
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 15 March 2023
Sec. Pharmacoepidemiology
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1129730
This article is part of the Research TopicImmune-related Adverse Events (irAEs) of BiopharmaceuticalsView all 7 articles
 Shuang Xia1,2,3
Shuang Xia1,2,3 Hui Gong1,2,3
Hui Gong1,2,3 Yi-kun Wang1,2,3
Yi-kun Wang1,2,3 Ling Liu4
Ling Liu4 Yi-chang Zhao1,2,3
Yi-chang Zhao1,2,3 Lin Guo1,2,3
Lin Guo1,2,3 Bi-kui Zhang1,2,3
Bi-kui Zhang1,2,3 Mayur Sarangdhar5,6,7
Mayur Sarangdhar5,6,7 Yoshihiro Noguchi8
Yoshihiro Noguchi8 Miao Yan1,2,3*
Miao Yan1,2,3*Background: Pneumocystis jirovecii pneumonia (PJP) has been reported with ICIs but limited to case reports. The clinical features of PJP with ICIs remain mostly unknown. This study aims to investigate the association of PJP with ICIs and describe clinical features.
Methods: Reports of PJP recorded in FAERS (January 2004–December 2022) were identified through the preferred term “Pneumocystis jirovecii pneumonia”. Demographic and clinical features were described, and disproportionality signals were assessed through the Reporting Odds Ratio (ROR) and Information Component (IC), using traditional chemotherapy and targeted therapy as comparators, and adjusting signals by excluding contaminant immunosuppressive drugs and pre-existing diseases. A systematic literature review was conducted to describe clinical features of published PJP reports with ICIs. Bradford Hill criteria was adopted for global assessment of the evidence.
Results: We identified 677 reports of PJP associated with ICIs, in which 300 (44.3%) PJP cases with fatal outcome. Nivolumab (IC025 2.05), pembrolizumab (IC025 1.88), ipilimumab (IC025 1.43), atezolizumab (IC025 0.36), durvalumab (IC025 1.65), nivolumab plus ipilimumab (IC025 1.59) have significant signals compared to other drugs in FAERS database. After excluding pre-existing diseases and immunosuppressive agents which may increase susceptibility of PJP, the signals for PJP associated with nivolumab, pembrolizumab, durvalumab, nivolumab plus ipilimumab remained robust (IC025 > 0). When compared to other anticancer regimens, although all ICIs showed a lower disproportionate signal for PJP than chemotherapy, nivolumab (IC025 0.33, p < 0.001), pembrolizumab (IC025 0.16, p < 0.001), both PD-1 inhibitors, presented a higher signal for PJP than targeted therapy. Male gender (IC025 0.26, p < 0.001) and age >65 years (IC025 0.38, p < 0.001) were predominant in PJP cases associated with across all ICIs. In literature, 15 PJP cases associated with ICIs were reported in 10 published case reports. 12 of 15 (80.0%) of cases received PD-1 inhibitors before PJP was diagnosed.
Conclusion: By the combined analysis of post-marketing data from FAERS and published case reports, we identified ICIs may be associated with PJP, especially in males aged >65years. After accounting for confounders, PD-1 inhibitors emerged with a robust disproportionality signal when compared to PD-L1/CTLA-4 inhibitors as well as targeted therapy. Further research is warranted to validate our findings.
Immune checkpoint inhibitors (ICIs) have dramatically changed the treatment landscape of many types of malignancies. As of November 2022, FDA has approved 10 ICI agents including PD-1 inhibitors: nivolumab, pembrolizumab, cemiplimab, dostarlimab, PD-L1 inhibitors: atezolizumab, avelumab, durvalumab, CTLA-4 inhibitors: ipilimumab and tremelimumab, LAG-3 inhibitors: relatlimab. These ICIs generate durable efficacy in certain types of cancer, however, there is a significant concern ICIs-mediated toxicities. Toxic effects from these ICI agents are related to removing nodes of self-tolerance and unleashing autoimmune-like phenomena (Postow et al., 2018). Although usually manageable with corticosteroid and immunosuppressants administration, clinically severe events leading to morbidity and even mortality may complicate ICI treatment (Wang et al., 2018).
Pneumocystis jirovecii pneumonia is a form of pneumonia that is caused by the yeast-like fungus Pneumocystis jirovecii. A recent study (Lee et al., 2019) showed that the 3-month mortality rate of lung cancer patients with the PJP was as high as 61.6%, suggesting the severity of PJP infection in lung cancer patients. Moreover, the Infectious Diseases Working Party (AGIHO) of the German Society for Hematology and Medical Oncology (DGHO) recommended that primary prophylaxis of Pneumocystis jirovecii pneumonia in patients with hematologic malignancies and solid tumors are warranted (Classen et al., 2021). To the best of our knowledge, only a few case reports/series have described Pneumocystis jirovecii pneumonia (PJP) during pembrolizumab (Liu et al., 2020; Sadek et al., 2020; Rath et al., 2021; Si et al., 2021; Hiba et al., 2022; Sanka and Hsu, 2023), nivolumab (Schwarz et al., 2019), ipilimumab (Arriola et al., 2015; Finbar Slevin et al., 2016; Sadek et al., 2020), nivolumab plus ipilimumab (Moujaess et al., 2020) and other anti-PD-1 inhibitors (Liu et al., 2020; Feng et al., 2021).
However, case reports can only provide a partial epidemiological perspective. There are no meta-analysis or systematic reviews to investigate the association of PJP and ICIs. In addition, the clinical manifestations of PJP in patients on ICIs are not well known. The analysis of spontaneous reporting databases, such as the FDA Adverse Even Reporting System (FAERS) and WHO Vigibase, allows a broader perspective by collecting unpublished reports of adverse events submitted all over the world occurring in real-world unselected subjects with comorbidities, poly-pharmacotherapy and complex anticancer combination regimens (Raschi et al., 2020), which ensures rapid detection of even rare adverse events. Previous disproportionality analysis based on the FAERS database indicated that interstitial lung disease (Guo et al., 2023), pulmonary tuberculosis (Zhu et al., 2022a) and pneumonitis (Cui et al., 2022) were found to be significantly associated with ICIs exposure. But it is still unclear if ICIs were associated with increased reporting frequency of PJP in the real-world clinical setting.
Herein, we conducted a large-scale pharmacovigilance study by using the US Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) database to investigate the link between PJP and ICIs. A systematic literature review was conducted to incorporate the information from published papers and compare with post-marketing data from FAERS database.
