
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 16 June 2023
Sec. Pharmacology of Infectious Diseases
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1128142
This article is part of the Research Topic Three decades with Lyme disease: Can we see the light at the end of the tunnel? View all 4 articles
 Giusto Trevisan1*
Giusto Trevisan1* Maurizio Ruscio2
Maurizio Ruscio2 Marina Cinco1
Marina Cinco1 Katiuscia Nan2
Katiuscia Nan2 Patrizia Forgione3
Patrizia Forgione3 Nicola Di Meo1,2
Nicola Di Meo1,2 Paolo Tranchini3
Paolo Tranchini3 Massimo Nacca4
Massimo Nacca4 Silvana Trincone5
Silvana Trincone5 Sara Giordana Rimoldi6
Sara Giordana Rimoldi6 Vania Giacomet7
Vania Giacomet7 Michela Ricci7
Michela Ricci7 Davide Melandri5
Davide Melandri5 Stefania Artioli8
Stefania Artioli8 Patrizia Monteforte9
Patrizia Monteforte9 Giuseppe Stinco10
Giuseppe Stinco10 Serena Bonin1
Serena Bonin1Lyme borreliosis (LB) is the most common vector-borne zoonotic inflammatory disease in the Northern Hemisphere. In Italy, the first case was diagnosed in 1985 in a woman in Liguria, while the second, in 1986 in Friuli-Venezia Giulia, documenting the infection in northern Italy. Both diagnoses were confirmed by serological assessment by an indirect immunofluorescence (IFI) technique. Borrelia cultivation from both Ixodes ricinus ticks and human lesions in Trieste (Friuli-Venezia Giulia) identified Borrelia afzelii as the prevalent genospecies; nevertheless, Borrelia garinii, Borrelia burgdorferi (sensu stricto), and Borrelia valaisiana (VS116 Group) were also detected, although less frequently. LB was also documented in other Italian regions: in Tuscany (1991), Trentino–Alto Adige (1995–1996), Emilia-Romagna (1998), Abruzzo (1998), and more recently, Lombardy. Nevertheless, data on LB in other Italian regions, especially in southern Italy and islands, are poor. The aim of this study is to document the spread of LB in Italy through the collection of data from LB patients in eight Italian hospitals located in different Italian regions. Diagnostic criteria for LB diagnosis are as follows: i) the presence of erythema migrans (EM) or ii) a clinical picture suggestive of LB, confirmed by serological tests and/or PCR positivity for Borrelia detection. In addition, data also included the place of residence (town and region) and the place where patients became infected. During the observation period, 1,260 cases were gathered from the participating centers. Although different in extent from northern Italy to central/southern Italy, this study shows that LB is widespread throughout Italy.
Lyme borreliosis (LB) is an anthropozoonotic infection transmitted by hard ticks. It is widespread mainly in the Northern Hemisphere (Trevisan et al., 2021). In Europe, LB is transmitted mainly by Ixodes ricinus ticks. Nevertheless, other vectors could be implicated in its transmission, such as Ixodes gibbosus in Abruzzi in Italy (Khoury et al., 1994). In Italy, the first case of LB was diagnosed in a woman in north-western Italy in Liguria in 1985 (north-western Italy) by Crovato et al. (1985), while the second case was described 1 year later in a young woman in Friuli-Venezia Giulia by Trevisan (1986), supporting the presence of this vector-borne disease even in north-eastern Italy. Both cases were confirmed by serological assessment, which was carried out in Bari by Prof. Fumarola, by an indirect immunofluorescence technique (Fumarola et al., 1985). The first study on the epidemiology of LB in Italy was presented in the “Second International Symposium on Lyme Disease and Related Disorders” in 1985 in Vienna (Trevisan et al., 1987). Further epidemiological studies were carried out, especially in Friuli-Venezia Giulia (Cinco et al., 1993) and Liguria (Cimmino et al., 1992). In Trieste, Borrelia (Friuli-Venezia Giulia) culture in the BSK medium was also carried out, both from Ixodes ticks (Cinco et al., 1989) and patients’ biopsies, namely, erythema migrans (EM) (Cinco et al., 1992) and annular/roseolar erythema (Trevisan et al., 1992), and from the human myocardium (Lardieri et al., 1993). The isolation and identification of Borreliae from ticks identified Borrelia afzelii as the most widespread genospecies in Friuli-Venezia Giulia, where Borrelia garinii, Borrelia burgdorferi (sensu stricto) (Ciceroni et al., 2001), and Borrelia valaisiana (VS116 Group) could also be found (Cinco et al., 1998).
The Regional Centre (Friuli-Venezia Giulia) of reference for Lyme disease (resolution of the Regional Council No. 1956/1993) appointed by the Ministry of Health (Note No. 1400.2/26.N/2445 of 9 April 1997) was established at the Dermatology Clinic in Trieste on 22 April 1993 as a supra-regional center. Patients from all over Italy have come there, gathering information on the spread of LB throughout Italy.
In 1991, Lyme Borreliae were detected for the first time in Veneto (Trevisan et al., 1991), where they were subsequently isolated from more than 50 patients (Ciceroni et al., 2001). Since that time LB has been endemic in that region (Beltrame et al., 2021).
