhibitors in the field of lung cancer have adopted paclitaxel in combination with platinum as the basis of chemotherapy regimen. Therefore, there are high-level safety data and evidence-based medical evidence for the combination of docetaxel and PD-1 inhibitors. Considering the patient’s economic burden, the relatively low-priced toripalimab was selected as the second-line treatment among the available immunotherapy checkpoint inhibitors.
Management principles of SJS include urgent inpatient evaluation/specialist support, prognostication with tools such as SCORTEN, withdrawal of culprit drug, and supportive care (Saw et al., 2017). The ideal management of severe anti-PD-1-related skin toxicities needs to be clarified. Intravenous prednisone/methylprednisolone 1–2 mg/kg/day and intravenous immunoglobulin are necessary.
The concomitant EGFR T790M/C797S in trans and cis is rare, with a poor prognosis (Liu et al., 2019). Previous studies showed that patients harboring EGFR C797S in trans with T790M are sensitive to a combination of first- and third-generation EGFR TKIs (Wang et al., 2017). However, patients harboring EGFR C797S in cis with T790M are resistant to combination therapy or every single reagent. In our case, the patient was sensitive to the combination of gefitinib and osimertinib, and the PFS was 8 m, indicating that gefitinib plus osimertinib might be an effective therapy for patients with EGFR T790M/C797S in trans and cis.
In the present case report, we provided timely treatment and discontinued the use of toripalimab when the diagnosis of SJS was made. However, we cannot fully exclude the possibility that SJS was caused by the administration of docetaxel. In the future, clinical usage of PD-1/PD-L1 antibodies in NSCLC patients harboring EGFR mutations needs to be cautious, and close attention should be paid to identify potential severe adverse events.
We reported the first case of SJS induced by toripalimab in a patient with lung adenocarcinoma harboring multiple EGFR mutations, and the TKI treatment after SJS was well tolerated and effective. Our case report gave additional cautions to observe possible life-threatening cutaneous reactions to toripalimab therapy in NSCLC patients with EGFR mutations.
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
The study was approved by The Ethics Committee of Changzheng Hospital, Naval Medical University. The study was conducted in accordance with the local legislation and institutional requirements. The participant provided his written informed consent to participate in this study. Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article.
All authors participated in study conceptualization, data collection and analysis, and manuscript draft and revision. HT provided project supervision. All authors contributed to the article and approved the submitted version.
This work was supported by grants from the General Program of the National Nature Science Foundation of China (No. 82070036).
The authors thank the patient for agreeing to publish this report and thank Mr. Mingjun Zhu, and Drs. Chunxiao Pan and Xiao Zou from Burning Rock Biotech for their help in sequencing. The authors also thank Shanghai Municipal Hospital Respiratory and Critical Care Medicine Specialist Alliance.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1131703/full#supplementary-material
Berner, F., Bomze, D., Diem, S., Ali, O. H., Fassler, M., Ring, S., et al. (2019). Association of checkpoint inhibitor-induced toxic effects with shared cancer and tissue antigens in non-small cell lung cancer. JAMA Oncol. 5, 1043–1047. doi:10.1001/jamaoncol.2019.0402
Chen, C. B., Wu, M. Y., Ng, C. Y., Lu, C. W., Wu, J., Kao, P. H., et al. (2018). Severe cutaneous adverse reactions induced by targeted anticancer therapies and immunotherapies. Cancer Manag. Res. 10, 1259–1273. doi:10.2147/CMAR.S163391
Chirasuthat, P., and Chayavichitsilp, P. (2018). Atezolizumab-induced stevens-johnson syndrome in a patient with non-small cell lung carcinoma. Case Rep. Dermatol 10, 198–202. doi:10.1159/000492172
Deng, Y., Li, S., Zhang, L., Jin, H., and Zou, X. (2018). Association between HLA alleles and lamotrigine-induced cutaneous adverse drug reactions in Asian populations: a meta-analysis. Seizure 60, 163–171. doi:10.1016/j.seizure.2018.06.024
Dika, E., Ravaioli, G. M., Fanti, P. A., Piraccini, B. M., Lambertini, M., Chessa, M. A., et al. (2017). Cutaneous adverse effects during ipilimumab treatment for metastatic melanoma: a prospective study. Eur. J. Dermatol 27, 266–270. doi:10.1684/ejd.2017.3023