FAERS is one of the largest publicly available databases designed to support the United States Food and Drug Administration (FDA) post-marketing safety surveillance program, which gathering more than 20 million reports worldwide, including the United States, Europe, and Asia. It allows for the signal detection and quantification of the association between drugs and reporting of AEs. AERSMine is a validated multi-cohort analyzing application designed to mine data across millions of patient reports (currently 19,089,556) from the FDA’s Adverse Event Reporting System (Sarangdhar et al., 2016).A recent high-impact study which combined clinical cardiotoxicity of kinase inhibitors with cell line-derived transcriptomic datasets to identify a gene signature that can predict risk of cardiotoxicity by leveraging AERSMine to visit FAERS data (van Hasselt et al., 2020). Firstly, we performed a retrospective disproportionality analysis of PJP cases with ICIs using data from the AERSMine. Secondly, we conducted a systematic literature review to confirm whether there is an association between PJP and ICIs therapies and provide a comprehensive clinical description of PJP induced by ICIs. The flow chart of this study was displayed in Figure 1. Ethical approval was not required because this study was conducted by using deidentified data.
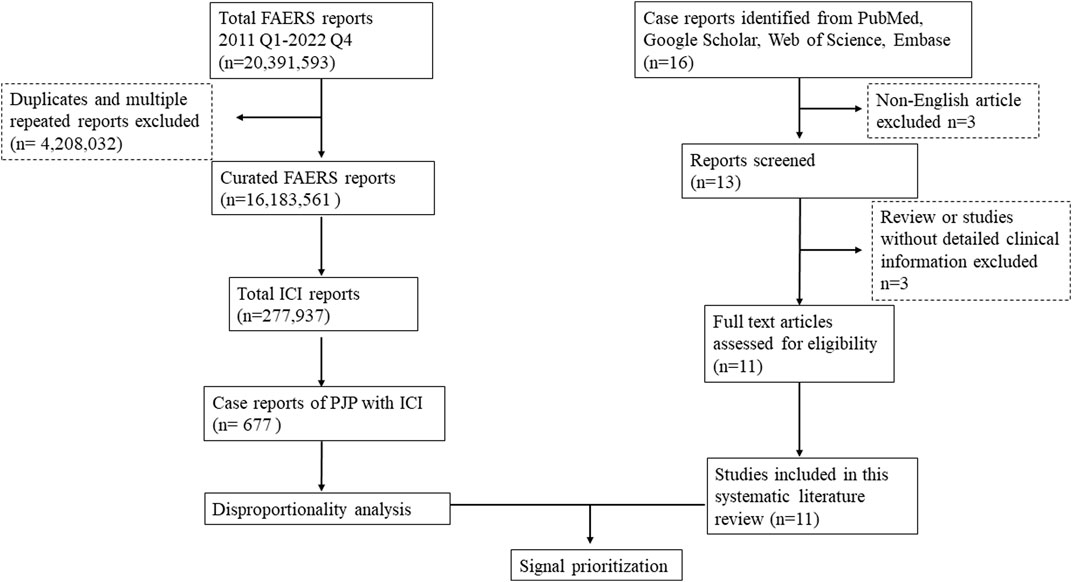
FIGURE 1. Flow chart to combine the FAERS disproportionality analysis with the systematic literature review. FAERS, FDA Adverse Event Reporting System; ICI, immune checkpoint inhibitor; Q1, quarter 1; PJP, Pneumocystis jirovecii pneumonia.
A pharmacovigilance study was conducted from 2011 Quarter 1 (Q1, ipilimumab was approved by FDA on 25 March 2011) to 2022 Q4 with the FAERS data in AERSMine to evaluate the disproportionate signal of PJP correlated with ICIs in a large-scale population. We included FDA approved 10 ICI agents (nivolumab, pembrolizumab, ipilimumab, atezolizumab, avelumab, durvalumab, dostarlimab, cemiplimab, tremelimumab, relatlimab) and one ICI combination therapies (nivolumab plus ipilimumab). PJP cases were identified by searching the Medical Dictionary for Regulatory Activities (MedDRA) (version 25.0), for preferred term “Pneumocystis jirovecii pneumonia”. Only case number more than 5 were included in this study. We collected the following information for each detected PJP reports: report year, demographic information (gender, age), drugs, indications, and co-administrated drugs. The demographics features including age and gender distribution of PJP cases associated with ICIs were investigated by comparing with all other adverse events of ICIs.
We performed the case/non-case analysis, a validated concept in pharmacovigilance, to investigate whether suspected PJP (cases) are differentially reported with immune check point inhibitors as compared to other adverse events (non-cases) (Faillie, 2019). We used two different disproportionate indicators, reporting odds ratios (RORs) and information components (IC) to reduce likelihood of false positive signals (Noguchi et al., 2021). When the lower limit of the 95% confidence interval of ROR (ROR025) >1 (Rothman et al., 2004) or the lower limit of the 95% confidence interval of IC (IC025) >0 (Bate et al., 1998), significant adverse events were detected. If the proportion of adverse events of interest is greater in patients exposed to a specific drug (cases) than in patients not exposed to this drug (non-cases), a disproportionality signal emerges, and subsequent analytical investigations are usually required before taking regulatory actions (Raschi et al., 2020). Both approaches were firstly conducted by using all other drugs in the FAERS database as a comparator, a common exploratory disproportionality analysis method.
Considering confounders such as comorbidities may affect the safety signal of PJP, we conducted primary sensitivity analyses to confirm the PJP safety signals correlated with ICIs. We excluded diseases (“renal transplant” “liver transplant” “stem cell transplant” “hiv infection” “bone marrow transplant” “inflammatory bowel disease” “organ transplant” “chronic obstructive pulmonary disease”) as PJP may occur preferentially in those immunocompromised patients with aforementioned conditions. We also excluded some drugs that cause an immunosuppression state which may further induce infection (“corticosteroids” “glucocorticoids” “immunosuppressants”). To assess the robustness of disproportionality signals and account for underlying confounders of the drug-event association, we conducted secondary sensitivity analysis by using anticancer drugs, such as traditional chemotherapy and molecular targeted therapy, as a comparator (to reduce confounding by indication and provide a clinical perspective). Firstly, we identified relevant National Comprehensive Cancer Network (NCCN) guidelines (Supplementary Figure S1), according to FDA-approved indications of ICIs. Then we extracted different cancer regimens from those selected NCCN guidelines and compared the safety signal of PJP between ICIs and traditional chemotherapy/molecular targeted therapy. Differences in categorical variables were assessed using a chi-squared test of independence performed on a 2 × 2 contingency table with Yates’ continuity correction or fisher exact test. Significance was assumed when the p-value less than 0.05. Data analyses were conducted by using the IBM SPSS (26.0) and Microsoft Excel (2021).