Certain cases of LB have also been reported in Tuscany (Stefanelli et al., 1994), with the following identification of Borrelia lusitaniae (Bertolotti et al., 2006), and in Trentino (Merler et al., 1996) and Alto Adige (South Tirol) (Cacciapuoti et al., 1995), where B. burgdorferi (sensu stricto), B. garinii, and Borrelia group VS461 were identified. Additional endemic areas in Italy were notified in Emilia-Romagna (Gaddoni et al., 1998), Abruzzi (Fazii et al., 2000), and Lombardy (Rimoldi et al., 2020). Data from southern Italy and islands are limited (Zanet et al., 2020). Sporadic cases have been reported in Sicily (Rinaldi et al., 1991), Sardinia (Ruata et al., 1992), Lazio (Frediani et al., 1993), and Calabria (Santino et al., 1996).
Over the past 15 years, LB cases have considerably increased in endemic regions of Europe and have emerged in new geographic areas (Stark et al., 2023). In the United States, Lyme disease is highly endemic in the Northeast, Middle Atlantic, and Upper Midwest regions, and the incidence is increasing in neighboring states (Schwartz et al., 2017). In Europe, the highest incidence of LB is in Estonia, Slovenia, Switzerland, Holland (Stark et al., 2023), and Norway (Eliassen et al., 2017) with more than 100 cases/100,000 inhabitants/year. In Lithuania, the incidence is 99.9/100,000 inhabitants per year (Petrulioniene et al., 2020), in Finland 99.6, and in Germany and Poland 37. However, the highest rate is in Sweden, in Blekinge, with 632/100,000 inhabitants/year (Vandekerckhove et al., 2021).
The aim of the present study is to document the spread of LB in Italy through the collection of data from LB patients in eight Italian hospitals located in different Italian regions.
Patients’ data were gathered from 01/01/2010 to 30/08/2022 in the participating centers in Friuli-Venezia Giulia, Liguria, Lombardy, Emilia-Romagna, and Campania, as shown in Figure 1. During the COVID pandemic, telemedicine has also been used (Trevisan et al., 2022) for diagnosis, especially for EM lesions. In each center, the following diagnostic criteria were applied for LB diagnosis:
1. The presence of an EM lesion that is pathognomonic for LB
2. A clinical picture suggestive of LB, confirmed by two-titer serological tests and/or by direct detection methods such as PCR and Borrelia culture. In addition to EM, symptoms suggestive of LB were conjunctivitis, migratory arthralgia, myalgia, arrhythmia, headache, involvement of cranial nerves, and poly-meningo-radicolo-neuritis, when appearing after the tick bite.
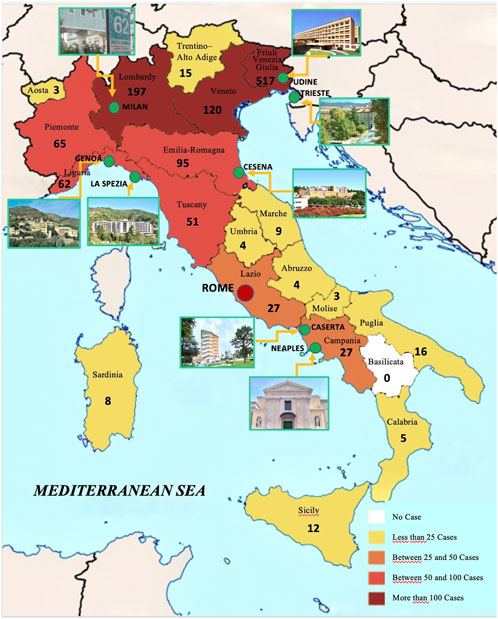
FIGURE 1. Map of Italy with participating centers and number of cases detected in each region during the observation period.
For each patient, the following data were gathered: age at diagnosis, gender, the year of diagnosis, the Italian region of residence, the geographical area of infection if different from residence, the recollection of a tick bite, the anatomical site of EM occurrence, other symptoms, and the antibiotic treatment. The following associated symptoms were recorded: fever, lymphadenopathy, migratory arthralgias, arthritis; at the skin level (in addition to EM), annular multiple erythema, Borrelia lymphocytoma, primary cutaneous B-cell marginal zone lymphoma, and acrodermatitis chronica atrophicans; at the muscular system, asthenia and myositic symptoms; at the nervous system, headache, meningo-encephalitis, facial palsy and other cranial nerves, poly-meningo-radiculus-neuritis (Miele et al., 2022), paresthesia, cognitive disorders, anxiety, and depression; at the cardiac level, rhythm disorders, myocarditis, pericarditis, and POT’s syndrome; at the ocular level, conjunctivitis, optic neuritis, uveitis, and neuroretinitis; dizziness; and Jarisch–Herxeimer reaction.
According to the residence of patients, cases were grouped in the following categories:
1- Northern Italy for patients living in Valle d'Aosta, Piemonte, Lombardy, Trentino-Alto Adige, Veneto, Friuli-Venezia Giulia, Liguria, and Emilia-Romagna.