Frantz, R., Huang, S., Are, A., and Motaparthi, K. (2021). Stevens-johnson syndrome and toxic epidermal necrolysis: a review of diagnosis and management. Med. Kaunas. 57, 895. doi:10.3390/medicina57090895
Han, B., Li, K., Wang, Q., Zhang, L., Shi, J., Wang, Z., et al. (2018). Effect of anlotinib as a third-line or further treatment on overall survival of patients with advanced non-small cell lung cancer: the ALTER 0303 phase 3 randomized clinical trial. JAMA Oncol. 4, 1569–1575. doi:10.1001/jamaoncol.2018.3039
Hellmann, M. D., Paz-Ares, L., Bernabe Caro, R., Zurawski, B., Kim, S. W., Carcereny Costa, E., et al. (2019). Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N. Engl. J. Med. 381, 2020–2031. doi:10.1056/NEJMoa1910231
Hosomi, Y., Morita, S., Sugawara, S., Kato, T., Fukuhara, T., Gemma, A., et al. (2020). Gefitinib alone versus gefitinib plus chemotherapy for non-small-cell lung cancer with mutated epidermal growth factor receptor: NEJ009 study. J. Clin. Oncol. 38, 115–123. doi:10.1200/JCO.19.01488
Hwang, S. J., Carlos, G., Wakade, D., Byth, K., Kong, B. Y., Chou, S., et al. (2016). Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: a single-institution cohort. J. Am. Acad. Dermatol 74, 455–461. doi:10.1016/j.jaad.2015.10.029
Jiang, T., Wang, P., Zhang, J., Zhao, Y., Zhou, J., Fan, Y., et al. (2021). Toripalimab plus chemotherapy as second-line treatment in previously EGFR-TKI treated patients with EGFR-mutant-advanced NSCLC: a multicenter phase-II trial. Signal Transduct. Target Ther. 6, 355. doi:10.1038/s41392-021-00751-9
Liu, C., Li, J., Liu, H., Du, R., Fang, N., Zhang, J., et al. (2019). The concomitant EGFR T790M/C797S in trans and cis in three osimertinib-resistant lung adenocarcinoma patients. J. Clin. Oncol. 37, e20105. doi:10.1200/jco.2019.37.15_suppl.e20105
Nakatani, K., Ueta, M., Khor, S.-S., Hitomi, Y., Okudaira, Y., Masuya, A., et al. (2019). Identification of HLA-A*02:06:01 as the primary disease susceptibility HLA allele in cold medicine-related Stevens-Johnson syndrome with severe ocular complications by high-resolution NGS-based HLA typing. Sci. Rep. 9, 16240. doi:10.1038/s41598-019-52619-2
Reck, M., Rodríguez-Abreu, D., Robinson, A. G., Hui, R., Csőszi, T., Fülöp, A., et al. (2016). Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 375, 1823–1833. doi:10.1056/NEJMoa1606774
Saw, S., Lee, H. Y., and Ng, Q. S. (2017). Pembrolizumab-induced Stevens-Johnson syndrome in non-melanoma patients. Eur. J. Cancer 81, 237–239. doi:10.1016/j.ejca.2017.03.026
Schoenfeld, A. J., Arbour, K. C., Rizvi, H., Iqbal, A. N., Gadgeel, S. M., Girshman, J., et al. (2019). Severe immune-related adverse events are common with sequential PD-(L)1 blockade and osimertinib. Ann. Oncol. 30, 839–844. doi:10.1093/annonc/mdz077
Soria, J. C., Ohe, Y., Vansteenkiste, J., Reungwetwattana, T., Chewaskulyong, B., Lee, K. H., et al. (2018). Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N. Engl. J. Med. 378, 113–125. doi:10.1056/NEJMoa1713137
Wang, Z., Wu, L., Li, B., Cheng, Y., Li, X., Wang, X., et al. (2023). Toripalimab plus chemotherapy for patients with treatment-naive advanced non–small-cell lung cancer: a multicenter randomized phase III trial (CHOICE-01). J. Clin. Oncol. 41, 651–663. doi:10.1200/JCO.22.00727
Wang, Z., Yang, J. J., Huang, J., Ye, J. Y., Zhang, X. C., Tu, H. Y., et al. (2017). Lung adenocarcinoma harboring EGFR T790M and in trans C797S responds to combination therapy of first- and third-generation EGFR TKIs and shifts allelic configuration at resistance. J. Thorac. Oncol. 12, 1723–1727. doi:10.1016/j.jtho.2017.06.017
Keywords: Stevens–Johnson syndrome, adverse reaction, toripalimab, non-small-cell lung cancer, EGFR tyrosine kinase inhibitor
Citation: Chen Y, Hong H, Bao S and Tang H (2023) Stevens–Johnson syndrome induced by toripalimab in a previously EGFR-TKI-treated advanced lung adenocarcinoma patient harboring EGFR mutations 19 del/T790M/C797S in trans and cis: a case report. Front. Pharmacol. 14:1131703. doi: 10.3389/fphar.2023.1131703
Received: 26 December 2022; Accepted: 25 October 2023;
Published: 14 November 2023.
Edited by:
Mounir Tilaoui, Waterford Institute of Technology, IrelandReviewed by:
Siu Fun Wong, Chapman University, United StatesCopyright © 2023 Chen, Hong, Bao and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao Tang, dGFuZ2hhb18wOTIxQDEyNi5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.