A comprehensive literature review was conducted through PubMed, Google Scholar, Web of Science, Embase from inception to 20 February 2023 (We have registered our protocol in PROSPERO with a number CRD42022376162 before the formal search). The search strategy included the keywords (“immunotherapy OR immune checkpoint inhibitors OR PD-1 inhibitors OR PD-L1 inhibitors OR CTLA-4 inhibitors OR nivolumab OR pembrolizumab OR cemiplimab OR dostarlimab OR atezolizumab OR avelumab OR durvalumab OR ipilimumab OR tremelimumab OR relatlimab”) AND (“Pneumocystis jirovecii pneumonia” OR “Pneumocystis pneumonia” OR “Pneumocystosis”). Mesh terms (“Immune Checkpoint Inhibitors” [Mesh]) AND “Pneumonia, Pneumocystis” [Mesh] were also used in the search process of PubMed. We only included literature written in English. Meeting abstracts were excluded. Case reports case series, case-control studies, observational studies, single-arm studies with detailed clinical information of PJP were retained in our final analyses. Two reviewers independently searched the literature and examined the relevant studies for further assessment of data, and collected clinical characteristics including age, gender, indication, absolute lymphocyte count, comorbidities, first/second-line regimens, immune-related adverse events, immunosuppressive agents, and outcome of PJP cases associated with ICIs. The quality of the reports was assessed following the recommended guidelines for publishing an adverse event report (Kelly et al., 2007).
Although disproportionality analysis per se is not an estimate, it could be evaluated and used for signal prioritization, which further provides clues for regimens management clinically and regulatory actions. Previous research (Pacurariu et al., 2017) concluded criteria used for signal prioritization and of the associated decision support frameworks, including strength of evidence, public health impact, novelty and general public and media attention. This study adopted novelty of the drug event association, seriousness, and disproportionate reporting as criteria for the signal prioritization. Moreover, a causal relationship appraisal was carried out on the entire body of evidence by using adapted Bradford Hill criteria used in epidemiology that have been applied to pharmacovigilance data (van Hunsel et al., 2018; Raschi et al., 2022), including biological plausibility, strength, consistency, specificity, coherence, and analogy. The rechallenge/de-challenge item were not analyzed in this study due to missing data.
From Q1, 2011 to Q4, 2022, we detected 677 patients on ICIs with PJP. In the primary analysis, we found that nivolumab, pembrolizumab, ipilimumab, atezolizumab, durvalumab, nivolumab plus ipilimumab had a significant safety signal (ROR025 > 1, IC025 > 0) for PJP (Table 1). After the sensitivity analyses, the above six ICI regimens consistently emerged with strong disproportionality, through both, ROR and IC approaches. (Table 2). When compared with all other anti-cancer drugs, nivolumab (IC025 0.09, p = 0.001) showed significant higher safety signal of PJP. Then we found that across all ICIs (IC025 0.18, p < 0.001), nivolumab (IC025 0.33, p < 0.001), pembrolizumab (IC025 0.16, p < 0.001), durvalumab (IC025–0.01, p = 0.019) presented a significant signal for PJP compared to targeted therapy. However, all ICIs showed a low signal of disproportionate reporting for PJP when compared to traditional chemotherapy (Figure 2).
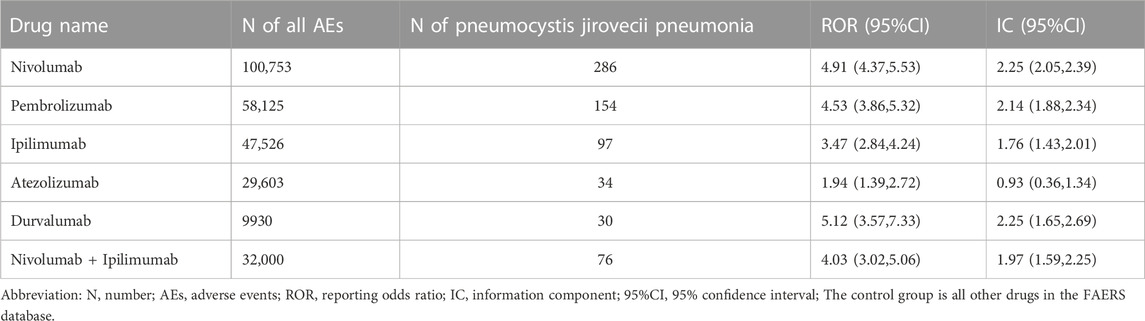
TABLE 1. Pneumocystis jirovecii pneumonia signals of different immune checkpoint inhibitors (Primary analysis).
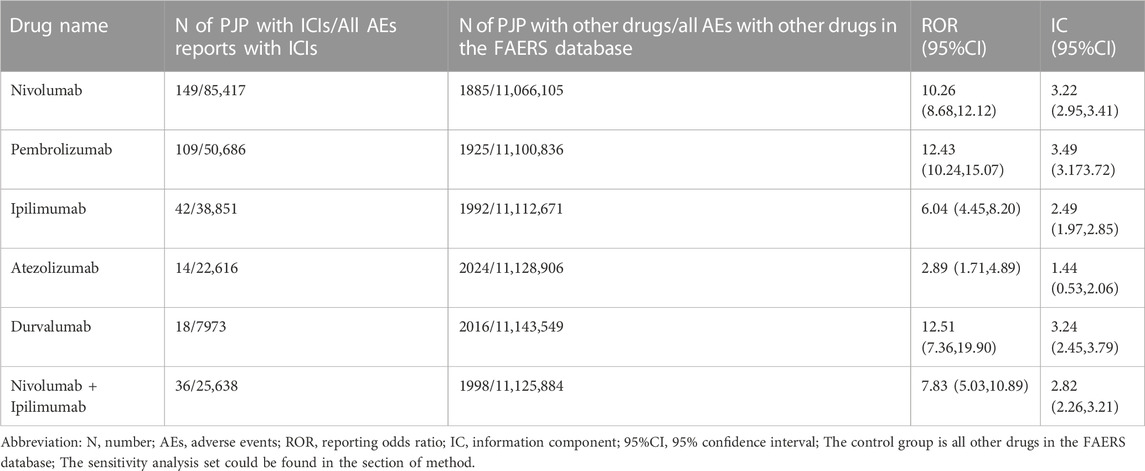
TABLE 2. Pneumocystis jirovecii pneumonia signals of different immune checkpoint inhibitors after sensitivity analysis.