2- Central Italy for patients living in Abruzzo, Lazio, Marche, Tuscany, and Umbria.
3- Southern Italy and islands for patients living in Apulia, Basilicata, Calabria, Campania, Molise, Sardinia, and Sicily.
Detection of antibodies against the B. burgdorferi sensu lato complex was performed by conventional two-tiered serologic testing, according to European and North American guidelines (Eldin et al., 2019). The first step was the ELISA test or the chemiluminescence test (CLIA), which were confirmed in the case of positivity or doubtful results by immunoblot. Serological tests were carried out in all patients except those with manifest EM. ELISA, CLIA, and immunoblots were provided by EUROIMMUN.
PCR analysis was carried out in DNA obtained by tissue biopsies or synovial fluid or blood amplifying two Borrelia targets, namely, a fragment of OspA and flagellin gene, as already reported (Bonin et al., 2016; di Meo et al., 2015; Ivacic et al., 2007; Jenkins et al., 2012; Pauluzzi et al., 2004).
Data were gathered in a database and submitted to statistical analyses. A descriptive analysis for each individual variable considered in this study was carried out. Continuous variables were described with a mean value and standard deviation. Associations were tested by the chi-squared test or the Fisher exact test. A p-value less than 0.05 was considered significant. Statistical analysis was carried out using the Stata/SE 16.0 package (StataCorp, College Station, TX, United States).
During the observation period, 1,260 patients were diagnosed with LB in Italy in the clinical centers participating in the study. Cases were grouped according to the region of residence, as shown in Figure 1; Table 1. The overall distribution of patients per gender did not differ across Italy, as reported in Table 1 (p = 0.6).
The mean age at diagnosis was 43 years (S.D. 20.0). Age at diagnosis varied slightly per gender with a higher age at diagnosis for women (44 years, 95% CI: 43.0–46.0) vs. men (42 years, 95% CI: 40.5–43.9) (p = 0.04), as shown in Figure 2A. Age at diagnosis also varied with the geographical area of residence (p = 0.01); notably, patients living in northern Italian regions were significantly older than those living in central Italy. In addition, a decreasing trend in age from northern to southern Italy was observed (p = 0.001). Accordingly, the mean age of patients from northern Italy was 44 years (95% CI: 42.8–45.3), from central Italy was 38 years (95% CI: 34.5–42.1), and from southern Italy and islands was 41 years (95% CI: 37.0–44.3).
A trend (p = 0.001) of growing cases across the years of observation was recorded in all geographical areas. A maximum of cases was observed during 2020 with 211 diagnoses.
Regarding the tick bite, 936 of the 1,260 patients recalled a tick bite (74%), while 324 did not do so (26%), with any difference between genders (p = 0.4). On the contrary, the recollection of the tick bite was associated with the geographical area of residence (p = 0.02) with a significantly higher number of patients recalling a tick bite in southern Italy and islands and a considerably lower number in central Italy. The development of EM was also associated with the recollection of a tick bite (p < 0.001); notably, 86% of patients recalling a tick bite developed EM. Anyway, the development of EM did not differ with the geographical area (p = 0.3) and genders (p = 0.2).
The body sites where EM developed were grouped in the i) head and neck; ii) trunk; iii) higher limbs; and iv) lower limbs. Of the entire cohort, 943 (75%) patients had an EM, as shown in Table 2. In 2% of cases, the location of the EM was not included in clinical records. As shown in Table 2, the lower limbs were the preferential site for EM, with 54% of EM in this site. The trunk was the second most common site, accounting for 25% of the total cases. Anyway, the distribution of the anatomical sites of EM did not differ between genders (p = 0.8).
In 120 patients, EM developed in pediatric patients (aged <14 years). In those patients, the most frequent site for EM was the trunk (32% of EM), followed by the head and neck (30% of pediatric EM) and lower limbs (28% of EM in children), but none of them prevailed, as shown in Table 2. As for the overall cohort, the location of EM did not differ between genders (p = 0.4).
Taken those observations, patients’ age varied significantly with the EM location (p < 0.0001), with the lowest age for the head and neck (26 years) and the highest for lower limbs (46 years).
LB manifested only with EM in 380 patients (30%), while in 563 patients (45%), other symptoms occurred in addition to EM. The presence of associated symptoms in patients with EM differed significantly with respect to genders; female patients had, indeed, a higher rate of associated symptoms than males (p = 0.02), Table 3. Furthermore, patients with EM rash at the head and neck had a higher rate of associated symptoms (79%, p < 0.001), even considering only adult patients (p < 0.001) with 90% of patients with EM at the head and neck developing associated symptoms.
Patients without EM, with other symptoms suggestive of LB, were 317 (25%). Considering the place of residence, the presence of associated symptoms was higher in patients from central Italy (85%) than that in northern Italy (68%) (p = 0.002), as depicted in Figure 3. In northern Italy, 348 (32%) patients had EM without associated symptoms, while they dropped down to 15% (14 out of 96) in central Italy and 25% (18 out of 71) in southern Italy (p = 0.001).