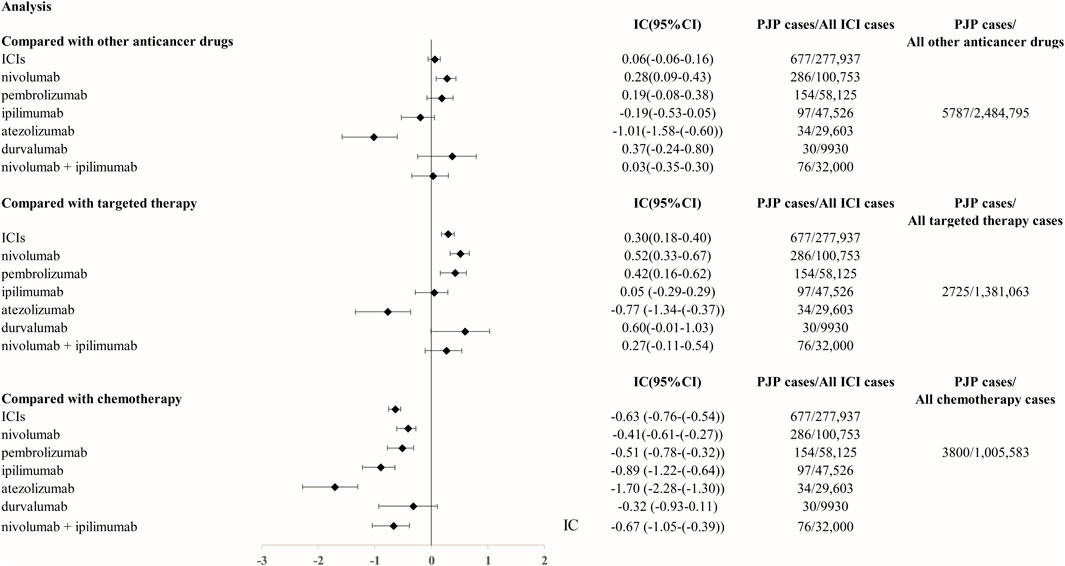
FIGURE 2. The comparison of Pneumocystis jirovecii pneumonia signal between ICIs and targeted therapy/chemotherapy in FAERS database. The list of control group (targeted therapy and chemotherapy) was extracted from NCCN guidelines for FDA approved indication of ICIs. Abbreviation: PJP, Pneumocystis jirovecii pneumonia. ROR, reporting odds ratio. IC, information component. 95%CI, 95% confidence interval. N, number. AEs, adverse events. ICIs, immune checkpoint inhibitors. NCCN, National Comprehensive Cancer Network.
We analyzed the clinical characteristics of PJP cases correlated with ICIs in the FAERS database. A total of 85.3% of PJP reports associated with ICIs were reported from 2018 to 2022.68.5% of cases were reported by health professionals. 300 of 677 (44.3%) of PJP cases died and 123 of 677 (18.2%) PJP cases experienced life-threatening situation. 66.3% (403 of 677) were elderly, age >65 years. A total of 77.0% (502/677) of PJP cases related to ICIs were males. To assess whether age >65 years is a factor may increase reporting of PJP in patients on ICIs, we calculated the ROR and IC by comparing age >65 years PJP cases/all PJP cases with age >65 years cases/all adverse events cases on ICIs. We found that elderly whose age more than 65 years old was predominant in PJP reports of overall ICIs (IC025 0.38, p < 0.001), nivolumab (IC025 0.31, p < 0.001), pembrolizumab (IC025 0.01, p = 0.001), ipilimumab (IC025 0.32, p < 0.001), durvalumab (IC025–0.13, p = 0.008), nivolumab plus ipilimumab (IC025 0.26, p < 0.001). Similarly, we also assessed the gender distribution in PJP associated with ICIs. Males is the predominance in PJP case reports of across all ICIs (IC025 0.26, p < 0.001), nivolumab (IC025 0.20, p < 0.001), pembrolizumab (IC025 0.23, p < 0.001), ipilimumab (IC025–0.13, p = 0.015), nivolumab plus ipilimumab (IC025–0.18, p = 0.029). (Figure 3). The top indications in PJP cases were lung cancer (171/667, 40.1%) and melanoma (160/677, 37.6%). We further analyzed the co-administrated regimens of ICIs in PJP cases. A total of 41.4% (280/677) cases reported to receiving glucocorticoids or corticosteroids during ICIs therapy. 69 out of 677 (10.2%) cases received immunosuppressants when they were on ICIs. Additional details of clinical features of PJP cases associated with specific ICIs regimens are shown in Table 3.
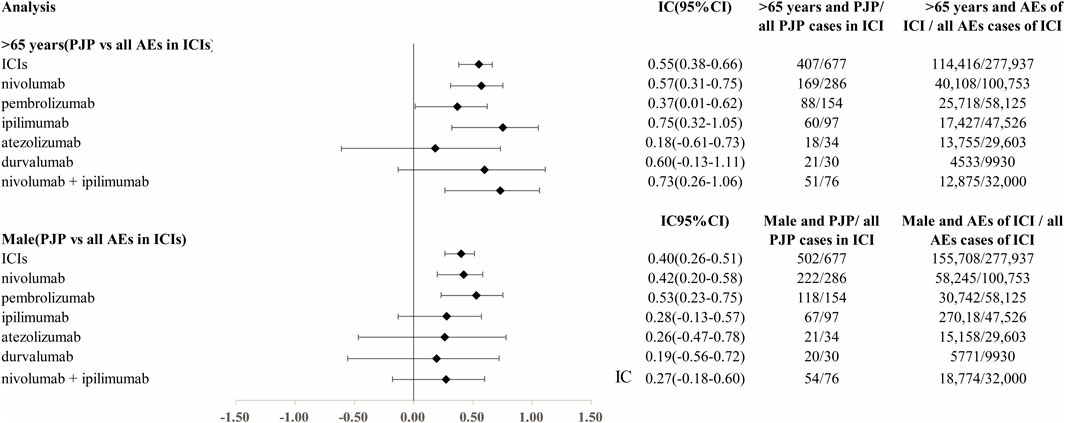
FIGURE 3. Age and gender distribution in Pneumocystis jirovecii pneumonia signal cases of ICIs. Abbreviation: PJP, Pneumocystis jirovecii pneumonia. ROR, reporting odds ratio. IC, information component. 95%CI, 95% confidence interval. N, number. AEs, adverse events. ICIs, immune checkpoint inhibitors.