FIGURE 3. Distribution of patients according to the presence of associated symptoms per geographical areas, namely, northern, central, and southern Italy with islands.
Overall, in 880 patients (70%), associated symptoms occurred as reported in Table 3.
In patients with associated symptoms, skin lesions other than EM were significantly higher in patients with EM (p < 0.001), while articular (p < 0.001), muscular (p < 0.001), and neurological (p < 0.001) symptoms were significantly higher in patients without EM. On the contrary, fever (p = 0.7), ocular (p = 0.4), and cardiac symptoms (p = 0.07) did not seem to be associated with the development of EM.
Serological tests were carried out in 947 patients (75% of the entire cohort). The rate of positive serology (both IgG or IgM) did not vary with gender (p = 0.3) and with the patients’ residence (p = 0.1). Serological tests were performed in 687 out of 943 patients with EM (73%) and in 303 out of 313 (96%) patients without EM. Since EM is pathognomonic for LB, there is no need to perform serological tests when EM is diagnosed (Schriefer, 2015). This is the main reason why serological tests were not performed in a certain number of patients with EM rashes. The results on serology are reported in Table 4.
In patients with EM, the duration of the disease varied significantly concerning serological results (p = 0.02) with a mean value of 2 months in those with negative serological tests. The same test in patients without EM did not show any significant result (p = 0.7).
In 14 patients (12 women and 2 men) without EM and negative serological results, LB diagnosis was based on a positive Borrelia detection by PCR. In seven cases, PCR was carried out in DNA from blood, in one from eye swab, in two cases from CSF, and in four from skin biopsies, namely, two primary cutaneous B-cell marginal zone lymphoma (PCMZL) (Gatti et al., 2014), one roseolar erythema, and one interstitial granulomatous dermatitis (IGD) (di Meo et al., 2015).
The therapeutical regimen was based on the use of the following antibiotics given per os: amoxicillin (1 g 3 times die, 14–21 days); POM penicillin (1*106 UI 3 times die, 14–21–28 days); doxycycline (100 mg 2 times die, 14–21–28 days); minocycline (100 mg 2 times die, 14–21-28 days); cefuroxime (500 mg 2 times die, 14–21–28 days); azithromycin (500 mg 1 die, 7–9 days); clarithromycin (500 mg 2 times die, 14–21–28 days). Ceftriaxone (2–3 g 1 die, 14–21–28 days) and penicillin G (5–6*106 UI 4 times a die, 14 days) were given intravenously. The distribution of the therapeutical regimen differed in patients with EM from those without EM, as shown in Table 5 (p < 0.001). EM patients were mostly treated with amoxicillin and doxycycline, while those without EM had a higher rate of intravenous therapy.
The therapeutical regimen differed concerning the geographic area of residence, as shown in Table 6 (p < 0.001). This is mostly due to the occurrence of associated symptoms that varied significantly with the geographic area of residence. Nevertheless, in early Lyme disease, amoxicillin was usually preferred in northern Italy, while doxycycline, in central and southern Italy. This difference was maintained even by the exclusion of pediatric patients for whom doxycycline is not recommended. In northern Italy, amoxicillin (40%) continued to prevail, while doxycycline, in central (42%) and southern Italy (28%). Data on adult patients also showed the highest rate in the use of intravenous therapy in central and southern Italy as a sign of late Lyme disease treatment.
This study is based on the collection of data from LB patients in different clinical centers for Lyme disease in Italy to obtain a picture of the disease throughout Italy. The centers involved in the study are located in northern Italy (6 centers: Friuli-Venezia Giulia, 2; Lombardy and Liguria, 2; Emilia-Romagna, 1) and southern Italy (Campania, 2) to cover the entire Italian territory. The observation period spanned between 2010 and 2022 to harmonize the collection of data since in southern Italy, in previous years, there was no particular surveillance on LB. In Europe, the most common tick-borne disease is LB (Marques et al., 2021). Its incidence is increasing in different Western European countries together with a geographical expansion of the disease into previously non-endemic areas (Beltrame et al., 2021). Although we are not describing the incidence of the disease, cases increased in the observation period with a maximum of observations in 2020, possibly due to the COVID pandemic and the use of telemedicine in diagnosing EM (Trevisan et al., 2022). In Italy, during the pandemic, patients could not reach hospitals for dermatological evaluation, and after a telephone interview with the doctors, they sent their pictures of the lesions by e-mail or showed them on a video call.
Most patients with LB in our cohort came from northern Italy, where LB has been reported with higher frequencies (Rimoldi et al., 2020; Beltrame et al., 2021). Nevertheless, certain cases were also diagnosed in central and southern Italy in agreement with the presence of Ixodes vectors and Borreliae in those areas (Stefanelli et al., 1994). Although the number of cases in central and southern Italy was significantly lower, our study highlights the presence of Lyme group Borrelia even there. Patients who became infected in different regions, where they lived, were very limited in our study (39), and 17 of them lived in southern Italy. This means that 24% of LB patients diagnosed in southern Italy in our cohort were infected in other countries (9) or in different Italian regions, but the residual 76% became infected in their region, documenting the presence of Lyme group Borreliae. Although not in humans, seroprevalence of B. burgdorferi in stray dogs, as sentinel animals for tick-borne infection, has been described in Sicily (Galluzzo et al., 2020) and B. burgdorferi sensu stricto and B. afzelii were identified in Ixodes ticks in southern Italy and islands (Zanet et al., 2020) and in central Italy (Pascucci and Cammà, 2010; Mancini et al., 2019) in agreement with our findings. Regarding the clinical manifestations, 85% of patients from central Italy had LB with associated symptoms documenting LB at least in an early disseminated stage. It is, indeed, well recognized that in non-endemic areas, LB diagnosis could be elusive (Maxwell, 2020) with the possible consequence of the dissemination of the disease from a local stage to an early or late disseminated phase.
Another significant geography-related difference in this study is the therapeutic regimen: in northern Italy, most patients were treated with amoxicillin, while in central and southern Italy, with doxycycline, and this was confirmed even by the exclusion of pediatric patients for whom doxycycline is not recommended (Wormser et al., 2019). The difference in the antibiotic regimen is possibly related to the diffusion in central and southern Italy of the Rickettsia species (Scaffidi, 1981; Selmi et al., 2017), for which the treatment of choice is doxycycline (Spernovasilis et al., 2021). Furthermore, patients treated intravenously with penicillin G or ceftriaxone were considerably higher in central (19%) and southern Italy (28%) when compared to northern Italy (10%), supporting for a diagnosis of late LB. Patients presenting with EM as the only LB symptom prevailed, indeed, in northern Italy. Therefore, difficulties in the diagnosis in non-endemic areas together with the lack of information on LB among inhabitants could explain those results. On the contrary, the recollection of the tick bite was higher in inhabitants of southern Italy and islands. Patients also differed for age at diagnosis, with older patients in northern Italy than central and southern Italy, which is in line with the differences in age among Italian region inhabitants (http://www.comuni-italiani.it/statistiche/eta.html, accessed 16/12/2022 2022). The mean age in northern Italy is, indeed, reported to be higher than that in southern Italy, explaining our findings. The body location of EM rashes confirmed previous reports with the prevailing site in adults being the lower limbs, followed by the trunk (Rebman et al., 2021). Anyway, in pediatric patients, EM rashes were differently distributed with similar occurrences in the head and neck, trunk, and lower limbs, as already found (Backman and Skogman, 2018).
EM rashes at the head and neck resulted in a higher risk of developing additional symptoms in LB in our cohort in agreement with other reports, both in children (Backman and Skogman, 2018) and adults (Ogrinc et al., 2022).
Overall, the collection of data from eight clinical centers in different geographic areas in Italy documented the presence of LB even in southern Italy and islands, although with considerably lower rates than northern Italy, where LB is endemic in certain regions. Nevertheless, we acknowledge that the main limitation to this study is the incomplete information underestimating LB in Italy, especially for certain regions where LB is endemic (i.e., Trentino-Alto Adige, Piemonte, and Valle d’Aosta).
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
GT, MR, KN, PF, ND, PT, MN, ST, SG, VG, MR, DM, SA, PM, and GS, data collection and manuscript revision; GT and SB, data curation and manuscript drafting; SB, statistical analyses. All authors contributed to the article and approved the submitted version.
The authors thank the “Associazione Lyme Italia e coinfezioni” for the technical support and Dr. Nausicaa De Rosa for drawing Figure 1.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Backman, K., and Skogman, B. H. (2018). Occurrence of erythema migrans in children with Lyme neuroborreliosis and the association with clinical characteristics and outcome - a prospective cohort study. BMC Pediatr. 18 (1), 189. doi:10.1186/s12887-018-1163-2
Beltrame, A., Rodari, P., Mauroner, L., Zanella, F., Moro, L., Bertoli, G., et al. (2021). Emergence of Lyme borreliosis in the province of verona, northern Italy: Five-years of sentinel surveillance. Ticks Tick. Borne Dis. 12 (2), 101628. doi:10.1016/j.ttbdis.2020.101628
Bertolotti, L., Tomassone, L., Tramuta, C., Grego, E., Amore, G., Ambrogi, C., et al. (2006). Borrelia lusitaniae and spotted fever group rickettsiae in Ixodes ricinus (Acari: Ixodidae) in Tuscany, central Italy. J. Med. Entomol. 43 (2), 159–165. doi:10.1603/0022-2585(2006)043[0159:blasfg]2.0.co;2
Bonin, S., Stinco, G., Patriarca, M. M., Trevisini, S., di Meo, N., and Trevisan, G. (2016). Could co-infection with Anaplasma play a role in Borrelia-associated primary cutaneous marginal zone B-cell lymphomas? Indian J. Dermatol Ve 82 (1), 81–84. doi:10.4103/0378-6323.171011
Cacciapuoti, B., Ciceroni, L., Ciarrocchi, S., Khoury, C., and Simeoni, J. (1995). Genetic and phenotypic characterization of Borrelia burgdorferi strains isolated from Ixodes ricinus ticks in the Province of Bolzano, Italy. Italy. new Microbiol. 18 (2), 169–181.