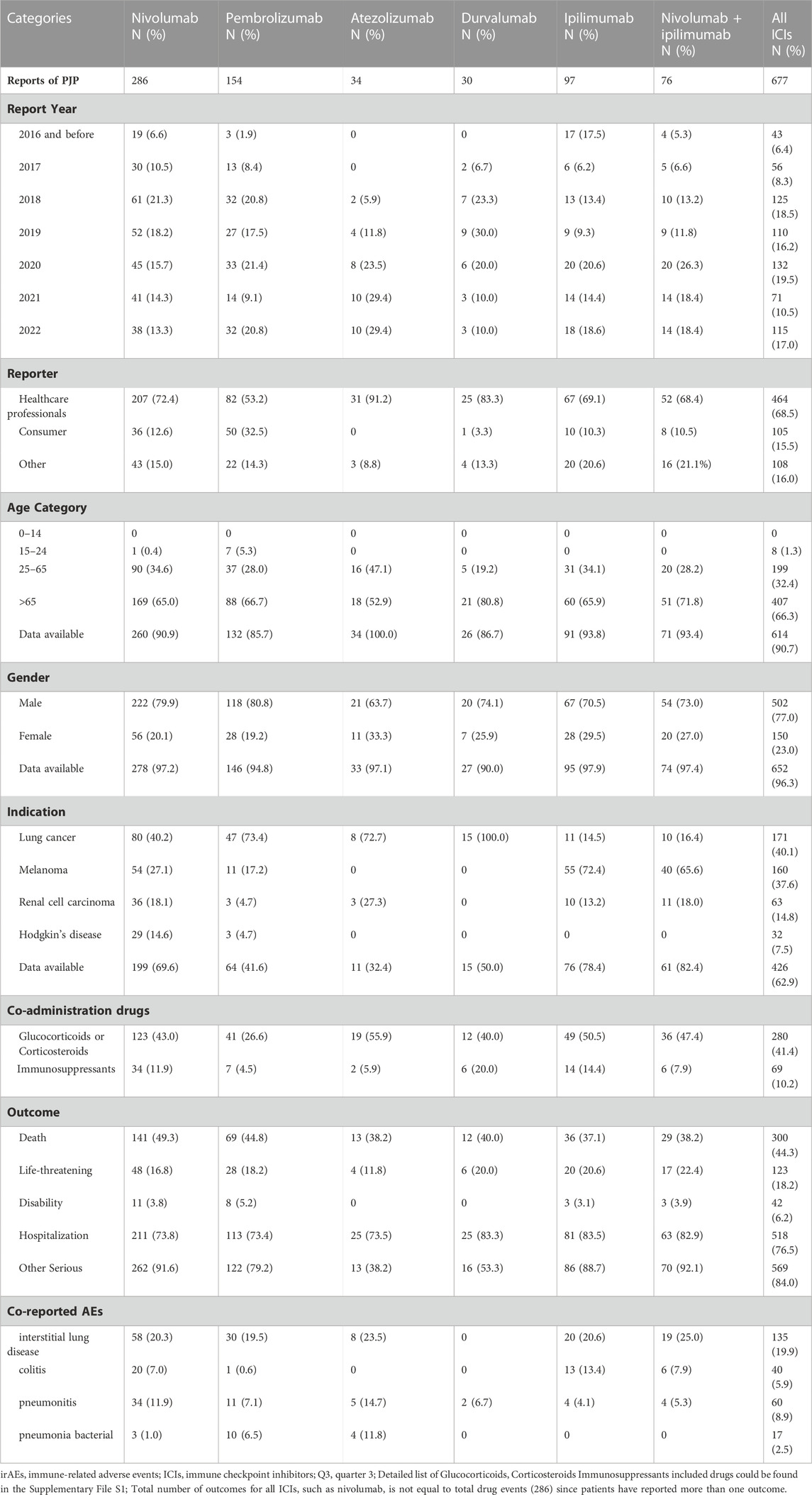
TABLE 3. Patient characteristics of PJP reports with immune checkpoint inhibitors in FAERS database.
Our literature review identified 15 PJP cases related to ICIs. 8 of 15 (53.3%) cases were lung cancer and 4 of 15 (26.7%) cases were melanoma. 8 cases (53.3%) were male while 7 cases (46.7%) were female. 8 PJP cases (53.3%) were patients with an age >60 years old. 7 of 15 (46.7%) PJP cases were co-reported with other immune-related adverse events such as colitis and hepatitis. 12 of 15 (80.0%) of cases received PD-1 inhibitors before PJP was diagnosed. 11 of 15 (73.3%) of PJP cases received glucocorticoids, corticosteroids or other immunosuppressants before PJP occurred. 5 of 15 PJP patients (33.3%) died. Additional details about ICIs regimens (and previous treatments) are listed in Table 4. The quality appraisal of the cases identified from the literature are summarized in Supplementary Figure S2.
By the combined analysis from post-marketing adverse reports submitted to FAERS and published case reports, this study detected novel signals of PJP with ICIs and provided comprehensive evidence for the signal prioritization. Bradford Hill criteria were fulfilled, as indicated by the strength of disproportionality and its consistency throughout the analyses, thus supporting a likely causal association between PJP and ICIs (The signal priority and causal relationship appraisal could be found in Supplementary Table S2).
To the best of our knowledge, this is the first large-scale pharmacovigilance study to investigate the association of PJP and ICIs by combining FAERS data mining and literature review. There are three main findings of this study:
Firstly, our post-marketing pharmacovigilance analysis showed that PJP was significantly associated with ICIs. Previous case reports (Arriola et al., 2015; Finbar Slevin et al., 2016; Schwarz et al., 2019; Liu et al., 2020; Moujaess et al., 2020; Sadek et al., 2020; Feng et al., 2021; Rath et al., 2021; Si et al., 2021; Hiba et al., 2022) showed that PJP may be a complication in immunotherapy. But the sample size is small. We retrospectively analyzed 677 case reports in FAERS database, conducted primary analysis and sensitivity analysis and found that all ICIs had significant higher disproportionate signals for PJP than other drugs in the FAERS database. We subsequently compared the signals of PJP between ICIs and other anticancer regimens, including targeted therapy, and chemotherapy, both were extracted from NCCN’s guideline for ICIs’ indications in order to enhance the clinical perspective of this signal comparison. Although all ICIs were detected a lower signal of PJP than chemotherapy, we found that nivolumab and pembrolizumab, both PD-1 inhibitors, showed significant higher reporting frequency for PJP than targeted therapy. And our case collections also showed that use of PD-1 inhibitors was correlated with PJP cases (12 of 15, 80.0%). However, CTLA-4 inhibitors or PD-L1 inhibitors were not associated with increased PJP reports compared with other anticancer drugs from FAERS data analysis. A previous meta-analysis (Su et al., 2019) showed that PD-L1 but not PD-1/CTLA4 inhibitors increased the risk of pneumonia compared to chemotherapy/placebo. However, another recent study (Tong et al., 2021) showed that both PD-1 and PD-L1 inhibitors significantly increase the risk of all-grade and high-grade pneumonia in NSCLC patients compared to conventional chemotherapy. Considering the conflicting research evidence, our pharmacovigilance analysis and literature collections support that PD-1 inhibitors may carry a clear potential for PJP. Further research is warranted to explore this clinical association of ICIs and PJP.