Ciceroni, L., Ciarrochi, S., Ciervo, A., Mondarini, V., Guzzo, F., Caruso, G., et al. (2001). Isolation and characterization of Borrelia burgdorferi sensu lato strains in an area of Italy where Lyme borreliosis is endemic. J. Clin. Microbiol. 39 (6), 2254–2260. doi:10.1128/JCM.39.6.2254-2260.2001
Cimmino, M. A., Fumarola, D., Sambri, V., and Accardo, S. (1992). The epidemiology of Lyme borreliosis in Italy. Microbiologica 15 (4), 419–424.
Cinco, M., Balanzin, D., Trevisan, G., and Benussi, P. (1993). Seroprevalence and incidence of Lyme borreliosis in forestry workers in friuli-venezia Giulia (northern Italy). Alpe Adria Microbiol. J. 2, 91–98.
Cinco, M., Banfi, E., Trevisan, G., and Stanek, G. (1989). Characterization of the first tick isolate of Borrelia burgdorferi from Italy. APMIS 97 (4), 381–382. doi:10.1111/j.1699-0463.1989.tb00804.x
Cinco, M., Padovan, D., Murgia, R., Poldini, L., Frusteri, L., van de Pol, I., et al. (1998). Rate of infection of Ixodes ricinus ticks with Borrelia burgdorferi sensu stricto, Borrelia garinii, Borrelia afzelii and group VS116 in an endemic focus of Lyme disease in Italy. Eur. J. Clin. Microbiol. Infect. Dis. official Publ. Eur. Soc. Clin. Microbiol. 17 (2), 90–94. doi:10.1007/BF01682162
Cinco, M., Trevisan, G., and Agolzer, A. (1992). Isolation of Borrelia burgdorferi from a Lyme seronegative patient in northern Italy: Expression of OspB immunodominant proteins on the isolated strain. Microbiologica 15 (2), 95–98.
Crovato, F., Nazzari, G., Fumarola, D., Rovetta, G., Cimmino, M. A., and Bianchi, G. (1985). Lyme disease in Italy: First reported case. Ann. rheumatic Dis. 44 (8), 570–571.
di Meo, N., Stinco, G., and Trevisan, G. (2015). Interstitial granulomatous dermatitis due to borreliosis. Indian J. Dermatol Venereol. Leprol. 81 (3), 327. doi:10.4103/0378-6323.154783
Eldin, C., Raffetin, A., Bouiller, K., Hansmann, Y., Roblot, F., Raoult, D., et al. (2019). Review of European and American guidelines for the diagnosis of Lyme borreliosis. Med. Mal. Infect. 49 (2), 121–132. doi:10.1016/j.medmal.2018.11.011
Eliassen, K. E., Berild, D., Reiso, H., Grude, N., Christophersen, K. S., Finckenhagen, C., et al. (2017). Incidence and antibiotic treatment of erythema migrans in Norway 2005-2009. Ticks Tick. Borne Dis. 8 (1), 1–8. doi:10.1016/j.ttbdis.2016.06.006
Fazii, P., Ballone, E., Ippolito, N., Cosentino, L., Clerico, L., Galella, G., et al. (2000). Survey of Lyme disease in Abruzzo (Italy). Int. J. Immunopathol. Pharmacol. 13 (3), 151–156.
Frediani, T., Lucarelli, S., Ferruzzi, M. F., De Gregorio, P., D'Eufemia, P., Barbato, M., et al. (1993). Lyme disease in central Italy. Description of a case of a 3-year-old girl. Pediatr. Med. Chir. 15 (5), 531–533.
Fumarola, D., Munno, I., Miragliotta, G., and Marcuccio, C. (1985). Lyme arthritis: Does endotoxin play a role? Eur. J. Clin. Microbiol. 4 (4), 440. doi:10.1007/BF02148711
Gaddoni, G., Benini, F., Martinelli, M., Pavan, W. O., Marangoni, A., and Sambri, V. (1998). Lyme borreliosis in the emilia-romagna region (Italy): Identification of the etiological agent. J. Eur. Acad. Dermatol Venereol. 10 (1), 86–87. doi:10.1111/j.1468-3083.1998.tb00936.x
Galluzzo, P., Grippi, F., Di Bella, S., Santangelo, F., Sciortino, S., Castiglia, A., et al. (2020). Seroprevalence of Borrelia burgdorferi in stray dogs from southern Italy. Microorganisms 8 (11), 1688. doi:10.3390/microorganisms8111688
Gatti, A., Stinco, G., Trevisini, S., di Meo, N., Signoretto, D., Leonardo, E., et al. (2014). Electrochemotherapy as a novel treatment for primary cutaneous marginal zone B-cell lymphomas. Dermatol. Ther. 27 (4), 244–247. doi:10.1111/dth.12128
Ivacic, L., Reed, K. D., Mitchell, P. D., and Ghebranious, N. (2007). A LightCycler TaqMan assay for detection of Borrelia burgdorferi sensu lato in clinical samples. Diagn Microbiol. Infect. Dis. 57 (2), 137–143. doi:10.1016/j.diagmicrobio.2006.08.005
Jenkins, A., Hvidsten, D., Matussek, A., Lindgren, P. E., Stuen, S., and Kristiansen, B. E. (2012). Borrelia burgdorferi sensu lato in Ixodes ricinus ticks from Norway: Evaluation of a PCR test targeting the chromosomal flaB gene. Exp. Appl. Acarol. 58 (4), 431–439. doi:10.1007/s10493-012-9585-2
Khoury, C., Manilla, G., and Maroli, M. (1994). Parasitic horse ticks in Italy. Observations on their distribution and pathogenic role. Parassitologia 36 (3), 273–279.
Lardieri, G., Salvi, A., Camerini, F., Cinco, M., and Trevisan, G. (1993). Isolation of Borrelia burgdorferi from myocardium. Lancet 342 (8869), 490. doi:10.1016/0140-6736(93)91612-p
Mancini, F., Vescio, M. F., Toma, L., Di Luca, M., Severini, F., Caccio, S. M., et al. (2019). Detection of tick-borne pathogens in ticks collected in the suburban area of Monte Romano, Lazio Region, Central Italy. Ann. Ist. Super. Sanita 55 (2), 143–150. doi:10.4415/ANN_19_02_06
Marques, A. R., Strle, F., and Wormser, G. P. (2021). Comparison of Lyme disease in the United States and Europe. Emerg. Infect. Dis. 27 (8), 2017–2024. doi:10.3201/eid2708.204763
Maxwell, S. P. (2020). The elusive understanding of Lyme disease in non-endemic geographic areas: An exploratory survey of patients with chronic symptoms in Texas. J. Patient Exp. 7 (6), 1621–1626. doi:10.1177/2374373520926821
Merler, S., Furlanello, C., Chemini, C., and Nicolini, G. (1996). Classification tree methods for analysis of mesoscale distribution of Ixodes ricinus (Acari:Ixodidae) in Trentino, Italian Alps. J. Med. Entomol. 33 (6), 888–893. doi:10.1093/jmedent/33.6.888
Miele, A., Tozza, S., Trevisan, G., Bonin, S., Sansone, M., Salvatore, P., et al. (2022). Garin-bujadoux-bannwarth meningoradiculoneuritis: A case report. Ann. Case Rep. 7 (5), 933. doi:10.29011/2574-7754.100933
Ogrinc, K., Kastrin, A., Lotric-Furlan, S., Bogovic, P., Rojko, T., Maraspin, V., et al. (2022). Colocalization of radicular pain and erythema migrans in patients with bannwarth Syndrome suggests a direct spread of Borrelia into the central nervous system. Clin. Infect. Dis. official Publ. Infect. Dis. Soc. Am. 75 (1), 81–87. doi:10.1093/cid/ciab867
Pascucci, I., and Cammà, C. (2010). Lyme disease and the detection of Borrelia burgdorferi genospecies in Ixodes ricinus ticks from central Italy. Veterinaria Ital. 46 (2), 173–180, 181-188.
Pauluzzi, P., Bonin, S., Gonzalez Inchaurraga, M. A., Stanta, G., and Trevisan, G. (2004). Detection of spirochaetal DNA simultaneously in skin biopsies, peripheral blood and urine from patients with erythema migrans. Acta dermato-venereologica 84 (2), 106–110. doi:10.1080/00015550310006815
Petrulioniene, A., Radzisauskiene, D., Ambrozaitis, A., Caplinskas, S., Paulauskas, A., and Venalis, A. (2020). Epidemiology of Lyme disease in a highly endemic European zone. Med. Kaunas. 56 (3), 115. doi:10.3390/medicina56030115
Rebman, A. W., Yang, T., Mihm, E. A., Novak, C. B., Yoon, I., Powell, D., et al. (2021). The presenting characteristics of erythema migrans vary by age, sex, duration, and body location. Infection 49 (4), 685–692. doi:10.1007/s15010-021-01590-0
Rimoldi, S. G., Merli, S., Bestetti, G., Giacomet, V., Cislaghi, G., Grande, R., et al. (2020). Occurrence of Lyme disease infection in a non-endemic area in Northern Italy. G. Ital. Dermatol Venereol. 155 (3), 320–324. doi:10.23736/S0392-0488.18.05941-2
Rinaldi, R., Gabellini, A. S., Procaccio, L., Benassi, G., and D'Alessandro, R. (1991). Lyme disease. First reported case in Sicily. Ital. J. Neurol. Sci. 12 (1), 105–107. doi:10.1007/BF02337622
Ruata, G., Roggia, F., De Angelis, M. S., Piras, M. R., D'Onofrio, M., and Mutani, R. (1992). Neuroborreliosis: A Sardinian case with cerebellar symptoms. Ital. J. Neurol. Sci. 13 (3), 271–274. doi:10.1007/BF02224403
Santino, I., Dastoli, F., Lavorino, C., Navazio, M., Nicosia, R., Oliveti, A., et al. (1996). Determination of antibodies to Borrelia burgdorferi in the serum of patients living in Calabria, southern Italy. Panminerva Med. 38 (3), 167–172.