Secondly, we systematically investigated the clinical features of PJP cases associated with ICIs. Across all ICIs (which included regimens in table 1), our data analysis from FAERS database showed that males had a higher PJP report frequency than female (IC025 0.26, p < 0.001), elderly patients >65 years had more reports than younger patients (<65 years) on ICIs (IC025 0.38, p < 0.001). This is consistent with our literature review on published case reports (53.3% PJP cases associated with ICIs were male and age more than 65).
Thirdly, our study provides more evidence to support that PJP may be unmasked in cancer patients in the process of immune reconstitution induced by immune checkpoint inhibitors. An earlier review (Morelli et al., 2022) showed that PJP, could be categorized into a kind of opportunistic infections, and maybe associated with irAE treatment (corticosteroid, infliximab, etc.). However, there were also case reports (Inthasot et al., 2020; Feng et al., 2021) of severe pulmonary infections induced by Mycobacterium tuberculosis, Aspergillus fumigatus and Pneumocystis jirovecii were outside the context of immunosuppressive therapy. Our FAERS data anallysis showed that only 41.4% and 10.2% of cases received glucocorticoids/corticosteroids, or immunosuppressants during ICIs therapy, respectively. Our literature review also showed that 26.7% (4 of 15) of cases did not receive glucocorticoids/corticosteroids, infliximab, mycophenolate mofetil or other immunosuppressive agents before the occurrence of PJP. Both the FAERS data and published case reports showed that PJP cases associated with ICIs may be independent of immunosuppression. The subclinical colonization of Pneumocystis jirovecii may unmask and progress into PJP in non-HIV-infected immunosuppressed populations (Morris and Norris, 2012). Previous research (Wu et al., 2004) showed that Pneumocystis jiroveci pneumonia will be unmasked during reversal of immunosuppression in non-HIV infected patients. Emerging research (Feng et al., 2021; Lin et al., 2021) has reported that immune-related pneumonitis could be induced by immune reconstitution inflammatory syndrome. Previous preclinical study (Kauffman et al., 2021) showed that immune checkpoint blockade may cause an exaggerated immune response to fungal colonisation, which could promote fungal growth similar to recent studies in Mycobacterium tuberculosis infection. With the data from FAERS and published case reports, as well as previous research on mechanism of immune-related pneumonitis, we believe immune checkpoint inhibitors especially PD-1 inhibitors, may “unmask” low level PJP colonisation in the same way that antiretroviral therapy can reveal subclinical tuberculosis as immune reconstitution occurs. This is inherently counter-intuitive as developing PJP (a disease of immunocompromise) in association with ICI (which boosts immunity) but indeed be a possible mechanism for PJP.
Another study (Mansharamani et al., 2000) indicated that in immunosuppressed persons without HIV infection, CD4 + counts may be a useful clinical marker to identify specific individuals at particularly high clinical risk for PJP. Previous prospective study (Agrawal et al., 2016) showed that Absolute Lymphocyte Count <1643 μl but not 1200 μl could be the cost-effective surrogate marker for CD4 cell counts <200 cells/μL in monitoring HIV infected individuals. Moreover, a recent meeting abstract (Joshi et al., 2019) in 2019 ASCO Annual Meeting indicated that low ALC (750/μL, median) and prolonged steroid therapy are more likely to result in PJP infection as opposed to steroid therapy alone. Our case series identified 9 PJP cases with ALC amount, 7 of 9 (77.8%) had an ALC <1200 μL. The above literature provided literature evidence of monitoring ALC value as an indicator of PJP infection when CD4 T Cells amount is not accessible.
To increase the robustness of disproportionate signals detected from spontaneous reporting systems, some researchers tried to combine pharmacovigilance analysis with literature review. Wang et al. investigated fatal toxic effects associated with immune checkpoint inhibitors by using data from large academic medical centers, global WHO pharmacovigilance data, and all published ICI clinical trials (Wang et al., 2018). Stevens-Johnson syndrome/toxic epidermal necrolysis (Zhu et al., 2021) and type 1 diabetes (Zhu et al., 2022b) were found highly associated with immune checkpoint inhibitors by analyzing data from clinical trials and post-marketing data from the FAERS database. Raschi et al. (2019) confirmed that ICIs are associated with a multitude of irAEs, especially respiratory, endocrine, and hepatic toxicities by conducting parallel approach through contemporary post-marketing analysis and overview of systematic reviews. With the above research experience, this study presented that ICIs were strongly associated with PJP by the combined analysis of pharmacovigilance and systematic literature review.
Our study has several limitations. Firstly, FAERS database is a spontaneous reporting system whose data source is heterogeneous (both non-health care and healthcare practitioners) and therefore reporting bias exists. Secondly, FAERS data could not be used to calculate the incidence of PJP because of the under-reporting phenomenon and not having the full number of patients who have received the drug. Thirdly, detailed clinical information such as previous treatment regimens and stages of cancer are missing, so the reports do not confirm causality of the drug-induced event. However, this large-scale pharmacovigilance analysis did provide comprehensive information about the link between PJP and ICIs.
Our literature and FAERS analysis indicated that ICIs may be associated with a safety signal of PJP, especially in males aged >65years. PD-1 inhibitors emerged with a robust disproportionality signal when compared to PD-L1/CTLA-4 inhibitors as well as targeted therapy, even accounting for confounders such as concomitant immunosuppressive drugs. Anti-PD-1 therapy may unmask low level Pneumocystis jirovecii colonization and cause PJP infection in patients. More pre-clinical or clinical studies are warranted to confirm the association of PJP and ICIs and explore the potential new mechanism of ICIs-related PJP.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
SX: formal analysis, data curation, writing—original draft, review and editing, and visualization. MS and YN: software, methodology, and writing—review and editing. LL, LG and YCZ: resources. HG and Y-KW: software. B-KZ: supervision. MY: conceptualization, methodology and supervision. All authors participated in the interpretation of the results. The final manuscript was read, checked, and approved by all authors.
This research was funded by Health Commission of Hunan Province, grant number 202113012480.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1129730/full#supplementary-material
Agrawal, P. B., Rane, S. R., and Jadhav, M. V. (2016). Absolute lymphocyte count as a surrogate marker of CD4 count in monitoring HIV infected individuals: A prospective study. J. Clin. Diagn Res. 10 (5), EC17–9. doi:10.7860/JCDR/2016/19263.7765
Arriola, E., Wheater, M., Krishnan, R., Smart, J., Foria, V., and Ottensmeier, C. (2015). Immunosuppression for ipilimumab-related toxicity can cause pneumocystis pneumonia but spare antitumor immune control. Oncoimmunology 4 (10), e1040218. doi:10.1080/2162402X.2015.1040218
Bate, A., Lindquist, M., Edwards, I. R., Olsson, S., Orre, R., Lansner, A., et al. (1998). A Bayesian neural network method for adverse drug reaction signal generation. Eur. J. Clin. Pharmacol. 54 (4), 315–321. doi:10.1007/s002280050466
Classen, A. Y., Henze, L., von Lilienfeld-Toal, M., Maschmeyer, G., Sandherr, M., Graeff, L. D., et al. (2021). Primary prophylaxis of bacterial infections and pneumocystis jirovecii pneumonia in patients with hematologic malignancies and solid tumors: 2020 updated guidelines of the infectious diseases working party of the German society of Hematology and medical Oncology (AGIHO/DGHO). Ann. Hematol. 100 (6), 1603–1620. doi:10.1007/s00277-021-04452-9
Cui, C., Deng, L., Wang, W., Ren, X., Wang, Y., and Cui, W. (2022). Respiratory system toxicity induced by immune checkpoint inhibitors: A real-world study based on the FDA adverse event reporting system database. Front. Oncol. 12, 941079. doi:10.3389/fonc.2022.941079
Faillie, J. L. (2019). Case-non-case studies: Principle, methods, bias and interpretation. Therapie 74 (2), 225–232. doi:10.1016/j.therap.2019.01.006
Feng, Y., Chen, C., Zhao, L., Zhu, X., Zhu, X., and Li, Q. (2021). A potential mechanism of the onset of immune-related pneumonitis triggered by anti-PD-1 treatment in a patient with advanced adenocarcinoma lung cancer: Case report. BMC Pulm. Med. 21 (1), 291. doi:10.1186/s12890-021-01649-6
Finbar Slevin, C. M., Marples, M., and Marples, M. (2016). Pneumocystis jirovecii pneumonia in a patient with melanoma treated with infliximab and corticosteroids for ipilimumab-associated colitis. Glob. Dermatol. 3, 1000193. doi:10.15761/god.1000193
Guo, X. J., Cai, X. T., Rong, Z. X., Zhang, Y. P., Wen, Y. X., Bai, X., et al. (2023). Interstitial pneumonitis associated with combined regimen of immunotherapy and conventional therapies-pharmacovigilance database analysis with real-world data validation. BMC Med. 21 (1), 6. doi:10.1186/s12916-022-02713-6
Hiba, Z., Abdelmoughit, H., Zaynab, I. H., Hounaida, J., Rachida, L., and Youssef, O. (2022). Pneumocystis pneumonia in patient with lung adenocarcinoma: Early side effects from pembrolizumab. Radiol. Case Rep. 17 (10), 3979–3981. doi:10.1016/j.radcr.2022.07.083
Inthasot, V., Bruyneel, M., Muylle, I., and Ninane, V. (2020). Severe pulmonary infections complicating nivolumab treatment for lung cancer: A report of two cases. Acta Clin. Belg 75 (4), 308–310. doi:10.1080/17843286.2019.1629078
Joshi, K. A., Atwal, D., Siegel, E. R., Jillella, A., Konda, M., and Parikh, R. (2019). Absolute lymphocyte count (ALC) as predictor of Pneumocystis Jiroveci Pneumonia (PCP) infection in patients on immune checkpoint inhibitors (ICPi). J. Clin. Oncol. 37, e14255. doi:10.1200/JCO.2019.37.15_suppl.e14255
Kauffman, K. D., Sakai, S., Lora, N. E., Namasivayam, S., Baker, P. J., Kamenyeva, O., et al. (2021). PD-1 blockade exacerbates Mycobacterium tuberculosis infection in rhesus macaques. Sci. Immunol. 6 (55), eabf3861. doi:10.1126/sciimmunol.abf3861
Kelly, W. N., Arellano, F. M., Barnes, J., Bergman, U., Edwards, R. I., Fernandez, A. M., et al. (2007). Guidelines for submitting adverse event reports for publication. Drug Saf. 30 (5), 367–373. doi:10.2165/00002018-200730050-00001
Lee, E. H., Kim, E. Y., Lee, S. H., Roh, Y. H., Leem, A. Y., Song, J. H., et al. (2019). Risk factors and clinical characteristics of Pneumocystis jirovecii pneumonia in lung cancer. Sci. Rep. 9 (1), 2094. doi:10.1038/s41598-019-38618-3
Lin, X., Lu, T., Li, S., Xie, X., Chen, X., Jiang, J., et al. (2021). Cytomegalovirus infection as an underestimated trigger for checkpoint inhibitor-related pneumonitis in lung cancer: A retrospective study. Clin. Transl. Oncol. 23 (2), 389–396. doi:10.1007/s12094-020-02432-5
Liu, Z., Liu, T., Zhang, X., Si, X., Wang, H., Zhang, J., et al. (2020). Opportunistic infections complicating immunotherapy for non-small cell lung cancer. Thorac. Cancer 11 (6), 1689–1694. doi:10.1111/1759-7714.13422
Mansharamani, N. G., Balachandran, D., Vernovsky, I., GaRland, R., and Koziel, H. (2000). Peripheral blood CD4 + T-lymphocyte counts during Pneumocystis carinii pneumonia in immunocompromised patients without HIV infection. Chest 118 (3), 712–720. doi:10.1378/chest.118.3.712
Morelli, T., Fujita, K., Redelman-Sidi, G., and Elkington, P. T. (2022). Infections due to dysregulated immunity: An emerging complication of cancer immunotherapy. Thorax 77 (3), 304–311. doi:10.1136/thoraxjnl-2021-217260
Morris, A., and Norris, K. A. (2012). Colonization by Pneumocystis jirovecii and its role in disease. Clin. Microbiol. Rev. 25 (2), 297–317. doi:10.1128/CMR.00013-12
Moujaess, E., El Haddad, E., and Joseph, K. (2020). Pneumocystis jiroveci mimicking COVID-19 pneumonia in a patient who is receiving ipilimumab and nivolumab combination therapy: A case report. Eurasian J. Med. Oncol. 2020, 89845. doi:10.14744/ejmo.2020.89845
Noguchi, Y., Tachi, T., and Teramachi, H. (2021). Detection algorithms and attentive points of safety signal using spontaneous reporting systems as a clinical data source. Brief. Bioinform 22 (6), bbab347. doi:10.1093/bib/bbab347
Pacurariu, A. C., Coloma, P. M., Gross-Martirosyan, L., Sturkenboom, M. C., and Straus, S. M. (2017). Decision making in drug safety-a literature review of criteria used to prioritize newly detected safety issues. Pharmacoepidemiol Drug Saf. 26 (3), 327–334. doi:10.1002/pds.4128
Postow, M. A., Sidlow, R., and Hellmann, M. D. (2018). Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 378 (2), 158–168. doi:10.1056/NEJMra1703481
Raschi, E., Fusaroli, M., Giunchi, V., Repaci, A., Pelusi, C., Mollica, V., et al. (2022). Adrenal insufficiency with anticancer tyrosine kinase inhibitors targeting vascular endothelial growth factor receptor: Analysis of the FDA adverse event reporting system. Cancers (Basel). 14 (19), 4610. doi:10.3390/cancers14194610
Raschi, E., Gatti, M., Gelsomino, F., Ardizzoni, A., Poluzzi, E., and De Ponti, F. (2020). Lessons to be learnt from real-world studies on immune-related adverse events with checkpoint inhibitors: A clinical perspective from pharmacovigilance. Target Oncol. 15 (4), 449–466. doi:10.1007/s11523-020-00738-6
Raschi, E., Mazzarella, A., Antonazzo, I. C., Bendinelli, N., Forcesi, E., Tuccori, M., et al. (2019). Toxicities with immune checkpoint inhibitors: Emerging priorities from disproportionality analysis of the FDA adverse event reporting system. Target Oncol. 14 (2), 205–221. doi:10.1007/s11523-019-00632-w
Rath, P., Bashir, H. I., Diwakar, A., and Murray, T. (2021). Pneumocystis jiroveci pneumonitis: A rare pneumonia in a patient treated with chemoimmunotherapy. Am. J. Respir. Crit. Care Med. 203, 1277676.