Scaffidi, V. (1981). Current endemic expansion of boutonneuse fever in Italy. Minerva Med. 72 (31), 2063–2070.
Schriefer, M. E. (2015). Lyme disease diagnosis: Serology. Clin. Lab. Med. 35 (4), 797–814. doi:10.1016/j.cll.2015.08.001
Schwartz, A. M., Hinckley, A. F., Mead, P. S., Hook, S. A., and Kugeler, K. J. (2017). Surveillance for Lyme disease - United States, 2008-2015. MMWR Surveill. Summ. 66 (22), 1–12. doi:10.15585/mmwr.ss6622a1
Selmi, M., Ballardini, M., Salvato, L., and Ricci, E. (2017). Rickettsia spp. in dermacentor marginatus ticks: Analysis of the host-vector-pathogen interactions in a northern mediterranean area. Exp. Appl. Acarol. 72 (1), 79–91. doi:10.1007/s10493-017-0132-z
Spernovasilis, N., Markaki, I., Papadakis, M., Mazonakis, N., and Ierodiakonou, D. (2021). Mediterranean spotted fever: Current knowledge and recent advances. Trop. Med. Infect. Dis. 6 (4), 172. doi:10.3390/tropicalmed6040172
Stark, J. H., Pilz, A., Jodar, L., and Moisi, J. C. (2023). The epidemiology of Lyme borreliosis in Europe: An updated review on a growing public Health issue. Vector Borne Zoonotic Dis. 23 (4), 139–141. doi:10.1089/vbz.2022.0068
Stefanelli, S., Paladini, A., Conforti, P. L., Leoncini, F., Viganò, S., De Giovannini, R., et al. (1994). Isolation of Borrelia burgdorferi in Tuscany (Italy). new Microbiol. 17 (4), 333–336.
Trevisan, G., Cinco, M., and Agolzer, A. (1992). Roseolar lesions in Lyme disease: Isolation of the causative agent. Int. J. Dermatol 31 (7), 507–508. doi:10.1111/j.1365-4362.1992.tb02703.x
Trevisan, G., Cinco, M., Trevisini, S., di Meo, N., Chersi, K., Ruscio, M., et al. (2021). Borreliae Part 1: Borrelia Lyme Group and Echidna-Reptile Group. Biology 10 (10), 1036. doi:10.3390/biology10101036
Trevisan, G., Crovato, F., Marcuccio, C., Fumarola, D., and Scarpa, C. (1987). Lyme disease in Italy. Zentralbl Bakteriol. Mikrobiol. Hyg. Abt 1 Orig. A 263 (3), 459–463. doi:10.1016/s0176-6724(87)80108-6
Trevisan, G., Nan, K., di Meo, N., and Bonin, S. (2022). The impact of telemedicine in the diagnosis of erythema migrans during the COVID pandemic: A comparison with in-person diagnosis in the pre-COVID era. Pathogens 11 (10), 1122. doi:10.3390/pathogens11101122
Trevisan, G., Pavan, W., and Rorai, E. (1991). Malattia di Lyme: Segnalazione di un caso a Cavarzere (Venezia). G. Ital. Dermatol. Venereologia 126, 435–437.
Trevisan, G. (1986). Malattia di Lyme: A propositi di un caso. Ann. Ital. Dermatol. Clinica Sper. 40, 91–95.
Vandekerckhove, O., De Buck, E., and Van Wijngaerden, E. (2021). Lyme disease in western Europe: An emerging problem? A systematic review. Acta Clin. Belg 76 (3), 244–252. doi:10.1080/17843286.2019.1694293
Wormser, G. P., Wormser, R. P., Strle, F., Myers, R., and Cunha, B. A. (2019). How safe is doxycycline for young children or for pregnant or breastfeeding women? Diagn Microbiol. Infect. Dis. 93 (3), 238–242. doi:10.1016/j.diagmicrobio.2018.09.015
Keywords: Lyme borreliosis, erythema migrans, associated symptoms, antibiotic therapy, Lyme in Italy
Citation: Trevisan G, Ruscio M, Cinco M, Nan K, Forgione P, Di Meo N, Tranchini P, Nacca M, Trincone S, Rimoldi SG, Giacomet V, Ricci M, Melandri D, Artioli S, Monteforte P, Stinco G and Bonin S (2023) The history of Lyme disease in Italy and its spread in the Italian territory. Front. Pharmacol. 14:1128142. doi: 10.3389/fphar.2023.1128142
Received: 20 December 2022; Accepted: 07 June 2023;
Published: 16 June 2023.
Edited by:
Joanna Zajkowska, Medical University of Bialystok, PolandReviewed by:
Juan Carlos Sepúlveda-Arias, Technological University of Pereira, ColombiaCopyright © 2023 Trevisan, Ruscio, Cinco, Nan, Forgione, Di Meo, Tranchini, Nacca, Trincone, Rimoldi, Giacomet, Ricci, Melandri, Artioli, Monteforte, Stinco and Bonin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giusto Trevisan, dHJldmlzYW5AdW5pdHMuaXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.