Rothman, K. J., Lanes, S., and Sacks, S. T. (2004). The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol Drug Saf. 13 (8), 519–523. doi:10.1002/pds.1001
Sadek, M., Loizidou, A., Drowart, A., Van den Wijngaert, S., Gomez-Galdon, M., and Aspeslagh, S. (2020). Pneumocystis infection in two patients treated with both immune checkpoint inhibitor and corticoids. J. Immunother. Precis. Oncol. 3 (1), 27–30. doi:10.4103/JIPO.JIPO_23_19
Sanka, P., and Hsu, A. (2023). A case of pneumocystis jirovecci in a patient with non-small cell lung cancer treated with immunotherapy. R I Med. J. (2013) 106 (1), 11–13.
Sarangdhar, M., Tabar, S., Schmidt, C., Kushwaha, A., Shah, K., Dahlquist, J. E., et al. (2016). Data mining differential clinical outcomes associated with drug regimens using adverse event reporting data. Nat. Biotechnol. 34 (7), 697–700. doi:10.1038/nbt.3623
Schwarz, M., Kocher, F., Niedersuess-Beke, D., Rudzki, J., Hochmair, M., Widmann, G., et al. (2019). Immunosuppression for immune checkpoint-related toxicity can cause pneumocystis jirovecii pneumonia (PJP) in non-small-cell lung cancer (NSCLC): A report of 2 cases. Clin. Lung Cancer 20 (3), e247–e250. doi:10.1016/j.cllc.2018.12.006
Si, S., Erickson, K., Evageliou, N., Silverman, M., and Kersun, L. (2021). An usual presentation of pneumocystis jirovecii pneumonia in a woman treated with immune checkpoint inhibitor. J. Pediatr. Hematol. Oncol. 43 (2), e163–e164. doi:10.1097/MPH.0000000000001757
Su, Q., Zhu, E. C., Wu, J. B., Li, T., Hou, Y. L., Wang, D. Y., et al. (2019). Risk of pneumonitis and pneumonia associated with immune checkpoint inhibitors for solid tumors: A systematic review and meta-analysis. Front. Immunol. 10, 108. doi:10.3389/fimmu.2019.00108
Tong, Z. Q., Wu, D. Y., Liu, D., and Dong, M. (2021). Incidence risk of PD-1/PD-L1-related pneumonia and diarrhea in non-small cell lung cancer (NSCLC) patients: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Clin. Pharmacol. 77 (8), 1079–1088. doi:10.1007/s00228-020-03083-9
van Hasselt, J. G. C., Rahman, R., Hansen, J., Stern, A., Shim, J. V., Xiong, Y., et al. (2020). Transcriptomic profiling of human cardiac cells predicts protein kinase inhibitor-associated cardiotoxicity. Nat. Commun. 11 (1), 4809. doi:10.1038/s41467-020-18396-7
van Hunsel, F., van de Koppel, S., van Puijenbroek, E., and Kant, A. (2018). Vitamin B(6) in health supplements and neuropathy: Case series assessment of spontaneously reported cases. Drug Saf. 41 (9), 859–869. doi:10.1007/s40264-018-0664-0
Wang, D. Y., Salem, J. E., Cohen, J. V., Chandra, S., Menzer, C., Ye, F., et al. (2018). Fatal toxic effects associated with immune checkpoint inhibitors: A systematic review and meta-analysis. JAMA Oncol. 4 (12), 1721–1728. doi:10.1001/jamaoncol.2018.3923
Wu, A. K., Cheng, V. C., Tang, B. S., Hung, I. F. N., Lee, R. A., Hui, D. S., et al. (2004). The unmasking of pneumocystis jiroveci pneumonia during reversal of immunosuppression: Case reports and literature review. BMC Infect. Dis. 4 (1), 57. doi:10.1186/1471-2334-4-57
Zhu, J., Chen, G., He, Z., Zheng, Y., Gao, S., Li, J., et al. (2021). Stevens-johnson syndrome/toxic epidermal necrolysis in patients treated with immune checkpoint inhibitors: A safety analysis of clinical trials and FDA pharmacovigilance database. EClinicalMedicine 37, 100951. doi:10.1016/j.eclinm.2021.100951
Zhu, J., He, Z., Liang, D., Yu, X., Qiu, K., and Wu, J. (2022). Pulmonary tuberculosis associated with immune checkpoint inhibitors: A pharmacovigilance study. Thorax 77 (7), 721–723. doi:10.1136/thoraxjnl-2021-217575
Keywords: immune checkpoint inhibitors, pneumocystis jirovecii pneumonia, pharmacovigilance, FAERS, systematic literature review
Citation: Xia S, Gong H, Wang Y-k, Liu L, Zhao Y-c, Guo L, Zhang B-k, Sarangdhar M, Noguchi Y and Yan M (2023) Pneumocystis jirovecii pneumonia associated with immune checkpoint inhibitors: A systematic literature review of published case reports and disproportionality analysis based on the FAERS database. Front. Pharmacol. 14:1129730. doi: 10.3389/fphar.2023.1129730
Received: 22 December 2022; Accepted: 03 March 2023;
Published: 15 March 2023.
Edited by:
Alexander Batista Duharte, Maimonides Biomedical Research Institute of Cordoba (IMIBIC), SpainReviewed by:
Fakhri Hassouneh, Maimonides Biomedical Research Institute of Cordoba (IMIBIC), SpainCopyright © 2023 Xia, Gong, Wang, Liu, Zhao, Guo, Zhang, Sarangdhar, Noguchi and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Miao Yan, eWFubWlhb0Bjc3UuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.